Abstract
Objective: To analyze the efficacy of sequential treatment with high-flow nasal cannula (HFNC) in chronic obstructive pulmonary disease (COPD) concomitant with respiratory failure. Methods: A total of 100 COPD patients concomitant with respiratory failure requiring invasive mechanical ventilation from June 2019 to May 2020 in our hospital were enrolled and then divided into two groups according to the random number table, with 50 in each group. Pulmonary infection control window (PIC) was used as a switching point for sequential ventilation. The control group (CNG) received non-invasive positive pressure ventilation (NIPPV), while the study group (SG) underwent HFNC. The efficacy, complications and 48 h reintubation rate of the two groups were statistically analyzed. The respiratory parameters, diaphragmatic parameters, diaphragmatic excursion during quiet breathing (DEq), diaphragmatic rapid shallow breathing index (D-RSBI), COPD score (CAT), 6-min walk test (6 MWT) score (Borg), General Comfort Questionnaire (GCQ), sputum viscosity, and serum factors were observed before intubation and after 48 hours of intubation. Results: The overall response rate (94.00%) in SG was higher than that in CNG (80.00%) (P < 0.05); SG had lower RR, PaCO2 and D-RSBI at 48 hours after extubation and higher PaO2/FiO2 and DEd than CNG (P < 0.05); SG exhibited lower CAT and Borg at 48 hours after extubation and higher GCQ score than CNG (P < 0.05); SG had lower sputum viscosity at 48 hours after extubation than CNG (P < 0.05); SG showed lower ET-1, NLR and NT-proBNP levels at 48 hours after extubation than CNG (P < 0.05). Conclusion: HFNC sequential therapy is effective and safe in the treatment of COPD concomitantly with respiratory failure. It can improve respiratory function and diaphragmatic function, reduce dyspnea and fatigue, reduce sputum viscosity, regulate serum factors, and make patients enjoy higher comfort.
Keywords: Chronic obstructive pulmonary disease, respiratory failure, high-flow nasal cannula, sequential mechanical ventilation
Introduction
Chronic obstructive pulmonary disease (COPD) is a type of chronic disease characterized by persistent airflow limitation and small airway damage, and it also has the features of high morbidity, high mortality and long duration of disease [1,2]. The pathophysiological changes of this disease include respiratory mucosal congestion and edema, tracheal inflammation and small airway spasm. Pulmonary function tests suggest dual disorders of ventilation function and pulmonary ventilation function, and patients often experience symptoms such as respiratory fatigue, increased resistance and poor breathing [3,4]. Affected by factors such as airway obstruction, respiratory tract infection, and respiratory muscle fatigue, COPD patients have gradually aggravated conditions, which can induce various types of respiratory syndromes, and some patients will concomitantly experience respiratory failure and pulmonary hypertension, of which respiratory failure is the main cause of death in COPD patients [5]. The principle of clinical treatment for COPD concurrent with respiratory failure is to reduce complications, delay disease progression and reduce clinical symptoms, while the key points in treatment lie in ensuring the body’s oxygen supply, improving ventilation function, maintaining acid-base and electrolyte balance, and reducing hypercapnia [6].
Invasive positive pressure ventilation (IPPV) is a common treatment regimen for COPD concurrent with respiratory failure, which can effectively drain sputum and control infection, but it is easy to induce complications such as respiratory muscle fatigue and ventilator-associated pneumonia, affecting the therapeutic effect [7]. In recent years, with the increasing update of mechanical ventilation technology, invasive-non-invasive sequential ventilation therapy has been widely used in clinical setting, but positive pressure ventilation (NIPPV) has poor tolerance and many contraindications, and it affects the removal of airway secretions, so its further application is limited. High-flow nasal cannula (HFNC), as a novel type of non-invasive ventilation oxygen therapy, can accurately control the volume fraction of oxygen inhalation and play a role in promoting the excretion of nitrogen dioxide, thus antagonizing endogenous positive end-expiratory pressure and supporting airway opening, without affecting airway secretion and drainage, with a higher degree of comfort [8]. The Invasive-Noninvasive Sequential Mechanical Ventilation Multicenter Study Collaborative Group has proposed that sequential ventilation with the pulmonary infection control window (PIC) as a switching point can reduce the occurrence of complications and shorten the length of hospital stay and invasive ventilation duration [9]. In view of this, we used PIC as a switching point and NIPPV as a control to assess the feasibility and safety of HFNC in treating COPD concurrent with respiratory failure.
Material and methods
Clinical data
A total of 100 COPD patients with respiratory failure requiring invasive mechanical ventilation from June 2019 to May 2020 in Ganzhou People’s Hospital and the First Affiliated Hospital of Gannan Medical University were enrolled and then divided into two groups according to the random number table. There were 50 patients in the control group (CNG), with 30 males and 20 females, aged 52-78 years, with a mean age of 68.39 ± 5.22 years; duration of COPD ranged from 5 to 15 years, with a mean duration of 7.65 ± 2.14 years; smoking history > 10 years in 29 cases. The study group (SG) consisted of 50 patients, 32 males and 18 females, aged 51-80 years, with a mean age of 69.22 ± 6.13 years; duration of COPD of 3-15 years, with a mean duration of 7.11 ± 2.09 years; and smoking history of > 10 years in 25 cases. The above data of SG were well-balanced compared with those of CNG (P > 0.05), with comparability.
Inclusion criteria
Inclusion criteria: Having met the diagnostic criteria for COPD in the Guidelines for Primary Diagnosis and Treatment of Chronic Obstructive Pulmonary Disease (Practice Edition 2018) [10], and having been diagnosed with type II respiratory failure by blood gas analysis and other examinations; the circulatory and respiratory conditions were relatively stable; there was no mental disorder; this study was approved by The First Affiliated Hospital of Gannan Medical University and the patients voluntarily signed the informed consent form. Exclusion criteria: Contraindications to mechanical ventilation or severe nasofacial trauma; having received glucocorticoid therapy in recent two weeks; concurrent with severe heart, liver, brain, kidney and other organ dysfunction; concurrent with active pulmonary tuberculosis, pneumothorax and other lung lesions; coagulopathy, systemic infectious diseases; thoracic deformity, pleural effusion, pleural disease; immunosuppression; severe respiratory failure [partial pressure of oxygen (PaO2) < 40 mmHg, partial pressure of carbon dioxide (PaCO2) > 65 mmHg].
Method
All patients were intubated on the machine, followed by the adoption of the pressure support ventilation (PSV) + synchronized intermittent mandatory ventilation (SIMV) pattern, and the ventilator parameters were adjusted according to the patient’s blood gas analysis as well as ventilation status and tolerance. At the same time of mechanical ventilation, conventional treatment was implemented, including drainage of airway secretions, expectoration induction, empirical anti-infection, and relief of bronchospasm. Under the condition of using PIC as a switching point sequential ventilation therapy, HFNC was performed in the SG: after extubation, the patients were given oxygen inhalation using Mindray EV300 nasal catheter oxygen therapy system, and the appropriate nasal plug model was selected according to the size of the patient’s nostrils, generally < 50% of the nostril diameter. Initial parameter settings: The oxygen concentration (FiO2) was 30-50%, the flow rate was 50 L/min, the temperature was 37°C, and the target value of saturation (SpO2) was maintained at 88-92%; if FiO2 ≤ 30%, the flow rate ≤ 30 L/min, the arterial partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) ≥ 300 was maintained for 12 h, and SpO2 was still within the target value, then it was changed to common nasal catheter oxygen inhalation. The patients in the CNG received NIPPV: assisted ventilation with Philips V60 was given after extubation, with the following initial parameter settings: FiO2 was 30-50%, expiratory pressure was 4-5 mmH2O, inspiratory pressure was 10-12 mmH2O, inspiratory/expiratory ratio was 1:1.5-2.0, and the target value of SpO2 was maintained at 88-92%; if the inspiratory pressure ≤ 10 mmH2O, FiO2 ≤ 30%, PaO2/FiO2 ≥ 300 was maintained for 12 h, and SpO2 was still within the target value, it was changed to common nasal catheter oxygen inhalation.
Evaluation parameters
(1) Efficacy. Marked efficacy: after treatment, blood gas analysis parameters returned to normal, and dyspnea, lung wet rales and other clinical symptoms disappeared; Response: after treatment, hypoxia symptoms were relieved, clinical symptoms were improved, lung wet rales were reduced; No response: blood gas test parameters and clinical symptoms were not improved, or the condition was aggravated [11]. Overall response = marked response + response.
(2) Respiratory parameters. Three milliliters of arterial blood was collected from patients before and 48 h after extubation. PaCO2, FiO2, and PaO2 were measured using the American GEM3500 automatic blood gas analyzer, and PaO2/FiO2 was calculated.
(3) Diaphragmatic parameters. Bedside ultrasonography was performed before and 48 h after extubation to measure diaphragmatic excursion during deep breathing (DEd), diaphragmatic excursion during quiet breathing (DEq), and RR. Diaphragmatic rapid shallow breathing index (D-RSBI) = RR/DEq.
(4) COPD score (CAT), 6-min walk test (6 MWT) score (Borg) and subjective comfort score (GCQ). There are a total of 8 items in the CAT scale, including energy, cough, exercise endurance, mood, chest tightness, and daily exercise, with a total of 40 points, using the 1-to-5 rating scale, with a score of < 10 indicating mild disease, 10 < score ≤ 20 indicating moderate disease, 20 < score ≤ 30 indicating severe disease, and > 30 indicating very severe disease. At the end of the 6 MWT, the patients were assessed using the Borg dyspnea and fatigue score out of a scale from 0 to 10, with 10 indicating the most intense respiratory distress and fatigue, and 0 indicating no respiratory distress and fatigue. The patient’s overall comfort was assessed according to the Comfort Status Scale (GCQ), which included 4 dimensions (29 items): psycho-mental, physical, environmental, and sociocultural, using the Likert 1-6 rating scale. A higher score means a higher degree of comfort. The assessment time of the above parameters was before extubation and 48 h after extubation.
(5) Sputum viscosity. After sputum suctioning, no sputum was retained on the inner wall of glass joint, and the degree of sputum such as foamy or rice soup-like was grade I; after sputum suctioning, a small amount of sputum was observed on the inner wall of glass joint, which was not easy to be washed away by water, and the appearance observation showed a viscosity of grade II; after sputum suctioning, a large amount of sputum was observed on the inner wall of glass joint, which was not easy to be washed away by water, and yellow phlegm, the viscosity was determined as grade III.
(6) Serum parameters. Peripheral blood lymphocyte count and neutrophils were measured by BC-5000 automatic hematology analyzer produced by Shenzhen Mindray Bio-Medical Electronics Co., Ltd., followed by the calculation of NLR values. Endothelin (ET) -1 and plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were measured by electrochemiluminescence sandwich immunoassay method.
(7) Complications and reintubation rate. A statistical analysis was performed on whether the patient had discomfort symptoms such as pneumothorax, facial trauma, and nasal injury, with the recording of the 48 h reintubation rate. Reintubation criteria: ① Mental status changes: Somnolence, unconsciousness; ② Refractory hypoxemia, i.e.PaO2/FiO2 ≤ 100; ③ Gradually increased partial pressure of carbon dioxide (PaCO2), pH ≤ 7.2; ④ Hemodynamic instability or sudden cardiac arrest; ⑤ Severe dyspnea [respiratory rate (RR) > 35 beats/min] or apnea or paradoxical respiration. If one of the above conditions occurred or persisted, reintubation was performed.
Statistical analysis
SPSS 23.0 statistical analysis software was used. GraphPad Prism 8.2 software was applied to draw the statistical charts. Measurement data were tested for normal distribution. Measurement data conforming to normal distribution were expressed as mean ± standard deviation (x̅ ± sd). Independent sample t-test was used for intergroup comparison. Paired sample t-test was used for intergroup comparison. Enumeration data were expressed as % using the χ2 test. Rank sum test was used for ranked data. P < 0.05 was considered statistically significant.
Results
Efficacy
The overall response rate (94.00%) in SG was higher than that (80.00%) in CNG (P < 0.05). It could be concluded that HFNC sequential treatment had higher efficacy in COPD concurrent with respiratory failure and could promote the relief of clinical symptoms. See Table 1.
Table 1.
Comparison of efficacy between the two groups [n (%)]
| Group | Number of subjects | Marked efficacy | Response | No response | Overall response |
|---|---|---|---|---|---|
| Control group | 50 | 11 (22.00) | 29 (58.00) | 10 (20.00) | 40 (80.00) |
| Study group | 50 | 20 (40.00) | 27 (54.00) | 3 (6.00) | 47 (94.00)# |
Note: Compared with control group;
P < 0.05.
Respiratory parameters
The differences in RR, PaCO2 and PaO2/FiO2 before extubation between the SG and the CNG had no statistical significance (P > 0.05). At 48 h after extubation, RR and PaCO2 were decreased in both groups, while PaO2/FiO2 was increased in both groups (P < 0.05). The RR and PaCO2 at 48 h after extubation in the SG were lower than those in the CNG, and PaO2/FiO2 was higher than that in the CNG (P < 0.05), indicating that compared with NIPPV treatment, HFNC sequential treatment could improve the respiratory function and blood gas analysis parameters in COPD patients concomitant with respiratory failure. See Figure 1.
Figure 1.
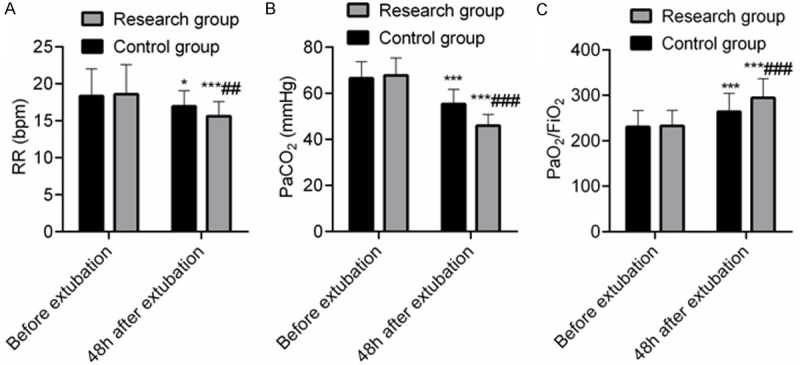
Comparison of respiratory parameters between the two groups (x̅ ± sd). Note: A: RR; B: PaCO2; C: PaO2/FiO2. Compared with concurrent control group, ##P < 0.01, ###P < 0.001; compared with that before extubation, *P < 0.05, ***P < 0.001.
Diaphragmatic parameters
There was no significant difference in DEd, DEq and D-RSBI before extubation between the SG and the CNG (P > 0.05); the D-RSBI at 48 h after extubation in the SG was lower than that in the CNG, and the DEd was higher than that in the CNG (P < 0.05), indicating that compared with NIPPV treatment, HFNC sequential treatment could improve diaphragmatic function in COPD patients concomitant with respiratory failure. See Figure 2.
Figure 2.
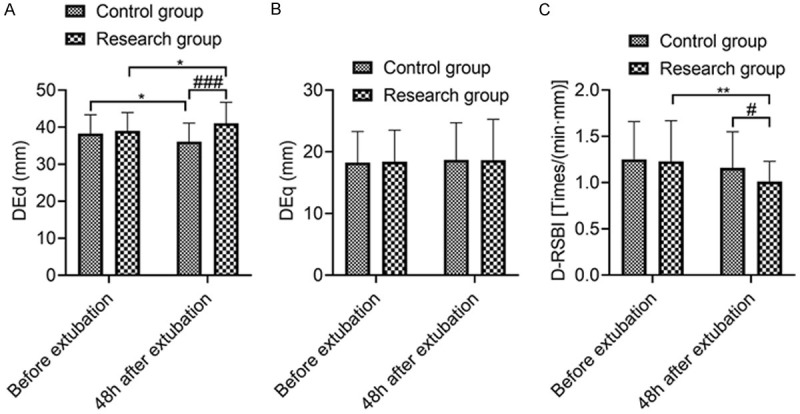
Comparison of diaphragmatic parameters between the two groups. Note: A: DEd; B: DEq; C: D-RSBI. Compared with concurrent control group, #P < 0.05, ###P < 0.001; compared with that before extubation, *P < 0.05, **P < 0.01.
CAT, 6 MWT and GCQ scores
There was no significant difference in CAT, Borg, and GCQ score before extubation between the SG and the CNG (P > 0.05). At 48 h after extubation, CAT and Borg were decreased, while GCQ score was increased in both groups (P < 0.05). The CAT and Borg at 48 h after extubation in the SG were lower than those in the CNG, and GCQ score was higher than that in the CNG (P < 0.05), indicating that compared with NIPPV treatment, HFNC sequential treatment could improve the subjective comfort of COPD patients concomitant with respiratory failure and relieve the condition and fatigue. See Figure 3.
Figure 3.
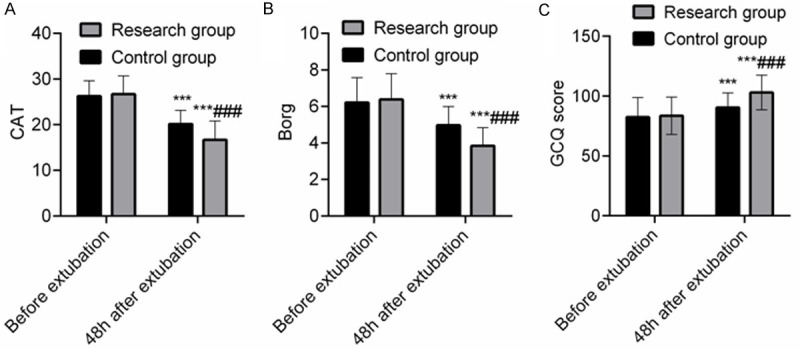
Comparison of CAT, 6 MWT and GCQ scores between the two groups (points). Note: A: CAT; B: Borg; C: GCQ score. Compared with concurrent control group, ###P < 0.001; compared with that before extubation in this group, ***P < 0.001.
Sputum viscosity
There was no significant difference in sputum viscosity before extubation between the SG and the CNG (P > 0.05). At 48 h after extubation, the sputum viscosity was reduced in both groups (P < 0.05). The sputum viscosity at 48 h after extubation in the SG was lower than that in the CNG (P < 0.05), indicating that compared with NIPPV treatment, sequential treatment with HFNC could promote the reduction of sputum viscosity in COPD patients concomitant with respiratory failure. See Table 2.
Table 2.
Comparison of sputum viscosity between the two groups [n (%)]
| Group | Number of subjects | Time | Grade I | Grade II | Grade III |
|---|---|---|---|---|---|
| Control group | 50 | Before extubation | 0 | 15 (30.00) | 35 (70.00) |
| 48 hours after extubation | 9 (18.00) | 22 (44.00) | 19 (38.00)*** | ||
| Study group | 50 | Before extubation | 0 | 17 (34.00) | 33 (66.00) |
| 48 hours after extubation | 16 (32.00) | 24 (48.00) | 10 (20.00)***,### |
Note: Compared with concurrent control group;
P < 0.001.
Compared with that before extubation in this group;
P < 0.001.
ET-1, NLR, and NT-proBNP
There was no significant difference in the levels of ET-1, NLR and NT-proBNP before extubation between the SG and the CNG (P > 0.05). At 48 h after extubation, the levels of ET-1, NLR and NT-proBNP were all decreased in both groups (P < 0.05). The levels of ET-1, NLR and NT-proBNP 48 h after extubation in SG were lower than those in CNG (P < 0.05), indicating that compared with NIPPV treatment, sequential treatment with HFNC in COPD patients concomitant with respiratory failure could promote the reduction of ET-1, NLR and NT-proBNP levels. See Figure 4.
Figure 4.
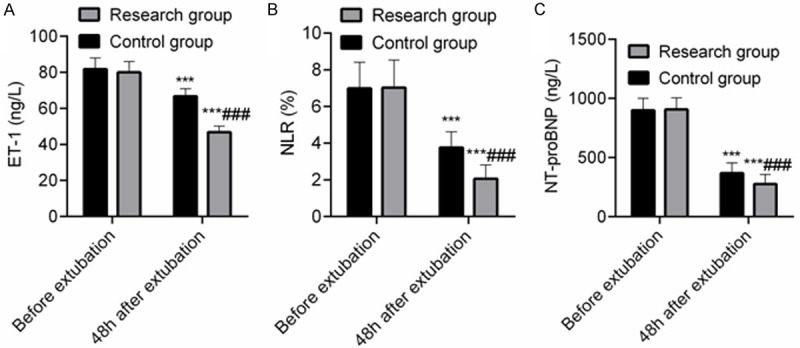
Comparison of ET-1, NLR, and NT-proBNP levels between the two groups. Note: A: ET-1; B: NLR; C: NT-proBNP. Compared with concurrent control group, ###P < 0.001; compared with that before extubation in this group, ***P < 0.001.
Complications and reintubation rate within 48 h
The overall incidence rate of complications and reintubation rate within 48 h in SG (10.00% and 2.00%, respectively) were slightly lower than those in CNG (16.00% and 6.00%, respectively), but the differences had no statistical significance (P > 0.05), indicating that compared with NIPPV treatment, HFNC sequential treatment had a certain safety profile, and the reintubation rate within 48 h was low. See Table 3.
Table 3.
Comparison of complications and reintubation rate within 48 hours between the two groups [n (%)]
| Group | Number of subjects | Pneumothorax | Facial trauma | Nasal injury | Total | Reintubation rate within 48 hours |
|---|---|---|---|---|---|---|
| Control group | 50 | 2 (4.00) | 3 (6.00) | 3 (6.00) | 8 (16.00) | 3 (6.00) |
| Study group | 50 | 0 | 2 (4.00) | 3 (6.00) | 5 (10.00) | 1 (2.00) |
Discussion
Airway trapping and airway remodeling caused by repeated internal exudation and chronic inflammation of small airways in COPD patients result in hyperinflation of lung tissue and limitation of exhaled airflow; at the same time, excessive residual gas at the end of expiration in alveoli can induce endogenous positive end-expiratory pressure, increase respiratory muscle work and expiratory load, and then cause respiratory failure [12,13]. HFNC can deliver high-flow air-oxygen mixed gas at a certain oxygen volume fraction to patients through temperature-humidification via a nasal plug catheter without sealing, which can provide gas with a relative humidity of 100% at 37°C and a flow rate of up to 60 L/min, and the evidence of its superiority is gradually increasing [14,15]. In this study, we intended to use PIC as a switching point to perform sequential ventilation therapy by taking the advantages of accurate regulation of FiO2 concentration with HFNC as well as warming and humidification, so as to provide a more scientific and safe mode of respiratory support for COPD patients concomitant with respiratory failure.
In this study, compared with the CNG, the SG had higher overall response rate, lower CAT, Borg score and sputum viscosity, ET-1, NLR, NT-proBNP levels, and there was no significant difference in the incidence rate of complications between the two groups, indicating that the sequential treatment of HFNC in COPD concurrent with respiratory failure could improve the efficacy, reduce dyspnea and fatigue, reduce sputum viscosity, regulate serum factors, reduce inflammatory factor levels, and inhibit disease progression, with a higher safety profile. Exudation and migration of neutrophils in the trachea of COPD patients can aggravate the inflammatory response, destroy the ciliary delivery system of columnar epithelial cells, increase airway mucosal secretion, increase the difficulty of sputum excretion, and then aggravate the degree of respiratory failure [16]. The HFNC can deliver warmed and humidified gas, avoid the loss of water and heat in the respiratory mucosa, maintain the good function of the mucociliary transport system, reduce the degree of respiratory mucosal damage, and then help to maintain airway patency and improve respiratory function. The gas temperature and humidification effect can enhance the mucociliary clearance ability and mucociliary system function, promote sputum excretion and secretion drainage, weaken upper respiratory tract airway resistance, and then reduce respiratory function and body metabolism, and improve lung compliance and air conductivity [17,18]. The pathological changes of COPD concurrent with respiratory failure can lead to fixed airway obstruction and airway stenosis, while mucosal cell fibrosis and increased endotracheal mucus cause increased exhaled airflow resistance, increased lung residual volume, and reduced respiratory muscle efficiency in patients [19]. The formation of endogenous positive end-expiratory pressure can increase the expiratory load of COPD concurrent with respiratory failure and increase respiratory muscle work, leading to respiratory muscle fatigue. While HFNC can stimulate the respiratory center and the regulation of the imbalance of ventilation/perfusion ratio in the respiratory tract, contributing to gas exchange and improvement of respiratory conditions. In addition, it can also rinse the nasopharyngeal anatomical dead space, reduce the volume of respiratory dead space and airway secretions, and reduce work of breathing; at the same time, HFNC can produce a low level (4-10 cmH2O) of positive end-expiratory pressure by providing a constant flow rate of gas, which can correct hypoxemia, and improve oxygen and capacity as well as oxygen ventilation function. In addition, high-flow gas can meet the needs of patients for inspiratory flow, reduce the respiratory anatomical dead space, reduce inspiratory resistance, and then reduce respiratory muscle fatigue symptoms [20,21].
Respiratory acidosis, hypercapnia and hypoxemia are the main causes of organ dysfunction in COPD patients concomitant with respiratory failure. The increase of PaCO2 level can affect the circulatory system and respiratory system of patients, mainly manifested as increased cardiac load and cardiac output, increased respiratory rate and heart rate; however, when PaCO2 level increases to a certain extent, the above effects change from excitation to inhibition, and in severe cases, it can increase complications such as pulmonary heart disease and pulmonary encephalopathy [22,23]. In this study, the RR, PaCO2, and D-RSBI were lower and the PaO2/FiO2 and DEd were higher in the SG than those in the CNG at 48 h after extubation, indicating that sequential treatment with HFNC could promote the improvement of respiratory function and diaphragmatic function, weaken the excitatory effect, correct hypercapnia and hypoxemia, and reduce the condition in COPD patients concomitant with respiratory failure. In this study, the GCQ score in the SG was higher than that in the CNG, which showed that HFNC sequential treatment could bring high comfort and could be tolerated by patients in COPD concurrent with respiratory failure. Yue et al. [24] also confirmed in a meta-analysis that compared with traditional oxygen therapy, the comfort level and patient acceptance of HFNC were similar to the results of this study. The underlying justification is that treating NIPPV with nasal mask is often affected by factors such as limited access to water and food, respiratory disorders caused by airflow, headband discomfort and tight mask, resulting in low patient tolerance and comfort. HFNC can provide humidifying and warming gas, reduce the discomfort caused by dry and cold gas to a certain extent, reduce the degree of mucosal dryness and airway sensitivity, and reduce complications such as facial trauma. Additionally, patients can wear nasal plug for a long time, with high comfort. The study carried out by Deng et al. [25] found that the reintubation rate of 4.48% in the HFNC group was significantly lower than that of 14.92% in the conventional oxygen inhalation group, but there was no significant difference in the reintubation rate within 48 h between the two groups in this study, which might be related to the small sample size included in the study.
In summary, HFNC sequential therapy is effective and safe in the treatment of COPD concomitant with respiratory failure. It can improve respiratory function and diaphragmatic function, reduce dyspnea and fatigue, reduce sputum viscosity, regulate serum factors, and make patients enjoy higher comfort. However, the sample size of this study was small, and the changes of the parameters were not dynamically analyzed, so future studies are needed to further investigate and confirm them.
Disclosure of conflict of interest
None.
References
- 1.Sehgal IS, Kalpakam H, Dhooria S, Aggarwal AN, Prasad KT, Agarwal R. A randomized controlled trial of noninvasive ventilation with pressure support ventilation and adaptive support ventilation in acute exacerbation of COPD: a feasibility study. Copd. 2019;16:168–173. doi: 10.1080/15412555.2019.1620716. [DOI] [PubMed] [Google Scholar]
- 2.Devi P, Raja R, Kumar R, Shah A, Ansari SI, Kumar B. Invasive versus non-invasive positive pressure ventilation in chronic obstructive pulmonary disease complicated by acute respiratory failure. Cureus. 2019;11:e5418. doi: 10.7759/cureus.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatti H, Ramdass A, Cury JD, Jones LM, Shujaat A, Louis M, Seeram V, Bajwa AA. Operator dependent factors implicated in failure of non-invasive positive pressure ventilation (NIPPV) for respiratory failure. Clin Respir J. 2017;11:901–905. doi: 10.1111/crj.12434. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61:529–541. doi: 10.4187/respcare.04577. [DOI] [PubMed] [Google Scholar]
- 5.Bruni A, Garofalo E, Cammarota G, Murabito P, Astuto M, Navalesi P, Luzza F, Abenavoli L, Longhini F. High flow through nasal cannula in stable and exacerbated chronic obstructive pulmonary disease patients. Rev Recent Clin Trials. 2019;14:247–260. doi: 10.2174/1574887114666190710180540. [DOI] [PubMed] [Google Scholar]
- 6.Cirio S, Piran M, Vitacca M, Piaggi G, Ceriana P, Prazzoli M, Paneroni M, Carlucci A. Effects of heated and humidified high flow gases during high-intensity constant-load exercise on severe COPD patients with ventilatory limitation. Respir Med. 2016;118:128–132. doi: 10.1016/j.rmed.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Sun J, Li Y, Ling B, Zhu Q, Hu Y, Tan D, Geng P, Xu J. High flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: an observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:1229–1237. doi: 10.2147/COPD.S206567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest. 2015;148:253–261. doi: 10.1378/chest.14-2871. [DOI] [PubMed] [Google Scholar]
- 9.Collaborating Research Group for Sequential Invasive to Noninvasive Ventilation. [Application of pulmonary infection control window as switching point for sequential invasive to noninvasive ventilation in treatment of severe respiratory failure of chronic obstructive pulmonary disease: a randomized controlled study] . Zhonghua Jie He He Hu Xi Za Zhi. 2006;29:14–18. [PubMed] [Google Scholar]
- 10.Chinese Medical Association. Guidelines for primary diagnosis and treatment of chronic obstructive pulmonary disease (Practice Edition 2018) Chinese Journal of General Practitioners. 2018;17:871–877. [Google Scholar]
- 11.Ishfaq N, Gul N, Zaka N. Outcome of early use of non-invasive positive pressure ventilation in patients with acute exacerbation of chronic obstructive pulmonary disease. Pak J Med Sci. 2019;35:1488–1492. doi: 10.12669/pjms.35.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansari SF, Memon M, Brohi N, Tahir A. Noninvasive positive pressure ventilation in patients with acute respiratory failure secondary to acute exacerbation of chronic obstructive pulmonary disease. Cureus. 2019;11:e5820. doi: 10.7759/cureus.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Struik FM, Lacasse Y, Goldstein RS, Kerstjens HA, Wijkstra PJ. Nocturnal noninvasive positive pressure ventilation in stable COPD: a systematic review and individual patient data meta-analysis. Respir Med. 2014;108:329–337. doi: 10.1016/j.rmed.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Zayed Y, Barbarawi M, Kheiri B, Haykal T, Chahine A, Rashdan L, Dhillon H, Khaneki S, Bachuwa G, Seedahmed E. Initial noninvasive oxygenation strategies in subjects with de novo acute hypoxemic respiratory failure. Respir Care. 2019;64:1433–1444. doi: 10.4187/respcare.06981. [DOI] [PubMed] [Google Scholar]
- 15.Lee M, Kim JH, Jeong IB, Son JW, Na MJ, Kwon SJ. Protecting postextubation respiratory failure and reintubation by high-flow nasal cannula compared to low-flow oxygen system: single center retrospective study and literature review. Acute Crit Care. 2019;34:60–70. doi: 10.4266/acc.2018.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ocal S, Ortac Ersoy E, Ozturk O, Hayran M, Topeli A, Coplu L. Long-term outcome of chronic obstructive pulmonary disease patients with acute respiratory failure following intensive care unit discharge in Turkey. Clin Respir J. 2017;11:975–982. doi: 10.1111/crj.12450. [DOI] [PubMed] [Google Scholar]
- 17.Rittayamai N, Phuangchoei P, Tscheikuna J, Praphruetkit N, Brochard L. Effects of high-flow nasal cannula and non-invasive ventilation on inspiratory effort in hypercapnic patients with chronic obstructive pulmonary disease: a preliminary study. Ann Intensive Care. 2019;9:122. doi: 10.1186/s13613-019-0597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee MK, Choi J, Park B, Kim B, Lee SJ, Kim SH, Yong SJ, Choi EH, Lee WY. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018;12:2046–2056. doi: 10.1111/crj.12772. [DOI] [PubMed] [Google Scholar]
- 19.Wilson ME, Dobler CC, Morrow AS, Beuschel B, Alsawas M, Benkhadra R, Seisa M, Mittal A, Sanchez M, Daraz L, Holets S, Murad MH, Wang Z. Association of home noninvasive positive pressure ventilation with clinical outcomes in chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2020;323:455–465. doi: 10.1001/jama.2019.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rittayamai N, Tscheikuna J, Praphruetkit N, Kijpinyochai S. Use of high-flow nasal cannula for acute dyspnea and hypoxemia in the emergency department. Respir Care. 2015;60:1377–1382. doi: 10.4187/respcare.03837. [DOI] [PubMed] [Google Scholar]
- 21.Okuda M, Tanaka N, Naito K, Kumada T, Fukuda K, Kato Y, Kido Y, Okuda Y, Nohara R. Evaluation by various methods of the physiological mechanism of a high-flow nasal cannula (HFNC) in healthy volunteers. BMJ Open Respir Res. 2017;4:e000200. doi: 10.1136/bmjresp-2017-000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim ES, Lee H, Kim SJ, Park J, Lee YJ, Park JS, Yoon HI, Lee JH, Lee CT, Cho YJ. Effectiveness of high-flow nasal cannula oxygen therapy for acute respiratory failure with hypercapnia. J Thorac Dis. 2018;10:882–888. doi: 10.21037/jtd.2018.01.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pisani L, Betti S, Biglia C, Fasano L, Catalanotti V, Prediletto I, Comellini V, Bacchi-Reggiani L, Fers SN. Effects of high-flow nasal cannula in patients with persistent hypercapnia after an acute COPD exacerbation: a prospective pilot study. BMC Pulm Med. 2020;20:12. doi: 10.1186/s12890-020-1048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yue W, Zhang Z, Zhang C, Yang L, He J, Hou Y, Tang Y, Tian J. High-flow nasal cannulae oxygen in patients with respiratory failure: a meta-analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29:396–402. doi: 10.3760/cma.j.issn.2095-4352.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Deng K, Guo C. Effects of high-flow nasal cannulae on the rate of tracheal intubation after extubation. Chinese Journal of Practical Nursing. 2016;32:2684–2686. [Google Scholar]


