Abstract
Cellulite is characterized by dimpled contour alterations of the skin and is present in approximately 85% to 90% of postpubertal females. Although the pathophysiology of cellulite remains to be fully elucidated, experimental evidence indicates a multifactorial process involving the number and types of fibrous septae, microvascular dysfunction, subcutaneous inflammation, decreased dermal thickness with age, and fat deposition. Cellulite is a major cosmetic concern for many women, and a number of both noninvasive (eg, massage, cosmeceuticals, laser therapy) and minimally invasive techniques (eg, subcision, collagenase injection) have been evaluated to improve the appearance of the affected skin. However, evidence for many of these treatments is limited, largely due to the lack of a validated, convenient tool for the standardized evaluation of cellulite severity. Various imaging modalities have been employed to characterize cellulite severity and the impact of treatment, but only 2-dimensional and 3-dimensional digital photography have been adequately validated. However, in many cases, imaging findings do not correlate with subjective measures of cellulite severity. A number of cellulite rating scales have been developed; some provide only a qualitative measure, whereas others do not fully capture all clinically relevant aspects of cellulite, including the perspective of the patient. There remains an unmet need for global adoption of a validated scale that can be utilized easily by clinicians and patients in clinical and research settings. We propose features that should be included in an ideal rating scale for assessment of cellulite severity.
The term cellulite describes dimpled contour alterations of the skin that are present in approximately 85% to 90% of postpubertal females.1 Although the term was first used in 1873,2 the first clinical description of cellulite was not published until 1920, when it was described as a noninflammatory mesenchymal disorder attributable to abnormal water metabolism.1,3 Despite the high prevalence of cellulite in adult women, controversy persisted for several decades over whether cellulite represented a normal physiologic process or a pathologic condition. This debate was complicated by frequent and inappropriate interchangeable use of the terms “cellulite” and “cellulitis” (a potentially serious skin infection) to describe the same condition.2-4 However, in 1978, Nürnberger and Müller4 concluded in a landmark paper that cellulite formation was a natural, gender-linked, physiologic process. This conclusion was based on detailed anatomic studies of the skin in females and males and on epidemiologic data indicating that signs of cellulite are present in women of all racial groups.4
Many synonyms have been employed to describe cellulite. In their classic description, Nürnberger and Müller4 concluded that the term dermo-panniculosis was most appropriate from a histopathologic perspective, because the characteristic histologic features of cellulite are confined to the collagen and elastica but occur in the hypodermis and the corium. Other potential synonyms include adiposis edematosa, status protusus cutis (incipient cellulite), nodular liposclerosis, gynoid lipodystrophy, and edematous fibrosclerotic panniculopathy.5
Anatomy and Etiology of Cellulite
Cellulite primarily affects the thighs and buttocks and sometimes the lower legs and abdomen, and it is characterized by skin alterations that have been described as “orange peel,” “cottage cheese,” or “mattress-like” in appearance.1,5 In contrast to obesity, which is characterized by hypertrophy and hyperplasia of adipocytes with no specific anatomic location, cellulite results from a variety of ultrastructural, inflammatory, histochemical, and biochemical changes in the dermis, microcirculation, and adipocytes.5,6 The pathophysiology of cellulite formation remains to be fully elucidated; however, it appears to be a multifactorial process in which the number and types of fibrous septae, microvascular dysfunction, subcutaneous inflammation and fibrosis, decreased dermal thickness with age, and adipose tissue deposition may all play a role.1,5,7-9 For example, estrogen, oxidative stress, and inflammation may promote fluid retention by altering local vascular and lymphatic drainage, resulting in edema.6 In addition, due to increased fibrogenesis and collagen deposition, the number of subdermal collagen cords (septae) is reduced in areas of cellulite and arranged perpendicular to the skin surface. By contrast, in skin without cellulite, there are more septae connections arranged tangentially to the surface.5,9,10 Due to this spatial distribution, thinning of the septae, and a reduced number of septae connections, increased pressure forces subcutaneous fat into the interface between the dermis and hypodermis, resulting in the dimpled skin appearance.3,9,11 A “2-hit” hypothesis has been proposed, whereby impaired microcirculation in the gluteofemoral fat (the first hit) leads to hypoxia (the second hit), which in turn leads to fibrosis of the subcutaneous connective tissue.7 Cellulite is also associated with adipocyte hypertrophy and increased subcutaneous fat,1,9,11,12 but, as noted above, the presence of microvascular changes and structural changes in the dermis distinguish it from obesity.6 However, obesity may exacerbate the skin changes associated with cellulite.1,3,5,9,13
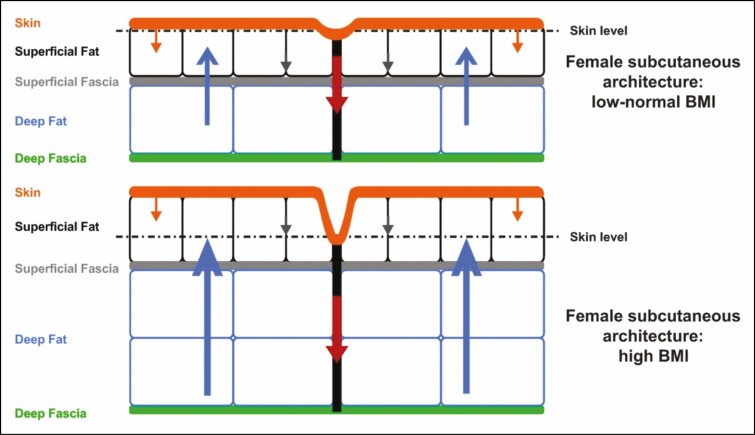
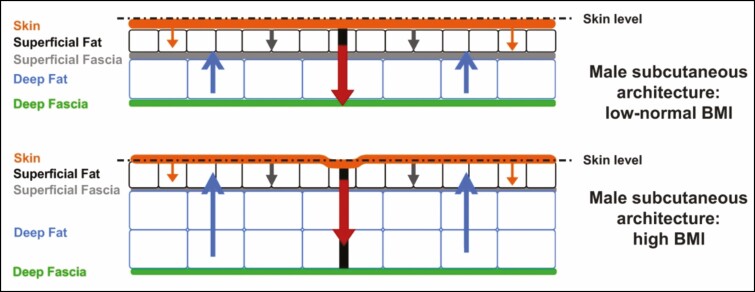
The pathophysiologic processes responsible for the development of cellulite may be modulated by a number of potential risk factors that include gender, race, and lifestyle factors.5 Gender differences in fascial architecture have been observed from birth (Figures 1 and 2).4,9 There is also evidence to support roles for both genetic predisposition and ethnicity: although cellulite is seen in women of all races,4 it tends to be more common in white women.5 In addition, lifestyle factors such as a high-carbohydrate diet or sedentary habits may promote cellulite formation via effects on body fat content or vascular stasis, respectively.5 In some women, cellulite becomes apparent with age.14 Contour irregularities (eg, loss of elasticity and sagging), which develop as a woman ages, are likely the result of increased skin laxity, which has been shown to be highly correlated with increasing age and body mass index.15 The association of contour irregularities and age is also supported by anatomical studies4 and histological studies demonstrating that skin exhibiting laxities shows dermal atrophy because of loss of collagen and reduced collagen biosynthesis, degradation of elastin fiber, and loss of hydration.16-18 The development of late-onset cellulite may be a factor of underlying anatomical structure that is prone to cellulite, with good skin surface tension (eg, elasticity) masking the underlying defect when women are younger. The loss of skin elasticity that occurs through the normal aging process may then “unmask” cellulite.
Figure 1.
Fascial architecture of females.9 Structure and arrangement of skin and subcutaneous tissue of individuals with low to normal BMI (upper panel) or high BMI (lower panel) are shown. The arrows demonstrate the interplay of biomechanical forces (blue arrows: outward force of fat lobules; red and grey arrows: inward tethering force of the septal network, with illustrated dimorphism between the numerous short and thin septae [grey arrows] vs the fewer long and thick septae, which have greater stability [red arrows]; orange arrows: inward containment force of the dermis). Reprinted with permission from Rudolph et al.9 BMI, body mass index.
Figure 2.
Fascial architecture of males.9 Structure and arrangement of skin and subcutaneous tissue of individuals with low to normal BMI (upper panel) or high BMI (lower panel) are shown. The arrows demonstrate the interplay of biomechanical forces (blue arrows: outward force of fat lobules; red and grey arrows: inward tethering force of the septal network, with illustrated dimorphism between the numerous short and thin septae [grey arrows] vs the fewer long and thick septae, which have greater stability [red arrows]; orange arrows: inward containment force of the dermis). Decreased probability of a mattress-like skin appearance at the skin surface in men may be due to the greater number of fibrous connections between the superficial fascia and the dermis, providing greater stability. Reprinted with permission from Rudolph et al.9 BMI, body mass index.
Evaluating Cellulite
Cellulite is a notable cosmetic concern for many women, and numerous treatment approaches have been employed in attempts to improve the appearance of affected skin.19 Potential treatment strategies include noninvasive interventions such as mechanical stimulation to improve lymphatic drainage; topical therapy to improve microvascular circulatory flow or to reduce fat deposition or inflammation; acoustic wave therapy (AWT); or the application of laser, light, or radiofrequency energy and minimally invasive techniques, such as subcision or collagenase clostridium histolyticum injection.1,19 However, high-quality evidence to support the use of many of these treatments is limited. A 2015 systematic review found that the majority of the 67 studies reviewed had methodologic flaws, such as a failure to assess cellulite severity as an end point or insufficient statistical analyses.19 This finding highlights a key unmet need in both clinical practice and research: a validated and convenient tool for the standardized evaluation of cellulite severity and treatment efficacy.19 Although various scales and other techniques have been developed to assess cellulite severity (Table 1),4,6,10-13,20-43 these are not universally employed in clinical trials.19 Most of the scales currently available do not feature a patient-reported component, which the US Food and Drug Administration (FDA) now recommends.44 In addition, there are currently no validated biophysical techniques to evaluate cellulite; most studies have utilized surrogate measures, such as imaging or biomechanical assessments.19 This paper reviews the rating scales and imaging techniques currently available for evaluating cellulite.
Table 1.
Cellulite Severity Scales and Techniques to Evaluate Efficacy in Clinical Trials
| • Cellulite severity rating scales –Nürnberger-Müller classification4 –Cellulite Severity Scale20,21 and Modified Cellulite Severity Scale22 –Curri scale6 –DiBernardo scoring system23 –Cellulite Dimples—At Rest and Cellulite Dimples—Dynamic scales43 –Clinician Reported and Patient Reported Photonumeric Cellulite Severity Scales24,25,42 –Investigator and Subjective Global Aesthetic Improvement Scales26 • Imaging techniques –2D or 3D digital photography12,22,23,27-33 –3D laser skin surface scanner34 –Ultrasonography12,27,34-36 –Laser doppler flowmetry37 –Thermography38,39 –Magnetic resonance imaging10,11,13,40 –Computed axial tomography41 • Measurement of skin biomechanics –Skin elasticity12,27,32,34 –Surface roughness32,34 –Skin surface profile (waviness)35 |
2D, 2-dimensional; 3D, 3-dimensional.
CELLULITE SEVERITY RATING SCALES
A number of cellulite severity rating scales have been developed, each of which has particular advantages and limitations (Table 2).4,6,20-28,31,42,43,45
Table 2.
Cellulite Severity Rating Scales
| Scale | Validated | Cellulite severity description | Advantages | Limitations |
|---|---|---|---|---|
| Nürnberger-Müller classification4 | No | • Grade 0: Skin is smooth in both lying-down and standing positions; • Grade I: Skin is smooth at rest but shows a mattress-like appearance on pinching; • Grade II: Skin is smooth at rest but has a mattress-like appearance on standing; • Grade III: Skin has a mattress-like appearance in both lying-down and standing positions; • Grades II and III can be subdivided into mild, moderate, or severe27,31 |
Uses pinch test (ie, easy to administer without need of a visual tool/scale) | • Qualitative • Not validated • Does not describe specific features of cellulite23 • Different clinical aspects or severities may be classified to same grade20 |
| CSS20,21 | Yes | 5 items, scored 0-3: • Number of evident depressions • Depth of depressions • Morphologic appearance of skin surface alterations • Grade of laxity, flaccidity, or sagging skin • Nürnberger-Müller classification • Mild cellulite: total score 1-5 • Moderate cellulite: total score 6-10 • Severe cellulite: total score 11-15 |
• Validated scale • Includes specific features of cellulite potentially amenable to therapeutic interventions • Qualitative and quantitative |
• Based on evaluation of thighs and buttocks; other areas not validated • Grading of laxity, flaccidity, and sagging of skin may reduce reliability of scale • May be cumbersome to use in clinical practice because it incorporates multiple ratings • Does not capture the patient perspective |
| Modified CSS22 | No | Similar to original CSS, except Nürnberger-Müller staging was omitted | • Includes specific features of cellulite potentially amenable to therapeutic interventions • Qualitative and quantitative |
• Lack of validation • Includes skin laxity |
| Curri scale6 | No | • Grade I: asymptomatic; areolar layer may be thickened, with increased capillary permeability; • Grade II: pallor, decreased temperature, and decreased elasticity after skin compression; periadipocyte hyperplasia and hypertrophy, with capillary dilatation, microhemorrhages, and increased capillary basement membrane thickness; • Grade III: padded skin or orange-peel appearance, with pain on palpation, decreased elasticity, pallor, and decreased temperature; histopathologically, collagen fibril neogenesis, sclerosis of internal layer of small artery walls, venous dilation, obliteration of border between dermis and SC tissue, and adipocyte inclusion within deep connective tissue; • Grade IV: similar to grade III; greater number of visible, palpable, painful nodules; wavy appearance of skin surface. Histopathologically, some nodules encapsulated by dense connective tissue and lobular fatty tissue have disappeared; microscopically, diffuse liposclerosis, telangiectasias, microvarices and varices, and epidermal atrophy |
Incorporates both clinical and histopathological features of cellulite | • Qualitative • Unclear if validated • Histopathologic assessment unlikely to be feasible in routine clinical practice |
| DiBernardo scoring system23 | Yes | Number of dimples and contour undulations severity graded 0-4 | Combines elements of both general (Nürnberger-Müller) and specific (CSS) scoring systems | Does not measure exact depth or volume of cellulite dimples to quantify cellulite condition |
| Cellulite Dimples—At Rest and Cellulite Dimples—Dynamic scales43 | Yes | Cellulite dimples graded on a 5-point scale from 0 (no dimples) to 4 (severe dimples [≥17 depressions]) in static (at rest) or dynamic state | • Scales are robust; no training is needed to administer them • Dimples are assessed in the static and dynamic states • Easy to use |
• Specific for cellulite dimples and not all cellulite-related skin deformities • Patient perspective not determined |
| CR-PCSS and PR-PCSS24,25 | Yes | Cellulite severity graded on a 5-point scale from 0 (none) to 4 (severe) | • Developed in accordance with FDA guidance on patient-reported outcome measures42 • Single-item scale facilitates broad use • Allows assessment from both clinician and patient perspective • Correlate with established measures of cellulite severity (CSS, I-GAIS, S-GAIS) |
Limited to buttocks and thighs |
| I-GAIS and S-GAIS26,42 | Not reported | • Based on before-and-after digital photographs • Severity expressed on 7-point scale ranging from +3 (very much improved) to −3 (very much worse)24 |
• Commonly used • Scores correlate with established measures of cellulite severity (CSS) |
Potential risk of response bias45 |
CR-PCSS, Clinician Reported Photonumeric Cellulite Severity Scale; CSS, Cellulite Severity Scale; FDA, US Food and Drug Administration; I-GAIS, Investigator Global Aesthetic Improvement Scale; PR-PCSS, Patient Reported Photonumeric Cellulite Severity Scale; S-GAIS, Subjective Global Aesthetic Improvement Scale; SC, subcutaneous.
The Nürnberger-Müller Classification
The oldest cellulite classification system was proposed by Nürnberger and Müller.4 This categorizes skin appearance into 4 grades (0-III).4 In grades I and II, the skin is smooth at rest but shows a mattress-like appearance on pinching or standing, respectively; in grade III, the skin has a mattress-like appearance in both the lying and standing positions. Grades II and III can be subclassified into mild, moderate, or severe.46 This classification is based on a simple pinch test (pinching skin between thumb and index finger), but it is purely qualitative and has not been validated (Table 2). Furthermore, it does not describe specific features of cellulite23; therefore, different clinical aspects or severities may be assigned to the same grade.20
The Cellulite Severity Scale
In contrast to the Nürnberger-Müller classification, the Cellulite Severity Scale (CSS) was developed to incorporate specific clinical and morphologic aspects of cellulite that may affect severity level.20 The CSS is a widely employed photonumeric scale developed from a photographic study of 55 women participating in clinical trials of cellulite therapies.20 It consists of 5 items, including the Nürnberger-Müller classification, each of which is rated on a scale from 0 to 3; scores of 1 to 5 indicate mild cellulite, 6 to 10 indicate moderate cellulite, and 11 to 15 indicate severe cellulite.20 Although this scale provides both qualitative and quantitative measures of cellulite severity, it applies only to cellulite located on the buttocks and thighs; it has not been validated for areas less commonly affected by cellulite, such as the arms or abdomen.20
An initial validation showed good intraclass correlations and consistency for both thighs and buttocks,20 and subsequent validation in a Spanish female population also showed good intraobserver and interobserver reliability and internal consistency.21 However, the latter study also reported that the degree of laxity, flaccidity, or sagging skin did not contribute significantly to the consistency of the scale, raising questions about the clinical relevance of this component.21 A modified version of the CSS was utilized in a study of the efficacy of AWT in the treatment of cellulite.22 This version omitted the Nürnberger-Müller staging, which requires physical contact with the patient, because assessments were based on blinded evaluation of 2-dimensional (2D) photographs.22 However, the skin laxity component of the CSS was retained.
Curri Scale
An alternative classification, originally proposed by Curri, classifies cellulite into 4 grades on the basis of clinical and histopathological changes (Table 2).6 Although this system evaluates multiple aspects of cellulite, it provides only a qualitative measure of severity and is largely based on histopathologic evaluation, which is not feasible in routine clinical practice.
DiBernardo Scoring System
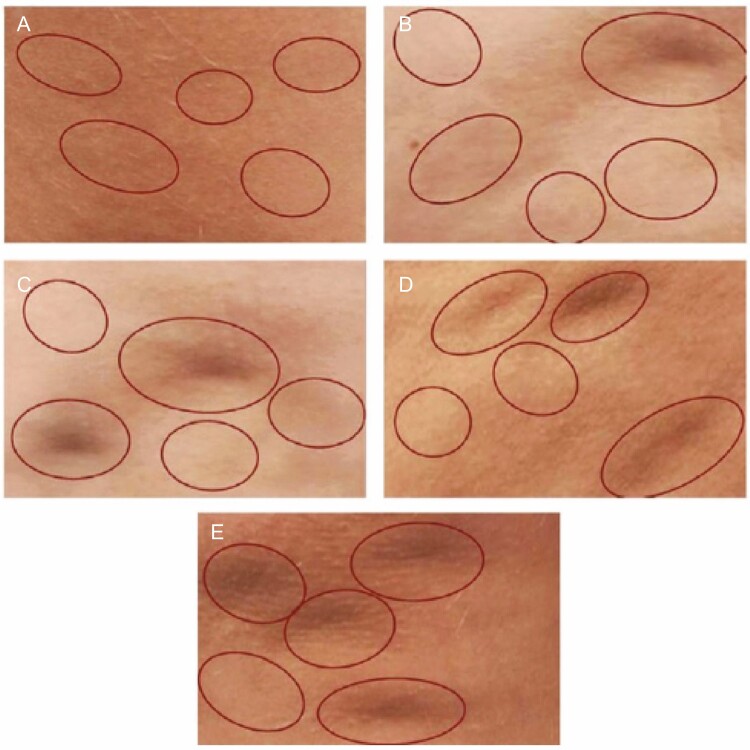
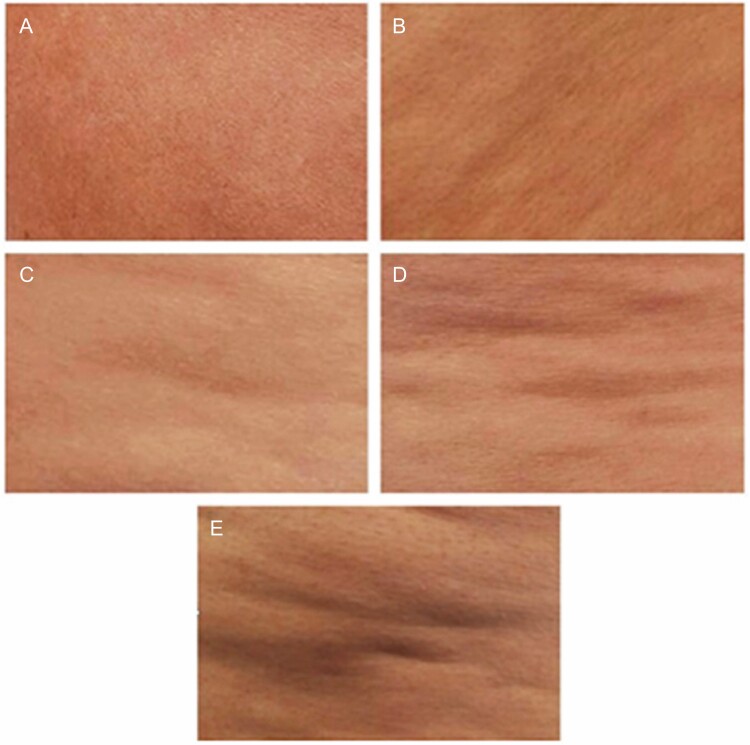
DiBernardo et al developed a photonumeric scale in which the number of evident dimples and the severity of linear undulations (contour irregularities) are graded from 0 to 4 (Figures 3 and 4).23 This scale combines elements of both the general (qualitative) Nürnberger-Müller staging and the more detailed (quantitative) CSS.23
Figure 3.
The DiBernardo scoring system for evaluating cellulite dimples in women.23 The number of evidence dimples are rated on a 0 to 4 scale. Each photo is marked with 5 circles, which may or may not contain a dimple. This ensures that the evaluator is not confused by nondimpling irregularities and avoids bias by not being explicitly told where dimples are located. Photos show (A) score 0 (no dimples); (B) score 1 (1 dimple); (C) score 2 (2 dimples); (D) score 3 (3 dimples); (E) score 4 (4 or more dimples). Reprinted with permission from DiBernardo et al.23
Figure 4.
The DiBernardo scoring system for evaluating cellulite contour irregularities in women.23 Contour irregularities are rated on a 0 to 4 scale. Photos show (A) score 0 (none: no depressions or raised areas); (B) score 1 (superficial irregularities: generalized, small depressions with no protuberances; (C) score 2 (mild irregularities: pattern of mild linear undulations with alternating areas of protuberances and depressions); (D) score 3 (moderate irregularities: pattern of moderate linear undulations with alternating areas of protuberances and depressions); (E) score 4 (severe irregularities: severe generalized linear undulations with alternating areas of protuberances and depressions). Reprinted with permission from DiBernardo et al.23
Cellulite Dimples—At Rest and Cellulite Dimples—Dynamic Scales
Cellulite Dimples—At Rest and Cellulite Dimples—Dynamic scales were developed to assess the severity of cellulite dimples on the thigh and buttocks in the static state (eg, at rest) or dynamic state.43 Both scales utilize photographs that facilitate assessment of the severity of cellulite dimples on a 5-point scale ranging from 0 (no depressions) to 4 (very severe depressions). For both intrarater and interrater reliability, intraclass correlation coefficients were ≥0.81 (almost perfect) for the static state scale, and between 0.61 and 0.80 (substantial) for the dynamic state scale.
Clinician Reported and Patient Reported Photonumeric Cellulite Severity Scales
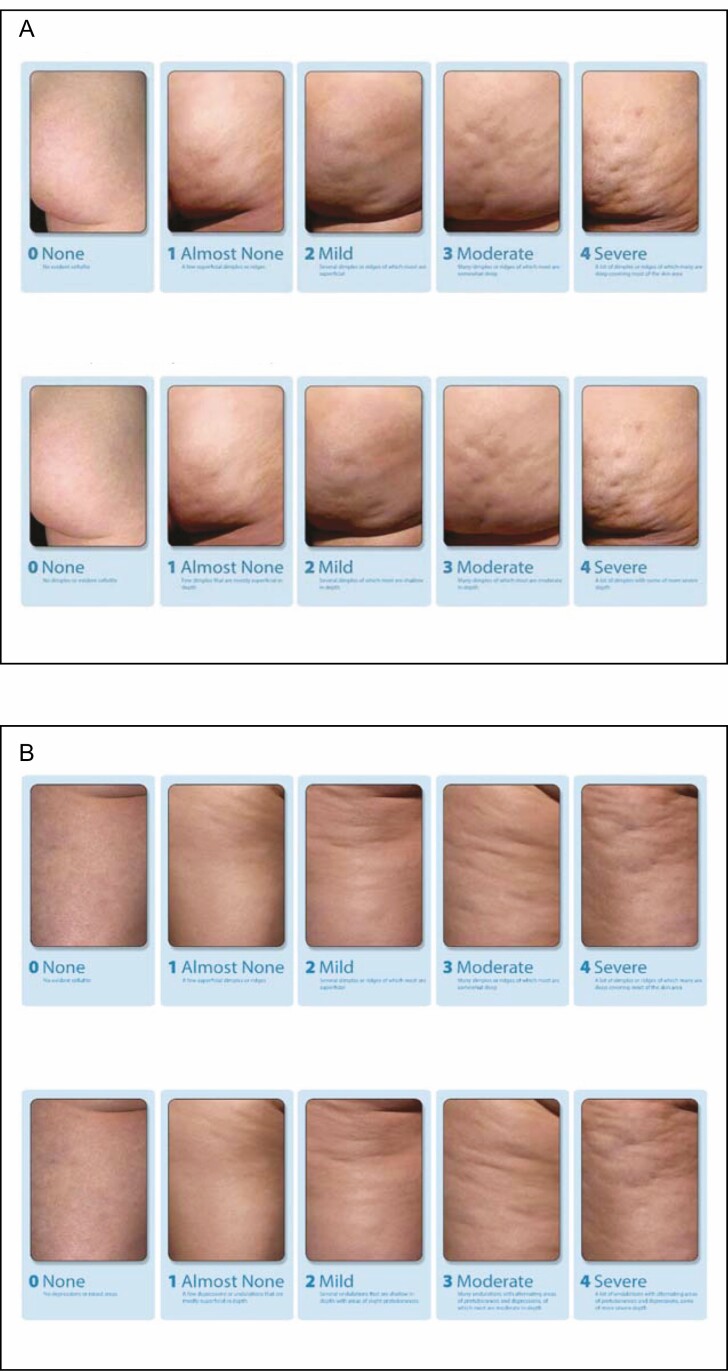
The Clinician Reported Photonumeric Cellulite Severity Scale (CR-PCSS) and the Patient Reported Photonumeric Cellulite Severity Scale (PR-PCSS) were developed to assess cellulite severity on the buttocks (Figure 5A) or thighs (Figure 5B) and allow assessment from the clinician (CR-PCSS) and patient perspectives (PR-PCSS).24,25 These scales utilize photographs in which cellulite severity is rated as a single item on a 5-point scale, ranging from 0 (none) to 4 (severe).24 In an evaluation of data from 375 women enrolled in a Phase 2 trial, good correlation was shown between the CR-PCSS and PR-PCSS overall and in each area rated (buttocks and thighs).24 In 2 phase 3 studies, the CR-PCSS and PR-PCSS for buttocks were utilized to assess improvement in cellulite severity.47 As single-item scales, the CR-PCSS and PR-PCSS may be easier to utilize in routine clinical practice than multi-item scales such as the CSS.24 However, at present, these 2 scales have been validated only for the buttocks and thighs.
Figure 5.
PR-PCSS and CR-PCSS for assessment of cellulite severity in women for the (A) buttocks and (B) thigh. CR-PCSS, Clinician Reported Photonumeric Cellulite Severity Scale; PR-PCSS, Patient Reported Photonumeric Cellulite Severity Scale. ©2017 Auxilium Pharmaceuticals, LLC. All rights reserved.
Investigator and Subjective Global Aesthetic Improvement Scales
The Global Aesthetic Improvement Scale is a 5-point scale rating global aesthetic change in appearance compared with pretreatment.26 The Investigator Global Aesthetic Improvement Scale and the Subjective Global Aesthetic Improvement Scale have been utilized to rate changes in the appearance of cellulite based on before-and-after digital photographs.42 Assessments are based on a 7-point scale ranging from +3 (very much improved) to −3 (very much worse).24 These scales are widely employed in clinical trials, but the utilization of subjective ratings of before-and-after images may introduce response bias.45
Features of an Ideal Rating Scale
Standardization and implementation of a validated scale to assess cellulite are important to allow comparisons of treatment results across clinical trials. However, at present, no single, standardized, universally accepted measurement of cellulite severity has been recommended or endorsed by regulatory authorities or professional societies for utilization in clinical trials.42 Furthermore, not all scales adequately address all clinically relevant aspects of cellulite. For example, in addition to dimples, some patients may show elongated horizontal streaks, particularly in the medial thigh.48 This is an important clinical consideration, because different skin defects will require different treatment approaches. Scales are needed to represent and, ultimately, treatments are needed to address all aspects of the cellulite defect. Conversely, some cellulite features captured in certain scales may not provide clinically useful information about cellulite severity. For example, several studies have shown that although skin laxity is included in the CSS, it is not correlated with cellulite severity; 3,27,49 thus, it should not be considered as part of a scale to assess cellulite severity. Ideally, rating scales to assess cellulite severity should combine reproducible results with published documentation of validation, ease of utilization, and inclusion of the patient perspective (Table 3).4,6,20-26,43
Table 3.
Features of an Ideal Rating Scale to Assess Cellulite Severity
| Scale | Demonstrated reliability | Validated | Ease of use | Skin laxity assessment included | Availability of clinician and patient scales |
|---|---|---|---|---|---|
| Nürnberger-Müller classification4 | No20,23 | No | Yes | No | No |
| CSS20,21 | Yes | Yes | No | Yes | No |
| Modified CSS22 | Yes | No | No | Yes | No |
| Curri scale6 | No | No | No | No | No |
| DiBernardo scoring system23 | Yes | Yes | Yes | No | No |
| Cellulite Dimples—At Rest and Cellulite Dimples—Dynamic scales43 | Yes | Yes | Yes | No | No |
| CR-PCSS; PR-PCSS24,25 | Yes | Yes | Yes | No | Yes |
| I-GAIS; S-GAIS26 | No | Not reported | Yes | No | Yes |
CR-PCSS, Clinician Reported Photonumeric Cellulite Severity Scale; CSS, Cellulite Severity Scale; I-GAIS, Investigator Global Aesthetic Improvement Scale; PR-PCSS, Patient Reported Photonumeric Cellulite Severity Scale; S-GAIS, Subjective Global Aesthetic Improvement Scale.
Since 2009, the FDA has required that outcome assessments utilized in clinical trials be validated to support labeling claims.44 Such validation is important to reduce bias and establish confidence in the objectivity and reproducibility of the measurement.50 It is recommended that measurement reliability be demonstrated by multiple live assessments and assessment of interrater and intrarater reproducibility.44 Patients may have different perceptions of treatment response than clinicians do, and, although barriers remain,51 there is increasing awareness of a need for more patient-centric approaches to treatment. For example, the 21st Century Cures Act emphasizes the need for patient engagement in drug development and requires regulatory agencies to create standards and guidance on the utilization of patient-reported outcomes52; FDA guidance recommends that treatment evaluations include the patient’s perspective.44 It is noteworthy that although at least 3 cellulite severity rating scales (CSS, DiBernardo, and CR-PCSS/PR-PCSS) have been clinically validated,3,20,23,25 only the CR-PCSS/PR-PCSS includes the patient’s perspective on cellulite appearance.24,25 To ensure acceptance of a given scale and its widespread adoption into clinical practice, it will be important to demonstrate ease of utilization by both clinicians and patients.42 As regulatory bodies begin to require patient-reported outcomes in drug development, future research will need to resolve discrepancies in representation of the clinical expectations of patients, particularly in aesthetic medicine.
IMAGING TECHNIQUES EMPLOYED TO ASSESS CELLULITE SEVERITY
Several imaging techniques have been used to evaluate cellulite (Table 1), including 2D or 3-dimensional (3D) digital photography, ultrasonography, and magnetic resonance imaging (MRI). Key features of these techniques are summarized in Table 4.6,10-13,20-23,25,28,30,34,35,39,41,49,53,54
Table 4.
Imaging Techniques Used to Assess Cellulite Severity
| Technique | Measurement of cellulite severity | Used in clinical practice? | Used in research? | Comments |
|---|---|---|---|---|
| 2D/3D photography20-23,25,28,30,49 | Measures: • Waviness of skin surface • Dimple depression and elevation |
2D photography is primarily used to document cellulite severity 3D photography is used where available |
Yes | • Photographic assessment is the only validated method of measuring cellulite severity, and the use of photonumeric scales is recommended by the FDA for the evaluation of new treatments • Standardized camera settings, lighting conditions, and patient positioning are important for reproducibility • In research studies, evaluators should ideally be blinded and independent |
| Ultrasonography12,13,34,35 | Provides direct visualization of the epidermis and dermal thickness | Very little clinical use Used in research to show fat herniation into the dermis |
Yes | • Operator technique is important for image quality • Dermis–hypodermis and dermis– subcutaneous length correlate with cellulite severity |
| Thermographic techniques6,39,53 | Uses measurements of skin temperature to grade cellulite severity | No | Yes | • Considered subjective because skin temperature can be affected by multiple factors, including sun exposure, fever, smoking, and menstrual cycle phase in women • Can be combined with less subjective and more quantitative techniques (eg, photonumeric scales) • Cellulite must be accompanied by edema for adequate assessment |
| MRI10,11,13 | Used to visualize skin architecture of the dermis and hypodermis | No | Yes | • Primarily used in research setting |
| Computed axial tomography41,54 | Used to assess adipose tissue thickness | No | Yes | • Primarily used in research setting • Good correlation with MRI results |
2D, 2-dimensional; 3D, 3-dimensional; FDA, US Food and Drug Administration; MRI, magnetic resonance imaging.
2D or 3D Digital Photography
2D or 3D digital photography has been utilized to evaluate efficacy in studies of both noninvasive22,31-33 and minimally invasive23,27-30,55 treatments of cellulite on the thighs or buttocks. 3D digital photography utilizes algorithms to transform 2D images to 3D images that can provide information about skin morphology and volumes of depressions or elevations.32,33 Noninvasive treatments evaluated by digital photography include AWT,22,32 radiofrequency,31 and a dual-wavelength laser/suction device.33 In a randomized, controlled trial of 17 patients treated with AWT on the thighs and buttocks, 2D digital photography evaluated by 4 blinded observers showed statistical improvement in CSS scores at 3 months compared with the untreated leg. 3D assessment also showed significant (P = 0.01) improvements in skin surface waviness and dimple depression and elevation at 3 months.22 A second randomized clinical trial involving 25 patients treated with AWT on the thighs and buttocks and assessed by 3D digital photography also showed significant improvement compared with the untreated leg in both dimple depressions (treated, 50.0%; untreated, 20.0%; P = 0.02) and elevations (treated, 55.0%; untreated, 15.0%; P = 0.002) at 3 months.32
In an observational study involving 20 patients treated with a dual-wavelength (650 and 915 nm) laser-suction and massage device, 3D digital photography showed improvements in skin surface irregularities at 1, 3, and 6 months, but no statistical analysis of these changes was performed.33 In a further study, 50 patients were treated with bipolar fractional radiofrequency, and cellulite severity was assessed employing 2D digital photographs evaluated by 3 blinded dermatologists utilizing the Nürnberger-Müller classification.31 Treatment success was defined as ≥1-point improvement in the treatment measures in accordance with FDA guidance.31 This study showed a statistically significant mean (± standard error of the mean) reduction in the number of dimples (1.1 ± 0.1, P < 0.0001) and an improvement in undulation severity measured on a 5-point scale (0.6 ± 0.1, P < 0.0001) at 6 months.31
Minimally invasive treatments assessed by 2D or 3D digital photography in clinical studies include 1440-nm neodymium-doped yttrium-aluminum-garnet (Nd:YAG) laser23,27,30,56 and manual or vacuum-assisted subcision.28,29,55,57 In a prospective study of 25 patients treated with the 1440-nm Nd:YAG laser and side-firing fiber, 2 independent evaluators utilized 2D digital photographs to assess treatment efficacy at 2 years according to the Nürnberger-Müller classification.27 Improvements in skin appearance were reported, but no statistical analysis was performed.27 A second study, involving 15 patients treated with a similar device, noted significant improvement at 6 months in dimple and contour irregularities (P < 0.005) assessed by 2D digital photography evaluated by independent, blinded individuals utilizing the photonumeric 5-point DiBernardo scale.30 In addition, 3D digital photography showed significant improvement in dimple depth at both 3 and 6 months (P = 0.0002 and P = 0.0003, respectively).30
Utilizing 2D and 3D digital photography, DiBernardo reported improvements in skin appearance in 10 women with moderate-to-severe cellulite treated with a side-firing 1440-nm pulsed laser.56 Subsequently, an observational study in 57 patients treated with the same device at 5 centers demonstrated significant improvement in mean dimple number at 2, 3, and 6 months (P < 0.001), as evaluated by independent observers utilizing the 5-point DiBernardo scoring system.23 In an observational study of 55 patients treated with vacuum-assisted subcision and assessed by 2D digital photography evaluated by independent blinded physicians, there were significant improvements in mean CSS scores at 3 months (2.1 ± 0.7 points; P < 0.0001) and 1 year (2.0 ± 0.8 points; P < 0.0001).28,29 These improvements were durable for up to 3 years (n = 45; 2.0 ± 1.0 points; P < 0.0001) and 5 years (n = 37; 1.8 points; P < 0.0001).29,57
Technical Considerations With Digital Photography
Both 2D and 3D photography are widely utilized in aesthetics research. In clinical practice, 3D photography is the preferred choice where available, but 2D photography is primarily utilized to document cellulite severity. At the time of writing, 3D capture devices capable of stitching multiple images together in a reproducible manner are new to the market. Ultimately, the 3D photography model should prevail for 2 reasons: lighting requirements are eliminated from the equation, and accurate, volumetric measurements of the defects can be quantified.
Obtaining reproducible results with digital photography is another challenge. Tangential lighting and the utilization of standardized distances and camera settings and positioning are essential for reliable results.23,28,30,58 The type of lighting is also important, and although soft boxes may be utilized to diffuse light and minimize shadows, direct light is preferred in most situations. In addition, it has been recommended that the same photographer take all photographs, particularly in clinical studies.23 The majority of the studies described above utilized methods for standardizing camera settings, lighting conditions, and patient positions for 2D digital photography.22,23,27,28,30,31,56 Similarly, the majority of studies utilized independent or blinded evaluators to review 2D photographs.22,23,27,28,30,31 In general, analyses are considered more reliable when evaluators are blinded and independent from the study.49
One advantage of 3D digital photography is that it can provide information about dimple depth,30,33,56 whereas 2D photography can flatten the skin surface, obscuring surface detail.49 Photographic assessment of cellulite is currently the only validated technology for determination of cellulite severity,3,20,25 and thus, the FDA has recommended that assessments of efficacy in trials of cellulite treatments should utilize photonumeric scales. In a small study of 26 healthy women with grade I to III cellulite (Nürnberger-Müller classification), a positive correlation (R = 0.77; P < 0.01) was reported between dermatologist photographic evaluation of cellulite severity and the photonumeric CSS; however, correlations between clinical evaluations and objective measures of skin thickness or elasticity were poor.49
Ultrasonography
High-frequency ultrasonography measures the acoustic signal recorded for a digital soundwave reflected from biological tissues and provides direct visualization of epidermal and dermal thickness (Figure 6).49,53,56 This type of imaging can measure dermal or adipose tissue thickness and provides information about changes in fibrosclerotic tissue that could affect the appearance of cellulite.36 However, image quality is highly dependent on operator technique.13 Ultrasonography has been utilized to characterize cellulite12,34 and to evaluate treatment efficacy in clinical studies.27,35,56 In 1 study, 94 healthy women aged 20 to 60 years with Nürnberger-Müller grade 0 or II cellulite had epidermal and total skin thickness assessed by ultrasound.12 Irrespective of age, a 30% increase in total skin thickness was seen in women with grade II cellulite compared with those without cellulite (P < 0.001).12 The length of the dermis–hypodermis interface was also significantly (P < 0.001) longer in women with cellulite in all age groups compared with those without cellulite (grade 0), reflecting herniation of the hypodermis into the dermis.12
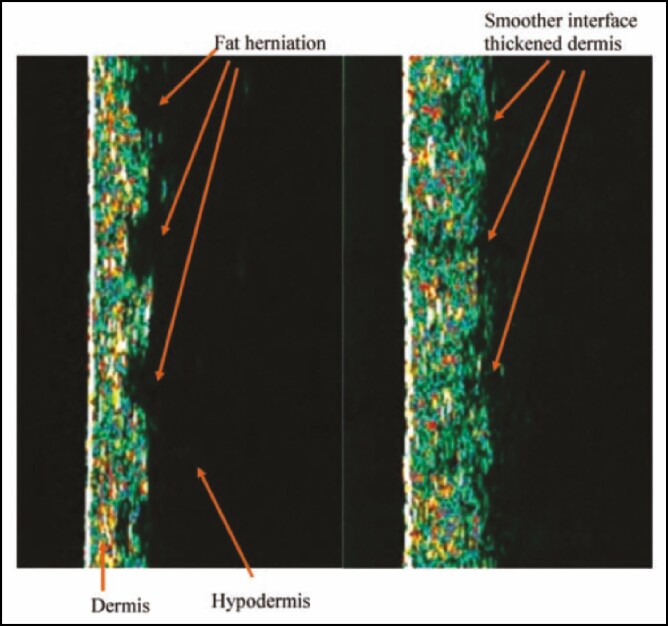
Figure 6.
Ultrasonography of the dermis of a woman presenting with cellulite.56 Images are of the dermis (green), hypodermis (black), and dermal–hypodermal interface showing fat herniations into the dermis at baseline (left) and 6 months after treatment with a 1440-nm pulsed laser (right). The vertical measured length is 12 mm in each image. Reprinted with permission from DiBernardo et al.56
In another study, 51 women with thigh cellulite were assessed utilizing ultrasound.34 Cellulite severity (scale 0-9) was determined by independent observers from images obtained utilizing a 3D skin surface laser scanner. There was a significant correlation (R ≥ 0.4; P < 0.05) between cellulite severity and the area of the dermal–hypodermal border, which could be predicted from percentage fat (P < 0.001) and the dermal–subcutaneous surface area (R = 0.7; P = 0.02).34
A double-blind, placebo-controlled, clinical study of 105 women with moderate cellulite (Nürnberger-Müller grade II-III) treated with bioactive collagen peptides showed a strengthening of connective tissue following peptide treatment.35 The peptide treatment group experienced a significant increase in relative dermis density from baseline (7.3) to 6 months (7.7; P < 0.05), whereas the placebo group showed a progressive loss of dermis density from baseline (6.8) to 6 months (6.4; P < 0.01).35 Furthermore, the length of the border between the dermis and hypodermis was significantly (P < 0.05) shortened in the peptide treatment group compared with the placebo group, and the length of this border correlated with cellulite severity.35 The mean (± standard deviation) CSS was improved from baseline in peptide-treated patients (treatment, 2.4 ± 0.4; placebo, 2.4 ± 0.4) to 6 months (treatment, 2.1 ± 0.4; placebo, 2.2 ± 0.5; P < 0.05), but the FDA criterion of a 1-point improvement was not met.59
In a prospective study of 25 patients treated with the 1440-nm Nd:YAG laser and side-firing fiber, ultrasound images demonstrated a more compact and dense dermis and a more defined border between the dermis and hypodermis compared with baseline.27 However, the changes were not statistically analyzed, and no correlation with cellulite severity was reported.27
In summary, based on ultrasound assessments, only the dermis–hypodermis or dermis–subcutaneous interface length has been shown to correlate with cellulite severity.12,34,35 Moreover, in treatment studies,27,35 ultrasound has been employed to characterize skin architecture after treatment, but not to establish efficacy. It also should be noted that a study has reported a poor correlation between ultrasonographic findings and clinical rating scales.49 For these reasons, ultrasonography has a limited role in clinical practice.
Thermographic Methods
Thermography evaluates the temperature of the skin to create a “map” of different colors, reflecting different temperatures on the skin surface. A homogenous green or rosy color indicates a lack of cellulite, whereas dark spots (referred to as “black holes” or “leopard skin”) indicate more advanced grades of cellulite.6,38 However, factors such as sun exposure, fever, smoking, and menstrual cycle phase can affect skin temperature6 and, hence, thermography is considered a subjective measure of cellulite severity.39 Another limitation of thermography is that cellulite must be accompanied by edema for adequate assessment.39,53
Thermography has been utilized primarily in research settings38,39 rather than in the clinic. In 1 study involving 39 healthy women with cellulite graded according to the Curri scale,38 initial findings showed that skin temperature variability was influenced by time of day (P = 0.0007), body position (P = 0.02), and consumption of a hot beverage (P = 0.05), but not by acclimatization time; subsequent experience confirmed the reproducibility of thermographic measurements.38 Thermal parameters relating to temperature homogeneity showed significant correlations with cellulite grade.38
The combination of thermography with image analysis and processing techniques can offer less subjective and more quantitative assessments than thermography alone.39 For example, in 1 study, 10 women were evaluated by thermography before and after treatment with a transdermal cosmeceutical product.39 The Grey Level Co-occurrence Matrix method was utilized to determine the difference between adjacent temperature fields and successfully demonstrated cellulite improvement (ie, reduction in mean Grey Level Co-occurrence Matrix contrast) following treatment. However, a validated CSS was not utilized, and no statistical analysis of the data was performed.39
Magnetic Resonance Imaging
The architecture of the dermis and hypodermis can be visualized utilizing high-resolution in vivo MRI. However, because the technology is not readily available, it has been employed primarily in the research setting and has been utilized to investigate cellulite-related changes in skin architecture in a number of studies.10,11,13,60 In a study involving 67 healthy volunteers with or without cellulite, women with cellulite had a thicker dermis (P < 0.01), thicker adipose tissue (P = 0.0001), and a higher percentage of perpendicular fibrous septae (P < 0.001) compared with men or women without cellulite.11 In a smaller study involving 18 participants (12 females, 6 males), the percentile of adipose tissue compared with connective tissue in a given volume of hypodermis and the percentile of hypodermic extrusions inside the dermis were significantly (P < 0.05) correlated with visually assessed cellulite grade.13 In another study that involved 30 women with cellulite in the buttocks, septae were visualized in 96.7% of areas with cellulite depressions compared with 16.7% of areas with no cellulite (P < 0.001); there was no relationship between cellulite severity (CSS score) and septae thickness.10
One study utilized MRI to evaluate changes in cellulite following manual subcision in 2 women with severe cellulite of the buttocks.60 The CSS grade improved to moderate at 1 month after subcision, and these improvements were maintained for an additional 7 months.60 MRI confirmed that the septae were severed following subcision (Figure 7), but MRI was not utilized to grade cellulite severity.60
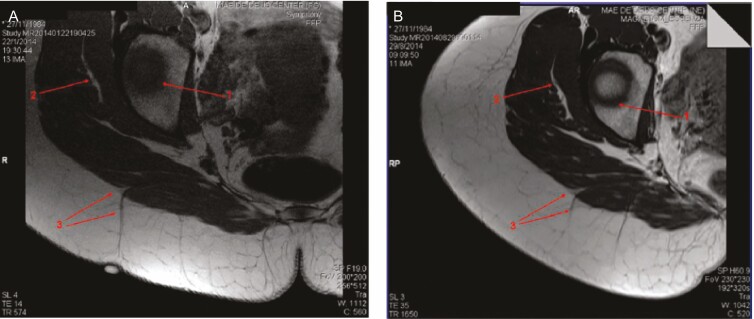
Figure 7.
Magnetic resonance imaging of cellulite from this 29-year-old woman at (A) baseline and (B) after subcision.60 Baseline image (A) shows a clear spot on the top of the depressed lesion with a perpendicular thick fibrous septum associated with this lesion and (B) the same area 7 months after subcision, showing the severed septum. Arrows 1 and 2 indicate anatomic structures utilized as a guide to obtain the same slices of bone and muscle layer, respectively. Arrow 3 points to the septum arising from the muscle. Reprinted with permission from Hexsel et al.60
Computed Axial Tomography
Similar to MRI, computed axial tomography has been utilized primarily in the research setting as a cellulite diagnostic tool. Computed axial tomography can only measure fatty tissue thickness41; it cannot characterize the dermis or microcirculation.6 Another concern that has limited the application of this imaging technique is patient exposure to radiation from the x-ray.54 Of note, quantitation of total, visceral, and subcutaneous fat area with MRI vs computed axial tomography was similar between the 2 techniques, and results were strongly correlated in 7 healthy patients (4 men, 3 women; mean age ± standard deviation, 27.6 ± 7.3).41
MEASUREMENT OF SKIN BIOMECHANICS
Measurements of skin biomechanics have been utilized to evaluate cellulite-related changes in the physical properties of skin. Such biomechanical measures include skin elasticity, surface roughness, and skin waviness (Table 5).3,27,34,35,49,53 Skin elasticity can be measured by utilizing a controlled vacuum from a suction probe to assess skin traction and relaxation.49 This approach has been utilized in a number of studies both to evaluate skin elasticity in relation to cellulite severity12,34 and to assess treatment efficacy.27,32 A study involving 62 females and 10 males showed no significant correlation between cellulite severity (assessed by independent observers on a 0-9 scale; based on 3D skin surface laser scanning) and skin laxity.34 Similarly, in a study involving 94 healthy women with Nürnberger-Müller grade 0 or II cellulite, skin elasticity decreased with age (P = 0.003) and between age groups (21-30 years vs 31-40 years; P = 0.02), but the effect did not correlate with cellulite grade.12
Table 5.
Techniques Used to Measure Skin Biomechanics in Women With Cellulite
| Technique | Measurement of cellulite severity | Used in clinical practice? | Used in research? | Comments |
|---|---|---|---|---|
| Measurement of skin elasticity3,27,49 | Skin elasticity measured by suction probe and combined with objective measures of cellulite severity | No | Yes | To date, no studies have shown skin elasticity to be a relevant assessment tool for measurement of cellulite severity |
| Measurement of skin surface roughness34 | Measured by 3D imaging and combined with objective measures of cellulite severity | No | Yes | Correlations reported between surface roughness and severity of cellulite |
| Measurement of skin surface profile (waviness)35 | Measured by 3D scanning and combined with objective measures of cellulite severity | No | Yes | Improvements in skin waviness have not been reported to correlate with cellulite severity |
| Optical computed tomography53 | Measured by reflecting infrared light from internal structures in the skin | No | Rarely | • Offers good resolution due to high frequency of infrared waves • However, infrared waves can only penetrate approximately 1000 μm into the skin; hence, only the epidermis and papillary dermis can be adequately visualized |
3D, 3-dimensional.
In a prospective study of 25 patients treated with the 1440-nm Nd:YAG laser and side-firing fiber, skin elasticity was significantly increased compared with baseline values (P < 0.01), but a correlation with cellulite severity was not performed.27 A randomized clinical trial involving 25 patients treated with AWT who were assessed by 3D digital photography demonstrated a significant improvement in elasticity for the treated leg compared with the untreated leg (treated, 33.3%; untreated, 5.6%; P = 0.03) 1 week after treatment, but a relationship with cellulite severity was not demonstrated.32 To date, no studies have demonstrated the relevance of skin laxity as an assessment tool to evaluate cellulite severity.21,27,49
A few studies have investigated skin surface roughness as a measure of cellulite severity. In 1 study, cellulite severity (scale 0-9) showed significant correlations with 2 key measures of surface roughness: the mean maximum profile depth over the entire skin (Svm, R = 0.86; P = 0.008) and the ratio between the roughness surface area and flat surface area (Sdr, R = 0.86; P = 0.002).34 In a randomized clinical trial with 25 patients treated with AWT, significant improvement in surface roughness was observed 1 week after treatment in the treated leg compared with the untreated leg (treated, 30.0%; untreated, 5.0%; P = 0.04).32
In the study of bioactive collagen peptides discussed previously,35 a significant 8% reduction (P < 0.05) in skin waviness was observed in peptide-treated patients at 6 months compared with placebo.35 However, although mean cellulite severity (measured utilizing the pinch test) was significantly improved from baseline, the FDA criterion of 1-point improvement in cellulite severity was not met, and no relationship between the improvements in skin surface profile and cellulite severity was observed.35
Optical coherence tomography can provide detailed images of the skin architecture. This technique involves measuring infrared light waves reflected from internal structures within the tissue; the high frequency and bandwidth of infrared light results in improved resolution compared with other imaging modalities such as ultrasound.53 However, a limitation of this technique is that infrared light can only penetrate approximately 1000 μm into the skin; as a result, the hypodermis cannot be visualized adequately.53
CONCLUSIONS
The appearance of cellulite reflects changes in both the dermis and hypodermis. As a result, numerous scales and imaging technologies have been utilized to quantify and assess cellulite. Strengths of this review included that the information provided is the most comprehensive evaluation of scales to assess cellulite severity to date. The presentation of the strengths and weaknesses of scales currently utilized in clinical practice and research allows for an improved paradigm in cellulite assessment based on previous experience and the literature. A limitation is that the key features of an ideal scale may differ depending on the application of the scale (eg, research or clinical) and language and/or ethnic considerations of the users. Furthermore, the weaknesses in a scale for this purpose are inherent because of the multifactorial nature of cellulite (eg, presentation, structure, form, and etiology). As our understanding of these parameters increases, the scale and imaging techniques can be optimized for assessment of a particular parameter. Many of the scales currently in use have limitations, such as a purely qualitative nature or a failure to capture clinically relevant features of cellulite. The CR-PCSS and PR-PCSS may be considered the best available cellulite rating scales currently because they are validated and, when utilized together, provide both clinician and patient perspectives. However, there remains an unmet need for a validated scale that can be utilized easily by the clinician and the patient, in both the clinical and research settings, to assess cellulite affecting a range of body areas. Similarly, there is a need for reliable imaging techniques to better characterize cellulite and treatment efficacy. Currently, photography is the only technology that has been validated as a component of cellulite severity assessments. Ultrasonography, MRI, thermography, and skin biomechanics have been employed in studies of the pathophysiology of cellulite and in interventional studies, but to date, results obtained with these techniques have not been shown to correlate with cellulite severity. Further research into the assessment of cellulite severity is warranted.
Acknowledgments
Technical editorial and medical writing assistance was provided, under the direction of the authors, by Mary Beth Moncrief, PhD, and Michael Shaw, PhD (Synchrony Medical Communications, LLC, West Chester, PA).
Disclosures
Dr Young reports serving as an investigator for Alder Biopharmaceuticals (Bothell, WA), Allergan plc (Dublin, Ireland), Amgen Inc. (Thousand Oaks, CA), Ardea Biosciences (San Diego, CA), Boehringer Ingelheim (Ingelheim am Rhein, Germany), Celgene Corporation (Summit, NJ), Daiichi Sankyo Co., Ltd. (Tokyo, Japan), DalCor Pharmaceuticals (Montréal, Canada), Derma Sciences Inc. (Princeton, NJ), Eisai Co., Ltd. (Tokyo, Japan), Eli Lilly and Company (Indianapolis, IN), Endo Pharmaceuticals, Inc. (Malvern, PA), Evidera (Bethesda, MD), Evolus, Inc. (Newport Beach, CA), Halscion, Inc. (Duluth, GA), Janssen Pharmaceuticals, Inc. (Beerse, Belgium), Kythera Biopharmaceuticals (Agoura Hills, CA), MedImmune (Gaithersburg, MD), Merck & Co., Inc. (Kenilworth, NJ), Neothetics, Inc. (San Diego, CA), Pfizer Inc. (New York, NY), RXi Pharmaceuticals (Marlborough, MA), Sanofi (Paris, France), Takeda Pharmaceuticals U.S.A., Inc. (Tokyo, Japan), and Therakos, Inc. (Raritan, NJ); serving as a consultant for AirXpanders, Inc. (San Jose, CA), miRagen Therapeutics (Boulder, CO), Regeneron Pharmaceuticals (Tarrytown, NY), and Sanofi; and receiving registration and travel expenses from Endo Pharmaceuticals, Inc. Dr DiBernardo reports serving as an investigator for Apyx Medical (Clearwater, FL), Cynosure (Hologic Company; Westford, MA), Endo Pharmaceuticals, Inc., Invasix (Irvine, CA), Medicel (Thal, Switzerland), Merz North America (Raleigh, NC), Nutraceutical Wellness Inc. (Miami, FL), Sciton, Inc. (Palo Alto, CA), Stratpharma AG (Basel, Switzerland), Thermi (Almirall Company; Irving, TX), and Under Skin USA. (San Diego, CA); serving as a consultant for Canfield Scientific (Parsippany, NJ), ELux Medical (La Jolla, CA), Revo Aesthetics, Sinclair Medical (London, United Kingdom), ZO Skin Health, Inc. (Irvine, CA); and being a stockholder of HintMD (Pleasanton, CA), RealSelf (Seattle, WA), and Strathspey Crown (Newport Beach, CA).
Funding
Writing and editorial assistance was provided to the authors by Dr Timothy Ryder (Biological Communications Limited, London, UK) and funded by Allergan (Dublin, Ireland) at the request of the investigator. Neither honoraria nor payments were made for authorship.
REFERENCES
- 1. Hexsel D, Mazzuco R. Cellulite. In: Tosti A, Hexsel D, eds. Update in Cosmetic Dermatology. Berlin, Germany: Springer-Verlag; 2013:21-32. [Google Scholar]
- 2. Ghigi R. The female body between science and guilt. Work Gender Soc. 2004;2(12):55-75. [Google Scholar]
- 3. De La Casa Almeida M, Suarez Serrano C, Rebollo Roldán J, Jiménez Rejano JJ. Cellulite’s aetiology: a review. J Eur Acad Dermatol Venereol. 2013;27:273-278. [DOI] [PubMed] [Google Scholar]
- 4. Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4(3):221-229. [DOI] [PubMed] [Google Scholar]
- 5. Khan MH, Victor F, Rao B, Sadick NS. Treatment of cellulite: part I. Pathophysiology. J Am Acad Dermatol. 2010;62(3):361-370; quiz 371-372. [DOI] [PubMed] [Google Scholar]
- 6. Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol. 2000;14(4):251-262. [DOI] [PubMed] [Google Scholar]
- 7. Emanuele E. Cellulite: advances in treatment: facts and controversies. Clin Dermatol. 2013;31(6):725-730. [DOI] [PubMed] [Google Scholar]
- 8. Pereira de Godoy JM, Guerreiro de Godoy MF. Physiopathological hypothesis of cellulite. Open Cardiovasc Med J. 2009;3:96-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rudolph C, Hladik C, Hamade H, et al. Structural gender dimorphism and the biomechanics of the gluteal subcutaneous tissue: implications for the pathophysiology of cellulite. Plast Reconstr Surg. 2019;143(4):1077-1086. [DOI] [PubMed] [Google Scholar]
- 10. Hexsel DM, Abreu M, Rodrigues TC, Soirefmann M, do Prado DZ, Gamboa MM. Side-by-side comparison of areas with and without cellulite depressions using magnetic resonance imaging. Dermatol Surg. 2009;35(10): 1471-1477. [DOI] [PubMed] [Google Scholar]
- 11. Querleux B, Cornillon C, Jolivet O, Bittoun J. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8(2):118-124. [DOI] [PubMed] [Google Scholar]
- 12. Ortonne JP, Zartarian M, Verschoore M, Queille-Roussel C, Duteil L. Cellulite and skin ageing: is there any interaction? J Eur Acad Dermatol Venereol. 2008;22(7):827-834. [DOI] [PubMed] [Google Scholar]
- 13. Mirrashed F, Sharp JC, Krause V, Morgan J, Tomanek B. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10(3):161-168. [DOI] [PubMed] [Google Scholar]
- 14. Lorencini M, Camozzato F, Hexsel D. Skin aging and cellulite in women. In: Farage M, Miller K, Maibach H, eds. Textbook of Aging Skin. Berlin: Springer; 2015:1-9. [Google Scholar]
- 15. Kaminer MS, Casabona G, Peeters W, et al. Validated assessment scales for skin laxity on the posterior thighs, buttocks, anterior thighs, and knees in female patients. Dermatol Surg. 2019;45(Suppl 1):S12-S21. [DOI] [PubMed] [Google Scholar]
- 16. Uitto J, Fazio MJ, Olsen DR. Molecular mechanisms of cutaneous aging. Age-associated connective tissue alterations in the dermis. J Am Acad Dermatol. 1989;21(3 Pt 2):614-622. [PubMed] [Google Scholar]
- 17. Uitto J, Bernstein EF. Molecular mechanisms of cutaneous aging: connective tissue alterations in the dermis. J Investig Dermatol Symp Proc. 1998;3(1):41-44. [PubMed] [Google Scholar]
- 18. Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7(2 Suppl):s12-s16. [PubMed] [Google Scholar]
- 19. Luebberding S, Krueger N, Sadick NS. Cellulite: an evidence-based review. Am J Clin Dermatol. 2015;16(4):243-256. [DOI] [PubMed] [Google Scholar]
- 20. Hexsel DM, Dal’forno T, Hexsel CL. A validated photonumeric cellulite severity scale. J Eur Acad Dermatol Venereol. 2009;23(5):523-528. [DOI] [PubMed] [Google Scholar]
- 21. De La Casa Almeida M, Suarez Serrano C, Jiménez Rejano JJ, et al. Intra- and inter-observer reliability of the application of the cellulite severity scale to a Spanish female population. J Eur Acad Dermatol Venereol. 2013;27(6):694-698. [DOI] [PubMed] [Google Scholar]
- 22. Russe-Wilflingseder K, Russe-Wilfingsleder K, Russe E, et al. Placebo controlled, prospectively randomized, double-blinded study for the investigation of the effectiveness and safety of the acoustic wave therapy (AWT®) for cellulite treatment. J Cosmet Laser Ther. 2013;15(3):155-162. [DOI] [PubMed] [Google Scholar]
- 23. DiBernardo B, Sasaki G, Katz BE, Hunstad JP, Petti C, Burns AJ. A multicenter study for a single, three-step laser treatment for cellulite using a 1440-nm Nd:YAG laser, a novel side-firing fiber, and a temperature-sensing cannula. Aesthet Surg J. 2013;33(4):576-584. [DOI] [PubMed] [Google Scholar]
- 24. Young VL, Sadick N, Liu G, et al. Comparisons of clinician-reported and patient-reported cellulite severity scales with existing scales for measurement of cellulite severity. Paper presented at Northeastern Society of Plastic Surgeons 34th Annual Meeting; September 8-10, 2017; Newport, RI. [Google Scholar]
- 25. Kirby MT, Lenderking WR, Bender RH, et al. Assessing cellulite severity: test-retest reliability of and concordance between new clinician reported and patient reported photonumeric scales. Paper presented at American Society for Dermatologic Surgery Annual Meeting; October 11-14, 2018; Phoenix, AZ. [Google Scholar]
- 26. Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29(6):588-595. [DOI] [PubMed] [Google Scholar]
- 27. Sasaki GH. Single treatment of grades II and III cellulite using a minimally invasive 1,440-nm pulsed Nd:YAG laser and side-firing fiber: an institutional review board-approved study with a 24-month follow-up period. Aesthetic Plast Surg. 2013;37(6):1073-1089. [DOI] [PubMed] [Google Scholar]
- 28. Kaminer MS, Coleman WP 3rd, Weiss RA, Robinson DM, Coleman WP 4th, Hornfeldt C. Multicenter pivotal study of vacuum-assisted precise tissue release for the treatment of cellulite. Dermatol Surg. 2015;41(3):336-347. [DOI] [PubMed] [Google Scholar]
- 29. Kaminer MS, Coleman WP 3rd, Weiss RA, Robinson DM, Grossman J. A multicenter pivotal study to evaluate tissue stabilized-guided subcision using the cellfina device for the treatment of cellulite with 3-year follow-up. Dermatol Surg. 2017;43(10):1240-1248. [DOI] [PubMed] [Google Scholar]
- 30. Katz B. Quantitative & qualitative evaluation of the efficacy of a 1440 nm Nd:YAG laser with novel bi-directional optical fiber in the treatment of cellulite as measured by 3-dimensional surface imaging. J Drugs Dermatol. 2013;12(11):1224-1230. [PubMed] [Google Scholar]
- 31. Alexiades M, Munavalli G, Goldberg D, Berube D. Prospective multicenter clinical trial of a temperature-controlled subcutaneous microneedle fractional bipolar radiofrequency system for the treatment of cellulite. Dermatol Surg. 2018;44(10):1262-1271. [DOI] [PubMed] [Google Scholar]
- 32. Adatto M, Adatto-Neilson R, Servant JJ, Vester J, Novak P, Krotz A. Controlled, randomized study evaluating the effects of treating cellulite with AWT/EPAT. J Cosmet Laser Ther. 2010;12(4):176-182. [DOI] [PubMed] [Google Scholar]
- 33. Kulick MI. Evaluation of a noninvasive, dual-wavelength laser-suction and massage device for the regional treatment of cellulite. Plast Reconstr Surg. 2010;125(6):1788-1796. [DOI] [PubMed] [Google Scholar]
- 34. Smalls LK, Lee CY, Whitestone J, Kitzmiller WJ, Wickett RR, Visscher MO. Quantitative model of cellulite: three-dimensional skin surface topography, biophysical characterization, and relationship to human perception. J Cosmet Sci. 2005;56(2):105-120. [PubMed] [Google Scholar]
- 35. Schunck M, Zague V, Oesser S, Proksch E. Dietary supplementation with specific collagen peptides has a body mass index-dependent beneficial effect on cellulite morphology. J Med Food. 2015;18(12):1340-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mlosek RK, Dębowska RM, Lewandowski M, Malinowska S, Nowicki A, Eris I. Imaging of the skin and subcutaneous tissue using classical and high-frequency ultrasonographies in anti-cellulite therapy. Skin Res Technol. 2011;17(4):461-468. [DOI] [PubMed] [Google Scholar]
- 37. Bertin C, Zunino H, Pittet JC, et al. A double-blind evaluation of the activity of an anti-cellulite product containing retinol, caffeine, and ruscogenine by a combination of several non-invasive methods. J Cosmet Sci. 2001;52(4):199-210. [PubMed] [Google Scholar]
- 38. Nkengne A, Papillon A, Bertin C. Evaluation of the cellulite using a thermal infra-red camera. Skin Res Technol. 2013;19(1):e231-e237. [DOI] [PubMed] [Google Scholar]
- 39. Wilczyński S, Koprowski R, Deda A, Janiczek M, Kuleczka N, Błońska-Fajfrowska B. Thermographic mapping of the skin surface in biometric evaluation of cellulite treatment effectiveness. Skin Res Technol. 2017;23(1):61-69. [DOI] [PubMed] [Google Scholar]
- 40. Hexsel D, Camozzato FO, Silva AF, Siega C. Acoustic wave therapy for cellulite, body shaping and fat reduction. J Cosmet Laser Ther. 2017;19(3):165-173. [DOI] [PubMed] [Google Scholar]
- 41. Seidell JC, Bakker CJ, van der Kooy K. Imaging techniques for measuring adipose-tissue distribution–a comparison between computed tomography and 1.5-T magnetic resonance. Am J Clin Nutr. 1990;51(6):953-957. [DOI] [PubMed] [Google Scholar]
- 42. Sadick NS, Goldman MP, Liu G, et al. Collagenase clostridium histolyticum for the treatment of edematous fibrosclerotic panniculopathy (Cellulite): a randomized trial. Dermatol Surg. 2019;45(8):1047-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hexsel D, Fabi SG, Sattler G, et al. Validated assessment scales for cellulite dimples on the buttocks and thighs in female patients. Dermatol Surg. 2019;45(Suppl 1):S2-S11. [DOI] [PubMed] [Google Scholar]
- 44. US Department of Health and Human Services, Food and Drug Administration. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Accessed April 23, 2019.
- 45. Rosenman R, Tennekoon V, Hill LG. Measuring bias in self-reported data. Int J Behav Healthc Res. 2011;2(4):320-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Friedmann DP, Vick GL, Mishra V. Cellulite: a review with a focus on subcision. Clin Cosmet Investig Dermatol. 2017;10:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaufman J, Joseph JH, Kaminer M, et al. Two randomized, double-blind, placebo-controlled, phase 3, trials of collagenase clostridium histolyticum (CCH) for the treatment of cellulite (edematous fibrosclerotic panniculopathy). Paper presented at American Academy of Dermatology Annual Meeting; March 1-5, 2019; Washington, DC. [Google Scholar]
- 48. DiBernardo BE, DiBernardo G, Pozner JN. Subsurface laser and radiofrequency for face and body rejuvenation. Clin Plast Surg. 2016;43(3):527-533. [DOI] [PubMed] [Google Scholar]
- 49. Soares JL, Miot HA, Sanudo A, Bagatin E. Cellulite: poor correlation between instrumental methods and photograph evaluation for severity classification. Int J Cosmet Sci. 2015;37(1):134-140. [DOI] [PubMed] [Google Scholar]
- 50. Sanchez MM, Binkowitz BS. Guidelines for measurement validation in clinical trial design. J Biopharm Stat. 1999;9(3):417-438. [DOI] [PubMed] [Google Scholar]
- 51. Lowe MM, Blaser DA, Cone L, et al. Increasing patient involvement in drug development. Value Health. 2016;19(6):869-878. [DOI] [PubMed] [Google Scholar]
- 52. 114th Congress. 21st Century Cures Act. Public Law 114–255. Washington, DC: 114th Congress. 2016:1-312. [Google Scholar]
- 53. Callaghan T, Wilhelm KP. An examination of non-invasive imaging techniques in the analysis and review of cellulite. J Cosmet Sci. 2005;56(6):379-393. [PubMed] [Google Scholar]
- 54. Leszko M. Cellulite in menopause. Prz Menopauzalny. 2014;13(5):298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Amore R, Amuso D, Leonardi V, et al. Treatment of dimpling from cellulite. Plast Reconstr Surg Glob Open. 2018;6(5):e1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. DiBernardo BE. Treatment of cellulite using a 1440-nm pulsed laser with one-year follow-up. Aesthet Surg J. 2011;31(3):328-341. [DOI] [PubMed] [Google Scholar]
- 57. Kaminer MS. Multi-center pivotal study of the safety and effectiveness of a tissue stabilized-guided subcision procedure for the treatment of cellulite - 5-year update. Paper presented at American Society for Dermatologic Surgery Annual Meeting; October 11-14, 2018; Phoenix, AZ. [Google Scholar]
- 58. Nikolis A, Enright KM. Methods of standardizing photography for cellulite in the buttocks and thighs. Dermatol Surg. 2019;45(9):1208-1210. [DOI] [PubMed] [Google Scholar]
- 59. DiBernardo BE, Sasaki GH, Katz BE, Hunstad JP, Petti C, Burns AJ. A multicenter study for cellulite treatment using a 1440-nm Nd:YAG wavelength laser with side-firing fiber. Aesthet Surg J. 2016;36(3):335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hexsel D, Dal Forno T, Hexsel C, et al. Magnetic resonance imaging of cellulite depressed lesions successfully treated by subcision. Dermatol Surg. 2016;42(5): 693-696. [DOI] [PubMed] [Google Scholar]