Abstract
Osteoarthritis (OA) is the most frequent and disabling disease in developed countries. The progressive degeneration of articular cartilage characterized as thinner and erosive. Inflammation is well-known to be involved in OA development. However, there are no effective therapeutic strategies to cure it. Xanthohumol (XH) is a natural prenylflavonoid isolated from hops and beer. The protective activity of XH against OA chondrocytes inflammation and ECM degradation is unclear. In this article, we found that XH significantly inhibited inflammatory responses, attenuated catabolic enzymes expression, and ameliorated ECM degradation, as showed by decreased production of NO, PGE2, TNFα, and IL-6, decreased expression of MMP-3/-13 and ADAMTS-4/-5, and increased expression of collagen-II and aggrecan. In addition, XH activated HO-1 signaling and attenuated IL-1β-induced C/EBPβ. XH promoted the interaction between HO-1 and C/EBPβ, inhibiting the nuclear translocation of C/EBPβ. HO-1 knockdown could abrogate the protective effects of XH in IL-1β-treated chondrocytes. Collectively, XH attenuated inflammatory responses and ECM degradation by mediating HO-1 and C/EBPβ signaling pathways in osteoarthritis chondrocytes.
Keywords: xanthohumol, osteoarthritis, ECM degradation, C/EBP, HO-1, mmp-13
Introduction
Osteoarthritis (OA), one of the age-related debilitating and degenerative diseases, is often clinically characterized by joint pain, limited movement, and transient morning stiffness (Shamdani et al., 2020). Mechanistically, cartilage degradation, synovial inflammation, and subchondral bone remodeling are involved in the pathological development of OA (Dai et al., 2017). However, the underlying mechanisms are still unclear, and no effective treatments are available to cure OA (Altman and Barthel, 2011).
Inflammation and inflammatory responses have been considered as the main factors contributing to the pathological development of OA. Inflammatory cytokines, such as IL-1β and TNFα, are closely involved in the aberrant metabolism and promote the catabolic activities of cartilage tissues in OA (Nambi, 2020). Expectedly, IL-1β dramatically contributes to the cartilage destruction of OA, which is associated with up regulation of C/EBPβ in chondrocytes (Nishimura et al., 2020). Increasing evidence shows that IL-1β suppresses the production of the component of extracellular matrix (ECM), synthesized by the unique chondrocytes. The possible mechanisms might be associated with the inductive activity of IL-1β on the expression of matrix metalloproteinases (MMPs), thrombin sensitive protein motifs (ADAMTS), cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS), which are the main catabolic factors for the destruction of articular cartilage (Wu et al., 2019). Nuclear factor-erythroid 2-related factor (Nrf2)/HO-1 is the critical factor mediating the expression of anti-oxidative enzymes and balancing the redox homeostasis (Zhao et al., 2020). Up regulation of NRF2/HO-1 expression may ameliorate IL-1β-induced inflammation and induction of ECM degradation (Gu et al., 2020). Promisingly, NRF2/HO-1 has become the potential target for the therapeutic management of OA.
Non-steroidal anti-in flammatory drugs (NSAIDs) have been widely used for treating OA, alleviating clinical symptoms, such as pain and swelling. However, they cannot inhibit the progression of OA development but produce many serious adverse effects (Bournia et al., 2017). It is emerging to explore the potential candidates for OA treatment. Recently, morroniside, a bioactive compound isolated from Cornus officinalis, has been showed to protect osteoarthritic cartilage by attenuation of inflammation in mice chondrocytes (Park et al., 2021). Engeletin (dihydrokaempferol 3-rhamnoside) is reported to decrease TNFα-induced chondrocytes apoptosis and the production of ROS in rat knee cartilage (Wang et al., 2021). Acacetin can significantly ameliorate IL-1β-induced matrix metalloproteinases expression in chondrocytes by inhibiting NF-κB signaling pathways in mice (Chen et al., 2020a). The therapeutic single compounds for osteoarthritis treatment have been reviewed recently (Lee et al., 2021). Xanthohumol (XH), a natural prenylflavonoid isolated from hops and beer, has been preventing hyaluronan overproduction and subsequent response in the early stage of OA (Stracke et al., 2011). Recently, XH has been reported to reduce the nephrotoxicity induced by cisplatin by activating Nrf2/HO-1 and attenuating NF-κB signaling pathways (Li et al., 2018). However, the biological activity of XH in osteoarthritic chondrocytes is still unclear.
Materials and Methods
General
This study was approved by the Institutional Animal Care and Use Committee of Taizhou People’s Hospital and performed in strict accordance with the guideline of Animal Care and Use (No. TZPH2006381962), according to the Declaration of Helsinki Principles.
Chemicals and Reagents
Xanthohumol (XH) (purity98%) (Cat. no. D108095) was obtained from BIOBW (Beijing, China). Collagenase type II (Cat. no. C2-28) and dimethylsulfoxide (DMSO) (Cat. no. D8418) were purchased from Sigma Aldrich (St Louis, MO, United States). The primary antibodies against C/EBPβ (Cat. no. 3082), HO-1 (Cat. no. 43966), MMP-3 (Cat. no. 14351), GAPDH (Cat. no. 5174S), and the horseradish peroxidase-labelled secondary antibody (Cat. no. 7074S) were purchased from Cell Signaling Technology. MMP-13 (Cat. no. 701287), ADAMTS-4 (Cat. no. PA5-69140), ADAMTS-5 (Cat. no. PA5-32142), collagen-II (Cat. no. MA5-12789), and aggrecan (Cat. no. MA3-16888) were obtained from Invitrogen. Recombinant rat IL-1β (Cat. no. ab9788) purchased from Abcam.
Rat Knee OA Models
Rat knee OA models were duplicated using a classic osteoarthritic destabilization of the medial meniscus (DMM) model through surgery (Glasson et al., 2007). Simply, eight-week-old male Sprague-Dawley rats (200 g) were anesthetized with 3% (w/v) pentobarbital (30 mg/kg) intraperitoneally. Then, surgery was conducted to cut the medial meniscus tibial ligament through the medial patellar tendon of the capsule in the right knee joints. The sham group was performed without cutting off the medial meniscus tibial ligament in the left knee joints. After eight weeks of post-operation, rats were sacrificed, and joint cartilages were collected for gross observation, histochemical examination, and immunohistochemical evaluation. The low and high doses of XH for treating rats OA were 5.64 mg/kg and 16.9 mg/kg, respectively, which were reported to be corresponding to the values of 60 mg and 180 mg XH, respectively, for humans with a body weight of 66 kg (Legette et al., 2012). Different doses of XH were dissolved in 0.5% DMSO. The sham group received the same volumes of dosing vehicle as the treating groups.
Cell Culture
Under sterile conditions, the articular cartilage of the articulatio genus, terminal femur, and upper tibia was collected, cut into small pieces, and digested with 0.25% pancreatic enzyme and 0.2% collagenase II at 37°C for 4 h. Cells were cultured in Dulbecco’s modified Eagle’s minimum essential medium (DMEM) (low glucose) (Life Technologies, NY, United States) supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin (Life Technologies) at 37°C with 5% CO2. 24 h later, the fresh complete culture medium was used for replacement. The cells employed for all the experiments were the second or the third generation of the primary cartilage cells, ensuring consistent cell phenotypes. XH dissolved in DMSO was prepared, and the final concentration of DMSO was 0.1% in the culture medium.
Cell Viability Assays
The CCK8 kit (Cat. no. C0038) (Beyotime, Shanghai, China) was used for testing the chondrocytes viability according to the manufacturer’s instructions. Chondrocytes were grown in 96-well plates at a density of 5000 cells/well for 24 h. Different concentrations of XH (0, 5, 10, 20, 40, and 80 μM) were used to pretreat the chondrocytes for 24 and 48 h, respectively. Then, CCK-8 solution (10 μl) was added into each well and incubated at 37°C for 4 h. After that, the absorbance was detected at the wavelength of 450 nm by using a microplate system (Leica microsystems, Germany).
Detection of NO, PGE2, TNFα, and IL-6
Chondrocytes were grown in 6-well plates, and XH was added to treat for 24 h. Then, cells were treated with the recombinant IL-1β for another 24 h. The levels of NO metabolite nitrite were detected as the production of NO by using sodium nitrite as the standard (Au et al., 2007). Simply, 100 μl of the culture supernatant was permitted to react with Griess reagent in an equal volume in 96-well microplate at room temperature for 10 min in the dark. The absorbance was detected at the wavelength of 540 nm by using a microplate system (Leica microsystems, Germany). The productions of PGE2, TNFα, and IL-6 were determined by employment of ELISA kits (Beyotime, Shanghai, China) according to the manufacturer’s instructions.
Cell Transfection
HO-1 siRNA and scramble siRNA (negative control), obtained from RiboBio (Guangzhou, China), were used for investigating the roles of HO-1 knockdown in the inflammatory responses in chondrocytes. Cells were cultured in 6-well plates at a density of 1×105 cells/well. Once they reached 60% confluence, cells were used for the subsequent transfections. According to the manufacturer’s instructions, transfections of HO-1 siRNA and negative control were performed by using lipofectamine 3000 reagent (Thermo Fisher Scientific, Inc.) for 36 h at 50 nM. Western blot assays were used for determining the transfection efficiency.
Western Blotting
RIPA lysis buffer (Beyotime Institute of Biotechnology) containing 1% PMSF was used to lyse cells. BCA protein assay kit (cat. no. 23250) (Pierce Biotechnology) was employed to quantify the total protein. 25 μg protein/lane was separated via gel electrophoresis and transferred to polyvinylidene fluoride membrane (Bio-Rad, CA, United States). It was blocked by tris-buffered saline supplemented with 5% nonfat milk for 1 h at room temperature. After washing three times with TBST (Tris-buffered saline containing Tween 20), the membrane was cut and incubated with the corresponding primary antibody at 4°C overnight. Then, it was washed with TBST for three times and incubated with horseradish peroxidase-labelled secondary antibody for 1 h at room temperature. Protein bands were detected using the enhanced chemiluminescence detection system (Bio-Rad Laboratories) and Quantity One software v4.6.2 (Bio-Rad Laboratories).
Co-immunoprecipitation Assay
Chondrocytes were harvested by the immunoprecipitation lysis buffer. Then, they were centrifugated at 12,000 rpm for 30 min 10% of chondrocytes lysates were kept and used as the input. The remained proteins were immunoprecipitated by incubating with normal goat IgG or HO-1 in immunoprecipitation washing buffer at 4°C overnight. Next, they were incubated with the pre-cleared protein A-sepharose beads at 4°C for 2 h. The eluted samples were then subjected to Western blot as mentioned above.
Immunofluorescence
Chondrocytes were grown on glass coverslips for 24 h. After rinsing with PBS for three times, cells were fixed with 4% paraformaldehyde for 15 min at room temperature and rinsed with PBS again. 0.1% Triton X-100 was used to infiltrate cell and nuclear membranes for 5 min at room temperature. Then, cells were blocked by 5% protease-free bovine serum albumin (BSA) for 1 h at room temperature, rinsed with PBS, and incubated with the corresponding primary antibody at 4°C overnight. Cells were washed with PBS and incubated with fluorescein-conjugated goat anti-rabbit IgG antibody for 1 h at room temperature. Subsequently, cells were mounted in medium containing DAPI (Invitrogen) after washing with PBS. A confocal laser scanning microscope (Leica Microsystems) was employed to observe the slides, and the fluorescence intensity was detected by using ImageJ software 2.1 (Bethesda, MD, United States).
Statistical Analysis
All experiments were performed in triplicate and data are presented as the mean ± standard error of the mean. SPSS 20.0 software (IBM Corp., Armonk, NY, United States) was used for statistical analysis. One-way ANOVA and followed by Tukey’s post hoc test was used to make statistical comparisons between multiple groups. An unpaired Student’s t-test was used to make statistical comparisons between two groups. p < 0.05 was considered to indicate a statistically significant difference.
Results
XH Exhibited Protective Activity Against OA Development in Rats
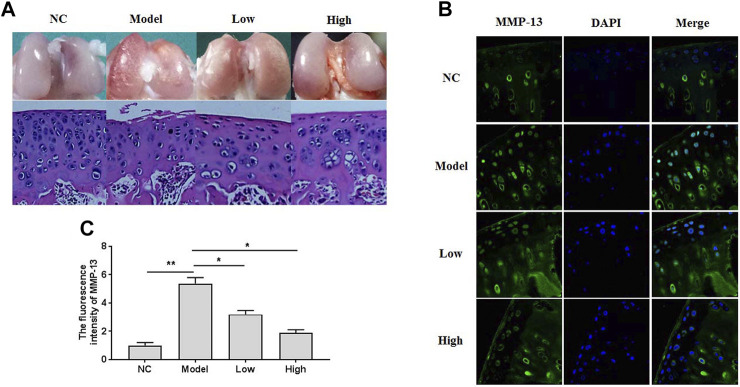
To investigate the effects of XH on OA development, a surgical rat DMM model was duplicated and treated with XH (5.64 mg/kg and 16.9 mg/kg) for 8 weeks after operation by oral administration. As showed in Figure 1A, gross observation and histomorphological examination were studied. No obvious damages were seen in the negative control group. In contrast, the rough surface of cartilage with some erosion was observed in the model group. Consistently, the decreased number and disorder distribution of chondrocytes and decreased thickness of articular cartilage were found in the model group by hematoxylin-eosin (HE) staining. XH could protect articular cartilage from damage, as indicated by better cartilage surface, increased cell number, and thicker cartilage dose dependently. The immunofluorescence study of MMP-13 in situ (Figure 1B) showed that oral administration of XH might significantly decrease the fluorescence intensity (Figure 1C) in a dose-dependent manner, compared with that in the model group. These indicated that XH exhibited protective effects against de-structure in articular cartilage, which might be possibly associated with down regulation of MMP-13 expression in chondrocytes.
FIGURE 1.
The gross observation, histomorphological examination, and immunofluorescence assays in the knee articular cartilage. (A) The gross observation and histomorphological examination (HE staining) of articular cartilage. (B) The immunofluorescence study of MMP-13 in cartilage. (C) The summary data of fluorescence intensity of MMP-13 in situ. All experiments were performed in triplicate and data are presented as the mean ± standard deviation. * p < 0.05 and **p < 0.01. NC, negative control; Model, the model group; Low, the group treated with XH (5.64 mg/kg); High, the group treated with XH (16.9 mg/kg).
Effects of XH on Cell Viability
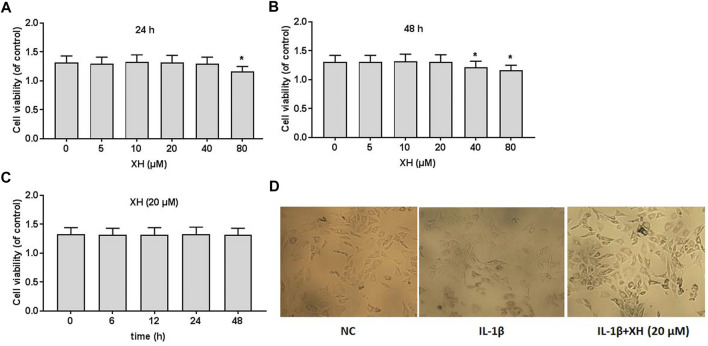
To detect the effects of XH on the viability of rat chondrocytes, the different doses XH (0, 5, 10, 20, 40, and 80 μM) were administered to the cultured cells for 24 and 48 h, respectively, before cell viability was detected using CCK8 assays. As indicated in Figures 2A,B XH did not produce any cytotoxicity when the concentration of XH was less than 20 μM. After 48 h incubation, XH at the dose of 40 μM slightly reduced the viability of chondrocytes, compared with that after 24 h incubation. At the dose of 20 μM, XH did not exhibit toxic effects on chondrocytes in 48 h (Figures 2C,D).
FIGURE 2.
Cell viability of XH on rat chondrocytes. CCK8 assays were conducted to determine the effects of XH (0, 5, 10, 20, 40, and 80 μM) in 24 h (A) and 48 h (B), respectively. (C) The effects of XH (20 μM) on cell viability in 48 h. (D) The cell morphology was observed after administration with IL-1β (10 ng/ml) with or without XH (20 μM) in 48 h. All experiments were performed in triplicate and data are presented as the mean ± standard deviation. * p < 0.05 and **p < 0.01.
XH Decreased the Productions of Inflammatory Cytokines in IL-1β-Treated Chondrocytes
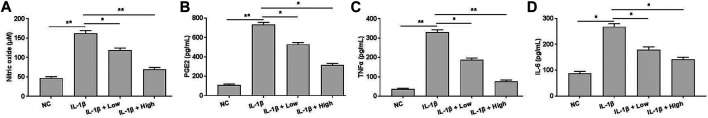
To investigate the effects of XH on chondrocytes regarding inflammation, cells were pretreated with IL-1β (10 ng/ml) and XH (5 and 20 μM) for 24 h. The productions of inflammatory cytokines, including NO, PGE2, TNFα, and IL-6 were determined. As shown in Figure 3, IL-1β could significantly stimulate the expression of NO (Figure 3A), PGE2 (Figure 3B), TNFα (Figure 3C), and IL-6 (Figure 3D). In contrast, XH exhibited protective activity and compromised the effects of IL-1β, decreasing the productions of these inflammatory cytokines in chondrocytes.
FIGURE 3.
Effects of XH on the production of NO, PGE2, TNFα, and IL-6 in IL-1β-treated chondrocytes. (A) The production of NO was determined. (B) The production of PGE2 was determined. (C) The production of TNFα was determined. (D) The production of IL-6 was determined. All experiments were performed in triplicate and data are presented as the mean ± standard deviation. *p < 0.05 and **p < 0.01. NC, negative control; Low, XH (5 μM); High, XH (20 μM).
XH Ameliorated IL-1β-Induced ECM Degradation in Chondrocytes
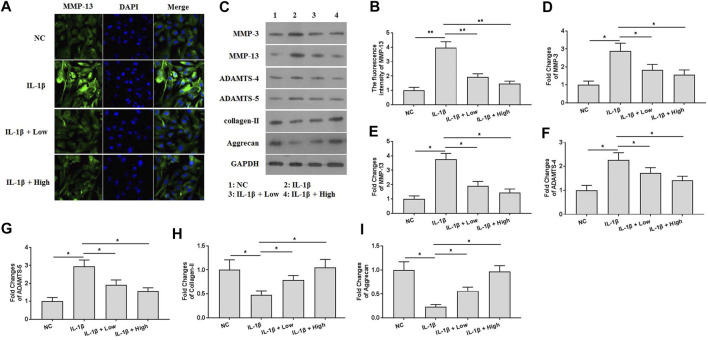
To explore the protective effects of XH against ECM degradation in vitro, immunofluorescence and western blotting assays were performed. The results of immunofluorescence assays (Figures 4A,B) showed that XH could effectively attenuate the expression of MMP-13, which was significantly induced by IL-1β. Consistently, IL-1β also increased the expression of the proteins of MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 and decreased the expression of collagen-II and aggrecan in chondrocytes (Figures 4C,D). Administration of XH could ameliorate IL-1β-induced up regulation of MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 expression and down regulation of collagen-II and aggrecan expression in chondrocytes.
FIGURE 4.
XH attenuated ECM degradation in IL-1β-treated chondrocytes. (A) The immunofluorescence study of MMP-13 in IL-1β-treated chondrocytes. (B) The summary data of fluorescence intensity of MMP-13. (C) The proteins expression of MMP-3, MMP-13, ADAMTS-4, ADAMTS-5, collagen-II, and aggrecan were detected by western blotting. (D–I) were the quantified values of tested proteins. All experiments were performed in triplicate and data are presented as the mean ± standard deviation. *p < 0.05 and **p < 0.01. NC, negative control; Low, XH (5 μM); High, XH (20 μM).
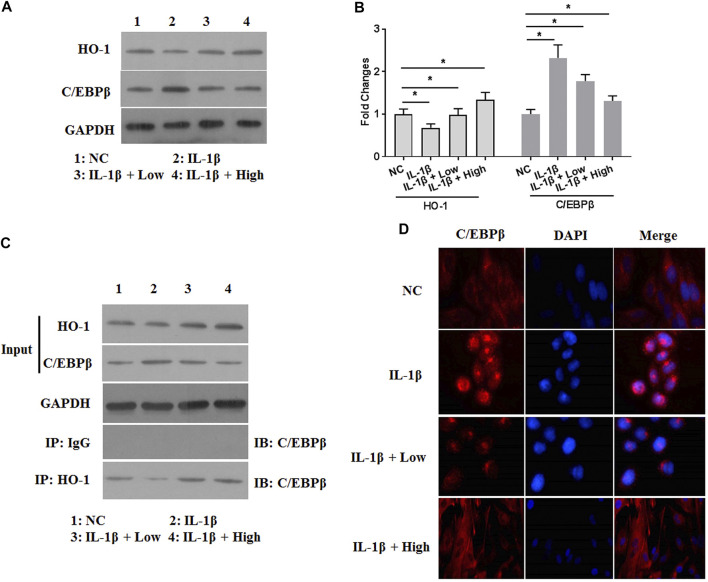
XH Inhibited the Activity of C/Ebpβ by Stimulating HO-1 Expression in IL-1β-Treated Chondrocytes
To further investigate the catabolic responses of ECM in IL-1β-treated chondrocytes, the effects of XH on the activity of IL-1β-activated C/EBPβ were involved. Interestingly, the expression of C/EBPβ was increased by IL-1β (Figures 5A,B). XH could effectively attenuate IL-1β-activated C/EBPβ expression in chondrocytes. XH has been reported to be an activator for NRF2/HO-1 signaling (Zimmermann et al., 2015). Whether HO-1 expression was involved in the protective activity of XH against IL-1β-induced C/EBPβ in chondrocytes, western blotting assays were conducted. The protein expression of HO-1 was detected (Figures 5A,B). IL-1β decreased HO-1 expression. XH reversed it effectively. Co-immunoprecipitation assays were conducted. XH promoted the interaction between HO-1 and C/EBPβ (Figure 5C). The results of immunofluorescence assays (Figure 5D) demonstrated that IL-1β promoted translocation of C/EBPβ into the nucleus, and XH could block C/EBPβ translocation induced by IL-1β in chondrocytes. These suggested that XH inhibited the activity of C/EBPβ by stimulating HO-1 expression in IL-1β-treated chondrocytes.
FIGURE 5.
XH increased the expression of HO-1 and attenuated the expression of C/EBPβ in IL-1β-treated chondrocytes. (A) The proteins expression of HO-1 and C/EBPβ were detected by western blotting. (B) The quantified values of tested proteins were indicated. (C) The co-immunoprecipitation of HO-1 and C/EBPβ was studied. (D) The immunofluorescence study of C/EBPβ in IL-1β-treated chondrocytes. All experiments were performed in triplicate and data are presented as the mean ± standard deviation. *p < 0.05 and **p < 0.01. NC, negative control; Low, XH (5 μM); High, XH (20 μM).
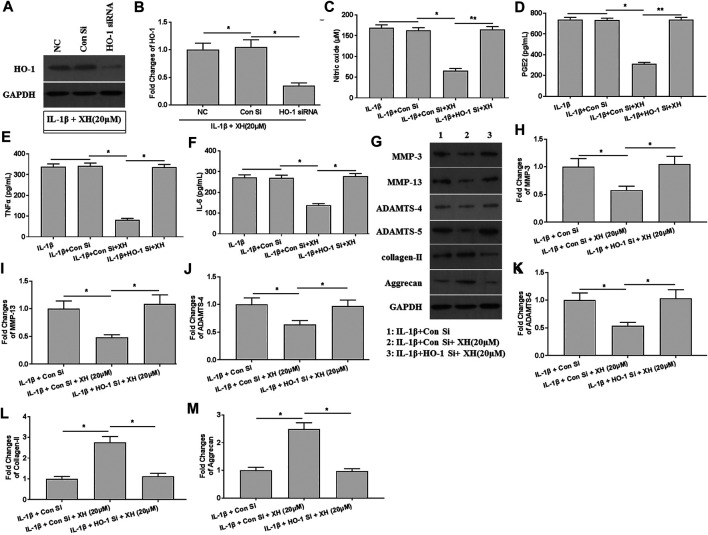
XH Ameliorated IL-1β-Induced ECM Degradation by Stimulating HO-1 Activity in Chondrocytes
To investigate the roles of XH-mediated HO-1 expression in IL-1β-induced ECM degradation, HO-1 was knockdown by siRNA. The decreased expression of HO-1 was observed, indicating successful transfection (Figures 6A,B). In HO-1-knockdown chondrocytes, the productions of NO, PGE2, TNFα, and IL-6 induced by IL-1β were not significantly down regulated by XH (Figures 6C–F). Similarly, the expression of the proteins of MMP-3, MMP-13, ADAMTS-4, ADAMTS-5, collagen-II, and aggrecan in IL-1β-treated chondrocytes showed no difference those in the negative control group (Figures 6G–M). These suggested that the protective mechanisms of XH against ECM degradation in cartilage might be associated with attenuation of inflammation and C/EBPβ expression induced by IL-1β.
FIGURE 6.
XH attenuated ECM degradation induced by IL-1β via up regulation of HO-1 in chondrocytes. (A) The expression of HO-1 was detected in HO-1 siRNA-transfected chondrocytes. (B) The quantified values of tested protein were indicated. The productions of inflammatory cytokines NO (C), PGE2 (D), TNFα (E), and IL-6 (F) in HO-1 siRNA-transfected chondrocytes were determined by ELISA. (G) The proteins expression of MMP-3, MMP-13, ADAMTS-4, ADAMTS-5, collagen-II, and aggrecan were detected in HO-1 siRNA-transfected chondrocytes. (H–M) were the quantified values of tested proteins. All experiments were performed in triplicate and data are presented as the mean ± standard deviation. * p < 0.05 and **p < 0.01. NC, negative control; Con Si, control siRNA.
Discussion
Maintenance of homeostasis in articular cartilage is tightly mediated by the anabolic and catabolic players in the metabolism of cartilage matrix. Recently, several inflammatory mediators have been demonstrated to be the major players in joint articular matrix degradation by up regulating the expression of MMPs (Nguyen et al., 2017), which are the important proteolytic enzymes degrading ECM and contributing the turnover of cartilage. Three main therapeutic agents including NSAIDs, disease-modifying OA drugs, and steroid and biological response modifiers have been developed for the main treatment strategy of OA (Saeki et al., 2012). However, long-term administration of these agents may produce serious side effects. Natural sources have been interested scientifically for developing novel managing strategy (Jayakumar et al., 2020). In this study, we found that XH could effectively decrease the productions of inflammatory cytokines NO, PGE2, TNFα, and IL-6, stimulate HO-1 expression, attenuate the activity of C/EBPβ, promote the interaction of HO-1 and C/EBPβ, and inhibit C/EBPβ nuclear translocation, resulting in amelioration of catabolic enzymes expression in IL-1β-treated chondrocytes.
Chronic, low-grade, and unregulated inflammation has been involved in the pathophysiological development of several human diseases, including osteoarthritis. Many studies showed that high levels of inflammatory responses are associated with OA development in human or experimental animal models and administration of anti-inflammatory agents is clinically implicated in OA treatment (Ansari et al., 2020). IL-1β and TNFα are often used to be stimulators to primary chondrocytes or cartilage explants, mimicking pathological conditions in vivo (Daheshia and Yao, 2008). Often, they up regulate the expression of many catabolic factors, such as COX-2, PGE2, IL-6, iNOS, MMPs, and ADAMTSs, and down regulate the expression of collagen-II and aggrecan (Ansari and Haqqi, 2016). In our study, we employed IL-1β as the stimulator to the primary isolated chondrocytes to duplicate cell models. As expected, IL-1β up regulated the expression of NO, PGE2, IL-6, MMP3, MMP-13, and ADAMTS-4/5 in chondrocytes and down regulated the expression of collagen-II and aggrecan.
MMPs are the collagenases to degrade collagen in the cartilage and bone. Aberrant expression of MMPs is linked to OA progression, and MMPs have become the potential targets for developing specific inhibitors implicated in clinic (Li et al., 2017). MMP-13 is the major enzyme to induce the degradation of collagen, particularly collagen (Knäuper et al., 1997). MMP-13 is the factor responsible for the early onset of OA, and its increased expression remained elevated throughout the 10 weeks study (Pickarski et al., 2011). Interestingly, MMP-13 acts as a biomarker to initiate the degradation of various downstream matrix and collagen components, and it has been comprehensively reviewed (Li et al., 2017). ADAMTS-5 is the major aggrecanase, a critical factor associating with OA pathogenesis, and it is much more active than ADAMTS-4 catalytically (Fushimi et al., 2008). In our study, we found that the expression of MMP-13 was significantly elevated in vivo and in vitro by immunofluorescence and western blotting assays. The proteins expression of ADAMTS-4 and ADAMTS-5 were also increased in IL-1β-treated chondrocytes.
Mechanistically, the regulation of the inflammatory cytokines, COX-2, iNOS, MMPs, and ADAMTSs is tightly mediated by inflammatory signaling pathways (Sun et al., 2020). Treatment with IL-1β may trigger a cascade of events and induce the activation of C/EBPβ expression in chondrocyte (Tsushima et al., 2012). It has been reported that C/EBPβ in the nucleus can bind to the promoter region of MMP-13 and induce its expression (Hayashida et al., 2009). Thus, IL-1β up regulated MMP-13 expression by promoting the recruitment of C/EBPβ to the promoter of MMP-13. In our study, we consistently demonstrated that IL-1β activated C/EBPβ expression and promoted C/EBPβ nuclear translocation in rat chondrocytes.
Natural products have gained increasing attention due to novel structures for drug development. β-Hydroxyisoamylshikonin, a natural naphthoquinone, has been reported to exhibit anti-inflammatory and anti-oxidative activity. β-Hydroxyisoamylshikonin can decrease the expression of NO, PEG2, IL-6, TNFα, iNOS, ADAMTS-5, and MMP13 in chondrocytes by down regulation of NF-κB pathway and up regulation of NRF2/HO-1 pathway (Chen et al., 2020b). Hyperoside also decrease the productions of inflammatory cytokines, down regulates the expression of MMPs and ADAMTS-5, and mediates the activity of NF-κB/MAPK and NRF2/HO-1 signaling pathways (Sun et al., 2021). XH is a prenylated chalcone isolated from the hop plant and shows anti-inflammatory, anti-oxidative, anti-proliferative, and anti-angiogenic activity (Harikumar et al., 2009; Krajka-Kuźniak et al., 2020). Consistently, our study showed that XH could decrease the productions of the inflammation cytokines. Interestingly, XH has also been an activator of NRF2/HO-1 signaling (Bai et al., 2019; Krajka-Kuźniak et al., 2020). XH exhibits free radicals scavenging activity by activating NRF2/HO-1 signaling, preventing neuro-degeneration (Wang et al., 2019). Our study found that XH increased the expression of HO-1 in chondrocytes.
It has been reported that the expression of NRF2/HO-1 is showed to be negatively correlated with C/EBPβ in neonatal satellite cell (Zecchini et al., 2019). In HO-1 knockout mice, the expression of C/EBPβ is significantly increased in myelocytes (Bukowska-Strakova et al., 2017). These indicate that C/EBPβ might be a downstream factor of HO-1. Our study revealed that XH promoted the binding of HO-1 to C/EBPβ and inhibited C/EBPβ nuclear translocation. To further investigate the roles of XH in protection against IL-1β-induced inflammatory responses and ECM degradation, HO-1 siRNA was employed to knockdown the expression of HO-1. Our study showed that HO-1 knockdown could abrogate the protective effects of XH on IL-1β-treated chondrocytes.
However, there are some limitations in this study. The selected doses were not obtained by our previous study but by the reference from Legette et al. (2012), who calculated them according to the FDA-approved Guidance for Industry. It is necessary to re-confirm them under the different environment. Recently, it has been demonstrated that DMSO exhibits anti-inflammatory and anti-oxidative activities (Kim et al., 2006; Cheleschi et al., 2018). In our study, DMSO was used as the vehicle to dissolve XH. The negative control group in rat OA models was applied to administration with the same volume vehicle. In vitro, the effects of 0.1% DMSO on the productions of inflammatory cytokines were investigated, and they showed no significant changes (Supplementary Figure S1). This might be associated with its low concentration. Then, the remaining experiments were conducted without consideration of DMSO influence. However, DMSO still exhibited potential activity on inflammatory and oxidative stress. More efforts are still needed for further confirmation.
Conclusion
Collectively, XH ameliorated IL-1β-induced inflammatory responses and ECM degradation by activation of HO-1 expression and inhibition of C/EBPβ activity in chondrocytes.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Taizhou People’s Hospital.
Author Contributions
WW and FP provided the idea of this paper. MZ, RZ, TZ, ZC, and GJ conducted the experiments and revised and finalized the paper. All authors approved the final paper.
Funding
This study was financially supported by the National Natural Science Foundation of China (81960883 and 81860261), the National Science Foundation of Jiangxi Province (20202BABL206122 and 20202BABL206134), Scientific Research Fund of Jiangxi Provincial Education Department (GJJ190789, GJJ190791, and GJJ190824), and Team Construction Projects from Gannan Medical University (TS202002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Altman R. D., Barthel H. R. (2011). Topical Therapies for Osteoarthritis. Drugs 71 (10), 1259–1279. 10.2165/11592550-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Ansari M. Y., Haqqi T. M. (2016). Interleukin-1β Induced Stress Granules Sequester COX-2 mRNA and Regulates its Stability and Translation in Human OA Chondrocytes. Sci. Rep. 6, 27611. 10.1038/srep27611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M. Y., Ahmad N., Haqqi T. M. (2020). Oxidative Stress and Inflammation in Osteoarthritis Pathogenesis: Role of Polyphenols. Biomed. Pharmacother. 129, 110452. 10.1016/j.biopha.2020.110452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au R. Y., Al-Talib T. K., Au A. Y., Phan P. V., Frondoza C. G. (2007). Avocado Soybean Unsaponifiables (ASU) Suppress TNF-α, IL-1β, COX-2, iNOS Gene Expression, and Prostaglandin E2 and Nitric Oxide Production in Articular Chondrocytes and Monocyte/macrophages. Osteoarthritis and Cartilage 15 (11), 1249–1255. 10.1016/j.joca.2007.07.009 [DOI] [PubMed] [Google Scholar]
- Bai F., Zhang B., Hou Y., Yao J., Xu Q., Xu J., et al. (2019). Xanthohumol Analogues as Potent Nrf2 Activators against Oxidative Stress Mediated Damages of PC12 Cells. ACS Chem. Neurosci. 10 (6), 2956–2966. 10.1021/acschemneuro.9b00171 [DOI] [PubMed] [Google Scholar]
- Bournia V-K., Kitas G., Protogerou A. D., Sfikakis P. P. (2017). Impact of Non-steroidal Anti-inflammatory Drugs on Cardiovascular Risk: Is it the Same in Osteoarthritis and Rheumatoid Arthritis? Mod. Rheumatol. 27 (4), 559–569. 10.1080/14397595.2016.1232332 [DOI] [PubMed] [Google Scholar]
- Bukowska-Strakova K., Ciesla M., Szade K., Nowak W. N., Straka R., Szade A., et al. (2017). Heme Oxygenase 1 Affects Granulopoiesis in Mice through Control of Myelocyte Proliferation. Immunobiology 222 (3), 506–517. 10.1016/j.imbio.2016.10.018 [DOI] [PubMed] [Google Scholar]
- Cheleschi S., Fioravanti A., De Palma A., Corallo C., Franci D., Volpi N., et al. (2018). Methylsulfonylmethane and Mobilee Prevent Negative Effect of IL-1β in Human Chondrocyte Cultures via NF-Κb Signaling Pathway. Int. Immunopharmacology 65, 129–139. 10.1016/j.intimp.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Chen J., Wang C., Huang K., Chen S., Ma Y. (2020a). Acacetin Suppresses IL-1β-Induced Expression of Matrix Metalloproteinases in Chondrocytes and Protects against Osteoarthritis in a Mouse Model by Inhibiting NF-Κb Signaling Pathways. Biomed. Res. Int. 2020, 1–12. 10.1155/2020/2328401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Gu M., Jin J., Ren C., Pan Z., Wu Y., et al. (2020b). β-Hydroxyisovalerylshikonin Inhibits IL-1β-induced Chondrocyte Inflammation via Nrf2 and Retards Osteoarthritis in Mice. Food Funct. 11 (11), 10219–10230. 10.1039/d0fo02192j [DOI] [PubMed] [Google Scholar]
- Daheshia M., Yao J. Q. (2008). The Interleukin 1β Pathway in the Pathogenesis of Osteoarthritis. J. Rheumatol. 35 (12), 2306–2312. 10.3899/jrheum.080346 [DOI] [PubMed] [Google Scholar]
- Dai X., Song R., Xiong Y. (2017). The Expression of ERK and JNK in Patients with an Endemic Osteochondropathy, Kashin-Beck Disease. Exp. Cel Res. 359 (2), 337–341. 10.1016/j.yexcr.2017.08.015 [DOI] [PubMed] [Google Scholar]
- Fushimi K., Troeberg L., Nakamura H., Lim N. H., Nagase H. (2008). Functional Differences of the Catalytic and Non-catalytic Domains in Human ADAMTS-4 and ADAMTS-5 in Aggrecanolytic Activity. J. Biol. Chem. 283 (11), 6706–6716. 10.1074/jbc.M708647200 [DOI] [PubMed] [Google Scholar]
- Glasson S. S., Blanchet T. J., Morris E. A. (2007). The Surgical Destabilization of the Medial Meniscus (DMM) Model of Osteoarthritis in the 129/SvEv Mouse. Osteoarthritis and Cartilage 15 (9), 1061–1069. 10.1016/j.joca.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Gu M., Jin J., Ren C., Chen X., Gao W., Wang X., et al. (2020). Akebia Saponin D Suppresses Inflammation in Chondrocytes via the NRF2/HO-1/nf-Κb axis and Ameliorates Osteoarthritis in Mice. Food Funct. 11 (12), 10852–10863. 10.1039/d0fo01909g [DOI] [PubMed] [Google Scholar]
- Harikumar K. B., Kunnumakkara A. B., Ahn K. S., Anand P., Krishnan S., Guha S., et al. (2009). Modification of the Cysteine Residues in IκBα Kinase and NF-Κb (P65) by Xanthohumol Leads to Suppression of NF-Κb-Regulated Gene Products and Potentiation of Apoptosis in Leukemia Cells. Blood 113 (9), 2003–2013. 10.1182/blood-2008-04-151944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida M., Okazaki K., Fukushi J., Sakamoto A., Iwamoto Y. (2009). CCAAT/ENHANCER Binding Protein β Mediates Expression of Matrix Metalloproteinase 13 in Human Articular Chondrocytes in Inflammatory Arthritis. Arthritis Rheum. 60 (3), 708–716. 10.1002/art.24332 [DOI] [PubMed] [Google Scholar]
- Jayakumar T., Saravana Bhavan P., Sheu J-R. (2020). Molecular Targets of Natural Products for Chondroprotection in Destructive Joint Diseases. Ijms 21 (14), 4931. 10.3390/ijms21144931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L. S., Axelrod L. J., Howard P., Buratovich N., Waters R. F. (2006). Efficacy of Methylsulfonylmethane (MSM) in Osteoarthritis Pain of the Knee: a Pilot Clinical Trial. Osteoarthritis and Cartilage 14 (3), 286–294. 10.1016/j.joca.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Knäuper V., Cowell S., Smith B., López-Otin C., O’Shea M., Morris H., et al. (1997). The Role of the C-Terminal Domain of Human Collagenase-3 (MMP-13) in the Activation of Procollagenase-3, Substrate Specificity, and Tissue Inhibitor of Metalloproteinase Interaction. J. Biol. Chem. 272 (12), 7608–7616. 10.1074/jbc.272.12.7608 [DOI] [PubMed] [Google Scholar]
- Krajka-Kuźniak V., Cykowiak M., Szaefer H., Kleszcz R., Baer-Dubowska W. (2020). Combination of Xanthohumol and Phenethyl Isothiocyanate Inhibits NF-Κb and Activates Nrf2 in Pancreatic Cancer Cells. Toxicol. Vitro 65, 104799. 10.1016/j.tiv.2020.104799 [DOI] [PubMed] [Google Scholar]
- Lee H., Zhao X., Son Y-O., Yang S. (2021). Therapeutic Single Compounds for Osteoarthritis Treatment. Pharmaceuticals 14 (2), 131. 10.3390/ph14020131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legette L., Ma L., Reed R. L., Miranda C. L., Christensen J. M., Rodriguez-Proteau R., et al. (2012). Pharmacokinetics of Xanthohumol and Metabolites in Rats after Oral and Intravenous Administration. Mol. Nutr. Food Res. 56 (3), 466–474. 10.1002/mnfr.201100554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang D., Yuan Y., Min J. (2017). New Insights on the MMP-13 Regulatory Network in the Pathogenesis of Early Osteoarthritis. Arthritis Res. Ther. 19 (1), 248. 10.1186/s13075-017-1454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Yao Y., Huang H., Hao H., Ying M. (2018). Xanthohumol Attenuates Cisplatin-Induced Nephrotoxicity through Inhibiting NF-Κb and Activating Nrf2 Signaling Pathways. Int. Immunopharmacology 61, 277–282. 10.1016/j.intimp.2018.05.017 [DOI] [PubMed] [Google Scholar]
- Nambi G. (2020). Does Low Level Laser Therapy Has Effects on Inflammatory Biomarkers IL-1β, IL-6, TNF-α, and MMP-13 in Osteoarthritis of Rat Models-A Systemic Review and Meta-Analysis. Lasers Med. Sci. 36, 475–484. 10.1007/s10103-020-03124-w [DOI] [PubMed] [Google Scholar]
- Nguyen L., Sharma A., Chakraborty C., Saibaba B., Ahn M-E., Lee S-S. (2017). Review of Prospects of Biological Fluid Biomarkers in Osteoarthritis. Ijms 18 (3), 601. 10.3390/ijms18030601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R., Hata K., Takahata Y., Murakami T., Nakamura E., Ohkawa M., et al. (2020). Role of Signal Transduction Pathways and Transcription Factors in Cartilage and Joint Diseases. Ijms 21 (4), 1340. 10.3390/ijms21041340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Lee C. G., Han S. J., Yun S. H., Hwang S., Jeon H., et al. (2021). Antiosteoarthritic Effect of Morroniside in Chondrocyte Inflammation and Destabilization of Medial Meniscus-Induced Mouse Model. Ijms 22 (6), 2987. 10.3390/ijms22062987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickarski M., Hayami T., Zhuo Y., Duong L. T. (2011). Molecular Changes in Articular Cartilage and Subchondral Bone in the Rat Anterior Cruciate Ligament Transection and Meniscectomized Models of Osteoarthritis. BMC Musculoskelet. Disord. 12, 197. 10.1186/1471-2474-12-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki Y., Matsui T., Saisho K., Tohma S. (2012). Current Treatments of Rheumatoid Arthritis: from the 'NinJa' Registry. Expert Rev. Clin. Immunol. 8 (5), 455–465. 10.1586/eci.12.35 [DOI] [PubMed] [Google Scholar]
- Shamdani S., Chantepie S., Flageollet C., Henni-Chebra N., Jouan Y., Eymard F., et al. (2020). Heparan Sulfate Functions Are Altered in the Osteoarthritic Cartilage. Arthritis Res. Ther. 22 (1), 283. 10.1186/s13075-020-02352-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke D., Schulz T., Prehm P. (2011). Inhibitors of Hyaluronan Export from Hops Prevent Osteoarthritic Reactions. Mol. Nutr. Food Res. 55 (3), 485–494. 10.1002/mnfr.201000210 [DOI] [PubMed] [Google Scholar]
- Sun Y., Zuo Z., Kuang Y. (2020). An Emerging Target in the Battle against Osteoarthritis: Macrophage Polarization. Ijms 21 (22), 8513. 10.3390/ijms21228513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Luo J., Jing X., Xiang W., Guo J., Yao X., et al. (2021). Hyperoside Ameliorates the Progression of Osteoarthritis: An In Vitro and In Vivo Study. Phytomedicine 80, 153387. 10.1016/j.phymed.2020.153387 [DOI] [PubMed] [Google Scholar]
- Tsushima H., Okazaki K., Hayashida M., Ushijima T., Iwamoto Y. (2012). CCAAT/enhancer Binding Protein β Regulates Expression of Matrix Metalloproteinase-3 in Arthritis. Ann. Rheum. Dis. 71 (1), 99–107. 10.1136/annrheumdis-2011-200061 [DOI] [PubMed] [Google Scholar]
- Wang X., Ho S-L., Poon C-Y., Yan T., Li H-W., Wong M. S. (2019). Amyloid-β Aggregation Inhibitory and Neuroprotective Effects of Xanthohumol and its Derivatives for Alzheimer's Diseases. Car 16 (9), 836–842. 10.2174/1567205016666190827123222 [DOI] [PubMed] [Google Scholar]
- Wang H., Jiang Z., Pang Z., Qi G., Hua B., Yan Z., et al. (2021). Engeletin Protects against TNF-α-Induced Apoptosis and Reactive Oxygen Species Generation in Chondrocytes and Alleviates Osteoarthritis In Vivo . Jir 14, 745–760. 10.2147/jir.S297166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Lin Z., Yan Z., Wang Z., Fu X., Yu K. (2019). Sinomenine Contributes to the Inhibition of the Inflammatory Response and the Improvement of Osteoarthritis in Mouse-Cartilage Cells by Acting on the Nrf2/HO-1 and NF-Κb Signaling Pathways. Int. Immunopharmacology 75, 105715. 10.1016/j.intimp.2019.105715 [DOI] [PubMed] [Google Scholar]
- Zecchini S., Giovarelli M., Perrotta C., Morisi F., Touvier T., Di Renzo I., et al. (2019). Autophagy Controls Neonatal Myogenesis by Regulating the GH-IGF1 System through a NFE2L2- and DDIT3-Mediated Mechanism. Autophagy 15 (1), 58–77. 10.1080/15548627.2018.1507439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y-L., Zhao W., Liu M., Liu L., Wang Y. (2020). TBHQ-overview of Multiple Mechanisms against Oxidative Stress for Attenuating Methamphetamine-Induced Neurotoxicity. Oxidative Med. Cell Longevity 2020, 1–10. 10.1155/2020/8874304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K., Baldinger J., Mayerhofer B., Atanasov A. G., Dirsch V. M., Heiss E. H. (2015). Activated AMPK Boosts the Nrf2/HO-1 Signaling Axis-A Role for the Unfolded Protein Response. Free Radic. Biol. Med. 88 (Pt B), 417–426. 10.1016/j.freeradbiomed.2015.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.