Abstract
Objective: To investigate the effects of combined anesthesia with dexmedetomide, propofol and remifentanil on perioperative inflammatory response and pulmonary function in patients with lung cancer. Methods: 90 patients with lung cancer admitted to our hospital from April 2017 to April 2019 were selected. According to different anesthesia schemes, patients undergoing combined anesthesia with propofol and remifentanil were included in group A (GA), and patients receiving combined anesthesia with dexmedetomidine, propofol and remifentanil were included in group B (GB). The blood gas, pulmonary function index, inflammatory factor level in serum, anesthetic effect and complications were compared between the two groups. Results: HR indexes at T1 and T2 in GB were significantly lower than those in GA (P<0.001). There was no significant fluctuation in PaCO2 and PaO2 indexes in the two groups at different time points (P>0.05). At T0, T1 and T2, RV/TLC levels in serum increased significantly in the two groups. (MVV-VE)/FEV1 and MVV/FEV levels were significantly decreased (all P<0.05). The fluctuation levels of RV/TLC, (MVV-VE)/FEV1 and MVV/FEV levels in serum of GB were significantly lower than those of GA at T1 and T2 (P<0.05). At T0, T1 and T2, the levels of inflammatory factors in serum were significantly decreased in the two groups (P<0.05), but the levels of inflammatory factors in serum of GB were significantly lower than those of GA at T1 and T2 (P<0.05). The VAS scores of GB were significantly lower than those of GA at 1 hour and 4 hours after operation (P<0.05). Ramsay scores of GB were significantly higher than those of GA at 1 hour and 4 hours after operation (P<0.05). The restlessness score and choking cough score in GB were lower than those in GA (P<0.05). Perioperative complications in GB were better than those in GA (P<0.05). Conclusion: On the basis of propofol and remifentanil anesthesia, the combination of dexmedetomidine for anesthesia induction can achieve satisfactory anesthesia effect. On the basis of propofol and remifentanil anesthesia combined with dexmedetomidine for anesthesia induction, it can significantly inhibit the inflammatory response of lung cancer patients during perioperative period and it can more effectively stabilize the blood gas microcirculation and lung function of patients.
Keywords: Dexmedetomidine, propofol, remifentanil, anesthesia, perioperative period in patients with lung cancer, inflammatory response
Introduction
Lung cancer is a common tumor disease, accounting for 7% of all cancers worldwide, with more than 700,000 deaths per year [1]. Due to the lack of effective biomarkers in early primary lung cancer and limited treatment options in late stage, it has become one of the deadliest cancers in the world, and an extremely concerned disease in the medical community [2,3]. Surgical resection is widely accepted as the choice for the treatment of lung cancer, which is the first and main treatment method for lung cancer, and it is also the only treatment method that can cure lung cancer [4,5]. Surgical resection takes a long time and causes a lot of trauma. Relevant inflammatory cytokines activate the inflammatory effect during lung cancer resection to activate and maintain the inflammatory response. Patients with severe inflammatory reactions suffer from pain and discomfort and poor surgical prognosis, so the selection of appropriate and safe narcotic drugs is very critical [6].
Dexmedetomidine, propofol and remifentanil are anesthetic drugs that are often combined in clinical application [7]. Studies have shown that propofol alone can make patients prone to respiratory depression, hypotension and body movements, which affects the clinical application of propofol alone [8,9]. Remifentanil is a new analgesic drug [10]. It has little effect on cardiovascular system, and it does not affect the postoperative recovery of patients when applied in large doses [11]. Dexmedetomidine is a highly selective new α2 adrenoceptor agonist with special pharmacological effects, and it is currently commonly used in clinical anesthesia [12]. At present, there are few related reports on the application of combined anesthesia with dexmedetomidine, propofol and remifentanil in perioperative inflammation of lung cancer patients. This experiment was designed to comprehensively compare the effects of combined anesthesia with dexmedetomidine, propofol and remifentanil on perioperative inflammatory response and pulmonary function of lung cancer patients, with a view to providing reference opinions for the implementation of anesthesia scheme in clinic.
Methods and materials
Patients’ data
90 patients with lung cancer admitted to Qinghai Provincial People’s Hospital from April 2017 to April 2019 were selected. According to different anesthesia schemes, patients undergoing combined anesthesia with propofol and remifentanil were included in GA, aged 45-75 years old, with an average age of 62.00±2.10 years. Patients undergoing combined anesthesia with dexmedetomidine, propofol and remifentanil were included in GB, aged 48-78 years old, with an average age of 62.00±1.20 years.
Inclusion criteria: patients had complete cases; patients did not receive relevant treatment in other hospitals.
Exclusion criteria: patients with severe liver and kidney dysfunction were excluded; patients with coagulation disorders were excluded; patients who did not cooperate with the examination were excluded; patients with cognitive impairment and communication impairment were excluded.
All subjects voluntarily participated in the experiment and signed informed consent form, and cooperated with medical staff to complete relevant diagnosis and treatment work. There was no allergy to drugs that used in surgical treatment. This study has been approved by the medical ethics committee of our hospital.
Methods and outcome measures
Anesthesia methods
In the two groups, the anesthetic dosage of patients was adjusted according to the weight and age of the patients. In this study, the dosage of anesthetic drugs was strictly controlled according to the standard clinical operation [13]. Medical staff created venous channels for patients. All patients were connected to monitors to closely monitor the clinical indicators after entering the consulting room.
Before anesthesia, medical staff infused lactated ringer’s solution to the patient with a dosage of 6 mL/kg. The medical staff sequentially injected 4 ug/kg fentanyl and 3 ug/mL propofol to the patient by target-controlled infusion for anesthesia induction. Since BIS value was less than 60, rocuronium was infused for 2 min, and mechanical ventilation was performed after intubation. The tidal volume was 8-10 ml/kg, respiratory frequency was 12-14 BPM, and PETCO2 was 35-45 mmHg. Target controlled infusion of cisatracurium, remifentanil and propofol was administered to the patients to maintain the anesthetic effect. Cisatracurium was discontinued when the abdomen was closed, and remifentanil and propofol were discontinued when surgery was completed. During the operation, the dosage of remifentanil and propofol was adjusted according to the clinical indexes of patients.
GB: Intravenous infusion of 0.5 g/kg dexmedetomidine was administered to patients before induction of anesthesia for 10 min, and continued pumping until the end of the operation. The dosage was 0.4 g/(kg·h). During the operation, the dosage of remifentanil and propofol was adjusted according to the clinical indexes of patients. The drug was stopped before the completion of the operation for 40 min. During operation, infusion heater was used to adjust the infusion speed to about 4 ml/min. The temperature did not exceed 43°C, and the operating room temperature was maintained at 24~26°C.
Outcome measures
The infusion volume, blood loss, anesthetic dosage and operation time were recorded in the two groups. 10 ml venous blood was collected from the patients at each time point before induction of anesthesia (T0), during the operation (T1) and 30 min after the operation (T2) for detection. Specific outcome measures were as follows:
Blood gas and pulmonary function indexes: HR, PaCO2, PaO2, RV/TLC, (MVV-VE)/FEV1 and MVV/FEV levels at T0, T1 and T2. Inflammation indexes: TNF-α, IL-8 and IL-10 levels in serum at T0, T1 and T2. Observation of anesthetic effect after drug administration: VAS score [14] and Ramsay score [15] at 1 hour and 4 hours after operation. Awakening quality after anesthesia: awakening time, extubation time and respiratory recovery time; Restlessness score [16] and choking cough score [17]. Perioperative complications of patients: bradycardia, nausea and vomiting, intraoperative movement, restlessness during extubation and postoperative hypotension and shivering.
Statistical methods
SPSS 19.1 (Beijing Sichuangweita Information Technology Co., Ltd.) was used for statistical analysis. The counting data were expressed by percentage [n (%)], and the difference between the two groups was compared by chi-square test. Measurement data were expressed by mean number ± standard deviation, and the difference between the two groups was compared by t test. The comparison of multiple time points in the group was conducted by repetitive measurement and analysis of variance. The difference was statistically significant with P<0.05.
Results
Comparison of patients’ clinical data
In order to make the experimental results accurate and reliable, the general data of patients were compared in the two groups, and there was no significant difference (P>0.05), indicating that patients in the two groups were comparable. Details of baseline data of patients are shown in Table 1.
Table 1.
Baseline data of patients in GA and GB [n (%)]
| GA (n=42) | GB (n=48) | X2 | P | |
|---|---|---|---|---|
| Gender | 0.231 | 0.631 | ||
| Male | 24 (57.14) | 25 (52.08) | ||
| Female | 18 (42.86) | 23 (47.92) | ||
| Age/year(s) old | 1.959 | 0.162 | ||
| ≤62 | 10 (23.81) | 18 (37.50) | ||
| >62 | 32 (76.19) | 30 (62.50) | ||
| Weight/kg | 0.000 | 1.000 | ||
| ≤60 | 21 (50.00) | 24 (50.00) | ||
| >60 | 21 (50.00) | 24 (50.00) | ||
| Nation | 0.885 | 0.347 | ||
| Han nationality | 42 (100.00) | 47 (97.92) | ||
| Minority nationality | 0 (0.00) | 1 (2.08) | ||
| Smoking | 0.000 | 1.000 | ||
| Yes | 42 (100.00) | 48 (100.00) | ||
| No | 0 (0.00) | 0 (0.00) | ||
| Alcoholism | 0.095 | 0.759 | ||
| Yes | 40 (95.24) | 45 (93.75) | ||
| No | 2 (4.76) | 3 (6.25) | ||
| Number of tumors | 0.989 | 0.320 | ||
| Single | 10 (23.81) | 16 (33.33) | ||
| Multiple | 32 (76.19) | 32 (66.67) | ||
| Differentiation | 1.367 | 0.242 | ||
| Well/middle differentiated | 15 (35.71) | 23 (47.92) | ||
| Poorly differentiated | 27 (64.29) | 25 (52.08) | ||
| TNM stage | 1.894 | 0.169 | ||
| I/II | 3 (7.14) | 8 (16.67) | ||
| III/IV | 39 (92.86) | 40 (83.33) |
Blood gas and pulmonary function indexes
Analysis of blood gas index at each time point in GB and GA
There was no significant difference in HR, PaCO2 and PaO2 indexes between the two groups at T0 point (P>0.05). The HR indexes at T1 and T2 in GB were significantly lower than those in GA (P<0.001). There was no significant fluctuation in PaCO2 and PaO2 indexes in the two groups at different time points (P>0.05) (Figure 1).
Figure 1.
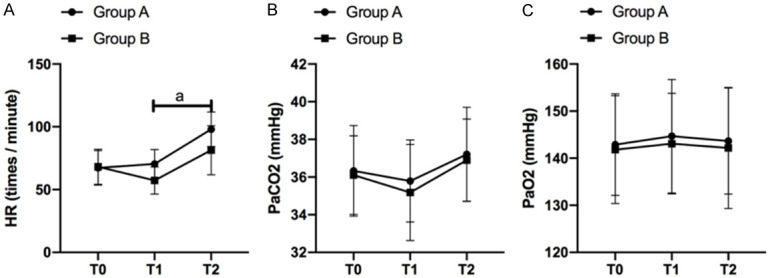
Analysis of blood gas index at each time point in GB and GA. A. The difference of blood gas indexe HR between GB and GA at each time points. B. The difference of blood gas index PaCO2 between GB and GA at each time points. C. The difference of blood gas index PaO2 between GB and GA at each time points. a means P<0.05.
Pulmonary function fluctuation of patients at each time points
At T0, T1 and T2, RV/TLC levels in serum increased significantly in the two groups. (MVV-VE)/FEV1 and MVV/FEV levels decreased significantly (all P<0.05). At T0, there was no difference in RV/TLC, (MVV-VE)/FEV1 and MVV/FEV levels between the two groups (P>0.05), but the fluctuation levels of RV/TLC, (MVV-VE)/FEV1 and MVV/FEV levels in serum of GB were significantly lower than those of GA at T1 and T2 (P<0.05) (Figure 2).
Figure 2.
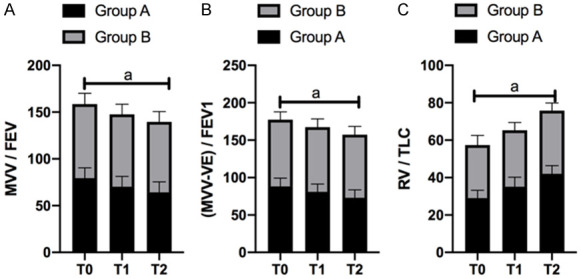
Pulmonary function fluctuation of patients at each time points. A. The fluctuation level of RV/TLC in serum of GB at T1 and T2 was significantly lower than that of GA (P<0.05). B. The fluctuation level of (MVV-VE)/FEV1 in serum of GB at T1 and T2 was significantly lower than that in GA (P<0.05). C. The fluctuation level of MVV/FEV in serum of GB at T1 and T2 was significantly lower than that of GA (P<0.05). a means P<0.05.
Changes of inflammatory factor levels in serum at different time points
The changes of serum TNF-α levels at different time points in the two groups
In the two groups, the levels of TNF-α in serum decreased significantly at T0, T1 and T2 (P<0.05). At T0, there was no difference between the two groups (P>0.05), but the levels of TNF-α in serum of GB were significantly lower than those of GA at T1 and T2 (P<0.05). More details are shown in Figure 3A.
Figure 3.
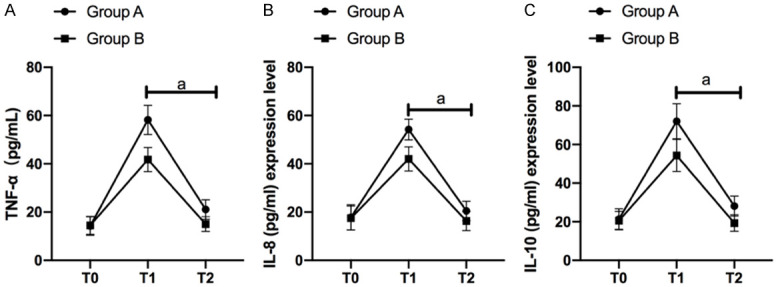
Inflammatory factor levels. In the two groups, the levels of TNF-α (A), IL-8 (B) and IL-10 (C) in serum decreased significantly at T0, T1 and T2 (P<0.05). At T0, there was no difference between the two groups (P>0.05), but the levels of TNF-α (A), IL-8 (B) and IL-10 (C) in serum of GB were significantly lower than those of GA at T1 and T2 (P<0.05).
The changes of serum IL-8 levels at different time points in the two groups
In the two groups, the levels of IL-8 in serum decreased significantly at T0, T1 and T2 (P<0.05). At T0, there was no difference between the two groups (P>0.05), but the levels of IL-8 in serum of GB were significantly lower than those of GA at T1 and T2 (p<0.05). More details are shown in Figure 3B.
The changes of serum IL-10 levels at different time points in the two groups
In the two groups, the levels of IL-10 in serum decreased significantly at T0, T1 and T2 (p<0.05). At T0, there was no difference between the two groups (p>0.05), but the levels of IL-10 in serum of GB were significantly lower than those of GA at T1 and T2 (P<0.05). More details are shown in Figure 3C.
Comparison of anesthesia effect between the GB and GA
VAS score and Ramsay score
The anesthetic effect was observed in the two groups at 1 hour and 4 hours after operation. The VAS scores of GA were 3.20±0.79 and 2.30±0.68 at 1 hour and 4 hours after operation respectively, while Ramsay scores of GA were 1.64±0.69 and 1.90±0.72 at 1 hour and 4 hours after operation respectively. The VAS scores of GB were 1.80±0.62 and 1.10±0.54 at 1 hour and 4 hours after operation respectively, while Ramsay scores of GB were 2.89±0.64 and 2.10±0.58 at 1 hour and 4 hours after operation respectively. Compared with the two groups, the VAS scores of GB were significantly lower than those of GA at 1 hour and 4 hours after operation (P<0.05), and the Ramsay scores of GB were significantly higher than those of GA at 1 hour and 4 hours after operation (P<0.05) (Figure 4).
Figure 4.
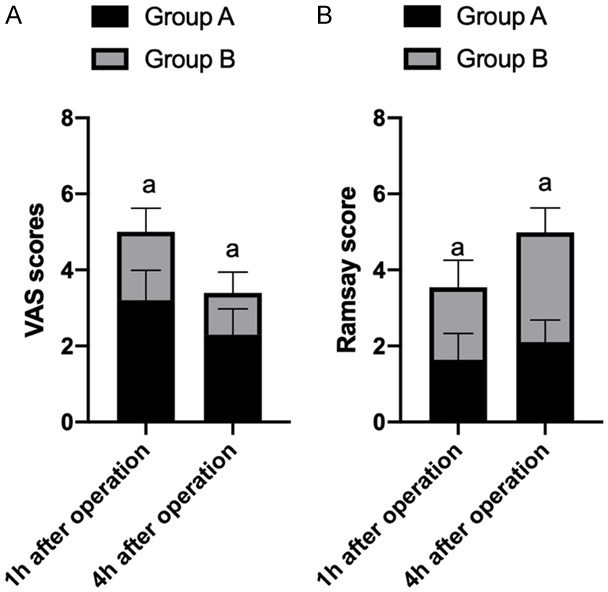
The VAS scores of the two groups were significantly down-regulated at 4 hours after surgery. The down-regulation level of the VAS scores in group B was significantly greater than that in group A (P<0.05) (A); the Ramsay scores at 4 hours after surgery in both groups were significantly up-regulated. The up-regulation level of Ramsay score in the last 4 hours was significantly greater than that of group A (P<0.05) (B). a means P<0.05.
Quality of awakening after anesthesia
In GA, the awakening time was 15.20±4.20 min, extubation time was 14.88±3.20 min and respiratory recovery time was 11.65±4.73 min. In GB, the awakening time was 14.90±4.24 min, extubation time was 14.00±3.30 min and respiratory recovery time was 10.20±3.59 min (Figure 5). In GA, the restlessness score was 3.20±1.02 and choking cough score was 2.99±1.50. In GB, the restlessness score was 1.34±0.50 and choking cough score was 1.53±0.47. Compared with the two groups, the awakening time, extubation time and respiratory recovery time in GB were shorter than those in GA, but the difference was not statistically significant (P>0.05). The restlessness score and choking cough score in GB were lower than those in GA (P<0.05) (Figures 5, 6).
Figure 5.
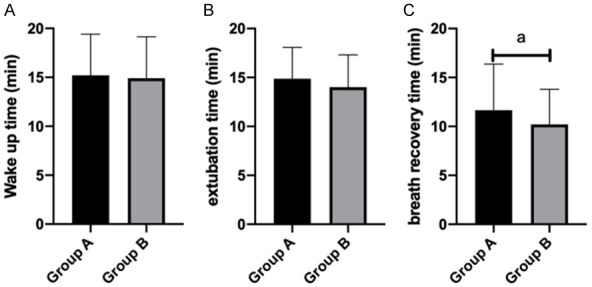
Quality of awakening after anesthesia wake up time (A), extubation time (B) and respiratory recovery time (C).
Figure 6.
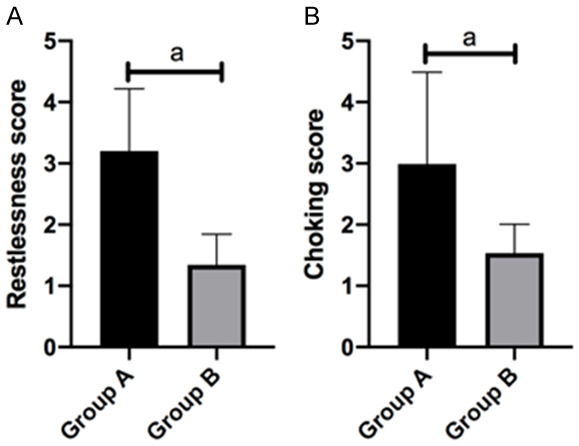
Restlessness and choking cough scoring. Restlessness score (A) and choking cough score (B). a means P<0.05.
Comparison of perioperative complications between the GB and GA
Perioperative complications in GB were better than those in GA (P<0.05) (Table 2).
Table 2.
Comparison of adverse reactions between the GB and GA [n (%)]
| GA (n=42) | GB (n=48) | X2 | P | |
|---|---|---|---|---|
| Bradycardia | 4 | 3 | - | - |
| Nausea and vomiting | 6 | 3 | - | - |
| Intraoperative body movement | 2 | 1 | - | - |
| Restlessness during extubation | 1 | 1 | - | - |
| Postoperative hypotension | 2 | 1 | - | - |
| Shivering | 2 | 1 | - | - |
| Total | 17 (40.48) | 10 (20.83) | 4.116 | 0.043 |
Discussion
There are still global differences in lung cancer rates. The 5-year survival rate of patients with advanced lung cancer is less than 10% [18]. Inflammatory reactions that are induced during lung cancer resection will activate inflammatory cells and release pro-inflammatory mediators such as TNF-α. Regulating inflammation and stress response during lung cancer resection and maintaining normal circulatory stability have great influence on the treatment and prognosis of lung cancer patients [19,20]. Dexmedetomidine, propofol and remifentanil are commonly used anesthetic drugs in lung cancer resection surgery. They can reduce the intensity of stress response and the incidence of complications in lung resection surgery. However, these two types of anesthesia are still controversial in the strength of inhibiting inflammatory response in lung cancer resection at present [21]. This study was designed to compare the effects of dexmedetomidine, propofol combined with remifentanil anesthesia on perioperative inflammatory response and pulmonary function of lung cancer patients. The selection of anesthetic drugs is the key to stabilize the vital signs of patients and reduce postoperative adverse reactions.
In this study, the anesthesia dose of patients was adjusted in the two groups according to the weight and age of the patients, and the intraoperative blood gas and pulmonary function indexes of the patients were observed. It was found that the PaCO2 and PaO2 indexes of patients had no obvious fluctuation in the two groups at each time points. The HR index of patients undergoing induction and maintenance of anesthesia with dexmedetomidine during surgery was significantly lower than that of patients undergoing propofol combined with remifentanil anesthesia. RV/TLC levels in serum of the two groups increased significantly during perioperative period. The levels of (MVV-VE)/FEV1 and MVV/FEV decreased obviously, but the fluctuation levels of RV/TLC, (MVV-VE)/FEV1 and MVV/FEV in serum of patients induced and maintained by dexmedetomidine anesthesia at T1 and T2 were significantly lower than those of patients undergoing propofol combined remifentanil anesthesia. Studies have shown that dexmedetomidine can effectively reduce the heart rate and blood pressure of perioperative patients and regulate hemodynamic changes such as tachycardia by inhibiting neuronal excitation. It also acts on corresponding receptors in spinal cord to exert analgesic effect [22].
Then, we analyzed the changes of serum inflammatory factor levels in patients during perioperative period. It was found that the levels of TNF-α, IL-8 and IL-10 in the two groups were significantly decreased during perioperative period, but the levels of TNF-α in the patients induced and maintained by dexmedetomidine anesthesia were significantly lower than those of patients undergoing propofol combined with remifentanil anesthesia at the beginning of the operation. Studies by Sánchez-Pedrosa et al. have shown that during surgery, abnormal expression of inflammatory cytokines is induced during wound cutting and suturing, which leads to inflammatory reaction in the body. In severe cases, it may lead to acute lung damage [23]. IL-8 and IL-10 both have significant pro-inflammatory effects, and the fluctuation of expression level is proportional to the damage degree of pulmonary function to a certain extent [24,25]. Therefore, we believed that anesthesia induction combined with dexmedetomidine on the basis of propofol and remifentanil anesthesia had significant inhibitory effect on serum inflammatory factors in lung cancer patients during perioperative period, and it could reduce vascular inflammatory reaction in patients during perioperative period and improved microcirculation of patients.
Finally, we compared the anesthesia effect and perioperative complications between GB and GA, and found that the VAS scores of GB were significantly lower than those of GA at 1 hour and 4 hours after operation, and the Ramsay scores of GB were significantly higher than those of GA at 1 hour and 4 hours after operation. The awakening time, extubation time, respiratory recovery time, restlessness score and choking cough score in GB were lower than those in GA. The complications were compared between the two groups. It showed that the two anesthesia schemes have little effect on complications such as bradycardia, restlessness during extubation, postoperative hypotension and shivering during perioperative period. A large number of studies have shown that dexmedetomidine exerts analgesic effect through α2 receptor of presynaptic membrane in postsynaptic and posterior horn of spinal cord interneurons, which can reduce sympathetic nerve tension and play a better sedative effect on restlessness of patients during extubation. In the study of Chen et al., dexmedetomidine, an anesthetic, had a better regulating effect on hypotension and bradycardia in patients undergoing radical gastrectomy for gastric cancer [26]. According to other studies, dexmedetomidine-assisted general anesthesia can effectively control shivering and play the greatest role in preventing shivering and reducing the side effects of drugs during perioperative period to a certain extent [27].
In this experiment, due to the limited medical resources in our hospital and the small base of the selected research subjects, there may be some contingency in the results, and it is not excluded that there are differences in the responses to anesthesia by different genders or age stages. We will conduct a longer-term follow-up investigation on the research subjects and continuously improve our experiment in the future to achieve the best experimental results.
To sum up, on the basis of propofol and remifentanil anesthesia, the combination of dexmedetomidine for anesthesia induction can achieve satisfactory anesthesia effect. On the basis of propofol and remifentanil anesthesia combined with dexmedetomidine for anesthesia induction, it can significantly inhibit the inflammatory response of lung cancer patients during perioperative period and it can more effectively stabilize the blood gas microcirculation and lung function of patients.
Disclosure of conflict of interest
None.
References
- 1.Sanchez-Carpintero Abad M, Sanchez-Salcedo P, de-Torres JP, Alcaide AB, Seijo LM, Pueyo J, Bastarrika G, Zulueta JJ, Campo A. Prevalence and burden of bronchiectasis in a lung cancer screening program. PLoS One. 2020;15:e0231204. doi: 10.1371/journal.pone.0231204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mella JM, Bledel I, Mora Nunez A, Romero Caimi G, Castano G, Nigro C, Pedreira S, Cimmino D, Boerr L. Lung cancer diagnosed by transesophageal ecoendoscopy and fine needle aspiration biopsy. Medicina (B Aires) 2020;80:173–176. [PubMed] [Google Scholar]
- 3.Yang WC, Hsu FM, Chen YH, Shih JY, Yu CJ, Lin ZZ, Lu SH, Yang JC, Cheng AL, Kuo SH. Clinical outcomes and toxicity predictors of thoracic re-irradiation for locoregionally recurrent lung cancer. Clin Transl Radiat Oncol. 2020;22:76–82. doi: 10.1016/j.ctro.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Geffen WH, Lamote K, Costantini A, Hendriks LEL, Rahman NM, Blum TG, van Meerbeeck J. The electronic nose: emerging biomarkers in lung cancer diagnostics. Breathe (Sheff) 2019;15:e135–e141. doi: 10.1183/20734735.0309-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tu C, Ren X, He J, Li S, Qi L, Duan Z, Wang W, Li Z. The predictive value of lncRNA MIR31HG expression on clinical outcomes in patients with solid malignant tumors. Cancer Cell Int. 2020;20:115. doi: 10.1186/s12935-020-01194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan Z, Yang Q, Xue M, Wang S, Hong W, Gao X. YY1-induced lncRNA ZFPM2-AS1 facilitates cell proliferation and invasion in small cell lung cancer via upregulating of TRAF4. Cancer Cell Int. 2020;20:108. doi: 10.1186/s12935-020-1157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tachibana S, Tanaka S, Yamakage M. Successful anesthetic management using dexmedetomidine sequentially with propofol in the asleep-awake-asleep technique for elderly patients undergoing awake craniotomy. Case Rep Anesthesiol. 2020;2020:6795363. doi: 10.1155/2020/6795363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raimann FJ, Adam E, Strouhal U, Zacharowski K, Seifert V, Forster MT. Dexmedetomidine as adjunct in awake craniotomy - improvement or not? Anaesthesiol Intensive Ther. 2020;52:15–22. doi: 10.5114/ait.2020.93043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabertanha A, Shakhsemampour B, Ekrami M, Allahyari E. Comparison of infusion of propofol and ketamine-propofol mixture (Ketofol) as anesthetic maintenance agents on blood pressure of patients undergoing orthopedic leg surgeries. Anesth Pain Med. 2019;9:e96998. doi: 10.5812/aapm.96998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Zhang K, Qi X, Yang G, Wang H, Zhang Z, Yang B. Effects of propofol on LC3II and mTOR/p-mTOR expression during ischemia-reperfusion myocardium injury in rats with type 2 diabetes mellitus. Exp Ther Med. 2020;19:2441–2448. doi: 10.3892/etm.2020.8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang LY, Zhang YH, Shen J, Luo Y. Effects of dexmedetomidine on post-operative recovery and mental status in patients receiving robotic-assisted thoracic surgery. Ann Palliat Med. 2019;8:469–475. doi: 10.21037/apm.2019.08.09. [DOI] [PubMed] [Google Scholar]
- 12.Elgebaly AS, Fathy SM, Sallam AA, Elbarbary Y. Cardioprotective effects of propofol-dexmedetomidine in open-heart surgery: a prospective double-blind study. Ann Card Anaesth. 2020;23:134–141. doi: 10.4103/aca.ACA_168_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao F, Xu S, Zhang W, Xiong H, Han J, Zhu A. Impacts of different administration modes of dexmedetomidine with 0.5% ropivacaine on intercostal nerve block. Ann Palliat Med. 2020;9:447–450. doi: 10.21037/apm.2020.03.25. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Pan J, Yang W, Lin J, Han Y, Lan K, Zeng L, Liang G, Liu J. First case report of Cryptococcus laurentii knee infection in a previously healthy patient. BMC Infect Dis. 2020;20:681. doi: 10.1186/s12879-020-05401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang X, Zhou H, Wu Y, Fang J, Lian Y. Impact of supraglottic device with assist ventilation under general anesthesia combined with nerve block in uniportal video-assisted thoracoscopic surgery. Medicine (Baltimore) 2020;99:e19240. doi: 10.1097/MD.0000000000019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mei X, Tong J. The plasma levels of brain-derived neurotrophic factor are positively associated with emergence agitation in the elderly after gastrointestinal surgery. J Anesth. 2016;30:811–816. doi: 10.1007/s00540-016-2212-3. [DOI] [PubMed] [Google Scholar]
- 17.Ishibashi C, Hayashida M, Sugasawa Y, Yamaguchi K, Tomita N, Kajiyama Y, Inada E. Effects of dexmedetomidine on hemodynamics and respiration in intubated, spontaneously breathing patients after endoscopic submucosal dissection for cervical esophageal or pharyngeal cancer. J Anesth. 2016;30:628–636. doi: 10.1007/s00540-016-2175-4. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Huang Q, Lin D, Zhao L, Ma J. Effect of lung protective ventilation on coronary heart disease patients undergoing lung cancer resection. J Thorac Dis. 2018;10:2760–2770. doi: 10.21037/jtd.2018.04.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo YB, Xu JD, Ji XX, Zhang JX, Liang JX, Zhou GB. Protective effect of dexmedetomidine against perioperative inflammation and on pulmonary function in patients undergoing radical resection of lung cancer. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37:1673–1677. doi: 10.3969/j.issn.1673-4254.2017.12.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jungraithmayr W, Frings C, Zissel G, Prasse A, Passlick B, Stoelben E. Inflammatory markers in exhaled breath condensate following lung resection for bronchial carcinoma. Respirology. 2008;13:1022–1027. doi: 10.1111/j.1440-1843.2008.01391.x. [DOI] [PubMed] [Google Scholar]
- 21.Ahn HJ, Park M, Kim JA, Yang M, Yoon S, Kim BR, Bahk JH, Oh YJ, Lee EH. Driving pressure guided ventilation. Korean J Anesthesiol. 2020;73:194–204. doi: 10.4097/kja.20041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Liu M, Yang Y, Cao J, Mi W. Dexmedetomidine exerts a protective effect on ischemia-reperfusion injury after hepatectomy: a prospective, randomized, controlled study. J Clin Anesth. 2020;61:109631. doi: 10.1016/j.jclinane.2019.109631. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Pedrosa G, Vara Ameigeiras E, Casanova Barea J, Rancan L, Simon Adiego CM, Garutti Martinez I. Role of surgical manipulation in lung inflammatory response in a model of lung resection surgery. Interact Cardiovasc Thorac Surg. 2018;27:870–877. doi: 10.1093/icvts/ivy198. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L, Zhu Y, Chen Z, Xu H, Zhou J, Tang S, Xu Z, Kong F, Li X, Zhang Y, Li X, Zhang J, Jia G. Cardiopulmonary effects induced by occupational exposure to titanium dioxide nanoparticles. Nanotoxicology. 2018;12:169–184. doi: 10.1080/17435390.2018.1425502. [DOI] [PubMed] [Google Scholar]
- 25.Moodley Y, Sturm M, Shaw K, Shimbori C, Tan DB, Kolb M, Graham R. Human mesenchymal stem cells attenuate early damage in a ventilated pig model of acute lung injury. Stem Cell Res. 2016;17:25–31. doi: 10.1016/j.scr.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Shao DH, Ma XD, Mao ZM. Dexmedetomidine aggravates hypotension following mesenteric traction during total gastrectomy: a randomized controlled trial. Ann Saudi Med. 2020;40:183–190. doi: 10.5144/0256-4947.2020.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bi YH, Wu JM, Zhang YZ, Zhang RQ. Effect of different doses of intrathecal dexmedetomidine as an adjuvant combined with hyperbaric ropivacaine in patients undergoing cesarean section. Front Pharmacol. 2020;11:342. doi: 10.3389/fphar.2020.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]


