Abstract
Objective: To investigate the effect of a low temperature plasma knife on the treatment of chronic tonsillitis and its effect on T lymphocyte subsets. Methods: A total of 70 patients diagnosed with tonsillitis from March 2017 to October 2018 were selected as research subjects. Among them, patients treated by routine surgery were placed into the control group (33 cases), and patients treated by low temperature plasma knife were placed in the observation group (37 cases). The clinical efficacy, intraoperative blood loss, operative time, time of complete white membrane coverage, time of complete white membrane shedding, pain scoring, adverse reactions and the influence on T cell subsets between the two groups were compared. Results: The clinical treatment efficacy of the observation group was significantly higher than that of the control group (P<0.05). The operative time, intraoperative blood loss, time of complete white membrane coverage and time of complete white membrane shedding in the observation group were significantly lower than those in the control group. The pain score of patients in the observation group was significantly lower than in the control group on 1 d, 3 d and 5 d after surgery (P<0.05), while there was no significant difference in pain score between the two groups 7 d after surgery (P>0.05). There was no significant difference between the observation group and the control group in postoperative blood loss, torus tubarius injury and adverse nasal adhesion (P>0.05). The total curative effective rate of the control group was significantly lower than that of the observation group (P<0.05). Conclusion: To sum up, a low temperature plasma knife has good effects in the treatment of chronic tonsillitis, which can alleviate the pain response of patients without increasing the incidence of adverse reactions, and as such it is worthy of clinical promotion.
Keywords: Low temperature plasma knife, chronic tonsillitis, CD3+, CD4+, CD8+
Introduction
Chronic tonsillitis is a common inflammatory disease found in the respiratory tract. It is caused by the involvement of various immune cells and cytokines, thus leading to chronic inflammatory reactions in the tonsils [1,2]. Symptoms of chronic tonsillitis are comprised of sore throat, blocked throat, foreign body sensation and other discomfort, which affects the health and quality of life of patients. Clinical treatment of patients with chronic tonsillitis is mostly surgical treatment [3-5]. However, conventional blunt tonsillectomy, hemostasis with compression by cotton balls or local ligation has been used in previous surgeries. Postoperative scar tissue will aggravate the patients’ sensation of a foreign body in the throat. Therefore, it is of great significance to explore appropriate treatment methods for chronic tonsillitis to improve patients’ quality of life [6,7].
In recent years, the emergence of low temperature plasma technology has come into focus, and its application in tonsillectomy and adenoidectomy is satisfactory. Due to its extremely low operating temperature, the cutting head is very small and the ablation depth can be precisely controlled, bringing little damage to the surrounding tissue during the operation [8,9]. In addition, as the tonsil is part of the portal of the oropharynx, it produces marked effects on promoting children’s immune development. For patients with simple tonsil hypertrophy, plasma technology can also be used to complete partial tonsil bursa resection to retain the maximum immune function, which is unmatched by conventional tonsillectomy [10,11]. According to reports by Mitic et al. [12], plasma adenoidectomy and tonsillectomy can reduce the hospital stay, parents’ care days for children, as well as social costs. T lymphocytes can be involved in the body’s ability to identify antigens, produce cytokines, and participate in cellular immune defense, regulation and health monitoring. Therefore, the number of T lymphocytes and their subsets can be used as indicators reflecting the body’s immune regulatory function [13,14]. CD4+ and CD8+ cells play an important role in the immune regulation and anti-infection of the body and are the main functional T lymphocytes in the T lymphocyte subsets. CD4+ is the helper cell of the immune system, while CD8+ cells have the effect of killing T cells [15,16].
Previous studies have shown that a low temperature plasma knife has significant benefits for the recovery of patients with chronic tonsillitis [17], but there are few studies on the changes of T lymphocyte subsets in patients with chronic tonsillitis treated with a low temperature plasma knife. Based on the study of the immune function of tonsils, this study explored the different roles of CD3+, CD4+, CD8+ in the development of chronic tonsillitis, discussed its possible effects, and speculated about their value in clinical intervention and immunological treatment of chronic tonsillitis through detecting the differential effects and distribution characteristics of CD3+, CD4+, CD8+ in the serum of two groups of patients.
Data and methods
General data
A total of 70 patients diagnosed with tonsillitis from March 2017 to October 2018 were elected as research subjects. Among them, patients treated by routine surgery were included in the control group (33 cases), and patients treated by a low temperature plasma knife were enrolled in the observation group (37 cases). Inclusion criteria were as follows: All patients’ family members were informed and signed the fully informed consent. All the patients met the diagnostic criteria of chronic tonsillitis, which manifested as excessive tonsil hypertrophy affecting breathing, swallowing or sleep apnea hypopnea syndrome. The study followed ethical requirements of our hospital, and the research plan was submitted to the ethics committee of the hospital for review and approval before implementation. Exclusion criteria were as follows: Patients complicated with autoimmune diseases. Patients with a history of splenectomy or with intolerance to treatment. Patients with severe dysfunction of the lung, kidney, liver or heart. Patients with malignant tumors. Patients with severe systemic diseases. Patients with severe mental retardation or multiple seizures that could not cooperate with treatment. Patients complicated with hematopoietic system diseases and coagulation dysfunction. Female patients before menstruation, during menstruation or pregnancy. Patients with other throat lesions.
Therapeutic schedule
After admission to the hospital, patients in the observation group received treatment of tonsillectomy with a low temperature plasma radiofrequency knife: combined anesthesia was administered intravenously, with routine disinfection and drape operations. The operating area was exposed with the Davis opener. The palatopharyngeal arch and mucous membrane were cut open with a low temperature plasma radiofrequency knife starting from the upper part of the tonsil. When the upper part of the tonsil was fully exposed, the tonsil was detached with a skin forceps, and hemostasis was performed. Patients in the control group were treated with conventional tonsillectomy: the palatopharyngeal arch and mucous membrane were cut open with a sickle knife, the tonsils were removed from the upper pole through a snare, and gauze balls were used to stop bleeding during the operation.
Outcome measures
The operative indicators of the two groups were analyzed, including intraoperative blood loss, operative time, time of complete white membrane coverage and time of complete white membrane shedding. The operative time started from the first incision of mucosa to complete hemostasis. Intraoperative blood loss calculation method was as follows: oropharyngeal secretion was sucked out and the initial value of the fluid in the suction bottle was recorded (A), the final value of the fluid in the suction bottle was recorded at the end of the operation (B), the estimated intraoperative blood absorption of cotton ball (C). Intraoperative blood loss = B+C-A. Postoperative pain assessment (children asked by parents) was scored using visual analogue scale (VAS). Peripheral venous blood was extracted from the patients in the observation group before and 3 months after the operation, and that of the control group was extracted on the second day of enrollment. T lymphocyte subset level was detected by flow cytometer. The percentage of CD3+, CD4+ T lymphocytes, CD8+ T lymphocytes, and CD4+/CD8+ ratios were calculated in the two groups. Efficacy assessment: the clinical efficacy and postoperative infection rate of different treatment modes in the two groups of patients. (1) Markedly effective: disappearance of longterm sore throat, fever, pharyngeal stenosis, not easy to catch cold. (2) Effective: symptoms improved compared with before treatment. (3) Ineffective: symptoms did not disappear, and even worsened [18].
Detection methods
In the observation group, 5 ml of fasting peripheral venous blood of patients was collected in the morning at two time points (1 day before the operation and 3 months after the operation), and anticoagulation was performed with conventional heparin. First, the blood was centrifuged at 3000 r/min for 5 min, and the supernatant was discarded, and then the remaining portion was centrifuged at 3000 r/min for 10 min. The middle suspension cell was taken and the lymphocyte suspension was prepared. The T lymphocyte subsets were identified by the T lymphocyte subset detection kit provided by Shanghai Huasheng Biotechnology Co., Ltd., and the cells isolated by peroxidase method were stained with Filoll, and CD4+ T lymphocytes and CD8+ T lymphocytes were counted under a 40-fold binocular microscope. Subjects in the control group received the same treatment after peripheral blood was collected on an empty stomach in the morning.
Statistical methods
SPSS 22.0 (IBM Corp, Armonk, NY, USA) was used for statistical treatment. Comparison of rates was performed by chi-square test, and measurement data were expressed as mean ± standard deviation. Comparison of measurement data between groups was qualified by independent sample t-test, and comparison before and after in group was qualified by paired t-test. P<0.05 was considered statistically significant.
Results
General data of the two groups
There was no significant difference between the observation group and the control group in terms of general clinical data such as gender, age, BMI, height, heart rate, urea nitrogen, place of residence, smoking history, exercise habits and drinking history (P>0.05). More details are shown in Table 1.
Table 1.
General data of the two groups
| Class | Observation group (n=37) | Control group (n=33) | t/χ2 | P |
|---|---|---|---|---|
| Gender | 0.892 | 0.345 | ||
| Male | 21 (56.76) | 15 (45.45) | ||
| Female | 16 (43.24) | 18 (54.55) | ||
| Age (years old) | 30.36±11.42 | 30.17±10.37 | 0.072 | 0.942 |
| BMI (kg/m2) | 24.37±1.28 | 23.89±1.46 | 1.466 | 0.147 |
| Heart rate (beats) | 105.66±11.49 | 105.81±11.58 | 0.054 | 0.957 |
| Urea nitrogen (mmol/L) | 13.14±3.55 | 13.16±3.73 | 0.022 | 0.982 |
| Place of residence | 0.1269 | 0.356 | ||
| Cities | 14 (37.84) | 13 (39.39) | ||
| Countryside | 18 (48.65) | 20 (60.61) | ||
| Smoking history | 1.266 | 0.260 | ||
| with | 6 (16.22) | 9 (27.27) | ||
| without | 31 (83.78) | 24 (72.73) | ||
| Exercise habit | 0.016 | 0.899 | ||
| with | 14 (37.84) | 12 (36.36) | ||
| without | 23 (62.16) | 21 (63.64) | ||
| History of alcoholism | 0.425 | 0.515 | ||
| with | 6 (16.22) | 8 (24.24) | ||
| without | 31 (83.78) | 28 (84.85) |
Operation conditions between the two groups
Compared with the operation conditions of the two groups, the operative time, intraoperative blood loss, time of complete white membrane coverage and time of complete white membrane shedding in the observation group were significantly lower than those of the control group. More details are shown in Table 2 and Figure 1.
Table 2.
Comparison of operation conditions between the two groups
| Groups | Observation group (n=37) | Control group (n=33) | t | P |
|---|---|---|---|---|
| Operative time (min) | 18.28±7.54 | 26.13±11.51 | 3.41 | <0.05 |
| Intraoperative blood loss (mL) | 5.56±4.21 | 8.97±6.21 | 2.714 | <0.05 |
| Time of complete white membrane coverage (h) | 40.38±10.27 | 47.45±11.62 | 2.702 | <0.05 |
| Time of complete white membrane shedding (d) | 7.98±3.76 | 9.87±3.39 | 2.198 | <0.05 |
Figure 1.
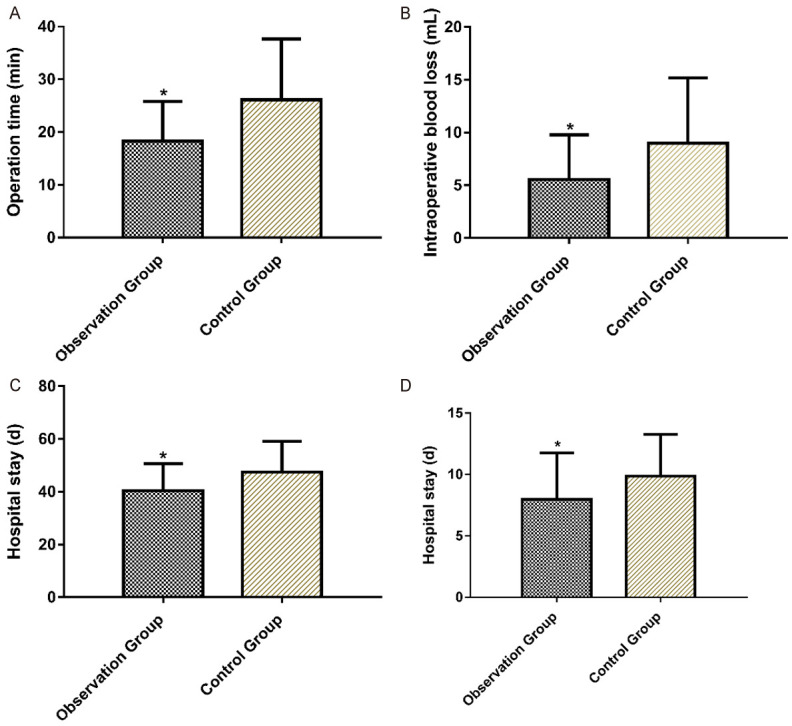
Comparison of operative time, intraoperative blood loss, time of complete white membrane coverage and time of complete white membrane shedding between the two groups. Note: Compared with the control group, *P<0.05.
Comparison of postoperative pain scoring between the two groups
Postoperative pain scores of patients in the control group were significantly higher than those in the observation group 1 d, 3 d and 5 d after surgery (P<0.05), and there was no significant difference in pain scores between the two groups on 7 d after surgery (P>0.05). More details are shown in Table 3 and Figure 2.
Table 3.
Comparison of postoperative pain scoring between the two groups
| Groups | Observation group (n=37) | Control group (n=33) | t | P |
|---|---|---|---|---|
| 1 d after surgery | 1.77±0.89 | 2.29±1.21 | 2.063 | 0.043 |
| 3 d after surgery | 1.34±0.51 | 1.62±0.54 | 2.23 | 0.029 |
| 5 d after surgery | 0.67±0.42 | 0.94±0.47 | 2.538 | 0.013 |
| 7 d after surgery | 0.41±0.26 | 0.42±0.29 | 0.125 | 0.880 |
Figure 2.
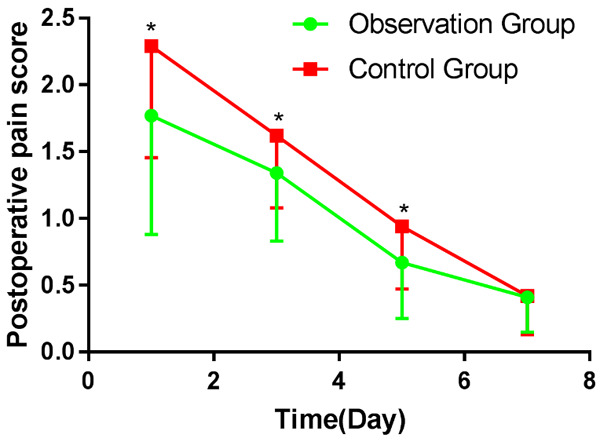
Comparison of postoperative pain scoring between the two groups. Note: Compared with the control group, *P<0.05.
Clinical efficacy between the two groups
After treatment, there were 29 cases of markedly effective (78.38%), 7 cases of effective (18.92%) and 1 case of ineffective (2.7%), with the total effective rate of treatment of 94.59%. There were 18 cases of markedly effective (54.55%), 9 cases of effective (18.92%) and 6 cases of ineffective (2.7%), with the total effective rate of treatment of 81.82%. The total effective rate of the control group was significantly lower than that of the observation group (P<0.05). More details are shown in Table 4.
Table 4.
Comparison of clinical efficacy between the two groups
| Category [case (%)] | Observation group (n=37) | Control group (n=33) | χ2 value | P value |
|---|---|---|---|---|
| Significant effect | 29 (78.38) | 18 (54.55) | 4.491 | 0.034 |
| Effective | 7 (18.92) | 9 (27.27) | 0.690 | 0.406 |
| Invalid | 1 (2.7) | 6 (18.18) | 4.482 | 0.034 |
| Total effective rate of treatment | 35 (94.59) | 27 (81.82) | 4.482 | 0.034 |
Adverse reactions in the two groups
In the observation group, there were 3 cases of blood loss (8.11%), 1 case of torus tubarius injury (2.7%), and 1 case of nasal adhesion (2.7%), with a total adverse reaction incidence of 13.51%. In the control group, there were 2 cases of hemorrhage (6.06%), 2 cases of torus tubarius injury (6.06%), and 1 case of nasal adhesion (3.03%), with the total adverse reaction incidence of 15.15%. There was no significant difference between the observation group and the control group in postoperative hemorrhage, torus tubarius injury and nasal adhesion (P>0.05). More details are shown in Table 5.
Table 5.
Adverse reactions in the two groups
| Class | Observation group (n=37) | Control group (n=33) | χ2 value | P value |
|---|---|---|---|---|
| Postoperative blood loss | 3 (8.11) | 2 (6.06) | 0.110 | 0.740 |
| Torus tubarius injury | 1 (2.7) | 2 (6.06) | 0.480 | 0.489 |
| Nasal adhesion | 1 (2.7) | 1 (3.03) | 0.007 | 0.935 |
| Total incidence rate | 5 (13.51) | 5 (15.15) | 0.038 | 0.845 |
Comparison of serum scores of T-lymphocyte subsets between the two groups before and after treatment
The levels of serum T lymphocyte subsets before and after treatment were compared between the two groups. Before treatment, there was no significant difference in the levels of serum CD3+, CD4+, CD8+, CD4+/CD8+ T lymphocyte subset between the two groups (P>0.05). After treatment, the serum levels of CD3+, CD4+ and CD4+/CD8+ in both groups were significantly increased (P<0.05). The values of CD3+, CD4+ and CD4+/CD8+ were higher in the observation group, but there was no statistical difference compared with the control group (P>0.05). More details are shown in Table 6 and Figure 3.
Table 6.
Comparison of serum scores of T lymphocyte subsets between the two groups before and after treatment
| Groups | CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Observation group (n=37) | 57.37±6.21 | 65.89±6.64* | 28.45±4.21 | 33.66±5.47* | 24.39±2.47 | 24.84±2.22 | 1.14±0.31 | 1.28±0.27* |
| Control group (n=33) | 57.84±5.82 | 63.13±7.21* | 28.77±4.72 | 33.13±5.16* | 24.41±2.38 | 24.06±2.24 | 1.12±0.28 | 1.26±0.24* |
| t value | 0.336 | 1.618 | 0.036 | 0.405 | 0.972 | 1.461 | 0.291 | 0.318 |
| P | 0.738 | 0.111 | 0.972 | 0.687 | 0.816 | 0.149 | 0.772 | 0.752 |
Note: compared with before treatment;
P<0.05.
Figure 3.
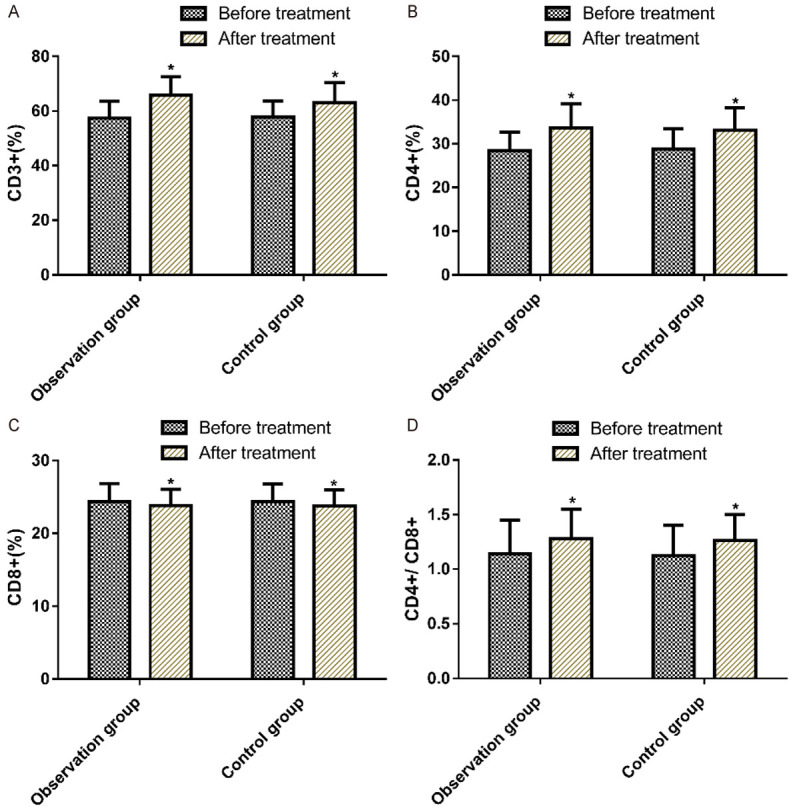
Comparison of serum score T-lymphocyte subset level between the control group and the observation group before and after treatment. Note: Compared with the control group, *P<0.05.
Discussion
Chronic tonsillitis is one of the most common diseases seen in the clinic, which is mostly caused by the development of acute tonsillitis. Recurrent episodes of acute tonsillitis may result from poor crypt drainage, crypts bacteria, and virus causing infection which then evolves into chronic tonsillitis. The clinical manifestations of adult chronic tonsillitis are mainly pharyngitis, fever, halitosis, irritating cough, etc. [19,20]. Recurrent inflammation is prone to a variety of complications, such as rheumatic fever, glomerulonephritis, secretory otitis media and sleep apnea hypopnea syndrome [21,22].
Surgery is one of the main methods to cure chronic tonsillitis. The tonsillectomy and squeezing under traditional cold instruments has a long operative time, with great intraoperative damage, and high risks during the operation [23]. The low temperature plasma knife consists of a conductive medium (salt) that forms a large concentration of plasma in the periphery of the electrode, which is composed of highly ionized particles [24,25]. Such particles have sufficient energy to dissociate the molecular chains in the material, which in turn separates various molecules and reduces the tissue, since the current does not generate a large amount of heat through the tissue and does not cause visual damage to the tissue. At the same time, the characteristics of an ultra-low temperature, strong plasma hemostatic effects, can completely cut off the diseased tonsils [26,27]. This study compared the surgical situation of patients undergoing routine surgery with that of patients treated with a low temperature plasma knife for chronic tonsillitis. The results showed that the operative time, intraoperative blood loss, time of complete white membrane coverage, and time of complete white membrane detachment were significantly lower in the observation group than in the control group. The pain scores of patients in the observation group were significantly lower than those in the control group at 1 d, 3 d, and 5 d after surgery. The total effective rate after treatment in the control group was significantly lower than that in the observation group. There was no significant difference in adverse reactions between the two groups. Omrani et al. [28] used a double-blind randomized controlled trial to report the operative time, intraoperative bleeding, and postoperative pain in 94 patients. The pain and recovery after low temperature plasma ablation were better than traditional tonsillectomy. These results suggest that a low temperature plasma knife is a feasible treatment for chronic tonsillitis, which can reduce the patient’s pain response and may be more effective than conventional dissection.
The tonsil is the largest lymphatic tissue in the human pharynx, and its main function is to act as the first line of defense against bacteria, viruses and food antigens entering the digestive tract and upper respiratory tract [29,30]. When various antigens from the blood and epidermis are accepted by the tonsils, the tonsils are stimulated to produce messenger lymphocytes and antibodies that mobilize the immune defenses of body. Therefore, some studies have pointed out that tonsillectomy may damage the body’s defense against microorganisms and immune surveillance function [31,32]. T lymphocytes can participate in the body’s recognition of antigens, production of cytokines, and cellular immune response, etc. Hence, their cell subsets can be used as indicators reflecting the immune regulatory function of body [33]. In this study, the levels of T lymphocyte subsets in peripheral blood of patients with chronic tonsillitis with surgical indications before and after surgical removal of tonsils were compared. It was found that serum CD3+, CD4+ and CD4+/CD8+ were elevated in the observation group before treatment, with statistical significance (P<0.05), indicating that the immune function of patients after tonsillectomy was significantly improved compared with preoperative conditions.
In this study, subjects were screened strictly according to the inclusion and exclusion criteria. There was no significant difference in general clinical data such as age and gender between the two groups of patients, which ensured the preciseness and reliability of the study. However, there are still some limitations in this study. First of all, this study is a non-randomized controlled experiment, and bias inevitably occurs during the experiment. Secondly, we did not study the specific mechanism of changes in T-lymphocyte subset before and after treatment, which will be supplemented in future studies.
To sum up, the low temperature plasma knife has a good effect in the treatment of chronic tonsillitis, which can reduce the pain response of patients without increasing the incidence of adverse reactions, and is worthy of clinical promotion.
Disclosure of conflict of interest
None.
References
- 1.Souza DL, Cabrera D, Gilani WI, Campbell RL, Carlson ML, Lohse CM, Bellolio MF. Comparison of medical versus surgical management of peritonsillar abscess: a retrospective observational study. Laryngoscope. 2016;126:1529–1534. doi: 10.1002/lary.25960. [DOI] [PubMed] [Google Scholar]
- 2.Choi KY, Lee BS, Kim JH, Kim JJ, Jang Y, Choi JW, Lee DJ. Assessment of volatile sulfur compounds in adult and pediatric chronic tonsillitis patients receiving tonsillectomy. Clin Exp Otorhinolaryngol. 2018;11:210–215. doi: 10.21053/ceo.2017.01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhuravskii SG, Shakhnazarov AE, Yukina GY, Samusenko IA, Kryzhanovskaya EA. Reaction of the palatine tonsillar immunocompetent cells to irrigation of crypts by suspension of silica nanoparticles under conditions of chronic tonsillitis. Bull Exp Biol Med. 2019;167:396–399. doi: 10.1007/s10517-019-04535-8. [DOI] [PubMed] [Google Scholar]
- 4.Yenigun A. The efficacy of tonsillectomy in chronic tonsillitis patients as demonstrated by the neutrophil-to-lymphocyte ratio. J Laryngol Otol. 2015;129:386–391. doi: 10.1017/S0022215115000559. [DOI] [PubMed] [Google Scholar]
- 5.Saltanova ZE. Chronic tonsillitis, etiological and pathogenetic aspects of the development of metatonsillar complications. Vestn Otorinolaringol. 2015;80:65–70. doi: 10.17116/otorino201580365-70. [DOI] [PubMed] [Google Scholar]
- 6.Bender B, Blassnigg EC, Bechthold J, Kral F, Riccabona U, Steinbichler T, Riechelmann H. Microdebrider-assisted intracapsular tonsillectomy in adults with chronic or recurrent tonsillitis. Laryngoscope. 2015;125:2284–2290. doi: 10.1002/lary.25265. [DOI] [PubMed] [Google Scholar]
- 7.Arzamazov CG, Ivanets IV. Paratonsillar abscess in the patients presenting with the non-anginous form of chronic tonsillitis. Vestn Otorinolaringol. 2013:25–28. [PubMed] [Google Scholar]
- 8.Carreiro AFP, Delben JA, Guedes S, Silveira EJD, Janal MN, Vergani CE, Pushalkar S, Duarte S. Low-temperature plasma on peri-implant-related biofilm and gingival tissue. J Periodontol. 2019;90:507–515. doi: 10.1002/JPER.18-0366. [DOI] [PubMed] [Google Scholar]
- 9.Bafoil M, Le Ru A, Merbahi N, Eichwald O, Dunand C, Yousfi M. New insights of low-temperature plasma effects on germination of three genotypes of Arabidopsis thaliana seeds under osmotic and saline stresses. Sci Rep. 2019;9:8649. doi: 10.1038/s41598-019-44927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacic A, Prgomet D, Janjanin S. Tonsil-derived mesenchymal stem cells exert immunosuppressive effects on T cells. Croat Med J. 2019;60:12–19. doi: 10.3325/cmj.2019.60.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton MJ, Perera R. A pilot randomised controlled trial of coblation tonsillectomy versus dissection tonsillectomy with bipolar diathermy. Clin Otolaryngol. 2007;32:495–496. doi: 10.1111/j.1749-4486.2007.01576.x. [DOI] [PubMed] [Google Scholar]
- 12.Mitic S, Tvinnereim M, Lie E, Saltyte BJ. A pilot randomized controlled trial of coblation tonsillectomy versus dissection tonsillectomy with bipolar diathermy haemostasis. Clin Otolaryngol. 2007;32:261–267. doi: 10.1111/j.1365-2273.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- 13.Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M, Han M, Thornton AM, Zhang H, Egger M, Luo J, Felsher DW, McVicar DW, Weber A, Heikenwalder M, Greten TF. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253–257. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Gonzalez P, Ubilla-Olguin G, Catalan D, Schinnerling K, Aguillon JC. Tolerogenic dendritic cells for reprogramming of lymphocyte responses in autoimmune diseases. Autoimmun Rev. 2016;15:1071–1080. doi: 10.1016/j.autrev.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 15.Hughes RA, May MT, Tilling K, Taylor N, Wittkop L, Reiss P, Gill J, Schommers P, Costagliola D, Guest JL, Lima VD, d’Arminio Monforte A, Smith C, Cavassini M, Saag M, Castilho JL, Sterne JAC. Long terms trends in CD4+ cell counts, CD8+ cell counts, and the CD4+: CD8+ ratio. AIDS. 2018;32:1361–1367. doi: 10.1097/QAD.0000000000001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baudoux T, Husson C, De Prez E, Jadot I, Antoine MH, Nortier JL, Hougardy JM. CD4(+) and CD8(+) T cells exert regulatory properties during experimental acute aristolochic acid nephropathy. Sci Rep. 2018;8:5334. doi: 10.1038/s41598-018-23565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao YC, Wang XY, Xu WW, Li JD, Yu QH. The effects of tonsillectomy by low-temperature plasma on the growth development and psychological behavior in children with obstructive sleep apnea hypopnea syndrome. Medicine (Baltimore) 2018;97:e13205. doi: 10.1097/MD.0000000000013205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu Bakar M, McKimm J, Haque SZ, Majumder MAA, Haque M. Chronic tonsillitis and biofilms: a brief overview of treatment modalities. J Inflamm Res. 2018;11:329–337. doi: 10.2147/JIR.S162486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siupsinskiene N, Katutiene I, Jonikiene V, Janciauskas D, Vaitkus S. Helicobacter pylori in the tonsillar tissue: a possible association with chronic tonsillitis and laryngopharyngeal reflux. J Laryngol Otol. 2017;131:549–556. doi: 10.1017/S0022215117000597. [DOI] [PubMed] [Google Scholar]
- 20.Erratum: chronic tonsillitis and biofilms: a brief overview of treatment modalities [Corrigendum] J Inflamm Res. 2018;11:375. doi: 10.2147/JIR.S187032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sancaktar ME, Celebi M, Yildirim M, Can E, Akgul G, Agri I, Unal A, Yilmaz F. Safety of outpatient admission and comparison of different surgical techniques in adult tonsillectomy. Eur Arch Otorhinolaryngol. 2019;276:1211–1219. doi: 10.1007/s00405-019-05334-7. [DOI] [PubMed] [Google Scholar]
- 22.Thompson RW, Gungor A. Immune thrombocytopenia of childhood responsive to tonsillectomy in the setting of chronic tonsillitis: a case report and literature review. Am J Otolaryngol. 2017;38:639–641. doi: 10.1016/j.amjoto.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell RB, Archer SM, Ishman SL, Rosenfeld RM, Coles S, Finestone SA, Friedman NR, Giordano T, Hildrew DM, Kim TW, Lloyd RM, Parikh SR, Shulman ST, Walner DL, Walsh SA, Nnacheta LC. Clinical practice guideline: tonsillectomy in children (update)-executive summary. Otolaryngol Head Neck Surg. 2019;160:187–205. doi: 10.1177/0194599818807917. [DOI] [PubMed] [Google Scholar]
- 24.Sadura I, Libik-Konieczny M, Jurczyk B, Gruszka D, Janeczko A. Plasma membrane ATPase and the aquaporin HvPIP1 in barley brassinosteroid mutants acclimated to high and low temperature. J Plant Physiol. 2019;244:153090. doi: 10.1016/j.jplph.2019.153090. [DOI] [PubMed] [Google Scholar]
- 25.Chuangsuwanich A, Assadamongkol T, Boonyawan D. The healing effect of low-temperature atmospheric-pressure plasma in pressure ulcer: a randomized controlled trial. Int J Low Extrem Wounds. 2016;15:313–319. doi: 10.1177/1534734616665046. [DOI] [PubMed] [Google Scholar]
- 26.Bie X, Wang J, Sun X, Sun K, Tang Y. Combined application of endoscope and low-temperature plasma knife in the excision of nasal septal schwannoma. Ear Nose Throat J. 2020;99:111–113. doi: 10.1177/0145561319837883. [DOI] [PubMed] [Google Scholar]
- 27.Hallissey CM, Heyderman RS, Williams NA. Human tonsil-derived dendritic cells are poor inducers of T cell immunity to mucosally encountered pathogens. J Infect Dis. 2014;209:1847–1856. doi: 10.1093/infdis/jit819. [DOI] [PubMed] [Google Scholar]
- 28.Omrani M, Barati B, Omidifar N, Okhovvat AR, Hashemi SA. Coblation versus traditional tonsillectomy: a double blind randomized controlled trial. J Res Med Sci. 2012;17:45–50. [PMC free article] [PubMed] [Google Scholar]
- 29.Yorita K, Nakagawa H, Miyazaki K, Ishitani Y, Arisawa Y. Lymphoid papillary hyperplasia arising from the upper portion of the left palatine tonsil: a case report and literature review. Indian J Otolaryngol Head Neck Surg. 2019;71:835–838. doi: 10.1007/s12070-019-01668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin AJ, Rao YJ, Chin RI, Campian J, Mullen D, Thotala D, Daly M, Gay H, Oppelt P, Hallahan D, Adkins D, Thorstad W. Post-operative radiation effects on lymphopenia, neutrophil to lymphocyte ratio, and clinical outcomes in palatine tonsil cancers. Oral Oncol. 2018;86:1–7. doi: 10.1016/j.oraloncology.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Osorio Y, Ghiasi H. Comparison of adjuvant efficacy of herpes simplex virus type 1 recombinant viruses expressing TH1 and TH2 cytokine genes. J Virol. 2003;77:5774–5783. doi: 10.1128/JVI.77.10.5774-5783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assadian F, Sandstrom K, Laurell G, Svensson C, Akusjarvi G, Punga T. Efficient isolation protocol for B and T lymphocytes from human palatine tonsils. J Vis Exp. 2015:53374. doi: 10.3791/53374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JY, Han AR, Lee DR. T lymphocyte development and activation in humanized mouse model. Dev Reprod. 2019;23:79–92. doi: 10.12717/DR.2019.23.2.079. [DOI] [PMC free article] [PubMed] [Google Scholar]


