Abstract
Secondary hemophagocytic lymphohistiocytosis (sHLH) is an excessive inflammatory response syndrome caused by immune abnormalities. Up to date, the risk factors for cytokines causing early death in sHLH patients have not been elucidated. Our study reviewed the cytokine expression levels in peripheral blood of 50 sHLH patients. Through Cox proportional hazard model analysis, we found that IL-17F ≥2.835 pg/mL (HR = 5.922, 95% CI = 1.793-19.558, P = 0.004) was an independent death risk factor in sHLH patients, and it was also 30 days (Cutoff-value = 2.890 pg/mL, HR = 16.568, 95% CI = 1.917-143.195, P = 0.011), 60 days (Cutoff-value = 2.890 pg/mL, HR = 7.559, 95% CI = 1.449-39.423, P = 0.016), 90 day death risk factor (Cutoff-value = 2.835 pg/mL, HR = 7.649, 95% CI = 1.965-29.778, P = 0.003); IL-10 ≥16.730 pg/mL (HR = 4.821, 95% CI = 1.151-20.116, P = 0.031) is not only a death risk factor within 90 days, but also within 10 days (Cutoff-value = 944.350 pg/mL, HR = 13.321, 95% CI = 1.123-158.03, P = 0.027); and IL-5 ≥2.495 pg/mL (HR = 15.687, 95% CI = 1.377-178.645, P = 0.04) was also a death risk factor within 10 days. Besides, IL-17F, IL-10, IL-5, and the previously reported common risk factors Age, platelets, activated partial thromboplastin time, triglyceride, and lactate dehydrogenase were analyzed together. It was found that the patient age ≥56 years-old is was an important risk factor for death within 30 days, IL-17 ≥2.89 pg/mL and IL-10 ≥16.73 pg/mL are important risk factors for patient death. In summary, our data indicate that age, IL-10 and IL-17F are important risk factors for early death in sHLH patients.
Keywords: Secondary hemophagocytic lymphohistiocytosis, risk factors for early death, cytokine, interleukin 17F, interleukin 10, age
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a rare life-threatening immune disease. It includes major types caused by genetic defects and immunodeficiency (such as PRF1, Munc13-4, Roquin-1, STX11, STXPB2, ITK, XIAP, LYST, SRGN), and secondary Hemophagocytic lymphohistiocytosis (sHLH) types caused mainly by tumors, infections, and rheumatic diseases [1-3]. In HLH patients, due to inflammatory response targets that cannot be eliminated, the persistence of inflammatory responses leads to uncontrolled activation of macrophages [4]. The primary hemophagocytic syndrome occurs more commonly in children and adolescents. The etiology of sHLH is complicated, and the clinical manifestations and laboratory indicators lack characteristics. Currently, it is believed that the uncontrolled regulation of the immune system may be the pathogenesis of sHLH. The imbalance of Th1/Th2 cells leads to intensive activation of cytotoxic T cells (CD8+ cells). Furthermore, cytokines are also produced in high concentration and stimulate the proliferation and activation of tissue macrophages. This in turn leads to high fever, liver function damage, hyperlipidemia, coagulopathy, phagocytosis and decreased NK cell activity [5,6]. Macrophage colony-stimulating factor (M-CSF), TNF-α, IL-1, and IL-6 are produced after excessive activation of tissue macrophages, which further aggravates cytokinineemia [5,7,8], activated macrophages engulf blood cells, IFN-α, TNF-α, and other factors to inhibit bone marrow hematopoiesis, accompanied by abnormal consumption of coagulation function, thus resulting in pancytopenia [9,10]. The causes of sHLH include infections, tumors, and autoimmune diseases. Secondary HLH is most commonly characterized by an infection caused by the Epstein-Barr virus (EBV), especially in Asia [11]. In the past few decades, HLH has been considered a genetic disease in children. But accumulating evidence indicates that the disease is susceptible to occur at any age, and about 40% of the patients are adults [12]. The cancer-related mortality rate of adult patients is generally higher, about 20%-60% [13]. HLH represents only 1-2 months median survival time, while there was only a 5% 1-year survival rate in the 1980s [14]. However, the current research shows that HLH-94 or HLH-04 treatment regimens have the potential to increase the disease response rate from less than 10% in the past to about 70% [15,16]. Unfortunately, the HLH-94 study exhibited only a 54% 5-year survival rate, while it was 62% for the HLH-04 study [17]. For patients with HLH-94 and HLH-04 schemes that are ineffective and refractory to relapse, there is currently no unified treatment plan. In addition to combined chemotherapy, a traditional treatment plan for the allogeneic hematopoietic stem cell transplantation, with the continuous deepening of the pathogenesis of HLH, a series of targeted drugs have been gradually applied in clinical practice. At present, it is relatively certain that Emapalumab (human anti-interferon-γ antibody) is an effective targeted therapy for primary HLH [18,19]. But most of the applications of these drugs are currently case reports, like JAK1/2 inhibitor Ruxolitinib was used as first-line treatment for sHLH [20,21], and the cytokine storm and multiorgan failure rapidly reversed, and sHLH patients’ condition improved after treatment with Tocilizumab. These targeted drugs can really treat sHLH patients. Despite this, there is no definitive study reporting that these targeted therapies can reduce the early mortality of sHLH. The early deaths rate of sHLH patients within one month still remains about 50% [22].
Previous studies on the relationship between cytokines and HLH risk mostly focused on children, which proved that cytokines had a predictive effect. There are few studies on the relationship between cytokines and sHLH risk (For example, IL-10 is a risk factor for early death in children) [23,24]. Based on the role of cytokines in predicting the risk of HLH related deaths in children, combined with the analysis of cytokine expression levels may be beneficial to predict the risk of sHLH related deaths. In this study, we tested serum Th1/Th2 cytokines including interferon (IFN)-γ, interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17A, IL-17F, IL-22, tumor necrosis factor (TNF)-α, and tumor necrosis factor (TNF)-β in 50 sHLH patients. Cox proportional hazards model was used for investigating the risk factor of described cytokines for early deaths in the sHLH patients. Then, four different groups of patients were prepared according to their different survival rates: (1) Survival time 1-10 days and >10 days, (2) Survival time 1-30 days and >30 days, (3) Survival time 1-60 days and >60 days, (4) Survival time 1-90 days and >90 days. The risk of deaths in different time zone was analyzed by the Cox proportional hazard model for the analysis of the risk factors associated with the early deaths of sHLH.
Statistical analysis
Interquartile range (IQR) and median were used to describe continuous variables, while categorical variables were shown as percentages and frequencies (%, n). The student’s t-test was used to differentiate the survivors and non-survivors. The parameters optimal cutoff values were analyzed with the help of receiver operating characteristic (ROC). The difference between the two sides of the Cutoff-value was determined by the student’s t-test. The Kaplan-Meier method and the Log-Rank Test was utilized to analyze the survival curves. Cox proportional hazard model-based analysis (univariate and multivariate) was performed. Statistical software package IBM SPSS 21.0 (SPSS, USA) was used for all statistical calculations. P<0.05 means there is statistical difference.
Patients and data collection
In the five years from January 2014 to February 2019, the sHLH patients in the First People’s Hospital of Yunnan Province were analyzed retrospectively. Diagnosis for sHLH was conducted by using the HLH-2004 protocol of the International Histiocyte Society [16,25]. Those patients were enrolled in the study that met at least five of the eight given criteria: (1) Fever for more than 1 week, with a peak of more than 38.5°C. (2) Splenomegaly. (3) Pancytopenia. (4) Fibrinogen <1.5 g/L and/or Triglyceride ≥2.0 mmol/L. (5) Hemophagocytosis in lymph nodes, spleen, or bone marrow. (6) Reduced or lacking activity of NK cell. (7) Ferritin ≥500 ug/L and (8) Soluble CD25 ≥2400 U/mL. The patients were excluded who possessed concurrent malignancy, previous history of macrophage activation syndrome, or immunosuppressive therapy. Through reviewing the patient’s medical records, laboratory results and clinical features were obtained at the time of diagnosis. Follow-up of the study was accomplished by making phone calls or examining medical records and the results were recorded on the last follow-up day. We measured the overall survival (OS) of patients from the diagnosis of HLH to the last follow-up or death for any cause. The preliminary research content of this project strictly follows the Declaration of Helsinki, the International Code of Ethics for Biomedical Research Involving Humans jointly formulated by the World Health Organization and the Council of International Medical Science Organizations, and the relevant regulations of the Ethics Committee of the First People’s Hospital of Yunnan Province (2014YXLH077).
Cytokine determination
Quantitative determination of serum cytokines levels (Th1/Th2) including Interferon (IFN)-γ, Interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17A, IL-17F, IL-22, Tumor necrosis factor (TNF)-α and TNF-β, was carried out using the Aimplex Cytokine (QuantoBio, Tianjing, China) with the detection range of 1-2500 pg/mL. Data of cytokines were collected for all patients during their diagnosis.
Results
Patient characteristics
Fifty sHLH patients [25 males and 25 females; with 40.5-year median age (range: 16-76 years)] were selected in this study. In this cohort, the OS rate was 24% (12/50), the 30-day OS rate was 56% (28/50), and the 60-day OS rate was 42% (21/50). All patients had a high prolonged fever and ferritin >500 ng/mL. Other commonly occurring clinical manifestations included hyperferritinemia 96% (48/50), and splenomegaly 84% (42/50). Laboratory examination abnormalities included leukopenia 70% (35/50), anemia 88% (44/50), and thrombocytopenia 80% (40/50). In HLH patients, the triggering factors were classified as: malignancies 34% (17/50), followed by Epstein-Barr virus infection 24% (12/50), autoimmune disorders 6% (3/50), and unknown triggers 36% (18/50) (Table 1). Laboratory and clinical indicators between survivors and non-survivors were compared and the overall mortality rate for this study was observed as 76% (38/50). Comparisons of clinical and laboratory parameters for survivors and non-survivors are shown in Tables 1 and 2. The age of non-survivors (median age 42.5 years) was generally higher than survivors (median age 28 years), which is statistically significant (P = 0.047) (Table 1). Similarly, no statistical difference in cytokines level exists between the two groups.
Table 1.
Basic patient cohort information
| Parameters | Survivors (n = 12) | Non-survivors (n = 38) |
|---|---|---|
| Sex (Male/Female) | 4/8 | 21/17 |
| Nationality | 10/2 | 8/30 |
| Lymphoma | 25% | 36.80% |
| Epstein-Barr virus infection | 25% | 23.70% |
| Autoimmune disorders | 8.30% | 5.30% |
| Unknown triggers | 41.70% | 34.20% |
| Age (years) Median (IQR) | 28 (16.0-54.0) | 42.5 (16.0-76.0) |
| Temperature (°C) Median (IQR) | 39.45 (36.20-42.00) | 39.5 (36.50-41.00) |
| WBC (×109/L) Median (IQR) | 2.25 (1.40-10.60) | 3.07 (0.50-25.90) |
| HGB (g/L) Median (IQR) | 109.5 (71.00-163.00) | 98 (45.00-600.00) |
| PLT (×109/L) Median (IQR) | 76.5 (15.00-354.00) | 35 (8.00-177.00) |
The proportion of patients with lymphoma, Epstein-Barr virus infection, and autoimmune diseases in the study cohort. And the distribution of patient age, gender, ethnicity, body temperature at the first visit, WBC, HGB, PLT in the study cohort.
Table 2.
Comparison of cytokines between survivors and non-survivors
| Parameters (pg/mL) | Survivors (n = 12) | Non-survivors (n = 38) | ||
|---|---|---|---|---|
|
|
|
|||
| mean-value | Median (IQR) | mean-value | Median (IQR) | |
| IL-1β | 1.889 | 1.78 (0.590-4.920) | 23.903 | 2.365 (0.050-499.870) |
| IL-2 | 31.64 | 2.54 (0.100-345.480) | 33.69 | 2.24 (0.000-697.930) |
| IL-4 | 3.388 | 3.53 (0.140-6.870) | 2.648 | 1.755 (0.330-9.780) |
| IL-5 | 1.499 | 1.35 (0.400-4.100) | 1.805 | 1.76 (0.900-3.100) |
| IL-6 | 26.847 | 7.81 (1.680-93.090) | 155.693 | 66.76 (1.870-1081.870) |
| IL-8 | 36.076 | 14.24 (4.540-133.950) | 111.364 | 41.775 (2.550-996.410) |
| IL-10 | 231.775 | 14.875 (2.300-1434.000) | 181.687 | 31.955 (1.510-1719.440) |
| IL-12P70 | 4.96 | 4.445 (0.5-20.040) | 4.273 | 3.85 (0.230-10.970) |
| IL-17A | 1.792 | 1.4 (0.100-4.330) | 3.09 | 2.32 (0.980-7.900) |
| IL-17F | 2.611 | 1.1 (0.50-013.700) | 2.835 | 2.88 (0.400-5.500) |
| IL-22 | 6.425 | 1.4 (0.440-32.550) | 2.542 | 1.7 (0.150-9.600) |
| IFN-γ | 42.141 | 2.195 (1.210-464.200) | 54.297 | 5.66 (0.430-860.510) |
| TNF-α | 7.307 | 1.21 (0.630-57.390) | 6.753 | 3.42 (1.120-42.280) |
| TNF-β | 2.52 | 3.025 (0.790-3.900) | 4.153 | 2.93 (0.720-20.803) |
Continuous variables are presented as median with interquartile range (IQR).
Univariate analysis for risk factors
Due to the high early mortality rate of HLH, about 50% HLH patients died within 30 days. To explore whether there are risk factors for cytokines between survivors and non-survivors, we set Cutoff-value. Table 3 and Supplementary Table 1 show the outcomes of univariate analysis of the potential predictors of death in patients with HLH. The IL-2 (P = 0.0351), IL-4 (P<0.0001), IL-5 (P = 0.0009), IL-6 (P = 0.0024), IL-10 (P = 0.0321), IL-12P70 (P≤0.0001), IL-17A (P = 0.0002), IL-17F (P = 0.0009), IL-22 (P = 0.0106), Age (P<0.0001), Temperature (P = 0.0028), WBC (P = 0.0002), HGB (P<0.0001), and PLT (P<0.0001) were significantly different, but no significant differences were found in other cytokines. Besides, in Figure 1A-E and Supplementary Figure 1A, the Kaplan-Meier method described the HLH patient’s survival curve with different risk factors between survivors and non-survivors. As for cumulative survival probability of patients, IL-1β <2.695 pg/mL was greater than IL-1β ≥2.695 pg/Ml (P = 0.047), IL-17A <1.930 pg/mL was greater than IL-17A ≥1.930 pg/mL (P = 0.015), IL-17F <2.835 pg/mL was greater than IL-17F ≥2.835 pg/mL (P = 0.001), IFN-γ <2.605 pg/mL was greater than IFN-γ ≥2.605 (P = 0.023), and TNF-α <1.263 pg/mL was greater than TNF-α ≥1.263 pg/mL (P = 0.032). Similarly, patients whose age was less than 54.5 years had a survival rate greater than ≥54.5 years-old patients (P = 0.027).
Table 3.
The Cutoff-value of cytokines between survivors and non-survivors
| Parameters (pg/mL) | Cutoff-value | P-value | 95% Confidence Interval |
|---|---|---|---|
| IL-1β | 2.695 | 0.1005 | - |
| IL-2 | 3.35 | 0.0351 | 6.986-181.200 |
| IL-4 | 4.835 | <0.0001 | 2.806-5.551 |
| IL-5 | 1.245 | 0.0009 | 0.527-1.758 |
| IL-6 | 38.68 | 0.0024 | 82.410-349.200 |
| IL-8 | 18.005 | 0.0608 | - |
| IL-10 | 16.725 | 0.0321 | 28.360-595.500 |
| IL-12P70 | 3.53 | <0.0001 | 2.833-6.745 |
| IL-17A | 1.93 | 0.0002 | 1.358-3.751 |
| IL-17F | 2.835 | 0.0009 | 1.744-5.765 |
| IL-22 | 1.685 | 0.0106 | 2.090-14.090 |
| IFN-γ | 2.605 | 0.2307 | - |
| TNF-α | 1.265 | 0.1045 | - |
| TNF-β | 1.82 | 0.0775 | - |
The Cutoff-value of cytokines was determined by the ROC curve, and the difference between the two sides was tested by the student’s t-test.
Figure 1.
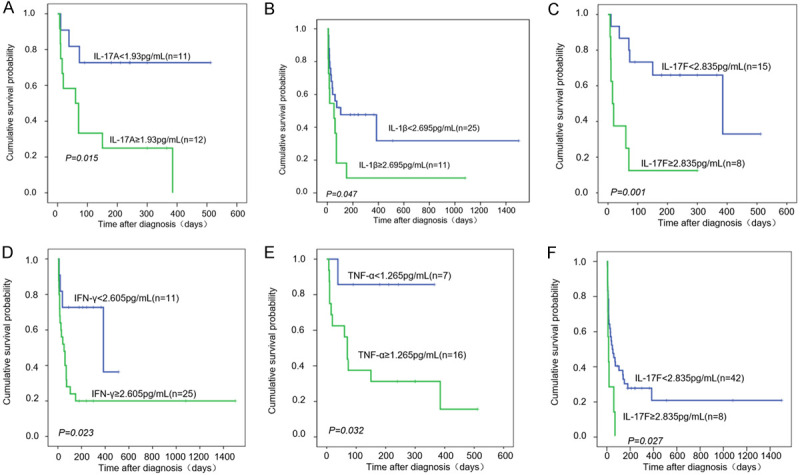
Kaplan-Meier survival curve of HLH patients with Cytokine’s different risk between survivors and non-survivors. Kaplan-Meier curve of patients Overall survival (OS) with (A) IL-17A ≥1.93 pg/mL or IL-17A <1.93 pg/mL, (B) IL-1β <2.695 pg/mL or IL-1β ≥2.695 pg/mL, (C) IL-17F <2.835 pg/mL or IL-17F ≥2.835 pg/mL, (D) INF-γ <2.605 pg/mL or INF-γ ≥2.605 pg/mL, (E) TNF-α ≥1.265 pg/mL or TNF-α <1.265 pg/mL, (F) IL-17F <46.35 pg/mL or IL-17F ≥46.35 pg/mL.
Multivariate analysis
Based on the 9 important cytokines determined by univariate analysis, forward conditional Cox region model analysis was accomplished to investigate the independent predictors of death in HLH patients. We found that only IL-17F (Cutoff-value = 2.835 pg/mL, HR = 5.922, 95% CI = 1.793-19.558, P = 0.004) had significant difference (Table 4). Besides, Figure 1F describes the different IL-17F levels containing the HLH patent’s survival curve using the Kaplan-Meier method.
Table 4.
Multiple factor analysis of cytokines for Risk factors of different long-term death in sHLH
| Duration of survival | Parameters (pg/mL) | Adverse factor | P-value | Hazard ratio |
|---|---|---|---|---|
| Survivors and non-survivors | IL-17F | ≥2.835 | 0.004 | 5.922 |
| 1-10 days and >10 days | IL-5 | ≥2.495 | 0.04 | 13.321 |
| IL-10 | ≥944.35 | 0.027 | 15.687 | |
| 1-30 days and >30 days | IL-17F | ≥2.890 | 0.011 | 16.568 |
| 1-60 days and >60 days | IL-17F | ≥2.890 | 0.016 | 7.559 |
| 1-90 days and >90 days | IL-10 | ≥16.73 | 0.031 | 4.812 |
| IL-17F | ≥2.835 | 0.003 | 7.649 |
Through multivariate analysis of risk factors, significantly different level of IL-17F was observed in survivors and non-survivors with a hazard ratio of 5.922. Serum levels of IL-10 and IL-5 are also significantly different among patients with survival time of 1 to 10 days and >10 days, and the hazard ratios were 15.687 and 13.321 respectively. Furthermore, IL-17F also significantly different among patients with survival time of 1-30 days and >30 days, with a Hazard ratio of 16.568. Patients with survival time of 1-60 days and >60 days had significant differences in IL-17F, the hazard ratios were 7.559. IL-10 and IL-17F exhibited significant differences between 1-90 days and >90 days, with hazard ratios of 4.812 and 7.649 respectively.
In our study, we found that IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-17A, IL-17F, IL-22 are different between survivors and non-survivors. To further analyze the difference of these cytokines and their effect on early death in HLH patients, according to the survival time, we divided HLH patients into four groups: (1) Survival time 1-10 days and >10 days, (2) Survival time 1-30 days and >30 days, (3) Survival time 1-60 days and >60 days, (4) Survival time 1-90 days and >90 days. Through multivariate analysis of cytokines by forward conditional Cox regression model, we found that: (1) IL-10 (Cutoff-value = 944.350 pg/mL, HR = 13.321, 95% CI = 1.123-158.033, P = 0.027) and IL-5 (Cutoff-value = 2.495 pg/mL, HR = 15.687, 95% CI = 1.377-178.645, P = 0.04) were risk factors for death within 10 days (Table 4, Supplementary Table 2). (2) IL-17F was risk factors for death within 30 days (Cutoff-value = 2.890 pg/mL, HR = 16.568, 95% CI = 1.917-143.195, P = 0.011), and also the risk factors for death within 60 days, the Cutoff-values are (HR = 7.559, 95% CI = 1.449-39.423, P = 0.016) (Table 4, Supplementary Tables 3, 4). (3) IL-17F (Cutoff-value = 2.835 pg/mL, HR = 7.649, 95% CI = 1.965-29.778, P = 0.003) and IL-10 (Cutoff-value = 16.730 pg/mL, HR = 4.821, 95% CI = 1.151-20.116, P = 0.031) were risk factors for death within 90 days (Table 4, Supplementary Table 5).
Next, we performed Kaplan-Meier survival curve and forward conditional Cox region model analysis on IL-17F, IL-10, IL-5, and the previously reported common risk factors age, platelets (PLT), activated partial thromboplastin time (APTT), triglyceride, and lactate dehydrogenase (LDH) [4,5,26,27]. The results show that APTT showed a significant difference in the Kaplan-Meier survival curve of patients on both sides of 33.7 s (P = 0.038) (Supplementary Figure 2G). The Kaplan-Meier survival curve of patients with LDH ≥1996 U/L and LDH <1996 U/L tends to be significantly different (P = 0.095) (Supplementary Figure 2F), regardless of death within 30 days or death within 60 days. Although ATPP and LDH did not show superiority in our forward conditional Cox region model analysis (Supplementary Table 6), there are significant differences or tend to be significant differences in the Kaplan-Meier survival curve of patients on both sides of the Cutt-off value. Kaplan-Meier survival curve for patients aged ≥56 years-old and patients <56 years-old who died within 30 days tended to be significantly different (P = 0.061) (Supplementary Figure 2A). Kaplan-Meier survival curve for patients ≥49.5 years-old and <49.5 years-old who died within 60 days There was a significant difference (P = 0.064) (Supplementary Figure 2B). Also, forward conditional Cox region model analysis shows that age ≥56 years-old is an important risk factor for death in sHLH patients within 30 days (Supplementary Table 6), which is similar to previous studies reporting the risk index of age greater than 54 years-old [8]. The results of IL-17F analysis are similar to those that did not include Age, APTT, LDH, PLT, and TRIG (Table 4, Supplementary Table 6; Figure 1C, Supplementary Figure 2H), and IL-10 ≥16.73 pg/mL is the risk factor for death in the patients (HR = 4.762, P = 0.05) (Table 4, Supplementary Table 6).
Generally speaking, our research has similar results with other people’s research work, but there are also different contents. This may be attributed to the following reasons: 1. The area where patients are collected is different, 2. The characteristics of patients is different (including age range, primary disease proportion, degree of infection, regulation of the autogenous immune system, etc.), 3. The included detection indicators are inconsistent.
Discussion
The early mortality rate of sHLH remains the main challenge for clinicians. In our cohort, 44% (22/50) of patients died within 30 days. The most common cause was malignant tumors (34%, 17/50) and EBV infection (24%, 12/50). It was consistent with the results of most retrospective sHLH studies. Our results are similar to those previously reported that age ≥56 years-old is a risk factor for sHLH (Their results show age ≥54 years-old as a risk factor for early deaths [5]) (Supplementary Table 6).
It has been found in a previous study that the TNF and IFN-γ enhanced in children with familial HLH [28]. Subsequently, accumulating evidence indicates that multiple cytokines including IL-1β, IL-2, IL-6, IL-10, IL-12, IL-16, IL-18, TNF-α, and IFN-γ are elevated in patients with HLH [29,30]. The increased level of IL-10 indicates that the patient does retain the mechanism that inhibits the activation of monocytes, macrophages, and T lymphocytes (although we are not yet clear) [24,31]. Among the cytokines with a sharp increase in HLH, IFN-γ is the noteworthy one. Increased IFN-γ level results in the activation of macrophage which subsequently increases the other pro-inflammatory cytokines production. In the analysis of hematopoietic kinetics in the sHLH mouse model, it was found that the positive regulatory factors encoding myelopoiesis (granulocyte colony stimulating factor (G-CSF) and granulocyte macrophage colony stimulating factor (GM-CSF)) were increased [32]. In clinical analysis, it was also found that GM-CSF expression was increased in sHLH patients [33]. While CD8+ T cells and IL-33/ST2 axis are still important mediators of HLH, IFN-γ regulated CD8+ T cell expression of GM-CSF and neutrophil survival [34,35]. In a mouse model with perforin deficiency developed by inducing HLH via lymphocytic choriomeningitis virus infection, the HLH phenotype uniquely required a combination of CD8+ T cells and IFN-γ, indicating that the inhibition of IFN-γ can be considered as a potential target for HLH therapy [36,37], such as Emapalumab [18,19]. Although IFN-γ is not required for the fulminant HLH development, using therapies that focus to target the CD8+ T cells activators (upstream), i.e. IL-33/ST2 signaling, may be an effective treatment with more universal applicability [38]. In our research, the cumulative survival probability of IFN-γ ≥2.605 pg/mL is significantly lower than the cumulative survival probability of IFN-γ <2.605 pg/mL (Figure 1D). Elevated early cytokine i.e. IFN-γ, IL-6, and IL-10 and are the models of childhood cases, while an independent early death prognostic factor in HLH children is an increased level of IL-10 [24,39]. The important anti-inflammatory factor (IL-10) that protects the host from overreactions of pathogens and microbiota, is also significantly involved in wound healing, autoimmune response, cancer, and homeostasis maintenance [40]. Th2 cells are the very first cellular source of IL-10 [41]. However, lymphoid and myeloid cells, and some non-hematopoietic cells (including tumor cells) also secrete IL-10, in response to different stimulation [42,43]. In our study cohort, the death risk factor is IL-10 ≥944.35 pg/mL within 10 days (HR = 15.687) while IL-10 ≥16.73 pg/mL within 90 days (HR = 4.812) (Table 4). Even if other risk indicators are included, IL-10 ≥16.73 pg/mL is still a risk factor for death in sHLH patients (HR = 4.762) (Supplementary Table 6; Supplementary Figure 2E). This may be related to most patients with lymphoma in our cohort. So, IL-10 is not only an independent risk factor for early childhood death, but also a death risk factor for sHLH.
IL-5 is the only cytokine involved in differentiation, and plays a substantial role in eosinophils induction and proliferation. IL-5 regulates various inflammatory responses, thus promotes the frequent clearance of pathogens. Meanwhile, it contributes to the pathology of chronic inflammation, overproduction of IL-5 will lead to the rise of eosinophils [44,45]. Anti-IL5 therapy is considered an effective drug intervention for the treatment of eosinophilia. At present, it is mainly used to treat asthma. An important humanized monoclonal antibody, mepolizumab, can rapidly cause the neutralization of IL-5 which leads to eosinophil reduction both in tissues and circulation [46-48]. Besides, Anti-IL-5 therapy with mepolizumab can effectively inhibit the middle ear recruitment of eosinophil in patients having eosinophilic otitis media (EOM) [49]. IL-5 has been widely reported in many immune diseases, but its relationship with HLH is rarely reported. In our study, IL-5 ≥2.495 pg/mL was a risk factor for sHLH patient’s death within 10 days (HR = 13.321) (Table 4). Although IL-5 does not show an advantage after the inclusion of other risk indicators (Supplementary Table 6), it is still necessary to pay attention to the abnormal expression of IL-5 in sHLH patients.
Along with IL-10 and IL-5 mentioned above, IL-17F is the most important cytokine in our research. The IL-17A-F 6 cytokines are the family members of IL-17 which can be used as new targets for drug intervention in certain inflammatory and immune diseases [50]. To treat colitis, IL-17F was reported as an effective target as it can inhibit the development of colitis [51]. Moreover, IL-17F overexpression can induce airway inflammation and can be used as a diagnostic indicator for patients with allergic asthma [52]. The hallmark pro-inflammatory cytokines of CD4+ cells included IL-17A and IL-17F while IL-17A production or signal transduction disorder often leads to an autoimmune response and tissue destruction, however, its mechanism is not clear [53]. IL-17A and IL-17F possess 50% sequence homology and overlapping biological functions, they are up-regulated in various inflammatory tissues and synergistically enhance the inflammatory response along with the rest of the pro-inflammatory cytokines i.e. TNF [54-56]. In our study cohort, the cumulative survival probability of IL-17F ≥2.835 pg/mL was significantly lower than that of IL-17F <2.835 pg/mL, and IL-17A ≥1.93 pg/mL was considerably lower as compared to IL-17A <1.93 pg/mL, the cumulative survival probability of TNF-α ≥1.265 pg/mL was significantly lower than that of TNF-α <1.265 pg/mL (Figure 1A, 1C, 1E). IL-1 is considered an important component to differentiate Th17 cells and is capable of stimulating a variety of responses associated with T cells, including CD8+ T cells [57]. Whether or not T cell receptor (TCR) binds IL-1α and IL-1β, IL-23 can synergistically support the production of the IL-17A by T cells in both mice and humans [58]. Obtained results revealed a significant difference in the cumulative survival probability between the two sides of the IL-1β threshold (Figure 1B). This may be associated with the synergistic impact of cytokines IL-17A, IL-17F, and TNF-α, but the mechanism is still not known. IL-17F is included in various immune diseases, however, the relationship between IL-17F and sHLH is rarely reported. IL-17 is a highly versatile pro-inflammatory cytokine in itself and is important for host defense, tissue repair, the pathogenesis of inflammatory diseases, and cancer progression [52]. The main predisposing factors for sHLH are infection, tumors, and autoimmune diseases. TH17 cells are the main instigator of autoimmune pathology [50,59]. There are many conditions for the production of IL-17 by TH-17 cells, but IL-23 and/or IL-1 are usually implicated, in conjunction with signals from pathogen derived PAMPs (pathogen-associated molecular patterns) or the inflammatory environment [60]. In sHLH patients with immune disorder and organ injury, a variety of immune cells and cytokines are not controlled and interacted, and the immune network among them has not been clarified. In our study, IL-17F was found a very important risk factor. IL-17F ≥2.835 pg/mL is an independent risk factor for deaths in sHLH patients (HR = 5.922) and is also a death risk factor of sHLH patients within 90 days (HR = 7.649). In addition, IL-17F ≥2.890 pg/mL is an independent risk factor for sHLH patients who died within 30 days (HR = 16.568) and 60 days (HR = 7.559) (Table 4). We were surprised to find that no matter what period the patient died, the Cutoff-value of IL-17F was approximately 2.890 pg/mL. We were surprised to find that regardless of the length of time the patient died and whether other risk indicators were included, the cut-off value of IL-17F was about 2.890 pg/mL (Supplementary Table 6, Table 4). Therefore, IL-17F is an important death risk factor of sHLH patients. But We did not test the patient’s immune cells, so our data do not support information about which immune cells are represented by abnormal cytokine expression.
Conclusion
In this retrospective study, we found that the cytokine risk factors for the early death of sHLH patients are IL-17F and IL-10. These results may help clinicians make better treatment decisions for the sHLH patients having abnormally elevated cytokines to reduce their early mortality. At the same time, they may help the development of clinical drugs for abnormal sHLH cytokines. However, it is not clear how these cytokines are involved in the process of sHLH disease, thus further research is needed for its understanding on a molecular level.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82070173, 81700164 and 82060810), the Yunnan Applied Basic Research Projects Foundation (2018FB112), the Medical Reserve Talents Cultivation Project of Yunnan Province (H2017012), the Science and Technology Department of Yunnan Province-Kunming Medical University Applied Basic Research Joint Special Fund Young Doctor Project [2018FE001(-156)], the Doctor Research Fund of Yunnan First People’s Hospital (KHBS-2020-007), the Open Project of Yunnan Blood Clinical Medical Center (2019LCZXKF-XY02, 2020LCZXKF-XY14 and 2019LCZXKF-XY11).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Tavernier SJ, Athanasopoulos V, Verloo P, Behrens G, Staal J, Bogaert DJ, Naesens L, De Bruyne M, Van Gassen S, Parthoens E, Ellyard J, Cappello J, Morris LX, Van Gorp H, Van Isterdael G, Saeys Y, Lamkanfi M, Schelstraete P, Dehoorne J, Bordon V, Van Coster R, Lambrecht BN, Menten B, Beyaert R, Vinuesa CG, Heissmeyer V, Dullaers M, Haerynck F. A human immune dysregulation syndrome characterized by severe hyperinflammation with a homozygous nonsense Roquin-1 mutation. Nat Commun. 2019;10:4779. doi: 10.1038/s41467-019-12704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, Strauss J, Kasper B, Nürnberg G, Becker C, Maul-Pavicic A, Beutel K, Janka G, Griffiths G, Ehl S, Hennies HC. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009;85:482–492. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mo W, Wei W, Sun Y, Yang Y, Guan Z, Li M, Zhu P, Chi Z. Application of blood and immunodeficiency gene detection in the diagnosis of hemophagocytic lymphohistiocytosis patients. Exp Hematol. 2019;78:62–69. doi: 10.1016/j.exphem.2019.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Luo ZB, Chen YY, Xu XJ, Zhao N, Tang YM. Prognostic factors of early death in children with hemophagocytic lymphohistiocytosis. Cytokine. 2017;97:80–85. doi: 10.1016/j.cyto.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Lu D, Ma S, Li L, Zhu J, Zhou D, Zheng Y, Yang X, Zhu L, Zhu M, Xie M, Sun J, Ye X, Xie W. Risk factors of early death in adult patients with secondary hemophagocytic lymphohistiocytosis: a single-institution study of 171 Chinese patients. Hematology. 2019;24:606–612. doi: 10.1080/16078454.2019.1660458. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki K, Uehara J, Iinuma S, Doi H, Honma M, Toki Y, Ishida-Yamamoto A. Hemophagocytic lymphohistiocytosis associated with dabrafenib and trametinib combination therapy following pembrolizumab administration for advanced melanoma. Ann Oncol. 2018;29:1602–1603. doi: 10.1093/annonc/mdy175. [DOI] [PubMed] [Google Scholar]
- 7.Kuriyama T, Takenaka K, Kohno K, Yamauchi T, Daitoku S, Yoshimoto G, Kishimoto J, Abe Y, Harada N, Miyamoto T, Iwasaki H, Teshima T, Akashi K. Engulfment of hematopoietic stem cells caused by down-regulation of CD47 is critical in the pathogenesis of hemophagocytic lymphohistiocytosis. Blood. 2012;120:4058–4067. doi: 10.1182/blood-2012-02-408864. [DOI] [PubMed] [Google Scholar]
- 8.Zinter MS, Hermiston ML. Calming the storm in HLH. Blood. 2019;134:103–104. doi: 10.1182/blood.2019001333. [DOI] [PubMed] [Google Scholar]
- 9.Shimada A, Kato M, Tamura K, Hirato J, Kanegane H, Takechi Y, Park MJ, Sotomatsu M, Hatakeyama S, Hayashi Y. Hemophagocytic lymphohistiocytosis associated with uncontrolled inflammatory cytokinemia and chemokinemia was caused by systemic anaplastic large cell lymphoma: a case report and review of the literature. J Pediatr Hematol Oncol. 2008;30:785–787. doi: 10.1097/MPH.0b013e318180bb33. [DOI] [PubMed] [Google Scholar]
- 10.Arceci RJ. When T cells and macrophages do not talk: the hemophagocytic syndromes. Curr Opin Hematol. 2008;15:359–367. doi: 10.1097/MOH.0b013e3282f97f88. [DOI] [PubMed] [Google Scholar]
- 11.Ishii E. Hemophagocytic lymphohistiocytosis in children: pathogenesis and treatment. Front Pediatr. 2016;4:47. doi: 10.3389/fped.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 13.Riviere S, Galicier L, Coppo P, Marzac C, Aumont C, Lambotte O, Fardet L. Reactive hemophagocytic syndrome in adults: a retrospective analysis of 162 patients. Am J Med. 2014;127:1118–1125. doi: 10.1016/j.amjmed.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Janka GE. Familial hemophagocytic lymphohistiocytosis. Eur J Pediatr. 1983;140:221–230. doi: 10.1007/BF00443367. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Huang W, Hu L, Cen X, Li L, Wang J, Shen J, Wei N, Wang Z. Multicenter study of combination DEP regimen as a salvage therapy for adult refractory hemophagocytic lymphohistiocytosis. Blood. 2015;126:2186–2192. doi: 10.1182/blood-2015-05-644914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Rosée P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N, Birndt S, Gil-Herrera J, Girschikofsky M, Jordan MB, Kumar A, van Laar JAM, Lachmann G, Nichols KE, Ramanan AV, Wang Y, Wang Z, Janka G, Henter JI. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133:2465–2477. doi: 10.1182/blood.2018894618. [DOI] [PubMed] [Google Scholar]
- 17.Bergsten E, Horne A, Aricó M, Astigarraga I, Egeler RM, Filipovich AH, Ishii E, Janka G, Ladisch S, Lehmberg K, McClain KL, Minkov M, Montgomery S, Nanduri V, Rosso D, Henter JI. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130:2728–2738. doi: 10.1182/blood-2017-06-788349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locatelli F, Jordan MB, Allen C, Cesaro S, Rizzari C, Rao A, Degar B, Garrington TP, Sevilla J, Putti MC, Fagioli F, Ahlmann M, Dapena Diaz JL, Henry M, De Benedetti F, Grom A, Lapeyre G, Jacqmin P, Ballabio M, de Min C. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. 2020;382:1811–1822. doi: 10.1056/NEJMoa1911326. [DOI] [PubMed] [Google Scholar]
- 19.Vallurupalli M, Berliner N. Emapalumab for the treatment of relapsed/refractory hemophagocytic lymphohistiocytosis. Blood. 2019;134:1783–1786. doi: 10.1182/blood.2019002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albeituni S, Verbist KC, Tedrick PE, Tillman H, Picarsic J, Bassett R, Nichols KE. Mechanisms of action of ruxolitinib in murine models of hemophagocytic lymphohistiocytosis. Blood. 2019;134:147–159. doi: 10.1182/blood.2019000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slostad J, Hoversten P, Haddox CL, Cisak K, Paludo J, Tefferi A. Ruxolitinib as first-line treatment in secondary hemophagocytic lymphohistiocytosis: a single patient experience. Am J Hematol. 2018;93:E47–E49. doi: 10.1002/ajh.24971. [DOI] [PubMed] [Google Scholar]
- 22.Bergsten E, Horne A, Arico M, Astigarraga I, Egeler RM, Filipovich AH, Ishii E, Janka G, Ladisch S, Lehmberg K, McClain KL, Minkov M, Montgomery S, Nanduri V, Rosso D, Henter JI. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130:2728–2738. doi: 10.1182/blood-2017-06-788349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shabrish S, Desai M, Saxena V, Kelkar M, Madkaikar M. IFN-g: IL-10 ratio: a putative predictive biomarker to discriminate HLH from severe viral infections. J Clin Immunol. 2019;39:135–137. doi: 10.1007/s10875-019-00601-y. [DOI] [PubMed] [Google Scholar]
- 24.Tang Y, Xu X, Song H, Yang S, Shi S, Wei J, Pan B, Zhao F, Liao C, Luo C. Early diagnostic and prognostic significance of a specific Th1/Th2 cytokine pattern in children with haemophagocytic syndrome. Br J Haematol. 2008;143:84–91. doi: 10.1111/j.1365-2141.2008.07298.x. [DOI] [PubMed] [Google Scholar]
- 25.Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 26.Goldman J, Desai MS, McClain KL, Tcharmtchi MH, Kennedy CE, Thompson K, Lam F, Bashir DA, Chinn IK, Goldberg BR, Allen CE, Nguyen TC. Hepatobiliary dysfunction and disseminated intravascular coagulation increase risk of mortality in pediatric hemophagocytic lymphohistiocytosis. Pediatr Crit Care Med. 2018;19:e522–e530. doi: 10.1097/PCC.0000000000001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barba T, Maucort-Boulch D, Iwaz J, Bohe J, Ninet J, Hot A, Lega JC, Guerin C, Argaud L, Broussolle C, Jamilloux Y, Richard JC, Sève P. Hemophagocytic lymphohistiocytosis in intensive care unit: a 71-case strobe-compliant retrospective study. Medicine (Baltimore) 2015;94:e2318. doi: 10.1097/MD.0000000000002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henter JI, Elinder G, Söder O, Hansson M, Andersson B, Andersson U. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991;78:2918–2922. [PubMed] [Google Scholar]
- 29.Fujiwara F, Hibi S, Imashuku S. Hypercytokinemia in hemophagocytic syndrome. Am J Pediatr Hematol Oncol. 1993;15:92–98. doi: 10.1097/00043426-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Takada H, Nomura A, Ohga S, Hara T. Interleukin-18 in hemophagocytic lymphohistiocytosis. Leuk Lymphoma. 2001;42:21–28. doi: 10.3109/10428190109097673. [DOI] [PubMed] [Google Scholar]
- 31.Osugi Y, Hara J, Tagawa S, Takai K, Hosoi G, Matsuda Y, Ohta H, Fujisaki H, Kobayashi M, Sakata N, Kawa-Ha K, Okada S, Tawa A. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997;89:4100–4103. [PubMed] [Google Scholar]
- 32.Tsuboi I, Harada T, Hirabayashi Y, Aizawa S. Dynamics of hematopoiesis is disrupted by impaired hematopoietic microenvironment in a mouse model of hemophagocytic lymphohistiocytosis. Ann Hematol. 2020;99:1515–1523. doi: 10.1007/s00277-020-04095-2. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Wang J, Wang Y, Wu L, Wang Z. Dectection of erythrocyte life and analysis of its effect on the anemia in patients with hemophagocytic syndrome. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020;28:652–656. doi: 10.19746/j.cnki.issn.1009-2137.2020.02.049. [DOI] [PubMed] [Google Scholar]
- 34.Burn TN, Weaver L, Rood JE, Chu N, Bodansky A, Kreiger PA, Behrens EM. Genetic deficiency of interferon-gamma reveals interferon-gamma-independent manifestations of murine hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2020;72:335–347. doi: 10.1002/art.41076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshihara S, Li Y, Xia J, Danzl N, Sykes M, Yang YG. Posttransplant hemophagocytic lymphohistiocytosis driven by myeloid cytokines and vicious cycles of T-cell and macrophage activation in humanized mice. Front Immunol. 2019;10:186. doi: 10.3389/fimmu.2019.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104:735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 37.Humblet-Baron S, Franckaert D, Dooley J, Ailal F, Bousfiha A, Deswarte C, Oleaga-Quintas C, Casanova JL, Bustamante J. IFN-γ and CD25 drive distinct pathologic features during hemophagocytic lymphohistiocytosis. J Allergy Clin Immunol. 2019;143:2215–2226. e2217. doi: 10.1016/j.jaci.2018.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burn TN, Weaver L, Rood JE, Chu N, Bodansky A, Kreiger PA, Behrens EM. Genetic deficiency of interferon-γ reveals interferon-γ-independent manifestations of murine hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2020;72:335–347. doi: 10.1002/art.41076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bin Q, Gao JH, Luo JM. Prognostic factors of early outcome in pediatric hemophagocytic lymphohistiocytosis: an analysis of 116 cases. Ann Hematol. 2016;95:1411–1418. doi: 10.1007/s00277-016-2727-6. [DOI] [PubMed] [Google Scholar]
- 40.Saraiva M, Vieira P, O’Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2020;217:e20190418. doi: 10.1084/jem.20190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouyang W, O’Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50:871–891. doi: 10.1016/j.immuni.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Lobo-Silva D, Carriche GM, Castro AG, Roque S, Saraiva M. Balancing the immune response in the brain: IL-10 and its regulation. J Neuroinflammation. 2016;13:297. doi: 10.1186/s12974-016-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dougan M, Dranoff G, Dougan SK. GM-CSF, IL-3, and IL-5 family of cytokines: regulators of inflammation. Immunity. 2019;50:796–811. doi: 10.1016/j.immuni.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 45.Yanagibashi T, Satoh M, Nagai Y, Koike M, Takatsu K. Allergic diseases: from bench to clinic - contribution of the discovery of interleukin-5. Cytokine. 2017;98:59–70. doi: 10.1016/j.cyto.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Peng H, Ning H, Wang Q, Lu W, Chang Y, Wang TT, Lai J, Kolattukudy PE, Hou R, Hof DF, Dykewicz MS, Liu J. Monocyte chemotactic protein-induced protein 1 controls allergic airway inflammation by suppressing IL-5-producing TH2 cells through the Notch/Gata3 pathway. J Allergy Clin Immunol. 2018;142:582–594. e510. doi: 10.1016/j.jaci.2017.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Passalacqua G. Anti-interleukin 5 therapies in severe asthma. Lancet Respir Med. 2017;5:537–538. doi: 10.1016/S2213-2600(17)30206-0. [DOI] [PubMed] [Google Scholar]
- 48.Kelly EA, Esnault S, Liu LY, Evans MD, Johansson MW, Mathur S, Mosher DF, Denlinger LC, Jarjour NN. Mepolizumab attenuates airway eosinophil numbers, but not their functional phenotype, in Asthma. Am J Respir Crit Care Med. 2017;196:1385–1395. doi: 10.1164/rccm.201611-2234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iino Y, Takahashi E, Ida S, Kikuchi S. Clinical efficacy of anti-IL-5 monoclonal antibody mepolizumab in the treatment of eosinophilic otitis media. Auris Nasus Larynx. 2019;46:196–203. doi: 10.1016/j.anl.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Ruiz de Morales JMG, Puig L, Daudén E, Cañete JD, Pablos JL, Martín AO, Juanatey CG, Adán A, Montalbán X, Borruel N, Ortí G, Holgado-Martín E, García-Vidal C, Vizcaya-Morales C, Martín-Vázquez V, González-Gay MÁ. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: an updated review of the evidence focusing in controversies. Autoimmun Rev. 2020;19:102429. doi: 10.1016/j.autrev.2019.102429. [DOI] [PubMed] [Google Scholar]
- 51.Hu Q, Khanna P, Ee Wong BS, Lin Heng ZS, Subhramanyam CS, Thanga LZ, Sing Tan SW, Baeg GH. Oxidative stress promotes exit from the stem cell state and spontaneous neuronal differentiation. Oncotarget. 2018;9:4223–4238. doi: 10.18632/oncotarget.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL. IL-17 receptor-based signaling and implications for disease. Nat Immunol. 2019;20:1594–1602. doi: 10.1038/s41590-019-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hatta M, Surachmanto EE, Islam AA, Wahid S. Expression of mRNA IL-17F and sIL-17F in atopic asthma patients. BMC Res Notes. 2017;10:202. doi: 10.1186/s13104-017-2517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50:892–906. doi: 10.1016/j.immuni.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glatt S, Baeten D, Baker T, Griffiths M, Ionescu L, Lawson ADG, Maroof A, Oliver R, Popa S, Strimenopoulou F, Vajjah P, Watling MIL, Yeremenko N, Miossec P, Shaw S. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523–532. doi: 10.1136/annrheumdis-2017-212127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hot A, Miossec P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann Rheum Dis. 2011;70:727–732. doi: 10.1136/ard.2010.143768. [DOI] [PubMed] [Google Scholar]
- 57.Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity. 2019;50:778–795. doi: 10.1016/j.immuni.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mills KH, Dungan LS, Jones SA, Harris J. The role of inflammasome-derived IL-1 in driving IL-17 responses. J Leukoc Biol. 2013;93:489–497. doi: 10.1189/jlb.1012543. [DOI] [PubMed] [Google Scholar]
- 59.Onishi RM, Park SJ, Hanel W, Ho AW, Maitra A, Gaffen SL. SEF/IL-17R (SEFIR) is not enough: an extended SEFIR domain is required for il-17RA-mediated signal transduction. J Biol Chem. 2010;285:32751–32759. doi: 10.1074/jbc.M110.121418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaffen S, Moutsopoulos N. Regulation of host-microbe interactions at oral mucosal barriers by type 17 immunity. Sci Immunol. 2020;5:eaau4594. doi: 10.1126/sciimmunol.aau4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


