Abstract
Different progestogens are widely used in hormonal therapy and mediate their therapeutic actions via the progesterone receptor (PR-B). Little published data exist on their relative efficacies and potencies via PR-B, while those available may be confounded by off-target receptors, different methodologies and model systems. We performed dose-response analysis to investigate the efficacies and potencies for transcription of several progestins widely used in contraception and P4 via human PR-B. We compared responses using three different cell lines and two different transient transfection conditions. Results show that in vitro biological responses via PR-B for the select progestogens can vary significantly in biocharacter, rank order and absolute values for efficacies and potencies, depending on the cell line and transfection condition. Progestogen rank orders for published relative binding affinities are mostly different to those for relative efficacies and potencies. These in vitro differences suggest that rank orders and absolute values of the efficacies and potencies of the progestogens are likely to vary in vivo in a cell-specific and progestogen-specific manner, and cannot easily be extrapolated from in vitro data, as is usually the practice. While obtaining such data in vivo is not possible, these in vitro data show proof of concept for likely significant cell- and progestogen-specific PR-B effects.
1. Introduction
Synthetic progestogens (progestins) are used for hormonal therapy to mimic the actions of progesterone (P4), by binding to and activating the progesterone receptor (PR) [1]. However, some progestins are associated with side-effects such as increased risk of breast cancer, cardiovascular disease and HIV-1 acquisition [2].
Development of therapeutic progestins requires determination of affinities, efficacies (maximal response a progestin can elicit) and potencies (EC50; the concentration that can elicit half the maximal response) for transcriptional regulation in cell line models expressing the PR, or potential off-target steroid receptors (SRs). This provides important information predictive of clinical relevance and side-effects [1,3]. The PR regulates transcription of specific target genes via multiple mechanisms including direct binding to progesterone response elements (PREs) in the promoter region of these genes or tethering to various DNA-bound transcription factors [4]. To determine potencies and efficacies for transcription via a particular receptor, dose-response analysis is usually performed using promoter-reporter constructs in cell lines overexpressing that receptor, where the promoter contains a SR binding site such as a PRE, ideally in a cell line deficient in competing receptors [5,6]. While the physiological relevance of such models could be disputed, they do yield direct evidence of relative receptor-specific effects of different progestins when performed in parallel experiments, which is almost impossible to otherwise obtain. Although preclinical animal and clinical models are more physiologically relevant, they have several limitations, including confounding factors due to species- and gene-specific effects, metabolism and off-target SR effects.
As it is thought that the off-target biological activity of progestins via SRs other than the PR are associated with side-effects [1,7], we have previously determined the efficacies and potencies of progestins via the glucocorticoid receptor (GR) [8], androgen receptor (AR) [9], mineralocorticoid [10] and estrogen receptors [9] in HEK293 or COS-1 cells with overexpressed SRs. Similar published studies assessing the relative efficacies and potencies of different progestins via the PR are surprisingly limited, and only a few [5,6,11–14] have investigated multiple progestins in parallel in the same model system. Notably, potencies reported for the PR and determined using in vitro models show a wide range of values between studies for progestins widely used in contraception in sub-Saharan Africa (Supplementary Table 1).
This study thus aimed to directly compare the transcriptional activities of the selected progestins medroxyprogesterone acetate (MPA), norethisterone (NET), levonorgestrel (LNG) and etonogestrel (ETG), relative to each other, P4 and the PR-specific synthetic agonist promegestone (R5020) via exogenously expressed human PR-B in COS-1 cells. As different model systems are often used when studying the transcriptional activity of progestins, we also sought to determine whether cell line and transfection conditions could be confounding factors influencing reported efficacies and potencies.
2. Materials and Methods
2.1. Cell lines and materials
COS-1 monkey kidney and U2OS human bone osteosarcoma cells obtained from the ATCC (USA), and the MDA-MB-231 human breast adenocarcinoma cells received from Adrienne Edkins (Rhodes University, RSA), were maintained as previously described [22]. Only mycoplasma-negative cells were used for experiments. P4, MPA, NET, LNG and ETG were purchased from Sigma-Aldrich, RSA, and R5020 from PerkinElmer Life and Analytical Science, RSA. The human PR-B (pSG5-hPR-B) expression vector [23] and the pTAT-2xPRE-E1b-luciferase [24] construct were received from Eric Kalkhoven (University Medical Centre Utrecht, The Netherlands) and Guido Jenster (Erasmus University of Rotterdam, Netherlands), respectively. The pSG5 empty vector [25] was obtained from Gunnar Mellgren (University of Bergen, Norway).
2.2. Reporter assays
Promoter-reporter assays were performed essentially as previously described [9], with a few modifications. Briefly, COS-1 or MDA-MB-231 cells were seeded at a density of 2 × 106 cells, while U2OS cells were seeded at 1.5 × 106 cells into 10 cm dishes. Cells were transiently transfected, using XtremeGene HP (Roche Molecular Biochemicals), as follows: Transfection condition #1: 900 ng of the pSG5 empty vector or pSG5-hPR-B and 9000 ng of the pTAT-2xPRE-E1b-luciferase construct; Transfection condition #2: 3500 ng pSG5 or pSG5-hPR-B and 1410 ng pTAT-2xPRE-E1b-luciferase. The following day, the transfected cells were reseeded into 96-well plates at a density of 1 × 104 cells per well and subsequently treated with vehicle (0.1% EtOH) or increasing concentrations of the test compounds for 24 hours in either serum-free medium (COS-1 and U2OS), or medium containing charcoal-stripped fetal calf serum (FCS) (MDA-MB-231). Luciferase activity was measured and normalized as previously described [9]
2.3. Immunoblotting
Protein samples were prepared as previously described [26] and 20 μg separated on a 10% SDS-polyacrylamide gel before transfer to nitrocellulose membranes (Amersham) and blocking in 10% fat-free milk powder. Membranes were probed with anti-PR (PGR-312-L-CE, Leica Biosystems, UK) or anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 0411, Santa Cruz Biotechnology, USA) (loading control) followed with the HRP-conjugated secondary goat anti-mouse antibody (Santa Cruz Biotechnology, USA). Proteins were visualised using enhanced chemiluminescence (Bio-Rad Laboratories, Inc., USA) and a MyECL Imager (Pierce Thermo Scientific Inc., USA), and expression levels quantified using ImageJ (Version 1.49).
2.4. Data and statistical analysis
Graph Pad Prism® software version 7 was used for data analysis. Non-linear regression and sigmoidal dose-response were used with the slope was set to one. One- or two-way ANOVA (analysis of variance) and the Bonferroni (compares all pairs of columns) post-test were used for statistical analysis when multiple ligands were tested in parallel, while unpaired t-tests were used when ligands were not tested in parallel. The error bars represent the standard error of the mean (SEM) of at least three independent experiments, each performed in triplicate.
3. Results
3.1. Some progestogens have different efficacies and potencies via PR-B.
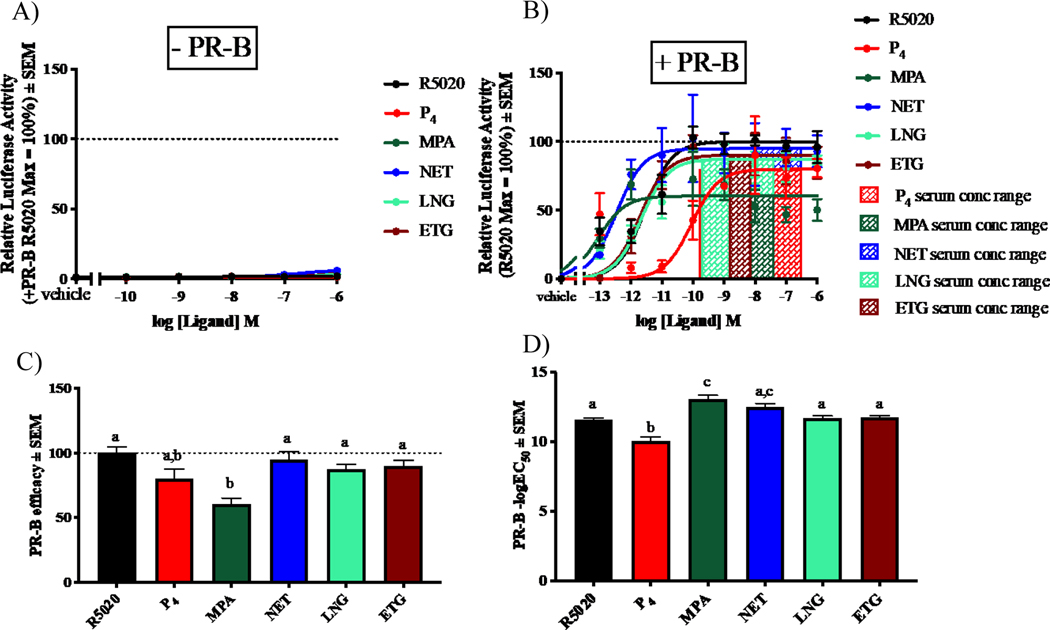
To compare the efficacies and potencies of select progestogens, promoter-reporter and dose-response analyses were performed in COS-1 cells exogenously expressing human PR-B (Fig. 1). This cell line was selected due to negligible endogenous expression of SRs [3]. Relative to the R5020 response in PR-B transfected cells, negligible transactivation by all the progestogens was observed in the absence of transfected PR-B (Fig. 1A). In PR-B-transfected cells, all progestogens except MPA were full agonists for transactivation (Fig. 1B–C). MPA was significantly less efficacious than all progestogens investigated except P4 (Fig. 1C), suggesting it is a partial agonist relative to R5020, NET, ETG and LNG. Interestingly, although MPA displayed a similar potency to NET, it was significantly more potent than all the other progestogens investigated, while P4 was the least potent ligand (Fig. 1D).
Fig. 1. Some progestogens display different efficacies and potencies via PR-B.
COS-1 cells transiently transfected with (A) 900 ng pSG5-empty vector or (B-D) pSG5-hPR-B expression vector and 9000 ng pTAT-2xPRE-E1b luciferase reporter (transfection condition #1), were treated with 0.1% EtOH (vehicle) or increasing concentrations of each ligand for 24 hours. Luciferase activity was measured and normalized to protein concentration. (A-B) Relative luciferase activity is shown with PR-B R5020 (maximal response) set as 100% and all other response relative to this. The shaded bars in B indicate the reported serum concentrations of progestogens in women. (C) Efficacy and (D) −logEC50 values ± SEM of the ligands via PR-B were plotted and analysed using one-way ANOVA with a Bonferroni post-test. Different letters denote statistically significant differences while the same letters do not.
3.2. Relative and absolute efficacies and potencies are cell-specific.
To investigate whether the efficacies and potencies of the progestogens via human PR-B are influenced by the model system, we also performed experiments in the MDA-MB-231 breast cancer (Fig. 2A) and U2OS bone osteosarcoma (Fig. 2B) cell lines. These cell lines do not express endogenous PR, while low endogenous GR or AR levels are sometimes detectable [28,29]. However, we observed negligible reporter transactivation in the absence of exogenous PR-B (Supplementary Fig. 1). When the efficacies of the progestogens via PR-B were compared between the cell lines, the only statistically significant differences were seen with P4, MPA and ETG (Fig. 2C). Both ETG and MPA were significantly more efficacious in U2OS cells compared to MDA-MB-231 cells, with MPA also being more efficacious compared to the COS-1 cells, while P4 was significantly less efficacious in U2OS cells compared to MDA-MB-231 cells (Fig. 2C). The efficacy and potency rank orders were not conserved between cell lines (Supplementary Fig. 2). A greater number of statistically significant differences was observed for potencies compared to efficacies across the three cell lines (Fig. 2D). All the progestogens investigated via PR-B in the three cell lines had potency and efficacy values falling within the serum concentration range found in contraceptive users (Fig. 1B, 2A–B, Supplementary Table 2). Although all cell lines were transfected with 900 ng PR-B (transfection condition #1), the MDA-MB-231 cells expressed much less PR-B compared to both COS-1 and U2OS cells, while U2OS cells expressed the most PR-B (Supplementary Fig. 3G).
Fig. 2. Progestogen efficacies and potencies via PR-B are cell line-specific.
(A) MDA-MB-231 and (B) U2OS cells were transfected and treated as for Fig 1B–D, while results were analysed as in Fig 1A–B. (C) The bar graph shows the efficacies ± SEM for the COS-1 (Fig 1), MDA-MB-231and U2OS cell-lines, while (D) shows the −logEC50 values ± SEM. Relative efficacies and −logEC50 were analysed using unpaired t-tests with *, **, *** denoting p<0.05, 0.01 and 0.001, respectively. One-way ANOVA with a Bonferroni post-test was performed to determine statistical differences within each cell line (COS-1, no symbol, MDA-MB-231 # and U2OS §) where different letters denote statistically significant differences while the same letters do not.
3.3. Relative and absolute efficacies and potencies are mostly not significantly affected by transfection conditions.
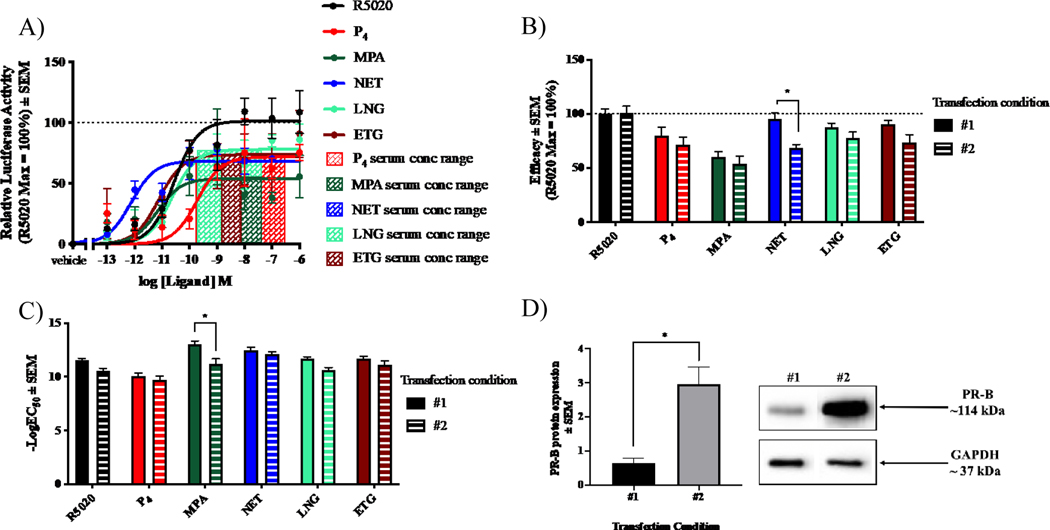
Next, we investigated whether the efficacies and potencies of the progestogens via PR-B are influenced by the transfection conditions. When comparing the first (Fig. 1B–D) to the second (Fig. 3A–C) transfection condition, very few significant differences were detected. Only NET displayed a significantly lower efficacy via condition 2 (Fig. 3B), while only MPA was more potent via condition 1 (Fig. 3C). The progestogen responses via PR-B using condition 2 were also investigated in MDA-MB-231 and U2OS cells (Supplementary Fig. 3A–B). No significant differences in efficacy were observed in MDA-MB-231 and U2OS cells (Supplementary Fig. 3C–D), while similar to COS-1 cells, MPA was more potent via condition 1 in the MDA-MB-231 cells, however more potent via condition 2 in the U2OS cells (Supplementary Fig. 3E–F). NET was more potent via condition 2 in the MDA-MB-231 cells (Supplementary Fig. 3E). Interestingly, about 4-fold more PR-B was expressed under condition 2 compared to condition 1 in COS-1 cells (Fig. 3D). A similar increase in PR-B expression under condition 2 was observed for MDA-MB-231 cells, while the increase for U2OS cells was negligible (Supplementary Fig. 3G).
Fig. 3. Progestogen efficacies and potencies via PR-B are minimally influenced by transfection conditions.
COS-1 cells, transiently transfected as for Fig.1 (transfection condition #1) or 3500 ng pSG5-hPR-B expression vector and 1410 ng pTAT-2xPRE-E1b-luciferase (transfection condition #2), were treated with the 0.1% EtOH (vehicle) or increasing concentrations of each ligand for 24 hours. Luciferase activity was measured and normalized to the protein concentration. (A) Relative luciferase activity is shown with R5020 set as 100% and all other responses relative to this. (B) Efficacy and (C) −logEC50 values ± SEM of the ligands via PR-B using transfection condition #1 vs #2 were plotted. (D) Total protein was harvested and a representative western blot of the PR-B expression levels between the two different transfection conditions is shown. Two-way ANOVA with a Bonferroni post-test (B and C) and unpaired t-tests (D) were performed to determine statistical differences * denoting p<0.05.
4. Discussion
Our study is the first to determine efficacies and potencies of these progestogens in parallel within the same model system. We show that all progestogens, except MPA, are full agonists for transactivation via human PR-B in the COS-1 cell line. While MPA displays similar potency to NET, it is significantly more potent than R5020, LNG, ETG and P4. Only two studies have previously investigated the potencies of P4, NET, LNG, ETG in parallel [13,14], one of which included MPA [14], via the human PR. Our results are not directly comparable since Bain and co-workers performed experiments in U2OS cells stably expressing multiple copies of the PRE and did not specify the PR isoform or include R5020 and MPA [13], while the study by Bray and co-workers included MPA and was conducted in T47D cells expressing both PR isoforms [14]. The absolute EC50 values for PR-B (Supplementary Table 1) are lower than those reported in both studies [13,14]. However, in agreement with our study, P4 is the least potent progestogen and the rank order is very similar to that determined for PR-B in the U2OS cells. Other studies obtained similar potencies with overexpressed PR-B for R5020 in HeLa cells [16] and P4 and LNG in HEK293 cells [6], to those we obtained for those progestins in COS-1 cells, while other studies have reported greater potencies for P4 [5,12]. In terms of MPA and NET, much lower potencies were obtained for human PR-B in some other studies [5,6] compared to our study. Clearly the absolute and relative values obtained for progestogen potencies in different studies are highly dependent on the model system used. Thus, while data are consistent across studies, differences in absolute and relative progestogen potencies between studies could be due to multiple factors, including differences in cell type, promoter-reporter constructs, expression levels of the PR, method of dose-response analysis or the PR isoform(s) investigated.
Given these apparent discrepancies, we investigated whether efficacies and potencies of the progestogens for human PR-B are sensitive to the model system used. We found that MPA acts as a full agonist only in the U2OS cells, but a partial agonist in the other 2 cell lines, while most other progestogens are full agonists in all 3 cell lines. We show that relative potencies of the progestogens are more sensitive than efficacies to the cell line model system used. A possible explanation could be differential metabolism [22], either to decrease the effective concentration of select progestogens, and/or production of a metabolite that is active via the PR. Different cell lines could express different types and/or expression levels of co-regulators which may play a role in PR-B-mediated transcriptional regulation, as different ligands may cause differential recruitment of coregulators [30], and are sensitive to which progestogen is bound to the PR. Indeed, it has been shown that the ratio of coactivators and corepressors could modulate the inhibitory or stimulatory effects of the PR antagonist, RU486 [31]. All the cell line models used contain the downstream factors necessary to support PR-mediated transcription as evident from the potent responses. It is possible that some in vivo cofactors in cells that express endogenous PR are not present in some or all of our cell line models which may result in different relative potencies and efficacies in vivo. When investigating the effects of changing both PR-B levels and DNA reporter template levels in all three cell lines, our results show that both efficacies and potencies of most progestogens are not significantly affected by the change in transient transfection conditions. However, the biocharacter of NET changes from being a full to a partial agonist, suggesting that NET in complex with PR-B is particularly sensitive to changing the concentrations and/or ratio of PR-B to DNA template.
In summary, our results show that biological responses via the PR for different progestogens in vitro can vary in rank order, biocharacter, and absolute values for efficacies and potencies, depending mainly on the cell line and to only a limited extent on transient transfection conditions. One of the key findings of our work is that it is difficult to establish statistically significant differences by dose-response analysis for efficacy and potency even in vitro, when multiple ligands are investigated in parallel. Thus caution should be used when drawing conclusions about differences between ligands without any statistical analysis of significance of difference. We established that significant differences are detected in our assays between the efficacies and potencies of several progestogens. Moreover, we show that the rank order for progestogen efficacies and potencies sometimes but not always correlate with rank order for relative binding affinities for PR-B (Supplementary Table 2 and Supplementary Fig. 4), confirming that affinity is not proportional to biological activity [8]. While steroid efficacy and potency are affected by affinity for the receptor, they are also affected by precise conformation induced by the steroid, as well as cell-specific cofactors and SR expression levels [32,33]. Our results suggest several of these factors may play a role in the progestogen-specific and cell-specific efficacies and potencies of the progestins via the PR-B.
The physiological significance of our results is to suggest that rank order and absolute values for efficacy and potency, and even biocharacter, of progestogens are likely to vary in different cells and tissues in vivo and cannot easily be predicted from in vitro dose-response assays or receptor binding affinities. Nevertheless, the in vitro results show valuable proof of concept effects and present viable strategies to further directly investigate mechanisms of such effects. Our findings showing that the EC50 values of the progestogens are well below the reported serum levels found in women using contraceptives containing these progestogens (Supplementary Table 2). Although this suggests that these progestogens are likely to elicit similar effects in vivo, we show that absolute potency values change depending on assay conditions, suggesting that these may vary in vivo in a cell-specific manner. Further studies to understand mechanisms of progestogens in vitro on endogenous genes via PR-B, as well as clinical studies investigating specific biological responses to progestogens, would allow a more comprehensive understanding of the benefit/side-effect profiles of these clinically-significant steroids, and facilitate choice and dose of progestin for use in hormonal therapy.
Supplementary Material
Acknowledgements
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development [R01HD083026]. We thank John Woodland for intellectual contributions.
Abbreviations:
- AR
Androgen receptor
- ETG
Etonogestrel
- GR
Glucocorticoid receptor
- LNG
Levonorgestrel
- MPA
Medroxyprogesterone Acetate
- NET
Norethindrone/Norethisterone
- P4
Progesterone
- PR
Progesterone receptor
- PRE
Progesterone response element
- R5020
Promegestone
- RBA
Relative binding affinity
References
- [1].Stanczyk F, Hapgood J, Winer S. et al. Progestogens Used in Postmenopausal Hormone Therapy: Differences in Their Pharmacological Properties, Intracellular Actions, and Clinical Effects, Endocr. Rev 34 (2013) 171–208. doi: 10.1210/er.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hapgood J, Kaushic C, Hel Z Hormonal Contraception and HIV-1 Acquisition: Biological mechanisms, Endocr. Rev 39 (2018) 36–78. doi: 10.1210/er.2017-00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Africander D, Verhoog N, Hapgood J Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception, Steroids. 76 (2011) 636–652. doi: 10.1016/j.steroids.2011.03.001. [DOI] [PubMed] [Google Scholar]
- [4].Proietti C, Cenciarini M, Elizalde P Revisiting progesterone receptor (PR) actions in breast cancer: Insights into PR repressive functions, Steroids. 133 (2018) 75–81. doi: 10.1016/j.steroids.2017.12.015. [DOI] [PubMed] [Google Scholar]
- [5].Sasagawa S, Shimizu Y, Kami H. et al. Dienogest is a selective progesterone receptor agonist in transactivation analysis with potent oral endometrial activity due to its efficient pharmacokinetic profile, Steroids. 73 (2008) 222–231. doi: 10.1016/j.steroids.2007.10.003. [DOI] [PubMed] [Google Scholar]
- [6].Kumar N, Fagart J, Liere P. et al. Nestorone® as a novel progestin for nonoral contraception: Structure-activity relationships and brain metabolism studies, Endocrinology. 158 (2017) 170–182. doi: 10.1210/en.2016-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moore N, Hickey T, Butler L. et al. Multiple nuclear receptor signaling pathways mediate the actions of synthetic progestins in target cells., Mol. Cell. Endocrinol 357 (2012) 60–70. doi: 10.1016/j.mce.2011.09.019. [DOI] [PubMed] [Google Scholar]
- [8].Ronacher K, Hadley K, Avenant C. et al. Ligand-selective transactivation and transrepression via the glucocorticoid receptor: Role of cofactor interaction, Mol. Cell. Endocrinol 299 (2009) 219–231. doi: 10.1016/j.mce.2008.10.008. [DOI] [PubMed] [Google Scholar]
- [9].Louw-du Toit R, Perkins M, Hapgood J. et al. Comparing the androgenic and estrogenic properties of progestins used in contraception and hormone therapy, Biochem. Biophys. Res. Commun 491 (2017) 140–146. doi: 10.1016/j.bbrc.2017.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Louw-du Toit R, Hapgood J, Africander D A direct comparison of the transcriptional activities of progestins used in contraception and menopausal hormone therapy via the mineralocorticoid receptor, Biochem. Biophys. Res. Commun 526 (2020) 466–471. doi: 10.1016/j.bbrc.2020.03.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Attardi B, Burgenson J, Hild S. et al. CDB-4124 and its putative monodemethylated metabolite, CDB-4453, are potent antiprogestins with reduced antiglucocorticoid activity : in vitro comparison to mifepristone and CDB-2914, Mol. Cell. Endocrinol 188 (2002) 111–123. [DOI] [PubMed] [Google Scholar]
- [12].Attardi B, Koduri S, Hild S Relative progestational and androgenic activity of four progestins used for male hormonal contraception assessed in vitro in relation to their ability to suppress LH secretion in the castrate male rat, Mol. Cell. Endocrinol 328 (2010) 16–21. doi: 10.1016/j.mce.2010.06.010. [DOI] [PubMed] [Google Scholar]
- [13].Bain P, Kumar A, Ogino Y. et al. Nortestosterone-derived synthetic progestogens do not activate the progestogen receptor of Murray-Darling rainbowfish (Melanotaenia fluviatilis) but are potent agonists of androgen receptors alpha and beta, Aquat. Toxicol 163 (2015) 97–101. doi: 10.1016/j.aquatox.2015.03.021. [DOI] [PubMed] [Google Scholar]
- [14].Bray J, Jelinsky S, Ghatge R. et al. Quantitative analysis of gene regulation by seven clinically relevant progestins suggests a highly similar mechanism of action through progesterone receptors in T47D breast cancer cells., J. Steroid Biochem. Mol. Biol 97 (2005) 328–41. doi: 10.1016/j.jsbmb.2005.06.032. [DOI] [PubMed] [Google Scholar]
- [15].Lim C, Baumann C, Htunt H. et al. Differential Localization and Activity of the A- and B-Forms of the Human Progesterone Receptor Using Green Fluorescent Protein Chimeras, Mol. Endocrinol 13 (1999) 366–375. doi: 10.1210/mend.13.3.0247. [DOI] [PubMed] [Google Scholar]
- [16].Abdel-Hafiz H, Dudevoir M, Horwitz K Mechanisms underlying the control of progesterone receptor transcriptional activity by SUMOylation, J. Biol. Chem 284 (2009) 9099–9108. doi: 10.1074/jbc.M805226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Escande A, Servant N, Rabenoelina F. et al. Regulation of activities of steroid hormone receptors by tibolone and its primary metabolites, J. Steroid Biochem. Mol. Biol 116 (2009) 8–14. doi: 10.1016/j.jsbmb.2009.03.008. [DOI] [PubMed] [Google Scholar]
- [18].Tegley C, Zhi L, Marschke K. et al. 5-Benzylidene 1,2-Dihydrochromeno[3,4-F]Quinolines, a Novel Class of Nonsteroidal Human Progesterone Receptor Agonists, J. Med. Chem 41 (1998) 4354–4359. doi: 10.1021/jm980366a. [DOI] [PubMed] [Google Scholar]
- [19].Austin R, Maschera B, Walker A. et al. Mometasone furoate is a less specific glucocorticoid than fluticasone propionate, Eur. Respir. J 20 (2002) 1386–1392. doi: 10.1183/09031936.02.02472001. [DOI] [PubMed] [Google Scholar]
- [20].Zhang Z, Olland A, Zhu Y. et al. Molecular and pharmacological properties of a potent and selective novel nonsteroidal progesterone receptor agonist tanaproget, J. Biol. Chem 280 (2005) 28468–28475. doi: 10.1074/jbc.M504144200. [DOI] [PubMed] [Google Scholar]
- [21].Madauss K, Deng S, Austin R. et al. Progesterone receptor ligand binding pocket flexibility: Crystal structures of the norethindrone and mometasone furoate complexes, J. Med. Chem 47 (2004) 3381–3387. doi: 10.1021/jm030640n. [DOI] [PubMed] [Google Scholar]
- [22].Skosana S, Woodland J, Cartwright M. et al. Differential metabolism of clinically-relevant progestogens in cell lines and tissue: Implications for biological mechanisms, J. Steroid Biochem. Mol. Biol 189 (2019) 145–153. doi: 10.1016/j.jsbmb.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kastner P, Bocquel M, Turcotte B. et al. Transient expression of human and chicken progesterone receptors does not support alternative translational initiation from a single mRNA as the mechanism generating two receptor isoforms, J. Biol. Chem 265 (1990) 12163–12167. [PubMed] [Google Scholar]
- [24].Jenster G, Spencer T, Burcin M. et al. Steroid receptor induction of gene transcription: a two-step model., Proc. Natl. Acad. Sci 94 (1997) 7879–7884. doi: 10.1073/pnas.94.15.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Green S, Issemann I, Sheer E A versatile in vivo and in vitro eukaryotic expression vector for protein engineering, Nucleic Acids Res. 16 (1988) 369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Louw-du Toit R, Hapgood J, Africander D Medroxyprogesterone Acetate Differentially Regulates Interleukin (IL)-12 and IL-10 in a Human Ectocervical Epithelial Cell Line in a Glucocorticoid Receptor (GR)-dependent Manner, J. Biol. Chem 289 (2014) 31136–49. doi: 10.1074/jbc.M114.587311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Africander D, Storbeck K, Hapgood J A comparative study of the androgenic properties of progesterone and the progestins, medroxyprogesterone acetate (MPA) and norethisterone acetate (NET-A), J. Steroid Biochem. Mol. Biol 143 (2014) 404–415. doi: 10.1016/j.jsbmb.2014.05.007. [DOI] [PubMed] [Google Scholar]
- [28].Horwitz K, Zava D, Thilagar A. et al. Steroid Receptor Analyses of Nine Human Breast Cancer Cell Lines, Cancer Res. 38 (1978) 2434–2437. [PubMed] [Google Scholar]
- [29].Hadley K An investigation into the role of acetylation and ligand-dependent nuclear localisation in glucocorticoid receptor transcriptional regulation, (2010). Doctor of Philosophy Doctoral Thesis, University of Cape Town. http://hdl.handle.net/11427/10607. [Google Scholar]
- [30].Scarpin K, Graham J, Mote P. et al. Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression, Nucl. Recept. Signal 7 (2009) e009. doi: 10.1621/nrs.07009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu Z, Auboeuf D, Wong J. et al. Coactivator/corepressor ratios modulate PR-mediated transcription by the selective receptor modulator RU486, Proc. Natl. Acad. Sci 99 (2002) 7940–7944. doi: 10.1016/j.purol.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Simons S, Chow C The road less travelled: new views of steroid receptor action from the path of dose-response curves, Mol. Cell. Endocrinol 348 (2012) 373–382. doi: 10.1038/mp.2011.182.doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Simons S Glucocorticoid receptor co-factors as therapeutic targets, Curr. Opin. Pharmacol 10 (2010) 613–619doi: 10.1038/mp.2011.182.doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.