Abstract
Objective: Renal cell carcinoma (RCC) is one of the most common and life-threatening cancers in the world. Accumulating evidence suggest propofol inhibits the initiation and development of cancers. The main focus of the study was to explore the effect of propofol on RCC and its mechanism of action. Methods: In this study, different doses of propofol were used to treat human RCC cell lines i.e., OSRC-2 and SW839. Western blot and trans-well assays were used for the evaluation of RCC cell invasion, proliferation, migration, and transition of epithelial to mesenchymal (EMT). RCC cells following 5 μmol/L propofol treatment for 24 h were applied in the subsequent experiments. Expression of MicroRNAs-363 (miR-363) in cells with or without propofol treatment were analyzed. The expression of Snail1, Vimentin, N-cadherin, and E-cadherin in RCC cells was measured, and then the effect of loss-of-function of miR-363 and gain-of-function of Snail on RCC cells were analyzed. The targeted relationship between miR-363 and Snail1 was investigated using luciferase assay and RIP, RNA pull down. Results: Propofol reduced the migration, proliferation, invasion and EMT of RCC cells in a dose-dependent way. Propofol elevated miR-363 expression but reduced Snail1 expression, and it reduced Vimentin and N-cadherin but increased E-cadherin expression in RCC cells. miR-363 directly bounds to Snail1. miR-363 inhibition or Snail1 promotion reversed propofol-inhibited malignant behaviors of RCC cells. Conclusion: Our study found that propofol could inhibit invasion, migration, proliferation and EMT of RCC cells by promoting miR-363 expression and suppressing Snail1 expression.
Keywords: Propofol, microRNA-363, Snail1, cancer metastasis, renal cell carcinoma
Introduction
Renal cell carcinoma (RCC) is one of the most familiar types of tumors, originates from renal tubular epithelium and encompasses a heterogeneous group of cancers. RCC is among the top ten most common cancers globally and is the most deadly genitourinary cancers [1]. In recent years, the incidence of RCC has been increasing by an average of 2% to 4% every year [1]. Thus, investigating effective therapeutic measures is urgent and crucial for the global management of RCC.
Propofol has been commonly used in clinical anesthesia as an intravenous anesthetic [2]. A number of studies have shown that propofol inhibited the cancer occurrence and development in a variety of cancers [1-3]. For instance, one previous study showed that propofol suppressed cell proliferation and metastasis in glioma, pancreatic cancer and hepatocellular carcinoma [3-5]. Nevertheless, there is no studies so far have reported the role of propofol on renal cell carcinoma (RCC) yet and the mechanism of propofol in RCC requires further investigation.
MicroRNAs (miRNAs) are small endogenous non-coding RNA molecules comprising around 22 nucleotides, which possess a potential role in the regulation of gene expression such as inhibition of post-transcriptional gene expression via binding to the 3’untranslated regions (3’UTRs) of the mRNA [6]. Previous studies have reported its key role in the regulation of gene expression, cell proliferation, pathogenesis and progression of various cancers [7]. For instance, accumulating evidence suggests the important role of microRNA-363 (miR-363) in cancer progression and metastasis in gastric cancer, colorectal cancer and RCC [8-10]. In addition, a zinc-finger transcription factor (Snail1) can be targeted by miRNA and has been reported to stimulates EMT and promote metastasis in RCC [11].
The purpose of current study is to identify the mechanisms underlying anti-tumor effect of propofol on RCC. Propofol effect in the RCC cell lines, including SW839 and OSRC-2, was analyzed against cell proliferation, migration, invasion, and transition of epithelial to mesenchymal (EMT). The regulatory effect of propofol on miR-363 was studied to reveal the potential mechanism and signaling pathways of the anti-cancer effect of propofol.
Materials and methods
Cell culture
Human cell lines (i.e., OSRC-2 and SW839), were procured from the Cell Bank of the Chinese Academy of Science. Cells were maintained with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), followed by cells culturing at 37°C, using a humidified incubator with (gas mixture: 5% CO2 and 95% air).
Propofol was obtained from Corden Pharma S.p.A, while the culture media used for the incubation of the cells contained various doses of propofol (i.e., 0, 1, 5, 10 μmol/L), for a range of hours (0, 24 h, 48 h, 72 h).
Cell viability assay
In order to measure the cell viability, a standard CCK-8 kit (Abcam) was used. OSRC-2 and SW839 cells were sown into 96-well plate with 1 * 104 cells per well and then, treated with 0, 1, 5, 10 μmol/L propofol for 0, 24 h, 48 h, and 72 h. Subsequently, cells were maintained for 1 h at 37°C by adding 10 μl of CCK-8 solution. Absorbance was recorded at 450 nm by using Microplate Reader (Bio-Rad, USA). Cell viability in the propofol treated group was presented in mean absorbance/mean absorbance in the control group * 100%.
Cell migration and invasion assays
To measure cell migration as well as their invasion ability, trans-well assays were performed. Cultured cells with 70-80% confluency were digested for the next experiments.
For cell migration, it was performed by cell invasion chambers that carry membrane with 8 μmpores in 24-well plates (Corning). The cell suspensions was added into the upper chamber. Serum-free medium was transferred into the upper compartment, and medium with 10% FBS was added to the lower compartment. Cells were incubated for 24 h at 37°C and after 24 h of incubation, the upper chamber’s cells were fixed by adding methanol for 10 min followed by 0.1% crystal violet solution staining. Cells attached to the upper-membranes were removed, while lower-membranes cells were counted in five random fields by using a microscope. To assess cell invasion, the upper chambers were coated with Matrigel (BD Biosciences, Bedford, MA), before the addition of cells suspension.
Quantitative real-time PCR (qRT-PCR)
Trizol reagent was used in the total RNA extraction from the given two cell lines, such as OSRC-2 and SW839 cells, in accordance with the manufacturer’s instructions. Concentration and quality of the RNA were examined by measuring OD260 and the OD260/OD280 ratio. Then RNA was converted to cDNA with the primers using PrimeScript RT reagent Kit (Takara, Kusatsu, Japan) and then the resulted RNA was stored at -80°C. qRT-PCR was then carried out by using a standard 7900HT Fast Real-Time PCR System (Applied Biosystems, USA). A well-known standard method Ct was used to calculate and investigate the relative expression level ratio of the desired gene and the efficiency was compared with a reference gene. GAPDH was used as the reference of mRNA, and U6 was used as the reference of miRNA. The primers’ sequences used in this study, have been listed in Table 1.
Table 1.
Primer sequences for qRT-PCR
| Gene | Primer sequence (5’-3’) |
|---|---|
| miR-363 | Forward primer: GCGGCCAATTGCACGGTAT |
| Reverse primer: GTGCAGGGTCCGAGGTATTC | |
| U6 | Forward primer: CTCGCTTCGGCAGCACA |
| Reverse primer: AACGCTTCACGAATTTGCGT | |
| snail1 | Forward primer: ATGCCGCGCTCTTTCCTCGTC |
| Reverse primer: TCAGCGGGGACATCCTGAGC | |
| GAPDH | Forward primer: GCACCGTCAAGGCTGAGAAC |
| Reverse primer: ATGGTGGTGAAGACGCCAGT |
Western blot analysis
The procedure of western blot was the same as the previous paper [12]. Total proteins from OSRC-2 and SW839 cells were extracted. For total protein extraction, Mammalian Protein Extraction Reagent (M-PER™) was utilized, while the concentration of the desired protein was quantified using a Bradford protein assay kit. Protein (50 µg) was electrophoresed in polyacrylamide gels as well as electro-transferred to nitrocellulose membranes. Relevant antibodies were used for membrane probing. The blots and primary antibodies probe formation was followed by the HRP-conjugated secondary antibody. All the primary and secondary antibodies were purchased from Abcam. The primary antibodies used in the current study are listed as follows: Beta-actin (1:5000), N-cadherin (1:1000), (1:1000), E-cadherin and Vimentin (1:1000). The secondary antibody was 1:5000 dilution.
Luciferase reporter assays
To perform luciferase reporter assays, a Dual-luciferase reporter system was used. The 3’UTR sequences of the wide-type or mutant of Snail1 (Figure 5A) were introduced into a pmir-GLO Dual-luciferase vector (Promega) to form the pmirGLO-snail1-wild type (Snail1-3’UTR-WT) or pmirGLO-Snail1-mutant (snail1-3’UTR-MUT) reporter vector. Co-transfection of Snail1-3’UTR-MUT or Snail1-3’UTR-WT was performed with miR-363 mimics or negative control into OSRC-2 and SW839 cells. After 48 h transfection, the relative luciferase activities were assessed in accordance with manufacturer’s instructions.
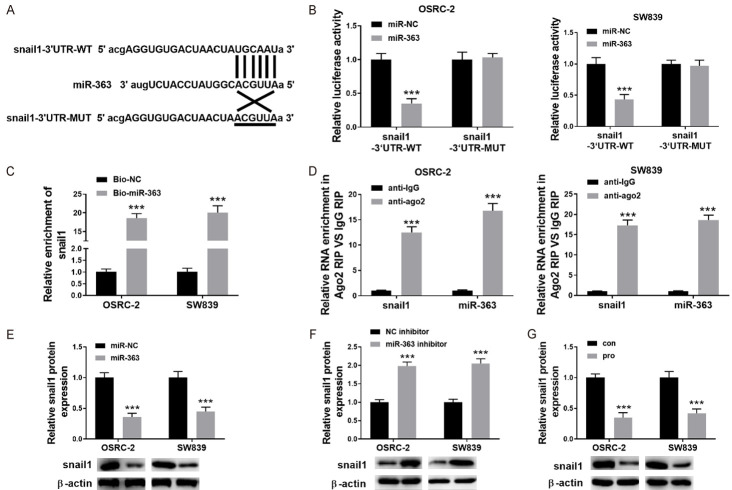
Figure 5.
miR-363 directly targeted Snail1 in RCC cells. A: To clone 3’-UTR sequences of wild type and mutant Snail1 pGL3-promoter luciferase vector was used. To justify the putative binding-sites between 3’-UTR and miR-363, matched base pairs were presented with solid lines and unmatched with cross lines. B: miRNA control (miR-NC), or miR363 mimic, Snail1-Mut reporter and wild-type (WT) Snail1 can be used for co-transfection with OSRC-2 and SW839 cells. Then, 48 hours post-transfection the luciferase activity was detected; C: The validation of the interaction between Snail1 and miR-363 in OSRC-2 cells and SW839 cells were conducted with RNA pull-down assays; D: Ago2 or IgG antibody were used in RNA-immunoprecipitation assays to investigate the enrichment of snail1 and miR-363 in Ago2 or IgG immunoprecipitation complexes of OSRC-2 cells and SW839 cells. The input group is a positive control; E: To detect the expression level of Snail1 in RCC cells after overexpressing miR-363; western blot was performed. F: Western blot was used for the measurement of Snail expression in RCC cells after knocked out of miR-363; G: Expression level of a snail after treatment with propofol was measured in SW839 and OSRC-2 cells. Data are presented in mean ± S.E.M. ***P<0.001, while comparing with control (ANOVA and post hoc test).
RNA pulldown assay
RNA pulldown assay was performed with no changes as described in previous studies [13]. Briefly, OSRC-2 and SW839 cells were quantitatively evaluated and then treated with 1 mL of cell lysis buffer for 72 h. Before the cells centrifugation at 4°C overnight, 1.5 μL of RNase inhibitor was added, followed by the addition of 500 pM antisense oligos of miR-363 (Thermofisher) and 10 μL of streptavidin agarose beads to OSRC-2 and SW839 cells. qRT-PCR was performed for the total RNA quantification.
Ago2 immunoprecipitation assay
The MagnaRIP RNA-Binding Protein Immunoprecipitation Kit was used to perform the Ago2 immunoprecipitation assay. Briefly, the transfected cells were lysed on ice for 30 minutes, by adding RIPA lysis buffer (150 mM NaCl, 20 nM Tris-HCl (PH7.5), 2.5 mM sodium pyrophosphate, 1% NP-40, 1 mM Na2EDTA, 1% sodium deoxycholate, 1 mM EGTA, 1 μg/mL leupetin, 1 mM beta-glycerophosphate) 1 mM Na3VO4. In the following step, the suspension of the cells was centrifuged at 14000 rpm for 15 min. After the addition of 10 μL of beads (Thermofisher) and 2 μL of Ago2 antibody (Abcam), the supernatant was rotated at 4°C overnight. A lysis buffer was used for the mixture washing. RNA extraction was performed using Trizol reagent and then subjected to qRT-PCR analysis.
Statistical analysis
Data was processed in mean ± S.E.M. For the comparison of independent groups One-way (ANOVA) was used to analyze the data, and Tukey’s post-hoc test was also performed. P<0.05 were considered as statistical significance.
Results
Propofol inhibited the proliferation of RCC cells
Firstly, a CCK-8 assay was performed to investigate the propofol effect on the RCC cell proliferation. Figure 1A, our acquired results indicated that propofol exhibits a significant inhibitory effect on the proliferation of OSRC-2 and SW839 cells in a dose-dependent way. It is worth noting that the cell viability was robustly decreased when propofol concentration increased from 1 μmol/L to 5 μmol/L, however, the viability of the cells was decreased slightly when the concentration of propofol increased from 5 μmol/L to 10 μmol/L, indicating 5 μmol/L propofol a threshold and sufficient to inhibit the cell viability of different RCC cell lines. So, 5 μmol/L propofol was chosen for subsequent experiments. It’s shown in Figure 1B that the inhibition of cell proliferation also significantly increased with the elongation of propofol treatment time, of which, 24 h was sufficient enough and elonged time only had mildly increased effects. Finally, Propofol concentration of 5 μmol/L treated for 24 h were chosen as propofol treatment for subsequent experiments.
Figure 1.
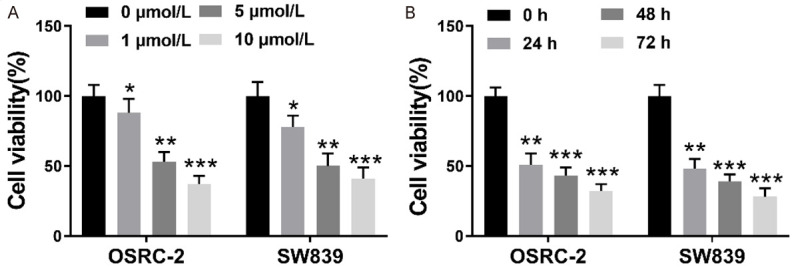
Propofol inhibited the proliferation of kidney cancer cells. A: Human OSRC-2 cells and SW839 cells were exposed to diverse concentrations of propofol (0, 1, 5, and 10 μmol/L). After 24 h, to measure the viability of the cells a standard CCK-8 assay was used; B: Human OSRC-2 and SW839 cells were exposed to 5 μmol/L propofol for 0, 24 h, 48 h, 72 h. The cell viability was assessed at different time points using CCK-8 assay. Data with the given parameters is shown in means ± S.E.M. *P<0.05, **P<0.01, ***P<0.001, while comparing to control (Student t test, ANOVA and post hoc test).
Propofol inhibited the migration, invasion and EMT of RCC cells
Subsequently, we measured the effect of propofol on OSRC-2 and SW839 cell migration and invasion using Transwell assays. Figure 2A and 2B displayed that in contrast to control group, the relative invasion and migration of OSRC-2 and SW839 cells were both significantly reduced in propofol group (P<0.01, P<0.001). Additionally, EMT of SW839 and OSRC-2 cells was also estimated by performing western blot (Figure 2C). These results indicated that a significant increase was observed in the level of E-cadherin protein (P<0.01), while in contrast a significant decrease was observed in the expression level of Vimentin, N-cadherin (P<0.01, P<0.001) in OSRC-2 and SW839 cells after being treated with propofol. Our current results revealed that propofol is apparently involved in the inhibition of RCC cell migration, invasion, and EMT.
Figure 2.
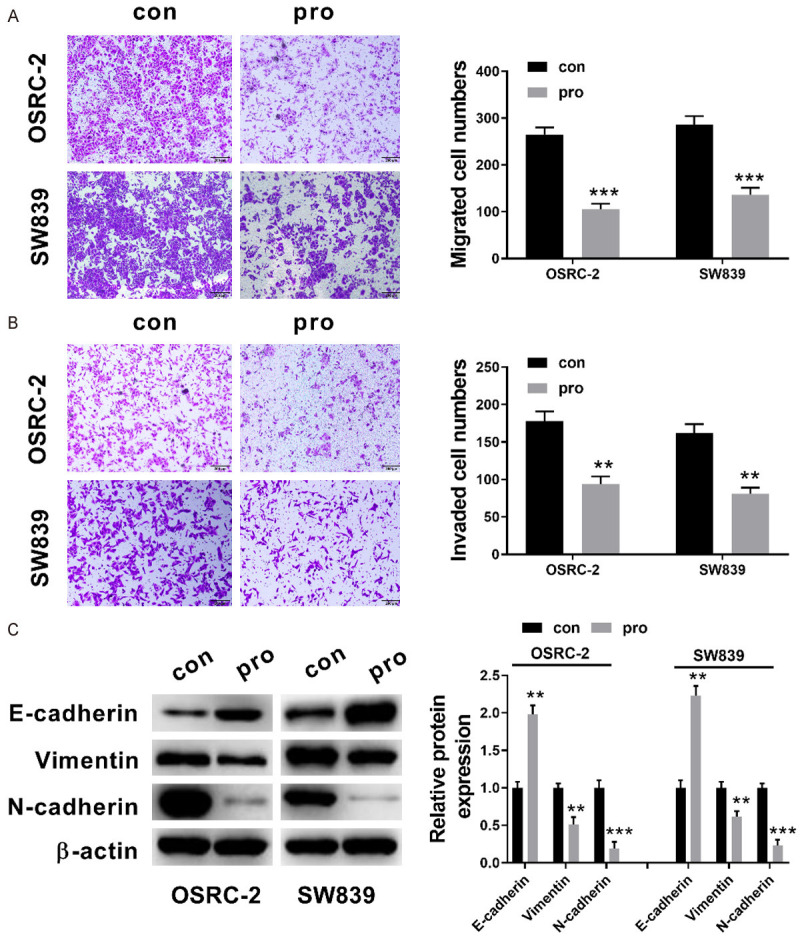
Propofol inhibited the invasion, migration and EMT of RCC cells. A: The migration of OSRC-2 and SW839 cells after 5 μmol/L propofol treatment for 24 h (transwell assays, ×100); B: The invasion of OSRC-2 and SW839 cells after 5 μmol/L propofol treatment for 24 h (transwell assays, ×100); C: Relative expression levels of the proteins including, Vimentin, E-cadherin, N-cadherin after 5 μmol/L propofol treatment for 24 h (western blot analysis). Data with the given parameters is shown in means ± S.E.M. **P<0.01; ***P<0.001, while comparing to control (Student t test, ANOVA and post hoc test).
Propofol upregulated the expression of miR-363 in RCC cells
We had noticed that miR-363 were significantly decreased in a majority of human RCC cells including SW839, SN12-PM6, and A498, OSRC-2, Caki-1 cells, especially in OSRC-2 and SW839 cells (P<0.001, P<0.001), in contrast with Human normal kidney cell line HK-2 (Figure 3A). Previous researches have revealed that miR-363 is crucial for cancer progression and metastasis [8-10]. Other studies show that propofol induces neurotoxicity by increasing the expression of miR-363 [14,15]. Therefore, we hypothesized if propofol is responsible for the inhibition of cell proliferation, migration, invasion, and EMT of RCC cells by up-regulating miR-363. The treatment of propofol significantly increased the relative expression levels of miR-363 in both OSRC-2 and SW839 cells (P<0.001, P<0.001; Figure 3B and 3C). This indicated that the potential anti-cancer mechanism of propofol in RCC cells might be in part by up-regulating miR-363.
Figure 3.
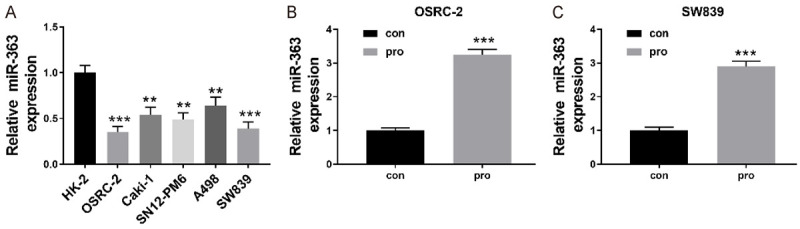
Propofol upregulated the expression of miR-363. A: Relative expression levels of miR-363 in human RCC cells including, SW839 Caki-1, A498, SN12-PM6, and OSRC-2 cells as well as normal kidney cell lines of Human HK-2; B and C: After 5 μmol/L propofol treatment for 24 h, a relative of miR-195 expression level in OSRC-2 and SW839 cells was measured using qRT-PCR. Data with the given parameters is shown in means ± S.E.M. **P<0.01; ***P<0.001, while comparing to control (Student t test, ANOVA and post hoc test).
Upregulation of miR-363 was responsible for propofol-inhibited proliferation, invasion, migration and EMT in RCC cells
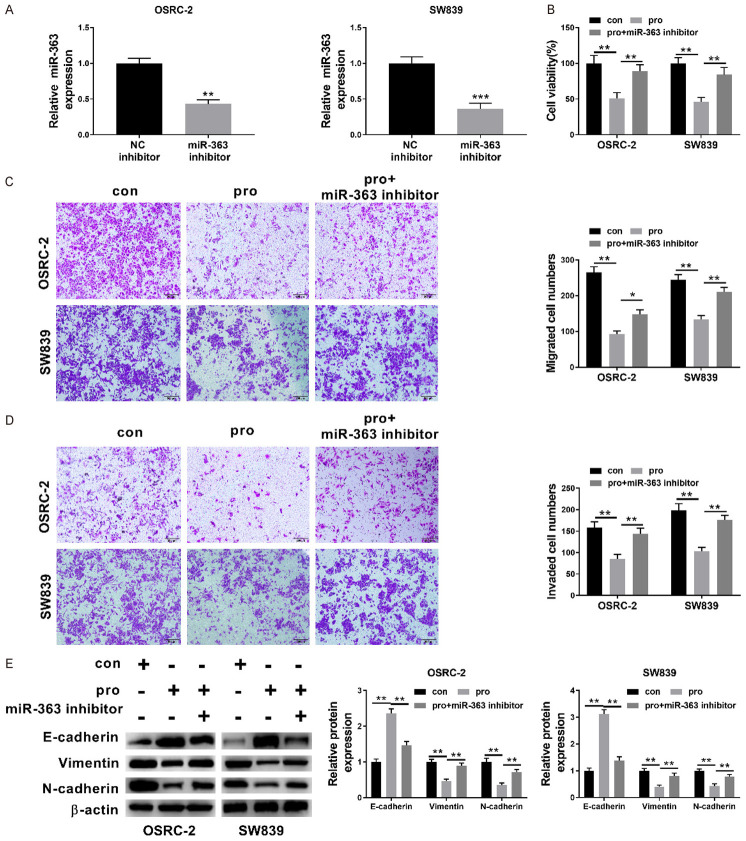
To further investigate the role of miR-363 in the propofol-induced RCC cells proliferation, migration, invasion as well as EMT, the miR-363 inhibitor was transfected into SW839 and OSRC-2 cells. Figure 4A clearly indicated that miR-363 inhibitor transfection is responsible for the significant decrease in the relative expression level of miR-363 in OSRC-2 and SW839 cell (P<0.01, P<0.001). Figure 4B showed that a significant increase in the OSRC-2 and SW839 cell was observed in the group treated with propofol and miR-195 inhibitor, in contrast to the single propofol treated group with (P<0.01, P<0.01). Relative invasion and migration of OSRC-2 and SW839 were significantly increased in the group treated with propofol + miR-195 inhibitor (all P<0.05) while comparing with the single propofol treated group (Figure 4C and 4D). Western blot acquired data were analyzed for the comparison of expression levels cell EMT-related protein. These results revealed that miR-363 inhibitor transfection was responsible for the significant attenuation in the expression level of propofol-induced E-cadherin and Vimentin and N-cadherin in the OSRC-2 and SW839 cells (Figure 4E, all P<0.05). Besides this, our obtained data further identified the crucial role of miR-363 in propofol’s effect on OSRC-2 and SW839 cell proliferation, migration, invasion and EMT.
Figure 4.
Upregulation of miR-363 was responsible for propofol-inhibited proliferation, invasion, migration and EMT in renal cancer cells. A: Relative expression level of miR-363 in OSRC-2 and SW839 cell after miR-363 inhibitor transfection was measured using qRT-PCR; B: The cell viability of OSRC-2 and SW839 cell was investigated after no treatment, propofol treatment and propofol treatment along with miR-363 inhibitor transfection was measured by applying CCK-8 assay; C and D: The relative invasion and migration of SW839 and OSRC-2 cells measured after no treatment, propofol treatment and propofol treatment + miR-363 inhibitor transfection (×100); E: Western blot analysis was performed to investigate the levels of expression of various proteins, such as E-cadherin, Vimentin, N-cadherin after no treatment, treated with propofol and propofol treatment along with miR-363 inhibitor transfection. Data are presented in mean ± S.E.M. *P<0.05; **P<0.01; ***P<0.001, while comparing with control (ANOVA and post hoc test).
miR-363 directly targeted Snail1 in RCC cells
The relationship between miR-363 and EMT regulators Snail1 was also studied. According to the Starbase database search, Snail1 has putative miR-363 binding sites and one binding site of miR-363 is in its 3’-UTR (Figure 5A). To confirm the actual binding site and target of miR-363 in OSRC-2 and SW839 cells, wild-type (with target site) and mutant-type (without target-site) vectors were constructed. As is shown in Figure 5B, the overexpression of miR-363 in SW839 and OSRC-2 cells significantly decreased. On the other hand, relative luciferase activity in the Snail1 3’-UTR wild-type constructs (Figure 5C, P<0.001, P<0.001), which was not observed in the Snail1 3’-UTR mutant constructs. Moreover, RIP assays demonstrating that snail1 and miR-363 expressions were considerably enriched by the Ago2 antibody in OSRC-2 and SW839 cells as shown in (Figure 5D, P<0.001). The overexpression of miR-363 in SW839, and in OSRC-2 cells resulted in a decrease of Snail1 (Figure 5E, P<0.001). Consistent with this, knocking down miR-363 in SW839, and OSRC-2 cells and resulted in an increase of Snail1 (Figure 5F, P<0.001). The Snail1 expression in SW839 and OSRC-2 cells were also determined after the treatment of propofol, which indicated the propofol led to the decrease of snail1 in both RCC cells (Figure 5G, P<0.001).
Overexpression of snail1 partially reversed the propofol’s inhibitory effect on RCC cells
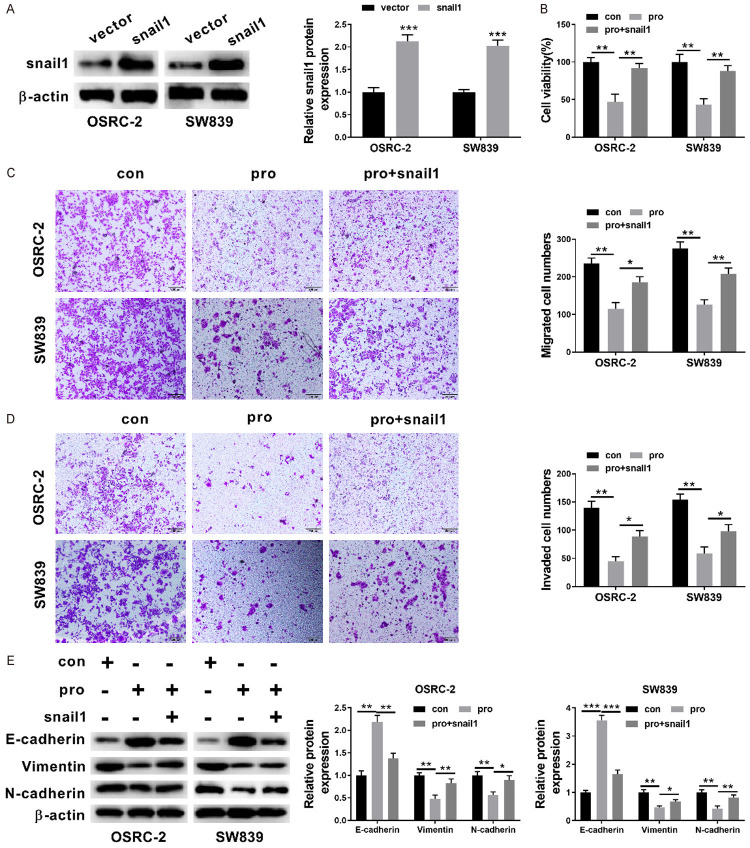
To assess whether Snail1 is involved in the inhibition of RCC cell proliferation, migration, invasion and EMT by miR-363, a Snail1 construct which lacks a 3’-UTR, or empty vector was used to transfect OSRC-2 cells and SW839 cells. The expression of Snail protein in the RCC cells was subsequently evaluated by western blot analysis which showed that in contrast with cells transfected with the empty vector, those transfected with Snail1 construct had significantly increased Snail protein expression (Figure 6A, P<0.001, P<0.001). The CCK-8 assay revealed that Snail1 induction significantly increased cell proliferation in OSRC-2 and SW839 cells as described in the given (Figure 6B, P<0.01, P<0.01). The Transwell assays indicated that the restored expression of Snail1 partially reversed the miR-363 mediated suppression of the migration and invasion of RCC cells (Figure 6C and 6D, all P<0.05). The protein expression levels of the aforementioned cell EMT-related protein (E-cadherin, Vimentin, N-cadherin) were also compared using the western blot analysis. Similarly, Figure 6E showed that Snail1 partially reversed the propofol-induced the increased E-cadherin expression level and decreased Vimentin, N-cadherin expression level in OSRC-2 and SW839 cells (Figure 6E, all P<0.05). Our obtained results further supported that Snail1 was also engaged in the role of propofol on OSRC-2 and SW839 cell proliferation, invasion, migration and EMT.
Figure 6.
Overexpression of snail1 partially reversed the inhibitory effect of propofol on RCC cells. A: The expression of Snail1 protein in RCC cells which were transfected with the empty vector and those transfected with Snail1 construct; B: The cell viability of OSRC-2 and SW839 cell after no treatment, propofol treatment and propofol treatment + Snail1 transfection were measured by CCK-8 assay; C and D: The relative invasion and migration of OSRC-2 cells and SW839 cells after no treatment, propofol treatment and propofol treatment + Snail1 transfection (×100); E: Western blot analysis was carried out to assess the protein expression levels of E-cadherin, Vimentin, N-cadherin after no treatment, propofol treatment and propofol treatment + Snail1 transfection. Data are shown as mean ± S.E.M. *P<0.05; **P<0.01; ***P<0.001, compared to control (ANOVA and post hoc test).
Discussion
The poor prognosis in most RCC patients is due to its high proliferation, migration and invasion ability. In current study, we revealed that propofol significantly inhibited RCC OSRC-2 and SW839 cells proliferation, invasion, migration and EMT. Moreover, after the treatment of propofol the relative expression level of miR-363 was upregulated in OSRC-2 and SW839 cells. Also, the propofol-induced RCC cell migration, proliferation, invasion, and EMT inhibition was significantly reversed by the suppression of miR-363. We also showed that miR-363 directly targeted EMT-regulator Snail1 in RCC cells and the overexpression of Snail1 partially reversed the suppression effect of miR-363 on propofol-induced RCC cell proliferation, invasion, migration and EMT inhibition.
Propofol is well-known and frequently used, intravenous anesthetic medicine. Apart from its anesthetic effect, propofol also exerts anti-cancer features in multiple cancer types including gastric cancer, lung cancer and pancreatic cancer [16-21]. The benefits of propofol as the potential chemotherapy candidate drug is its cost-effectivity for clinical trial and little side effect. However, the effect of propofol on RCC hasn’t yet been studied. We demonstrated that propofol suppressed RCC cell proliferation in a dose-dependent manner, which has also been reported for gastric cancer [16,17]. Furthermore, in concordance with previous research, we found that propofol significantly suppressed RCC cell migration, invasion and EMT in our study. This is crucial for the management and treatment of RCC metastasis in patients. These findings confirmed that the anti-cancer effect of propofol on RCC specifically.
The microRNAs are small molecules that play an important role in the regulation of multiple signaling networks. Several research studies have reported the low expression and anti-cancer effect of miR-363 in cancer. One study on RCC reported that patients with higher TNM stage and higher Fuhrman grade expressed lower level of miR-363 [10]. Similarly, the downregulation of miR-363 was observed in colorectal cancer tissues. Then it was negatively related with the advanced stage of colorectal cancer [22]. Consistent findings were also revealed in our current study that miR-363 expression level in RCC cells level was significantly decreased. Besides, our study identified that miR-363 intervention reversed the protective events of propofol in RCC cells, which further validated that miR-363 was responsible for these events.
The Snail family transcriptional repressor 1 (Snail1) has been reported to promote metastasis in RCC, however the mechanism is largely unexplored [11]. We revealed that Snail1 is a potential miR-363 target gene in the current study. Our findings showed that miR-363 might directly targeting the 3’-UTR of Snail1 mRNA in RCC cells and thus negatively regulate Snail1 expression. Snail1 is a transcription factor first identified in Drosophila, and zinc-finger motif as well as the E-cadherin promoter E-box region (sequence CAGGTG) are located on the main chain [23,24]. One important function of Snail1 is to suppress E-cadherin expression, which directly leads to EMT, altered cell morphology, instability in cellular structure thus leading to cell metastasis, migration and invasion from the original cancer site [25]. Additionally, previous studies have identified that Snail1 expression level could a potential prognostic indicator for poor prognosis and low survival rate in RCC patients [11]. Our study provided further evidence for the role of Snail1 in RCC metastasis mediated by miR-363.
In summary, the results supported that propofol inhibits the proliferation, invasion, migration and EMT of RCC cells by targeting microRNA-363/Snail1 pathway, which helps clarify the mechanisms underlying anti-cancer effect of propofol on RCC and identify new biomarker and therapeutic targets. Furthermore, the therapeutic effect of propofol on RCC worth deep research.
Disclosure of conflict of interest
None.
References
- 1.Cairns P. Renal cell carcinoma. Cancer Biomark. 2011;9:461–473. doi: 10.3233/CBM-2011-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Zhou SH, Liu R, Xu CX, Guo Q, Xiao DH, Huang LH, Wang F, Tang WL, Shen SR, Jiang XW, Wang XY. Safety of propofol/midazolam sedation for upper gastrointestinal endoscopy in patients with co-morbidities. Int J Clin Exp Med. 2018;11:12514–12522. [Google Scholar]
- 3.Liu ZM, Zhang J, Hong GC, Quan JP, Zhang L, Yu MQ. Propofol inhibits growth and invasion of pancreatic cancer cells through regulation of the miR-21/Slug signaling pathway. Am J Transl Res. 2016;8:4120–4133. [PMC free article] [PubMed] [Google Scholar]
- 4.Ou W, Lv J, Zou X, Yao Y, Wu J, Yang J, Wang Z, Ma Y. Propofol inhibits hepatocellular carcinoma growth and invasion through the HMGA2-mediated Wnt/β-catenin pathway. Exp Ther Med. 2017;13:2501–2506. doi: 10.3892/etm.2017.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang XY, Li YL, Wang HY, Zhu M, Guo D, Wang GL, Gao YT, Yang Z, Li T, Yang CY, Chen YM. Propofol inhibits invasion and proliferation of C6 glioma cells by regulating the Ca2+ permeable AMPA receptor-system xc - pathway. Toxicol Vitro. 2017;44:57–65. doi: 10.1016/j.tiv.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 7.Chang TC, Yu DN, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, Mendell JT. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang PF, Sheng LL, Wang G, Tian M, Zhu LY, Zhang R, Zhang J, Zhu JS. miR-363 promotes proliferation and chemo-resistance of human gastric cancer via targeting of FBW7 ubiquitin ligase expression. Oncotarget. 2016;7:35284–35292. doi: 10.18632/oncotarget.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu F, Min J, Cao XN, Liu L, Ge ZQ, Hu JB, Li XL. MiR-363-3p inhibits the epithelial-to-mesenchymal transition and suppresses metastasis in colorectal cancer by targeting Sox4. Biochem Biophys Res Commun. 2016;474:35–42. doi: 10.1016/j.bbrc.2016.04.055. [DOI] [PubMed] [Google Scholar]
- 10.Xie Y, Chen LC, Gao Y, Ma X, He WY, Zhang Y, Zhang F, Fan Y, Gu LY, Li P, Zhang X, Gou X. miR-363 suppresses the proliferation, migration and invasion of clear cell renal cell carcinoma by downregulating S1PR1. Cancer Cell Int. 2020;20:227. doi: 10.1186/s12935-020-01313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu WS, Liu YD, Liu HO, Zhang WJ, An HM, Xu JJ. Snail predicts recurrence and survival of patients with localized clear cell renal cell carcinoma after surgical resection. Urol Oncol. 2015;33:69, e1–10. doi: 10.1016/j.urolonc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Mahmood T, Yang PC. Western blot: technique, theory, and trouble shooting. N Am J Med Sci. 2012;4:429–434. doi: 10.4103/1947-2714.100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang KF, Jin W, Song Y, Fei X. LncRNA RP11-436H11.5, functioning as a competitive endogenous RNA, upregulates BCL-W expression by sponging miR-335-5p and promotes proliferation and invasion in renal cell carcinoma. Mol Cancer. 2017;16:166. doi: 10.1186/s12943-017-0735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang CS, Logan S, Yan YS, Inagaki Y, Arzua T, Ma PZ, Lu SM, Bosnjak ZJ, Bai XW. Signaling network between the dysregulated expression of microRNAs and mRNAs in propofol-induced developmental neurotoxicity in mice. Sci Rep. 2018;8:14172. doi: 10.1038/s41598-018-32474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Y, Zhang JJ. Propofol induces oxidative stress and apoptosis in vitro via regulating miR-363-3p/CREB signalling axis. Cell Biochem Funct. 2020;38:1119–1128. doi: 10.1002/cbf.3572. [DOI] [PubMed] [Google Scholar]
- 16.Yang C, Gao J, Yan N, Wu BL, Ren YQ, Li H, Liang JM. Propofol inhibits the growth and survival of gastric cancer cells in vitro through the upregulation of ING3. Oncol Rep. 2017;37:587–593. doi: 10.3892/or.2016.5218. [DOI] [PubMed] [Google Scholar]
- 17.Zeng RF, Li Y, Wu QX, Qi L, Li HS, Wang XC, Lian QQ, Yang JP. Premedication of butorphanol benefits gastrointestinal endoscopy screening under sedation: a randomized, controlled, double-blinded clinical trial. Int J Clin Exp Med. 2019;12:283–290. [Google Scholar]
- 18.Cui WY, Liu Y, Zhu YQ, Song T, Wang QS. Propofol induces endoplasmic reticulum (ER) stress and apoptosis in lung cancer cell H460. Tumour Biol. 2014;35:5213–5217. doi: 10.1007/s13277-014-1677-7. [DOI] [PubMed] [Google Scholar]
- 19.Liu WZ, Liu N. Propofol inhibits lung cancer A549 cell growth and epithelial-mesenchymal transition process by upregulation of MicroRNA-1284. Oncol Res. 2018;27:1–8. doi: 10.3727/096504018X15172738893959. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Chen XY, Wu QC, You L, Chen SS, Zhu MM, Miao CH. Propofol attenuates pancreatic cancer malignant potential via inhibition of NMDA receptor. Eur J Pharmacol. 2017;795:150–159. doi: 10.1016/j.ejphar.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Gao YT, Yu XD, Zhang FX, Dai J. Propofol inhibits pancreatic cancer progress under hypoxia via ADAM8. J Hepato-Biliary Pancreat Sci. 2019;26:219–226. doi: 10.1002/jhbp.624. [DOI] [PubMed] [Google Scholar]
- 22.Dong JL, Geng JS, Tan WW. MiR-363-3p suppresses tumor growth and metastasis of colorectal cancer via targeting SphK2. Biomed Pharmacother. 2018;105:922–931. doi: 10.1016/j.biopha.2018.06.052. [DOI] [PubMed] [Google Scholar]
- 23.Hemavathy K, Ashraf SI, Ip YT. Snail/slug family of repressors: slowly going into the fast lane of development and cancer. Gene. 2000;257:1–12. doi: 10.1016/s0378-1119(00)00371-1. [DOI] [PubMed] [Google Scholar]
- 24.Ohkubo T, Ozawa M. The transcription factor snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci. 2004;117:1675–1685. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- 25.Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, De Herreros AG. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]