Abstract
Endomyocardial biopsy (EMB) is an invaluable and underused diagnostic tool for myocardial disease. The primary indications are surveillance of cardiac allograft rejection and the diagnosis of inflammatory and infiltrative cardiomyopathies. EMB is typically performed by sampling the right ventricular septum via the right internal jugular vein using fluoroscopic guidance. The diagnostic yield of EMB is improved by sampling both ventricles and with the use of guidance from imaging or electroanatomic mapping. The risk of major cardiac complications is operator dependent and < 1% in experienced centres. EMB is the gold standard and most common form of cardiac allograft rejection surveillance, whereas advanced cardiac imaging and donor-specific antibody quantification provide complementary information. Gene expression profiling is an alternative surveillance strategy to EMB for low-risk patients. EMB is recommended for myocarditis and can guide therapy for giant-cell myocarditis, necrotizing eosinophilic myocarditis, sarcoidosis, and immune checkpoint inhibitor myocarditis. There is growing interest in using EMB to guide therapy for viral myocarditis, although the uptake of this approach is limited to specialized centres. EMB has been replaced as a first-line test for infiltrative cardiomyopathy by nonbiopsy diagnostic techniques, but is still useful to clarify the diagnosis or disease subtype. The miniaturization of bioptomes and advances in laboratory techniques such as microarrays promises to improve the safety and yield of EMB. We review the contemporary use of EMB for cardiac allograft rejection, inflammatory cardiomyopathy, and infiltrative cardiomyopathy.
Résumé
La biopsie endomyocardique est un outil diagnostique sous-utilisé et très utile en cas de maladie myocardique. Ses principales indications sont la surveillance du rejet d’une allogreffe cardiaque et le diagnostic des cardiomyopathies restrictives (infiltrantes) et inflammatoires. La biopsie endomyocardique est généralement réalisée en prélevant un échantillon du septum ventriculaire droit par la veine jugulaire interne droite, sous guidage fluoroscopique. Le succès diagnostique de la biopsie endomyocardique est amélioré en prélevant un échantillon des deux ventricules et en utilisant le guidage par imagerie ou cartographie électroanatomique. Le risque de complications cardiaques majeures varie en fonction de la personne qui effectue l’intervention, et est de moins de 1 % dans les centres où cette intervention est courante. La biopsie endomyocardique est la norme de référence et la forme la plus courante de surveillance du rejet d’une allogreffe cardiaque, tandis que les techniques avancées d’imagerie cardiaque et la quantification des anticorps spécifiques au donneur fournissent de l’information complémentaire. Le profil d’expression génétique est une stratégie de surveillance qui peut être utilisée plutôt que la biopsie endomyocardique chez les patients à faible risque. La biopsie endomyocardique est recommandée pour la myocardite et peut guider le traitement dans les cas de myocardite à cellules géantes, de myocardite nécrosante éosinophilique, de sarcoïdose et de myocardite induite par les inhibiteurs du point de contrôle immunitaire. La biopsie endomyocardique est de plus en plus utilisée pour guider le traitement de la myocardite virale, même si l’adoption de cette approche n’est observée que dans les centres spécialisés. En première intention, la biopsie endomyocardique a été remplacée par des techniques diagnostiques n’ayant pas recours à la biopsie dans les cas de cardiomyopathie restrictive, mais demeure néanmoins utile pour préciser le diagnostic ou le sous-type de maladie. La miniaturisation des bioptomes et les progrès en matière de techniques de laboratoire, comme les microréseaux, devraient améliorer l’innocuité et l’efficacité de la biopsie endomyocardique. Nous avons passé en revue l’utilisation faite à l’heure actuelle de la biopsie endomyocardique en ce qui concerne le rejet de l’allogreffe cardiaque, la cardiomyopathie inflammatoire et la cardiomyopathie restrictive.
Endomyocardial biopsy (EMB) is an invasive procedure to sample myocardium, and is standard practice in the management of myocardial disease. The primary indications for EMB are the surveillance of cardiac allograft rejection and the diagnosis of inflammatory and infiltrative cardiomyopathies (Table 1).1,2 These indications were on the basis of the utility of EMB at the time of a joint guideline by the American Heart Association (AHA), American College of Cardiology, and European Society of Cardiology (ESC) established in 2007.2 Since then, the utility of EMB has improved because modern laboratory techniques such as immunohistochemistry and viral genome detection offer information complementary to histopathologic analysis. Conversely, alternative diagnostic modalities including advanced imaging can now establish a nonbiopsy diagnosis in many cases of inflammatory and infiltrative cardiomyopathies. We review the contemporary use of EMB for the diagnosis of allograft rejection, inflammatory cardiomyopathies, and infiltrative cardiomyopathies.
Table 1.
Common indications for endomyocardial biopsy
| Heart transplant recipient14,59 |
| Surveillance for allograft rejection |
| Suspected allograft rejection (eg, allograft dysfunction, ventricular arrhythmias) |
| Monitoring of rejection treatment |
| Cardiomyopathy2,30,35,36,60 |
| Acute heart failure with hemodynamic compromise |
| Subacute heart failure |
| Ventricular arrhythmias |
| Advanced conduction disease |
| Poor response to therapy |
| Suspected autoimmune or toxic myocarditis |
| Giant-cell myocarditis |
| Acute necrotizing eosinophilic myocarditis |
| Immune checkpoint inhibitor myocarditis |
| Anthracycline cardiomyopathy |
| Restrictive cardiomyopathy |
| Infiltrative cardiomyopathy |
| Miscellaneous2 |
| Cardiac tumours |
EMB Procedure
How to perform EMB
EMB is typically performed by sampling the right ventricular (RV) septum via the right internal jugular vein using fluoroscopic guidance, although alternative sample sites, vascular access, and procedural guidance may be used (Fig. 1, and see Videos 1-3
 ; view videos online). A sheath-dilator is inserted into the right internal jugular vein using the standard Seldinger technique using ultrasound guidance. If a rigid bioptome is used, it is inserted through the venous sheath into the right atrium and guided past the tricuspid valve toward the RV septum. If a flexible bioptome is used, a preformed long sheath is first inserted through the venous sheath and positioned past the tricuspid valve directed toward the septum with the aid of a guidewire or balloon-tipped catheter. The flexible bioptome is then advanced within the long sheath past the tricuspid valve, thereby avoiding the risk of trauma by the bioptome on the tricuspid valve apparatus. With fluoroscopy, the left anterior oblique view is used to ensure positioning along the ventricular septum. To reduce the risk of cardiac perforation, the bioptome forceps are opened immediately upon exiting the sheath and remain open until contact is made with the ventricular wall and the bioptome shaft buckles slightly. The bioptome forceps are closed and the operator waits for 2-3 cardiac cycles before withdrawing the bioptome. A slight release of traction is often sensed as the specimen separates from the ventricular wall. The bioptome is removed from the sheath and the sheath is flushed. The bioptome may be repositioned to sample different areas of myocardium. This procedure is repeated until the desired number of specimens are obtained, which is typically 3-5.
; view videos online). A sheath-dilator is inserted into the right internal jugular vein using the standard Seldinger technique using ultrasound guidance. If a rigid bioptome is used, it is inserted through the venous sheath into the right atrium and guided past the tricuspid valve toward the RV septum. If a flexible bioptome is used, a preformed long sheath is first inserted through the venous sheath and positioned past the tricuspid valve directed toward the septum with the aid of a guidewire or balloon-tipped catheter. The flexible bioptome is then advanced within the long sheath past the tricuspid valve, thereby avoiding the risk of trauma by the bioptome on the tricuspid valve apparatus. With fluoroscopy, the left anterior oblique view is used to ensure positioning along the ventricular septum. To reduce the risk of cardiac perforation, the bioptome forceps are opened immediately upon exiting the sheath and remain open until contact is made with the ventricular wall and the bioptome shaft buckles slightly. The bioptome forceps are closed and the operator waits for 2-3 cardiac cycles before withdrawing the bioptome. A slight release of traction is often sensed as the specimen separates from the ventricular wall. The bioptome is removed from the sheath and the sheath is flushed. The bioptome may be repositioned to sample different areas of myocardium. This procedure is repeated until the desired number of specimens are obtained, which is typically 3-5.
Figure 1.
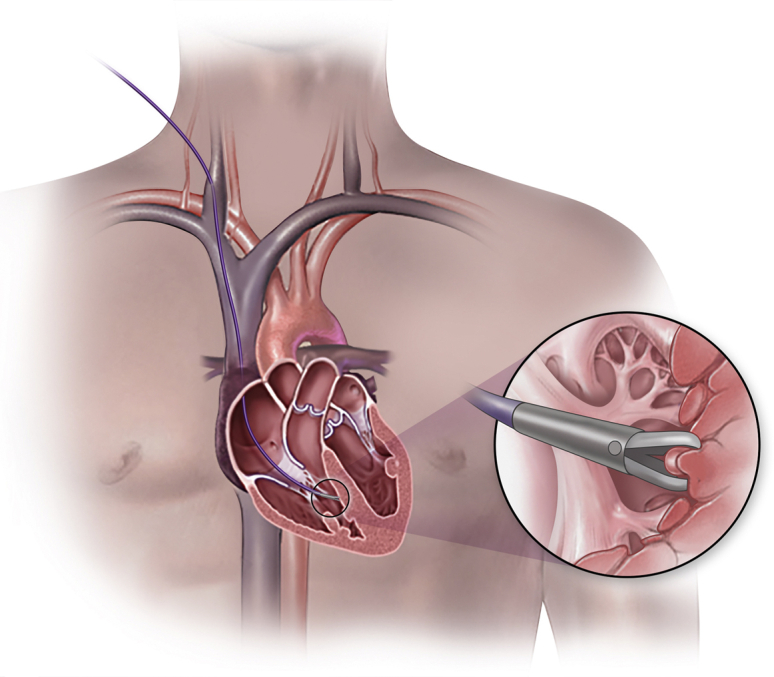
Endomyocardial biopsy is an invasive procedure to sample myocardium. Venous access is obtained, the bioptome is advanced to the right ventricular septum, and the bioptome forceps are used to separate a tissue sample from the myocardium.
We prefer fluoroscopy instead of echocardiography to guide EMB because it provides visualization of the course of the bioptome, contour of the shaft, and position of the forceps head. We combine echocardiography and fluoroscopy to guide the biopsy of cardiac tumours, because echocardiography provides better visualization of masses (see Video 4
 ; view video online). We prefer the use of a long sheath/catheter across the tricuspid valve to reduce the risk of bioptome-related damage to tricuspid valve apparatus. This also provides the opportunity to obtain invasive hemodynamics.
; view video online). We prefer the use of a long sheath/catheter across the tricuspid valve to reduce the risk of bioptome-related damage to tricuspid valve apparatus. This also provides the opportunity to obtain invasive hemodynamics.
How to improve diagnostic yield
The first major limitation of EMB is the low diagnostic yield, particularly for patchy or isolated disease.3 Sampling error can be reduced by a number of techniques, including biventricular biopsy, imaging, or electroanatomic guidance, and modern laboratory analysis.
The comparative yield of RV, left ventricular (LV), or biventricular biopsy was studied in a series of 755 patients who underwent diagnostic EMB. Diagnostic results were more common using biventricular EMB (79.3%) compared with isolated RV or LV biopsy (67.3%; P < 0.001).4 The major complication rate was similar for LV (0.64%) and RV (0.82%) biopsy.4 Among those with a diagnostic biventricular EMB, myocarditis was most often detected in both ventricles (73.4%) and less commonly isolated to the left (18.7%) or right (7.9%; P < 0.002) ventricle.4 In a separate series of 4221 patients who underwent imaging-guided diagnostic EMB, the diagnostic yield was closely tied to the disease etiology and presence of structural or functional abnormalities.5 When imaging abnormalities were limited to the left ventricle, the yield of LV biopsy was 97.8% compared with 53% for RV biopsy.5 Conversely, when abnormalities of the right ventricle were present, the diagnostic yield of LV biopsy was 98.1% compared with 96.5% for RV biopsy.5 There was a large discrepancy in diagnostic yield for myocarditis, which might involve focal or patchy disease. In contrast, histopathologic findings of infiltrative cardiomyopathy were always detected in both ventricles.5
Guided EMB using electroanatomic mapping, cardiac magnetic resonance imaging (CMR), or positron emission tomography have been used to sample areas of affected myocardium and improve diagnostic yield.6, 7, 8 However, these techniques are infrequently performed and not widely available.
Modern laboratory techniques, such as viral genome analysis and immunohistochemistry, improve the diagnostic yield of EMB compared with histopathologic analysis alone. In a study of 181 patients with clinically suspected viral myocarditis who underwent EMB, immunohistochemistry for inflammatory infiltrate was more sensitive (50%) than viral genome polymerase chain reaction (44%) or histopathology using Dallas criteria (38%).9 Immunohistochemistry, but not viral genome polymerase chain reaction or Dallas criteria, was predictive of the outcome of cardiac death or heart transplantation.9
The risk of complications
The second major limitation of EMB is the risk of complications, which can be classified as relating to vascular access vs the biopsy procedure. Complications from the biopsy procedure include arrhythmia, conduction abnormalities, valvular damage, embolism, cardiac perforation, and death. These complication rates are highly dependent on operator experience. High-volume specialized centres report major complication rates of < 1%,4,5 and as low as 0.0-0.3%.3 EMB for cardiomyopathies is associated with a higher rate of cardiac complications and arrhythmias/atrioventricular block compared with EMB for surveillance of cardiac allograft rejection, presumably because of the higher risk of cardiac perforation with a thinned and dilated right ventricle often seen in patients with cardiomypathies.1,10 Conversely, heart transplant recipients are more likely to develop tricuspid regurgitation (TR). Repeated EMB results in moderate to severe TR from biopsy-induced trauma in approximately one-third of transplant recipients at 4.5 years after transplantation, and is associated with significant morbidity.11, 12, 13
EMB for Select Conditions
Cardiac allograft rejection
EMB-based surveillance
Cardiac allograft rejection is a common and an important cause of morbidity and mortality after heart transplantation.14 Allograft rejection is caused by cellular or antibody-mediated (AMR) processes, and is most frequent in the first 6 months after transplantation.14 The symptoms of allograft rejection are nonspecific and consequences of untreated rejection are potentially severe. Therefore, heart transplant recipients undergo routine surveillance for allograft rejection.14 EMB is the gold standard and most common method of rejection surveillance (Fig. 2).14 EMB specimens are graded according to internationally standardized histologic criteria that serve as a therapeutic target and surrogate for outcomes, including cardiac allograft vasculopathy and graft loss.15
Figure 2.
Recommendations for initial surveillance strategy for cardiac allograft rejection.
Although EMB remains the gold standard, it is limited by patient discomfort, weak reliability, low cost-effectiveness, and a small but important risk of complications.14,16 Heart transplant recipients typically undergo at least 10 EMB procedures during the first year after transplantation, with a minimum of 3 samples per procedure.15 Repeated EMB sampling results in scarring of the right ventricle, which makes it increasingly difficult to obtain adequate specimens, as well as moderate to severe TR, as discussed previously. There is significant interobserver variability among pathologists, and the positive agreement for moderate or greater rejection is < 30%.16 The specificity of a positive result is approximately 80% compared with histologic analysis of the whole heart.17 For these reasons, alternatives to EMB have been developed for the purposes of allograft rejection surveillance.
Noninvasive surveillance
A number of noninvasive techniques for surveillance of cardiac allograft rejection have been studied. High-sensitivity troponin and B-type natriuretic peptide might increase with significant allograft rejection, but lack the accuracy required of a surveillance strategy.18, 19, 20 Advanced cardiac imaging with speckle-tracking echocardiography and CMR are more promising, but still lack standardization and validation in the first 6 months after transplantation.21, 22, 23 Accordingly, their use is limited to the evaluation of cardiac allograft function and injury.14
Donor-specific antibodies (DSAs) are quantified before transplantation and are routinely surveilled thereafter. The development of de novo DSAs is associated with negative outcomes after heart transplantation, including AMR, cardiac allograft vasculopathy, and allograft failure.24 DSA quantification is recommended as an adjunct to the primary rejection surveillance strategy, but not as a replacement.14
Gene expression profiling (GEP) of peripheral blood samples provides a quantitative assessment of mononuclear-cell derived RNAs that are upregulated during rejection. In low-risk patients who had received a cardiac transplant at least 6 months earlier, a strategy of monitoring for rejection with GEP was noninferior and resulted in sixfold fewer biopsies per person-year (0.5 vs 3.0; P < 0.001) compared with routine EMB surveillance.25 A subsequent study showed that low-risk patients could be safely surveilled with GEP as early as 2 months after cardiac transplantation.26 A commercial GEP assay is widely available and calibrated as a rule-out test with a negative predictive value of > 98%. EMB is still required to confirm rejection.27 Canadian guidelines support the use of GEP as an alternative initial surveillance strategy in low-risk patients beyond 6 months post transplantation (Fig. 2).14
Donor-derived cell-free DNA (dd-cfDNA) is actively investigated as an initial surveillance strategy alternative to EMB. dd-cfDNA is released from damaged donor heart cells and readily detectable in peripheral blood. Elevated dd-cfDNA levels correlate with the presence and severity of rejection.28 In contrast with GEP, dd-cfDNA is not affected by immunosuppression and therefore can been used as early as 2 weeks after heart transplantation for the detection of allograft rejection.28 In a multicentre study of 760 patients who were > 55 days post heart transplantation, the median dd-cfDNA was 0.07% in patients without rejection and 0.17% in patients with acute rejection. Using a threshold of 0.2%, dd-cfDNA had a positive predictive value of 8.9% and a negative predictive value of 97.1%.29 The combination of dd-cfDNA and GEP as a strategy for rejection surveillance is promising, but currently not recommended in Canadian guidelines.14
EMB remains the bedrock of allograft rejection surveillance among heart transplant recipients. Alternative strategies can reduce the frequency of EMB and aid in characterization of rejection type and severity, but do not replace the role of EMB.
Myocarditis
Myocarditis is an inflammatory cardiomyopathy caused by a wide range of infectious and noninfectious etiologies. Although EMB is required for a definitive diagnosis of myocarditis and is supported by guideline recommendations, it is infrequently used.2,30 In practice, CMR has largely replaced EMB for diagnostic, prognostic, and disease monitoring purposes.30 The emergence of CMR was in part because of the deficiencies of EMB, because it suffered from high sampling error (> 25%) and inter-reader variability, and yielded diagnostic information (according to Dallas criteria) in only 10%-20% of cases.31, 32, 33, 34
The primary purpose of EMB for myocarditis is to diagnose autoimmune or toxic myocarditis, such as giant-cell myocarditis (GCM), acute necrotizing eosinophilic myocarditis (NEM), and immune checkpoint inhibitor myocarditis. These conditions are associated with a poor prognosis and respond to immunosuppression. Joint guidelines by the AHA, American College of Cardiology, and ESC recommend EMB when there is a high probability of these diseases, including: (1) unexplained, new-onset heart failure of < 2-week duration with hemodynamic compromise; and (2) unexplained, new-onset subacute heart failure with a dilated left ventricle and new bradyarrhythmia, new ventricular arrythmias, or a failure to respond to therapy within 1-2 weeks of diagnosis (Fig. 3).2,35 These recommendations were later strengthened by the ESC to include EMB for all patients with clinical suspected myocarditis30 and by the AHA to consider EMB in heart failure that is rapidly progressing when there is a suspicion that the cause can be confirmed only using biopsy.36
Figure 3.
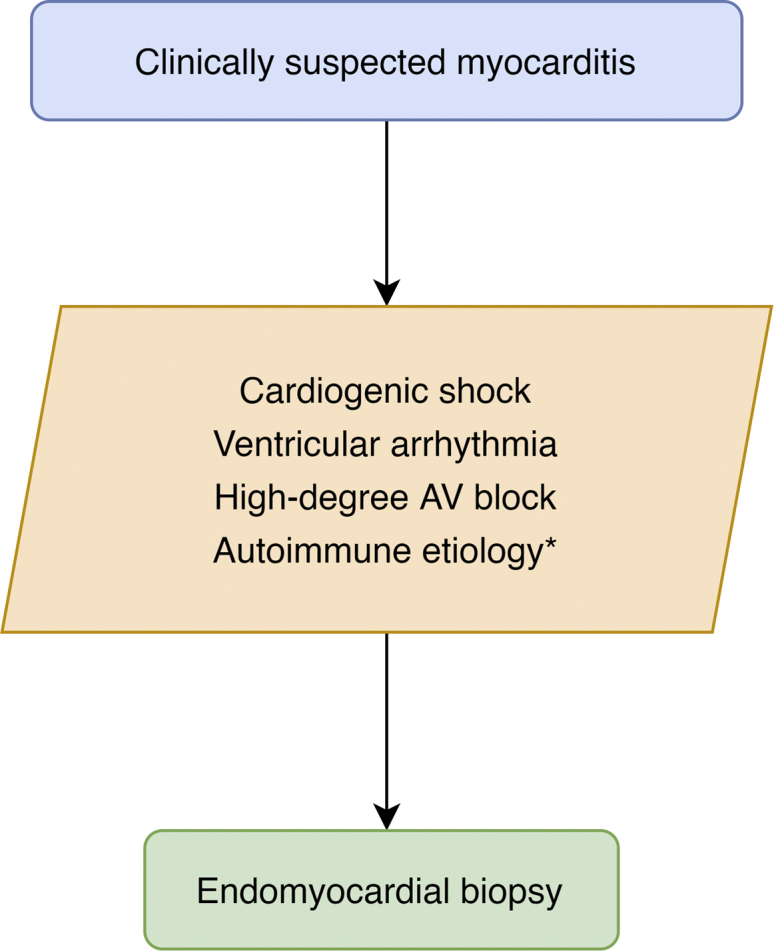
Recommendations for endomyocardial biopsy for unexplained acute cardiomyopathy include high-risk features that might suggest autoimmune myocarditis.36 AV, atrioventricular. ∗ Including suspected giant-cell myocarditis, acute necrotizing eosinophilic myocarditis, and, if it affects treatment and cardiac magnetic resonance imaging is uncertain, immune-checkpoint inhibitor myocarditis.
EMB is practical and logical in most of these cases. For example, a patient with unexplained cardiogenic shock requires emergency coronary angiography and right heart catheterization. EMB is readily performed at this time if angiography is negative and myocarditis suspected. Furthermore, CMR is not feasible for such a patient in cardiogenic shock. Despite strong recommendations in all major practice guidelines for the use of EMB for myocarditis, the use of EMB in clinical practice is declining.1 Limitations to the use of EMB include a low index of suspicion, high perceived risk, and lack of operator availability.
Myocarditis is classified by the inciting event as infectious, toxic, and autoimmune.35 The initial insult causes direct or indirect myocardial injury, which results in inflammation from the innate immune response. Myocarditis can either resolve or enter a phase of driven by the adaptive immune response that results in auto- or cross-reactivity to viral or self antigens. EMB can characterize the inflammatory response and identify the presence of a viral genome to determine the phase of disease. In theory, this allows for a nuanced understanding of myocarditis and the provision of tailored therapy.30,35,37 The uptake of this approach is limited to specialized centres, perhaps in part because large clinical trials of this approach are lacking.2,30,36,38, 39, 40
Noninfectious myocarditis
GCM is the prototypical disease for EMB-guided therapy. If untreated, transplantation-free survival is abysmal at 10% at 1 year.41 Conversely, when treated with combination immunosuppression the outcome is dramatically improved to 50% survival at 5 years, which is similar to that for lymphocytic myocarditis.42,43 EMB is required for a definitive diagnosis and, because of the diffuse inflammatory infiltrate caused by GCM, has a sensitivity of 80%.41 In some cases, serial biopsy is required to differentiate GCM from acute NEM and sarcoidosis, because there is overlap in histopathologic findings and characteristic giant cells take 1-2 weeks to develop. In addition to immunosuppression, GCM identified using EMB might prompt the rapid initiation of biventricular mechanical circulatory support and consideration for transplantation, because of the severity of disease.35 EMB is similarly required to diagnose and guide therapy for many cases of acute NEM, fulminant lymphocytic myocarditis, and immune checkpoint inhibitor myocarditis.29,44 In contrast, cardiac sarcoidosis is often diagnosed using extracardiac biopsy and advanced cardiac imaging, because the focal nature cardiac involvement reduces the sensitivity of EMB to < 25%.44 Although immunosuppression is life-saving for these select forms of myocarditis, the effect of immunosuppression is neutral when used to treat all-comers with myocarditis of an unknown etiology.43 This reinforces the value of EMB to diagnose and guide therapy for select cases of myocarditis.
Infectious myocarditis
There is growing interest in the use of EMB-guided immunosuppression for chronic viral myocarditis in patients who are genome-negative or have inflammatory infiltrate.45 The rationale for this approach is on the basis of the hypothesized mechanism of disease. Acute myocarditis (days to weeks) is marked by (typically) viral entry and replication that results in cardiomyocyte necrosis, cytokine release, and an innate immune response.33,37,46 The subacute phase results from activation of the adaptive immune response (humoral and cellular) and lasts from weeks to months and results in myocardial dysfunction.33,37,46 In fact, the severity of cardiac dysfunction correlates with the degree of T-cell infiltrate.33,37,46 The immune response and cardiac function generally resolve as the virus is cleared. However, a subset of patients have persistent viral genome replication or develop an autoimmune response that persists after viral clearance, resulting in chronic myocarditis (months to years).33,37,46 Dilated cardiomyopathy might result from persistent viral replication and cardiomyocyte injury, autoimmune-mediate injury, and/or maladaptive neurohormonal responses to myocardial dysfunction. EMB can define the immune response and the presence of a viral genome in a patient with subacute or chronic myocarditis.30 It is proposed that immunosuppressive therapy might benefit patients who are viral genome-negative but have a persistent inflammatory cardiomyopathy (Fig. 4); however, large-volume randomized clinical trial of this approach are lacking.30,45 Of note, although there is interest in using EMB to guide immunomodulatory therapy using antiviral therapies or high-dose intravenous immunoglobulin, these therapies have not been adequately studied or shown to improve outcomes in patients with acute myocarditis.30,37,47,48
Figure 4.
A simplified algorithm of endomyocardial biopsy-guided management of suspected myocarditis.
Infiltrative cardiomyopathies
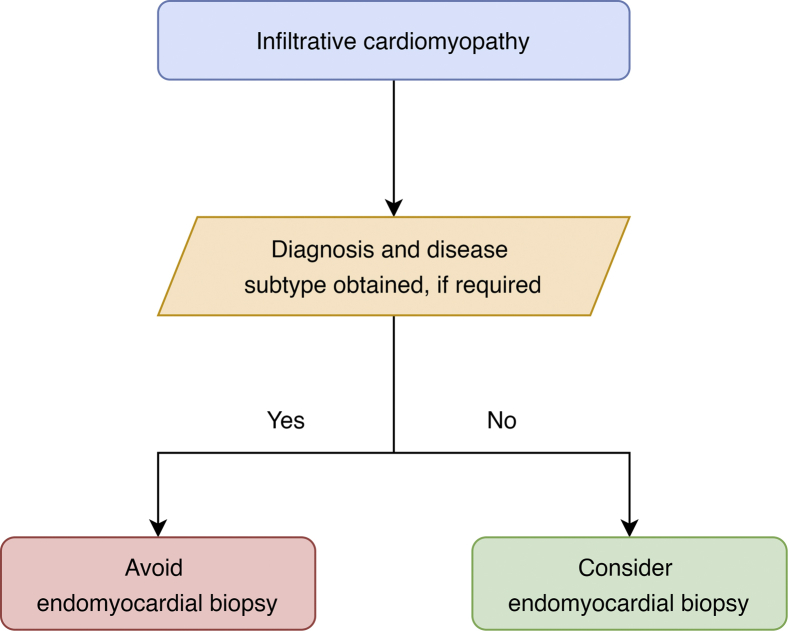
Infiltrative cardiomyopathies are a heterogeneous group of cardiac disorders characterized by the abnormal deposition of substances in the heart tissue, and include conditions such as cardiac amyloidosis, iron-overload cardiomyopathy, and glycogen storage disease. They share a clinical phenotype of heart failure with restrictive diastolic function and can be challenging to diagnose. Although EMB is recommended for infiltrative cardiomyopathies, the diagnostic yield is low and the rate of complications higher compared with EMB for transplant recipients.1 In a series of patients with heart failure with an LV ejection fraction > 50%, the diagnostic yield of EMB for cardiac amyloidosis was only 14%.49 In a separate series of patients with restrictive cardiomyopathy, the diagnostic yield of EMB for an infiltrative cardiomyopathy was 29%.3
Noninvasive techniques have therefore replaced EMB as the diagnostic approach for most infiltrative cardiomyopathies. In particular, CMR with T1, T2, and extracellular volume mapping can provide a clinical diagnosis in most cases. An example of this is cardiac transthyretin amyloidosis, for which a nonbiopsy diagnosis using peripheral blood and a pyrophosphate scan is now recommended.50,51 EMB should be reserved for cases in which tissue is required to subtype the amyloid deposits (ie, exclude concomitant light chain amyloid cardiomyopathy in the presence of a positive pyrophosphate scan and plasma dyscrasia), a negative pyrophosphate scan despite a high clinical suspicion, and unavailability of pyrophosphate scanning (Fig. 5).36,50, 51, 52, 53 Similarly, EMB is avoided in many cases of light chain amyloid cardiomyopathy, the most common form of cardiac amyloidosis, if a diagnosis can be made with laboratory testing, cardiac imaging, and extracardiac (eg, abdominal fat pad) tissue biopsy.54 The noninvasive diagnosis of iron-overload cardiomyopathy, hypertrophic cardiomyopathy, and glycogen storage disease such as Fabry disease is achievable with peripheral blood, genetic testing, and advanced cardiac imaging. This avoids the need for EMB for most cases of infiltrative cardiomyopathy.
Figure 5.
Simplified algorithm for the use of endomyocardial biopsy in the workup of infiltrative cardiomyopathies.
Cardiac tumours
Cardiac biopsy is infrequently used for cardiac tumours1 but might be reasonable if: (1) a nonbiopsy diagnosis (eg, using CMR) or noncardiac biopsy diagnosis is not possible; (2) tissue diagnosis will alter management; and (3) an experienced operator is available to perform cardiac biopsy with a high chance of success.2 Accordingly, cardiac biopsy of tumours is most frequently performed for lymphoma and discouraged for myxomas, which have a typical appearance and high risk of embolization with manipulation.2 Cardiac biopsy of tumours is typically performed with combined fluoroscopic and echocardiographic guidance (see Video 4
 ; view video online).
; view video online).
On the Horizon
Advances in procedural and analytic techniques continue to improve the safety and utility of EMB. For example, the use of microarrays to measure mRNAs associated with a specific disease or immune response is a novel and promising technique.55, 56, 57 When configured for cardiac allograft rejection, the assay output includes a probability of cellular and AMR processes and, in some instances, might obviate the need for histopathology.55, 56, 57 Although this work is being pioneered for allograft rejection, the development of these microarrays might allow for precision diagnostics and tailored therapy for other cardiomyopathies. Investigators have also proposed a “micro-bioptome” to address the shortcomings of EMB, namely the risk of complications and low diagnostic yield.55, 56, 57 A smaller bioptome acquires smaller endomyocardial specimens (0.4 mm vs 5.0 mm), and would theoretically reduce the risk of cardiac perforation.58 This permits the operator to safely obtain a higher number of samples and to sample nontraditional locations, such as the RV free wall, to increase diagnostic yield. These examples show how EMB continues to evolve as an increasingly safe and useful diagnostic tool.
Conclusion
EMB is an invaluable and underused diagnostic tool that is standard of care for select inflammatory and infiltrative cardiomyopathies, but remains underused. The diagnostic yield of EMB is improved by increased sampling and/or guided biopsies, and rate of complications low when performed by experienced operators. Despite advances in noninvasive techniques, EMB remains the gold standard for most instances of cardiac allograft rejection surveillance and significant myocarditis. In contrast, EMB is now a second-line test for most infiltrative cardiomyopathies and used when noninvasive testing is inconclusive or does not provide the subtype of the disease. The development of miniaturized bioptomes and microarrays might improve the safety and utility of EMB, and better our understanding of myocardial disease.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: Research reported has adhered to the relevant ethical guidelines.
See page 529 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.11.017.
Supplementary Material
Fluoroscopic imaging showing endomyocardial biopsy of the right ventricular septum by advancing a bioptome through the right atrium and tricuspid valve via the right internal jugular vein.
Fluoroscopic imaging showing endomyocardial biopsy of the right ventricular septum by advancing a bioptome through the right atrium and tricuspid valve via a long sheath in the left internal jugular vein.
Fluoroscopic imaging showing endomyocardial biopsy of the right ventricular septum by advancing a bioptome through the right atrium and tricuspid valve via a long sheath in the right femoral vein.
Transthoracic echocardiogram (modified apical 4-chamber view) showing: (1) a large mass occupying the right ventricle; (2) positioning of a bioptome; and (3) acquisition of a specimen from the mass using bioptome forceps.
References
- 1.Singh V., Mendirichaga R., Savani G.T. Comparison of utilization trends, indications, and complications of endomyocardial biopsy in native versus donor hearts (from the Nationwide Inpatient Sample 2002 to 2014) Am J Cardiol. 2018;121:356–363. doi: 10.1016/j.amjcard.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Cooper L.T., Baughman K.L., Feldman A.M. The role of endomyocardial biopsy in the management of cardiovascular disease. Circulation. 2007;116:2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 3.Bennett M.K., Gilotra N.A., Harrington C. Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000-2009. Circ Heart Fail. 2013;6:676–684. doi: 10.1161/CIRCHEARTFAILURE.112.000087. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz A., Kindermann I., Kindermann M. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation. 2010;122:900–909. doi: 10.1161/CIRCULATIONAHA.109.924167. [DOI] [PubMed] [Google Scholar]
- 5.Chimenti C., Frustaci A. Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies: a retrospective study over a 28-year period. Circulation. 2013;128:1531–1541. doi: 10.1161/CIRCULATIONAHA.13.001414. [DOI] [PubMed] [Google Scholar]
- 6.Vaidya V.R., Abudan A.A., Vasudevan K. The efficacy and safety of electroanatomic mapping-guided endomyocardial biopsy: a systematic review. J Interv Card Electrophysiol. 2018;53:63–71. doi: 10.1007/s10840-018-0410-7. [DOI] [PubMed] [Google Scholar]
- 7.Omote K., Naya M., Koyanagawa K. 18F-FDG uptake of the right ventricle is an important predictor of histopathologic diagnosis by endomyocardial biopsy in patients with cardiac sarcoidosis. J Nucl Cardiol. 2020;27:2135–2143. doi: 10.1007/s12350-018-01541-7. [DOI] [PubMed] [Google Scholar]
- 8.Unterberg-Buchwald C., Ritter C.O., Reupke V. Targeted endomyocardial biopsy guided by real-time cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2017;19:45. doi: 10.1186/s12968-017-0357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingrid K., Michael K., Reinhard K. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–648. doi: 10.1161/CIRCULATIONAHA.108.769489. [DOI] [PubMed] [Google Scholar]
- 10.Fiorelli A.I., Benvenuti L., Aielo V. Comparative analysis of the complications of 5347 endomyocardial biopsies applied to patients after heart transplantation and with cardiomyopathies: a single-center study. Transplant Proc. 2012;44:2473–2478. doi: 10.1016/j.transproceed.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Chan M.C.Y., Giannetti N., Kato T. Severe tricuspid regurgitation after heart transplantation. J Heart Lung Transplant. 2001;20:709–717. doi: 10.1016/s1053-2498(01)00258-3. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen V., Cantarovich M., Cecere R., Giannetti N. Tricuspid regurgitation after cardiac transplantation: how many biopsies are too many? J Heart Lung Transplant. 2005;24(7 suppl):S227–S231. doi: 10.1016/j.healun.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Alharethi R., Bader F., Kfoury A.G. Tricuspid valve replacement after cardiac transplantation. J Heart Lung Transplant. 2006;25:48–52. doi: 10.1016/j.healun.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Chih S., Mcdonald M., Dipchand A. Canadian Cardiovascular Society/Canadian Cardiac Transplant Network position statement on heart transplantation: patient eligibility, selection, and post-transplantation care. Can J Cardiol. 2020;36:335–356. doi: 10.1016/j.cjca.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Stewart S., Winters G.L., Fishbein M.C. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Crespo-Leiro M.G., Zuckermann A., Bara C. Concordance among pathologists in the second cardiac allograft rejection gene expression observational study (CARGO II) Transplantation. 2012;94:1172–1177. doi: 10.1097/TP.0b013e31826e19e2. [DOI] [PubMed] [Google Scholar]
- 17.Zerbe T.R., Arena V. Diagnostic reliability of endomyocardial biopsy for assessment of cardiac allograft rejection. Hum Pathol. 1988;19:1307–1314. doi: 10.1016/s0046-8177(88)80286-7. [DOI] [PubMed] [Google Scholar]
- 18.O’Neill J.O., Mcrae A.T., Troughton R.W. Brain natriuretic peptide levels do not correlate with acute cellular rejection in de novo orthotopic heart transplant recipients. J Heart Lung Transplant. 2005;24:416–420. doi: 10.1016/j.healun.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Damodaran A., Dardas T., Wu A.H. Changes in serial B-type natriuretic peptide level independently predict cardiac allograft rejection. J Heart Lung Transplant. 2012;31:708–714. doi: 10.1016/j.healun.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Patel P.C., Hill D.A., Ayers C.R. High-sensitivity cardiac troponin I assay to screen for acute rejection in patients with heart transplant. Circ Heart Fail Heart Fail. 2014;7:463–469. doi: 10.1161/CIRCHEARTFAILURE.113.000697. [DOI] [PubMed] [Google Scholar]
- 21.Butler C.R., Savu A., Bakal J.A. Correlation of cardiovascular magnetic resonance imaging findings and endomyocardial biopsy results in patients undergoing screening for heart transplant rejection. J Heart Lung Transplant. 2015;34:643–650. doi: 10.1016/j.healun.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Bonnemains L., Cherifi A., Girerd N., Odille F., Felblinger J. Design of the DRAGET study: a multicentre controlled diagnostic study to assess the detection of acute rejection in patients with heart transplant by means of T2 quantification with MRI in comparison to myocardial biopsies. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-008963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antończyk K., Niklewski T., Antończyk R. Speckle-tracking echocardiography for monitoring acute rejection in transplanted heart. Transplant Proc. 2018;50:2090–2094. doi: 10.1016/j.transproceed.2018.03.112. [DOI] [PubMed] [Google Scholar]
- 24.Tran A., Fixler D., Huang R. Donor-specific HLA alloantibodies: impact on cardiac allograft vasculopathy, rejection, and survival after pediatric heart transplantation. Int Soc Heart Lung Transplant. 2016;35:87–91. doi: 10.1016/j.healun.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Pham M.X., Teuteberg J.J., Kfoury A.G. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362:1890–1900. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 26.Kobashigawa J., Patel J., Azarbal B. Randomized pilot trial of gene expression profiling versus heart biopsy in the first year after heart transplant: Early Invasive Monitoring Attenuation Through Gene Expression Trial. Circ Heart Fail. 2015;8:557–564. doi: 10.1161/CIRCHEARTFAILURE.114.001658. [DOI] [PubMed] [Google Scholar]
- 27.Deng M.C., Eisen H.J., Mehra M.R. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant. 2006;6:150–160. doi: 10.1111/j.1600-6143.2005.01175.x. [DOI] [PubMed] [Google Scholar]
- 28.De Vlaminck I., Valantine H.A., Snyder T.M. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med. 2014;6:241ra77. doi: 10.1126/scitranslmed.3007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khush K.K., Patel J., Pinney S. Noninvasive detection of graft injury after heart transplant using donor-derived cell-free DNA: a prospective multicenter study. Am J Transplant. 2019;19:2889–2899. doi: 10.1111/ajt.15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caforio A.L.P., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 31.Chow L.H., Radio S.J., Sears T.D., Mcmanus B.M. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. 1989;14:915–920. doi: 10.1016/0735-1097(89)90465-8. [DOI] [PubMed] [Google Scholar]
- 32.Baughman K.L. Diagnosis of myocarditis. Circulation. 2006;113:593–595. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- 33.Kindermann I., Barth C., Mahfoud F. Update on myocarditis. J Am Coll Cardiol. 2012;59:779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 34.Magnani J.W., Dec G.W. Myocarditis. Circulation. 2006;113:876–890. doi: 10.1161/CIRCULATIONAHA.105.584532. [DOI] [PubMed] [Google Scholar]
- 35.Kociol R.D., Cooper L.T., Fang J.C. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141:e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 36.Bozkurt B., Colvin M., Cook J. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016;134:e579–e646. doi: 10.1161/CIR.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 37.Tschöpe C., Cooper L.T., Torre-Amione G., Van Linthout S. Management of myocarditis-related cardiomyopathy in adults. Circ Res. 2019;124:1568–1583. doi: 10.1161/CIRCRESAHA.118.313578. [DOI] [PubMed] [Google Scholar]
- 38.Ezekowitz J.A., O’Meara E., McDonald M.A. 2017 Comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol. 2017;33:1342–1433. doi: 10.1016/j.cjca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Leone O., Veinot J.P., Angelini A. 2011 Consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol. 2012;21:245–274. doi: 10.1016/j.carpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 41.Cooper L.T., Berry G.J., Shabetai R. Idiopathic giant-cell myocarditis — natural history and treatment. N Engl J Med. 1997;336:1860–1866. doi: 10.1056/NEJM199706263362603. [DOI] [PubMed] [Google Scholar]
- 42.Kandolin R., Lehtonen J., Salmenkivi K. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail. 2013;6:15–22. doi: 10.1161/CIRCHEARTFAILURE.112.969261. [DOI] [PubMed] [Google Scholar]
- 43.Mason J.W., O’Connell J.B., Herskowitz A. A clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 44.Birnie D.H., Sauer W.H., Bogun F. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1304–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 45.Merken J., Hazebroek M., Van Paassen P. Immunosuppressive therapy improves both short- and long-term prognosis in patients with virus-negative nonfulminant inflammatory cardiomyopathy. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.117.004228. [DOI] [PubMed] [Google Scholar]
- 46.Pollack A., Kontorovich A.R., Fuster V., Dec G.W. Viral myocarditis—diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12:670–680. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 47.Caforio A.L.P., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 48.Turgeon P.Y., Massot M., Beaupré F. Effect of acute immunosuppression on left ventricular recovery and mortality in fulminant viral myocarditis: a case series and review of literature. CJC Open. 2021;3:292–302. doi: 10.1016/j.cjco.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hahn V.S., Yanek L.R., Vaishnav J. Endomyocardial biopsy characterization of heart failure with preserved ejection fraction and prevalence of cardiac amyloidosis. JACC Heart Fail. 2020;8:712–724. doi: 10.1016/j.jchf.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gillmore J.D., Maurer M.S., Falk R.H. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 51.Fine N.M., Davis M.K., Anderson K. Canadian Cardiovascular Society/Canadian Heart Failure Society joint position statement on the evaluation and management of patients with cardiac amyloidosis. Can J Cardiol. 2020;36:322–334. doi: 10.1016/j.cjca.2019.12.034. [DOI] [PubMed] [Google Scholar]
- 52.Maurer M.S., Bokhari S., Damy T. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019;12:1–11. doi: 10.1161/CIRCHEARTFAILURE.119.006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kittleson M.M., Maurer M.S., Ambardekar A.V. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020;142:e7–e22. doi: 10.1161/CIR.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 54.Witteles R.M., Liedtke M. AL amyloidosis for the cardiologist and oncologist. JACC CardioOncology. 2019;1:117–130. doi: 10.1016/j.jaccao.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mengel M., Sis B., Kim D. The molecular phenotype of heart transplant biopsies: relationship to histopathological and clinical variables. Am J Transplant. 2010;10:2105–2115. doi: 10.1111/j.1600-6143.2010.03182.x. [DOI] [PubMed] [Google Scholar]
- 56.Halloran P.F., Potena L., Van Huyen J.P.D. Building a tissue-based molecular diagnostic system in heart transplant rejection: the heart Molecular Microscope Diagnostic (MMDx) system. J Heart Lung Transplant. 2017;36:1192–1200. doi: 10.1016/j.healun.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 57.Shah K.S., Kittleson M.M., Kobashigawa J.A. Updates on heart transplantation Curr Heart Fail Rep. 2019;16:150–156. doi: 10.1007/s11897-019-00432-3. [DOI] [PubMed] [Google Scholar]
- 58.Grankvist R., Chireh A., Sandell M. Myocardial micro-biopsy procedure for molecular characterization with increased precision and reduced trauma. Sci Rep. 2020;10:1–11. doi: 10.1038/s41598-020-64900-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costanzo M.R., Dipchand A., Starling R. The International Society of Heart and Lung Transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–956. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 60.Brahmer J.R., Lacchetti C., Schneider B.J. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluoroscopic imaging showing endomyocardial biopsy of the right ventricular septum by advancing a bioptome through the right atrium and tricuspid valve via the right internal jugular vein.
Fluoroscopic imaging showing endomyocardial biopsy of the right ventricular septum by advancing a bioptome through the right atrium and tricuspid valve via a long sheath in the left internal jugular vein.
Fluoroscopic imaging showing endomyocardial biopsy of the right ventricular septum by advancing a bioptome through the right atrium and tricuspid valve via a long sheath in the right femoral vein.
Transthoracic echocardiogram (modified apical 4-chamber view) showing: (1) a large mass occupying the right ventricle; (2) positioning of a bioptome; and (3) acquisition of a specimen from the mass using bioptome forceps.