Abstract
Background
Cardiovascular disease (CVD) is the leading cause of death in women globally. In recent years, attention has turned to infertility and pregnancy-related events as potential markers for early mortality and future CVD.
Methods
The Study of Women’s Health Across the Nation (SWAN) is an ongoing longitudinal cohort study of women’s health. Women aged 42-52 years with a uterus and ≤ 1 intact ovary, a menstrual period, and no hormone medications within 3 months before enrollment were eligible. Infertility was self-reported and defined as the inability to achieve pregnancy after 12 months of trying to conceive, or use of fertility medications for > 1 month. Outcomes included development of metabolic syndrome over a 7-year follow-up, and any atherosclerotic CVD event (ie, stroke, angina, myocardial infarction) over a 10-year follow-up. Cox proportional hazards models were used to calculate hazard ratios (HRs) for metabolic syndrome and CVD events in participants with infertility, with adjustment for relevant covariates. Participants without infertility were used as the comparison group.
Results
We included 2370 participants in the analysis of metabolic syndrome risk, and 2809 participants were included in the analysis of CVD event risk. Participants with self-reported infertility did not have a higher risk of developing metabolic syndrome (HR, 0.91; 95% confidence interval, 0.71-1.15) or experiencing CVD events (HR, 0.79; 95% confidence interval, 0.52-1.21) after adjusting for relevant covariates.
Conclusions
Infertility was not associated with development of metabolic syndrome or CVD events in women; further research is required to investigate the effects of specific causes of infertility and fertility treatments on CVD outcomes.
Résumé
Introduction
Les maladies cardiovasculaires (MCV) sont la principale cause de décès chez les femmes dans le monde. Au cours de dernières années, l’infertilité et les complications de la grossesse ont retenu l’attention, à savoir qu’ils constituent des marqueurs potentiels de la mortalité précoce et des MCV futures.
Méthodes
La Study of Women’s Health Across the Nation (l’étude SWAN) qui constitue une étude de cohorte longitudinale sur la santé des femmes est en cours. Les femmes âgées de 42 à 52 ans qui ont un utérus et ≤ 1 ovaire intact, une période menstruelle et qui ne prenaient aucun médicament hormonal 3 mois avant le recrutement étaient admissibles. L’infertilité était autodéclarée et définie comme l’incapacité à être enceinte après 12 mois de tentatives de conception ou l’utilisation de médicaments pour traiter l’infertilité durant > 1 mois. L’issue était la suivante : la survenue du syndrome métabolique au cours du suivi de 7 ans ou de tout événement lié à la MCV athérosclérotique (c.-à-d. l’accident vasculaire cérébral, l’angine, l’infarctus du myocarde) au cours du suivi de 10 ans. Nous avons utilisé les modèles de risques proportionnels de Cox pour calculer les rapports de risque (RR) du syndrome métabolique et des événements liés aux MCV chez les participantes infertiles par l’ajustement des covariables pertinentes. Les participantes fertiles constituaient le groupe témoin.
Résultats
Nous avons recruté 2 370 participantes pour l’analyse du risque de syndrome métabolique, et 2 809 participantes pour l’analyse du risque d’événements liés aux MCV. Les participantes qui avaient autodéclaré leur infertilité n’avaient pas de risque plus élevé de souffrir du syndrome métabolique (RR, 0,91 ; intervalle de confiance à 95 %, 0,71-1,15) ou de subir des événements liés aux MCV (RR, 0,79 ; intervalle de confiance à 95 %, 0,52-1,21) après l’ajustement des covariables pertinentes.
Conclusions
L’infertilité n’était pas associée à la survenue du syndrome métabolique ou des événements liés aux MCV chez les femmes. D’autres recherches qui porteront sur les effets des causes particulières de l’infertilité et des traitements favorisant la fertilité sur l’évolution des MCV sont nécessaires.
Globally, cardiovascular disease (CVD) is the leading cause of death in women,1 highlighting the need for examination of sex-specific risk factors. Although CVD rates have decreased globally, cardiovascular mortality has stagnated in women younger than 55 years of age.2,3 Approximately 36% of women in the United States aged 20 and older live with CVD, which translates to a staggering 47 million people; non-Hispanic black women experience even higher risk of CVDs, with a prevalence of 47.7%.4 Infertility affects approximately 15% of women in the United States and has recently been highlighted as a potential risk factor for CVD.5,6 The potential relationship between infertility and future CVD might be specifically related to the underlying causes of infertility (eg, polycystic ovarian syndrome, endometriosis), risks incurred by treatment of fertility, and/or a lack of risk reduction from a healthy pregnancy.6 To date, the body of evidence on infertility and CVD is inconclusive and lacking in longitudinal analyses with large sample sizes, especially those that follow women until ages at which cardiovascular events could be expected to happen.7, 8, 9, 10, 11, 12, 13 The reproductive period provides an opportunity to identify early markers of CVD in women, with potential for low-cost and implementable screening and primary prevention strategies (eg, routine blood pressure, glucose, and cholesterol checks), as well as lifestyle modifications before and during subsequent pregnancies (eg, increased physical activity, healthy diet).6
Our objectives were to examine whether self-reported infertility in women was associated with the development of: (1) cardiovascular risk factors (metabolic syndrome, a validated marker of CVD risk14); and (2) CVD events (stroke, myocardial infarction, angina), using data from the Study of Women’s Health Across the Nation (SWAN), a multisite, prospective, epidemiologic study on women’s health.
Methods
Study design and data source
We performed a secondary analysis of a prospective cohort study using data from baseline through the 10th annual follow-up visit for women enrolled at all sites included in the publicly available SWAN study data set. SWAN is a multi-site, longitudinal, epidemiological study that collects data on the physical, biological, psychological, and social health of women in their middle years, with the goal of understanding how midlife experiences affect quality of life and health during aging.15 Details of SWAN methodology have been published elsewhere.15 Longitudinal data collection began in 1996 when 3302 premenopausal women were enrolled at 7 sites across the United States. Eligible participants were 42-52 years old at recruitment with a uterus and at least 1 intact ovary, a menstrual period within 3 months before enrollment, and had not taken oral contraceptives or postmenopausal hormone therapy in the previous 3 months. Participants self-identified from the following 5 options for racial/ethnic backgrounds: Caucasian/white non-Hispanic, black/African American, Japanese/Japanese American, Hispanic, and Chinese/Chinese American. Participants were assessed annually with interviewer- and self-administered questionnaires, physical examination measurements (ie, weight, height, blood pressure), and fasting morning blood tests.
Ethical approval
All participants signed informed consent and the institutional review board at each site approved the study protocol.
Exposures
Infertility, defined as the inability to achieve a clinical pregnancy for a period of > 12 months of trying to conceive16 or use of fertility medications for > 1 month, was self-reported at baseline. Unexposed participants were those who did not self-report infertility. Participants were excluded from all analyses if they had missing data on infertility or pregnancy history, or if they reported no attempts to conceive.
Outcomes
The primary outcome was metabolic syndrome, a validated marker of CVD risk14 that might be even more pronounced in women.17 Metabolic syndrome is a well established cluster of inter-related risk factors that have been shown to be a precursor of CVD.18 Participants were classified as having metabolic syndrome if they had 3 or more of the following 5 cardiovascular risk factors, as measured by study staff: elevated waist circumference (≥ 80 cm if the participant identified as Chinese/Chinese American or Japanese/Japanese American; ≥ 88 cm if the participant identified as Caucasian/white non-Hispanic, Hispanic, or black/African American); elevated triglycerides (≥ 1.7 mmol/L); reduced high-density lipoprotein cholesterol (< 1.3 mmol/L); elevated blood pressure (systolic blood pressure ≥ 130 mm Hg, diastolic blood pressure ≥ 85 mm Hg, or use of antihypertensive medications); and elevated fasting glucose (≥ 6.1 mmol/L and/or diabetes). This definition is based on harmonized guidelines for the identification and definition of metabolic syndrome19 and has been used as a proxy for CVD risk in previous studies using SWAN data.13,20, 21, 22 Participants were excluded from the analysis of metabolic syndrome risk if they had diabetes or metabolic syndrome at baseline. Components of metabolic syndrome were measured in SWAN until the 7th annual follow-up visit.
CVD events self-reported in SWAN were stroke, myocardial infarction, and angina. Participants were excluded from analysis of CVD event risk if they had missing data on all CVD events at all follow-up visits. CVD events were measured at all visits for which data is publicly available (10 annual follow-up visits).
Participants were excluded from both analyses (metabolic syndrome and CVD) if they had previous stroke, myocardial infarction, angina, or reported taking any cholesterol-lowering or cardiac medication at baseline.
Covariates
Covariates included age, race/ethnicity, education, insurance, marital status, income, body mass index (BMI), birth control pills or other female hormones used continuously from age 25-35 years, family history of CVD events, hypertension, diabetes, menopausal status, any postmenopausal hormone use, smoking, and alcohol consumption. Income was categorized according to total family income before taxes (≤ $19,999; $20,000-$49,999; $50,000-$99,999; ≥ $100,000). Marital status was determined by participants reporting being married or in a committed relationship at each follow-up visit. BMI was calculated from metric height and weight measures at each follow-up visit, and further categorized using race-specific cutoffs: Caucasian/white non-Hispanic, Hispanic, and black/African American participants were categorized as underweight or average weight (BMI < 25), overweight (BMI 25-29.9), or obese (BMI ≥ 30); and Chinese/Chinese American or Japanese/Japanese American participants were categorized as underweight or average weight (BMI < 23), overweight (BMI 23-24.9), or obese (BMI ≥ 25).22,23 Participants were considered to have a family history of CVD events if any immediate family members had a history of stroke, myocardial infarction, or other heart disease. Menopausal status was categorized at each follow-up visit as premenopausal (pregnant, breastfeeding, or premenopausal), perimenopausal (early or late perimenopausal), or postmenopausal (natural or nonsurgical postmenopausal). Smoking was recorded at each follow-up visit as current, former, or never. Participants were considered to consume alcohol if they had consumed any beer, wine, liquor, or mixed drinks since their previous follow-up visit.
Statistical methods
Categorical variables, stratified according to fertility status were described using frequencies and percentages. Modified Poisson regression was used to calculate risk ratios (RRs) for: (1) metabolic syndrome development; and (2) any CVD event in women who had infertility compared with women who never had infertility (the referent). Cox proportional hazards models were then used to calculate hazard ratios for: (1) time to development of metabolic syndrome; and (2) time to first CVD event in women who had infertility. These women were compared with women who never had infertility (the referent). A sensitivity analysis was conducted among participants who had infertility, comparing metabolic syndrome development in those who reported using fertility medications, compared with those who had never used fertility medications. A forwards modelling strategy was used to find the most parsimonious model, in which univariate analyses were used to identify candidates for the multivariate model using a liberal P value cutoff of < 0.25, including clinically or epidemiologically significant variables. A multivariable model was then fit including all covariates identified as potentially related. Variables with a P > 0.05 in this model were then removed and assessed for their change in β. If > 10% change in β occurred, the excluded variable was deemed important and included in the model. Potential covariates were continuous (age), categorical (race/ethnicity, education, insurance, family history of CVD events, birth control pills or other female hormones used continuously from age 25-35 years, postmenopausal hormone use), and time-varying (marital status, income, BMI, hypertension, diabetes, menopausal status, smoking, and alcohol consumption). Diabetes and hypertension development were only included as potential covariates in analysis for CVD event risk, because diabetes and hypertension are 2 of 5 variables included in the measurement of metabolic syndrome. The last observation carried forward was used to account for missing in time-varying covariates.24,25 An α of 0.05 was used for all analyses. Analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Descriptive analyses
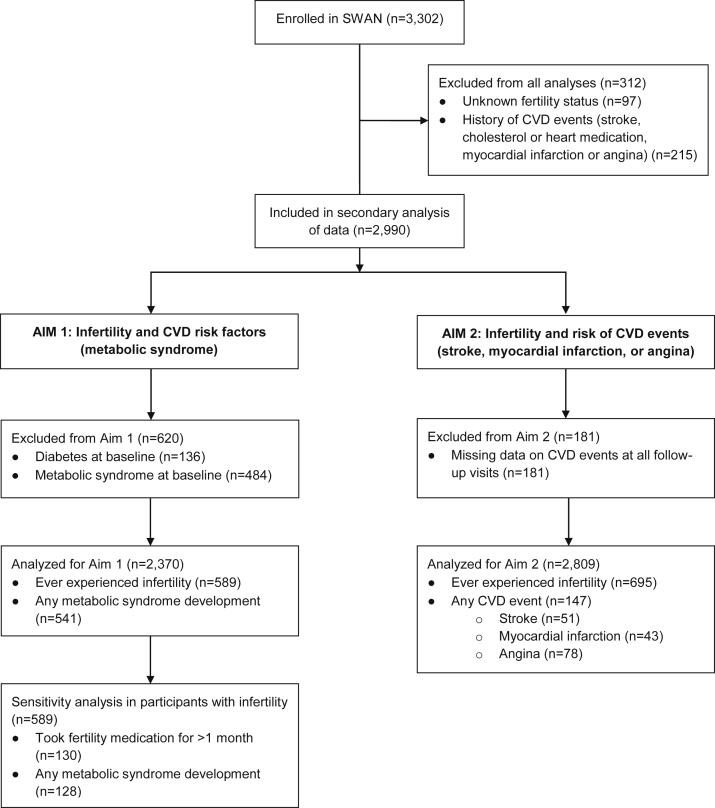
A total of 2990 participants were enrolled after excluding those with unknown fertility status (n = 97) and a history of CVD events at baseline (n = 215; Fig. 1). To assess the development of metabolic syndrome, an additional 620 participants were excluded because of preexisting diabetes (n = 136) and metabolic syndrome (n = 484). Of the remaining 2370 participants, 24.9% (n = 589) self-reported infertility, and 22.8% (n = 541) developed metabolic syndrome over 7 years of available follow-up data. For the sensitivity analysis, 22% (n = 130) of the participants who had infertility reported using fertility medications for > 1 month. To assess CVD event risk, 181 participants were excluded because of missing data on CVD events at all follow-up visits. Of the remaining 2809 participants, 24.7% (n = 695) self-reported infertility and 5.2% (n = 147) experienced a CVD event over 10 years of available follow-up. Because of low event rates, a sensitivity analysis of fertility medications and CVD event risk could not be completed in this sample.
Figure 1.
Flow diagram of sample selection. CVD, cardiovascular disease; SWAN, Study of Women’s Health Across the Nation.
Baseline characteristics of the final study population are described in Table 1. Overall, 7.3% were Japanese/Japanese American, 8.1% were Chinese/Chinese American, 8.1% were Hispanic, 28.4% were black/African American, and 48.1% were Caucasian/white non-Hispanic. Approximately 25% of participants had a high school education or less. At baseline, 53% of participants were premenopausal, whereas the remaining 46% were in perimenopause.
Table 1.
Baseline characteristics of SWAN participants with and without infertility (N = 2990)
| Characteristic | Infertility (n = 738) | No infertility (n = 2252) |
|---|---|---|
| Mean age (SD), years | 45.7 (2.7) | 45.8 (2.7) |
| Race/ethnicity | ||
| Black/African American | 187 (25.3) | 661 (29.4) |
| Chinese/Chinese American | 51 (6.9) | 191 (8.5) |
| Japanese/Japanese American | 70 (9.5) | 149 (6.6) |
| Caucasian/white non-Hispanic | 375 (50.8) | 1063 (47.2) |
| Hispanic | 55 (7.5) | 188 (8.4) |
| Education | ||
| Less than high school | 38 (5.2) | 171 (7.6) |
| High school diploma | 118 (16.0) | 405 (18.0) |
| Some college/technical school | 248 (33.6) | 700 (31.1) |
| College degree | 149 (20.2) | 451 (20.0) |
| Postgraduate education | 180 (24.4) | 511 (22.7) |
| Missing | 5 (0.7) | 14 (0.6) |
| Insurance | ||
| Private | 655 (88.8) | 1874 (83.2) |
| Government (Medicare, Medicaid, veteran) | 25 (3.4) | 96 (4.3) |
| Other | 15 (2.0) | 95 (4.2) |
| No insurance | 41 (5.6) | 174 (7.7) |
| Missing | 2 (0.3) | 13 (0.6) |
| Married or in a committed relationship | ||
| Yes | 626 (84.8) | 1696 (75.3) |
| No | 112 (15.2) | 556 (24.7) |
| Income | ||
| Less than $19,999 | 80 (10.8) | 334 (14.8) |
| $20,000-$49,999 | 225 (30.5) | 777 (34.5) |
| $50,000-$99,999 | 294 (39.8) | 775 (34.4) |
| $100,000 or more | 118 (16.0) | 314 (13.9) |
| Missing | 21 (2.9) | 52 (2.3) |
| Body mass index | ||
| Underweight or average weight | 269 (36.5) | 834 (37.0) |
| Overweight | 200 (27.1) | 595 (26.4) |
| Obese | 258 (35.0) | 784 (34.8) |
| Missing | 11 (1.5) | 39 (1.7) |
| Birth control or female hormones used continuously from 25-35 years | ||
| Yes | 51 (6.9) | 193 (8.6) |
| No | 686 (93.0) | 2058 (91.4) |
| Missing | 1 (0.1) | 1 (0.04) |
| Family history of CVD events | ||
| Yes | 402 (54.5) | 1251 (55.6) |
| No | 233 (31.6) | 681 (30.2) |
| Missing | 103 (14.0) | 320 (14.2) |
| Hypertension | ||
| Yes | 132 (17.9) | 409 (18.2) |
| No | 604 (81.8) | 1841 (81.8) |
| Missing | 2 (0.3) | 2 (0.1) |
| Diabetes | ||
| Yes | 36 (4.9) | 100 (4.4) |
| No | 701 (95.0) | 2150 (95.5) |
| Missing | 1 (0.1) | 2 (0.1) |
| Menopausal status | ||
| Premenopausal | 405 (54.9) | 1189 (52.8) |
| Perimenopausal | 330 (44.7) | 1045 (46.4) |
| Missing | 3 (0.4) | 18 (0.8) |
| Postmenopausal hormone use | ||
| Yes | 181 (24.5) | 504 (22.4) |
| No | 557 (75.5) | 1748 (77.6) |
| Smoking | ||
| Current | 110 (14.9) | 395 (17.5) |
| Former | 204 (27.6) | 538 (23.9) |
| Never | 421 (57.1) | 1.300 (57.7) |
| Missing | 3 (0.4) | 19 (0.8) |
| Alcohol | ||
| Yes | 379 (51.4) | 1057 (46.9) |
| No | 333 (45.1) | 1076 (47.8) |
| Missing | 26(3.5) | 119 (5.3) |
Data are presented as n (%) except where otherwise noted.
CVD, cardiovascular disease.
Risk of development of metabolic syndrome
Results of univariate and adjusted analyses for hazard ratios of the development of metabolic syndrome are presented in Table 2. Covariates included in the final model for metabolic syndrome risk were family history of CVD events, any postmenopausal hormone use, marital status, income, BMI, smoking, and alcohol consumption. After adjustment for covariates, the hazard ratio for time to metabolic syndrome development in women with infertility, compared with those without infertility, was 0.91 (95% confidence interval [CI], 0.71-1.15). BMI, any postmenopausal hormone use, income, and alcohol consumption were most strongly associated with metabolic syndrome development.
Table 2.
Univariate and adjusted HRs and 95% CIs for the development of metabolic syndrome (N = 2370)
| Variable | Univariate |
Adjusted |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Infertility | ||||
| No | 1.00 (Referent) | 0.49 | 1.00 (Referent) | 0.42 |
| Yes | 0.93 (0.76-1.14) | 0.91 (0.71-1.15) | ||
| Age | 1.04 (1.01-1.07) | 0.01 | – | – |
| Race/Ethnicity | ||||
| Caucasian/white non-Hispanic | 1.00 (Referent) | < 0.0001 | – | – |
| Chinese/Chinese American | 0.94 (0.67-1.33) | |||
| Japanese/Japanese American | 1.07 (0.76-1.50) | |||
| Black/African American | 1.63 (1.35-1.98) | |||
| Hispanic | 1.48 (1.08-2.03) | |||
| Education | ||||
| High school diploma | 1.00 (Referent) | 0.0005 | – | – |
| Less than high school | 1.07 (0.75-1.53) | |||
| Some college/technical school | 0.91 (0.72-1.16) | |||
| College degree | 0.64 (0.49-0.85) | |||
| Postgraduate education | 0.66 (0.50-0.86) | |||
| Insurance | ||||
| Private | 1.00 (Referent) | 0.009 | – | – |
| Government | 1.59 (1.07-2.36) | |||
| Other | 1.68 (1.15-2.46) | |||
| No insurance | 1.05 (0.75-1.48) | |||
| Family history of CVD events | ||||
| No | 1.00 (Referent) | 0.002 | 1.00 (Referent) | 0.27 |
| Yes | 1.36 (1.12-1.64) | 1.13 (0.91-1.41) | ||
| Birth control or female hormones used continuously from 25-35 years | ||||
| No | 1.00 (Referent) | 0.43 | – | – |
| Yes | 1.13 (0.84-1.51) | |||
| Any postmenopausal hormone use | ||||
| No | 1.00 (Referent) | 0.15 | 1.00 (Referent) | 0.02 |
| Yes | 1.15 (0.95-1.40) | 1.32 (1.05-1.65) | ||
| Married or in a committed relationship∗ | 0.82 (0.68-1.00) | 0.04 | 1.21 (0.94-1.56) | 0.15 |
| Income∗ | 0.85 (0.77-0.93) | 0.0003 | 0.86 (0.76-0.97) | 0.02 |
| Body mass index∗ | 3.16 (2.80-3.57) | < 0.0001 | 3.18 (2.74-3.67) | < 0.0001 |
| Menopausal status∗ | 1.24 (1.06-1.45) | 0.007 | – | – |
| Smoking∗ | 1.10 (0.99-1.23) | 0.09 | 1.11 (0.97-1.27) | 0.14 |
| Alcohol∗ | 0.80 (0.67-0.96) | 0.02 | 0.79 (0.64-0.98) | 0.03 |
CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
Included as a time-varying covariate.
There was no increase in relative risk of metabolic syndrome development over the entire follow-up period (RR, 0.93; 95% CI, 0.73-1.20) for participants who had infertility, compared with those who never had infertility, after adjusting for the same covariates included in the time-to-event model.
Risk of CVD events
Results of univariate and adjusted analyses for hazard ratios of CVD events are presented in Table 3. Covariates included in the final model for CVD event risk were race/ethnicity, family history of CVD events, birth control pills or other female hormones used continuously from age 25-35 years, hypertension, diabetes, BMI, and smoking. After adjustment for covariates, the hazard ratio for time to first CVD event was 0.79 (95% CI, 0.52-1.21). Similar to development of metabolic syndrome, hypertension, BMI, and smoking were strongly associated with CVD events, as was diabetes. Race/ethnicity, specifically black/African American and Hispanic races/ethnicities, was strongly associated with outcomes.
Table 3.
Univariate and adjusted HRs and 95% CIs for any CVD event (stroke, myocardial infarction, or angina; N = 2809)
| Variable | Univariate |
Adjusted |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Infertility | ||||
| No | 1.00 (Referent) | 0.27 | 1.00 (Referent) | 0.27 |
| Yes | 0.80 (0.54-1.19) | 0.79 (0.52-1.21) | ||
| Age | 1.07 (1.01-1.13) | 0.03 | – | – |
| Race/ethnicity | ||||
| Caucasian/white non-Hispanic | 1.00 (Referent) | < 0.0001 | 1.00 (Referent) | 0.006 |
| Chinese/Chinese American | 0.67 (0.31-1.48) | 0.92 (0.39-2.18) | ||
| Japanese/Japanese American | 0.20 (0.05-0.83) | 0.24 (0.06-0.97) | ||
| Black/African American | 2.16 (1.52-3.07) | 1.63 (1.11-2.38) | ||
| Hispanic | 2.07 (1.11-3.87) | 2.03 (1.02-4.03) | ||
| Education | ||||
| High school diploma | 1.00 (Referent) | 0.02 | – | – |
| Less than high school | 1.69 (0.90-3.17) | |||
| Some college/technical school | 1.07 (0.67-1.69) | |||
| College degree | 0.61 (0.34-1.07) | |||
| Postgraduate education | 0.72 (0.43-1.21) | |||
| Insurance | ||||
| Private | 1.00 (Referent) | 0.0002 | – | – |
| Government | 3.19 (1.83-5.57) | |||
| Other | 1.71 (0.80-3.67) | |||
| No insurance | 1.83 (1.03-3.26) | |||
| Family history of CVD events | ||||
| No | 1.00 (Referent) | 0.0009 | 1.00 (Referent) | 0.01 |
| Yes | 1.99 (1.33-2.99) | 1.71 (1.12-2.61) | ||
| Birth control or female hormones used continuously from 25-35 | ||||
| No | 1.00 (Referent) | 0.21 | 1.00 (Referent) | 0.08 |
| Yes | 0.64 (0.31-1.30) | 0.51 (0.24-1.09) | ||
| Any postmenopausal hormone use | ||||
| No | 1.00 (Referent) | 0.38 | – | – |
| Yes | 1.18 (0.82-1.68) | |||
| Married or in a committed relationship∗ | 0.59 (0.43-0.83) | 0.002 | – | – |
| Income∗ | 0.66 (0.56-0.78) | < 0.0001 | – | – |
| Body mass index∗ | 1.85 (1.49-2.31) | < 0.0001 | 1.44 (1.12-1.84) | 0.004 |
| Hypertension∗ | 2.65 (1.90-3.69) | < 0.0001 | 1.61 (1.10-2.35) | 0.01 |
| Menopausal status∗ | 1.43 (1.05-1.95) | 0.03 | – | – |
| Smoking∗ | 1.59 (1.30-1.94) | < 0.0001 | 1.48 (1.20-1.84) | 0.0003 |
| Alcohol∗ | 0.67 (0.48-0.93) | 0.02 | – | – |
| Diabetes∗ | 4.03 (2.72-5.98) | < 0.0001 | 2.50 (1.62-3.87) | < 0.0001 |
CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
Included as a time-varying covariate.
There was no increase in relative risk of any CVD event over the entire follow-up period (RR, 0.78; 95% CI, 0.51-1.21) for participants who had infertility, compared with those who never had infertility, after adjusting for the same covariates included in the time-to-event model.
Risk of metabolic syndrome in participants who used fertility medications
Results of the sensitivity analysis are presented in Table 4. Covariates included in the final model were income, BMI, and smoking. After adjustment for covariates, the hazard ratio for time to metabolic syndrome development in participants who reported using fertility medications was 0.80 (95% CI, 0.46-1.38), compared with those who reported never using fertility medications.
Table 4.
Univariate and adjusted HRs and 95% CIs for the development of metabolic syndrome among women who experienced infertility and used fertility medications, compared with those who experienced infertility but did not use fertility medications (N =589)
| Variable | Univariate |
Adjusted |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Fertility medication used > 1 month | ||||
| No | 1.00 (Reference) | 0.005 | 1.00 (Reference) | 0.42 |
| Yes | 0.47 (0.28-0.80) | 0.80 (0.46-1.38) | ||
| Age | 1.04 (0.97-1.10) | 0.27 | – | – |
| Race/ethnicity | ||||
| Caucasian/white non-Hispanic | 1.00 (Reference) | 0.01 | – | – |
| Chinese/Chinese American | 0.88 (0.42-1.85) | |||
| Japanese/Japanese American | 0.61 (0.29-1.27) | |||
| Black/African American | 1.76 (1.19-2.59) | |||
| Hispanic | 1.28 (0.63-2.59) | |||
| Education | ||||
| High school diploma | 1.00 (Reference) | 0.06 | – | – |
| Less than high school | 1.75 (0.80-3.85) | |||
| Some college/technical school | 1.21 (0.72-2.03) | |||
| College degree | 0.87 (0.48-1.56) | |||
| Postgraduate education | 0.66 (0.36-1.21) | |||
| Insurance | ||||
| Private | 1.00 (Reference) | 0.47 | – | – |
| Government | 1.85 (0.82-4.21) | |||
| Other | 1.46 (0.46-4.59) | |||
| No insurance | 1.02 (0.45-2.31) | |||
| Family history of CVD events | ||||
| No | 1.00 (Reference) | 0.009 | – | – |
| Yes | 1.70 (1.14-2.53) | |||
| Birth control or female hormones used continuously from 25-35 years | ||||
| No | 1.00 (Reference) | 0.71 | – | – |
| Yes | 1.13 (0.59-2.16) | |||
| Any postmenopausal hormone use | ||||
| No | 1.00 (Reference) | 0.03 | – | – |
| Yes | 1.51 (1.05-2.19) | |||
| Married or in a committed relationship∗ | 0.70 (0.46-1.07) | 0.10 | – | – |
| Income∗ | 0.72 (0.59-0.86) | 0.0005 | 0.85 (0.68-1.07) | 0.16 |
| Body mass index∗ | 3.56 (2.73-4.63) | < 0.0001 | 3.80 (2.79-5.17) | < 0.0001 |
| Menopausal status∗ | 1.17 (0.84-1.63) | 0.34 | – | – |
| Smoking∗ | 1.34 (1.08-1.68) | 0.009 | 1.44 (1.11-1.87) | 0.007 |
| Alcohol∗ | 0.88 (0.60-1.28) | 0.51 | – | – |
CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
Included as a time-varying covariate.
Discussion
In this 10-year nationally representative cohort study of 2990 middle-aged women, we examined the association between infertility and development of cardiovascular risk factors and events. Of almost 3000 participants, approximately one-quarter reported experiencing infertility, 23% developed metabolic syndrome, and 5% experienced CVD events. After adjusting for relevant covariates, no association was observed between infertility and time-to-event of metabolic syndrome development or CVD event (stroke, myocardial infarction, or angina), nor was there an association between the use of fertility medications in participants with infertility and time-to-event of metabolic syndrome development.
Rates of infertility in SWAN were higher than the reported averages for the United States and North America,5 whereas rates of metabolic syndrome26 and CVD events were lower than rates reported in the literature.4 Although SWAN was designed to be nationally representative in its selection of participants with varying racial, economic, and educational backgrounds, these results indicate potential for selection bias,5 and should be used in caution with respect to generalizing the results to a broader population. Our results align with much of the existing literature on infertility and CVD risk, although several cross-sectional studies have reported higher rates of cardiovascular risk factors and events in women with infertility7, 8, 9,12 as well as in women with diminished ovarian reserve.11 However, a longitudinal study of 116,430 women followed > 18 years in the United States showed no increased risk of hypertension for women with self-reported infertility, except for in those with tubal disease infertility.27 Likewise, in a nationwide cohort study of women in Denmark who received medically assisted reproduction, rates of hospitalization for CVD were no higher than in women not diagnosed with infertility.13 Results of our study suggest that in a longitudinal rather than cross-sectional study, and after the inclusion of relevant covariates, there is no association between infertility and CVD risk.
Our study has several limitations. First, we were not able to ascertain the cause of infertility, which might be relevant to the underlying pathophysiology of CVD in women. For example, women with polycystic ovarian syndrome have been reported to be more likely to develop CVD than the general population,28 and women with tubal disease infertility have been reported to have increased risk of hypertension.27 Furthermore, male-factor infertility is solely responsible for approximately one-third of infertility and contributes to 50% of infertility.29 We expect that up to half of the infertility reported in our study was related to male-factor infertility, which might bias the results toward the null. Because of small sample size, we could not complete a sensitivity analysis in women who had infertility and did not go on to have any children, which might also be a relevant factor in the relationship between infertility and CVD risk; in a population-based study of 28,442 women who received fertility therapy, the rate of cardiovascular events or death was 21% higher in women who did not ultimately give birth compared with those who did.10 We were also not able to measure the dose of fertility treatments that women underwent, or the type of treatment used, the guidelines for which might have changed over the course of this long-term prospective study. Moderate and severe ovarian hyperstimulation syndrome, a rare but serious condition associated with assisted reproductive technologies, has been identified as a potential connection between infertility and vascular health and was not measured in this study.6,30 This association should be investigated in future studies on the topic.
This study also relies on several self-report measures. Outcome measurement in our study was not fully inclusive, because there are many CVD events not captured in SWAN (eg, transient ischemic attacks, heart failure, and peripheral artery disease), and further relies on self-reported outcomes not confirmed by medical records. Although data on life-threatening events (eg, myocardial infarction and stroke) are well identified through self-report, data on chronic cardiovascular disorders including angina are captured with less reliability.31 Similarly, the use of self-reported infertility as an exposure measure likely overestimates the prevalence in the population. Together, these measurement errors might act to bias the results toward the null. Finally, SWAN presents a relatively small sample size, limiting statistical power.
In conclusion, in a longitudinal cohort study of women in their middle years, no association was observed between self-reported infertility and metabolic syndrome or cardiovascular events. Further studies should examine the specific causes of infertility and aim to select a sample broader than only those undergoing fertility treatment, because this reduces generalizability. Although infertility in general might not be associated with CVD, there might be specific groups at increased risk of CVD who might benefit from targeted CVD risk factor screening and prevention strategies.
Acknowledgements
We thank the participants and study staff who contributed to SWAN. K.N. acknowledges Heart & Stroke as well as the Canadian Institute for Health Research for the Women’s Heart and Brain Health Mid-Career Research Chair.
Funding Sources
The authors report no funding sources.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This research has adhered to all relevant guidelines. All participants signed informed consent and the institutional review board at each site approved the study protocol.
See page 407 for disclosure information.
References
- 1.Roth G.A., Johnson C., Abajobir A. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaccarino V. Myocardial infarction in young women: an unrecognized and unexplained epidemic. Circulation. 2019;139:1057–1059. doi: 10.1161/CIRCULATIONAHA.118.039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilmot K.A., O’Flaherty M., Capewell S., Ford E.S., Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation. 2015;132:997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin E.J., Virani S.S., Callaway C.W. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 5.Thoma M.E., McLain A.C., Louis J.F. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–13231.e1. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senapati S. Infertility: a marker of future health risk in women? Fertil Steril. 2018;110:783–789. doi: 10.1016/j.fertnstert.2018.08.058. [DOI] [PubMed] [Google Scholar]
- 7.Gleason J.L., Shenassa E.D., Thoma M.E. Self-reported infertility, metabolic dysfunction, and cardiovascular events: a cross-sectional analysis among US women. Fertil Steril. 2019;111:138–146. doi: 10.1016/j.fertnstert.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Kurabayashi T., Mizunuma H., Kubota T., Hayashi K. Ovarian infertility is associated with cardiovascular disease risk factors in later life: a Japanese cross-sectional study. Maturitas. 2016;83:33–39. doi: 10.1016/j.maturitas.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Mahalingaiah S., Sun F., Cheng J.J. Cardiovascular risk factors among women with self-reported infertility. Fertil Res Pract. 2017;3:7. doi: 10.1186/s40738-017-0034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Udell J.A., Lu H., Redelmeier D.A. Failure of fertility therapy and subsequent adverse cardiovascular events. CMAJ. 2017;189:E391–E397. doi: 10.1503/cmaj.160744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verit F.F., Keskin S., Omer B., Yalcinkaya S., Sakar N. Is there any relationship between cardiovascular risk markers and young women with diminished ovarian reserve? Gynecol Endocrinol. 2014;30:697–700. doi: 10.3109/09513590.2014.922948. [DOI] [PubMed] [Google Scholar]
- 12.Verit F.F., Yildiz Zeyrek F., Zebitay A.G., Akyol H. Cardiovascular risk may be increased in women with unexplained infertility. Clin Exp Reprod Med. 2017;44:28–32. doi: 10.5653/cerm.2017.44.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bungum A.B., Glazer C.H., Arendt L.H. Risk of hospitalization for early onset of cardiovascular disease among infertile women: a register-based cohort study. Hum Reprod. 2019;34:2274–2281. doi: 10.1093/humrep/dez154. [DOI] [PubMed] [Google Scholar]
- 14.Mottillo S., Filion K.B., Genest J. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 15.Sowers M.F., Crawford S.L., Sternfeld B. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R.A., Kelsey J.L., Marcus R., editors. Menopause: Biology and Pathobiology. Academic Press; San Diego: 2000. pp. 175–188. [Google Scholar]
- 16.Smith S., Pfeifer S.M., Collins J.A. Diagnosis and management of female infertility. JAMA. 2003;290:1767–1770. doi: 10.1001/jama.290.13.1767. [DOI] [PubMed] [Google Scholar]
- 17.Kangas P., Tikkakoski A., Kettunen J. Changes in hemodynamics associated with metabolic syndrome are more pronounced in women than in men. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-54926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson P.W., D’Agostino R.B., Parise H., Sullivan L., Meigs J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 19.Alberti K., Eckel R.H., Grundy S.M. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 20.Polotsky A.J., Allshouse A., Crawford S.L. Relative contributions of oligomenorrhea and hyperandrogenemia to the risk of metabolic syndrome in midlife women. Int J Clin Endocrinol Metab. 2012;97:e868–e877. doi: 10.1210/jc.2011-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton-Tyrrell K., Wildman R.P., Matthews K.A. Sex hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 22.Ylitalo K.R., Karvonen-Gutierrez C., McClure C. Is self-reported physical functioning associated with incident cardiometabolic abnormalities or the metabolic syndrome? Diabetes Metab Res Rev. 2016;32:413–420. doi: 10.1002/dmrr.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 24.McClure C.K., El Khoudary S.R., Karvonen-Gutierrez C.A. Prospective associations between inflammatory and hemostatic markers and physical functioning limitations in mid-life women: longitudinal results of the Study of Women’s Health Across the Nation (SWAN) Exp Gerentol. 2014;49:19–25. doi: 10.1016/j.exger.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong J.Y., Chang P.Y., Gold E.B., Johnson W.O., Lee J.S. Environmental tobacco smoke and risk of late-diagnosis incident fibroids in the Study of Women’s Health across the Nation (SWAN) Fertil Steril. 2016;106:1157–1164. doi: 10.1016/j.fertnstert.2016.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ervin R.B. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index; United States, 2003-2006. Natl Health Stat Rep. 2009;13:1–7. [PubMed] [Google Scholar]
- 27.Farland L.V., Grodstein F., Srouji S.S. Infertility, fertility treatment, and risk of hypertension. Fertil Steril. 2015;104:391–397. doi: 10.1016/j.fertnstert.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanson B., Johnstone E., Dorais J. Female infertility, infertility-associated diagnoses, and comorbidities: a review. J Assist Reprod Genet. 2017;34:167–177. doi: 10.1007/s10815-016-0836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vander Borght M., Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Practical Committee of the American Society for Reproductive Medicine Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106:1634–1647. doi: 10.1016/j.fertnstert.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 31.Okura Y., Urban L.H., Mahoney D.W., Jacobsen S.J., Rodeheffer R.J. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]