Abstract
Objective: The present study aimed to evaluate the safety and benefits of N-acetylcysteine (NAC) for chronic kidney disease (CKD) through a systematic review and meta-analysis. Methods and materials: We performed a literature search until 5 May 2020 in the CENTRAL, MEDLINE, EMBASE, CINAHL, Clinical Trials Registry Platform and CBM. Two reviewers independently identified eligible articles and extract data. The risk of bias and publication bias were evaluated in all included trials and Cochrane Collaboration’s RevMan5.3 software was used for data analysis. Fifteen trials (20 articles) involving a total of 768 patients were included. Results: The summary results of the studies showed that NAC did reduce cardiovascular events among people with CKD, the RR was 0.60, and the number that needs to be treated (NNT) was 5.29. Pooled date of estimated glomerular filtration rate (eGFR) and serum creatinine (Scr) in the NAC group were better than those in the placebo group. No patients in all studies were terminated due to side effect. Subgroup analysis also showed that inflammatory cytokines and homocysteine were significantly lower in NAC group. Conclusion: These results suggested that NAC appears to be safe without obvious adverse events, which can also benefit kidney function, relieve inflammation and reduce cardiovascular events among people with CKD.
Keywords: N-acetylcysteine, chronic kidney disease, oxidative stress, systematic review, meta-analysis
Introduction
Chronic kidney disease (CKD) is a chronic disease that has an abnormal structure or function of the kidney that lasts more than 3 months and affects health [1]. Chronic kidney disease is an independent risk factor for cardiovascular disease, which in turn worsens CKD, leading to a vicious cycle [2]. Compared with the general population, the damaging factors of the high incidence of cardiovascular disease (CVD) in patients with CKD not only include traditional factors, but also non-traditional risk factors such as oxidative stress (OS) and inflammation [3]. CKD is associated with OS which probably makes a significant contribution to the excess cardiovascular burden in CKD patients [4]. Markers of inflammation, such as interleukin (IL)-6, tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP), are also elevated in CKD patient which might be triggered by OS, and thus accelerating the renal injury progression [5]. Meanwhile, high level of homocysteine (HCY) and uremic toxin are also related to vascular endothelial injury [6,7]. There is accumulating evidence which suggests that antioxidative treatment may reduce OS.
N-acetylcysteine (NAC) is a thiol compound with anti-oxidant effect, which can reduce the production of oxygen free radicals [8] and reduce the levels of pro-inflammatory cytokines [9], and is widely used in clinics [10-12]. It is well tolerated without any serious side effects [13]. Several studies have speculated that NAC can reduce serum creatinine (Scr), increase the clearance of endogenous creatinine, and improve the ultrastructure of podocytes, which can delay the deterioration progress of renal function [14-17]. It has been demonstrated that NAC could significantly reduce urinary protein in diabetic rat model and possibly delay the occurrence and development of diabetic nephropathy [18].
Despite potential effectiveness and increasing interest, there is uncertainty whether NAC is effective for CKD treatment. Therefore, we performed a systematic review to fill this knowledge gap.
Methods and materials
Study selection and outcome assessment
We included randomized controlled trials (RCTs) of adults, in which at least one of the treatment groups received NAC, administered orally or intravenously at any dose for any length of time.
Participants with CKD, including those who needed renal replacement therapy (dialysis), had a functioning kidney transplant, or whose kidney function was impaired (defined as a reduced estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2), and other markers of kidney damage such as proteinuria (KDOQI stages 1 to 5), or an elevated Scr level (Scr > 120 µmol/L) was appeared. Data from subgroups of participants with CKD within studies with broader inclusion criteria (e.g. people from the general population, patients with diabetes, patients with CVD) were also included.
The primary measured outcomes were eGFR, Scr, all-cause mortality, cardiovascular and cerebrovascular diseases and adverse events. The secondary outcomes included blood urea nitrogen (BUN), endogenous creatinine clearance (Ccr), serum cystatin C (CysC), serum HCY, hemoglobin (Hb), IL-6, IL-1 and CRP.
Data sources and study search strategy
A systematic and comprehensive literature search was carried out to identify eligible RCTs using the following sources: MEDLINE (1946 to May 5, 2020), EMBASE (1974 to May 5, 2020), CINAHL (1937 to May 5, 2020), CENTRAL (Cochrane Central Register of Controlled Trials, May, 2020), World Health Organization International Clinical Trials Registry Platform (May 5, 2020). Key words used to search included ‘N-acetylcysteine OR NAC’ and ‘chronic renal insufficiency OR chronic kidney disease OR chronic renal failure OR end stage renal disease OR CKD OR ESRD’. We identified additional studies by hand-searching the references cited in relevant publications.
Selection of studies
Publications retrieved from CENTRAL, MEDLINE, EMBASE, CINAHL and the Clinical Trials Registry Platform were imported in a reference management software (EndNote X5). After removing the duplicate results, non blinded trial reports were reviewed independently by LS and YM according to the selection criteria. Disagreements were resolved by consensus and by recourse to the third review author whenever not resolved by consensus.
Date extraction and management
Two authors independently read the full text of extracted articles and included studies that met the inclusion criteria. The same independent authors used standardized data forms to extract data on: type of trial, sample size, diagnostic input, intervention, length of follow-up, outcome variables, and results. We entered continuous data (e.g. eGFR, Scr) as means and standard deviations (SDs), and dichotomous outcomes (e.g. response, improvement) as number of events.
Assessment of risk of bias in included studies
The risk of bias of included trials were independently assessed by the review authors, using a data collection form. All included studies were assessed by looking at standard quality domains using the risk of bias assessment tool [19]. There were six domains: sequence generation, allocation concealment, blinding of outcome assessment, completeness of outcome data, selective reporting and other potential sources of bias. We made a judgment about risk of bias according to the criteria described in the Cochrane Handbook [19]. Risk of bias was categorized as ‘Low risk’, ‘High risk’ or ‘Unclear risk’. We explored the impact of bias by undertaking sensitivity analyses. The publication bias was analysed by funnel plot.
Data analysis
We analyzed only the available data for continuous data. For dichotomous data, we used intention-to-treat (ITT) analysis. Meta-analysis was performed when the participants and interventions were sufficiently homogeneous, using Review Manager 5.3 [20]. For continuous data, weighted mean differences (MD) and 95% confidence interval (CI), and for dichotomous data, weighted risk ratios (RR) and 95% CI were calculated. All comparisons were two-sided and a P-value < 0.05 was considered statistically significant. Heterogeneity was explored by the chi-squared test (Chi2 test) with significance set at P value 0.10. The quantity of heterogeneity was measured by I2 with I2 ≥ 50% as substantial heterogeneity. A random-effect model was used to combine the results where heterogeneity was significant. Otherwise, a fixed-effect model was used.
Sub-group analysis was performed to assess if the route of administration and different duration of NAC influenced the outcome variable. Sensitivity analyses were undertaken to explore the robustness of findings to key decisions in the review process. Two sensitivity analyses were performed according to whether: 1) the allocation to intervention or control groups was truly randomized, to explore the potential selection bias; 2) the outcome assessment was blinded, to explore the potential assessment bias associated with knowledge of the intervention.
Results
Results of the search
6957 potentially relevant references were searched from MEDLINE, EMBASE, CINAHL, CENTRAL and the Clinical Trials Registry Platform. After title and abstract Screening, 39 full-text articles were selected. Fifteen individual studies published in 20 papers met the inclusion criteria for the systematic review [21-35]. Other 19 records were excluded because they were not original articles, not RCTs, or had irrelevant outcomes (Figure 1).
Figure 1.
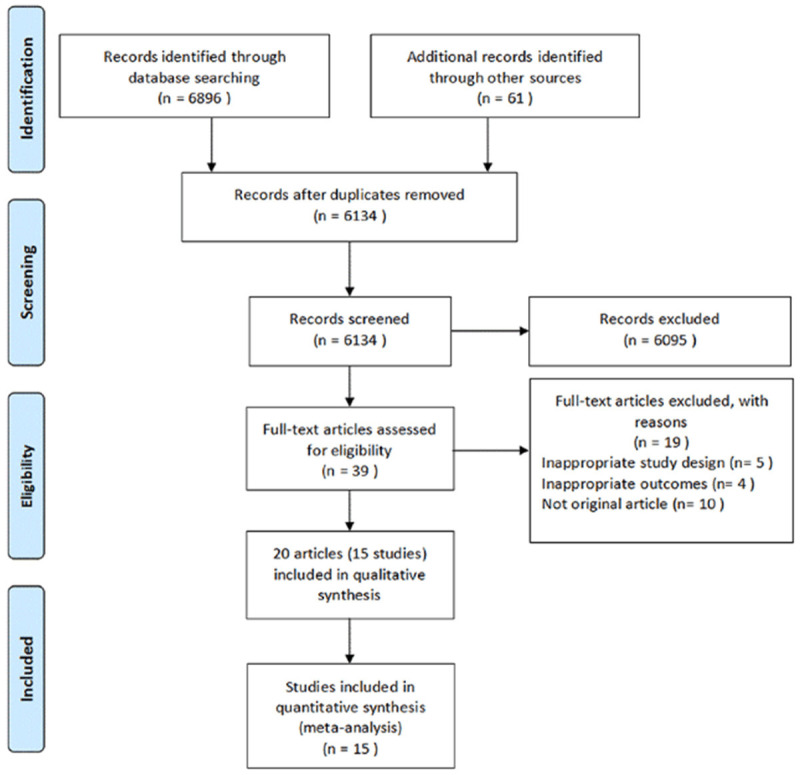
Flow diagram of the literature search conducted in the present study.
Characteristics of included studies
Characteristics of included trials and patients were available in the Table 1. Fifteen RCTs (20 papers) [21-35] met the inclusion criteria, including 13 randomized parallel controlled trials [21-27,29-31,33-35] (15 papers) and 2 randomized crossover trials [28,32] (5 papers). For the randomized parallel controlled trials, the median number of study participants was 47 (range, 32-134). There were 19 and 20 patients in the two crossover trials, respectively. A total of 786 patients were included. All these patients fulfilled the diagnostic criteria of CKD and were over 18 years old. None of the patients received any antioxidant drugs before the trial. Patients in 10 trials [21,22,25,29-35] (565 participants) received chronic hemodialysis with end-stage renal disease. Seventy-two patients from two studies [29,32] were on continuous ambulatory peritoneal dialysis (CAPD).
Table 1.
Characteristics of included studies
| Authors (year) (Ref.) | Study type (RCT) | Sample size | Age (years) | Treatment protocol | Included criteria | Follow-up time | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| NAC | Control | NAC | Control | NAC | Control | |||||
| Oral NAC | ||||||||||
| Ahmadi et al (2017) [21] | Parallel | 26 | 21 | 57.5±13.3 | 59.1±12.7 | Oral NAC 1,200 mg twice/day | N/A* | ESRD | 12 weeks | eGFR, Kt/V, 24-hour urine volume |
| Bashardoust et al (2018) [22] | Parallel | 26 | 25 | 65.5±11.05 | 62.76±14.47 | Oral NAC 1,200 mg/day | Placebo capsules | ESRD | 4 weeks | Hb, Ferritin, hs-CRP, ALP, P, Ca, AE |
| Friedman et al (2003) [23] | Parallel | 18 | 17 | 68±3 | 70±4 | Oral NAC 1,200 mg twice/day | Placebo capsules | ESRD | 4 weeks | HCY, AE |
| Hashemi et al (2012) [24] | Parallel | 35 | 35 | 60.2±10.1 | 63.4±6.4 | Oral NAC 600 mg twice/day | N/A | DN | 2 months | 24-hour urine protein |
| Larki et al (2019) [25] | Parallel | 21 | 19 | 60.61±16.61 | 61.05±19.09 | Oral NAC 600 mg/12 hour | Placebo capsules | ESRD | 8 weeks | SCr, BUN, ALB, Hb, Ca, P, PTH, ESR, CRP, IL-6 |
| Moist et al (2010) [26] | Parallel | 30 | 30 | 68.6±12.5 | 71.6±9.1 | Oral NAC 1,200 mg/12 hour | Placebo capsules | CCR 30-60 mL/min | 2 days | SCr, eGFR, 24-hour urine protein, CysC |
| Purwanto et al (2012) [27] | Parallel | 16 | 16 | 45.79±7.59 | 42.54±6.79 | Oral NAC 600 mg twice/day | Placebo capsules | ESRD | 8 weeks | PCT, TNF-α, hs-CRP, IL-6, IL-1 |
| Renke et al (2010) [28] | Cross-over | 19 | 19 | N/A | N/A | Oral NAC 1,200 mg/day | Placebo capsules | non-DN | 8 weeks | SCr, eGFR, 24-hour urine protein, HCY, Blood pressure, AE |
| Tepel et al (2003) [29] | Parallel | 64 | 70 | 63±14 | 62±18 | Oral NAC 600 mg twice/day | Placebo capsules | ESRD | 2 years | cardiac events, ischemic stroke, total mortality, AE |
| Vural et al (2018) [30] | Parallel | 23 | 17 | 46±15 | 49±13 | Oral NAC 600 mg twice/day | Placebo capsules | ESRD | 54 weeks | TNF-α, IL-6 |
| IVdrop NAC | ||||||||||
| Perna et al (2012) [31] | Parallel | 47 | 48 | 65.8±1.83 | 58.5±2.70 | IV NAC 5 g in 5% glucose, MTHF | 5% glucose solution alone | ESRD | 10 dialysis sessions | HCY, AE |
| Scholze et al (2004) [32] | Cross-over | 20 | 20 | N/A | N/A | IV NAC 5 g in 5% glucose | 5% glucose solution alone | ESRD | one dialysis sessions | HCY, BP |
| Thaha et al (2006) [33] | Parallel | 30 | 30 | 48.1±11.08 | 52.6±10.3 | IV NAC 5 g in 5% glucose | 5% glucose solution alone | ESRD | one dialysis sessions | HCY, BP, AE |
| Thaha et al (2008) [34] | Parallel | 20 | 20 | 44.8±12.7 | 45.8±11.05 | IV NAC 5 g in 5% glucose | 5% glucose solution alone | ESRD | one dialysis sessions | AE |
| Tsai et al (2010) [35] | Parallel | 22 | 21 | 56.45±15.67 | 55.05±14.87 | IV NAC 5 g in normal saline 250 mL | normal saline 250 mL | ESRD | one dialysis sessions | HCY, TNF-α, hs-CRP |
Note: AE, adverse events; ALB, albumin; ALP, alkaline phosphatase; BP, blood pressure; BUN, blood urea nitrogen; Ca, calcium; CAPD, continuous ambulatory peritoneal dialysis; CCR, endogenous creatinine clearance; CKD, chronic kidney disease; CRP, C-reactive protein; CysC, serum cystatin C; DN, diabetic nephropathy; eGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; ESRD, end-stage renal disease; Hb, hemoglobin; HCY, homocysteine; hs-CRP, high sensitivity C reactive protein; IL, interleukin; IVdrop, intravenous drop infusion; MTHF, Methylfolate; NAC, N-acetylcysteine; P, phosphorus; PCT, procalcitonin; PTH, parathyroid hormone; RCT, randomized controlled trials; SCr, serum creatinine; TNF-α, tumor necrosis factor-α;
N/A, No data provided.
All of included trials compared NAC with placebo or no medication. NAC was administered orally in ten studies [21-30], with doses and frequency of 1200 mg/day, 1200 mg twice/day and 600 mg twice/day, respectively. Five studies [31-35] applied NAC intravenously within 4 hours during hemodialysis (NAC 5 g in 500 ml 5% glucose solution or 500 ml normal saline). In Renke’s study, patients were randomly assigned to 1 of 2 treatment sequences, NAC/washout/placebo or placebo/washout/NAC [28]. Clinical evaluation and laboratory tests were performed at the randomization point and after each period of the study. In another crossover trial [32], each patient received NAC (5 g in 5% glucose solution for 4 hours) during a single hemodialysis session and 5% glucose solution alone for placebo control during another hemodialysis session.
The primary outcome of eGFR, Scr, cardiovascular and cerebrovascular diseases, and adverse events were measured in these trials. More than 20 other outcomes were assessed, such as CysC, HCY, IL-6, IL-1 and CRP. But unfortunately, none of these trials reported all-cause mortality.
Risk of bias in included studies
The risks of bias of all trials were summarized in Table 2.
Table 2.
Risk of bias summary
| Authors (year) (Ref.) | Random sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|
| Ahmadi et al (2017) [21] | Low risk | High risk | High risk | Low risk | Low risk | Low risk |
| Bashardoust et al (2018) [22] | Low risk | Low risk | Low risk | Unclear risk | Low risk | Unclear risk |
| Friedman et al (2003) [23] | Unclear risk | Low risk | Low risk | Low risk | Low risk | Unclear risk |
| Hashemi et al (2012) [24] | Unclear risk | High risk | High risk | Low risk | Low risk | Unclear risk |
| Larki et al (2019) [25] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Moist et al (2010) [26] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Purwanto et al (2012) [27] | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Renke et al (2010) [28] | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Tepel et al (2003) [29] | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Vural et al (2018) [30] | Low risk | High risk | High risk | Low risk | Low risk | Low risk |
| Perna et al (2012) [31] | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Scholze et al (2004) [32] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Thaha et al (2006) [33] | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk |
| Thaha et al (2008) [34] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Tsai et al (2010) [35] | Low risk | High risk | High risk | Low risk | High risk | Unclear risk |
Allocation (selection bias). All trials mentioned randomization, and there were 9 trials [21,22,25,26,28,30,32-34] that clearly showed that the random sequence was generated by the random number table, so the these trials were judged as ‘low risk’. The random methods of other 6 trials [23,24,27,29,31,35] were unknown. After contacting with the authors, it was known that, the random sequences were generated by experiment-coordinated researcher and computer in Perna’s trial and Tsai’s trial [31,35], respectively. Therefore, both of them were also judge as low risk and other 4 trials [23,24,27,29] were judged as unclear risk of bias. For allocation concealment, no control drug was used in two trials, and both of Perna’s trial [31] and Vural’s trial [30] were open-label trials, thus all of these trials were judged as high risk in this item.
Blindness (performance bias and detection bias). After contacting with the authors, patients in 7 trials [22,23,25,26,32-34] were blinded and judged as low risk of bias. Two open-label trials [30,31] and two no placebo trials [19,22] were judged as high risk of bias. The other 4 studies [27-29,31] were judged as unclear risk.
Incomplete outcome data (attrition bias). Eleven patients were excluded after lost to follow-up and ITT analysis was no used in the Bashardoust’s trial [22], which was judged as high risk. In Friedman’s trial [23], 2 patients in treatment group missed treatment doses and 1 patient in control group fell off after the start of the trial. Three patients in the Moist’s trial [26] missed treatment doses. ITT were used in both trials. No loss of follow-up occurred in other trials. Therefore, we determined that the bias in data integrity of these studies was low risk.
Selective reporting (reporting bias). All trials had protocols, and all but Vual’s trial [35] were available for pre-specified outcomes, account for its data of hs-CRP has not reported in the section of result.
Other potential sources of bias. All the included trials appeared to be free of other sources of bias and therefore judged as low risk of bias.
Publication bias. The funnel plot showed that there was low publication bias (Figure 2).
Figure 2.
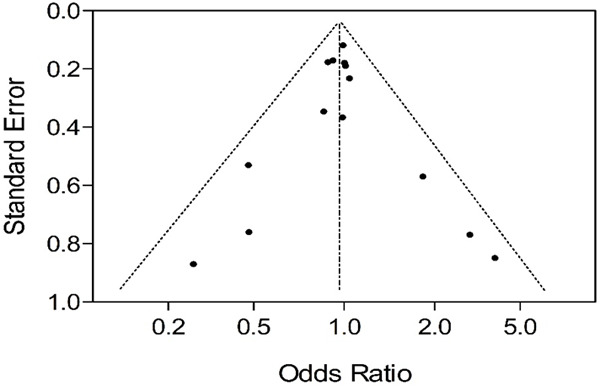
The funnel plot showed that there was low publication bias.
Effects of interventions
Because of multiple sources of heterogeneity, we could not perform meta-analyses in all outcomes. Instead, we presented a narrative summary of pertinent findings from the individual studies. Different routes of administration and duration of treatment were performed in these trials. Therefore, we pooled all 15 studies firstly, and then sub-group analysis was performed according to administration and duration of treatment. NAC has no clear long-term or short-term treatment limit, so we tried to conduct sub-group analysis with a one-month limit. No data were available in these trials for all-cause mortality.
General summary
NAC vs. placebo
Three trials [21,26,28] had reported the endpoint value of eGFR which were calculated by Cockcroft-Gault formula, but only two trials reported the change from baseline value. No significant heterogeneity was found among these trials and fixed effect model was used. NAC was better than the placebo both in the end point date and change from baseline date. MDs were 138 (95% CI 0.92 to 1.84) and -1.86 ml/min (95% CI -3.58 to -0.15), respectively (Figure 3).
Figure 3.
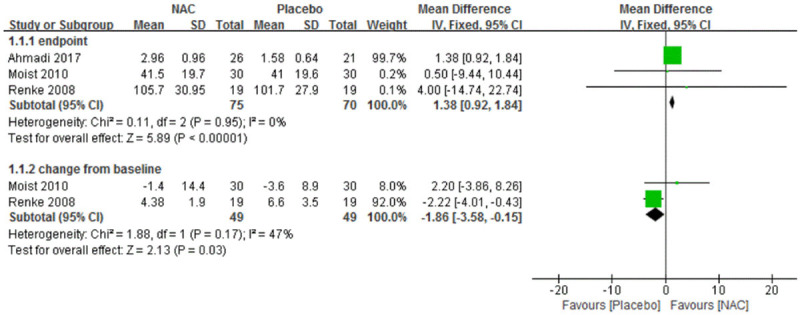
Forest plot for eGFR in general summary of NAC vs. Placebo. eGFR, estimated glomerular filtration rate. NAC, N-acetylcysteine. SD, standard deviation. IV, inverse variance. Fixed, fixed effect. CI, confidence interval.
For Scr, three trials [25,26,28] found statistically significant differences between intervention groups, favoring NAC over placebo. A similar result was found in the pooled data. No significant heterogeneity was found among these trials and fixed effect model was used. MD of change from baseline was -0.04 mg/dL, 95% CI -0.07 to -0.01. MD of end point was -0.04 mg/dL, 95% CI -0.07 to -0.01 mg/dL (Figure 4).
Figure 4.
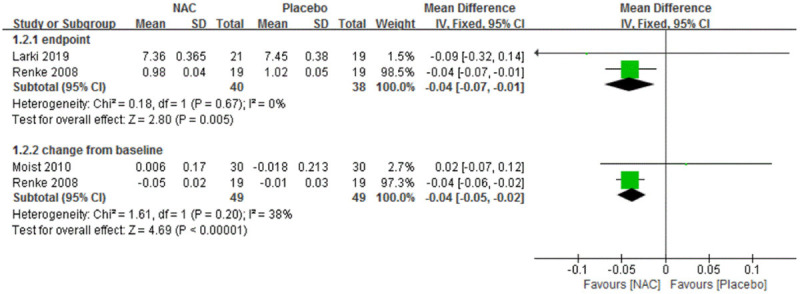
Forest plot for Scr in general summary of NAC vs. Placebo. Scr, serum creatinine. NAC, N-acetylcysteine. SD, standard deviation. IV, Inverse Variance. Fixed, fixed effect. CI, confidence interval.
Only one trial assessed cardiovascular and cerebrovascular diseases evens [29]. Fifty-one cardiovascular events occurred in all 134 patients, including 18 in NAC group and 33 in placebo group. NAC significantly reduced cardiovascular events than placebo group. Pooled RR was 0.60 (95% CI: 0.38 to 0.95), and the number need to treat (NNT) was 5.29. Cardiovascular death events were also reported in this trial, 9 persons were dead in NAC group and 8 in placebo group. No statistically significant difference was found between two groups.
A total of 7 of 15 trials [22,23,28,29,31,33,34] reported adverse events. Gastrointestinal discomfort was reported in five studies. Statistically significant difference was found between two treatment groups. Pooled RR was 3.28 (95% CI 1.45 to 7.43). More adverse events occurred in NAC group, but no patients were terminated due to adverse reactions in all these 6 studies (Figure 5).
Figure 5.
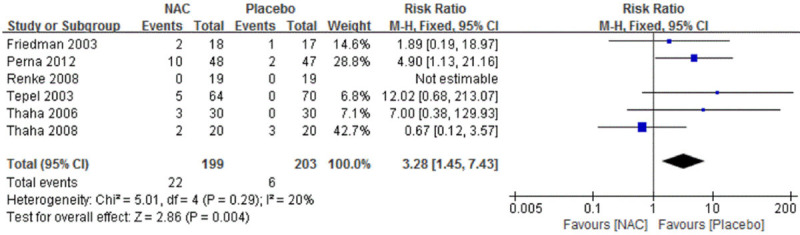
Forest plot for adverse events in general summary of NAC vs. Placebo. NAC, N-acetylcysteine. M-H, mantel haenszel. Fixed, fixed effect. CI, confidence interval.
There was only one trial that assessed BUN [25], and the trial found no significant difference between intervention and control group (39.83±18.76 vs. 50.31±20.33, 95% CI: -22.64 to 1.68; P=0.09). Three trials assessed 24-hour urinary protein quantification [24,26,28]. Both changes from baseline data and endpoint date were reported in Renke’s and Moist’s trials, and only endpoint date was reported in Hashemi’s trial. Strangely, the baseline of 24-hour urinary protein quantification in NAC group was much higher than that in control group. As expected, endpoint date pooled MD was 98.36 mg/24 h (95% CI 36.50 to 160.23) and -74.87 mg/24 h (95% CI: -168.29 to 18.54) for change from baseline date, NAC groups were no better than placebo groups. Six studies showed lower HCY endpoint date in the NAC compared with placebo group [25,30,33-35,37]. This statistically significant difference was confirmed in pooled analysis with weighted MD as -5.95 μmol/L (95% CI: -9.57 to -2.15). Nonetheless, two of these trials also reported change from baseline date. Pooled MD was 0.61 μmol/L (95% CI: -1.99 to 3.21), and no statistically significant difference was found between intervention groups. In the Moist’s trial [26], CysC levels were measured at 4 hour, 24 hour and 48 hour after administration in both groups. Changes in CysC levels between baseline and post-treatment did not differ significantly between treatment groups. Two trials [22,25] analyzed Hb and found no difference between the NAC and placebo groups, neither did our pooled result. Purwanto’s trial [27] assessed PCT and IL-1, and found the NAC group was better than the placebo group (MD -0.47, 95% CI: -0.76 to -0.18; MD -0.15, 95% CI: -0.25 to -0.05). Purwanto’s trial [27] and other two trials (25, 30) also assessed IL-6. MD of change from baseline was -1.33 pg/ml (95% CI -1.85 to -0.81). MD of end point was 0.05 pg/ml (95% CI -0.39 to 0.49, not statistically significant). Three trials [27,30,35] assessed TNF-α and hs-CRP, respectively. Pooled analysis showed significant heterogeneity among these trials. None of them found a statistically significant difference between the NAC and placebo groups in the analysis of either end point data or change from baseline data. Systolic blood pressure and diastolic blood pressure were recorded in three trials [28,32,33]. Meanwhile, two of them reported pulse pressure. Pooled result found no significant difference in these outcomes.
Sub-group summary
Oral, NAC vs. placebo
Oral administration was used in 10 of 15 trials [21-30] (528 patients). The date of eGFR, Scr, cardiovascular events and cardiovascular death events, 24-hour urinary protein quantification, Hb, PCT, IL-1, IL-6 and CysC for this sub-group were exactly the same as General summary. We present only inconsistent indicators in this part.
The number of patients with adverse events were available in four of the six trials [22,23,28,29]. In Friedman’s trial [23], one patient in the placebo group and 2 patients in the NAC group reported nausea and gastric upset. Five patients (8%) reported gastrointestinal discomfort during treatment with NAC during Tepel’s trial [29]. No major side effects were observed in other two trials [22,28]. Pooled RR was 5.51 (95% CI: 0.94 to 27.83). Adverse reactions were slightly higher in the NAC group.
HCY was available in two trials [22,28]. There was no statistically significant difference between the two groups in the analysis of either end point data or change from baseline data. For change from baseline data, two trials [27,30] showed lower TNF-α in the NAC compared with placebo group. This statistically significant difference was confirmed in pooled analysis with weighted MD as -0.88 pg/ml. For hs-CRP [22,27], statistically significant differences was found between intervention groups, favoring NAC over placebo. MD of change from baseline was -23.10 mg/L, 95% CI -28.87 to -17.33 mg/L. MD of end point was -3.97 mg/L, 95% CI: -5.39 to -2.55. The blood pressure was available for one of these six studies [28] and showed no statistically significant difference between two groups.
Intravenous drip infusion, NAC vs. placebo
NAC was administered intravenous in 5 of 15 trials [31-35] (258 patients). Patients received a 4-hour intravenous infusion of NAC (5 g in 5% glucose solution or normal saline) or placebo during a dialysis session. The included trials reported adverse events, HCY, blood pressure, TNF-α and hs-CRP.
Adverse events were reported in three [31,33,34] of the five trials, including hypotension, tachycardia, and allergy. Significantly higher rates of adverse reactions were found in the NAC group compared with the placebo group. Pooled RR was 2.79 (95% CI: 1.09 to 7.12). HCY was analyzed in four trials [31-33,35]. There was significant heterogeneity among the trials (P < 0.000001). The beneficial effect of NAC continued to be statistically significant when using random effects but showed larger confidence intervals. Pooled MD was -8.58 μmol/L (95% CI: -13.31 to -3.85). The change of systolic pressure, diastolic pressure and pulse pressure before and after dialysis were available for two [32,33] of these five studies. However, a statistically significant difference was not found between two groups. Only Tsai’s trial assessed TNF-α and hs-CRP, and no significant difference was found between the intervention groups (35).
Short-course therapy (less than 1 month)
Treatment time of eight [22,23,26,31-35] in fifteen trials is less than 1 month. NAC was significantly greater than placebo for HCY (MD -7.25 μmol/L; 95% CI: -11.44 to -3.06). More adverse reactions were reported in NAC group (RR 2.65, 95% CI: 1.11 to 6.30). There was no statistical difference in other outcomes between the two groups, such as eGFR, Scr, adverse events, 24-hour urinary protein quantification, TNF-α, hs-CRP and blood pressure.
Long-course therapy (more than 1 month)
Treatment time of seven [21,24,25,27-30] in fifteen trials is more than 1 month. No statistical difference was found between the two groups, such as adverse events, hs-CRP, IL-1, PCT, HCY and blood pressure.
Two [21,28] of seven trials assessed eGFR and none of them found a statistically significant difference between the NAC and the placebo groups in the analysis of either end point data or change from baseline data. Interestingly, pooled analysis of end point data showed an opposite result, MD was 1.11 ml/min, 95% CI: 0.65 to 1.57 ml/min. Similar result was found in 24-hour urinary protein quantification as there were statistically significant differences between intervention groups, favoring NAC over placebo (Figure 6).
Figure 6.
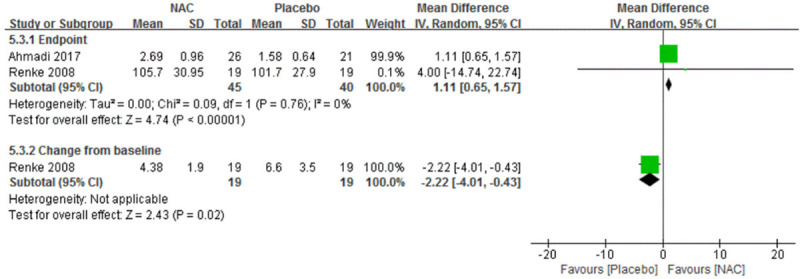
Forest plot for eGFR in sub-group summary of long-course therapy NAC vs. Placebo. eGFR, estimated glomerular filtration rate. NAC, N-acetylcysteine. SD, standard deviation. IV, inverse variance. Fixed, fixed effect. CI, confidence interval.
There were two trials each reported Scr [25,28] and hs-CRP [22,27]. All these trials found statistically significant differences between intervention groups, favoring NAC over placebo. MD of changes from baseline were -0.04 mg/dL, 95% CI: -0.07 to -0.01 mg/dL, and -3.97 mg/L, 95% CI: -5.39 to -2.25 mg/L, respectively. MD of end point were -0.04 mg/dL, 95% CI: -0.06 to -0.02 mg/dL and -23.10 mg/L, 95% CI: -31.74 to -14.46 mg/L, respectively (Figure 7). For TNF-α, Purwanto’s trial [27] found a statistically significant difference between intervention groups about change from baseline data, favoring NAC over placebo. But the difference of end point data in Vural’s trial [30] was statistically insignificant.
Figure 7.
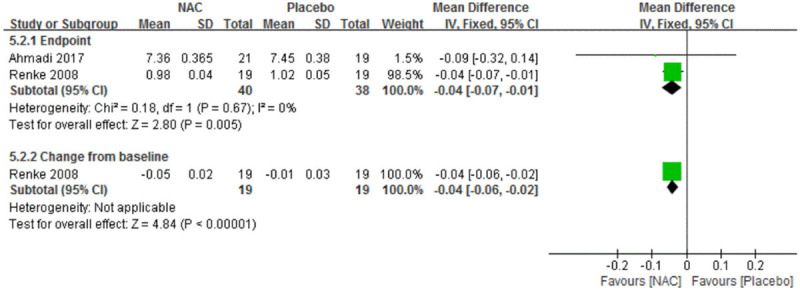
Forest plot for Scr in sub-group summary of long-course therapy NAC vs. Placebo. Scr, serum creatinine. NAC, N-acetylcysteine. SD, standard deviation. IV, Inverse Variance. Fixed, fixed effect. CI, confidence interval.
Sensitivity analysis
We did not perform a sensitivity analysis against random method because we did not rank any included trials at high risk of bias. Four studies [21,24,30,35] were identified as high risk of bias in concealment. After exclusion of Ahmadi and Hashemi’s trial [21,24], the pooled difference of eGFR (end point) and 24-hour urinary protein (end point) became insignificant, respectively. When excluded Vural’s trial [30] which was an open-label trial, the pooled difference of TNF-α (change from baseline) became statistically significant (weighted MD -0.87 pg/ml, 95% CI -1.3 to -0.44 pg/ml), while other results remained similar. The blind method of the Tsai’s trial [35] was judged as a high risk of bias. For continuous outcome data, HCY, exclusion of this trial from the analysis showed a similar result. No dichotomous outcome was available in this trial.
Discussion
We conducted a meta-analysis to analyze the efficacy and safety of NAC in the treatment of CKD. Collectively, for major efficacy evaluation outcomes, both the pooled analysis and a subgroup analysis showed that NAC did reduce cardiovascular events among people with CKD. Pooled date also showed that eGFR and Scr were found to be statistically significantly better in the NAC group compared with the placebo group. None of the included studies assessed all-cause mortality or total number of withdrawals due to adverse events. With regard to the safety of NAC, no patients in all studies were terminated due to side effect, and no adverse events occurred in the oral treatment group. Contrarily, gastrointestinal discomfort was reported in five intravenous administration studies, the total number of adverse events was higher in the NAC group than in the placebo group.
Subgroup analysis of administration pattern showed that hs-CRP, TNF-α, PCT, IL-6 and IL-1 were significantly lower in NAC group by oral than in placebo. Significant benefits for HCY could also be achieved when NAC were applied by intravenous. For subgroup analysis of course of treatment, the longer treatment period of NAC, the more significant reduction of inflammatory cytokines was found.
NAC was well tolerated in most of the included studies. However, higher rates of adverse reactions were observed when patients were treated with intravenous NAC than placebo during the course of hemodialysis session. Higher dose of NAC increased side effects (> 3 g/day). The most frequent side effects with NAC were mild gastrointestinal reactions, such as nausea and vomiting [36]. According to a previous study [37], gastrointestinal reactions also occurred in patients without dialysis after receiving NAC by intravenous. Slowing down the drip rate can reduce the generation of side effects and no statistically significant difference in the number of adverse events between two groups.
Patients with CKD have high cardiovascular mortality and morbidity [38,39]. Excessive OS is thought to play a major role in elevating these risks [40], OS has been identified as one important cause of vascular injury in several studies [41-43]. In our review, we were excited to find that NAC group significantly reduced eGFR, Scr, hs-CRP, TNF-α, PCT, IL-6, IL-1 and cardiovascular events. Subgroup analysis also found that the efficacy of NAC depends on the course of treatment. There was no statistically significant difference in the reduction of inflammatory mediators between the NAC group and the placebo group in the study with a course of treatment of less than 1 month, but the results were reversed in the group with a course of treatment of more than 1 month.
In addition, seven and three RCTs in this systematic review provided NAC through oral administration with the dose of 1200 mg per day and 2400 mg per day, respectively. Further analysis found that, NAC has dose-effect relationship in reducing plasma homocysteine [44]. The plasma homocysteine was lower in the high dose group than in the low dose group.
The mechanism for how NAC protects the kidney is not yet clear. It may protect tubular from injury, reduce renal cell apoptosis, promote cell repair, and increase expression of nitric oxide synthase (NOS) through direct antioxidant effect and indirect antioxidant effect of glutathione, thereby reducing vasoconstriction, improving renal flow, and reducing renal injury [45,46]. The conclusions of our review are consistent with some previously published studies. Briguori et al [47] found that NAC can reduce Scr, but not improve eGFR and reduce the concentration of CysC. Dittmann’s research [48] has shown that NAC can reduce blood HCY. Results of Jun M’s study [49] also showed that antioxidant therapies including NAC did not reduce the total death events, with the RR was 0.93 (0.76, 1.14). Another study [50] showed that inflammatory mediators were significantly decreased by the treatment of NAC, which significantly reduce the expression of inflammatory factors and improve the OS state. Romano’s research [51] found that the effect of NAC in the treatment of contrast nephropathy was dose-dependent. Tian et al [52] found that NAC could improve eGFR in hypertensive rats. Liu et al [17] also found that NAC may reduce urinary protein by up-regulating the expression level of nephrin protein in foot cells and maintaining the structural integrity of the hiatus membrane in vivo. Both of these two studies were based on model rats, but the conclusion is the same as our review.
While this review included all RCTs of NAC treat for CKD, the data were incomplete in several areas. First of all, no head-to-head comparisons of antioxidant agents were available in patients with CKD or those requiring hemodialysis. meanwhile, none of the included studies assessed all-cause mortality, and only one or two trials reported the major, validated outcomes such as Scr, eGFR or cardiovascular events. Moreover, the majority of these trials did not clearly clarify the causes of CKD. In addition, the study population in the included trials involved 768 CKD participants who fulfilled the KDOQI or KDIGO criteria of CKD. All of these participants were adults, in stages CKD3 to CKD5. The duration of follow-up was 2 days to 2 years in the oral administration group and 1 to 10 dialyses duration in the intravenous administration group. Therefore, the evidence was not applicable to CKD patients who are minors or with early disease onset.
The quality of evidence found in the trials included in this review appears to be moderate. All trials were randomized and controlled, and more than half of them were explicitly described the methods of allocation concealment, sequence generation and adequate methods of blindness. ITT analysis was applied to all major outcome indicators. The directivity of all indicators was basically consistent, and there were no cases of withdrawal due to adverse reactions or ineffective treatment.
However, this study does have some limitations. Firstly, most of the pooled data in the present review included only a few trials and participants which might introduce a risk of false-negative results because of low statistical power, and overlook the potential benefit of NAC. Secondly, for quality of evidence, one of eleven trials has no blindness, and the existence of statistical heterogeneity in the several outcome analyses might have affected our results, although we attempted to address these through the use of sensitivity analysis and random-effects models, respectively. Thirdly, the cause of CKD, CKD stage, and basic treatment were not identical among subjects in each trial, and the dose, duration of follow-up and delivery mode of NAC were also different in these trials, which mighty confuse the result. Therefore, these results should be interpreted with caution.
In conclusion, our systematic review has shown that NAC appears to be safe without obvious adverse events, also could improve eGFR, reduce the level of Scr and cardiovascular events in CKD patients. The longer treatment period of NAC, the more significant reduction of HCY and inflammatory cytokines was also found. RCTs of larger sample sizes and with longer duration are needed. It is also important to subgroup appropriately for the cause of CKD, CKD stage and method of administration of NAC.
Acknowledgements
This work was supported by the Startup Fund for Scientific Research, Fujian Medical University (2019QH1122).
Disclosure of conflict of interest
None.
References
- 1.Tonelli M, Muntner P, Lloyd A, Manns BJ, James MT, Klarenbach S, Quinn RR, Wiebe N, Hemmelgarn BR. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011;154:12–21. doi: 10.7326/0003-4819-154-1-201101040-00003. [DOI] [PubMed] [Google Scholar]
- 2.Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, Stengel B, Houillier P, Froissart M, Boutouyrie P. Arterial remodeling associates with CKD progression. J Am Soc Nephrol. 2011;22:967–974. doi: 10.1681/ASN.2010080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008;3:505–521. doi: 10.2215/CJN.03670807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hambali Z, Ahmad Z, Arab S, Khazaai H. Oxidative stress and its association with cardiovascular disease in chronic renal failure patients. Indian J Nephrol. 2003;21:21–25. doi: 10.4103/0971-4065.75218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kones R. Rosuvastatin, inflammation, C-reactive protein, JUPITER, and primary prevention of cardiovascular disease-a perspective. Drug Des Devel Ther. 2010;4:383–413. doi: 10.2147/DDDT.S10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malyszko J. Mechanism of endothelial dysfunction in chronic kidney disease. Clin Chim Acta. 2010;411:1412–20. doi: 10.1016/j.cca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Tossios P, Bloch W, Huebner A, Raji MR, Dodos F, Klass O, Suedkamp M, Kasper SM, Hellmich M, Mehlhorn U. N-acetylcysteine prevents reactive oxygen species-mediated myocardial stress in patients undergoing cardiac surgery: results of a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg. 2003;126:1513–1520. doi: 10.1016/s0022-5223(03)00968-1. [DOI] [PubMed] [Google Scholar]
- 9.Durham J, Caputo C, Dokko J, Zaharakis T, Pahlavan M, Keltz J, Dutka P, Marzo K, Maesaka JK, Fishbane S. A randomized controlled trial of N-acetylcysteine to prevent contrast nephropathy in cardiac angiography. Kidney Int. 2002;62:2202–2207. doi: 10.1046/j.1523-1755.2002.00673.x. [DOI] [PubMed] [Google Scholar]
- 10.Zachwieja J, Zaniew M, Bobkowski W, Stefaniak E, Warzywoda A, Ostalska-Nowicka D, Dobrowolska-Zachwieja A, Lewandowska-Stachowiak M, Siwińska A. Beneficial in vitro effect of N-acetyl-cysteine on oxidative stress and apoptosis. Pediatr Nephrol. 2005;20:725–731. doi: 10.1007/s00467-004-1806-4. [DOI] [PubMed] [Google Scholar]
- 11.Marian AJ, Tan Y, Li L, Chang J, Syrris P, Hessabi M, Rahbar MH, Willerson JT, Cheong BY, Liu CY, Kleiman NS, Bluemke DA, Nagueh SF. Hypertrophy regression with N-AcetyLcysTeine in hypertrophic CardioMyopathy (HALT-HCM): a randomized placebo controlled double blind pilot study. Circ Res. 2018;122:1109–1118. doi: 10.1161/CIRCRESAHA.117.312647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang N, Qian P, Kumar S, Yan TD, Phan K. The effect of N-acetylcysteine on the incidence of contrast-induced kidney injury: a systematic review and trial sequential analysis. Int J Cardiol. 2016;209:319–327. doi: 10.1016/j.ijcard.2016.02.083. [DOI] [PubMed] [Google Scholar]
- 13.Bakker J, Zhang H, Depierreux M, van Asbeck S, Vincent JL. Effects of N-acetylcysteine in endotoxic shock. J Crit Care. 1994;9:236–243. doi: 10.1016/0883-9441(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 14.Tian N, Rose R, Jordan S, Dwyer TM, Hughson MD, Manning RD Jr. N-acetylcysteine improves renal dysfunction, ameliorates kidney damage and decreases blood pressure in salt-sensitive hypertension. J Hypertens. 2006;24:2263–2270. doi: 10.1097/01.hjh.0000249705.42230.73. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann U, Fischereder M, Kruger B, Drobnik W, Krämer BK. The value of N-acetylcycsteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15:407–410. doi: 10.1097/01.asn.0000106780.14856.55. [DOI] [PubMed] [Google Scholar]
- 16.Safirstein R, Andrade L, Vieira J. Acetylcycsteine and nephrotoxic effects of radiographic contrast agents: a new use for an old drug. N Engl J Med. 2000;343:210–212. doi: 10.1056/NEJM200007203430311. [DOI] [PubMed] [Google Scholar]
- 17.Liu TQ, Feng X, Li JJ, Tang JH, Gong SF. The protective effects of N-acetylcysteine on podocytes of diabetic nephropathy rats. Chin J Clin Med. 2009;10:863–866. [Google Scholar]
- 18.Feng X, Liu TQ, Li JJ. The effects of N-acetylcysteine on oxidative stress of the kidneys of diabetic rats. Clinical Medical Journal of China. 2008;15:842–843. [Google Scholar]
- 19.Higgins J, Green S. Cochrane handbook for systematic reviews of intervention. Version 5.1.0. Accessed January. 2013:2. [Google Scholar]
- 20.Review Manager (RevMan) (Computer program). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2012. [Google Scholar]
- 21.Ahmadi F, Abbaszadeh M, Razeghi E, Maziar S, Khoidaki SD, Najafi MT, Lessan-Pezeshki M. Effectiveness of N-acetylcysteine for preserving residual renal function in patients undergoing maintenance hemodialysis: multicenter randomized clinical trial. Clin Exp Nephrol. 2017;21:342–349. doi: 10.1007/s10157-016-1277-5. [DOI] [PubMed] [Google Scholar]
- 22.Bashardoust B, Alaei R, Kebar SM, Hasani S, Habibzadeh A. The effect of oral N-acetylcysteine on serum high sensitive CRP and plasma hemoglobin levels in end-stage renal disease patients under routine hemodialysis; a randomized placebocontrolled clinical trial. J Nephropathol. 2018;7:268–272. [Google Scholar]
- 23.Friedman A, Bostom A, Laliberty P, Selhub J, Shemin D. The effect of N-acetylcysteine on plasma total homocysteine levels in hemodialysis: a randomized, controlled study. Am J Kidney Dis. 2003;41:442–446. doi: 10.1053/ajkd.2003.50054. [DOI] [PubMed] [Google Scholar]
- 24.Rasi Hashemi S, Noshad H, Tabrizi A, Mobasseri M, Tayebi Khosroshahi H, Heydarnejad M, Khalaj MR, Aghamohammadzadeh N. Angiotensin receptor blocker and N-Acetyl Cysteine for reduction of proteinuria in patients with type 2 diabetes mellitus. Iran J Kidney Dis. 2012;6:39–43. [PubMed] [Google Scholar]
- 25.Larki R, Panahi A, Manzouri L, Sedaghattalab M. Effect of N-acetylcysteine on inflammatory and biochemical markers of hemodialysis patients: a randomized controlled trial. Acta Med Iran. 2019;7:57–62. [Google Scholar]
- 26.Moist L, Sontrop JM, Gallo K, Mainra R, Cutler M, Freeman D, House AA. Effect of N-acetylcysteine on serum creatinine and kidney function: results of a randomized controlled trial. Am J Kidney Dis. 2010;56:643–650. doi: 10.1053/j.ajkd.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Purwanto B, Prasetyo D. Effect of oral N-acetylcysteine treatment on immune system in continuous ambulatory peritoneal dialysis patients. Acta Med Indones. 2012;44:140–144. [PubMed] [Google Scholar]
- 28.Renke M, Tylicki L, Rutkowski P, Larczynski W, Neuwelt A, Aleksandrowicz E, Łysiak-Szydłowska W, Rutkowski B. The effect of N-acetylcysteine on blood pressure and markers of cardiovascular risk in non-diabetic patients with chronic kidney disease: a placebo-cotrolled, randomized, cross-over study. Med Sci Monit. 2010;16:PI13–PI18. [PubMed] [Google Scholar]
- 29.Tepel M, van der Giet M, Statz M, Jankowski J, Zidek W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: a randomized, controlled trial. Circulation. 2003;107:992–995. doi: 10.1161/01.cir.0000050628.11305.30. [DOI] [PubMed] [Google Scholar]
- 30.Vural A, Koçyiğit İ, Şan F, Eroğlu E, Ketenci İ, Ünal A, Tokgöz B, Ünlü Y. Long-term protective effect of N-acetylcysteine against amikacin-induced ototoxicity in end-stage renal disease: a randomized trial. Perit Dial Int. 2018;38:57–62. doi: 10.3747/pdi.2017.00133. [DOI] [PubMed] [Google Scholar]
- 31.Perna AF, Violetti E, Lanza D, Sepe I, Bellinghieri G, Savica V, Santoro D, Satta E, Cirillo G, Lupo A, Abaterusso C, Raiola I, Raiola P, Coppola S, Di Iorio B, Tirino G, Cirillo M, Ingrosso D, De Santo NG. Therapy of hyperhomocysteinemia in hemodialysis patients: effects of folates and N-acetylcysteine. J Ren Nutr. 2012;22:507–514. doi: 10.1053/j.jrn.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Scholze A, Rinder C, Beige J, Riezler R, Zidek W, Tepel M. Acetylcysteine reduces plasma homocysteine concentration and improves pulse pressure and endothelial function in patients with end-stage renal failure. Circulation. 2004;109:369–374. doi: 10.1161/01.CIR.0000109492.65802.AD. [DOI] [PubMed] [Google Scholar]
- 33.Thaha M, Yogiantoro M, Tomino Y. Intravenous N-acetylcysteine during haemodialysis reduces the plasma concentration of homocysteine in patients with end-stage renal disease. Clin Drug Investig. 2006;26:195–202. doi: 10.2165/00044011-200626040-00003. [DOI] [PubMed] [Google Scholar]
- 34.Thaha M, Widodo , Pranawa W, Yogiantoro M, Tomino Y. Intravenous N-acetylcysteine during hemodialysis reduces asymmetric dimethylarginine level in end-stage renal disease patients. Clin Nephrol. 2008;69:24–32. doi: 10.5414/cnp69024. [DOI] [PubMed] [Google Scholar]
- 35.Tsai JP, Yang FL, Wang CH, Fang TC, Lee RP, Hsu BG. Effect of intravenous N-acetylcysteine on plasma total homocysteine and inflammatory cytokines during high flux hemodialysis. Tzu Chi Medical Journal. 2010;22:90–95. [Google Scholar]
- 36.Grandjean E, Berthet P, Ruffman R, Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind placebo-controlled clinical trials. Clin Ther. 2000;22:209–221. doi: 10.1016/S0149-2918(00)88479-9. [DOI] [PubMed] [Google Scholar]
- 37.He JJ, Huang F. The observation of clinical effectiveness to treat chronic liver failure by using intravenous acetylcysteine. Chinese Journal of Modern Applied Pharmacy. 2000;17:327–329. [Google Scholar]
- 38.US Renal Data System. USRDS: “Atlas of end-stage renal disease in the United States”, Annual Data Report. Bethesda, Md, USA: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009. [Google Scholar]
- 39.Manjunath G, Tighiouart H, Coresh J. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63:1121–1129. doi: 10.1046/j.1523-1755.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- 40.Becker BN, Himmelfarb J, Henrich WL, Hakim RM. Reassessing the cardiac risk profile in chronic hemodialysis patients: a hypothesis on the role of oxidant stress and other non-traditional cardiac risk factors. J Am Soc Nephrol. 1997;8:475–486. doi: 10.1681/ASN.V83475. [DOI] [PubMed] [Google Scholar]
- 41.Clermont G, Lecour S, Lahet J, Siohan P, Vergely C, Chevet D. Alteration in plasma antioxidant capacities in chronic renal failure and hemodialysis patients: a possible explanation for increased cardiovascular risk in these patients. Cardiovasc Res. 2000;18:618–623. doi: 10.1016/s0008-6363(00)00117-6. [DOI] [PubMed] [Google Scholar]
- 42.Miyazaki H, Matsuoka H, Itabe H, Usui M, Ueda S, Okuda S, Imaizumi T. Hemodialysis impairs endothelial function via oxidative stress: effects of vitamin E-coated dialyzer. Circulation. 2000;101:102–106. doi: 10.1161/01.cir.101.9.1002. [DOI] [PubMed] [Google Scholar]
- 43.Mezzano D, Pais EO, Aranda E, Panes O, Downey P, Ortiz M, Tagle R, González F, Quiroga T, Caceres MS, Leighton F, Pereira J. Inflammation, not hyper homocysteinemia, is related to oxidative stress and hemostatic and endothelial dysfunction in uremia. Kidney Int. 2001;60:1844–1850. doi: 10.1046/j.1523-1755.2001.00996.x. [DOI] [PubMed] [Google Scholar]
- 44.Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Bush AI. N-acetylcysteine for depressive symptoms in bipolar disorder--a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:468–475. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 45.Nogueira GB, Punaro GR, Oliveira CS, Maciel FR, Fernandes TO, Lima DY, Rodrigues AM, Mouro MG, Araujo SRR, Higa EMS. N-acetylcysteine protects against diabetic nephropathy through control of oxidative and nitrosative stress by recovery of nitric oxide in rats. Nitric Oxide. 2018;8:22–31. doi: 10.1016/j.niox.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Xing Y, Wei RB, Tang L, Yang Y, Zheng XY, Wang ZC, Gao YW. Protective effect of salidroside on contrast-induced nephropathy in comparison with N-acetylcysteine and its underlying mechanism. Chin J Integr Med. 2015;21:266–273. doi: 10.1007/s11655-015-2137-y. [DOI] [PubMed] [Google Scholar]
- 47.Briguori C, Visconti G, Rivera N, Focaccio A, Golia B, Giannone R, Castaldo D, Micco De F, Ricciardelli B, Colombo A. Cystatin C and contrast-induced acute kidney injury. Circulation. 2010;121:2117–2122. doi: 10.1161/CIRCULATIONAHA.109.919639. [DOI] [PubMed] [Google Scholar]
- 48.Dittmann S, Seemüller F, Schwarz MJ, Kleindienst N, Stampfer R, Zach J, Born C, Bernhard B, Fast K, Grunze H, Engel RR, Severus E. Association of cognitive deficits with elevated homocysteine levels in euthymic bipolar patients and its impact on psychosocial functioning: preliminary results. Bipolar Disord. 2007;9:63–70. doi: 10.1111/j.1399-5618.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 49.Jun M. Antioxidants for chronic kidney disease. Nephrology (Carlton) 2013;18:576–578. doi: 10.1111/nep.12103. [DOI] [PubMed] [Google Scholar]
- 50.Xu L, Luo Q. Effects of N-acetylcysteine on inflammation factor in maintenance hemodialysis patients. Journal of Taishan Medical College. 2011;32:91–93. [Google Scholar]
- 51.Romano G, Briguori C, Quintavalle C, Zanca C, Rivera NV, Colombo A, Condorelli G. Contrast agents and renal cell apoptosis. Eur Heart J. 2008;29:2569–2576. doi: 10.1093/eurheartj/ehn197. [DOI] [PubMed] [Google Scholar]
- 52.Tian N, Rose R, Jordan S, Dwyer TM, Hughson MD, Manning RD Jr. N-acetylcysteine improves renal dysfunction, ameliorates kidney damage and decreases blood pressure in salt-sensitive hypertension. J Hypertens. 2006;24:2263–2270. doi: 10.1097/01.hjh.0000249705.42230.73. [DOI] [PubMed] [Google Scholar]


