Abstract
Objective: To evaluate the diagnostic and prognostic value of circulating long non-coding RNA H19 (H19) in sepsis. Methods: A total of 104 septic patients admitted to our hospital from June 2018 to April 2019 were enrolled as the disease group, and another 92 healthy individuals were selected as the control group. The relative expression of H19 in peripheral blood was quantified by quantitative real-time polymerase chain reaction, and the diagnostic value in sepsis was assessed by receiver operating characteristic curve analysis. Pearson correlation coefficient was used to analyze the correlation between H19 and other inflammatory markers. Results: Compared with the control group, the expression of peripheral blood H19 in the disease group was significantly down-regulated. The area under the curve (AUC) of H19 for diagnosing sepsis was 0.849. The expression of H19 in the survival group was significantly up-regulated compared with that in the death group, and the AUC in the survival group was 0.865. The relative expression of H19 was negatively correlated with PCT, CRP, IL-6, TNF-α, CK-MB, and cTnI. Multivariate logistic regression showed that patients with high lactic acid, coagulation dysfunction, high levels of PCT, CRP, IL-6, TNF-α, CK-MB, and cTnI, but low H19 expression had an increased risk of sepsis. Conclusion: Peripheral blood H19 may be used for early diagnosis, clinical assessment, and prognosis of sepsis.
Keywords: lncRNA-H19, sepsis, peripheral blood, prognosis, diagnosis, markers
Introduction
Sepsis is a common systemic inflammatory response in the aging population, that can lead to the aggravation of infection and has a serious impact on patients with cancer and underlying immunosuppression [1]. Sepsis can also lead to organ dysfunction, which can be life-threatening. An unstable immune response induced by invasive pathogens contributes to the development of a pathologic syndrome characterized by persistent inflammation and immunosuppression [2,3]. At present, antibiotics, respiratory support, and cardiovascular support are the preferred therapies for sepsis clinically; however, the morbidity and mortality remain high, and the quality of life of survivors is likely to be poor [4]. Therefore, it is necessary to seek novel therapeutic and diagnostic targets to clarify the pathogenesis of sepsis and improve clinical outcomes.
Long non-coding RNAs (lncRNAs) are non-coding transcripts involved in a variety of key biologic processes and human diseases [5]. LncRNAs have also been reported to act as regulators of gene expression networks and play a significant role in epigenetic regulation of gene expression [6]. A previous study has shown that lncRNAs regulate gene expression at multiple levels, and are closely related to tumor formation, inflammatory damage, viral replication, and other pathologic processes [7]. In addition, they are involved in the inflammatory response to sepsis [8]. H19 is a key imprinted oncofetal gene in carcinogenesis [9], which is expressed in most human cancers, such as liver cancer, rectal cancer, and ovarian cancer. [10]. Geng et al. have proven that H19 is an evolutionarily conserved and maternally expressed imprinted lncRNA, which can improve reconstruction and homeostasis of intestinal epithelial cells (IECs). Also, it is an inflammatory lncRNA induced by IL-22, that antagonizes negative regulators of IECs and maintains intestinal epithelial regeneration under an inflammatory condition [11]. However, there have been few studies on the role of H19 in sepsis. The myocardial toxicity caused by sepsis is mainly related to the reduction of cardiac energy and the production of inflammatory cytokines, so the treatment of sepsis focuses on optimizing myocardial function and alleviating inflammatory response in vivo [12]. Therefore, markers of myocardial injury and inflammatory response are important in sepsis.
In the present study, we quantified the expression of H19 in the peripheral blood of patients with sepsis to explore the role of H19 in the diagnosis and prognosis of sepsis.
Materials and methods
General data
A total of 104 septic patients admitted to our hospital from June 2018 to April 2019 were enrolled as the disease group (DG), including 57 males and 47 females, aged 45-64 years, with a mean age of (57.23±12.27) years; infection sites: circulatory system (26 cases), skin-soft tissue (33 cases), abdominal infection (18 cases), and respiratory infection (27 cases). In addition, 92 healthy individuals undergoing physical examination were selected as the control group (CG), including 55 males and 37 females, aged 25-73 years, with a mean age of (56.76±12.22) years. The guardians of these participants signed the informed consent form. This study was approved by the Ethics Committee of Weihai Hosptial of Traditional Chinese Medicine.
Inclusion and exclusion criteria
Inclusion criteria: patients in the DG met the diagnostic criteria for sepsis [13]; all patients had complete clinical data and were age 25-73 years; laboratory testing results were systolic blood pressure (SBP) <90 mmHg, mean arterial pressure (MAP) <70 mmHg, white blood cell (WBC) count >12×109/L, cardiac index (CI) >3.5 L/(min·m2), mixed venous oxygen saturation (SvO2) >70%. Exclusion criteria: patients with congenital heart disease or previous cardiac surgery; patients who had autoimmune diseases, acute pulmonary embolism, cerebrovascular diseases, coronary heart disease, acute pulmonary heart disease, mental diseases, acute coronary syndrome, malignant tumor, congestive heart failure, liver and renal insufficiency; patients who refused to follow-up or had an expected survival time less than 1 month; patients who were lost to follow up. The inclusion criteria were applicable to the patients in the DG.
Detection methods
Fasting venous blood (5 mL) sample was collected and centrifuged at 5000 rpm for 15 min to separate serum. Enzyme-linked immunosorbent assay (ELISA) [14] was employed to quantify procalcitonin (PCT) (Huijia Biotechnology Co., Ltd., Xiamen, China, ATHJMP00030HU), Creactive protein (CRP) (Hengfei Biotechnology Co., Ltd., Shanghai, China, K001607P), interleukin-6 (IL-6) (Elabscience Biotechnology Co., Ltd., Wuhan, China, E-EL-H0102c), tumor necrosis factor-alpha (TNF-α) (Hengfei Biotechnology Co., Ltd., Shanghai, China, bs-215OR-2), creatine kinase-MB isoenzyme (CK-MB) (Yiyan Biotechnology Co., Ltd., Shanghai, China, EY-D9125), and cardiac troponin I (cTnI) (Wondfo Biotechnology Co., Ltd., Guangzhou, China, A217). The tests were carried out in strict accordance with the kit instructions. A sample well, a standard well, and a blank well were set up. Approximately 50 μL of sample was added to the sample well, and 50 μL of standard was added to the standard well, while the blank well was kept empty. Horseradish peroxidase-conjugated antibody was added to the sample well and the standard well, sealed and incubated at 37°C for 60 min. Afterwards, the liquid was discarded and spin-dried, followed by washing 5 times. The substrates A and B were fully mixed at 1:1 volume; 100 μL of the mixture was added to all wells. The plate was sealed and incubated at 37°C for 15 min. Stop solution (50 μL) was added to each well. The optical density (OD) value at 450 nm was read with a fully-automatic microplate reader (Chenlian Biotechnology Development Co., Ltd., Shanghai, China, M15), and the levels of PCT, CRP, IL-6, TNF-α, CK-MB, and cTnI were calculated.
Real-time quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted using the Ambion kit (Thermo Scientific, Wilmington , DE, USA). The concentration and purity were measured by an ultraviolet spectrophotometer (PKUCare Industrial Park Technology Co., Ltd., Beijing, China, UV-1100), and the integrity was measured by 1% agarose gel electrophoresis. The RNA was reverse-transcribed into cDNA with a reverse transcription kit (Protein Innovation Co., Ltd., Beijing, China, BPI01030), and the sample obtained was stored at -80°C. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Upstream of H19: 5’-GCCTTGACGTGCTGGATCT-3’; Downstream of H19: 5’-TCCGATGCTTTACTCAAGAAGTT-3’; Upstream of GAPDH: 5’-GGGAAACTGTGGCGTGAT-3’, downstream of GAPDH: 5’-GAGTGGGTGTCGCTGTTGA-3’. The PCR was carried out on a real-time PCR instrument (Sangshai Biotechnology Co., Ltd., Shanghai, China, TOWER3G). Amplification conditions: pre-denaturation at 95°C for 30 s, 40 cycles of denaturation and annealing at 95°C for 40 min, then left at 55°C for 1 min, and final extension at 40°C for 5 min. The relative expression of the target gene was calculated by 2-ΔΔCT with the data obtained after 3 independent tests.
Statistical analysis
SPSS 22.0 (IBM Corp, Armonk, NY, USA) was used for statistical analysis, and GraphPad Prism 8 (IBM CORP, Armonk, NY, USA) was used for drawing graphs. Counting data and measurement data were expressed as [n (%)] and mean ± standard deviation (x ± SD), respectively. The comparison of counting data and measurement data between groups was analyzed by using Chi-square test and t-test, separately. The diagnostic value of H19 in sepsis was determined by the logistic regression equation and receiver operating characteristic curve. Pearson correlation analysis was performed to obtain the correlations between peripheral blood H19 and levels of PCT, CRP, IL-6, TNF-α, CK-MB, and cTnI. Cox regression equation tested the independent prognostic factors of sepsis. P<0.05 represents significance.
Result
General data
There was no significant difference in baseline data of sex, age, residence, nationality, education, smoking history, drinking history, exercise history, SBP, and diastolic blood pressure (DBP) between the two groups (P>0.05), while there was a significant difference in lactic acid and coagulation dysfunction (P<0.05) (Table 1).
Table 1.
General data [N (%)] (x ± SD)
| Classification | Disease group (n=104) | Control group (n=92) | t/χ2 | P |
|---|---|---|---|---|
| Sex | 0.493 | 0.482 | ||
| Male | 57 (54.81) | 55 (59.78) | ||
| Female | 47 (45.19) | 37 (40.22) | ||
| Age (years) | 57.23±12.27 | 56.76±12.22 | 0.268 | 0.788 |
| Residence | 0.178 | 0.673 | ||
| Urban | 63 (60.58) | 53 (57.61) | ||
| Rural | 41 (39.42) | 39 (42.39) | ||
| Nationality | 2.782 | 0.095 | ||
| Han | 61 (58.65) | 43 (46.74) | ||
| Minority | 43 (41.35) | 49 (53.26) | ||
| Education | 2.451 | 0.117 | ||
| ≥ High school | 58 (55.77) | 41 (44.57) | ||
| < High school | 46 (44.23) | 51 (55.43) | ||
| Smoking history | 0.981 | 0.321 | ||
| Yes | 66 (63.46) | 52 (56.52) | ||
| No | 38 (36.54) | 40 (43.48) | ||
| Drinking history | 0.750 | 0.386 | ||
| Yes | 64 (61.54) | 51 (55.43) | ||
| No | 40 (38.46) | 41 (44.57) | ||
| Exercise history | 0.388 | 0.533 | ||
| Yes | 60 (57.69) | 49 (53.26) | ||
| No | 44 (42.31) | 43 (46.74) | ||
| SBP (mmHg) | 115.02±8.51 | 114.62±8.22 | 0.333 | 0.739 |
| DBP (mmHg) | 74.14±6.18 | 73.75±6.13 | 0.442 | 0.658 |
| Infection site | - | - | ||
| Blood system | 26 (25.00) | - | ||
| Skin and soft tissue | 33 (31.73) | - | ||
| Abdominal infection | 18 (17.31) | - | ||
| Respiratory infection | 27 (25.96) | - | ||
| Lactic acid (mmol/L) | 2.82±1.21 | 1.31±0.29 | 11.671 | <0.001 |
| Coagulation dysfunction | 84.111 | <0.001 | ||
| Yes | 78 (75.00) | 9 (9.78) | ||
| No | 26 (25.00) | 83 (90.22) |
Expression of peripheral blood H19 and its diagnostic value in sepsis
The expression of peripheral blood H19 in the DG was significantly down-regulated compared to that in the CG (0.36±0.08 vs 0.57±0.17) (P<0.05). ROC curve demonstrated that the AUC, sensitivity, and specificity of H19 in the diagnosis of sepsis were 0.849, 81.73%, and 76.08%, respectively (Table 2 and Figure 1).
Table 2.
ROC markers of peripheral blood H19 in sepsis
| Diagnostic index | AUC | 95% CI | Standard error | Cut-off | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| H19 | 0.849 | 0.793-0.905 | 0.028 | 0.425 | 81.73 | 76.08 |
Figure 1.

Expression of peripheral blood H19 and its diagnostic value in sepsis. A. Relative expression of peripheral blood H19 in the disease group was significantly lower than that in the control group (P<0.05). B. ROC curve of peripheral blood H19 in the diagnosis of sepsis.
Expression of peripheral blood H19 in survival and death groups, and its prognostic value in sepsis
Patients in the DG were assigned into the survival group (n=73) and the death group (n=31) after 28 days. Compared with the death group, expression of peripheral blood H19 was up-regulated in the survival group (0.52±0.13 vs 0.35±0.08) (P<0.05). ROC curve demonstrated that the AUC, sensitivity, specificity , and the optimal cut-off for peripheral blood H19 in the diagnosis of sepsis were 0.865, 69.86%, 90.32%, and 0.448, respectively (Table 3 and Figure 2).
Table 3.
Prognostic value of peripheral blood H19 in sepsis
| Diagnostic index | AUC | 95% CI | Standard error | Cut-off | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| H19 | 0.865 | 0.797-0.934 | 0.034 | 0.448 | 69.86 | 90.32 |
Figure 2.
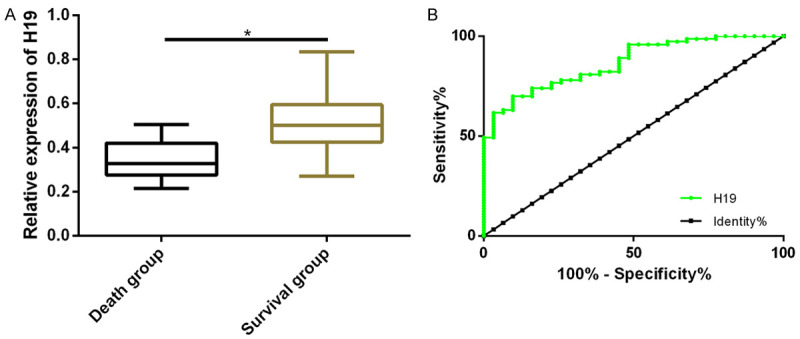
Expression of peripheral blood H19 in survival and death groups, and its prognostic value in sepsis. A. Relative expression of peripheral blood H19 in the survival group was significantly higher than that in the death group (P<0.05). B. ROC curve of peripheral blood H19 in sepsis prognosis.
Levels of inflammatory response markers and myocardial injury indicators
Compared with the CG, levels of PCT, CRP, IL-6, TNF-α, CK-MB, and cTnI in the DG were significantly increased (P<0.05) (Figure 3).
Figure 3.

Expression of inflammatory response markers and myocardial injury indicators. A. PCT level in the disease group was significantly higher than that in the control group (P<0.05). B. CRP level in the disease group was significantly higher than that in the control group (P<0.05). C. IL-6 level in the disease group was significantly higher than that in the control group (P<0.05). D. TNF-α level in the disease group was significantly higher than that in the control group (P<0.05). E. CK-MB level in the disease group was significantly higher than that in the control group (P<0.05). F. cTnI level in the disease group was significantly higher than that in the control group (P<0.05).
Correlation of peripheral blood H19 with inflammatory response markers and myocardial injury indicators
Pearson correlation analysis showed that the relative expression of peripheral blood H19 was negatively correlated with PCT (r=0.699), CRP (r=0.629), IL-6 (r=0.666), TNF-α (r=0.617), CK-MB (r=0.649), and cTnI (r=0.698) (P<0.001) (Figure 4).
Figure 4.

Correlation between peripheral blood H19 and PCT, CRP, IL-6, TNF-α, CK-MB, and cTnI in disease group. A-F. Peripheral blood H19 was negatively correlated with PCT, CRP, IL-6, TNF-α, CK-MB, and cTnI (r=0.699, P<0.001; r=0.629, P<0.001; r=0.666, P<0.001; r=0.617, P<0.001; r=0.649, P<0.001; r=0.698, P<0.001).
Prognostic factors in patients with sepsis
The factors with differences were analyzed by multivariate logistic regression. Lactic acid (P=0.001), coagulation dysfunction (P=0.018), PCT (P=0.011), CRP (P=0.005), IL-6 (P=0.001), TNF-α (P=0.004), CK-MB (P=0.001), cTnI (P=0.001), and H19 (P≤0.001) were independent risk factors for sepsis. Patients with high lactic acid, coagulation dysfunction, high levels of PCT, CRP, IL-6, TNF-α, CK-MB, and cTnI, and low H19 were more likely to develop sepsis (Tables 4, 5).
Table 4.
Multivariate logistic regression assignment
| Factor | Variable | Assignment |
|---|---|---|
| Lactic acid | X1 | Continuous variable |
| Coagulation dysfunction | X2 | No =0, Yes =1 |
| PCT | X3 | Continuous variable |
| CRP | X4 | Continuous variable |
| IL-6 | X5 | Continuous variable |
| TNF-α | X6 | Continuous variable |
| CK-MB | X7 | Continuous variable |
| cTnI | X8 | Continuous variable |
| H19 | X9 | Continuous variable |
Table 5.
Multivariate logistic regression of the incidence of sepsis
| Variable | B | S.E | Wals | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Lactic acid | 0.543 | 0.519 | 2.145 | 0.001 | 2.707 | 0.571-6.152 |
| Coagulation dysfunction | 0.674 | 0.480 | 2.011 | 0.018 | 1.497 | 0.748-2.994 |
| PCT | 0.131 | 0.473 | 1.745 | 0.011 | 1.503 | 0.751-3.006 |
| CRP | 0.248 | 0.248 | 1.935 | 0.005 | 1.198 | 1.599-2.396 |
| IL-6 | 0.684 | 0.619 | 1.145 | 0.001 | 1.556 | 0.778-3.112 |
| TNF-α | 0.735 | 0.613 | 1.732 | 0.004 | 2.133 | 1.066-4.266 |
| CK-MB | 1.145 | 0.515 | 8.617 | 0.001 | 4.014 | 2.007-8.028 |
| cTnI | 1.314 | 0.449 | 11.658 | 0.001 | 5.105 | 2.552-10.210 |
| H19 | 2.235 | 0.915 | 13.214 | <0.001 | 6.793 | 3.396-13.586 |
Discussion
Sepsis is a series of serious organic manifestations caused by infection, that can lead to the aggravation of inflammation and oxidative stress, as well as poor prognosis [15]. It is also the main cause of death in the ICU [16], causing fever or hypothermia, changes in WBCs, and tachycardia [17]. In the early stage of sepsis, activated innate immunity ignites the cascade of immune responses in cells, and abnormal immune function generally leads to automatic amplification of cytokines, causing organ dysfunction [18]. Therefore, it is of great significance to seek biomarkers for early diagnosis of sepsis.
LncRNAs have been increasingly reported to act as major regulators of gene expression and cell signaling pathways, which are also abnormally expressed in serum of septic patients [8]. Yong et al. revealed that lncRNAs functioned as novel gene regulators and prognostic markers in sepsis [19]. Further, a study by Wei et al. demonstrated that lncRNA NEAT1 (nuclear enriched abundant transcript 1) reduced inflammation in myocardial cell injury by regulating the interaction between nuclear factor (NF)-kB signal pathway and miR-144-3p [20]. In this study, the expression of peripheral blood H19 in the DG was lower than that in the CG, indicating the involvement of H19 in sepsis. The ROC curve showed that the AUC, sensitivity, and specificity of H19 in the diagnosis of sepsis were 0.849, 81.73%, and 76.08%, respectively, suggesting that H19 could be an ideal biomarker to predict the early diagnosis of sepsis. Fang et al. reported that the low expression of H19 in septic mice aggravated inflammatory response and myocardial dysfunction [21]. Thus, we speculated that H19 might affect the prognosis of septic patients. By assigning patients in the diseased group into survival and death groups, it was found that H19 had certain predictive value for the death of septic patients. Therefore, it is concluded that H19 may be used for early diagnosis and prognosis assessment of sepsis.
Inflammatory factors (IL-6, PCT, CRP) released excessively by sepsis are associated with cardiac insufficiency [22,23]. cTnl and CK-MB are markers of myocardial injury, and the serum levels of cTnl and CK-MB in patients are significantly increased [24]. The serum of septic rats has demonstrated increased myocardial injury markers and inflammatory factors [25], indicating their vital role in sepsis. These revealed that H19 might be associated with inflammatory factors and myocardial injury markers in sepsis. Multivariate logistic regression showed that patients with high lactic acid, coagulation dysfunction, high levels of PCT, CRP, IL-6, TNF-α, CK-MB, and cTnI, and low H19 expression were more likely to develop sepsis, and this probability increased with decreased expression of H19. Therefore, elevated H19 expression may reduce the risk of sepsis.
This study confirmed the role of H19 in the early diagnosis and prognosis assessment of sepsis, but there are still some limitations. First, no basic experiments have been conducted. Second, the specific regulatory mechanism of H19 in sepsis remains unclear. We will address these limitations to support our findings.
In conclusion, peripheral blood H19 can be used for the early diagnosis, clinical assessment, and prognosis of sepsis.
Disclosure of conflict of interest
None.
References
- 1.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. doi: 10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 2.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 3.Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319:62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonioli L, Blandizzi C, Fornai M, Pacher P, Lee HT, Haskó G. P2X4 receptors, immunity, and sepsis. Curr Opin Pharmacol. 2019;47:65–74. doi: 10.1016/j.coph.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing MiRNA-LncRNA interactions. Methods Mol Biol. 2016:271–286. doi: 10.1007/978-1-4939-3378-5_21. [DOI] [PubMed] [Google Scholar]
- 6.Huang YA, Chen X, You ZH, Huang DS, Chan KC. ILNCSIM: improved lncRNA functional similarity calculation model. Oncotarget. 2016;7:25902–25914. doi: 10.18632/oncotarget.8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Li L, Yan J. Progress in relationship of the long non-coding RNA and sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29:181–183. doi: 10.3760/cma.j.issn.2095-4352.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Wei S, Liu Q. Long noncoding RNA MALAT1 modulates sepsis-induced cardiac inflammation through the miR-150-5p/NF-κB axis. Int J Clin Exp Pathol. 2019;12:3311–3319. [PMC free article] [PubMed] [Google Scholar]
- 9.Peng F, Li TT, Wang KL, Xiao GQ, Wang JH, Zhao HD, Kang ZJ, Fan WJ, Zhu LL, Li M, Cui B, Zheng FM, Wang HJ, Lam EW, Wang B, Xu J, Liu Q. H19/let-7/LIN28 reciprocal negative regulatory circuit promotes breast cancer stem cell maintenance. Cell Death Dis. 2017;8:e2569. doi: 10.1038/cddis.2016.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S, Wang Y, Wang T, Hou Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8:3932–3948. doi: 10.7150/thno.25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geng H, Bu HF, Liu F, Wu L, Pfeifer K, Chou PM, Wang X, Sun J, Lu L, Pandey A, Bartolomei MS, De Plaen IG, Wang P, Yu J, Qian J, Tan XD. In inflamed intestinal tissues and epithelial cells, interleukin 22 signaling increases expression of H19 long noncoding RNA, which promotes mucosal regeneration. Gastroenterology. 2018;155:144–155. doi: 10.1053/j.gastro.2018.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Bei Y, Shen S, Huang P, Shi J, Zhang J, Sun Q, Chen Y, Yang Y, Xu T, Kong X, Xiao J. miR-21-3p controls sepsis-associated cardiac dysfunction via regulating SORBS2. J Mol Cell Cardiol. 2016;94:43–53. doi: 10.1016/j.yjmcc.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority - a WHO resolution. N Engl J Med. 2017;377:414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- 14.Hornbeck PV. Enzyme-linked immunosorbent assays. Curr Protoc Immunol. 2015;110:2.1.1–2.1.23. doi: 10.1002/0471142735.im0201s110. [DOI] [PubMed] [Google Scholar]
- 15.Brito LF, Oliveira HBM, das Neves Selis N, E Souza CLS, Júnior MNS, de Souza EP, Silva LSCD, de Souza Nascimento F, Amorim AT, Campos GB, de Oliveira MV, Yatsuda R, Timenetsky J, Marques LM. Anti-inflammatory activity of β-caryophyllene combined with docosahexaenoic acid in a model of sepsis induced by staphylococcus aureus in mice. J Sci Food Agric. 2019;99:5870–5880. doi: 10.1002/jsfa.9861. [DOI] [PubMed] [Google Scholar]
- 16.Vishnupriya K, Falade O, Workneh A, Chandolu S, Landis R, Elizabeth K, Iyengar R, Wright S, Sevransky J. Does sepsis treatment differ between primary and overflow intensive care units? J Hosp Med. 2012;7:600–605. doi: 10.1002/jhm.1955. [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian R, Wang X, Pan T, Li R, Wang J, Liu Z, Chen E, Mao E, Tan R, Chen Y, Liu J, Qu H. Plasma PTX3, MCP1 and Ang2 are early biomarkers to evaluate the severity of sepsis and septic shock. Scand J Immunol. 2019;90:e12823. doi: 10.1111/sji.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yong H, Wu G, Chen J, Liu X, Bai Y, Tang N, Liu L, Wei J. lncRNA MALAT1 accelerates skeletal muscle cell apoptosis and inflammatory response in sepsis by decreasing BRCA1 expression by recruiting EZH2. Mol Ther Nucleic Acids. 2020;19:97–108. doi: 10.1016/j.omtn.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei JL, Wu CJ, Chen JJ, Shang FT, Guo SG, Zhang XC, Liu H. LncRNA NEAT1 promotes the progression of sepsis-induced myocardial cell injury by sponging miR-144-3p. Eur Rev Med Pharmacol Sci. 2020;24:851–861. doi: 10.26355/eurrev_202001_20069. [DOI] [PubMed] [Google Scholar]
- 21.Fang Y, Hu J, Wang Z, Zong H, Zhang L, Zhang R, Sun L. LncRNA H19 functions as an Aquaporin 1 competitive endogenous RNA to regulate microRNA-874 expression in LPS sepsis. Biomed Pharmacother. 2018;105:1183–1191. doi: 10.1016/j.biopha.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Han D, Li X, Li S, Su T, Fan L, Fan WS, Qiao HY, Chen JW, Fan MM, Li XJ, Wang YB, Ma S, Qiu Y, Tian ZH, Cao F. Reduced silent information regulator 1 signaling exacerbates sepsis-induced myocardial injury and mitigates the protective effect of a liver X receptor agonist. Free Radic Biol Med. 2017;113:291–303. doi: 10.1016/j.freeradbiomed.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Weidhase L, Wellhöfer D, Schulze G, Kaiser T, Drogies T, Wurst U, Petros S. Is Interleukin-6 a better predictor of successful antibiotic therapy than procalcitonin and C-reactive protein? A single center study in critically ill adults. BMC Infect Dis. 2019;19:150. doi: 10.1186/s12879-019-3800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastro F, Guida P, Scrascia G, Rotunno C, Amorese L, Carrozzo A, Capone G, Paparella D. Cardiac troponin I and creatine kinase-MB release after different cardiac surgeries. J Cardiovasc Med (Hagerstown) 2015;16:456–464. doi: 10.2459/JCM.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Zeng Z, Wu C, Liu H. Tropisetron inhibits sepsis by repressing hyper-inflammation and regulating the cardiac action potential in rat models. Biomed Pharmacother. 2019;110:380–388. doi: 10.1016/j.biopha.2018.11.142. [DOI] [PubMed] [Google Scholar]


