Abstract
Introduction:
[111In]XYIMSR-01 is a promising single-photon emission computed tomography (SPECT) imaging agent for identification of tumors that overexpress carbonic anhydrase IX. To translate [111In]XYIMSR-01 to phase I trials, we performed animal toxicity and dosimetry studies, determined the maximum dose for human use, and completed the chemistry, manufacturing and controls component of a standard regulatory application.
Methods:
The production process, quality control testing, stability studies, and specifications for sterile drug product release were based on United States Pharmacopeia chapters <823> and <825>, FDA 21 CFR part 212. Toxicity was evaluated by using non-radioactive [113/115In]XYIMSR-01 according to 21 CFR part 58 guidelines. OLINDA/EXM was used to calculate the maximum single dose for human studies.
Results:
Three process validation runs at starting radioactivities of ~ 800 MBq were completed with a minimum concentration of 407 MBq/mL and radiochemical purity of ≥ 99 % at the end-of-synthesis. A single intravenous dose of 55 μg/mL of [113/115In]XYIMSR-01 was well-tolerated in male and female Sprague-Dawley rats. The calculated maximum single dose for human injection from dosimetry studies was 390.35 MBq of [111In]XYIMSR-01.
Conclusion:
We have completed toxicity and dosimetry studies as well as validated a manufacturing process to test [111In]XYIMSR-01 in a phase I clinical trial.
Keywords: carbonic anhydrase 9, clear cell renal cell carcinoma, SPECT, clinical trials, molecular imaging
Graphical Abstract
Introduction
A total of 73,750 new cases of kidney cancer, representing 5% of all diagnosed malignancies, are estimated to be diagnosed in the United States in 2020.1 Additionally, 14,830 deaths are estimated from this disease. The clear cell subtype of renal cell carcinoma (ccRCC) accounts for up to 70% of all kidney cancers.2 Although computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound are useful non-invasive tools for detecting and staging kidney cancer, they are unable to reliably distinguish ccRCC from less aggressive tumors subtypes.3 Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) combined with new biomarkers have the potential to overcome those limitations.
Carbonic anhydrase IX (CAIX) is a membrane-associated member of the carbonic anhydrase (CA) family. CAIX is up-regulated in multiple different cancers including ccRCC. CAIX is over-expressed in 95% of ccRCC tumor specimens and has very low expression in normal tissues and other renal tumor sybtypes4. Therefore, CAIX is a potential target for ccRCC imaging and therapy. Although anti-CAIX monoclonal antibodies (mAbs) radiolabeled with 124I, 89Zr and 111In for non-invasive PET and SPECT imaging of ccRCC have shown promising results5–7, they suffer from slow blood and non-target tissue clearance and non-specific organ uptake.3, 8 As opposed to mAbs, low-molecular-weight (LMW) CAIX inhibitors have rapid pharmacokinetics and have the advantages of potential same day imaging and lower radiation exposure to the patient.3
We have reported dual-motif targeting LMW CAIX ligands [111In]XYIMSR-018, 9, [64Cu] XYIMSR-0610 and [177Lu]XYIMSR-0111 for SPECT and PET imaging and therapy of ccRCC, respectively. Those agents demonstrated high selectivity for CAIX in vitro and in vivo, with the potential to image both metastatic and localized ccRCC. Here we endeavored to evaluate the radiation dosimetry and acute toxicity in mice and to complete the chemistry, manufacturing and controls component (CMC) of the investigational new drug application (IND) in compliance with current good manufacturing practices (cGMP) found in Food and drug administration (FDA) 21 code of regulation (CFR) part 212 for radiopharmaceutical drugs. Those items are needed to obtain FDA approval to translate [111In]XYIMSR-01 to a phase I clinical trial.
Materials and methods
General
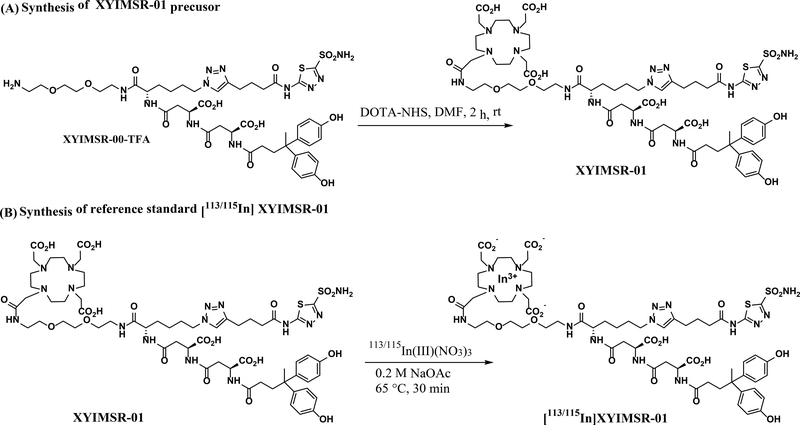
Raw materials, reference standards, solvents, and media with valid manufacturer certificates of analysis (COA) were released for cGMP use as per standard operating procedures (SOPs) from the Center for Translational Molecular Imaging (CTMI), a Johns Hopkins cGMP facility. cGMP grade 111InCl3 was purchased from Nordion, Inc. (Ottawa, ON, Canada), and identity testing was performed at CTMI before release. High purity XYIMSR-01 precursor and the reference standard [113/115In]XYIMSR-01 at research scale were synthesized at CTMI according to Scheme 18 and were characterized by visual inspection for physical appearance, proton nuclear magnetic resonance (1H-NMR), and electrospray ionization (ESI) mass analysis for identity, and reverse phase high performance liquid chromatography (RP-HPLC) for purity.
Scheme 1.
General scheme for the synthesis of XYIMSR-01 precursor and [113/115In]XYIMSR-01 reference standard.
Pharmacokinetic studies and human dosimetry projections
Animal experiments were performed in accordance with protocols approved by the Johns Hopkins Animal Care and Use Committee (ACUC). Bio-distribution studies for human dosimetry calculations used six-week-old female NOD/SCID mice (n = 25). Animals were sacrificed at 1, 4, 8, 24, and 48 h post-injection, with organs removed and processed as in Yang et al.8 Residence times for human organs, including bone marrow, heart, lungs, pancreas, spleen, brain, muscle, small intestine, liver, stomach, kidneys, and urinary bladder, were estimated from mouse bio-distribution data. Those data were used as input to Organ Level INternal Dose Assessment/EXponential Modeling (OLINDA/EXM)12 to obtain the absorbed organ dose for 111In and adult reference human male phantom. The residence time for the tumor was calculated assuming a tumor mass of 1 g using the same conversion from mouse to human.
Acute toxicology study of [113/115In]XYIMSR-01 in Sprague Dawley rats
Toxicology was conducted in compliance with FDA Good Laboratory Practices (GLP) as found in title 21 CFR part 58. Groups of ten male and ten female Sprague-Dawley rats were dosed with [113/115In]XYIMSR-01 at 11 and 55 μg/kg. An additional group of ten males and ten females served as controls and received the vehicle, 6.67% ethanol in 0.9% sterile saline. All rats received a dose volume of 1 mL/kg. The rats were dosed intravenously once on Study Day 1. Five male and five female rats from each group were bled on Study Day 3 and the remaining rats were bled on Study Day 15. Animals were euthanized and necropsied following blood collection. Parameters evaluated for test article effect included survival, clinical observation, body weight, clinical pathology, gross pathology, urinalysis, organ weights, and microscopic pathology.
Process validation, manufacturing, and process controls of [111In]XYIMSR-01
All activities were carried out at the CTMI cGMP facility equipped with controlled environment rooms, ISO 5 Class hot cells, and state-of-the-art equipment for testing. Indium-111 has very low gamma energy, hence the entire process can be run manually inside a hot cell with adequate shielding. Prior to process validation (PV), personnel were qualified for aseptic processing through training and by successfully completing three consecutive media fill simulations of the aseptic process. PV involved the preparation and testing of three consecutive batches of [111In]XYIMSR-01 conforming to prescribed specifications. Those batches were prepared in accordance with approved master batch records. The process of manufacturing [111In]XYIMSR-01 consists of four general steps: 1) radiolabeling XYIMSR-01 precursor with 111InCl3, 2) RP-HPLC column purification of crude, radiolabeled [111In]XYIMSR-01, 3) removal of RP-HPLC solvent via C-18 Sep-Pak with formulation, and, finally, 4) aseptic filtration and dispensing.
Briefly, 500 μL of 0.5M NaOAc buffer (pH ~ 5.6) was added to a vial containing 111InCl3. The activity in buffer was then transferred to a 2 mL reaction vial containing 200 μg of XYIMSR-01 precursor. A further 500 μL of buffer was added to the same 2 mL reaction vial. The 2 mL vial containing the reaction mixture was transferred to a thermomixer and incubated at 61°C while undergoing rotation at 350 rpm for a period of 31 ± 1 minutes. After cooling to room temperature, the reaction mixture containing crude [111In]XYIMSR-01 was purified via chromatographic separation using RP-HPLC [Phenomenex 250 × 10 mm column (part # 00G-4253-N0), eluted with 17% acetonitrile with 0.1% formic acid and 83% water at a flow rate 10 mL/min]. The column effluent was monitored using UV (254 nm) and radiometric detectors connected in series. The [111In]XYIMSR-01 peak was collected in a clean glass bottle with 150 mL of sterile water for irrigation and passed through an activated Sep-Pak C-18 plus cartridge by N2 flow attached to a 5-gang manifold to capture the product on the cartridge. The cartridge was washed with sterile water for injection (20 mL) to remove trace amounts of acetonitrile and formic acid (from the RP-HPLC mobile phase). The product was then eluted with 1 mL of ethanol and 14 mL of 0.9% saline solution into a 30 mL sterile vial. The drug product vial was transferred into the ISO Class 5 hot cell located in a cleanroom area. The product was processed aseptically through a 0.2 μm sterile filter into the final 30 mL sterile vial within the ISO Class 5 hot cell. Samples for quality control (QC) testing, sterility, and retention were obtained from the final 30 mL vial. The remaining activity in the final vial was the source of activity for administration to the patient. Sterile contact agar plates were used for air quality, gloved fingertip sampling of the operator, and surface monitoring during the aseptic operation. Test methods and release specifications for [111In]XYIMSR-01 injectable drug product are listed in Supplementary Table 1. The specifications, where possible, are based on existing current guidelines (USP monographs, ICH, and FDA) for radiopharmaceutical, dosimetry and toxicity data. 13–16
Validation of the analytical methods and procedures for [111In]XYIMSR-01 product release
The HPLC and gas chromatography (GC) methods were evaluated and validated in terms of linearity, accuracy, robustness, limit of detection (LOD), limit of quantification (LOQ), precision (repeatability and reproducibility conditions), and system suitability. The radiochemical purity, radiochemical identity, and chemical purity were tested using an analytical Phenomenex 150 × 4.6 mm column (Part # 00F-4252-E0). The quality control (QC) testing samples were eluted with 80% water with 0.1% TFA and 20% acetonitrile with 0.1% TFA for 20 min at a flow rate of 1.5 mL/min. A GC method was developed to analyze residual solvent in the final formulation of [111In]XYIMSR-01 drug product. The product identity was determined by the percentage difference of peak retention times of the radiodetector chromatogram of [111In]XYIMSR-01 and the [113/115In]XYIMSR-01 from the UV chromatogram. Endotoxin was quantified with an Endosafe-PTS instrument (Charles River Laboratories, Wilmington, MA) with 1:100 dilution of final drug product in endotoxin-free water. In addition to the vendor’s COA, the gamma ray spectrum was collected from a multichannel analyzer (MCA) with a NaI detector for 111In radionuclidic identity and purity. In addition to the filter integrity test, a direct inoculation method was used for sterility testing. Drug product samples were incubated for 14 days at 20 – 25°C in tryptic soy broth (TSB) and at 30 – 35°C in fluid thioglycollate medium (FTM) along with positive [BIOBALL (BioMérieux, Marcy-l’Étoile, FRA)] and negative (unopened TSB and FTM media tubes) controls. The pH of the final drug product was measured by two pH indicator strips in the range of 4.0 – 7.0 and 5.0 – 10.0, respectively.
Container closure system, formulation and stability
Three batches that met all specifications and were released by quality assurance were used to demonstrate the stability of sterile [111In]XYIMSR-01. Stability testing was performed in a 30 mL clear borosilicate type 1 glass vial with a chlorobutyl-type rubber stopper at controlled room temperature (15 – 30°C) with a final formulation of 1 mL of ethanol and 14 mL of 0.9% saline. The vial was upright and inverted to assure contact of the drug product solution to both the container and closure. An 8 h stability protocol was carried out to validate an 8 h shelf-life of the product. The stability protocol consisted of intermediate time-points (2 h and 4 h) after end-of-synthesis (EOS) as well as an 8 h time point.
Results and Discussion
Pharmacokinetics and radiation dosimetry estimates
According to the bio-distribution results, bone marrow and the majority of the organs, including heart, lungs, pancreas, small-intestine, liver, kidneys, and urinary bladder, exhibited a two-phase (rapid and slow) clearance; spleen, fat, muscle, stomach, and tumor showed fast clearance to T2 (4 h), then uptake to T3 (8 h) and a slow clearance at the later time-points. The uptake and clearance kinetics of all mouse organs are given in Supplementary Table 2. The projections for human organ and tumor residence times and human organ absorbed doses of [111In]XYIMSR-01 are given in Table 2.
Table 2.
Human organ and tumor residence times
| Organ | Organ in OLINDA | Organ Mass in OLINDA (g) | Residence Time (h) |
|---|---|---|---|
| Red Marrow | Red Marrow | 1,120 | 0.012 |
| Heart | Heart Wall | 316 | 0.067 |
| Lungs | Lungs | 1,000 | 1.958 |
| Pancreas | Pancreas | 94.3 | 0.012 |
| Spleen | Spleen | 183 | 0.033 |
| Brain | Brain | 1,420 | 0.107 |
| Muscle | Muscle | 28,000 | 20.984 |
| Small Intestine | Small intestine | 677 | 0.213 |
| Liver | Liver | 1,910 | 0.700 |
| Stomach | Stomach | 158 | 0.085 |
| Kidneys | Kidneys | 299 | 0.745 |
| Bladder | Bladder | 47.6 | 0.007 |
| Tumor | N/A | N/A | 0.003 |
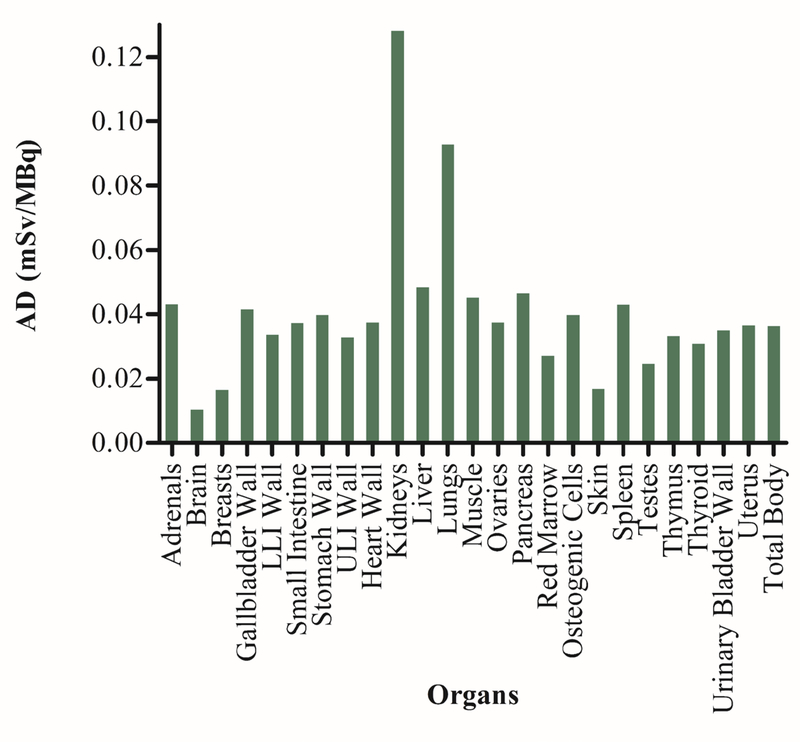
The projected human organ absorbed doses of [111In]XYIMSR-01 in mSv/MBq are shown in Figure 1. The organs receiving the highest dose were the kidneys (1.28E-01 mSv/MBq), followed by the lungs (9.28E-02 mSv/MBq) and liver (4.84E-02 mSv/MBq). The estimated tumor absorbed dose for a sphere mass of 1.0 g was 6.34E-02 mGy/MBq. Therefore, assuming the kidneys are the dose-limiting organ and following the guidelines provided by FDA 21 CFR 361.1 for radioactive drugs for imaging with an annual limit of 5 rem, the maximum [111In]XYIMSR-01 activity that can be administered to a human subject is 390.35 MBq (10.55 mCi).
Figure 1.
Projected human organ absorbed doses for [111In]XYIMSR-01. Organ absorbed doses were calculated by the residence times as input in OLINDA for an adult male reference human and 111In as the radionuclide. AD = Adsorbed doses.
Toxicology
All rats survived to the scheduled termination and no abnormal findings were identified during cage-side or hands-on observation. Slight variations in body weight were noted, particularly among Day 3 cohort rats, but the changes were either not of a biologically meaningful degree (i.e., < 10% change in almost all instances) or were attributable to pre-necropsy fasting. The body weights for the males treated with 11 μg/mL [113/115In]XYIMSR-01 were significantly less (P < 0.05) than for the vehicle control males at Days 1 and 3 in the Day 3 cohort. The body weights in the females treated with 11 μg/mL [113/115In]XYIMSR-01 were significantly less than in the vehicle controls on Days 1, 2, and 3 in the Day 3 cohort. As there were no statistically significant effects on Group 3, 55 μg/mL [113/115In]XYIMSR-01 rats, those changes are not considered to be toxicologically meaningful. In the Day 15 cohort, there were no statistically significant body weight differences between rats treated with vehicle control and rats treated with [113/115In]XYIMSR-01. Female rats treated with 11 μg/mL and 55 μg/mL [113/115In]XYIMSR-01 had a significant (P < 0.05) decrease in absolute lymphocyte count (Day 3 cohort) when compared to the vehicle control. Female rats treated with 55 μg/mL [113/115In]XYIMSR-01 had a statistically significant increase of calcium (Day 15 cohort) and triglycerides (Day 3 cohort) when compared to the vehicle control. Male rats treated with 11 μg/mL and 55 μg/mL [113/115In]XYIMSR-01 had a statistically significant decrease in urine specific gravity (Day 3 cohort) when compared to the vehicle control. Male rats treated with 55 μg/mL [113/115In]XYIMSR-01 had a statistically significant increase of white blood cells (Day 15 cohort) when compared to the vehicle control. In summary, a single intravenous dose of 11 or 55 μg/mL [113/115In]XYIMSR-01 was well-tolerated in male and female Sprague-Dawley rats. There were no consistent or treatment-related effects on endpoints including survival, clinical signs, body weights, clinical chemistry, hematology, urinalysis parameters, absolute or relative organ weights, or histopathology.
Chemistry manufacturing and controls (CMC)
Three consecutive PV batches with starting activities of 777 MBq, 1,173 MBq and 1,147 MBq were successfully validated and evaluated on all aspects and stages of the manufacturing process to establish that the process was capable of consistently delivering quality product of [111In]XYIMSR-01. The crude drug product was purified by a highly efficient preparative RP-HPLC method that removed non-radiolabeled 111In (III), excess precursor, and other impurities (Supplementary Figure 1). The radiolabeling yields for the PV batches were 53.4%, 47.9% and 50.4%, respectively. The QC results for all three PV batches are summarized in Table 1. The specific radioactivities for the corresponding PV runs were 174 GBq/μmol, 246 GBq/μmol, and 248 GBq/μmol, respectively. The radiochemical purities of all three PV batches were > 99%. The representative chromatograms for RP-HPLC purification of crude drug product and radiochemical purity of injectable [111In]XYIMSR-01 are shown in Supplementary Figure 1. The HPLC quality control analytical data for all three PV batches provided documented evidence that neither XYIMSR-01 precursor nor other impurities carried over into the final drug product (Figure 1). The chemical purities for all three PV batches were 100% (Figure 1 UV data ). According to the GC results, all three PV batches were free of acetonitrile. The pH of the final drug product was ~ 5.0. All three PV batches passed 14-day sterility and endotoxin tests. The analytical RP-HPLC methods for UV and radiodetector channels and GC met USP <1225> requirements and validation results for the radiodetector are summarized in Supplementary Table 3. Summaries of the stability results in Supplementary Table 4 show that the [111In]XYIMSR-01 drug product in its final formulation was stable for 8 h at room temperature.
Table 1.
Quality control results for the three process validation batches.
| Test | Acceptance criteria | PV Batch-1 results | PV Batch-2 results | PV Batch-3 results |
|---|---|---|---|---|
| Appearance | Clear, colorless, particulate-free | Pass | Pass | Pass |
| pH | 4.0–8.0 | 4.7 and 5.0 | 5.0 and 5.0 | 5.0 and 5.0 |
| Radionuclidic ID | Major peaks @ 245 (± 3) keV and 171 (±3) keV | 244 and 173 | 246 and 170 | 244 and 171 |
| Radiochemical purity | ≥ 95.0 % | 100% | 99.7% | 99.1% |
| Chemical purity | Impurities ≤ 3 μg/mL | 0 | 0 | 0 |
| Radiochemical ID | % difference of retention time < 5% | 0.0% | 0.3% | 2.6% |
| Bacterial endotoxins | < 175 EU/dose volume | 3.71 | 4.37 | 5.12 |
| Residual solvents | Acetonitrile <0.25 mg/mL Ethanol < 10% (v/v) | Pass | Pass | Pass |
| Filter integrity test | > 50 psi | 60 | 55 | 52 |
| Specific Activity | ≥ 661 mCi/μmol | 4706 | 6657 | 6702 |
| Retrospective Tests | ||||
| Sterility | Sterile (no growth after 14 days) | Pass | Pass | Pass |
Environmental analysis requirements
[111In]XYIMSR-01 injection prepared as a unit dose results in a total mass of active drug that does not exceed 10 μg. Based on the validation runs the total mass for [111In]XYIMSR-01 was below the UV channel LOD (0.5 μg/mL) and the total mass in 15 mL at the LOD concentration (7.5 μg). Accordingly, it is reasonable to expect that the amount of the waste to enter the environment is nontoxic.
Safety evaluation of adventitious agents
There are no recognized or potential adventitious agents found in [111In]XYIMSR-01 injection that require safety evaluation over and above the pharmacology evaluations directed to the drug substance itself.
Conclusion
The results for pharmacokinetics, radiation dosimetry and acute toxicity studies conducted in compliance with USP and FDA guidelines indicate that [111In]XYIMSR-01 injectable drug has no adverse effects with tested amounts of the agent. The three PV batches demonstrated that the process we have developed is capable of consistently delivering sterile product of [111In]XYIMSR-01 at suitable scale for clinical trials. With our stability testing result at room temperature, a shelf-life of 8 h was established for the [111In]XYIMSR-01 drug product in the final formulation of ~ 7% ethanol and 93% saline in a 30 mL vial. In summary, here we provide the dosimetry, toxicity, and CMC component results of the IND application to translate the new SPECT imaging radiopharmaceutical drug product [111In]XYIMSR-01 to phase I clinical trials.
Supplementary Material
Figure 2.
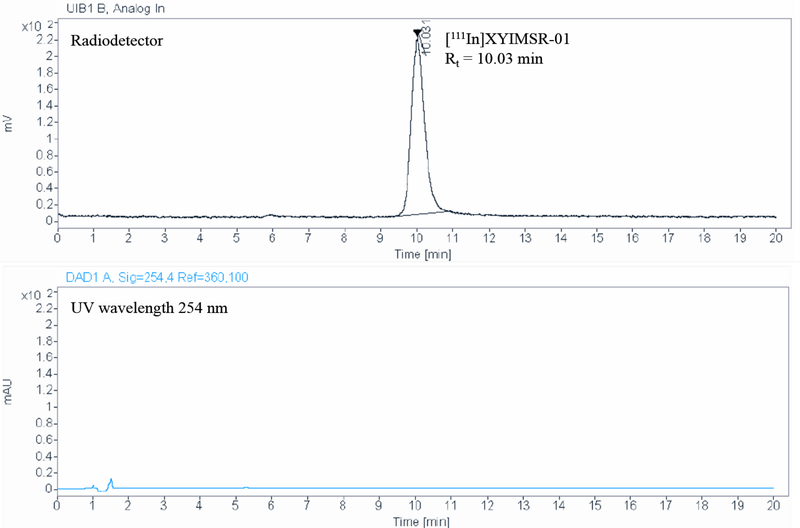
(A) Preparative RP-HPLC chromatogram for [111In]XYIMSR-01 purification. Radiodetector peak with 32.78 min (top) and UV peak at 26.80 min (bottom) retention times corresponding to [111In]XYIMSR-01 and XYIMSR-01 precursor, respectively. (B) QC RP-HPLC chromatogram for radiochemical and chemical purity. Phenomenex 150 × 4.6 mm column (part # 00F-4252-E0) was eluted with 80% water with 0.1% TFA and 20% acetonitrile with 0.1% TFA for 20 min at a flow rate of 1.5 mL/min. Radiodetector peak with 10.03 min (top) retention times corresponding to [111In]XYIMSR-01 drug product in final formulation.
Funding information
This research was supported, in part, by EB024495 and CA134675.
Conflicts of Interest
M.A.G., R.C.M., I.M., M.E.A., X.Y., S.P.R., and M.G.P. are co-inventors on a U.S. patent covering [111In]XYIMSR-01, and as such are entitled to a portion of any licensing fees and royalties generated by this technology. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. S.P.R. and M.G.P. own equity in, receive research funding from, and serve as consultants to Precision Molecular, Inc., a subsidiary of D&D Pharmatech, the licensee of [111In]XYIMSR-01. X.Y. serves as a consultant to Precision Molecular, Inc. No other potential conflict of interest relevant to this article was reported.
References
- 1.Henley SJ, Ward EM, Scott S, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer. 2020;126(10):2225–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng J-h, Lu W, Liang L, et al. Prognosis of clear cell renal cell carcinoma (ccRCC) based on a six-lncRNA-based risk score: an investigation based on RNA-sequencing data. Journal of translational medicine. 2019;17(1):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindenberg L, Mena E, Choyke PL, Bouchelouche K. PET imaging in renal cancer. Current opinion in oncology. 2019;31(3):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nature reviews Drug discovery. 2008;7(2):168–181. [DOI] [PubMed] [Google Scholar]
- 5.van Es SC, Brouwers AH, Mahesh SV, et al. 89Zr-bevacizumab PET: potential early indicator of everolimus efficacy in patients with metastatic renal cell carcinoma. Journal of Nuclear Medicine. 2017;58(6):905–910. [DOI] [PubMed] [Google Scholar]
- 6.Divgi CR, Uzzo RG, Gatsonis C, et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. Journal of clinical oncology. 2013;31(2):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muselaers CH, Boerman OC, Oosterwijk E, Langenhuijsen JF, Oyen WJ, Mulders PF. Indium-111–labeled girentuximab immunoSPECT as a diagnostic tool in clear cell renal cell carcinoma. European urology. 2013;63(6):1101–1106. [DOI] [PubMed] [Google Scholar]
- 8.Yang X, Minn I, Rowe SP, et al. Imaging of carbonic anhydrase IX with an 111In-labeled dual-motif inhibitor. Oncotarget 2015;6(32):33733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Minn I, Rowe S, et al. Nuclear imaging and radiotherapeutics agents targeting carbonic anhydrase ix and uses thereof. Google Patents; 2019. [Google Scholar]
- 10.Minn I, Koo SM, Lee HS, et al. [64Cu] XYIMSR-06: A dual-motif CAIX ligand for PET imaging of clear cell renal cell carcinoma. Oncotarget. 2016;7(35):56471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minn I, Lee HS, Koo SM, et al. [177Lu] XYIMSR-01, a Theranostic for Targeting Carbonic Anhydrase IX. Journal of Nuclear Medicine. 2016;57(supplement 2):53–53. [Google Scholar]
- 12.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. Journal of nuclear medicine. 2005;46(6):1023–1027. [PubMed] [Google Scholar]
- 13.Sgouros G, Bodei L, McDevitt MR, Nedrow JR. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nature Reviews Drug Discovery. 2020:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantel E, Williams J. An introduction to newer PET diagnostic agents and related therapeutic radiopharmaceuticals. Journal of Nuclear Medicine Technology. 2019;47(3):203–209. [DOI] [PubMed] [Google Scholar]
- 15.Kolenc Peitl P, Rangger C, Garnuszek P, et al. Clinical translation of theranostic radiopharmaceuticals: Current regulatory status and recent examples. Journal of Labelled Compounds and Radiopharmaceuticals. 2019;62(10):673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sally Schwarz MSB. FDA cGMP requirements for PET drugs. The Journal of Nuclear Medicine. 2011;52(5):16N. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.