Abstract
Objective: To analyze the effects of botulinum toxin type A (BtA) in the treatment of patients with Parkinson’s disease and depression. Method: 89 patients with Parkinson’s disease and depression were assigned into control group and observation group by random number table method, of which 44 patients in the control group were treated with sertraline and 45 patients in the observation group were treated with BtA. The two groups were compared in terms of mood, cognitive function and adverse reactions. Results: The Hamilton Depression Self-Assessment Scale (HAMD) scores of the two groups following treatment were lower than those before treatment while the Mini-Mental State Examination (MMSE) scores were higher than those before treatment (P<0.05). The incidence rate of adverse events was 11.11% in the observation group and 29.55% in the control group (P<0.05). The Pittsburgh sleep quality index (PSQI) scores and 39-item PD Questionnaire (PDQ-39) scores after 2 and 3 months of treatment and 2 months after completion of treatment were lower than those before treatment (P<0.05). Conclusion: Patients with Parkinson’s disease and depression receiving BtA treatment can gain treatment effects similar to those of the sertraline, with less adverse reactions.
Keywords: Parkinson’s disease, depression, botulinum toxin type A, treatment, mood, cognition
Introduction
Parkinson’s disease (PD) is one of the most prevalent neurological disorders, with the elderly being the predominant population and the incidence increasing with age [1]. Patients with PD exhibit a variety of motor and non-motor symptoms, which include postural instability, rest tremor, muscle tone, and slowness of movement, while non-motor symptoms commonly include decreased sleep quality, pain, fatigue, autonomic dysfunction, and depression [2,3].
Depression is the most prevalent type of non-motor symptoms in patients with PD. Statistics show that the incidence rate of depression in patients with PD is about 40%, most of whom are mildly depressed, but a very small proportion of patients will experience severe depression [4]. Studies have shown that risk factors for depression in patients with PD include gender, gait abnormalities, and severe movement disorders [5]. It has been found that depression can occur early or late in the course of PD and some patients even have depressive symptoms before the onset of PD, which is considered to be one prodromal manifestation of PD [6]. Patients with PD combined with depressive symptoms may experience irritability, loss of pleasure, persistent depressed mood, pessimism, and some may even show suicidal tendencies, posing a serious threat to their health and safety. Therefore, it is very important for patients with PD to prevent and control depressive symptoms. Selective serotonin reuptake inhibitors are widely prescribed, and sertraline is the representative drug. However, this drug has a lot of adverse reactions and needs to be used continuously for a longer period of time, so it is difficult to ensure patient compliance [7]. Botulinum toxin type A (BtA) was mostly used in cosmetic industry and neurology treatment, but it was gradually found to have some improvement effects on depression [8]. Studies using BtA to treat multiple cases of depression have shown remission rates of over 90% [9]. A randomized controlled study found that BtA was effective in controlling primary depression [10]. However, whether this drug has the same therapeutic effect on depression caused by PD has not been confirmed by studies.
In this study, 89 patients with PD and depression were enrolled to analyze the effectiveness of BtA in their treatment to explore more feasible treatment options for PD and depression.
Materials and methods
Data
The 89 patients with PD and depression admitted to our hospital from January 2019 to December 2019 were divided into a control group (CNG) and an observation group (OG) by random numerical table method, with 44 and 45 patients in each group, respectively. Inclusion criteria: patients who met diagnostic criteria of PD [11] and depression [12]; patients who had been receiving regular antiparkinson drugs and had not received other antidepressant medication within 1 month prior to the study. All the patients signed informed consent. This study had obtained the ethical approval of Zhangqiu District People’s Hospital. Exclusion criteria: patients who were taking antipsychotic medication prior to study, with family history of psychiatric disorders, co-morbidities of other important organs and systems, depression not caused by PD; with allergic reactions to study medication; inability to complete the full follow-up.
Methods
In addition to the regular treatment for PD, the CNG received sertraline hydrochloride tablets (H20060316, Shanxi Qianyuan Pharmaceutical Group Co., Ltd.) for antidepressant treatment, q.d.1-2 tablets for 3 months. BtA (S20030099, Allergan Ireland Pharmaceutical Company) was administrated in the OG. 100 U of BtA was diluted with 2 ml of 0.9% saline, and it was then injected into 20 different sites in the temporalis, frowning muscle, lateral canthus of the eyes, and frontalis muscle using a 1 ml syringe, q.d for 3 months (Figure 1).
Figure 1.

Schematic diagram of BtA injection sites.
Observation indicators
Hamilton Depression Self-Assessment Scale (HAMD) [13]: Eight items are scored on a 5-point scale, ranging from 0 = not present to 4 = severe. Nine are scored from 0-2. The total scale score ranged 0-76, with a score of 35 or more indicating severe depression, a score of 20-35 indicating mild or moderate depression, and a score of less than 8 indicating no depressive symptoms.
Cognitive function was assessed using the Mini-Mental State Examination (MMSE) [14], which includes temporal orientation (5 points), spatial orientation (5 points), immediate memory (3 points), attention/concentration (5 points), delayed recall (3 points), naming (2 points), verbal repetition (1 points), verbal comprehension (3 points), writing (1 points), reading a sentence (1 points), and constructional praxis (1 points). 0 point stands for incorrect or unknown answers. Any score of 27 or more (out of 30) indicates a normal cognition. Lower scores can indicate severe (≤ 9 points), moderate (10-20 points) or mild (21-26 points) cognitive impairment.
Adverse reactions including a feeling of brow muscle stiffness, headache, dizziness, gastrointestinal distress, and dry mouth were recorded.
Sleep quality was evaluated by the Pittsburgh sleep quality index (PSQI) [15] scale, which contains 19 self-rated questions and 5 questions rated by the bed partner or roommate (if one is available). With a total score of 0-21, the higher the score, the worse the sleep quality.
Quality of life (QOL) was evaluated using the 39-item PD Questionnaire (PDQ-39) [16], which assesses how often people affected by Parkinson’s disease experience difficulties across 8 dimensions of daily living including relationships, social situations and communication. With 156 points in total, the higher the score, the lower the patient’s QOL.
The above scales were all evaluated before treatment, 1 month after treatment, 2 months after treatment, 3 months after treatment, and 2 months after completion of treatment.
Statistical methods
Statistical analysis was performed with SPSS 23.0, count data were expressed as [n (%)] and examined by X2 test; Measurement data were expressed as (x̅ ± sd) and compared using t test; Multi-point comparisons were analyzed with ANVOA with post hoc F-test. Figures were drawn with Graphpad Prism 8. P<0.05 was considered statistically significant.
Results
General information
There were no significant differences in the proportion of males and females (P>0.05), mean age, duration of PD, duration of depressive symptoms, mean body mass index (BMI), levodopa equivalent daily dose, and Hoehn and Yahr scale scores between the OG and the CNG (P>0.05) (Table 1).
Table 1.
Comparison of baseline data between the two groups (x̅ ± sd)/[n (%)]
| Data | Observation group (n=45) | Control group (n=44) | t/X2 | P | |
|---|---|---|---|---|---|
| Gender | Male | 26 (57.78) | 28 (63.64) | 0.320 | 0.572 |
| Female | 19 (42.22) | 16 (36.36) | |||
| Age (years) | 68.75±11.43 | 67.52±10.19 | 0.535 | 0.594 | |
| Duration of Parkinson’s disease (years) | 5.62±2.75 | 5.81±2.83 | 0.321 | 0.749 | |
| Duration of depressive symptoms (months) | 5.32±0.59 | 5.41±0.62 | 0.702 | 0.485 | |
| BMI (kg/m2) | 22.34±1.19 | 21.98±1.05 | 1.512 | 0.134 | |
| Levodopa equivalent daily dose | 581.36±152.16 | 569.45±147.21 | 0.375 | 0.708 | |
| Hoehn-yahrrating (points) | 3.16±1.86 | 3.22±1.69 | 0.159 | 0.874 | |
BtA reduced HAMD score
There was no significant difference in HAMD scores between the observation group and the control group before treatment (P>0.05). The HAMD scores after 1, 2, and 3 months of treatment and 2 months after completion of treatment were lower in both groups than before treatment (P<0.05). There was no significant difference in HAMD scores between the observation group and the control group after 1, 2, and 3 months of treatment and 2 months after completion of treatment (Figure 2).
Figure 2.
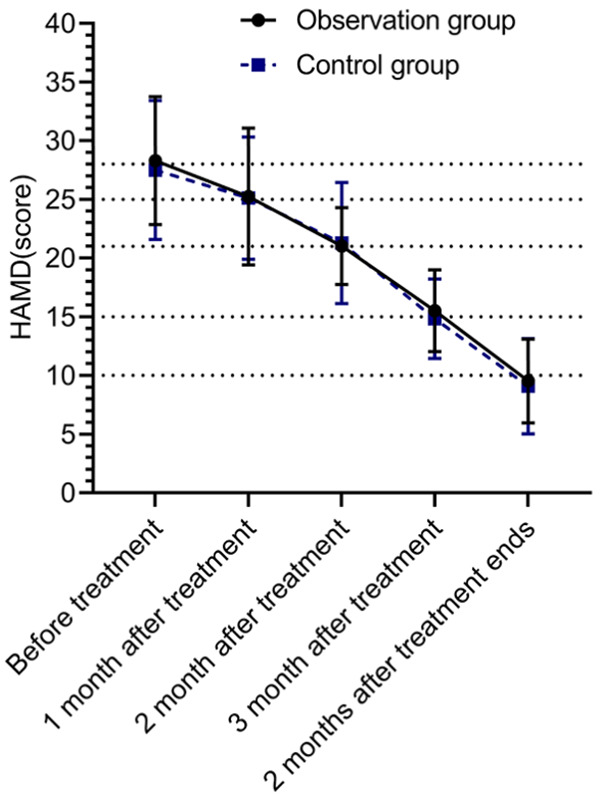
Comparison of HAMD scores. There was no significant difference in HAMD scores between the control group and the observation group before treatment, after 1, 2 and 3 months of treatment, and 2 months after completion of treatment (P>0.05). The HAMD scores after 1, 2, and 3 months of treatment and 2 months after completion of treatment were lower in both groups than those before treatment (P<0.05).
BtA improved MMSE score
The MMSE scores did not differ between the two groups before and after treatment (P>0.05), and the MMSE scores were increased significantly in both groups after treatment (P<0.05) (Figure 3).
Figure 3.
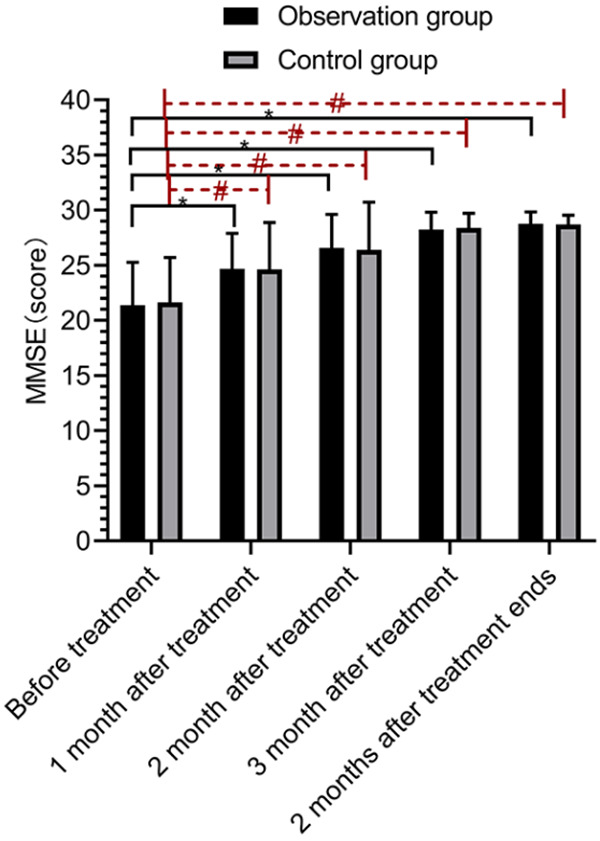
Comparison of cognitive function. There was no significant difference in cognitive function scores between the control group and the observation group before treatment, after 1, 2 and 3 months of treatment, and 2 months after completion of treatment (P>0.05). Compared with before treatment, both the observation group and the control group exhibited higher cognitive function scores after 1, 2 and 3 months of treatment and 2 months after completion of treatment (P<0.05). * indicates comparisons within the observation group, P<0.05, # indicates comparisons within the control group, P<0.05.
BtA reduced adverse reactions
The incidence rate of adverse events during BtA treatment was 11.11%, which was significantly lower compared to the 29.55% of the CNG receiving sertraline (P<0.05) (Table 2).
Table 2.
Comparison of the incidence of adverse reactions during treatment in the two groups [n (%)]
| Grouping | Stiffness in the brow muscles | Headaches | Dizziness | Gastrointestinal discomfort | Dry mouth | Total incidence rate |
|---|---|---|---|---|---|---|
| Observation group (n=45) | 2 (4.44) | 2 (4.44) | 1 (2.22) | 0 (0.00) | 0 (0.00) | 5 (11.11) |
| Control group (n=44) | 0 (0.00) | 2 (4.55) | 3 (6.82) | 4 (9.09) | 4 (9.09) | 13 (29.55) |
| X2 | 4.686 | |||||
| P | 0.030 |
BtA reduced sleep quality scores
The PSQI scores of the two groups after 2 and 3 months of treatment and 2 months following completion of treatment were lower than those before treatment (P<0.05) (Figure 4).
Figure 4.
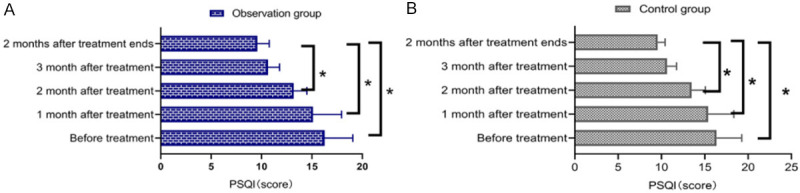
Comparison of sleep quality. There was no significant difference in sleep quality scores between the control group and the observation group before treatment, after 1, 2 and 3 months of treatment, and 2 months after completion of treatment (P>0.05). Compared with those before treatment, both the observation group (A) and the control group (B) exhibited lower sleep quality scores after 2 and 3 months of treatment and 2 months after completion of treatment (P<0.05). *P<0.05 indicates the comparison between the two groups at different times, P<0.05.
BtA reduced QOL score
There was no significant difference in PDQ-39 scores between the OG and the CNG before and after treatment (P>0.05), and the PDQ-39 scores of the two groups after 3 months of treatment and 2 months after the end of treatment were lower than those before treatment (P<0.05) (Figure 5).
Figure 5.
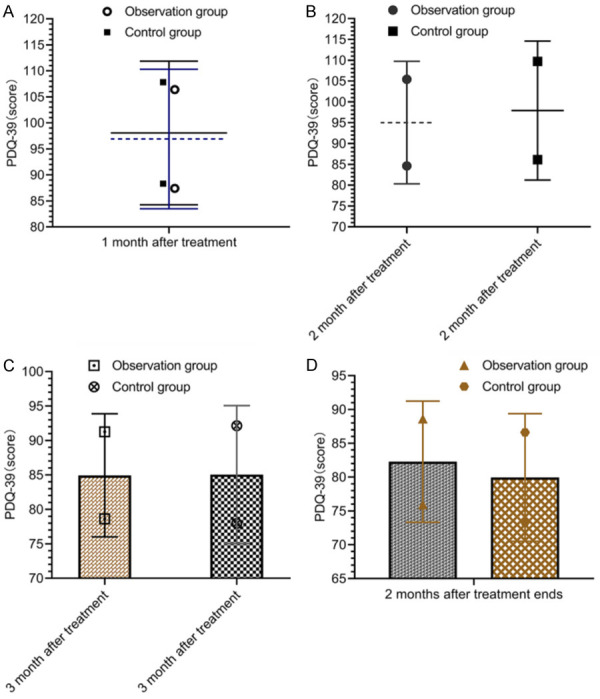
Comparison of quality of life. There was little difference in sleep quality scores between the control group and the observation group after 1 month of treatment (A), 2 months of treatment (B), 3 months of treatment (C), and 2 months after the end of treatment (D) (P>0.05).
Discussion
Depression, as a type of symptom that is prevalent in PD, is correlated with cognitive impairment and motor dysfunction, and negatively affects QOL of patients. The occurrence of depression in patients with PD was decided by medical, psychosocial, neurobiological, and genetic factors [17].
The BtA of this study is a class of neurotoxin produced by Clostridium botulinum, which can exert neurophilic effects [18]. There are seven main types of botulinum toxin, named type A-G. The BtA used in this study is the most powerful one and have shown good results in the treatment of migraine, torticollis, and blepharospasm [19,20]. BtA specifically binds to the presynaptic membrane of the somatic motor neurons at the neuromuscular junction, controls the release of acetylcholine from cholinergic nerve endings, induces muscle relaxation and paralysis, and thus relieves muscle spasm [21]. However, there is no unanimous conclusion on whether this drug can improve depressive symptoms in patients with PD and depression. Some studies have concluded that it can improve depression, while others showed contrary results [22,23]. In this study, the HAMD scores and MMSE scores were improved in both groups after treatment (P<0.05), suggesting that BtA showed similar efficacy to the traditional antidepressant sertraline in the treatment of depressive symptoms. A similar study administrating botulinum toxin in patients with spasmodic dysphonia showed a significant reduction in depressive symptoms in addition to an improvement in spasmodic dysphonia [24]. A low-sample, single-group study using BtA in 12 PD patients with depression exhibited a remission rate of more than 80% after 2 months. Although this study lacked a controlled analysis and the sample size was too small, it still confirmed the effect of BtA in relieving depressive symptoms [25]. The mechanism of the improvement of depressive symptoms by BtA may be that facial expressions and emotional responses are controlled by the brain, and changes in facial expressions and muscles will also provide emotional feedback to the brain. BtA, which is injected between the eyebrows, temporarily paralyzes the muscles and prevents the face from expressing emotions such as fear, anger and depression, thus inhibiting the feedback of depressive emotions and relieving depressive symptoms [26]. Moreover, the depressive symptoms are related to the severity of PD, and the patients show depressive emotions out of worries about the disease. Treatment with BtA can significantly reduce the symptoms of PD, eliminate the patient’s worries, and reduce depression accordingly.
Some studies have suggested that sleep quality is closely linked to a person’s emotional state [27]. Sleep quality is positively correlated with QOL, and the improvement of sleep quality can contribute to the improvement of QOL [28]. In this study, PSQI scores of two groups only started to improve significantly after 2 months of treatment, the QOL scores started to improve significantly after 3 months of treatment, and the comparison between the two groups at different time points was not statistically significant, suggesting that both BtA and sertraline can improve the sleep quality and enhance the QOL of patients with PD and depression. However, the timing to improvement of sleep quality and QOL started later than the improvements of HAMD and MMSE scores. The reason may be that the sleep quality and QOL could be influenced by many factors, including depressive symptoms and cognitive function. When the depressive symptoms are relieved, the cognitive function is improved, the factors influencing the sleep quality are reduced, and the sleep quality can be gradually improved. The quality of sleep has a direct impact on the QOL, and when the quality of sleep is improved, the QOL is improved accordingly. This study also showed that the incidence rate of adverse reactions after treatment with BtA was 11.11%, which was significantly lower than that of 29.55% in the CNG (P<0.05), indicating that compared with the traditional antidepressant drug, BtA not only achieve similar antidepressant effects, but also show high safety. Similar studies have shown that the incidence of adverse reactions in patients treated with BtA does not differ much from those treated with sertraline [29], and differences may lie in the choice of injection sites, the dose of the drug, and condition of study subjects.
In conclusion, BtA can achieve an effect close to that of sertraline, and can also reduce adverse reactions in patients with PD and depression. However, this study included a small number of subjects, and the results obtained are not sufficiently representative and scientific, and the the mechanism of BtA should be explored further.
Disclosure of conflict of interest
None.
References
- 1.Dorsey ER, Sherer T, Okun MS, Bloem BR. The emerging evidence of the parkinson pandemic. J Parkinsons Dis. 2018;8:S3–s8. doi: 10.3233/JPD-181474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puschmann A. New genes causing hereditary parkinson’s disease or parkinsonism. Curr Neurol Neurosci Rep. 2017;17:66. doi: 10.1007/s11910-017-0780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrag A, Taddei RN. Depression and anxiety in parkinson’s disease. Int Rev Neurobiol. 2017;133:623–655. doi: 10.1016/bs.irn.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Timmer MHM, van Beek MHCT, Bloem BR, Esselink RAJ. What a neurologist should know about depression in Parkinson’s disease. Pract Neurol. 2017;17:359–368. doi: 10.1136/practneurol-2017-001650. [DOI] [PubMed] [Google Scholar]
- 5.Dallé E, Mabandla MV. Early life stress, depression and parkinson’s disease: a new approach. Mol Brain. 2018;11:18. doi: 10.1186/s13041-018-0356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan M, Eatmon CV, Slevin JT. Drug treatment strategies for depression in Parkinson disease. Expert Opin Pharmacother. 2019;20:1351–1363. doi: 10.1080/14656566.2019.1612877. [DOI] [PubMed] [Google Scholar]
- 7.Gilliam FG, Black KJ, Carter J, Freedland KE, Sheline YI, Tsai WY, Lustman PJ. A trial of sertraline or cognitive behavior therapy for depression in epilepsy. Ann Neurol. 2019;86:552–560. doi: 10.1002/ana.25561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasyanju Carrero LM, Ma WW, Liu HF, Yin XF, Zhou BR. Botulinum toxin type A for the treatment and prevention of hypertrophic scars and keloids: Updated review. J Cosmet Dermatol. 2019;18:10–15. doi: 10.1111/jocd.12828. [DOI] [PubMed] [Google Scholar]
- 9.Rojewska E, Piotrowska A, Popiolek-Barczyk K, Mika J. Botulinum toxin type A-A modulator of spinal neuron-glia interactions under neuropathic pain conditions. Toxins (Basel) 2018;10:145. doi: 10.3390/toxins10040145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan M, Kim E, Koren G, Bozzo P. Botulinum toxin type A in pregnancy. Can Fam Physician. 2013;59:1183–1184. [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes MT. Parkinson’s disease and parkinsonism. Am J Med. 2019;132:802–807. doi: 10.1016/j.amjmed.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Barbano AC, van der Mei WF, deRoon-Cassini TA, Grauer E, Lowe SR, Matsuoka YJ, O’Donnell M, Olff M, Qi W, Ratanatharathorn A, Schnyder U, Seedat S, Kessler RC, Koenen KC, Shalev AY. Differentiating PTSD from anxiety and depression: lessons from the ICD-11 PTSD diagnostic criteria. Depress Anxiety. 2019;36:490–498. doi: 10.1002/da.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the hamilton depression rating scale. J Affect Disord. 2013;150:384–388. doi: 10.1016/j.jad.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Trivedi D. Cochrane Review Summary: Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Prim Health Care Res Dev. 2017;18:527–528. doi: 10.1017/S1463423617000202. [DOI] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Hagell P, Nilsson MH. The 39-item parkinson’s disease questionnaire (PDQ-39): is it a unidimensional construct? Ther Adv Neurol Disord. 2009;2:205–214. doi: 10.1177/1756285609103726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh L. Depression and Parkinson’s disease: current knowledge. Curr Neurol Neurosci Rep. 2013;13:409. doi: 10.1007/s11910-013-0409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan X, Lan J, Liu Y, Miao J. Efficacy and safety of botulinum toxin type a in spasticity caused by spinal cord injury: a randomized, controlled trial. Med Sci Monit. 2018;24:8160–8171. doi: 10.12659/MSM.911296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boozalis E, Sheu M, Selph J, Kwatra SG. Botulinum toxin type A for the treatment of localized recalcitrant chronic pruritus. J Am Acad Dermatol. 2018;78:192–194. doi: 10.1016/j.jaad.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Khalifeh M, Mehta K, Varguise N, Suarez-Durall P, Enciso R. Botulinum toxin type A for the treatment of head and neck chronic myofascial pain syndrome: a systematic review and meta-analysis. J Am Dent Assoc. 2016;147:959–973.e1. doi: 10.1016/j.adaj.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Araujo JP, Cruz J, Oliveira JX, Canto AM. Botulinum Toxin Type-A as an alternative treatment for gummy smile: a case report. Dermatol Online J. 2018;24 13030/qt75f0h8kz. [PubMed] [Google Scholar]
- 22.Ochudlo S, Bryniarski P, Opala G. Botulinum toxin improves the quality of life and reduces the intensification of depressive symptoms in patients with blepharospasm. Parkinsonism Relat Disord. 2007;13:505–508. doi: 10.1016/j.parkreldis.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Tyślerowicz M, Kiedrzyńska W, Adamkiewicz B, Jost WH, Sławek J. Cervical dystonia - improving the effectiveness of botulinum toxin therapy. Neurol Neurochir Pol. 2020;54:232–242. [Google Scholar]
- 24.Yang KY, Kim MJ, Ju JS, Park SK, Lee CG, Kim ST, Bae YC, Ahn DK. Antinociceptive effects of botulinum toxin type A on trigeminal neuropathic pain. J Dent Res. 2016;95:1183–1190. doi: 10.1177/0022034516659278. [DOI] [PubMed] [Google Scholar]
- 25.Stearns TP, Shad MU, Guzman GC. Glabellar botulinum toxin injections in major depressive disorder: a critical review. Prim Care Companion CNS Disord. 2018;20 doi: 10.4088/PCC.18r02298. 18r02298. [DOI] [PubMed] [Google Scholar]
- 26.Lu X, Chen G, Ren P, Yang Y, Fan F. Progress on botulinum toxin type a-induced pain relief in the field of plastics. J Craniofac Surg. 2017;28:2045–2052. doi: 10.1097/SCS.0000000000003981. [DOI] [PubMed] [Google Scholar]
- 27.Martin CK, Bhapkar M, Pittas AG, Pieper CF, Das SK, Williamson DA, Scott T, Redman LM, Stein R, Gilhooly CH, Stewart T, Robinson L, Roberts SB. Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults: the CALERIE 2 randomized clinical trial. JAMA Intern Med. 2016;176:743–752. doi: 10.1001/jamainternmed.2016.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karatas A, Canakci E, Turkmen E. Comparison of sleep quality and quality of life indexes with sociodemographic characteristics in patients with chronic kidney disease. Niger J Clin Pract. 2018;21:1461–1467. doi: 10.4103/njcp.njcp_146_18. [DOI] [PubMed] [Google Scholar]
- 29.Matak I, Lacković Z, Relja M. Botulinum toxin type A in motor nervous system: unexplained observations and new challenges. J Neural Transm (Vienna) 2016;123:1415–1421. doi: 10.1007/s00702-016-1611-9. [DOI] [PubMed] [Google Scholar]


