Abstract
Acute myocardial infarction (AMI) seriously threatens human life. In this study we aimed to systemically analyze the function of key gene modules in human platelets in AMI. We used weighted gene co-expression network analysis (WGCNA) to construct a co-expression module, and analyzed the relationship between potential modules and clinical characteristics based on platelet RNA-seq RPKM count reads of 16 ST-segment elevation myocardial infarction (STEMI) patients and 16 non-STEMI (NSTEMI) patients provided by the GEO database. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were performed with the DAVID tool. Hub genes were calculated by the Cytohubba package. A total of 3653 genes was selected to construct the co-expression modules. A significant correlation between BMI and the module with color of sky-blue in STEMI. In NSTEMI, there was a significant correlation between the sky blue module and CAD, the Salmon module and HT, and the Cyan module and HT. In STEMI, the Hub genes were mainly enriched in functions related to cell membrane signal transduction including Aqp1, Armcx1, Gsta4, Hist3h2a and Il17re. In NSTEMI, the Hub genes are related mainly to energy metabolism in the sky-blue module including Olr1, Nap1l3, Gfer, Dohh, Crispld1 and Ccdc8b; they are mainly related to extracellular space and calcium binding in the Cyan module, including Clec12b, Chd4, Asgr1, Armcx4, Chid1 and Alkbh7. The hub genes in the Salmon module include Ell3, Aldh1b1, Cavin4, Cabp4, Eif1ay and Dus3l. Our results provide a framework for co-expression gene modules in STEMI and NSTEMI patients, and identify key targets as biomarkers for patients with different subtypes of AMI.
Keywords: RNA-seq, RPKM count reads data, co-expression network, Hub gene, coronary atherosclerosis
Introduction
The characteristic pathological changes of acute myocardial infarction (AMI) are acute myocardial ischemia and necrosis, which are caused mainly by coronary atherosclerosis [1]. According to electrocardiogram characteristics, AMI can be divided into two types: ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI). Because of the several differences in clinical manifestations and complications between STEMI and NSTEMI, the specific treatment and prognosis for these AMI variants are also different [2].
High platelet reactivity (HPR) is associated with the occurrence of acute myocardial infarction [3,4]. Platelet activity is the main factor of intracoronary thrombosis [5]. Platelets may play an important role in the development of atherosclerosis by regulating the inflammatory response [6]. Plaque rupture of coronary atherosclerosis and percutaneous coronary intervention (PCI) could damage vascular endothelium and promote activation and aggregation of platelets on the endothelial surface, which may lead to AMI or the formation of acute thrombosis in stents [7]. Several investigators have reported that platelet activation occurs during thrombosis and plays a role in cardiovascular diseases and events [6,8]. Differences in platelet transcription levels may reveal causal or reactive mechanisms that lead to platelet reactions and the occurrence and/or prognosis of various cardiovascular events. Other studies have reported that HPR is associated with adverse outcomes in STEMI and NSTEMI patients undergoing surgical treatment [9]. Platelet reactivity and antiplatelet resistance in STEMI patients are higher than those in NSTEMI patients. The differences in platelet reactivity and disease progression between STEMI and NSTEMI patients may be due to differences in gene expression. Identifying transcripts of the two AMI clinical subtypes will facilitate identification of the pathways relevant to STEMI and NSTEMI and development of specially tailored therapies for NSTEMI and STEMI patients.
Weighted correlation network analysis, also known as weighted gene co-expression network analysis (WGCNA) [10], has been widely used in genomics and in explaining expression patterns in disease transcriptomes. It has also been used in AMI research [11]. Hub genes such as Cd81 and T-cell receptor CD3ζ were identified by WGCNA in blood transcriptional profiles of the Toll-like receptor (TLR), TCR, and B-cell receptor (BCR) signaling pathways in asymptomatic atherosclerosis, acute ischemic stroke, and myocardial infarction patients [12]. WGCNA of microarray expression profiling of peripheral blood in patients with AMI identified TBX21 and PRF1 as potential diagnostic biomarker candidates and as possible regulatory targets in AMI [13]. In recent research, WGCNA was used to identify the critical genes in development of heart failure after AMI [14]. WGCNA is designed to identify higher-order correlation between gene products, while standardization analysis with DEG aims to detect the individual genes associated with disease. Cluster analysis and classification analysis of biological networks can more accurately reflect the network characteristics of biological systems, which is obviously superior to one-dimensional differential expression analysis [15]. Gene co-expression network analysis constructs gene modules based on all human coding genes, and the data are all from published literature, with a small bias [16]. The WGCNA algorithm can greatly simplify the unavoidable multiple detection problem in microarray expression profile analysis.
In this study, we used WGCNA to analyze the different gene modules of platelet RNA-seq in NSTEMI and STEMI in AMI patients, and analyzed the effects of the main functions and pathways of genes based on different modules; Our analysis identified key genes in the modules that are significantly correlated with HPR in AMI patients.
Material and methods
Expression analysis of RNA-seq RPKM count reads
RNA sequence data and patient clinic traits were obtained from GEO (http://www.ncbi.nlm.nih.gov/geo; accession number was GSE65705). The cohort was comprised of 16 patients with STEMI and 16 with NSTEMI. 32 samples of the platelet transcriptome in arterial blood were profiled using the chip-based platform GPL11154 Illumina HiSeq 2000 (Homo sapiens). The number of exons, annotated transcripts, protein domains, coding sequence size, homology information, and GC percent content were retrieved using the biomaRt R package [17]. After the gene expression data were read, those genes with larger variance are selected according to the variance interquartile range. The WGCNA algorithm was used to evaluate gene expression. In addition, flashClust tools in R language were used for cluster analysis of samples with the appropriate threshold value [16].
Analysis of co-expression module construction
The power value is filtered out in module construction by the WGCNA algorithm. The independence and average connectivity of different modules with different power values (between 1 and 30) were tested by the gradient method. When the degree of independence reached 0.8, the appropriate power value was determined, and the WGCNA algorithm was then used to construct the module. The corresponding gene information of each module was extracted. In order to ensure high reliability of the results, the minimum number of genes was set to 30. The WGCNA algorithm was used to identify the co-expression module, and the Heatmap toolkit was used to analyze the intensity of interaction. Genes that could not be included in any modules were sorted into the grey module and removed in subsequent analysis.
Construction of module-trait relationships
The correlation between Eigengene module and phenotype (clinical traits) was used to estimate the module-trait association, and facilitate identification of highly phenotypically related expression sets (modules). For each expression profile, gene saliency (GS) was calculated as the absolute value of the correlation between the expression profile and each feature; module member (mm) is defined as the correlation between the expression profile and each module feature value [16].
Functional enrichment analysis of co-expression modules
To analyze the correlation between clinical traits and modules, the genes in modules with a P value less than 0.05 were selected for GO and KEGG analysis. The analysis tool was DAVID Functional Annotation Bioinformatics Microarray Analysis (https://david.ncifcrf.gov/). GO annotations were divided into three categories: Molecular Function (MF), Biological Process (BP), and Cellular Components (CC). According to the number of genes contained in each item, the functional modules whose P value is less than 0.05 after enrichment analysis were selected for tabular display and visualization. If more than ten records were recorded, the first ten records were extracted. Interested modules were calculated by Cytohubba package in Cytoscape software, and the Hub genes in the module analyzed [18].
Results
Construction of co-expression modules of STEMI and NSTEMI
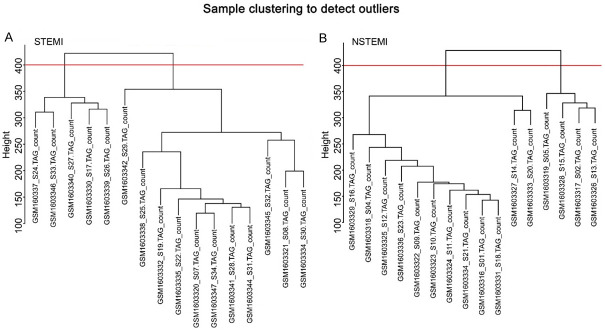
Gene co-expression networks of STEMI and NSTEMI samples were constructed using WGCNA software package tools. Gene expression variance was calculated and 3653 genes (top 25% of expression value) were selected for WGCNA analysis. These samples were clustered using the flashClust toolkit; results are shown in Figure 1A and 1B. In construction of co-expression modules, there were no outlier samples. All sample were divided into two clusters; we chose cluster I. Finally, there were 10 samples in STEMI and 12 samples in NSTEMI. We identified a module containing highly related genes and used sample clustering to detect abnormal samples (Figure 2A and 2B).
Figure 1.
Sample clustering to detect outliers. The top 25% gene expression (3563 genes) out of 14610 genes from different samples were clustered. A. STEMI samples can be separated into two clusters: one includes 5 samples and the other includes 11 samples; B. NSTEMI samples can be separated into two clusters: one with 12 samples and the other with 4 samples. Red lines indicate the outlier samples. The threshold was set as 400; no outlier samples were found.
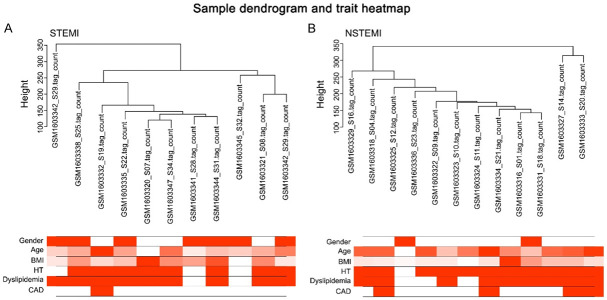
Figure 2.
Sample dendrogram and trait heatmap. A. STEMI; B. NSTEMI. No abnormal samples were detected by sample clustering.
Power value is one of the most critical parameters in construction of the WGCNA model, which mainly affects the independence and average connectivity of the co-expression module. First, the power value is filtered out to establish a scale-free distribution (Figure 3A and 3B). In the STEMI samples, when the power value is equal to 20, the degree of independence can reach 0.85 with high average connectivity. In NSTEMI samples, when the power value is equal to 18, the degree of independence can reach 0.85 with high average connectivity. After determining the appropriate power value, the genes were clustered and the co-expression module was constructed. Seven gene co-expression modules were established in STEMI, and 10 gene co-expression modules were established in NSTEMI. The heatmap describes the topological overlap matrix (Tom) between all the genes in the analysis. Light colors indicate low overlap, while darker colors indicate high overlap. The dark block along the diagonal line is the module. Genotype maps and module assignments are also shown on the left and top (Figure 4A-D).
Figure 3.
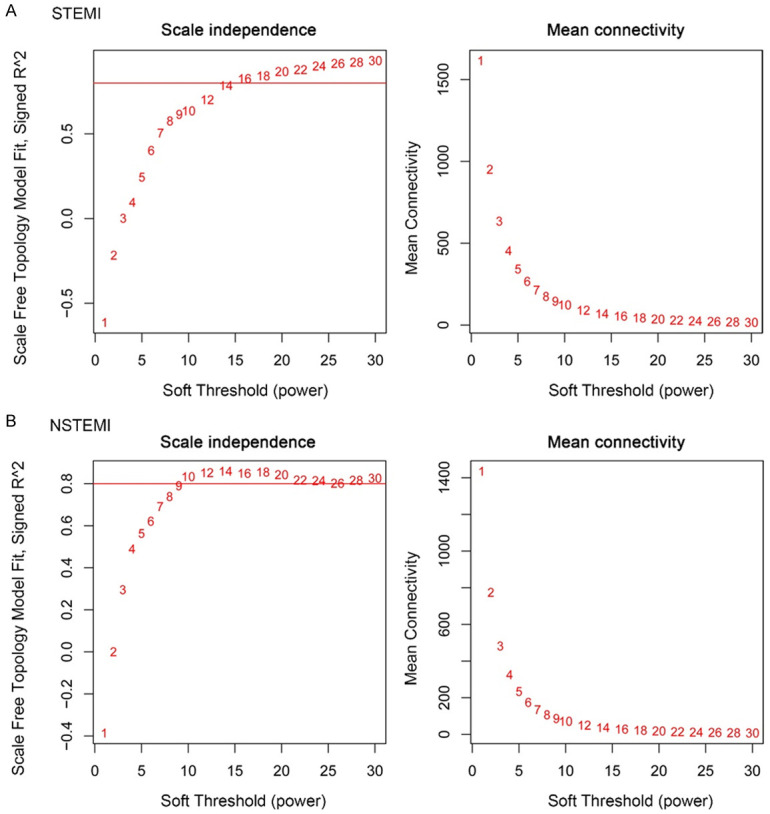
Analysis of network topology for various soft-thresholding powers. A. STEMI; B. NSTEMI. Both scale independence and mean connectivity are shown.
Figure 4.
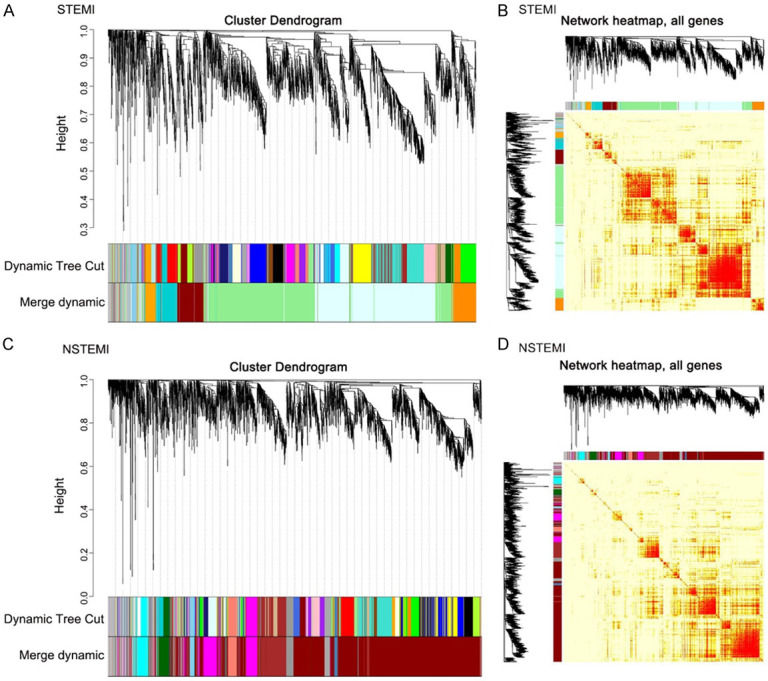
Cluster dendrogram and gene network heatmap plot. A. Co-expression module in STEMI; B. Topological overlap matrix (Tom) among all the genes in the STEMI analysis; C. Co-expression module in NSTEMI; D. Topological overlap matrix among all the genes in the NSTEMI analysis.
Construct module-trait relationships
The clinical features were provided by GSE65705 RNA-seq in GEO database (GSE65705_RNA-seq_ClinicalVars_GEO_13Nov2014.xls). We selected data on risk factors related to myocardial infarction, including gender, age, body mass index (BMI), hypertension (HT), dyslipidemia and coronary artery disease (CAD). The co-expression modules associated with specific features were analyzed (Figure 5A and 5B). The correlation between genes and associated traits in the module was validated by using an Eigengene adjacency Heatmap and Module Membership scatter plot (Figure 6). In STEMI, the genes in the sky-blue module were significantly correlated with clinical characteristics of BMI, with a correlation coefficient of 0.67 (P=0.02). In NSTEMI, the genes in the sky-blue module were significantly correlated with clinical characteristics of CAD, with a correlation coefficient of -0.63 (P=0.03); the genes in the cyan module were significantly correlated with HT clinical traits, with a correlation coefficient of 0.82 (P=0.001); and the genes in salmon modules were significantly correlated with HT clinical traits with a correlation coefficient of 0.64 (P=0.02).
Figure 5.
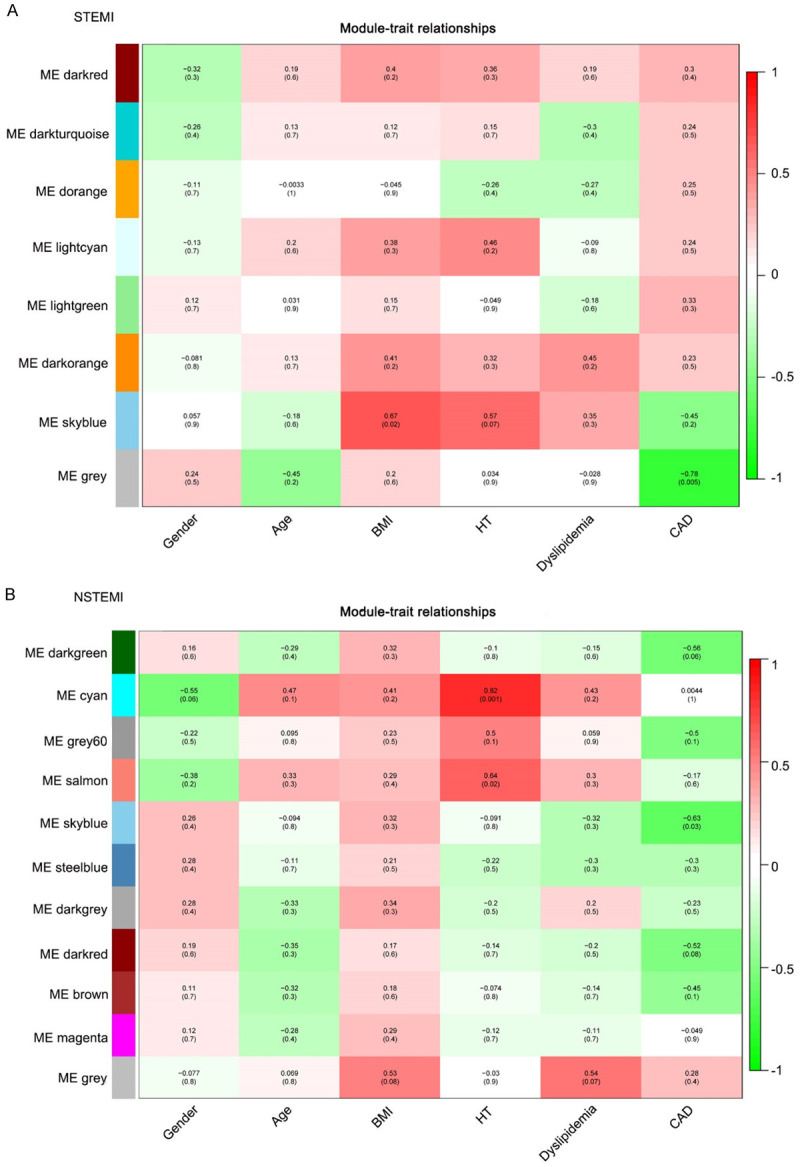
Module-trait relationship. Each row corresponds to a module eigengene, each column corresponds to a clinical trait. Each cell contains the corresponding correlation and p-value. The table is color-coded by correlation according to the color legend.
Figure 6.
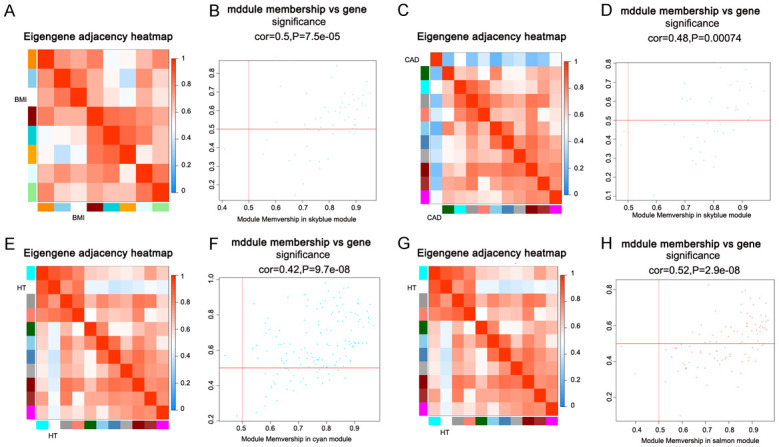
The eigengene dendrogram and heatmap identify groups of correlated eigengenes termed meta-modules. As a result, (A, B) the sky-blue module is highly correlated with BMI in STEMI patients, (C, D) the sky-blue module is highly correlated with CAD in NSTEMI patients, (E, F) the cyan module is highly correlated with HT in NSTEMI patients, and (G, H) the salmon module is also highly correlated with HT in NSTEMI patients. The heatmap in the panel indicates eigengene adjacency.
Functional enrichment analysis of co-expression modules
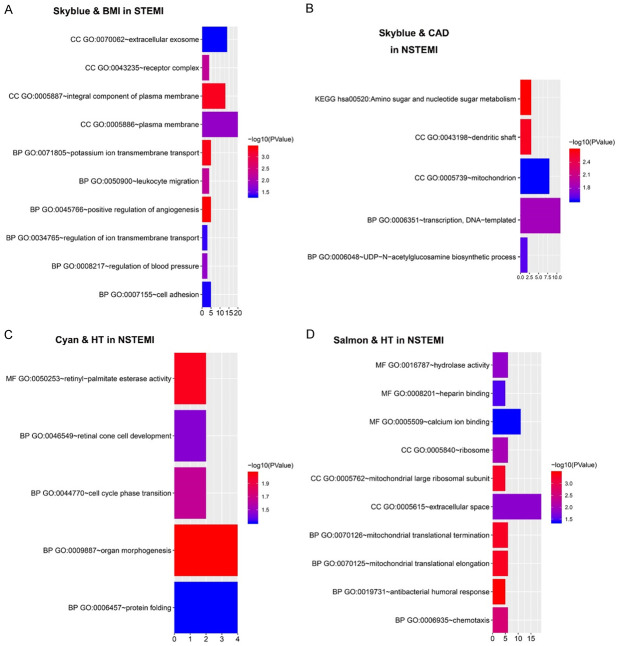
GO enrichment analysis and KEGG analysis were performed on genes in STEMI (Table 1 and Figure 7) and NSTEMI (Table 2 and Figure 7) modules that were significantly correlated with clinical traits [19]. In sky-blue modules in STEMI, CC was enriched mainly in the GO: 0005886~plasma membrane, GO: 0070062~extracellular exosome, and GO: 0005887~integral component of plasma membrane, BP was enriched mainly in the GO: 0045766~positive component, regulation of angiogenesis, GO: 0071805~potassium transmembrane transport, and GO: 0007155~cell adhesions. In sky-blue modules in NSTEMI, genes were concentrated mainly in GO: 0006351~transcription and DNA-templated, GO: 0005739. In the mitochondrion, the main pathways were enriched in hsa00520: Amino sugar and nucleotide sugar metabolism. In cyan modules in NSTEMI, genes were mainly enriched in GO: 0005615~extracellular space and GO: 0005509~calcium binding. In salmon modules in NSTEMI, the gene was mainly enriched in BP: GO: 0009887~organ morphogenesis, GO: 0006457~protein folding, GO: 0044770~cell cycle phase transition, and MF: GO: 0050253~retinyl-palmitate esterase activity.
Table 1.
GO enrichment analysis of genes in co-expression modules of STEMI
| Module | Category | Term | Count | P | Genes |
|---|---|---|---|---|---|
| Sky-blue | CC | GO: 0005886~plasma membrane | 20 | 0.015 | Ret, Kcnc3, Ptprf, C3, Gpr63, Gnrhr, Itga3, Il17re, Ptpru, Npr3, Aqp1, Xpnpep2, Kcnk10, Ddr1, Kcnq4, Arhgap33, Cd34, Kcnh2, Traf4, Lct |
| Sky-blue | CC | GO: 0070062~extracellular exosome | 14 | 0.049 | Ddr1, Bhlhb9, Ptprf, Nit2, C3, Sh3d21, Ephx2, Cd276, Hist3h2a, Itga3, Npr3, Aqp1, Ecm1, Xpnpep2 |
| Sky-blue | CC | GO: 0005887~integral component of plasma membrane | 13 | <0.001 | Ddr1, Ret, Ptprf, Cd34, Gnrhr, Il17re, Npr3, Ptpru, Aqp1, Gpr1, Lct, Dcstamp, Kcnk10 |
| Sky-blue | BP | GO: 0045766~positive regulation of angiogenesis | 5 | <0.001 | Cd34, C3, Aqp1, Ecm1, Angpt4 |
| Sky-blue | BP | GO: 0071805~potassium ion transmembrane transport | 5 | <0.001 | Kcnq4, Kcnc3, Kcnh2, Aqp1, Kcnk10 |
| Sky-blue | BP | GO: 0007155~cell adhesion | 5 | 0.047 | Ddr1, Ptprf, Cd34, Itga3, Ptpru |
| Sky-blue | BP | GO: 0050900~leukocyte migration | 4 | 0.006 | Cd34, Itga3, Mmp1, Angpt4 |
| Sky-blue | CC | GO: 0043235~receptor complex | 4 | 0.006 | Ddr1, Ret, Gpr63, Itga3 |
| Sky-blue | BP | GO: 0008217~regulation of blood pressure | 3 | 0.016 | Cd34, Ephx2, Npr3 |
| Sky-blue | BP | GO: 0034765~regulation of ion transmembrane transport | 3 | 0.043 | Kcnq4, Kcnc3, Kcnk10 |
Abbreviations: CC, cellular components; BP, biological process.
Figure 7.
GO enrichment analysis of genes in modules associated with clinical traits. A. Sky-blue modules in STEMI. B. Sky-blue modules in NSTEMI. C. Cyan modules in NSTEMI. D. Salmon modules in NSTEMI.
Table 2.
GO enrichment analysis of genes in co-expression modules of NSTEMI
| Module | Category | Term | Count | P | Genes |
|---|---|---|---|---|---|
| Sky-blue | BP | GO: 0006351~transcription, DNA-templated | 11 | 0.014 | Sbno2, Znf593, Rasl11a, Ing2, Zscan22, Znf697, Znf649, Znf511, Mad2l2, Znf219, Kank2 |
| Sky-blue | CC | GO: 0005739~mitochondrion | 8 | 0.031 | Maff, Atpaf2, Myom2, Gfer, Romo1, Ntsr1, Abcb6, Kank2 |
| Sky-blue | CC | GO: 0043198~dendritic shaft | 3 | 0.002 | Jph4, Gper1, Ntsr1 |
| Sky-blue | BP | GO: 0006048~UDP-N-acetylglucosamine biosynthetic process | 2 | 0.026 | Renbp, Uap1l1 |
| cyan | CC | GO: 0005615~extracellular space | 19 | 0.016 | Cmtm2, Ltbp2, Rnase3, Enpp2, Podxl, Il1rn, Plbd1, Igf2, Pomc, Tcn2, C1qc, Cxcl10, Chid1, Defa1b, Adm, Lrg1, Vegfa, Ltf, Defa1 |
| cyan | MF | GO: 0005509~calcium ion binding | 11 | 0.042 | Fkbp9, Prrg4, Lrp1, Ltbp2, Enpp2, Mex3b, Rph3al, Ryr1, Dsc2, Celsr3, Cabyr |
| cyan | BP | GO: 0070125~mitochondrial translational elongation | 6 | <0.001 | Mrpl53, Mrpl2, Mrpl23, Mrpl14, Mrps22, Mrpl54 |
| cyan | BP | GO: 0070126~mitochondrial translational termination | 6 | <0.001 | Mrpl53, Mrpl2, Mrpl23, Mrpl14, Mrps22, Mrpl54 |
| cyan | BP | GO: 0006935~chemotaxis | 6 | 0.002 | Defa1b, Cmtm2, Enpp2, Znf580, Defa1, Cxcl10 |
| cyan | CC | GO: 0005840~ribosome | 6 | 0.009 | Mrpl53, Mrpl2, Mrpl23, Mrpl14, Mrps22, Mrpl54 |
| cyan | MF | GO: 0016787~hydrolase activity | 6 | 0.014 | Psmc3, Enpp2, Nudt7, Abhd17c, Dhx58, Afmid |
| cyan | BP | GO: 0019731~antibacterial humoral response | 5 | <0.001 | Defa1b, Adm, Rnase3, Ltf, Defa1 |
| cyan | CC | GO: 0005762~mitochondrial large ribosomal subunit | 5 | <0.001 | Mrpl53, Mrpl2, Mrpl23, Mrpl14, Mrpl54 |
| cyan | MF | GO: 0008201~heparin binding | 5 | 0.032 | Ltbp2, Vegfa, Ltf, Mdk, Cxcl10 |
| salmon | BP | GO: 0009887~organ morphogenesis | 4 | 0.009 | Hras, Gmnn, Gamt, Bhlhe41 |
| salmon | BP | GO: 0044770~cell cycle phase transition | 2 | 0.023 | Timeless, Tipin |
| salmon | BP | GO: 0046549~retinal cone cell development | 2 | 0.036 | Cabp4, Gnat2 |
| salmon | BP | GO: 0006457~protein folding | 4 | 0.049 | Tbcc, Ppil6, Hscb, Gnat2 |
| salmon | MF | GO: 0050253~retinyl-palmitate esterase activity | 2 | 0.01 | Plb1, Pnpla4 |
Abbreviations: CC, cellular components; BP, biological process; MF, molecular function.
The connectivity of the module and hub gene was analyzed by Cytoscape software using the Cytohubba package. The hub genes obtained by the 12 algorithms are shown in Tables 3, 4, 5 and 6. The hub genes in the sky-blue modules in STEMI were Aqp1, Armcx1, Gsta4, Hist3h2a, and Il17re (Table 3). Hub genes in the sky-blue module of NSTEMI were Olr1, Nap1l3, Gfer, Dohh, and Crispld1 (Table 4); hub genes in the cyan module were Clec12b, Chchchd4, Asgr1, Armcx4, Chid1, and Alkbh7 (Table 5); hub genes in the salmon module were Ell3, Aldh1b1, Cavin4, Cabp4, Eif1ay, and Dus3l (Table 6).
Table 3.
Hub genes in the modules of Sky-blue in STEMI
| Gene | MCC | DMNC | MNC | Degree | EPC | BN | EcCentricity | Closeness | Radiality | Betweenness | Stress | CC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C3 | 2 | 0.308 | 2 | 2 | 5.358 | 1 | 0.127 | 7.95 | 4.293 | 0 | 0 | 1 |
| Hist3h2a | 3 | 0.308 | 2 | 3 | 5.714 | 2 | 0.127 | 8.317 | 4.257 | 34 | 38 | 0.333 |
| Gsta4 | 5 | 0.308 | 2 | 5 | 7.264 | 9 | 0.158 | 10.5 | 4.68 | 108 | 190 | 0.2 |
| Armcx1 | 4 | 0.308 | 2 | 4 | 6.511 | 1 | 0.127 | 8.817 | 4.293 | 17 | 34 | 0.167 |
| Aqp1 | 5 | 0.308 | 2 | 5 | 6.738 | 5 | 0.127 | 9.7 | 4.469 | 102 | 220 | 0.1 |
| Il17re | 7 | 0 | 1 | 7 | 7.479 | 19 | 0.127 | 11.45 | 4.714 | 173 | 330 | 0 |
| Ccdc28b | 3 | 0 | 1 | 3 | 4.95 | 4 | 0.106 | 8.7 | 4.328 | 94 | 154 | 0 |
| Ecm1 | 2 | 0 | 1 | 2 | 4.274 | 3 | 0.106 | 7.317 | 4.011 | 64 | 136 | 0 |
| Ddr1 | 2 | 0 | 1 | 2 | 2.942 | 2 | 0.09 | 6.06 | 3.483 | 34 | 70 | 0 |
| Cd276 | 2 | 0 | 1 | 2 | 5.805 | 1 | 0.158 | 8.417 | 4.433 | 20 | 64 | 0 |
| Ephx2 | 2 | 0 | 1 | 2 | 5.566 | 1 | 0.158 | 8.417 | 4.433 | 20 | 64 | 0 |
| Ccdc103 | 2 | 0 | 1 | 2 | 3.337 | 2 | 0.090 | 6.71 | 3.8 | 34 | 54 | 0 |
| Gpr1 | 2 | 0 | 1 | 2 | 5.674 | 1 | 0.106 | 8.033 | 4.257 | 5 | 16 | 0 |
| Bhlhb9 | 2 | 0 | 1 | 2 | 5.558 | 1 | 0.106 | 8.033 | 4.257 | 5 | 16 | 0 |
| Cd34 | 3 | 0 | 1 | 3 | 2.581 | 6 | 0.067 | 3.833 | 0.88 | 14 | 14 | 0 |
| Dcstamp | 2 | 0 | 1 | 2 | 2.447 | 6 | 0.1 | 3.5 | 0.88 | 12 | 12 | 0 |
| Gtf2h4 | 2 | 0 | 1 | 2 | 2.239 | 2 | 0.067 | 3.167 | 0.8 | 8 | 8 | 0 |
| Gnrhr | 1 | 0 | 1 | 1 | 3.991 | 1 | 0.106 | 7.117 | 4.117 | 0 | 0 | 0 |
| Irak1bp1 | 1 | 0 | 1 | 1 | 2.92 | 1 | 0.09 | 6.043 | 3.73 | 0 | 0 | 0 |
| Guca1b | 1 | 0 | 1 | 1 | 3.626 | 1 | 0.106 | 5.85 | 3.659 | 0 | 0 | 0 |
| Angpt4 | 1 | 0 | 1 | 1 | 2.325 | 1 | 0.079 | 5.068 | 3.202 | 0 | 0 | 0 |
| Fam86c1 | 1 | 0 | 1 | 1 | 2.218 | 1 | 0.079 | 4.677 | 2.885 | 0 | 0 | 0 |
| Arhgap33 | 1 | 0 | 1 | 1 | 1.951 | 1 | 0.05 | 2.583 | 0.72 | 0 | 0 | 0 |
| Bspry | 1 | 0 | 1 | 1 | 1.931 | 1 | 0.05 | 2.583 | 0.72 | 0 | 0 | 0 |
| Casp12 | 1 | 0 | 1 | 1 | 1.797 | 1 | 0.05 | 2.333 | 0.64 | 0 | 0 | 0 |
| Atp6v1g2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fam187a | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gpr63 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eppk1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ac020613.1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: MCC, maximal clique centrality; DMNC, density of maximum neighborhood; MNC, maximum neighborhood component; EPC, edge percolated component; BN, bottleneck; CC, clustering coefficient.
Table 4.
The hub genes in the modules of Sky-blue in NSTEMI
| Gene | MCC | DMNC | MNC | Degree | EPC | BN | EcCentricity | Closeness | Radiality | Betweenness | Stress | CC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Olr1 | 4 | 0.308 | 2 | 4 | 3.794 | 4 | 0.08 | 6.617 | 2.582 | 34 | 44 | 0.167 |
| Nap1l3 | 3 | 0 | 1 | 3 | 3.361 | 4 | 0.1 | 6.167 | 2.582 | 28 | 38 | 0 |
| Gfer | 3 | 0 | 1 | 3 | 3.492 | 4 | 0.1 | 6.25 | 2.618 | 50 | 64 | 0 |
| Dohh | 3 | 0.308 | 2 | 3 | 3.58 | 4 | 0.1 | 6.25 | 2.618 | 32 | 40 | 0.333 |
| Crispld1 | 3 | 0.308 | 2 | 3 | 3.387 | 3 | 0.08 | 6.117 | 2.545 | 36 | 36 | 0.333 |
| Jph4 | 2 | 0 | 1 | 2 | 2.226 | 2 | 0.067 | 4.483 | 2.109 | 20 | 24 | 0 |
| Atpaf2 | 2 | 0 | 1 | 2 | 2.732 | 3 | 0.08 | 5.2 | 2.4 | 36 | 44 | 0 |
| Abcb6 | 2 | 0 | 1 | 2 | 2.564 | 2 | 0.067 | 4.867 | 2.255 | 20 | 20 | 0 |
| Ntsr1 | 1 | 0 | 1 | 1 | 2.082 | 1 | 0.08 | 4.233 | 2.218 | 0 | 0 | 0 |
| Mad2l2 | 1 | 0 | 1 | 1 | 1.68 | 1 | 0.057 | 3.41 | 1.745 | 0 | 0 | 0 |
| Catip | 1 | 0 | 1 | 1 | 1.875 | 1 | 0.057 | 3.626 | 1.891 | 0 | 0 | 0 |
| C2orf66 | 1 | 0 | 1 | 1 | 2.293 | 1 | 0.067 | 4.367 | 2.218 | 0 | 0 | 0 |
| Nanp | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kank2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mln | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fzd5 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Plpp7 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myo7a | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Insl3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gper1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arl10 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pfkfb1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myot | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myom2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maff | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ing2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Glb1l | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fam206a | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Depp1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cenpm | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: MCC, maximal clique centrality; DMNC, density of maximum neighborhood; MNC, maximum neighborhood component; EPC, edge percolated component; BN, BottleNeck; CC, clustering coefficient.
Table 5.
The hub genes in the modules of Cyan in NSTEMI
| Gene | MCC | DMNC | MNC | Degree | EPC | BN | EcCentricity | Closeness | Radiality | Betweenness | Stress | CC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Armcx4 | 7 | 0.309 | 3 | 6 | 12.248 | 1 | 0.173 | 12.867 | 4.125 | 75.476 | 148 | 0.133 |
| Chid1 | 4 | 0.309 | 3 | 3 | 10.882 | 1 | 0.144 | 10.617 | 3.744 | 4 | 12 | 0.667 |
| Asgr1 | 7 | 0.308 | 2 | 7 | 12.599 | 20 | 0.289 | 14.833 | 4.576 | 269.881 | 412 | 0.048 |
| Clec12b | 5 | 0.308 | 2 | 5 | 12.011 | 7 | 0.217 | 13.5 | 4.403 | 145 | 204 | 0.1 |
| Cabyr | 4 | 0.308 | 2 | 4 | 10.377 | 3 | 0.173 | 11.483 | 3.952 | 62.5 | 100 | 0.167 |
| C1qc | 4 | 0.308 | 2 | 4 | 11.01 | 2 | 0.144 | 11.15 | 3.779 | 31.952 | 60 | 0.167 |
| Arhgap22 | 4 | 0.308 | 2 | 4 | 10.327 | 2 | 0.217 | 11.75 | 4.056 | 55.024 | 94 | 0.167 |
| Adm | 4 | 0.308 | 2 | 4 | 10.842 | 3 | 0.173 | 12.15 | 4.091 | 78.333 | 124 | 0.167 |
| Aldh1a1 | 3 | 0.308 | 2 | 3 | 9.281 | 3 | 0.217 | 11.167 | 3.987 | 92 | 128 | 0.333 |
| Afmid | 2 | 0.308 | 2 | 2 | 9.65 | 1 | 0.144 | 9.817 | 3.605 | 0 | 0 | 1 |
| Chchd4 | 8 | 0.284 | 4 | 6 | 12.323 | 5 | 0.173 | 13.283 | 4.229 | 88.524 | 164 | 0.2 |
| Bcl2l12 | 3 | 0 | 1 | 3 | 9.445 | 3 | 0.217 | 11.083 | 3.987 | 47.809 | 92 | 0 |
| Alkbh7 | 3 | 0 | 1 | 3 | 10.089 | 3 | 0.217 | 11.917 | 4.195 | 107.238 | 160 | 0 |
| Abhd17c | 3 | 0 | 1 | 3 | 10.732 | 1 | 0.173 | 11.617 | 4.091 | 27.833 | 62 | 0 |
| Clp1 | 2 | 0 | 1 | 2 | 5.825 | 2 | 0.173 | 8.533 | 3.224 | 48 | 66 | 0 |
| Clec4a | 2 | 0 | 1 | 2 | 9.652 | 3 | 0.217 | 11.25 | 4.125 | 23.238 | 52 | 0 |
| Cfap157 | 2 | 0 | 1 | 2 | 7.902 | 1 | 0.173 | 9.183 | 3.467 | 4.5 | 10 | 0 |
| Cdc42ep4 | 2 | 0 | 1 | 2 | 9.629 | 2 | 0.217 | 11 | 4.056 | 15.238 | 44 | 0 |
| Cchcr1 | 2 | 0 | 1 | 2 | 6.944 | 2 | 0.144 | 8.933 | 3.328 | 6.5 | 16 | 0 |
| Catsper1 | 2 | 0 | 1 | 2 | 5.854 | 2 | 0.173 | 8.933 | 3.432 | 48 | 60 | 0 |
| C1orf116 | 2 | 0 | 1 | 2 | 7.079 | 1 | 0.144 | 8.6 | 3.189 | 3.167 | 8 | 0 |
| Art3 | 2 | 0 | 1 | 2 | 8.286 | 1 | 0.173 | 9.6 | 3.605 | 15.786 | 32 | 0 |
| Arhgef40 | 2 | 0 | 1 | 2 | 8.291 | 1 | 0.173 | 9.95 | 3.744 | 18 | 32 | 0 |
| Cinp | 1 | 0 | 1 | 1 | 3.38 | 1 | 0.144 | 6.517 | 2.392 | 0 | 0 | 0 |
| C20orf96 | 1 | 0 | 1 | 1 | 1.485 | 1 | 0.067 | 1 | 0.2 | 0 | 0 | 0 |
| Borcs8 | 1 | 0 | 1 | 1 | 3.838 | 1 | 0.144 | 6.767 | 2.6 | 0 | 0 | 0 |
| Bcl7c | 1 | 0 | 1 | 1 | 1.485 | 1 | 0.067 | 1 | 0.2 | 0 | 0 | 0 |
| Arhgef10l | 1 | 0 | 1 | 1 | 6.506 | 1 | 0.173 | 8.9 | 3.571 | 0 | 0 | 0 |
| Celsr3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apoa2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: MCC, maximal clique centrality; DMNC, density of maximum neighborhood; MNC, maximum neighborhood component; EPC, edge percolated component; BN, BottleNeck; CC, clustering coefficient.
Table 6.
The hub genes in the modules of Salmon in NSTEMI
| Gene | MCC | DMNC | MNC | Degree | EPC | BN | EcCentricity | Closeness | Radiality | Betweenness | Stress | CC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldh1b1 | 7 | 0 | 1 | 7 | 9.385 | 23 | 0.193 | 14.65 | 7.561 | 408.857 | 736 | 0 |
| Ell3 | 4 | 0 | 1 | 4 | 8.52 | 18 | 0.193 | 12.983 | 7.423 | 256.586 | 528 | 0 |
| Cavin4 | 4 | 0 | 1 | 4 | 8.366 | 15 | 0.193 | 11.983 | 7.181 | 182.362 | 470 | 0 |
| Cabp4 | 5 | 0.308 | 2 | 5 | 7.964 | 3 | 0.138 | 11.342 | 6.49 | 124.167 | 306 | 0.1 |
| Dus3l | 3 | 0 | 1 | 3 | 7.499 | 2 | 0.161 | 11.6 | 7.077 | 89.776 | 210 | 0 |
| Bhlhe41 | 3 | 0 | 1 | 3 | 7.043 | 2 | 0.161 | 11.3 | 6.974 | 103.333 | 210 | 0 |
| C8orf58 | 3 | 0 | 1 | 3 | 7.029 | 6 | 0.161 | 10.883 | 6.836 | 162.143 | 328 | 0 |
| Eif1ay | 2 | 0 | 1 | 2 | 6.986 | 7 | 0.161 | 11.283 | 7.181 | 173.638 | 278 | 0 |
| Fam107a | 3 | 0 | 1 | 3 | 6.961 | 7 | 0.138 | 10.96 | 6.87 | 161.971 | 260 | 0 |
| Gamt | 2 | 0 | 1 | 2 | 6.93 | 5 | 0.161 | 10.3 | 6.801 | 57.181 | 178 | 0 |
| C7orf43 | 3 | 0.308 | 2 | 3 | 6.782 | 1 | 0.121 | 9.751 | 6.076 | 24.5 | 44 | 0.333 |
| Camkk2 | 2 | 0 | 1 | 2 | 6.682 | 1 | 0.161 | 10.3 | 6.801 | 57.181 | 178 | 0 |
| Fbxl6 | 3 | 0 | 1 | 3 | 6.498 | 2 | 0.121 | 10.301 | 6.387 | 71.805 | 152 | 0 |
| Cltb | 3 | 0 | 1 | 3 | 6.244 | 3 | 0.121 | 9.885 | 6.283 | 78.5 | 104 | 0 |
| Best3 | 3 | 0 | 1 | 3 | 6.2 | 3 | 0.161 | 10.833 | 6.767 | 106 | 170 | 0 |
| Fdxacb1 | 2 | 0.308 | 2 | 2 | 6.082 | 1 | 0.121 | 8.58 | 5.662 | 0 | 0 | 1 |
| Bola1 | 1 | 0 | 1 | 1 | 5.414 | 1 | 0.161 | 9.5 | 6.629 | 0 | 0 | 0 |
| Akr1e2 | 2 | 0 | 1 | 2 | 5.237 | 2 | 0.161 | 9.583 | 6.56 | 54 | 76 | 0 |
| Cenps | 1 | 0 | 1 | 1 | 5.225 | 1 | 0.161 | 9.5 | 6.629 | 0 | 0 | 0 |
| Cope | 2 | 0 | 1 | 2 | 4.569 | 3 | 0.138 | 8.786 | 6.042 | 104 | 196 | 0 |
| Dync2li1 | 1 | 0 | 1 | 1 | 4.182 | 1 | 0.138 | 8.26 | 6.145 | 0 | 0 | 0 |
| Emilin1 | 1 | 0 | 1 | 1 | 4.162 | 1 | 0.138 | 8.093 | 6.042 | 0 | 0 | 0 |
| Atp23 | 1 | 0 | 1 | 1 | 4.01 | 1 | 0.107 | 7.255 | 5.351 | 0 | 0 | 0 |
| Gmnn | 1 | 0 | 1 | 1 | 3.788 | 1 | 0.138 | 7.819 | 5.835 | 0 | 0 | 0 |
| Gnat2 | 1 | 0 | 1 | 1 | 3.706 | 1 | 0.138 | 7.819 | 5.835 | 0 | 0 | 0 |
| C16orf95 | 1 | 0 | 1 | 1 | 3.673 | 1 | 0.107 | 7.472 | 5.455 | 0 | 0 | 0 |
| Ddx39a | 2 | 0 | 1 | 2 | 3.369 | 2 | 0.121 | 7.421 | 5.179 | 54 | 100 | 0 |
| C17orf51 | 1 | 0 | 1 | 1 | 3.264 | 1 | 0.138 | 7.269 | 5.627 | 0 | 0 | 0 |
| C8orf37 | 1 | 0 | 1 | 1 | 2.492 | 1 | 0.107 | 5.903 | 4.246 | 0 | 0 | 0 |
| Alpl | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: MCC, maximal clique centrality; DMNC, density of maximum neighborhood; MNC, maximum neighborhood component; EPC, edge percolated component; BN, BottleNeck; CC, clustering coefficient.
Discussion
Gene co-expression analysis and genome module network detection enable deep exploration of inter-gene relationships [20]. WGCNA aims to distinguish high-order relationships among gene products. By focusing on the correlation between co-expression modules and clinical characteristics, the results of WGCNA analysis have higher reliability and biological significance [21]. The identified biologically related modules and hub genes may eventually serve as biomarkers for detection or treatment of AMI. In this study, the WGCNA method was used to analyze 3653 genes from RNA-seq data of 16 NSTEMI and 16 STEMI samples. Correlation between genes and clinical traits was analyzed by establishing a co-expression module combined with clinical traits. We established seven gene co-expression modules in STEMI and 10 gene co-expression modules in NSTEMI.
The hub genes in the sky-blue module in STEMI are closely associated with cardiovascular disease. The sky-blue module in STEMI is significantly correlated with BMI. A large number of recent studies have shown that a high percentage of obese people are associated with some types of cardiovascular disease, especially with coronary heart disease [22,23]. Our analysis suggests that high BMI is a high-risk factor for cardiovascular events. In this study, the results of GO enrichment analysis of genes significantly correlated with BMI suggest that the genes in this module are mainly related to Cellular Component. The exosomes are closely associated with endothelial cell function [24]. The functions of these genes are mainly involved in cell membrane transport. Earlier studies [25] found that regulatory proteins on endothelial cell membranes play an important role in the occurrence of thromboembolic disease. For example, thrombomodulin acts as a cofactor of thrombin-mediated protein C activation, and impaired function of thrombomodulin cofactors may also lead to abnormal thrombosis in thromboembolic diseases. The Hub genes of this module were Aqp1, Armcx1, Gsta4, Hist3h2a, and Il17re. AQP1 is a key member of the aquaporins (AQPs) family, which plays an important role in promoting water transport by regulating osmotic pressure and enhancing the membrane permeability to water. AQP protein plays a potential pathophysiological role in myocardial edema [26,27]. GSTA4 plays an important role in regulating oxidative stress in human atherosclerosis [28]. IL-17 is widely involved in the regulation of myocardial inflammation, and has been reported in acute coronary syndrome (CAD) and atherosclerosis [29,30]. HIST3H2A is also involved in atherosclerosis [31,32]. The roles of the remaining HUB genes in cardiovascular disease have not been further studied. We carried out transcriptome analysis in AMI patients compared to CAD patients with no history of MI, and showed that hub genes might contribute to the recovery from AMI [33]. Our results showed the hub genes might involve in both CAD and AMI.
The sky-blue module in NSTEMI is significantly correlated with CAD. Functional enrichment analysis shows that this module mainly enriched in genes associated with energy metabolism. The heart is a high energy-consuming organ, which needs sufficient blood oxygen to provide energy to maintain its normal function [34]. Hub genes in this module include Olr1, Nap1l3, Gfer, Dohh, and Crispld1. OLR1 is induced by atherosclerotic stimulation and inflammatory cytokines [35], which are up-regulated in rats with ischemia-reperfusion injury [36]. The Olr1 gene encodes the endothelial lectin-like oxidized low density lipoprotein (oxLDL) receptor, which is involved in oxLDL binding, internalization, and protein hydrolysis degradation, suggesting that this receptor may play an important role in atherosclerosis [37]. Crispld1 rs12115090 polymorphism plays an important regulatory role in the antiplatelet effect of clopidogrel in Han Chinese patients with coronary heart disease [38]. The remaining HUB genes have received little study with relation to in cardiovascular disease.
Genes in the cyan module and salmon module were significantly correlated with HT in NSTEMI. The functions of the cyan module group, such as GO: 0005615~extracellular space and GO: 0005509~calcium binding, play important roles in the regulation of HT [39,40]. The Hub genes in this module were Clec12b, Chchd4, Asgr1, Armcx4, Chid1, and Alkbh7. The main function enrichment in the salmon module includes GO: 0009887~organ morphogenesis.
Numerous studies have suggested that elastin is related to arterial hypertension [41,42], suggesting that those genes affecting arterial elasticity affect the occurrence of hypertension and lead to the occurrence of adverse cardiovascular events. The Hub genes in this module were Ell3, Aldh1b1, Cavin4, Cabp4, Eif1ay, and Dus3l. It has been reported that Asgr1 variation is associated with a reduced risk of coronary heart disease [43]. Decrease in ASGR1 expression has been observed in the peripheral blood mononuclear cells of diabetic atherosclerosis patients [44]. It has been reported that MURC/Cavin-4 can be a therapeutic target for cardiac I/R injury [45]. In the mouse model of ischemia-reperfusion injury, decrease in MURC/Cavin-4 can reduce infarct size and preserve cardiac function [46]. EIFAY was found by variational Bayesian Gaussian mixture model analysis in screening target genes of microRNAs in coronary heart disease [47].
The results or our study provide a framework for co-expression gene modules in STEMI and NSTEMI patients, and identify key regulatory targets. Although only a few studies on human platelet RNA-seq have been conducted thus far, our study suggests the value of further investigation of the functions of these potential hub genes. Thus far, we found only one RNA-seq dataset of platelets from MI patients that included 16 NSTEMI and 16 STEMI samples. WGCNA algorithm (which requires a sample size greater than 15), we plan watch for to new RNA-seq data to verify the candidate target genes identified here, although that dataset met the minimum sample size requirement for use of the WGCNA.
Data availability statement
This study is a re-analysis of existing data, which are openly available at the locations cited in the reference section. Further documentation about data processing during the current study are available from the corresponding author upon request.
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (No. 81570310&81770337), The Natural Science Foundation of Xinjiang Province (No. 2020D01C138) and Natural Science Foundation of Hunan Province (No. 2019JJ50893).
Disclosure of conflict of interest
None.
References
- 1.National Heart Lung, Blood Institute % J Bethesda: National Heart Lung and Institute Blood. What are the signs and symptoms of coronary heart disease. 2016 [Google Scholar]
- 2.McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;124:40–47. doi: 10.1016/j.amjmed.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matetzky S, Shenkman B, Guetta V, Shechter M, Beinart R, Goldenberg I, Novikov I, Pres H, Savion N, Varon D, Hod H. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–3175. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- 4.Snoep JD, Roest M, Barendrecht AD, De Groot PG, Rosendaal FR, Van Der Bom JG. High platelet reactivity is associated with myocardial infarction in premenopausal women: a population-based case-control study. J Thromb Haemost. 2010;8:906–913. doi: 10.1111/j.1538-7836.2010.03786.x. [DOI] [PubMed] [Google Scholar]
- 5.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 6.Gawaz M, Stellos K, Langer HF. Platelets modulate atherogenesis and progression of atherosclerotic plaques via interaction with progenitor and dendritic cells. J Thromb Haemost. 2008;6:235–242. doi: 10.1111/j.1538-7836.2008.02867.x. [DOI] [PubMed] [Google Scholar]
- 7.Parodi G, Marcucci R, Valenti R, Gori AM, Migliorini A, Giusti B, Buonamici P, Gensini GF, Abbate R, Antoniucci D. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215–1223. doi: 10.1001/jama.2011.1332. [DOI] [PubMed] [Google Scholar]
- 8.Kottke-Marchant K. Importance of platelets and platelet response in acute coronary syndromes. Cleve Clin J Med. 2009;76(Suppl 1):S2–7. doi: 10.3949/ccjm.76.s1.01. [DOI] [PubMed] [Google Scholar]
- 9.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, Bhatt DL, Cattaneo M, Collet JP, Cuisset T, Gachet C, Montalescot G, Jennings LK, Kereiakes D, Sibbing D, Trenk D, Van Werkum JW, Paganelli F, Price MJ, Waksman R, Gurbel PA Working Group on High On-Treatment Platelet Reactivity. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 10.Pei G, Chen L, Zhang W. WGCNA application to proteomic and metabolomic data analysis. Methods Enzymol. 2017;585:135–158. doi: 10.1016/bs.mie.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Liu W, Liu X, Qi J, Deng C. Biomarkers identification for acute myocardial infarction detection via weighted gene co-expression network analysis. Medicine (Baltimore) 2017;96:e8375. doi: 10.1097/MD.0000000000008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barr TL, VanGilder RL, Seiberg R, Petrone A, Chantler PD, Huang CC. Systemic transcriptional alterations of innate and adaptive immune signaling pathways in atherosclerosis, ischemia stroke, and myocardial infarction. J Bioanal Biomed. 2015;7:029–34. doi: 10.4172/1948-593X.1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Yu L, Zhang S, Chen X. Network analysis-based approach for exploring the potential diagnostic biomarkers of acute myocardial infarction. Front Physiol. 2016;7:615. doi: 10.3389/fphys.2016.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niu X, Zhang J, Zhang L, Hou Y, Pu S, Chu A, Bai M, Zhang Z. Weighted gene co-expression network analysis identifies critical genes in the development of heart failure after acute myocardial infarction. Front Genet. 2019;10:1214. doi: 10.3389/fgene.2019.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinkel D, Albertson DG. Array comparative genomic hybridization and its applications in cancer. Nat Genet. 2005;37(Suppl):S11–17. doi: 10.1038/ng1569. [DOI] [PubMed] [Google Scholar]
- 16.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc. 2009;4:1184–1191. doi: 10.1038/nprot.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wisniewski N, Cadeiras M, Bondar G, Cheng RK, Shahzad K, Onat D, Latif F, Korin Y, Reed E, Fakhro R, Deng MC. Weighted Gene Coexpression Network Analysis (WGCNA) modeling of multiorgan dysfunction syndrome after mechanical circulatory support therapy. J Heart Lung Transplant. 2013;32:S223. [Google Scholar]
- 19.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 20.Stuart JM, Eran S, Daphne K, Kim SK. A gene-coexpression network for global discovery of conserved genetic modules. Science. 2003;302:249–255. doi: 10.1126/science.1087447. [DOI] [PubMed] [Google Scholar]
- 21.Chou WC, Cheng AL, Brotto M, Chuang CY. Visual gene-network analysis reveals the cancer gene co-expression in human endometrial cancer. BMC Genomics. 2014;15:300. doi: 10.1186/1471-2164-15-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KS, Owen WL, Williams D, Adams-Campbell LL. A comparison between BMI and conicity index on predicting coronary heart disease: the framingham heart study. Ann Epidemiol. 2000;10:424–431. doi: 10.1016/s1047-2797(00)00065-x. [DOI] [PubMed] [Google Scholar]
- 23.Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith GD. Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet. 1996;348:1478. doi: 10.1016/S0140-6736(96)03482-4. [DOI] [PubMed] [Google Scholar]
- 24.Zeng Y, Yao X, Liu X, He X, Li L, Liu X, Yan Z, Wu J, Fu B. Anti-angiogenesis triggers exosomes release from endothelial cells to promote tumor vasculogenesis. J Extracell Vesicles. 2019;8:1629865. doi: 10.1080/20013078.2019.1629865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohlin AK, Morser J, Ohlin H. Soluble thrombomodulin antigen in plasma is increased in patients with acute myocardial infarction treated with thrombolytic therapy. Thromb Res. 1996;82:313–322. doi: 10.1016/0049-3848(96)00081-3. [DOI] [PubMed] [Google Scholar]
- 26.Frigeri A, Nicchia GP, Verbavatz JM, Valenti G, Svelto M. Expression of aquaporin-4 in fast-twitch fibers of mammalian skeletal muscle. J Clin Invest. 1998;102:695–703. doi: 10.1172/JCI2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang B, Miao H, Yuan Y, Qiu F, Liu X, Liu Z, Zhang H, Zhao Q, Wang M, Dong H, Zhang Z. PEDF decreases cardiomyocyte edema during oxygenglucose deprivation and recovery via inhibiting lactate accumulation and expression of AQP1. Int J Mol Med. 2019;43:1979–1990. doi: 10.3892/ijmm.2019.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Yang Y, Trent MB, He N, Lick SD, Zimniak P, Awasthi YC, Boor PJ. Glutathione-S-transferase A4-4 modulates oxidative stress in endothelium: possible role in human atherosclerosis. Atherosclerosis. 2004;173:211–221. doi: 10.1016/j.atherosclerosis.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Su SA, Ma H, Shen L, Xiang MX, Wang JA. Interleukin-17 and acute coronary syndrome. J Zhejiang Univ Sci B. 2013;14:664–669. doi: 10.1631/jzus.BQICC701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shuang C, Crother TR, Moshe A. Emerging role of IL-17 in atherosclerosis. J Innate Immun. 2010;2:325–333. doi: 10.1159/000314626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdullah MH, Othman Z, Noor HM, Arshad SS, Yusof AK, Jamal R, Rahman AR. Peripheral blood gene expression profile of atherosclerotic coronary artery disease in patients of different ethnicity in Malaysia. J Cardiol. 2012;60:192–203. doi: 10.1016/j.jjcc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Cao Y, Li R, Li Y, Zhang T, Wu N, Zhang J, Guo Z. Identification of transcription factor-gene regulatory network in acute myocardial infarction. Heart Lung Circ. 2017;26:343–353. doi: 10.1016/j.hlc.2016.06.1209. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Ma C, Gu J, Yu M. Potential biomarkers of acute myocardial infarction based on weighted gene co-expression network analysis. Biomed Eng Online. 2019;18:9. doi: 10.1186/s12938-019-0625-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salem JE, Saidel GM, Stanley WC, Cabrera ME. Mechanistic model of myocardial energy metabolism under normal and ischemic conditions. Ann Biomed Eng. 2002;30:202–216. doi: 10.1114/1.1454133. [DOI] [PubMed] [Google Scholar]
- 35.Mehta JL, Li D. Identification, regulation and function of a novel lectin-like oxidized low-density lipoprotein receptor. J Am Coll Cardiol. 2002;39:1429–1435. doi: 10.1016/s0735-1097(02)01803-x. [DOI] [PubMed] [Google Scholar]
- 36.Li D, Williams V, Liu L, Chen H, Sawamura T, Romeo F, Mehta JL. Expression of lectin-like oxidized low-density lipoprotein receptors during ischemia-reperfusion and its role in determination of apoptosis and left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1048–1055. doi: 10.1016/s0735-1097(02)02966-2. [DOI] [PubMed] [Google Scholar]
- 37.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–7. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 38.Wang JY, Zhang YJ, Li H, Hu XL, Li MP, Song PY, Ma QL, Peng LM, Chen XP. CRISPLD1 rs12115090 polymorphisms alters antiplatelet potency of clopidogrel in coronary artery disease patients in Chinese Han. Gene. 2018;678:226–232. doi: 10.1016/j.gene.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 39.Nagasawa K, Zimmermann R, Munkel B, Linz W, Scholkens B, Schaper J. Extracellular matrix deposition in hypertensive hearts antifibrotic effects of ramipril. Eur Heart J. 1995;16(Suppl C):33–37. doi: 10.1093/eurheartj/16.suppl_c.33. [DOI] [PubMed] [Google Scholar]
- 40.Postnov YV, Orlov SN, Shevchenko A, Adler AM. Altered sodium permeability, calcium binding and Na-K-ATPase activity in the red blood cell membrane in essential hypertension. Pflugers Arch. 1977;371:263–269. doi: 10.1007/BF00586267. [DOI] [PubMed] [Google Scholar]
- 41.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 42.Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res. 2012;5:264–273. doi: 10.1007/s12265-012-9349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huynh K. Genetics: variants in ASGR1 linked to reduced CAD risk. Nat Rev Cardiol. 2016;13:442. doi: 10.1038/nrcardio.2016.90. [DOI] [PubMed] [Google Scholar]
- 44.Hamledari H, Sajjadi SF, Alikhah A, Boroumand MA, Behmanesh M. ASGR1 but not FOXM1 expression decreases in the peripheral blood mononuclear cells of diabetic atherosclerotic patients. J Diabetes Complications. 2019;33:539–546. doi: 10.1016/j.jdiacomp.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Mirizzi AM, Demarchi A, Crimi G, Ferlini M, Ruffinazzi M, Camporotondo R, Ravera A, Ferrario M, Oltrona-Visconti L, De Ferrari GM. Role of hypoalbuminemia in myocardial reperfusion after primary PCI in patients with ST-elevation myocardial infarction. Eur Heart J. 2018;39(Suppl 1):P1686. [Google Scholar]
- 46.Nishi M, Ogata T, Nakanishi N, Kasahara T, Maruyama N, Higuchi Y, Matoba S. Abstract 12941: MURC/Cavin-4 deficiency reduces infarct size and preserves cardiac function in a mouse model of ischemia-reperfusion injury. Circulation. 2016;134:A12941. [Google Scholar]
- 47.Ma XL, Yang X, Fan R. Screening of miRNA target genes in coronary artery disease by variational Bayesian Gaussian mixture model. Exp Ther Med. 2019;17:2129–2136. doi: 10.3892/etm.2019.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study is a re-analysis of existing data, which are openly available at the locations cited in the reference section. Further documentation about data processing during the current study are available from the corresponding author upon request.