Abstract
Background
Tartrate-resistant acid phosphatase type 5b (TRACP5b) is derived from osteoclasts, and has been used as a marker of osteoporosis (bone resorption). Although heart failure (HF) is associated with catabolic bone remodelling, serum TRACP5b levels have not been rigourously examined in patients with HF.
Methods
We conducted a prospective observational study of 688 decompensated HF patients who had been discharged and whose TRACP5b had been measured. These patients were divided into tertiles on the basis of serum TRACP5b levels: first (TRACP5b < 316 mU/dL, n = 229), second (TRACP5b 316-489 mU/dL, n = 229), and third (TRACP5b ≥ 490 mU/dL, n = 230). We compared the patient baseline characteristics, exercise capacity, and their postdischarge prognosis, including cardiac mortality and cardiac events such as cardiac death and worsening HF.
Results
Age was significantly higher, and prevalence of female sex and anemia was significantly higher in the third tertile than in the first and second tertiles (P < 0.05, respectively). Circulating TRACP5b levels were correlated with peak breath-by-breath oxygen consumption, but not with left ventricular ejection fraction. In the Kaplan-Meier analysis (mean follow-up, 426 days), cardiac mortality and cardiac event rates progressively increased from the first to the third tertiles (P < 0.05, respectively). In the multivariable Cox proportional hazard analysis, the third tertile was an independent predictor of cardiac mortality and cardiac events (cardiac mortality hazard ratio, 2.493; P = 0.040; cardiac events hazard ratio, 1.687; P = 0.030).
Conclusions
High serum levels of TRACP5b, a marker of bone resorption, are associated with high cardiac mortality and cardiac events, accompanied by impaired exercise capacity.
Résumé
Introduction
La TRACP5b (de l’anglais, tartrate-resistant acid phosphatase type 5b, soit l’isoforme 5 b de la phosphatase acide résistante au tartrate) qui est dérivée des ostéoclastes a été utilisée comme marqueur de l’ostéoporose (la résorption de l’os). Bien que l’insuffisance cardiaque (IC) soit associée au remodelage osseux catabolique, les concentrations sériques de TRACP5b n’ont pas été rigoureusement examinées chez les patients atteints d’IC.
Méthodes
Nous avons mené une étude prospective observationnelle auprès de 688 patients atteints d’IC décompensée qui avaient obtenu leur sortie de l’hôpital et pour lesquels nous avions les mesures de TRACP5b. Nous avons réparti ces patients en tertiles en fonction des concentrations sériques de TRACP5b : le premier (TRACP5b < 316 mU/dL, n = 229), le deuxième (TRACP5b 316-489 mU/dL, n = 229) et le troisième (TRACP5b ≥ 490 mU/dL, n = 230). Nous avons comparé les caractéristiques initiales des patients, leur capacité à l’effort et leur pronostic après la sortie de l’hôpital, à savoir la mortalité d’origine cardiaque et les événements cardiaques tels que la mort cardiaque et l’aggravation de l’IC.
Résultats
L’âge était significativement plus élevé, et la prévalence du sexe féminin et de l’anémie était significativement plus élevée dans le troisième tertile que dans les premier et deuxième tertiles (P < 0,05, respectivement). Les concentrations circulantes de TRACP5b corrélaient avec la consommation d’oxygène maximale « respiration par respiration », mais non avec la fraction d’éjection ventriculaire gauche. Dans l’analyse de Kaplan-Meier (durée moyenne de suivi, 426 jours), les taux de mortalité d’origine cardiaque et d’ événements cardiaques augmentaient progressivement du 1er tertile au 3e tertile (P < 0,05, respectivement). Dans l’analyse multivariable selon le modèle des risques proportionnels de Cox, le 3e tertile était un prédicteur indépendant de la mortalité d’origine cardiaque et des événements cardiaques (rapport de risque de mortalité d’origine cardiaque, 2,493; P = 0,040; rapport de risque d’événements cardiaques, 1,687; P = 0,030).
Conclusions
Les concentrations sériques élevées de TRACP5b, un marqueur de la résorption de l’os, sont associées à la hausse des taux de mortalité d’origine cardiaque et d’ événements cardiaques, accompagnés de la diminution de la capacité à l’effort.
Heart failure (HF) has become a significant public health problem as a major cause of death among the elderly population in many countries.1, 2, 3 However, osteoporosis is a multifactorial skeletal disease, which is characterized by low bone mass and microarchitectural deterioration of bone tissue determined according to bone mineral density (BMD). Approximately 21% of women and 6% of men aged 50 years or older have osteoporosis, which is a major public health concern.4,5
Tartrate-resistant acid phosphate (TRACP) is known as type-5 acid phosphatase and purple acid phosphatase. TRACP is an iron-containing glycoprotein expressed in high amounts by bone-resorbing osteoclasts, inflammatory macrophages, and dendritic cells. There are 2 forms of TRACP in the circulating human blood. TRACP type 5a is known as a biomarker of the systemic inflammatory burden in patients with chronic inflammatory diseases such as sarcoidosis and rheumatoid arthritis. TRACP type 5b (TRACP5b), secreted by osteoclasts, is elevated in patients with osteoporosis,6 and is used as a specific and sensitive marker of bone resorption and bone remodelling.6,7
Osteoporosis is prevalent in patients with HF and might associate with the pathogenesis of HF.8 The prevalence of HF and osteoporosis increases with aging, and osteoporosis increases cardiovascular risks.4 Low BMD, namely, osteoporosis, increases the risk of development of cardiovascular diseases, and is a novel biomarker that indicates increased HF risk, independent of established risk factors.4 HF and osteoporosis share common pathophysiology, including increased parathyroid hormone levels, activation of the renin-angiotensin-aldosterone system, and oxidative stress.4,9 In addition, loop diuretic use is associated with increased loss in BMD in men, and with fractures in postmenopausal women.9 Osteoporosis might be of substantial significance in patients with HF. It has been reported that lower BMD is associated with increased mortality, need for implantation of a left ventricular assist device, and inotrope dependency in patients with HF.9, 10, 11 However, the association between serum TRACP5b levels and the prognosis in HF patients remains unclear.
In the present study, we aimed to investigate the effect of serum levels of TRACP5b on HF prognosis, underlying clinical background, cardiac function, and exercise capacity.
Methods
This was a prospective observational study of 688 consecutive decompensated HF patients who were discharged alive from Fukushima Medical University Hospital between 2016 and 2019. The diagnosis of decompensated HF was made by each patient’s attending cardiologist and finally confirmed by the chief physician group on the basis of the HF guidelines.1, 2, 3 Namely, HF was characterized by typical symptoms (eg, breathlessness, ankle swelling, and fatigue) that might be accompanied by signs (eg, elevated jugular venous pressure, pulmonary crackles, and peripheral edema) caused by a structural and/or functional cardiac abnormality.12,13 Blood samples were obtained when the patients were at stable condition before hospital discharge each morning. Patients with acute coronary syndrome and dialysis were excluded. Patients were divided into tertiles on the basis of serum TRACP5b levels: first (TRACP5b < 316 mU/dL, n = 229), second (TRACP5b 316-489 mU/dL, n = 229), and third (TRACP5b ≥ 490 mU/dL, n = 230).
We compared the patient baseline characteristics (eg, blood pressure, heart rate, New York Heart Association [NYHA] classification, comorbidity, laboratory data, echocardiography) at stable condition before hospital discharge and their postdischarge prognosis. The patients were followed-up until January 2020 for cardiac death, and cardiac events defined as composites of cardiac death or unplanned rehospitalization for HF treatment. For patients who experienced 2 or more events, only the first event was included in the analysis. Cardiac death was classified by experienced cardiologists as death caused by worsened HF in accordance with the Framingham criteria, ventricular tachyarrhythmia documented using electrocardiogram or implantable devices, acute coronary syndrome, or sudden cardiac death. Worsening HF was defined as unplanned hospitalization because of worsening HF.14 The status and/or dates of death of all patients were obtained from the patient medical records or the attending physicians at the patient’s referring hospital. Because these patients visited their referring hospital monthly or bimonthly, we were able to follow-up on all patients. Survival time was calculated from the date of hospitalization until the date of death or last follow-up. Written informed consent was obtained from all study subjects at discharge. This study complied with the Declaration of Helsinki and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.15,16
We evaluated several comorbidities (ie, hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease [CKD], anemia), with patients in a stable condition before hospital discharge, which often coexist and are associated with prognosis in HF patients. Comorbidities were defined in accordance with our previous studies.17, 18, 19, 20, 21 Hypertension was defined as the previous use of antihypertensive drugs for treatment of hypertension, and/or a systolic blood pressure of ≥ 140 mm Hg, and/or a diastolic blood pressure of ≥ 90 mm Hg. Diabetes mellitus was defined as the previous use of antidiabetic dugs, a fasting glucose value of ≥ 126 mg/dL, a casual glucose value of ≥ 200 mg/dL, and/or a hemoglobin A1c percentage of ≥ 6.5% (National Glycohemoglobin Standardization Program). Dyslipidemia was defined as the previous use of cholesterol-lowering drugs, a triglyceride value of ≥ 150 mg/dL, a low-density lipoprotein cholesterol value of ≥ 140 mg/dL, and/or a high-density lipoprotein cholesterol value of < 40 mg/dL. CKD was defined as estimated glomerular filtration rate of < 60 mL/min/1.73 m2 using a 3-variable Japanese equation.22,23 Anemia was defined as hemoglobin levels of < 12.0 g/dL in women and < 13.0 g/dL in men.3 Atrial fibrillation was identified using an electrocardiogram performed during hospitalization and/or from medical records.24
Measurement of blood samples
Blood samples were obtained with the patient in stable condition before hospital discharge in morning. Serum TRACP5b was measured using fragment-absorbed immunocapture enzymatic assay using an Osteolinks TRACP5b kit (Nittobo Medical, Tokyo, Japan). Sensitivity of this assay is 19.2 mU/dL, the interassay coefficient of variation (CV), intraassay CV, and overall CV of this measurement are 7.3%, 4.9% and 8.8%, respectively.25 Serum total procollagen type 1 N propeptide (TP1NP) was measured using electrochemiluminescence immunoassay with an Eclusis total P1NP kit (Roche Diagnostics, Tokyo, Japan), and interassay analytical CV was less than 1.1% over the reference interval.26,27 Plasma osteoprotegerin (OPG) was measured using an enzyme-linked immunosorbent assay with a human OPG ELISA kit (ab100617; Abcam, Cambridge, United Kingdom). Sensitivity of this assay is 1 pg/mL,28 and the interassay and intra-assay CV for this assay is < 12%.29 Serum alkaline phosphatase was measured using the enzyme reaction rate method using a Quick Auto Neo ALP-JSⅡ kit (Shino-test Corp, Sagamihara, Japan). Serum calcium and phosphorus were measured using enzyme-linked immunosorbent assay using a Aqua Auto Kainos Calcium kit and Aqua Auto Kainos IP-KⅡ kit, respectively (Kainos Laboratories, Inc, Tokyo, Japan). Serum C-reactive protein was measured using a latex turbidimetric assay with an N-assay LA CRP-S kit (Nittobo Medical, Tokyo, Japan). B-type natriuretic peptide (BNP) levels were measured using a specific immunoradiometric assay with a Shionoria BNP kit (Shionogi, Osaka, Japan).
Echocardiography
Echocardiography was performed blindly by experienced echocardiographers using standard techniques with the patient in stable condition before hospital discharge.30 The echocardiographic parameters investigated included left ventricular end-diastolic volume, left ventricular end-systolic volume, left ventricular ejection fraction (LVEF), interventricular septum, posterior wall, left ventricular mass index (LVMI), left atrial volume, tricuspid valve regurgitation pressure gradient, inferior vena cava, right ventricular diastolic area, right ventricular systolic area, and right ventricular fractional area change. The LVEF was calculated using Simpson’s method in 4-chamber view. LVEF < 40% was considered reduced LVEF, 40%-49% LVEF was considered midrange LVEF, and LVEF ≥ 50% was considered preserved LVEF.2,31 The right ventricular fractional area change, defined as (end diastolic area − end systolic area)/end diastolic area × 100, was used as a measure of right ventricular systolic function. All measurements were performed using ultrasound systems (Acuson Sequoia; Siemens Medical Solutions USA, Inc, Mountain View, CA).
Cardiopulmonary exercise testing
The patients underwent incremental symptom-limited exercise testing while in a stable condition before hospital discharge, using an upright cycle ergometer with a ramp protocol (Strength Ergo 8; Fukuda Denshi Co Ltd, Tokyo, Japan). Breath-by-breath oxygen consumption (VO2), carbon dioxide production (VCO2), and minute ventilation (VE) were measured during exercise using an AE-300S respiratory monitor (Minato Medical Science, Osaka, Japan).32 Peak VO2 was measured as an average of the last 30 seconds of exercise. Ventilatory response to exercise (slope of the relationship between ventilation and VCO2 [VE/VCO2 slope]) was calculated as the regression slope relating VE to CO2 from the start of exercise until the respiratory compensation point (the time at which ventilation is stimulated by CO2 output and end-tidal CO2 tension begins to decrease).32 The ventilatory anaerobic threshold was calculated with the V-slope method.
Statistical analysis
Parametric variables are presented as mean ± SD, nonparametric variables (eg, C-reactive protein and BNP) are presented as median and interquartile range, and categorical variables are expressed as numbers and percentages. The χ2 test was used for comparisons of categorical variables. We used the analysis of variance for continuous variables, followed by Bonferroni post hoc test. We performed multiple regression analysis, allowing for interaction between serum TRACP5b level and clinical confounding factors: age, sex, NYHA functional class, presence of ischemic etiology, hypertension, diabetes, dyslipidemia, CKD, anemia, and atrial fibrillation. Correlations between serum TRACP5b levels and other parameters (eg, laboratory data, echocardiography, and cardiopulmonary exercise test) were assessed using Spearman correlation analysis. Kaplan-Meier analysis was used for presenting the cardiac death and cardiac events, and the log rank test was used for initial comparisons. The prognostic value was tested using univariable and multivariable Cox proportional hazard analyses. In the multivariable Cox proportional hazard analysis, to prepare for potential confounding, because of small event and sample size and presence of multicollinearity between TRACP5b and other variables (eg, BNP), we considered the following clinical factors: age, sex, NYHA class, blood pressure, ischemic etiology, reduced LVEF, hypertension, diabetes, CKD, anemia, atrial fibrillation, and TRACP5b. Univariable parameters with P values of < 0.10 were included in the multivariable analysis. A P value of < 0.05 was considered statistically significant for all comparisons. All analyses were performed using a statistical software package (SPSS version 24.0; IBM Corp, Armonk, NY).
Results
The average TRACP5b level of the present study’s population was 443.9 ± 235.1 mU/dL (range, 68-1500). The comparisons of the clinical features among tertiles are shown in Table 1. Although age was significantly higher, the prevalence of female sex and anemia, and the use of diuretics were significantly higher in the third tertile; no significant differences in blood pressure, heart rate, NYHA class III or IV, ischemic etiology, other comorbidities, or medications were observed among the tertiles. In the laboratory data (Table 2), white blood cell count, hemoglobin, estimated glomerular filtration rate (eGFR), and chloride were lowest, and blood urea nitrogen, creatinine, alkaline phosphatase, magnesium, phosphorus, TP1NP, and BNP were highest in in the third tertile. In contrast, iron, ferritin, total protein, albumin, calcium, corrected calcium, sodium, and C-reactive protein did not differ among the groups. Echocardiographic parameters, except for LVMI, showed no statistical differences among the tertiles (Table 2). The available data of cardiopulmonary exercise testing (n = 250) are shown in Table 2. The peak VO2 was lowest and VE/VCO2 slope was highest in the third tertile.
Table 1.
Comparisons of clinical characteristics of patients (N = 688)
| Characteristic | TRACP5b < 316 (n = 229) | TRACP5b 316-489 (n = 229) | TRACP5b ≥ 490 (n = 230) | P |
|---|---|---|---|---|
| TRACP5b, mU/dL | 229.1 ± 54.6 | 393.9 ± 49.6∗ | 707.5 ± 204.9∗,† | < 0.001 |
| Age, years | 64.2 ± 15.1 | 67.3 ± 15.2 | 71.9 ± 12.8∗,† | < 0.001 |
| Female sex | 81 (35.4) | 99 (43.2) | 116 (50.4) | 0.005 |
| Systolic blood pressure, mm Hg | 126.7 ± 27.2 | 126.3 ± 24.9 | 122.1 ± 25.8 | 0.110 |
| Heart rate, bpm | 78.6 ± 23.8 | 77.9 ± 25.5 | 75.9 ± 21.2 | 0.454 |
| NYHA class III/IV | 16 (7.0) | 12 (5.2) | 19 (8.3) | 0.436 |
| Ischemic etiology | 52 (22.7) | 56 (24.5) | 40 (17.4) | 0.159 |
| LVEF reduced/midrange/preserved | 78 (34.1)/21 (9.2)/130 (56.8) | 84 (36.7)/23 (10.0)/122 (53.3) | 97 (42.2)/18 (7.8)/115 (50.0) | 0.446 |
| Comorbidity | ||||
| Hypertension | 141 (61.6) | 138 (60.3) | 142 (61.7) | 0.939 |
| Diabetes | 84 (36.7) | 83 (36.2) | 90 (39.1) | 0.789 |
| Dyslipidemia | 154 (67.2) | 161 (70.3) | 138 (60.0) | 0.057 |
| CKD | 117 (51.1) | 122 (53.3) | 141 (61.3) | 0.068 |
| Anemia | 96 (41.9) | 107 (46.7) | 126 (54.8) | 0.021 |
| AF | 74 (32.3) | 76 (33.2) | 94 (40.9) | 0.108 |
| Treatment | ||||
| RAS inhibitors | 155 (67.7) | 160 (69.9) | 146 (63.5) | 0.334 |
| β-Blockers | 145 (63.3) | 162 (70.7) | 152 (66.1) | 0.234 |
| Diuretics | 150 (65.5) | 164 (71.6) | 177 (77.0) | 0.025 |
| Inotropic | 34 (14.8) | 27 (11.8) | 26 (11.3) | 0.465 |
| CCBs | 78 (34.1) | 82 (35.8) | 76 (33.0) | 0.820 |
| Statins | 92 (40.2) | 98 (42.8) | 99 (43.0) | 0.788 |
| Implantable devices | 52 (22.7) | 55 (24.0) | 71 (30.9) | 0.100 |
Data are presented as n (%) or mean (SD), except where otherwise noted.
AF, atrial fibrillation; CCB, calcium channel blocker; CKD, chronic kidney disease; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; TRACP5b, tartrate-resistant acid phosphatase type 5b; RAS, renin-angiotensin-aldosterone system.
P < 0.01 vs first tertile.
P < 0.01 vs second tertile.
Table 2.
Comparisons of parameters of laboratory data, echocardiography and cardiopulmonary exercise tests (N = 688)
| Characteristic | TRACP5b < 316 (n = 229) | TRACP5b 316-489 (n = 229) | TRACP5b ≥ 490 (n = 230) | P |
|---|---|---|---|---|
| Laboratory data | ||||
| WBC, × 103/μL | 7.7 ± 4.2 | 7.2 ± 3.1 | 6.4 ± 2.2∗,† | < 0.001 |
| Hemoglobin, g/dL | 13.3 ± 2.1 | 13.0 ± 2.0 | 12.7 ± 2.3∗ | 0.003 |
| Iron, μg/dL | 82.3 ± 40.9 | 81.2 ± 42.7 | 79.5 ± 42.7 | 0.830 |
| Ferritin, ng/mL | 179.7 ± 334.8 | 287.9 ± 1698.4 | 376.8 ± 3112.1 | 0.696 |
| UIBC, μg/dL | 239.1 ± 75.7 | 234.7 ± 72.8 | 244.0 ± 78.5 | 0.534 |
| BUN, mg/dL | 19.7 ± 9.2 | 19.0 ± 9.1 | 23.0 ± 13.7∗,‡ | < 0.001 |
| Creatinine, mg/dL | 1.0 ± 0.5 | 1.0 ± 0.4 | 1.1 ± 0.9‡ | 0.009 |
| eGFR, mL/min/1.73 cm2 | 60.3 ± 22.3 | 59.8 ± 18.9 | 54.7 ± 23.5†,§ | 0.013 |
| Total protein, g/dL | 7.0 ± 0.8 | 7.0 ± 0.7 | 7.0 ± 0.8 | 0.897 |
| Albumin, g/dL | 3.9 ± 0.6 | 3.8 ± 0.6 | 3.8 ± 0.6 | 0.217 |
| ALP, IU/L | 237.0 ± 133.3 | 258.3 ± 87.4 | 300.2 ± 130.6 | < 0.001 |
| Magnesium, mg/dL | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.8 ± 0.2∗,‡ | < 0.001 |
| Calcium, mg/dL | 9.0 ± 0.7 | 9.0 ± 0.6 | 9.1 ± 0.6 | 0.113 |
| Corrected calcium, mg/dL | 9.3 ± 0.5 | 9.4 ± 0.6 | 9.4 ± 1.0 | 0.453 |
| Phosphorus, mEq/L | 3.5 ± 0.7 | 3.6 ± 0.7 | 3.7 ± 0.6§ | 0.049 |
| TP1NP, μg/L | 35.2 ± 20.4 | 50.7 ± 52.9∗ | 77.5 ± 68.8∗,‡ | < 0.001 |
| OPG, pg/mL | 207.1 ± 136.9 | 219.0 ± 157.6 | 265.3 ±178.2 | 0.051 |
| Sodium, mmol/L | 139.4 ± 3.6 | 140.0 ± 3.0 | 139.2 ± 4.4 | 0.117 |
| Potassium, mmol/L | 4.3 ± 0.5 | 4.2 ± 0.5 | 4.2 ± 0.5 | 0.202 |
| Chloride, mmol/L | 104.1 ± 4.0 | 104.2 ± 3.6 | 103.1 ± 4.7† | 0.014 |
| CRP, mg/dL | 0.2 (0.1-0.9) | 0.2 (0.1-0.8) | 0.1 (0.1-0.6) | 0.495 |
| BNP, pg/mL | 163.8 (72.9-485.1) | 256.8 (93.3-562.1) | 338.9 (126.6-652.8) | 0.003 |
| Echocardiography | ||||
| LVEDV, mL | 119.2 ± 60.0 | 117.4 ± 64.2 | 118.4 ± 67.1 | 0.969 |
| LVESV, mL | 62.7 ± 49.0 | 63.9 ± 54.0 | 68.0 ± 55.0 | 0.658 |
| LVEF, % | 53.0 ± 16.6 | 51.4 ± 16.7 | 48.6 ± 16.8 | 0.081 |
| IVS, mm | 10.5 ± 2.4 | 10.4 ± 2.5 | 10.6 ± 2.7 | 0.775 |
| PW, mm | 10.5 ± 2.4 | 10.4 ± 2.0 | 10.7 ± 2.3 | 0.500 |
| LVMI, g/m2 | 121.2 ± 41.7 | 123.8 ± 43.0 | 132.0 ± 46.3 | 0.044 |
| Left atrial volume, mL | 72.4 ± 43.0 | 69.9 ± 45.2 | 73.9 ± 39.7 | 0.730 |
| TR-PG, mm Hg | 27.9 ± 16.3 | 29.2 ±17.4 | 29.9 ± 17.4 | 0.534 |
| IVC, mm | 15.5 ± 4.5 | 15.6 ± 4.6 | 15.2 ± 5.3 | 0.651 |
| RV FAC, % | 41.5 ± 17.2 | 38.0 ± 12.8 | 38.8 ± 13.3 | 0.278 |
| Cardiopulmonary exercise test, n = 250 | ||||
| Peak VO2, mL/kg/min | 15.6 ± 4.5 | 15.5 ± 5.0 | 13.9 ± 4.5 | 0.033 |
| VE/VCO2 slope | 33.1 ± 7.7 | 34.8 ± 9.3 | 36.7 ± 9.0§ | 0.034 |
Data are presented as mean ± SD or median (interquartile range) except where otherwise noted.
ALP, alkaline phosphatase; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; CRP, C-reactive protein; eGFR; estimated glomerular filtration rate; IVC, inferior vena cava; IVS, interventricular septum; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVMI, left ventricular mass index; OPG, osteoprotegerin; PW, posterior wall; RV FAC, right ventricular fractional area change; TP1NP, total procollagen type I intact N-terminal propeptide; TRACP5b, tartrate-resistant acid phosphatase type 5b; TR-PG, tricuspid regurgitation pressure gradient; UIBC, unsaturated iron binding capacity; VE/VCO2 slope, slope of the relationship between ventilation and carbon dioxide production; VO2, breath-by-breath oxygen consumption; WBC, white blood cell count.
P < 0.01 vs first tertile.
P < 0.05.
P < 0.01 vs second tertile.
P < 0.05.
Regarding factors that might affect serum TRACP5b levels, multiple regression analysis (Table 3) showed that age was an independent predictor of serum TRACP5b levels (β = 0.123; P = 0.003). Additionally, correlation analyses with the serum TRACP5b levels and other parameters are presented in Table 4. There were significant correlations between serum TRACP5b levels and hemoglobin, eGFR, alkaline phosphatase, phosphorus, TP1NP, OPG, BNP, LVMI, peak VO2, and VE/VCO2 slope.
Table 3.
Multiple regression analysis to determine serum TRACP5b levels
| Factor | Univariable |
Multivariable |
||
|---|---|---|---|---|
| β coefficient | P | β coefficient | P | |
| Age | 0.207 | < 0.001 | 0.168 | < 0.001 |
| Female sex | 0.108 | 0.005 | 0.073 | 0.055 |
| NYHA functional class Ⅲ/ Ⅳ | −0.023 | 0.546 | ||
| Ischemic etiology | −0.016 | 0.666 | ||
| Hypertension | −0.005 | 0.887 | ||
| Diabetes | 0.025 | 0.658 | ||
| Dyslipidemia | −0.031 | 0.421 | ||
| Chronic kidney disease | 0.104 | 0.006 | 0.032 | 0.426 |
| Anemia | 0.117 | 0.002 | 0.066 | 0.086 |
| Atrial fibrillation | 0.055 | 0.148 | ||
NYHA, New York Heart Association; TRACP5b, tartrate-resistant acid phosphatase type 5b.
Table 4.
Correlation analysis with TRACP5b and other parameters
| Parameter | R | P |
|---|---|---|
| Laboratory data | ||
| Hemoglobin | −0.150 | < 0.001 |
| eGFR | −0.129 | 0.001 |
| Total protein | 0.007 | 0.854 |
| Albumin | −0.073 | 0.071 |
| Alkaline phosphatase | 0.247 | < 0.001 |
| Magnesium | 0.072 | 0.122 |
| Calcium | 0.062 | 0.108 |
| Corrected calcium | 0.037 | 0.367 |
| Phosphorus | 0.100 | 0.009 |
| TP1NP | 0.400 | < 0.001 |
| OPG | 0.204 | 0.001 |
| Sodium | −0.041 | 0.291 |
| Potassium | −0.021 | 0.584 |
| Chloride | −0.113 | 0.004 |
| Log CRP | −0.060 | 0.071 |
| Log BNP | 0.188 | < 0.001 |
| Echocardiography | ||
| LVEF | −0.077 | 0.109 |
| LVMI | 0.090 | 0.025 |
| Left atrial volume | 0.017 | 0.733 |
| TR-PG | 0.025 | 0.564 |
| IVC | −0.047 | 0.246 |
| RV FAC | 0.015 | 0.816 |
| Cardiopulmonary exercise test | ||
| Peak VO2 | −0.159 | 0.012 |
| VE/VCO2 slope | 0.180 | 0.004 |
BNP, B-type natriuretic peptide; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; IVC, inferior vena cava; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; OPG, osteoprotegerin; RV FAC, right ventricular fractional area change; TP1NP, total procollagen type I intact N-terminal propeptide; TRACP5b, tartrate-resistant acid phosphatase type 5b; TR-PG, tricuspid regurgitation pressure gradient; VE/VCO2 slope, slope of the relationship between ventilation and carbon dioxide production; VO2, breath-by-breath oxygen consumption.
In the follow-up period (range 4-1109, mean 426 days), there were 39 cardiac deaths and 113 cardiac events (39 cardiac deaths and 74 unplanned rehospitalizations because of worsening HF). In the Kaplan-Meier analysis (Fig. 1), cardiac mortality and cardiac event rates progressively increased from the first to the third tertiles (cardiac mortality, 3.1%, 5.2%, and 8.7%, log rank P = 0.024; cardiac event rates, 11.8%, 15.3%, and 22.2%, log rank P = 0.010). In the Cox proportional hazard analysis (Table 5), after adjusting for other confounding factors, high TRACP5b level was an independent predictor of cardiac mortality (hazard ratio, 2.493; 95% confidence interval, 1.041-5.974; P = 0.040) and cardiac event rates (hazard ratio, 1.687; 95% confidence interval, 1.051-2.707; P = 0.030) in HF patients. Even in cases of quartile or quintile models, high TRACP5b levels were associated with high incidence of cardiac mortality and cardiac event rates (log rank P < 0.05, respectively).
Figure 1.
Accumulated event rates of cardiac deaths and cardiac events stratified according to serum tartrate-resistant acid phosphatase type 5b (TRACP5b) levels.
Table 5.
Cox proportional hazard model of cardiac mortality and cardiac events in patients with heart failure
| Risk factor | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Cardiac mortality (39 events/N = 688) | ||||||
| Age ≥ 75 years, yes = 1 | 1.033 | 1.007-1.059 | 0.013 | 1.545 | 0.795-3.002 | 0.200 |
| Female sex, yes = 1 | 0.667 | 0.342-1.297 | 0.233 | |||
| NYHA functional class III/IV | 2.366 | 1.241-4.512 | 0.009 | 1.956 | 1.011-3.785 | 0.046 |
| Systolic blood pressure, mm Hg | 0.995 | 0.982-1.008 | 0.429 | |||
| Ischemic etiology, yes = 1 | 1.499 | 0.759-2.962 | 0.244 | |||
| Reduced EF ≤ 40%, yes = 1 | 2.217 | 1.176-4.178 | 0.013 | 1.727 | 0.893-3.340 | 0.105 |
| Hypertension, yes = 1 | 0.979 | 0.514-1.867 | 0.949 | |||
| Diabetes, yes = 1 | 2.639 | 1.384-5.033 | 0.003 | 2.115 | 1.093-4.093 | 0.026 |
| Chronic kidney disease, yes = 1 | 2.959 | 1.403-6.241 | 0.004 | 2.140 | 0.987-4.640 | 0.054 |
| Anemia, yes = 1 | 1.309 | 0.697-2.457 | 0.403 | |||
| Atrial fibrillation, yes = 1 | 1.567 | 0.835-2.942 | 0.162 | |||
| TRACP5b | ||||||
| First tertile | Reference | |||||
| Second tertile | 1.651 | 0.650-4.193 | 0.292 | |||
| Third tertile | 2.977 | 1.258-7.044 | 0.013 | 2.493 | 1.041-5.974 | 0.040 |
| Cardiac events (113 events/n = 688) | ||||||
| Age ≥ 75, yes = 1 | 1.032 | 1.016-1.048 | < 0.001 | 1.835 | 1.237-2.724 | 0.003 |
| Female sex, yes = 1 | 0.980 | 0.664-1.447 | 0.920 | |||
| NYHA functional class Ⅲ/Ⅳ | 1.657 | 1.128-2.435 | 0.010 | 1.302 | 0.891-1.902 | 0.173 |
| Systolic blood pressure, mm Hg | 0.995 | 0.988-1.003 | 0.246 | |||
| Ischemic etiology, yes = 1 | 0.936 | 0.589-1.489 | 0.781 | |||
| Reduced EF ≤ 40%, yes = 1 | 1.115 | 0.765-1.626 | 0.570 | |||
| Hypertension, yes = 1 | 1.401 | 0.924-2.125 | 0.112 | |||
| Diabetes, yes = 1 | 1.609 | 1.095-2.365 | 0.015 | 1.663 | 1.144-2.418 | 0.008 |
| Chronic kidney disease, yes = 1 | 1.576 | 1.056-2.351 | 0.026 | 1.184 | 0.785-1.788 | 0.420 |
| Anemia, yes = 1 | 2.353 | 1.565-3.536 | < 0.001 | 1.441 | 0.963-2.157 | 0.076 |
| Atrial fibrillation, yes = 1 | 1.703 | 1.159-2.503 | 0.007 | 1.529 | 1.051-2.226 | 0.027 |
| TRACP5b | ||||||
| First tertile | Reference | |||||
| Second tertile | 1.243 | 0.736-2.097 | 0.416 | 1.128 | 0.681-1.870 | 0.639 |
| Third tertile | 1.997 | 1.229-3.245 | 0.005 | 1.687 | 1.051-2.707 | 0.030 |
CI, confidence interval; EF, ejection fraction; HR, hazard ratio; NYHA, New York Heart Association; TRACP5b, tartrate-resistant acid phosphatase type 5b.
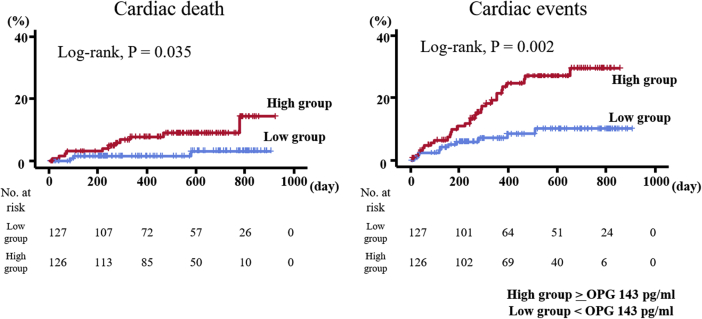
Additionally, OPG levels were partly measured in 255 of the 688 patients (Table 2). High OPG levels in HF patients were associated with high incidence of cardiac mortality and cardiac event rates (Fig. 2; log rank P < 0.05, respectively).
Figure 2.
Accumulated event rates of cardiac deaths and cardiac events stratified according to serum osteoprotegerin (OPG) levels.
Discussion
To our knowledge, the present study is the first to report that high TRACP5b levels in patients with HF are associated with high cardiac mortality and cardiac events, accompanied by high OPG levels, left ventricular hypertrophy, and impaired exercise capacity.
Bone remodelling is a tightly regulated process that requires the resorption of old and defective bone by osteoclasts, followed by the formation of new bone by osteoblasts. There are several biochemical markers for estimating osteoporosis, such as serum/urine N-terminal cross-linked telopeptides of type Ⅰ collagen, C-terminal cross-linked telopeptides of type Ⅰ collagen, deoxypyridinoline, and TRACP5b as bone resorption markers; bone alkaline phosphatase, intact osteocalcin and TP1NP as bone formation markers; and receptor activator of nuclear factor κB ligand (RANKL) as a regulator of bone turnover. In addition, RANKL and OPG as regulators of bone turnover are very important factors in bone remodelling. These are members of the tumour necrosis factor superfamily that are critical regulators in bone metabolism, and appear also to be involved in immune response.33 In addition, impaired renal function is associated with anemia, increased parathyroid hormone concentrations, and osteoporosis.34 Thus, TRACP5b levels seemed to be correlated with TP1NP, OPG, alkaline phosphatase, hemoglobin, and eGFR in the present study.
Regarding associations of cardiovascular disease with biochemical markers for osteoporosis, OPG and RANKL have been reported. OPG activates tumour necrosis factor α and progresses vascular atherosclerosis and calcification.7 The plasma concentration of OPG increases with age in a healthy population, and is elevated in patients with cardiac hypertrophy, myocardial infarction, and HF.8 LVMI is gradually increased with reduced BMD.35 Concordant with previous reports,8,35 TRACP5b levels were correlated with LVMI in the present study. In addition, OPG is independently related to the incidence of HF hospitalization in patients with ischemic HF.36 Furthermore, higher OPG levels predict poor prognosis such as higher all-cause mortality and hospitalization for worsening of HF in patients with HF.8,11 Regarding exercise capacity, it has been reported that exercise capacity determined using cardiopulmonary exercising testing (ie, peak VO2 and exercise duration) was impaired in patients who had osteoporosis for > 5 years than in those who had osteoporosis for < 5 years.37 In addition, osteoporosis is associated with kyphosis-related respiratory dysfunction, muscle weakness, and leads to impaired exercise capacity.37 Concordant with these data, in the present study, high TRACP5b levels were associated with LVMI and impaired exercise capacity (lower peak VO2 and higher VE/VCO2 slope).
RANKL is a chemotactic factor that stimulates chemokine release and matrix metalloproteinase activity, promotes inflammatory response in T cells, is required for normal development of lymph nodes,38 and causes left ventricular remodelling.4,33 OPG and RANKL have recently been considered as new signalling molecules, mediators, and potential biomarkers for atherosclerosis, and it is reported that the serum levels of OPG and RANKL are higher in HF patients.33,38 However, it is difficult to measure OPG and RANKL in daily clinical settings. On the contrary, measuring TRACP5b is used in daily clinical settings for screening for osteoporosis or evaluating therapeutic effects on osteoporosis. Serum TRACP5b activity has a low diurnal variability, its level is not affected by feeding,39 and it does not accumulate in the circulation in cases of renal or hepatic failure,39 which are often complicated with HF; thus, in the serum samples TRACP5b might be easier to measure than other osteoporotic biomarkers especially in HF patients. Although there have been no reports on the relationships among OPG, RANKL, and TRACP5b in HF patients, it has been reported that serum TRACP5b levels are positively correlated with plasma OPG levels in patients with coronary artery disease.7 Concordant with the results,7 we first presented that TRACP5b levels were positively correlated with plasma OPG levels in HF patients. In addition, the serum levels of C-terminal cross-linked telopeptides of type Ⅰ collagen, which is a marker of bone resorption as well as TRACP5b, are positively correlated with the bone marrow plasma levels of RANKL in HF patients.40 In these regards, the serum TRACP5b levels might reflect OPG and RANKL, indicating bone remodelling, and could affect adverse prognosis in HF patients.
Study strengths and limitations
Our study has several strengths. For example, this is the first study, to our knowledge, to show the association of increased serum TRACP5b with adverse prognosis in HF patients, taking into consideration multifaceted clinical backgrounds such as laboratory tests, echocardiography, and exercise capacity. Second, we were able to follow-up on all patients and we enrolled more study subjects than previous studies.33,38
The present study has several limitations. First, because it was a single-centre study with a relatively small number of study subjects, the present results might not necessarily be representative of a general HF population. Although we performed multivariate Cox proportional hazard analysis, we cannot rule out residual confounding factors. Second, although blood samples were obtained when the patients were in stable condition before hospital discharge in the present study, sampling at hospital admission without any therapy might be preferable to examine the prognostic effect of TRACP5b. Third, in the present study we included several variables during hospitalization, without taking into consideration changes in any parameters and treatments during the postdischarge follow-up period. Fourth, we could not necessarily perform cardiopulmonary exercise testing in all of the patients because of various reasons (eg, medical reasons, patient refusal, etc), and potential selection bias might exist in these measurements. Fifth, we could not examine the dual-energy x-ray absorptiometry in all patients, which is standard for osteoporosis, because of implantable devices. Sixth, we did not examine markers of osteoporosis, other than TRACP5b, TP1NP, and OPG in the present study. Seventh, because this was a cross-sectional and prospective observational study without any intervention for osteoporosis, the causal relationships between increased TRACP5b and worse prognosis could not be fully explained. Therefore, the present results should be viewed as preliminary, and further studies are needed.
Conclusions
Increased serum levels of TRACP5b, a marker of bone resorption, is associated with adverse prognosis, accompanied by impaired exercise capacity, in HF patients.
Acknowledgements
The authors thank Ms Kumiko Watanabe, Ms Yumi Yoshihisa, and Ms Tomiko Miura for their technical assistance.
Funding Sources
This work was supported in part by a grant-in-aid for Scientific Research (20K07828 and 20K17157) from the Japan Society for the Promotion of Science, Tokyo, Japan.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This study complied with the Declaration of Helsinki and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
See page 477 for disclosure information.
References
- 1.Tsutsui H., Isobe M., Ito H. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure–digest version. Circ J. 2019;83:2084–2184. doi: 10.1253/circj.CJ-19-0342. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Pfister R., Michels G., Sharp S.J. Low bone mineral density predicts incident heart failure in men and women: the EPIC (European Prospective Investigation into Cancer and Nutrition)-Norfolk prospective study. JACC Heart Fail. 2014;2:380–389. doi: 10.1016/j.jchf.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Kuo T.R., Chen C.H. Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark Res. 2017;5:18. doi: 10.1186/s40364-017-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lv Y., Wang G., Xu W. Tartrate-resistant acid phosphatase 5b is a marker of osteoclast number and volume in RAW 264.7 cells treated with receptor-activated nuclear kappaB ligand. Exp Ther Med. 2015;9:143–146. doi: 10.3892/etm.2014.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morisawa T., Nakagomi A., Kohashi K., Kusama Y., Shimizu W. Serum tartrate-resistant acid phosphatase-5b levels are associated with the severity and extent of coronary atherosclerosis in patients with coronary artery disease. J Atheroscler Thromb. 2017;24:1058–1068. doi: 10.5551/jat.39339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao Y., Tsuruda T., Sekita-Hatakeyama Y. Cardiac hypertrophy is exacerbated in aged mice lacking the osteoprotegerin gene. Cardiovasc Res. 2016;110:62–72. doi: 10.1093/cvr/cvw025. [DOI] [PubMed] [Google Scholar]
- 9.Aluoch A.O., Jessee R., Habal H. Heart failure as a risk factor for osteoporosis and fractures. Curr Osteoporos Rep. 2012;10:258–269. doi: 10.1007/s11914-012-0115-2. [DOI] [PubMed] [Google Scholar]
- 10.Terrovitis J., Zotos P., Kaldara E. Bone mass loss in chronic heart failure is associated with secondary hyperparathyroidism and has prognostic significance. Eur J Heart Fail. 2012;14:326–332. doi: 10.1093/eurjhf/hfs002. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y.H., Wu Y.W., Yang W.S. Relationship between bone mineral density and serum osteoprotegerin in patients with chronic heart failure. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 13.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 14.McKee P.A., Castelli W.P., McNamara P.M., Kannel W.B. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 15.Rickham P.P. Human rxperimentation. Code of ethics of the World Medical Association. Declaration of Helsinki. Br Med J. 1964;2:177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Elm E., Altman D.G., Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshihisa A., Ichijo Y., Watanabe K. Prior history and incidence of cancer impacts on cardiac prognosis in hospitalized patients with heart failure. Circ J. 2019;83:1709–1717. doi: 10.1253/circj.CJ-19-0279. [DOI] [PubMed] [Google Scholar]
- 18.Yoshihisa A., Sato Y., Kanno Y. Prognostic impacts of changes in left ventricular ejection fraction in heart failure patients with preserved left ventricular ejection fraction. Open Heart. 2020;7 doi: 10.1136/openhrt-2019-001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshihisa A., Takiguchi M., Shimizu T. Cardiovascular function and prognosis of patients with heart failure coexistent with chronic obstructive pulmonary disease. J Cardiol. 2014;64:256–264. doi: 10.1016/j.jjcc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Sato A., Yoshihisa A., Kanno Y. Associations of dipeptidyl peptidase-4 inhibitors with mortality in hospitalized heart failure patients with diabetes mellitus. ESC Heart Fail. 2016;3:77–85. doi: 10.1002/ehf2.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshihisa A., Watanabe S., Kanno Y. The CHA2 DS2-VASc score as a predictor of high mortality in hospitalized heart failure patients. ESC Heart Fail. 2016;3:261–269. doi: 10.1002/ehf2.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey A.S., Eckardt K.U., Tsukamoto Y. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo S., Imai E., Horio M. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 24.Yoshihisa A., Sato Y., Sato T. Better clinical outcome with direct oral anticoagulants in hospitalized heart failure patients with atrial fibrillation. BMC Cardiovasc Disord. 2018;18:11. doi: 10.1186/s12872-018-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iki M., Fujita Y., Tamaki J. Design and baseline characteristics of a prospective cohort study for determinants of osteoporotic fracture in community-dwelling elderly Japanese men: the Fujiwara-kyo osteoporosis risk in men (FORMEN) study. BMC Musculoskelet Disord. 2009;10:165. doi: 10.1186/1471-2474-10-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumsohn A., Marin F., Nickelsen T. Early changes in biochemical markers of bone turnover and their relationship with bone mineral density changes after 24 months of treatment with teriparatide. Osteoporos Int. 2011;22:1935–1946. doi: 10.1007/s00198-010-1379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garnero P., Vergnaud P., Hoyle N. Evaluation of a fully automated serum assay for total N-terminal propeptide of type I collagen in postmenopausal osteoporosis. Clin Chem. 2008;54:188–196. doi: 10.1373/clinchem.2007.094953. [DOI] [PubMed] [Google Scholar]
- 28.Sawicka-Powierza J., Jablonska E., Ratajczak-Wrona W. Bone metabolism markers and bone mineral density in patients on long-term acenocoumarol treatment: a cross-sectional study. J Clin Med. 2018;7:372. doi: 10.3390/jcm7100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim B.J., Hamrick M.W., Yoo H.J. The detrimental effects of kynurenine, a tryptophan metabolite, on human bone metabolism. J Clin Endocrinol Metab. 2019;104:2334–2342. doi: 10.1210/jc.2018-02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang R.M., Bierig M., Devereux R.B. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Yoshihisa A., Ichijo Y., Sato Y. Comprehensive clinical characteristics of hospitalized patients with mid-range left ventricular ejection fraction. Eur J Prev Cardiol. 2020;27:2084–2088. doi: 10.1177/2047487319859689. [DOI] [PubMed] [Google Scholar]
- 32.Kanno Y., Yoshihisa A., Watanabe S. Prognostic significance of insomnia in heart failure. Circ J. 2016;80:1571–1577. doi: 10.1253/circj.CJ-16-0205. [DOI] [PubMed] [Google Scholar]
- 33.Ueland T., Yndestad A., Oie E. Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation. 2005;111:2461–2468. doi: 10.1161/01.CIR.0000165119.62099.14. [DOI] [PubMed] [Google Scholar]
- 34.Chen H., Lips P., Vervloet M.G., van Schoor N.M., de Jongh R.T. Association of renal function with bone mineral density and fracture risk in the Longitudinal Aging Study Amsterdam. Osteoporos Int. 2018;29:2129–2138. doi: 10.1007/s00198-018-4592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C., Li S. Correlation research between osteoporosis and left ventricular hypertrophy in older men. Arch Med Sci. 2016;12:1220–1224. doi: 10.5114/aoms.2016.62910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueland T., Dahl C.P., Kjekshus J. Osteoprotegerin predicts progression of chronic heart failure: results from CORONA. Circ Heart Fail. 2011;4:145–152. doi: 10.1161/CIRCHEARTFAILURE.110.957332. [DOI] [PubMed] [Google Scholar]
- 37.Ordu Gokkaya N.K., Koseoglu F., Albayrak N. Reduced aerobic capacity in patients with severe osteoporosis: a cross sectional study. Eur J Phys Rehabil Med. 2008;44:141–147. [PubMed] [Google Scholar]
- 38.Raaz-Schrauder D., Schrauder M.G., Stumpf C. Plasma levels of sRANKL and OPG are associated with atherogenic cytokines in patients with intermediate cardiovascular risk. Heart Vessels. 2017;32:1304–1313. doi: 10.1007/s00380-017-0998-z. [DOI] [PubMed] [Google Scholar]
- 39.Shiraki M., Kuroda T., Shiraki Y. Urinary pentosidine and plasma homocysteine levels at baseline predict future fractures in osteoporosis patients under bisphosphonate treatment. J Bone Miner Metab. 2011;29:62–70. doi: 10.1007/s00774-010-0191-2. [DOI] [PubMed] [Google Scholar]
- 40.Leistner D.M., Seeger F.H., Fischer A. Elevated levels of the mediator of catabolic bone remodeling RANKL in the bone marrow environment link chronic heart failure with osteoporosis. Circ Heart Fail. 2012;5:769–777. doi: 10.1161/CIRCHEARTFAILURE.111.966093. [DOI] [PubMed] [Google Scholar]