Abstract
Coronary heart disease (CHD) is one of the most vital reasons for death and disability all over the world. miRNA, as a plasma index, is quite valuable for disease screening and prognosis prediction in CHD. Mining the molecular mechanism behind miRNA is also helpful for us to find molecular therapeutic strategies. In this research, we found that the expression of plasma miR-30c-5p in CHD patients was obviously lower than that in the control group (CG), which had a high differential value for CHD. We also discovered that miR-30c-5p was obviously correlated with clinical characteristics of CHD patients such as age, NYHA grade, smoking history, hypertension, hyperlipidemia, etc. In prognosis analysis, the miR-30c-5p expression in patients with poor prognosis was dramatically lower than that in those with good one, and the AUC for predicting poor prognosis of CHD was not lower than 0.850. In addition, we also induced myocardial ischemia/reperfusion (I/R) injury model of H9C2 cells through hypoxia/reoxygenation, and found that H9C2 cells also had abnormally down-regulated miR-30c-5p and up-regulated BCL2-like 11 (BCL2L11). Up-regulating miR-30c-5p or down-regulating BCL2L11 were helpful to improve proliferation and apoptosis of I/R injury model. Mechanically, BCL2L11 was also negatively regulated by miR-30c-5p, and up-regulating the former could cancel the in vitro protective effect of up-regulating the latter on H9C2 cell I/R injury model. In vivo research, up-regulating miR-30c-5p or down-regulating BCL2L11 can improve myocardial injury, histopathological changes and apoptosis in rat I/R model.
Keywords: Coronary heart disease, miR-30c-5p, BCL2L11, screening/prognosis, myocardial ischemia/reperfusion injury
Introduction
Coronary heart disease (CHD) is a global risk factor for human death and disability. The main inducement is myocardial ischemia/reperfusion (I/R) injury [1]. According to American Heart Association, the incidence of CHD increases with age in the United States. More than 15.5 million people aged not less than 20 suffer from CHD. What’s more, on average, one person suffers from myocardial infarction every 42 s [2]. Early diagnosis is the key cornerstone for CHD patients to receive timely treatment and improve prognosis [3]. More and more evidences show that miRNA in plasma, as short-chain non-coding RNA, has potential screening and prognosis prediction potential for cardiovascular diseases including CHD. The main reason is that miRNA takes part in the molecular mechanism in CHD pathogenesis and is highly correlated with the disease process [4,5]. It is understood that under normal physiological conditions, this miRNA can also be secreted in plasma, and its abnormal disorder may help to reflect disease occurrence and development. As for the secretion mechanism, we speculate that it may be due to coagulation reaction, and the substances released after the rupture of platelets or red blood cells contain miRNA [6,7]. This study mainly explores the screening and prognostic value of miR-30c-5p as a plasma marker in CHD and its regulatory mechanism in myocardial I/R injury, aiming to provide new insights for CHD treatment and prevention, which has important value for improving the morbidity and prognosis of patients.
miR-30c-5p, a member of miRNA family, is proved to regulate I/R injury such as kidney and myocardium, mediating the disease process [8,9]. Zou et al. [8] reported that it could be employed as a urine marker and was highly responsive to acute renal I/R injury process in rats, and it showed higher diagnostic value compared with miR-192-5p. Chen et al. [9] confirmed that it promoted the severity of disease development and apoptosis by activating NF-κB pathway in rat myocardial I/R injury model. Besides, it was also reported that it would participate in the in vitro protection mechanism of panax notoginseng saponins in myocardial I/R injury. Up-regulating its expression showed a positive side, which was mainly reflected in increasing cell vitality and reducing apoptosis level [10]. We discovered that miR-30c-5p’s role in myocardial I/R injury was controversial. Chen et al. [9] believed that it promoted the disease process of the in vivo model of myocardial I/R injury, while Wang et al. [10] claimed that it had certain protective effect. It is well known that miRNA mediates gene silencing mainly by binding with target mRNA, and eventually leads to mRNA cleavage or translation inhibition [11]. We hereby verify it by establishing an in vivo model of myocardial I/R injury model, and explore its potential downstream mRNA to further supplement its regulatory mechanism. Through biological analysis, we found that BCL2-like 11 (BCL2L11) had potential conservative binding sites with BCL2-like 11. It is understood that it is also known as BIM, and as an apoptosis promoter, it can mediate the molecular mechanism of sodium tanshinone IIA sulfonate in rat myocardial I/R injury model, in which the former reduces dramatically after treatment [12].
We believe that miR-30c-5p has certain screening and prognostic value in CHD, and it can regulate the disease process of BCL2L11 affecting myocardial I/R injury, which is hereby verified and reported.
Data and methods
Sample collection
This research was approved by the ethics committee of Heart Center of Henan Province People’s Hospital. The subjects and their guardians were informed, and they signed a fully informed consent form. The plasma of 100 CHD patients (CHD group) and 80 healthy subjects (HC group) admitted to Heart Center of Henan Province People’s Hospital from April 2010 to April 2015 were taken into consideration. The CHD group was diagnosed as CHD [13], and all met the classification standard of New York Heart Association (NYHA) [14], excluding those with malignancy or other serious organ dysfunction, and those who could not be followed up for 5 years.
Follow-up
The CHD group was followed up for 5 years, mainly by means of electric interview and medical record inquiry. The follow-up frequency was once every 3 months. The total survival time (OS) was the period from the day of diagnosis to the last day of follow-up or the day of death.
Cell culture
H9C2 cells (Aolu Biotechnology Co., Ltd., Shanghai, China, XB-3141) were purchased, and then cultured in Dulbecco modified Eagle Medium (DMEM) (Chreagen Biotechnology Co., Ltd., Beijing, China, 120002). Next, we added 10% fetal bovine serum (FBS) (Lianshuo Biotechnology Co., Ltd., Shanghai, China, A3160802 10×50 ml/ box), and adjusted the cell density in the 6-well plate to 1.5×105 cells/well and the environmental parameters to 5% CO2, 37°C.
Establishment of myocardial I/R injury model
The myocardial I/R injury model of H9C2 cells was induced by hypoxia/reoxygenation (H/R) [15]. The cells were first exposed to hypoxia for 24 h in a 1% oxygen environment, and environmental parameters of 37°C, 94% N2 and 5% CO2 were set in a modular incubator. After that, it was oxidized for another 24 h, and the environmental parameters were set at 37°C, 5% CO2. The cells in normal oxygen environment were used as control group.
Cell transfection
Transfections were transiently transfected into H9C2 cells by Lipofectamine 3000 kit (Qiming Biotechnology Co., Ltd., Shanghai, China, RF(m)11471), respectively. Forty-eight hours later, the test was started after the cells were collected. The above process was strictly conducted in accordance with the instructions. According to the experimental purpose, the transfectants mainly include: miR negative control (miR-NC), miR-30c-5p mimics (miR-30c-5p), inhibitor (inhibitor), si negative control (si-NC), BCL2L11 targeted inhibition (si-BCL2L11), BCL2l1 over-expression vector (pcDNA3.1-BCL2L11), pcDNA3.1 empty vector (pcDNA3.1), all of which were bought from Shanghai Meixuan Biotechnology Co., Ltd.
RT-PCR
The total RNA was extracted by TRIzol reagent (Mairuibo Biotech Co., Ltd., Beijing, China, M NR0002). Afterwards, it was reverse transcribed into cDNA through reverse transcription kit (Baiaolaibo Technology Co., Ltd., Beijing, China, BTN60906-PYW) and then amplified. All primers were synthesized by Shanghai Jikai Gene Medical Technology Co., Ltd. mRNA employed GAPDH as internal reference, while miRNA employed U6. The relative expression was determined by 2-ΔΔct.
Western blot analysis
The total protein was extracted by RIPA pyrolysis method, and the protein concentration was tested via BCA kit (Chreagen Biotechnology Co., Ltd., Beijing, China, 120982). The dilution ratio of primary antibodies such as BCL2L11, Caspase-3, Bax, Bcl-2 and GAPDH were all 1: 1000. Each membrane was rinsed 3 times with phosphate buffered saline (PBS) (Keshun Biotechnology Co., Ltd., Shanghai, China, 383855000) and then incubated 1 h with horseradish peroxidase labeled goat anti-rabbit secondary antibody. All antibodies were bought from Beijing Solarbio Technology Co., Ltd. Excess liquid on the membrane was absorbed by filter paper, ECL was used for visualization, and the gray value was assessed by ImageJ (Easybio Biotechnology Co., Ltd., Beijing, China).
Cell damage assessment
To evaluate cell damage, we measured the LDH and MDA concentrations in H9C2 cells in culture medium with lactate dehydrogenase (LDH) detection kit and lipid peroxidation malondialdehyde (MDA) detection kit (Yuanye Biotechnology Co., Ltd., Shanghai, China, R24020, R21869-).
Cell proliferation assay
Cell proliferation was tested via CCK-8 kit (Xinyu Biotechnology Co., Ltd., Shanghai, China, XY1759), and it was strictly in accordance with the instructions. Cell proliferation at 0, 24, 48 and 72 h was mainly evaluated. H9C2 cells were washed with PBS, cultured in CCK-8 containing 10 μL and 90 μL serum-free medium (Hengfei Biotechnology Co., Ltd., Shanghai, China, N6010) for 2 h at 37°C, 5% CO2, and optical density (OD) values of cells in each group were measured at 450 mm wavelength.
Determination of apoptosis
Cell apoptosis was evaluated by flow cytometry and apoptosis kit (Chreagen Biotechnology Co., Ltd., Beijing, China, YZ0176). Cells were prepared into 1×106/mL suspension, digested, and washed with 0.25% trypsin (Hengfei Biotechnology Co., Ltd., Shanghai, China, CC0128). We added AnnexinV-FITC and PI in sequence, each 10 μL, and they were cultured 5 min at indoor temperature in the dark, and tested with ACEA NovoCyte flow cytometry (Beamdiag Biotechnology Co., Ltd., Changzhou, China, 1026).
Dual-luciferase report
To authenticate the targeted relationship between miR-30c-5p and BCL2L11, biological analysis was conducted through (http://www.targetscan.org), miRwalk (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/), miRDB (http://www.mirdb.org/miRDB/), respectively. BCL2L11-Wt (wild type), BCL2L11-Mut (mutant type), miR-30c-5p and miR-NC were co-transfected into H9C2 cells using Lipofectamine™ 3000 kit, and luciferase activity was detected using dual-luciferase reporter gene detection kit (Solarbio Technology Co., Ltd., Beijing, China, D0010).
Construction of rat I/R model
Fifty four male Sprague-Dawley rats (Focus Biotechnology Co., Ltd., Guangzhou, China) with an average mass of (275±25) g were purchased and fed in cages with food and water. This animal experiment scheme has been approved by the Animal Nursing and Use Committee of our hospital, and it was operated in strict accordance with the guidelines. The rats were randomized into groups: sham, I/R, I/R+miR-NC, I/R+miR-30c-5p, I/R+si-NC, I/R+si-BCL2L11, 9 in each group. For group I/R, rats were anesthetized by intraperitoneal injection of 3% pentobarbital sodium (Xinya Pharmaceutical Co., Ltd., Shanghai, China, H31021725). Then, the left anterior descending (LAD) was occluded with 4-silk suture for 30 min and they were reperfused for 2 h. In the sham group, LAD was not ligated. In the latter four groups, miR-NC, miR-30c-5p and si-NC, si-BCL2L11 were injected into caudal vein of rats by adeno-associated virus (AAV) system (2×1011 vector genome particles/vg per rat) and RNA interference Technology (RNAi). AAV and RNAi were designed and constructed by Shanghai Hanheng Biotechnology Co., Ltd.
Assessment of myocardial function
After I/R, we collected the coronary effluent and detected the activity of lactate dehydrogenase (LDH) and creatine kinase (CK) by LDH and CK kits (Future Industrial Co., Ltd., Shanghai, China, 15323783212532240037).
Histopathological assessment
The rat heart was fixed with 10% formalin (Yuanye Biotechnology Co., Ltd., Shanghai, China, R30420) and embedded in paraffin wax. Next, 5 μm thick sections were harvested, and the myocardial tissue morphology was observed under optical microscope after HE staining. Cardiomyocyte apoptosis was detected by Tunel kit (Bangjing Industrial Co., Ltd., Shanghai, China, BJ-10511).
Statistical methods
All our experiments were conducted independently for at least 3 times. The data were represented as mean ± standard deviation and assessed statistically, and pictures were generated through GraphPad 6. We compared the differences between groups through independent-samples T test, one-way analysis of variance (ANOVA), LSD-t test, repeated measures ANOVA, Bonferroni test, etc. Value of screening and prognosis prediction was evaluated by receiver operating characteristic curve (ROC), and the correlation between miR-30c-5p and NYHA grade was evaluated by Spearman correlation coefficient. P<0.05 was considered to be statistically remarkable.
Results
Plasma miR-30c-5p down-regulates in CHD
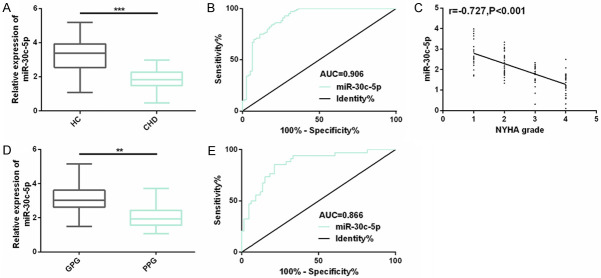
To explore whether miR-30c-5p, as a plasma biological indicator, responds to the occurrence and progression of CHD diseases, we detected the expression of subjects in the two groups. As it turned out, the expression of plasma miR-30c-5p in CHD patients was dramatically lower than that of control subjects, and ROC’s AUC was 0.906, which was also obviously negatively correlated with the NYHA grade (r=-0.727, P<0.001). It meant that plasma miR-30c-5p participated in the CHD process, and it might be used as a biomarker for screening CHD and also be related to disease severity. To understand the potential prognostic value of miR-30c-5p, we evaluated its prognosis. All 100 CHD patients were successfully followed up for 5 years. We took 34 CHD patients who died or suffered from disease recurrence or disease progression as the poor prognosis group (PPG), and the remaining 66 patients as the good prognosis group (GPG). The miR-30c-5p expression in the PPG was obviously lower than that in the GPG, and the AUC for predicting poor prognosis of CHD was 0.866. In Cox analysis, miR-30c-5p (P<0.001) was also an independent prognostic factor affecting their poor prognosis, including NYHA grade (P=0.005) (Figure 1; Table 1).
Figure 1.
Expression and clinical significance of plasma miR-30c-5p in CHD. A. The miR-30c-5p expression in plasma of subjects in the two groups is detected by real-time quantitative PCR; B. The value of plasma miR-30c-5p in distinguishing CHD is evaluated by drawing ROC curve; C. The relationship between miR-30c-5p and NYHA grade in CHD patients is assessed through Spearman correlation analysis. D. Plasma miR-30c-5p expression of CHD patients in the poor prognosis group and the good prognosis group; E. ROC curve is drawn to evaluate the predictive value of plasma miR-30c-5p for CHD prognosis. Note: **represents the comparison between the two groups (P<0.01), ***represents the comparison between the two groups (P<0.001).
Table 1.
Cox analysis on poor prognosis of CHD patients
| Parameter | Single-factor | Multiple factors | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender | 1.733 (0.318-9.652) | 0.527 | ||
| Age | 1.578 (0.952-2.643) | 0.076 | ||
| NYHA grade | 3.546 (0.437-25.900) | 0.044 | 3.652 (0.854-6.396) | 0.005 |
| CHD family history | 1.068 (0.813-1.359) | 0.632 | ||
| Smoking history | 2.981 (1.045-8.491) | 0.040 | 1.209 (0.791-2.165) | 0.214 |
| Hypertension | 1.847 (1.036-3.278) | 0.035 | 1.473 (0.872-2.490) | 0.148 |
| Hyperlipidemia | 1.795 (1.214-2.656) | 0.023 | 1.103 (0.890-1.542) | 0.097 |
| miR-30c-5p | 2.756 (1.394-5.337) | 0.002 | 4.259 (2.653-7.011) | <0.001 |
Relationship between plasma miR-30c-5p and clinical characteristics of CHD patients
We analyzed the relationship between plasma miR-30c-5p and clinical characteristics of CHD patients. The results manifested that it had no remarkable difference with gender, CHD family history of patients, but it was dramatically correlated with their clinical characteristics such as age, NYHA grade, smoking history, hypertension, hyperlipidemia and so on, suggesting that miR-30c-5p might have certain clinical guiding function (Table 2).
Table 2.
Relationship between plasma miR-30c-5p and clinical characteristics of CHD patients (mean ± SD)
| Parameter | n | miR-30c-5p | t/F | P |
|---|---|---|---|---|
| Gender | 0.617 | 0.539 | ||
| Male | 60 | 1.80±0.69 | ||
| Female | 40 | 1.89±0.75 | ||
| Age (years) | 3.460 | <0.001 | ||
| ≤60 | 45 | 2.03±0.64 | ||
| >60 | 55 | 1.60±0.60 | ||
| NYHA grade | 15.901 | <0.001 | ||
| I | 20 | 2.43±0.73 | ||
| II | 30 | 2.06±0.65 | ||
| III | 22 | 1.65±0.60 | ||
| IV | 28 | 1.28±0.48 | ||
| CHD family history | 0.564 | 0.574 | ||
| No | 64 | 1.90±0.74 | ||
| Yes | 36 | 1.82±0.56 | ||
| Smoking history | 3.316 | 0.001 | ||
| No | 52 | 2.04±0.70 | ||
| Yes | 48 | 1.60±0.62 | ||
| Hypertension | 2.102 | 0.038 | ||
| No | 61 | 1.96±0.69 | ||
| Yes | 39 | 1.68±0.58 | ||
| Hyperlipidemia | 2.461 | 0.016 | ||
| No | 56 | 1.98±0.76 | ||
| Yes | 44 | 1.63±0.63 |
Abnormal expression levels of miR-30c-5p and BCL2L11 exist in H9C2 cell myocardial I/R injury model
A myocardial I/R injury model of H9C2 cells was established to explore CHD’s molecular mechanism. We found that in those cells, miR-30c-5p with abnormal down-regulation and BCL2L11 (transcription and protein level) were also found in H/R group compared with CG. This indicated that miR-30c-5p and BCL2L11 could respond to CHD occurrence and progression (Figure 2).
Figure 2.
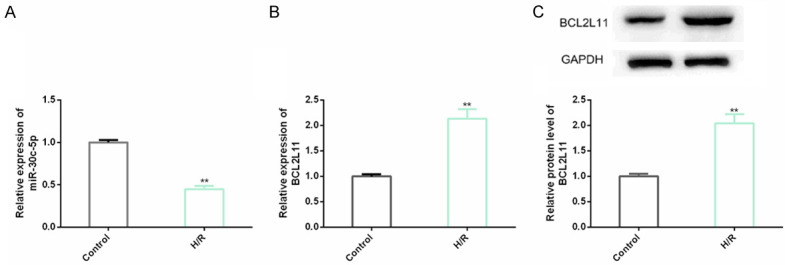
Levels of miR-30c-5p and BCL2L11 in H9C2 cell myocardial I/R injury model. A, B. The miR-30c-5p and BCL2L11 expression levels are detected by real-time quantitative PCR in H9C2 cells; C. BCL2L11 protein level in H9C2 cells is detected through western blot analysis and its protein map is shown. Note: compared with Control, **indicates P<0.01.
Up-regulating miR-30c-5p can improve proliferation and apoptosis of H9C2 cell myocardial I/R injury model
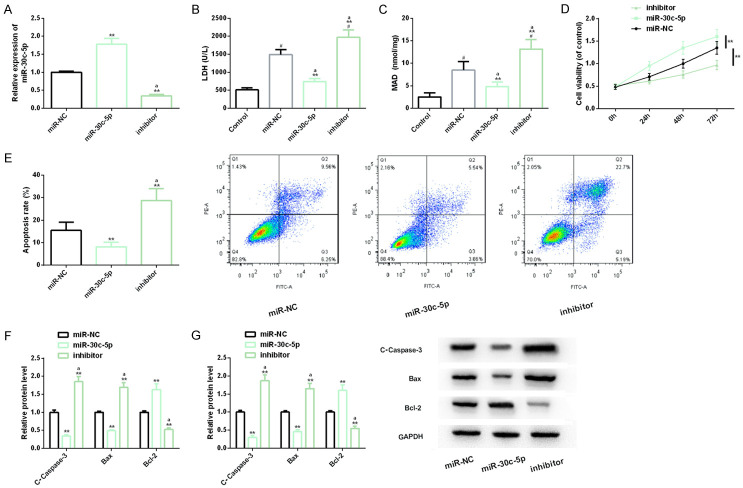
To probe into miR-30c-5p’s molecular mechanism in CHD, we up-regulated and down-regulated it by transfecting miR-30c-5p and inhibitor separately. It should be added that LDH is a marker of CHD, and its excessive up-regulation is related to CHD progression [16]. MDA is an indicator of lipid peroxidation, and its level is inversely proportional to the body’s antioxidant capacity [17]. The measurement of LDH and MDA indexes is helpful for us to evaluate the I/R damage of H9C2 cells. Besides, Caspase-3 and Bax are well-known apoptosis-promoting factors, while Bcl-2 has anti-apoptosis properties. Dynamic monitoring of the three levels is helpful for us to understand apoptosis development [18]. Our cell tests showed that up-regulating miR-30c-5p dramatically reduced LDH and MDA levels in H9C2 cell myocardial I/R injury model, and it increased cell viability and inhibited apoptosis level. In addition, the levels of Caspase-3 and Bax in apoptosis markers were also obviously down-regulated under the influence of miR-30c-5p over-expression, while Bcl-2 was dramatically up-regulated. However, down-regulating miR-30c-5p was obviously contrary to the above results. The above research showed that up-regulating miR-30c-5p was helpful to inhibit disease progression in CHD in vitro model (Figure 3).
Figure 3.
Effect of miR-30c-5p on myocardial I/R injury model of H9C2 cells. A. The transfection efficiency of miR-30c-5p is detected by real-time quantitative PCR; B. The effect of miR-30c-5p on LDH level of H9C2 cells is detected by LDH kit; C. The effect of miR-30c-5p on MDA level of H9C2 cells is detected by MDA detection kit; D, E. The effect of miR-30c-5p on proliferation and apoptosis level of H9C2 cell I/R injury model is detected via CCK-8 method and flow cytometry, and its flow cytometry is shown; F, G. The effect of miR-30c-5p on the expression of apoptosis factor and protein level is detected via real-time quantitative PCR and western blot analysis, and its protein map is shown. Note: #indicates the comparison with Control (P<0.05); **indicates the comparison with miR-NC (P<0.01); aindicates the comparison with miR-30c-5p (P<0.01).
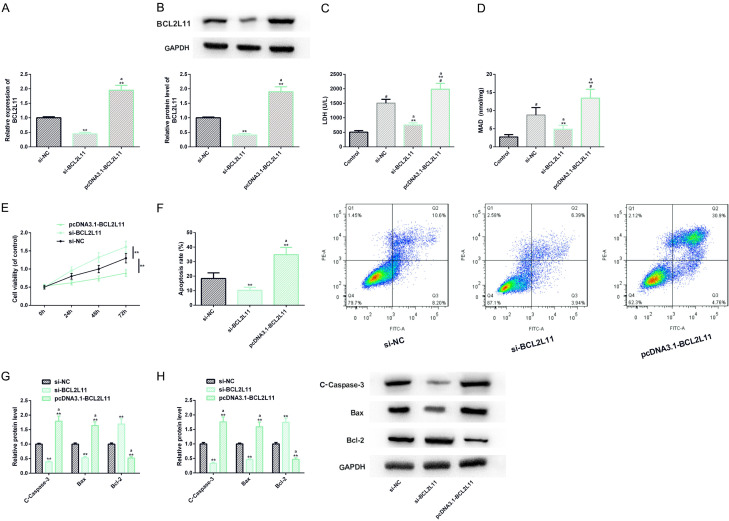
Down-regulating BCL2L11 can also improve proliferation and apoptosis of H9C2 cell myocardial I/R injury model
Similarly, we also transfected si-BCL2L11 and BCL2L11 into H9C2 cell myocardial I/R injury model, and realized down-regulation and up-regulation treatment respectively. In cell analysis, down-regulating BCL2L11 also showed one side that was conducive to the inhibition of CHD process, i.e. the levels of LDH and MDA reduced, while the cell vitality was enhanced and apoptosis was inhibited, similar to the results of previous research on transfection of miR-30c-5p. Furthermore, up-regulating BCL2L11 showed the opposite negative side to the previous results, suggesting that down-regulating it had certain efficacy on CHD (Figure 4).
Figure 4.
Effect of BCL2L11 on myocardial I/R injury model of H9C2 cells. A, B. The transfection efficiency of BCL2L11 is detected by real-time quantitative PCR and western blot analysis, and its protein map is shown. C, D. LDH and MDA test kits are used to detect the effect of BCL2L11 on LDH and MDA levels of H9C2 cells injured by I/R; E, F. The effect of BCL2L11 on the proliferation and apoptosis level of H9C2 cell I/R injury model is detected by CCK-8 method and flow cytometry, and its flow cytometry is shown; G, H. The effect of BCL2L11 on the expression of apoptosis factor and protein level is detected by real-time quantitative PCR and western blot analysis, and its protein map is shown. Note: #indicates the comparison with Control (P<0.05); **indicates the comparison with si-NC (P<0.01); aindicates the comparison with si-BCL2L11 (P<0.01).
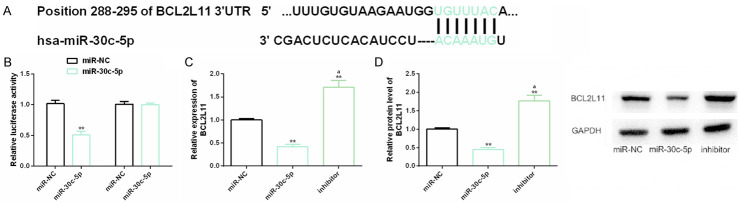
BCL2L11 is the downstream target of miR-30c-5p
We further explored the relationship between miR-30c-5p and BCL2L11, and discovered that they had potential conservative binding sites through biological analysis. In the dual-luciferase gene report results, miR-30c-5p only down-regulated BCL2L11-Wt, but had no marked effect on BCL2L11-Mut. In addition, BCL2L11 was also negatively regulated by miR-30c-5p, i.e. up-regulating miR-30c-5p dramatically down-regulated BCL2L11 expression and protein level. All the above results revealed that BCL2L11 was a downstream target of miR-30c-5p and was negatively regulated by it (Figure 5).
Figure 5.
Relationship between miR-30c-5p and BCL2L11. A. Bioinformatics analysis reveals that miR-30c-5p and BCL2L11 have conserved binding sites; B. Dual luciferase gene report results; C, D. The effect of miR-30c-5p on BCL2L11 expression and protein level is detected by real-time quantitative PCR and western blot analysis, and its protein map is shown. Note: **indicates the comparison with miR-NC (P<0.01); aindicates the comparison with miR-30c-5p (P<0.01).
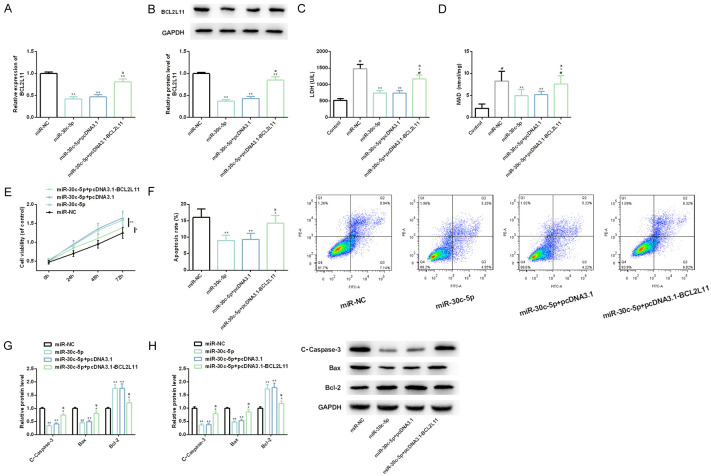
Up-regulating BCL2L11 can weaken the improvement of miR-30c-5p up-regulation on myocardial I/R injury
Through co-transfection of BCL2L11 and miR-30c-5p, we also found that up-regulating the former could weaken the improvement of over-expressing the latter on myocardial I/R injury: LDH and MDA, which had been reduced, began to increase dramatically, while the previously increased cell viability and anti-apoptosis decreased dramatically. This showed that BCL2L11 could also react on miR-30c-5p, threatening its protection in CHD (Figure 6).
Figure 6.
Effect of BCL2L11 up-regulation on myocardial I/R injury model of H9C2 cells transfected with miR-30c-5p. A, B. The BCL2L11 level in myocardial I/R injury models of H9C2 cells in each group is detected by real-time quantitative PCR and western blot analysis, and its protein map is shown. C, D. LDH and MDA detection kits are used to detect LDH and MDA levels of myocardial I/R injury models of H9C2 cells in each group, respectively. E, F. CCK-8 method and flow cytometry are used to detect the proliferation and apoptosis levels of myocardial I/R injury models of H9C2 cells in each group, respectively, and its flow cytometry is shown. G, H. Real-time quantitative PCR and western blot analysis are used to detect the expression and protein level of apoptosis factor in H9C2 cell myocardial I/R injury models of each group, and its protein map is shown. Note: #indicates the comparison with Control (P<0.05); **indicates the comparison with miR-NC (P<0.01); aindicates the comparison with miR-30c-5p (P<0.01).
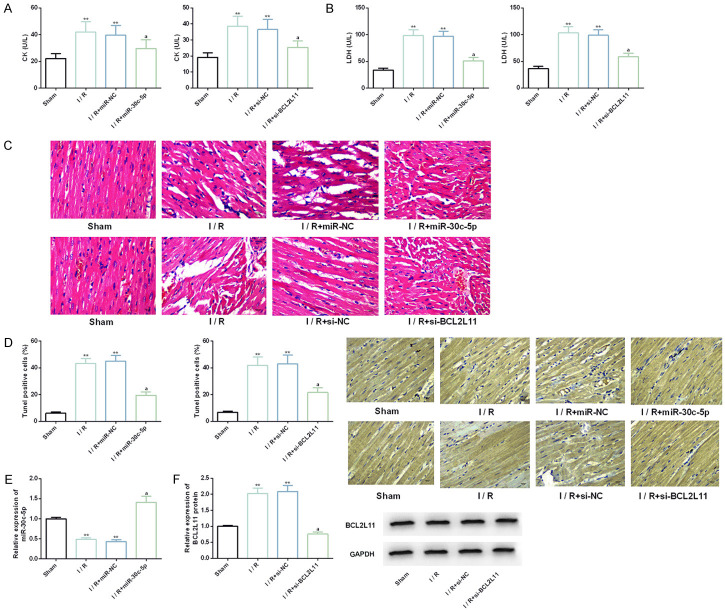
Potential efficacy of up-regulating miR-30c-5p or down-regulating BCL2L11 on rat I/R model
We established the rat I/R model by I (30 min)/R (2 h) intervention, and on this basis, we injected miR-30c-5p or si-BCL2L11 intraperitoneally. Further studies showed that CK and LDH were obviously up-regulated in the rat I/R model, and the positive rate of Tunel cells increased markedly. The muscle fibers of myocardial tissue were loosely and irregularly arranged and even necrotic. But in the model injected with miR-30c-5p or si-BCL2L11, the above adverse phenomena were dramatically improved. All these suggest that up-regulating miR-30c-5p or down-regulating BCL2L11 has potential efficacy on rat I/R model, which is helpful to improve myocardial injury, inhibit apoptosis and repair myocardial fiber (Figure 7).
Figure 7.
Effects of up-regulating miR-30c-5p or down-regulating BCL2L11 on rat I/R model. A, B. The coronary effluent of rat I/R model is collected and the activities of CK and LDH in each group are detected by CK and LDH kits. C. The myocardial tissue morphology is evaluated by HE staining. D. The apoptosis rate of rats in each group is evaluated by TUNEL assay. E, F. The expression or protein levels of miR-30c-5p and BCL2L11 are detected by real-time PCR and Western blot. Note: **indicates the comparison with sham (P<0.01); aindicates the comparison with I/R (P<0.01).
Discussion
In this research, we mainly found that plasma miR-30c-5p could be employed as a screening and prognosis indicator for CHD patients, and up-regulating its expression could also improve myocardial I/R injury by targeted inhibition of BCL2L11. CHD is a manifestation of cardiac dysfunction, and its etiology is tied to atherosclerotic stenosis or coronary artery occlusion [19]. New cases and deaths of CHD are increasing, which may be attributed to the trend of cardiovascular disease risk factors still spreading [20]. Therefore, exploring the molecular mechanism of CHD and increasing the understanding of CHD pathogenesis are quite practical for formulating new therapeutic strategies or preventive measures related to CHD.
The miR-30c-5p-BCL2L11 axis is a novel molecular regulatory network for CHD pathogenesis that we have discovered for the first time. miR-30c-5p is first reportedly dramatically down-regulated in the plasma of patients with type 2 diabetes complicated with CHD, and it is negatively correlated with the severity of coronary artery lesions. It can also regulate PAI-1-VN interaction, suggesting that it participates in the regulation process of such CHD complex diseases and may be used as a biomarker for such complex diseases [21]. Secondly, Urbich et al. [22] pointed out that BCL2L11, as an apoptosis-promoting factor, mediated endothelial progenitor cell apoptosis signal transduction and responded to atorvastatin’s anti-apoptosis and anti-oxidative stress effects in CHD. However, there are still few reports on the molecular mechanism of miR-30c-5p-BCL2L11 axis. We discovered that miR-30c-5p, as a plasma index, expressed obviously lower in CHD patients than in the control group. It also had great screening value for CHD, specifically, ROC’s AUC was as high as 0.906, and it was also dramatically negatively correlated with the NYHA grade of CHD patients, which indicated that it might be a highly responsive factor for CHD progression and had great potential as a screening index. In addition, it was also dramatically correlated with their clinical characteristics such as age, NYHA grade, smoking history, hypertension, hyperlipidemia, etc., further confirming that it might be helpful to indicate the clinical characteristics of patients in these aspects. Furthermore, we also found that miR-30c-5p had an AUC of 0.866 for predicting poor prognosis of CHD, and its potential as a prognostic indicator was not low. Moreover, miR-30c-5p and NYHA grade were independent prognostic factors that affected poor prognosis of patients, which further confirmed its prognostic value.
On the other hand, we also simulated the myocardial I/R injury model of H9C2 cells through H/R, and found that the abnormal expression of miR-30c-5p and BCL2L11 were similar to clinical results, and the former was down-regulated while the latter was up-regulated. It is understood that the down-regulation mechanism of miR-30c-5p in H9C2 myocardial injury cell model may be related to the damage to the protection or defense mechanism of myocardial injury, thus promoting the injury and apoptosis of myocardial cells [10]. What’s more, the relationship between the down-regulation of miR-30c-5p in cells and plasma may prove that miR-30c-5p is CHD specific in both serological and cellular molecular levels, which reflects that it is a highly responsive indicator of CHD and may be its biomarker. Many studies have also pointed out that miR-30c-5p is low expressed in cryptococcus neoformans infection, nonalcoholic fatty liver disease, gastric cancer and other diseases, while increasing its expression obviously inhibits disease progression through different mechanisms of action [23-25]. In addition, BCL2L11 has also been reported by many scholars to show abnormally high expression during disease development and progression. For example, Wei et al. [26] confirmed that down-regulating the expression in Parkinson’s Disease had positive significance for inhibiting neuronal apoptosis. Other studies have shown that it is pathogenic in ischemic stroke. Targeted down-regulation of its expression has certain therapeutic effect on restoring neuronal function [27]. In our CHD disease background, up-regulating miR-30c-5p or down-regulating BCL2L11 is helpful to increase the cell viability and inhibit apoptosis of H9C2 cell I/R injury model, and we can also reduce cell injury by down-regulating LDH and MDA. Hu et al. [28] reported that miR-30c-5p had shown therapeutic effect in ox-LDL induced atherosclerosis in vitro model, i.e. up-regulating its expression was helpful to inhibit apoptosis and inflammatory state. In addition, Li et al. [29] confirmed that BCL2L11 took part in the protective mechanism of Glutaredoxin 1 against atherosclerosis development, and its remarkable down-regulation under the intervention of Glutaredoxin 1 was quite marked to prevent apoptosis. Atherosclerosis is one of the inducing factors of CHD, and the above research shows certain similarity with our research results. Mechanically, BCL2L11 is also the direct target of miR-30c-5p, and up-regulating the former can cancel the in vitro protective effect of up-regulating the latter on I/R damage model of H9C2 cells, which indicates that the two have interaction in CHD process. Finally, we also explored the effects of miR-30c-5p and BCL2L11 on rat I/R model in vivo. The data showed that up-regulating miR-30c-5p or down-regulating BCL2L11 could improve myocardial index, histopathological changes of myocardial tissue and apoptosis level of rat I/R model. It had certain efficacy on I/R in vivo model, which improved myocardial function, inhibited myocardial lesion and strengthened anti-apoptosis. The above research reveals that developing miR-30c-5p expression promoter or BCL2L11 inhibitor may have molecular therapeutic effect on CHD patients.
Although our research has proved miR-30c-5p-BCL2L11 axis’s regulatory mechanism in CHD in vitro model, as well as the screening and prognostic value of miR-30c-5p. However, there is still some room for improvement. First of all, we think that the miR-30c-5p-BCL2L11 axis probably has upstream regulatory factors. Finding upstream factors is helpful to expand the molecular regulatory network. Secondly, we can supplement the performance of miR-30c-5p-BCL2L11 axis in CHD animal models, further improving the accuracy and comprehensiveness of the research results. Finally, we can also focus on whether the axis responds to the efficacy of anti-CHD drugs and comprehensively supplement the potential clinical effect of the axis.
In short, plasma miR-30c-5p has screening and prognostic value in CHD, and over-expressing it can improve I/R injury and apoptosis of H9C2 cells by down-regulating BCL2L11.
Acknowledgements
Project supported by the Fund of Comprehensive Surgical Treatment of End-stage Heart Disease (No. 201601011).
Disclosure of conflict of interest
None.
References
- 1.Kong F, Jin J, Lv X, Han Y, Liang X, Gao Y, Duan X. Long noncoding RNA RMRP upregulation aggravates myocardial ischemia-reperfusion injury by sponging miR-206 to target ATG3 expression. Biomed Pharmacother. 2019;109:716–725. doi: 10.1016/j.biopha.2018.10.079. [DOI] [PubMed] [Google Scholar]
- 2.Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4:256. doi: 10.21037/atm.2016.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ke-Gang J, Zhi-Wei L, Xin Z, Jing W, Ping S, Xue-Jing H, Hong-Xia T, Xin T, Xiao-Cheng L. Evaluating diagnostic and prognostic value of plasma miRNA133a in acute chest pain patients undergoing coronary angiography. Medicine (Baltimore) 2016;95:e3412. doi: 10.1097/MD.0000000000003412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, Gao W, Long QQ, Zhang J, Li YF, Liu DC, Yan JJ, Yang ZJ, Wang LS. Increased plasma levels of lncRNA H19 and LIPCAR are associated with increased risk of coronary artery disease in a Chinese population. Sci Rep. 2017;7:7491. doi: 10.1038/s41598-017-07611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busch A, Eken SM, Maegdefessel L. Prospective and therapeutic screening value of non-coding RNA as biomarkers in cardiovascular disease. Ann Transl Med. 2016;4:236. doi: 10.21037/atm.2016.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Li HH, Yang R, Yang BJ, Gao ZY. Association between circulating microRNA-208a and severity of coronary heart disease. Scand J Clin Lab Invest. 2017;77:379–384. doi: 10.1080/00365513.2017.1328740. [DOI] [PubMed] [Google Scholar]
- 7.Corduan A, Ple H, Laffont B, Wallon T, Plante I, Landry P, Provost P. Dissociation of SERPINE1 mRNA from the translational repressor proteins Ago2 and TIA-1 upon platelet activation. Thromb Haemost. 2015;113:1046–1059. doi: 10.1160/TH14-07-0622. [DOI] [PubMed] [Google Scholar]
- 8.Zou YF, Wen D, Zhao Q, Shen PY, Shi H, Zhao Q, Chen YX, Zhang W. Urinary MicroRNA-30c-5p and MicroRNA-192-5p as potential biomarkers of ischemia-reperfusion-induced kidney injury. Exp Biol Med (Maywood) 2017;242:657–667. doi: 10.1177/1535370216685005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Zhang M, Zhang S, Wu J, Xue S. Rno-microRNA-30c-5p promotes myocardial ischemia reperfusion injury in rats through activating NF-kappaB pathway and targeting SIRT1. BMC Cardiovasc Disord. 2020;20:240. doi: 10.1186/s12872-020-01520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Chen X, Wang Y, Zhao L, Zhao X, Wang Y. MiR-30c-5p mediates the effects of panax notoginseng saponins in myocardial ischemia reperfusion injury by inhibiting oxidative stress-induced cell damage. Biomed Pharmacother. 2020;125:109963. doi: 10.1016/j.biopha.2020.109963. [DOI] [PubMed] [Google Scholar]
- 11.Hu J, Li L, Chen H, Zhang G, Liu H, Kong R, Chen H, Wang Y, Li Y, Tian F, Lv X, Li G, Sun B. MiR-361-3p regulates ERK1/2-induced EMT via DUSP2 mRNA degradation in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9:807. doi: 10.1038/s41419-018-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang MQ, Zheng YL, Chen H, Tu JF, Shen Y, Guo JP, Yang XH, Yuan SR, Chen LZ, Chai JJ, Lu JH, Zhai CL. Sodium tanshinone IIA sulfonate protects rat myocardium against ischemia-reperfusion injury via activation of PI3K/Akt/FOXO3A/Bim pathway. Acta Pharmacol Sin. 2013;34:1386–1396. doi: 10.1038/aps.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Infante T, Forte E, Schiano C, Cavaliere C, Tedeschi C, Soricelli A, Salvatore M, Napoli C. An integrated approach to coronary heart disease diagnosis and clinical management. Am J Transl Res. 2017;9:3148–3166. [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Zhang F, Zhou H, Hu Y, Guo D, Fang X, Chen Y. Interplay of TNF-alpha, soluble TNF receptors and oxidative stress in coronary chronic total occlusion of the oldest patients with coronary heart disease. Cytokine. 2020;125:154836. doi: 10.1016/j.cyto.2019.154836. [DOI] [PubMed] [Google Scholar]
- 15.Yuan Y, Wang YY, Liu X, Luo B, Zhang L, Zheng F, Li XY, Guo LY, Wang L, Jiang M, Pan YM, Yan YW, Yang JY, Chen SY, Wang JN, Tang JM. KPC1 alleviates hypoxia/reoxygenation-induced apoptosis in rat cardiomyocyte cells though BAX degradation. J Cell Physiol. 2019;234:22921–22934. doi: 10.1002/jcp.28854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng H, Wang Z, Wang C, Zhu X, Liu Z, Liu H, Guo M, Hou Q, Chu Z. Effect of furostanol saponins from allium macrostemon bunge bulbs on platelet aggregation rate and PI3K/Akt pathway in the rat model of coronary heart disease. Evid Based Complement Alternat Med. 2019;2019:9107847. doi: 10.1155/2019/9107847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Q, Liu Z, Huang B, Yuan Y, Liu X, Zhang H, Qiu F, Zhang Y, Li Y, Miao H, Dong H, Zhang Z. PEDF improves cardiac function in rats subjected to myocardial ischemia/reperfusion injury by inhibiting ROS generation via PEDFR. Int J Mol Med. 2018;41:3243–3252. doi: 10.3892/ijmm.2018.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu CT, Liu S, Tang M. Downregulation of linc00961 contributes to promote proliferation and inhibit apoptosis of vascular smooth muscle cell by sponging miR-367 in patients with coronary heart disease. Eur Rev Med Pharmacol Sci. 2019;23:8540–8550. doi: 10.26355/eurrev_201910_19168. [DOI] [PubMed] [Google Scholar]
- 19.Shams A, Morley JE. Editorial: autonomic neuropathy and cardiovascular disease in aging. J Nutr Health Aging. 2018;22:1028–1033. doi: 10.1007/s12603-018-1097-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G, Wang S, Gu Y, Song L, Yu S, Feng X. Tai Chi improves coronary heart disease risk by inactivating MAPK/ERK pathway through serum miR-126. Evid Based Complement Alternat Med. 2020;2020:4565438. doi: 10.1155/2020/4565438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo M, Wang G, Xu C, Zeng M, Lin F, Wu J, Wan Q. Circulating miR-30c as a predictive biomarker of type 2 diabetes mellitus with coronary heart disease by regulating PAI-1/VN interactions. Life Sci. 2019;239:117092. doi: 10.1016/j.lfs.2019.117092. [DOI] [PubMed] [Google Scholar]
- 22.Urbich C, Knau A, Fichtlscherer S, Walter DH, Bruhl T, Potente M, Hofmann WK, de Vos S, Zeiher AM, Dimmeler S. FOXO-dependent expression of the proapoptotic protein Bim: pivotal role for apoptosis signaling in endothelial progenitor cells. FASEB J. 2005;19:974–976. doi: 10.1096/fj.04-2727fje. [DOI] [PubMed] [Google Scholar]
- 23.Jin Y, Yao G, Wang Y, Teng L, Wang Y, Chen H, Gao R, Lin W, Wang Z, Chen J. MiR-30c-5p mediates inflammatory responses and promotes microglia survival by targeting eIF2alpha during Cryptococcus neoformans infection. Microb Pathog. 2020;141:103959. doi: 10.1016/j.micpath.2019.103959. [DOI] [PubMed] [Google Scholar]
- 24.Fan J, Li H, Nie X, Yin Z, Zhao Y, Chen C, Wen Wang D. MiR-30c-5p ameliorates hepatic steatosis in leptin receptor-deficient (db/db) mice via down-regulating FASN. Oncotarget. 2017;8:13450–13463. doi: 10.18632/oncotarget.14561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao JM, Li GZ, Han M, Xu HL, Huang KM. MiR-30c-5p suppresses migration, invasion and epithelial to mesenchymal transition of gastric cancer via targeting MTA1. Biomed Pharmacother. 2017;93:554–560. doi: 10.1016/j.biopha.2017.06.084. [DOI] [PubMed] [Google Scholar]
- 26.Wei M, Cao LJ, Zheng JL, Xue LJ, Chen B, Xiao F, Xu CS. MicroRNA-181c functions as a protective factor in a 1-methyl-4-phenylpyridinium iodide-induced cellular Parkinson’s disease model via BCL2L11. Eur Rev Med Pharmacol Sci. 2017;21:3296–3304. [PubMed] [Google Scholar]
- 27.Wei N, Xiao L, Xue R, Zhang D, Zhou J, Ren H, Guo S, Xu J. MicroRNA-9 mediates the cell apoptosis by targeting Bcl2l11 in ischemic stroke. Mol Neurobiol. 2016;53:6809–6817. doi: 10.1007/s12035-015-9605-4. [DOI] [PubMed] [Google Scholar]
- 28.Hu WN, Duan ZY, Wang Q, Zhou DH. The suppression of ox-LDL-induced inflammatory response and apoptosis of HUVEC by lncRNA XIAT knockdown via regulating miR-30c-5p/PTEN axis. Eur Rev Med Pharmacol Sci. 2019;23:7628–7638. doi: 10.26355/eurrev_201909_18886. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Ren M, Wang X, Cui X, Zhao H, Zhao C, Zhou J, Guo Y, Hu Y, Yan C, Berk B, Wang J. Glutaredoxin 1 mediates the protective effect of steady laminar flow on endothelial cells against oxidative stress-induced apoptosis via inhibiting Bim. Sci Rep. 2017;7:15539. doi: 10.1038/s41598-017-15672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]