Abstract
Objective: The overexpression of transcription factor Sine oculis homeobox 1 (SIX1) is discovered in various malignant tumors and has been known to be closely associated with tumorigenesis, progression and prognosis. This study aims to determine the role of SIX1 in endometrial cancer (EC). Methods: In this study, we analyzed the SIX1 expression profile and the correlation with the corresponding clinical characteristics of EC samples from the Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO) and Clinical Proteomic Tumor Analysis Consortium (CPTAC) databases. Wilcoxon signed-rank test was applied to analyze the difference between tumor group and control group. The potential biological processes or signaling pathways related to SIX1 activity in EC was also assessed. Results: The results showed that SIX1 was overexpressed in EC tissues compared to normal tissues (P=2.029e-15, P=6.25e-6). The SIX1 level was correlated with tumor grade (P=2.91e-4), peritoneal cytology (P=0.005), and the subsequent tumor surgery (P=1.169e-4). SIX1 expression was negatively associated with overall survival rate (P=4.241e-4, P=0.000241) and served as an independent factor that affected EC overall survival rate (P=0.005063), similar to other factors such as age, Figo stage, and tumor (T) stage. SIX1 participates in cancer pathogenesis through gene regulation that involves PI3K/AKT/MTOR signaling, mitotic spindle, G2M checkpoint, E2F targets, NOTCH signaling, glycolysis, cholesterol homeostasis, DNA repair and early estrogen response. Conclusions: Our data demonstrate that SIX1 is overexpressed in EC and associated with adverse clinicopathological outcomes, which can function as an independent factor for EC prognosis.
Keywords: Endometrial cancer, SIX1, clinicopathological factors, prognosis
Introduction
Endometrial carcinoma (EC) represents a type of common malignant disease in women globally [1] and the incidence has been increasing in recent years [2], along with a markedly high mortality. There are approximately 20% of patients who eventually succumb to the devastating disease [2,3].
Transcription factor Sine oculis homeobox 1 (SIX1) belongs to SIX family [4]. Aberrant expression of SIX1 plays important roles in both occurrence and development of tumor [5,6]. SIX1 contributes to a cancer-protective factor and modulates the biological activities of tumor cells [7-9]. Also, the overexpression of SIX1 was observed in several kinds of cancers, including endometrial cancer [10], esophageal squamous cell carcinoma [11], acute myeloid leukemia [12], cervical cancer [13], and thyroid carcinoma [14]. It is believed that SIX1 is associated with malignant tumor progression such as metastasis and poor survival [15]. However, whether SIX1 is involved in EC remains unclear. The purpose of our study was to systematically investigate SIX1 expression and determine its prognostic value in EC patients. In addition, we aimed to explore the possible biological pathways or signaling pathways related to SIX1 in the pathogenesis of EC.
Material and methods
Dataset collection
The gene expression profiles (587 cases, Workflow Type: HTSeq-FPKM) and clinical data were downloaded from TCGA Genomic Data Commons data portal.
Validation using the gene expression omnibus (GEO) and clinical proteomic tumor analysis consortium (CPTAC) databases
GSE17025 from the GEO database was used to validate SIX1 expression. The dataset included 91 EC patients at stage I and 12 age-matched normal endometrial samples. CPTAC integrated genomic and proteome data to identify and describe all kinds of proteins from tumor and adjacent normal tissues to identify possible candidates as biomarkers of tumors. In addition, the expressions of SIX1 protein between normal and EC tissues were compared by using UALCAN [16] from the CPTAC database.
Clinicopathological features investigation
All samples from TCGA were sub-grouped as high and low level based on the median SIX1 level.
Gene set enrichment analysis (GSEA)
GSEA [17], is a computationally efficient algorithm designated for gene study. EC samples obtained from TCGA were separated into SIX1 high expression group and SIX1 low expression group as mentioned above followed by statistical analysis of relevant indicators using GSEA v3.0.
Statistical method
Data in this study were presented as mean ± standard deviation. Statistical Product and Service Solutions (SPSS) 19.0 software (SPSS Inc., Chicago, IL, USA) was used for data processing. T test was used for the comparison between two groups, and continuous data from multiple groups were analyzed by using one-way ANOVA, with the Tukey’s post hoc test. Gehan-Breslow-Wilcoxon test was used to determine the survival difference between two groups. Kruskal test and the logistic regression test evaluated the association using R software (V.3.6.2). Clinical factors related to overall survival rate of EC patients were identified by Cox regression assay and Kaplan-Meier method. P-values <0.05 were considered statistically significant.
Results
SIX1 level in EC patients
SIX1 expressions in EC and normal samples were evaluated from TCGA (Tumor =552, Normal =35) and the result showed that SIX1 level was significantly elevated in EC compared to that in normal control (P=2.029e-15) (Figure 1A). Similar statistical difference was also detected in the paired tumor and its adjacent normal tissue (P=1.354e-5) (Figure 1B). The significantly higher SIX1 expression was also assessed in EC tissues obtained from GEO study GSE17025 as presented in Figure 1C (P=6.25e-6). In addition, SIX1 protein expression was also significantly higher in EC tissues than that in normal tissues from CPTAC database as shown in Figure 1D (P=4.033e-5), confirming our findings at the mRNA level.
Figure 1.
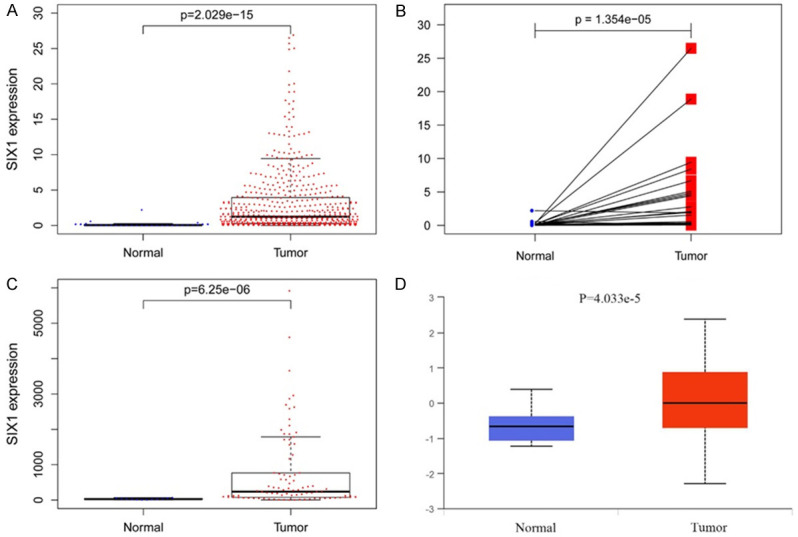
SIX1 is highly expressed in EC tissues. A. SIX1 expression between EC and normal tissues from TCGA database (normal =35, tumor =552). B. SIX1 expression between matched EC and APC tissue from TCGA database (normal =35, tumor =35). C. SIX1 expression between EC and normal tissues from GEO database (normal =12, tumor =91). D. SIX1 expression between EC and normal tissues from CPTAC database (normal =31, tumor =100). SIX1, Sine oculis homeobox 1; EC, endometrial cancer; TCGA, The Cancer Genome Atlas; APC, adjacent para-cancer; GEO, Gene Expression Omnibus; CPTAC, Clinical Proteomic Tumor Analysis Consortium.
The association of SIX1 expression with EC clinical characteristics
The clinical characteristics of EC patients, the clinical data of 548 patients from TCGA were collected and grouped based on Figo stage, TNM stage, tumor grade, histology, menopause status, peritoneal cytology, surgical approach and the characteristics of the surgery (tumor-free). The Kruskal test results revealed that the SIX1 level was associated with tumor grade (P=2.91e-4), peritoneal cytology (P=0.005), and the characteristics of the surgery (P=1.169e-4) (Figure 2). Notably, the increase of SIX1 was related to the elevation of tumor grade. The SIX1 expression of positive ascites cytology was found higher than that of negative ascites cytology. The SIX1 expression of patients with surgical residual was markedly higher than those with free tumor. Meanwhile, our data revealed no significant correlation between the Figo stage, TNM stage and histology, and the level of SIX1 expression (Figure 3). SIX1 level was related to adverse prognostic variables (Table 1). High SIX1 level in EC was statistically associated with tumor grade (P=0.001143322 for G3 vs G1; P=0.006300469 for G3 vs G2), peritoneal cytology (P=0.00216459 for positive vs negative), and the characteristics of the surgery (P=0.001500783 for with tumor vs tumor free) (Table 1). These findings revealed association of high SIX1 level with poor prognosis of EC.
Figure 2.
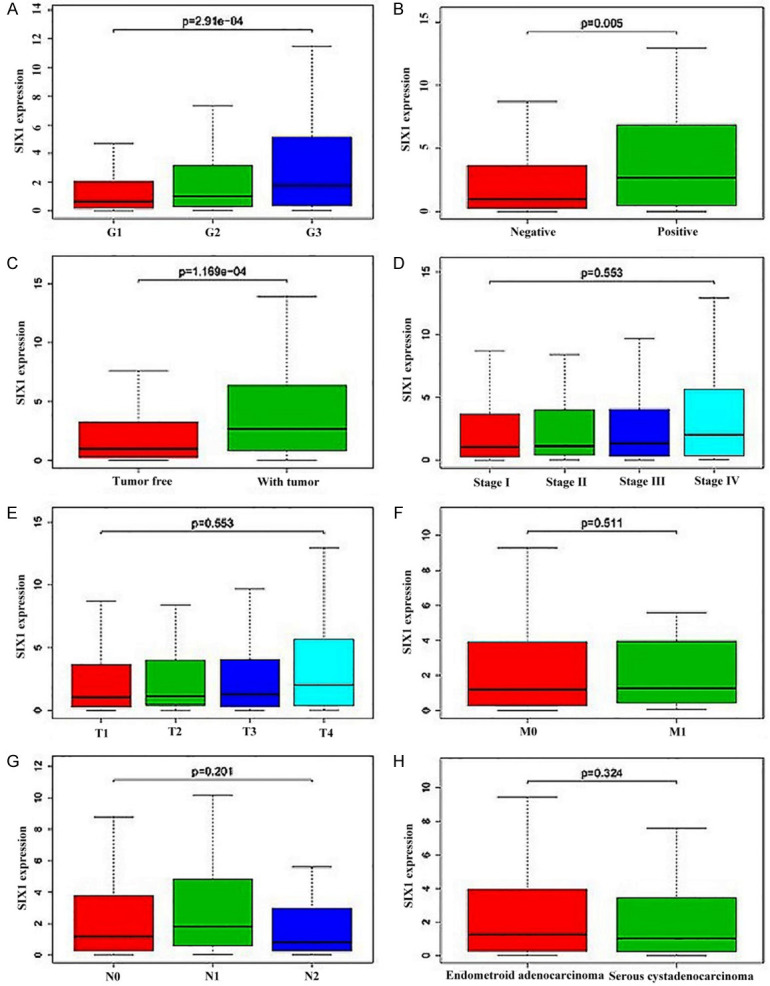
Association of SIX1 expression with clinical variables in EC from TCGA database. A. Tumor grade. B. Ascites cytology status. C. Whether the operation is tumor-free. D. Figo stage. E. T stage. F. Distant metastasis. G. Lymph node metastasis. H. Histology subtype. SIX1, Sine oculis homeobox 1; EC, endometrial cancer; TCGA, The Cancer Genome Atlas.
Figure 3.
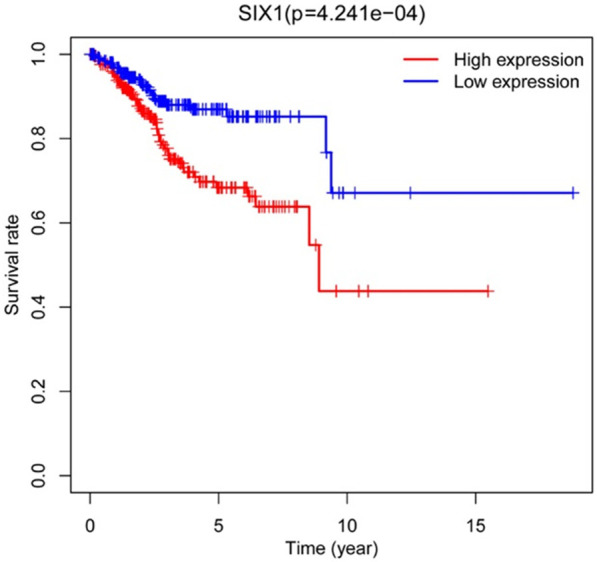
Prognostic significance of SIX1 in EC, assessed by Kaplan-Meier plotter. SIX1, Sine oculis homeobox 1; EC, endometrial cancer.
Table 1.
Association between SIX1 expression and clinical pathological characteristics of the EC patients
| characteristics | Total (N) | Odds ratio for SIX1 | 95% CI | P-value |
|---|---|---|---|---|
| Figo/T stage | ||||
| (II vs I) | 390 | 1.026032 | 0.5673105-1.852573 | 0.9318377 |
| (III vs I) | 463 | 1.102059 | 0.7300798-1.664599 | 0.643452 |
| (IV vs I) | 368 | 2.027439 | 0.9340816-4.66036 | 0.08135141 |
| Grade | ||||
| (G2 vs G1) | 218 | 1.409091 | 0.8182054-2.442822 | 0.2182168 |
| (G3 vs G1) | 423 | 0.2182168 | 1.366031-3.472236 | 0.001143322 |
| (G3 vs G2) | 445 | 1.812057 | 1.186491-2.788371 | 0.006300469 |
| M stage | ||||
| (M1 vs M0) | 543 | 1.079799 | 0.5084594-2.306641 | 0.8406878 |
| N stage | ||||
| (N1+N2 vs N0) | 535 | 1.062827 | 0.666531-1.69686 | 1.69686 |
| Histology | ||||
| (serous vs endometroid) | 537 | 0.8760947 | 0.5933435-1.291668 | 0.5044681 |
| Menopause status | ||||
| (post vs peri) | 461 | 1.135181 | 0.4265455-3.074648 | 0.7978767 |
| (pre vs peri) | 52 | 1.0625 | 0.331042-3.448223 | 0.9184371 |
| (pre vs post) | 479 | 0.9444444 | 0.4709182-1.886675 | 0.8707381 |
| Peritoneal cytology | ||||
| (Positive vs negative) | 407 | 2.535787 | 1.41898-4.691813 | 0.00216459 |
| With tumor vs tumor free | 462 | 2.290503 | 1.385172-3.868607 | 0.001500783 |
| Surgical approach | ||||
| (open vs mini-invisive) | 519 | 0.8044139 | 0.5641882-1.145442 | 0.2279554 |
SIX1, Sine oculis homeobox 1; EC, endometrial cancer; CI, confidence interval; Post, prior bilateral ovariectomy or >12 months since last menstrual period with no prior hysterectomy; Pre, <6 months since last menstrual period and no prior bilateral ovariectomy and not on estrogen replacement; Peri, 6-12 months since last menstrual period; P-values in bold print indicate statistically significant differences.
The correlation of SIX1 level with EC prognosis
The correlation between the prognosis of endometrial cancer patients and the SIX1 expression levels was explored using Kaplan-Meier analysis. As illustrated in Figure 3, the survival rate of SIX1 high level group (5-year survival: 68.4%) was significantly lower than the low level group (85.2%) (P=4.241e-4).
Cox analyses of OS among EC patients
Univariate Cox analysis showed a statistical association of high SIX1 level with poor overall survival rate [hazard ratio (HR): 1.354607; 95% CI: 1.151992-1.592858; P=0.000241]. We also identified some other clinical factors that might be associated with relatively low survival rate, including age, Figo stage, histology, tumor grade and TNM stage (Table 2). Multivariate Cox analysis demonstrated high SIX1 level to be an independent risk factor for overall survival rate among EC patients with a HR of 1.255019 (95% CI: 1.070705-1.47106, P=0.005063) age (HR: 1.023688, 95% CI: 1.000126-1.047805, P=0.04877), Figo and T stage (HR: 1.786826, 95% CI: 1.279625-2.495065, P=0.000656), and tumor grade (HR: 0.023641, 95% CI: 1.075222-2.748215, P=0.023641) (Table 2).
Table 2.
Univariable and multivariable Cox analyses of overall survival of EC patients
| Parameter (N=527) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age | 1.031969 | 1.009505-1.054934 | 0.005073 | 1.023688 | 1.000126-1.047805 | 0.048771 |
| Grade | 2.622629 | 1.704185-4.036053 | 1.17e-05 | 1.718994 | 1.075222-2.748215 | 0.023641 |
| Figo stage | 1.985491 | 1.616599-2.43856 | 6.15e-11 | 1.786826 | 1.279625-2.495065 | 0.000656 |
| T stage | 1.985491 | 1.616599-2.43856 | 6.15e-11 | 1.786826 | 1.279625-2.495065 | 0.000656 |
| M stage | 4.388336 | 2.25142-8.553486 | 1.40e-05 | 0.842492 | 0.335826-2.113576 | 0.714946 |
| N stage | 3.218735 | 1.98479-5.219823 | 2.15e-06 | 1.117473 | 0.602219-2.073573 | 0.724735 |
| Histology | 2.780334 | 1.777736-4.348374 | 7.42e-06 | 1.275626 | 0.771201-2.109982 | 0.343075 |
| SIX1 | 1.354607 | 1.151992-1.592858 | 0.000241 | 1.255019 | 1.070705-1.47106 | 0.005063 |
SIX1, Sine oculis homeobox 1; HR, hazard ratio; CI, confidence interval; P-values in bold print indicate statistically significant differences.
Biological processes or pathways associated with SIX1 expression
To identify the SIX1-related biological processes or pathways involved in the pathogenesis of EC, GSEA assay was conducted and the result showed that SIX1 associated biological processes or signaling pathways include ‘PI3K/AKT/MTOR signaling’, ‘mitotic spindle’, ‘G2M checkpoint’, ‘E2F targets’, ‘NOTCH signaling’, ‘glycolysis’, ‘cholesterol homeostasis’, ‘DNA repair’ and ‘early estrogen response’. These biological processes and signaling pathways were changed with aberrant SIX1 level (Table 3 and Figure 4). The above results suggested the involvement of SIX1 in the development of EC.
Table 3.
Biological processes or signaling pathways associated with SIX1 expression
| Gene set | ES | NES | NOM P-value | FDR q-value |
|---|---|---|---|---|
| PI3k/AKT/MTOR signaling | -0.50862 | -1.85568 | 0.004 | 0.044654 |
| Mitotic spindle | -0.60529 | -2.06665 | 0.004024 | 0.022918 |
| G2M checkpoint | -0.72909 | -2.05715 | 0.008032 | 0.01855 |
| E2F targets | -0.75283 | -1.96647 | 0.014 | 0.03592 |
| NOTCH signaling | -0.55541 | -1.8054 | 0.01417 | 0.055925 |
| Glycolysis | -0.45734 | -1.68532 | 0.024242 | 0.064034 |
| Cholesterol homeostasis | -0.48201 | -1.63903 | 0.027944 | 0.073193 |
| DNA repair | -0.53234 | -1.72811 | 0.041257 | 0.082555 |
| Estrogen response early | -0.41188765 | -1.5964891 | 0.04499 | 0.080924 |
SIX1, Sine oculis homeobox 1; ES, enrichment score; NES, normalized enrichment score; NOM P-val, nominal P value; FDR q-val, false discovery rate q value.
Figure 4.
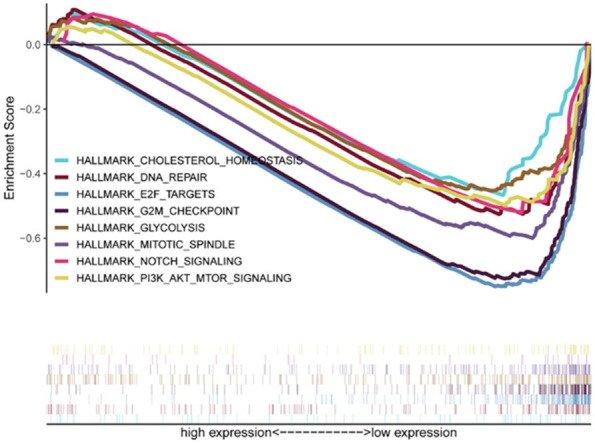
GSEA analysis. The possible biological processes or signaling pathways of SIX1 as determined by GSEA. GSEA, Gene set enrichment analysis; SIX1, Sine oculis homeobox 1.
Discussion
Endometrial cancer represents a common type of gynecological malignancies in developed countries [18]. Furthermore, its incidence has been rapidly increasing in the USA recently [19]. Early diagnosis facilitates the prognosis of the disease [20]. Up to date, there have been no reliable methods for early diagnosis and effective therapy against this disease.
SIX1 belongs to the sine oculis homeobox family [21] and plays a well-established role in tumorigenesis [15]. For example, Six1 overexpression can affect the proliferation of colorectal cancer cells by activating Wnt/β-catenin signaling [22]. Additionally, SIX1 promoted osteosarcoma cell growth by activating PI3K/AKT signaling [23]. However, whether SIX1 is involved in the progression of EC remains unknown. We found significant upregulation of SIX1 in endometrial cancer tissues compared to normal endometrial tissues and the para-cancer tissues, in agreement with the results of GEO study GSE17025. SIX1 level in EC tissues was also significantly elevated. Similar results were also found in breast, cervical and ovarian carcinomas [24-26]. SIX1 was also upregulated in pancreatic ductal adenocarcinoma samples [27].
Further, we demonstrated that SIX1 level was significantly associated with tumor grade, peritoneal cytology status and the characteristics of EC surgery (tumor-free). The present results indicated that SIX1 expression in EC was implicated in tumor progression. The aberrant elevation of SIX1 in glioma tissues was found along with high malignancy grades, which was in line with our results [28]. Our result unraveled an association of high SIX1 level with EC poor outcomes which was in accordance with previous studies in osteosarcoma [29], esophageal cancer [30], hepatocellular carcinoma [31], prostate cancer [32] and colorectal cancer [33]. All these studies demonstrated that SIX1 overexpression was a prognostic factor for poor survival. Moreover, it is noted that SIX1 knockdown could suppress DDP resistance in non-small-cell lung cancer cells (NSCLSs) [34] and enhance paclitaxel sensitivity of hepatocellular carcinoma cells [35]. Therefore, it is proposed that SIX1 may also be a potential target for treating EC.
SIX1 contributes to the regulation of cancer cells proliferation [36]. Additionally, by regulating ERK, SIX1 regulates tumor cell invasion [37]. SIX1 can influence tumor microenvironment, as well as promote tumor development and progression [9]. Upregulated SIX1 can also promote HCC cell growth and invasion by regulating MMP-9 [8]. We performed GSEA using TCGA data to further evaluate the roles of SIX1 in EC, and as a result, SIX1 may participate in regulating the proliferation of tumor cells such as mitotic spindle and G2M checkpoint. Moreover, our data suggested that SIX1 is closely related to multiple signaling pathways such as PI3K/AKT/MTOR signaling, NOTCH signaling, E2F targets, DNA repair and early estrogen response. The biological processes related to glycolysis and cholesterol homeostasis can affect the material metabolism of tumor cells and promote tumor cell growth. While PI3K/AKT/MTOR signaling was reported as a key factor in EC development [38], AKT inhibitor significantly decreased SIX1’s effect on cell growth rate in endometrial cancer cells [10]. Abnormal endometrial epithelial cells with high expression of SIX1 can be found in the newborn mice model of endometrial cancer induced by synthetic estrogen [39], indicating that PI3K/AKT/MTOR signaling and early estrogen response are closely related to EC. However, further experiments are required to confirm our findings in the future. The limitation in the study still exists that our preliminary finding needs further validation in future clinical tests and the role of SIX1, along with the potential mechanisms, requires exploration by in vivo assays.
In conclusion, our data demonstrate that high expression of SIX1 functions as an independent predictor of poor prognosis in endometrial cancer, which provides insights for the diagnosis and treatment against EC in the future.
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Vogel RI, Pulver T, Heilmann W, Mooneyham A, Mullany S, Zhao X, Shahi M, Richter J, Klein M, Chen L, Ding R, Konecny G, Kommoss S, Winterhoff B, Ghebre R, Bazzaro M. USP14 is a predictor of recurrence in endometrial cancer and a molecular target for endometrial cancer treatment. Oncotarget. 2016;7:30962–30976. doi: 10.18632/oncotarget.8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawakami K, Sato S, Ozaki H, Ikeda K. Six family genes--structure and function as transcription factors and their roles in development. Bioessays. 2000;22:616–26. doi: 10.1002/1521-1878(200007)22:7<616::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Towers CG, Guarnieri AL, Micalizzi DS, Harrell JC, Gillen AE, Kim J, Wang CA, Oliphant MUJ, Drasin DJ, Guney MA, Kabos P, Sartorius CA, Tan AC, Perou CM, Espinosa JM, Ford HL. The Six1 oncoprotein downregulates p53 via concomitant regulation of RPL26 and microRNA-27a-3p. Nat Commun. 2015;6:10077. doi: 10.1038/ncomms10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Liang Y, Kang L, Liu Y, Gao S, Chen S, Li Y, You W, Dong Q, Hong T, Yan Z, Jin S, Wang T, Zhao W, Mai H, Huang J, Han X, Ji Q, Song Q, Yang C, Zhao S, Xu X, Ye Q. Transcriptional regulation of the warburg effect in cancer by SIX1. Cancer Cell. 2018;33:368–385. e367. doi: 10.1016/j.ccell.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Z, Rong Z, Luo Z, Yu Z, Zhang J, Qiu Z, Huang C. Circular RNA circNHSL1 promotes gastric cancer progression through the miR-1306-3p/SIX1/vimentin axis. Mol Cancer. 2019;18:126. doi: 10.1186/s12943-019-1054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Wang S, Liu Z, Yang L, Liu J, Xiu M. Increased Six1 expression in macrophages promotes hepatocellular carcinoma growth and invasion by regulating MMP-9. J Cell Mol Med. 2019;23:4523–4533. doi: 10.1111/jcmm.14342. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Xu H, Zhang Y, Pena MM, Pirisi L, Creek KE. Six1 promotes colorectal cancer growth and metastasis by stimulating angiogenesis and recruiting tumor-associated macrophages. Carcinogenesis. 2017;38:281–292. doi: 10.1093/carcin/bgw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xin X, Li Y, Yang X. SIX1 is overexpressed in endometrial carcinoma and promotes the malignant behavior of cancer cells through ERK and AKT signaling. Oncol Lett. 2016;12:3435–3440. doi: 10.3892/ol.2016.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Z, Li G, Tang L, Li Y. SIX1 overexpression predicts poor prognosis and induces radioresistance through AKT signaling in esophageal squamous cell carcinoma. Onco Targets Ther. 2017;10:1071–1079. doi: 10.2147/OTT.S125330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu Y, Chen Y, Li M, Shi D, Wang B, Lian Y, Cheng X, Wang X, Xu M, Cheng T, Shi J, Yuan W. Six1 regulates leukemia stem cell maintenance in acute myeloid leukemia. Cancer Sci. 2019;110:2200–2210. doi: 10.1111/cas.14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li YM, Li XJ, Yang HL, Zhang YB, Li JC. MicroRNA-23b suppresses cervical cancer biological progression by directly targeting six1 and affecting epithelial-to-mesenchymal transition and AKT/mTOR signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:4688–4697. doi: 10.26355/eurrev_201906_18050. [DOI] [PubMed] [Google Scholar]
- 14.Kong D, Li A, Liu Y, Cui Q, Wang K, Zhang D, Tang J, Du Y, Liu Z, Wu G, Wu K. SIX1 activates STAT3 signaling to promote the proliferation of thyroid carcinoma via EYA1. Front Oncol. 2019;9:1450. doi: 10.3389/fonc.2019.01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu W, Ren Z, Li P, Yu D, Chen J, Huang R, Liu H. Six1: a critical transcription factor in tumorigenesis. Int J Cancer. 2015;136:1245–1253. doi: 10.1002/ijc.28755. [DOI] [PubMed] [Google Scholar]
- 16.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, Sessa C ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978-2013. J Natl Cancer Inst. 2018;110:354–361. doi: 10.1093/jnci/djx214. [DOI] [PubMed] [Google Scholar]
- 20.Makker V, Green AK, Wenham RM, Mutch D, Davidson B, Miller DS. New therapies for advanced, recurrent, and metastatic endometrial cancers. Gynecol Oncol Res Pract. 2017;4:19. doi: 10.1186/s40661-017-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar JP. The sine oculis homeobox (SIX) family of transcription factors as regulators of development and disease. Cell Mol Life Sci. 2009;66:565–583. doi: 10.1007/s00018-008-8335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song W, Ma J, Lei B, Yuan X, Cheng B, Yang H, Wang M, Feng Z, Wang L. Sine oculis homeobox 1 promotes proliferation and migration of human colorectal cancer cells through activation of Wnt/beta-catenin signaling. Cancer Sci. 2019;110:608–616. doi: 10.1111/cas.13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu C, Zhang B, Li YL, Yu XR. SIX1 reduces the expression of PTEN via activating PI3K/AKT signal to promote cell proliferation and tumorigenesis in osteosarcoma. Biomed Pharmacother. 2018;105:10–17. doi: 10.1016/j.biopha.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Radisky DC. Defining a role for the homeoprotein Six1 in EMT and mammary tumorigenesis. J Clin Invest. 2009;119:2528–2531. doi: 10.1172/JCI40555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng XH, Liang PH, Guo JX, Zheng YR, Han J, Yu LL, Zhou YG, Li L. Expression and clinical implications of homeobox gene Six1 in cervical cancer cell lines and cervical epithelial tissues. Int J Gynecol Cancer. 2010;20:1587–1592. [PubMed] [Google Scholar]
- 26.Behbakht K, Qamar L, Aldridge CS, Coletta RD, Davidson SA, Thorburn A, Ford HL. Six1 overexpression in ovarian carcinoma causes resistance to TRAIL-mediated apoptosis and is associated with poor survival. Cancer Res. 2007;67:3036–3042. doi: 10.1158/0008-5472.CAN-06-3755. [DOI] [PubMed] [Google Scholar]
- 27.Jin A, Xu Y, Liu S, Jin T, Li Z, Jin H, Lin L, Lin Z. Sineoculis homeobox homolog 1 protein overexpression as an independent biomarker for pancreatic ductal adenocarcinoma. Exp Mol Pathol. 2014;96:4. doi: 10.1016/j.yexmp.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Xu R. Six1 expression is associated with a poor prognosis in patients with glioma. Oncol Lett. 2017;13:1293–1298. doi: 10.3892/ol.2017.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao L, Liu J, Zhao D. Increased Six1 expression is associated with poor prognosis in patients with osteosarcoma. Oncol Lett. 2017;13:2891–2896. doi: 10.3892/ol.2017.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimura T, Tamaoki M, Komatsuzaki R, Oue N, Taniguchi H, Komatsu M, Aoyagi K, Minashi K, Chiwaki F, Shinohara H, Tachimori Y, Yasui W, Muto M, Yoshida T, Sakai Y, Sasaki H. SIX1 maintains tumor basal cells via transforming growth factor-beta pathway and associates with poor prognosis in esophageal cancer. Cancer Sci. 2017;108:216–225. doi: 10.1111/cas.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen K, Wei H, Pan J, Chen Z, Pan D, Gao T, Huang J, Huang M, Ou M, Zhong W. Six1 is negatively correlated with poor prognosis and reduces 5-fluorouracil sensitivity via attenuating the stemness of hepatocellular carcinoma cells. Eur J Pharmacol. 2019;861:172599. doi: 10.1016/j.ejphar.2019.172599. [DOI] [PubMed] [Google Scholar]
- 32.Zeng J, Shi R, Cai CX, Liu XR, Song YB, Wei M, Ma WL. Increased expression of Six1 correlates with progression and prognosis of prostate cancer. Cancer Cell Int. 2015;15:63. doi: 10.1186/s12935-015-0215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahlert C, Lerbs T, Pecqueux M, Herpel E, Hoffmeister M, Jansen L, Brenner H, Chang-Claude J, Blaker H, Kloor M, Roth W, Pilarsky C, Rahbari NN, Scholch S, Bork U, Reissfelder C, Weitz J, Aust D, Koch M. Overexpression of SIX1 is an independent prognostic marker in stage I-III colorectal cancer. Int J Cancer. 2015;137:2104–2113. doi: 10.1002/ijc.29596. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Zhou X, Chen Y, Huang Y, He J, Luo H. miR-186-5p targeting SIX1 inhibits cisplatin resistance in non-small-cell lung cancer cells (NSCLCs) Neoplasma. 2020;67:147–157. doi: 10.4149/neo_2019_190511N420. [DOI] [PubMed] [Google Scholar]
- 35.Li B, Zhao S, Geng R, Huo Z, Zhang H. The sineoculis homeobox homolog 1 (SIX1) gene regulates paclitaxel resistance by affecting reactive oxygen species and autophagy in human hepatocellular carcinoma cell line HepG2. Med Sci Monit. 2018;24:2271–2279. doi: 10.12659/MSM.906361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kingsbury TJ, Kim M, Civin CI. Regulation of cancer stem cell properties by SIX1, a member of the PAX-SIX-EYA-DACH network. Adv Cancer Res. 2019;141:1–42. doi: 10.1016/bs.acr.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Xie Y, Jin P, Sun X, Jiao T, Zhang Y, Li Y, Sun M. SIX1 is upregulated in gastric cancer and regulates proliferation and invasion by targeting the ERK pathway and promoting epithelial-mesenchymal transition. Cell Biochem Funct. 2018;36:6. doi: 10.1002/cbf.3361. [DOI] [PubMed] [Google Scholar]
- 38.Barra F, Evangelisti G, Ferro Desideri L, Di Domenico S, Ferraioli D, Vellone VG, De Cian F, Ferrero S. Investigational PI3K/AKT/mTOR inhibitors in development for endometrial cancer. Expert Opin Investig Drugs. 2019;28:131–142. doi: 10.1080/13543784.2018.1558202. [DOI] [PubMed] [Google Scholar]
- 39.Suen AA, Jefferson WN, Williams CJ, Wood CE. Differentiation patterns of uterine carcinomas and precursor lesions induced by neonatal estrogen exposure in mice. Toxicol Pathol. 2018;46:574–596. doi: 10.1177/0192623318779326. [DOI] [PMC free article] [PubMed] [Google Scholar]


