Abstract
STUDY QUESTION
Do parental characteristics and treatment with ART affect perinatal outcomes in singleton pregnancies?
SUMMARY ANSWER
Both parental and ART treatment characteristics affect perinatal outcomes in singleton pregnancies.
WHAT IS KNOWN ALREADY
Previous studies have shown that singleton pregnancies resulting from ART are at risk of preterm birth. ART children are lighter at birth after correction for duration of gestation and at increased risk of congenital abnormalities compared to naturally conceived children. This association is confounded by parental characteristics that are also known to affect perinatal outcomes. It is unclear to which extent parental and ART treatment characteristics independently affect perinatal outcomes.
STUDY DESIGN, SIZE, DURATION
All IVF clinics in the Netherlands (n = 13) were requested to provide data on all ART treatment cycles (IVF, ICSI and frozen-thawed embryo transfers (FET)), performed between 1 January 2000, and 1 January 2011, which resulted in a pregnancy. Using probabilistic data-linkage, these data (n = 36 683) were linked to the Dutch Perinatal Registry (Perined), which includes all children born in the Netherlands in the same time period (n = 2 548 977).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Analyses were limited to singleton pregnancies that resulted from IVF, ICSI or FET cycles. Multivariable models for linear and logistic regression were fitted including parental characteristics as well as ART treatment characteristics. Analyses were performed separately for fresh cycles and for fresh and FET cycles combined. We assessed the impact on the following perinatal outcomes: birth weight, preterm birth below 37 or 32 weeks of gestation, congenital malformations and perinatal mortality.
MAIN RESULTS AND THE ROLE OF CHANCE
The perinatal outcomes of 31 184 out of the 36 683 ART treatment cycles leading to a pregnancy were retrieved through linkage with the Perined (85% linkage). Of those, 23 671 concerned singleton pregnancies resulting from IVF, ICSI or FET. Birth weight was independently associated with both parental and ART treatment characteristics. Characteristics associated with lower birth weight included maternal hypertensive disease, non-Dutch maternal ethnicity, nulliparity, increasing duration of subfertility, hCG for luteal phase support (compared to progesterone), shorter embryo culture duration, increasing number of oocytes retrieved and fresh embryo transfer. The parental characteristic with the greatest effect size on birth weight was maternal diabetes (adjusted difference 283 g, 95% CI 228–338). FET was the ART treatment characteristic with the greatest effect size on birth weight (adjusted difference 100 g, 95% CI 84–117) compared to fresh embryo transfer. Preterm birth was more common among mothers of South-Asian ethnicity. Preterm birth was less common among multiparous women and women with ‘male factor’ as treatment indication (compared to ‘tubal factor’).
LIMITATIONS, REASONS FOR CAUTION
Due to the retrospective nature of our study, we cannot prove causality. Further limitations of our study were the inability to adjust for mothers giving birth more than once in our dataset, missing values for several variables and limited information on parental lifestyle and general health.
WIDER IMPLICATIONS OF THE FINDINGS
Multiple parental and ART treatment characteristics affect perinatal outcomes, with birth weight being influenced by the widest range of factors. This highlights the importance of assessing both parental and ART treatment characteristics in studies that focus on the health of ART-offspring, with the purpose of modifying these factors where possible. Our results further support the hypothesis that the embryo is sensitive to its early environment.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by Foreest Medical School, Alkmaar, the Netherlands (grants: FIO 1307 and FIO 1505). B.W.M. reports grants from NHMRC and consultancy for ObsEva, Merck KGaA, iGenomics and Guerbet. F.B. reports research support grants from Merck Serono and personal fees from Merck Serono. A.C. reports travel support from Ferring BV. and Theramex BV. and personal fees from UpToDate (Hyperthecosis), all outside the remit of the current work. The remaining authors report no conflict of interests.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: ART, IVF, ICSI, subfertility, perinatal outcomes, ART safety
Introduction
During the late 1970s, IVF was developed as a fertility treatment for women with tubal occlusion (Steptoe and Edwards, 1978). Since then, the range of indications for ART treatment has been broadened. Technique innovations have included the introduction of ovarian stimulation (Steptoe et al., 1986), freezing and thawing of gametes and embryos (Zeilmaker et al., 1984; Downing et al., 1985), ICSI (Palermo et al., 1992) and the introduction of several commercially available embryo culture media. These changes aimed to improve ongoing pregnancy and live birth rates per aspiration cycle. Strikingly, these changes almost without exception were introduced with little to no preclinical or clinical trials to assess the effects on pregnancy complications and neonatal outcomes (Braakhekke et al., 2014).
Evidence is growing that ART treatment characteristics may have a negative impact on perinatal outcomes including low birth weight for gestational age and a higher risk of preterm birth, not only in multiple pregnancies but also in singletons (Pinborg et al., 2013; Marino et al., 2014; Qin et al., 2016). Data suggest that birth weight is affected by subfertility itself and by several ART treatment characteristics such as the use of frozen embryos for transfer, the supraphysiological levels of estrogen resulting from ovarian stimulation, the choice of embryo culture medium and the transfer of multiple embryos (De Sutter et al., 2006; Dumoulin et al., 2010; Pelkonen et al., 2010; Sazonova et al., 2011a, 2012; Hu et al., 2014; Kleijkers et al., 2016; Seggers et al., 2016). Furthermore, the transfer of multiple embryos may increase the risk of perinatal mortality and preterm birth in singleton pregnancies (De Sutter et al., 2006; Sullivan et al., 2012). Studies on the effects of excessive ovarian stimulation (more than 20 oocytes yielded) on perinatal outcomes have shown conflicting results (Sunkara et al., 2015; Magnusson et al., 2018). Thus far, there is no consensus on which parental and ART treatment characteristics independently affect perinatal outcomes and to which extent.
Studies on the effects of ART treatment on perinatal outcomes often focus on a single aspect of the ART treatment. As ART treatment characteristics are highly dependable on parental characteristics, this study aimed to focus on contextualising the independent effects of the parental as well as the ART treatment features that could determine perinatal outcome. We addressed this question by linking clinical and laboratory data of all ART treatments performed in Dutch IVF clinics from 2000 until 2011 to the Dutch Perinatal Registry (Perined) to create a large retrospective national cohort.
Materials and methods
Data sources, study population and available variables
National ART dataset
All 13 Dutch IVF clinics were requested to provide retrospective data for each ART treatment cycle that led to a pregnancy between 1 January 2000 and 1 January 2011. Pregnancy was defined as the presence of hCG hormone tested in a urine or blood sample following ART treatment. Treatment cycles were excluded if pregnancy loss or termination was registered and had occurred prior to 20 weeks of gestation since this is the earliest gestational age for which perinatal outcomes are registered in the Dutch Perinatal Registry (Perined).
Information on patient characteristics and ART treatment information was retrieved from the clinic’s electronic patient records system (EPR) in which embryologists and physicians register treatment specifics for each patient receiving ART treatment. The information retrieved from the EPR contained maternal and paternal age, duration of subfertility, the indication for ART treatment and the medication regimen used for the treatment (pituitary downregulation, stimulation and luteal phase support). Treatment indications were divided in categories, namely ‘tubal factor’ (unilateral or bilateral), ‘cervical factor’, ‘combination’, ‘endometriosis’, ‘failed insemination’ (with sperm of partner or sperm donor), ‘male factor’, ‘female non-tubal factor’ (comprising ovulatory disorders such as polycystic ovary syndrome, hypopituarism and imminent ovarian failure), ‘unexplained’ and ‘not registered’. The information on the ART treatment laboratory phase provided by the IVF clinics included data on number of oocytes retrieved at the time of aspiration, fertilisation method (IVF, ICSI), transfer of fresh or frozen embryos and the number of embryos transferred. Information on used culture medium and usage period was reported separately by each IVF clinic. The number of oocytes retrieved was used as a proxy for intensity of ovarian stimulation in the absence of information on estrogen levels. The information on indication for treatment included the category ‘not registered’ (25–30.5%) which was included in the analyses but because of the lack of information regarding it, we abstained from interpreting the related results.
Dutch perinatal registry (Perined)
Information on maternal characteristics such as ethnicity and parity, singleton or multiple births, pregnancy complications and perinatal outcomes were extracted from the Perined (In Dutch: Stichting Perinatale Registratie Nederland). This is a validated population-based probabilistically linked dataset containing information on pregnancy complications, delivery, neonatal outcomes and neonatal hospital readmission up to 28 days. Perined comprises ∼96% of all deliveries in the Netherlands from 20 gestational weeks onwards (Tromp et al., 2008; Kok et al., 2014). Méray et al. (2007) previously described the development and validation of the Perined dataset: perinatal care providers record the information during gestation, at the time of delivery or during neonatal readmission to the hospital. The information provided does not include information on previous pregnancies, with the exception of parity. Depending on the situation, a perinatal care provider could be a paediatrician, a midwife (attending a hospital or a home delivery), a general practitioner attending a home delivery or an obstetrician or a registrar attending a delivery.
Maternal ethnicity in Perined is based on race and country of birth and categorised in ‘Dutch’, ‘Mediterranean’, ‘Other European’, ‘African’, ‘South-Asian’, ‘East-Asian’ and ‘Mixed/Other’. ‘Mediterranean’ comprises non-European women from countries around the Mediterranean Sea, ‘African’ comprises women of (originally) Sub-Saharan African origin, ‘South-Asian’ comprises women originally from the Indian subcontinent (Schaaf et al., 2012).
Data linkage and validation
In order to comply with the Dutch patient privacy protection law (in Dutch: ‘Wet Bescherming Persoonsgegevens’ (WBP)), neither of the datasets included unique personal identifying numbers.
To combine the data from the National IVF and the Perined datasets, it was necessary to determine, for all possible pairs of observations, whether they belonged to the same pregnancy (i.e. matches) or not (i.e. non-matches). Therefore, the two databases were combined by probabilistic linkage (Fellegi and Sunter, 1969) by using the following variables: the first set of digits and the second set of digits of the postal code, the maternal day, month and year of birth, and the child’s day, month and year of birth, if available. The threshold for identifying a record pair as a match was set at a probability of match of 0.9. Validation of the dataset was performed by comparing birth date, neonatal sex and singleton/multiple pregnancy between hospital records and our dataset in a sample of 105 records. See the Supplementary File S1 for a detailed description of the data retrieval, linkage and validation of the dataset.
Ethical concerns and data handling
The research protocol for this study was reviewed by a Data Protection Officer for aspects involving patient identifying information in order for this study to comply with Dutch patient privacy protection laws. To ensure patient privacy, case records, provided by the Dutch IVF clinics, were stripped of directly identifying information (names, addresses, personal patient hospital number and citizen service number). Indirectly identifying information necessary for data linkage (birth date of mother and child and four digits of the postal code) was only used for the linkage purpose and later removed from all files to make the linked dataset anonymous. The institutional review boards of participating clinics issued a waiver for the Medical Research Act (in Dutch: Wet medisch-wetenschappelijk onderzoek met mensen (WMO)). Perined gave approval for the data linkage and the use of data for the purpose of this study (approval number 12.43).
Outcomes
Perined provided information on the following perinatal outcomes of interest: birth weight (reported in grams), duration of gestation (days), the presence of congenital malformations and the occurrence of perinatal mortality (stillbirths after 20 weeks of gestation, intrapartum death or neonatal death within 28 days after birth). Furthermore, it provided information on maternal hypertensive disease (pre-existing as well as pregnancy-induced hypertension), maternal diabetes mellitus (pre-existing diabetes mellitus as well as gestational diabetes), delivery mode and spontaneous or medically indicated start of delivery. Using birth weight and duration of gestation, neonates small for gestational age (SGA) or large for gestational age (LGA) were identified, defined, respectively, as a birth weight below the 5th percentile or above the 95th percentile on Dutch reference curves for birth weight by gestational age, parity and sex which are based on the entire population in the Netherlands without exclusion of ethnic minorities (Visser et al., 2009). We defined preterm birth as delivery before 37 weeks of gestation and early preterm birth as delivery before 32 weeks of gestation. Congenital abnormalities in the Perined are registered using a predefined list of diagnoses based on ICD-codes (Hindori-Mohangoo, 2014) and are registered in live births as well as in case of a still birth. We defined congenital malformations as the presence of a minor or major birth defect as registered in the Perined database after birth or in the first 28 days thereafter.
Sample selection, missing values and statistical analysis
For this study, we used only pregnancies of singletons resulting from ART treatment cycles with IVF, ICSI or frozen-thawed embryo transfer (FET) (IVF and ICSI). Ovulation induction and intrauterine insemination treatments, treatment cycles where pre-implantation genetic diagnosis (PGD) was applied and treatments with the use of donor oocytes were excluded after linkage to the Perined database. Treatments where testicular or epididymal sperm aspiration (TESE, PESA or MESA) had been applied were excluded due to the small number of records in the dataset.
Missing values from variables in the linked dataset were imputed in R 3.3 (R Core Team, 2013) using multivariate imputation by chained equation (MICE) (Van Buuren and Groothuis-Oudshoorn, 2011). Resulting values were pooled from 10 imputed datasets.
Because of considerable number of missing values on stimulation regime and culture medium for FET (e.g. culture medium missing in 75% for FET cycles in comparison to 6% missing for fresh cycles), we chose to create two separate imputed datasets to optimise multiple imputation reliability (Dong and Peng, 2013).
The first dataset included only ART treatments with fresh embryo transfers, FET cycles were excluded. For this dataset, maternal and paternal age, duration of subfertility, indication for treatment, maternal hypertensive disease and diabetes, delivery mode, spontaneous or medically indicated start of delivery, treatment type, downregulation, stimulation and luteal phase medication, culture medium, number of embryos transferred, neonatal sex and perinatal and neonatal outcomes could be imputed. Further on we shall refer to this dataset as the ‘fresh transfer dataset’.
A second dataset included treatments with fresh embryo transfers as well as FET treatments. For this dataset, maternal and paternal age, indication for treatment, maternal hypertensive disease and diabetes, delivery mode, spontaneous or medically indicated start of delivery, treatment type, number of embryos transferred, neonatal sex and perinatal and neonatal outcomes were imputed. Further on we shall refer to this dataset as the ‘fresh + frozen transfer dataset’.
To assess the quality of the linked dataset, we compared baseline characteristics of the linked patient records with that of the non-linked records (records where linkage had failed).
Furthermore, we compared the perinatal outcomes of the children born from fresh embryo transfers and children born after FET to the singletons born in the general population in the Netherlands between 2009 and 2011. For this, we used the overviews provided by Perined of the general population born in the Netherlands in 2009, 2010 and 2011 as perinatal outcomes are recorded using the same method and therefor guarantee equivalent data type and quality (Stichting Perinatale Registratie Nederland, 2013a,b,c).
We fitted multivariable linear and logistic regression models to assess the impact of parental and ART treatment characteristics on perinatal outcomes (birth weight, occurrence of preterm and very preterm birth, occurrence of congenital malformation and perinatal mortality). These analyses were performed in the fresh transfer dataset and in the fresh + frozen transfer dataset separately. Association between parental and ART treatment characteristics and outcome was defined as P < 0.001.
For analysis in the fresh transfer dataset, the model included the following predictive variables: parental age, maternal ethnicity (reference: Dutch), nulliparity, duration of subfertility (years), indication for treatment (reference: ‘tubal factor’), maternal hypertensive disease and diabetes, culture medium (reference: HTF medium), fertilisation method (reference: IVF), duration of embryo culture (reference: 3 days), pituitary downregulation medication (reference: GnRH agonist), ovarian stimulation medication (reference: recombinant FSH), luteal phase support medication (reference: progesterone), number of oocytes retrieved at time of aspiration, duration of gestation, single or multiple embryo transfer (reference: single embryo transfer), duration of gestation (excluded in logistic regression analysis for preterm birth) and neonatal sex (reference: boy). ‘Tubal factor’ was selected as the reference group for indication of treatment as other indications (e.g. ‘female non-tubal factor’ or ‘male factor’) may be associated to a larger extent with endocrine or medical disorders which in turn may influence perinatal outcomes.
For analysis with the fresh + frozen transfer dataset, the models included the following predictive variables: parental age, maternal ethnicity (reference: Dutch), nulliparity, indication for treatment (reference: ‘tubal factor’), maternal hypertensive disease and diabetes, FET or fresh embryo transfer (reference: fresh embryo transfer), single or multiple embryo transfer (reference: single embryo transfer), duration of gestation (excluded in logistic regression analysis for preterm birth) and neonatal sex (reference: boy).
Logistic regression for preterm and early preterm birth was performed separately in the group with spontaneous delivery and in the group with medically indicated delivery.
Results
Study characteristics
The 13 Dutch clinics provided information on 37 487 ART treatment cycles leading to a pregnancy, comprising cycles with ovulation induction, intrauterine insemination, IVF, ICSI, FET, oocyte donation treatments, PGD, TESA, PESA and MESA. Of these, 33 110 consisted of IVF ICSI and FET cycles (characteristics of these cycles are presented in Table I).
Table I.
Data characteristics for each ART Clinic prior to selection for linkage, linkage and imputation for IVF, ICSI and FET cycles only.
| Clinic | 1 | 2a | 2b | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycles. n | 1519 | 1906 | 2718 | 3305 | 2510 | 532 | 1695 | 5341 | 2971 | 2254 | 3705 | 2492 | 756 | 1406 | |
| Period (month-year) | 09-1999/ 09-2010 | 01-2008/ 01-2011 | 03-1999/ 12-2007 | 01-1999/ 05-2011 | 01-2000/ 01-2011 | 01-2006/ 01-2011 | 01-2000/ 01- 2011 | 01- 2000/ 02-2012 | 11-2000/ 01-2011 | 01-2000/ 01-2011 | 01-1999/ 01-2011 | 01-2000/ 01-2011 | 04-2001/ 01-2011 | 02-2002/ 01-2011 | |
| Duration subfertility (years) | |||||||||||||||
| Missing | 100.0% | 13.0% | 1.9% | 61.0% | 4.0% | 31.0% | 1.4% | 16.3% | 9.9% | 100.0% | 32.7% | 34.0% | 4.0% | 42% | |
| Mean (SD) | 3.2 (2.2) | 3.7 (2.4) | 4.3 (6.9) | 3.5 (2.3) | 3.7 (2.1) | 2.9 (3.2) | 4.1 (2.7) | 4 (8.13) | 3.5 (2.2) | 2.8 (2.2) | 3.4 (2.0) | 3.4 (2.1) | |||
| Indication for fertility treatment | |||||||||||||||
| Missing | 0.0% | 9.1% | 1.3% | 0.0% | 13.5% | 0.0% | 0.0% | 0.0% | 0.0% | 12.7% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Tubal factor | 14.2% | 11.3% | 17.8% | 0.2% | 12.4% | 0.6% | 13.2% | 11.5% | 8.9% | 9.8% | 3.8% | 8.1% | 7.4% | 6.0% | |
| Cervical factor | 1.3% | 0.6% | 1.1% | 0.0% | 3.3% | 0.0% | 0.0% | 0.4% | 1.4% | 0.0% | 1.2% | 3.2% | 0.0% | 1.6% | |
| Combination | 6.8% | 9.5% | 9.0% | 0.3% | 0.0% | 77.3% | 0.0% | 13.3% | 0.0% | 0.0% | 3.1% | 12.2% | 41.7% | 9.0% | |
| Endometriosis | 1.1% | 5.7% | 6.5% | 0.2% | 8.2% | 0.0% | 6.7% | 1.4% | 4.5% | 2.8% | 1.2% | 2.7% | 2.1% | 2.0% | |
| Failed insemination | 1.3% | 3.3% | 0.0% | 0.0% | 1.5% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Male factor | 35.6% | 38.9% | 45.0% | 0.5% | 42.2% | 1.9% | 58.9% | 31.2% | 60.9% | 39.8% | 17.9% | 35.5% | 36.8% | 34.7% | |
| Female non-tubal factor | 2.8% | 3.3% | 2.6% | 0.1% | 4.3% | 0.0% | 3.2% | 1.9% | 4.5% | 5.1% | 3.7% | 3.2% | 0.0% | 4.1% | |
| Unexplained | 20.1% | 15.6% | 13.5% | 0.3% | 13.0% | 0.4% | 14.0% | 17.1% | 17.2% | 21.5% | 10.0% | 7.7% | 10.7% | 10.6% | |
| Unknown | 16.1% | 2.6% | 3.2% | 98.4% | 0.5% | 19.9% | 3.9% | 23.2% | 2.4% | 8.3% | 59.0% | 27.4% | 1.3% | 32.0% | |
| Other | 0.7% | 0.0% | 0.0% | 0.0% | 1.1% | 0.0% | 0.0% | 0.0% | 0.2% | 0.0% | 0.1% | 0.0% | 0.0% | 0.1% | |
| Treatment type | |||||||||||||||
| IVF | 45.8% | 35.9% | 51.4% | 56.5% | 46.4% | 24.4% | 38.3% | 62.0% | 37.9% | 43.7% | 40.4% | 42.6% | 26.2% | 46.40% | |
| ICSI | 40.8% | 36.1% | 42.9% | 37.2% | 40.2% | 59.6% | 55.8% | 27.2% | 52.5% | 30.3% | 41.8% | 39.7% | 52.1% | 47.20% | |
| FET | 13.4% | 28.0% | 5.7% | 6.3% | 13.3% | 16.0% | 5.8% | 10.7% | 9.6% | 26.1% | 17.8% | 17.7% | 21.7% | 6.50% | |
| Downregulation type | |||||||||||||||
| Missing | 100.0% | 30.5% | 8.5% | 60.8% | 33.7% | 23.9% | 35.7% | 74.8% | 11.5% | 56.6% | 51.7% | 21.2% | 100.0% | 100.00% | |
| Agonist | 67.7% | 91.5% | 25.9% | 46.3% | 74.8% | 35.3% | 16.6% | 88.5% | 42.3% | 47.8% | 78.6% | ||||
| Antagonist | 1.8% | 0.0% | 13.3% | 20.0% | 1.3% | 29.0% | 8.7% | 0.0% | 1.1% | 0.4% | 0.2% | ||||
| Gonadotropin | |||||||||||||||
| Missing | 100.0% | 30.2% | 6.7% | 67.0% | 15.4% | 28.2% | 7.1% | 81.0% | 11.5% | 56.4% | 77.9% | 42.6% | 100.0% | 41.50% | |
| Rec-FSH | 62.5% | 90.9% | 33.0% | 27.6% | 71.8% | 89.7% | 19.0% | 88.5% | 43.1% | 21.4% | 57.0% | 58.30% | |||
| hMG | 7.3% | 2.1% | 0.0% | 56.9% | 0.0% | 3.1% | 0.0% | 0.0% | 0.5% | 0.6% | 0.0% | 0.00% | |||
| Luteal phase support | |||||||||||||||
| Missing | 100% | 0.2% | 0.8% | 53.2% | 13.9% | 41.5% | 2.2% | 42.5% | 100.0% | 56.7% | 38.1% | 35.9% | 100.0% | 100.0% | |
| Progesteron | 69.0% | 90.3% | 45.4% | 33.0% | 57.1% | 42.4% | 35.8% | 11.4% | 52.6% | 52.6% | |||||
| hCG | 5.3% | 4.1% | 0.5% | 50.2% | 0.8% | 53.9% | 21.0% | 0.2% | 7.1% | 1.0% | |||||
| Estrogen/progesteron | 0.5% | 0.0% | 0.3% | 2.9% | 0.0% | 0.0% | 0.2% | 0.0% | 0.3% | 3.2% | |||||
| hCG/progesteron | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 31.4% | 0.0% | 0.0% | |||||
| None | 25.0% | 4.9% | 0.5% | 0.0% | 0.6% | 1.5% | 0.4% | 0.3% | 1.9% | 7.2% | |||||
| Ooocytes retrieved | |||||||||||||||
| Missing | 13.4% | 28.0% | 6.1% | 8.3% | 13.4% | 26.5% | 8.1% | 12.6% | 9.7% | 26.1% | 23.3% | 20.4% | 21.7% | 7.40% | |
| Mean (SD) | 11.3 (5.8) | 10.4 (5.8) | 10 (5.5) | 9.6 (5.6) | 10.8 (6.7) | 8.1 (4.9) | 7.9 (6.6) | 9.2 (5.2) | 11.4 (5.4) | 9.6 (5.3) | 10.8 (5.5) | 9.8 (5.3) | 9.4 (4.9) | 10.5 (5.8) | |
| n embryos transferred | |||||||||||||||
| Missing | 0.0% | 0.2% | 100.0% | 2.1% | 0.0% | 7.7% | 3.5% | 2.5% | 9.7% | 0.0% | 6.3% | 4.9% | 0.3% | 2.30% | |
| 1 | 13.6% | 64.7% | 45.2% | 24.5% | 42.1% | 39.1% | 37.4% | 24.6% | 48.8% | 25.1% | 39.2% | 42.3% | 26.90% | ||
| >=2 | 86.4% | 35.0% | 52.5% | 75.5% | 50.2% | 57.0% | 59.9% | 65.7% | 51.2% | 68.0% | 55.9% | 56.3% | 70.50% | ||
% unless otherwise stated.
FET, frozen embryo transfer.
Clinic 2 provided information from two subsequent Electronic Patient Records (EPR, 2a and 2b).
Of all the provided cycles (n = 37 487), 36 683 ART treatment cycles were selected for probabilistic linkage as they comprised maternal birth date, postal code and a date of treatment. After probabilistic linkage, the corresponding Perined record was found for 31 184 (85%) ART treatments. Analyses were limited to pregnancies leading to singleton births (n = 26 403; 84.7% of 31 184). The following ART treatments were excluded: ovulation induction, intrauterine insemination, PGD, TESA, PESA MESA and oocyte donation cycles (n = 2732) leaving IVF, ICSI and FET cycles for analysis. The total linked database before imputation thus consisted of 23 671 mother–child records, including 20 264 fresh embryo transfer cycles and 3407 FET cycles. Parental and ART treatment characteristics were compared between the linked and non-linked records (data not shown). In the non-linked records, women were more often nulliparous compared to the linked records for fresh embryo transfer and less often for FET cycles (fresh embryo transfer group: 68.4% versus 67.4%, FET group: 51.6% versus 56.9%), had a lower rate of ‘male-factor’ as indication (fresh embryo transfer group: 33.9% versus 35.9%, FET group: 27.9% versus 31.7%) and a lower rate of single embryo transfer (fresh embryo transfer group: 32.2% versus 35.1%, FET group: 48.6% versus 53.3%). ART treatment records where no embryos were transferred were excluded (n = 38) as these would have concerned a natural conception after an oocyte retrieval and not an IVF/ICSI pregnancy and as because could not exclude an administrative mistake in the reported number of embryos transferred. In order for multiple imputation to be representative, we removed 129 records with extremely rare covariate values, leaving 23 504 linked mother–child records available for imputation and analysis (Van Buuren and Groothuis-Oudshoorn, 2011).
ART data description
The 13 IVF clinics delivered their data from their EPR; one clinic delivered the data from two subsequent EPR systems. When considering treatments with IVF, ICSI or FET, the number of patient records delivered varied between 532 and 5341. The period over which records were delivered varied per clinic with a period range between January 1999 and February 2012, since fiver clinics provided data for a wider period as this was easily available into their electronic patient records. An overview of the quality of the data concerning IVF, ICSI and FET cycles prior to selection for linkage, linkage and imputation of missing values is shown in Table I.
Parental and neonatal characteristics
Baseline characteristics of the study population (n = 23 504) are shown in Table II. Compared to the singletons from the general Dutch population in 2009–2011 (n = 520 371 singletons), children born from fresh embryo transfer cycles have lower birth weight (3347 g versus 3437–3448 g in the general population), a higher incidence of preterm birth (preterm birth: 8.8% compared to 6.0% in the general population; early preterm birth: 1.8% versus 1.1% in the general population), a higher incidence of congenital malformations (3.4% versus 3.0% in the general population) and a higher perinatal mortality rate (1.2% versus 0.8% in the general population).
Table II.
Characteristics of parents, fertility treatment and neonates stratified by treatment cycles.
| Fresh transfer cycles |
Frozen transfer (FET) cycles |
||||
|---|---|---|---|---|---|
| n = 20 147 | n = 3357 | ||||
| Parental characteristics | Missing % | Missing % | |||
| Age mother (years) a | 34.0 (4.1) | 0.0% | 34.2 (4.1) | 0.0% | |
| Age father (years) a | 36.7 (5.6) | 18.3% | 36.8 (5.4) | 31.8% | |
| Nulliparity | 13 549 (67.3%) | 0.0% | 1795 (53.4%) | 0.0% | |
| Ethnicity mother | 0.6% | 0.5% | |||
| Dutch | 17 260 (85.7%) | 2760 (82.2%) | |||
| Mediterranean | 976 (4.8%) | 208 (6.2%) | |||
| Other European | 610 (3%) | 112 (3.3%) | |||
| African | 250 (1.2%) | 59 (1.8%) | |||
| South-Asian | 200 (1%) | 52 (1.5%) | |||
| East-Asian | 295 (1.5%) | 65 (1.9%) | |||
| Mixed/other | 432 (2.1%) | 84 (2.5%) | |||
| Duration subfertility (years) a | 3.6 (2.3) | 28.6% | 3.6 (2.3) | 42.8% | |
| Indication for fertility treatment | 3% | 3.6% | |||
| Tubal factor | 1804 (9%) | 306 (9.1%) | |||
| Cervical factor | 226 (1.1%) | 25 (0.7%) | |||
| Combination | 1544 (7.7%) | 225 (6.7%) | |||
| Endometriosis | 652 (3.2%) | 102 (3%) | |||
| Failed insemination | 75 (0.4%) | 19 (0.6%) | |||
| Male factor | 7070 (35.1%) | 1034 (30.8%) | |||
| Female non-tubal factor | 609 (3%) | 100 (3%) | |||
| Unexplained | 2640 (13.1%) | 400 (11.9%) | |||
| Not registered | 5033 (25%) | 1025 (30.5%) | |||
| Maternal hypertensive disease | 2221 (11%) | 0.0 | 389 (11.6%) | 0.0% | |
| Maternal diabetes | 254 (1.3%) | 0.0% | 39 (1.2%) | 0.0% | |
| Fertility treatment | |||||
| Downregulation type | 39.9% | – | 93.9% | ||
| Agonist | 10 553 (52.4%) | ||||
| Antagonist | 1548 (7.7%) | ||||
| Stimulation type | 40.3% | – | 93.8% | ||
| Recombinant FSH | 10 842 (53.8%) | ||||
| hMG | 1183 (5.9%) | ||||
| Luteal phase support | 40.6% | – | 71.4% | ||
| Progesterone | 8376 (41.6%) | ||||
| hCG | 2704 (13.4%) | ||||
| Oestrogen/progesterone | 49 (0.2%) | ||||
| hCG/progesterone | 544 (2.7%) | ||||
| None | 300 (1.5%) | ||||
| Number of oocytes at retrieval | 9.8 (5.7) | 1.9% | – | 99.9% | |
| ICSI | 9425 (46.8%) | 0% | – | – | |
| Medium | 5.6% | – | 75.2% | ||
| HTF | 11 051 (54.9%) | ||||
| Cook | 80 (0.4%) | ||||
| Universal IVF medium | 1140 (5.7%) | ||||
| Gain | 1409 (7%) | ||||
| Global | 308 (1.5%) | ||||
| GZ | 2140 (10.6%) | ||||
| In-vitro care | 798 (4%) | ||||
| Sage | 886 (4.4%) | ||||
| Scandinavian IVF Science | 35 (0.2%) | ||||
| Vitrolife | 1118 (5.5%) | ||||
| Zeilmaker | 52 (0.3%) | ||||
| Duration embryo culture | 13.8% | 100% | |||
| 1–2 days | 2436 (12.1%) | ||||
| 3 days | 11 366 (56.4%) | ||||
| 4 days | 3331 (16.5%) | ||||
| 5–6 days | 237 (1.2%) | ||||
| Multiple embryo transfer | 10 517 (52.2%) | 10.4% | 1218 (36.3%) | 13.5% | |
| Pregnancy | |||||
| Pregnancy duration (days) a | 275 (16.4) | 0.7% | 276 (15.2) | 0.7% | |
| Preterm delivery <32 weeks | 369 (1.8%) | 44 (1.3%) | |||
| Preterm delivery <37 weeks | 1763 (8.8%) | 241 (7.2%) | |||
| Medically indicated delivery | 8985 (44.6%) | 3.0% | 1585 (47.2%) | 3.6% | |
| Neonatal characteristics | |||||
| Birth weight (g) a | 3347 (644) | 0.0% | 3505 (620) | 0.0% | |
| Girl | 9813 (48.7%) | 0.0% | 1666 (49.6%) | 0.0% | |
| Perinatal infant mortality | 246 (1.2%) | 0.0% | 28 (0.8%) | 0.0% | |
| Congenital malformation | 685 (3.4%) | 0.0% | 110 (3.3%) | 0.0% | |
n and % unless otherwise stated.
mean (SD).
Children born from FET cycles have a higher birth weight (3505 g versus 3437–3448 g in the general population), a higher incidence of preterm birth (preterm birth: 7.2% compared to 6.0% in the general population; early preterm birth: 1.3% versus 1.1% in the general population), a higher incidence of congenital malformations (3.3% versus 3.0% in the general population) and similar perinatal mortality (both 0.8%) compared to the general Dutch population born in 2009–2011.
We present all parental and ART treatment characteristics that were associated after multivariable linear and logistic regression analysis for each of the five perinatal outcomes. The full results showing the impact of each parental and ART treatment characteristics are shown in Figs 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 and 14.
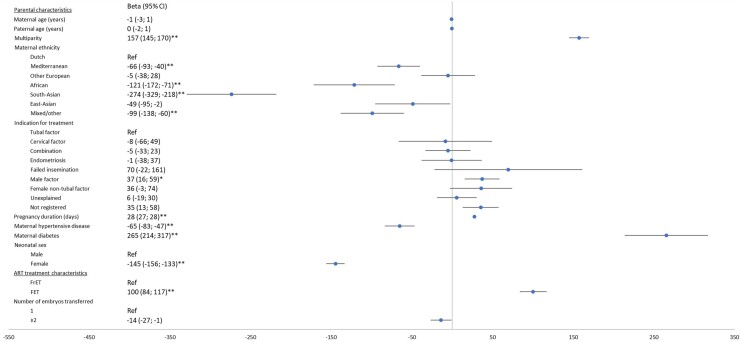
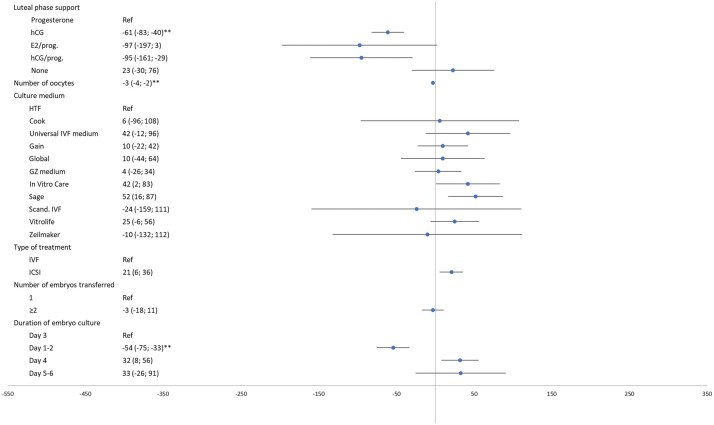
Figure 1.
Multivariable linear regression analysis of parental and treatment characteristics effect on birth weight for fresh transfer cycles only (n = 20 147). Fresh: analysis in the fresh transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, duration of subfertility, indication for treatment, maternal hypertensive and diabetic disease, culture medium, IVF or ICSI use, duration of embryonal culture, downregulation medication, ovarian stimulation medication, luteal phase support medication, number of oocytes retrieved at time of aspiration, single or multiple embryo transfer, duration of gestation and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. Rec. FSH, recombinant FSH.
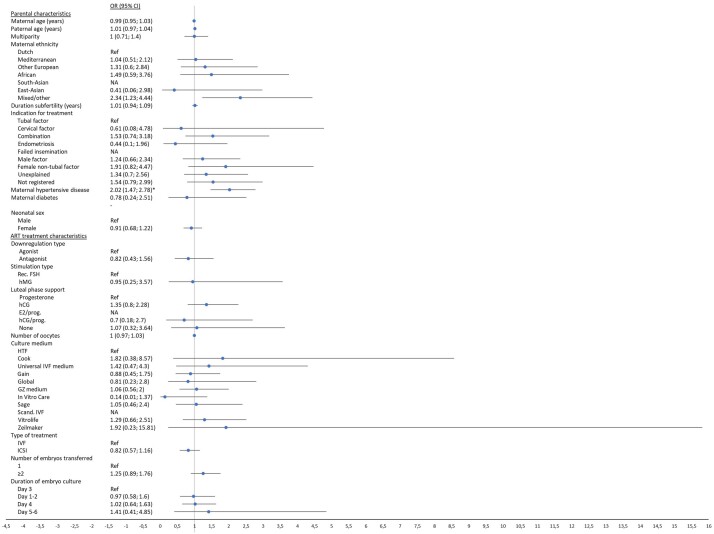
Figure 2.
Multivariable linear regression analysis of parental and treatment characteristics effect on birth weight for fresh and frozen transfer treatments combined (n = 23 504). Fresh + frozen transfer analysis in the fresh + frozen transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, indication for treatment, maternal hypertensive and diabetic disease, fresh or frozen embryo transfer, single or multiple embryo transfer, duration of gestation and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. FrET, fresh embryo transfer; FET, frozen embryo transfer.
Figure 3.
Multivariable logistic regression analysis of parental and treatment characteristics effect on spontaneous preterm birth (<37 weeks of gestation) for fresh transfer cycles only (n = 20 147). Fresh: analysis in the fresh transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, duration of subfertility, indication for treatment, maternal hypertensive and diabetic disease, culture medium, IVF or ICSI use, duration of embryonal culture, downregulation medication, ovarian stimulation medication, luteal phase support medication, number of oocytes retrieved at time of aspiration, single or multiple embryo transfer and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. Rec. FSH, recombinant FSH; NA, not applicable.
Figure 4.
Multivariable logistic regression analysis of parental and treatment characteristics effect on spontaneous preterm birth (<37 weeks of gestation) for fresh and frozen transfer treatments combined (n = 23 504). Fresh + frozen transfer analysis in the fresh + frozen transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, indication for treatment, maternal hypertensive and diabetic disease, fresh or frozen embryo transfer, single or multiple embryo transfer and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. FrET, fresh embryo transfer; FET, frozen embryo transfer.
Figure 5.
Multivariable logistic regression analysis of parental and treatment characteristics effect on spontaneous early preterm birth (<32 weeks of gestation) for fresh transfer cycles only (n = 20 147). Fresh: analysis in the fresh transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, duration of subfertility, indication for treatment, maternal hypertensive and diabetic disease, culture medium, IVF or ICSI use, duration of embryonal culture, downregulation medication, ovarian stimulation medication, luteal phase support medication, number of oocytes retrieved at time of aspiration, single or multiple embryo transfer and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. Rec. FSH, recombinant FSH; NA, not applicable.
Figure 6.
Multivariable logistic regression analysis of parental and treatment characteristics effect on spontaneous early preterm birth (<32 weeks of gestation) for fresh and frozen transfer treatments combined (n = 23 504). Fresh + frozen transfer analysis in the fresh + frozen transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, indication for treatment, maternal hypertensive and diabetic disease, fresh or frozen embryo transfer, single or multiple embryo transfer and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. FrET, fresh embryo transfer; FET, frozen embryo transfer; NA, not applicable.
Figure 7.
Multivariable logistic regression analysis of parental and treatment characteristics effect on indicated preterm birth (<37 weeks of gestation) for fresh transfer cycles only (n = 20 147). Fresh: analysis in the fresh transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, duration of subfertility, indication for treatment, maternal hypertensive and diabetic disease, culture medium, IVF or ICSI use, duration of embryonal culture, downregulation medication, ovarian stimulation medication, luteal phase support medication, number of oocytes retrieved at time of aspiration, single or multiple embryo transfer and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. Rec. FSH, recombinant FSH.
Figure 8.
Multivariable logistic regression analysis of parental and treatment characteristics effect on indicated preterm birth (<37 weeks of gestation) for fresh and frozen transfer treatments combined (n = 23 504). Fresh + frozen transfer analysis in the fresh + frozen transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, indication for treatment, maternal hypertensive and diabetic disease, fresh or frozen embryo transfer, single or multiple embryo transfer and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. FrET, fresh embryo transfer; FET, frozen embryo transfer.
Figure 9.
Multivariable logistic regression analysis of parental and treatment characteristics effect on indicated early preterm birth (<32 weeks of gestation) for fresh transfer cycles only (n = 20 147). Fresh: analysis in the fresh transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, duration of subfertility, indication for treatment, maternal hypertensive and diabetic disease, culture medium, IVF or ICSI use, duration of embryonal culture, downregulation medication, ovarian stimulation medication, luteal phase support medication, number of oocytes retrieved at time of aspiration, single or multiple embryo transfer and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. Rec. FSH, recombinant FSH; NA, not applicable.
Figure 10.
Multivariable logistic regression analysis of parental and treatment characteristics effect on indicated early preterm birth (<32 weeks of gestation) for fresh and frozen transfer treatments combined (n = 23 504). Fresh + frozen transfer analysis in the fresh + frozen transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, indication for treatment, maternal hypertensive and diabetic disease, fresh or frozen embryo transfer, single or multiple embryo transfer and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. FrET, fresh embryo transfer; FET, frozen embryo transfer; NA, not applicable.
Figure 11.
Multivariable logistic regression analysis of parental and treatment characteristics effect on congenital malformations for fresh transfer cycles only (n = 20 147). Fresh: analysis in the fresh transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, duration of subfertility, indication for treatment, maternal hypertensive and diabetic disease, culture medium, IVF or ICSI use, duration of embryonal culture, downregulation medication, ovarian stimulation medication, luteal phase support medication, number of oocytes retrieved at time of aspiration, single or multiple embryo transfer, duration of gestation and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. Rec. FSH, recombinant FSH.
Figure 12.
Multivariable logistic regression analysis of parental and treatment characteristics effect on congenital malformations for fresh and frozen transfer treatments combined (n = 23 504). Fresh + frozen transfer analysis in the fresh + frozen transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, indication for treatment, maternal hypertensive and diabetic disease, fresh or frozen embryo transfer, single or multiple embryo transfer, duration of gestation and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. FrET, fresh embryo transfer; FET, frozen embryo transfer.
Figure 13.
Multivariable logistic regression analysis of parental and treatment characteristics effect on perinatal mortality for fresh transfer cycles only (n = 20 147). Fresh: analysis in the fresh transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, duration of subfertility, indication for treatment, maternal hypertensive and diabetic disease, culture medium, IVF or ICSI use, duration of embryonal culture, downregulation medication, ovarian stimulation medication, luteal phase support medication, number of oocytes retrieved at time of aspiration, single or multiple embryo transfer, duration of gestation and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. Rec. FSH, recombinant FSH; NA, not applicable.
Figure 14.
Multivariable logistic regression analysis of parental and treatment characteristics effect on perinatal mortality for fresh and frozen transfer treatments combined (n = 23 504). Fresh + frozen transfer analysis in the fresh + frozen transfer dataset. Multivariable model includes: parental age, maternal ethnicity, multiparity, indication for treatment, maternal hypertensive and diabetic disease, fresh or frozen embryo transfer, single or multiple embryo transfer, duration of gestation and neonatal sex. *Uncorrected P-value <0.001. **Uncorrected P-value <0.0001. FrET, fresh embryo transfer; FET, frozen embryo transfer; NA, not applicable.
Figure 1.
Continued.
Birth weight
Parental and ART treatment characteristics associated with birth weight are shown in Figs 1 and 2. Multivariable linear regression analysis in the fresh transfer dataset (n = 20 147) showed that the parental characteristics with an effect on birth weight were, in order of effect size: maternal diabetes (adjusted difference (adj. β) 283 g, 95% CI 228–338), maternal ethnicity (South-Asian, African and Mediterranean ethnicity as compared to Dutch ethnicity, which resulted in an adj. β of −255 g (95% CI −318 to −193), adj. β −124 g (95% CI −180 to −69) and adj. β −66 g (95% CI −95 to −36), respectively), multiparity (adj. β 159 g, 95% CI 145–173), maternal hypertensive disease (adj. β −74 g, 95% CI −94 to −54) and duration of subfertility with −6 g for each added year (95% CI −10 to −3).
ART treatment characteristics with an effect on birth weight were, in order of effect size: hCG as luteal phase support as compared to progesterone (adj. β −61 g 95% CI −83 to −40), shorter duration of embryo culture (adj. β −54 g, 95% CI −75 to −33 compared to 3 days) and a larger number of oocytes yielded (adj. β −3 g per oocyte, 95% CI −4 to −2). Multivariable linear regression analysis in the Fresh + FET dataset (n = 23 504) showed a birth weight difference between FET versus fresh embryo transfer (adj. β 100 g, 95% CI 84–117). Repeating the multivariable linear regression analysis in the fresh + frozen transfer dataset did not alter the above-mentioned findings.
Indication for treatment ‘Male factor’ produced higher birth weight compared to ‘Tubal factor’ in the fresh + frozen transfer dataset, but this difference was diminished in the fresh transfer dataset (adj. β 37 g, 95% CI 16–59 and adj. β 28 g, 95% CI 2–53, respectively).
Preterm and early preterm birth
Parental and ART treatment characteristics associated with spontaneous preterm and early preterm birth are shown in Figs 3, 4, 5 and 6.
Parental characteristics with an effect on spontaneous preterm birth were, in order of effect size: maternal hypertensive disease (adj. OR 1.99, 95% CI 1.56–2.55), indication for treatment (‘male factor’ adj. OR 0.61, 95% CI 0.47–0.79 and ‘unexplained’ adj. OR 0.62, 95% CI 0.47–0.81, respectively, as compared to ‘tubal factor’) and multiparity (adj. OR 0.72, 95% CI 0.62–0.85).
Analysis in the fresh-frozen transfer dataset showed an effect of ‘male factor’ as indication for treatment on spontaneous early preterm birth compared to ‘tubal factor’, however, this effect was diminished in the fresh transfer dataset (adj. OR 0.4, 95% CI 0.25–0.65 and adj. OR 0.51, 95% CI 0.29–0.88).
No ART treatment characteristics were associated with spontaneous preterm or early preterm birth.
Maternal hypertensive disease was associated with medically indicated preterm and early preterm birth (adj. OR 3.01, 95% CI 2.57–3.51 and adj. OR 2.02, 95% CI 1.47–2.78, respectively), Figs 7, 8, 9 and 10.
Congenital malformations
There were no associations between parental and ART treatment characteristics and congenital malformations (Figs 11 and 12).
Perinatal mortality
There were no associations between parental characteristics and ART treatment characteristics and perinatal mortality (Figs 13 and 14).
Discussion
Main outcome
We found that both parental characteristics and ART treatment characteristics independently affected perinatal outcome, including small effects on birth weight and preterm birth. Although the effect size would not directly have clinical consequences, it does warrant further research on the underlying mechanism and potential long-term effect on the health of ART children. In our cohort of 23 504 ART conceived singletons, maternal hypertensive disease or diabetes, ethnicity and parity in particular, had the largest effect size on perinatal outcomes. Findings of our study that are in accordance with previous studies are, among others, the influence of maternal ethnicity on birth weight and the risk of preterm birth (Schaaf et al., 2012; Crawford et al., 2017) and higher birth weight after FET compared to a fresh embryo transfer (Wennerholm et al., 2013). A new finding is the role of luteal support medication on perinatal outcomes.
Strengths and weaknesses
The size of our study’s database, its population-based nature, the wide variety of parental and cycle-specific ART treatment characteristics available and the linkage procedure used to retrieve the perinatal outcomes of interest are strengths of this study.
This study comprises data from all the IVF clinics in the Netherlands for a 10-year period. In the Netherlands, ART is non-commercial and universally available to couples with a medical indication based on duration of subfertility or underlying pathology. Our study was population based, representing all ART patients treated in the Netherlands in the studied time slot, minimising selection bias.
Validation demonstrated that the probabilistic linkage technique we used was a reliable method of linking maternal and neonatal files in absence of a unique identifier. Failure to link maternal and neonatal files with high level of certainty would have occurred mainly due to termination or loss of pregnancy between a positive pregnancy test (inclusion criteria for the ART dataset) and 20 weeks of gestation (inclusion criteria for Perined), as there is a 15.5–19.8% chance of pregnancy loss following ART treatment (De Geyter et al., 2018). Other reasons for linkage failure include alteration of postal code and human error of the fertility or obstetric caregiver. This is not considered as a failure of the linkage procedure. For the remaining ‘true’ missings, we assumed missingness at random. Because of this, selection bias is not considered to be an issue. A similar linkage technique has been used in the American State Monitoring ART Collaborative studies to link ART records with birth records in several states using only data from ascertained ongoing pregnancies (Mneimneh et al., 2013). The linkage probability of the American State Monitoring ART Collaborative studies is similar to the linkage probability seen in our dataset for clinics who provided birth date of child, confirming the robustness of the method we used.
The fact that no unique identifier for mothers was available that several variables in the ART database had missing values, the lack of information on pregnancy outcomes prior to the 20th gestational week, the lack of information available on lifestyle and general health of parents and the limited period of follow-up of children provided by the Perined (the first 28 days after birth or at neonatal hospital readmission) are weaknesses of our study. Not having information on pregnancy outcomes prior to the 20th week of gestation may cause a lower reporting of outcomes such as congenital malformations for which a pregnancy may have been terminated.
The lack of a unique identifier was addressed by using several identifying characteristics (birth date mother and child and postal code) for the probabilistic linkage procedure, a validated method (Fellegi and Sunter, 1969). However, we could not control for mothers present in the dataset more than once in subsequent pregnancies, which may result in an underestimation of the precision of the effect of some maternal characteristics on perinatal outcomes.
We chose to address missing values in the ART database by imputing missing values using the MICE method so as to maintain the original characteristics of the population (Van Buuren and Groothuis-Oudshoorn, 2011). This method takes the uncertainty of data into account, as is reflected by a higher standard error when uncertainty is high (Azur et al., 2011).
Due to the fact that several ART treatment characteristics in the FET cycles were missing in 55–100% of the cases, it was not possible to apply multiple imputation for those in FET cycles. We were therefore unable to assess the independent impact of several ART treatment characteristics (i.e. pituitary downregulation and ovarian stimulation medication) on perinatal outcomes in this specific group. In order to examine the effect of freezing and thawing of the embryo, we chose to consider the FET group as a separate group.
Several factors which could impact perinatal outcome, such as smoking, body mass index or use of medication, were not available for analysis. In addition, data provided by Perined on the presence of congenital malformations and maternal disease may be under-registered (Broekhuijsen et al., 2015; Fleurke-Rozema et al., 2016). However, we have no reason to believe that this was differently distributed between the IVF, ICSI or FET pregnancies and we assumed this information as missing at random. Baseline characteristics of the study population were compared to the baseline characteristics of the general population using overviews provided by the Perined for three consecutive years (2009–2011), as an overview for the total period of our cohort was not available and as overviews of previous years reported outcomes in a different format without reporting average birth weight and congenital malformations rendering it impossible to pool the data. Despite this limitation, we are confident that the differences found between our ART population and the general Dutch population were not influenced by this as we expect no drastic differences to have occurred in the general population of the Netherlands during the period of 2000–2011.
Finally, ongoing innovation, changes in treatment protocols and changes in population characteristics in ART cycles may have led to interactions with treatment over time on perinatal outcome, which we did not study. It is possible that the absence of more recent innovative ART techniques from our database could limit the representativeness of our study findings for more contemporary cohorts. Nonetheless, many of the ART features investigated in this study are still commonly employed, confirming our study relevance to present day clinicians.
Interpretation of results and relation to other studies
We demonstrated that some ART treatment characteristics, including oocyte yield and choice of luteal support, independently impact birth weight. A possible explanation might be an endocrine effect on the endometrium, which, in turn, affects embryo implantation and early placentation. A study by Magnusson et al. (2018) showed no impact of number oocytes yielded on chance of being SGA or preterm birth which is in concordance with our findings (data not shown). We, however, found a negative effect of increasing number of oocytes yielded on birth weight when analysing birth weight as a continuous variable. Ovarian stimulation leads to supra-physiological estrogen levels during the follicular phase of the cycle. Previous studies had conflicting findings on the effects of estrogen levels on perinatal outcomes. In a study by Hu et al. (2014) high estrogen level were associated with a low mean birth weight and a higher risk for being SGA, although the authors did not study the role of ART treatment regimen on estrogen levels and birth outcomes. Previous studies have shown a difference in levels of vascular endothelial growth factor, insulin-like growth factors and their binding proteins in the follicular fluid (Choi et al., 2006; Ferrari et al., 2006) depending on the GnRH agonist or antagonist of choice. Whether these alterations in the microenvironment of the oocyte result in a difference in birth weight is not known, but there is a growing body of evidence to suggest that such environmental influences induce epigenetic alterations in the oocyte and future embryo (Gardner and Lane, 2005; Sirard, 2017). We found that luteal support with hCG was associated with lower birth weight, compared to progesterone. Luteal support in ART cycles with fresh embryo transfer is focused on optimising progesterone levels after a prolonged period of pituitary downregulation. This can be achieved by externally administered progesterone or by administration of hCG which in turn stimulates the existing corpora luteal and results in an increase in endogenous progesterone (Fatemi, 2009). Progesterone and hCG have been demonstrated to exert differential effects on gene expression patterns of the endometrium (Zhao et al., 2012), which could contribute to the environment during early placentation, which in turn may affect foetal growth.
We found that, of all ART treatment characteristics available, embryo cryopreservation had the largest effect on birth weight and increased the risk of being born LGA (data not shown). This finding is in accordance with previous studies where children born after FET had higher birth weight and a higher chance of being LGA compared to children born after fresh embryo transfer (Wennerholm et al., 2013; Zhao et al., 2016). Here again the effect is unlikely to be due to residual confounding, and might be mediated by the effect of ovarian stimulation on the endometrium, which is absent in FET cycles. However, the mechanism behind these differences remains unknown (Maheshwari et al., 2016).
We did not find that embryo culture medium was strongly associated with birth weight or other perinatal outcomes. This does not necessarily mean that culture medium could not have an effect on these outcomes and these findings may be inherent to the method we used for our statistical analysis. In a previous large randomised trial, a difference in birth weight was found when two particular media which had different composition were compared (Kleijkers et al., 2016). In other non-randomised comparative studies where the composition of the media possibly was more similar, no difference was found (Zandstra et al., 2015). Our analysis consisted of a collection of multiple media, diluting a possible effect of distinct media.
No association was found between transfer of multiple embryos with poorer perinatal outcomes in singleton pregnancies. This is in contrast with previous studies where transfer of multiple embryos was associated with a higher risk of low birth weight and preterm birth, possibly due to vanishing twin phenomenon (Sazonova et al., 2011b; Kamath et al., 2018).
Conclusion
This study has shown that several parental and ART treatment characteristics affect perinatal outcomes to different extents. This highlights the sensitivity of the embryo to its early environment. To what extent these findings, however, small, are relevant for the growth, development and health of the offspring, remains to be determined, as are the mechanisms underlying these associations.
Data availability
The data underlying this article cannot be shared publicly without prior consent from Perined. The data will be shared on reasonable request to the corresponding author and after the use of the data is approved by Perined.
Supplementary Material
Acknowledgements
We thank the Foundation of the Netherlands Perinatal Registry for permission to use their registry data (approval number 12.43). We thank G.P. Kroon and H.W.W. van Leeuwen for their assistance in collecting the necessary IVF data. Furthermore, we thank the medical informatics students A. Wong for the first deterministic data linkage and S. Wortel for assisting in the database validation process. In addition, we thank all care providers for the registration of the perinatal data as well as the IVF laboratory data.
Authors’ roles
All authors fulfil the criteria for authorship. T.J.R., S.R., R.C.P. and B.W.M. initiated the study. M.P., R.C.P. and T.J.R. obtained funding and coordinated the data collection. M.P. prepared the first draft of the manuscript and performed part of the analysis. M.H.H. performed the linkage and analysis. A.C.J.R. supervised the linkage and provided the Perined data. A.T.S., E.H.K., E.S., A.E.P.C., L.A.J.W., W.I., J.C.M.D., L.R., E.B.B., D.C., F.J.M.B., P.M.R. and M.H.J.M.C. provided the IVF data. A.J.A. provided support with digital data extraction. M.P., S.M., R.C.P. and T.J.R. interpreted the data. All authors commented on the drafts and have seen and approved a final version.
Funding
This study was funded by Foreest Medical School, Alkmaar, the Netherlands (grants: FIO 1307 and FIO 1505).
Conflict of interest
Dr B.W.M. reports grants from NHMRC and consultancy for ObsEva, Merck KGaA, iGenomics and Guerbet. Dr F.B. reports research support grants from Merck Serono and personal fees from Merck Serono. Dr A.C. reports travel support from Ferring BV. and Theramex BV. and personal fees from UpToDate (Hyperthecosis), all outside the remit of the current work. The remaining authors report no conflict of interests.
References
- Azur MJ, Stuart EA, Frangakis C, Leaf PJ.. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011;20:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakhekke M, Kamphuis EI, Rumste MM. V, Mol F, Veen F V D, Mol BW.. How are neonatal and maternal outcomes reported in randomised controlled trials (RCTs) in reproductive medicine? Hum Reprod 2014;29:1211–1217. [DOI] [PubMed] [Google Scholar]
- Broekhuijsen K, Ravelli AC, Langenveld J, van Pampus MG, van den Berg PP, Mol BW, Franssen MT.. Maternal and neonatal outcomes of pregnancy in women with chronic hypertension: a retrospective analysis of a national register. Acta Obstet Gynecol Scand 2015;94:1337–1345. [DOI] [PubMed] [Google Scholar]
- Choi YS, Ku SY, Jee BC, Suh CS, Choi YM, Kim JG, Moon SY, Kim SH.. Comparison of follicular fluid IGF-I, IGF-II, IGFBP-3, IGFBP-4 and PAPP-A concentrations and their ratios between GnRH agonist and GnRH antagonist protocols for controlled ovarian stimulation in IVF-embryo transfer patients. Hum Reprod 2006;21:2015–2021. [DOI] [PubMed] [Google Scholar]
- Crawford S, Joshi N, Boulet SL, Bailey MA, Hood ME, Manning SE, McKane P, Kirby RS, Kissin DM, Jamieson DJ, for the States Monitoring Assisted Reproductive Technology (SMART) Collaborative. Maternal racial and ethnic disparities in neonatal birth outcomes with and without assisted reproduction. Obstet Gynecol 2017;129:1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, Scaravelli G, Smeenk J, Vidakovic S, Goossens V, European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2014: results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod 2018;133:1586–1601.30032255 [Google Scholar]
- De Sutter P, Delbaere I, Gerris J, Verstraelen H, Goetgeluk S, Van der Elst J, Temmerman M, Dhont M.. Birthweight of singletons after assisted reproduction is higher after single- than after double-embryo transfer. Hum Reprod 2006;21:2633–2637. [DOI] [PubMed] [Google Scholar]
- Dong Y, Peng CJ.. Principled missing data methods for researchers. SpringerPlus 2013;2:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing BG, Mohr LR, Trounson AO, Freeman LE, Wood C.. Birth after transfer of cryopreserved embryos. Med J Aust 1985;142:409–411. [DOI] [PubMed] [Google Scholar]
- Dumoulin JC, Land JA, Van Montfoort AP, Nelissen EC, Coonen E, Derhaag JG, Schreurs IL, Dunselman GA, Kester AD, Geraedts JP. et al. Effect of in vitro culture of human embryos on birthweight of newborns. Hum Reprod 2010;25:605–612. [DOI] [PubMed] [Google Scholar]
- Fatemi HM. Assessment of the luteal phase in stimulated and substituted cycles. Facts Views Vis Obgyn 2009;1:30–46. [PMC free article] [PubMed] [Google Scholar]
- Fellegi IP, Sunter AB.. A theory of record linkage. J Am Stat Assoc 1969;64:1183–1210. [Google Scholar]
- Ferrari B, Pezzuto A, Barusi L, Coppola F.. Follicular fluid vascular endothelial growth factor concentrations are increased during GnRH antagonist/FSH ovarian stimulation cycles. Eur J Obstet Gynecol Reprod Biol 2006;124:70–76. [DOI] [PubMed] [Google Scholar]
- Fleurke-Rozema JH, van de Kamp K, Bakker MK, Pajkrt E, Bilardo CM, Snijders RJ.. Prevalence, diagnosis and outcome of cleft lip with or without cleft palate in The Netherlands. Ultrasound Obstet Gynecol 2016;48:458–463. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M.. Ex vivo early embryo development and effects on gene expression and imprinting. Reprod Fertil Dev 2005;17:361–370. [DOI] [PubMed] [Google Scholar]
- Hindori-Mohangoo AD, Schönbeck Y, Van der Pal-de Bruin KM. TNO-rapport, TNO/CH 2014 R 11308; 2014. Aangeboren afwijkingen in Nederland 2001-2012: gebaseerd op de landelijke perinatale registraties. https://www.tno.nl/media/4521/2014-r-11308-rapportage-aangeboren-afwijkingen-2001-2012-def.pdf (1 August 2020, date last accessed).
- Hof MHP, Zwinderman AH.. Methods for analyzing data from probabilistic linkage strategies based on partially identifying variables. Statist Med 2012;31:4231–4242. [DOI] [PubMed] [Google Scholar]
- Hu XL, Feng C, Lin XH, Zhong ZX, Zhu YM, Lv PP, Lv M, Meng Y, Zhang D, Lu XE. et al. High maternal serum estradiol environment in the first trimester is associated with the increased risk of small-for-gestational-age birth. J Clin Endocrinol Metab 2014;99:2217–2224. [DOI] [PubMed] [Google Scholar]
- Kamath MS, Antonisamy B, Selliah HY, Sunkara SK.. Perinatal outcomes of singleton live births with and without vanishing twin following transfer of multiple embryos: analysis of 113 784 singleton live births. Hum Reprod 2018;33:2018–2022. [DOI] [PubMed] [Google Scholar]
- Kleijkers SH, Mantikou E, Slappendel E, Consten D, van Echten-Arends J, Wetzels AM, van Wely M, Smits LJ, van Montfoort AP, Repping S. et al. Influence of embryo culture medium (G5 and HTF) on pregnancy and perinatal outcome after IVF: a multicenter RCT. Hum Reprod 2016;31:2219–2230. [DOI] [PubMed] [Google Scholar]
- Kok N, Ruiter L, Hof M, Ravelli A, Mol B, Pajkrt E, Kazemier B.. Risk of maternal and neonatal complications in subsequent pregnancy after planned caesarean section in a first birth, compared with emergency caesarean section: a nationwide comparative cohort study. BJOG: Int J Obstet Gy 2014;121:216–223. [DOI] [PubMed] [Google Scholar]
- Magnusson Å, Wennerholm UB, Källén K, Petzold M, Thurin-Kjellberg A, Bergh C.. The association between the number of oocytes retrieved for IVF, perinatal outcome and obstetric complications. Hum Reprod 2018;33:1939–1947. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Raja EA, Bhattacharya S.. Obstetric and perinatal outcomes after either fresh or thawed frozen embryo transfer: an analysis of 112,432 singleton pregnancies recorded in the Human Fertilisation and Embryology Authority anonymized dataset. Fertil Steril 2016;106:1703–1708. [DOI] [PubMed] [Google Scholar]
- Marino JL, Moore VM, Willson KJ, Rumbold A, Whitrow MJ, Giles LC, Davies MJ.. Perinatal outcomes by mode of assisted conception and sub-fertility in an Australian data linkage cohort. PLoS One 2014;9:e80398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méray N, Reitsma JB, Ravelli ACJ, Bonsel GJ.. Probabilistic record linkage is a valid and transparent tool to combine databases without a patient identification number. J Clin Epidemiol 2007;60:883–891. [DOI] [PubMed] [Google Scholar]
- Mneimneh AS, Boulet SL, Sunderam S, Zhang Y, Jamieson DJ, Crawford S, McKane P, Copeland G, Mersol-Barg M, Grigorescu V. et al. States Monitoring ART (SMART) Collaborative. States Monitoring Assisted Reproductive Technology (SMART) Collaborative: data collection, linkage, dissemination, and use. J Womens Health (Larchmt) 2013;22:571–577. [DOI] [PubMed] [Google Scholar]
- Palermo G, Joris H, Devroey PV.. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992;340:17–18. [DOI] [PubMed] [Google Scholar]
- Pelkonen S, Koivunen R, Gissler M, Nuojua-Huttunen S, Suikkari A-M, Hyden-Granskog C, Martikainen H, Tiitinen A, Hartikainen A-L.. Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995-2006. Hum Reprod 2010;25:914–923. [DOI] [PubMed] [Google Scholar]
- Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Söderström-Anttila V, Nygren KG, Hazekamp J, Bergh C.. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update 2013;19:87–104. [DOI] [PubMed] [Google Scholar]
- Qin J, Liu X, Sheng X, Wang H, Gao S.. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertil Steril 2016;105:73–85. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. http://www.R-project.org/ (1 August 2020, date last accessed). [Google Scholar]
- Sazonova A, Källen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C.. Obstetric outcome after in vitro fertilization with single or double embryo transfer. Hum Reprod 2011a;26:442–450. [DOI] [PubMed] [Google Scholar]
- Sazonova A, Källen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C.. Factors affecting obstetric outcome of singletons born after IVF. Hum Reprod 2011b;26:2878–2886. [DOI] [PubMed] [Google Scholar]
- Sazonova A, Kallen K, Thurin-Kjellberg A, Wennerholm U-B, Bergh C.. Obstetric outcome in singletons after in vitro fertilization with cryopreserved/thawed embryos. Hum Reprod 2012;27:1343–1350. [DOI] [PubMed] [Google Scholar]
- Schaaf JM, Mol BW, Abu-Hanna A, Ravelli AC.. Ethnic disparities in the risk of adverse neonatal outcome after spontaneous preterm birth. Acta Obstet Gynecol Scand 2012;91:1402–1408. [DOI] [PubMed] [Google Scholar]
- Seggers J, Pontesilli M, Ravelli ACJ, Painter RC, Hadders-Algra M, Heineman MJ, Repping S, Mol BWJ, Roseboom TJ, Ensing S.. Effects of in vitro fertilization and maternal characteristics on perinatal outcomes: a population-based study using siblings. Fertil Steril 2016;105:590–598. [DOI] [PubMed] [Google Scholar]
- Sirard MA. The influence of in vitro fertilization and embryo culture on the embryo epigenetic constituents and the possible consequences in the bovine model. J Dev Orig Health Dis 2017;8:411–417. [DOI] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RG.. Birth after the reimplantation of a human embryo. Lancet 1978;312:366. [DOI] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RG, Walters DE.. Observations on 767 clinical pregnancies and 500 births after human in-vitro fertilization. Hum Reprod 1986;1:89–94. [DOI] [PubMed] [Google Scholar]
- Stichting Perinatale Registratie Nederland. Perinatale Zorg in Nederland 2009. Utrecht: Stichting Perinatale Registratie Nederland, 2013a. https://assets.perined.nl/docs/7a350c76-57ab-46c5-9e42-b67374083bda.pdf (1 August 2020, date last accessed). [Google Scholar]
- Stichting Perinatale Registratie Nederland. Perinatale Zorg in Nederland 2010. Utrecht: Stichting Perinatale Registratie Nederland, 2013b. assets.perined.nl/docs/d5e0359b-6030-4b01-b40b-0c0dc6959b19.PDF (1 August 2020, date last accessed). [Google Scholar]
- Stichting Perinatale Registratie Nederland. Perinatale Zorg in Nederland 2011. Utrecht: Stichting Perinatale Registratie Nederland, 2013c. https://assets.perined.nl/docs/ec7434e3-c899-4008-8a04-b3068f285b86.PDF (1 August 2020, date last accessed). [Google Scholar]
- Sullivan EA, Wang YA, Hayward I, Chambers GM, Illingworth P, McBain J, Norman RJ.. Single embryo transfer reduces the risk of perinatal mortality, a population study. Hum Reprod 2012;27:3609–3615. [DOI] [PubMed] [Google Scholar]
- Sunkara SK, La Marca A, Seed PT, Khalaf Y.. Increased risk of preterm birth and low birthweight with very high number of oocytes following IVF: an analysis of 65 868 singleton live birth outcomes. Hum Reprod 2015;30:1473–1480. [DOI] [PubMed] [Google Scholar]
- Tromp M, Ravelli ACJ, Méray N, Reitsma JB, Bonsel GJ.. An efficient validation method of probabilistic record linkage including readmissions and twins. Methods Inf Med 2008;47:356–363. [DOI] [PubMed] [Google Scholar]
- Van Buuren S, Groothuis-Oudshoorn K.. Mice: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- Visser GH, Eilers PH, Elferink-Stinkens PM, Merkus HM, Wit JM.. New Dutch reference curves for birthweight by gestational age. Early Hum Dev 2009;85:737–744. [DOI] [PubMed] [Google Scholar]
- Wennerholm UB, Henningsen AKA, Romundstad LB, Bergh C, Pinborg A, Skjaerven R, Forman J, Gissler M, Nygren KG, Tiitinen A.. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod 2013;28:2545–2553. [DOI] [PubMed] [Google Scholar]
- Zandstra H, Van Montfoort AP, Dumoulin JC.. Does the type of culture medium used influence birthweight of children born after IVF? Hum Reprod 2015;30:530–542. [DOI] [PubMed] [Google Scholar]
- Zeilmaker GH, Alberda AT, van Gent I, Rijkmans CM, Drogendijk AC.. Two pregnancies following transfer of intact frozen-thawed embryos. Fertil Steril 1984;42:293–296. [DOI] [PubMed] [Google Scholar]
- Zhao J, Xu B, Zhang Q, Li YP.. Which one has a better obstetric and perinatal outcome in singleton pregnancy, IVF/ICSI or FET?: a systematic review and meta-analysis. Reprod Biol Endocrinol 2016;14:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zacur H, Cheadle C, Ning N, Fan J, Vlahos NF.. Effect of luteal-phase support on endometrial microRNA expression following controlled ovarian stimulation. Reprod Biol Endocrinol 2012;10:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly without prior consent from Perined. The data will be shared on reasonable request to the corresponding author and after the use of the data is approved by Perined.