Abstract
Objective: To observe the effects of baicalin capsules combined with α-lipoic acid on nerve conduction velocity, oxidative stress and inflammatory injury in patients with diabetic peripheral neuropathy. Methods: A total of 96 patients with diabetic peripheral neuropathy who received treatment in our hospital were divided into the control group (CG) and the research group (SG) by a randomized double-blind method, with 48 cases in each group. In the CG, patients were treated with α-lipoic acid. In the SG, patients were treated with baicalin capsules combined with α-lipoic acid. The clinical efficacy, symptoms (neuropathic subjective symptom questionnaire (TSS) score), nerve conduction velocity, oxidative stress indicators (superoxide dismutase (SOD) and malondialdehyde (MDA)), inflammatory injury indicators (hs-C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor (TNF-α)) and adverse reactions were compared between the two groups. Results: The clinical efficacy in the SG was higher than that in the CG (P<0.05). After treatment, the TSS score and the levels of serum SOD, MDA, TNF-α, IL-6 and CRP decreased in both groups, and the SG was lower than the CG (P<0.05). After treatment, the motor conduction velocity (MCV) and sensory conduction velocity (SCV) velocities of the tibial nerve and common peroneal nerve increased in both groups, and the SG was higher than the CG (P<0.05). There was no statistical difference in adverse drug reactions between the two groups (P>0.05). Conclusion: Compared with α-lipoic acid monotherapy, baicalin capsules combined with α-lipoic acid are effective in treating patients with diabetic peripheral neuropathy, this treatment can obviously relieve the clinical symptoms of patients, improve nerve conduction velocity, reduce oxidative stress and inflammatory injury, and it does not increase adverse reactions. Therefore, it is worthy of promotion.
Keywords: Diabetic peripheral neuropathy, nerve conduction velocity, oxidative stress, inflammatory injury, α-lipoic acid, baicalin capsule
Introduction
Diabetic peripheral neuropathy (DPN) is a distal symmetric polyneuropathy induced by long-term poor control of blood sugar. Patients often develop acroparesthesia, limb numbness, limb pain and other uncomfortable symptoms, and the incidence rate can reach 30%-90% [1]. Modern medicine believes that diabetes patients with long-term hyperglycemia will turn into metabolism disorders of the blood lipids, thickening of capillary basement membranes, vascular intima hyperplasia, and swelling of blood vessels and endothelial cells, which causes glycoprotein deposition on the blood vessel walls resulting in stenosis and microcirculation disturbance, and finally lead to ischemia and hypoxia of nerve tissue which induces DPN [2,3]. α-lipoic acid, as a common therapeutic drug for diabetes, it can inhibit aldose reductase, prevent protein glycosylation and inhibit the conversion of glucose into sorbitol by inhibiting lipid oxidation in nerve tissue, thus preventing neuropathy caused by hyperglycemia [4]. α-lipoic acid can effectively relieve the clinical symptoms of patients with DPN, but it is not effective in some patients. Some studies by Zhang Liyun et al. have revealed that the total effective rate of improvement in DPN patients treated with α-lipoic acid wis only 74.19%, which is far lower than that of patients treated with Jiawei Buyang Huanwu Decoction combined with α-lipoic acid (91.93%) [5].
The main component of baicalin capsules is baicalin, which is a flavonoid compound. It is antibacterial, diuretic, anti-inflammatory and can inhibit aldose reductase. It can also dilate blood vessels, promote insulin secretion, lower blood sugar, regulate endocrine function, improve body immunity and enhance antiviral ability [6]. Baicalin can also inhibit the precipitation of melanin in the body, prevent oxidation and aging, scavenge free radicals, and reduce the damage of reactive oxygen species to the human body [7]. Studies by Zhang Yingying et al. have revealed that baicalin capsules can effectively regulate the nerve function of mice with acute ocular hypertension and optic nerve injury [8]. At present, there is no report on the treatment of DPN with baicalin capsules. In view of this, this study aimed to analyze the effects of α-lipoic acid combined with baicalin capsules on nerve conduction velocity, oxidative stress and inflammatory injury in patients with DPN, so as to provide a reference for a better clinical treatment development plan for DPN.
Materials and methods
Baseline data
From August 2018 to May 2019, 96 patients with diabetic peripheral neuropathy who were admitted to Chongqing Traditional Chinese Medicine Hospital were randomly divided into the control group (CG) and research group (SG) by a randomized double-blind method, with 48 cases in each group. This study was approved by the Medical Ethics Committee in Chongqing Traditional Chinese Medicine Hospital. The included patients were informed and gave their consent.
Inclusion criteria
Inclusion criteria: All of them met the diagnostic criteria of diabetes in the Chinese Guidelines for Prevention and Treatment of Type 2 Diabetes (2013 Edition), and they were diagnosed with peripheral neuropathy by electromyography, which was mainly characterized by body cooling, weakened tendon reflex, limb numbness, stinging pain, etc. The patient’s mental cognition was normal [9].
Exclusion criteria were as follows: type I diabetes; peripheral neuropathy induced by other reasons; comorbid with severe organ dysfunction; comorbid with edema, foot ulcer and infection; patients who had taken neurotrophic drugs within two weeks; patients who were allergic to the research drugs; abnormal coagulation function or immune system; patients who did not follow through to the end of the study.
Methods
In both groups, patients received routine glucose-lowering treatment according to the doctor’s advice. Patients were subcutaneously injected with insulin (Hefei Tianmai Biotechnology Development Co., Ltd., China) for blood glucose control (FPG <7.0 mmol/L, 2 hpg <10.0 mmol/L). The specific dosage was changed according to the patients’ blood sugar control. If necessary, anti-infection treatment was conducted, and a reasonable dietary plan and exercise plan were formulated for the patients.
In the two groups, patients were treated with drugs on this basis. CG: In the CG, patients were treated with α-lipoic acid (Jiangsu Shenlong Pharmaceutical Co., Ltd., China), and normal saline (250 mL) was added into the injection (20 mL) for intravenous drip, once a day. SG: On the basis of the CG, patients in the SG were given baicalin capsules (Dongguan Jinmeiji Pharmaceutical Co., Ltd., China), orally, 2 capsules/time, 3 times/day. In the two groups, patients were treated continuously for 8 weeks.
Outcome measures
Main outcome measures
(1) After treatment for 8 weeks, the clinical efficacy was evaluated according to Consensus on the Diagnosis and Treatment of Diabetic Peripheral neuropathy [10]. Cure: The clinical symptoms and signs disappeared completely, the tendon reflex returned to normal, and the blood sugar decreased by 30%. Markedly effective: The clinical symptoms and signs improved obviously, the tendon reflex returned to normal, and the blood sugar decreased by 20%. Effective: The clinical symptoms and signs did not disappear completely, the tendon reflex recovered generally, and the blood sugar decreased by 10%. Ineffective: The clinical symptoms and signs were not improved but aggravated, the tendon reflex did not recovered, and the reduction of blood sugar did not reach the recovery standard. Total effective rate = (cure + markedly effective + effective)/total cases × 100%.
(2) Before and after treatment for 8 weeks, the neuropathic subjective symptom questionnaire (TSS) scores were assessed by the TSS, which included four symptoms: paresthesia of lower limbs and feet, numbness, burning heat sensation and pain [11]. According to the seizure frequency, severity and duration of each symptom, a score of 0-3.66 was applied. The higher the score was, the more serious the symptoms.
(3) Nerve conduction velocity before and after treatment for 8 weeks: The nerve conduction velocity of patients (either left or right side) was measured by electromyography (Shanghai Hanfei Medical Equipment Co., Ltd., China), including motor conduction velocity (MCV) and sensory conduction velocity (SCV) of the ulnar nerve, median nerve and common peroneal nerve. Examination process: The electrode was inserted into the muscle, and the biological current was amplified under the resting state of muscle contraction, and MCV and SCV were displayed by cathode ray oscilloscope.
Secondary outcome measures
Before and after treatment for 8 weeks, fasting venous blood (4 mL) was drawn from patients and centrifuged at 3000 r/min for approximately 5 min. The serum was separated and stored for examination.
(1) Oxidative stress indexes: The levels of superoxide dismutase (SOD) and malondialdehyde (MDA) were detected by chemical colorimetry, and the kits were all purchased from Shanghai Yanhui Biotechnology Co., Ltd., China.
(2) Inflammatory injury indexes: The level of serum C-reactive protein (CRP) was measured by immune scattering turbidimetry with automatic biochemical analyzer (Shenyang Wan Tai Medical Equipment Co., Ltd., China). The level of interleukin-6 (IL-6) was detected by radioimmunoassay with radioimmunoassay counter (Anhui Zhongke Zhongjia Scientific Instrument Co., Ltd., China). The level of tumor necrosis factor (TNF-α) was determined by enzyme-linked immunosorbent assay (ELISA) with IAMMGE (Beckman, USA).
(3) Adverse reactions: The adverse reactions of all patients were recorded during treatment, including headache, dyspnea, convulsions and other adverse reactions.
Statistical methods
SPSS 21.0 was used for statistical analysis. The measurement data were expressed in (x̅ ± sd). The independent sample t test was used for comparison between groups. The paired sample t test was used for the comparison before and after the experiment within the group. The counting data were expressed in percentage and tested by χ2 test. The Rank sum test was used for ranked data. The difference was statistically significant with P<0.05.
Results
Baseline data
There was no statistically significant difference in terms of gender, age, course of diabetes, course of diabetic peripheral neuropathy and complications between the two groups (P>0.05). This revealed that the baseline data were comparable in the two groups. See Table 1.
Table 1.
Baseline data ((x̅ ± sd), %)
| Group | Control group (n=48) | Research group (n=48) | χ2/t | P |
|---|---|---|---|---|
| Gender (male/female) | 27/21 | 25/23 | χ2=0.168 | 0.682 |
| Age (years) | 68.2±5.3 | 68.4±5.1 | t=0.188 | 0.851 |
| Course of diabetes (years) | 10.6±2.3 | 10.5±2.1 | t=0.222 | 0.824 |
| Course of diabetic peripheral neuropathy (years) | 4.6±0.4 | 4.6±0.5 | t=0.000 | 1.000 |
| Complications (%) | ||||
| Hypertension | 29 (60.42) | 27 (56.25) | χ2=0.171 | 0.679 |
| Hyperlipidemia | 24 (50.00) | 24 (50.00) | χ2=0.000 | 1.000 |
Clinical efficacy
The total effective rate in the SG (97.92%) was higher than that in the CG (83.33%) (P=0.014). This revealed that α-lipoic acid combined with baicalin capsules was helpful to improve the clinical treatment efficacy of patients with diabetic peripheral neuropathy. See Table 2.
Table 2.
Clinical efficacy (n, %)
| Group | Control group (n=48) | Research group (n=48) | Z/χ2 | P |
|---|---|---|---|---|
| Cure | 8 (16.67) | 14 (29.17) | Z=3.274 | 0.001 |
| Markedly effective | 20 (41.67) | 30 (62.50) | ||
| Effective | 12 (25.00) | 3 (6.25) | ||
| Ineffective | 8 (16.67) | 1 (2.08) | ||
| The total effective rate | 40 (83.33) | 47 (97.92) | χ2=6.008 | 0.014 |
TSS score
Before treatment, there was no statistically significant difference in TSS score between the two groups (P>0.05). After treatment, the scores of paresthesia, numbness, burning heat sensation and pain in the two groups were significantly lower than those before treatment (P<0.001), and the above indexes in the SG were lower than those in the CG after treatment (P<0.01). This revealed that α-lipoic acid combined with baicalin capsules was more beneficial to improve the clinical symptoms of patients with diabetic peripheral neuropathy. See Table 3 and Figure 1.
Table 3.
Comparison of TSS score ((x̅ ± sd), score)
| Group | Before treatment | After treatment | t | P |
|---|---|---|---|---|
| Paresthesia of lower limbs and feet | ||||
| Control group (n=48) | 2.05±0.62 | 1.05±0.46 | 8.974 | <0.001 |
| Research group (n=48) | 2.17±0.69 | 0.75±0.31 | 13.006 | <0.001 |
| t | 0.896 | 3.747 | ||
| P | 0.373 | <0.001 | ||
| Numbness | ||||
| Control group (n=48) | 2.75±0.85 | 1.28±0.54 | 10.113 | <0.001 |
| Research group (n=48) | 2.66±0.78 | 0.84±0.34 | 14.819 | <0.001 |
| t | 0.540 | 4.777 | ||
| P | 0.590 | <0.001 | ||
| Burning heat sensation | ||||
| Control group (n=48) | 1.79±0.59 | 0.97±0.41 | 7.907 | <0.001 |
| Research group (n=48) | 1.73±0.57 | 0.67±0.28 | 11.564 | <0.001 |
| t | 0.507 | 4.186 | ||
| P | 0.613 | <0.001 | ||
| Pain | ||||
| Control group (n=48) | 2.45±0.69 | 1.01±0.45 | 12.111 | <0.001 |
| Research group (n=48) | 2.49±0.70 | 0.74±0.31 | 15.837 | <0.001 |
| t | 0.282 | 3.423 | ||
| P | 0.779 | 0.001 |
Note: TSS: neuropathic subjective symptom questionnaire.
Figure 1.
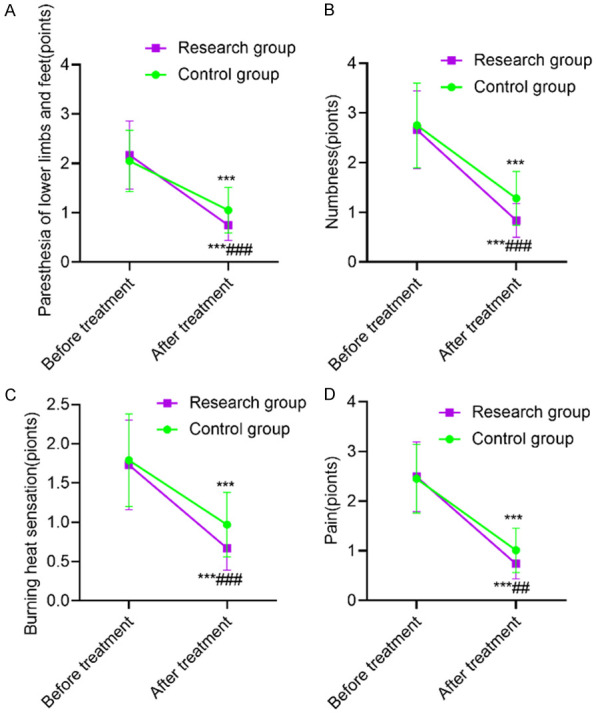
Comparison of TSS score. A: Paresthesia of lower limbs and feet; B: Numbness; C: Burning heat sensation; D: Pain. Compared with the same group before treatment, ***P<0.001; compared with the control group after treatment, ###P<0.001. TSS: neuropathic subjective symptom questionnaire.
Nerve conduction velocity
Before treatment, there was no statistically significant difference in nerve conduction velocity between the two groups (P>0.05). After treatment, the MCV and SCV velocities of the tibial nerve and common peroneal nerve in the SG were significantly higher than those before treatment in the same group (P<0.05), and the above indexes in the SG were higher than those in the CG after treatment (P<0.001). This revealed that α-lipoic acid combined with baicalin capsules was more beneficial to improve nerve conduction velocity of patients with diabetic peripheral neuropathy. See Table 4 and Figure 2.
Table 4.
Comparison of nerve conduction velocity ((x̅ ± sd), m/s)
| Group | Before treatment | After treatment | t | P | |
|---|---|---|---|---|---|
| Tibial nerve | |||||
| MCV | Control group (n=48) | 39.58±4.64 | 42.34±5.22 | 2.738 | 0.007 |
| Research group (n=48) | 40.33±5.77 | 47.68±6.78 | 5.720 | <0.001 | |
| t | 0.702 | 4.324 | |||
| P | 0.484 | <0.001 | |||
| SCV | Control group (n=48) | 37.65±4.72 | 40.42±4.98 | 2.797 | 0.006 |
| Research group (n=48) | 37.47±4.31 | 46.52±5.12 | 9.369 | <0.001 | |
| t | 0.195 | 5.917 | |||
| P | 0.846 | <0.001 | |||
| Common peroneal nerve | |||||
| MCV | Control group (n=48) | 41.21±3.43 | 43.21±4.43 | 2.473 | 0.015 |
| Research group (n=48) | 42.42±4.65 | 48.49±7.65 | 4.698 | <0.001 | |
| t | 1.451 | 4.138 | |||
| P | 0.150 | <0.001 | |||
| SCV | Control group (n=48) | 40.91±5.64 | 43.39±5.56 | 2.152 | 0.034 |
| Research group (n=48) | 40.16±5.73 | 47.24±6.63 | 5.598 | <0.001 | |
| t | 0.646 | 5.598 | |||
| P | 0.520 | <0.001 |
Note: MCV: motor conduction velocity; SCV: sensory conduction velocity.
Figure 2.
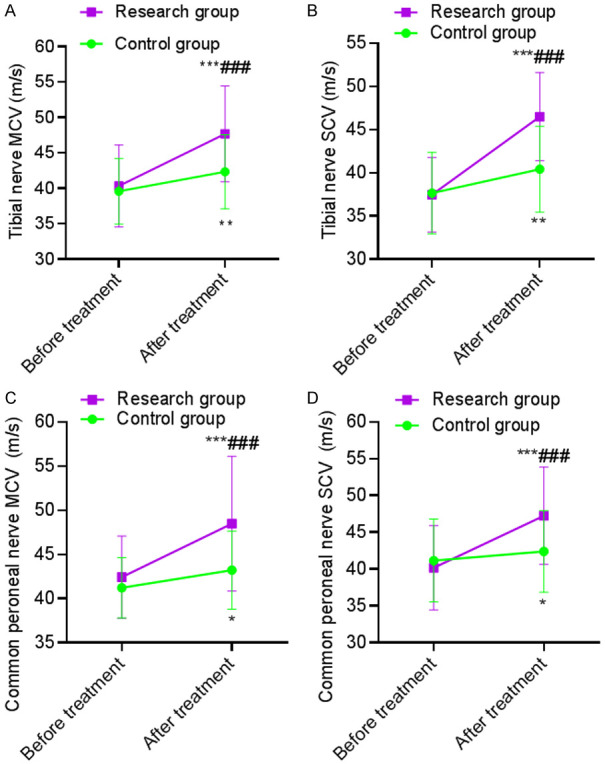
Comparison of nerve conduction velocity. A: MCV of tibial nerve; B: SCV of tibial nerve; C: MCV of common peroneal nerve; D: SCV of common peroneal nerve. Compared with the same group before treatment, ***P<0.001; compared with the control group after treatment, ###P<0.001. MCV: motor conduction velocity; SCV: sensory conduction velocity.
Oxidative stress indicators
Before treatment, there was no statistically significant difference in the levels of oxidative stress between the two groups (P>0.05). After treatment, the levels of SOD and MDA in the two groups were significantly lower than those before treatment in the same group (P<0.001), and the above indicators in the SG were lower than those in the CG after treatment (P<0.001). This revealed that α-lipoic acid combined with baicalin capsules was more beneficial to improve the oxidative stress response of patients with diabetic peripheral neuropathy. See Table 5 and Figure 3.
Table 5.
Comparison of oxidative stress indicators (x̅ ± sd)
| Group | Control group (n=48) | Research group (n=48) | t | P |
|---|---|---|---|---|
| SOD (U/mL) | ||||
| Before treatment | 71.65±10.52 | 70.36±9.14 | 0.641 | 0.523 |
| After treatment | 58.37±10.51*** | 47.74±9.46*** | 5.208 | <0.001 |
| MDA (U/L) | ||||
| Before treatment | 8.62±1.22 | 8.48±1.13 | 0.583 | 0.561 |
| After treatment | 6.74±0.74*** | 5.55±0.61*** | 8.597 | <0.001 |
Note: Compared with the same group before treatment;
P<0.001.
SOD: superoxide dismutase; MDA: malondialdehyde.
Figure 3.
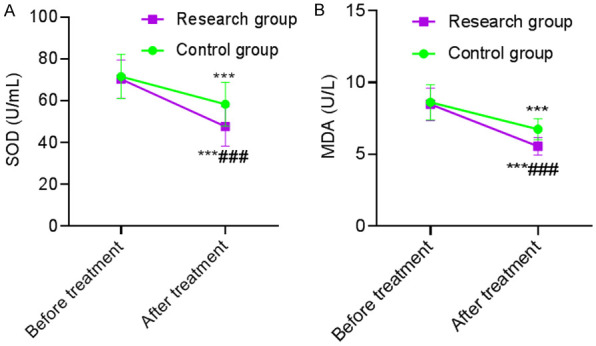
Comparison of oxidative stress indicators. A: SOD score; B: MDA score. Compared with the same group before treatment, ***P<0.001; compared with the control group after treatment, ###P<0.001. SOD: superoxide dismutase; MDA: malondialdehyde.
Inflammatory injury indexes
Before treatment, there was no statistically significant difference in the levels of inflammatory injury between the two groups (P>0.05). After treatment, the levels of TNF-α, IL-6 and CRP in the two groups were significantly lower than those before treatment in the same group (P<0.001), and the above indexes in the SG were lower than those in the CG after treatment (P<0.001). This revealed that α-lipoic acid combined with baicalin capsules were more beneficial to improve the inflammatory reaction of patients with diabetic peripheral neuropathy. See Table 6 and Figure 4.
Table 6.
Comparison of inflammatory injury indexes (x̅ ± sd)
| Group | Before treatment | After treatment | t | P |
|---|---|---|---|---|
| TNF-α (μg/mL) | ||||
| Control group (n=48) | 17.47±4.36 | 11.98±3.74 | 6.621 | <0.001 |
| Research group (n=48) | 17.50±4.31 | 8.36±3.04 | 12.006 | <0.001 |
| t | 0.034 | 5.204 | ||
| P | 0.973 | <0.001 | ||
| IL-6 (μg/mL) | ||||
| Control group (n=48) | 14.71±3.46 | 10.05±2.63 | 7.429 | <0.001 |
| Research group (n=48) | 14.93±3.52 | 6.08±2.53 | 14.144 | <0.001 |
| t | 0.309 | 7.537 | ||
| P | 0.758 | <0.001 | ||
| CRP (μg/mL) | ||||
| Control group (n=48) | 12.78±3.59 | 5.45±1.61 | 12.907 | <0.001 |
| Research group (n=48) | 13.69±3.86 | 3.61±1.05 | 17.458 | <0.001 |
| t | 1.196 | 6.632 | ||
| P | 0.235 | <0.001 |
Note: CRP: C-reactive protein; IL-6: interleukin-6; TNF-α: tumor necrosis factor.
Figure 4.
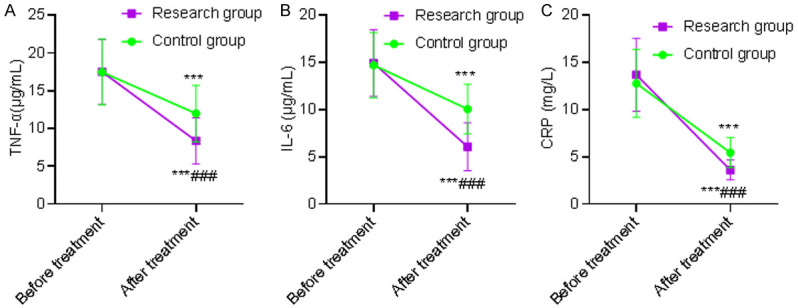
Comparison of inflammatory injury indexes. A: TNF-α; B: IL-6; C: CRP. Compared with the same group before treatment, ***P<0.001; compared with the control group after treatment, ###P<0.001. CRP: C-reactive protein; IL-6: interleukin-6; TNF-α: tumor necrosis factor.
Adverse reactions
There was no statistically significant difference in adverse drug reactions between the two groups (P>0.05). This revealed that α-lipoic acid combined with baicalin capsules was safe in the treatment of diabetic peripheral neuropathy. See Table 7.
Table 7.
Comparison of adverse reactions (n, %)
| Group | Control group (n=48) | Research group (n=48) | χ2 | P |
|---|---|---|---|---|
| Headache | 1 (2.08) | 1 (2.08) | 0.511 | 0.475 |
| Dyspnea | 1 (2.08) | 0 (0.00) | 0.000 | 1.000 |
| Convulsions | 0 (0.00) | 1 (2.08) | 0.000 | 1.000 |
| Other adverse reactions | 1 (2.08) | 1 (2.08) | 0.511 | 0.475 |
| Total incidence | 3 (6.25) | 3 (6.25) | 0.511 | 0.475 |
Discussion
In recent years, the number of patients with DPN is increasing in China, which seriously affects the quality of life of patients. How to treat this disease effectively is the focus of clinical work at present. In this study, α-lipoic acid combined with baicalin capsules was used to treat DPN. The results showed that the clinical efficacy in the SG was higher than that in the CG, the TSS score was lower than that in the CG, and the nerve conduction velocity was higher than that in the CG. This suggested that α-lipoic acid combined with baicalin capsules was more effective in the treatment of patients with DPN, and they had complementary advantages, which can effectively relieve clinical symptoms and improve nerve conduction velocity.
Clinical findings show that oxidative stress plays an important role in the development and progression of diabetic peripheral neuropathy. By alleviating oxidative stress, it can effectively alleviate patients’ illness and promote physical recovery [12,13]. Diabetic patients will have deposits of protein kinase C, advanced glycation end products and other substances on the blood vessel wall with long-term hyperglycemia, thus inducing oxidative stress reactions. The oxidative stress reaction can activate the apoptosis pathway of stress response element cells and growth factors, inhibit glucose metabolism and force cell apoptosis [14]. In this process, the transcription factors will be oxidized and modified to increase the expression of pro-apoptotic proteins, thus activating oxidative stress and redox sensitivity signaling pathways, and finally causing peripheral neuropathy or other chronic complications. When this oxidative stress reaction weakens the antioxidant system of the body, it will also cause the endocardium to produce more oxidative stress substances under the stimulation of high sugar, which cannot be excreted in time, and then produce toxic effects in the body, thus directly affecting nerve tissue, causing damage to patients’ neurons and slowing down nerve conduction velocity [15]. The results of this study showed that the levels of SOD and MDA decreased in both groups after treatment, and the levels in the SG were lower than those in the CG. This suggested that baicalin capsules combined with α-lipoic acid can effectively reduce oxidative stress in the treatment of patients with diabetic peripheral neuropathy.
The reason is that α-lipoic acid, as an oxidant, can improve the utilization rate of glucose in the body, inhibit nerve injury caused by hyperglycemia by suppressing lipid oxidation, repair damaged nerves at the same time, reduce pathological progress, and relieve clinical symptoms such as limb paresthesia, limb numbness and limb pain [16]. In addition, α-lipoic acid can inhibit oxidative stress reactions in the body by promoting the regeneration of vitamin C and vitamin E, improve nerve conduction velocity, increase blood flow of neurotrophic vessels, improve the function of vascular endothelial cells, and repair nerve function by enhancing nerve Na+-K+-ATPase activity and correcting neuropeptide defects [17,18]. Baicalin is the main component of baicalin capsules, and it is believed to have many functions, such as scavenging oxygen free radicals, promoting apoptosis and alleviating tissue ischemia-reperfusion injury. The free radical scavenging activity is obviously related to the number of phenolic hydroxyl groups. Compared with Scutellaria baicalensis Georgi, baicalin contains more phenolic hydroxyl groups, so its free radical scavenging activity is stronger [19]. It can eliminate hydroxyl radical and alkyl radicals, inhibit mitochondrial lipid peroxidation and lecithin plastid metabolism caused by the former two, and resist cell damage induced by hydrogen peroxide. In addition, baicalin phospholipid complex can slow down the excretion rate of baicalin in vivo, further enhance the oxygen radical scavenging capacity and reduce the content of MDA [20]. Therefore, baicalin capsules can treat DPN related to oxidative stress.
Clinical findings show that the inflammatory response is closely related to the development and progression of DPN [21]. As a pro-inflammatory cytokine, TNF-α can accelerate lipolysis, increase the production of hepatic glycogen, inhibit the expression of signal molecules and proteins in adipocytes, lead to insulin resistance, damage microvessels, cause nerve ischemia and hypoxia, and then cause nerve injury. Because patients with DPN are affected by long-term hyperglycemia, large amounts of IL-6 are secreted by islet cells and lymphocytes. On the one hand, it can aggravate the inflammatory reaction and damage the peripheral nervous system. On the other hand, it can be combined with TNF-α, CRP and other inflammatory factors to produce cytotoxicity, thus killing islet B cells and aggravating the patient’s condition [22]. CRP is a typical inflammatory factor, which can promote the secretion and release of a large number of endothelial factors by endothelial cells, increase the thickness of microvascular basement membrane, lead to the decrease of blood flow in nerve tissue, cause ischemia and necrosis of nerve tissue, and then damage peripheral nerves [23]. In this study, the levels of TNF-α, IL-6 and CRP decreased in both groups after treatment, and the levels in the SG were lower than those in the CG. Studies by Chen Zhonghua et al. have revealed that baicalin can effectively reduce the level of inflammatory factors in patients with acute cerebral infarction complicated with type 2 diabetes, which is basically consistent with the results of this study [24]. The results showed that baicalin capsules combined with α-lipoic acid could effectively reduce inflammatory injury in treating patients with diabetic peripheral neuropathy. The reason is that baicalin flavonoid compounds can effectively inhibit or induce the growth and apoptosis of tumor cells such as TNF-α, so as to improve oxidative stress and inflammatory reaction and treat peripheral neuropathy [25]. The combination of the two drugs can promote the recovery of peripheral nerve function through different pharmacology. In addition, this study revealed that there was no significant difference in adverse reactions between the combination of the two drugs and the single drug, which indicated that baicalin capsules were safe and easily tolerated by patients.
To sum up, baicalin capsules combined with α-lipoic acid is effective in treating patients with diabetic peripheral neuropathy, which can obviously relieve the clinical symptoms of patients, improve nerve conduction velocity, reduce oxidative stress and inflammatory injury, and does not increase adverse reactions. Therefore, it is worthy of promotion. However, there are still some shortcomings in this study. Only the treatment group of baicalin capsules combined with α-lipoic acid was tested, while the treatment group of baicalin capsules alone was not tested. So it was not possible to analyze the efficacy of baicalin capsules alone in the treatment of DPN. In the next study, this treatment group will be set up to analyze the curative effect. At the same time, the number of cases included in this study was small, and the observation time was short. The long-term efficacy of the combination therapy needs to be studied more widely to obtain more research results in the future.
Disclosure of conflict of interest
None.
References
- 1.Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep. 2019;19:86. doi: 10.1007/s11892-019-1212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iqbal Z, Azmi S, Yadav R, Ferdousi M, Kumar M, Cuthbertson DJ, Lim J, Malik RA, Alam U. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther. 2018;40:828–849. doi: 10.1016/j.clinthera.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, Tesfaye S. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7:938–948. doi: 10.1016/S2213-8587(19)30081-6. [DOI] [PubMed] [Google Scholar]
- 4.Chukanova EI, Chukanova AS. Alpha-lipoic acid in the treatment of diabetic polyneuropathy. Zh Nevrol Psikhiatr Im S S Korsakova. 2018;118:103–109. doi: 10.17116/jnevro201811811103-109. [DOI] [PubMed] [Google Scholar]
- 5.Zhang LY, Yang JS. Clinical study of Jiawei Buyang Huanwu decoction combined with α-lipoic acid in treating diabetic peripheral neuropathy of Qi deficiency and blood stasis type and urinary NAG and DPN. Shaanxi J Tradit Chin Med. 2018;39:1414–1416. [Google Scholar]
- 6.Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig. 2017;8:646–655. doi: 10.1111/jdi.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghav A, Singh P, Ahmad J. New insights into bioelectronic medicines: a new approach to tackle diabetic peripheral neuropathy pain in clinics. Diabetes Metab Syndr. 2019;13:1011–1014. doi: 10.1016/j.dsx.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YY, Li ZD, Jiang N, Wan PX, Deng CB, Su WR, Zhuo YH. Study on the role and mechanism of baicalin in acute optic nerve injury in mice with high intraocular pressure. Chin J Ophthalmol. 2020;56:376–382. doi: 10.3760/cma.j.cn112142-20200107-00011. [DOI] [PubMed] [Google Scholar]
- 9.Chinese Medical Association Diabetes Branch. Chinese guidelines for the prevention and treatment of Type 2 diabetes (2013 edition) Chin J Endocrinol Metabol. 2014;30:893–942. [Google Scholar]
- 10.Electromyography and Clinical Neuroelectrophysiology Group, Chinese Society of Neurology and Neuromusculopathy Group, Neurology Branch, Chinese Medical Association. Diagnostic and therapeutic consensus of diabetic peripheral neuropathy. Chin J Neurol. 2013;46:787–789. [Google Scholar]
- 11.Wang XT, Lin HX, Xu SA, Lu YK. Lipoic acid combined with epalrestat versus lipoic acid in treating diabetic peripheral neuropathy: a meta-analysis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017;39:656–664. doi: 10.3881/j.issn.1000-503X.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Fu QW, Yang H, Zhang LZ, Liu Y, Li XR, Dai ML, Yang YP, Yang SS, Xie Y, Liu Y, Fu L, Liu ZQ, Zhang QX. Traditional Chinese medicine foot bath combined with acupoint massage for the treatment of diabetic peripheral neuropathy: a systematic review and meta-analysis of 31 RCTs. Diabetes Metab Res Rev. 2020;36:e3218. doi: 10.1002/dmrr.3218. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Liang XC. Effects of mitochondrial dysfunction via AMPK/PGC-1α signal pathway on pathogenic mechanism of diabetic peripheral neuropathy and the protective effects of Chinese medicine. Chin J Integr Med. 2019;25:386–394. doi: 10.1007/s11655-018-2579-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhan P, Xi GM, Liu HB, Liu YF, Xu WJ, Zhu Q, Zhou ZJ, Miao YY, Wang XX, Jin JJ, Lv TF, Song Y. Protein regulator of cytokinesis-1 expression: prognostic value in lung squamous cell carcinoma patients. J Thorac Dis. 2017;9:2054–2060. doi: 10.21037/jtd.2017.06.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun L, Hei J, Sun T, Wang WP. Effects of baicalin on the inhibition of lipopolysaccharide induced uveitis in rats. J Ningxia Med Univ. 2018;40:28–30. [Google Scholar]
- 16.Han YJ, Wang M, Shen J, Zhang Z, Zhao M, Huang J, Chen YM, Chen Z, Hu YL, Wang YB. Differential efficacy of methylcobalamin and alpha-lipoic acid treatment on symptoms of diabetic peripheral neuropathy. Minerva Endocrinol. 2018;43:11–18. doi: 10.23736/S0391-1977.16.02505-0. [DOI] [PubMed] [Google Scholar]
- 17.Pieralice S, Vari R, Minutolo A, Maurizi AR, Fioriti E, Napoli N, Pozzilli P, Manfrini S, Maddaloni E. Biomarkers of response to alpha-lipoic acid ± palmitoiletanolamide treatment in patients with diabetes and symptoms of peripheral neuropathy. Endocrine. 2019;66:178–184. doi: 10.1007/s12020-019-01917-w. [DOI] [PubMed] [Google Scholar]
- 18.Zhang K, Tang QL, Gao C. Non-pharmacologic treatments for symptoms of diabetic peripheral neuropathy: a systematic review-methodological issues are a matter for concern. Curr Med Res Opin. 2019;35:1319–1320. doi: 10.1080/03007995.2019.1598135. [DOI] [PubMed] [Google Scholar]
- 19.Dimitrova A, Murchison C, Oken B. The case for local needling in successful randomized controlled trials of peripheral neuropathy: a follow-up systematic review. Med Acupunct. 2018;30:179–191. doi: 10.1089/acu.2018.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu JY, Gao HY. Combined treatment of traditional Chinese medicine and western medicine in the treatment of diabetic peripheral neuropathy. J Biol Regul Homeost Agents. 2018;32:945–949. [PubMed] [Google Scholar]
- 21.Huo J, Liu LS, Jian WY, Zeng JP, Duan JG, Lu XJ, Yin S. Stationary treatment compared with individualized chinese medicine for type 2 diabetes patients with microvascular complications: study protocol for a randomized controlled trial. Chin J Integr Med. 2018;24:728–733. doi: 10.1007/s11655-018-2987-1. [DOI] [PubMed] [Google Scholar]
- 22.Waldfogel JM, Nesbit SA, Dy SM, Sharma R, Zhang A, Wilson LM, Bennett WL, Yeh HC, Chelladurai Y, Feldman D, Robinson KA. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: a systematic review. Neurology. 2017;88:1958–1967. doi: 10.1212/WNL.0000000000003882. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Lin H, Xu S, Jin Y, Zhang R. Alpha lipoic acid combined with epalrestat: a therapeutic option for patients with diabetic peripheral neuropathy. Drug Des Devel Ther. 2018;12:2827–2840. doi: 10.2147/DDDT.S168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen ZH, Yi XL, Li LJ. Effect of baicalin on neurological function, blood glucose and inflammatory factors in patients with acute cerebral infarction complicated with type 2 diabetes. Guide Chin Med. 2019;17:12–13. 16. [Google Scholar]
- 25.Yang J, Guan K, Wang JZ. Clinical study on the arthroscopic refreshing treatment of anterior cruciate ligament injury combined with stable medial meniscus ramp injury. J Musculoskelet Neuronal Interact. 2017;17:108–113. [PMC free article] [PubMed] [Google Scholar]


