Abstract
Objectives: This study aimed to investigate the therapeutic effects of two different blood purification treatments combined with immunosuppressants on patients with lupus nephritis (LN) and their effects affecting granulocyte-macrophage colony-stimulating factor (GM-CSF) and CXC chemokine ligand-16 (CXCL16) levels. Methods: Ninety patients with LN admitted to our hospital were enrolled and randomly assigned into groups A and B. Group A was treated with continuous veno-venous hemofiltration (CVVH) combined with conventional medical treatment (CMT, n = 40, including 23 females and 17 males), whereas group B was treated with intermittent hemodialysis combined with conventional medical treatment (n = 50, including 35 females and 15 males). Both groups received prednisone and cyclophosphamide. Results: GM-CSF and CXCL16 levels in the two groups were significantly reduced after treatment (P < 0.05); and GM-CSF level in group A was significantly lower than that in group B (P < 0.05, -1.261 to -0.8395), and CXCL16 level in group A was significantly lower than that in group B (P < 0.05, -0.5745 to -0.4355). There was no significant difference in general data between the two groups (P > 0.05). After treatment, the SLEDAI scores were significantly decreased in both groups, and were significantly lower in group A than in group B (P < 0.05, -1.816 to -0.1241). Conclusion: CVVH combined with conventional medical treatment is more effective than intermittent hemodialysis combined with conventional medical treatment, and is easier to remove GM-CSF and CXCL16.
Keywords: Blood purification treatment, immunosuppressant, lupus nephritis, GM-CSF, CXCL16
Introduction
Lupus nephritis (LN) is a kidney disease generally associated with autoantibodies [1,2]. As a serious complication of systemic lupus erythematosus (SLE), LN may have significant toxicity and a low complete remission rate [3]. Approximately 50% of patients with SLE develop LN each year, which increases the risks of renal failure, cardiovascular diseases, and even death [4,5]. LN is easy to recur; hence, there is a need for continuous follow-up and monitoring as well as changes in treatment methods according to the patient’s condition [6].
Continuous veno-venous hemofiltration (CVVH) promotes hemodynamic stability in the treatment of multiple organ failure [7,8]. It has fewer complications and the ability to completely control the levels of metabolic wastes; therefore, it is the preferred method for continuous renal replacement therapy [9]. Meanwhile, hemodialysis is often used in the treatment of kidney-related diseases, but it often ignores the urinary clearance of urea and creatinine and thus has a significant correlation with mortality in patients with end-stage renal disease [10].
Cyclophosphamide is an immunosuppressant and has been widely used in the treatment of treatment LN [11]. Another choice of drug is the steroid prednisone, which has excellent efficacy in preventing renal deterioration and lowering the adverse reaction rate when combined with cyclophosphamide [12]; it is also beneficial for achieving a long-term prognosis [13].
This study aimed to explore the efficacy of two different blood purification treatments, namely, CVVH and hemodialysis, combined with immunosuppressants and their effects affecting the levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) and CXC chemokine ligand-16 (CXCL16) in patients with LN.
Materials and methods
General data
Ninety patients with LN admitted to our hospital from February 2017 to October 2018 were enrolled in this study and assigned into groups A and B. Group A included 40 patients who received CVVH combined with conventional medical treatment, comprising 23 females and 17 males, with an average age of (30.38 ± 8.13) years, an average weight of (66.59 ± 11.49 kg), and a disease course of (2.18 ± 1.49) years. Group B included 50 patients who received intermittent hemodialysis combined with conventional medical treatment, comprising 35 females and 15 males, with an average age of (29.77 ± 7.42) years, an average weight of (68.23 ± 10.32) kg, and a disease course of (2.48 ± 1.53) years.
Exclusion and inclusion criteria
Inclusion criteria: all enrolled subjects were patients with lupus nephritis whose serum endogenous creatinine clearance was ≤ 15 mL/min (or blood creatinine ≥ 707 μmol/L) and no significant reduction in the kidneys was observed by color Doppler ultrasound [14].
The patients and their families were informed of this study and signed the informed consent forms. This study was approved by the Medical Ethics Committee of the Tongxiang First People’s Hospital.
Exclusion criteria: patients with previous treatment history, mental disorders, malignant tumors, severe infections, dysfunctions, severe hematological diseases, and drug allergy; and patients with a high degree of crescent formation and glomerular necrosis.
Methods
After admission, both groups received 1 mg/kg/day of prednisone (Henan Topfond Pharmaceutical Co., Ltd.; SFDA Approval No. H41020283) for consecutive 8 weeks. Subsequently, the dosage was decreased by 5 mg/day every 2 weeks to 20 mg/day and further decreased to 2.5 mg/day every week to 6-9 mg/day for long-term treatment. The patients were also treated with cyclophosphamide pulse therapy (0.6-1.0 g) (Shanxi Powerdone Pharmaceutical Co., Ltd.; SFDA Approval No. H14023686), which lasted for half a year and was changed to once every 3 months, with a cumulative total amount of 150 ≤ 150 mg. Blood purification treatment was then performed. Group A received CVVH combined with conventional medical treatment, which lasted for 8 hours per session for three times a week. Group B received intermittent hemodialysis combined with conventional medical treatment, which lasted for 4 hours per session for three times a week lasting for 3 months. Vital signs of patients were monitored throughout the process to adjust inhibitors in time.
Patient and public involvement
Patients’ priorities, experiences and preferences had no effect on the study. Patients were communicated and informed of this study, and signed the informed consents. Patients were involved in the recruitment and conduct of the study. The hospitalized participants were notified the results verbally, and discharged participants were notified the results through E-mail or telephone.
Outcome measures
All indicators were measured 1 day before admission and 3 months after treatment.
(1) The disease activity index (DAI) was monitored in the two groups by means of the systemic lupus erythematosus DAI (SLEDAI) score [15].
(2) The changes in the levels of of anti-double stranded DNA (anti-dsDNA), complement C3, and serum creatinine (Scr) as well as in the erythrocyte sedimentation rate (ESR) were observed.
(3) Treatment efficacy was evaluated as complete, partial, or no remission [16]. Complete remission (CR): urine protein excretion of < 0.3 g per day, normal urine sediment and serum albumin concentration, and creatinine clearance rate not exceeding 15% of the baseline value; partial remission (PR): urine protein excretion of 0.3-2.9 g per day; no remission (NR): no improvement in indices. Total effective rate = (CR+PR)/total cases × 100%.
(4) Fasting blood (5 mL) was extracted before and after treatment, and the changes in the levels of serum GM-CSF and CXCL16 were observed using enzyme-linked immunosorbent assay (ELISA) (Shanghai Yanzai Biotechnology Co., Ltd.). Standard sample addition: set standard sample holes and sample holes, add 50 μL of standard products of different concentrations to each standard hole. Add samples: set up blank wells (the blank control wells do not add samples and enzyme-labeled reagents, the other steps are the same), the sample to be teste. Pin hole. Add 40 μl of sample diluent, and then add 10 μl of sample to be tested into the sample well of the enzyme-labeled coating plate (The final dilution of the sample is 5 times). Add sample: Add the sample to the bottom of the well of the microtiter plate, try not to touch the wall of the well, gently shake Move and mix well. Add enzyme: add 100 μl of enzyme-labeled reagent to each well, except for blank wells. Incubation: Seal the plate with a sealing film and incubate at 37°C for 60 minutes. Liquid preparation: Dilute the 20-fold concentrated washing solution with distilled water 20-fold before use. Washing: Carefully remove the sealing film, discard the liquid, spin dry, fill each well with washing liquid, let it stand for 30 seconds and then discard it. Repeat 5 times and pat dry. Color development: add 50 μl of developer A and 50 μl of developer B to each well, shake and mix gently, and avoid light at 37°C. Color for 15 minutes. Stop: add 50 μl stop solution to each well to stop the reaction (the blue will turn to yellow at this time). Determination: Adjust the blank hole to zero, and measure the absorbance (OD value) of each hole in sequence at 450 nm wavelength. Stop solution Within 15 minutes.
Statistical methods
Statistical analysis was conducted using the SPSS 21.0. Graphpad Prism 8 was used to draw the graphs. The measurement data were expressed as mean ± SD, and the intergroup comparison was conducted using the t test. The counting data were expressed as cases (percentage) [n (%)], and the intergroup comparison was conducted using the Chi-square test. P < 0.05 was considered to be statistically significant.
Results
General data
Group A included 23 females and 17 males, with an average age of (30.38 ± 8.13) years, while group B included 35 females and 15 males, with an average age of (29.77 ± 7.42) years. The two groups were not significantly different in terms of sex, age, weight, educational background, smoking, drinking, obesity, and organ damage (P > 0.05) (Table 1).
Table 1.
General data [n (%)] (mean ± SD)
| Classification | Group A (n = 40) | Group B (n = 50) | t/χ2 value | P value |
|---|---|---|---|---|
| Sex | 1.515 | 0.218 | ||
| Female | 23 (57.50) | 35 (70.00) | ||
| Male | 17 (42.50) | 15 (30.00) | ||
| Age (years) | 30.38 ± 8.13 | 29.77 ± 7.42 | 0.371 | 0.711 |
| Weight (kg) | 66.59 ± 11.49 | 68.23 ± 10.32 | 0.712 | 0.478 |
| Course of disease (years) | 2.18 ± 1.49 | 2.48 ± 1.53 | 0.935 | 0.352 |
| Residence | 2.246 | 0.133 | ||
| Urban | 21 (52.50) | 34 (68.00) | ||
| Rural | 19 (47.50) | 16 (32.00) | ||
| Education | 0.01 | 1.000 | ||
| ≥ high school | 32 (80.00) | 40 (80.00) | ||
| < high school | 8 (20.00) | 10 (20.00) | ||
| Smoking history | 0.720 | 0.396 | ||
| Yes | 18 (45.00) | 27 (54.00) | ||
| No | 22 (55.00) | 23 (46.00) | ||
| Drinking history | 0.058 | 0.809 | ||
| Yes | 25 (62.50) | 30 (60.00) | ||
| No | 15 (37.50) | 20 (40.00) | ||
| History of diabetes | 0.750 | 0.386 | ||
| Yes | 14 (35.00) | 22 (44.00) | ||
| No | 26 (65.00) | 28 (56.00) | ||
| History of hypertension | 0.984 | 0.321 | ||
| Yes | 19 (47.50) | 29 (58.00) | ||
| No | 21 (52.50) | 21 (42.00) | ||
| Obesity | 0.035 | 0.850 | ||
| Yes | 20 (50.00) | 26 (52.00) | ||
| No | 20 (50.00) | 24 (48.00) | ||
| Organ damage | 0.607 | 0.435 | ||
| Nervous system disease | 13 (32.50) | 17 (34.00) | ||
| Digestive system disease | 17 (42.50) | 12 (24.00) | ||
| Blood system disease | 5 (12.50) | 14 (28.00) | ||
| Skin disease | 5 (12.50) | 7 (14.00) |
SLEDAI scores in both the groups
The SLEDAI scores before and after treatment were (9.38 ± 3.48) and (5.79 ± 1.29) in group A and (9.98 ± 3.59) and (7.13 ± 2.34) in group B, respectively. The scores were decreased significantly after treatment (P < 0.05), and group A had significantly lower scores than group B (P < 0.05) (Table 2).
Table 2.
SLEDAI score (mean ± SD)
| Group | Group A (n = 40) | Group B (n = 50) | t | P value |
|---|---|---|---|---|
| Before treatment | 9.38 ± 3.48 | 9.98 ± 3.59 | 0.798 | 0.426 |
| After treatment | 5.79 ± 1.29 | 7.13 ± 2.34 | 3.246 | 0.001 |
| t | 6.118 | 4.703 | - | - |
| P | < 0.001 | < 0.001 | - | - |
Index observation
The anti-dsDNA levels before and after treatment were (40.34 ± 9.44) and (23.59 ± 4.29) IU/L in group A and (41.69 ± 10.24) and (27.25 ± 5.71) IU/L in group B, respectively. The C3 levels before and after treatment were (0.53 ± 0.21) and (0.73 ± 0.20) g/L in group A and (0.54 ± 0.25) and (0.63 ± 0.23) g/L in group B, respectively. Furthermore, the ESR values before and after treatment were (65.38 ± 10.43) and (26.23 ± 8.44) mm/h in group A and (66.34 ± 11.26) and (31.39 ± 9.30) mm/h in group B, respectively. Lastly, the Scr levels before and after treatment were (759.24 ± 103.48) and (428.52 ± 64.45) μmol/L in group A and (784.23 ± 108.30) and (593.39 ± 72.24) μmol/L in group B, respectively. These indices were improved after treatment (P < 0.05); the improvement in group A was more significant than that in group B (P < 0.05) (Figure 1).
Figure 1.
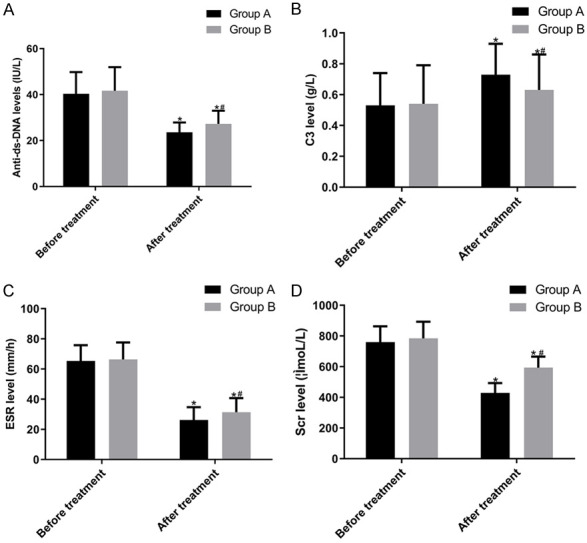
Comparison of index observation. A: anti-dsDNA levels; B: C3 levels; C: ESR values; D: Scr levels. The anti-dsDNA levels, C3 levels, ESR values and Scr levels were not significantly different between the two groups before treatment (P > 0.05). However, anti-dsDNA levels, ESR values and Scr levels decreased significantly after treatment (P < 0.05), with group A having a significantly lower level than group B (P < 0.05); C3 levels increased significantly after treatment (P < 0.05), with group A having a significantly higher level than group. Note: Compared with before treatment in the same group, *P < 0.05; compared with after treatment in group A, #P < 0.05.
Comparison of efficacy
In group A, there were 20 cases of CR, 17 cases of PR, and 3 cases of NR, with a total effective rate of 92.50%. In group B, there were 18 cases of CR, 20 cases of PR, and 12 cases of NR, with a total effective rate of 76%. Therefore, the total effective rate in group A was higher than that in group B (P = 0.036).
Comparison of adverse reactions
Group A had 2 cases of edema, 3 cases of headache, 1 case of tinnitus, 2 case of dysphoria with feverish sensation in the chest, palms, and soles, 1 case of infection and 0 case of heart failure, while the cases of adverse reactions above in group B were 3, 4, 5, 4, 3, and 4, respectively. Furthermore, the total incidence of adverse reactions in group A (22.5%) was significantly lower than that in group B (46%) (P = 0.02).
Changes in the levels of GM-CSF and CXCL16
The GM-CSF levels before and after treatment were (10.69 ± 0.78) and (5.38 ± 0.54) μg/L in group A and (10.93 ± 0.79) and (7.24 ± 0.69) μg/L in group B, respectively. The CXCL16 levels before and after treatment were (4.68 ± 0.28) and (2.02 ± 0.13) mg/L in group A and (4.74 ± 0.32) and (2.97 ± 0.14) mg/L in group B, respectively. The GM-CSF and CXCL16 levels in both groups were significantly decreased after treatment (P < 0.05), but those in group A were significantly lower than those in group B (P < 0.05) (Figure 2).
Figure 2.
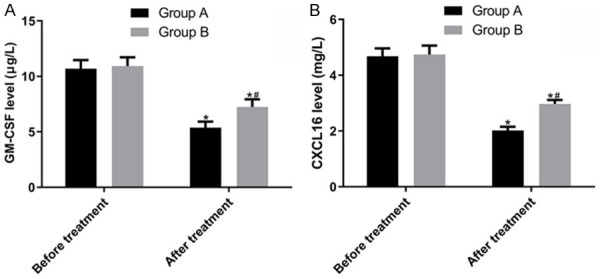
Comparison of the GM-CSF and CXCL16 levels. A: The GM-CSF levels were not significantly different between the two groups before treatment (P > 0.05). However, they decreased significantly after treatment (P < 0.05), with group A having a significantly lower level than group B (P < 0.05). B: The CXCL16 levels were not significantly different between the two groups before treatment (P > 0.05). However, they decreased significantly after treatment (P < 0.05), with group A having a significantly lower level than group B (P < 0.05). Note: Compared with before treatment in the same group, *P < 0.05; compared with after treatment in group A, #P < 0.05.
Discussion
LN is an inflammatory condition that affects the kidneys and comprises various renal diseases, including glomerular and tubulointerstitial lesions [17]. A key feature of LN is the deposition of immune complexes containing nucleic acids bound to nucleic acids and autoantibodies that recognize these molecules [18]. Despite advances in treatment, LN is still a major cause of mortality and morbidity [19]. LN is a heterogeneous disease, and the heterogeneity brings difficulties to its effective diagnosis and treatment [20]. Therefore, searching for a feasible therapy is extremely necessary.
There is no significant difference of the general data between the two groups in our study. However, the SLEDAI [21] scores were proportional to the DAI scores. and the SLEDAI score in group A was significantly lower than that in group B after treatment, suggesting that the treatment of CVVH combined with CMT in group A was more effective than that in group B that was treated with intermittent hemodialysis combined with CMT. Furthermore, we observed the levels of anti-dsDNA, C3, and Scr as well as ESR and compared the efficacy between the two groups. After treatment, all the indices in group A were better than those in group B, and the total effective rate in group A was clearly higher than that in group B, indicating that CVVH combined with conventional medical treatment was superior to intermittent hemodialysis combined with conventional medical treatment. We also compared the adverse reactions of the two treatments, and the results showed that the total incidence in group A was significantly lower than that in group B, suggesting that CVVH had fewer adverse reactions. Currently, no studies have directly shown the effect of the two blood purification treatments on LN. Nonetheless, the effects of various renal replacement therapies on the chronic dialysis rate in adults with traumatic intracranial hemorrhage and acute renal injury were studied; compared with intermittent hemodialysis, CVVH yielded better renal outcomes [22]. Another study found that CVVH was safe and feasible in eliminating myoglobin, supporting multiple organ functions, and modulating the systemic inflammatory response syndrome [23]. Combining the results of the study above with ours, CVVH achieved better curative effects, fewer side effects, and possibly better renal prognosis than intermittent hemodialysis.
GM-CSF is a growth factor that induces the differentiation and proliferation of granulocytes and macrophages derived from hematopoietic progenitor cells [24]. It also acts as a communication conduit between tissue-invading lymphocytes and myeloid cells in inflammation [25]. It plays a key role in human innate immune effector function. CXCL16 is a proinflammatory chemokine [26], and its expression is elevated in LN; it is expected to be an effective biomarker for disease activity, renal injury, and pathological activity, and its continous elevation may increase kidney damage. In our study, the GM-CSF and CXCL16 levels were significantly decreased in both groups after treatment, with group A showing significantly lower levels than group B. Therefore, the two blood purification treatments effectively decreased the levels of inflammatory factors, but CVVH could better eliminate such inflammatory factors than intermittent hemodialysis. Although few studies have compared the two treatment methods and their effects on GM-CSF and CXCL16 levels, there is evidence that CVVH reduces acute inflammation and signaling molecules by removing proinflammatory factors [27]. Combined with the results of this study, CVVH was more effective in eliminating inflammations, which may be closely related to its better therapeutic effect.
However, our study has some limitations. We should have used more blood purification treatments and immunosuppressants for comparison. Moreover, the experimental data are not sufficiently inclusive. Therefore, an extensive research should be conducted to supplement our study.
In conclusion, CVVH combined with conventional medical treatment has better efficacy, easier removal of proinflammatory factors, and fewer adverse reactions than intermittent hemodialysis combined with conventional medical treatment, thus promoting the treatment of LN.
Acknowledgements
The contributions of the patient advisers are important and greatly appreciated.
Disclosure of conflict of interest
None.
References
- 1.Alba P, Bento L, Cuadrado M, Karim Y, Tungekar M, Abbs I, Khamashta M, D’Cruz D, Hughes G. Anti-dsDNA, anti-Sm antibodies, and the lupus anticoagulant: significant factors associated with lupus nephritis. Ann Rheum Dis. 2003;62:556–560. doi: 10.1136/ard.62.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lech M, Anders HJ. The pathogenesis of lupus nephritis. J Am Soc Nephrol. 2013;24:1357–1366. doi: 10.1681/ASN.2013010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginzler EM, Wax S, Rajeswaran A, Copt S, Hillson J, Ramos E, Singer NG. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res Ther. 2012;14:R33. doi: 10.1186/ar3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, Boletis J, Cervera R, Dörner T, Doria A. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis. 2012;71:1771–1782. doi: 10.1136/annrheumdis-2012-201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saxena R, Mahajan T, Mohan C. Lupus nephritis: current update. Arthritis Res Ther. 2011;13:240. doi: 10.1186/ar3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz N, Rubinstein T, Burkly LC, Collins CE, Blanco I, Su L, Hojaili B, Mackay M, Aranow C, Stohl W. Urinary TWEAK as a biomarker of lupus nephritis: a multicenter cohort study. Arthritis Res Ther. 2009;11:R143. doi: 10.1186/ar2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koo JR, Kim JC, Yoon JW, Kim GH, Jeon RW, Kim HJ, Chae DW, Noh JW. Failure of continuous venovenous hemofiltration to prevent death in paraquat poisoning. Am J Kidney Dis. 2002;39:55–59. doi: 10.1053/ajkd.2002.29880. [DOI] [PubMed] [Google Scholar]
- 8.Jiang HL, Xue WJ, Li DQ, Yin AP, Xin X, Li CM, Gao JL. Influence of continuous veno-venous hemofiltration on the course of acute pancreatitis. World J Gastroenterol. 2005;11:4815. doi: 10.3748/wjg.v11.i31.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macias WL, Mueller BA, Scarim SK, Robinson M, Rudy DW. Continuous venovenous hemofiltration: an alternative to continuous arteriovenous hemofiltration and hemodiafiltration in acute renal failure. Am J Kidney Dis. 1991;18:451–458. doi: 10.1016/s0272-6386(12)80113-2. [DOI] [PubMed] [Google Scholar]
- 10.Shemin D, Bostom AG, Laliberty P, Dworkin LD. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis. 2001;38:85–90. doi: 10.1053/ajkd.2001.25198. [DOI] [PubMed] [Google Scholar]
- 11.Donadio JV Jr, Glassock RJ. Immunosuppressive drug therapy in lupus nephritis. Am J Kidney Dis. 1993;21:239–250. doi: 10.1016/s0272-6386(12)80741-4. [DOI] [PubMed] [Google Scholar]
- 12.Felson DT, Anderson J. Evidence for the superiority of immunosuppressive drugs and prednisone over prednisone alone in lupus nephritis: results of a pooled analysis. N Engl J Med. 1984;311:1528–1533. doi: 10.1056/NEJM198412133112402. [DOI] [PubMed] [Google Scholar]
- 13.Esdaile J, Joseph L, MacKenzie T, Kashgarian M, Hayslett J. The benefit of early treatment with immunosuppressive agents in lupus nephritis. J Rheumatol. 1994;21:2046–2051. [PubMed] [Google Scholar]
- 14.Yu C, Gershwin ME, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun. 2014;48:10–13. doi: 10.1016/j.jaut.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH, Austin A, Bell A, Bloch DA, Corey PN, Decker JL. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 16.Chan TM, Li FK, Tang CS, Wong RW, Fang GX, Ji YL, Lau CS, Wong AK, Tong MK, Chan KW. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. N Engl J Med. 2000;343:1156–1162. doi: 10.1056/NEJM200010193431604. [DOI] [PubMed] [Google Scholar]
- 17.Imran TF, Yick F, Verma S, Estiverne C, Ogbonnaya-Odor C, Thiruvarudsothy S, Reddi AS, Kothari N. Lupus nephritis: an update. Clin Exp Nephrol. 2016;20:1–13. doi: 10.1007/s10157-015-1179-y. [DOI] [PubMed] [Google Scholar]
- 18.Mistry P, Kaplan MJ. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin Immunol. 2017;185:59–73. doi: 10.1016/j.clim.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solé C, Cortés-Hernández J, Felip ML, Vidal M, Ordi-Ros J. miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol Dial Transplant. 2015;30:1488–1496. doi: 10.1093/ndt/gfv128. [DOI] [PubMed] [Google Scholar]
- 20.Der E, Ranabothu S, Suryawanshi H, Akat KM, Clancy R, Morozov P, Kustagi M, Czuppa M, Izmirly P, Belmont HM. Single cell RNA sequencing to dissect the molecular heterogeneity in lupus nephritis. JCI Insight. 2017;2:e93009. doi: 10.1172/jci.insight.93009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gladman DD, Urowitz MB, Kagal A, Hallett D. Accurately describing changes in disease activity in Systemic Lupus Erythematosus. J Rheumatol. 2000;27:377–379. [PubMed] [Google Scholar]
- 22.Tseng MF, Chou CL, Chung CH, Chien WC, Chen YK, Yang HC, Liao CY, Wei KY, Wu CC. Continuous veno-venous hemofiltration yields better renal outcomes than intermittent hemodialysis among traumatic intracranial hemorrhage patients with acute kidney injury: a nationwide population-based retrospective study in Taiwan. PLoS One. 2018;13:e0203088. doi: 10.1371/journal.pone.0203088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F, Song Q, Peng Z, Pan L, Kang H, Tang S, Yue H, Liu H, Xie F. Effects of continuous venous-venous hemofiltration on heat stroke patients: a retrospective study. J Trauma Acute Care Surg. 2011;71:1562–1568. doi: 10.1097/TA.0b013e31822a71c2. [DOI] [PubMed] [Google Scholar]
- 24.Wicks IP, Roberts AW. Targeting GM-CSF in inflammatory diseases. Nat Rev Rheumatol. 2016;12:37. doi: 10.1038/nrrheum.2015.161. [DOI] [PubMed] [Google Scholar]
- 25.Becher B, Tugues S, Greter M. GM-CSF: from growth factor to central mediator of tissue inflammation. Immunity. 2016;45:963–973. doi: 10.1016/j.immuni.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Collins DM, Madden SF, Eustace AJ, Toomey S, Kay EW, Fay J, O’Donovan N, Gallagher WM, Hennessy B, Crown J. Abstract P5-11-03: tumor CXCL16/CXCR6 expression and soluble CXCL16 in HER2+ breast cancer (BC) 2018 [Google Scholar]
- 27.Wu J, Ren J, Liu Q, Hu Q, Wu X, Wang G, Hong Z, Ren H, Li J. Effects of changes in the levels of damage-associated molecular patterns following continuous veno-venous hemofiltration therapy on outcomes in acute kidney injury patients with sepsis. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.03052. [DOI] [PMC free article] [PubMed] [Google Scholar]


