Abstract
Objective: The purpose of this study was to identify the optimal treatment plan for hospitalized patients with community-acquired pneumonia (CAP) by evaluating related studies on combination therapies of β-lactams/macrolides (BLM) and β-lactams/fluoroquinolones (BLFQ) in the treatment of CAP. Methods: A meta-analysis was performed on studies with mortality rates as the main result using PubMed, Scopus, Cochrane, and other journal databases. The literature was evaluated using GRADE and MiNORS. Results: A total of 17 studies were included. Various studies included the effects of combination therapy and mortality rates of β-lactam, fluoroquinolones and macrolides. The quality of currently available evidence was low. In the preliminary data analysis, the mortality rate of BLFQ was higher than that of BLM (RR = 1.33, 95% CI: 1.15-1.54, I2 = 28%). No difference was observed in patients with bacteremia and septic shock. In a meta-analysis with adjusted mortality rates, no significant difference was shown in two therapies (RR = 1.26, 95% CI: 0.95-1.67, I2 = 43%). Conclusion: The related studies on the relative effects of BLFQ and BLM therapies in the treatment of CAP hospitalized patients have low-quality evidence. The current data indicate that BLFQ combination therapy is associated with higher mortality rates.
Keywords: Azithromycin, moxifloxacin, penicillin, cephalosporin
Introduction
The incidence and mortality rates of community-acquired pneumonia (CAP) decreased as a result of the improvement of public health management and medical services. However, the morbidity and mortality rates of the disease remain relatively high [1]. It is an infectious disease that causes death in patients from both developed and developing countries, leading to huge disease burden and expenditure [2,3].
With the discovery and development of antibiotics, there are effective solutions to infectious diseases. Antibiotics are also adopted as the main treatment option in CAP management, and prompt use of antibodies can effectively reduce mortality rates in patients and improve prognosis [4]. In the currently published guidelines, three types of antibiotics (fluoroquinolones, macrolides, and β-lactams) are recommended for the treatment of CAP [5]. Several randomized controlled trials (RCT) studies have shown that three types of drugs are found to have good efficacy in patients with mild to moderate conditions [6]. A meta-analysis indicated that fluoroquinolones achieved similar outcomes of mortality rates compared to macrolides, β-lactams and their combination therapies had better clinical efficacy [7].
However, there is still a lack of evidence from RCT studies on severe CAP patients [8]. Results from previous retrospective studies indicated that β-lactams/macrolides (BLM) combination therapy had better outcome than β-lactam alone [8]. As a result, the guideline recommended the treatment plan with BLM. In addition, some studies also provided evidence which support the β-lactams/fluoroquinolones (BLFQ) therapy. In RCT studies on severe CAP patients, combination therapy with ceftriaxone, levofloxacin and moxifloxacin also achieved similar outcomes. Therefore, this study compared the therapeutic effects of these two combination therapies on severe CAP patients.
Materials and methods
Literature retrieval methods
Related studies up to 17 February 2018 were retrieved from PubMed, Scopus and Cochrane databases. The search strategy was (combination therapy OR dual therapy OR macrolide OR quinolone OR β-lactam) and (community acquired pneumonia OR CAP) and (treatment OR management). Studies with accessible data and full text were included. In addition, the reference was also retrieved.
Selection criteria for literature
Randomized, non-randomized and observational studies with data for mortality and all-cause mortality rates were included. Repeated studies based on pneumonia infections acquired in the hospital or from other medical procedures, with case reports of less than 10 patients, or treatment with single-agent antibiotics were excluded.
Data extraction and quality evaluation
Two researchers assessed the studies independently, extracted data and evaluated the risk of bias. The assessment included: research cohort, double-blind method, and data integrity. The opinion of a third researcher was included when disagreements were encountered. Methodological index for non-randomized studies (MINORS), which included 12 items with a maximum score of 24, were adopted to assess bias in included studies to determine selection bias and other sources of bias. The overall evaluation was also performed using the GRADE tool.
Statistical methods
The data analysis was performed using Review Manager 5.3. The included studies were inputted into a random-effects model. X2 test and I2 evaluation were performed to measure heterogeneity. A sensitivity analysis of the included risk of bias was conducted, and a score of less than 16 was considered as lower risk. The adjusted RR was expressed as aRR. If aRR was not provided in the study, the adjusted odds ratio was converted to aRR. If the adjusted hazard ratio (aHR) was provided in the studies with cumulative incidence rates, aHR was considered as aRR. If the adjusted effect size with 95% CI could not be acquired and there was no significant relationship between antibiotic combinations and mortality rate, the adjusted effect size was estimated to be 1, and the unadjusted standard deviation was used to indicate the degree of dispersion.
Results
Characteristics of included studies
The inclusion procedure is shown in Figure 1. A total of 17 studies, consisting of 11 retrospective studies and 6 prospective studies [9-25], involved a total of 16,684 patients, were selected from the original 140 studies. Besides, more data were obtained from 8 of these included studies [11-14,18,20-22]. The characteristics of included studies, including 11 retrospective studies and 6 prospective studies, were listed in Table 1.
Figure 1.

Document retrieval procedures.
Table 1.
Summary of included randomized controlled trials of the effect of NSAIDs on recurrent colorectal cancers
| Study, year | Study design | Study period | Study place | No. of evaluable patients | Treatment administered as empirical, definitive, both |
|---|---|---|---|---|---|
| Adrie et al. 2013 | MC prospective | 1996-2010 | France | BLM: 164 | both |
| BLFQ: 230 | |||||
| Bratzler et al. 2008 | retrospective | 1998-2001 | USA | BLM: 6830 | empirical |
| BLFQ: 1691 | |||||
| Capelastegui et al. 2005 | MC retrospective | 1998-1999 + 2000-2001 | Spain | BLM: 267 | empirical |
| BLFQ: 10 | |||||
| Capelastegui et al. 2006 | SC prospective | 2000-2004 | Spain | BLM: 125 | empirical |
| BLFQ: 39 | |||||
| Charles et al. 2008 | MC prospective | 2004-2006 | Australia | BLM: 681 | empirical |
| BLFQ: 3 | |||||
| Frei et al. 2006 | retrospective | 1999-2000 | USA | BLM: 255 | empirical |
| BLFQ: 68 | |||||
| Houck et al. 2001 | retrospective | 1992-1993, 1994-1995, 1996-1997 | USA | BLM: 1743 | empirical |
| BLFQ: 156 | |||||
| Karhu et al. 2013 | retrospective | 2000-2010 | Finland | BLM: 106 | empirical |
| BLFQ: 104 | |||||
| Mahboub et al. 2015 | prospective | 2009-2011 | UAE, Kuwait, Oman, Bahrain, Qatar | BLM: 48 | empirical |
| BLFQ: 77 | |||||
| Martin-Loeches et al. 2010 | MC prospective | NR | Europe | BLM: 46 | empirical |
| BLFQ: 54 | |||||
| Menendez et al. 2012 | MC prospective | 2005-2007 | Spain | BLM: 1073 | empirical |
| BLFQ: 488 | |||||
| Minhas et al. 2007 | retrospective | 2002-2005 | Canada | BLM: 18 | both |
| BLFQ: 6 | |||||
| Mongardon et al. 2012 | retrospective | 2001-2008 | France | BLM: 87 | empirical |
| BLFQ: 68 | |||||
| Mortensen et al. 2006 | retrospective | 1999-2002 | USA | BLM: 87 | empirical |
| BLFQ: 50 | |||||
| Naucler et al. 2013 | retrospective | 2007-2009 | Sweden | BLM: 26 | empirical |
| BLFQ: 31 | |||||
| Waterer et al. 2001 | retrospective | 1996-2000 | USA | BLM: 43 | empirical |
| BLFQ: 24 | |||||
| Wilson et al. 2012 | retrospective | 2001-2007 | USA | BLM: 1106 | empirical |
| BLFQ: 883 |
Note: BL, β-lactam; BLI, β-lactamase inhibitor; FQ, fluoroquinolone; M, macrolide; MC, multicenter.
Preliminary data analysis
The MINORS for 8 of the studies were higher than 16, with 3 studies scoring higher than 18 and a median of 15 (ranging from 10-19). The main items that were absent in all included studies were the blinded assessment of primary endpoint and prospective calculations of the study size, which resulted in relatively low scores. 11 of the studies had a relatively high risk of bias (median: 13) and 6 of the studies had a relatively low risk of bias (median: 13). Besides, the studies lacked the evidence from RCT studies, which resulted in a relatively high risk of bias, and were assessed as low-quality by GRADE.
The unadjusted mortality rates of the patients on BLFQ and BLM therapies were compared in 17 studies. The results indicated that BLFQ was relatively highly correlated with mortality rates (Figure 2, RR = 1.33, 95% CI: 1.15-1.54, I2 = 8%). The small sample size of the BLFQ combination therapy resulted in relatively obvious publication bias (Figure 3). Besides, no difference was observed in patients with bacteremia and septic shock.
Figure 2.

The risk ratio of initial mortality rates of BLM and BLFQ therapies. Note: the vertical line represents balanced points of no difference between the two therapies. The squares represent the adjusted risk ratios. The diamonds represent the combined adjusted risk ratios of all studies. The horizontal lines represent 95% CI.
Figure 3.

The funnel plot of the initial mortality risks of BLM and BLFQ therapies.
Risks of different treatment plans
The sensitivity analysis (Table 2) indicated that the mortality rate of BLFQ therapy was higher than that of BLM. The subgroup analysis showed less moderate heterogeneity of the results, while the moderate heterogeneity was found in both the study period and the analysis of mortality rate in BLM therapy. The relevant results also supported the combination therapy BLM.
Table 2.
The sensitivity analyses of BLM and BLFQ therapies
| Sensitivity analysis | Included studies, n | Patients with outcome, n | RR, 95% CI | P value | I2 |
|---|---|---|---|---|---|
| Quality of studies | |||||
| Lower risk for bias | 6 | 4729 | 1.30, 1.03-1.65 | 0.03 | 54% |
| Higher risk for bias | 11 | 11955 | 1.44, 1.24-1.67 | <0.001 | 0% |
| Mortality recording time (subgroup difference I2 = 0%) | |||||
| 30-day | 9 | 13935 | 1.25, 1.01-1.55 | 0.04 | 48% |
| In-hospital | 6 | 9481 | 1.47, 1.15-1.89 | 0.002 | 24% |
| ICU-treated | 8 | 4217 | 1.44, 1.08-1.91 | 0.01 | 77% |
| Study design (subgroup difference I2 = 0%) | |||||
| Retrospective | 11 | 13656 | 1.30, 1.06-1.59 | 0.01 | 44% |
| Prospective | 6 | 3028 | 1.44, 1.15-1.81 | 0.002 | 0% |
| Study period (subgroup difference I2 = 37.4%) | |||||
| Initiated before 1998 | 3 | 2360 | 1.53, 1.18-1.99 | 0.001 | 0% |
| Initiated after 1998 | 12 | 14224 | 1.25, 1.03-1.50 | 0.02 | 36% |
| Study BLM mortality (subgroup difference I2 = 60.5%) | |||||
| 0%-1.99% | 3 | 505 | 3.90, 1.14-13.34 | 0.03 | 0% |
| 2%-8.99% | 5 | 11107 | 1.50, 1.28-1.76 | <0.001 | 0% |
| >9% | 9 | 5072 | 1.23, 1.00-1.50 | 0.05 | 41% |
Note: ICU, intensive care unit; RCT, randomized controlled trial.
Analysis of adjusted mortality rate
A total of 5 studies [9,17,19,22,25] adjusted the mortality rate and provided relevant data; similar results were obtained after combining with the data from 3 other studies (Figure 4, aRR = 1.26, 95% CI: 0.95-1.67, I2 = 43%). There was no statistically significant difference in mortality rates of different combination therapies after the exclusion of aHR study.
Figure 4.
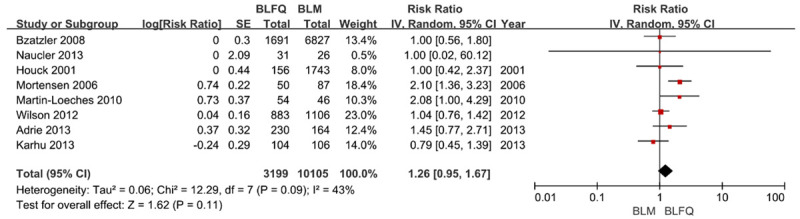
The risk ratios of adjusted initial mortality risks of BLM and BLFQ. Note: the vertical line represents balanced points of no difference between the two therapies. The squares represent the adjusted risk ratios. The diamonds represent the combined adjusted risk ratios of all studies. The horizontal lines represent 95% CI.
Discussion
The results for the analysis of unadjusted data indicated that the mortality rates of BLFQ combination therapy were higher than that of BLM combination therapy. Similar results were obtained using the sensitivity analysis. There was no difference between patients with bacteremia and septic shock in the subgroup analysis. Therefore, BLFQ therapy had a relatively higher mortality risk compared to BLM therapy.
Since there was no current data from RCT studies that directly compared these two treatment therapies, the data included in this study are relatively at low quality, which might result in difference between actual outcomes and trends as estimated in this study. A double-blinded RCT of 738 patients compared the effects of combination therapies of ceftriaxone/levofloxacin and moxifloxacin, and the outcome exhibited no significant difference either [26].
The main limitation of this study is that all the studies included were non-randomized studies and with low-quality. Besides, these studies did not focus on the comparison of the two therapies included in this study. As a result, the demographic data, medical history, and morbidity of the patients were not available. Only a few studies adjusted the data, and the adjustment performed was not standardized across different studies. This may affect the rconclusion of our study outcomes.
In addition, the differences could be due to the immunomodulatory properties of macrolides, which may be involved in the varied capacities of antibiotics to reduce the body’s inflammatory response to infectious stimuli [27], given the qualities of the included data have no impact on the outcomes. Despite their similar properties to macrolides, fluoroquinolones could lead to further repression in the immune response, including the reduction of interferon-γ levels, which hinders the restoration of immune functions after sepsis-induced immune paralysis. However, if the anti-inflammatory properties of macrolides were the main reasons for the difference in mortality rates between BLFQ and BLM therapies, a difference in therapeutic effects between fluoroquinolones and BLM therapy would be expected. However, this was not observed in other meta-analyses [28].
The second explanation is that fluoroquinolones are frequently applied to patients infected with multidrug-resistant pathogens, which may increase the mortality risks resulted from recurrent infections in patients requiring ICU care or long-term hospitalization. However, in individual studies with relevant data, BLFQ and BLM therapies had similar occurrence rates of such infection [9]. In addition, in most studies, the length of hospital stay was insufficient to cause such infection. Other possible explanations are that there is an antagonistic effect between the β-lactams and fluoroquinolones in the body, or there is an increase in the fatal adverse events (no related reports were found on combination therapies commonly prescribed in hospitals). Drug resistance to fluoroquinolones or a lack of coverage in atypical pneumonia is unlikely to lead to these outcomes [25].
In conclusion, the related studies on the relative effects of BLFQ and BLM therapies in the treatment of CAP hospitalized patients have low-quality evidence. The current data indicate that BLFQ combination therapy is associated with higher mortality rates. However, no treatment therapies should be advocated or rejected without randomized controlled trials. In addition, a subgroup of patients who could not benefit from BLFQ therapy was not defined. This study indicates that in unadjusted analyses using the BLFQ combination therapy as recommended by the guideline, the mortality rates are higher. Even though the findings are not conclusive, it is recommended to avoid this combination therapy for the treatment of severe CAP. CAP patients with suspected Pseudomonas aeruginosa, higher possibility of acquiring multi-drug resistant pathogens, or history of severe macrolides allergies could be better candidates for BLFQ therapy. A well-designed RCT maybe needed to compare the two therapies.
Disclosure of conflict of interest
None.
References
- 1.Ivanovska V, van DL, Mantel-Teeuwisse AK. Chapter 21. Priority medicines for children. Exploring age-appropriate medicines and antibiotic use in children. Utrecht: Utrecht University; 2017. pp. 21–38. [Google Scholar]
- 2.Personne V, Chevalier J, Buffel du Vaure C, Partouche H, Gilberg S, de Pouvourville G. CAPECO: cost evaluation of community acquired pneumonia managed in primary care. Vaccine. 2016;34:2275–2280. doi: 10.1016/j.vaccine.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Peyrani P, Mandell L, Torres A, Tillotson GS. The burden of community-acquired bacterial pneumonia in the era of antibiotic resistance. Expert Rev Respir Med. 2019;13:139–152. doi: 10.1080/17476348.2019.1562339. [DOI] [PubMed] [Google Scholar]
- 4.Lee JS, Giesler DL, Gellad WF, Fine MJ. Antibiotic therapy for adults hospitalized with community-acquired pneumonia: a systematic review. JAMA. 2016;315:593–602. doi: 10.1001/jama.2016.0115. [DOI] [PubMed] [Google Scholar]
- 5.Vardakas KZ, Trigkidis KK, Apiranthiti KN, Falagas ME. The dilemma of monotherapy or combination therapy in community-acquired pneumonia. Eur J Clin Invest. 2017;47 doi: 10.1111/eci.12845. [DOI] [PubMed] [Google Scholar]
- 6.Williams DJ, Edwards KM, Self WH, Zhu Y, Arnold SR, McCullers JA, Ampofo K, Pavia AT, Anderson EJ, Hicks LA, Bramley AM, Jain S, Grijalva CG. Effectiveness of β-lactam monotherapy vs macrolide combination therapy for children hospitalized with pneumonia. JAMA Pediatr. 2017;171:1184–1191. doi: 10.1001/jamapediatrics.2017.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang YQ, Zou SL, Zhao H, Zhang MM, Han CL. Ceftriaxone combination therapy versus respiratory fluoroquinolone monotherapy for community-acquired pneumonia: a meta-analysis. Am J Emerg Med. 2018;36:1759–1765. doi: 10.1016/j.ajem.2018.01.079. [DOI] [PubMed] [Google Scholar]
- 8.König R, Cao X, Oswald M, Forstner C, Rohde G, Rupp J, Witzenrath M, Welte T, Kolditz M, Pletz M CAPNETZ study group. Macrolide combination therapy for patients hospitalised with community-acquired pneumonia? An individualised approach supported by machine learning. Eur Respir J. 2019;54:1900824. doi: 10.1183/13993003.00824-2019. [DOI] [PubMed] [Google Scholar]
- 9.Adrie C, Schwebel C, Garrouste-Orgeas M, Vignoud L, Planquette B, Azoulay E, Kallel H, Darmon M, Souweine B, Dinh-Xuan AT, Jamali S, Zahar JR, Timsit JF. Initial use of one or two antibiotics for critically ill patients with community-acquired pneumonia: impact on survival and bacterial resistance. Crit Care. 2013;17:R265. doi: 10.1186/cc13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bratzler DW, Ma A, Nsa W. Initial antibiotic selection and patient outcomes: observations from the National Pneumonia Project. Clin Infect Dis. 2008;47(Suppl 3):S193–201. doi: 10.1086/591404. [DOI] [PubMed] [Google Scholar]
- 11.Capelastegui A, España PP, Quintana JM, Areitio I, Gorordo I, Egurrola M, Bilbao A. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J. 2006;27:151–157. doi: 10.1183/09031936.06.00062505. [DOI] [PubMed] [Google Scholar]
- 12.Capelastegui A, España PP, Quintana JM, Gorordo I, Martínez Urquiri A, Idoiaga I, Bilbao A. Patients hospitalized with community-acquired pneumonia: a comparative study of outcomes by medical specialty area. Arch Bronconeumol. 2005;41:300–306. doi: 10.1016/s1579-2129(06)60229-2. [DOI] [PubMed] [Google Scholar]
- 13.Charles PG, Whitby M, Fuller AJ, Stirling R, Wright AA, Korman TM, Holmes PW, Christiansen KJ, Waterer GW, Pierce RJ, Mayall BC, Armstrong JG, Catton MG, Nimmo GR, Johnson B, Hooy M, Grayson ML. The etiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis. 2008;46:1513–1521. doi: 10.1086/586749. [DOI] [PubMed] [Google Scholar]
- 14.Cherfan AJ, Bizri AR, Steitieh SW, Moukhachen OE. Management of community-acquired pneumonia at a tertiary care medical center in Lebanon. Am J Health Syst Pharm. 2003;60:934–938. doi: 10.1093/ajhp/60.9.934. [DOI] [PubMed] [Google Scholar]
- 15.Frei CR, Restrepo MI, Mortensen EM, Burgess DS. Impact of guideline-concordant empiric antibiotic therapy in community-acquired pneumonia. Am J Med. 2006;119:865–871. doi: 10.1016/j.amjmed.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Houck PM, MacLehose RF, Niederman MS, Lowery JK. Empiric antibiotic therapy and mortality among medicare pneumonia inpatients in 10 western states: 1993, 1995, and 1997. Chest. 2001;119:1420–1426. doi: 10.1378/chest.119.5.1420. [DOI] [PubMed] [Google Scholar]
- 17.Karhu J, Ala-Kokko TI, Ohtonen P, Syrjälä H. Severe community-acquired pneumonia treated with β-lactam-respiratory quinolone vs. β-lactam-macrolide combination. Acta Anaesthesiol Scand. 2013;57:587–593. doi: 10.1111/aas.12081. [DOI] [PubMed] [Google Scholar]
- 18.Mahboub B, Al Zaabi A, Al Ali OM, Ahmed R, Niederman MS, El-Bishbishi R. Real life management of community-acquired Pneumonia in adults in the Gulf region and comparison with practice guidelines: a prospective study. BMC Pulm Med. 2015;15:112. doi: 10.1186/s12890-015-0108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Loeches I, Lisboa T, Rodriguez A, Putensen C, Annane D, Garnacho-Montero J, Restrepo MI, Rello J. Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med. 2010;36:612–620. doi: 10.1007/s00134-009-1730-y. [DOI] [PubMed] [Google Scholar]
- 20.Menéndez R, Torres A, Reyes S, Zalacain R, Capelastegui A, Aspa J, Borderías L, Martín-Villasclaras JJ, Bello S, Alfageme I, de Castro FR, Rello J, Molinos L, Ruiz-Manzano J. Initial management of pneumonia and sepsis: factors associated with improved outcome. Eur Respir J. 2012;39:156–162. doi: 10.1183/09031936.00188710. [DOI] [PubMed] [Google Scholar]
- 21.Mongardon N, Max A, Bouglé A, Pène F, Lemiale V, Charpentier J, Cariou A, Chiche JD, Bedos JP, Mira JP. Epidemiology and outcome of severe pneumococcal pneumonia admitted to intensive care unit: a multicenter study. Crit Care. 2012;16:R155. doi: 10.1186/cc11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortensen EM, Restrepo MI, Anzueto A, Pugh J. The impact of empiric antimicrobial therapy with a β-lactam and fluoroquinolone on mortality for patients hospitalized with severe pneumonia. Crit Care. 2005;10:R8. doi: 10.1186/cc3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naucler P, Darenberg J, Morfeldt E, Ortqvist A, Henriques Normark B. Contribution of host, bacterial factors and antibiotic treatment to mortality in adult patients with bacteraemic pneumococcal pneumonia. Thorax. 2013;68:571–579. doi: 10.1136/thoraxjnl-2012-203106. [DOI] [PubMed] [Google Scholar]
- 24.Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med. 2001;161:1837–1842. doi: 10.1001/archinte.161.15.1837. [DOI] [PubMed] [Google Scholar]
- 25.Wilson BZ, Anzueto A, Restrepo MI, Pugh MJ, Mortensen EM. Comparison of two guideline-concordant antimicrobial combinations in elderly patients hospitalized with severe community-acquired pneumonia. Crit Care Med. 2012;40:2310–2314. doi: 10.1097/CCM.0b013e31825151a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parnham MJ. Immunomodulatory effects of antimicrobials in the therapy of respiratory tract infections. Curr Opin Infect Dis. 2005;18:125–131. doi: 10.1097/01.qco.0000160901.71813.fe. [DOI] [PubMed] [Google Scholar]
- 28.Vardakas KZ, Trigkidis KK, Falagas ME. Fluoroquinolones or macrolides in combination with β-lactams in adult patients hospitalized with community acquired pneumonia: a systematic review and meta-analysis. Clin Microbiol Infect. 2017;23:234–241. doi: 10.1016/j.cmi.2016.12.002. [DOI] [PubMed] [Google Scholar]


