Abstract
Objective: To investigate the roles and mechanisms of miR-195-5p and forkhead box K1 (FOXK1) in rat xenograft models of non-small cell lung cancer (NSCLC). Methods: Rat xenograft models of NSCLC were established. Evaluations of morphology of NSCLC cells and levels of Ki67 and P53 were detected by hematoxylin-eosin (HE) staining and immunohistochemistry (IHC), respectively. The miR-195-5p level in NSCLC was measured by quantitative real-time RT-PCR (qRT-PCR), and FOXK1, Bax, Caspase-3 and Bal-2 levels were quantified by Western blot. And the regulatory relation between miR-195-5p and FOXK1 was determined by dual-luciferase reporter (DLR) assay. Results: HE staining and IHC demonstrated successful establishment of NSCLC models in which miR-195-5p was downregulated and FOXK1 was upregulated. Pearson correlation showed that miR-195-5p and FOXK1 were inversely associated (r=0.551, P=0.012). DLR assay confirmed the targeted regulatory relation between miR-195-5p and FOXK1, and upregulation of miR-195-5p accelerated apoptosis of tumor cells. Conclusion: miR-195-5p is inversely associated with FOXK1 in NSCLC in rats. Upregulation of miR-195-5p suppresses FOXK1 and accelerates apoptosis of tumor cells, which may serve as an therapeutic target for the treatment of NSCLC.
Keywords: Non-small cell lung cancer, miR-195-5p, FOXK1, mechanism
Introduction
Lung cancer (LC) is the primary cause of cancer-related deaths worldwide [1], with the highest morbidity and mortality of all malignancies in China [2]. The global death toll from LC is expected to rise to 3 million by 2035 [3]. At present, chemotherapy, radiotherapy and radical resection are preferred treatments for non-small cell lung cancer (NSCLC) [4]. However, at least 30% of patients relapse within 5 years after surgical resection [5]. First-line platinum-based chemotherapy, the standard treatment for advanced NSCLC, can only maintain a median progression-free survival (PFS) of ~6 months and a 30% remission rate [6]. Therefore, more effective treatment to improve the prognosis of NSCLC become urgent.
MicroRNAs (miRs) are a class of non-coding short-chain RNAs that promote the degradation of transcripts by binding to sequences of 3’ untranslated region (3’UTR) of target mRNAs, which may affect cell proliferation, apoptosis and other biological functions [7]. miRs act as tumor suppressors or oncogenes to regulate their target genes, which becomes a new perspective for cancer treatment [8]. There is evidence that miR-195-5p expression is suppressed in renal clear cell carcinoma [9], melanoma [10] and breast cancer [11], and participates in their progression. A domestic study reveals the diagnostic and prognostic value of serum miR-195-5p in NSCLC [12]. Besides, Zheng reports that miR-195-5p is down-regulated in NSCLC tissues and cell lines, and its up-regulation inhibits the proliferation of those cells [13]. In addition, Ma et al. indicates that FOXK1 plays an important role in regulation of cell proliferation and invasion of NSCLC [14]. A previous study has also shown that miR-195 acts as a tumor suppressor in NSCLC and its overexpression slows down tumor growth [15]. Therefore, we speculate that miR-195-5p may function as a potential therapeutic target for CLC. However, its specific role and mechanism in NSCLC has been poorly dissected.In the present study, FOXK1 was found to be a target of miR-195-5p via targetscan.
Therefore, we established a xenograft rat model of NSCLC to investigate the roles of miR-195-5p and FOXK1 in NSCLC, so as to provide more therapeutic targets for its treatment.
Materials and methods
Materials and treatments
Laboratory animals
Sixty male SD rats [Vital River, Shanghai, China], aged 9-11 weeks, weighing ~210-255 g, were raised at 23-28°C for 7 days. All operations conform to the standards of the Laboratory Animal Ethics Committee.
Establishment of rat xenograft models of NSCLC
Sixty rats were allocated into control group (n=20), NSCLC group (n=20) and miR-195-5p-agomir group (agomir group, n=20). Suspensions (1×106/mL) of NSCLC cell line HCC827 (Procell Life Science & Technology Co., Ltd., Wunhan, China) were prepared with Hank’s solution, and a volume of 0.2 mL was injected intravenously into the back of rats in NSCLC group and agomir group, then they were fed for 7 consecutive days to establish models of NSCLC. Whereas controls were treated by same dose of normal saline. After modeling, agomir group was treated with 100 μL miR-195-5p-agomir (100 nmol/ml, normal saline as solvent) via tail vein, NSCLC group was injected with 100 μL miR-NC (100 nmol/mL, normal saline as solvent), and control group with same dose of normal saline, twice a week for 4 consecutive weeks. All rats were anesthetized in anesthesia induction chambers filled with 4% isoflurane (KEW Biotech, Nanjing, China, C002). Rats that fell over without attempting to restore their prone posture when gently shaking the chamber were considered fully anesthetized. Afterwards, all anesthetized rats were cervically dislocated and lung tissues were removed. Animal experiments were approved by animal experiment ethics committee.
Cell culture and transfection
Cell culture: NSCLC cell line HCC827 was cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37°C and 5% CO2. When reaching 80% confluence, cells were digested with 0.25% trypsin for passage. Cell transfection: miR-195-5p-mimics group, miR-NC group, si-FOXK1 group and si-NC group were treated with miR-195-5p-mimics, miR-NC, si-FOXK1 and si-NC by LipofectamineTM 2000 kit (Invitrogen, USA), respectively.
Detection methods
Quantitative real-time RT-PCR (qRT-PCR)
Purity and concentration of total RNAs extracted with Trizol were measured by an ultraviolet spectrophotometer. Then reverse transcription was performed on 5 μg of total RNAs. Reaction parameters: 37°C for 15 min, 42°C for 35 min and 70°C for 5 min. Amplification: 94°C for 45 sec, 40 cycles of 94°C for 10 sec and 60°C for 45 sec. The test was repeated for 3 times. Taking U6 as the internal reference, data analyses were performed with 2-ΔΔCT.
Hematoxylin-eosin (HE) staining
The tissues were embedded in paraffin and cut into thin slices; Afterwards, they were dewaxed with xylene, dehydrated with ethanol, stained with hematoxylin solution, dehydrated again with ethanol, and stained again with eosin, next, dehydrated with ethanol, cleared in xylene and mounted.
Western blot
The collected tissues and cells were lysed with radio immunoprecipitation assay (RIPA), and the concentration of total protein was tested by bicinchoninic acid (BCA) and kept at 4 μg/μL. After separation with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane, then stained in Ponceau S and immersed in phosphate buffered saline with Tween 20 (PBST) for 5 min. Afterwards, the membrane was washed and blocked with 5% non-fat milk powder for 2 h, followed by an overnight incubation with primary antibodies against Bax, Bcl-2, active caspase-3, FOXK1 and β-Actin (all 1:500) at 4°C. Subsequently, the membrane was washed again for the removal of primary antibodies, and HRP-coagulated goat anti-rabbit secondary antibodies (1:1000) were adopted for a 1 h incubation at 37°C, followed by three 5-min rinses with PBS. Protein bands were visualized using ECL reagent in the dark.
Immunohistochemistry (IHC)
Levels of Ki67 and P53 were measured by IHC. The primary and secondary antibodies and IHC kit were all purchased from Roche, Switzerland. Tumor tissues were embedded in paraffin and cut into thin slices, and hydrated in a gradient ethanol, immersed in 3% hydrogen peroxide for 10 min, rinsed with double distilled water, soaked in ethylenediamine tetraacetic acid (EDTA) solution for 2 min, then cooled in tap water for 20 min. Following three PBS washes, they were incubated with primary antibodies at 32°C for 40 min. PBS was used as negative control, and positive sections as positive controls. After three 5-min washes with PBS, another incubation was performed with 50 μL of anti-post primary block at 32°C for 15 min. Following repeating the above steps once, a 3-time PBS washing was performed again (5 min/time), and diaminobenzidine (DAB) was added for a 1-min color development. The tissues were re-stained with hematoxylin for 1 min, then washed and mounted for observation under a microscope.
Cell proliferation assay
The transfected cells prepared into a suspension were grown in a 96-well plate (100 μL/well). Each sample was tested in 3 repeated wells. CCK8 reagents were added at 24 h, 48 h, 72 h, 96 h, and cultured at 37°C and 5% CO2 for 2 h. The absorbance at 490 wavelength was measured and growth curves were plotted.
Cell migration and invasion assays
A total of 500 μL medium containing 10% FBS was added to the basolateral chamber, and 100 μL cells (2×105 cells/mL) were added to the apical chamber and cultured for 24 h. After removing cells in the apical chamber, 4% paraformaldehyde and 0.1% crystal violet were employed to dye the cells migrating to the basolateral chamber. After drying, the membrane was sealed, and the cells were counted under a microscope. The invading cells were counted under 5 visual fields.
Dual-luciferase reporter (DLR) assay
Targetscan7.2 was used to predict downstream target genes of miR-195-5p. FOXK1-3’UTR wild type (Wt), FOXK1-3’UTR mutant (Mut), miR-195-5p-mimics and miR-NC were separately transferred into HEK293T cells by the LipofectamineTM 2000 kit. A DLR assay kit (Promega, USA) evaluated the luciferase activity 48 h after transfection.
Statistical analysis
Statistical analyses were carried out with SPSS20.0 (IBM, Armonk, NY, USA), and figure plotting with GraphPad 7. Inter-group comparisons were performed by independent samples t test, multi-group comparisons by one-way ANOVA, and post-hoc pairwise comparisons by LSD-t test. Pearson correlation analyzed the correlation between miR-195-5p and FOXK1 in tumor tissues. Differences were statistically significant at P < 0.05.
Results
Rat models of NSCLC
Paraffin sections of lung tissues were stained with HE. In NSCLC group, a large number of tumor foci were formed in the lungs of rats, epithelial cells were damaged, and tumor cells were arranged in a flaky or stringy disorder. In contrast, in control group, no foci were found in the lungs, and lung cells were in normal morphology and orderly arranged. The IHC showed that the positive staining for Ki67 and P53 in tumor cells in the control group was significantly lower than that in the NSCLC group, indicating that the lung tumor in the NSCLC group was NSCLC indeed (Figure 1).
Figure 1.
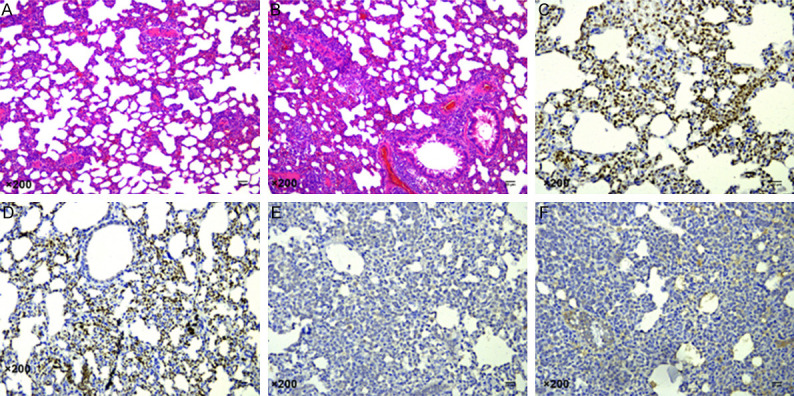
Microscopic images of lung tissues (×200). A. Lung tissues of normal rats. B. Lung tissues of NSCLC rats. C. Levels of Ki67 in lung tissues of normal rats. D. Levels of Ki67 in lung tissues of NSCLC rats. E. Levels of P53 in lung tissues of normal rats. F. Levels of P53 in lung tissues of NSCLC rats.
miR-195-5p and FOXK1 in NSCLC tissues
Compared with control group, NSCLC group presented dwonregulated miR-195-5p and upregulated FOXK1 in NSCLC tissues of rats (both P < 0.05). And miR-195-5p and FOXK1 were revealed to be inversely associated (r=-0.551, P=0.012) by Pearson correlation. The scatter plot demonstrated that FOXK1 decreased with the increase of miR-195-5p (Figure 2).
Figure 2.
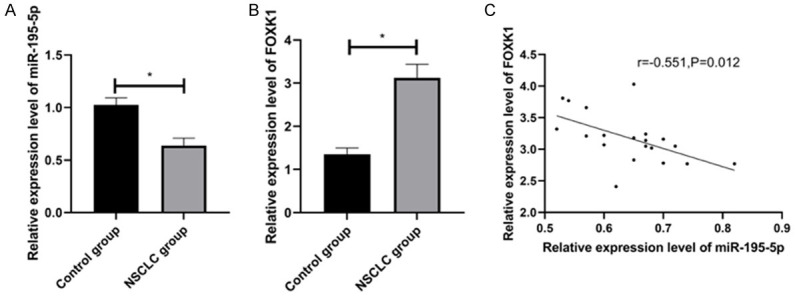
miR-195-5p and FOXK1 in NSCLC tissues. A. miR-195-5p in NSCLC tissues. B. FOXK1 in NSCLC tissues. C. Correlation between miR-195-5p and FOXK in NSCLC tissues. *P < 0.05.
miR-195-5p-agomir interferes with NSCLC tissues
NSCLC group had lower miR-195-5p and higher FOXK1 in NSCLC group than those in agomir group (P < 0.05). By detecting apoptosis-related proteins, it was found that compared with the control group and NSCLC group, Bcl-2 in agomir group was downregulated and Bax and Caspase-3 were upregulated (P < 0.05) (Figure 3).
Figure 3.
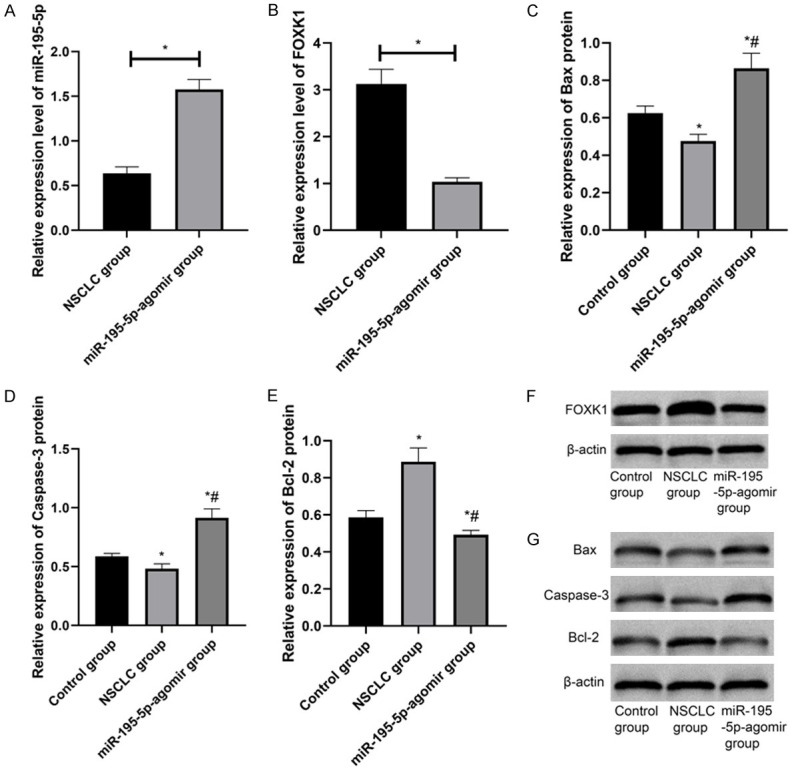
miR-195-5p-agomir interferes with NSCLC tissues. A. Effect of miR-195-5p-agomir intervention on miR-195-5p in tumor tissues of rats. B. Effect of miR-195-5p-agomir intervention on FOXK1 in tumor tissues of rats. C-E. Effect of miR-195-5p-agomir intervention on apoptosis-related proteins in tumor tissues of rats. F, G. WB. *P < 0.05 vs. control group, #P < 0.05 vs. NSCLC group.
Effects of miR-195-5p and FOXK1 on proliferation of HCC827 cells
Compared with miR-NC group, miR-195-5p in miR-195-5p-mimics group was evidently up-regulated. And FOXK in si-FOXK1 group was evidently down-regulated than that in si-NC group. Transfections of miR-195-5p-mimics and si-FOXK1 slowed the proliferation of HCC827 cells (Figure 4).
Figure 4.
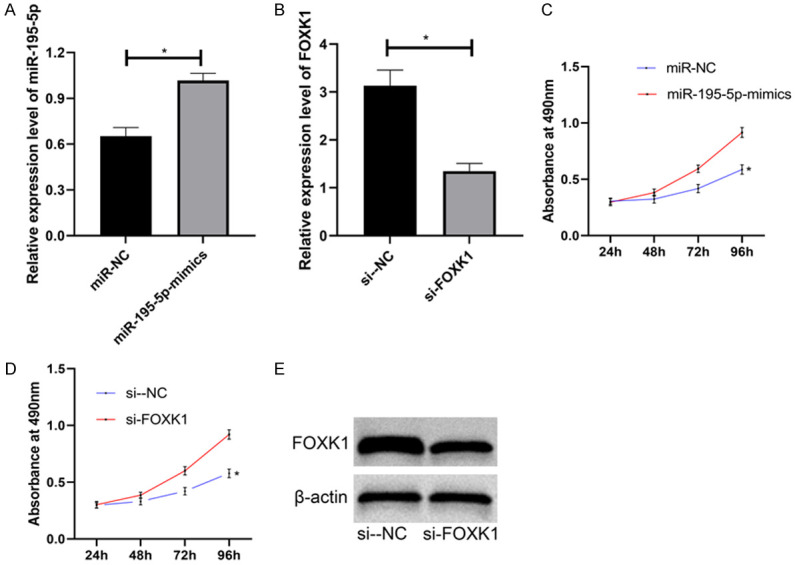
Effects of miR-195-5p and FOXK1 on proliferation of HCC827 cells. A, B. Relative expression of miR-195-5p and FOXK1 in HCC827 cells. C, D. Effects of miR-195-5p and FOXK1 on proliferation of HCC827 cells. E. WB.
Effects of miR-195-5p and FOXK1 on migration and invasion of HCC827 cells
After transfection, the migration and invasion of HCC827 cells in miR-195-5p-mimics group were weakened than those in miR-NC group, and in si-FOXK1 group were lower than those in si-NC group (P < 0.05) (Figure 5).
Figure 5.
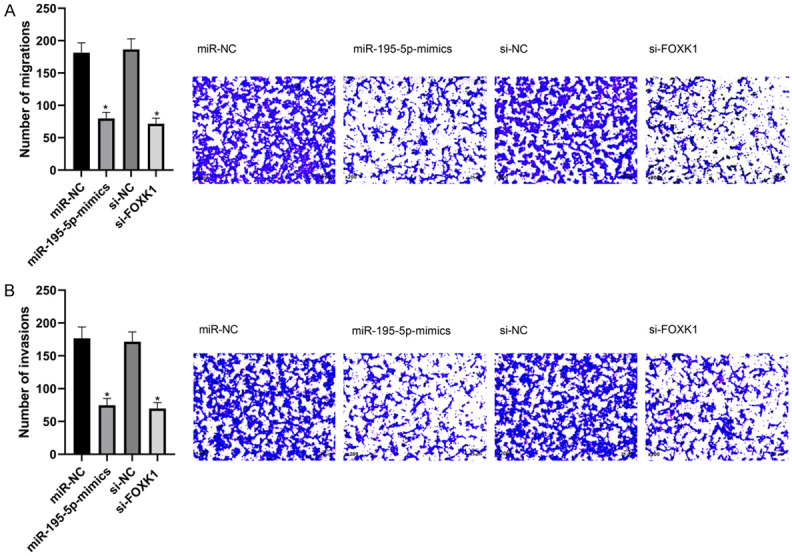
Effects of miR-195-5p and FOXK1 on migration and invasion of HCC827 cells. A. Effects of miR-NC, miR-195-5p-mimics, si-NC, and si-FOXK1 on migration of HCC827 cells. *P < 0.05. B. Effects of miR-NC, miR-195-5p-mimics, si-NC, and si-FOXK1 on invasion of HCC827 cells. *P < 0.05.
DLR assay
We used Targetscan to predict miR-195-5p’s downstream target genes. Targeted binding loci between miR-195-5p and FOXK1 were identified. Therefore, we carried out a DLR assay and found that overexpressing miR-195-5p suppressed FOXK1-3’UTR Wt luciferase activity (P < 0.05), while exerted no effects on FOXK1-3’UTR Mut (P > 0.05). As shown by western blot, FOXK1 in cells treated with miR-195-5p-mimics was reduced (P < 0.05) (Figure 6).
Figure 6.
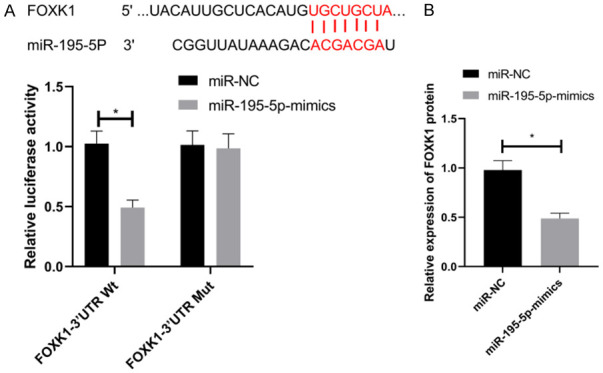
DLR assay. A. Effects of miR-195-5p on luciferase activity of FOXK1. B. Effects of FOXK1 in cells transfected with miR-195-5p-mimics. *P < 0.05.
Discussion
Although great progress has been made in the development of anti-cancer therapies, LC remains one of the most deadly malignant tumors, and patients in advanced stages still have a poor prognosis [16]. Only 16% of treated NSCLC patients achieved 5-year survival [17]. Therefore, it is urgent to understand the pathogenesis of NSCLC for the development of new therapeutic strategies.
MiRs are considered to be potential targets for the development of new anti-cancer drugs due to their roles as oncogenes or tumor suppressors [18]. miR-195 plays a pivotal regulatory role in multiple cancers including NSCLC [15]. It inhibits cell migration and invasion in cervical cancers by inhibiting ADP ribosylation factor-like protein 2 (Arl2) [19]. And by sponging miR-195-5p, circAGFG1 regulates cyclin E1 (CCNE1) and participates in triple negative breast cancer [9]. FOXK family is a subclass of Forkhead transcription factors which are known to regulate cell cycle, apoptosis, differentiation, metabolism, survival, and other cellular biological processes [20,21]. FOXK1 induces epithelial-mesenchymal transition (EMT) of tumor cells and regulates invasion and metastasis of cancers [22]. Besides, it accelerates cell proliferation, migration and invasion in glioma, gastric cancer, and LC [23]. As potential binding loci between miR-195-5p and FOXK1 were found by targetscan, we therefore investigated whether miR-195-5p affects FOXK1 in NSCLC. Firstly, rat models of NSCLC were established, and low level of miR-195-5p and high level of FOXK1 were shown in NSCLC tissues. Secondly, a negative correlation between the two was revealed by Pearson correlation. Therefore, miR-195-5p was closely related to FOXK1 in NSCLC by negatively regulating FOXK1. Wu et al. [24] reports that plasmacytoma variant translocation gene 1 (PVT1) can promote the proliferation of NSCLC cells by inhibiting miR-195, and knocking down PVT1 or overexpressing miR-195 improves the efficacy of in vitro radiotherapy in NSCLC.
To verify how miR-195-5p and FOXK1 participate in NSCLC, we upregulated miR-195-5p expression and found that FOXK1 was evidently reduced in NSCLC tissues. Bax and Bcl-2 are associated with cell apoptosis [25]. Enhancement of caspase-3 activity usually portends an apoptosis and is a positive indicator of treatment efficacy [26]. This study revealed that miR-195-5p decreased levels of Bcl-2 and increased levels of Bax and active caspase-3, indicating that miR-195-5p accelerates apoptosis and suppresses NSCLC progression by inhibiting FOXK1. The finding that miR-195-5p hinders biological ability of LC cells by regulating FOXK1 [27] confirms the reliability of our conclusions. Restricted proliferation, migration and invasion of HCC827 cells treated with miR-195-5p-mimics and si-FOXK1 demonstrated in this study are also consistent to above conclusions. Finally, we used DIR assay to detect the regulatory relationship between the two indicators. It showed that overexpression of miR-195-5p suppressed FOXK1-3’UTR Wt luciferase activity, and FOXK1 in cells was evidently reduced after miR-195-5p-mimics intervention. This indicates that miR-195-5p participates in NSCLC via regulating FOXK1. The present study suggests that FOXK1 has carcinogenic effect and is the direct target of miR-195-5p. Up-regulation of miR-195-5p suppresses FOXK1, while the absence of FOXK1 inhibits the growth and metastasis of NSCLC cells, thereby inhibiting the growth of the tumor.
To sum up, miR-195-5p promotes apoptosis of NSCLC cells in rats via regulating FOXK1, which may become potential therapeutic target for NSCLC. This manuscript can be improvement. Firstly, whether miR-195-5p has other targets for NSCLC remains unknown. Secondly, the possible downstream mechanism of FOXK1 is unclear. We will carry out more research to elaborate the mechanism of miR-195-5p in NSCLC in the future.
Acknowledgements
The Natural Science Foundation of Gansu Province, project number 17JR5RA169, Technical Research and Development Special Project of Gansu Province No. 1105TCYA019 and Scientific research project of colleges and universities in Gansu Province Project number 2016A-045.
Disclosure of conflict of interest
None.
References
- 1.Pinsky PF. Lung cancer screening with low-dose CT: a world-wide view. Transl Lung Cancer Res. 2018;7:234–242. doi: 10.21037/tlcr.2018.05.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Didkowska J, Wojciechowska U, Mańczuk M, Łobaszewski J. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med. 2016;4:150. doi: 10.21037/atm.2016.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, Escriu C, Peters S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 5.Cedrés S, Torrejon D, Martínez A, Martinez P, Navarro A, Zamora E, Mulet-Margalef N, Felip E. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol. 2012;14:864–869. doi: 10.1007/s12094-012-0872-5. [DOI] [PubMed] [Google Scholar]
- 6.Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII) Am J Cancer Res. 2015;5:2892–2911. [PMC free article] [PubMed] [Google Scholar]
- 7.Gareev IF, Novicova LB, Beylerli OA. Circulating microrPas as new potential biomarkers for the diagnosis of high-grade gliomas. Zh Nevrol Psikhiatr Im S S Korsakova. 2019;119:86–90. doi: 10.17116/jnevro201911905186. [DOI] [PubMed] [Google Scholar]
- 8.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 9.Yang R, Xing L, Zheng X, Sun Y, Wang X, Chen J. The circRNA circAGFG1 acts as a sponge of miR-195-5p to promote triple-negative breast cancer progression through regulating CCNE1 expression. Mol Cancer. 2019;18:4. doi: 10.1186/s12943-018-0933-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Wang K, Sun Y, Tao W, Fei X, Chang C. Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett. 2017;394:1–12. doi: 10.1016/j.canlet.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 11.Chai L, Kang XJ, Sun ZZ, Zeng MF, Yu SR, Ding Y, Liang JQ, Li TT, Zhao J. MiR-497-5p, miR-195-5p and miR-455-3p function as tumor suppressors by targeting hTERT in melanoma A375 cells. Cancer Manag Res. 2018;10:989–1003. doi: 10.2147/CMAR.S163335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Q, Wei C, Li X, Li J, Chen L, Huang Y, Song H, Li D, Fang L. MicroRNA-195-5p is a potential diagnostic and therapeutic target for breast cancer. Oncol Rep. 2014;31:1096–1102. doi: 10.3892/or.2014.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng J, Xu T, Chen F, Zhang Y. MiRNA-195-5p functions as a tumor suppressor and a predictive of poor prognosis in non-small cell lung cancer by directly targeting CIAPIN1. Pathol Oncol Res. 2019;25:1181–1190. doi: 10.1007/s12253-018-0552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma X, Yang X, Bao W, Li S, Liang S, Sun Y, Zhao Y, Wang J, Zhao C. Circular RNA circMAN2B2 facilitates lung cancer cell proliferation and invasion via miR-1275/FOXK1 axis. Biochem Biophys Res Commun. 2018;498:1009–1015. doi: 10.1016/j.bbrc.2018.03.105. [DOI] [PubMed] [Google Scholar]
- 15.Yu X, Zhang Y, Cavazos D, Ma X, Zhao Z, Du L, Pertsemlidis A. miR-195 targets cyclin D3 and survivin to modulate the tumorigenesis of non-small cell lung cancer. Cell Death Dis. 2018;9:193. doi: 10.1038/s41419-017-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 17.Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, Powderly J, Heist R, Sequist LV, Smith DC, Leming P, Geese WJ, Yoon D, Li A, Brahmer J. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J. Clin. Oncol. 2018;36:1675–1684. doi: 10.1200/JCO.2017.77.0412. [DOI] [PubMed] [Google Scholar]
- 18.Kleemann M, Schneider H, Unger K, Sander P, Schneider EM, Fischer-Posovszky P, Handrick R, Otte K. MiR-744-5p inducing cell death by directly targeting HNRNPC and NFIX in ovarian cancer cells. Sci Rep. 2018;8:9020. doi: 10.1038/s41598-018-27438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan SS, Zhou HE, Yu HY, Xu LH. MiR-195-5p inhibits the cell migration and invasion of cervical carcinoma through suppressing ARL2. Eur Rev Med Pharmacol Sci. 2019;23:10664–10671. doi: 10.26355/eurrev_201912_19764. [DOI] [PubMed] [Google Scholar]
- 20.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 21.Lam EW, Brosens JJ, Gomes AR, Koo CY. Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer. 2013;13:482–495. doi: 10.1038/nrc3539. [DOI] [PubMed] [Google Scholar]
- 22.Peng Y, Zhang P, Huang X, Yan Q, Wu M, Xie R, Wu Y, Zhang M, Nan Q, Zhao J, Li A, Xiong J, Ren Y, Bai Y, Chen Y, Liu S, Wang J. Direct regulation of FOXK1 by C-jun promotes proliferation, invasion and metastasis in gastric cancer cells. Cell Death Dis. 2016;7:e2480. doi: 10.1038/cddis.2016.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao F, Tian J. FOXK1, regulated by miR-365-3p, promotes cell growth and EMT indicates unfavorable prognosis in breast cancer. Onco Targets Ther. 2020;13:623–634. doi: 10.2147/OTT.S212702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu D, Li Y, Zhang H, Hu X. Knockdown of Lncrna PVT1 enhances radiosensitivity in non-small cell lung cancer by sponging Mir-195. Cell Physiol Biochem. 2017;42:2453–2466. doi: 10.1159/000480209. [DOI] [PubMed] [Google Scholar]
- 25.Zhao L, Gu Q, Xiang L, Dong X, Li H, Ni J, Wan L, Cai G, Chen G. Curcumin inhibits apoptosis by modulating Bax/Bcl-2 expression and alleviates oxidative stress in testes of streptozotocin-induced diabetic rats. Ther Clin Risk Manag. 2017;13:1099–1105. doi: 10.2147/TCRM.S141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 27.Long Z, Wang Y. miR-195-5p suppresses lung cancer cell proliferation, migration, and invasion via FOXK1. Technol Cancer Res Treat. 2020;19:1533033820922587. doi: 10.1177/1533033820922587. [DOI] [PMC free article] [PubMed] [Google Scholar]


