Abstract
Background
Heart failure is a complex clinical syndrome that usually presents with breathlessness, leg edema, and fatigue. Clinically measurable natriuretic neurohormones such as B-type natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) are elevated in people with heart failure. We conducted a health technology assessment of BNP and NT-proBNP tests for people with suspected heart failure, which included an evaluation of diagnostic accuracy, clinical impact, cost-effectiveness, the budget impact of publicly funding BNP and NT-proBNP tests, and patient preferences and values.
Methods
We performed a literature search of previously published systematic reviews of the clinical evidence. We conducted an overview of reviews and included only reviews with a low risk of bias as assessed using the Risk of Bias in Systematic Reviews tool (ROBIS). We excluded any reviews where we found 100% overlap of included primary studies and selected systematic reviews or health technology assessments published after 2006 for inclusion.
We performed an economic literature review of BNP and NT-proBNP testing in people with suspected heart failure. Medical and health economic databases were searched from database inception until July 25, 2019. Next, we assessed the cost-effectiveness of BNP and NT-proBNP based on the published economic literature. We transferred the cost-effectiveness results of two applicable, recent economic evaluations from the National Institute for Health and Care Excellence (NICE) to the Ontario setting in lieu of conducting de novo primary economic evaluations. We also estimated the budget impact of publicly funding BNP and NT-proBNP tests in people with suspected heart failure in Ontario over the next 5 years.
To contextualize the potential value of BNP and NT-proBNP testing, we spoke with people with suspected heart failure.
Results
We included eight systematic reviews in the clinical evidence review. B-type natriuretic peptides and NT-proBNP had a high pooled sensitivity (80% to 94% and 86% to 96%, respectively; strength of evidence: high) and a low pooled negative likelihood ratio (0.08–0.30 and 0.09–0.23, respectively; strength of evidence: not reported) within varying thresholds or cut points and settings, as reported in seven systematic reviews. In one systematic review, when BNP or NT-proBNP was used in the diagnosis of heart failure in the emergency department (ED), there was a decrease in the mean length of hospital stay (−1.22 days; confidence interval [CI] −2.31 to −0.14; Grading of Recommendations Assessment, Development, and Evaluation [GRADE] Working Group criteria: Moderate). B-type natriuretic peptide testing did not reduce hospital admission rates (odds ratio [OR]: 0.82; CI: 0.67–1.01; GRADE: Moderate), 30-day hospital readmission rates (OR: 0.88; CI: 0.64–1,20; GRADE: Moderate), or hospital mortality rates (OR: 0.96; CI: 0.65–1.41; GRADE: Moderate). No systematic review was identified that addressed the impact on clinical outcomes of BNP use in the community setting.
Our economic literature review found a total of 12 studies evaluating the cost-effectiveness of BNP or NT-proBNP testing in patients with suspected heart failure. The studies suggested that BNP or NT-proBNP tests, when used in addition to standard clinical investigations, were either dominant (less costly and more effective) or cost-effective across different countries (including Canada) and settings.
Two economic evaluations conducted by NICE were considered applicable to our research question and of high methodological quality. Based on the transferred results from the two NICE economic evaluations, we concluded that BNP and NT-proBNP were highly likely to be cost-effective in Ontario in the ED setting, and NT-proBNP was highly likely to be cost-effective in the community care setting.
Our budget impact analysis estimated that over the next 5 years, publicly funding BNP and NT-proBNP tests would result in an additional cost of $38 million in the ED (at a cost of $75 per test) and a cost savings of $20 million in community care (at a cost of $28 per test).
We received strong support from interview participants about BNP or NT-proBNP diagnostic testing. The main reason was the perceived potential benefit of receiving a speedier diagnosis. The overall process, from diagnosis to treatment, is a substantial emotional burden for patients and caregivers, and for those living further away from secondary or tertiary care settings. An earlier diagnosis could allow patients to receive treatment at a hospital better equipped to manage their potentially fatal symptoms and conditions.
Conclusions
B-type natriuretic peptide and NT-proBNP tests have high sensitivity and low negative likelihood ratio, suggesting that concentrations of either natriuretic peptides within the appropriate cut points can rule out the presence of heart failure with a high degree of confidence. Additionally, BNP or NT-proBNP testing along with usual care in an ED setting likely can reduce the length of hospital stay by at least 1 day but likely results in little to no difference in hospital mortality, 30-day readmission, or admission rates to hospital.
Based on the published economic literature, we expected BNP or NT-proBNP tests used in addition to standard clinical investigations to be cost-effective as a rule-out test in patients with suspected heart failure in Ontario. If BNP and NT-proBNP tests are publicly funded in Ontario, we estimated that there would be additional costs in the ED setting (due to increased detection of heart failure) and savings in community care (due to reduced referrals to echocardiography and cardiologists).
People we interviewed gave BNP and NT-proBNP testing strong support, citing the perceived benefits of quicker, more accurate diagnoses that could reduce misdiagnoses, stress, and the burden on patients and caregivers.
Objective
This health technology assessment evaluates the diagnostic accuracy, clinical impact, and cost-effectiveness of B-type natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) for adults with suspected heart failure. It also evaluates the budget impact of publicly funding BNP and NT-proBNP tests and the experiences, preferences, and values of people with suspected heart failure.
Background
Health Condition
Heart failure is a complex clinical syndrome in which abnormal cardiac function increases the risk of or results in clinical symptoms and signs of reduced cardiac output and/or pulmonary or systemic congestion.1,2 The syndrome can be acute or chronic and often develops after other conditions, such as hypertension, coronary artery disease, or diabetes mellitus, or behavioral factors such as heavy alcohol use, have damaged or weakened the heart.1,2 Common symptoms include breathlessness on exertion, difficulty breathing when lying flat (orthopnea), suddenly waking up from sleep with severe shortness of breath (paroxysmal nocturnal dyspnea), ankle or leg swelling (edema), and fatigue.1 Signs commonly associated with heart failure are elevated jugular venous pressure, pulmonary crackles on chest auscultation, and peripheral edema supported by evidence of pulmonary congestion on chest x-ray or structural abnormality on echocardiogram.1–3
Clinical Need and Target Population
The incidence of heart failure is approximately 1% to 2% among adults in developed nations and increases with age.1,2,4,5 In 2016, the Heart and Stroke Foundation reported that 50,000 Canadians are diagnosed with heart failure annually and about 600,000 are living with heart failure.3 According to a report by the Canadian Chronic Disease Surveillance System,2 the age-standardized incidence rates of heart failure in Ontario between 2012 and 2013 among men and women are 6.2 and 4.5 per 1,000 people, respectively; whereas the age-standardized prevalence of heart failure in Ontario between 2012 and 2013 is estimated to be about 3.3%.6 Other sources also indicate that the prevalence rate of heart failure among people aged 40 or older in Ontario is 3.9%.7
Diagnosis of Heart Failure
Heart failure is a major cause of mortality and morbidity. An accurate diagnosis of heart failure is important because the treatments are often specific and must be started as soon as possible. But heart failure can be challenging to diagnose because symptoms may be similar to those from other health conditions or comorbidities.1–3 The cardinal triad of edema (swelling), fatigue (tiredness), and dyspnea (shortness of breath) is not a sensitive or specific manifestation of heart failure.1,2 There is no single test to diagnose heart failure, and clinicians often agree that it is important to recognize atypical presentations.1,2 Diagnosis is based on medical history, clinical examination, and investigation. Different thresholds or cut points for the natriuretic peptides have been reported by the Canadian Cardiovascular Society guideline for the diagnosis of heart failure in various settings.2 Pragmatically, BNP levels less than 50 pg/mL and NT-proBNP levels ranging less than 125 pg/mL may be highly suggestive to rule out a diagnosis of heart failure.
Health Technology Under Review
Natriuretic peptides belong to a group of neurohormones that exist in three forms: A-, B-, and C-type natriuretic peptide (ANP, BNP, and CNP, respectively).8 A-type natriuretic peptide and BNP can be extracted from the myocardium, whereas CNP originates within endothelial cells. Clinically measurable natriuretic peptides include ANP, BNP, and NT-proBNP. However, BNP and NT-proBNP are preferred as biomarkers for the diagnosis of heart failure over ANP due to their prolonged biological half-life.8 BNP has a shorter half-life than NT-proBNP and is stable in whole blood at room temperature with the addition of ethylenediaminetetraacetic acid (EDTA) for at least 24 hours, compared with NT-proBNP, which is stable for at least 72 hours with EDTA.9 Clinicians can choose either BNP or NT-proBNP, depending on testing conditions.
Levels of BNP or NT-proBNP can be detected through a blood sample.10 Blood tests to detect natriuretic peptides, along with clinical examination and other blood work, are the primary means used to detect and diagnose heart failure.10 There are three different assay methods reported in literature. Firstgeneration assays are radioimmunoassays that require extraction and purification of a plasma sample.11,12 Second-generation assays are based on monoclonal antibodies and radioisotope labels and provide improved sensitivity and precision compared with the first generation. Commercial versions of the monoclonal antibody assay first appeared in 1994 and initially required 12 to 36 hours to complete.13,14 The third-generation assays use immunofluorescent methods and include point-of-care (POC) tests.15,16 They became available in 2000 and provide results in as little as 15 minutes.15,16 Both laboratory and POC testing are in routine clinical use in Ontario.
A BNP or NT-proBNP test may be ordered in a doctor's office when a person has signs and symptoms that could be due to heart failure.17 Testing may also be done in the emergency department when someone has findings that could indicate heart failure and health practitioners need to quickly determine if a person is suffering from heart failure or another medical problem that may have similar symptoms as heart failure. Results below established threshold levels suggest that the person has symptoms due to a cause other than heart failure. Values above threshold levels suggest that further investigation of heart failure is warranted.17
Regulatory Information
BNP and NT-proBNP are tested through assay kits. Some of the kits within the included studies are listed below with their approved licence numbers from Health Canada. An entire list of approved assay kits can be obtained from the Health Canada website.18
Siemens Advia Centaur CP System (Licence No: 73010)
Siemens Dimension Chemistry System (Licence No: 7757)
Quidel Triage BNP Test (Licence No: 38870)
Roche Elecsys ProBNP II (Licence No: 98340)
Abbott Architect “I” System (Licence No: 11491)
Abbott Alinity I BNP Assay (Licence No: 101345)
Ortho Clinical Diagnostics Vitros Immunodiagnostic Products NT-proBNP II Assay (Licence No: 104126)
Ontario, Canadian, and International Context
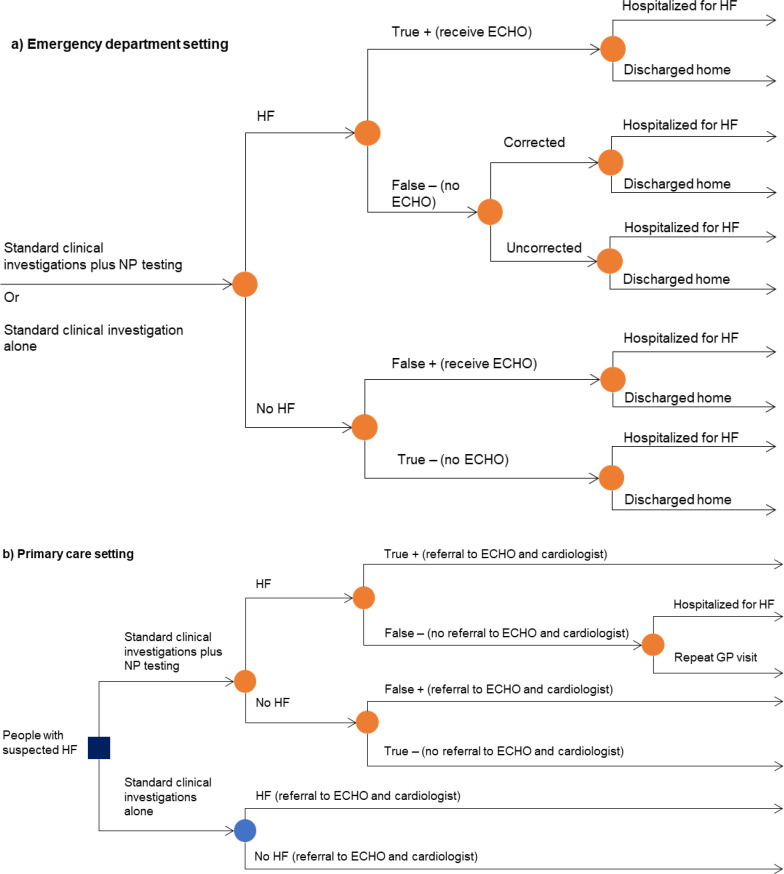
According to the Health Quality Ontario quality standard Heart Failure: Care in the Community for Adults, people with suspected heart failure should undergo an initial evaluation that includes, at minimum, a medical history, a physical examination, initial laboratory investigations, an electrocardiogram (ECG), and a chest x-ray. If appropriate, natriuretic peptide levels should be tested to help formulate a diagnosis. If heart failure is confirmed or still suspected after these tests, an echocardiogram (ECHO) is then performed.7
In Ontario, natriuretic peptide testing is a U-coded test, which means that it does not appear in the schedule of benefits for laboratory services. Patients pay for U-coded tests unless they are performed as an inpatient or outpatient hospital service. If a hospital doctor orders a U-coded test at their hospital laboratory, the expense is absorbed by the hospital's global budget. The hospitals do not receive a specific budget line for this test. There are 24 laboratories in Ontario currently licensed to perform natriuretic peptide testing, including 22 hospital laboratories and two community laboratories.
The National Institute for Health and Care Excellence (NICE) published a review in 2014 examining the diagnostic accuracy of BNP and NT-proBNP in an acute care setting.19 Another guideline released by NICE20 in 2018 has detailed methodology on a systematic search conducted to answer a research question in a community or outpatient setting.20 In addition, one recently published position paper from Europe recommends that natriuretic peptides be measured in all people with new onset or worsening heart failure as it facilitates either early diagnosis or exclusion of heart failure.21
Expert Consultation
We engaged with experts in the specialty areas of heart failure and laboratory investigations to help inform our understanding of aspects of the health technology and our methodologies and to contextualize the evidence surrounding the diagnostic accuracy and clinical impact of the test.
PROSPERO Registration
This health technology assessment has been registered in PROSPERO, the international prospective register of systematic reviews (CRD # 148036), available at https://www.crd.york.ac.uk/PROSPERO.
Clinical Evidence
Primary Research Question
What is the diagnostic accuracy of B-type natriuretic peptide (BNP) or N-terminal proBNP (NT-proBNP) tests for people with suspected heart failure?
Secondary Research Question
What is the impact of BNP or NT-proBNP testing on clinical outcomes in people with suspected heart failure?
Methods
Overview of Reviews Approach
Numerous recent systematic reviews and health technology assessments have been published evaluating the diagnostic accuracy of natriuretic peptides for suspected heart failure. To avoid duplication of prior work and to draw upon existing evidence, we planned to systematically search and identify systematic reviews or health technology assessments with high methodological quality that matched the scope of our project. The selection of systematic reviews and health technology assessments for final inclusion was based on a risk of bias assessment, recency, comprehensiveness of outcomes report, and relevance of the reviews.
Clinical Literature Search
We performed a systematic literature search on July 22, 2019, to retrieve studies published from database inception until the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, the Cochrane Database of Systematic Reviews, the Health Technology Assessment Database, and the National Health Service Economic Evaluation Database (NHS EED).
A medical librarian developed the search strategies using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. Methodological filters were used to limit retrieval to systematic reviews, meta-analyses, and health technology assessments. The final search strategies were peer-reviewed using the PRESS Checklist.22
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites as well as clinical trial and systematic review registries. The grey literature search was updated on January 14, 2020. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
STUDIES
Inclusion Criteria
English-language full-text publications
-
Systematic reviews and health technology assessments that include a systematic review
○ Research questions and PICO (population, intervention, comparator, outcomes) match or include the focus of our review
○ Provides information on their literature search methods including, at a minimum, the databases searched, search terms, and the search dates
○ Studies with prespecified eligibility criteria
Exclusion Criteria
Non-systematic reviews, primary studies, editorials, commentaries, case reports, conferences abstracts, letters
PARTICIPANTS
Inclusion Criteria
Adults (≥ 18 years) with suspected heart failure
Exclusion Criteria
People < 18 years of age
People undergoing chemotherapy or treatment for HIV (where the medication can cause heart failure) or who are pregnant (due to the unique physiology of pregnant people)
INTERVENTIONS
BNP or NT-proBNP blood tests (plasma and whole blood) at any reported threshold or cut point
REFERENCE STANDARD
Inclusion Criteria
Usual care, which includes history, physician exam, blood work, electrocardiograph (ECG), and chest x-ray
A clinical diagnosis by a specialist or family physician in patients with signs and symptoms of suspected heart failure
Echocardiography when used along with any of the reference standards above
DIAGNOSTIC ACCURACY OUTCOME MEASURES
Sensitivity
Specificity
Negative predictive value
Negative likelihood ratios
Positive predictive value
Positive likelihood ratios
Area under the curve
Diagnostic odds ratio
CLINICAL OUTCOME MEASURES
Length of hospital stay
Mortality outcomes as reported
Hospital admission rates
Quality of life
Other outcomes as reported
SETTING
Outpatients/community-based clinics
Emergency department (ED)/outpatient acute care
ICU/hospital inpatients (i.e., mixed settings)
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Covidence23 and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. A single reviewer then examined the full-text articles and selected reviews eligible for inclusion. A single reviewer also examined reference lists for any additional relevant studies not identified through the search.
Data Extraction
We extracted relevant data on study characteristics, such as the review methods (e.g., eligibility criteria—PICO, study types included, literature search information (e.g., date and databases searched), and number of studies included.
Evidence Synthesis
We provide a narrative synthesis of results analyzed and reported in the included studies. We present our findings in text and tabular formats, noting trends across systematic reviews. Where possible, we categorize findings into subgroups of interest: community/outpatient, ED, or mixed setting. Tables are also categorized into the different thresholds or cut offs as reported within the included studies. Where studies included multiple results, we used our best judgement to present the most appropriate and applicable results.
Critical Appraisal of Evidence
We used the Risk of Bias in Systematic Reviews tool (ROBIS) risk of bias assessment items (e.g., study screening methods, data extraction methods, evidence synthesis and statistical analysis methods, risk of bias assessment, quality of evidence assessment) to select systematic reviews or health technology assessments that scored high on ROBIS.24 We reported the risk of bias and the quality of evidence as reported within the selected reviews and health technology assessments.
Results
Clinical Literature Search
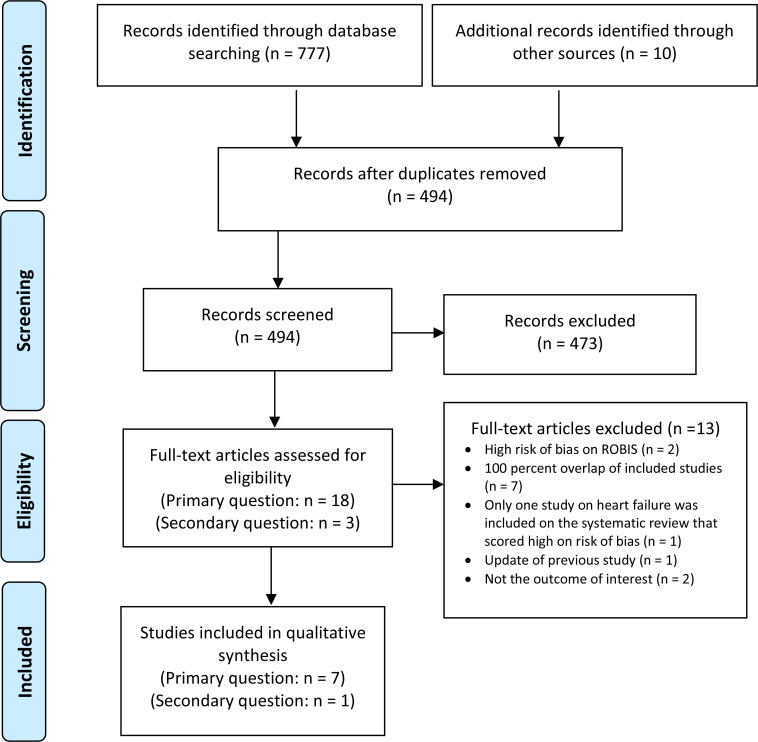
The database search of the clinical literature yielded 777 citations published from database inception until July 22, 2019. In total, we identified 10 additional studies from other sources. For our primary research question, we identified 18 systematic reviews that met our inclusion criteria.6,19,20,25–39 Two were excluded because they ranked high on risk of bias in ROBIS.30,40,41 Of the remaining 16, eight had almost 100 percent overlap with other recent systematic reviews. Within the eight remaining reviews, one examined point-of-care diagnostic accuracy testing for various health conditions and included only one study that examined the diagnostic accuracy of natriuretic peptides in heart failure and this primary study within the review ranked high on risk of bias.38 Hence, we also excluded it from our overview of reviews. Seven systematic reviews appropriately addressed our primary research question.6,19,20,25–39
For our secondary research question, we identified three systematic reviews that met our inclusion criteria.30,40,41 Two were excluded because they reported only clinical outcomes on natriuretic peptide–guided therapies or management in heart failure.30,40 One systematic review appropriately addressed our secondary research question.41 See Appendix 3, Table A5, for a list of selected studies excluded after full-text review. Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the clinical literature search.42
Figure 1: PRISMA Flow Diagram—Clinical Search Strategy.
Source: Adapted from Moher et al.42
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Characteristics of Included Studies
Table 1 summarizes the characteristics of the eight included systematic reviews.19,20,25–29,41 Seven assessed diagnostic accuracy through various outcome measures for BNP and NT-proBNP, except for one that assessed BNP alone.27 The systematic reviews presented in Table 1 are categorized based on the settings in which these tests were assessed. Two assessed the diagnostic accuracy outcome measures for BNP and NT-proBNP in the community or outpatient setting.20,25 One excluded studies with a sample size of < 100 and studies that used echocardiography alone as their reference standard.20 Of the two reviews26,28 that assessed diagnostic accuracy of natriuretic peptides in the emergency or urgent care setting, one focused on the emergency setting and included 57 studies with 52 unique cohorts28 while the other26 included 72 studies and included people with suspected heart failure presenting to the ED or to an urgent care setting. One review assessed point-of-care testing within all settings.29 One systematic review reporting clinical outcomes included five randomized controlled trials and examined the usefulness of using natriuretic peptides in the ED.41
Table 1:
Characteristics of Systematic Reviews of Diagnostic Accuracy of BNP and NT-proBNP in People With Suspected Heart Failure
| Author, Year | Country | Scope (Population, Index Test, Reference Standard) | Literature Search | N Studies | Outcomes Reported | Main Analyses |
|---|---|---|---|---|---|---|
| Diagnostic Accuracy Outcomes | ||||||
| Community/Outpatient Setting | ||||||
| NICE, 201820 | UK |
P: People with suspected HF in a community or outpatient setting I: BNP, NT-proBNP R: A clinical diagnosis based on the opinion of at least one cardiologist and objective evidence of cardiac dysfunction |
Databases searched: 5 Grey literature: ongoing, unpublished, and guidelines Search dates: update from 2009–Dec 2017 |
8 | Specificity, sensitivity, PPV/NPV, ROC curve, or AUC | Hierarchical bivariate meta-analysis and HSROC |
| Booth et al, 201425 | Canada |
P: People presenting to community care physicians with signs or symptoms of HF, or people who were at risk of HF at the time of presentation I: BNP, NT-proBNP R: Any comparator that was used in the primary studies |
Databases searched: 6 Grey literature: regulatory agency websites, clinical trials database, and conference sources Search dates: 1989–Jun 2012 |
32 | Sensitivity, specificity, PPV/NPV, DOR, ROC | DOR was calculated using GLM to bivariate meta-analysis Forest plots and HSROCs |
| Emergency Department/Acute Care Setting | ||||||
| Martindale et al, 201628 | USA |
P: Adults presenting to the ED with dyspnea as a primary complaint I: BNP, NT-proBNP R: History, symptoms, and physical examination |
Databases searched: 2 Grey literature: bibliographic review of reference lists Search dates: inception–Mar 2015 |
57, with 52 unique cohorts | Sensitivity, specificity, likelihood ratios | Descriptive only |
| Hill et al, 201426 | Canada |
P: Adults > 18 y who presented to an ED or UCC with signs or symptoms suggestive of HF I: BNP, NT-proBNP R: Comparator methods or prediction scores as used in the included studies |
Databases searched: 6 Search dates: 1989–Jun 2012 |
72 | Sensitivity, specificity, DOR, likelihood ratios, ROC/AUC | DOR was calculated using GLM to bivariate meta-analysis Forest plots and HSROCs |
| Mixed Settings | ||||||
| NICE, 201419 | UK |
P: Adults ≥ 18 y who have a diagnosis of acute HF, have possible acute HF, or are being investigated for acute HF, or people with dyspnea I: BNP, NT-proBNP R: Consensus of two senior ED physicians; Retrospective review by one or more cardiologists; clinical criteria or guidelines |
Databases searched: 4 Grey literature: abstracts from scientific forums, bibliographies of published articles Search dates: inception to Jan 2014 |
46 | Sensitivity, specificity, PPV, NPV, ROC curve | Bivariate method for the direct estimation of summary sensitivity and specificity using a random effects approach |
| Latour-Perez et al, 200627 | Spain |
P: Patients with suspected heart failure I: BNP R: Clinical diagnosis |
Databases searched: 4 Grey literature: hand search of reference lists Search dates: differed for different databases and ranged from 1966 to 2004 |
11 | Sensitivity, specificity, likelihood ratios, AUC, DOR | Random effects model for pooled analysis |
| Mixed Setting (Point-of-Care Testing) | ||||||
| Taylor et al, 201829 | UK |
P: Adults with suspected or confirmed HF, with a focus on ambulatory care (mixed setting) I: BNP, NT-proBNP R: Echocardiography, clinical examination or both |
Databases searched: 6 Grey literature: related search and clinical trials registry Search dates: inception to March 2017 |
40 | Sensitivity, Specificity, ROC curve | Bivariate meta-analysis Methods and the hierarchical summary receiver operating characteristic (HSROC) model |
| Clinical Outcomes | ||||||
| Lam et al, 201041 | Australia |
P: Adults presenting to the ED with acute shortness of breath I: BNP, NT-proBNP R: Routine testing, clinical examination or both |
Databases searched: 2 Grey literature: related search and reference lists were hand searched Search dates: 1996–Jul 2010 |
5 | Hospital admission rate, length of hospital stay, in-hospital mortality, intensive care unit admission rate, 30-d readmission rate | Random-effects models using inverse variance weighting for continuous outcomes Mantel–Haenszel methods for all dichotomous outcomes |
Abbreviations: AUC, area under the curve; BNP, B-type natriuretic peptide; DOR: diagnostic odds ratio; ED, emergency department; GLM, generalized linear model; HF, heart failure; HSROC, hierarchical summary receiver operating characteristic curve; NICE, National Institute for Health and Care Excellence; NT-proBNP, N-terminal proBNP; NPV, negative predictive value; PPV, positive predictive value; ROC, receiver operating characteristic curve; UCC, urgent care centre.
The measures of test performance reported by systematic reviews varied and included sensitivity, specificity, positive and negative predictive values, likelihood ratios, area under the curve, receiver operator curve characteristics, and diagnostic odds ratios. Heterogeneity within the included studies prevented many of the reviews from reporting a summary estimate. Three were authored by groups from the United Kingdom,19,20,29 two were from Canada,25,26 there was one each from the United States,28 Spain,27 and Australia.41
OVERLAP BETWEEN SYSTEMATIC REVIEWS ON DIAGNOSTIC ACCURACY
A total of 185 individual studies were included in the seven systematic reviews that answered our primary research question. There were 123 unique citations; the remainder of the studies overlapped between the reviews. This overlap is primarily explained by the literature search dates for each review and reflects variation in eligibility criteria such as setting, population, minimum sample sizes, and reference standards.
Risk of Bias in the Included Studies
The risk of bias of the systematic reviews was assessed using ROBIS.24 The risk of bias was judged to be low in five included systematic reviews20,25,26,28,29 and high or unclear in two,19,27 mainly owing to concerns with identification and selection of studies, data collection, and study appraisal (Table A1, Appendix 2).
Six of the seven systematic reviews reported the risk of bias of their included studies using the quality assessment of diagnostic accuracy studies (QUADAS-2) tool43 (Table A2, Appendix 2). Five reviews reported either low or unclear risk of bias for the domains of index test, reference standard, and timing and flow.19,20,25,26,28,29 Two reviews reported a high risk of bias for the domain on patient selection.26,28 Hill et al26 reported the risk to be either high or there was not enough information to make an assessment; whereas, Martindale et al28 considered excluding people with comorbidities, which does not accurately reflect the population and had the potential to inflate specificity of the test under study. There were either low or unclear concerns on applicability of the test as reported by majority of the reviews. Taylor et al29 had a high concern on the applicability of patient selection as the population was not representative of the ambulatory setting.29
Two systematic reviews assessed the strength of evidence using Agency for Healthcare Research and Quality (AHRQ) guidance and found the sensitivity evidence to be of high quality and the specificity evidence to be of moderate quality for the included studies for both natriuretic peptides (Table A3, Appendix 2).25,26 The strength of evidence was reported only for the outcomes of sensitivity and specificity. There was insufficient information reported in the reviews for us to assess the quality of the body of evidence within each systematic review using GRADE. For our secondary outcome on clinical utility, Lam and colleagues41 assessed the risk of bias as low, using the Cochrane Collaboration risk of bias tool.
Outcomes: Diagnostic Accuracy of BNP
Seven systematic reviews reported accuracy measures such as sensitivity and specificity of BNP at different thresholds.19,20,25–29 Specificity was considered important in order to avoid unnecessary referrals for echocardiography and specialist clinical assessment where heart failure was highly unlikely. Other accuracy statistics, such as positive and negative predictive values and area under the curve (AUC), were also reported. The AUC has a value between 0 and 1. A value of 1 denotes that the diagnostic test is accurate, a value of 0.5 denotes that the test is nondiscriminatory.44
COMMUNITY/OUTPATIENT SETTING
Two reviews reported outcomes in the community/outpatient setting.20,25 The different thresholds reported in one review by NICE20 ranged from 30 to 400 pg/mL, with sensitivity ranging from 6% to 97% and specificity ranging from 35% to 100%. The positive predictive value ranged from 43% to 100% and the negative predictive value ranged from 47% to 98%, whereas the AUC ranged from 69% to 96%.20 A cut point of BNP > 50 pg/mL has been recommended by the Canadian Cardiovascular Society clinical guideline for a diagnosis of heart failure in a community or outpatient setting.1 The National Institute for Health and Care Excellence reported a sensitivity ranging from 87% to 97% for a threshold of 65–77 pg/mL.20 Additionally, a sensitivity analysis conducted by the NICE reviewers that included studies with low risk of bias reported very similar sensitivity and specificity.20 Booth et al25 reported pooled sensitivity and specificity to be 80% and 61%, respectively, for an optimum cut point, as defined by the primary study authors25 but not reported in the studies (Table 2).
Table 2:
Diagnostic Accuracya of BNP as Reported in Included Systematic Reviews
| Author, Year | # Studies | Assays | Threshold/Cut Point in pg/mL | Sensitivity (95% CI) | Specificity (95%CI) | PPV or LR+ (95% CI) | NPV or LR-(95% CI) | AUC/ROC (95%CI) | log DOR |
|---|---|---|---|---|---|---|---|---|---|
| Community/Outpatient Setting | |||||||||
| NICE, 201820 | 5 | AxSym, Centaur | 30–400 | Range: 6–97 | Range: 35–100 | PPV Range: 43–100 | NPV Range: 47–98 | 0.69–0.96 | NR |
| 1 | 30 | 95 (89–98) | 35 (29–42) | PPV: 43 | NPV: 93 | 0.84 (0.79–0.89) | NR | ||
| Booth et al, 201425 | 26 | Triage, AxSym, Centaur | 30–500 | Range: 25–97 | Range: 23–92 | NR | NR | 0.62–0.93 | NR |
| 8 | Optimumb | 80 (71–89) | 61 (43–80) | 2.27 (1.59–3.24) | 0.30 (0.16–0.55) | 0.80 (0.71–0.90) | 2.07 (1.20–2.94) | ||
| Emergency Department/Acute Care Setting | |||||||||
| Martindale et al, 201628 | 19 | Triage, AxSym, iSTAT | 100 | 94 (93, 94) | 53 (52–54) | 2.20 (1.8–2.7) | 0.11 (0.07–0.16) | NR | NR |
| Hill et al, 201426 | 22 | Triage, AxSym, iStat, Centaur | 100 | 95 (93–96) | 66 (56–74) | 2.76 (2.12–3.59) | 0.08 (0.06–0.10) | 0.94 (0.92–0.96) | 3.55 (3.13–3.97) |
| 29 | Optimumb | 91 (88–94) | 80 (74–85) | 4.61 (3.49–6.09) | 0.11 (0.08–0.15) | 0.92 (0.91–0.94) | 3.74 (3.31–4.18) | ||
| Mixed Setting | |||||||||
| Taylor et al, 201829,c | 30 | Triage | 100 | 95 (90–98) | Range: 47–97 | NR | NR | 0.95 (0.92–0.97) | NR |
| NICE, 201419 | 19 | Triage | ≤ 100 | Range: 81–100 | Range: 31–94 | NR | NR | 0.95 (0.93–0.97) | NR |
| 20 | 100–500 | Range: 47–93 | Range: 61–100 | NR | NR | 0.85 (0.81–0.89) | NR | ||
| Latour-Perez et al, 200627 | 9d | Triage | 20–250 | Range: 53–97 | Range: 54–97 | NR | 0.11, (0.08–0.16) | 0.927 (SE:0.017) | 28.9 (20.6–40.5)e |
Abbreviations: AUC, area under the curve; BNP, B-type natriuretic peptide; CI, confidence interval; DOR: diagnostic odds ratio, LR, likelihood ratio; NPV, negative predictive value; NR, not reported; PPV, positive predictive value; ROC, receiver operating characteristic curve.
Results are presented as reported within the reviews.
Optimum cut point as defined by report authors.
Point-of-care testing.
As reported in the review, two studies excluded as outliers.
Diagnostic odds ratio.
EMERGENCY DEPARTMENT/ACUTE CARE SETTING
Two reviews reported a pooled estimate for diagnostic accuracy outcomes.26,28 For a cut point of 100 pg/mL, pooled sensitivity and pooled specificity ranged from 94% to 95% and 53% to 66%, respectively.26,28 The pooled positive likelihood ratio ranged from 2.20 to 2.76, while the pooled negative likelihood ratio ranged from 0.08 to 0.11.26,28 The AUC was 0.94 and log diagnostic odds ratio was 3.55 (Table 2).26 A log diagnostic odds ratio may be used in meta-analyses of diagnostic accuracy studies for non-parametric data or where data is not normally distributed.45
MIXED SETTING
Of the three reviews that included studies from mixed settings,19,27,29 one, Taylor et al,29 reported results of diagnostic accuracy using only point-of-care devices for a threshold of 100 pg/mL.29 The authors reported that pooled sensitivity and AUC were 95% each, while the specificity ranged from 47% to 97%.29 Depending on the thresholds, similar ranges were reported in the other two reviews (Table 2).19,27
Authors of two of the three included systematic reviews rated the certainty of the evidence for sensitivity as high and for specificity as moderate, downgrading for inconsistency (Table A3, Appendix 2).25,26
Outcomes: Diagnostic Accuracy of NT-proBNP
We included six reviews reporting the accuracy of NT-proBNP.19,20,25,26,28,29 Two reported diagnostic accuracy outcomes of NT-proBNP in the community/outpatient setting,20,25 two in emergency care setting,26,28 and two in mixed setting.19,29
COMMUNITY/OUTPATIENT SETTING
Two reviews reported a sensitivity of 2% to 100% and a specificity of 3% to 100% with varying thresholds for NT-proBNP in a community/outpatient setting.20,25 Results of sensitivity and specificity in the two reviews for an optimum cut point (defined by the authors) ranged from 86% to 96% and 48% to 58%, respectively.20,25 The pooled estimate for an optimum cut point for the positive and negative likelihood ratios were 2.18 and 0.23, respectively, and the log diagnostic odds ratio was 2.50 (Table 3).25
Table 3:
Diagnostic Accuracy of NT-proBNP as Reported in Included Systematic Reviews
| Author, Year | # Studies | Assays | Threshold/Cut Off, in pg/mL | Sensitivity (95% CI) | Specificity (95%CI) | PPV or LR+ (95% CI) | NPV or LR-(95% CI) | AUC/ROC (95%CI) | log DOR |
|---|---|---|---|---|---|---|---|---|---|
| Community/Outpatient Setting | |||||||||
| NICE, 201820 | 6 | Elecsys | 67–2000 | Range: 2–100 | Range: 27–100 | PPV range: 29–100 | NPV range: 71–100 | 0.74–0.94 | NR |
| 3 | 125 | 96 (72–100) | 48 (18–80) | PPV range: 38–48 | NPV range: 87–100 | 0.74–0.94 | NR | ||
| Booth et al, 201425 | 20 | Elecsys | 25–6180 | Range: 44–100 | Range: 3–97 | NR | NR | 0.70–0.98 | NR |
| 11 | Optimuma 87–424 | 86 (79–93) | 58 (42–75) | 2.18 (1.81–2.63) | 0.23 (0.16–0.34) | 0.85 (0.79–0.90) | 2.50 (1.87–3.13) | ||
| Emergency Department/Acute Care Setting | |||||||||
| Martindale et al, 201628 | 10 | Elecsys, Dimension | 300 | 90 (89–92) | 38 (36–40) | 1.8 (1.4–2.2) | 0.09 (0.03–0.34) | NR | NR |
| Hill et al, 201426 | 4 | Elecsys, Dimension | 125–450 | 91 (88–93) | 67 (50–80) | 2.74 (1.74–4.32) | 0.13 (0.10–0.19) | 0.87 (0.79–0.95) | 3.01 (2.34–3.69) |
| 19 | Optimuma | 90 (85–93) | 74 (67–81) | 3.49 (2.72–4.49) | 0.14 (0.10–0.20) | 0.90 (0.87–0.93) | 3.22 (2.80–3.64) | ||
| Mixed Setting | |||||||||
| Taylor et al, 201829,b | 7 | Cardiac Reader (POC) | 135 | 99 (57–100) | 60 (44–74) | NR | NR | 0.97 (0.57–1.00) | NR |
| NICE, 201419,c | 10 | Elecsys, Dimension | ≤ 300 | Range: 96–100 | Range: 5–93 | NR | NR | 0.99 (0.98–1.00) | NR |
| 13 | 300–1800 | Range: 75–98 | Range: 49–93 | NR | NR | 0.90 (0.85–0.94) | NR | ||
Abbreviations: AUC, area under the curve; CI, confidence interval; DOR, diagnostic odds ratio; LR, likelihood ratio; NPV, negative predictive value; NR, not reported; POC, point of care; PPV, positive predictive value; ROC, receiver operating characteristics curve.
The cut off points used by study authors varied among the different studies.
Included both primary and urgent care.
Included inpatient, intensive care unit, emergency department, and acute referral.
EMERGENCY DEPARTMENT/ACUTE CARE SETTING
Two systematic reviews contributed to the outcomes in this setting.26,28 For an NT-proBNP cut point of 300 pg/mL, or the optimum cut point adopted by the authors within each systematic review, to rule out a diagnosis of heart failure in an emergency or acute care setting, pooled sensitivity and pooled specificity ranged from 90% to 91% and 38% to 74%, respectively. The pooled positive and negative likelihood ratios ranged from 1.8 to 3.49 and 0.09 to 0.14, respectively. The pooled estimate for AUC ranged from 0.87 to 0.90 and the diagnostic odds ratio approximated 3 (Table 3).
MIXED SETTING
Two systematic reviews contributed to the outcomes for this setting.19,29 Taylor et al29 reported the outcomes of interest in point-of-care devices and showed a pooled sensitivity of 99%, specificity of 60%, and an AUC of 97%. Results of POC tests by NICE were very similar to those reported for laboratory tests (Table 3).19
Authors of the two included systematic reviews rated the certainty of the evidence for sensitivity as high and for specificity as moderate, downgrading for inconsistency (Table A3, Appendix 2).25,26
Outcomes: Clinical Outcomes for BNP or NT-proBNP
The systematic review by Lam et al41 examined the clinical outcomes of using BNP or NT-proBNP in the diagnosis of heart failure in an ED setting.41 The review included five randomized controlled trials that reported on outcomes such as length of hospital stay, critical care unit stay, admission rates, 30-day readmission rates and hospital mortality (see Table 4). All the included studies examined the use of natriuretic peptides as a diagnostic tool in people with acute dyspnea. The comparator groups received usual care with no BNP testing as reported by the included trials.
Table 4:
Clinical Outcomes as Reported in the Systematic Review by Lam et al41
| Study, Year | No of Studies, Design | BNP/NT proBNP | Admission Rates OR (95% CI) | Hospital Mortality OR (95% CI) | 30-Day Readmission Rates OR (95% CI) | Length of Hospital Stay, Days MD (95% CI) | Critical Care Unit Stay, Daysa MD (95% CI) |
|---|---|---|---|---|---|---|---|
| Lam et al, 201041,b | 5 RCTs | 3/2 | 0.82 (0.67–1.01) | 0.96 (0.65–1.41) | 0.88 (0.64–1.20) | –1.22 (–2.31 to –0.14) | –0.56 (–1.06 to –0.05) |
Abbreviations: BNP, B-type natriuretic peptide; CI, confidence interval; MD, mean difference; NT-proBNP; N-terminal proBNP; OR, odds ratio; RCT, randomized controlled trial.
Includes both intensive and coronary care units
BNP was examined in emergency setting with acute dyspnoea
Among the intervention group, admission rates were somewhat decreased, but all-cause hospital mortality rates and 30-day readmission rates were not affected.41 Overall, there was at least a 1 day decrease in the number of days at the hospital for the intervention group. There was a modest reduction in the number of days that the intervention group stayed in the critical care unit. Lam et al41 assessed the risk of bias as low, using the Cochrane Collaboration risk of bias tool. There is moderate quality evidence that natriuretic peptide testing to diagnose heart failure in people presenting to the ED with acute dyspnea does not significantly reduce mortality, hospital admission rates, or 30-day rehospitalization rates.46 Quality of evidence was downgraded due to risk of bias (Table A4, Appendix 2).
Ongoing Studies/Reviews
We are not aware of any ongoing reviews that have potential relevance to this review.
Discussion
Clinical assessment along with some initial laboratory testing is the current standard of care to diagnose individuals with suspected heart failure.7 We chose the best available systematic reviews with a low risk of bias to assess the evidence regarding the usefulness of natriuretic peptides (BNP and/or NT-proBNP) in the diagnosis of suspected heart failure.7 Using the cut point chosen according to the Canadian Cardiovascular Society guidelines in different settings,1 our review found that the sensitivity for both BNP and NT-proBNP are high and the negative likelihood ratios are low, suggesting that concentrations of both peptides below the decision cut points can rule out the presence of heart failure with a high degree of confidence. The benefit observed by testing for natriuretic peptides lies within the clinical context and does not eliminate the need for cardiac imaging in most situations.2,7,20 If either of the natriuretic peptide tests were positive in a person with suspected heart failure in a community care setting, they could potentially be referred to a cardiologist for further evaluation.20 Similarly, if the tests were positive in an emergency department setting, they could be managed as heart failure, keeping the limitations of the test in mind.28 If negative in either setting, alternative diagnoses for the clinical picture could be considered without any further delay. We also examined the clinical outcomes of conducting natriuretic peptide tests and found that BNP testing in an acute care setting could potentially decrease length of hospital stay.41 However, there was no difference in all-cause hospital mortality, hospital admission, or 30-day readmission rates between the comparator groups.41
The evidence suggests that low concentrations of natriuretic peptides in the heart are consistent with no heart failure, whereas high concentrations could potentially lead to further work up to confirm heart failure, especially in patients with an uncertain clinical diagnosis.47 While natriuretic peptide levels can be used to improve clinical outcomes, the specific thresholds for the tests remain uncertain. Different cut points were reported in the included studies, introducing clinical heterogeneity. Additionally, natriuretic peptide cut points could differ for the diagnosis of patients depending on the acute or community care setting. Specific thresholds or cut points for these tests have been provided by the Canadian Cardiovascular guidelines for the diagnosis of heart failure.1
All included studies focused on people who presented with clinical symptoms of heart failure, regardless of co-morbidities. We presented the summary of evidence for this population to maximize applicability and generalizability. For example, natriuretic peptides might be elevated in other cardiovascular conditions, such as valvular heart disease, ischemia, or uncontrolled hypertension.48 Both BNP and NT-proBNP concentrations may vary according to patient characteristics, such as age and renal function.20 Some reviews reported the diagnostic performance of an age-specific threshold for the natriuretic peptides.19,20 The results from studies that reported an age-specific threshold were consistent with the combined overall estimate of the diagnostic performance.49,50
We conducted an overview of reviews of the diagnostic accuracy of natriuretic peptides in diagnosing heart failure using reference standards as reported within the individual reviews. However, heart failure is a clinical diagnosis and the reference standard against which the natriuretic peptides were compared varied among the studies within the reviews. Few studies used echocardiogram along with a cardiologist opinion as the reference standard, and some studies used the criteria of different working groups. This variability in reference standards may have contributed to the broad range of estimates in the diagnostic performance of the natriuretic peptides and did not allow us to present with a meaningful pooled estimate for sensitivity and specificity in some scenarios. Despite the variability, the estimates were remarkably consistent within specific ranges as recommended by the Canadian Cardiovascular Society guidelines.1
We identified one systematic review that discussed clinical outcomes in an ED setting41 and none in a community setting.41 Outside of our overview of reviews, we identified two primary studies reporting on clinical outcomes. One randomized controlled study reported that BNP increased the diagnostic certainty (defined by the need for further diagnostic work-up) and accelerated the initiation of the appropriate treatment.51 Other outcomes, such as number of days in hospital, number of hospitalizations, and mortality, did not differ between comparator groups.51 Another retrospective cohort study concluded that patients not on the pathway recommended by NICE (which included BNP in their diagnostic clinical pathway) were at greater risk for heart failure admission.52 However, there was no significant difference in mortality between the groups.52
Our review avoided duplication of prior work and leveraged knowledge of existing reviews. Because we relied on results from other reviews and health technology assessments, it is possible that relevant reviews were missed or not reported or that variations in the interpretation of the evidence may exist. Despite these potential limitations, we remain confident in the results presented as all other identified systematic reviews and assessments had similar conclusions.
Conclusions
Based on the results from the reported systematic reviews included in this report, both BNP and NT-proBNP are useful diagnostic tools in people with suspected heart failure. Natriuretic peptides can be used to rule out heart failure with a high degree of confidence both in the community and acute care settings (strength of evidence: High). Additionally, using BNP or NT-proBNP as a diagnostic test along with usual care likely reduces the length of hospital stay by at least 1 day (GRADE: Moderate), but likely results in little to no difference in all-cause hospital mortality, hospital admission, or 30-day readmission rates
(GRADE: Moderate).
Economic Evidence
Research Question
What is the cost-effectiveness of B-type natriuretic peptide (BNP) or N-terminal proBNP (NT-proBNP) tests used in addition to standard clinical investigations compared with standard clinical investigations alone for people with suspected heart failure?
Methods
We performed an economic literature search on July 25, 2019, to retrieve studies published from database inception until the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic and costing filter applied.
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites, clinical trial and systematic review registries, and the Tufts Cost-Effectiveness Analysis Registry. The grey literature search was updated on January 14, 2020. See Clinical Literature Search, above, for further details on methods used. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
INCLUSION CRITERIA
English-language full-text publications
Studies published from database inception until July 25, 2019
Studies in adults with suspected heart failure
Studies in the emergency department (ED) or community care setting
Studies comparing BNP or NT-proBNP to usual care (which includes standard clinical investigations such as history, physical exam, blood work, electrocardiograph [ECG] and chest x-ray; or clinical diagnosis by a specialist or family physician based on signs and symptoms; or echocardiography when used along with any of the reference standards above)
Studies comparing both the costs and outcomes (e.g., quality-adjusted life years [QALY], life years, mortality, hospitalizations, length of stay, number of diagnoses, incremental cost-effectiveness ratio [ICER])
Cost–utility, cost-effectiveness, cost-benefit, or cost-consequence analyses
EXCLUSION CRITERIA
Narrative reviews, letters/editorials, case reports, commentaries, conference abstracts, posters, unpublished studies
Studies using echocardiography alone as reference standard
Studies reporting only costs
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Covidence23 and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. The reviewer then examined the full-text articles and selected studies eligible for inclusion. The reviewer also examined reference lists for any additional relevant studies not identified through the search.
Data Extraction
We extracted relevant data on study characteristics and outcomes to collect information about the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, analytic technique, perspective, time horizon, population, interventions, comparators)
Outcomes (e.g., health outcomes, costs, incremental cost-effectiveness ratios)
We contacted study authors to provide clarification as needed.
Study Applicability and Limitations
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform the development of NICE's clinical guidelines.53 We modified the wording of the questions to remove references to guidelines and to make it specific to Ontario. Next, we separated the checklist into two sections. In the first section, we assessed the applicability of each study to the research question (directly, partially, or not applicable). In the second section, we assessed the limitations (minor, potentially serious, or very serious) of the studies that we found to be directly applicable.
Results
Economic Literature Search
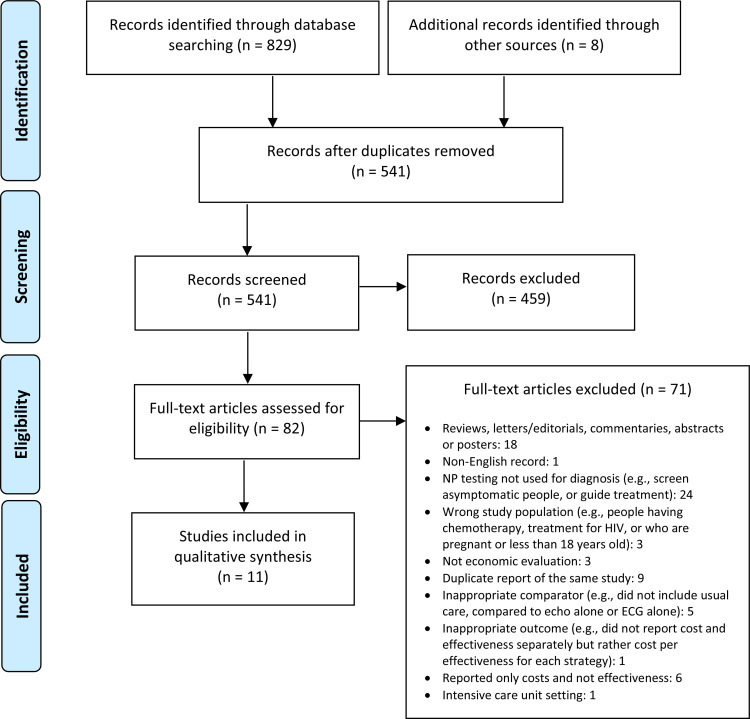
The database search of the economic literature yielded 829 citations published from database inception until July 25, 2019. We identified eight additional studies from other sources, for a total of 541 studies after duplicates removed. Figure 2 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search.
Figure 2: PRISMA Flow Diagram—Economic Search Strategy.
Source: Adapted from Moher et al, 2009.42
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Overview of Included Economic Studies
We identified a total of 11 economic studies that met our inclusion criteria. The studies were conducted in the United States, United Kingdom, Canada, Australia, Netherlands, Switzerland, Germany, and Norway. The use of natriuretic peptide tests was evaluated in different health care settings:
ED setting: seven studies
Community care setting: four studies
We summarized the characteristics and results of the included studies by setting in Table A6 (Appendix 4).
EMERGENCY DEPARTMENT
In the ED setting, a total of seven studies were included: one study evaluated both BNP and NT-proBNP,54 three evaluated NT-proBNP alone,55–57 and three evaluated BNP alone.58–60 Overall, all studies found that adding a BNP or NT-proBNP test to standard clinical investigations was either a dominant strategy (less costly and more effective)55–60 or cost-effective (ICER: £11,656 GBP per QALY gained, 95% CI: £4,641–£23,774),54 when compared to standard clinical investigations alone.
Six of seven studies found that a BNP or NT-proBNP test reduced the total cost of patient management in the short-term (from 30 days to 1 year). The cost savings ranged from a few hundred dollars up to $2,604 per patient (2003 USD). This was mainly driven by reduced acute care burden due to fewer admissions, fewer re-admissions, and shorter length of stay.
Griffin et al54 was the only study that found that natriuretic peptide testing strategies have slightly higher costs than standard clinical investigations, based on a decision-analytic model.54 The authors conservatively assumed that clinicians would maximize specificity to increase the certainty of ruling out heart failure (a sensitivity of 80% and a specificity of 77%). In this case, standard clinical investigation alone was assumed to have lower sensitivity (true positive rate) and higher specificity (true negative rate) than the BNP strategy. As a result, the model predicted that the BNP strategy would be associated with fewer false negatives (2.3% vs. 9.4%) and more false positives (19.8% vs. 12.2%). However, when the analysis used higher sensitivity and lower specificity for standard clinical investigations (a sensitivity of 95% and a specificity of 30%), Griffin et al54 found BNP to be cost saving (BNP became the dominant strategy).
Some of the included studies found similar mortality between strategies (with or without natriuretic peptide testing),55–58 whereas some found short-term all-cause mortality to be slightly lower in people managed by the BNP strategy.59,60
Five of the seven included studies were based on randomized controlled trials (RCTs) evaluating the use of BNP or NT-proBNP.55,56,58–60 The trial-based cost-effectiveness analyses measured health outcomes in natural units, such as all-cause mortality, initial hospitalization rate, rehospitalization rate, and hospital length of stay. Three of the studies were based on the same RCT for BNP (the BASEL study),61 but used costs and outcomes from different follow-up timepoints (30, 180, 360, and 720 days).58–60 Notably, there was one Canadian cost-effectiveness analysis based on an RCT for NT-proBNP (the IMPROVE-CHF study). Moe et al56 found that NT-proBNP testing significantly reduced the duration of ED visits by 21% (6.3 vs. 5.6 hours, P = 0.031), the proportion of patients re-hospitalized by 60 days (13% vs. 20%, P = 0.0463), and the total direct medical costs by 15% (from $6,129 to $5,180 per person, P = 0.023). There were no statistically significant differences in other clinical outcomes between NT-proBNP and standard clinical investigations groups, including the initial hospitalization rate, the initial intensive care unit admission rate, the length of stay, and the initial in-hospital and 60-day mortality rates.
There were two model-based economic evaluations (one cost-effectiveness analysis57 and one cost-utility analysis54). Siebert et al57 developed a simple decision tree model based on a prospective blinded single-armed NT-proBNP study (PRIDE) and quantified costs and health outcomes over a 60-day follow-up period.57 The authors found that, compared to standard clinical investigations alone, adding NT-proBNP to standard clinical investigations reduced the risk of serious adverse events by 1.6% and direct medical costs by 9.4% (a savings of $474 per patient). NT-proBNP could also reduce the use of echocardiography by up to 58%, prevent 13% of initial hospitalization, and reduce hospital days by 12%.
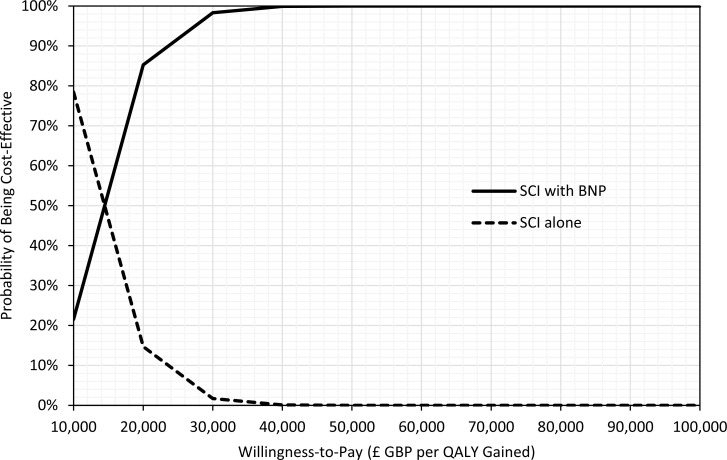
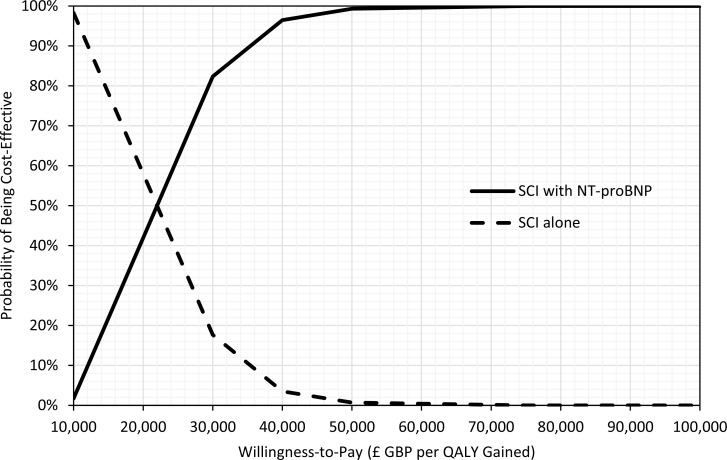
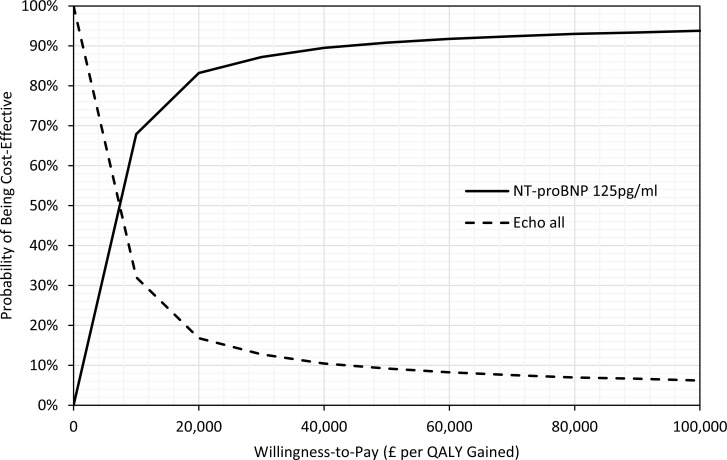
As part of the 2014 NICE guideline on acute heart failure, Griffin et al54 conducted a cost–utility analysis using a decision tree plus Markov model. The study estimated total costs, life years, and QALYs over a 4-year time horizon. The BNP test was cost-effective versus no test (standard clinical investigations), with an ICER of £11,656 GBP per QALY gained (95% CI: £4,641–£23,774). When NT-proBNP was used instead of BNP, the ICER was slightly higher at £19,778 GBP per QALY gained. In the context of specialist management, the BNP test was also cost-effective versus no test, with an ICER of £7,914 GBP per QALY gained (95% CI £4,007–£14,554).
COMMUNITY CARE
In the community care setting, four studies met our inclusion criteria. One study evaluated BNP,51 and three studies evaluated NT-proBNP.20,40,62
Burri et al51 conducted a trial-based cost-effectiveness analysis and found that the use of BNP increased total medical costs, but the difference was not statistically significant (median of $1,655 [interquartile range, IQR: 850–3,331] vs. $1,541 [IQR: 859–2,827] at 3 months, P = 0.68). The use of BNP also improved diagnostic certainty as defined by the need for further diagnostic workup (33% vs. 45%, P = 0.02) and time to appropriate treatment (13 vs. 25 days, P = 0.01).
There were three model-based studies of NT-proBNP (one 12-month cost-effectiveness analysis40 and two lifetime cost–utility analyses20,62).
Bugge et al40 compared clinical diagnosis plus NT-proBNP test (both point-of-care and hospital laboratory–based tests) with clinical diagnosis alone using a decision tree model. From a health care payer perspective, the total costs per patient at 1 year were €379, €344, and €397 EUR for clinical diagnosis, point-of-care NT-proBNP test, and hospital NT-proBNP test, respectively. Point-of-care NT-proBNP had a lower cost due to fewer revisits with the general practitioner (GP) and less use of spirometry. Compared with clinical diagnosis alone, fewer patients had a delayed diagnosis with point-of-care and hospital NT-proBNP tests (22% vs. 38%, respectively).
Monahan et al62 developed a decision tree model to assess the cost-effectiveness of using the MICE (Male, Infarction, Crepitations, Edema) decision rule compared with other diagnostic strategies (NT-proBNP < 125 pg/mL, NT-proBNP < 400 pg/mL, echocardiography for all, and do nothing over a lifetime horizon. The study found that the MICE rule was more expensive and less effective than the other comparators (i.e., the MICE rule was dominated by other strategies). At a willingness-to-pay of £20,000 GBP per QALY, the NT-proBNP 400 pg/mL strategy was the most cost-effective (£4,400 vs. do nothing). However, if the proportion of patients who had heart failure with a reduced ejection fraction was higher, the NT-proBNP 125 pg/mL strategy would be more cost-effective.
As part of the NICE guideline on chronic heart failure,20 a cost–utility analysis was conducted to determine whether natriuretic peptide tests are cost-effective and, if so, what the cost-effective diagnostic threshold should be. A decision tree plus Markov model was used to estimate the lifetime costs and QALYs associated with NT-proBNP at three thresholds (400, 125, and 280 pg/mL), versus echocardiography for all. The NT-proBNP 400 pg/mL strategy was found to be the most cost-effective (£5,468 GBP per QALY when compared with echocardiography for all).
Applicability and Quality of the Included Studies
Results of the applicability and quality assessment checklists of the included studies can be found in Tables A7 and A8 (Appendix 4).
EMERGENCY DEPARTMENT
One Canadian cost-effectiveness analysis (Moe et al56) was deemed directly applicable to our research question (same population, intervention, and perspective). Four studies were considered partially applicable because they evaluated natriuretic peptide cut-off values relevant for Canada, but used non-Canadian perspectives.54,58–60 Two studies were considered not applicable because they did not evaluate natriuretic peptide cut-off values relevant for Canada.55,57
We then assessed the quality of the directly and partially applicable studies. We found that two (Griffin et al54 and Mueller et al59) had minor limitations, and three (Moe et al,56 Breidthardt et al,58 and Medical Services Advisory Committee60) had potentially serious limitations.
COMMUNITY CARE
Three of the four included studies (Bugge et al,40 Monahan et al,62 NICE20) were considered partially applicable. The fourth (Burri et al51) was considered not applicable because it did not evaluate natriuretic peptide cut-off values relevant for Canada. We assessed the quality of the partially applicable studies. We found that the NICE assessment20 had minor limitations while Bugge et al40 and Monahan et al62 had potentially serious limitations.
Discussion
Setting
Based on the literature, natriuretic peptide tests are most commonly used in the ED and community care settings. The effect of natriuretic peptide testing on medical costs seems to depend on the setting. In the ED, six of seven studies reported that BNP or NT-proBNP testing led to considerable cost savings. However, in the community care setting, the additional use of BNP or NT-proBNP did not always reduce total medical costs. Some researchers suggested that the cost savings observed in the ED were largely due to a reduction in hospitalizations and time to discharge.51 However, in the community care setting it was difficult for natriuretic peptide testing to impact on cost-intensive management decisions because of the lower rate of hospitalizations seen in this setting. According to clinical experts, people who present to community care tend to have less severe symptoms (or more gradual onset of symptoms) compared to those who present to the ED (Robert McKelvie, MD, email communication, September 2019; Lisa Mielniczuk, MD, email communication, September 2019).
Point-of-Care Versus Laboratory Testing
The cost of BNP or NT-proBNP tests in the included studies ranged from $20 to $80. In the community care setting, some studies evaluated both laboratory and point-of-care testing. A point-of-care test can produce results much more quickly than a laboratory assay, but may have slightly higher costs.63
Optimal Threshold
Some of the included studies evaluated natriuretic peptide tests using different diagnostic thresholds. Historically, the optimal thresholds for the community care setting recommended by the European and UK guidelines were 100 pg/mL for BNP and 400 pg/mL for NT-proBNP. However, in 2016, the European Society of Cardiology guideline2,5 lowered the threshold to 35 pg/mL for BNP and 125 pg/mL for NT-proBNP due to concerns that previously recommended thresholds were too high and may have resulted in a delayed diagnosis for some patients.20 Similarly, the 2017 update of the Canadian guidelines2 recommended still lower thresholds (50 pg/mL for BNP and 125 pg/mL for NT-proBNP).
BNP Versus NT-proBNP
It is worth noting that the 2018 NICE assessment20 excluded BNP testing from the economic analysis for several reasons. First, the NICE clinical review demonstrated that NT-proBNP has a greater sensitivity over a range of thresholds compared to BNP. Second, on a practical level, NT-proBNP has a longer stability than BNP in blood samples (days vs. hours). Therefore, it would be more suitable for use in the community care setting, where samples need to be transported from GP's office to the laboratory. Lastly, a new heart failure drug (Sacubitril/Valsartan) is found to interfere with BNP measurement in the body. In this case where a patient is being treated with this drug, it may be preferable to use the NT-proBNP test.20
Type of Economic Studies
We found a mix of trial- and model-based economic evaluations in our literature review. The advantage of trial-based economic evaluations is that all health care resource use, costs, and effects are collected from the clinical trial.64 Therefore, it is a direct observation of the impact of the technology on costs and effects. However, the potential limitations are that (1) the economic results observed in a trial may not truly reflect real world results, and (2) the time horizon of a trial is usually short, so long-term costs and effects are not included. The advantages of model-based economic evaluations include (1) models can extrapolate costs and effects beyond trial duration, (2) models can use QALYs as an outcome measure instead of natural units, and (3) models can vary different parameter values and assumptions and assess their impact on the cost-effectiveness results.
Conclusions
Our economic evidence review found a total of 12 studies evaluating the cost-effectiveness of natriuretic peptide testing in patients with suspected heart failure. The studies found that natriuretic peptide testing was either dominant (less costly and more effective) or cost-effective, across different countries (including Canada) and settings.
Primary Economic Evaluation
Our economic evidence review found a total of 12 studies evaluating the cost-effectiveness of natriuretic peptide tests in patients with suspected heart failure (including two economic evaluations performed in the United Kingdom). Although the included studies differ in several aspects (such as the study perspective, modelling approach, and time horizon), they reached similar conclusions. The studies found that natriuretic peptide tests were either dominant (less costly and more effective) or cost-effective, across different countries (including Canada) and settings. Most studies conducted sensitivity analyses and results remained robust.
In the ED setting, we identified one directly applicable, Canadian trial-based cost-effectiveness analysis (Moe et al56). It showed that NT-proBNP testing significantly reduced the duration of ED visits by 21% (6.3 vs. 5.6 h, P = 0.031), the number of patients re-hospitalized by 60 days (13% vs. 20%, P = 0.0463), and total direct medical costs by 15% (from $6,129 to $5,180 USD per person, P = 0.023). However, this study has some limitations. First, it only considered costs and clinical outcomes during a short follow-up period (60 days), which may not reflect all differences in costs and outcomes. Second, it did not measure health effects using QALYs, which would allow broad comparison of various technologies and the allocation of resources across different conditions.
In addition to the Canadian analysis, we identified two economic evaluations conducted by NICE19,20 that are partially applicable to our research question (similar population, intervention, comparator, and clinical pathways, but with a non-Canadian perspective) and also have high methodological quality. One of the economic evaluations was subsequently published in a peer-reviewed journal (Griffin et al54). The studies found that natriuretic peptide tests are cost-effective in the ED and community care settings in the United Kingdom. Subsequently, NICE guidelines recommended natriuretic peptide testing for the diagnosis of heart failure in both acute and ambulatory care settings.
We decided to leverage the two NICE studies20,54 instead of conducting de novo primary economic evaluations. To assess how the economic evaluation results can be transferred from the United Kingdom to Ontario, we followed the methods described in a well-known transferability publication by Welte et al.65
Transferability of Economic Evaluation Results
Research Questions
What is the cost-effectiveness of B-type natriuretic peptide (BNP) or N-terminal proBNP (NT-proBNP) tests used in addition to standard clinical investigations, compared with standard clinical investigations alone for people with suspected heart failure?
In the emergency department (ED) setting
In the community care setting
Methods
Through our economic literature review, we have identified two studies that are applicable to our research question and are of high methodological quality. We followed the Welte transferability method,65 and used the following four steps to evaluate the transferability of the two NICE studies20,54 to Ontario. We also contacted study authors to provide clarification as needed. We transferred the overall conclusions of the study of interest (e.g., whether the technology is cost-effective and what are the uncertainties), rather than focusing on the specific value of the ICER.
Step 1: General Knock-Out Criteria
First, we assessed the transferability potential of the identified economic studies20,54 using three general knock-out criteria. If any of the following criteria were met, the study results would not be transferrable.
-
1.
The evaluated technology is not comparable to the technology that will be used in the decision country
-
2.
The comparator is not comparable to the standard of care technology used in the decision country
-
3.
The study quality is not acceptable (i.e., it does not live up to the standards required in the decision country)
Step 2: Specific Knock-Out Criteria
Next, we assessed whether there were any specific knock-out criteria that would make the identified economic studies unsuitable for use. Welte et al65 identified a total of 14 transferability factors, divided into three categories:
-
Methodological characteristics
○ Perspective
○ Discount rate
○ Medical cost approach
○ Productivity cost approach
-
Health care system characteristics (supply of technology)
○ Absolute and relative prices in health care
○ Practice pattern
○ Technology availability
-
Population characteristics (demand for technology)
○ Disease incidence/prevalence
○ Case mix
○ Life expectancy
○ Health status preferences
○ Acceptance, compliance, and incentives to patients
○ Productivity and work-loss time
○ Disease spread
Each of the transferability factors can become a knock-out criterion. If any of the transferability factors cannot be assessed (due to a lack of data), or if there is low correspondence between the study country and decision country, the study results would not be transferrable.
Step 3: Necessity of Modelling Adjustment
We then assessed if any modelling adjustment is necessary. According to Welte et al,65 modelling adjustments are needed when there are relevant differences between the study country and the decision country with respect to practice pattern, prices, or incidence/prevalence of the target disease.
Step 4: Adjusting the Results to the Canadian Context
If no modelling adjustment is needed, the study results can be deemed qualitatively transferrable (i.e., the order of magnitude can be transferred). To make the study results more comparable for decision makers in Ontario, we applied the Welte transferability checklist and adjusted the results from £GBP to ĆD.
-
The Welte transferability checklist includes a total of 14 transferability factors. For each factor, we determined:
○ To what extent it is relevant for the investigated technology
○ The correspondence between the study country (United Kingdom) and the decision country (Ontario, Canada)
○ The likely effect of the transferability factor on the estimated ICER
For correction of price inflation, we adjusted the costs to 2019 CAD using the health component of the UK consumer price index (95.5 in 2013, 107.4 in 2018, and 110.6 in 2019).66
For currency conversion, we applied the purchasing power parity index from 2018, the most recent year (Canada: 1.245; United Kingdom: 0.700).67
Results
Emergency Department
TRANSFERABILITY CHECK
We applied the Welte transferability method to the NICE economic evaluation in the ED setting (Griffin et al54). First, we determined that the study passed the general knock-out criteria test (similar intervention, similar comparator, and high methodological quality). Nine of the transferability factors were deemed to have high or very high relevance to our current research question. The estimated correspondence between the United Kingdom and Canada were also judged as high for these transferability factors (e.g., similar health care systems, epidemiology, relative prices, and population characteristics; see Table 5 for details). Five factors were deemed to have very low relevance (e.g., discount rate) or no relevance (e.g., productivity cost approach, disease spread), but none make the study unsuitable for use.
Table 5:
Transferability of the Cost-Effectiveness Results in the Emergency Department Setting from the United Kingdom to Ontario
| Transferability Factor | Estimated Relevancea | Estimated Correspondence Between Study and Decision Countrya | Estimation of ICER of Decision Country Based on the ICER of Study Countryb |
|---|---|---|---|
| Methodological Characteristics | |||
| Perspective | Very high | Very high (Public health care payer perspective for both UK and Ontario) | Unlikely to have a large bias |
| Discount rate | Very low | Medium (UK 3.5%;54 Ontario 1.5%68) | Unlikely to have a large bias |
| Medical cost approach | High | Very high (Direct medical costs are estimated using charges, fees, average bed day, etc.54 The same costing approach is also recommended by the Canadian economic evaluation guidelines68) | Unlikely to have a large bias |
| Productivity cost approach | Not relevant (no productivity costs measured) | — | — |
| Health Care System Characteristics (Supply of Technology) | |||
| Absolute and relative prices in health care | Very high | High UK: BNP: £28.13 GBP per test (∼$50 CAD); ECHO £63.60 GBP per test (∼$111 CAD); hospital bed day £232 GBP (∼$412 CAD); GP visit £37 GBP (∼$66 CAD)54 Canada: BNP $18–$75 per test,69–71 ECHO $209 per test,72 hospital bed $815 per day,73 GP visit $7272 |
Could be slightly higher (since hospital day cost is higher in Canada, potentially there are more savings from hospital length of stay reduced) |
| Practice pattern | Very high | High Clinical management guidelines are similar2,19 Standard clinical investigations in both countries included history, physical examination, laboratory investigations, ECG, and chest x-ray. Echocardiography is performed if heart failure is confirmed or still suspected after standard clinical investigations2,19 Same cut-off values used to rule out HF in the acute care setting (BNP: < 100 pg/mL; NT-proBNP: < 300 pg/mL)2,19 |
Unlikely to have a large bias |
| Technology availability | Very high | High (Heart failure treatments are similar in UK and Ontario, e.g., pharmacotherapy, surgery2,19) | Unlikely to have a large bias |
| Population Characteristics (Demand for Technology) | |||
| Disease incidence/prevalence | Very high | Very high Incidence of HF is 3.3 per 1,000 in UK, for people ≥ 16 y;74 incidence of HF is 3.06 per 1,000 in Ontario, for people ≥ 2075 |
Unlikely to have a large bias |
| Case-mix | High | Very high UK cohort: 56% male, average age 77 y; most common comorbidities are diabetes (33%) and COPD (20%)54,76 Canadian cohort: 52% male, 93% Caucasian, average age 71 y, most common comorbidities are diabetes (27%) and COPD (31%)56 |
Unlikely to have a large bias |
| Life expectancy | High | Very high General life expectancy: UK: 18.8 y for men and 21.2 y for women aged 65 y77 Canada: 18.5 y for men and 21.6 y for women aged 65 y78 HF-specific life expectancy: for patients receiving standard care ∼30% are still alive at 4 years after discharge from index admission in both UK and Canada54,79 |
Unlikely to have a large bias |
| Health-status preferences | High | Very high UK: acute HF 0.688; chronic HF 0.75254 Canada: heart diseases 0.719 (95% CI: 0.705–0.732; 2013/14 Canadian Community Health Survey) |
Unlikely to have a large bias |
| Acceptance, compliance, incentives to patients | Very low | Very high | Unlikely to have a large bias |
| Productivity and work-loss time | Not relevant (no productivity costs measured) | – | – |
| Disease spread | Not relevant (not infectious disease) | – | – |
Abbreviations: BNP, B-type natriuretic peptide; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ECG, electrocardiogram; ECHO, echocardiogram; GP, general practitioner; HF, heart failure; ICER, incremental cost-effectiveness ratio; NT-proBNP, N-terminal proBNP.
Response options: “Very high,” “High,” “Low,” “Very low” and “Not relevant.”65
Response options: “Unbiased (unlikely to have a large bias),” “Too high,” “Slightly too high,” “Too low,” and “Slightly too low.”65
Next, we evaluated the model structures, parameters, and assumptions used by Griffin et al.54 The model structure was considered suitable and appropriately constructed for the decision problem. A decision tree was first used to estimate the proportion of patients with true positive, true negative, false positive, and false negative diagnostic results. Markov models were then used to estimate the long-term outcomes. We compared the clinical parameters used by Griffin et al (e.g., diagnostic accuracy of BNP or NT-proBNP test) to those identified in our clinical evidence review, and they were similar. The health-state preference weights were considered comparable between the United Kingdom and Canada. We also compared the key cost parameters (e.g., natriuretic peptide test, echocardiography, hospital stay, GP visit, ED visit) and they were mostly similar between contexts (Table 5).
Based on the Welte criteria, the Griffin et al54 study was adequate for Ontario and no modelling adjustment was needed. As a result, the study results were deemed qualitatively transferrable.
ADJUSTED COST-EFFECTIVENESS RESULTS
According to Griffin et al,54 over a 4-year time horizon the total per-patient costs associated with standard clinical investigations alone and standard clinical investigations with BNP were £2,664 (95% CI: 1,445–4,517) and £2,714 (95% CI: 1,495–4,553) GBP, respectively (Table 6). A break-down of costs were provided in the 2014 NICE guidelines (Appendix M),19 and it showed that about 75% of the total cost was associated with the initial hospital admission, about 11% with re-admissions, about 11% with drugs and doctor's visits, and only a very small proportion with diagnostic workup.
Table 6:
Original and Adjusted Cost-Effectiveness Results in the Emergency Department Setting—Standard Clinical Investigations With BNP Versus Standard Clinical Investigations Alone
| SCI With BNP, Mean (95% CI) | SCI Alone, Mean (95% CI) | |
|---|---|---|
| Total Costs per Patient | ||
| 2013 GBP | £2,714 (1,495–4,553) | £2,664 (1,445–4,517) |
| 2019 CAD | $5,590 (3,079–9,378) | $5,487 (2,976–9,304) |
| QALYs | 2.2136 (2.1392–2.2861) | 2.2099 (2.1350–2.2817) |
| Incremental Cost | ||
| 2013 GBP | £49.23 | |
| 2019 CAD | $101.40 | |
| Incremental QALY | 0.0037 | |
| ICER per QALY Gained | ||
| 2013 GBP | £13,357 (5,585–28,034) | |
| 2019 CAD | $27,513 (11,504–57,744) | |
Abbreviations: BNP, B-type natriuretic peptide; CI, confidence interval; SCI, standard clinical investigations; QALY, quality-adjusted life year.
Note: results presented in this table differ slightly from those presented in the original publication. The 95% CIs around costs and QALYs were not available in the publication, so we contacted the authors54 to obtain the complete probabilistic sensitivity analysis results. The analysis was re-run to generate the 95% CI.
The QALYs associated with standard clinical investigations alone and standard clinical investigations with BNP were estimated to be 2.2099 (95% CI: 2.1350–2.2817) and 2.2136 (95% CI: 2.1392–2.2861), respectively. The life years associated with standard clinical investigations alone and standard clinical investigations with BNP were 3.154 and 3.159, respectively. This result was comparable to an Ontario cost-effectiveness study of heart failure patients, which found the projected life expectancy of this population in Ontario is about 3.21 years.79
When comparing standard clinical investigations with BNP to standard clinical investigations alone in the United Kingdom, the ICER was £13,357 GBP per QALY gained (95%CI: 5,585–28,034). After adjusting for inflation and currency, the estimated ICER in Ontario was $27,513 CAD per QALY gained (95% CI 11,504–57,744). When comparing standard clinical investigations with NT-proBNP to standard clinical investigations alone in the United Kingdom, the ICER was £21,298 GBP per QALY gained (95% CI 10,754– 41,498). After adjusting for inflation and currency, the estimated ICER in Ontario was $43,869 per QALY gained (95% CI 22,151–85,477). The estimated ICERs were below the commonly used willingness-to-pay values of $50,000 to $100,000 per QALY. Therefore, BNP and NT-proBNP tests are likely to be cost-effective in the ED setting in Ontario.
The original and adjusted results of standard clinical investigations with NT-proBNP compared to standard clinical investigations alone are shown in Table 7.
Table 7:
Original and Adjusted Cost-Effectiveness Results in the Emergency Department Setting–Standard Clinical Investigations With NT-proBNP Versus Standard Clinical Investigations Alone
| SCI With NT-proBNP Mean (95% CI) | SCI Alone Mean (95% CI) | |
|---|---|---|
| Total Costs per Patient | ||
| 2013 GBP | £2,774 (1,538–4,533) | £2,675 (1,444–4,399) |
| 2019 CAD | $5,714 (3,168–9,337) | $5,510 (2,974–9,061) |
| QALYs | 2.2156 (2.1440–2.2957) | 2.2109 (2.1405–2.2907) |
| Incremental Cost | ||
| 2013 GBP | £98.84 | |
| 2019 CAD | $203.59 | |
| Incremental QALY | 0.0046 | |
| ICER (per QALY gained) | ||
| 2013 GBP | £21,298 (10,754–41,498) | |
| 2019 CAD | $43,869 (22,151–85,477) | |
Abbreviations: CI, confidence interval; NT-proBNP, N-terminal pro–B-type natriuretic peptide; QALY, quality-adjusted life year; SCI, standard clinical investigations.
Note: results presented in this table differ slightly from those presented in the original publication. The 95% CIs around costs and QALYs were not available in the publication, so we contacted the authors54 to obtain the complete probabilistic sensitivity analysis results. The analysis was re-run to generate the 95% CI.
UNCERTAINTY
Griffin et al54 conducted comprehensive sensitivity analyses, both deterministically and probabilistically. For the probabilistic analysis results, we contacted the authors and obtained the 95% CIs around the costs, QALYs, and ICER, as well as the cost-effectiveness acceptability curve (numbers here are slightly different from the published numbers because the authors re-ran the analysis). The analysis found that standard clinical investigations with BNP or NT-proBNP was highly likely to be cost-effective at a willingness-to-pay value of £20,000 GBP per QALY gained (Figures 3 and 4). Extensive univariate sensitivity analyses were conducted by varying all key input parameters by ±10% and the results were shown in a tornado diagram (2014 NICE guidelines,19 Appendix M, Figure 232). The ICER was most sensitive to changes in the sensitivity of BNP and of standard clinical investigations alone. To explore structural uncertainty and other parameter uncertainty, the authors also conducted 19 scenario analyses (2014 NICE guidelines, Appendix M, Table 24019). The results remained robust in all analyses (the ICER remained under £22,271 GBP per QALY gained). Overall, Griffin et al54 used very conservative assumptions for the reference case analysis. For example, the time horizon (4 years) was conservatively short. When the time horizon was extended to 10 years, the ICER was reduced by half.
Figure 3: Cost-Effectiveness Acceptability Curve–Standard Clinical Investigations With BNP Versus Standard Clinical Investigations Alone in the Emergency Department Setting.
Figure 4: Cost-Effectiveness Acceptability Curve–Standard Clinical Investigations With NT-proBNP Versus Standard Clinical Investigations Alone in the Emergency Department Setting.
Although the Griffin et al study was conducted in 2014, we are not aware of any new clinical evidence that would change the economic results. Our economic literature review also did not identify any economic studies with contradicting results. While economic studies in other countries all found that natriuretic peptide testing resulted in net savings, Griffin et al was the only study that found an increase in overall cost. This is because the authors conservatively assumed that clinicians in this situation would maximize specificity to increase the certainty of ruling out heart failure (a sensitivity of 80% and a specificity of 77%). Therefore, standard clinical investigations alone were assumed to have lower sensitivity (true positive rate) and higher specificity (true negative rate) than the BNP strategy. As a result, the model predicted that the BNP strategy would be associated with fewer false negatives (2.3% vs. 9.4%) and more false positives (19.8% vs. 12.2%). However, when the analysis used higher sensitivity and lower specificity for standard clinical investigations (a sensitivity of 95% and a specificity of 30%), Griffin et al also found BNP to be cost savings (BNP became the dominant strategy).
In conclusion, we followed the Welte transferability method and found the Griffin et al study54 suitable for use in Ontario. Based on the transferred cost-effectiveness results, BNP and NT-proBNP tests are likely cost-effective in the ED setting in Ontario when used in addition to standard clinical investigations.
Community Care
TRANSFERABILITY CHECK
We applied the Welte transferability method to the 2018 NICE economic evaluation20 in the community care setting. First, we determined that the study passed the general knock-out criteria test (similar intervention, similar comparator, high methodological quality). We also did not identify any transferability factors that would make the NICE study unsuitable for our use. Nine of the transferability factors were deemed to have high or very high relevance to our current research question. The estimated correspondence between the United Kingdom and Canada was also judged as high for these transferability factors (e.g., similar health care systems, epidemiology, relative prices, and population characteristics; Table 8). Five factors were deemed to have very low relevance (e.g., discount rate) or no relevance (e.g., productivity cost approach, disease spread).
Table 8:
Transferability of the Cost-Effectiveness Results of 2018 NICE Assessment20 to Ontario
| Transferability Factor | Estimated Relevancea | Estimated Correspondence Between Study and Decision Countrya | Estimation of ICER of Decision Country Based on the ICER of Study Countryb |
|---|---|---|---|
| Methodological Characteristics | |||
| Perspective | Very high | Very high Public health care payer perspective for both UK and Ontario |
Unlikely to have a large bias |
| Discount rate | Very low | Medium UK 3.5%;54 Ontario 1.5%68 |
Unlikely to have a large bias |
| Medical cost approach | High | Very high The UK study estimated direct medical costs using charges, fees, average bed day, etc.54 The same costing approach is also recommended by the Canadian economic evaluation guidelines68 |
Unlikely to have a large bias |
| Productivity cost approach | Not relevant (no productivity costs measured) | — | — |
| Health Care System Characteristics (Supply of Technology) | |||
| Absolute and relative prices in health care | Very high | High UK: NT-proBNP £26.07 GBP per test (∼$46.4 CAD); ECHO £83.2 GBP per test (∼$148 CAD); GP visit £36 GBP (∼$64 CAD); cardiologist visit £156 GBP (∼$277 CAD); HF hospitalization £2,849 GBP (∼$5,071 CAD) Canada: BNP $18–$75 per test;69–71 ECHO $209 per test;72 hospital bed day $815;73 GP visit $72; cardiologist visit $15772 HF hospitalization $9,38780 |
Could be slightly lower (since HF hospitalization cost is higher in Canada, potentially there are more additional cost from hospitalization due to false negative natriuretic peptide test results) |
| Practice pattern | Very high | High Clinical management guidelines are similar.2,19 Standard clinical investigations in both countries included history, physical examination, laboratory investigations, ECG, and chest x-ray. ECHO is performed if heart failure is confirmed or still suspected after standard clinical investigations.2,19 For the outpatient setting, NICE recommends a higher threshold than does Canada (NT-proBNP: < 400 pg/mL vs. < 125 pg/mL), but the economic analysis included both of these thresholds2,19 |
Unlikely to have a large bias |
| Technology availability | Very high | High Heart failure treatments are similar in UK and Ontario (e.g., pharmacotherapy, surgery)2,19 |
Unlikely to have a large bias |
| Population Characteristics (Demand for Technology) | |||
| Disease incidence/prevalence | Very high | Very high Incidence of HF is 3.3 per 1,000 in UK, for people aged ≥ 1674; incidence of HF is 3.06 per 1,000 in Ontario, for people aged ≥2075 |
Unlikely to have a large bias |
| Case mix | High | Very high UK cohort: 50.6% male, average age 77 y, proportion of HFPEP 86.5%; most common comorbidities are diabetes (33%) and COPD (20%)20,76 Canadian cohort: 52% male, 93% Caucasian, average age 71 y; most common comorbidities are diabetes (27%) and COPD (31%)56 |
Unlikely to have a large bias |
| Life expectancy | High | Very high General life expectancy: UK: 18.8 y for men and 21.2 y for women aged 6577 Canada: 18.5 y for men and 21.6 y for women aged 6578 HF-specific life expectancy: For patients receiving standard care, ∼30% still alive at 4 y after discharge from index admission in both UK and Canada54,79 |
Unlikely to have a large bias |
| Health status preferences | High | High UK: HF 0.581; other conditions 0.573 Canada: heart diseases 0.719 (95% CI: 0.705–0.732); COPD 0.649 (95% CI: 0.633–0.665) (2013/14 Canadian Community Health Survey) |
Could be slightly lower or higher |
| Acceptance, compliance, incentives to patients | Very low | Very high | Unlikely to have a large bias |
| Productivity and work-loss time | Not relevant (no productivity costs measured) | — | — |
| Disease spread | Not relevant (not infectious disease) | — | — |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; ECG, electrocardiogram; ECHO, echocardiogram; HF, heart failure; HFPEP, heart failure with preserved ejection fraction; ICER, incremental cost-effectiveness ratio; NT-proBNP, N terminal–pro BNP.
Response options: “Very high,” “High,” “Low,” “Very low” and “Not relevant.”.65
Response options: “Unbiased (unlikely to have a large bias),” “Too high,” “Slightly too high,” “Too low,” and “Slightly too low.”65
Next, we evaluated the model structures, parameters, and assumptions used by NICE.20 The model structure was considered suitable and appropriately constructed for the decision problem. A decision tree was first used to estimate the proportion of people with true positive, true negative, false positive, and false negative diagnostic results. Markov models were then used to estimate the long-term outcomes. We compared the clinical parameters used by NICE (e.g., the diagnostic accuracy of natriuretic peptide testing) to those identified in our clinical evidence review and they were similar. The health-state preference weights were considered comparable between the United Kingdom and Canada. We also compared the key cost parameters (e.g., natriuretic peptide tests, echocardiography, hospital stay, GP visit, ED visit) and they were also mostly similar (Table 8).
Based on the Welte criteria, the NICE study was adequate for Ontario and therefore no modelling adjustment was needed. The study results were deemed qualitatively transferrable.
ADJUSTED COST-EFFECTIVENESS RESULTS
According to the NICE study,20 over a lifetime horizon, the total per-patient costs associated with standard care alone (echocardiography for all) and standard care with NT-proBNP 125 pg/mL were £1,682 GBP (95% CI: 1,297–2,143) and £2,080 GBP (95% CI: 1,654–2,557), respectively (Table 9).
Table 9:
Original and Adjusted Cost-Effectiveness Results in the Community Care Setting
| NT-proBNP Mean (95% Crl) | ECHO All Mean (95% Crl) | |
|---|---|---|
| Total Cost per Patient | ||
| (2018 GBP) | £2,080 (1,654–2,557) | £1,682 (1,297 2,143) |
| (2019 CAD) | $3,810 (3,029–4,683) | $3,081 (2,376–3,925) |
| QALYs | 4.96 (0.86–8.45) | 4.894 (0.85–8.33) |
| Incremental Cost | ||
| (2018 GBP) | £398.00 | |
| (2019 CAD) | $728.96 | |
| Incremental QALY | 0.066 | |
| ICER (per QALY Gained) | ||
| (2018 GBP) | £6,030.00 | |
| (2019 CAD) | $11,045.00 | |
Abbreviations: CrI, credible interval; ECHO, echocardiogram; ICER, incremental cost-effectiveness ratio; NT-proBNP, N-terminal pro–B-type natriuretic peptide; QALY, quality-adjusted life year.
The authors also concluded that the QALYs associated with standard care alone (echocardiography for all) and standard care with NT-proBNP 125 pg/mL were 4.894 (95% CI: 0.85–8.33) and 4.960 (95% CI: 0.86–8.45), respectively. They provided disaggregated QALYs for each comparator:
Echocardiography for all: 0.9978 in the heart failure population, 3.8968 in the non-heart failure population
NT-proBNP < 125 pg/mL: 0.9937 in the heart failure population, 3.9668 in the non-heart failure population
When comparing standard care with NT-proBNP to standard care alone (echocardiography for all), the ICER was £6,030 GBP per QALY gained. After adjusting for inflation and currency, the estimated ICER was $11,045 CAD per QALY gained in Ontario. The estimated ICER was below the commonly used willingness-to-pay values of $50,000 and $100,000 per QALY. Therefore, NT-proBNP testing is likely cost-effective in the community care setting in Ontario. The NICE study excluded BNP tests from the economic analysis because the researchers considered NT-proBNP more appropriate for use in the community care setting.
UNCERTAINTY
The authors of the NICE study20 conducted comprehensive sensitivity analyses, both deterministically and probabilistically. Besides NT-proBNP at 125 pg/mL cut-off, the analysis also included two additional cut-off values (400 and 280 pg/mL) to assess what the most cost-effectiveness cut-off value should be (this is beyond the scope of our assessment). We considered only results that are relevant to our research question (cut-off values recommended by the Canadian clinical guideline). For the probabilistic analysis results, the authors presented only the probability of each treatment being the most cost-effective option at £20,000 GBP per QALY gained for all four comparators. Therefore, we contacted the authors to obtain the 95% confidence intervals around the costs, QALYs, and ICER, as well as the cost-effectiveness acceptability curve. The cost-effectiveness acceptability curve showed that NT-proBNP 125 pg/mL was highly likely to be cost-effective at a willingness-to-pay value of £20,000 GBP per QALY gained (Figure 5).
Figure 5: Cost-Effectiveness Acceptability Curve—NT-proBNP 125 pg/mL Versus Echocardiography for All in the Community Care Setting.
To explore structural and parameter uncertainty, the NICE authors also conducted 16 scenario analyses (Appendix O, Table 100).20 The results remained robust in all analyses.
We are not aware of any new clinical evidence that would change the economic results and our economic literature review did not identify any studies with contradicting results. The results of the NICE study20 were driven by the cost reductions and QALY benefits of diagnosing other non–heart failure conditions earlier, and consequently the model results are driven by the specificity rather than the sensitivity of the strategy. According to the authors, this suggests that the benefits of diagnosing COPD and myocardial ischemia, which are more common than heart failure and can be well-treated, are greater than those for early diagnosis of heart failure. The negative effects of missing the COPD and myocardial ischemia are greater than missing people with heart failure.
Conclusions
We transferred the ICER results from two UK studies to the Ontario setting, and the results indicated that natriuretic peptide tests are generally cost-effective in the ED and community care settings. We anticipate that a de novo cost-effectiveness analysis for Ontario would produce similar results.
Budget Impact Analysis
Research Question
What is the potential 5-year budget impact for the Ontario Ministry of Health of publicly funding B-type natriuretic peptide (BNP) or N-terminal proBNP (NT-proBNP) tests for people with suspected heart failure?
In the emergency department (ED) setting
In the community care setting
Methods
Analytic Framework
We estimated the budget impact of publicly funding BNP or NT-proBNP tests using the cost difference between two scenarios: (1) the current clinical practice without public funding for BNP or NT-proBNP tests (standard clinical investigations alone, or the current scenario), and (2) the anticipated clinical practice with public funding for BNP or NT-proBNP tests (the new scenario). Figure 6 shows the budget impact model schematic.
Figure 6: Budget Impact Model Schematic.
Key Assumptions
Either test works well to rule out (but not to rule in) the diagnosis of heart failure (i.e., both BNP and NT-proBNP tests have high sensitivity and low negative likelihood ratios)
If natriuretic peptide tests are publicly funded, both BNP and NT-proBNP would be available to patients
An eligible patient would receive either BNP or NT-proBNP test, but not both (at the same time)
We considered only short-term costs related to the diagnostic process (e.g., costs of BNP or NT-proBNP test, echocardiography, referral to cardiologists, and increased hospitalizations or ED visits due to uncorrected false negative diagnoses). The impact on long-term clinical outcomes (e.g., mortality) was not included
Echocardiography plus standard clinical assessment is close to 100% accurate (since this is the current reference standard)
Target Population
The target population is adults who present with suspected heart failure (e.g., symptoms of unclear cause, such as dyspnea). More specifically, there are two populations of interest that may require natriuretic peptide testing:
Population A: people with no history of heart failure (to rule out new-onset heart failure)
Population B: people with history of heart failure who experience worsening symptoms that are of unclear cause (testing is indicated to rule out heart failure decompensation)
POPULATION A: PEOPLE WITH NO HISTORY OF HEART FAILURE (DE NOVO CASES)
We estimated the size of this population using the incidence of heart failure (Figure 7). The epidemiological inputs we used are presented in Table 10.
Figure 7: Flow Diagram for Estimating the Number of People With No History of Heart Failure.
Table 10:
Epidemiological Inputs Used to Derive the Target Population
| Variable | Mean | Reference |
|---|---|---|
| Population Size | ||
| Number of people aged ≥ 40 y | Ontario Ministry of Finance82 | |
| 2020 | 7,493,324 | |
| 2021 | 7,611,943 | |
| 2022 | 7,728,926 | |
| 2023 | 7,847,524 | |
| 2024 | 7,967,102 | |
| Prevalence and Incidence | ||
| Prevalence rate of HF in people aged ≥ 40 y | 3.9% | Health Quality Ontario, 20197 |
| Annual change in prevalence rate | 0% | Public Health Agency of Canada |
| Number of people living with HF in 2020 | 292,240 | Calculated based on prevalence rate and population size |
| Incident rate of HF in people aged ≥ 40 y | 0.52% | Health Quality Ontario, 20197 |
| Annual change in incident rate | –3.1% | Public Health Agency of Canada |
| Probability of HF in People Present With Suspected HF | ||
| Emergency department setting | 50% | Clinical expert opinion; Martindale et al, 201628; NICE guideline, 201419 |
| Community care setting | 35% | Clinical expert opinion; NICE guideline, 201820; Burri et al, 201251; Scott et al, 200863 |
Abbreviation: HF, heart failure.
The incident rate of heart failure among individuals aged ≥ 40 years was 0.52% in 2017.7 This is comparable to the incidence rate reported by Public Health Agency of Canada,81 which is 0.53%, with an annual average percentage change of –3.1%.81 We also obtained the 5-year population projections for those aged ≥ 40 from the Ontario Ministry of Finance.82 Based on these values, we estimated that there would be 38,965 people newly diagnosed with heart failure in Year 1 and 36,526 in Year 5 (Table 11).
Table 11:
Number of People Presenting With Suspected Heart Failure With No History of Heart Failure (New Cases)
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Population aged ≥ 40 y | 7,493,324 | 7,611,943 | 7,728,926 | 7,847,524 | 7,967,102 |
| Incident casesa | 38,965 | 38,355 | 37,737 | 37,128 | 36,526 |
| Emergency Department | |||||
| Incident casesa | 19,483 | 19,178 | 18,869 | 18,564 | 18,263 |
| Number of people with suspected HF and needing NP testing | 38,965 | 38,355 | 37,737 | 37,128 | 36,526 |
| Community Care | |||||
| Incident casesa | 19,483 | 19,178 | 18,869 | 18,564 | 18,263 |
| Number of people with suspected HF and needing NP testing | 55,665 | 54,793 | 53,910 | 53,041 | 52,180 |
Abbreviations: HF, heart failure; NP, natriuretic peptide.
Number of newly diagnosed cases of HF.
Yeung et al75 examined the incidence of heart failure in Ontario using administrative databases of hospital discharge abstracts and physician health insurance claims from 1997 to 2007. They found that about half of the incident cases were diagnosed in an outpatient setting and the other half in an inpatient setting.75 Therefore, we estimated that in Year 1, about 19,483 new heart failure cases would be diagnosed in ED, and another 19,483 in community care.
Based on the literature19,20,28,51,54,63 as well as clinical expert opinion (Robert McKelvie, MD, phone communication, January 2020; Lisa Mielniczuk, MD, phone communication, January 2020), the probability of heart failure in people who present with suspected heart failure was about 50% in ED and 35% in community care. Therefore, the number of people who may require natriuretic peptide testing was estimated to be 38,965 (19,483/50%) in ED and 55,665 (19,483/35%] in community care in Year 1 (Table 11).
POPULATION B: PEOPLE WITH HISTORY OF HEART FAILURE WHO EXPERIENCE WORSENING SYMPTOMS THAT ARE OF UNCLEAR CAUSE
Next, we estimated the size of population B using Ontario administrative data for ED visits.83 These are people living with heart failure who experience worsening symptoms and the reason for the deterioration is unclear (e.g., symptoms could be due to COPD, pneumonia, or heart failure). Natriuretic peptide tests may help clinicians with the diagnosis and decrease uncertainty.
Figure 8 shows the 2018 numbers (the most recent data available at the time of writing). First, we identified all ED visits with heart failure as a pre-existing chronic condition (n = 59,621). Next, we identified which of these ED visits included shortness of breath and/or leg swelling (edema) among the top three patient complaints (n = 33,474). Based on clinical expert opinion (Robert McKelvie, MD, email communication, January 2020; Lisa Mielniczuk, MD, email communication, January 2020), people who present with both shortness of breath and leg swelling are considered highly likely to have heart failure if they have already been diagnosed with heart failure. We excluded these cases (n=57) because BNP or NT-proBNP tests should only be used when the presence of heart failure is uncertain (i.e., only one symptom suggestive of heart failure is present). With this adjustment, the number of ED visits in 2018 with heart failure as a pre-existing condition and requiring natriuretic peptide testing was estimated to be 33,417.
Figure 8: Flow Diagram for Estimating the Number of People With History of Heart Failure.
Source: Ambulatory visits with heart failure as a chronic condition in 2017, Ontario Ministry of Health, IntelliHealth Ontario, Date Extracted: January 14, 2020.83
Abbreviations: ED, emergency department; HF, heart failure; NP, natriuretic peptide; SOB, shortness of breath.
- Recognition of diagnostic uncertainly given the co-morbidity burden of people with HF that may also contribute to shortness of breath or leg swelling
- Recognition of provider confidence with diagnosing heart failure in the setting of uncertainty
- Recognition of provider knowledge of benefits/limitations of natriuretic peptide tests
IntelliHealth data from the most recent 10 years (2009 to 2018) showed that the number of ED visits with heart failure as a pre-existing chronic condition has been increasing at about 3% per year.83 We estimated the number of ED visits for the next 5 years extrapolating from this trend (Table 12). We then multiplied the number of ED visits with heart failure as a pre-existing chronic condition by 56% (n = 33,417/59,621) to get the number of ED visits that would require natriuretic peptide testing.
Table 12:
Number of People With History of Heart Failure and Requiring NP Testing (Prevalent Cases)
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| ED visits with heart failure as a pre-existing chronic condition | 63,273 | 65,181 | 67,148 | 69,174 | 71,260 |
| ED visits with heart failure as a pre-existing chronic condition and requiring NP testing | 35,461 | 36,530 | 37,632 | 38,768 | 39,937 |
Abbreviations: ED, emergency department; NP, natriuretic peptide.
Note: 63,273 = 59,621 × (1+3%)∧2; 35,461 = 63,273 × 56%.
We did not include people who may receive early intervention and not require an ED visit. We assumed that these numbers are relatively small, as most community care providers do not have the necessary resources to confidently manage patients with existing heart failure who develop worsening symptoms when the etiology is unclear (Robert McKelvie, MD, phone communication, January 2020; Lisa Mielniczuk, MD, phone communication, January 2020).
TOTAL POPULATION BY SETTINGS (POPULATIONS A AND B)
The total number of people with suspected heart failure in the ED setting (both with and without a history of heart failure) was estimated to range from 74,426 in Year 1 to 76,463 in Year 5 (Table 13). The total number of people with suspected heart failure in the community care setting was estimated to range from 55,665 in Year 1 to 52,180 in Year 5.
Table 13:
Total Number of People With Suspected Heart Failure in the Emergency Department and Community Care Settings
| People With Suspected HF | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Emergency Department | |||||
| Presenting with no history of HF | 38,965 | 38,355 | 37,737 | 37,128 | 36,526 |
| Presenting with history of HF | 35,461 | 36,530 | 37,632 | 38,768 | 39,937 |
| TOTAL | 74,426 | 74,885 | 75,370 | 75,896 | 76,463 |
| Community Care | |||||
| Presenting with no history of HF | 55,665 | 54,793 | 53,910 | 53,041 | 52,180 |
| TOTAL | 55,665 | 54,793 | 53,910 | 53,041 | 52,180 |
Abbreviation: HF, heart failure.
Current Intervention Mix
Currently, there is no programmatic funding for BNP or NT-proBNP tests in Ontario. Therefore, we assumed all people with suspected heart failure would receive standard clinical investigations in the current scenario. This usually includes medical history, physical examination, blood work, electrocardiograph (ECG), and chest x-ray. If heart failure is confirmed or still suspected after these investigations, an echocardiography (ECHO) is then performed. In community care, all patients suspected of heart failure are referred for echocardiography plus cardiologist assessment.
Uptake of the New Intervention and New Intervention Mix
We estimated the uptake of natriuretic peptide testing based on clinical expert opinion (Robert McKelvie, MD, phone communication, December 2019; Lisa Mielniczuk, MD, phone communication, December 2019). We expect natriuretic peptide tests to be adopted quickly once they are publicly funded and therefore estimate the uptake rate in the ED to be 90% in Year 1 and reach 100% by Year 2. The uptake rate in community care is expected to be slightly lower (50% in Year 1, 75% in Year 2, and 100% in Year 3). For the reference case, we assumed half of the eligible people would receive BNP tests and half would receive NT-proBNP (Andrew Don-Wauchope, MD, email communication, December 2019). In our scenario analyses, we assumed either 100% BNP or 100% NT-proBNP. Table 14 shows the number of people expected to receive each diagnostic strategy in the current and new scenarios.
Table 14:
Number of People Receiving Different Strategies in the Current and New Scenarios
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Emergency Department | |||||
| Current scenario | |||||
| SCI | 74,426 | 74,885 | 75,370 | 75,896 | 76,463 |
| New scenario | |||||
| SCI | 7,443 | — | — | — | — |
| SCI with BNP | 33,492 | 37,443 | 37,685 | 37,948 | 38,231 |
| SCI with NT-proBNP | 33,492 | 37,443 | 37,685 | 37,948 | 38,231 |
| Community Care | |||||
| Current scenario | |||||
| SCI | 55,665 | 54,793 | 53,910 | 53,041 | 52,180 |
| New scenario | |||||
| SCI | 27,832 | 13,698 | — | — | — |
| SCI with BNP | 13,916 | 20,547 | 26,955 | 26,520 | 26,090 |
| SCI with NT-proBNP | 13,916 | 20,547 | 26,955 | 26,520 | 26,090 |
Abbreviations: BNP, B-type natriuretic peptide; NT-proBNP, N-terminal proBNP; SCI, standard clinical investigations.
Model Structure
We used two simple decision tree models to represent the clinical pathways and estimate the cost per person associated with each diagnostic strategy (Figure 8). Different categories of results (i.e., true positive, false negative, false positive, or true negative) were calculated using test sensitivity, test specificity, and disease prevalence. Where possible, we used the same conservative assumptions from the NICE economic evaluations20,54 to keep the budget impact analysis consistent.
The clinical utility of BNP and NT-proBNP tests has been demonstrated in the literature. Based on a meta-analysis by Lam et al,41 BNP testing in the ED for people presenting with acute dyspnea decreased hospital length of stay by about 1 day and possibly reduced admission rates (odds ratio: 0.82; 95% CI: 0.67–1.01), but did not conclusively affect hospital mortality rates (odds ratio: 0.96; 95% CI: 0.65–1.41).
In the ED:
People with symptoms suggestive of heart failure may or may not have the condition. Based on the literature,28,54 as well as clinical expert opinion, the probability of heart failure in people who present in the ED with suspected heart failure is about 50%
-
Next, people who receive a positive test result (above threshold) would be diagnosed and managed as patients with heart failure. Also, they would be referred to echocardiography to confirm the diagnosis of heart failure
○ True positive results: those who have heart failure would receive timely treatment for their condition. Some patients may be hospitalized immediately, and some may be stabilized in the ED and discharged home. Based on data from IntelliHealth Ontario, about 63% of people diagnosed with heart failure are hospitalized following the ED visit. This is consistent with observations from clinical trials conducted in the ED setting55,56
○ False positive results: those who do not have heart failure (their symptoms are caused by some other condition) would receive echocardiography. Their true conditions are presumed to be identified early and, therefore, we assumed there would be no delay in treatment. Based on data from IntelliHealth Ontario, about 47% of people diagnosed with other conditions are hospitalized following an ED visit
-
People who receive a negative result (below threshold) would not be referred to echocardiography. They would be investigated for other conditions8
○ False negative results: we applied the same assumptions from Griffin et al54 (the NICE economic evaluation) to those who do have heart failure but receive a negative test result. We assumed that 80% of false negative diagnoses would be corrected during admission, resulting in no delay in treatment. The remaining 20% of patients would experience a delay in treatment. These patients would eventually be hospitalized for heart failure, but we assumed their hospital length of stay would be increased by 2 days
In community care:
People with symptoms suggestive of heart failure may or may not be experiencing heart failure. Based on the literature20,51,63 and expert opinion, the probability of heart failure in people who present with suspected heart failure was about 35% in community care
When natriuretic peptide tests are not available (standard clinical investigations alone), all patients with suspected heart failure would be referred for echocardiography and cardiologist assessment
-
When natriuretic peptide tests are available, patients who receive a positive test result would be referred for echocardiography and cardiologist assessment
○ True positive results: those who have heart failure would receive timely treatment for their condition
○ False positive results: those who do not have heart failure (their symptoms are caused by some other condition) would receive echocardiography. Their true conditions are presumed to be identified early and, therefore, we assumed there would be no delay in treatment
-
People who receive a negative result would not be referred for echocardiography and cardiologist assessment. Community care providers would consider alternative diagnoses.8
○ False negative results: those with heart failure would be at risk of a heart failure hospitalization. Based on the NICE economic evaluation,20 the annual hospitalization rate of untreated heart failure is 0.044. If a hospitalization does not occur, the authors assumed that the patient would re-present to their GP after 6 months.
Figure 9: Model Structures–Natriuretic Peptide Testing for Suspected Heart Failure.
Legend: The square represents a decision node, and the circles represent chance nodes.
Clinical Parameters—Diagnostic Accuracy
To keep consistency, the sensitivity and specificity of BNP and NT-proBNP tests were obtained from the NICE economic evaluations (Table 15). We compared the values used by the NICE authors with those identified in our clinical evidence review and they were similar. We used the following cut-off values for ruling out heart failure based on the Canadian guidelines2:
Table 15:
Clinical Parameters for the Budget Impact Analysis
| Variable | Mean | Reference |
|---|---|---|
| Emergency Department Setting | ||
| Standard clinical investigations | ||
| Sensitivity | 0.80 | Griffin et al 2017;54 NICE Acute HF guideline19 |
| Specificity | 0.77 | Griffin et al 2017;54 NICE Acute HF guideline19 |
| BNP ≤ 100 pg/mL | ||
| Sensitivity | 0.95 | Griffin et al 2017;54 NICE Acute HF guideline19 |
| Specificity | 0.63 | Griffin et al 2017;54 NICE Acute HF guideline19 |
| NT-proBNP ≤ 300 pg/mL | ||
| Sensitivity | 0.99 | NICE Acute HF guideline19 |
| Specificity | 0.43 | NICE Acute HF guideline19 |
| Community Care Setting | ||
| BNP ≤ 50 pg/mL | ||
| Sensitivity | 0.80 | Clinical evidence review |
| Specificity | 0.61 | Clinical evidence review |
| NT-proBNP ≤ 125 pg/mL | ||
| Sensitivity | 0.843 | NICE Chronic HF guideline20 |
| Specificity | 0.419 | NICE Chronic HF guideline20 |
Abbreviations: BNP, B-type natriuretic peptide; HF, heart failure; NT-proBNP, N-terminal proBNP.
BNP ≤ 100 pg/mL and NT-proBNP ≤ 300 pg/mL in the ED
BNP ≤ 50 pg/mL and NT-proBNP ≤ 125 pg/mL in community care
Resources and Costs
We included the following types of short-term costs in our analysis (Table 16):
Table 16:
Cost and Probability Parameters
| Variable | Value | Reference |
|---|---|---|
| Costs | ||
| BNP | ||
| Hospital lab | $75.00 | Ontario hospital websites70 |
| Community lab | $28.14 | BC Schedule of Fees, 201969 |
| Point-of-care test | $40.00 | Chuck and Jacobs, 200585 |
| NT-proBNP | ||
| Hospital lab | $82.50 | Assume unit cost to be 10% higher than BNP |
| Community lab | $28.14 | BC Schedule of Fees, 201969 |
| Point-of-care test | $44.00 | Assume unit cost to be 10% higher than BNP |
| Echocardiography | $208.80 | Schedule of Benefit (G570 technical component: $112.60; G571 professional component: $96.20)72 |
| GP consultation | $77.20 | Schedule of Benefit, 2019 (A005)72 |
| Cardiology consultation | $157.00 | Schedule of Benefit, 2019 (A605)72 |
| ED visit | $454.00 | Ontario Case Costing, 2017/1880 |
| Hospitalization for HF | $9,387.00 | Ontario Case Costing, 2017/1880 (LOS 8.8 d) |
| Hospitalization for other conditions | $5,460.00 | CIHI 2017/18 (LOS 6.7 d)73 |
| Hospital bed day | $815.00 | Estimated as $5,460.00/6.7 |
| Probabilities | ||
| % FN corrected during admission | 80% | Griffin et al, 2017;54 NICE Acute HF guideline19 |
| Additional LOS if FN is uncorrected | 2.00 | Griffin et al, 2017;54 NICE Acute HF guideline19 |
| % ED HF patients hospitalized | 63% | IntelliHealth Ontario83 |
| % ED non-HF patients hospitalized | 47% | IntelliHealth Ontario83 |
| Annual % hospitalization of untreated HF in community care | 4% | NICE Chronic HF guideline20 |
Abbreviations: BNP, B-type natriuretic peptide; ED, emergency department; FN, false negative; GP, general practitioner; HF, heart failure; LOS, length of stay; NT-proBNP, N-terminal proBNP.
BNP or NT-proBNP test (includes labour, equipment, and supplies)
Increased hospitalizations due to uncorrected false-negative diagnoses
Echocardiography (includes labour, equipment, and supplies)
Referral to cardiologist for diagnosis
Increased ED visits due to uncorrected false-negative diagnoses
The costs of standard clinical investigations (e.g., history, physical examination, ECG, and chest x-ray) were not included because they are common to both strategies.
In the ED setting, the unit cost of BNP in an Ontario hospital lab was $75 per test. We could not find published information on the cost of NT-proBNP. N terminal–proBNP typically has higher reagent costs since it is patented technology, while the BNP test is available from multiple vendors (Andrew Don-Wauchope, MD, email communication, December 2019). Therefore, we assume the unit cost of NT-proBNP test in a hospital lab is about 10% higher than BNP. In the reference case analysis, we assumed the unit costs of BNP and NT-proBNP would maintain at the current level. However, the NT-proBNP test is due to come off patent in 2024, so the price may potentially go down. Therefore, we included a sensitivity analysis that assumes the unit costs of BNP and NT-proBNP will be lower.
In the community care setting, we found several published costs (or list prices) for the test. In British Columbia in 2015, the cost of BNP was $42.56 per test,84 and in 2019 the cost of BNP or NT-proBNP was $28.14 per test based on the schedule of fees for outpatient laboratory services. According to Alberta Health Services, the reference median cost of BNP from six Canadian diagnostic laboratories was $18 in 2016.71 The unit cost of the test is lower in community laboratories, potentially due to larger volumes compared to hospital laboratories. Also, like many routine tests, the unit cost tends to decrease over time due to improved technology. We assumed that, for the reference case analysis, publicly funded tests in Ontario would have a unit cost in community care (through community laboratories) that is similar to that in British Columbia ($28.14 per test). We also included a scenario analysis where we used the current Ontario unit cost ($75 per test) without considering any cost reduction.
In an Alberta study on point-of-care BNP testing, the cost per assay was about $40 (including equipment and supplies). The cost of a portable BNP analyzer (Biosite Triage) was about $5,500 if it is purchased, or no charge if acquired under a reagent rental plan (for a volume of ≥ 300 tests per year). The cartridges cost approximately $35 each under a reagent rental plan or $30 each if under a capital purchase plan, and come in a kit of 25 assays. Other costs include quality control, which is required every 30 days at a cost of $155, and calibration verification control, which is required every 6 months at a cost of $125.
Analysis
We conducted a reference case analysis and sensitivity analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. In sensitivity analyses, we explored the impact of input parameters and model assumptions on the budget impact results (Table 17).
Table 17:
Parameters Varied in the Sensitivity Analyses
| Parameter | Reference Case | Sensitivity Analysis |
|---|---|---|
| % of BNP and NT-proBNP | 50% BNP and 50% NT-proBNP | 100% BNP or 100% NT-proBNP |
| % living with HF needing NP testing | 56% based on IntelliHealth Ontario data83 | 30% to 80%a |
| % of incident HF cases diagnosed in ED | 50% in ED | 75% in ED or 25% in ED |
| Uptake of BNP and NT-proBNP | ED: 90% in Year 1, 100% in Years 2–5 Community care: 50% in Year 1, 75% in Year 2, 100% in Years 3–5 | Faster uptake: 100% right away in both ED and community care Slower uptake: ED: 70%, 80%, 90%, 100%, 100% in Years 1–5, respectively Community care: 25%, 50%, 75%, 100%, 100% in Years 1–5, respectively |
| % patients receiving BNP or NT-proBNP tests in the ED in the current scenario | Assume 0% since there is no programmatic funding for BNP and NT-proBNP tests in Ontario | Assume 50% to 70% of patients are currently receiving BNP or NT-proBNP tests for the diagnosis of HF in the ED (since the tests are partially funded through the hospital's global budget) |
| Additional LOS for uncorrected FN diagnoses | 2 d, based on Griffin et al54 | 0 and 4 d |
| Unit cost of BNP and NT-proBNP | ED: $75, based on current Ontario cost Community care: $28, based on BC schedule of fees |
ED: assume same as BC cost ($28) Community care: $75 for BNP and $82.5 for NT-proBNP, based on current Ontario cost |
| Sample collection fee for BNP and NT-proBNP test conducted in the community care setting | Excluded (when multiple tests are ordered for the same patient, for the same day, only one sample collection fee is allowed. For this population, they usually receive several different tests at a time, so the sample collection fee is not considered to avoid double counting) | A sample collection fee of $10.76 per test (L700)86 was included for one-third of patientsb |
| Diagnostic accuracy of standard clinical investigations alone (ED setting) | Sensitivity: 80% Specificity: 77% |
Match to BNP sensitivity: Sensitivity 95%, specificity 30% Match to BNP specificity: Sensitivity 87%, specificity 63% |
| % FN diagnoses corrected during hospital stay | 80% | 100% |
| Prevalence of HF in ED | 50% | 29% to 79%, according to Martindale et al 201628 (a systematic review) |
| Point-of-care testing | Laboratory-based test | Apply cost of point-of-care testing (assume similar accuracy as laboratory-based tests) |
Abbreviations: BNP, B-type natriuretic peptide; ED, emergency department; FN, false negative; HF, heart failure; LOS, length of stay; NP, natriuretic peptide; NT-proBNP, N-terminal proBNP.
Robert McKelvie, MD, email communication, January 2020; Lisa Mielniczuk, MD, email communication, January 2020.
Andrew Don-Wauchope, MD, email communication, December 2019.
Internal Validation
Formal internal validation was conducted by the secondary health economist. This included checking for errors and accuracy of parameter inputs and equations in the budget impact analysis.68
Results
Reference Case
COST PER PERSON
Using the decision tree models, we estimated the cost per person related to each testing strategy (Table 18). In the ED, the total cost per person was approximately $4,912 for standard clinical investigations with BNP, $4,940 for standard clinical investigations with NT-proBNP, and $4,822 for standard clinical investigations alone. Compared to standard clinical investigations alone, using BNP or NT-proBNP would increase the cost of diagnostic workup (BNP or NT-proBNP and echo), which is only partially offset by the cost of hospitalization, resulting in an overall increase in cost per person.
Table 18:
Average Cost per Person
| Emergency Department | |||||
|---|---|---|---|---|---|
| Cost | SCI with BNP | SCI with NT-proBNP | SCI | ΔCost of BNP vs. SCI | ΔCost of NT-proBNP vs. SCI |
| Total | 4,911.95 | 4,940.40 | 4,822.07 | 89.87 | 118.32 |
| NP testing | 75.00 | 82.50 | — | 75.00 | 82.50 |
| ECHO | 137.81 | 162.86 | 107.53 | 30.28 | 55.33 |
| ED visits | 454.00 | 454.00 | 454.00 | 0.00 | 0.00 |
| Hospitalization | 4,245.14 | 4,241.03 | 4,260.54 | –15.40 | –19.51 |
| Community Care | |||||
| Cost | SCI with BNP | SCI with NT-proBNP | SCI (ECHO all) | ΔCost of BNP | ΔCost of NT-proBNP |
| Total | 333.95 | 377.68 | 443.00 | –109.05 | –65.32 |
| Initial GP visit | 77.20 | 77.20 | 77.20 | 0.00 | 0.00 |
| NP testing | 28.14 | 28.14 | — | 28.14 | 28.14 |
| ECHO | 111.39 | 140.46 | 208.80 | –97.41 | –68.34 |
| Cardiologist | 83.76 | 105.61 | 157.00 | –73.24 | –51.39 |
| Hospitalization | 28.29 | 22.20 | — | 28.29 | 22.20 |
| Repeat GP visit | 5.17 | 4.06 | — | 5.17 | 4.06 |
Abbreviations: BNP, B-type natriuretic peptide; ED, emergency department; ECHO, echocardiography; GP, general practitioner; NP, natriuretic peptide; NT-proBNP, N-terminal proBNP; SCI, standard clinical investigations.
In community care, the total cost per person was approximately $334 for standard clinical investigations with BNP, $378 for standard clinical investigations with NT-proBNP, and $443 for standard clinical investigations alone. Compared to standard clinical investigations alone, using BNP or NT-proBNP would increase the cost of hospitalization and repeat GP visits, but reduce referrals to ECHO and cardiology, resulting in an overall savings per person.
BUDGET IMPACT
We estimated the budget impact by multiplying the cost per person associated with each strategy by the number of people expected to receive that strategy (Table 19). In the ED, we estimated that publicly funding BNP and NT-proBNP tests for people with suspected heart failure would result in an additional cost of $38.47 million to the provincial budget over the next 5 years. The cost of the tests alone was estimated to be about $29.11 million. In community care, we estimated that publicly funding BNP and NT-proBNP tests for people with suspected heart failure would result in a savings of $19.88 million over the next 5 years. The cost of the tests alone was estimated to be about $6.42 million.
Table 19:
Budget Impact of BNP and NT-proBNP Test (Reference Case)—All Costs (in Millions)
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
|---|---|---|---|---|---|---|
| Emergency Department | ||||||
| Current scenario | ||||||
| SCI | 358.89 | 361.10 | 363.44 | 365.98 | 368.71 | 1818.11 |
| New scenario | ||||||
| SCI | 35.89 | — | — | — | — | 35.89 |
| SCI with BNP | 164.51 | 183.92 | 185.11 | 186.40 | 187.79 | 907.72 |
| SCI with NT-proBNP | 165.46 | 184.98 | 186.18 | 187.48 | 188.88 | 912.98 |
| Budget impact (all costs) | 6.97 | 7.80 | 7.85 | 7.90 | 7.96 | 38.47 |
| Test costs only | 5.27 | 5.90 | 5.94 | 5.98 | 6.02 | 29.11 |
| Community Care | ||||||
| Current scenario | ||||||
| SCI | 24.66 | 24.27 | 23.88 | 23.50 | 23.12 | 119.43 |
| New scenario | ||||||
| SCI | 12.33 | 6.07 | — | — | — | 18.40 |
| SCI with BNP | 4.65 | 6.86 | 9.00 | 8.86 | 8.71 | 38.08 |
| SCI with NT-proBNP | 5.26 | 7.76 | 10.18 | 10.02 | 9.85 | 43.07 |
| Budget impact (all costs) | –2.43 | –3.58 | –4.70 | –4.62 | –4.55 | –19.88 |
| Test cost only | 0.78 | 1.16 | 1.52 | 1.49 | 1.47 | 6.42 |
Abbreviations: BNP, B-type natriuretic peptide; NT-proBNP, N-terminal proBNP; SCI, standard clinical investigations.
Sensitivity Analysis
Results of the sensitivity analyses are presented in Tables 20 and 21. In the ED setting, publicly funding BNP and NT-proBNP would result in an additional cost to the provincial budget. The cost increased significantly when we assumed the following:
Table 20:
Sensitivity Analysis Results—Emergency Department Setting (in Millions)
| Costs | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total |
|---|---|---|---|---|---|---|
| Reference Case | ||||||
| Total | 6.97 | 7.80 | 7.85 | 7.90 | 7.96 | 38.47 |
| Test only | 5.27 | 5.90 | 5.94 | 5.98 | 6.02 | 29.11 |
| 100% BNP (Only BNP Is Funded) | ||||||
| Total | 6.02 | 6.73 | 6.77 | 6.82 | 6.87 | 33.22 |
| Test only | 5.02 | 5.62 | 5.65 | 5.69 | 5.73 | 27.72 |
| 100% NT-proBNP (Only NT-proBNP Is Funded) | ||||||
| Total | 7.93 | 8.86 | 8.92 | 8.98 | 9.05 | 43.73 |
| Test only | 5.53 | 6.18 | 6.22 | 6.26 | 6.31 | 30.49 |
| 80% Living With HF Needing NP Testing | ||||||
| Total | 8.39 | 9.42 | 9.52 | 9.63 | 9.74 | 46.70 |
| Test only | 6.35 | 7.13 | 7.20 | 7.28 | 7.37 | 35.33 |
| 30% Living With HF Needing NP Testing | ||||||
| Total | 5.43 | 6.03 | 6.03 | 6.03 | 6.03 | 29.54 |
| Test only | 4.11 | 4.56 | 4.56 | 4.56 | 4.56 | 22.34 |
| 75% of Incident HF Cases Diagnosed in ED | ||||||
| Total | 8.80 | 9.79 | 9.81 | 9.83 | 9.86 | 48.09 |
| Test only | 6.66 | 7.41 | 7.42 | 7.44 | 7.46 | 36.38 |
| 25% of Incident HF Cases Diagnosed in ED | ||||||
| Total | 5.15 | 5.80 | 5.88 | 5.97 | 6.06 | 28.85 |
| Test only | 3.89 | 4.39 | 4.45 | 4.51 | 4.58 | 21.83 |
| Faster Uptake (100% in Year 1) | ||||||
| Total | 7.75 | 7.80 | 7.85 | 7.90 | 7.96 | 39.25 |
| Test only | 5.86 | 5.90 | 5.94 | 5.98 | 6.02 | 29.69 |
| Slower uptake (70% in Year 1, 80% in Year 2, 90% in Year 3, 100% in Years 4&5) | ||||||
| Total | 5.42 | 6.24 | 7.06 | 7.90 | 7.96 | 34.58 |
| Test only | 4.10 | 4.72 | 5.34 | 5.98 | 6.02 | 26.16 |
| 50% Patients Receiving BNP or NT-proBNP Tests in the ED in the Current Scenario | ||||||
| Total | 3.10 | 3.90 | 3.92 | 3.95 | 3.98 | 18.85 |
| Test only | 2.34 | 2.95 | 2.97 | 2.99 | 3.01 | 14.26 |
| 70% Patients Receiving BNP or NT-proBNP Tests in the ED in the Current Scenario | ||||||
| Total | 1.55 | 2.34 | 2.35 | 2.37 | 2.39 | 11.00 |
| Test only | 1.17 | 1.77 | 1.78 | 1.79 | 1.81 | 8.32 |
| FN 0 Day Additional LOS | ||||||
| Total | 8.14 | 9.10 | 9.16 | 9.23 | 9.29 | 44.93 |
| Test only | 5.27 | 5.90 | 5.94 | 5.98 | 6.02 | 29.11 |
| FN 4 Days Additional LOS | ||||||
| Total | 5.80 | 6.49 | 6.53 | 6.58 | 6.62 | 32.02 |
| Test only | 5.27 | 5.90 | 5.94 | 5.98 | 6.02 | 29.11 |
| Lower Unit Cost of NP (Same as BC: $28.14 Per Test) | ||||||
| Total | 3.58 | 4.01 | 4.03 | 4.06 | 4.09 | 19.77 |
| Test only | 1.88 | 2.11 | 2.12 | 2.14 | 2.15 | 10.40 |
| Point-of-Care Test ($40 for BNP and $44 for NT-proBNP) | ||||||
| Total | 4.51 | 5.04 | 5.08 | 5.11 | 5.15 | 24.89 |
| Test only | 2.81 | 3.15 | 3.17 | 3.19 | 3.21 | 15.52 |
| Diagnostic Accuracy of SCI Alone, Matched to NP Sensitivity (95%, 30%) | ||||||
| Total | 3.67 | 4.10 | 4.13 | 4.16 | 4.19 | 20.24 |
| Test only | 5.27 | 5.90 | 5.94 | 5.98 | 6.02 | 29.11 |
| Diagnostic Accuracy of SCI Alone, Matched to NP Specificity (87%, 63%) | ||||||
| Total | 5.99 | 6.69 | 6.74 | 6.78 | 6.83 | 33.03 |
| Test only | 5.27 | 5.90 | 5.94 | 5.98 | 6.02 | 29.11 |
| 100% FN Corrected During Hospital Stay | ||||||
| Total | 8.14 | 9.10 | 9.16 | 9.23 | 9.29 | 44.93 |
| Test only | 5.27 | 5.90 | 5.94 | 5.98 | 6.02 | 29.11 |
| Prevalence of HF in ED (Lower Range of the Literature: 29%28) | ||||||
| Total | 10.58 | 11.75 | 11.76 | 11.77 | 11.78 | 57.64 |
| Test only | 7.27 | 8.08 | 8.09 | 8.09 | 8.10 | 39.65 |
| Prevalence of HF in ED (Upper Range in the Literature: 79%28) | ||||||
| Total | 4.86 | 5.46 | 5.52 | 5.59 | 5.66 | 27.08 |
| Test only | 4.26 | 4.79 | 4.84 | 4.90 | 4.97 | 23.76 |
Abbreviations: BNP, B-type natriuretic peptide; ED, emergency department; FN, false negative; HF, heart failure; NP, natriuretic peptide; NT-proBNP, N-terminal proBNP; SCI, standard clinical investigations; LOS, length of stay.
Table 21:
Sensitivity Analysis Results—Community Care Setting (in Millions)
| Costs | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total |
|---|---|---|---|---|---|---|
| Reference Case | ||||||
| Total | –2.43 | –3.58 | –4.70 | –4.62 | –4.55 | –19.88 |
| Test only | .78 | 1.16 | 1.52 | 1.49 | 1.47 | 6.42 |
| 100% BNP (Only BNP Is Funded) | ||||||
| Total | –3.04 | –4.48 | –5.88 | –5.78 | –5.69 | –24.87 |
| Test only | .78 | 1.16 | 1.52 | 1.49 | 1.47 | 6.42 |
| 100% NT-proBNP (Only NT-proBNP Is Funded) | ||||||
| Total | –1.82 | –2.68 | –3.52 | –3.46 | –3.41 | –14.90 |
| Test only | .78 | 1.16 | 1.52 | 1.49 | 1.47 | 6.42 |
| 75% of Incident HF Cases Diagnosed in ED | ||||||
| Total | –1.21 | –1.79 | –2.35 | –2.31 | –2.27 | –9.94 |
| Test only | .39 | .58 | .76 | .75 | .73 | 3.21 |
| 25% of Incident HF Cases Diagnosed in ED | ||||||
| Total | –3.64 | –5.37 | –7.05 | –6.94 | –6.82 | –29.83 |
| Test only | 1.17 | 1.73 | 2.28 | 2.24 | 2.20 | 9.63 |
| Faster Uptake (100% in Year 1) | ||||||
| Total | –4.85 | –4.78 | –4.70 | –4.62 | –4.55 | –23.50 |
| Test only | 1.57 | 1.54 | 1.52 | 1.49 | 1.47 | 7.59 |
| Slower uptake (70% in Year 1, 80% in Year 2, 90% in Year 3, 100% Years 4&5) | ||||||
| Total | –1.21 | –2.39 | –3.53 | –4.62 | –4.55 | –16.30 |
| Test only | .39 | .77 | 1.14 | 1.49 | 1.47 | 5.26 |
| Higher Unit Cost of NP ($75 for BNP and $82.5 for NT-proBNP) | ||||||
| Total | –1.02 | –1.50 | –1.97 | –1.94 | –1.91 | –8.34 |
| Test only | 2.19 | 3.24 | 4.25 | 4.18 | 4.11 | 17.96 |
| Point-of-Care Test ($40 for BNP and $44 for NT-proBNP) | ||||||
| Total | –2.04 | –3.01 | –3.95 | –3.89 | –3.83 | –16.72 |
| Test only | 1.17 | 1.73 | 2.26 | 2.23 | 2.19 | 9.58 |
| Prevalence of HF in Community Care (Lower Range: –10%) | ||||||
| Total | –4.21 | –6.21 | –8.15 | –8.02 | –7.89 | –34.48 |
| Test only | 1.10 | 1.62 | 2.12 | 2.09 | 2.06 | 8.98 |
| Prevalence of HF in Community Care (Upper Range: +10%) | ||||||
| Total | –1.44 | –2.12 | –2.78 | –2.74 | –2.69 | –11.77 |
| Test only | .61 | .90 | 1.18 | 1.16 | 1.14 | 4.99 |
| Including Sample Collection Fee ($10.76 Per Test for a Third of Patients) | ||||||
| Total | –2.33 | –3.44 | –4.51 | –4.43 | –4.36 | –19.07 |
| Test only | .88 | 1.30 | 1.71 | 1.68 | 1.66 | 7.24 |
Abbreviations: BNP, B-type natriuretic peptide; HF, heart failure; ED, emergency department; NP, natriuretic peptide; NT-proBNP, N-terminal proBNP.
Only NT-proBNP is funded (i.e., 100% NT-proBNP)
80% of people who present to ED require natriuretic peptide testing
75% of incident heart failure cases are diagnosed in ED
No additional hospital length of stay for uncorrected false-negative diagnoses
All false-negative diagnoses are corrected during the hospital stay
The prevalence of heart failure in the ED (i.e., probability of heart failure among those who present with suspected heart failure) is 29% instead of 50%
In the community care setting, publicly funding BNP and NT-proBNP would result in cost savings. The cost savings decreased significantly when we assumed the following:
75% of incident heart failure cases are diagnosed in the ED
The unit cost of BNP remains at the current level ($75 per test)
The prevalence of heart failure in community care (i.e., probability of heart failure among those who present with suspected heart failure) is 45% instead of 35%
Discussion
Heart failure is a common chronic condition, especially among the elderly. With an aging population, we expect the number of people affected by heart failure to increase in the future. In the present analysis, we estimated the number of people with suspected heart failure using Ontario-specific data from the published literature and administrative databases. We found that about 130,000 people could be eligible for natriuretic peptide testing each year. The size of the target population is one of the key drivers of the budget impact.
Another key driver of the budget impact is the unit cost of BNP and NT-proBNP tests. Compared to other Canadian provinces, such as British Columbia ($28 per test) and Alberta ($18 per test), the cost per test is much higher in Ontario ($75 per test). We are uncertain what has caused such a large difference. A potential factor may be the total test volume. Currently, BNP and NT-proBNP are publicly funded in British Columbia and Alberta, but not in Ontario. Because Ontario has the largest population in Canada,87 if BNP and NT-proBNP tests were to become publicly funded in Ontario, we would expect the unit cost to go down. We also expect the unit cost to go down due to technology advancement and automation. If the unit cost remained at the current level ($75 per test), there would be less savings in the community care setting ($19.88 million under current unit costs, compared with $8.34 million with reduced unit costs; test costs alone would be $17.96 million, compared with $6.42 million).
Another important consideration is how physicians would use these tests in actual practice. Since BNP and NT-proBNP tests are simple blood tests and easy to order, there is a potential for physicians to over test. Based on clinical expert opinion, BNP and NT-proBNP should only be used when the diagnosis of heart failure is uncertain. However, due to variation in health care provider experience with diagnosing heart failure and knowledge of benefits/limitations of natriuretic peptide tests, we might see the tests being both overused and underused in different clinical settings. To capture this potential large variation in our analysis, we varied the proportion of ED visits with a history of heart failure requiring natriuretic peptide testing from 30% to 80% (reference case value 56%).
Also, BNP and NT-proBNP tests could be used for other purposes, such as informing prognosis, guiding treatment, and monitoring patients. If the tests are made available for the purpose of diagnosis, physicians may expand the uses beyond diagnosis. It might be helpful to place some restrictions on testing if it becomes publicly funded in Ontario. For example, in British Columbia, the following restrictions are listed in the schedule of fees for outpatient laboratory services regarding BNP or NT-proBNP tests69:
-
1.
Tests are payable for assessment of symptomatic patients where the diagnosis of heart failure remains in doubt after standard assessment.
-
2.
Repeat testing is payable once annually. Additional testing may be requested by the practitioner for a new clinical episode suspicious for heart failure or in the tertiary cardiac care outpatient setting for prognostic stratification of heart failure.
-
3.
Repeat testing for monitoring therapy is not payable.
In the current analysis, we assumed that 50% of the incident heart failure cases were diagnosed in the ED and 50% were diagnosed in community care.75 If the test became available and was more widely used in community care, we might expect more people to be diagnosed earlier and given proper treatment. Therefore, fewer people will go to the ED with more acute symptoms. In this case, the 5-year budget impact would be reduced in the ED setting (from $38 million to $28 million of additional cost), and the savings would increase in the community care setting (from $19.88 million to $29.83 million in savings).
There may be some variation in the magnitude of savings. In the reference case analysis, we estimated the budget impact with consideration of the potential benefits of BNP and NT-proBNP tests (i.e., reducing the use of ECHO, reducing hospitalization, and reducing length of stay). Overall, BNP and NT-proBNP testing has the potential for considerable cost savings while improving health outcomes (e.g., improving the accuracy of diagnosis and reducing hospitalization and length of stay). The clinical utility of natriuretic peptide testing in the ED setting has been demonstrated in several randomized controlled trials, including in Canada.55–59 In the community care setting, a retrospective cohort study based on real-world data from the UK general practices found that patients who were not put on the NICE-recommended pathway (BNP testing and/or echocardiography, or specialist referral) had a significantly higher risk of heart failure admission and also non-significant higher risk of death than other patients who were.52 Despite these potential benefits, publicly funding BNP and NT-proBNP tests would still require an initial added cost, while the savings may not be as apparent and may not occur right away. If the potential savings in hospitalization were not considered in the ED setting, the 5-year budget impact would increase from $38.47 million to $44.93 million. If the potential savings in ECHO and cardiologist referrals were not considered in the community care setting, the 5-year budget impact would become a $6.42 million cost instead of a $19.88 million savings.
Currently, patients in the community setting have to pay out of pocket for this test. Testing in the hospital is controlled by the hospital laboratory, which means the cost for all testing comes from the hospital's global budget. However, the cost of natriuretic peptide testing is not factored into the hospital's global budget. If hospitals choose to do the test, it can significantly impact their budget. Our analysis estimated that it would cost about $6 million per year to provide BNP and NT-proBNP testing for diagnosing heart failure in the ED. According to clinical experts, the lack of funding for these tests could lead to inequities in care. Currently, not all hospitals in Ontario allow equal access to these tests for diagnosing heart failure. Our reference case analysis conservatively overestimated the budget impact in the ED setting by assuming that there is no funding for BNP and NT-proBNP tests in the current scenario. When we assumed that 50% to 70% of patients are currently receiving a BNP or NT-proBNP test for the diagnosis of heart failure in the ED (since the tests are partially funded through the hospital's global budget), the 5-year budget impact was reduced to $19 million and $11 million, respectively.
Conclusions
Our budget impact analysis estimated that about 130,000 people could be eligible for natriuretic peptide testing each year in Ontario. Under the most likely scenario, over the next 5 years, publicly funding BNP and NT-proBNP would result in an additional cost of $38 million in the ED setting (at a cost of $75 per test) and a savings of $20 million in community care (at a cost of $28 per test).
Preferences and Values Evidence
Objective
The objective of this analysis was to explore the underlying values, needs, and priorities of those who have lived experience of receiving diagnostic assessments for heart failure, as well as the preferences and perceptions of both patients and caregivers for B-type natriuretic peptide (BNP) or N-terminal proBNP (NT-proBNP) tests.
Background
Exploring patient preferences and values provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat that health condition. It includes the impact of the condition and its treatment on the person with the health condition, their family and other caregivers, and the person's personal environment. Engagement also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., outcomes important to those with lived experience that are not reflected in the literature).88–90 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, preferences, priorities, and values of those with lived experience in Ontario are important to consider to understand the impact of the technology in peoples lives, we may speak directly with people who live with a given health condition, including those with experience of the technology or intervention we are exploring.
For this analysis, we used direct engagement methods to examine the preferences and values of people who had undergone diagnostic assessments for heart failure.
Direct Patient Engagement
Methods
PARTNERSHIP PLAN
The partnership plan for this health technology assessment focused on consultation to examine the experiences of people who have gone through diagnostic testing for heart failure and those of their families and other caregivers. We engaged people via phone interviews and an online survey.
We used a qualitative interview, as this method of engagement allowed us to explore the meaning of central themes in the experiences of people who have undergone diagnostic testing for heart failure, as well as those of their families and caregivers.91 The sensitive nature of exploring people's experiences of a health condition and their quality of life are other factors that support our choice of interview and survey methodologies.
PARTICIPANT OUTREACH
We used an approach called purposive sampling,92–95 which involves actively reaching out to people with direct experience of the health condition or health technology being reviewed. We approached a variety of health clinics, cardiac rehabilitation facilities, heart failure community support groups, and health system partner organizations and associations to spread the word about this engagement activity and to contact people who have been assessed for heart failure, and their family members and caregivers.
Inclusion Criteria
Adults (≥ 18 years) with suspected heart failure
Exclusion Criteria
People < 18 years
People undergoing chemotherapy or treatment for HIV (where the medication can cause heart failure) or who are pregnant (due to the unique physiology of pregnant people)
Participants
For this project, we interviewed six people and received survey feedback from an additional 15. Six of the 21 participants had undergone diagnostic testing for heart failure, all of whom were diagnosed with heart failure. The remaining participants were family members and caregivers of patients who had received diagnostic testing for heart failure. One patient was from Vancouver, and the rest of the participants were from Ontario (15 lived in the Greater Toronto Area and five in Northern or Northwestern Ontario.
APPROACH
At the beginning of the interviews and surveys, we explained the role of Ontario Health, the purpose of this health technology assessment, the risks of participation, and how participants' personal health information would be protected. We gave this information to participants both verbally and in a letter of information (Appendix 5). We then obtained participants' verbal consent before starting the interview. With participants' consent, we audio-recorded and then transcribed the interviews. For individuals who completed surveys, each respondent remained anonymous.
Interviews lasted approximately 20 to 30 minutes. The interviews were loosely structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.96 Questions focused on the impact of diagnostic testing for heart failure on the quality of life of people with possible heart failure, their experiences with treatments to manage their heart failure if diagnosis was confirmed, their experiences with the process of being diagnosed with heart failure, and their perceptions of the benefits or limitations of getting diagnosed. For family members and caregivers, questions focused on their perceptions of the impact of diagnostic testing for heart failure and on the quality of life of the person with confirmed heart failure, as well as the impact of the person's diagnostic assessments, condition, and treatments on the family members and caregivers themselves. See Appendix 6 for our interview and survey guide.
DATA EXTRACTION AND ANALYSIS
We used a modified version of a grounded-theory methodology to analyze interview transcripts and survey results. The grounded-theory approach allowed us to organize and compare information on experiences across participants. This method consists of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.97,98 We used the qualitative data analysis software program NVivo99 to identify and interpret patterns in the data. The patterns we identified allowed us to highlight the impact of the diagnostic process for heart failure on the people with suspected heart failure, family members, and caregivers we interviewed and surveyed.
Results
EXPERIENCE WITH DIAGNOSTIC TESTING
Positive Experience
A majority of the participants we interviewed indicated that they experienced a fast, simple diagnostic process. Patients experienced symptoms for which they went to the emergency department (ED). After doing a few simple tests, most were given a diagnosis within a 1 to 3 days. Other patients received a diagnosis within a couple of weeks. Although some patients were frustrated at long wait times in the ED, they were relieved to receive a diagnosis and begin their treatment.
The process was simple. I had been taking amiodarone and realized something was wrong, so [I] went to the ER. I was diagnosed within 1 day.
I found the diagnosis process to be simple. They did a range of tests in the cardiologist's office and we saw the doctor to receive the diagnosis once the tests were complete.
Negative Experience
Participants emphasized the stress of having to go through diagnostic assessments for heart failure. This stress was primarily attributed to the length of time taken for both the diagnostic assessment and the remaining treatment pathway. Patients experienced different diagnostic processes depending on the severity of their condition. Some participants described their experience with the diagnosis process as complicated, frustrating, and lengthy. These were generally people who had symptoms that were not immediately recognized as related to a heart condition. In these cases, patients either had a known comorbidity to which their symptoms were mistakenly attributed, or the cause of their symptoms was misdiagnosed.
When he was first starting to feel unwell, and water filled up in his lungs around December or January … at that time, we still didn't know what was happening. It wasn't until June when the water in his lungs filled up again and they looked into why this was happening… we found out he had heart failure.
The whole process was complicated and frustrating. The heart specialist experimented with different drugs and refused to hear that symptoms were continuing.
Patients experienced long wait times in the ED. The busyness of ED settings was emphasized by people living in Northern and rural Ontario. People felt that the Greater Toronto Area has more hospitals that are better equipped for cardiac services. Though patients were able to get diagnosed within 1 to 2 days in the emergency settings of their hometowns, they said the lack of hospital staff made wait times longer. One caregiver described the difference between a hospital in Toronto and a hospital located in the caregiver's hometown, several hours away from Toronto.
He was in emergency care [in Northern Ontario] several times over, and I think what I noticed is that once my grandfather moved [from Toronto] back to this hospital … they seemed to be understaffed, under resourced, and overworked.
We've had the same GP for 30 years [and] like him very much. But he's overwhelmed with 3,500 patients. That's just the way it is up here [in Northern Ontario], there's a shortage. And so, you know, you get shortchanged on your care as well.
DAY-TO-DAY IMPACT OF HEART FAILURE DIAGNOSIS
Patients: Emotional Toll
A diagnosis of heart failure puts a big emotional toll on both patients and caregivers. Patients reported being unaware that diagnostic testing for heart failure had been done until they received a diagnosis. This was partly because it was not paid out of pocket; the test was overlooked as simply one of many they underwent. As a result, many patients and families said they were shocked, scared, and traumatized when they heard the news. In cases where diagnoses took longer, participants were left feeling stress and anxiety for a longer period. Almost all patients and caregivers were stressed by the diagnosis of heart failure. One patient with pre-existing mental health conditions reported that the diagnosis worsened their depression and anxiety. They described how it took a big emotional toll on their emotional health.
Not really knowing what was wrong. And then suddenly to hear the words, heart failure, was a total shock. In fact, I also have depression. Long-term, chronic. I'd been on antidepressants for 20 odd years. and it [dropped] me into a more moody state … it did affect my Mental health.
We were so overwhelmed. I remember crying and the doctor was telling about my father's heart failure. I couldn't respond to him. I was scared. My father doesn't talk much about his feelings, but I could tell by his face he was really scared too.
Patients: Motivated to Improve Health
People who received a diagnosis of heart failure generally seemed to want to work towards getting better and sticking to their treatment plans. It took some patients a long time before they received a diagnosis, and they were living with symptoms for which they did not know the cause. Finally getting a diagnosis motivated patients to follow their treatment plans and improve their health, whether this was by taking new medications, exercising and eating better foods, going to support groups, or agreeing to go through a high-risk surgery.
My grandfather's a fighter and when you receive that news, you're already in a poor state of health and your body is physically failing you, you want to fight for life. It was a very traumatizing experience to witness firsthand.
I started going to community support groups and started finding out about all the ways to take care of myself. I started walking more and eating better. I told all my family too because its hereditary.
Caregivers: Changing Priorities
Caregivers were also heavily impacted by the heart failure diagnosis. In all cases, caregivers felt anxious, worried, and scared after hearing their family members' diagnoses. Family members and caregivers said they made sure they were present for the patient. They spoke of shifting their priorities away from everyday life to focus on the patient and the management of their heart failure.
It was a very stressful experience. … One of those things where a family emergency or tragedy happens and your priorities shift. When it's your family member, other things like work, voluntary commitments, and your social life become less important because you're dealing with a critical issue where you [may] lose someone that you love very much.
The impact was huge as it meant more hospital visits. Exacerbation of symptoms also meant the whole family had to come around and care for him. And his eventual demise is a loss that cannot be overcome.
Caregivers: Relieved to Know Cause of Symptoms
Some caregivers talked about how they felt relieved to receive a diagnosis. In some cases, patients had been facing worsening symptoms for a long time without getting a proper diagnosis. Caregivers explained how it was a relief to find out the cause of the issue and to know that they can finally address the symptoms. They were happy to know that they could now treat the condition or prevent it from progressing and harming their family member.
[We were] relieved that we caught something prior to an actual heart attack [that] we don't think he would have survived. We had to fight to escalate for more tests.
We were relieved when we knew the cause of my family members symptoms because the symptoms had disappeared at that time, but if they ever came back then I knew the hospital was just down the road, so I could take him there in case of an emergency
BARRIERS TO DIAGNOSIS
Wait Times Due to Long Hospital Visits
The time it took for patients to get diagnosed varied depending on the complexity and severity of the symptoms. Some patients reported faster results than others. Patients who were facing severe symptoms and were examined at the ED generally took 1 to 3 days to get diagnosed. However, they often experienced long wait times at the hospital, primarily due to what they felt was an overburdened ED staff. One patient who received a diagnosis of heart failure within 1 to 3 days said:
There were not many barriers except for long wait times during ED visits, and long appointments
We thought the doctors … would find me when they're [ready]. Whatever they're doing, [they'd] come in, [we'd] give them an update, [and then] the waiting game … 2 to 3 hours, you just go by with no update.
Wait Times Due to Difficulty Getting Appointment With Heart Specialist
In cases where it took patients a few weeks to receive a diagnosis, delays were attributed to the time it took to get an appointment with a heart specialist. These patients would initially go to see a community care physician about their symptoms, and they would receive a referral to a heart specialist, but it could take weeks to get an appointment. One patient described their experience:
Getting to see the heart specialist is difficult. Wait times are very long. Once in the hospital, however, our family doctor was able to communicate by phone with our specialist and they cooperated well.
There were not many barriers except for long waits during hospital visits and long appointments.
Lack of Communication With Clinicians
Some patients reported a lack of communication with busy clinicians as a barrier to diagnosis. Several caregivers mentioned not being able to speak with a clinician to explain the entirety of symptoms that the patient was facing. Caregivers reported wanting to speak to clinicians, but had difficulty getting a hold of them. Caregivers explained how the difficulty with communication contributed to delayed diagnoses and mismanagement of their cases.
We wanted to talk to the doctors directly [about my father's symptoms], but we couldn't reach them—they wouldn't come back until the next day. They [office staff] wouldn't tell us when he would be getting his tests.
It took a long time before staff in the ED listened to me, my mother's caregiver, regarding her health. They were more interested in listening to my mother, who was unable to speak. As her caregiver, I knew exactly what was happening from previous ED visits. Due to this, my mother almost needed intubation before [I got] a cardiologist to consult with us.
Misdiagnosis Due to Age
Some patients did not fit the typical criteria for a heart failure patient. In one case, upon examining their symptoms, doctors did not consider heart failure due to a patients young age. This initial misdiagnosis led to incorrect medications and course of treatment. It added multiple trips to the hospital, and the patient's heart condition and related symptoms worsened.
I went to the doctor and he said, “It's probably your childhood asthma, maybe the flu. Here's an inhaler and be on your way.” So, like anyone else 23 years old, I went on my way. A week later the symptoms got progressively worse. It got to a point where I couldn't walk up my stairs without stopping because I was getting short of breath. I couldn't lie down in my bed because I kept coughing and then got chest pain, and I realized I'm [due] for a second opinion. I went to the emergency department. And then, after a few hours, the physician came back in and he told me I have dilated cardiomyopathy. He said, “Your heart's failing.”
Misdiagnosis Due to Comorbidity
Some patients and caregivers spoke of having multiple underlying conditions that their symptoms were often attributed to. One caregiver talked about how their father took about 6 months to receive his diagnosis of heart failure because clinicians were focused on his existing lung condition. Patients said a misdiagnosis here could lead to multiple visits to the hospital, delays in accurate diagnosis, and worsening of symptoms of both the heart condition and the comorbidity of the patient.
Something was wrong with his lungs and it kept recurring, so they probed further and found out his lungs were collapsing because his heart wasn't functioning as it should have been. … Initially, the focus was just on the lungs. There was no mention of the heart at that time.
My grandfather suffered from many underlying health conditions throughout this lifetime. Some conditions may include, but are not limited to, anxiety and depression, insomnia, mood disorders, [and] a triple heartbeat condition, which weakened his physical abilities.
Misdiagnosis Due to Missed Symptoms
Patients talked about how some of their symptoms were missed entirely and how this delayed diagnosis and/or treatment, for years in some cases. In some cases, symptoms would be caught by a community care clinician and missed by a secondary care clinician. In other cases, symptoms were missed altogether, either because the patient was reluctant to report their symptoms, or because the doctor confused the symptoms for something else. One patient died before a diagnosis could be made.
Five years ago, the doctor checked my blood pressure and said he did not hear any heart murmur. Then 2 years later my OB-GYN picked up on a heart murmur and told me to tell my GP, who said I was fine. So two times with no further action. Then, a year later when I had the pneumonia, a third doctor wrote the letter to my doctor to … follow up on the heart. So it took me 5 years from my first symptoms to the time that something really got followed up on.
We found out my father had heart failure after he died … Twice my father woke up with his head spinning and could not get out of bed. The doctor told him to stay in bed until he felt better. The doctor who performed the autopsy told us that these 2 episodes were mini heart attacks.
PERCEPTIONS OF BNP OR NT-proBNP DIAGNOSTIC TESTS
Perceived Timeliness: Timely Treatment
Patients and caregivers emphasized how important it was to get a diagnosis sooner rather than later, especially in communities where hospitals are not equipped to handle patients who need an immediate course of treatment after diagnosis of heart failure. Patients and caregivers living in these communities described how, after diagnosis, they had to be transported to hospitals in different cities to get the appropriate care because their local hospitals were not properly equipped. Patients and caregivers felt that by having a test that could help speed up the overall diagnostic process, they could get faster access to treatment.
The farther you are from a tertiary hospital setting, the more frustrating and difficult it is and the more important it is to be diagnosed quickly so that you can … get in to see the specialists. Even for my husband, it took at least half a year to be referred to [a specialist]. Everything would move a lot faster and a lot safer with the tests like [the BNP or NT-pro BNP diagnostic test].
I certainly believe early diagnosis of heart failure will enable early treatment and increased outcome and survival.
Perceived Timeliness: Allows for Preventative Versus Reactive Response
Patients felt that receiving an earlier diagnosis would allow them to begin treating their symptoms earlier and prevent their condition from progressing, whereas later diagnosis meant reacting to worsened symptoms and conditions. We saw in multiple cases, including some mentioned earlier, that patients who were not immediately diagnosed with heart failure were unable to manage their symptoms and ended up in the emergency department when faced with severe symptoms. When asked whether a patient thought the BNP or NT-pro BNP tests would be useful, they said,
Of course, it would be useful. You can know if someone has heart issues and can deal with them.
I think a test such as this would help diagnose heart failure earlier and hopefully stop the need for an ED visit.
Perceived Costs: Saved Trips to Hospital
Participants believed that the time saved in receiving a more timely diagnosis of heart failure could reduce the number of trips to the hospital trying to figure out the cause of their symptoms. In many cases, symptoms progressed and worsened during the delay. Patients and caregivers reported feeling that an earlier diagnosis would not only prevent additional trips to the hospital, but also reduce the decline in patient health.
If there's something that can tell you right then and there [whether or not you have heart failure], you can skip that whole 2 days it takes to find out what's wrong. And wouldn't it make them check it more often? And delay clinicians from waiting till the second admittance to come in and check if it's a heart issue? It would be even better if we had that [test] available.
Perceived Costs: Associated Cost Savings
Participants noted that fewer trips to the hospital would also mean less money patients and caregivers need to spend on travel, on parking at the hospital, and, in some cases, accommodation.
[I've had to pay a] lot[for] parking … because of us going in and out of the hospital.
It certainly was very expensive and very stressful to be away from your support team and everything, you know. To have to take care of somebody who's very critically ill, out of a hotel room. Also to get them on the plane and home safely and when to take them home, all of that. Yeah, stressful for sure.
Perceived Medical Benefits: Reduced Misdiagnosis
Along with a faster diagnosis, patients and caregivers felt this test could help to reduce misdiagnosis and make the diagnosis process more efficient. With misdiagnosis, patients said they were given the wrong treatment plan and that they had to go through additional testing and procedures and were prescribed medication they did not need.
It would be amazing if the [BNP or NT-pro BNP diagnostic] tests could be used early in the process of understanding one's symptoms. Perhaps it would prevent unnecessary procedures and guesswork when prescribing medication.
[The test] will leave no doubt as to what the diagnosis is. Tiredness and shortness of breath due to heart failure will not be misunderstood for another condition.
Perceived Medical Benefits: Associated Cost Savings
Patients and caregivers believe that a correct diagnosis would also reduce their costs by avoiding purchasing medications they did not need to treat conditions they did not have.
The medication costs were not included [in my coverage]. I remember paying [something like] $100 … for one of those medications.
Absolutely, I think proactive testing at a certain age is essential and will result in cost saving in the long term.
Perceived Impact on Quality of Life: Reduced Stress and Anxiety
We saw how much stress and anxiety patients and families experienced while going through the diagnostic process for their heart failure. Patients and caregivers felt that reducing diagnosis time would reduce wait times and help them establish a treatment plan sooner, reducing the time to implement treatment and the emotional toll caused by the entire process. They felt this would improve overall quality of life of patients and caregivers.
Taking a blood test [and] then having to wait 3 hours for another test is very hard on everyone, patient and family.
Prolonging a diagnosis, especially when it has to do with the heart, puts patients and families in a very difficult situation, so I'm behind any technology that can help the medical community.
I've been in this limbo for 2 years and it affects the quality of life, because you're not sure if you can take that. You know, I used to like to walk to the park. It's about a mile, it's a perfect walk, and now, I'm afraid.
Discussion
Both patients and caregivers were enthusiastic about participating in this health technology assessment, all with the hopes that their experience would help improve future outcomes. Respondents participated through phone or online survey.
The majority of people who participated in the interview were not initially aware of the BNP or NT-pro BNP diagnostic tests. Most patients and caregivers were also unaware of what the diagnostic process for congestive heart failure often includes. Those who remembered generally stated that they received an echocardiogram, ECG, x-ray, or blood work. Throughout the interview process, participants would often change the focus of the conversation to the treatments after the testing rather than the diagnostic process. For most patients, diagnostic testing for heart failure was done behind the scenes, along with diagnostic testing for other conditions and so they were not fully aware that the testing was done.
The major factors that patients and caregivers could recall were the complexity and timeliness of the process and their emotional state at the time. This was understandable, given the high potential mortality risk of the condition being discussed, and it gives us a sense of what patients and caregivers found most important throughout the diagnostic process for heart failure.
Participants were very impressed when told that the BNP and NT-pro BNP diagnostic tests could rule out heart failure and make the diagnostic process more efficient. They felt that the tests would allow for a more timely, simple, and low-stress diagnostic process. They believed that it would reduce misdiagnosis and its associated outcomes, such as unnecessary medications, procedures, trips to hospitals, and any associated costs. Although interviewees did not mention having to pay for any diagnostic testing they received, they did express the view that by reducing the time to diagnosis and by reducing misdiagnoses, the BNP and NT-pro BNP tests would reduce or eliminate indirect costs.
The focus of the interviews and online survey questions was on the process of heart failure, with the intent of speaking with those who both were and were not diagnosed with heart failure. However, all the patients we spoke with had experienced heart failure. This may have created some bias given that people with different conditions who may have been misdiagnosed with heart failure were not interviewed. This experience is important because the BNP and NT-proBNP tests rule out heart failure, which means diagnoses of other health conditions with similar symptoms to heart failure may be quicker. However, having that cohort within our sample would have likely resulted in further support for these diagnostic tests, given the perceived benefits seen through interviews.
Conclusions
We received strong support from interview participants about the BNP or NT-proBNP diagnostic test. The main reason for support from participants was the potential time saved by receiving a speedier diagnosis. The overall process, from diagnosis to treatment, is a substantial emotional burden for patients and caregivers. It also impacts them financially and disrupts their quality of life. Over time, without a correct diagnosis, patients' symptoms and condition worsen. For those living further away from secondary or tertiary care settings, an earlier diagnosis is even more important, especially in an emergent situation, as it may get patients to a hospital better equipped to treat them and manage their potentially fatal symptoms and conditions.
Conclusions of the Health Technology Assessment
BNP and NT-proBNP have a high sensitivity and low negative likelihood ratio, suggesting that concentrations of both natriuretic peptides within the appropriate cut points can rule out the presence of heart failure with a high degree of confidence. Additionally, natriuretic peptide testing in an emergency department setting likely can reduce the length of hospital stay by at least 1 day. However, natriuretic peptide testing likely results in little to no difference in all-cause hospital mortality, admission rates, or 30-day readmission rates to hospital.
Our economic evidence review found a total of 12 studies evaluating the cost-effectiveness of natriuretic peptide testing in patients with suspected heart failure. The studies found that natriuretic peptide testing was either dominant (less costly and more effective) or cost-effective across different countries (including Canada) and settings. We anticipate that a de novo cost-effectiveness analysis for Ontario would produce similar results. Our budget impact analysis estimated that over the next 5 years, publicly funding BNP and NT-proBNP in Ontario would result in an additional cost of $38 million in the ED setting (at a cost of $75 per test) and a savings of $20 million in community care (at a cost of $28 per test) with about 130,000 people eligible for testing each year in Ontario.
We received strong support for the BNP or NT-proBNP diagnostic test from interview participants. The main benefit given was the potential time saved by receiving a speedier diagnosis. The overall process, from diagnosis to treatment, is a substantial emotional burden for patients and caregivers. For those living further away from secondary or tertiary care settings, an earlier diagnosis could allow patients to receive treatment at a hospital better equipped to manage their potentially fatal symptoms and conditions.
Acknowledgments
This report was developed by a multidisciplinary team at Ontario Health. The clinical epidemiologist was Immaculate Nevis, the medical librarians were Melissa Walter and Caroline Higgins, the primary health economists were Chunmei Li and Yuan Zhang, the secondary health economist was Shawn Xie, and the patient and public partnership analyst was Aroma Akhund.
The medical editor was Tim Maguire. Others involved in the development and production of this report were Paul Kolodziej, Claude Soulodre, Susan Harrison, Saleemeh Abdolzahraei, Elisabeth Smitko, Sarah McDowell, Vivian Ng, David Wells, Andrée Mitchell, Charles de Mestral, and Nancy Sikich.
We would like to thank the following people and organizations for lending their expertise to the development of this report:
Andrew Don-Wauchope, McMaster University
Robert S. McKelvie, Western University
Lisa M. Mielniczuk, University of Ottawa Heart Institute
CorHealth Ontario
We also thank our lived experience participants who generously gave their time to share their stories with us for this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those we consulted.
Abbreviations
- ANP
A-type natriuretic peptide
- AUC
Area under the curve
- BNP
B-type natriuretic peptide
- CI
Confidence interval
- CNP
C-type natriuretic peptide
- ED
Emergency department
- EDTA
Ethylenediaminetetraacetic acid
- GP
General practitioner
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- ICER
Incremental cost-effectiveness ratio
- IQR
Interquartile range
- NICE
National Institute for Health and Care Excellence
- NT-proBNP
N-terminal pro–B-type natriuretic peptide
- PICO
Population, intervention, comparator, outcomes
- POC
Point of care
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- QALY
Quality-adjusted life years
- RCT
Randomized controlled trial
- ROBIS
Risk of Bias in Systematic Reviews tool
Glossary
- Adverse event
An adverse event is an unexpected medical problem that happens during treatment for a health condition. Adverse events may be caused by something other than the treatment.
- Area under the curve
Area under the curve (AUC) is a graphic representation that illustrates the ability of a diagnostic test at different thresholds to differentiate between the normal and diseased individual. The graph plots the true positive rates against the false positive rates. The AUC is given as a value between 0 and 1, with 1 being a perfect diagnostic test and 0.5 representing a nondiscriminating test.
- Budget impact analysis
A budget impact analysis estimates the financial impact of adopting a new health care intervention on the current budget (i.e., the affordability of the new intervention). It is based on predictions of how changes in the intervention mix will impact the level of health care spending for a specific population. Budget impact analyses are typically conducted for a short-term period (e.g., 5 years). The budget impact, sometimes referred to as the net budget impact, is the estimated cost difference between the current scenario (i.e., the anticipated amount of spending for a specific population without using the new intervention) and the new scenario (i.e., the anticipated amount of spending for a specific population following the introduction of the new intervention).
- Cost-effective
A health care intervention is considered cost-effective when it provides additional benefits, compared with relevant alternatives, at an additional cost that is acceptable to a decision-maker based on the maximum willingness-to-pay value.
- Cost-effectiveness acceptability curve
In economic evaluations, a cost-effectiveness acceptability curve is a graphical representation of the results of a probabilistic sensitivity analysis. It illustrates the probability of health care interventions being cost-effective over a range of willingness-to-pay values. Willingness-to-pay values are plotted on the horizontal axis of the graph, and the probability of the intervention of interest and its comparator(s) being cost-effective at corresponding willingness-to-pay values is plotted on the vertical axis.
- Cost-effectiveness analysis
Used broadly, “cost-effectiveness analysis” may refer to an economic evaluation used to compare the benefits of two or more health care interventions with their costs. It may encompass several types of analysis (e.g., cost-effectiveness analysis, cost–utility analysis). Used more specifically, “cost-effectiveness analysis” may refer to a type of economic evaluation in which the main outcome measure is the incremental cost per natural unit of health (e.g., life-year, symptom-free day) gained.
- Cost–utility analysis
A cost–utility analysis is a type of economic evaluation used to compare the benefits of two or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years, which capture both the quality and quantity of life. In a cost–utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Cut point
A cut point is a specific value used to divide continuous variables into discrete categories to assist diagnosis or classification of test results. The optimal cut point or points will correctly sort individuals according to whether they have the condition being tested.
- Decision tree
A decision tree is a type of economic model used to assess the costs and benefits of two or more alternative health care interventions. Each intervention may be associated with different outcomes, which are represented by distinct branches in the tree. Each outcome may have a different probability of occurring and may lead to different costs and benefits.
- Deterministic sensitivity analysis
Deterministic sensitivity analysis is an approach used to explore uncertainty in the results of an economic evaluation by varying parameter values to observe the potential impact on the cost-effectiveness of the health care intervention of interest. One-way sensitivity analysis accounts for uncertainty in parameter values one at a time, whereas multiway sensitivity analysis accounts for uncertainty in a combination of parameter values simultaneously.
- Diagnostic odds ratio
The diagnostic odds ratio is a measure of the effectiveness of a diagnostic test. It is defined as the ratio of the odds of the test being positive if the person has a disease relative to the odds of the test being positive if the person does not have the disease.
- Discounting
Discounting is a method used in economic evaluations to adjust for the differential timing of the costs incurred and the benefits generated by a health care intervention over time. Discounting reflects the concept of positive time preference, whereby future costs and benefits are reduced to reflect their present value. The health technology assessments conducted by Ontario Health use an annual discount rate of 1.5% for both future costs and future benefits.
- Dominant
A health care intervention is considered dominant when it is more effective and less costly than its comparator(s).
- Incremental cost
The incremental cost is the additional cost, typically per person, of a health care intervention versus a comparator.
- Incremental cost-effectiveness ratio (ICER)
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a health care consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental cost-effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- Log diagnostic odds ratio
The diagnostic odds ratio is a measure of the effectiveness of a diagnostic test. It is defined as the ratio of the odds of the test being positive if the person has a disease relative to the odds of the test being positive if the person does not have the disease. The log diagnostic ratio is the log of the diagnostic odds ratio. It is used when the data is not normally distributed.
- Markov model
A Markov model is a type of decision-analytic model used in economic evaluations to estimate the costs and health outcomes (e.g., quality-adjusted life-years gained) associated with using a particular health care intervention. Markov models are useful for clinical problems that involve events of interest that may recur over time (e.g., stroke). A Markov model consists of mutually exclusive, exhaustive health states. Patients remain in a given health state for a certain period of time before moving to another health state based on transition probabilities. The health states and events modelled may be associated with specific costs and health outcomes.
- Negative likelihood ratio
The negative likelihood ratio measures how likely a negative test result is accurate. The ratio represents the probability that a person who has the disease tested negative for the disease (false negative) divided by the probability that a person who does not have the disease tested negative for the disease (true negative).
- Negative predictive value
The negative predictive value is the probability that a person with a negative screening test does not have the disease.
- Positive likelihood ratios
The positive likelihood ratio measures how likely a positive test result is accurate. The ratio represents the probability of a person who has the disease testing positive divided by the probability of a person who does not have the disease testing positive.
- Positive predictive value
The positive predictive value is the probability that subjects with a positive screening test have the disease.
- Scenario analysis
A scenario analysis is used to explore uncertainty in the results of an economic evaluation. It is done by observing the potential impact of different scenarios on the cost-effectiveness of a health care intervention. Scenario analyses include varying structural assumptions from the reference case.
- Sensitivity
Sensitivity measures a test's ability to correctly generate a positive result for people who have the condition that is being tested for (also known as the “true positive” rate).
- Sensitivity analysis
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Societal perspective
The perspective adopted in an economic evaluation determines the types of costs and health benefits to include. The societal perspective reflects the broader economy and is the aggregation of all perspectives (e.g., health care payer and patient perspectives). It considers the full effect of a health condition on society, including all costs (regardless of who pays) and all benefits (regardless of who benefits).
- Specificity
Specificity measures a test's ability to correctly generate a negative result for people who don't have the condition that is being tested for (also known as the “true negative” rate).
- Time horizon
In economic evaluations, the time horizon is the time frame over which costs and benefits are examined and calculated. The relevant time horizon is chosen based on the nature of the disease and health care intervention being assessed, as well as the purpose of the analysis. For instance, a lifetime horizon would be chosen to capture the long-term health and cost consequences over a patient's lifetime.
- Utility
A utility is a value that represents a person's preference for various health states. Typically, utility values are anchored at 0 (death) and 1 (perfect health). In some scoring systems, a negative utility value indicates a state of health valued as being worse than death. Utility values can be aggregated over time to derive quality-adjusted life-years, a common outcome measure in economic evaluations.
- Willingness-to-pay value
A willingness-to-pay value is the monetary value a health care consumer is willing to pay for added health benefits. When conducting a cost–utility analysis, the willingness-to-pay value represents the cost a consumer is willing to pay for an additional quality-adjusted life-year. If the incremental cost-effectiveness ratio is less than the willingness-to-pay value, the health care intervention of interest is considered cost-effective. If the incremental cost-effectiveness ratio is more than the willingness-to-pay value, the intervention is considered not to be cost-effective.
Appendices
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Search date: July 22, 2019
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Database of Systematic Reviews <2005 to July 18, 2019>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2019 Week 29>, Ovid MEDLINE(R) ALL <1946 to July 19, 2019>
Search Strategy:
————————————————————————–
-
1
exp Heart Failure/ (570841)
-
2
((cardia* or heart or myocardial or coronary) adj2 (decompensat* or failure or incompetence or insufficien*)).ti,ab,kf. (454707)
-
3
(CHF or HF).ti,ab,kf. (147778)
-
4
cardiomyopathy, dilated/ (31132)
-
5
((dilated or congestive) adj2 cardiomyopath*).ti,ab,kf. (44778)
-
6
shock, cardiogenic/ (19104)
-
7
cardiogenic shock.ti,ab,kf. (28069)
-
8
cardiac output, low/ (9006)
-
9
(((low or impaired) adj2 cardiac output) or forward failure).ti,ab,kf. (8514)
-
10
Ventricular Dysfunction, Left/ (66223)
-
11
(left ventric* adj2 (failure or insufficien* or dysfunction*)).ti,ab,kf. (52515)
-
12
LVSD.ti,ab,kf. (1708)
-
13
or/1–12 (866007)
-
14
Natriuretic Peptides/ (7532)
-
15
Natriuretic Peptide, Brain/ (41002)
-
16
natriuretic peptide*.ti. (30058)
-
17
((b type or btype or brain or probrain or type b or pro b or prob or protype b or pro type b) adj3 (natriuretic peptide* or natriuretic pro peptide*)).ti,ab,kf. (40884)
-
18
(bnp or probnp or ntprobnp or b natriuretic peptide*).ti,ab,kf. (49190)
-
19
or/14–18 (88167)
-
20
13 and 19 (45778)
-
21
(Systematic Reviews or Meta Analysis).pt. (102936)
-
22
Systematic Review/ or Systematic Reviews as Topic/ or Meta-Analysis/ or exp Meta-Analysis as Topic/ or exp Technology Assessment, Biomedical/ (541302)
Annotation: add “Systematic Reviews as Topic” once it appears in MeSH tree
-
23
((systematic* or methodologic*) adj3 (review* or overview*)).ti,ab,kf. (369812)
-
24
(meta analy* or metaanaly* or met analy* or metanaly* or meta review* or metareview* or health technolog* assess* or HTA or HTAs or (technolog* adj (assessment* or overview* or appraisal*))).ti,ab,kf. (375853)
-
25
(evidence adj (review* or overview* or synthes#s)).ti,ab,kf. (14181)
-
26
(review of reviews or overview of reviews).ti,ab,kf. (1303)
-
27
umbrella review*.ti,ab,kf. (539)
-
28
GRADE Approach/ (185)
-
29
((pool* adj3 analy*) or published studies or published literature or hand search* or handsearch* or manual search* or ((database* or systematic*) adj2 search*) or reference list* or bibliograph* or relevant journals or data synthes* or data extraction* or data abstraction*).ti,ab,kf. (405318)
-
30
(medline or pubmed or medlars or embase or cinahl or web of science or ovid or ebsco* or scopus).ab. (419333)
-
31
cochrane.ti,ab,kf. (176817)
-
32
(meta regress* or metaregress*).ti,ab,kf. (17201)
-
33
(((integrative or collaborative or quantitative) adj3 (review* or overview* or synthes*)) or (research adj3 overview*)).ti,ab,kf. (23523)
-
34
(cochrane or (health adj2 technology assessment) or evidence report or systematic review*).jw. (61194)
-
35
((comparative adj3 (efficacy or effectiveness)) or relative effectiveness or ((indirect or indirect treatment or mixed-treatment) adj comparison*)).ti,ab,kf. (40983)
-
36
or/21–35 (1115110)
-
37
20 and 36 (1215)
-
38
exp Animals/ not Humans/ (17258003)
-
39
37 not 38 (630)
-
40
Case Reports/ or Comment.pt. or Editorial.pt. or (Letter not (Letter and Randomized Controlled Trial)).pt. or Congress.pt. (5287622)
-
41
39 not 40 (611)
-
42
limit 41 to english language [Limit not valid in CDSR; records were retained] (569)
-
43
42 use medall,cleed (308)
-
44
limit 20 to english language [Limit not valid in CDSR; records were retained] (43181)
-
45
44 use coch,clhta (20)
-
46
43 or 45 (328)
-
47
heart failure/ (330541)
-
48
exp congestive heart failure/ (204727)
-
49
((cardia* or heart or myocardial or coronary) adj2 (decompensat* or failure or incompetence or insufficien*)).tw,kw. (459642)
-
50
(CHF or HF).tw,kw. (148499)
-
51
congestive cardiomyopathy/ (44940)
-
52
((dilated or congestive) adj2 cardiomyopath*).tw,kw. (45208)
-
53
cardiogenic shock/ (30925)
-
54
cardiogenic shock.tw,kw. (28547)
-
55
forward heart failure/ (5317)
-
56
(((low or impaired) adj2 cardiac output) or forward failure).tw,kw. (8627)
-
57
exp heart left ventricle failure/ (30658)
-
58
(left ventric* adj2 (failure or insufficien* or dysfunction*)).tw,kw. (53099)
-
59
LVSD.tw,kw. (1721)
-
60
or/47–59 (736938)
-
61
natriuretic factor/ (6045)
-
62
exp *brain natriuretic peptide derivative/ (12028)
-
63
exp brain natriuretic peptide derivative/ (41129)
-
64
(biomarker* or bio marker*).ti. (155271)
-
65
63 and 64 (2821)
-
66
natriuretic peptide*.ti. (30058)
-
67
((b type or btype or brain or probrain or type b or pro b or prob or protype b or pro type b) adj3 (natriuretic peptide* or natriuretic pro peptide*)).tw,kw,dv. (41185)
-
68
(bnp or probnp or ntprobnp or b natriuretic peptide*).tw,kw,dv. (49590)
-
69
or/61–62,65–68 (79339)
-
70
60 and 69 (38494)
-
71
Systematic review/ or “systematic review (topic)”/ or exp Meta Analysis/ or “Meta Analysis (Topic)”/ or Biomedical Technology Assessment/ (534812)
Annotation: Added Systematic review/ or “systematic review (topic)”/ for thoroughness, but these may add many results. Will monitor
-
72
(meta analy* or metaanaly* or health technolog* assess* or systematic review*).hw. (529382)
-
73
((systematic* or methodologic*) adj3 (review* or overview*)).tw,kw. (381331)
-
74
(meta analy* or metaanaly* or met analy* or metanaly* or meta review* or metareview* or health technolog* assess* or HTA or HTAs or (technolog* adj (assessment* or overview* or appraisal*))).tw,kw. (402762)
-
75
(evidence adj (review* or overview* or synthes#s)).tw,kw. (14567)
-
76
(review of reviews or overview of reviews).tw,kw. (1491)
-
77
umbrella review*.tw,kw. (578)
-
78
((pool* adj3 analy*) or published studies or published literature or hand search* or handsearch* or manual search* or ((database* or systematic*) adj2 search*) or reference list* or bibliograph* or relevant journals or data synthes* or data extraction* or data abstraction*).tw,kw. (430566)
-
79
(medline or pubmed or medlars or embase or cinahl or web of science or ovid or ebsco* or scopus).ab. (419333)
-
80
cochrane.tw,kw. (180407)
-
81
(meta regress* or metaregress*).tw,kw. (18108)
-
82
(((integrative or collaborative or quantitative) adj3 (review* or overview* or synthes*)) or (research adj3 overview*)).tw,kw. (24403)
-
83
(cochrane or (health adj2 technology assessment) or evidence report or systematic review*).jw. (61194)
-
84
((comparative adj3 (efficacy or effectiveness)) or relative effectiveness or ((indirect or indirect treatment or mixed-treatment) adj comparison*)).tw,kw. (42630)
-
85
or/71–84 (1142125)
-
86
70 and 85 (1065)
-
87
(exp animal/ or nonhuman/) not exp human/ (10362894)
-
88
86 not 87 (1060)
-
89
Case Report/ or Comment/ or Editorial/ or (letter.pt. not (letter.pt. and randomized controlled trial/)) or conference abstract.pt. (10589689)
-
90
88 not 89 (850)
-
91
limit 90 to english language [Limit not valid in CDSR; records were retained] (788)
-
92
91 use emez (449)
-
93
46 or 92 (777)
-
94
93 use medall (307)
-
95
93 use coch (4)
-
96
93 use clhta (16)
-
97
93 use cleed (1)
-
98
93 use emez (449)
-
99
remove duplicates from 93 (497)
Economic Evidence Search
Search date: July 25, 2019
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <June 2019>, EBM Reviews – Cochrane Database of Systematic Reviews <2005 to July 24, 2019>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2019 Week 29>, Ovid MEDLINE(R) ALL <1946 to July 23, 2019>
Search Strategy:
————————————————————————–
-
1
exp Heart Failure/ (579139)
-
2
((cardia* or heart or myocardial or coronary) adj2 (decompensat* or failure or incompetence or insufficien*)).ti,ab,kf. (479992)
-
3
(CHF or HF).ti,ab,kf. (157864)
-
4
cardiomyopathy, dilated/ (31638)
-
5
((dilated or congestive) adj2 cardiomyopath*).ti,ab,kf. (45779)
-
6
shock, cardiogenic/ (19330)
-
7
cardiogenic shock.ti,ab,kf. (29034)
-
8
cardiac output, low/ (9362)
-
9
(((low or impaired) adj2 cardiac output) or forward failure).ti,ab,kf. (8862)
-
10
Ventricular Dysfunction, Left/ (68143)
-
11
(left ventric* adj2 (failure or insufficien* or dysfunction*)).ti,ab,kf. (55586)
-
12
LVSD.ti,ab,kf. (1788)
-
13
or/1–12 (897845)
-
14
Natriuretic Peptides/ (7567)
-
15
Natriuretic Peptide, Brain/ (42224)
-
16
natriuretic peptide*.ti. (31226)
-
17
((b type or btype or brain or probrain or type b or pro b or prob or protype b or pro type b) adj3 (natriuretic peptide* or natriuretic pro peptide*)).ti,ab,kf. (43662)
-
18
(bnp or probnp or ntprobnp or b natriuretic peptide*).ti,ab,kf. (53063)
-
19
or/14–18 (93673)
-
20
13 and 19 (48900)
-
21
economics/ (252855)
-
22
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (829651)
-
23
economics.fs. (421671)
-
24
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).ti,ab,kf. (885794)
-
25
exp “costs and cost analysis”/ (578844)
-
26
(cost or costs or costing or costly).ti. (263817)
-
27
cost effective*.ti,ab,kf. (325134)
-
28
(cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab,kf. (213623)
-
29
models, economic/ (12731)
-
30
markov chains/ or monte carlo method/ (80499)
-
31
(decision adj1 (tree* or analy* or model*)).ti,ab,kf. (42283)
-
32
(markov or markow or monte carlo).ti,ab,kf. (128750)
-
33
quality-adjusted life years/ (39775)
-
34
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (73438)
-
35
((adjusted adj1 (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).ti,ab,kf. (119676)
-
36
or/21–35 (2542649)
-
37
20 and 36 (1532)
-
38
Case Reports/ or Comment.pt. or Editorial.pt. or (Letter not (Letter and Randomized Controlled Trial)).pt. or Congress.pt. (5293899)
-
39
37 not 38 (1424)
-
40
exp Animals/ not Humans/ (17259105)
-
41
39 not 40 (900)
-
42
limit 41 to english language [Limit not valid in CDSR; records were retained] (825)
-
43
42 use medall,coch,cctr,clhta (353)
-
44
limit 20 to english language [Limit not valid in CDSR; records were retained] (45440)
-
45
44 use cleed (20)
-
46
43 or 45 (373)
-
47
heart failure/ (338609)
-
48
exp congestive heart failure/ (213025)
-
49
((cardia* or heart or myocardial or coronary) adj2 (decompensat* or failure or incompetence or insufficien*)).tw,kw. (486702)
-
50
(CHF or HF).tw,kw. (158587)
-
51
congestive cardiomyopathy/ (45446)
-
52
((dilated or congestive) adj2 cardiomyopath*).tw,kw. (46317)
-
53
cardiogenic shock/ (31151)
-
54
cardiogenic shock.tw,kw. (29671)
-
55
forward heart failure/ (5317)
-
56
(((low or impaired) adj2 cardiac output) or forward failure).tw,kw. (8975)
-
57
exp heart left ventricle failure/ (30658)
-
58
(left ventric* adj2 (failure or insufficien* or dysfunction*)).tw,kw. (56847)
-
59
LVSD.tw,kw. (1801)
-
60
or/47–59 (769904)
-
61
natriuretic factor/ (6045)
-
62
exp *brain natriuretic peptide derivative/ (12028)
-
63
exp brain natriuretic peptide derivative/ (41129)
-
64
(biomarker* or bio marker*).ti. (161082)
-
65
63 and 64 (2821)
-
66
natriuretic peptide*.ti. (31226)
-
67
((b type or btype or brain or probrain or type b or pro b or prob or protype b or pro type b) adj3 (natriuretic peptide* or natriuretic pro peptide*)).tw,kw,dv. (44081)
-
68
(bnp or probnp or ntprobnp or b natriuretic peptide*).tw,kw,dv. (53463)
-
69
or/61–62,65–68 (84697)
-
70
60 and 69 (41517)
-
71
Economics/ (252855)
-
72
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (128672)
-
73
Economic Aspect/ or exp Economic Evaluation/ (453843)
-
74
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw,kw. (911523)
-
75
exp “Cost”/ (578844)
-
76
(cost or costs or costing or costly).ti. (263817)
-
77
cost effective*.tw,kw. (337455)
-
78
(cost* adj2 (util* or efficac* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab,kw. (224684)
-
79
Monte Carlo Method/ (64083)
-
80
(decision adj1 (tree* or analy* or model*)).tw,kw. (46091)
-
81
(markov or markow or monte carlo).tw,kw. (133813)
-
82
Quality-Adjusted Life Years/ (39775)
-
83
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (77275)
-
84
((adjusted adj1 (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw,kw. (140385)
-
85
or/71–84 (2179398)
-
86
70 and 85 (1197)
-
87
Case Report/ or Comment/ or Editorial/ or (letter.pt. not (letter.pt. and randomized controlled trial/)) or conference abstract.pt. (10615727)
-
88
86 not 87 (885)
-
89
(exp animal/ or nonhuman/) not exp human/ (10364002)
-
90
88 not 89 (881)
-
91
limit 90 to english language [Limit not valid in CDSR; records were retained] (797)
-
92
91 use emez (456)
-
93
46 or 92 (829)
-
94
93 use medall (286)
-
95
93 use coch (1)
-
96
93 use cctr (62)
-
97
93 use clhta (4)
-
98
93 use cleed (20)
-
99
93 use emez (456)
-
100
remove duplicates from 93 (544)
Grey Literature Search
Performed on: July 2–19, 2019
Clinicaltrials.gov searched on September 09, 2019
Search Updated January 10–14, 2020
Websites searched:
HTA Database Canadian Repository, Alberta Health Evidence Reviews, BC Health Technology Assessments, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, Centre Hospitalier de l'Universite de Quebec-Universite Laval, Health Technology Assessment Database, Epistemonikos, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Council of Australian Governments Health Technologies, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, Health Technology Wales, Oregon Health Authority Health Evidence Review Commission, Veterans Affairs Health Services Research and Development, Italian National Agency for Regional Health Services (AGENAS), Australian Safety and Efficacy Register of New Interventional Procedures-Surgical (ASERNIP-S), Belgian Health Care Knowledge Centre, Ludwig Boltzmann Institute for Health Technology Assessment, Ministry of Health Malaysia Health Technology Assessment Section, Swedish Agency for Health Technology Assessment and Assessment of Social Services, ClinicalTrials.gov, PROSPERO, EUnetHTA, Tuft's Cost-Effectiveness Analysis Registry
Keywords used: BNP, proBNP, ntproBNP, natriuretic
Results from clinical search: (included in PRISMA): 10
Results from economic search: (included in PRISMA): 8
Ongoing systematic reviews (PROSPERO/EUnetHTA): 2
Ongoing clinical trials (ClinicalTrials.gov): 6
Appendix 2: Critical Appraisal of Clinical Evidence
Table A1:
Risk of Biasa Among Systematic Reviews (ROBIS Tool)
| Phase 2 | Phase 3 | ||||
|---|---|---|---|---|---|
| Author, Year | Study Eligibility Criteria | Identification and Selection of Studies | Data Collection and Study Appraisal | Synthesis and Findings | Risk of Bias in the Review |
| NICE, 201820 | Low | Low | Low | Low | Low |
| Taylor et al, 201829 | Low | Low | Low | Low | Low |
| Schols et al, 201838 | Low | Low | Low | Low | Low |
| Martindale et al, 201628 | Low | Low | Low | Low | Low |
| Roberts, 2015100 | Low | High | Low | Low | Low |
| Booth et al, 201425 | Low | Low | Low | Low | Low |
| Hill et al, 201426 | Low | Low | Low | Low | Low |
| NICE, 201419 | Low | High | High | Low | Low |
| Balion et al, 201330 | Low | Low | Low | Low | Low |
| Merlin et al, 200837 | Low | High | High | Low | Low |
| Worster et al, 200839 | Low | High | Low | Low | Low |
| Clerico et al, 200734 | Low | High | High | Unclear | High |
| Korenstein et al, 200736 | Low | Low | Low | Low | Low |
| Balion et al, 200631 | Low | High | High | Low | Low |
| Latour-Perez et al, 200627 | Low | High | Low | Low | Low |
| Battaglia et al, 200632 | Low | Low | Low | Low | Low |
| Doust et al, 200435 | Low | Low | Low | Low | Low |
| Cardarelli & Lumicao, 200333 | Low | High | High | Unclear | High |
Abbreviation: ROBIS, Risk of Bias in Systematic Reviews.
Possible risk of bias levels: low, high, unclear.
Table A2:
Risk of Biasa Among Diagnostic Accuracy Studies (QUADAS-2 Tool) as Reported Within Systematic Reviews
| Risk of Bias | Applicability Concerns | ||||||
|---|---|---|---|---|---|---|---|
| Author, Year | Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard |
| NICE, 201820 | Low/unclear | Low | Low | Low | Low | Low | Low |
| Taylor et al, 201829 | Low | Unclear | Low | Low | Highb | Unclearc | Low |
| Martindale et al, 201628 | Highd | Low | Low | Low | Highd | Low | Low |
| Booth et al, 201425 | Unclear | Low | Low | Low | Unclear | Low | Low |
| Hill et al, 201426 | High | High | Low/unclear | Low/Unclear | Low/unclear | Low/unclear | Low/unclear |
| NICE, 201419 | Unclear | Low | Low/unclear | Low/unclear | Unclear | Low | Low |
| Latour-Perez et al, 200627 | NR | NR | NR | NR | NR | NR | NR |
Abbreviations: QUADAS, Quality Assessment of Diagnostic Accuracy Studies, NR, not reported.
Possible risk of bias levels: low, high, unclear.
Population was not representative of ambulatory setting.
Not using prespecified thresholds.
Inflates specificity since people with renal comorbidities were excluded.
Table A3:
Strength of Evidence Profile for Outcomes of Sensitivity and Specificity as Reported Within the Two Systematic Reviews
| Test | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| BNPa | |||||||
| Sensitivity | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | High |
| Specificity | No serious limitations | Some serious limitations | No serious limitations | Some serious limitations | Undetected | None | Moderate |
| NT-proBNPb | |||||||
| Sensitivity | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | High |
| Specificity | No serious limitations | Some serious limitations | No serious limitations | Some serious limitations | Undetected | None | Moderate |
Table A4:
GRADE for Clinical Outcomes
| Test | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Natriuretic Peptides | |||||||
| 1 SR of 5 RCTs | Serious limitation | No serious limitations | No serious limitations | No serious limitations | Undetected | None | Moderate |
Abbreviations: RCT, randomized controlled trial; SR, systematic review.
Note: statistical heterogeneity was not reported, although some outcomes were stated to be heterogeneous (e.g., length of hospital stay). Studies were from different health care systems: Australia, Canada, Switzerland, United States, and Netherlands (first point of contact for the patient was different between studies; e.g., senior vs. junior physicians). All studies reported adequate sequence generation and allocation concealment for randomization except for one study. Four of the five RCTs reported no blinding of physicians. Two of the five RCTs reported blinding of participants, and three of the five RCTs reported blinding outcome assessors.
Appendix 3: Excluded Reviews
Table A5:
Selected Excluded Reviews—Clinical Evidence
| Citation | Primary Reason for Exclusion |
|---|---|
| Roberts E, Ludman AJ, Dworzynski K, Al-Mohammad A, Cowie MR, McMurray JJV, et al. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ. 2015;350:h910. | Studies overlapped |
| Schols AMR, Stakenborg JPG, Dinant GJ, Willemsen RTA, Cals JWL. Point-of-care testing in primary care patients with acute cardiopulmonary symptoms: a systematic review. Fam Pract. 2018;35(1):4–12. | High risk of bias |
| Clerico A, Fontana M, Zyw L, Passino C, Emdin M. Comparison of the diagnostic accuracy of brain natriuretic peptide (BNP) and the N-terminal part of the propeptide of BNP immunoassays in chronic and acute heart failure: a systematic review. Clin Chem. 2007;53(5):813–22. | High Risk of Bias |
| Balion C, Santaguida P, Hill S, Worster A, McQueen M, Oremus M, et al. Testing for BNP and NT-proBNP in the diagnosis and prognosis of heart failure. Evid Rep Technol Assess (Full Rep). 2006;142:1–147. | Updated information was available in 2013 |
| Cardarelli R, Lumicao TG. B-type natriuretic peptide: a review of its diagnostic, prognostic, and therapeutic monitoring value in heart failure for primary care. J Am Board Fam Pract: 2003. | High risk of bias |
| Merlin T, Moss J, Brooks A, Newton S, Hedayati H, Hiller J. B-type natriuretic peptide assays in the diagnosis of heart failure. Part A: in the hospital emergency setting. Part B: in the non-hospital setting (structured abstract). Health Technol Assess Database. 2008(4). | Studies overlap |
| Doust JA, Glasziou PP, Pietrzak E, Dobson AJ. A systematic review of the diagnostic accuracy of natriuretic peptides for heart failure. Arch Intern Med. 2004;164(18):1978–84. | Studies overlap |
| Battaglia M, Pewsner D, Juni P, Egger M, Bucher HC, Bachmann LM. Accuracy of B-type natriuretic peptide tests to exclude congestive heart failure. Arch Intern Med. 2006;166:1073–1080. | Studies overlap |
| Korenstein D, Wisnivesky JP, Wyer P, Adler R, Ponieman D, McGinn T. The utility of B-type natriuretic peptide in the diagnosis of heart failure in the emergency department: a systematic review. BMC Emerg Med. 2007;7:6. | Studies overlap |
| Worster A, Balion CM, Hill SA, Santaguida P, Ismaila A, McKelvie R, et al. Diagnostic accuracy of BNP and NT-proBNP in patients presenting to acute care settings with dyspnea: a systematic review. Clin Biochem. 2008;41:250–59. | Studies overlap |
Appendix 4: Economic Evidence Review
Table A6:
Summary of Included Economic Studies
| Author, Year, Country | Analytic Technique, Study Design, Perspective, Time Horizon | Population | Interventions and Comparators | Results | ||
|---|---|---|---|---|---|---|
| Health Outcomes | Costs | ICER | ||||
| ED Setting | ||||||
| BNP and NT-proBNP | ||||||
| Griffin et al, 201754 United Kingdom (NICE guideline 201419) |
• Cost–utility analysis • Decision tree and Markov model • Health care payer perspective (NHS) • Time horizon: 4 y |
• Patients with suspected acute HF • 47% true for acute HF • Age (mean): 75 y for males, 80 y for females • Male: 56% |
Interventions: • SCI with NP • Specialist management with NP Comparators: • SCI alone • Specialist management alone Reference case: BNP (< 100 ng/L) Scenario analysis: NT-proBNP (< 300 ng/L) |
Life years: Reference case • SCI alone: 3.154 • SCI with BNP: 3.159 • Specialist alone: 3.178 • Specialist with BNP: 3.188 QALYs: Reference case • SCI alone: 2.212 • SCI with BNP: 2.216 • Specialist alone: 2.229 • Specialist with BNP: 2.236 |
Total cost per patient (2013 £ GBP): Reference case • SCI alone: 2,654 • SCI with BNP: 2,698 • Specialist alone: 2,703 • Specialist with BNP: 2,759 |
Primary ICER (2013 £ GBP): Reference case • SCI with BNP vs. SCI alone: 11,656/QALY (95% CI: 4,641–23,774) • Specialist alone vs. SCI alone: 2,883/QALY (95% CI: 2,103–4,324) • Specialist with BNP vs. SCI alone: 4,350/QALY (95% CI: 2,976–6,788) • Specialist with BNP vs. specialist alone: 7,914/QALY (95% CI 4,007–14,554) Scenario analysis • SCI with NT-proBNP vs. SCI alone: 19,778/QALY • Specialist with NT-proBNP vs. SCI alone: 6,210/QALY Analysis of uncertainty: DSA and PSA conducted. Results remained robust |
| NT-proBNP | ||||||
| Rutten et al, 200855 Netherlands |
• Cost-effectiveness analysis • RCT • Public payer perspective: • Time horizon: 30 d • Discounting: NA |
• Patients presenting to ED with acute dyspnea (n = 477) • Age (mean): 59 y • Male: 54% |
Intervention: • SCI supplemented and guided by NT-proBNP (< 93 pg/mL for males and < 144 pg/mL for females to rule out; > 1,017 pg/mL to rule in) Comparator: • SCI alone |
30-d mortality, median: • SCI with NT-proBNP: 6% • SCI alone: 8% In-hospital mortality, median: • SCI with NT-proBNP: 6% • SCI alone: 6% Initial hospitalization rate, median: • SCI with NT-proBNP: 62% • SCI alone: 67% 30-d readmission rate, median: • SCI with NT-proBNP: 3% • SCI alone: 5% Admission to intensive care, median: • SCI with NT-proBNP: 16% • SCI alone: 16% ED admission, median: • SCI with NT-proBNP: 170 min • SCI alone: 172 min Time to discharge, median: • SCI with NT-proBNP: 1.9 d • SCI alone: 3.9 d P = 0.04 Duration of hospitalization, median: • SCI with NT-proBNP group: 7.8 d • SCI alone: 8.1 d |
Total cost per patient, mean (Costing year and currency not specified): • SCI with NT-proBNP: $4,984 • SCI alone: $6,352 Difference: −$1,364 (95% CI: −246 to 3,215) Cost components incorporated: Emergency and admitted patient care, including cardio-pulmonary investigations, outpatient care, and NT-proBNP test |
Primary ICER: NT-proBNP testing reduces the time to discharge (P = 0.04) and is associated with a trend toward cost reduction Secondary ICER: PSA using bootstrap sampling based on 30-d all-cause mortality showed that the point-estimate was most likely to lie in the less costly/lower mortality quadrant (probability not reported) Analysis of uncertainty: A post-hoc subgroup analysis indicated that the effect on costs is largest in patients with cardiac dyspnea compared to patients without cardiac dyspnea |
| Moe et al, 200756 Canada |
• Cost-effectiveness analysis • RCT (IMPROVE-CHF) • Public payer perspective: • Time horizon: 60 d • Discounting: NA |
• People presenting to ED with acute dyspnea (n = 500) • Age (mean): 70.5 y • Male: 52% |
Intervention: • SCI supplemented and guided by NT-proBNP (initially the cut-off values came from Roche Diagnostics, later from the PRIDE study: < 300 pg/mL to rule out; > 450 pg/mL for under 50 y, > 900 pg/mL for over 50 y to rule in) (n = 246) Comparator: • SCI alone (n = 254) |
60-d all-cause mortality: • SCI with NT-proBNP: 5.5% • SCI alone: 4.4% In-hospital mortality: • SCI with NT-proBNP: 4.5% • SCI alone: 2.4% Initial hospitalization from ED: • SCI with NT-proBNP: 57% • SCI alone: 58% Patients re-hospitalized by 60 d: • SCI with NT-proBNP: 13% • SCI alone: 20% P = 0.046 Duration of ED visit, median (IQR): • SCI with NT-proBNP: 5.6 (4.0–8.0) • SCI alone: 6.3 (4.3–8.6) P = 0.031 Duration of ICU stay, median (IQR): • SCI with NT-proBNP: 6 (1–11) • SCI alone: 5.5 (3–11) Hospital length of stay, median (IQR): • SCI with NT-proBNP: 6 (4–11) • SCI alone: 7 (4–13) |
Total cost per patient, median (IQR) (2005 $ USD): • SCI with NT-proBNP: 5,180 (3,005–8,416) • SCI alone: 6,129 (3,384–9991) Difference: −$949 P = 0.023 Cost components incorporated: Emergency and admitted patient care, including cardio-pulmonary investigations, outpatient care, and NT-proBNP test |
Primary ICER: NT-proBNP testing reduced the duration of ED visit by 21% (P = 0.031), the number of patients rehospitalized over 60 d by 35% (P = 0.046), and direct medical costs (P = 0.023) Analysis of uncertainty: None |
| Sibert et al, 200657 United States |
• Cost-effectiveness analysis • Decision tree model based on a prospective blinded single-armed study (PRIDE) • Hospital payer perspective • Time horizon: 60 d • Discounting: NA |
• People presenting to ED with acute dyspnea (n = 599) • Age (mean): 62 y • Male: 51% |
Intervention: • SCI supplemented and guided by NT-proBNP result (> 900 pg/mL to rule in; < 900 pg/mL to rule out) Comparator: • SCI alone |
Risk for SAE: • SCI with NT-proBNP: 0.254 • SCI alone: 0.258 Probability of correct HF diagnosis: • SCI with NT-proBNP: 0.957 • SCI alone: 0.967 Proportion of true+ CHF cases: • SCI with NT-proBNP: 0.328 • SCI alone: 0.320 Proportion of true- non-CHF cases: • SCI with NT-proBNP: 0.629 • SCI alone: 0.647 Number of ECHOs: • SCI with NT-proBNP: 0.105 • SCI alone: 0.251 Number of initial hospitalizations after ED: • SCI with NT-proBNP: 0.677 • SCI alone: 0.778 Average length of stay: • SCI with NT-proBNP: 3.88 d • SCI alone: 4.41 d |
Total cost per patient, mean (2005 $ USD): • SCI with NT-proBNP: 4,558 • SCI alone: 5,032 Difference: −474 Cost components incorporated: Emergency and admitted patient care, including cardio-pulmonary investigations, outpatient care, and NT-proBNP test |
Primary ICER: SCI with NT-proBNP dominates SCI alone (less costly, lower risk for SAE) Analysis of uncertainty: PSA of the incidence of SAEs found SCI with NT-proBNP to be dominant in 78% of the simulations Including effect of NT-proBNP on mortality yielded a relative reduction of 1% associated with NT-proBNP strategy When prevalence of true chronic HF varied from 20% to 80%, NT-proBNP strategy remained dominant NT-proBNP strategy remained dominant after 1) halving and doubling costs for NT-proBNP tests, echo, or hospitalization; 2) assuming each prevented echo saved only 1 d of hospital stay compared with 2.7 d in the reference case; 3) assuming a positive NT-proBNP result would not replace echo; 4) varying sensitivity and specificity of NT-proBNP across the 95% CI |
| BNP | ||||||
| Breidthardt et al, 200758 Switzerland |
• Cost-effectiveness analysis • RCT (BASEL) • Public payer perspective: • Time horizon: 360 d for cost; 720 d for health outcomes • Discounting: NA |
• People presenting to ED with acute dyspnea (n = 452) • Age (mean): 71 y • Male: 58% |
Intervention: • SCI supplemented and guided by BNP (100 pg/mL to rule out; 500 pg/mL to rule in; n = 225) Comparator: • SCI alone (n = 227) |
720-d all-cause mortality: • SCI with BNP: 37% • SCI alone: 36% Total days in-hospital, median (IQR): • SCI with BNP: 12 (2–28) • SCI alone: 16 (7–32) P = 0.025 Days in-hospital for dyspnea, median (IQR): • SCI with BNP: 11 (2–23) • SCI alone: 14 (6–26) P = 0.009 |
Total cost per patient, mean (2003 $ USD): • SCI with BNP: 10,144 • SCI alone: 12,748 Difference: −2,604 P = 0.008 Total cost per patient, median (IQR) (2003 $ USD): • SCI with BNP: 6,292 (2,309–13,262) • SCI alone: 8,643 (4,481–16,062) |
Primary ICER: SCI with BNP is less costly than SCI alone; no difference in long-term mortality, and fewer days spent in-hospital Analysis of uncertainty: PSA using bootstrap sampling found SCI with BNP to be dominant in 39.5% of the simulations, less costly/higher mortality in 59.1% of the simulations, more costly/lower mortality in 0.5% of the simulations, and more costly/higher mortality in 0.9% of the simulations The reduction in initial mortality observed in frail elderly patients was no longer evident at 720 d The reduction in days hospitalized was the major driver for a significant reduction in total treatment cost at 360 d |
| Mueller et al, 200659 Switzerland |
• Cost-effectiveness analysis • RCT (BASEL) • Public payer perspective • Time horizon: 180 d • Discounting: NA |
• People presenting to ED with acute dyspnea (n = 452) • Age (mean): 71 y • Male: 58% |
Intervention: • SCI supplemented and guided by BNP (100 pg/mL to rule out and 500 pg/mL to rule in; n = 225) Comparator: • SCI alone (n = 227) |
180-d all-cause mortality: • SCI with BNP: 20% • SCI alone: 23% Total days in-hospital, median (IQR): • SCI with BNP: 10 (2–24) • SCI alone: 14 (6–27) P = 0.005 Days in-hospital for dyspnea, median (IQR): • SCI with BNP: 9 (1–20) • SCI alone: 13 (6–24) P = 0.003 Initial hospitalization rate: • SCI with BNP: 0.75 • SCI alone: 0.85 Difference: −0.10 P = 0.008 Admission rate to ICU: • SCI with BNP: 0.15 • SCI alone: 0.24 Difference: −0.09 P = 0.01 |
Total cost per patient, mean (SD) (2003 $ USD): • SCI with BNP: 7,930 (8,805) • SCI alone: 10,053 (10,176) Difference: −2,123 P = 0.004 Cost components incorporated: Emergency and admitted patient care, including cardio-pulmonary investigations, outpatient care, and BNP test |
Primary ICER: SCI with BNP dominates SCI alone (less costly, fewer days in hospital) Analysis of uncertainty: PSA using bootstrap sampling found SCI with BNP to be dominant in 80.6% of the simulations, less costly/higher mortality in 19.3% of the simulations, more costly/lower mortality in 0.04% of the simulations, and more costly/higher mortality in 0.02% of the simulations The primary driver of lower costs in the BNP group is reduced time in hospital. Also fewer admissions and less intensive care Results remained robust in all DSAs Subgroup analysis showed that the effect on cost is largest in patients with a history of coronary artery disease or pulmonary disease |
| Medical Services Advisory Committee, 200760 Australia |
• Cost-effectiveness analysis • RCT (BASEL) • Public payer perspective • Time horizon: 30 d • Discounting: NA |
• People presenting to ED with acute dyspnea (n = 452) • Age (mean): 71 y • Male: 58% |
Intervention: • SCI supplemented and guided by BNP (100 pg/mL to rule out; 500 pg/mL to rule in; n = 225) Comparator: • SCI alone (n = 227) |
30-d all-cause mortality: • SCI with BNP: 0.10 • SCI alone: 0.12 Difference: −0.026 (95% CI −0.083 to −0.032) Time to discharge, median (IQR): • SCI with BNP: 8 (1–16) • SCI alone: 11 (5–18) Difference: −3 P = 0.001 Initial hospitalization rate: • SCI with BNP: 0.75 • SCI alone: 0.85 Difference: −0.10 P = 0.008 30-d readmission rate: • SCI with BNP: 0.12 • SCI alone: 0.10 Difference: 0.02 P = 0.63 |
Total cost per patient, mean (2005 $ AUD): • SCI with BNP: 3,756 • SCI alone: 4,094 Difference: −338 Cost components incorporated: Emergency and admitted patient care, including cardio-pulmonary investigations, outpatient care, and BNP test |
Primary ICER: SCI with BNP dominates SCI alone (less costly, lower mortality) Analysis of uncertainty: PSA using bootstrap sampling found SCI with BNP to be dominant in 78.8% of the simulations, less costly/higher mortality in 18.8% of the simulations, more costly/lower mortality in 1.9% of the simulations, and more costly/higher mortality in 0.5% of the simulations At 30 d, the primary cost saving element is the patient admission rate (initial plus re-admission) |
| Community care | ||||||
| BNP | ||||||
| Burri et al, 201251 Switzerland and Germany |
• Cost-effectiveness analysis • RCT (BASEL III) • Public payer perspective • Time horizon: 12 m • Discounting: NA |
• People presenting to community care with dyspnoea (n = 323) • Age (mean): 73 y • Male: 50% |
Intervention: • SCI supplemented and guided by BNP POC test (100 pg/mL to rule out; 400 pg/mL to rule in; n = 163) Comparator: • SCI alone (n = 160) |
Number of hospitalizations after 3 and 12 mo: • SCI with BNP: 28/163, 50/163 • SCI alone: 20/160, 42/160 Days in hospital at 3 and 12 mo (median): • SCI with BNP: 12.1 d, 14.3 d • SCI alone: 9.5 d, 14.9 d Mortality after 3 and 12 mo: • SCI with BNP: 3%, 6% • SCI alone: 2%, 6% Time to appropriate therapy, median (IQR): • SCI with BNP: 0.04 d (0.04–8) • SCI alone: 0.04 d (0.04–34) P = 0.01 Dyspnea after 3 mo: • SCI with BNP: improved in 55% of patients • SCI alone: improved in 54% of patients The need for further diagnostic workup: • SCI with BNP: 33% • SCI alone: 45% P = 0.02 |
Total cost per patient at 3 mo, median (IQR), ($ USD; year not specified): • SCI with BNP: 1,655 (850–3,331) • SCI alone: 1,541 (859–2,827) Difference: 114 P = 0.68 Total cost per patient at 12 mo, median (IQR), (, $ USD year not specified): • SCI with BNP: 6,153 (3,271–11,144) • SCI alone: 5,771 (2,840–9,524) Difference: 382 P = 0.45 Cost components incorporated: Hospitalizations for dyspnoea, outpatient visits to a physician, and medical treatment after study entry |
Primary ICER: The use of BNP did not reduce total medical cost, but improved some secondary endpoints, including diagnostic certainty and time to initiation of appropriate treatment Analysis of uncertainty: None |
| NT-proBNP | ||||||
| Bugge et al, 201840 Norway |
• Cost-effectiveness analysis • Decision tree model • Health care payer and society perspective • Time horizon: 12 mo • Discounting: NA |
• Patients with suspected HF |
Intervention: • Clinical diagnosis plus NT-proBNP POC test in GP's office (cut-off value 125 ng/L) • Clinical diagnosis plus NT-proBNP in hospital laboratory Comparator: • Clinical diagnosis only (no NT-proBNP) |
Proportion of initial incorrect diagnosis: • Clinical diagnosis plus NT-proBNP: 22.0% (95% CI: 16.1–31.0%) • Clinical diagnosis only: 38.0% (95% CI: 31.0–45.0%) |
Total cost per patient, mean (95% CI), health care payer perspective (2017 € Euro): • Clinical diagnosis plus NT-proBNP POC test: 344 (225–502) • Clinical diagnosis plus NT-proBNP hospital test: 397 (276–554) • Clinical diagnosis only: 379 (226–661) Total cost per patient, mean (95% CI), societal perspective (2017 € Euro): • Clinical diagnosis plus NT-proBNP POC test: 505 (375–674) • Clinical diagnosis plus NT-proBNP hospital test: 607 (469–780) • Clinical diagnosis only: 543 (378–767) |
Primary ICER: POC testing results in lower costs and earlier diagnosis Analysis of uncertainty: PSA was conducted |
| Monahan et al, 201762 United Kingdom |
• Cost-effectiveness and cost–utility analysis • Decision tree model based on a prospective, observational, diagnostic validation study of the MICE clinical decision rule (REFER) • Health care payer perspective (NHS) • Time horizon: lifetime • Discounting: 3.5% |
• People with suspected HF (n = 304) • Age (mean): 73.9 y • Male: 40.8% |
Intervention: • MICE strategy (upper cut-off) • MICE strategy (lower cut-off) • NICE strategy: NT-proBNP (400 pg/mL cut-off) • ECHO all • NT-proBNP (125 pg/mL cut-off) Comparator: • Do nothing |
QALY gained vs. do nothing: • NT-proBNP 400: 0.0051 • MICE upper cut-off: 0.0050 • MICE lower cut-off: 0.0057 • NT-proBNP 125: 0.0059 • ECHO all: 0.0063 Proportion of true HF detected: • Do nothing: 0% • NT-proBNP 400: 78.85% • MICE upper cut-off: 81.73% • MICE lower cut-off: 90.38% • NT-proBNP 125: 94.23% • ECHO all: 100% Proportion of not HF ruled out: • Do nothing: 100% • NT-proBNP 400: 63.5% • MICE upper cut-off: 84.00% • MICE lower cut-off: 45.50% • NT-proBNP 125: 49.00% • ECHO all: 0% |
Total cost per patient (2013/2014 £ GBP): • Do nothing: 119 • NT-proBNP 400: 142 • MICE upper cut-off: 167 • MICE lower cut-off: 191 • NT-proBNP 125: 196 • ECHO all: 241 Cost components incorporated: GP visits, HF hospitalization, echocardiography, and NT-proBNP test |
Primary ICER (2013/2014 £ GBP): • NT-proBNP 400 vs. do nothing: 4,400/QALY • MICE upper cut-off: dominated by NT-proBNP 400 • MICE lower cut-off: extended dominance • NT-proBNP 125 vs. NT-proBNP 400: 69,000/QALY • ECHO all vs. NT-proBNP 125: 125,100/QALY • NT-proBNP 125 vs. do nothing: 13,051/QALY (calculated based on reported data) Analysis of uncertainty: PSA was conducted. At 20,000/QALY, the likelihood of NT-proBNP 400 strategy being cost-effective is 99.9% DSA was conducted: doubling and halving the NT-proBNP cost; altering drug efficacies to lower and upper confidence interval; substituting in branded drug prices for generic drug prices; increasing the proportion of HFREF patients from 12% to 24%, 50%, and 100%. NT-proBNP 400 remained the most cost-effective option for each scenario, except where the proportion of HFREF changed to 50% and above. When the proportion of HFREF patients was 50%, NT-proBNP 125 became cost-effective. When the proportion of HFREF was 100%, echo all became cost-effective. |
| NICE, 201820 United Kingdom (NICE CHF Guideline) |
• Cost–utility analysis • Decision tree and Markov model • Health care payer perspective (NHS) • Time horizon: lifetime • Discounting: 3.5% |
• Patients with suspected HF • Age (mean): 77 y for people with HF, 72 y for other conditions • Male: 50.6% for people with HF, 36% for other conditions |
Intervention: • NT-proBNP (400 pg/mL cut-off) • NT-proBNP (280 pg/mL cut-off) • NT-proBNP (125 pg/mL cut-off) Comparator: • ECHO for all |
QALYs: • NT-proBNP 125 pg/mL: 4.960 • NT-proBNP 280 pg/mL: 5.004 • NT-proBNP 400 pg/mL: 5.018 • ECHO all: 4.894 |
Total cost per patient, mean (2018 £ GBP): • NT-proBNP 125 pg/mL: 2,080 • NT-proBNP 280 pg/mL: 2,297 • NT-proBNP 400 pg/mL: 2,360 • ECHO all: 1,682 |
Primary ICER vs. ECHO all (2018 £ GBP; calculated based on reported data): • NT-proBNP 125: 6,030/QALY • NT-proBNP 280: 5,590/QALY • NT-proBNP 400: 5,468/QALY Analysis of uncertainty: Probability most cost-effective option at 20,000 per QALY: • ECHO all: 14% • NT-proBNP 125: 1% • NT-proBNP 280: 8% • NT-proBNP 400: 77% Results remained robust in comprehensive sensitivity analysis |
Abbreviations: BNP, B-type natriuretic peptide; CI, confidence interval; DSA, deterministic sensitivity analysis; ECHO, echocardiography; ED, emergency department; GP, general practitioner; HF, heart failure; ICER, incremental cost-effectiveness ratio; ICU, intensive care unit; IQR, interquartile range; NA, not applicable; NHS, National Health Service; NP, natriuretic peptide; NT-proBNP, N-terminal proBNP; POC, point-of-care; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life years; RCT, randomized control trial; SAE, serious adverse events; SCI, standard clinical investigations.
Note: For trial-based economic evaluations, we reported P value if it is less than 0.05.
Table A7:
Assessment of the Applicability of Included Economic Studies
| Author, Year, Country | Is the study population similar to the question?a | Are the interventions similar to the question?b | Is the health care system studied sufficiently similar to Ontario?c | Were the perspectives clearly stated? If yes, what were they? | Are all direct effects included? Are all other effects included where they are material?d | Are all future costs and outcomes discounted? If yes, at what rate? | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued?e | Overall Judgmentf |
|---|---|---|---|---|---|---|---|---|---|
| Emergency Department | |||||||||
| Griffin et al, 2017,54 United Kingdom (NICE AHF guideline 2014) |
Yes | Yes | Partially | Yes, health care payer (NHS) | Partially | Yes, 3.5% | Yes | No | Partially applicable |
| Rutten et al, 2008,55 Netherlands |
Yes | Partially (different cut-off values) | Partially | No (likely public payer since only direct cost included) | Partially | Not applicable (time horizon < 1 y) | No | No | Not applicable |
| Moe et al, 2007,56 Canada |
Yes | Yes | Yes | Yes, health care payer (Canada) | Partially | Not applicable (time horizon < 1 y) | No | No | Directly applicable |
| Sibert et al, 2006,57 United States |
Yes | Partially (different cut-off values) | Partially | No (likely public payer since only direct cost included) | Partially | Not applicable (time horizon < 1 y) | No | No | Not applicable |
| Breidthardt et al, 2007,58 Switzerland |
Yes | Yes | Partially | No (likely public payer since only direct cost included) | Partially | No | No | No | Partially applicable |
| Mueller et al, 2006,59 Switzerland |
Yes | Yes | Partially | No (likely public payer since only direct cost included) | Partially | Not applicable (time horizon < 1 y) | No | No | Partially applicable |
| Medical Services Advisory Committee, 2007,60 Australia |
Yes | Yes | Partially | Yes, health care payer (Australia) | Partially | Not applicable (time horizon < 1 y) | No | No | Partially applicable |
| Community care | |||||||||
| Burri et al, 2012,51 Switzerland and Germany |
Yes | Partially (different cut-off values) | Partially | No (likely public payer since only direct cost included) | Partially | Not applicable (time horizon < 1 y) | No | No | Not applicable |
| Bugge et al, 2018,40 Norway |
Yes | Yes | Partially | Yes, health care perspective and societal perspective | Partially | Not applicable (time horizon < 1 y) | No | No | Partially applicable |
| Monahan et al, 2017,62 United Kingdom |
Yes | Yes | Partially | Yes, health care payer (NHS) | Partially | yes, 3.5% | Yes | No | Partially applicable |
| NICE, 2018,20 United Kingdom (NICE CHF guideline) |
Yes | Yes | Partially | Yes, health care payer (NHS) | Partially | yes, 3.5% | Yes | No | Partially applicable |
Abbreviations: BNP, B-type natriuretic peptide; NT-proBNP, N-terminal proBNP; NHS, national health services.
Note: response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Study population: answer “yes” if the study included patients with suspected heart failure.
Intervention: answer “yes” if the study included BNP and NT-proBNP and used the same cut-off values as recommended by the Canadian guidelines. Answer “partially” if the study included BNP and NT-proBNP, but used different cut-off values.
Health care system: answer “yes” if the study was conducted in Canada and was sufficiently recent to reflect recent Canadian system. Answer “partially” if the study was conducted in a public health care system similar to Canada.
Answer “yes” if the measure of health outcomes included mortality, hospitalization rate, length of stay, risk of serious adverse events, number of correct or incorrect diagnosis, and number of echocardiography. Answer “partially” if only some of these outcomes were included.
Answer “yes” if societal costs were included. Answer “no” if societal costs were not included.
- Not applicable: the study fails to meet one or more applicability criteria, and this is likely to change the conclusions about cost effectiveness. Such studies would be excluded from further consideration (e.g., studies conducted in non-Canadian settings, used different cut-off values).
- Partially applicable: the study fails to meet one or more applicability criteria and this could change the conclusions about cost effectiveness (e.g., studies conducted in non-Canadian settings, but used the same cut-off values as recommended by the Canadian guidelines).
- Directly applicable: the study meets all applicability criteria, or fails to meet one or more applicability criteria, but this is unlikely to change the conclusions about cost effectiveness (e.g., studies conducted in a Canadian setting).
Table A8:
Assessment of the Limitations of Included Economic Studies
| Author, Year Country | Does the model structure adequately reflect the nature of the health condition under evaluation? | Is the time horizon sufficiently long to reflect all important differences in costs and outcomes? | Are all important and relevant health outcomes included? | Are the clinical inputsa obtained from the best available sources? | Do the clinical inputsa match the estimates contained in the clinical sources? | Are all important and relevant (direct) costs included in the analysis? | Are the estimates of resource use obtained from the best available sources? | Are the unit costs of resources obtained from the best available sources? | Is an appropriate incremental analysis presented, or can it be calculated from the reported data? | Are all important and uncertain parameters subjected to appropriate sensitivity analysis? | Is there a potential conflict of interest? | Overall Judgmentb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Emergency | ||||||||||||
| Griffin et al, 2017,54 United Kingdom (NICE AHF guideline 2014) |
Yes | Yes (4 y) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Minor limitations |
| Moe et al, 2007,56 Canada |
NA (trial-based analysis) | No (1 mo) | Partially | Partially | Yes | Yes | Yes | Yes | Yes | Partially | Yes (industry funded) | Potentially serious limitations |
| Breidthardt et al, 2007,58 Switzerland |
NA (trial-based analysis) | Partially (1 y for costs, 2 y for health outcomes) | Partially | Partially | Yes | No (did not consider costs beyond 1 y) | Yes | Yes | No (use of different follow-up time for costs and outcomes may bias the results) | Partially | No | Potentially serious limitations |
| Mueller et al, 2006,59 Switzerland |
NA (trial-based analysis) | Partially (6 mo) | Partially | Partially | Yes | Yes | Yes | Yes | Yes | Partially | No | Minor limitations |
| Medical Services Advisory Committee, 2007,60 Australia |
NA (trial-based analysis) | No (1 mo) | Partially | Partially | Yes | Yes | Partially | Yes | Yes | Partially | No | Potentially serious limitations |
| Community care | ||||||||||||
| Bugge et al, 2018,40 Norway |
Partially | Partially | No | Partially | Yes | Yes | Yes | Yes | Yes | Yes | Yes (industry-funded) | Potentially serious limitations |
| Monahan et al, 2017,62 United Kingdom |
Partially | Yes | No | Partially | Yes | Yes | Yes | Yes | Yes | Yes | No | Potentially serious limitations |
| NICE, 2018,20 United Kingdom (NICE CHF guideline) |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Minor limitations |
Note: response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Clinical inputs include relative treatment effects, natural history, and utilities.
Overall judgment may be “minor limitations,” “potentially serious limitations,” or “very serious limitations.
Appendix 5: Letter of Information
Appendix 6: Interview Guide
Introduction
Explain Ontario Health purpose, health technology assessment process, and purpose of interview.
Lived-Experience
Background of condition or circumstances leading to heart failure and its diagnosis
Describe the diagnostic process. What was involved?
What was the impact on receiving/not receiving diagnosis and diagnostic testing on quality of life?
Impact on family/caregivers, work? (if applicable)
Barriers/Challenges to Receiving Diagnosis
What were the barriers or delays to receiving diagnosis? Cost? Geography? Any other inconveniences?
Lived- Experience
Previous information surrounding this diagnostic testing? (i.e., had you ever heard of it before?
In your opinion, would a diagnostic test, like the BNP test we are considering today (which could theoretically diagnose them more efficiently), be useful? In what ways?
Do you see any benefits to using the test? Limitations?
How would it impact your/your families/caregivers' quality of life?
Author contributions
This report was developed by a multidisciplinary team at Ontario Health. The clinical epidemiologist was Immaculate Nevis, the medical librarians were Melissa Walter and Caroline Higgins, the primary health economists were Chunmei Li and Yuan Zhang, the secondary health economist was Shawn Xie, and the patient and public partnership analyst was Aroma Akhund.
Key Messages
What Is This Health Technology Assessment About?
Heart failure is a major cause of illness and death. About 50,000 Canadians are diagnosed with heart failure each year, and an estimated 600,000 Canadians are living with heart failure. It is important that people with symptoms suggestive of heart failure are identified quickly and treated appropriately.
There is no single diagnostic test for heart failure, and diagnosis can be challenging since the symptoms of heart failure could be caused by other conditions. The levels of certain hormones—B-type natriuretic peptide (BNP) or N-terminal proBNP (NT-proBNP)—in the blood may indicate that a person is experiencing heart failure. Test kits have been developed that can measure the levels of these hormones and give guidance to health care practitioners diagnosing people who have conditions with symptoms similar to heart failure.
This health technology assessment looked at the diagnostic accuracy, clinical impact, and cost-effectiveness of BNP and NT-proBNP testing for adults suspected of having heart failure. It also looked at the budget impact of publicly funding tests and at the experiences, preferences, and values of people with suspected heart failure.
What Did This Health Technology Assessment Find?
The diagnostic accuracy of BNP and NT-proBNP tests was useful in ruling out heart failure in people with suspected heart failure. Conducting these tests in people with suspected heart failure in the emergency department setting could reduce length of hospital stay.
Our economic analysis found 12 studies evaluating the cost-effectiveness of BNP and NT-proBNP tests in people with suspected heart failure. These studies suggested that BNP or NT-proBNP tests, when used in addition to standard clinical investigations, were either dominant (less costly and more effective) or cost-effective. The estimated 5-year budget impact is an additional cost of about $38 million for emergency departments and a cost savings of about $20 million for community care.
People we interviewed gave BNP and NT-proBNP testing strong support, citing the potential benefits of quicker, more accurate diagnoses that could reduce misdiagnoses, stress, and the burden on patients and caregivers.
Contributor Information
Ontario Health (Quality):
Immaculate Nevis, Melissa Walter, Caroline Higgins, Chunmei Li, Yuan Zhang, Shawn Xie, and Aroma Akhund
About Us
Ontario Health is an agency of the Government of Ontario. Our mandate is to connect and coordinate our province's health care system in ways that have not been done before to help ensure that Ontarians receive the best possible care. We work to support better health outcomes, patient experiences, provider experiences and value for money spent.
For more information about Ontario Health, visit ontariohealth.ca.
References
- (1).Canadian Cardiovascular Society. Pocket guides [Internet]. Ottawa (ON): Canadian Cardiovascular Society; c2020. Available from: https://www.ccs.ca/en/resources/pocket-guides [Google Scholar]
- (2).Ezekowitz JA, O'Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, et al. 2017 comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol. 2017;33(11):1342–433. [DOI] [PubMed] [Google Scholar]
- (3).Heart and Stroke Foundation. 2016 report on the health of Canadians: the burden of heart failure [Internet]. Ottawa (ON): Heart & Stroke; 2016. Available from: https://www.heartandstroke.ca/-/media/pdf-files/canada/2017-heart-month/heartandstroke-reportonhealth-2016.ashx?la=en&hash=0478377DB7CF08A281E0D94B22BED6CD093C76DB [Google Scholar]
- (4).Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. Corrigendum to: ‘2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure’. Eur Heart J. 2018;39(14):1206. [DOI] [PubMed] [Google Scholar]
- (5).Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. [DOI] [PubMed] [Google Scholar]
- (6).Harris MH, DuBois SG, Glade Bender JL, Kim A, Crompton BD, Parker E, et al. Multicenter feasibility study of tumor molecular profiling to inform therapeutic decisions in advanced pediatric solid tumors: the individualized cancer therapy (iCat) study molecular profiling in pediatric tumorsmolecular profiling in pediatric tumors. JAMA Oncology. 2016;2(5):608–15. [DOI] [PubMed] [Google Scholar]
- (7).Health Quality Ontario. Heart failure: care in the community for adults [Internet]. Toronto (ON): Queen's Printer for Ontario; 2019. [cited 2019 Oct 29]. Available from: https://www.hqontario.ca/Portals/0/documents/evidence/quality-standards/qs-heart-failure-quality-standard-en.pdf [Google Scholar]
- (8).Don-Wauchope AC, McKelvie RS. Evidence based application of BNP/NT-proBNP testing in heart failure. Clin Biochem. 2015;48(4–5):236–46. [DOI] [PubMed] [Google Scholar]
- (9).Weber M, Hamm C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. 2006;92(6):843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Lee DS, Vasan RS. Novel markers for heart failure diagnosis and prognosis. Curr Opin Cardiol. 2005;20(3):201–10. [DOI] [PubMed] [Google Scholar]
- (11).Dhaliwal AS, Deswal A, Pritchett A, Aguilar D, Kar B, Souchek J, et al. Reduction in BNP levels with treatment of decompensated heart failure and future clinical events. J Card Fail. 2009;15(4):293–9. [DOI] [PubMed] [Google Scholar]
- (12).Hasegawa K, Fujiwara H, Doyama K, Miyamae M, Fujiwara T, Suga S, et al. Ventricular expression of brain natriuretic peptide in hypertrophic cardiomyopathy. Circulation. 1993;88(2):372–80. [DOI] [PubMed] [Google Scholar]
- (13).Del Ry S, Clerico A, Giannessi D, Andreassi MG, Caprioli R, Iascone MR, et al. Measurement of brain natriuretic peptide in plasma samples and cardiac tissue extracts by means of an immunoradiometric assay method. Scand J Clin Lab Invest. 2000;60(2):81–90. [DOI] [PubMed] [Google Scholar]
- (14).Yandle TG, Richards AM, Gilbert A, Fisher S, Holmes S, Espiner EA. Assay of brain natriuretic peptide (BNP) in human plasma: evidence for high molecular weight BNP as a major plasma component in heart failure. J Clin Endocrinol Metab. 1993;76(4):832–8. [DOI] [PubMed] [Google Scholar]
- (15).Wang J, Hong B, Kai J, Han J, Zou Z, Ahn CH, et al. Mini sensing chip for point-of-care acute myocardial infarction diagnosis utilizing micro-electro-mechanical system and nano-technology. Adv Exp Med Biol. 2009;645:101–7. [PubMed] [Google Scholar]
- (16).Yang Z, Min Zhou D. Cardiac markers and their point-of-care testing for diagnosis of acute myocardial infarction. Clin Biochem. 2006;39(8):771–80. [DOI] [PubMed] [Google Scholar]
- (17).Maalouf R, Bailey S. A review on B-type natriuretic peptide monitoring: assays and biosensors. Heart Fail Rev. 2016;21(5):567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Health Canada. Medical devices active licence listing (MDALL): your reference tool for licensed medical devices in Canada [Internet]. Ottawa (ON): Government of Canada. 2020. Available from: https://health-products.canada.ca/mdall-limh/index-eng.jsp [Google Scholar]
- (19).National Institute for Health and Care Excellence. Acute heart failure: diagnosis and management. Clinical guideline [CG187] [Internet]. London: NICE; 2014. Oct. Available from: https://www.nice.org.uk/guidance/cg187 [Google Scholar]
- (20).National Institute for Health and Care Excellence. Chronic heart failure in adults: diagnosis and management: NICE guideline [NG106] [Internet]. London: NICE; 2018. [cited 2018 Sept 19]. Available from: https://www.nice.org.uk/guidance/ng106 [PubMed] [Google Scholar]
- (21).Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21(6):715–31. [DOI] [PubMed] [Google Scholar]
- (22).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (23).Covidence systematic review software [Internet]. Melbourne (Australia): Veritas Health Innovation; c2020. [Available from: https://www.covidence.org/home [Google Scholar]
- (24).Whiting P, Savovic J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Booth RA, Hill SA, Don-Wauchope A, Santaguida PL, Oremus M, McKelvie R, et al. Performance of BNP and NT-proBNP for diagnosis of heart failure in primary care patients: a systematic review. Heart Fail Rev. 2014;19(4):439–51. [DOI] [PubMed] [Google Scholar]
- (26).Hill SA, Booth RA, Santaguida PL, Don-Wauchope A, Brown JA, Oremus M, et al. Use of BNP and NT-proBNP for the diagnosis of heart failure in the emergency department: a systematic review of the evidence. Heart Fail Rev. 2014;19(4):421–38. [DOI] [PubMed] [Google Scholar]
- (27).Latour-Perez J, Coves-Orts FJ, Abad-Terrado C, Abraira V, Zamora J. Accuracy of B-type natriuretic peptide levels in the diagnosis of left ventricular dysfunction and heart failure: a systematic review. Eur J Heart Fail. 2006;8(4):390–9. [DOI] [PubMed] [Google Scholar]
- (28).Martindale JL, Wakai A, Collins SP, Levy PD, Diercks D, Hiestand BC, et al. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23(3):223–42. [DOI] [PubMed] [Google Scholar]
- (29).Taylor KS, Verbakel JY, Feakins BG, Price CP, Perera R, Bankhead C, et al. Diagnostic accuracy of point-of-care natriuretic peptide testing for chronic heart failure in ambulatory care: systematic review and meta-analysis. BMJ. 2018;361:k1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Balion C, Don-Wauchope A, Hill S, Santaguida PL, Booth R, Brown JA, et al. Use of natriuretic peptide measurement in the management of heart failure. Comparative effectiveness review No. 126. Rockville (MD): Agency for Healthcare Research and Quality; 2013. 11. [PubMed] [Google Scholar]
- (31).Balion C, Santaguida PL, Hill S, Worster A, McQueen M, Oremus M, et al. Testing for BNP and NT-proBNP in the diagnosis and prognosis of heart failure. Evid Rep Technol Assess (Full Rep). 2006(142):1–147. [PMC free article] [PubMed] [Google Scholar]
- (32).Battaglia M, Pewsner D, Juni P, Egger M, Bucher HC, Bachmann LM. Accuracy of B-type natriuretic peptide tests to exclude congestive heart failure: Systematic review of test accuracy studies. Arch Intern Med. 2006;166(10):1073–80. [DOI] [PubMed] [Google Scholar]
- (33).Cardarelli R, Lumicao TG, Jr. B-type natriuretic peptide: a review of its diagnostic, prognostic, and therapeutic monitoring value in heart failure for primary care physicians. J American Board Fam Pract. 2003;16(4):327–33. [DOI] [PubMed] [Google Scholar]
- (34).Clerico A, Fontana M, Zyw L, Passino C, Emdin M. Comparison of the diagnostic accuracy of brain natriuretic peptide (BNP) and the N-terminal part of the propeptide of BNP immunoassays in chronic and acute heart failure: a systematic review. Clin Chem. 2007;53(5):813–22. [DOI] [PubMed] [Google Scholar]
- (35).Doust JA, Glasziou PP, Pietrzak E, Dobson AJ. A systematic review of the diagnostic accuracy of natriuretic peptides for heart failure. Arch Intern Med. 2004;164(18):1978–84. [DOI] [PubMed] [Google Scholar]
- (36).Korenstein D, Wisnivesky JP, Wyer P, Adler R, Ponieman D, McGinn T. The utility of B-type natriuretic peptide in the diagnosis of heart failure in the emergency department: a systematic review. BMC Emerg Med. 2007;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Merlin T, Moss J, Brooks A, Newton S, Hedayati H, Hiller J. B-type natriuretic peptide assays in the diagnosis of heart failure. Part A: in the hospital emergency setting. Part B: in the non-hospital setting (structured abstract). Health Technol Assess Database. 2008(4). [Google Scholar]
- (38).Schols AMR, Stakenborg JPG, Dinant GJ, Willemsen RTA, Cals JWL. Point-of-care testing in primary care patients with acute cardiopulmonary symptoms: a systematic review. Fam Pract. 2018;35(1):4–12. [DOI] [PubMed] [Google Scholar]
- (39).Worster A, Balion CM, Hill SA, Santaguida P, Ismaila A, McKelvie R, et al. Diagnostic accuracy of BNP and NT-proBNP in patients presenting to acute care settings with dyspnea: a systematic review. Clin Biochem. 2008;41(4–5):250–9. [DOI] [PubMed] [Google Scholar]
- (40).Bugge C, Sether EM, Pahle A, Halvorsen S, Kristiansen IS. Diagnosing heart failure with NT-proBNP point-of-care testing: lower costs and better outcomes. A decision analytic study. BJGP Open. 2018;2(3):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Lam LL, Cameron PA, Schneider HG, Abramson MJ, Muller C, Krum H. Meta-analysis: effect of B-type natriuretic peptide testing on clinical outcomes in patients with acute dyspnea in the emergency setting. Ann Intern Med. 2010;153(11):728–35. [DOI] [PubMed] [Google Scholar]
- (42).Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. [DOI] [PubMed] [Google Scholar]
- (44).Šimundić A.-M. Measures of diagnostic accuracy: basic definitions. EJIFCC. 2009;19(4):203–11. [PMC free article] [PubMed] [Google Scholar]
- (45).Böhning D, Holling H, Patilea V. A limitation of the diagnostic-odds ratio in determining an optimal cut-off value for a continuous diagnostic test. Stat Methods Med Res. 2011;20(5):541–50. [DOI] [PubMed] [Google Scholar]
- (46).McMartin K. B-type natriuretic peptide testing: a rapid review. 2013 Jan:18.
- (47).Moe GW. BNP in the diagnosis and risk stratification of heart failure. Heart Fail Monit. 2005;4(4):116–22. [PubMed] [Google Scholar]
- (48).Burke MA, Cotts WG. Interpretation of B-type natriuretic peptide in cardiac disease and other comorbid conditions. Heart Fail Rev. 2007;12(1):23–36. [DOI] [PubMed] [Google Scholar]
- (49).Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339(5):321–8. [DOI] [PubMed] [Google Scholar]
- (50).Spanaus KS, Kronenberg F, Ritz E, Schlapbach R, Fliser D, Hersberger M, et al. B-type natriuretic peptide concentrations predict the progression of nondiabetic chronic kidney disease: the Mild-to-Moderate Kidney Disease Study. Clin Chem. 2007;53(7):1264–72. [DOI] [PubMed] [Google Scholar]
- (51).Burri E, Hochholzer K, Arenja N, Martin-Braschler H, Kaestner L, Gekeler H, et al. B-type natriuretic peptide in the evaluation and management of dyspnoea in primary care. J Intern Med. 2012;272(5):504–13. [DOI] [PubMed] [Google Scholar]
- (52).Bottle A, Kim D, Aylin PP, Majeed FA, Cowie MR, Hayhoe B. Real-world presentation with heart failure in primary care: do patients selected to follow diagnostic and management guidelines have better outcomes? Open Heart. 2018;5(2):e000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).National Institute for Health and Care Excellence. Appendix I: Quality appraisal checklist—economic evaluations. 2012. [cited 2016 Jan]. In: Methods for the development of NICE public health guidance, 3rd ed [Internet]. London: The Institute. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations [PubMed]
- (54).Griffin EA, Wonderling D, Ludman AJ, Al-Mohammad A, Cowie MR, Hardman SMC, et al. Cost-effectiveness analysis of natriuretic peptide testing and specialist management in patients with suspected acute heart failure. Value Health. 2017;20(8):1025–33. [DOI] [PubMed] [Google Scholar]
- (55).Rutten JHW, Steyerberg EW, Boomsma F, van Saase JLCM, Deckers JW, Hoogsteden HC, et al. N-terminal pro-brain natriuretic peptide testing in the emergency department: Beneficial effects on hospitalization, costs, and outcome. Am Heart J. 2008;156(1):71–7. [DOI] [PubMed] [Google Scholar]
- (56).Moe GW, Howlett J, Januzzi JL, Zowall H. N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation. 2007;115(24):3103–10. [DOI] [PubMed] [Google Scholar]
- (57).Siebert U, Januzzi JL, Jr, Beinfeld MT, Cameron R, Gazelle GS. Cost-effectiveness of using N-terminal pro-brain natriuretic peptide to guide the diagnostic assessment and management of dyspneic patients in the emergency department. Am J Cardiol. 2006;98(6):800–5. [DOI] [PubMed] [Google Scholar]
- (58).Breidthardt T, Laule K, Strohmeyer AH, Schindler C, Meier S, Fischer M, et al. Medical and economic long-term effects of B-type natriuretic peptide testing in patients with acute dyspnea. Clin Chem. 2007;53(8):1415–22. [DOI] [PubMed] [Google Scholar]
- (59).Mueller C, Laule-Kilian K, Schindler C, Klima T, Frana B, Rodriguez D, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Arch Intern Med. 2006;166(10):1081–7. [DOI] [PubMed] [Google Scholar]
- (60).Medical Services Advisory Committee. B-type natriuretic peptide assays in the diagnosis of heart failure, part A – in the hospital emergency setting – November 2006; bart B – in the non-hospital setting – May 2007 [Internet]. Canberra (Australia): Commonwealth of Australia; 2007. Available from: http://www.msac.gov.au/internet/msac/publishing.nsf/Content/B68AAF8993749793CA25801000123B83/$File/1087-Assessment-Report.pdf [Google Scholar]
- (61).Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, et al. Use of B-Type Natriuretic Peptide in the Evaluation and Management of Acute Dyspnea. N Engl J Med. 2004;350(7):647–54. [DOI] [PubMed] [Google Scholar]
- (62).Monahan M, Barton P, Taylor CJ, Roalfe AK, Hobbs FDR, Cowie M, et al. MICE or NICE? An economic evaluation of clinical decision rules in the diagnosis of heart failure in primary care. Int J Cardiol. 2017;241:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Scott MA, Price CP, Cowie MR, Buxton MJ. Cost-consequences analysis of natriuretic peptide assays to refute symptomatic heart failure in primary care. British Journal of Cardiology. 2008;15(4):199–204. [Google Scholar]
- (64).Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health. 2015;18(2):161–72. [DOI] [PubMed] [Google Scholar]
- (65).Welte R, Feenstra T, Jager H, Leidl R. A decision chart for assessing and improving the transferability of economic evaluation results between countries. Pharmacoeconomics. 2004;22(13):857–76. [DOI] [PubMed] [Google Scholar]
- (66).Consumer price inflation, UK: October 2019. Price indices, percentage changes and weights for the different measures of consumer price inflation. [Internet]. London: Office for National Statistics; 2019. [cited 2019 Nov 14]. Available from: https://www.ons.gov.uk/economy/inflationandpriceindices/bulletins/consumerpriceinflation/october2019#data [Google Scholar]
- (67).Purchasing power parities (PPP) [Internet]. Paris: Organisation for Economic Co-operation and Development; (c)2018. [cited 2019 Nov 14]. Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm [Google Scholar]
- (68).Guidelines for the economic evaluation of health technologies: Canada [Internet]. Ottawa: CADTH; 2017. Available from: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf [Google Scholar]
- (69).Schedule of fees for the laboratory services: outpatient payment schedule [Internet]. Vancouver (BC): British Columbia Ministry of Health; 2019. [cited 2020 Jan 8]. Available from: https://www2.gov.bc.ca/assets/gov/health/practitioner-pro/laboratory-services/laboratory_services_schedule_of_fees.pdf [Google Scholar]
- (70).Non-OHIP insured tests [Internet]. Hearst (ON): Hospital Notre-Dame; 2017. [cited 2019 Dec 10]. Available from: http://www.ndh.on.ca/wp-content/uploads/2018/01/uninsured-lab-tests.pdf [Google Scholar]
- (71).Alberta Health Services. Laboratory bulletin: laboratory tests and associated costs [Internet]. Edmonton (AB): Alberta Health Services; 2016. [cited 2019 Nov 20]. Available from: https://www.albertahealthservices.ca/assets/wf/lab/wf-lab-bulletin-revised-laboratory-tests-and-associated-costs.pdf [Google Scholar]
- (72).Ontario Ministry of Health. Schedule of benefits: physician services under the health insurance act [Internet]. Toronto (ON): Queen's Printer for Ontario; 2019. Sep [cited 2020 Jan 7]. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20191001.pdf [Google Scholar]
- (73).Canadian Institute for Health Information. Cost of a standard hospital stay: details for Ontario [Internet]. Ottawa (ON): CIHI. (c)1996–2020. Available from: https://yourhealthsystem.cihi.ca/hsp/indepth?lang=en#/indicator/015/2/C5001/ [Google Scholar]
- (74).Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391(10120):572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Yeung DF, Boom NK, Guo H, Lee DS, Schultz SE, Tu JV. Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007. CMAJ. 2012;184(14):E765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).National Cardiac Audit Programme. National heart failure audit: 2016/17 summary report [Internet]. London: National Institute for Cardiovascular Outcomes Research; 2017. [cited 2019. Nov 20]. Available from: https://www.nicor.org.uk/wp-content/uploads/2018/11/Heart-Failure-Summary-Report-2016-17.pdf [Google Scholar]
- (77).Office for National Statistics. Life expectancy at birth and at age 65 by local areas in England and Wales: 2012 to 2014 [Internet]. London: Office for National Statistics; 2015. [cited 2020 Jan 10]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/lifeexpectancyatbirthandatage65bylocalareasinenglandandwales/2015-11-04#national-life-expectancy-at-age-65 [Google Scholar]
- (78).Statistics Canada. Life expectancy at birth and at age 65, by province and territory, three-year average [Internet]. Ottawa (ON): Statistics Canada. 2020. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310040901&pickMembers%5B0%5D=3.3 [Google Scholar]
- (79).Wijeysundera HC, Machado M, Wang X, Van Der Velde G, Sikich N, Witteman W, et al. Cost-effectiveness of specialized multidisciplinary heart failure clinics in Ontario, Canada. Value Health. 2010;13(8):915–21. [DOI] [PubMed] [Google Scholar]
- (80).Ministry of Health. Health data branch web portal – Ontario case costing [Internet]. Toronto (ON): Queen's Printer for Ontario. 2019. Available from: https://hsim.health.gov.on.ca/HDBPortal [Google Scholar]
- (81).Public Health Agency of Canada. Report from the Canadian chronic disease surveillance system: heart disease in Canada, 2018 [Internet]. Ottawa (ON): Her Majesty the Queen in Right of Canada; 2018. [cited 2019 Dec 19]. Available from: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/diseases-conditions/report-heart-disease-canada-2018/pub1-eng.pdf [Google Scholar]
- (82).Ontario Ministry of Finance. Ontario population projections, 2018–2046: Table 7: total population of Ontario by five-year age group, 2018–2046 – reference scenario [Internet]. Toronto (ON): Queen's Printer for Ontario. 2019. Available from: https://www.fin.gov.on.ca/en/economy/demographics/projections/table7.html [Google Scholar]
- (83).IntelliHealth Ontario. Ambulatory visits (2009–2018) [Internet]. Toronto (ON): Queen's Printer for Ontario. (c)2006–2015. [Google Scholar]
- (84).British Columbia Ministry of Health. Chronic heart failure – diagnosis and management: appendix A [Internet]. Vancouver: Province of British Columbia; 2015 Oct 28. [cited 2019 Oct 31]. Available from: https://www2.gov.bc.ca/assets/gov/health/practitioner-pro/bc-guidelines/chronicheartfailure_full.pdf [Google Scholar]
- (85).Chuck A, Jacobs J. Cost estimation of point of care B-type natriuretic peptide for the diagnosis of heart failure in the emergency department: application to Alberta [Internet]: Alberta Heritage Foundation for Medical Research; 2005. [cited 2020 Jan 22]. [Google Scholar]
- (86).Schedule of benefits for laboratory services [Internet]: Ministry of Health, Ontario Health Insurance Plan, Laboratories and Genetics Branch; 2020. [cited 2020 Jan 31]. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/lab/lab_mn2020.pdf [Google Scholar]
- (87).Canada at a Glance [Internet]. Statistics Canada; 2018. [cited 2020 Jan 22]. Available from: https://www150.statcan.gc.ca/n1/pub/12-581-x/2018000/pop-eng.htm [Google Scholar]
- (88).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (89).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (90).Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. Apr [cited 2018 Apr 30]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (91).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage; 1996. [Google Scholar]
- (92).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage; 1999. p. 33–45. [Google Scholar]
- (93).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods. Thousand Oaks (CA): Sage; 1994. p. 23–41. [Google Scholar]
- (94).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage; 2002. [Google Scholar]
- (95).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage; 1998. [Google Scholar]
- (96).Health Technology Assessment International. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2018 Apr 30]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (97).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (98).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage; 1994. p. 273–85. [Google Scholar]
- (99).NVivo qualitative data analysis software. QSR International. Doncaster, Victoria (Australia). Available at: https://www.qsrinternational.com/nvivo/home. [Google Scholar]
- (100).Roberts E, Ludman AJ, Dworzynski K, Al-Mohammad A, Cowie MR, McMurray JJV, et al. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ. 2015;350(h910). [DOI] [PMC free article] [PubMed] [Google Scholar]