Abstract
Sarcoidosis is an inflammatory multisystemic disease of unknown etiology characterized by the formation of noncaseating epithelioid cell granulomas. Cardiac sarcoidosis might be life-threatening and its diagnosis and treatment remain a challenge nowadays. The aim of this review is to provide an updated overview of cardiac sarcoidosis and, through 10 practical clinical questions and real-life challenging case scenarios, summarize the main clinical presentation, diagnostic criteria, imaging findings, and contemporary treatment.
Résumé
La sarcoïdose est une maladie inflammatoire multisystémique de cause inconnue, caractérisée par la formation de granulomes non caséeux composés de cellules épithélioïdes. La sarcoïdose cardiaque est une pathologie potentiellement mortelle qui demeure, à ce jour, difficile à diagnostiquer et à traiter. L'objectif de cet article est de présenter les données les plus à jour concernant la sarcoïdose cardiaque et de résumer, à l'aide de 10 questions cliniques et de cas réels, la présentation clinique, les critères diagnostiques, les trouvailles à l'imagerie et le traitement contemporain.
Sarcoidosis is an inflammatory multisystemic disease of unknown etiology characterized by the formation of noncaseating epithelioid cell granulomas that affects the lungs and the lymph nodes (LNs) in 90% of the cases but can also occur in many other organs such as the heart.1 In the United States, the annual incidence is approximately 10-40 per 100,000 people, African-American women between the age of 20 and 40 years being the most affected group.2 The hypothesis of past exposure to an antigen (infectious, organic, or inorganic agents) that triggered an abnormal immune response with the formation of granulomas in different organs has been proposed.3 Genetics and environmental factors have also been suggested as possible modulators on the occurrence of the disease.3, 4, 5 Although overlap frequently occurs, sarcoidosis is believed to have 2 distinct clinical stages. There is an acute inflammatory phase with the deposition of sarcoid granulomas in 1 or more organs. These granulomas might then persist, resolve, or lead to fibrosis.3 Fibrosis, representing the chronic phase, is not present in all patients.
Cardiac Sarcoidosis
Cardiac sarcoidosis (CS) can be fatal, especially when not recognized.6 Clinical manifestations of cardiac involvement are present in approximately 5% of patients with systemic sarcoidosis7; however, myocardial granulomas have been found in up to 27% of specimens in autopsy studies.6 Consistent with this number, cardiac involvement is also found in > 25% of patients when advanced cardiac imaging modalities such as positron emission tomography (PET) or cardiac magnetic resonance (CMR) are used.8
In the past 2 decades, the number of new cases of CS has markedly increased. In Finland, the detection rate of CS increased > 20-fold during the 25-year period of 1988-2012.9 As shown by the increasing publication of clinical studies and use of specific cardiac imaging modalities, awareness of CS has grown in recent years so that physicians are more prompt to diagnose this disease.
Although the literature on CS is constantly evolving, robust data are scarce and many questions remain unanswered. In this work we summarize the available published information on CS and we also present a retrospective analysis of 34 cases of histologically proven (n = 29) or clinically very probable (n = 5) CS diagnosed at our institution over the past 10 years. Through clinical questions and case presentations, we discuss the practical clinical key elements a cardiologist should know about CS. We also propose several algorithms to help clinicians properly manage this disease. Our algorithms are on the basis of the most recent literature but have not been validated prospectively.
1. What Are the 3 Most Frequent Clinical Manifestations of CS?
Cardiologists might face 3 different scenarios when suspecting CS. First, a patient might present with cardiac symptoms consistent with CS without known or clinically apparent extracardiac sarcoidosis on the basis of clinical examination. These patients can be classified as having a clinically isolated CS.9,10 Of note, most of these patients will have evidence of sarcoidosis outside the heart after investigations.9,11 Second, a patient with known extracardiac sarcoidosis might present with signs or symptoms consistent with cardiac involvement that will lead to the diagnosis of CS. Finally, a patient with extracardiac sarcoidosis who has no symptoms of cardiac involvement might have evidence of CS on advanced cardiac imaging (CMR or fluorodeoxyglucose [FDG]-PET). Clinically isolated CS represents approximately 60% of cases of CS and tends to have a more dramatic presentation than CS associated with extracardiac manifestations.9 The 3 most frequent initial cardiac presentations are discussed in the following sections. The prognosis of patients with CS is highly driven by the type of presentation, atrioventricular block (AVB) having a more benign outcome than heart failure (HF).9,12, 13, 14 Of notice, more than 1 feature of CS can be present at the time of diagnosis in > 20% of patients.12
Atrioventricular block
AVB is the most frequent presentation of symptomatic patients with CS. Up to 47% of patients will have clinically relevant AVB as their initial presentation of CS9,10,12,15,16 and up to 62% of patients will develop AVB during the course of the disease.17 Case 1 is an example.
Case 1
In 2014, a 52-year-old man who came to the emergency room (ER) for dyspnea evolving over weeks and a heart rate (HR) of 47 bpm at rest. The resting electrocardiogram (ECG) showed a 2:1 AVB and chest radiograph showed hilar adenopathy. Transthoracic echocardiogram (TTE) was normal. A treadmill test was performed showing 2:1 AVB, and failure to achieve maximal HR (50% of the predicted). Ischemia was ruled out using PET myocardial perfusion imaging. A computed tomography (CT) scan of the chest showed multiple hilar and mediastinal adenopathy compatible with sarcoidosis. A CMR image and an FDG-PET scan were compatible with pulmonary and CS. Finally, an endobronchial ultrasound (EBUS)-guided LN biopsy showed granulomas compatible with sarcoidosis.
AVB from CS tends to occur at a younger age than AVB from other etiologies.18 One prospective study of 32 patients aged 18-60 years who presented with unexplained Mobitz II or third-degree AVB with no history of sarcoidosis showed that 34% of these patients were diagnosed with CS.19 Also, in a Finnish retrospective study of 72 patients aged 18-55 years who underwent pacemaker implantation for an unexplained AVB, 19% of these patients eventually had a biopsy-proven CS.20
Complete AVB is usually associated with a positive uptake on nuclear test imaging suggesting that conduction abnormalities occur mainly in the active phase of CS.21 Also, granulomas and fibrosis from CS can affect virtually any parts of the conduction system leading to sinus node dysfunction or arrest, AVB of any degree, right or left bundle branch blocks (BBBs), incomplete BBB, hemiblocks, nonspecific interventricular delay, or fragmented QRS.17,22,23
Ventricular tachycardia and sudden cardiac death
Ventricular tachycardia (VT) is the initial presentation in 29% of patients9,10,12,15,16 and might develop in up to 42% of patients with CS.17 In a Canadian prospective study of 14 consecutive patients who presented with unexplained monomorphic VT, 4 (29%) had biopsy-confirmed CS.24 Also, an American prospective study of 103 patients with ventricular arrhythmias and unexplained reduced left ventricular ejection fraction (LVEF) < 55% showed that 18 (17%) had biopsy-proven CS.25
Multiple morphologies of inducible monomorphic VT is frequent and most of these VTs come from the right ventricle,26 sometimes mimicking arrhythmogenic right ventricular cardiomyopathy.27,28 In a Finnish registry, many cases of CS have also been initially misdiagnosed as giant cell myocarditis.15 CS might also be misdiagnosed as idiopathic VT especially in the absence of extracardiac symptoms.26 Polymorphic VT and cycle length variation during VT might be more in favour of CS.29 VT tends to occur in the fibrotic stage of CS and mainly results from reentry circuits through regions of scars.26 Another suggested mechanism for VT is abnormal automaticity from granulomas.26,30
Sudden cardiac death (SCD) might also occur as the initial presentation of CS in patients who previously did not present any signs or symptoms of CS. Ekstrom et al. showed that SCD is the initial presentation in 14% of patients with CS and is the mode of death in 80% of patients with CS.15 Supraventricular arrhythmias are usually not the main initial presentation in CS but might develop in up to 32% of patients.31,32
Heart failure
HF can be the initial CS presentation in 15%-18%9,10,12,15,16 and develop in up to 30% of patients with CS.17 Severe HF is probably the final stage of an unrecognized and long-time evolving CS. In a series of 346 patients who underwent heart transplantation (HT) for systolic HF, 10 (3%) had a diagnosis of CS made histologically at the time of transplant or left ventricular assist device (LVAD) implantation. None were diagnosed before the surgery.33 Another study of 177 patients who underwent LVAD implantation for severe systolic HF showed that 6 (3.4%) had non-necrotizing granulomatous inflammation compatible with CS in the myocardial wall.34 Table 1 shows the best evidence studies describing the initial presentation of CS.
Table 1.
Best evidence studies describing the initial presenting manifestation of cardiac sarcoidosis
| Reference | Patient group | Main initial presentation |
||||
|---|---|---|---|---|---|---|
| AVB | VT | HF | SCD | Others | ||
| Ekstrom et al.15 | Nationwide registries (1998-2015), n = 351 | 42 | 14 | 17 | 14 | 14 |
| Nordenswan et al.16 | Nationwide registries (1988-2015), n = 325 | 44 | 13 | 15 | 14 | 14 |
| Fussner et al.12 | 2 academic medical centres in Minnesota and Calgary (1994-2014), n = 91∗ | 34 | 24 | 52 | N/A | 15 |
| Kandolin et al.9 | 22 hospitals in Finland (1988-2012), n = 110 | 44 | 28 | 18 | 5 | 6 |
| Kandolin et al.10 | Single centre (2000-2010), n = 55 | 47 | 29 | 16 | 4 | 4 |
Data are given in percentages.
AVB, atrioventricular block; HF, heart failure; N/A, not available; SCD, sudden cardiac death; VT, ventricular tachycardia.
Twenty patients had more than 1 feature at presentation.
Our experience
At our institution, 34 patients were diagnosed with CS of which 17 (50%) had a clinically isolated CS. This is in accordance with Okura et al.35 and with Kandolin et al. who reported clinically isolated CS in 57% and 65% of patients, respectively.9
In our series, the initial presentation of clinically isolated CS was AVB in 9 (53%), VT in 3 (18%), HF in 2 (12%), SCD in 1 (6%), and other conduction defects in 2 (12%; Supplemental Table S1). These data are similar to the results reported by Kandolin et al.9 Of note, CS diagnosis was initially missed in 5 of our 9 patients who presented with AVB. These 5 patients were younger than the age of 65 years and had a dual-chamber pacemaker implanted for an idiopathic AVB without further investigation. They later presented with cardiac complications (VT, HF, or SCD) and were then diagnosed with CS.
Seventeen (50%) patients diagnosed with CS had extracardiac disease manifestations and presented with non-life-threatening conduction defects (41%) or arrhythmias (29%; Supplemental Table S1). This might suggest that CS in patients known for extracardiac sarcoidosis tends to be diagnosed at an earlier stage of the disease as reported in the literature.9
2. What Are the Diagnostic Criteria for CS?
The first criteria for CS were published in 1992 by the Japanese Ministry of Health and Welfare and were upgraded in 2006 and 2016.36 In parallel, the World Association of Sarcoidosis and Other Granulomatous Disorders published their criteria in 199937 with updates in 2014.38 Also, the Heart Rhythm Society (HRS) published their consensus statement on CS in 2014.39
According to the HRS criteria (Table 2), a histological confirmation must be made either from a cardiac or extracardiac site before confirming the diagnosis of CS.39 Histologic findings usually reveal non-necrotic granulomas but there are no unique histologic features that enable pathologists to differentiate sarcoidosis from other granulomatous disease.40 The differential diagnosis of non-necrotic granulomas is broad and includes infectious disease such as tuberculosis and histoplasmosis, malignancy such as lymphoma, and many other granulomatous diseases such as chronic beryllium disease41 or Wegener granulomatosis.40 Hence, a complete history and physical examination are important when sarcoidosis is considered.
Table 2.
CS criteria according to the Heart Rhythm Society39
| A. Definitive diagnosis |
|
| B. Probable diagnosis |
|
CMR, cardiac magnetic resonance; CS, cardiac sarcoidosis; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; PET, positron emission tomography; VT, ventricular tachycardia.
Clinically isolated CS
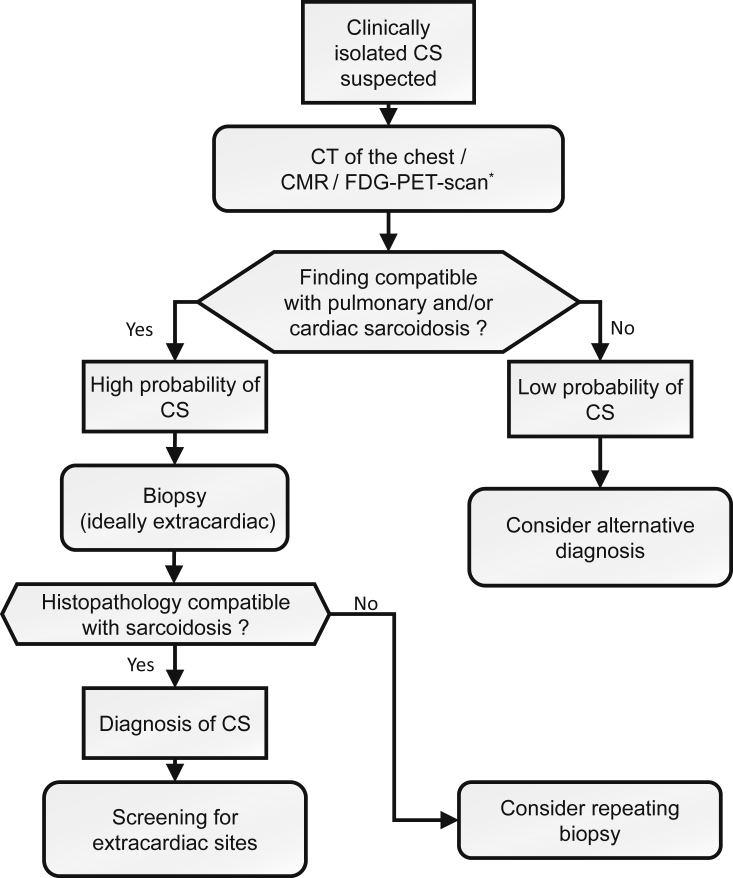
In patients in whom clinically isolated CS is suspected, chest CT scan with contrast for better assessment of adenopathy and advanced cardiac imaging should be performed.10,39,42 Performing CMR and FDG-PET scans should be considered because many believe performing both imaging modalities increases the diagnostic yield with the potential to reveal extracardiac disease and to guide for biopsies. Also, performing both tests can be used to assess the stage of the disease, fibrotic or inflammatory.43 If the chest CT and the advanced cardiac imaging are normal, there is a low probability of CS and an alternative diagnosis should be considered. Otherwise, if either the chest CT or the advanced cardiac imaging is abnormal, a biopsy, ideally extracardiac, should be performed.39 When the diagnosis of CS is made, screening of extracardiac sites should be performed. As per the American Thoracic Society (ATS) guidelines,40 patients with sarcoidosis should get a baseline skin and eye examination to screen for cutaneous and ocular sarcoidosis. Also, blood tests to screen for renal, hepatic, hematological, and calcium abnormalities are recommended.40 In Figure 1, we describe an algorithm to diagnose clinically isolated CS.
Figure 1.
Algorithm to diagnose clinically isolated CS. CMR, cardiac magnetic resonance; CS, cardiac sarcoidosis; CT, computed tomography; FDG, fluorodeoxyglucose; PET, positron emission tomography. ∗ Consider performing CMR and FDG-PET scan to increase diagnostic yield, to assess for fibrosis and inflammation and to guide biopsy. Consider a CT scan of the chest with contrast for optimal evaluation of adenopathy.
CS with known extracardiac manifestations
Optimal screening for cardiac involvement in patients with extracardiac sarcoidosis is still a matter of debate. Mehta et al. reported a 100% sensitivity and 87% specificity for CS when any of these 4 screening variables were abnormal: a history of cardiac symptoms, ECG, Holter monitoring, and echocardiogram.44 In contrast, Hena et al. reported that ECG, echocardiogram, and Holter monitoring remained normal in 56% of their patients who developed signs of CS on CMR, supporting the use of advanced cardiac imaging for screening of CS.45
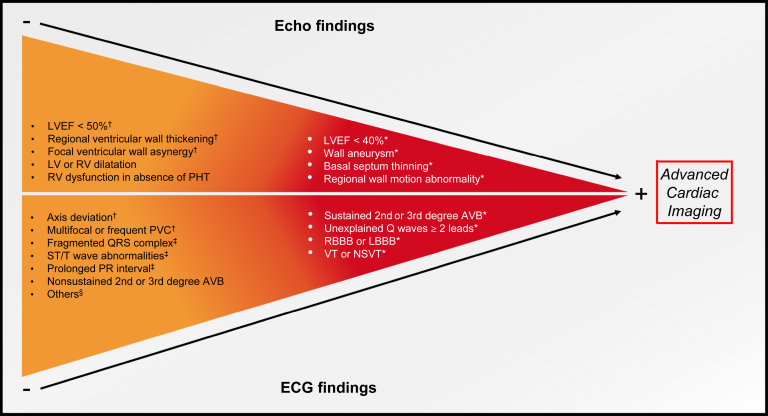
The HRS consensus recommends performing a careful history to identify palpitations, presyncope, or syncope, as well as doing an ECG and echocardiogram for all patients with extracardiac sarcoidosis to detect cardiac involvement.39 Advanced cardiac imaging should be made subsequently if initial screening is abnormal. Figure 2 summarizes red flags on initial screening that should warrant further investigations. Recently, the ATS suggested not performing routine baseline echocardiogram to screen for CS because of the lack of strong evidence supporting this practice. However, the panel recognizes that this test should be considered on a case-by-case basis.40 If initial screening is normal, it might be reasonable to follow these patients with a careful history and ECG every year because CS might develop during the course of the disease.
Figure 2.
Red flags that should warrant further investigation in patients with extracardiac sarcoidosis. Advanced cardiac imaging is recommended in the red panel and could be considered in the orange panel. AVB, atrioventricular block; ECG, electrocardiogram; Echo, echocardiography; HRS, Heart Rhythm Society; LBBB, left bundle branch block; LV, left ventricle; LVEF, left ventricular ejection fraction; NSVT, nonsustained ventricular tachycardia; PHT, pulmonary hypertension; PVC, premature ventricular contractions; RBBB, right bundle branch block; RV, right ventricle; VT, ventricular tachycardia. ∗According to the HRS consensus. † Criteria for cardiac involvement of sarcoidosis as per Japanese guidelines.36‡ Associated with increased risk of developing cardiac events.51§ Atrial arrhythmias, junctional rhythm, chronotropic incompetence, incomplete bundle branch block.
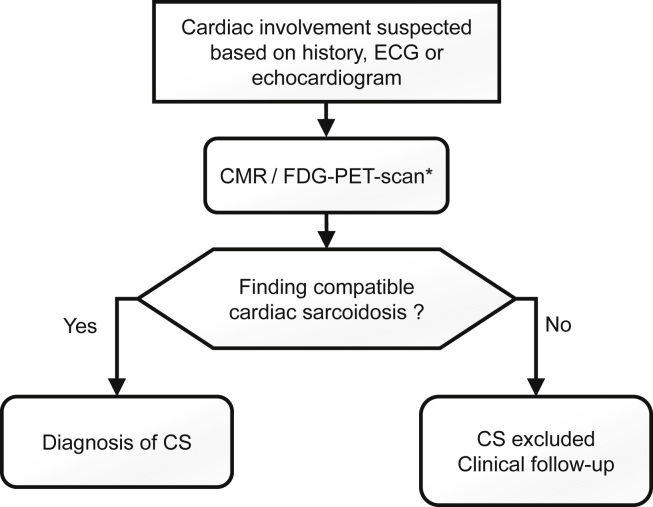
In 2017, the European Society of Cardiology (ESC) issued recommendations for advanced cardiac imaging in patients with extracardiac sarcoidosis in whom cardiac involvement was suspected on the basis of history, ECG, Holter monitoring, or echocardiogram.46 The ESC and the ATS recommend performing CMR before FDG-PET scan in patients with extracardiac sarcoidosis with suspected cardiac involvement.40,46 However, consideration should be made to include these 2 tests to increase diagnostic yield and to assess for fibrosis and inflammation. In Figure 3, an approach to patients with extracardiac sarcoidosis and suspected cardiac involvement is proposed.
Figure 3.
Algorithm to diagnose CS in patients with known extracardiac sarcoidosis. CMR, cardiac magnetic resonance; CS, cardiac sarcoidosis; ECG, electrocardiogram; FDG, fluorodeoxyglucose; PET, positron emission tomography. ∗ Consider performing CMR and FDG-PET scan to increase diagnostic yield and to assess for fibrosis and inflammation.
Case 2 is an example of a patient known for pulmonary sarcoidosis in whom CS was subsequently diagnosed.
Case 2
In 2018, a 63-year-old woman who was diagnosed with pulmonary and ocular sarcoidosis proven using an EBUS-guided LN biopsy. Her only complaint was dyspnea that evolved over 1 year. ECG showed sinus bradycardia with a 200-msec PR interval and a left anterior hemiblock. TTE was normal. Holter monitoring showed nonsustained VT and multiple episodes of ventricular and atrial premature beats. A CMR and FDG-PET scan confirmed CS.
3. In CS, What Are the Usual Findings on Chest CT, ECG, and Echocardiogram?
Chest CT scan
A CT scan of the chest with contrast is suggested as an initial investigation for patients in whom clinically isolated CS is suspected.39 Russo et al. showed a sensitivity and specificity of 94% and 86%, respectively, for chest CT in patients with suspected clinically isolated CS because most patients have lung or mediastinal involvement of sarcoidosis.47 However, clinically isolated CS might occasionally be missed by CT scan alone, especially in patients without extracardiac involvement.47,48
Electrocardiogram
The sensitivity and specificity of ECG to detect CS in patients with an extracardiac disease vary widely among different studies depending on the selected criteria. For example, Mehta et al.44 reported an 8% sensitivity and 97% specificity when complete BBB, hemiblocks, or AVB was used as criteria to predict CS whereas Schuller et al.49 reported a 90% sensitivity and 63% specificity using BBB and fragmented QRS (2 contiguous leads showing RSR’ patterns in the absence of BBB) as criteria to predict CS. In the absence of pathognomonic findings of CS on the ECG, most sarcoidosis experts consider second- or third-degree AVB, BBB, atrial arrhythmias, and ventricular ectopy to be ECG findings indicative of CS in selected populations.50 In addition, Nagao et al. showed that conduction disorders, ST segment/T wave abnormalities, and fragmented QRS complex are associated with an increased risk of developing cardiac events in asymptomatic patients with extracardiac sarcoidosis and that electrocardiographic abnormalities usually precede cardiac events.51
Other tools such as Holter monitoring,44,52 signal-averaged ECG,53 and Selvester QRS scoring54 have shown their usefulness to screen for CS although they are not routinely recommended in absence of symptoms such as palpitations, presyncope, or syncope.39
Transthoracic echocardiography
In patients with extracardiac sarcoidosis, TTE is a useful, noninvasive technique that might provide the first suspicion of CS.55,56 However, its utility for screening is controversial57 because it is not sensitive enough to detect mild or small localized abnormalities.58,59 Sensitivity and specificity have been reported to be between 25% and 69%44,57 and 29% to 95%,57 respectively. Of notice even if speckle tracking echocardiography seems to show incremental benefits, it is not recommended for screening.60,61
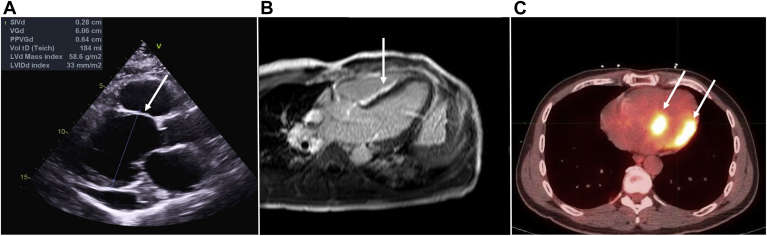
Although not pathognomonic nor frequently encountered, the most characteristic echocardiographic finding is basal septal thinning with hyperechogenicity (Fig. 4). The most frequent anomaly is still LVEF impairment. Many other abnormalities can be found, such as wall motion abnormalities, right ventricular dysfunction, chamber enlargements, aneurysm formation, diastolic dysfunction, or pericardial effusion.55,56,61, 62, 63 Despite the potential value of all of these TTE findings, patients with CS often have normal TTEs.64 Echocardiography is also useful because in patients with CS, left ventricle (LV) end-diastolic diameter and systolic dysfunction are independent predictors for mortality.64,65
Figure 4.
Findings on echocardiogram, cardiac magnetic resonance, and FDG-PET scan consistent with cardiac sarcoidosis. (A) Echocardiogram showing basal septum thinning. (B) Cardiac magnetic resonance showing subepicardial late gadolinium enhancement in the septum and right ventricle free wall. (C) FDG-PET scan showing focal uptake of FDG on the inferoseptal and lateral walls of the left ventricle. FDG, fluorodeoxyglucose; PET, positron emission tomography.
Our experience
Among our 17 patients with clinically isolated CS, 16 (94%) had abnormalities on their ECG at the time of their diagnosis. Among our 17 patients known for extracardiac sarcoidosis, 15 (88%) had abnormalities on their ECG when CS was diagnosed. Among them, 8 (53%) did not have any cardiac symptoms and were diagnosed after an abnormal ECG only (Supplemental Table S2).
Among our 17 patients with clinically isolated CS, 6 (35%) had normal TTEs (Supplemental Table S3). Interestingly, all patients who presented with AVB or conduction abnormalities had a normal TTE whereas all patients who presented with VT, HF, or SCD had an abnormal TTE. Among our 17 patients known for extracardiac sarcoidosis at the time of their diagnosis, 13 (76%) had a completely normal TTE (Supplemental Table S4). In our entire cohort, 19 patients of 34 (56%) had normal TTE when CS was diagnosed. Our results and studies from others9 support the fact that patients with clinically isolated CS tend to present with a more advanced disease.
4. In CS, What Are the Usual Findings on Advanced Cardiac Imaging?
Cardiac magnetic resonance
CMR is an excellent imaging modality for patients with suspected CS to evaluate the myocardium and the presence of fibrosis (Fig. 4).66 Zhang et al. reported in a meta-analysis that CMR had a 93% sensitivity and 85% specificity to detect CS.67 When studies before 2011 were excluded, the sensitivity and specificity increased to 95% and 92%, respectively, suggesting that the diagnostic accuracy of CMR has improved over time.67
CMR findings can vary and change with the evolution of the disease, being able to detect inflammation during the acute phase, and fibrosis in a more advanced stage, although these 2 features might frequently overlap.68 During the acute phase, inflammation and edema can be seen as areas of focal wall thickening associated with wall motion abnormalities, along with increased signal intensity areas in T2-weighted images. Hyperintense regions in early gadolinium enhancement phase might also be identified.62,64,69, 70, 71, 72
Furthermore, late gadolinium enhancement (LGE) and multiparametric magnetic resonance imaging (MRI) enables myocardial tissue characterization and visualization of myocardial fibrosis, methods that help differentiate an ischemic vs nonischemic etiology.66 Although subendocardial distribution has been described (mimicking ischemic disease), typical LGE pattern involves mid- and epicardial myocardium.71,73 The most commonly affected areas are the basal segments, particularly the septum and lateral segments, concordant with the TTE findings.74
Nuclear medicine
PET scan is the best available tool to detect the active inflammatory phase of sarcoidosis (Fig. 4) and has been shown to be superior to Gallium-67 scintigraphy.46,75 PET scan is also highly valuable to diagnose CS. A meta-analysis reported a sensitivity and specificity of 89% and 78%, respectively.76 However, the range of reported sensitivities might vary from 79% to 100%, reflecting the heterogeneity regarding the population and the threshold to diagnose CS among the different studies.76 It could be assumed that PET scan sensitivity is probably higher in the acute active phase of CS and lower in the chronic fibrotic phase. Of notice, 2 studies reported a higher sensitivity of PET scan compared with CMR to diagnose CS.44,77,78
The marker of choice is FDG, a glucose analogue that is uptaken by cells with high metabolic activity such as those found in granulomas.75 To reduce the physiologic uptake of FDG by cardiomyocytes, the patient should eat at least 2 high fat and low carbohydrate meals (Atkin’s diet) the day before the study and then fast for at least 12 hours.79 Nonadherence to this diet might lead to false positive results as described in case 3. In addition, heparin is often administered to reduce the physiologic FDG suppression.79
The FDG-PET images can be classified into 4 patterns: “none,” “diffuse,” “focal,” or “focal on diffuse.”80 “Focal” or “focal on diffuse” sometimes referred to as “patchy” or heterogeneous uptake is typical of CS and is mostly seen in the basal segment of the LV (septal and lateral walls). High standardized uptake value (SUV) count is also highly associated with CS.81 However, there is no pathognomonic pattern for CS and other pathologies might also show FDG uptake by the myocardium such as myocarditis, ischemia, or hibernating myocardium.
Case 3
In 2012, a 49-year-old woman who was diagnosed with pulmonary sarcoidosis, confirmed on EBUS-guided LN biopsy. Initial investigations did not show any evidence of cardiac involvement. Six years later, she developed a left BBB. The treating physician decided to screen for cardiac involvement using FDG-PET scan before CMR. The PET scan showed anterolateral wall hypermetabolism, a common false-positive variant. The patient later confessed not to have respected Atkin’s diet. CMR did not display edema nor LGE. A control PET scan with proper Atkin’s diet did not show any cardiac uptake, confirming that the first test was a false positive result.
CMR vs nuclear medicine
CMR and FDG-PET scan offer complementary information on the disease’s stage and prognosis and consideration should be given to perform both tests whenever possible in patients in whom CS is highly suspected or diagnosed.43 In a cohort of 51 patients suspected of having CS, hybrid PET/CMR imaging was superior for detecting CS than both tests alone.43 Moreover, combining both tests might allow the cardiologist to confirm the diagnosis in unclear situations.82
The PET scan is better at detecting the active and inflammatory phase of CS and, thus, helps in decision-making on whether or not an immunosuppressive treatment should be started.46 It is also better in detecting extracardiac activity, which is present in 97% of patients with CS.83 Furthermore, it is an excellent method to monitor therapy. Repeat PET scan allows for assessment of treatment response and can help to adjust immunosuppression.75,84, 85, 86 One study also showed that a reduction in FDG uptake was associated with an increase in LVEF.87 There are scarce data supporting the use of PET scan for risk stratification in patients with CS although one study showed a significant association between SUV count at the time of diagnosis and future cardiac events.88
CMR is the modality of choice to detect the chronic fibrotic phase and to quantify the degree of fibrosis and myocardial scar, which are independent predictors of cardiac death in patients with CS.89 In a meta-analysis of 760 patients with known or suspected CS, LGE on CMR was associated with increased mortality and ventricular arrhythmias.90 Another meta-analysis including 694 patients showed an 8.8% annualized incidence of death or ventricular arrhythmias among patients with LGE vs 0.6% in those without LGE, confirming the utility of CMR to assess prognosis.91
Our experience
In our cohort, 23 patients (68%) had CMR imaging and FDG-PET scan (Supplemental Tables S5 and S6). In 12 of them, CMR and cardiac FDG-PET scan showed complementary information; 8 patients had LGE on CMR consistent with CS in the absence of FDG uptake and 4 had normal CMR with abnormal FDG uptake consistent with CS. Cases 4 and 5 are 2 examples to illustrate the usefulness and limitations of the FDG-PET scan and CMR imaging in the diagnosis of CS. It also shows the importance of considering another advanced cardiac imaging method when there is a high clinical suspicion of the disease. In case 4, the patient was in the chronic fibrotic stage of CS and had developed VT due to myocardial scars. Thus, no inflammation was seen on the FDG-PET scan. In case 5, the patient was in the inflammatory phase of CS and had not yet developed fibrosis from his CS.
Case 4
In 2013, a 52-year-old woman was diagnosed with pulmonary sarcoidosis, confirmed using EBUS-guided LN biopsy. No treatment was administered. Four years later, in 2017, she presented with symptomatic VT at 200 bpm. CS was suspected and CMR showed intramyocardial LGE on the basal septal and inferior walls of the LV with a degree of fibrosis estimated at 7%. The FDG-PET scan did not show any evidence of active CS.
Case 5
In 2006, a 34-year-old man was diagnosed with pulmonary and pleural sarcoidosis confirmed using LN biopsy. Nine years later, in 2015, he presented with atypical chest pain and dyspnea. ECG showed nonspecific ST segment and T wave abnormalities. However, TTE, nuclear stress test for ischemia, and CMR were normal. FDG-PET scan showed multiple hypermetabolic adenopathy with a maximal SUV count of 12. There were also focal hypermetabolic uptakes in the lungs and a right pleural effusion. There were focal on diffuse FDG uptake in the heart mainly involving the lateral wall of the LV with a SUV count up to 6. On the basis of the FDG-PET scan findings, the patient was treated with immunosuppressive therapy.
5. What Should You Do When Clinically Isolated CS Is Highly Suspected With a Negative Biopsy Result?
One challenging situation that might occur is when a patient has a classical presentation of clinically isolated CS with imaging suggestive of extracardiac and CS but the biopsy comes back normal. Case 6 is an example.
Case 6
In 2017, 57-year-old woman who came to the ER for palpitations and sustained VT. The LVEF was 45% and the coronary angiogram was normal. FDG-PET scan showed multiple highly hypermetabolic hilar, mediastinal, and abdominal adenopathy with a hypermetabolic thyroid consistent with systemic sarcoidosis. There was no myocardial FDG uptake. CMR imaging showed edema and subepicardial LGE on the basal anterior and anterolateral walls of the LV. All biopsies were normal (3 EBUS-guided LN, 1 transbronchial, 1 thyroid, and 1 cardiac). The patient had a presumptive diagnosis of systemic sarcoidosis with cardiac involvement. Finally, she underwent a mediastinoscopy with biopsy of mediastinal LNs, which revealed sarcoidosis.
Case 6 illustrates the importance of repeating biopsies to increase its diagnostic yield when there is a high suspicion of CS. It also shows the possible usefulness of performing CMR and FDG-PET scan when CS is highly suspected.
Although endomyocardial biopsy (EMB) is mandatory for a definitive CS diagnosis, it has a low sensitivity (20%-30%).92,93 The low sensitivity is due to the patchy distribution of the granulomas and their usual location in the basal septum or the lateral wall of the LV whereas biopsies are usually taken on the distal septum or the lateral wall of the right ventricle.18 Because it has a low sensitivity and is associated with risks, extracardiac biopsies of low-risk sites (eg, LNs, lungs, skin) are favoured over EMB whenever possible.
Petek et al. showed that among a population of patients not known for sarcoidosis who presented cardiac manifestation and abnormal cardiac imaging consistent with CS (active inflammation on nuclear imaging or LGE on CMR imaging with the exclusion of other possible causes) in whom clinically isolated CS was suspected, a diagnosis of sarcoidosis was made in 58% of patients who underwent lung or LN biopsy irrespective of the degree of lung involvement on chest CT imaging.48 Moreover, bronchoalveolar cellular analyses were suggestive of sarcoidosis in 67% of their patients.48 Among patients with stage 0 sarcoidosis (normal CT scan), 40% had an abnormal transbronchial biopsy but none had EBUS-guided LN sampling performed.
In patients with a normal CT scan in whom clinically isolated CS is suspected, we suggest performing FDG-PET scan to find potential sites of biopsy. In the absence of extracardiac sites, transbronchial/endobronchial biopsies with or without EBUS-guided LN biopsy could be considered, although there is not strong evidence supporting this approach in the literature.48 In addition, bronchoalveolar lavage should be considered because lymphocytosis ≥ 25% with an increased CD4/CD8 ratio is associated with sarcoidosis.94 Also, performing biopsies before introducing immunosuppression probably decreases the chance of getting a false negative result.
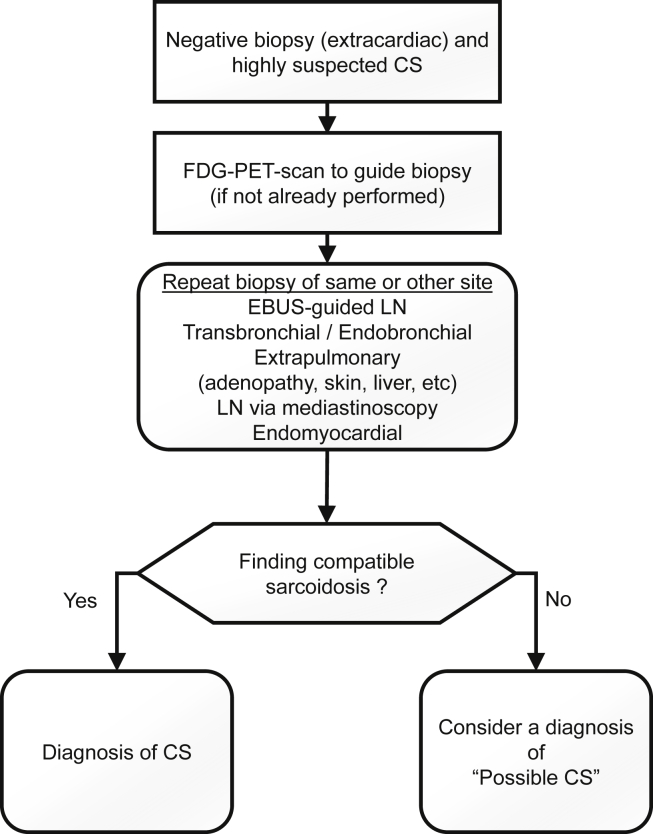
In case the initial extracardiac biopsy is normal, it could be reasonable to repeat the biopsy or to biopsy another easily accessible site guided by FDG-PET scan (Fig. 5). Another option is to perform a sampling of a mediastinal LN via mediastinoscopy that appears abnormal on PET imaging.40,95 In patients with 2 normal biopsy results in whom CS is highly suspected, it could be reasonable to rule for “possible CS.” Ruling out other causes is of prime importance because of the dramatic consequences that could occur if CS is missed. Of note, the recently updated Japanese guidelines on CS propose clinical criteria to pose the diagnosis of CS without tissue sampling.36
Figure 5.
Biopsy-negative patients with highly suspected clinically isolated CS. CS, cardiac sarcoidosis; EBUS, endobronchial ultrasound; FDG, fluorodeoxyglucose; LN, lymph node; PET, positron emission tomography.
Our experience
Among our 17 patients with clinically isolated CS, 16 had at least 1 biopsy and 1 refused a biopsy. Fifteen patients had an EBUS-guided LN biopsy and/or transbronchial/endobronchial biopsy of which 9 (60%) were consistent with sarcoidosis. Four patients had a cardiac biopsy of which 1 (25%) was consistent with CS. Mediastinoscopy with biopsy of adenopathy was performed in 1 patient and was consistent with sarcoidosis. One patient who had a EBUS-guided LN biopsy that was normal was finally diagnosed with CS at autopsy. Two patients had thyroid and liver biopsies that were normal. A diagnosis of “possible CS” was made in 5 patients despite normal biopsy results because their clinical presentation and cardiac imaging were highly suggestive of CS.
6. What Should You Do When Clinically Isolated CS Is Suspected in Patients Who Present With Unstable AVB Preventing Advanced Cardiac Imaging?
The management of patients with unstable AVB requiring emergency pacing is challenging because safe CMR and/or FDG-PET scan often cannot be performed before device implantation. Patients with CS have a class IIa recommendation for an implantable cardioverter-defibrillator (ICD) when a pacemaker is indicated whatever the ejection fraction. This stresses the importance of confirming CS whenever it is possible before device implantation.39,96 Case 7 is an example.
Case 7
In 2018, a 43-year-old man who was admitted for dyspnea and lightheadedness. ECG showed sinus bradycardia at 57 bpm with a 400 msec PR interval. Two episodes of Mobitz II were also seen on telemetry. TTE and coronary angiogram were normal. The patient eventually developed a high-grade AVB with 5 consecutive blocked P waves associated with hypotension. A temporary pacemaker was implanted followed by an emergency permanent MRI-safe pacemaker. Two days later, an FDG-PET scan was consistent with systemic and CS. The CMR imaging was to be delayed because the device implantation was too recent. A transbronchial biopsy revealed the presence of noncaseating granulomas. The final diagnosis was sarcoidosis with cardiac involvement.
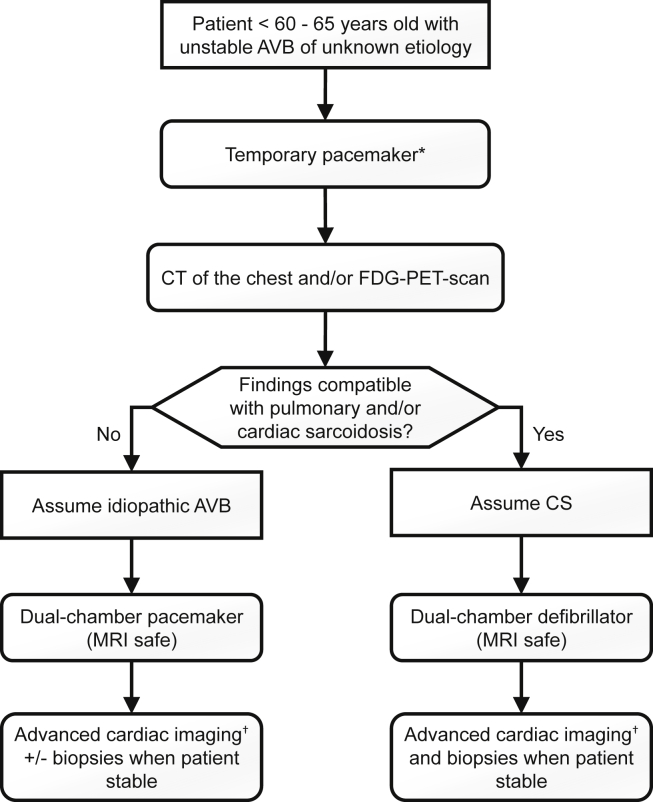
In a specific population (young age and other causes of AVB excluded), we suggest inserting a temporary pacemaker, ideally a screw-in-lead pacemaker for better stability, and to perform a chest CT scan with contrast and, when possible, an FDG-PET scan (Fig. 6). If FDG-PET scan and/or CT scan is compatible with sarcoidosis, a presumptive diagnosis of CS should be made even if the biopsy is to come and a permanent pacemaker-defibrillator MRI-safe should be implanted. If the CT scan and the FDG-PET scan are normal, it could be reasonably assumed that AVB is not caused by CS and inserting a permanent MRI-safe pacemaker is suggested. In both cases, an MRI-safe device should be implanted because CMR should be performed in the following weeks to definitely exclude CS. An EBUS-guided LN biopsy should also be performed when the patient is stabilized if the chest CT scan and/or the advanced cardiac imaging is consistent with sarcoidosis.
Figure 6.
Unstable atrioventricular block (AVB) and suspected clinically isolated cardiac sarcoidosis (CS). CT, computed tomography; FDG, fluorodeoxyglucose; MRI, magnetic resonance imaging; PET, positron emission tomography. ∗ Consider inserting a screw-in-lead temporary pacemaker and performing an FDG-PET scan to increase diagnostic yield. † Consider performing cardiac magnetic resonance imaging as soon as possible if the device is compatible and FDG-PET scan if not already performed.
7. What Is the Role of Immunosuppressive Therapy in CS?
Immunosuppression with prednisone is the gold standard for the treatment of CS although optimal medical management remains unknown because most studies are small and retrospective.8 Immunosuppression aims to improve clinical symptoms by controlling inflammation and preventing fibrosis and decline in cardiac function. Corticosteroids reduce inflammation and SUV count on FDG-PET scan and might improve conduction abnormalities.75,97 Yodogawa et al. reported that early initiation of corticosteroids resulted in AVB recovery in some patients.98 Similarly, Sadek et al. reported in a systematic review of 10 studies that 47% of patients treated with corticosteroids improved their atrioventricular conduction compared with 0% when not administered.99 The authors could not conclude on the effects of corticosteroids on LVEF and ventricular arrhythmias.99
The effect of corticosteroids on LV dysfunction and ventricular arrhythmias is not well established, because many studies showed conflicting results.99 Chiu et al. reported preservation of cardiac function with corticosteroids in patients with LVEF > 55%, improvement in cardiac function in patients with LVEF 30%-54%, and no effects in patients with LVEF < 30%.83 Likewise, Nagai et al. reported that corticosteroid therapy was associated with an increase in LVEF and fewer long-term adverse events such as cardiac death, symptomatic arrhythmias, and HF admission.97 In contrast, Kandolin et al. showed in a retrospective study of 110 patients with CS that immunosuppression improved cardiac function even when LVEF was severely impaired (LVEF < 35%).9 However, another 91 patients in a retrospective study by Fussner et al. did not show any benefits on LVEF nor on the composite end points consisting of ventricular assist device implantation, heart transplantation, or death in patients treated with corticosteroids.12 Conflicting results from different studies suggest that there must be a subgroup of patients with HF more likely to respond to therapy. Large prospective randomized controlled trials are necessary to identify this subgroup of patients.
Of notice, there is no clear evidence that corticosteroids reduce the burden of VT in patients with CS because most trials included patients who were receiving anti-arrhythmic drugs and corticosteroids.100,101 Moreover, Medor et al. reported a threefold increase in premature ventricular contraction and a significant increase in nonsustained VT after initiating corticosteroids compared with pretreatment in patients with symptomatic CS and active inflammation on PET scan.102 This was in accordance with the study of Banba et al.21 Of note, Yodoga et al. reported no significant difference in the number of premature ventricular contractions and nonsustained VT after initiating corticosteroids in their entire cohort but most patients included did not present active inflammation on nuclear imaging.103
Methotrexate can be used as a second-line therapy when corticosteroids are not effective or if there are significant side effects. One study showed that the combination of low-dose prednisolone with methotrexate stabilizes cardiac function.104 Whether methotrexate should be given systematically to all patients with CS to allow effective corticosteroid weaning, remains to be determined. Treatment with other immunosuppressive agents such as cyclophosphamide, infliximab, rituximab, and mycophenolate mofetil has been reported but the evidence supporting the use of these drugs is low and they should not be used as initial therapies.17,105, 106, 107, 108
It is unclear whether we should treat all patients with CS or only those with clinical manifestations.8 According to the HRS consensus, immunosuppression should be considered when there is evidence of myocardial inflammation and the patient either has a Mobitz II, third-degree AVB, frequent ventricular ectopy, nonsustained VT, or sustained ventricular arrhythmias (Table 3).8,39 In addition to these recommendations, Birnie et al. suggest considering immunosuppression in patients with left ventricular dysfunction and evidence of myocardial inflammation.8 More studies are needed to decide whether asymptomatic patients with CS who present only with mild ECG, TTE, or advanced cardiac imaging abnormalities should be treated. In our institution and others, patients with severe cardiac inflammation are generally treated with immunosuppression regardless of left ventricular function.
Table 3.
Specific recommendations regarding immunosuppression and implantable cardioverter-defibrillator for patients with CS according to the HRS39 and the AHA96
| Immunosuppression |
|
| Implantable cardioverter-defibrillator |
|
AHA, American Heart Association; AVB, atrioventricular block; CMR, cardiac magnetic resonance; CS, cardiac sarcoidosis; FDG, fluorodeoxyglucose; HF, heart failure; HRS, Heart Rhythm Society; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; PET, positron emission tomographic; RVEF, right ventricular ejection fraction; VF, ventricular fibrillation; VT, ventricular tachycardia.
On advanced cardiac imaging (FDG-PET-scan or CMR).
This recommendation is not supported by strong data.
Percentage of myocardial scar not specified.
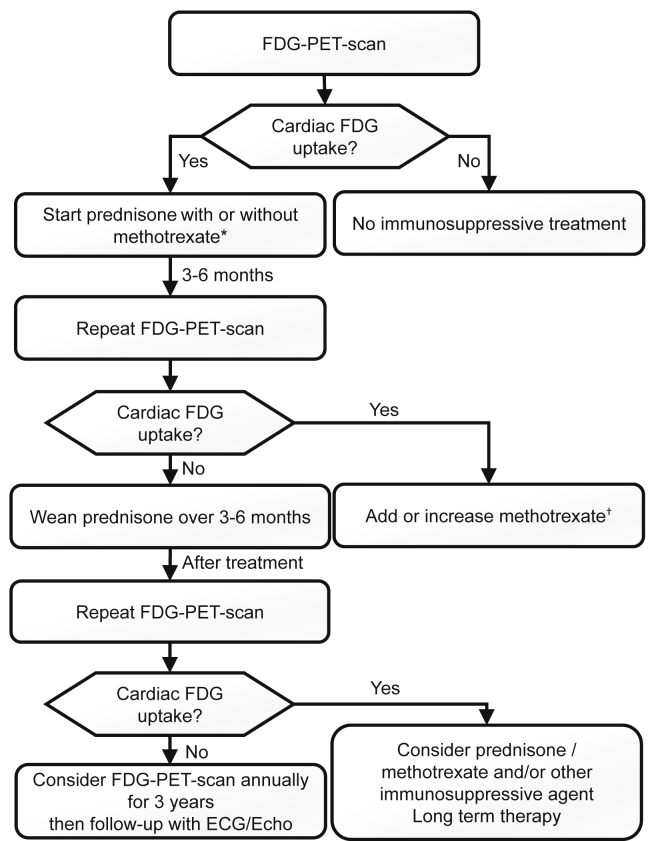
When treatment is indicated, early initiation of corticosteroids is associated with better outcomes on cardiac function and rhythm disturbances compared with delayed treatment.109 The optimal dose and duration of treatment with corticosteroids are not well established. Most experts recommend starting with a dose of 0.5 mg/kg/d with a maximum dose of 30-40 mg/d8 because no differences in outcomes have been reported with higher doses.110 Some studies suggest that long-term therapy in patients with CS is often necessary. Discontinuation of corticosteroids might be associated with poor clinical outcomes consisting of increased mortality and LV function deterioration.111 For patients in whom long-term therapy with prednisone is considered, the prevention of osteoporosis is important.112 Methotrexate is usually started at a dose of 10-15 mg orally or subcutaneous once per week and can be increased to 20-25 mg orally or subcutaneous once per week. Before introducing methotrexate, screening for tuberculosis and hepatitis B and C viruses should be performed and vaccines should be administered according to current guidelines.113 Also, complete blood count, liver function tests, and creatinine level should be obtained at baseline and monitored according to current guidelines.113 Figure 7 shows a practical approach to the treatment and follow-up of CS, adapted from Birnie et al.39 and the ESC recommendations.46 The next 2 cases illustrate the complexity of CS treatment.
Figure 7.
Proposed algorithm for treatment and follow-up of cardiac sarcoidosis patients. ECG, electrocardiogram; Echo, echocardiography; FDG, fluorodeoxyglucose; PET, positron emission tomography. ∗ Start immunosuppressive therapy in presence of Mobitz II or third-degree atrioventricular block, frequent ventricular ectopy or nonsustained ventricular tachycardia, sustained ventricular arrhythmias, or significant inflammation in the presence or absence of left ventricular dysfunction. Prednisone started at 0.5 mg/kg/d (maximum dose of 30-40 mg/d). Methotrexate could be considered as first-line therapy with prednisone in case of significant FDG uptake and severe ventricular dysfunction. Methotrexate started at 10 or 15 mg orally or subcutaneous injection once per week. † Methotrexate could be increased to 20-25 mg orally or subcutaneous injection once per week.
Case 8
In 2014, a 52-year-old man was diagnosed with cardiac and pulmonary sarcoidosis. Because the patient had an advanced AVB and an FDG-PET scan showing significant heart uptake and edema on CMR images, implantation of an ICD and immunosuppressive treatment were recommended. To taper off corticosteroids, methotrexate was initiated. During follow-up, only 1% ventricular pacing was recorded, consistent with AVB recovery. A control FDG-PET scan was performed 6 months after treatment, showing no metabolic activity and accordingly, down-titration of corticosteroids was continued. A year later, off treatment, FDG-PET scan showed recurrence of the disease with hypermetabolic adenopathy and heterogeneous FDG uptake by the myocardium. Methotrexate dosage was increased and corticosteroid treatment was resumed. A follow-up FDG-PET scan was normal.
Case 9
In 2016, a 73-year-old man was admitted for congestive HF and decreased LVEF. He had been diagnosed with idiopathic dilated cardiomyopathy 3 years earlier. At the time of admission, the ECG was normal. The chest radiograph and chest CT scan showed prominent adenopathy, therefore EBUS-guided LN biopsy was performed, confirming the diagnosis of pulmonary sarcoidosis. TTE showed a 35% LVEF. CMR showed diffuse intramyocardial LGE all along the anterior, septal, and inferior walls with a nonischemic pattern with no edema. Moreover, FDG-PET scan showed high metabolic uptake in supraclavicular, cervical, and mediastinal regions, and hilar adenopathy with no cardiac uptake. No immunosuppressive treatment was started but HF medication was initiated. During follow-up, his LVEF improved to 45% and to date no ventricular arrhythmias have been documented.
8. How to Manage AVB and/or Ventricular Arrhythmias in Patients With CS?
AVB
Although AVB might reverse with immunosuppression, the long-term outcome is unpredictable and recurrence can occur. Additionally, AVB in patients with CS has a worse prognosis compared with those with other etiologies. Nordenswan et al. showed a 5-year risk of 24% for SCD or VT in a group of 90 patients who presented solely with AVB as their initial CS manifestation.16 Similarly, a Finnish retrospective study of 72 patients showed 39% had major adverse cardiac events (MACE; ventricular fibrillation, VT, cardiac death, or heart transplantation) in the group with AVB caused by CS or giant cell myocarditis compared with 2% in the group of patients with idiopathic AVB.20 A prospective Canadian study of 32 patients who presented with AVB who were followed for 21 months showed that MACE (sustained VT, HF, heart transplantation, LVAD insertion) occurred in 27% of patients with AVB secondary to CS compared with 0% of patients with idiopathic AVB.19 In addition, among a Japanese cohort of 22 patients with AVB caused by CS, 55% had MACE (ventricular fibrillation, sustained VT, or HF) during follow-up.14 It is therefore recommended to implant a permanent pacemaker and an ICD in all patients with proven CS who have an indication for pacing even those with transient reversal of the AVB (Table 3).
Ventricular arrhythmias
CS might not behave like other cardiomyopathies and the risk of fatal arrhythmias remains high even though the LVEF is > 35%.114 A study of 235 patients with CS reported appropriate ICD therapies for secondary prevention indications in 61% of patients compared with 24% for primary prevention indication. Interestingly, these patients were properly treated with β-blockers, antiarrhythmic drugs, and/or immunosuppressive therapy.115 Another study of 29 patients with CS who had an ICD implanted for primary prevention (up to 10-year follow-up), showed that 27.6% had appropriate ICD therapies and of those, 41% had an LVEF > 35%.116
General guidelines regarding ICD implantation also apply to patients with CS. Patients with LVEF ≤ 35% despite optimal medical therapy (including a period of immunosuppression if there is active inflammation) and patients with spontaneous sustained ventricular arrhythmias including previous cardiac arrest have a class I indication for an ICD.39,96 Specific recommendations for patients with CS are summarized in Table 3.
In patients with normal LVEF, it could be reasonable to perform CMR and to implant an ICD if there is significant fibrosis because it predicts adverse outcomes.91,96,117 Kazmirczak et al. reported that an LGE cutoff of 5.7% in patients without a class I indication for an ICD predicts the composite end point of significant ventricular arrhythmias or SCD with an 83% sensitivity and a 95% specificity.118 However, more studies are needed to confirm these data. Equally, in patients with normal LVEF but significant fibrosis, it could be reasonable to perform an electrophysiological study and to implant an ICD if sustained ventricular arrhythmias are inducible.96,119,120
Antiarrhythmic drugs such as β-blockers, amiodarone, and sotalol might be considered, but care should be taken in patients with pulmonary sarcoidosis before initiating amiodarone, because of its pulmonary side effects.39 Class I antiarrhythmic drugs should be avoided because CS is often associated with myocardial scars.39 In some cases, catheter ablation has been shown to be effective101,121 but is associated with a significant recurrence rate.26,122
9. How to Manage Advanced HF in Patients With CS?
HF is seen in 10%-40% of CS cases,123,124 leading to a poor prognosis with a 10-year transplantation-free survival of 53%.9 Different consensus agreed that patients with HF due to CS should be addressed according to HF guidelines and the option of resynchronization therapy and HT should also be considered.125 Resynchronization therapy in patients with CS showed worse outcomes and lower responder rates (45% vs 79%) compared with patients with dilated cardiomyopathy.126 However, HT showed good results and should be considered in patients with severe HF refractory to medical therapy, especially in younger patients. Cardiac transplantation can sometimes be avoided if corticosteroid treatment is started before the occurrence of severe systolic dysfunction or in the presence of severe systolic dysfunction with significant inflammation in the absence of a heavy fibrosis burden.62
An interesting study was published recently by Crawford et al., who showed that 0.4% of patients waiting for an HT had CS. Of those, 23% received mechanical circulatory support as a bridge to transplantation, of whom only 59% afterward received a transplant. One-year survival for patients on the waiting list was similar for CS patients and for those without CS and freedom from mortality at 1 and 5 years was similar for those who had CS compared with patients without CS.127 Another study has reported an 80% 5-year survival post-transplantation.62
Patients who require HT have a good prognosis and CS recurrence is rare128,129 with different studies showing no recurrence at all130,131 and some case reports reporting CS recurrence in cardiac allografts especially at corticosteroids weaning.128,132 Thus, long-term corticosteroids might be considered in a subgroup of patients.127
Case 10
In 2010, a 37-year-old man was referred for newly diagnosed HF with reduced LVEF. The ECG showed a long PR interval with right BBB and the TTE showed a very severely dilated LV and an LVEF of 15%. A CMR image showed LGE with edema consistent with nonischemic cardiomyopathy. An EMB confirmed CS. Medical treatment was started along with immunosuppressive therapy with prednisone and infliximab. Six months later, there was no LVEF improvement and a primary prevention ICD was implanted. Over the next 2 years, he was admitted several times for decompensated HF and ventricular arrhythmias. Continuous milrinone infusion was started and the patient was listed for HT. In 2012 he received an HT, and 8 years later, no recurrence of his former CS occurred.
10. How Will Unrecognized CS Evolve?
Unrecognized CS might have dramatic consequences, the worst of all being SCD. This is generally caused by fatal ventricular arrhythmias or high-grade AVB. In a series of 351 cases, Ekstrom et al. reported that nearly two-thirds of deaths from CS were caused by undiagnosed cardiac involvement.15 The 2 cases that follow show the catastrophic presentations of 2 patients with unrecognized CS.
Case 11
In 2012, a 48-year-old man came to the ER for dyspnea that evolved over 3 months. ECG showed a first-degree AVB, Mobitz II and right BBB. On the treadmill, there was chronotropic incompetence with a maximal HR of 76 bpm. The TTE showed normal LVEF. A dual-chamber pacemaker was implanted and the patient was discharged. At this time the possible diagnosis of CS was not considered by the cardiologist taking care of the patient. One year after the patient was resuscitated from SCD. The initial rhythm was a polymorphic VT. A coronary angiogram showed nonsignificant lesions. The TTE showed global hypokinesia, 25% LVEF and a severe right ventricle dysfunction. CMR showed extensive LGE with a nonischemic pattern. The FDG-PET scan was consistent with systemic and active CS and the EBUS-guided LN biopsy showed sarcoidosis. Hence, his pacemaker was upgraded to a defibrillator. With immunosuppressive therapy consisting of prednisone and methotrexate, the LVEF improved to 45%.
Case 12
In 2015, a 56-year-old woman came to the ER for a presyncope. Third-degree AVB was noted on her ECG. TTE showed an LVEF of 63% with hypokinesia of the midseptal and apical inferior walls of the LV. The coronary angiogram was normal. A dual-chamber pacemaker was implanted with no further testing. Two years later, TTE showed a 15% LVEF, basal septal thinning, and right ventricle dysfunction. CMR could not be performed because her pacemaker was not MRI-compatible. She was then referred to our centre and FDG-PET scan was done and showed a result consistent with systemic and active CS; however, an EBUS-guided LN biopsy was normal. Her dual-chamber pacemaker was upgraded to an ICD. The patient declined HT evaluation consideration. Three weeks after the ICD upgrade, the patient died in her sleep. The postmortem interrogation of her device showed a slow VT (monitor zone) that eventually transformed into ventricular fibrillation with an appropriate ICD shock and conversion to sinus rhythm and electromechanical dissociation was the cause of death. At the autopsy, CS was diagnosed with no other cause to explain SCD.
Conclusion
Despite the increasing awareness of this potentially life-threatening illness, many questions remain unanswered and more clinical trials are needed to guide optimal CS therapy. In this review, we provided answers to the top 10 questions cardiologists should be able to answer about CS and summarized the key elements in Table 4. The ongoing CHASM-CS randomized controlled trial will give us more information about the effect of corticosteroid treatment on the clinical course of CS. We aimed to raise awareness within the medical community and to promote meaningful collaboration between lung specialists, internists, rheumatologists, and cardiologists to target appropriate investigation and optimal treatment for patients with CS.
Table 4.
Ten questions cardiologists should be able to answer about CS
|
|
|
|
|
|
|
|
|
|
AVB, atrioventricular block; BBB, bundle branch block; Bx, biopsy; CMR, cardiac magnetic resonance; CS, cardiac sarcoidosis; CT, computed tomography; ECG, electrocardiogram; FDG, fluorodeoxyglucose; HF, heart failure; HT, heart transplantation; ICD, implantable cardioverter-defibrillator; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction; PET, positron emission tomographic; SCD, sudden cardiac death; TTE, transthoracic echocardiogram; VT, ventricular tachycardia.
Acknowledgements
We acknowledge Michelle Dubois, BSc, RN, for her help in the drafting of the manuscript.
Funding Sources
Dr Massot’s fellowship is supported by a research grant from the Fundación Alfonso Martín Escudero (Madrid, Spain).
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: We certify that our research conforms to the ethical guidelines.
See page 544 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.11.022.
Supplementary Material
References
- 1.Baughman R.P., Teirstein A.S., Judson M.A. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 2.Rybicki B.A., Major M., Popovich J., Jr., Maliarik M.J., Iannuzzi M.C. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 3.Iannuzzi M.C., Rybicki B.A., Teirstein A.S. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 4.Rybicki B.A., Iannuzzi M.C., Frederick M.M. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS) Am J Respir Crit Care Med. 2001;164:2085–2091. doi: 10.1164/ajrccm.164.11.2106001. [DOI] [PubMed] [Google Scholar]
- 5.Deubelbeiss U., Gemperli A., Schindler C., Baty F., Brutsche M.H. Prevalence of sarcoidosis in Switzerland is associated with environmental factors. Eur Respir J. 2010;35:1088–1097. doi: 10.1183/09031936.00197808. [DOI] [PubMed] [Google Scholar]
- 6.Silverman K.J., Hutchins G.M., Bulkley B.H. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–1211. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 7.Iwai K., Takemura T., Kitaichi M., Kawabata Y., Matsui Y. Pathological studies on sarcoidosis autopsy. II. Early change, mode of progression and death pattern. Acta Pathol Jpn. 1993;43:377–385. doi: 10.1111/j.1440-1827.1993.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 8.Birnie D.H., Nery P.B., Ha A.C., Beanlands R.S. Cardiac sarcoidosis. J Am Coll Cardiol. 2016;68:411–421. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 9.Kandolin R., Lehtonen J., Airaksinen J. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 10.Kandolin R., Lehtonen J., Graner M. Diagnosing isolated cardiac sarcoidosis. J Intern Med. 2011;270:461–468. doi: 10.1111/j.1365-2796.2011.02396.x. [DOI] [PubMed] [Google Scholar]
- 11.Juneau D., Nery P., Russo J. How common is isolated cardiac sarcoidosis? Extra-cardiac and cardiac findings on clinical examination and whole-body (18)F-fluorodeoxyglucose positron emission tomography. Int J Cardiol. 2018;253:189–193. doi: 10.1016/j.ijcard.2017.09.204. [DOI] [PubMed] [Google Scholar]
- 12.Fussner L.A., Karlstedt E., Hodge D.O. Management and outcomes of cardiac sarcoidosis: a 20-year experience in two tertiary care centres. Eur J Heart Fail. 2018;20:1713–1720. doi: 10.1002/ejhf.1319. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y., Lower E.E., Li H.P. Cardiac sarcoidosis: the impact of age and implanted devices on survival. Chest. 2017;151:139–148. doi: 10.1016/j.chest.2016.08.1457. [DOI] [PubMed] [Google Scholar]
- 14.Takaya Y., Kusano K.F., Nakamura K., Ito H. Outcomes in patients with high-degree atrioventricular block as the initial manifestation of cardiac sarcoidosis. Am J Cardiol. 2015;115:505–509. doi: 10.1016/j.amjcard.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Ekstrom K., Lehtonen J., Nordenswan H.K. Sudden death in cardiac sarcoidosis: an analysis of nationwide clinical and cause-of-death registries. Eur Heart J. 2019;40:3121–3128. doi: 10.1093/eurheartj/ehz428. [DOI] [PubMed] [Google Scholar]
- 16.Nordenswan H.K., Lehtonen J., Ekstrom K. Outcome of cardiac sarcoidosis presenting with high-grade atrioventricular block. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.006145. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.S., Judson M.A., Donnino R. Cardiac sarcoidosis. Am Heart J. 2009;157:9–21. doi: 10.1016/j.ahj.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Lynch J.P., 3rd, Hwang J., Bradfield J. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med. 2014;35:372–390. doi: 10.1055/s-0034-1376889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nery P.B., Beanlands R.S., Nair G.M. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol. 2014;25:875–881. doi: 10.1111/jce.12401. [DOI] [PubMed] [Google Scholar]
- 20.Kandolin R., Lehtonen J., Kupari M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol. 2011;4:303–309. doi: 10.1161/CIRCEP.110.959254. [DOI] [PubMed] [Google Scholar]
- 21.Banba K., Kusano K.F., Nakamura K. Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart Rhythm. 2007;4:1292–1299. doi: 10.1016/j.hrthm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Sekhri V., Sanal S., Delorenzo L.J., Aronow W.S., Maguire G.P. Cardiac sarcoidosis: a comprehensive review. Arch Med Sci. 2011;7:546–554. doi: 10.5114/aoms.2011.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abeler V. Sarcoidosis of the cardiac conducting system. Am Heart J. 1979;97:701–707. doi: 10.1016/0002-8703(79)90004-8. [DOI] [PubMed] [Google Scholar]
- 24.Nery P.B., Mc Ardle B.A., Redpath C.J. Prevalence of cardiac sarcoidosis in patients presenting with monomorphic ventricular tachycardia. Pacing Clin Electrophysiol. 2014;37:364–374. doi: 10.1111/pace.12277. [DOI] [PubMed] [Google Scholar]
- 25.Tung R., Bauer B., Schelbert H. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: the potential role of occult inflammation in arrhythmogenesis. Heart Rhythm. 2015;12:2488–2498. doi: 10.1016/j.hrthm.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koplan B.A., Soejima K., Baughman K., Epstein L.M., Stevenson W.G. Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2006;3:924–929. doi: 10.1016/j.hrthm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Swanson N., Goddard M., McCann G., Ng G.A. Sarcoidosis presenting with tachy- and brady-arrhythmias. Europace. 2007;9:134–136. doi: 10.1093/europace/eul173. [DOI] [PubMed] [Google Scholar]
- 28.Ladyjanskaia G.A., Basso C., Hobbelink M.G. Sarcoid myocarditis with ventricular tachycardia mimicking ARVD/C. J Cardiovasc Electrophysiol. 2010;21:94–98. doi: 10.1111/j.1540-8167.2009.01479.x. [DOI] [PubMed] [Google Scholar]
- 29.Panda S., Kaur D., Lalukota K. Pleomorphism during ventricular tachycardia: a distinguishing feature between cardiac sarcoidosis and idiopathic VT. Pacing Clin Electrophysiol. 2015;38:694–699. doi: 10.1111/pace.12626. [DOI] [PubMed] [Google Scholar]
- 30.Furushima H., Chinushi M., Sugiura H. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol. 2004;27:217–222. doi: 10.1002/clc.4960270409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viles-Gonzalez J.F., Pastori L., Fischer A. Supraventricular arrhythmias in patients with cardiac sarcoidosis prevalence, predictors, and clinical implications. Chest. 2013;143:1085–1090. doi: 10.1378/chest.11-3214. [DOI] [PubMed] [Google Scholar]
- 32.Selan J.C., Michaelson M., Fanburg B.L., Estes N.A. Evaluation and management of heart rhythm disturbances due to cardiac sarcoidosis. Heart Lung Circ. 2014;23:1100–1109. doi: 10.1016/j.hlc.2014.07.065. [DOI] [PubMed] [Google Scholar]
- 33.Roberts W.C., Chung M.S., Ko J.M., Capehart J.E., Hall S.A. Morphologic features of cardiac sarcoidosis in native hearts of patients having cardiac transplantation. Am J Cardiol. 2014;113:706–712. doi: 10.1016/j.amjcard.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Segura A.M., Radovancevic R., Demirozu Z.T., Frazier O.H., Buja L.M. Granulomatous myocarditis in severe heart failure patients undergoing implantation of a left ventricular assist device. Cardiovasc Pathol. 2014;23:17–20. doi: 10.1016/j.carpath.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Okura Y., Dec G.W., Hare J.M. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol. 2003;41:322–329. doi: 10.1016/s0735-1097(02)02715-8. [DOI] [PubMed] [Google Scholar]
- 36.Terasaki F., Azuma A., Anzai T. JCS 2016 guideline on diagnosis and treatment of cardiac sarcoidosis- digest version. Circ J. 2019;83:2329–2388. doi: 10.1253/circj.CJ-19-0508. [DOI] [PubMed] [Google Scholar]
- 37.Judson M.A., Baughman R.P., Teirstein A.S., Terrin M.L., Yeager H., Jr. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A case control etiologic study of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:75–86. [PubMed] [Google Scholar]
- 38.Judson M.A., Costabel U., Drent M. The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27. [PubMed] [Google Scholar]
- 39.Birnie D.H., Sauer W.H., Bogun F. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 40.Crouser E.D., Maier L.A., Wilson K.C. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26–51. doi: 10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller-Quernheim J., Gaede K.I., Fireman E., Zissel G. Diagnoses of chronic beryllium disease within cohorts of sarcoidosis patients. Eur Respir J. 2006;27:1190–1195. doi: 10.1183/09031936.06.00112205. [DOI] [PubMed] [Google Scholar]
- 42.Otsuka K., Terasaki F., Eishi Y. Cardiac sarcoidosis underlies idiopathic dilated cardiomyopathy: importance of mediastinal lymphadenopathy in differential diagnosis. Circ J. 2007;71:1937–1941. doi: 10.1253/circj.71.1937. [DOI] [PubMed] [Google Scholar]
- 43.Wicks E.C., Menezes L.J., Barnes A. Diagnostic accuracy and prognostic value of simultaneous hybrid 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging. 2018;19:757–767. doi: 10.1093/ehjci/jex340. [DOI] [PubMed] [Google Scholar]
- 44.Mehta D., Lubitz S.A., Frankel Z. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133:1426–1435. doi: 10.1378/chest.07-2784. [DOI] [PubMed] [Google Scholar]
- 45.Hena K.M., Yip J., Jaber N. Clinical course of sarcoidosis in World Trade Center-exposed firefighters. Chest. 2018;153:114–123. doi: 10.1016/j.chest.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Writing group; Document reading group; EACVI Reviewers This document was reviewed by members of the EACVI Scientific Documents Committee for 2014–2016 and 2016–2018. A joint procedural position statement on imaging in cardiac sarcoidosis: from the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. Eur Heart J Cardiovasc Imaging. 2017;18:1073–1089. doi: 10.1093/ehjci/jex146. [DOI] [PubMed] [Google Scholar]
- 47.Russo J.J., Nery P.B., Ha A.C. Sensitivity and specificity of chest imaging for sarcoidosis screening in patients with cardiac presentations. Sarcoidosis Vasc Diffuse Lung Dis. 2019;36:18–24. doi: 10.36141/svdld.v36i1.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petek B.J., Rosenthal D.G., Patton K.K. Cardiac sarcoidosis: diagnosis confirmation by bronchoalveolar lavage and lung biopsy. Respir Med. 2018;144S:S13–S19. doi: 10.1016/j.rmed.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Schuller J.L., Olson M.D., Zipse M.M. Electrocardiographic characteristics in patients with pulmonary sarcoidosis indicating cardiac involvement. J Cardiovasc Electrophysiol. 2011;22:1243–1248. doi: 10.1111/j.1540-8167.2011.02099.x. [DOI] [PubMed] [Google Scholar]
- 50.Hamzeh N.Y., Wamboldt F.S., Weinberger H.D. Management of cardiac sarcoidosis in the United States: a Delphi study. Chest. 2012;141:154–162. doi: 10.1378/chest.11-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagao S., Watanabe H., Sobue Y. Electrocardiographic abnormalities and risk of developing cardiac events in extracardiac sarcoidosis. Int J Cardiol. 2015;189:1–5. doi: 10.1016/j.ijcard.2015.03.175. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T., Kanda T., Kubota S., Imai S., Murata K. Holter monitoring as a noninvasive indicator of cardiac involvement in sarcoidosis. Chest. 1994;106:1021–1024. doi: 10.1378/chest.106.4.1021. [DOI] [PubMed] [Google Scholar]
- 53.Schuller J.L., Lowery C.M., Zipse M. Diagnostic utility of signal-averaged electrocardiography for detection of cardiac sarcoidosis. Ann Noninvasive Electrocardiol. 2011;16:70–76. doi: 10.1111/j.1542-474X.2010.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sobue Y., Harada M., Koshikawa M. QRS-based assessment of myocardial damage and adverse events associated with cardiac sarcoidosis. Heart Rhythm. 2015;12:2499–2507. doi: 10.1016/j.hrthm.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Lewin R.F., Mor R., Spitzer S., Arditti A., Hellman C., Agmon J. Echocardiographic evaluation of patients with systemic sarcoidosis. Am Heart J. 1985;110:116–122. doi: 10.1016/0002-8703(85)90524-1. [DOI] [PubMed] [Google Scholar]
- 56.Kurmann R., Mankad S.V., Mankad R. Echocardiography in sarcoidosis. Curr Cardiol Rep. 2018;20:118. doi: 10.1007/s11886-018-1065-9. [DOI] [PubMed] [Google Scholar]
- 57.Ho J.S.Y., Chilvers E.R., Thillai M. Cardiac sarcoidosis - an expert review for the chest physician. Expert Rev Respir Med. 2019;13:507–520. doi: 10.1080/17476348.2018.1511431. [DOI] [PubMed] [Google Scholar]
- 58.Burstow D.J., Tajik A.J., Bailey K.R., DeRemee R.A., Taliercio C.P. Two-dimensional echocardiographic findings in systemic sarcoidosis. Am J Cardiol. 1989;63:478–482. doi: 10.1016/0002-9149(89)90323-8. [DOI] [PubMed] [Google Scholar]
- 59.Ayoub C., Pena E., Ohira H. Advanced imaging of cardiac sarcoidosis. Curr Cardiol Rep. 2015;17:17. doi: 10.1007/s11886-015-0572-1. [DOI] [PubMed] [Google Scholar]
- 60.Murtagh G., Laffin L.J., Patel K.V. Improved detection of myocardial damage in sarcoidosis using longitudinal strain in patients with preserved left ventricular ejection fraction. Echocardiography. 2016;33:1344–1352. doi: 10.1111/echo.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schouver E.D., Moceri P., Doyen D. Early detection of cardiac involvement in sarcoidosis with 2-dimensional speckle-tracking echocardiography. Int J Cardiol. 2017;227:711–716. doi: 10.1016/j.ijcard.2016.10.073. [DOI] [PubMed] [Google Scholar]
- 62.Bussinguer M., Danielian A., Sharma O.P. Cardiac sarcoidosis: diagnosis and management. Curr Treat Options Cardiovasc Med. 2012;14:652–664. doi: 10.1007/s11936-012-0208-3. [DOI] [PubMed] [Google Scholar]
- 63.Focardi M., Picchi A., Nikiforakis N. Assessment of cardiac involvement in sarcoidosis by echocardiography. Rheumatol Int. 2009;29:1051–1055. doi: 10.1007/s00296-009-0904-9. [DOI] [PubMed] [Google Scholar]
- 64.Blankstein R., Waller A.H. Evaluation of known or suspected cardiac sarcoidosis. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.113.000867. [DOI] [PubMed] [Google Scholar]
- 65.Angomachalelis N., Hourzamanis A., Vamvalis C., Gavrielides A. Doppler echocardiographic evaluation of left ventricular diastolic function in patients with systemic sarcoidosis. Postgrad Med J. 1992;68(suppl 1):S52–S56. [PubMed] [Google Scholar]
- 66.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J., Li Y., Xu Q., Xu B., Wang H. Cardiac magnetic resonance imaging for diagnosis of cardiac sarcoidosis: a meta-analysis. Can Respir J. 2018;2018:7457369. doi: 10.1155/2018/7457369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schatka I., Bengel F.M. Advanced imaging of cardiac sarcoidosis. J Nucl Med. 2014;55:99–106. doi: 10.2967/jnumed.112.115121. [DOI] [PubMed] [Google Scholar]
- 69.Yang Y., Safka K., Graham J.J. Correlation of late gadolinium enhancement MRI and quantitative T2 measurement in cardiac sarcoidosis. J Magn Reson Imaging. 2014;39:609–616. doi: 10.1002/jmri.24196. [DOI] [PubMed] [Google Scholar]
- 70.Timmers M., Claeys M.J., Vanhauwaert B., Rivero-Ayerza M., De Hondt G. Cardiac sarcoidosis: a diagnostic and therapeutic challenge. Acta Cardiol. 2018;73:1–6. doi: 10.1080/00015385.2017.1325633. [DOI] [PubMed] [Google Scholar]
- 71.Youssef G., Beanlands R.S., Birnie D.H., Nery P.B. Cardiac sarcoidosis: applications of imaging in diagnosis and directing treatment. Heart. 2011;97:2078–2087. doi: 10.1136/hrt.2011.226076. [DOI] [PubMed] [Google Scholar]
- 72.Tadamura E., Yamamuro M., Kubo S. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. AJR Am J Roentgenol. 2005;185:110–115. doi: 10.2214/ajr.185.1.01850110. [DOI] [PubMed] [Google Scholar]
- 73.Dubrey S.W., Sharma R., Underwood R., Mittal T. Cardiac sarcoidosis: diagnosis and management. Postgrad Med J. 2015;91:384–394. doi: 10.1136/postgradmedj-2014-133219. [DOI] [PubMed] [Google Scholar]
- 74.Kono T., Ogimoto A., Saito M. Cardiac magnetic resonance imaging for assessment of steroid therapy in a patient with cardiac sarcoidosis and a magnetic resonance-conditional pacemaker. Int J Cardiol. 2014;176:e89–91. doi: 10.1016/j.ijcard.2014.07.157. [DOI] [PubMed] [Google Scholar]
- 75.Gotthardt M., Bleeker-Rovers C.P., Boerman O.C., Oyen W.J. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med. 2010;51:1937–1949. doi: 10.2967/jnumed.110.076232. [DOI] [PubMed] [Google Scholar]
- 76.Youssef G., Leung E., Mylonas I. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and meta-analysis including the Ontario experience. J Nucl Med. 2012;53:241–248. doi: 10.2967/jnumed.111.090662. [DOI] [PubMed] [Google Scholar]
- 77.Skali H., Schulman A.R., Dorbala S. 18F-FDG PET/CT for the assessment of myocardial sarcoidosis. Curr Cardiol Rep. 2013;15:352. [PMC free article] [PubMed] [Google Scholar]
- 78.Ohira H., Tsujino I., Ishimaru S. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933–941. doi: 10.1007/s00259-007-0650-8. [DOI] [PubMed] [Google Scholar]
- 79.Chareonthaitawee P., Beanlands R.S., Chen W. Joint SNMMI-ASNC expert consensus document on the role of (18)F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Cardiol. 2017;24:1741–1758. doi: 10.1007/s12350-017-0978-9. [DOI] [PubMed] [Google Scholar]
- 80.Ishimaru S., Tsujino I., Takei T. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005;26:1538–1543. doi: 10.1093/eurheartj/ehi180. [DOI] [PubMed] [Google Scholar]
- 81.Yokoyama R., Miyagawa M., Okayama H. Quantitative analysis of myocardial 18F-fluorodeoxyglucose uptake by PET/CT for detection of cardiac sarcoidosis. Int J Cardiol. 2015;195:180–187. doi: 10.1016/j.ijcard.2015.05.075. [DOI] [PubMed] [Google Scholar]
- 82.Vita T., Okada D.R., Veillet-Chowdhury M. Complementary value of cardiac magnetic resonance imaging and positron emission tomography/computed tomography in the assessment of cardiac sarcoidosis. Circ Cardiovasc Imaging. 2018;11 doi: 10.1161/CIRCIMAGING.117.007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiu C.Z., Nakatani S., Zhang G. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–146. doi: 10.1016/j.amjcard.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 84.Shelke A.B., Aurangabadkar H.U., Bradfield J.S. Serial FDG-PET scans help to identify steroid resistance in cardiac sarcoidosis. Int J Cardiol. 2017;228:717–722. doi: 10.1016/j.ijcard.2016.11.142. [DOI] [PubMed] [Google Scholar]
- 85.Ning N., Guo H.H., Iagaru A. Serial cardiac FDG-PET for the diagnosis and therapeutic guidance of patients with cardiac sarcoidosis. J Card Fail. 2019;25:307–311. doi: 10.1016/j.cardfail.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 86.Kersey C.B., Flaherty K.R., Goldenthal I.L., Bokhari S., Biviano A.B. The use of serial cardiac (18)F-fluorodeoxyglucose positron emission tomography imaging to diagnose, monitor, and tailor treatment of cardiac sarcoidosis patients with arrhythmias: a case series and review. Eur Heart J Case Rep. 2019;3:1–7. doi: 10.1093/ehjcr/ytz188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Osborne M.T., Hulten E.A., Singh A. Reduction in (1)(8)F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21:166–174. doi: 10.1007/s12350-013-9828-6. [DOI] [PubMed] [Google Scholar]
- 88.Flores R.J., Flaherty K.R., Jin Z., Bokhari S. The prognostic value of quantitating and localizing F-18 FDG uptake in cardiac sarcoidosis. J Nucl Cardiol. 2020;27:2003–2010. doi: 10.1007/s12350-018-01504-y. [DOI] [PubMed] [Google Scholar]
- 89.Greulich S., Deluigi C.C., Gloekler S. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6:501–511. doi: 10.1016/j.jcmg.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 90.Coleman G.C., Shaw P.W., Balfour P.C., Jr. Prognostic value of myocardial scarring on CMR in patients with cardiac sarcoidosis. JACC Cardiovasc Imaging. 2017;10:411–420. doi: 10.1016/j.jcmg.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hulten E., Agarwal V., Cahill M. Presence of late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uemura A., Morimoto S., Hiramitsu S. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999;138:299–302. doi: 10.1016/s0002-8703(99)70115-8. [DOI] [PubMed] [Google Scholar]
- 93.Cooper L.T., Baughman K.L., Feldman A.M. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 94.Winterbauer R.H., Lammert J., Selland M. Bronchoalveolar lavage cell populations in the diagnosis of sarcoidosis. Chest. 1993;104:352–361. doi: 10.1378/chest.104.2.352. [DOI] [PubMed] [Google Scholar]
- 95.Trisolini R., Baughman R.P., Spagnolo P., Culver D.A. Endobronchial ultrasound-guided transbronchial needle aspiration in sarcoidosis: beyond the diagnostic yield. Respirology. 2019;24:531–542. doi: 10.1111/resp.13537. [DOI] [PubMed] [Google Scholar]
- 96.Al-Khatib S.M., Stevenson W.G., Ackerman M.J. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:e91–220. doi: 10.1016/j.jacc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 97.Nagai T., Nagano N., Sugano Y. Effect of corticosteroid therapy on long-term clinical outcome and left ventricular function in patients with cardiac sarcoidosis. Circ J. 2015;79:1593–1600. doi: 10.1253/circj.CJ-14-1275. [DOI] [PubMed] [Google Scholar]
- 98.Yodogawa K., Seino Y., Shiomura R. Recovery of atrioventricular block following steroid therapy in patients with cardiac sarcoidosis. J Cardiol. 2013;62:320–325. doi: 10.1016/j.jjcc.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 99.Sadek M.M., Yung D., Birnie D.H., Beanlands R.S., Nery P.B. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29:1034–1041. doi: 10.1016/j.cjca.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 100.Futamatsu H., Suzuki J., Adachi S. Utility of gallium-67 scintigraphy for evaluation of cardiac sarcoidosis with ventricular tachycardia. Int J Cardiovasc Imaging. 2006;22:443–448. doi: 10.1007/s10554-005-9043-x. [DOI] [PubMed] [Google Scholar]
- 101.Jefic D., Joel B., Good E. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multicenter registry. Heart Rhythm. 2009;6:189–195. doi: 10.1016/j.hrthm.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 102.Medor M.C., Spence S., Nery P.B. Treatment with corticosteroids is associated with an increase in ventricular arrhythmia burden in patients with clinically manifest cardiac sarcoidosis; insights from implantable cardioverter defibrillator diagnostics. J Cardiovasc Electrophysiol. 2020;31:2751–2758. doi: 10.1111/jce.14689. [DOI] [PubMed] [Google Scholar]
- 103.Yodogawa K., Seino Y., Ohara T. Effect of corticosteroid therapy on ventricular arrhythmias in patients with cardiac sarcoidosis. Ann Noninvasive Electrocardiol. 2011;16:140–147. doi: 10.1111/j.1542-474X.2011.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nagai S., Yokomatsu T., Tanizawa K. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med. 2014;53:427–433. doi: 10.2169/internalmedicine.53.0794. [DOI] [PubMed] [Google Scholar]
- 105.Uthman I., Touma Z., Khoury M. Cardiac sarcoidosis responding to monotherapy with infliximab. Clin Rheumatol. 2007;26:2001–2003. doi: 10.1007/s10067-007-0614-1. [DOI] [PubMed] [Google Scholar]
- 106.Kikuchi N., Nunoda S., Serizawa N. Combination therapy with corticosteroid and mycophenolate mofetil in a case of refractory cardiac sarcoidosis. J Cardiol Cases. 2016;13:125–128. doi: 10.1016/j.jccase.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krause M.L., Cooper L.T., Chareonthaitawee P., Amin S. Successful use of rituximab in refractory cardiac sarcoidosis. Rheumatology (Oxford) 2016;55:189–191. doi: 10.1093/rheumatology/kev309. [DOI] [PubMed] [Google Scholar]
- 108.Demeter S.L. Myocardial sarcoidosis unresponsive to steroids. Treatment with cyclophosphamide. Chest. 1988;94:202–203. doi: 10.1378/chest.94.1.202. [DOI] [PubMed] [Google Scholar]
- 109.Padala S.K., Peaslee S., Sidhu M.S., Steckman D.A., Judson M.A. Impact of early initiation of corticosteroid therapy on cardiac function and rhythm in patients with cardiac sarcoidosis. Int J Cardiol. 2017;227:565–570. doi: 10.1016/j.ijcard.2016.10.101. [DOI] [PubMed] [Google Scholar]
- 110.Yazaki Y., Isobe M., Hiroe M. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–1010. doi: 10.1016/s0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 111.Nagai T., Nagano N., Sugano Y. Effect of discontinuation of prednisolone therapy on risk of cardiac mortality associated with worsening left ventricular dysfunction in cardiac sarcoidosis. Am J Cardiol. 2016;117:966–971. doi: 10.1016/j.amjcard.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 112.Papaioannou A., Morin S., Cheung A.M. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182:1864–1873. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Singh J.A., Saag K.G., Bridges S.L., Jr. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:1–25. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 114.Birnie D., Ha A., Kron J. Which patients with cardiac sarcoidosis should receive implantable cardioverter-defibrillators: some answers but many questions remain. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.118.006685. [DOI] [PubMed] [Google Scholar]
- 115.Kron J., Sauer W., Schuller J. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace. 2013;15:347–354. doi: 10.1093/europace/eus316. [DOI] [PubMed] [Google Scholar]
- 116.Betensky B.P., Tschabrunn C.M., Zado E.S. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm. 2012;9:884–891. doi: 10.1016/j.hrthm.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 117.Ise T., Hasegawa T., Morita Y. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart. 2014;100:1165–1172. doi: 10.1136/heartjnl-2013-305187. [DOI] [PubMed] [Google Scholar]
- 118.Kazmirczak F., Chen K.A., Adabag S. Assessment of the 2017 AHA/ACC/HRS guideline recommendations for implantable cardioverter-defibrillator implantation in cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mehta D., Mori N., Goldbarg S.H. Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol. 2011;4:43–48. doi: 10.1161/CIRCEP.110.958322. [DOI] [PubMed] [Google Scholar]
- 120.Aizer A., Stern E.H., Gomes J.A. Usefulness of programmed ventricular stimulation in predicting future arrhythmic events in patients with cardiac sarcoidosis. Am J Cardiol. 2005;96:276–282. doi: 10.1016/j.amjcard.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 121.Stees C.S., Khoo M.S., Lowery C.M., Sauer W.H. Ventricular tachycardia storm successfully treated with immunosuppression and catheter ablation in a patient with cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2011;22:210–213. doi: 10.1111/j.1540-8167.2010.01826.x. [DOI] [PubMed] [Google Scholar]
- 122.Tokuda M., Tedrow U.B., Kojodjojo P. Catheter ablation of ventricular tachycardia in nonischemic heart disease. Circ Arrhythm Electrophysiol. 2012;5:992–1000. doi: 10.1161/CIRCEP.112.971341. [DOI] [PubMed] [Google Scholar]
- 123.Yazaki Y., Isobe M., Hiramitsu S. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;82:537–540. doi: 10.1016/s0002-9149(98)00377-4. [DOI] [PubMed] [Google Scholar]
- 124.Pereira N.L., Grogan M., Dec G.W. Spectrum of restrictive and infiltrative cardiomyopathies: part 1 of a 2-part series. J Am Coll Cardiol. 2018;71:1130–1148. doi: 10.1016/j.jacc.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 125.Birnie D.H., Kandolin R., Nery P.B., Kupari M. Cardiac manifestations of sarcoidosis: diagnosis and management. Eur Heart J. 2017;38:2663–2670. doi: 10.1093/eurheartj/ehw328. [DOI] [PubMed] [Google Scholar]
- 126.Yufu K., Kondo H., Shinohara T. Outcome of patients with cardiac sarcoidosis who received cardiac resynchronization therapy: comparison with dilated cardiomyopathy patients. J Cardiovasc Electrophysiol. 2017;28:177–181. doi: 10.1111/jce.13119. [DOI] [PubMed] [Google Scholar]
- 127.Crawford T.C., Okada D.R., Magruder J.T. A contemporary analysis of heart transplantation and bridge-to-transplant mechanical circulatory support outcomes in cardiac sarcoidosis. J Card Fail. 2018;24:384–391. doi: 10.1016/j.cardfail.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 128.Yager J.E., Hernandez A.F., Steenbergen C. Recurrence of cardiac sarcoidosis in a heart transplant recipient. J Heart Lung Transplant. 2005;24:1988–1990. doi: 10.1016/j.healun.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 129.Perkel D., Czer L.S., Morrissey R.P. Heart transplantation for end-stage heart failure due to cardiac sarcoidosis. Transplant Proc. 2013;45:2384–2386. doi: 10.1016/j.transproceed.2013.02.116. [DOI] [PubMed] [Google Scholar]
- 130.Sperry B.W., Oldan J., Hachamovitch R., Tamarappoo B.K. Insights into biopsy-proven cardiac sarcoidosis in patients with heart failure. J Heart Lung Transplant. 2016;35:392–393. doi: 10.1016/j.healun.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Theofilogiannakos E.K., Pettit S.J., Ghazi A. Heart transplantation for advanced heart failure due to cardiac sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:208–214. [PubMed] [Google Scholar]
- 132.Oni A.A., Hershberger R.E., Norman D.J. Recurrence of sarcoidosis in a cardiac allograft: control with augmented corticosteroids. J Heart Lung Transplant. 1992;11:367–369. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.