Abstract
Objective: The purpose of this study was to analyze the efficacy of platelet-rich plasma (PRP) injection combined with arthroscopic microfracture technique for knee cartilage injury. Methods: Seventy-nine patients with knee cartilage injury were randomly divided into a control group (CG, n=39) and an observation group (OBG, n=40). Both of the groups were treated with the arthroscopic microfracture technique, and the OBG was additionally treated with PRP injection. Results: The VAS scores for pain in the affected area of the OBG were lower than those of the CG at 1, 3, 5, and 7 days after surgery (P < 0.05). Knee flexion, hyperextension, and rotation angles in the OBG were greater than those in the CG at 1 month after surgery (P < 0.05). IKDC scores in the OBG were lower than those in the CG at 1, 2, and 3 weeks after surgery (P < 0.05). The Tegner and Lysholm scores in the OBG were higher than those in the CG at 1, 2, and 3 months after surgery (P < 0.05). The complication rate in the OBG was 10.00%, which was lower than that of 28.21% in the CG (P < 0.05). Conclusion: The efficacy of microfracture technique combined with PRP injection in the treatment of knee joint cartilage injury is significantly improved compared with that of microfracture technique alone, which can reduce postoperative complications and improve the range of motion and function of the knee joint.
Keywords: Knee joint cartilage injury, arthroscopy, microfracture technique, platelet-rich plasma, injection therapy
Introduction
The knee is the largest weight-bearing joint in the body. A great deal of twisting, turning, and rotating result in a high incidence of knee injuries among all joints [1]. Cartilage damage is somewhat different from other types of knee injuries. As a component of the joints, cartilage has no distribution of lymphatic vessels or nerves, and therefore it usually repairs itself after damage, but only if the defect is less than 3 mm in diameter, otherwise it cannot repair itself successfully and requires surgical treatment [2].
Arthroscopic microfracture technique is a kind of bone marrow stimulation technique that has mild trauma, low technical requirements, low surgical costs, high surgical safety, and rapid postoperative recovery, and has currently become an important method for the treatment of articular cartilage injuries [3]. However, although arthroscopic microfracture technique has its advantages, it has also shown some shortcomings in long-term use, such as the inability to generate the original hyaline cartilage, thus shortening the maintenance time. The cartilage usually deteriorates gradually within 18-36 months, and could not bear weight for a long period of time after surgery. Elderly patients may also experience spontaneous osteonecrosis [4]. In order to improve the long-term efficacy and safety of arthroscopic microfracture technique in the treatment of knee cartilage injuries, scholars have proposed that surgical treatment should be combined with drug treatment. Platelet-rich plasma (PRP) is widely used in the treatment of bone injuries, tendon injuries, and refractory traumas to accelerate the healing time [5]. In a previous study, patients with patellar tendinopathy were injected with PRP, and the results found significant improvement in the observed indicators after 2 years of follow-up [6].
Arthroscopic microfracture technique or PRP injection alone has s been administrated previously [7,8], but the effect and safety of the combined application of these two methods have not been extensively investigated. This study analyzed the value of combined therapy on 79 patients with knee articular cartilage injury.
Materials and methods
Data
Seventy-nine patients with knee cartilage injury in our hospital from January 2018 to January 2020 were enrolled as study subjects and were randomly divided into a control group (CG, n=39) and an observation group (OBG, n=40). The inclusion criteria: patients diagnosed as knee articular cartilage injury by MRI examination; physical examination showed varying degrees of tenderness in the joint space; X-ray examination showed normal knee valgus angle; patients aged ≥18 years; regardless of gender; patients were familiar with study procedures and signed the consent form, and this study has got the ethical approval of the First Affiliated Hospital of University of South China. Exclusion criteria: concomitant knee infection, meniscal or cruciate ligament injuries, obvious deformity of joints, gout, failure to successfully complete postoperative follow-up, psychiatric disorders, communication disorders, or cognitive impairment.
Methods
Arthroscopic microfracture treatment was performed as follows. The patients remained in supine posture. After combined spinal-epidural anesthesia was administered, the affected lower limbs were disinfected and sterile towels were laid. The 0.5-0.8 cm, transverse incision was made on the right and left sides of the patellar ligament at about 0.5 cm on the medial and lateral tibial plateau. Routine arthroscopic examination was performed first. The joint cavity was flushed with a large amount of normal saline to completely remove cartilage debris and particles, the free body in the joint cavity and the proliferating synovial tissue was thoroughly removed, and the defect edges of cartilage were appropriately modified. The cartilage defect was drilled with a 45 angled awl with a depth of 5 mm, hole spacing of 3 mm and hole diameter of 4 mm. At the end of perfusion, bone marrow should be leaked from the drilled site. The fluid was aspirated from the joint and wait until leaked bone marrow from the wound formed a blood clot. The cartilage defect should be thoroughly filled in; otherwise, drilling should be performed again. The normal saline was drained from the joint cavity and the drainage film was placed. After surgery, the affected limb was elevated for 24 h, and ice was applied to the affected knee. Patients with severe joint swelling were punctured and drawn as needed. At 1 day after surgery, bilateral ankle pumping exercises were conducted, followed by straight leg lift exercises for both lower limbs. The patient should not bear weight on the affected limb within 6 weeks, and the patient should not exercise vigorously within 3 months.
The CG received only the above surgical treatment, and the OBG additionally received PRP injection. Before surgery, 10 ml of venous blood was drawn and centrifuged at 4°C for 10 min (2000 r/min). The supernatant, platelet, and white blood cells were stored in a new tube. After another round of centrifugation under the same conditions, the supernatant, white blood cells, and platelets were retained. After suspension, calcium chloride was added to activate the platelets, and the preparation of PRP was completed. The knee joint of the affected limb was disinfected again. 2 ml of PRP was injected at the intersection of the upper border of the patella and the patellofemoral and knee joint space, and the affected knee was passively flexed 10 times. Patients should not touch water for 1 day after injection and the painful knees were treated with ice. Patients with postoperative pain score of > 5 were given NSAIDs orally. Diclofenac was selected (drug specification: 25 mg/tablet, approval number: H21021130, manufacturer: Shanxi Jinxin Shuanghe Pharmaceutical Co., Ltd.) and taken 75 mg-150 mg daily in 3 times. PRP injections were administered once every 7 days for a total of 6 cycles.
Outcome measurement
Pain level
Visual Analogue Scale/Score (VAS) [9] was adopted to evaluate the pain level, with 0 indicating no pain, 10 indicating severe pain, 1-3 as mild pain, 4-6 as moderate pain, and 7-9 as severe pain. The evaluation was performed before surgery, at 1, 3, 5 and 7 days after surgery, respectively.
Knee joint range of motion was measured before and at 1 month after surgery. The measurement was repeated for 3 times, and the highest value was recorded as the result. Normal range: 120 to 150 degrees of flexion; 5 to 10 degrees of hyperextension; 10 degrees of internal rotation and 20 degrees of external rotation in knee flexion.
Knee symptoms
The 11-item international knee documentation committee knee evaluation form (IKDC) [10] was used. Questions 2, 3, 9, 10, and 11 were scored 0-10, question 6 was scored 1-2, and questions 1, 4, 5, 7, and 8 were scored 1-5 for a total score of 0-77, with higher scores indicating more severe symptoms of knee ligament injury. Evaluations were performed before surgery, at 1, 2, and 3 weeks after surgery, respectively.
Motor function
The Tegner Knee Motor Function Rating Scale (postoperative) [11] was selected for evaluation, rated 0-10 and scored accordingly, with a higher score indicating a higher level of exercise that the patient could participate in, suggesting better motor function. Evaluations were performed before surgery, at 1, 2, and 3 months after surgery, respectively.
Knee function
Lysholm Knee Scale [12] consists of eight items to measure pain (25 points), instability (25 points), locking (15 points), swelling (10 points), limp (5 points), stair climbing (10 points), squatting (5 points), and need for support (5 points). Each question response has been assigned an arbitrary score on an increasing scale. The total score is the sum of each response to the eight questions, and may range from 0-100. Higher scores indicate a better outcome with fewer symptoms or disability. A score of > 95 indicates excellent knee function, 85-94 indicates good joint function, 65-84 indicates moderate joint function, and < 65 indicates poor joint function. Evaluations were performed before surgery, at 1, 2, and 3 months after surgery, respectively.
Complications
The incidence of muscle atrophy, joint stiffness, hematoma, thrombosis, and infection were recorded during and after surgery in the two groups.
Statistical analysis
Statistical analysis was performed using SPSS 23.0. Count data were expressed as [n (%)] and compared by X2 test. Measurement data were expressed as (x̅ ± s) and compared by t test. Multipoint comparisons were analyzed using ANVOA with post hoc F-test. Graphs were produced using Graphpad Prism 8. P < 0.05 was considered statistically significant.
Results
Baseline data
There was no significant difference in terms of mean age, mean disease duration, mean body mass, and mean body mass index (BMI), ratio of male versus female, and ratio of left versus right knee ligament injury between the two groups (P > 0.05) (Table 1).
Table 1.
Baseline data (x̅ ± s)/[n (%)]
| Data | Observation group (n=40) | Control group (n=39) | t/X2 | P | |
|---|---|---|---|---|---|
| Gender | Male | 23 (57.50) | 21 (53.85) | 0.107 | 0.744 |
| Female | 17 (42.50) | 18 (46.15) | |||
| Age (year) | 48.95±16.37 | 50.34±15.75 | 0.384 | 0.702 | |
| Duration of disease (year) | 0.82±0.39 | 0.86±0.42 | 0.439 | 0.662 | |
| Body mass (kg) | 61.68±7.91 | 63.45±8.42 | 0.963 | 0.338 | |
| BMI (kg/m2) | 23.42±1.77 | 24.18±1.81 | 1.887 | 0.063 | |
| Injury side | Left knee | 21 (52.50) | 23 (58.97) | 0.335 | 0.562 |
| Right knee | 19 (47.50) | 16 (41.03) | |||
Pain level
There was no significant difference in VAS scores for preoperative pain in the affected area between the two groups (P > 0.05). The VAS scores of pain in the affected area decreased in both groups at 1, 3, 5, and 7 days after surgery, and the VAS scores of pain in the OBG at 1, 3, 5, and 7 days after surgery were lower than those before surgery (P < 0.05). The VAS scores of pain in the affected area at 1 and 3 days after surgery in the CG exhibited no significant difference than those before surgery (P > 0.05), and the VAS scores of pain in the CG at 5 and 7 days after surgery were lower than before surgery (P < 0.05). The VAS scores for pain in the OBG at 1, 3, 5, and 7 days after surgery were lower than those in the CG (P < 0.05) (Figure 1).
Figure 1.
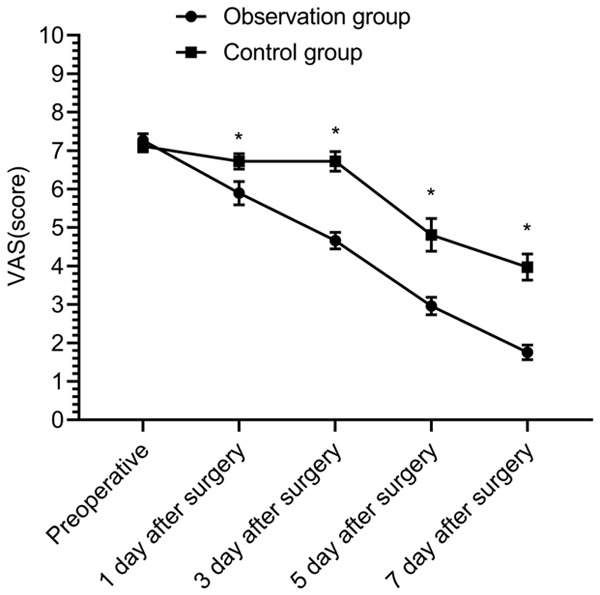
Pain level. Compared with the preoperative pain VAS score of the control group, the observation group showed little difference (P > 0.05); compared with the control group at 1, 3, 5, and 7 days after surgery, the observation group had lower pain VAS scores (P < 0.05). *P < 0.05.
Knee range of motion
There was no difference between the two groups in terms of the preoperative knee flexion, hyperextension, and rotation angles (P > 0.05), and these angles were increased in both groups at 1 month after surgery (P < 0.05), and were greater in the OBG than in the CG at 1 month after surgery (P < 0.05) (Figure 2).
Figure 2.
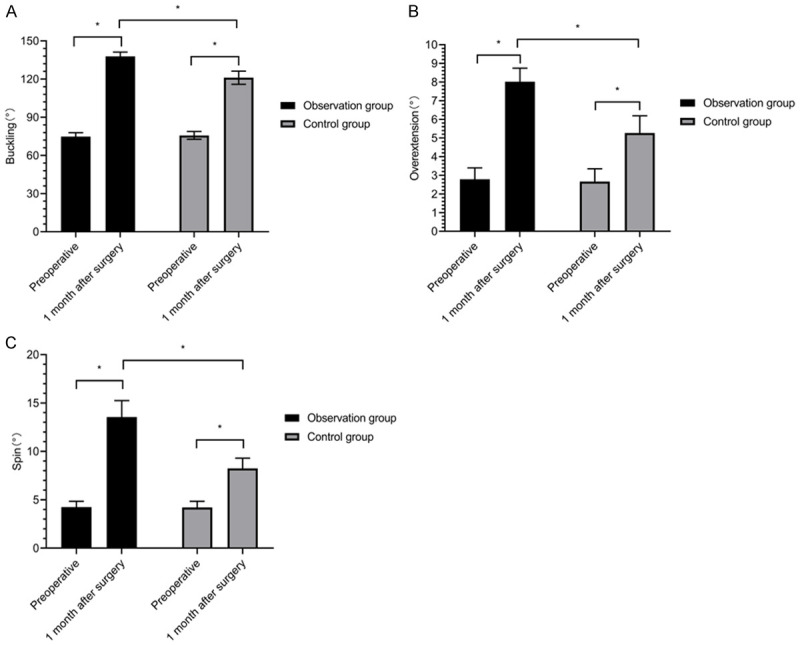
The range of motion of the knee joint. Knee flexion (A), hyperextension (B), and rotation (C) angles. *P < 0.05.
Knee symptoms
There was no significant difference in IKDC scores between the two groups before surgery (P > 0.05). The IKDC scores of the two groups at 1, 2 weeks, and 3 weeks after surgery were lower than those before surgery (P < 0.05), and the scores in the OBG were lower than those in the CG at 1, 2, and 3 weeks after surgery (P < 0.05) (Figure 3).
Figure 3.
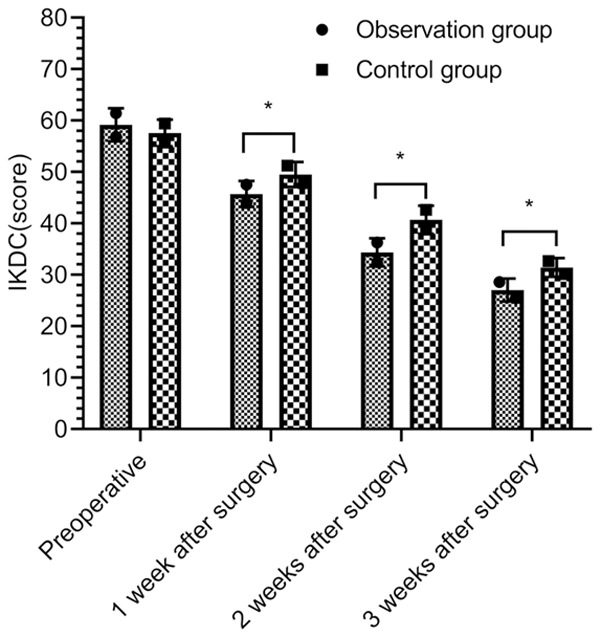
Comparison of the knee joint symptoms by IKDC score. *P < 0.05.
Motor function
There was no significant difference in preoperative Tegner scores between the two groups (P > 0.05). At 1, 2, and 3 months after surgery, Tegner scores were all increased in both groups (P < 0.05), and the Tegner scores in the OBG were higher than those in the CG at 1, 2, and 3 months after surgery (P < 0.05) (Figure 4).
Figure 4.
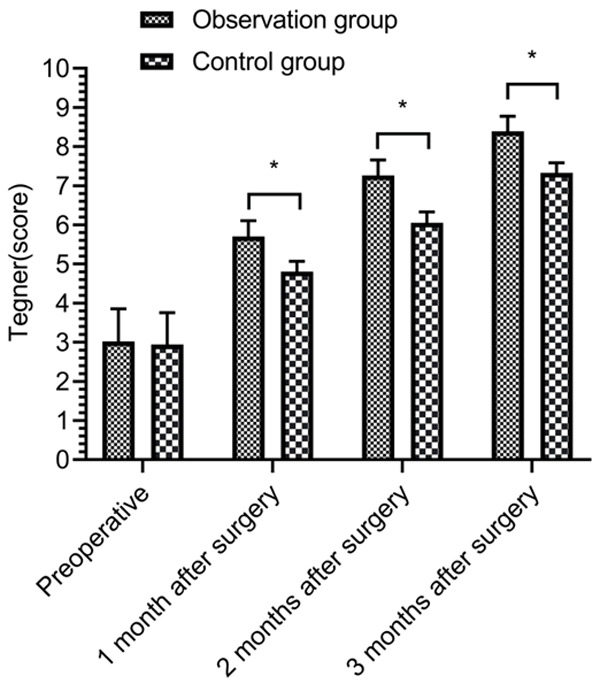
Comparison of knee joint function using Tegner score. *P < 0.05.
Knee function
There was no significant difference in preoperative Lysholm scores between the two groups (P > 0.05). Lysholm scores were improved in both groups at 1, 2, and 3 months after surgery (P < 0.05), and the scores in the OBG were higher than those in the CG at 1, 2, and 3 months after surgery (P < 0.05) (Figure 5).
Figure 5.
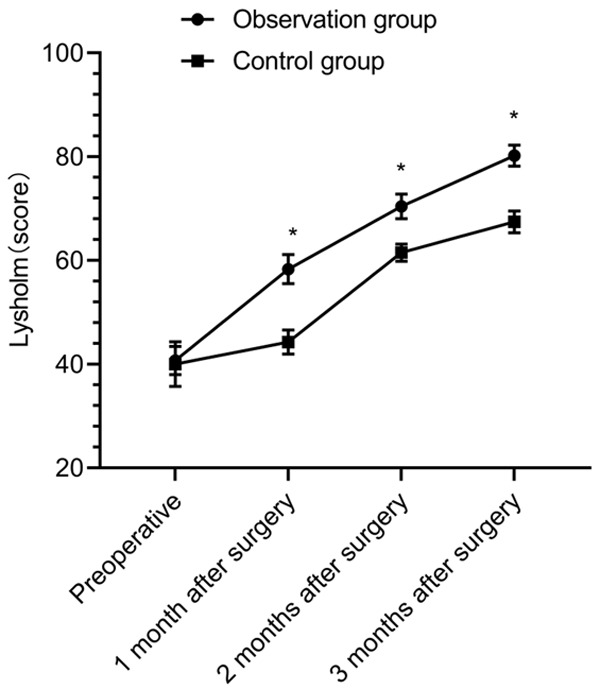
Comparison of knee joint function by Lysholm scores. Before surgery, 1 month after surgery, 2 months after surgery, 3 months after surgery, *P < 0.05.
Complications
The complication rate in the OBG (10%) was lower than that in the CG (28.21%) (P < 0.05) (Table 2).
Table 2.
Comparison of complications in the two groups [n (%)]
| Group | Muscle atrophy | Stiff joints | Hematoma | Thrombus | Infection | The incidence rate |
|---|---|---|---|---|---|---|
| Observation group (n=40) | 1 (2.50) | 2 (5.00) | 1 (2.50) | 0 (0.00) | 0 (0.00) | 4 (10.00) |
| Control group (n=39) | 2 (5.13) | 3 (7.69) | 2 (5.13) | 2 (5.13) | 2 (5.13) | 11 (28.21) |
| X2 | 4.255 | |||||
| P | 0.039 |
Discussion
Microfracture technique was first applied in the 1980s. Evidence showed that microfracture technique was effective in restoring cartilage defects in patients with postoperative subchondral bone hemorrhage and clot formation [13]. After subchondral microfracture, the mesenchymal stem cells of the cancellous bone are stimulated and proliferated. Thereafter, the mesenchymal stem cells flow with the blood along the pores, and the cartilage overflow turns into fibrous clots while the differentiated mesenchymal stem cells continue to proliferate and differentiate into cells with the morphological characteristics of chondrocytes, achieving the repair of cartilage defects [14,15]. The microfracture technique stimulates the differentiation of chondrocytes by filling fibrous clots of bone marrow to achieve the repair of cartilage defects [16].
However, the microfracture technique is not applicable to all patients, and its current indications can be summarized as the International Cartilage Repair Society (ICRS) score of 3, a total layer of cartilage defect, age < 50 years, defect size < 4 cm2, no bone loss, and no high demand for exercise [17,18]. In order to further improve the quality of cartilage defect repair, many scholars have attempted to combine adjuvant therapies with this surgery, and some studies have tried autologous or allogeneic osteochondral transplantation and achieved good results [19,20]. With the progress of medical technology, cartilage repair in vivo has been proposed, which has the advantages of simple operation, mild trauma, low cost, and rapid recovery. In the OBG of this study, PRP injection was performed based on surgery. After treatment, it was shown that the VAS score began to decrease statistically at 1 day after surgery, while the CG did not show similar improvement until 5 days after surgery. There was a statistically significant decrease before surgery. The VAS scores of the OBG were lower than those of the CG at all time points after surgery, suggesting that PRP injection combined with surgery can not only control pain more quickly, but also show a higher degree of pain relief. A similar study also showed that the VAS score of patients with knee cartilage injury treated with PRP injection combined with surgery at 1, 3 and 5 days after surgery was lower than that of patients treated with surgery alone (P < 0.05), which was consistent with this study [21]. A study has also shown that PRP injections as adjunctive therapy in orthopedic surgical procedures can further reduce pain levels and relieve patient discomfort [22].
PRP contains a high concentration of platelets, which can activate platelets during the repair process, thus promoting the conversion of fibrinogen to fibrin, playing a role in shrinking the wound and promoting the healing of injuries [23]. It has been found that PRP injection improves knee mobility to a greater extent and enhances knee function [24]. In this study, the knee flexion, hyperextension, and rotation angles in the OBG were greater than those in the CG at 1 month after surgery (P < 0.05). Another study also showed that the flexion, hyperextension and rotation angles of knee joint after arthroscopic surgery combined with PRP injection at 1 and 3 months after surgery were greater than those of the single arthroscopic surgery group (P < 0.05) [25]. This proved that microfracture technique could effectively improve the knee joint motion in patients with knee ligament injury, and PRP injection could further improve knee joint motion, approaching the normal range. In this study, IKDC score, Tegner score and Lysholm score in the OBG at 1, 2 and 3 months after surgery were better than those in the CG (P < 0.05). A previous study also confirmed that surgery combined with PRP injection could improve the knee joint function of patients to a greater extent [26]. This suggested that PRP injection on the basis of surgery can more effectively control symptoms and restore physical status, as well as more effectively accelerate the recovery of joint function and motor function after surgery. In terms of treatment safety, there was a difference in the incidence of complications between the OBG and the CG (10.00% vs. 28.21%), indicating that the combined therapy can improve the safety of surgical treatment, reduce treatment-related complications, resulting in a higher quality of rapid recovery. The PRP applied to the OBG contained several concentrated growth factors that, when combined with activators, induced the differentiation of hyaline cartilage in addition to accelerating cell proliferation. It has been proposed that the combination of the microscopic microfracture technique with PRP not only provides sufficient MSCs but also provides different concentrated growth factors as nutrients, thus achieving a better quality of cartilage defect repair [27].
In summary, arthroscopic microfracture combined with PRP injection is more effective in the treatment of knee cartilage injury. However, this study also has some shortcomings. The mechanism of arthroscopic microfracture technique combined with PRP is not thoroughly explored. It is not clear that other surgical methods combined with PRP therapy are equally effective and safe, and the dose of PRP injection has not been analyzed, and the most appropriate dose has not been clarified. These need to be studied in the future.
Disclosure of conflict of interest
None.
References
- 1.Grob K, Manestar M, Filgueira L, Kuster MS, Gilbey H, Ackland T. The interaction between the vastus medialis and vastus intermedius and its influence on the extensor apparatus of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2018;26:727–738. doi: 10.1007/s00167-016-4396-3. [DOI] [PubMed] [Google Scholar]
- 2.Rath B, Eschweiler J, Betsch M, Gruber G. Cartilage repair of the knee joint. Orthopade. 2017;46:919–927. doi: 10.1007/s00132-017-3463-x. [DOI] [PubMed] [Google Scholar]
- 3.Becher C, Malahias MA, Ali MM, Maffulli N, Thermann H. Arthroscopic microfracture vs. arthroscopic autologous matrix-induced chondrogenesis for the treatment of articular cartilage defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2019;27:2731–2736. doi: 10.1007/s00167-018-5278-7. [DOI] [PubMed] [Google Scholar]
- 4.Atilla HA, Luo TD, Stubbs AJ. Arthroscopic microfracture of hip chondral lesions. Arthrosc Tech. 2017;6:e2295–e2299. doi: 10.1016/j.eats.2017.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-Martínez A, Ruiz-Santiago F, García-Espinosa J. Platelet-rich plasma: myth or reality? Radiologia. 2018;60:465–475. doi: 10.1016/j.rx.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Emer J. Platelet-rich plasma (PRP): current applications in dermatology. Skin Therapy Lett. 2019;24:1–6. [PubMed] [Google Scholar]
- 7.Elghblawi E. Platelet-rich plasma, the ultimate secret for youthful skin elixir and hair growth triggering. J Cosmet Dermatol. 2018;17:423–430. doi: 10.1111/jocd.12404. [DOI] [PubMed] [Google Scholar]
- 8.Wan YTO, Lui TH. Arthroscopic debridement and microfracture of osteochondral lesion of the talar head. Arthrosc Tech. 2019;8:e969–e973. doi: 10.1016/j.eats.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung YT, Wu JS. The visual analogue scale for rating, ranking and paired-comparison (VAS-RRP): a new technique for psychological measurement. Behav Res Methods. 2018;50:1694–1715. doi: 10.3758/s13428-018-1041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: international knee documentation committee (IKDC) subjective knee evaluation form, knee injury and osteoarthritis outcome score (KOOS), knee injury and osteoarthritis outcome score physical function short form (KOOS-PS), knee outcome survey activities of daily living scale (KOS-ADL), lysholm knee scoring scale, oxford knee score (OKS), western ontario and mcmaster universities osteoarthritis index (WOMAC), activity rating scale (ARS), and tegner activity score (TAS) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S208–28. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DW, Yang SJ, Cho SI, Lee JH, Kim JG. Single-leg vertical jump test as a functional test after anterior cruciate ligament reconstruction. Knee. 2018;25:1016–1026. doi: 10.1016/j.knee.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Peng Q, Li XD, Cao GJ, Hu ZX, Zheng SQ, Shi CF. Treatment of degenerative medial meniscus injury of knee joint by arthroscopy combined with small needle knife to release superficial medial collateral ligament of knee joint. Zhongguo Gu Shang. 2019;32:1090–1093. doi: 10.3969/j.issn.1003-0034.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Freitag J, Ford J, Bates D, Boyd R, Hahne A, Wang Y, Cicuttini F, Huguenin L, Norsworthy C, Shah K. Adipose derived mesenchymal stem cell therapy in the treatment of isolated knee chondral lesions: design of a randomised controlled pilot study comparing arthroscopic microfracture versus arthroscopic microfracture combined with postoperative mesenchymal stem cell injections. BMJ Open. 2015;5:e009332. doi: 10.1136/bmjopen-2015-009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang HY, Lee KB. Arthroscopic microfracture for osteochondral lesions of the talus: second-look arthroscopic and magnetic resonance analysis of cartilage repair tissue outcomes. J Bone Joint Surg Am. 2020;102:10–20. doi: 10.2106/JBJS.19.00208. [DOI] [PubMed] [Google Scholar]
- 15.Eren TK, Ataoğlu MB, Eren A, Geylan DE, Öner AY, Kanatlı U. Comparison of arthroscopic microfracture and cell-free scaffold implantation techniques in the treatment of talar osteochondral lesions. Eklem Hastalik Cerrahisi. 2019;30:97–105. doi: 10.5606/ehc.2019.64401. [DOI] [PubMed] [Google Scholar]
- 16.Camp CL, Dines JS, Degen RM, Sinatro AL, Altchek DW. Arthroscopic microfracture for osteochondritis dissecans lesions of the capitellum. Arthrosc Tech. 2016;5:e477–81. doi: 10.1016/j.eats.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schallmo MS, Marquez-Lara A, Luo TD, Rosas S, Stubbs AJ. Arthroscopic treatment of hip chondral defect with microfracture and platelet-rich plasma-infused micronized cartilage allograft augmentation. Arthrosc Tech. 2018;7:e361–e365. doi: 10.1016/j.eats.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei M, Liu Y. Clinical effects of arthroscopic microfracture on osteochondral lesions of the talus. Zhongguo Gu Shang. 2017;30:751–754. doi: 10.3969/j.issn.1003-0034.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Redondo ML, Beer AJ, Yanke AB. Cartilage restoration: microfracture and osteochondral autograft transplantation. J Knee Surg. 2018;31:231–238. doi: 10.1055/s-0037-1618592. [DOI] [PubMed] [Google Scholar]
- 20.Southworth TM, Naveen NB, Nwachukwu BU, Cole BJ, Frank RM. Orthobiologics for focal articular cartilage defects. Clin Sports Med. 2019;38:109–122. doi: 10.1016/j.csm.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Shi W, Sun M, Hu X, Ren B, Cheng J, Li C, Duan X, Fu X, Zhang J, Chen H, Ao Y. Structurally and functionally optimized silk-fibroin-gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv Mater. 2017:29. doi: 10.1002/adma.201701089. [DOI] [PubMed] [Google Scholar]
- 22.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 23.Wu PI, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. 2016;27:825–853. doi: 10.1016/j.pmr.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Mlynarek RA, Kuhn AW, Bedi A. Platelet-rich plasma (PRP) in orthopedic sports medicine. Am J Orthop (Belle Mead NJ) 2016;45:290–326. [PubMed] [Google Scholar]
- 25.Zotti F, Albanese M, Rodella LF, Nocini PF. Platelet-rich plasma in treatment of temporomandibular joint dysfunctions: narrative review. Int J Mol Sci. 2019;20:277. doi: 10.3390/ijms20020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashim PW, Levy Z, Cohen JL, Goldenberg G. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239–242. [PubMed] [Google Scholar]
- 27.Cohen PR, Riahi RR. Platelet-rich plasma and genital rejuvenation. Skinmed. 2019;17:272–274. [PubMed] [Google Scholar]


