Abstract
Background
Some of the millions of women with silicone breast implants (SBIs) report a pattern of systemic complaints, known as ASIA syndrome. However, the association between these complaints and breast implants remains uncertain.
Objectives
This study aimed to evaluate the prevalence of complaints in women with breast implants and healthy controls, and to compare their health-related quality of life.
Methods
Four groups of subjects were requested to fill in a general and a diagnostic questionnaire, and the Short Form 36. Group 1 was recruited from the Dutch foundation for breast implant illness (BII). Two groups were recruited from Dutch hospitals, where they had been augmented or reconstructed with SBIs (group 2) or saline-filled and hydrogel implants (group 3). A control group without breast implants was recruited from friends of subjects from group 2.
Results
In total, 238 women completed the questionnaires. ASIA manifestations appeared in the majority of the respondents (72.3%-98.8%), with a latency period of 0 to 35 years. Adjusted for age, smoking, and comorbidities, typical symptoms only occurred significantly more frequently in group 1. The presence of a chronic disease was an independent predictor for ASIA syndrome. The health-related quality of life was lower in women with SBIs than in women without breast implants.
Conclusions
The adjusted prevalence of BII manifestations is not significantly higher in women with SBIs than in women without implants. The findings of this study suggest that results on BII are subject to selection bias. Further studies are needed to prove an association between self-reported complaints and SBIs.
Level of Evidence: 2

In the United States alone, nearly 330,000 breast augmentation procedures were performed in 2018.1 An estimated 3% of Dutch women between 20 and 70 years old have breast implants.2 Some of these women report a pattern of systemic health complaints of varying severity, including myalgia, arthralgia, fever, fatigue, dry eyes and mouth, as well as cognitive impairment.3,4 In 2011, Shoenfeld and Agmon-Levin5 proposed the existence of an autoimmune syndrome induced by adjuvants (eg, breast implants)—the ASIA syndrome. Many studies have investigated the possible health effects of silicone breast implants (SBIs); however, a clear association between breast implants and systemic or autoimmune diseases remains uncertain.6-8 The explanation for complaints in these patients is probably multifactorial. It is unclear whether these symptoms would have occurred if no implants had been placed. However, in cases where there is an association with implants, immunogenic factors such as pre-existing allergies and environmental aspects such as smoking may play a role in the development of SBI-induced health complaints, also referred to as breast implant illness (BII).9,10 Interestingly, there is a remarkable overlap with fibromyalgia and it cannot be excluded that it concerns the same disease.11-13
Studies on the prevalence of BII among women with SBIs show different figures, varying from nonspecific complaints in 2%14 to rheumatic symptoms after surgery in 37.4% of cases,12 and the development of a pattern of systemic complaints in 65% of women with SBIs.15 The Dutch Foundation for Women with Illness due to Breast Implants (Meldpunt Klachten Siliconen [MKS]) indicates that in 2014 and 2015, around 150 women reported breast-associated complaints.16 This is, however, a selected group and large epidemiologic studies are lacking.
The main objective of this study was to evaluate the prevalence of clinical manifestations related to ASIA syndrome in 4 different cohorts. The first cohort is a group of women with self-reported complaints, recruited from the MKS. The second and third cohorts are groups of unselected women with respectively silicone or saline/hydrogel (Monobloc; Laboratoires Arion, Mougins, France) breast implants. The fourth group is a control group of women without breast implants. In addition to the evaluation of typical complaints, health-related quality of life (HRQoL) survey results were evaluated and compared between these groups.
METHODS
Patient Selection
Four groups of subjects were included in this retrospective cohort study. Group 1 consisted of women with SBIs and self-reported complaints, recruited from the MKS. All women who were registered with MKS with address details were invited. Participants in groups 2 and 3 were women who had, based on surgery reports, undergone breast augmentation or breast reconstruction in 3 hospitals in the Netherlands (Maastricht University Medical Center, Maastricht; Maxima Medical Center, Eindhoven; and St Anna Hospital, Geldrop), between January 1997 and December 2004. This time span was chosen based on our previous study in which we found a median time between breast implantation and diagnosis of ASIA syndrome of 13 years.4 All women who received SBIs (group 2) or saline-filled/hydrogel implants (group 3) during this period were invited to participate in this study, provided that their address details were known. Any patient with silicone exposure before having an alternative implant was allocated to the silicone group (group 2). Patients in groups 2 or 3 who also reported to the MKS were excluded from these groups as they were already allocated to group 1. A fourth—control group—consisting of healthy women without breast implants, was recruited from close friends and family from responders of group 2 as they were most likely to be age-matched and to be of similar socioeconomic status. Having SBI and/or breast cancer, or a history of it, were exclusion criteria for the control group.
Written informed consent for participation in this study was obtained from all subjects. The study was approved by the local medical ethics board of Maastricht University Medical Center.
Questionnaires
All subjects were invited by post to complete a questionnaire after signing the informed consent form. The questionnaire consisted of a general questionnaire, the Dutch version of the 2010 American College of Rheumatology (ACR) Fibromyalgia Diagnostic Criteria, and the Dutch version of the Short Form 36 Health Survey (SF-36).
The general questionnaire contained items about the breast implants, health complaints, allergies, immune diseases, other chronic diseases, intoxications, and family history.
The 2010 ACR Fibromyalgia Diagnostic Criteria is a validated questionnaire for the diagnosis of fibromyalgia and measurement of symptom severity. It consists of 3 sections: pain areas, symptom severity, and other symptoms. This questionnaire was used to examine the appearance of “typical” clinical manifestations of ASIA syndrome. A minimum of 3 symptoms was required for the diagnosis of ASIA: arthralgia and/or myalgia, chronic fatigue and/or cognitive impairment, and pyrexia and/or sicca complaints. Subsequently, symptom severity is scaled from 0 to 6 (number of typical symptoms).
The SF-36 is a 36-item survey for evaluating HRQoL on 8 scales: physical functioning, physical role functioning, bodily pain, general health, vitality, social role functioning, emotional role functioning, and mental health.
Paper questionnaires were distributed by the clinical researcher (M.C.). They were coded with a unique number in advance in order to anonymize the data obtained (Appendix).
Statistical Analyses
Symptoms were reported as counts and percentages. Differences in percentages between groups were tested with Pearson’s chi-square test. Multivariable logistic regression was performed to identify factors associated with typical clinical manifestations, and to compute differences adjusted for potential confounding factors. The SF-36 outcomes were transformed into scores from 0 to 100, with higher values indicating better functioning and health status. The Pearson correlation coefficient was used to measure the linear correlation between age and HRQoL. One-way analysis of variance was performed to determine whether mean differences between the outcomes of the 4 groups were significant. Subsequently, Games-Howell post-hoc tests were executed. All analyses were performed in IBM SPSS Statistics version 25; an α level of 0.05 was considered significant.
RESULTS
Patient Characteristics and Medical History
The survey yielded an overall response rate of 48%; 68% of the healthy controls, 65% of the women from MKS, and 34% of the women from the hospital registries. In total, 238 women were included in this study. Eighty-five MKS-registered women (group 1), 83 women with—or with a history of—SBIs (group 2), 13 women with saline-filled or Monobloc implants (group 3), and 57 healthy women from the control group (group 4) completed the questionnaire.
The mean ages of the respondents were 52.7 years (range, 35-71 years), 57.1 years (range, 34-83 years), 50.2 years (range, 36-71 years), and 43.3 years (range, 19-75 years) in groups 1 to 4, respectively. Those in the healthy control group were significantly younger than women with silicone implants (P < 0.001). In the self-reported (MKS) group, there was a trend toward more active smokers in comparison with the healthy control group (31.8% vs 21.1%; P = 0.081).
In the vast majority, breast implants were placed bilaterally (88.4%) and for cosmetic reasons (71.8%). In group 1, implants were placed between 1971 and 2011 (median, 1999); in groups 2 and 3, implants were placed between 1972 and 2004 (median, 1998). Of the women from the MKS, 86% reported that they underwent at least 1 revision, whereas for 68.7% of group 2 and 61.5% of group 3 a second surgery was needed. Surgeries were most frequently performed in group 1. Implant rupture and capsular contracture were mentioned as the main causes for revision. In groups 1, 2, and 3, 36.5%, 10.8%, and 7.7% of the women underwent explantation of the SBI, respectively.
Comparison of the prevalence of comorbidities showed a significant difference between the 4 groups (Table 1). A significantly higher prevalence of chronic diseases (not specified), allergies, and irritable bowel syndrome (IBS) was found in women from the MKS compared with the control group; however, this was not found in women from groups 2 and 3. Chronic fatigue syndrome (CFS) was reported significantly more frequently in women with SBIs, and fibromyalgia (FM) significantly more frequently in women with all types of breast implants, compared with women without breast implants.
Table 1.
Prevalence of Comorbidities
| Group 1 | Group 2 | Group 3 | Group 4 | P value | |
|---|---|---|---|---|---|
| Chronic disease, % | 74.1a | 44.6 | 53.8 | 31.6 | <0.001 |
| Allergy, % | 56.5a | 32.5 | 30.8 | 35.1 | 0.006 |
| Fibromyalgia, % | 27.1a | 16.9a | 23.1a | 3.5 | 0.002 |
| CFS, % | 30.6a | 10.8a | 7.7 | 1.8 | <0.001 |
| IBS, % | 44.7a | 15.7 | 23.1 | 8.8 | <0.001 |
CFS, chronic fatigue syndrome; IBS, irritable bowel syndrome. aPrevalence is significantly higher compared with healthy controls.
Self-Reported Health Complaints
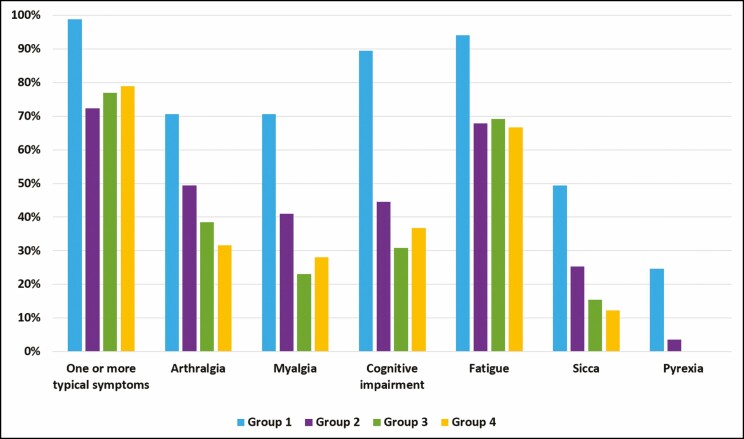
One or more typical clinical ASIA manifestations appeared in 98.8%, 72.3%, 76.9%, and 78.9% of the respondents of groups 1 to 4, respectively (Figure 1). The mean time between implant placement and the development of symptoms in group 1 was 4.9 years (range, 0-35 years). Women in groups 2 and 3 reported a latency period of 3.3 years (range, 0-10 years) and 7.8 years (range, 5-10 years) years, respectively.
Figure 1.
Prevalence (%) of typical clinical manifestations related to ASIA syndrome.
All symptoms were reported more frequently in group 1 than in the control group (P < 0.001). In group 2, more women reported arthralgia (P = 0.015) and sicca (P = 0.038) than in the control group. Between group 3 and the control, there were no major differences in the prevalence of reported complaints. Significantly more women in groups 1 and 2 met the criteria for the clinical diagnosis of ASIA syndrome, as described earlier, compared with the control group. There were no significant differences in ASIA prevalence found based on the reason for implant placement (cosmetic vs reconstructive) within groups 1 (83.6% vs 93.4%, P = 0.299), 2 (47.1% vs 46.2%, P = 0.940), and 3 (33.3% vs 66.7%, P = 0.523).
After adjusting for potential confounding variables (eg, age, smoking, and comorbidities), only the prevalence of myalgia and cognitive impairment was significantly higher in group 1 than in the control group (Table 2). There was a significant difference between the prevalence of myalgia, fatigue, and cognitive impairment between groups 1 and 2. Furthermore, myalgia and cognitive impairment were more common in group 1 than in group 3; the adjusted prevalence for groups 2, 3, and 4 did not differ significantly. The prevalence of ASIA syndrome remained significantly higher in group 1 compared with the control group after adjusting for potential confounders. The adjusted ASIA prevalence in group 2 did not differ significantly from the control group.
Table 2.
Prevalence of Self-Reported Manifestations Related to ASIA Syndrome
| Group 1 (%) | Adjusted P value | Group 2 (%) | Adjusted P value | Group 3 (%) | Adjusted P value | Group 4 (%) | |
|---|---|---|---|---|---|---|---|
| Arthralgia | 70.6 | 0.315 | 49.4 | 0.910 | 38.5 | 0.449 | 31.6 |
| Myalgia | 70.6 | 0.009 | 41 | 0.494 | 23.1 | 0.320 | 28.1 |
| Cognitive impairment | 89.4 | <0.001 | 44.6 | 0.492 | 30.8 | 0.919 | 36.8 |
| Fatigue | 94.1 | 0.183 | 67.8 | 0.375 | 69.2 | 0.569 | 66.7 |
| Sicca | 49.4 | 0.083 | 25.3 | 0.376 | 15.4 | 0.962 | 12.3 |
| Pyrexia | 24.7 | —a | 3.6 | —a | 0 | —a | 0 |
| ASIA (≥3 symptoms) | 84.7 | 0.003 | 46.2 | 0.700 | 38.5 | 0.931 | 28.1 |
Multivariable logistic regression analysis of self-reported symptoms in women with breast implants (groups 1, 2, and 3) compared with women without breast implants (group 4), adjusted for age, smoking, and comorbidities. aUnable to estimate due to too few events.
Multivariable logistic regression that included age, smoking, and comorbidities (chronic disease, allergy, FM, CFS, and IBS) as independent variables showed that age was an independent predictor for arthralgia, that the presence of a chronic disease (not specified) was a predictor for arthralgia, fatigue, and sicca, and that fibromyalgia was a predictor for both arthralgia and myalgia. The presence of a chronic disease was the only independent predictor for the clinical diagnosis of ASIA syndrome (Table 3).
Table 3.
Predictors of Typical Clinical Manifestations Related to ASIA Syndrome
| Predictor | Adjusted P value | |
|---|---|---|
| Arthralgia | Age | 0.039 |
| Chronic diseasea | 0.039 | |
| Fibromyalgia | 0.006 | |
| Myalgia | Fibromyalgia | 0.002 |
| Fatigue | Chronic diseasea | 0.002 |
| Sicca | Chronic diseasea | 0.003 |
| ASIA (≥3 symptoms) | Chronic diseasea | 0.015 |
aChronic diseases were not specified in the analysis. The presence of a chronic disease was scored binary.
SF-36
Patient-reported outcomes on health status measured by means of the SF-36 questionnaire were compared between all groups (Table 4). There was a statistically significant difference between the mean scores of the 4 groups on all subdomains (P < 0.001). Post-hoc tests showed that women with silicone exposure (groups 1 and 2) scored significantly lower on all domains of the SF-36 than the healthy control group, except for emotional role functioning, where the mean difference between groups 2 and 4 did not reach statistical significance (P = 0.159). No significant difference was found between the outcomes of group 3 and the healthy control group.
Table 4.
Mean SF-36 Scores per Group
| Group 1 | Group 2 | Group 3 | Group 4 | P value | |
|---|---|---|---|---|---|
| Physical functioning | 59.3a | 74.1a | 82.7 | 92.5 | <0.001 |
| Physical role functioning | 26.2a | 62.4a | 75.0 | 86.3 | <0.001 |
| Emotional role functioning | 49.6a | 77.6 | 92.3 | 89.5 | <0.001 |
| Vitality | 37.7a | 59.1a | 61.9 | 72.7 | <0.001 |
| Mental health | 56.4a | 73.9a | 75.7 | 81.4 | <0.001 |
| Social functioning | 43.9a | 75.3a | 84.7 | 90.0 | <0.001 |
| Pain | 45.8a | 66.0a | 74.8 | 81.0 | <0.001 |
| General health | 34.1a | 58.3a | 65.6 | 74.6 | <0.001 |
aMean difference is statistically significant compared with healthy controls.
The Pearson correlation showed that physical functioning, physical role functioning, general health, and bodily pain are associated with age (P < 0.01). No correlation was found between age and emotional role functioning, mental health and social functioning, and vitality.
DISCUSSION
This retrospective cohort study aimed to evaluate the prevalence of clinical manifestations related to ASIA syndrome in women with breast implants compared with women without breast implants. Furthermore, the HRQoL survey was evaluated and compared between these groups by means of the SF-36 questionnaire.
Three major outcomes arose from this study. First, this study showed that the adjusted prevalence of clinical manifestations related to ASIA syndrome was only significantly higher in women who reported to the MKS. Second, age, fibromyalgia, and having a chronic disease were found to be independent predictors for the development of typical clinical symptoms. Third, HRQoL was found to be significantly lower in women with SBI than in women with no breast implants.
From the many case reports about BII, the signal has been that adverse effects may occur as a result of the use of SBIs. Effects can be local—eg, an inflammatory response to silicone leakage—or systemic. However, case reports do not form a basis for demonstrating an association between SBIs and health complaints because there is selection bias, and outcomes are not generalizable. Moreover, results are usually not compared with healthy controls without implants.
In the current study, we were able to compare the symptoms of women with all types of breast implants to women without breast implants. We found a pattern of unexplained systemic symptoms consisting of fatigue, arthralgia, myalgia, cognitive problems, sicca complaints, and pyrexia, often reported in previous studies3,15,17 to be occurring more frequently in women with SBIs than in women without implants. Given the nonspecific nature of these complaints, it is crucial to compare the prevalence of these complaints with a control group, as these symptoms may occur independently from having breast implants. Our results showed that even in the general population, the prevalence of nonspecific complaints is high. Nevertheless, more women with SBI reported symptoms.
However, there may be confounders involved. In accordance with the findings of Maijers et al15 the majority of the women with self-reported complaints in our study reported allergies, almost half of the women had IBS, and there was a higher prevalence of fibromyalgia in women with breast implants. This high prevalence of fibromyalgia in women with SBIs has been repeatedly noticed,3,11,18,19 although evidence has failed to support an association.20 The complaints of women with SBIs have a substantial overlap with the aforementioned functional disorders.21,22 However, due to the retrospective design of this current study, it could not be verified whether these complaints were pre-existing, were the result of a functional disorder, or can genuinely be attributed to the breast implants. Therefore, adjustments were made for comorbidities, as well as for age and smoking, which may also play a role in the development of similar complaints.
Interestingly, adjustment for potential confounders showed that the prevalence of clinical symptoms was only higher in the group of the self-reported women. The adjusted prevalence in women with SBIs, recruited from the Dutch hospitals, did not differ from women without breast implants. This strongly suggests that results on the prevalence of health complaints in women with SBIs are subject to selection bias. Women who registered at MKS do not accurately reflect the population of women with SBIs; this group concerns a selection of women with the most severe complaints.
Moreover, age, fibromyalgia, and having chronic diseases were found to be independent predictors for the development of clinical manifestations related to ASIA syndrome. This means that the significantly older age and a more frequent occurrence of both fibromyalgia and chronic diseases in the MKS group have contributed to the development of typical complaints. This heterogeneity may cause a biased view on the development of health complaints due to SBIs.
In contrast to earlier findings, another major outcome of this study was the decreased HRQoL in women with SBIs. Previous studies showed an improvement in body image and QoL after breast augmentation surgery.23-26 This, in particular, seems to concern a psychological benefit. Alderman et al25 described a significantly improved QoL based on the subscales “satisfaction with breasts” and “psychosocial well-being” of the BREAST-Q. Conversely, Coriddi et al27 found a significant decrease in the “physical well-being” subscale in the short term. In accordance with the results of Murphy et al,28 we observed statistically significant decreases in SF-36 scores of women with SBIs. When interpreting these results, the potential selection bias must be taken into account. We do not have the data for the non-responders. It is, however, plausible that these are the ones with a lower QoL, meaning that the responders are not an accurate reflection of the invited group. Furthermore, age may have affected the QoL. Based on the Pearson correlation, however, only the physical domains of the SF-36 are associated with age. The psychological well-being of women with SBIs (groups 1 and 2) was found to be significantly lower, regardless of age. We are not certain, however, whether this developed as a result of the breast implants or was a pre-existing problem. One of many hypotheses is that somatization plays an important role in the development and progression of symptoms and complaints in some women with SBIs.22 According to this, BII may be mediated by stress, personality characteristics, and social context. People who have a higher rate of physical or psychological stress seem more susceptible to somatization.29 The higher prevalence of comorbidities that we found in women with SBIs may be stress factors. Psychological initiation of dysfunction and intensification of symptoms, in combination with poor coping responses, may have led to the decreased HRQoL observed in the women with self-reported complaints.22 We expect that, based on this hypothesis and the selection bias, the results of this study are an underestimate of the actual HRQoL of women with SBIs. We feel that additional research into personality characteristics and psychological well-being of women seeking breast augmentation surgery can contribute to understanding BII.
Despite the lack of evidence for causality, women have requested removal of their implants due to extensive worrying. Studies reported subjective improvement of patient-reported complaints after explantation of the SBIs.30-32 A recent literature review showed that 75% of the patients with silicone-related complaints experienced relief of their complaints.33 Although improved QoL was observed in more than 50% of the cases,34,35 correlating self-reported complaints to QoL remains difficult.35 Also in this regard, a patient’s psychological profile plays an important role.36 To our knowledge, this is the first study into the prevalence of self-reported complaints in women with SBIs compared with women without implants. However, we are aware that the design of our study may have several limitations. Due to selection bias, the outcomes of this study do not provide a representation of the total group of women with breast implants. Not only group 1, but also groups 2 and 3 are expected to contain women with more, or more severe, complaints than the general population with implants. Women with complaints are more likely to participate in this research than healthy women and may answer the questionnaires strategically, because they benefit from scientific research demonstrating the noxiousness of implants. This was reflected in the high response rate of group 1. Moreover, a retrospective survey research involves recall bias. Women may inaccurately remember the exact course of complaints, and therefore, it is not certain whether comorbidities developed before or after implant placement. Whenever women are convinced that their complaints are attributable to the implants, they may be reluctant to reconsider alternative causes. This can potentially exaggerate the association between the reported complaints and the breast implants. Furthermore, no data were available on preoperative QoL surveys. In order to correlate breast implants to a reduced QoL, knowledge of the preoperative physical and psychological status is required. Finally, the control group was not ideally matched. Although psychological well-being was not correlated to age, controls should properly match demographic characteristics in order to exclude potential confounders in future studies. This study shows no association between self-reported complaints and SBIs based on this study, and confirmation by means of large, prospectively controlled studies is necessary to establish causality.
CONCLUSIONS
The prevalence of self-reported health complaints related to ASIA syndrome, such as arthralgia, myalgia, chronic fatigue, cognitive impairment, pyrexia, and sicca complaints, was not significantly higher in women with SBIs than in women without breast implants, when adjusted for age, smoking, and comorbidities. Fibromyalgia and chronic fatigue syndrome were significantly more common in women with SBIs, and the presence of a chronic disease was found to be an independent predictor for ASIA syndrome. Furthermore, HRQoL was lower in women with SBIs than in women without breast implants. The findings of this study suggest that results on BII are subject to selection bias. Further studies are needed to prove an association between self-reported complaints and SBIs.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Supplementary Material
REFERENCES
- 1. The Aesthetic Society’s. Aesthet Surg J. 2019;39(Suppl_4):1-27. [DOI] [PubMed] [Google Scholar]
- 2. de Boer M, van Middelkoop M, Hauptmann M, et al. Breast implant prevalence in the Dutch female population assessed by chest radiographs. Aesthet Surg J. 2020;40(2):156-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watad A, Quaresma M, Bragazzi NL, et al. The autoimmune/inflammatory syndrome induced by adjuvants (ASIA)/Shoenfeld’s syndrome: descriptive analysis of 300 patients from the international ASIA syndrome registry. Clin Rheumatol. 2018;37(2):483-493. [DOI] [PubMed] [Google Scholar]
- 4. Colaris MJL, de Boer M, van der Hulst RR, Cohen Tervaert JW. Two hundreds cases of ASIA syndrome following silicone implants: a comparative study of 30 years and a review of current literature. Immunol Res. 2017;65(1):120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shoenfeld Y, Agmon-Levin N. ‘ASIA’—autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36(1):4-8. [DOI] [PubMed] [Google Scholar]
- 6. Balk EM, Earley A, Avendano EA, Raman G. Long-term health outcomes in women with silicone gel breast implants: a systematic review. Ann Intern Med. 2016;164(3):164-175. [DOI] [PubMed] [Google Scholar]
- 7. Janowsky EC, Kupper LL, Hulka BS. Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases. N Engl J Med. 2000;342(11):781-790. [DOI] [PubMed] [Google Scholar]
- 8. Karlson EW, Hankinson SE, Liang MH, et al. Association of silicone breast implants with immunologic abnormalities: a prospective study. Am J Med. 1999;106(1):11-19. [DOI] [PubMed] [Google Scholar]
- 9. Colaris MJL, van der Hulst RR, Tervaert JWC. Vitamin D deficiency as a risk factor for the development of autoantibodies in patients with ASIA and silicone breast implants: a cohort study and review of the literature. Clin Rheumatol. 2017;36(5):981-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen Tervaert JW, Colaris MJ, van der Hulst RR. Silicone breast implants and autoimmune rheumatic diseases: myth or reality. Curr Opin Rheumatol. 2017;29(4):348-354. [DOI] [PubMed] [Google Scholar]
- 11. Cohen Tervaert JW. Autoinflammatory/autoimmunity syndrome induced by adjuvants (ASIA; Shoenfeld’s syndrome): a new flame. Autoimmun Rev. 2018;17(12):1259-1264. [DOI] [PubMed] [Google Scholar]
- 12. Giltay EJ, Bernelot Moens HJ, Riley AH, Tan RG. Silicone breast prostheses and rheumatic symptoms: a retrospective follow up study. Ann Rheum Dis. 1994;53(3):194-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolfe F. “Silicone related symptoms” are common in patients with fibromyalgia: no evidence for a new disease. J Rheumatol. 1999;26(5):1172-1175. [PubMed] [Google Scholar]
- 14. Pool SMW, Wolthuizen R, Mouës-Vink CM. Silicone breast prostheses: a cohort study of complaints, complications, and explantations between 2003 and 2015. J Plast Reconstr Aesthet Surg. 2018;71(11):1563-1569. [DOI] [PubMed] [Google Scholar]
- 15. Maijers MC, de Blok CJ, Niessen FB, et al. Women with silicone breast implants and unexplained systemic symptoms: a descriptive cohort study. Neth J Med. 2013;71(10):534-540. [PubMed] [Google Scholar]
- 16. Zorginstituut Nederland. (2018). Standpunt verwijderen (explantatie) van siliconen borstimplantaten bij aanhoudende systemische klachten. https://www.zorginstituutnederland.nl/publicaties/standpunten/2018/05/31/standpunt-verwijderen-explantatie-van-siliconen-borstimplantaten-bij-aanhoudende-systemische-klachten. Accessed September 6, 2020. [Google Scholar]
- 17. Cohen Tervaert Nederland, Kappel RM. Silicone implant incompatibility syndrome (SIIS): a frequent cause of ASIA (Shoenfeld’s syndrome). Immunol Res. 2013;56(2-3):293-298. [DOI] [PubMed] [Google Scholar]
- 18. Brown SL, Pennello G, Berg WA, Soo MS, Middleton MS. Silicone gel breast implant rupture, extracapsular silicone, and health status in a population of women. J Rheumatol. 2001;28(5):996-1003. [PubMed] [Google Scholar]
- 19. Khoo T, Proudman S, Limaye V. Silicone breast implants and depression, fibromyalgia and chronic fatigue syndrome in a rheumatology clinic population. Clin Rheumatol. 2019;38(5):1271-1276. [DOI] [PubMed] [Google Scholar]
- 20. Lipworth L, Tarone RE, McLaughlin JK. Breast implants and fibromyalgia: a review of the epidemiologic evidence. Ann Plast Surg. 2004;52(3):284-287. [DOI] [PubMed] [Google Scholar]
- 21. Aaron LA, Buchwald D. A review of the evidence for overlap among unexplained clinical conditions. Ann Intern Med. 2001;134(9 Pt 2):868-881. [DOI] [PubMed] [Google Scholar]
- 22. Dush DM. Breast implants and illness: a model of psychological factors. Ann Rheum Dis. 2001;60(7):653-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Penaud A, De Mortillet S. [Evaluation of the psychological benefits of breast augmentation for aesthetic purposes. Results of a multicenter prospective study of a series of 181 patients]. Ann Chir Plast Esthet. 2013;58(1):10-17. [DOI] [PubMed] [Google Scholar]
- 24. Kalaaji A, Bjertness CB, Nordahl C, Olafsen K. Survey of breast implant patients: characteristics, depression rate, and quality of life. Aesthet Surg J. 2013;33(2):252-257. [DOI] [PubMed] [Google Scholar]
- 25. Alderman A, Pusic A, Murphy DK. Prospective analysis of primary breast augmentation on body image using the BREAST-Q: results from a nationwide study. Plast Reconstr Surg. 2016;137(6):954e-960e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coriddi M, Nadeau M, Taghizadeh M, Taylor A. Analysis of satisfaction and well-being following breast reduction using a validated survey instrument: the BREAST-Q. Plast Reconstr Surg. 2013;132(2):285-290. [DOI] [PubMed] [Google Scholar]
- 27. Coriddi M, Angelos T, Nadeau M, Bennett M, Taylor A. Analysis of satisfaction and well-being in the short follow-up from breast augmentation using the BREAST-Q, a validated survey instrument. Aesthet Surg J. 2013;33(2):245-251. [DOI] [PubMed] [Google Scholar]
- 28. Murphy DK, Beckstrand M, Sarwer DB. A prospective, multi-center study of psychosocial outcomes after augmentation with Natrelle silicone-filled breast implants. Ann Plast Surg. 2009;62(2):118-121. [DOI] [PubMed] [Google Scholar]
- 29. Barsky AJ, Borus JF. Functional somatic syndromes. Ann Intern Med. 1999;130(11):910-921. [DOI] [PubMed] [Google Scholar]
- 30. Godfrey PM, Godfrey NV. Response of locoregional and systemic symptoms to breast implant replacement with autologous tissues: experience in 37 consecutive patients. Plast Reconstr Surg. 1996;97(1):110-116. [DOI] [PubMed] [Google Scholar]
- 31. Melmed EP. A review of explantation in 240 symptomatic women: a description of explantation and capsulectomy with reconstruction using a periareolar technique. Plast Reconstr Surg. 1998;101(5):1364-1373. [DOI] [PubMed] [Google Scholar]
- 32. Rohrich RJ, Kenkel JM, Adams WP, Beran S, Conner WC. A prospective analysis of patients undergoing silicone breast implant explantation. Plast Reconstr Surg. 2000;105(7):2529-2537. [DOI] [PubMed] [Google Scholar]
- 33. de Boer M, Colaris M, van der Hulst RRWJ, Cohen Tervaert JW. Is explantation of silicone breast implants useful in patients with complaints? Immunol Res. 2017;65(1):25-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Svahn JK, Vastine VL, Landon BN, Dobke MK. Outcome of mammary prostheses explantation: a patient perspective. Ann Plast Surg. 1996;36(6):594-600. [DOI] [PubMed] [Google Scholar]
- 35. Rohrich RJ, Rathakrishnan R, Robinson JB Jr, Griffin JR. Factors predictive of quality of life after silicone-implant explanation. Plast Reconstr Surg. 1999;104(5): 1334-1337. [DOI] [PubMed] [Google Scholar]
- 36. Walden KJ, Thompson JK, Wells KE. Body image and psychological sequelae of silicone breast explantation: preliminary findings. Plast Reconstr Surg. 1997;100(5): 1299-1306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.