Abstract
Background
Among individuals with nonvalvular atrial fibrillation (AF), the prevalence of obstructive sleep apnea (OSA) can be as high as 85%. Continuous positive airway pressure treatment for moderate or severe OSA might improve AF outcomes and quality of life, so early identification of OSA might be of value. However, screening questionnaires for OSA are suboptimal because they are weighted toward tiredness and loud snoring, which might be absent in AF patients. NoSAS (Neck, Obesity, Snoring, Age, Sex) is a new OSA questionnaire that excludes these parameters. Acoustic pharyngometry (AP) is a potential novel screening technique that measures pharyngeal cross-sectional area, which is reduced in patients with OSA.
Methods
We prospectively compared the accuracy of the NoSAS, the STOP-BANG questionnaire (Snoring, Tiredness, Observed apnea, blood Pressure, Body mass index, Age, Neck circumference and Gender), and AP with home sleep apnea testing (HSAT) in consecutive patients with nonvalvular AF.
Results
Of 188 participants, 86% had OSA and 49% had moderate or severe OSA. Mean Epworth Sleepiness Scale scores were low; 5.9 (SD, 3.9), indicating that most participants were not sleepy. Receiver operating characteristic curves for comparisons of screening tests with HSAT showed suboptimal accuracy. For moderate plus severe and severe only groups respectively, the area under the curve was 0.50 (95% confidence interval [CI], 0.42-0.58) and 0.42 (95% CI, 0.34-0.52) for AP, 0.65 (95% CI, 0.58-0.73) and 0.63 (95% CI, 0.52-0.74) for the STOP-BANG questionnaire, and 0.68 (95% CI, 0.60-0.75) and 0.69 (95% CI, 0.59-0.80) for the NoSAS.
Conclusions
AP and NoSAS are not sufficiently accurate for screening AF patients for OSA. Because of the high rates of OSA in this cohort, the potential benefits of OSA treatment, and the suboptimal accuracy of current screening questionnaires, cardiologists should consider HSAT for AF patients.
Résumé
Contexte
Chez les sujets présentant une fibrillation auriculaire (FA) non valvulaire, la prévalence de l’apnée obstructive du sommeil (AOS) peut atteindre 85 %. En cas d’AOS modérée ou sévère, un traitement par ventilation spontanée en pression positive continue peut améliorer les résultats liés à la FA et la qualité de vie du patient; un diagnostic précoce d’AOS pourrait donc être utile. Les questionnaires de dépistage de l’AOS ne sont toutefois pas optimaux parce qu’ils accordent une grande importance à la fatigue et aux ronflements sonores, des symptômes qui ne se manifestent pas nécessairement en cas de FA. Le questionnaire NoSAS (de l’anglais Neck, Obesity, Snoring, Age, Sex) est un nouvel outil d’évaluation de l’AOS qui ne tient pas compte de ces paramètres. La pharyngométrie acoustique (PA) pourrait aussi constituer une nouvelle technique de dépistage; elle mesure l’aire de section transversale du pharynx, qui est réduite chez les patients souffrant d’AOS.
Méthodologie
Nous avons comparé de façon prospective la précision du score au questionnaire NoSAS, du score au questionnaire STOP-BANG (de l’anglais Snoring, Tiredness, Observed apnea, blood Pressure, Body mass index, Age, Neck circumference and Gender) et des résultats de la PA à celle du test d’apnée du sommeil à domicile (TASD) chez des patients consécutifs présentant une FA non valvulaire.
Résultats
Sur les 188 participants, 86 % présentaient une AOS et 49 % souffraient d’AOS modérée ou sévère. Le score moyen sur l’échelle de somnolence d’Epworth était faible et se situait à 5,9 (écart-type : 3,9), ce qui indique que la plupart des participants ne ressentaient pas de somnolence. La comparaison entre les questionnaires de dépistage et le TASD effectuée au moyen des courbes caractéristiques de la performance des tests a révélé une précision sous-optimale. Dans les groupes souffrant d’AOS modérée ou sévère et d’AOS sévère seulement, les aires sous la courbe étaient respectivement de 0,50 (intervalle de confiance [IC] à 95 % : de 0,42 à 0,58) et de 0,42 (IC à 95 % : de 0,34 à 0,52) pour la PA, de 0,65 (IC à 95 % : de 0,58 à 0,73) et de 0,63 (IC à 95 % : de 0,52 à 0,74) pour le questionnaire STOP-BANG, et de 0,68 (IC à 95 % : de 0,60 à 0,75) et de 0,69 (IC à 95 % : de 0,59 à 0,80) pour le questionnaire NoSAS.
Conclusions
La PA et le questionnaire NoSAS ne sont pas suffisamment précis pour dépister l’AOS chez les patients présentant une FA. Compte tenu de la forte prévalence de l’AOS dans cette cohorte, des bienfaits potentiels d’un traitement de l’AOS et de la précision sous-optimale des questionnaires de dépistage actuels, il conviendrait d’envisager un TASD chez les patients présentant une FA.
Atrial fibrillation (AF) is a recognized marker for obstructive sleep apnea (OSA), present in 21%-85% of AF patients1,2 but markedly underdiagnosed.3 In AF patients with OSA, multiple cohort studies and meta-analyses have shown that treatment with continuous positive airway pressure (CPAP), which maintains oropharyngeal patency,4,5 can help reduce AF recurrence, maintain sinus rhythm, and prevent the progression to more permanent forms of the arrhythmia.6, 7, 8, 9 CPAP treatment of OSA has also been shown to improve sleepiness, cognitive function, depression,5 quality of life,10 drowsy driving,11 and hypertension.12 Thus, earlier identification of OSA would be of value.
The current gold standard for OSA diagnosis is overnight polysomnography (PSG), conducted in a sleep laboratory; however, it can be costly, time-consuming, and relatively inaccessible, making it poorly suited for the screening of large numbers of patients.13 Home sleep apnea testing (HSAT), with a level III study (eg, Apnea Link [ResMed, San Diego, CA]), with a limited number of leads but no sleep recording, is often used instead of PSG because of its good diagnostic performance and availability.13 Apnea Link (ResMed) can detect central apneas and hypopneas, although confirmation with PSG is often required.14
In a population at risk for OSA, such as at an AF clinic, the identification of a reliable, user-friendly, and inexpensive clinic-based screening test has the potential to address the gap in OSA diagnosis. Such a test would not remove the need for formal OSA diagnostic testing (eg, with an HSAT) but could screen out those with no or mild OSA who need no treatment and identify those at risk for moderate and severe disease, who might benefit from CPAP. A number of screening tests for OSA exist13 but these might not be appropriate for all phenotypes of OSA. For example, the widely used STOP-BANG questionnaire (SBQ)—which is used to evaluate (Snoring, Tiredness, Observed apnea, blood Pressure, Body mass index, Age, Neck circumference and Gender)1 (Supplemental Table S1) might be of less value in patients who do not report sleepiness, are not obese, and have thin necks.15 In addition, although it has shown high sensitivity, it is challenged by its low specificity and high false-positive rate.15
A novel potential screening test is acoustic pharyngometry (AP), which uses acoustic waves to measure the cross-sectional area of the pharynx, which is usually reduced in patients with OSA.16 DeYoung et al.16 reported that measurement of minimum oropharyngeal cross-sectional area using AP, helped differentiate mild from moderate and severe OSA whereas Kamal17 reported a strong correlation between apnea index and pharyngeal area, measured using AP.
The NoSAS (Neck, Obesity, Snoring, Age, Sex) score is a recently validated questionnaire, which consists of 5 questions related to the patient’s Neck circumference, Obesity (BMI), Snoring status, Age, and Sex) (Supplemental Table S2).18,19 In contrast to the SBQ, the NoSAS score does not require differentiation between snoring and loud snoring and does not include sleepiness as a risk factor, which could be useful when screening a cardiac population, who are often asymptomatic.20,21
In this study we aimed to evaluate the accuracy of AP, the NoSAS score, and the SBQ as screening tests for OSA compared with HSAT. In addition, the value of other clinical parameters in combination with these screening methods in detecting OSA were also assessed.
Methods
Study population
Consecutive outpatients with paroxysmal, persistent, or permanent nonvalvular AF from a community cardiology clinic in Richmond, British Columbia were prospectively screened for OSA. The diagnosis of AF was made using electrocardiogram either at the time of evaluation or from documentation of previous episodes of AF on electrocardiogram.22 Paroxysmal AF was defined as AF that terminates spontaneously or with intervention within 7 days of onset.22 Persistent AF was defined as AF that is sustained ≥ 7 days.22 The term, “permanent AF” was used when the patient and clinician made a joint decision to stop further attempts to restore and or maintain sinus rhythm.22 Nonvalvular AF was defined as AF in the absence of rheumatic mitral stenosis, a mechanical or bioprosthetic heart valve, or mitral valve repair.22
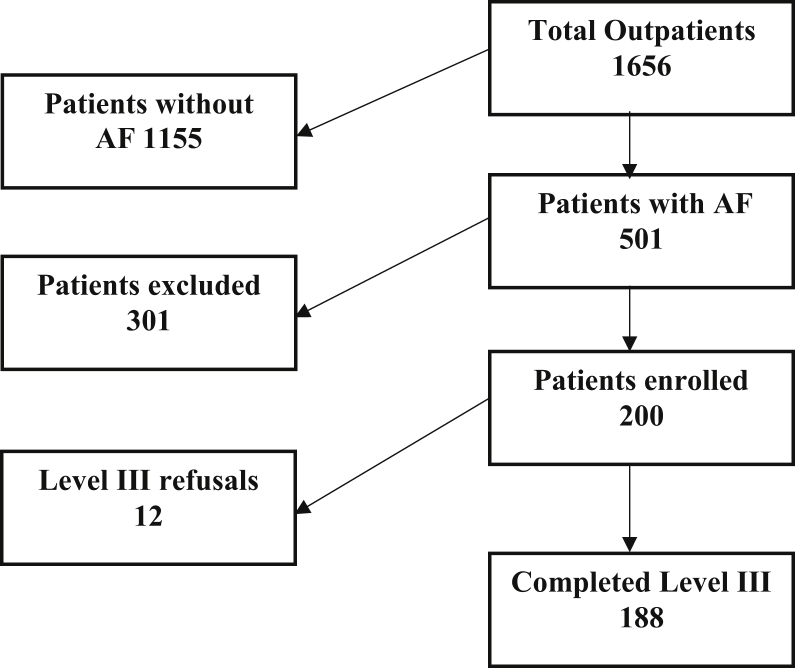
Inclusion and exclusion criteria were as listed in Table 1. Of the 301 patients excluded or who did not enter the study, 101 (33.5%) were 85 years of age or older, which was the most common reason for exclusion. Other common reasons for exclusion were left ventricular ejection fraction < 45% (20 patients), current treatment with CPAP (16 patients), and the presence of a mechanical valve (15 patients; Supplemental Table S3). Thus, 200 patients were enrolled in the study and 188 completed level III testing and were eligible for analysis (Fig. 1). Of those 188, 114 had paroxysmal AF, 55 had permanent AF, 12 had persistent AF, and 7 were undetermined. The study was approved by the Clinical Research and Ethics Board of the University of British Columbia, Canada.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| Paroxysmal, persistent or permanent, nonvalvular AF |
| Exclusion criteria |
| 85 years or older |
| Active antineoplastic therapy or terminal cancer |
| Alcoholic cardiomyopathy |
| Chronic dystonia |
| Congenital heart disease |
| Currently being treated with CPAP or BiPAP |
| Dementia/severe memory deficit |
| End-stage renal disease receiving dialysis |
| Facial abnormality that precludes AP testing |
| Hypertrophic cardiomyopathy |
| Implantable cardioverter defibrillator |
| Mechanical heart valve |
| Recently post surgery for aortic dissection |
| Severe anemia and shortness of breath |
| CHF (LVEF < 45%) |
| Severe interstitial/restrictive lung disease |
| Severe mobility limitations |
| Severe psychosis |
| Severe valvular heart disease |
| Uncontrolled hyperthyroidism |
AF, atrial fibrillation; AP, acoustic pharyngometry; BiPAP, bilevel positive airway pressure; CHF, congestive heart failure; CPAP, continuous positive airway pressure; LVEF, left ventricular ejection fraction.
Figure 1.
Patient participation in the study. AF, atrial fibrillation.
Study design
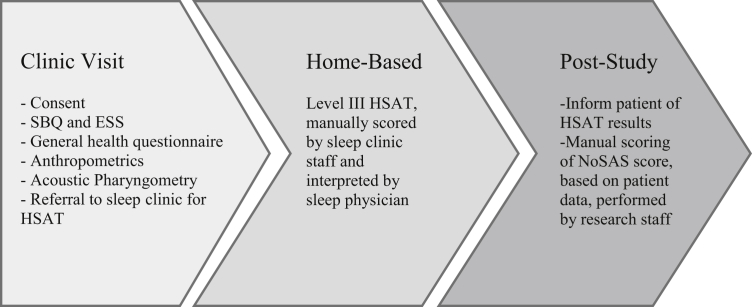
The study consisted of 3 phases: clinic visit, home-based level III testing, and post study assessment (Fig. 2). During the clinic visit, participants met with research assistants (RAs) who obtained informed consent; measured height, weight, and NC; calculated BMI; and administered the SBQ, Epworth Sleepiness Scale (ESS), a general health questionnaire (see Supplemental Appendix S1), and performed AP. After the clinic visit, patients were referred for HSAT using a level III device.
Figure 2.
Study flow diagram. ESS, Epworth Sleepiness Scale; HSAT, home sleep apnea test; NoSAS, NoSAS (Neck, Obesity, Snoring, Age, Sex) questionnaire; SBQ, STOP-BANG (Snoring, Tiredness, Observed apnea, blood Pressure, Body mass index, Age, Neck circumference and Gender) questionnaire.
AP
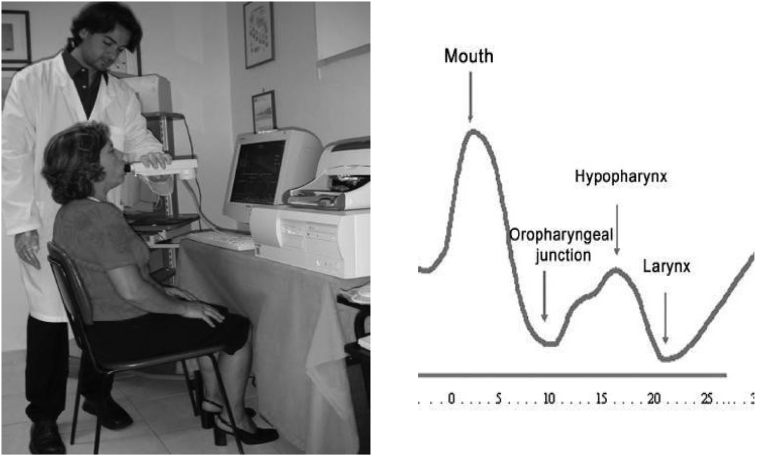
The acoustic pharyngometer (Eccovision Acoustic Pharyngometer, Sleep Group Solutions, Miami, FL) is a hand-held device that projects acoustic waves down a participant’s mouth and throat (Fig. 3). The reflected waves create a curve of distance from the mouthpiece (x-axis) against a cross-sectional area (y-axis) from which the positions of various oropharyngeal structures can be identified, including the measurement of minimal cross-sectional area (MCA; Fig. 3). Measurements were made in the erect position at functional residual capacity according to a standard technique described by Kamal17 and DeYoung et al.16 The tests were repeated 3 times and then averaged to provide a measure of MCA, with < 7% variation in MCA allowed between tests (see Supplemental Appendix S2). The RAs were trained on how to use the acoustic pharyngometer by a registered respiratory therapist (S.S.) who had experience in evaluating normal subjects and patients with OSA. The AP technique and measurements by the RAs were supervised by S.S. initially and then subsequently using intermittent spot checks. Telephone discussions were held with Dr Pamela DeYoung, who performed the MCA measurements in the latter study, to verify the AP technique. Several AP curves were sent to her to confirm the accuracy of MCA measurements.
Figure 3.
(Left) A demonstration of the acoustic pharyngometry technique. (Right) Graph generated from acoustic pharyngometry. The reflected acoustic waves create a curve of distance from the mouthpiece (x-axis) against cross-sectional area (y-axis) from which the positions of various oropharyngeal structures can be identified. Reproduced from Gelardi et al.23 with permission from lead author and under the Creative Commons Attribution 4.0 International Public License.
NoSAS score
The NoSAS score (Supplemental Table S2) was calculated by the RAs on the basis of data collected during the clinic visit for the SBQ.
HSAT
For the home-based portion of the study, participants were referred for HSAT using a level III device, Apnea Link (ResMed). Subjects received education on device set-up and procedure from registered respiratory therapists (R.J. or P.S.), who were also available via telephone for trouble-shooting during the night if required. Apnea Link (ResMed) is a battery-operated device consisting of 5 channels that measure snoring, oxygen saturation, pulse rate, and air flow (using a nasal cannula connected to a pressure transducer), and respiratory effort (using a respiratory effort belt around the patient’s chest).14
From PSG, the diagnosis of OSA is made on the basis of the number of apneas plus hypopneas measured per hour, with the apnea-hypopnea index (AHI) ≥ 5 per hour being diagnostic of OSA.24 An apnea is defined as a reduction in air flow by ≥ 90% for ≥ 10 seconds.24 A hypopnea is a reduction in air flow by ≥ 30% for ≥ 10 seconds associated with a decrease in oxygen saturation of ≥ 3% or an arousal.24 The definition from a level III study is similar except that for hypopnea, arousal cannot be detected. In our study we did not use arousal in the scoring definition for hypopnea.
Obstructive events (apnea and hypopnea) are characterized by paradoxical chest wall movements to overcome the obstruction; in contrast, central events have no such movements.7 Moderate and severe OSA are defined as 15≤AHI < 30 per hour (the value of the AHI must be greater than or equal to 15, and less than 30) and AHI ≥ 30 per hour, respectively. The scoring of HSAT was performed manually by 2 registered respiratory therapists (R.J. or P.S.) and interpreted by a sleep disorder physician (I.H.A.). Clinic staff were blinded to all other testing. When results were available, participants were informed of their sleep apnea status, and all patients with AHI ≥ 15 per hour were referred to a sleep disorder physician. The sleep disorder physician provided a comprehensive sleep evaluation, reviewed the raw data, and recommended nasal CPAP for all patients with AHI ≥ 15 per hour (93 patients in total).
Statistical methods
Descriptive statistics (mean, SD, count, and percent) were used to report on the screened cardiac population. The accuracy of each of the screening techniques—SBQ, NoSAS, ESS, and MCA (from AP)—to identify OSA, compared with level III testing, was evaluated by plotting a receiver operating curve (ROC) and determining the area under the curve (AUC) for each technique. An ROC curve is obtained by plotting the sensitivity, which is the rate of true positive results, against the rate of false positive results, and illustrates each test’s ability to correctly classify patients with OSA at various threshold values.
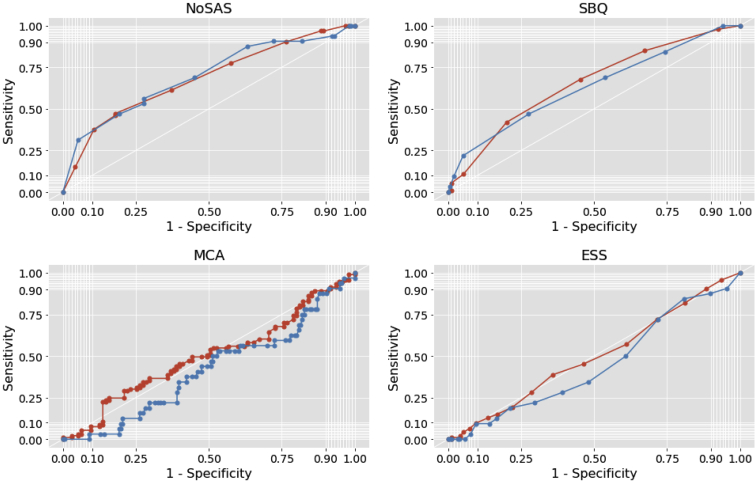
ROC curves were constructed for each of the NoSAS score, SBQ score, MCA (from AP), and ESS compared with AHI from HSAT to assess how well these screening tests could be used to detect OSA (Fig. 4).
Figure 4.
Receiver operating characteristic curves for NoSAS Questionnaire (Neck, Obesity, Snoring, Age, Sex), STOP-BANG (Snoring, Tiredness, Observed apnea, blood Pressure, Body mass index, Age, Neck circumference and Gender) questionnaire (SBQ), minimal circumferential area of the oropharynx (MCA), and Epworth Sleepiness Scale (ESS) compared with home sleep apnea test. Red indicates moderate and severe obstructive sleep apnea (apnea-hypopnea index ≥ 15); blue indicates severe obstructive sleep apnea (apnea-hypopnea index ≥ 30). For area under the curve values, see Table 3.
Additionally, an exhaustive search among all combinations and interactions of the desired tests (ie, SBQ, NoSAS, MCA) was performed to determine the best regression model, including only statistically significant predictors of AHI. For each model, variable selection was performed using forward and backward selection, which compares possible subsets of model coefficients using Aikake information criterion values. Aikake information criterion values provide a measure of each model’s performance relative to another. The following sets of variables were explored: (1) MCA, SBQ, NC, BMI, ESS, and NoSAS scores (to compare tests); (2) MCA, NC, BMI, SBQ, ESS, NoSAS, smoking status, the presence of diabetes, asthma, hypertension, and the need for cardioversion and/or ablation (to see if any other test would perform well enough to warrant inclusion in the model); and (3) NoSAS, smoking status, the presence of diabetes, asthma, hypertension, and the need for cardioversion and/or ablation (to see if any additional patient health information would warrant inclusion because we have a NoSAS score already). All of these regressions were used to finally choose a model that included only NoSAS and eliminated all other variables.
All statistical analysis was performed using the programming language “Python” (version 3.6). The package “matplotlib”25 was used to plot ROCs, whereas the package “scikit-learn”26 was used to compute AUCs and logistic regression coefficients.
Results
Study population
Characteristics of the study population, including BMI and ESS, are shown in Table 2. Using the cutoff AHI ≥ 5, 86% of the cohort had OSA. Rates for mild, moderate, and severe OSA were 36.7% (AHI 5.1-14.9), 32.4% (AHI 15-29.9), and 17% (AHI ≥ 30), respectively. Average ESS score for study participants was 5.9 (SD, 3.9), which is within the normal range. ESS scores ranged from 0 to 19 and only 17 subjects (9%) described excessive sleepiness (ESS > 11).
Table 2.
Patient characteristics
| Characteristic | All participants (N = 188; 100%) | OSA severity (AHI) |
|||
|---|---|---|---|---|---|
| None (< 4.9; n = 26; 18.8%) | Mild (5.0-14.9; n = 69; 36.7%) | Moderate (15.0-29.9; n = 61; 32.4%) | Severe (≥ 30; n = 32; 17.0%) | ||
| General characteristics | |||||
| Male sex | 115 (61.2) | 10 (38.5) | 38 (55.1) | 41 (67.2) | 26 (81.3) |
| Age, years | 69.0 ± 9.5 | 67.2 ± 12.2 | 69.6 ± 8.8 | 69.1 ± 9.1 | 68.9 ± 9.5 |
| OSA screening-related parameters | |||||
| ESS | 5.9 ± 3.9 | 5.2 ± 4.1 | 6.2 ± 4.0 | 6.1 ± 3.8 | 5.2 ± 3.4 |
| Neck circumference, cm | 38.5 ± 4.1 | 35.2 ± 3.7 | 38.1 ± 3.7 | 39.3 ± 3.7 | 40.7 ± 4.4 |
| MCA, cm2 | 2.4 ± 0.9 | 2.4 ± 0.7 | 2.4 ± 0.9 | 2.5 ± 1.1 | 2.2 ± 0.6 |
| NoSAS | 10.4 ± 3.9 | 8.1 ± 3.4 | 9.6 ± 3.7 | 11.2 ± 3.6 | 12.6 ± 4.1 |
| SBQ | 3.6 ± 1.5 | 2.9 ± 1.4 | 3.4 ± 1.4 | 3.9 ± 1.3 | 4.3 ± 1.6 |
| Snoring | 126 (67.0) | 12 (46.2) | 51 (73.9) | 39 (63.9) | 24 (75.0) |
| Cardiac risk factors | |||||
| Current or ex-smoker | 92 (48.9) | 10 (38.5) | 28 (40.6) | 34 (55.7) | 20 (62.5) |
| Stroke | 14 (7.4) | 3 (11.5) | 5 (7.2) | 5 (8.2) | 1 (3.1) |
| Diabetes | 25 (13.3) | 3 (11.5) | 8 (11.6) | 8 (13.1) | 6 (18.8) |
| Hypertension | 117 (62.2) | 15 (57.7) | 38 (55.1) | 42 (68.9) | 21 (65.6) |
| BMI | 28.8 ± 6.4 | 27.6 ± 6.9 | 27.3 ± 5.0 | 29.2 ± 5.2 | 32.1 ± 9.3 |
| Medications | |||||
| Oral anticoagulant | 141 (75.0) | 16 (61.5) | 53 (76.8) | 52 (85.2) | 20 (62.5) |
| Antiarrhythmic | 71 (37.8) | 11 (42.3) | 31 (44.9) | 20 (32.8) | 9 (28.1) |
| ACE inhibitor | 53 (28.2) | 5 (19.2) | 21 (30.4) | 18 (29.5) | 9 (28.1) |
| β-Blocker | 89 (47.3) | 8 (30.8) | 31 (44.9) | 28 (45.9) | 22 (68.8) |
| Cardiac procedures | |||||
| Ablation | 25 (13.3) | 5 (19.2) | 8 (11.6) | 8 (13.1) | 4 (12.5) |
| Cardioversion | 54 (28.7) | 8 (30.8) | 16 (23.2) | 21 (34.4) | 9 (28.1) |
| Coronary bypass | 4 (2.1) | 0 (0.0) | 2 (2.9) | 1 (1.6) | 1 (3.1) |
| Stent insertion | 11 (5.9) | 0 (0.0) | 2 (2.9) | 5 (8.2) | 4 (12.5) |
Values are presented as n (%) or mean ± SD.
ACE, angiotensin-converting enzyme; AHI, apnea-hypopnea index; BMI, body mass index; ESS, Epworth Sleepiness Scale; MCA, minimal circumferential area of the oropharynx; NoSAS, NoSAS score; OSA, obstructive sleep apnea; SBQ, STOP-BANG questionnaire, Snoring, Tiredness, Observed apnea, blood Pressure, Body mass index, Age, Neck circumference, and Gender.
Utility of questionnaires and AP to predict AHI
NoSAS performed best with an AUC of 0.69 (95% confidence interval [CI], 0.58-0.80) for classifying severe OSA patients (AHI ≥ 30), and 0.68 (95% CI, 0.60-0.75) for classifying moderate or severe OSA (AHI ≥ 15; Fig. 4; Table 3). The SBQ performed second best for classifying severe OSA patients, with an AUC of 0.63 (95% CI, 0.52-0.74; Table 3). Also, the final regression model included only the NoSAS score. For moderate or severe OSA (AHI ≥ 15), at a threshold of 6 points or higher, NoSAS had a sensitivity of 97% (95% CI, 93%-100%), specificity of 28% (95% CI, 6%-19%), positive predictive value of 52% (95% CI, 45%-60%), and negative predictive value of 79% (95% CI, 55%-100%). For severe OSA (AHI ≥ 30), at a threshold of 8 points or higher, NoSAS had a sensitivity of 91% (95% CI, 79%-100%), specificity of 28% (95% CI, 21%-35%), positive predictive value of 21% (95% CI, 14%-27%), and negative predictive value of 93% (95% CI, 85%-100%). For results at other NoSAS score thresholds, see Supplemental Table S4.
Table 3.
AUC (95% CI) for screening tests according to OSA severity
| Moderate and severe OSA (AHI ≥ 15) | Severe OSA (AHI ≥ 30) | |
|---|---|---|
| MCA | 0.50 (0.42-0.58) | 0.42 (0.34-0.52) |
| SBQ | 0.65 (0.58-0.73) | 0.63 (0.52-0.74) |
| NC | 0.68 (0.60-0.75) | 0.67 (0.57-0.79) |
| BMI | 0.65 (0.57-0.73) | 0.65 (0.54-0.76) |
| ESS | 0.50 (0.41-0.58) | 0.45 (0.34-0.55) |
| NoSAS | 0.68 (0.60-0.75) | 0.69 (0.59-0.80) |
| Regression models | Moderate and severe OSA | |
| NC, BMI, SBQ∗ | 0.70 (0.63-0.78) | |
| NoSAS, SBQ† | 0.70 (0.62-0.77) |
AHI, apnea-hypopnea index; AUC, area under the curve; BMI, body mass index; CI, confidence interval; ESS, Epworth Sleepiness Scale; MCA, minimal circumferential area of the oropharynx; NC, neck circumference; NoSAS, NoSAS (Neck, Obesity, Snoring, Age, Sex) questionnaire; OSA, obstructive sleep apnea; SBQ, STOP-BANG (Snoring, Tiredness, Observed apnea, blood Pressure, Body mass index, Age, Neck circumference and Gender) questionnaire.
(0.09 × NC) + (0.03 × BMI) + (0.24 × SBQ score).
(0.15 × NoSAS score) + (0.21 × SBQ score).
Discussion
This study shows that OSA is widely prevalent in a community cardiology population with AF and markedly underdiagnosed, consistent with other studies.1,2,6 In our study population, 49% had moderate or severe OSA (AHI ≥ 15) and 86% had OSA (AHI ≥ 5), which is similar to findings reported by Abumuamar et al. (54% of subjects had moderate or severe OSA [AHI ≥ 15] and 85% had OSA [AHI ≥ 5]).2 Of the 501 patients with AF, a mere 16 (3.7%) had already been diagnosed with OSA and were using CPAP. This is similar to the results of Costa et al.,3 who reported only 13 (3.1%) patients previously diagnosed with OSA in a group of 500 patients drawn from cardiac clinics in a tertiary institution. A possible explanation for the lack of previous OSA diagnoses in our study population is that they did not fit the usual clinical stereotype for OSA—OSA patients were not sleepy (mean ESS, 5.9), not obese (mean BMI, 28.8), without large necks (mean NC, 38.5 cm), and approximately one-third did not snore.
In this study, 51% of AF subjects had no or mild OSA on the basis of the level III study and were unlikely to require treatment.4 This supports the notion that a screening test is required to separate those who have moderate or severe OSA—and would need treatment on the basis of American Academy of Sleep Medicine guidelines—from those who have no or mild OSA. Notably, sleepiness, BMI, NC, and the presence of comorbidities did not separate these groups.
To our knowledge, this is the first study to examine the value of AP and NoSAS in screening for OSA in the AF population. ROC results and logistic regression support NoSAS as the best single screening test, although it still performed poorly compared with the HSAT. Further studies are required to see if NoSAS when combined with other parameters not measured in this study could become a better OSA screening test for AF. Although we concluded that questionnaires are not adequate for OSA screening in patients with AF, Farrehi et al. reported that high-risk SBQ scores (≥ 3) predicted recurrent AF in 247 AF patients post radiofrequency ablation, with an odds ratio of 3.7 (95% CI, 1.4-11.4).27
AP was not useful as a screening method for OSA in patients with AF, with an AUC of 0.50 and 0.42 for moderate and severe (AHI ≥ 15) and severe OSA (AHI ≥ 30), respectively. Our results resemble those of Kendzerska et al.,28 who compared AP with PSG in 576 patients suspected of OSA and reported only a fair accuracy with AHI, with an AUC of 0.6. The poor AP results might be related to variability in patient positioning and technique and the presence of other unmeasured factors (eg, ethnic differences in cephalometry, oropharyngeal collapsibility, and neuromuscular differences). However, changing body position or the level of inspiration when measuring upper airway cross-sectional area did not improve correlation with AHI in the study by Kendzerka et al.28 When Kim et al. added AP to their model of age, BMI, and sex for the diagnosis of OSA, they obtained an AUC of 0.74.29 However, they did not specifically assess patients with AF, and their population was younger (mean age, 42.8 years; SD, 10.6 years) with a lower BMI (26.5 ± 3.5).29
Other than the screening tests used in this study, another screening method is level IV testing with overnight oximetry at home. Linz and coworkers30 used a novel automated algorithm tracking oxygen desaturation and resaturation and reported it had high specificity, sensitivity, and negative predictive value for detecting moderate to severe or severe sleep-disordered breathing. Further research is required to confirm these results and to see if the algorithm used in this study can be included in commercially available home overnight oximetry monitors.
Because of the high OSA prevalence in the AF population, the absence of typical signs and symptoms of OSA, and the inadequacy of screening questionnaires (SBQ, NoSAS), objective evaluation with an HSAT should be considered for patients with AF, as supported by the review by Desteghe et al.6 We also agree with these author’s suggestion of an integrated cardiology clinic-based model linked to a sleep centre or clinic, with a target population of symptomatic AF patients who require drug therapy, direct cardioversion, or pulmonary vein isolation. Management strategies would also include lifestyle modifications, which are essential for the comprehensive management of AF.31
Limitations of our work include the recruitment of patients from a single centre and the use of level III testing rather than PSG for the diagnosis of OSA. According to a 2017 systematic review, for moderate and severe OSA (AHI ≥ 15 per hour) the diagnostic sensitivity of HSAT was 62%-94% and specificity 25%-97%, compared with PSG.13 A small validation trial using Apnea Link (ResMed), the same device as used in this study, showed a 93% sensitivity (95% CI, 80.9%-99.8%) and a 91.7% specificity (95% CI, 61.5%-99.8%).32 In addition, we used a level III device to make a definitive diagnosis of OSA on the basis of 1 night’s evaluation, although it is known that OSA severity can vary from night to night.33 It is possible that some of the patients diagnosed with OSA had a component of central sleep apnea, but we did not explore this further and all patients with AHI ≥ 15 per hour were referred to a sleep disorder physician and offered CPAP.
Large randomized trials are needed to confirm that OSA treatment with CPAP can improve AF outcomes as has been suggested in reports of cohort studies and meta-analyses.6, 7, 8, 9 Future studies are required to evaluate NoSAS for OSA screening in larger AF populations, and to identify ways to separate moderate to severe from the mild OSA group in patients with AF. Further studies should also evaluate nonsleepy AF OSA patients for other symptoms (eg, cognitive impairment) that might improve with CPAP therapy and evaluate AF outcomes in a lifestyle intervention trial that addresses OSA alongside other modifiable risk factors such as hypertension, obesity, and alcohol abuse.
In conclusion, in light of the findings of this study, cardiologists should be aware of the significant potential for moderate or severe OSA in their AF population, even in the absence of symptoms traditionally associated with the condition. HSAT would be an appropriate next step for screening, because of the low efficacy of in-office screening tests.
Acknowledgements
The authors thank Dr DeYoung for help with identifying MCA in the AP curves and all at Richmond Cardiology and the Richmond Hospital Sleep Lab.
Funding Sources
This work was self-funded.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The study was approved by the Clinical Research and Ethics board of the University of British Columbia, Canada. Informed consent was obtained from all participants included in the analyses.
See page 448 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.09.026.
Supplementary Material
References
- 1.Traaen G.M., Øverland B., Aakerøy L. Prevalence, risk factors, and type of sleep apnea in patients with paroxysmal atrial fibrillation. Int J Cardiol. 2020;26:100463. doi: 10.1016/j.ijcha.2019.100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abumuamar A.M., Dorian P., Newman D., Shapiro C.M. The prevalence of obstructive sleep apnea in patients with atrial fibrillation. Clin Cardiol. 2018;41:601–607. doi: 10.1002/clc.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa L.E., Uchôa C.H.G., Harmon R.R. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart. 2015;101:1288–1292. doi: 10.1136/heartjnl-2014-307276. [DOI] [PubMed] [Google Scholar]
- 4.Epstein L.J., Kristo D., Strollo P.J. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 5.Giles T.L., Lasserson T.J., Smith B. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3:CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Desteghe L., Hendriks J.M.L., McEvoy R.D. The why, when and how to test for obstructive sleep apnea in patients with atrial fibrillation. Clin Res Cardiol. 2018;107:617–631. doi: 10.1007/s00392-018-1248-9. [DOI] [PubMed] [Google Scholar]
- 7.Maeder M.T., Schoch O.D., Rickli H. A clinical approach to obstructive sleep apnea as a risk factor for cardiovascular disease. Vasc Health Risk Manag. 2016;12:85–103. doi: 10.2147/VHRM.S74703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linz D., McEvoy R.D., Cowie M.R. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment. JAMA Cardiol. 2018;3:532–540. doi: 10.1001/jamacardio.2018.0095. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y., Ning Y., Wen W. CPAP is associated with decreased risk of AF recurrence in patients with OSA, especially those younger and slimmer: a meta-analysis. J Interv Card Electrophysiol. 2020;58:369–379. doi: 10.1007/s10840-020-00738-6. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn E., Schwarz E.I., Bratton D.J., Rossi V.A., Kohler M. Effects of CPAP and mandibular advancement devices on health-related quality of life in OSA: a systematic review and meta-analysis. Chest. 2017;151:786–794. doi: 10.1016/j.chest.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Walia H.K., Thompson N.R., Pascoe M. Effect of positive airway pressure therapy on drowsy driving in a large clinic-based obstructive sleep apnea cohort. J Clin Sleep Med. 2019;15:1613–1620. doi: 10.5664/jcsm.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkataraman S., Vungarala S., Covassin N., Somers V.K. Sleep apnea, hypertension and the sympathetic nervous system in the adult population. J Clin Med. 2020;9:591. doi: 10.3390/jcm9020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapur V.K., Auckley D.H., Chowdhuri S. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collop N.A., Anderson W.M.D., Boehlecke B. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–747. [PMC free article] [PubMed] [Google Scholar]
- 15.Abumuamar A.M., Dorian P., Newman D., Shapiro C.M. The STOP-BANG questionnaire shows an insufficient specificity for detecting obstructive sleep apnea in patients with atrial fibrillation. J Sleep Res. 2018;27 doi: 10.1111/jsr.12702. [DOI] [PubMed] [Google Scholar]
- 16.DeYoung P.N., Bakker J.P., Sands S.A. Acoustic pharyngometry measurement of minimal cross-sectional airway area is a significant independent predictor of moderate-to-severe obstructive sleep apnea. J Clin Sleep Med. 2013;9:1161–1164. doi: 10.5664/jcsm.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamal I. Test-retest validity of acoustic pharyngometry measurements. Otolaryngol Head Neck Surg. 2004;130:223–228. doi: 10.1016/j.otohns.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Coutinho Costa J., Rebelo-Marques A., Machado J.N. Validation of NoSAS (Neck, Obesity, Snoring, Age, Sex) score as a screening tool for obstructive sleep apnea: analysis in a sleep clinic. Pulmonology. 2019;25:263–270. doi: 10.1016/j.pulmoe.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Marti-Soler H., Hirotsu C., Marques-Vidal P. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4:742–748. doi: 10.1016/S2213-2600(16)30075-3. [DOI] [PubMed] [Google Scholar]
- 20.Altmann D.R., Ullmer E., Rickli H. Clinical impact of screening for sleep related breathing disorders in atrial fibrillation. Int J Cardiol. 2012;154:256–258. doi: 10.1016/j.ijcard.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 21.Kadhim K., Middeldorp M.E., Elliott A.D. Self-reported daytime sleepiness and sleep-disordered breathing in patients with atrial fibrillation: SNOozE-AF. Can J Cardiol. 2019;35:1457–1464. doi: 10.1016/j.cjca.2019.07.627. [DOI] [PubMed] [Google Scholar]
- 22.January C.T., Wann L.S., Alpert J.S. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Gelardi M., Del Giudice A., Cariti F. Acoustic pharyngometry: clinical and instrumental correlations in sleep disorders. Braz J Otorhinolaryngol. 2007;73:257–265. doi: 10.1016/S1808-8694(15)31075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry R.B., Quan S.F., Abreu A.R. American Academy of Sleep Medicine; Darien, IL: 2020. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.6. [Google Scholar]
- 25.Hunter J.D. Matplotlib: a 2D graphics environment. Comput Sci Eng. 2007;9:90–95. [Google Scholar]
- 26.Pedregosa F., Varoquaux G., Gramfort A. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 27.Farrehi P.M., O’Brien L.M., Bas H.D. Occult obstructive sleep apnea and clinical outcomes of radiofrequency catheter ablation in patients with atrial fibrillation. J Interv Card Electrophysiol. 2015;43:279–286. doi: 10.1007/s10840-015-0014-4. [DOI] [PubMed] [Google Scholar]
- 28.Kendzerska T., Grewal M., Ryan C.M. Utility of acoustic pharyngometry for the diagnosis of obstructive sleep apnea. Ann Am Thorac Soc. 2016;13:2019–2026. doi: 10.1513/AnnalsATS.201601-056OC. [DOI] [PubMed] [Google Scholar]
- 29.Kim B.Y., Cho J.H., Kim D.H. Utility of acoustic pharyngometry for screening of obstructive sleep apnea. Auris Nasus Larynx. 2020;47:435–442. doi: 10.1016/j.anl.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Linz D., Kadhim K., Brooks A.G. Diagnostic accuracy of overnight oximetry for the diagnosis of sleep-disordered breathing in atrial fibrillation patients. Int J Cardiol. 2018;272:155–161. doi: 10.1016/j.ijcard.2018.07.124. [DOI] [PubMed] [Google Scholar]
- 31.Linz D., Kalman J.M., R Doug M., Sanders P. CPAP initiation in persistent atrial fibrillation: Have we overslept the alarm clock? Int J Cardiol. 2019;278:144–146. doi: 10.1016/j.ijcard.2018.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Nigro C.A., Dibur E., Malnis S., Grandval S., Nogueira F. Validation of ApneaLink Ox for the diagnosis of obstructive sleep apnea. Sleep Breath. 2013;17:259–266. doi: 10.1007/s11325-012-0684-4. [DOI] [PubMed] [Google Scholar]
- 33.Linz D., Brooks A.G., Elliott A.D. Nightly variation in sleep apnea severity as atrial fibrillation risk. J Am Coll Cardiol. 2018;72:2406–2407. doi: 10.1016/j.jacc.2018.08.2159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.