Abstract
Background
Major depression is one of the most diagnosed mental illnesses in Canada. Generally, people are treated successfully with antidepressants or psychotherapy, but some people do not respond to these treatments (called treatment-resistant depression [TRD]). Repetitive transcranial magnetic stimulation (rTMS) delivers magnetic pulses to stimulate the areas of the brain associated with mood regulation. Several modalities of rTMS exist (e.g., high frequency rTMS, intermittent theta burst stimulation [iTBS], deep transcranial magnetic stimulation). We conducted a health technology assessment of rTMS for people with TRD, which included an evaluation of effectiveness, safety, cost-effectiveness, the budget impact of publicly funding rTMS, and patient preferences and values.
Methods
We performed a systematic literature search of the clinical evidence. We assessed the risk of bias of each included study using the Risk of Bias in Systematic Reviews (ROBIS) tool and Cochrane Risk of Bias for Randomized Controlled Trials and the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We performed a systematic economic literature search and conducted a cost–utility analysis with a 3-year horizon from a public payer perspective. We also analyzed the 5-year budget impact of publicly funding rTMS for people with TRD in Ontario. To assess the potential value of rTMS, we spoke with people who have TRD. Seven rTMS modalities were considered: low-frequency (1 Hz) stimulation, high-frequency (10–20 Hz) stimulation, unilateral stimulation, bilateral stimulation, iTBS, continuous theta burst stimulation, and deep transcranial magnetic stimulation.
Results
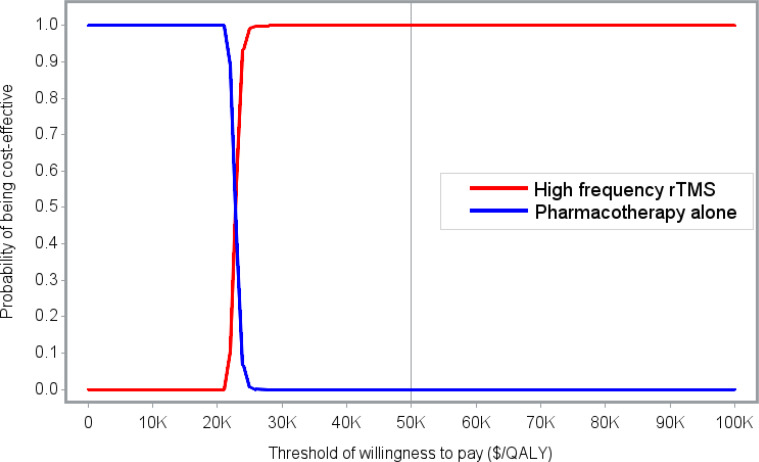
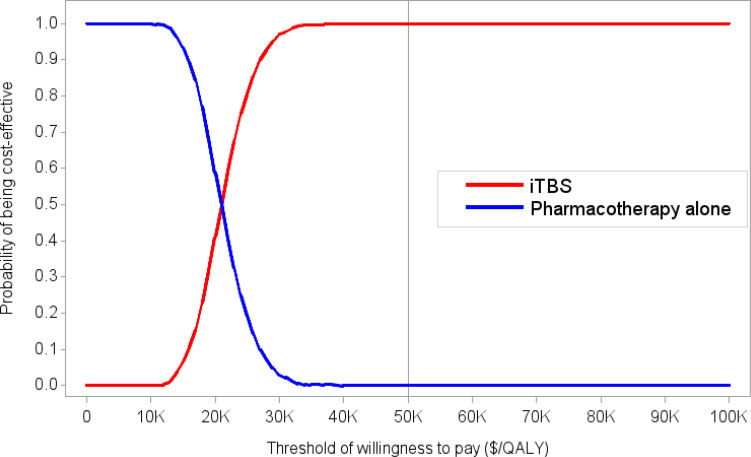
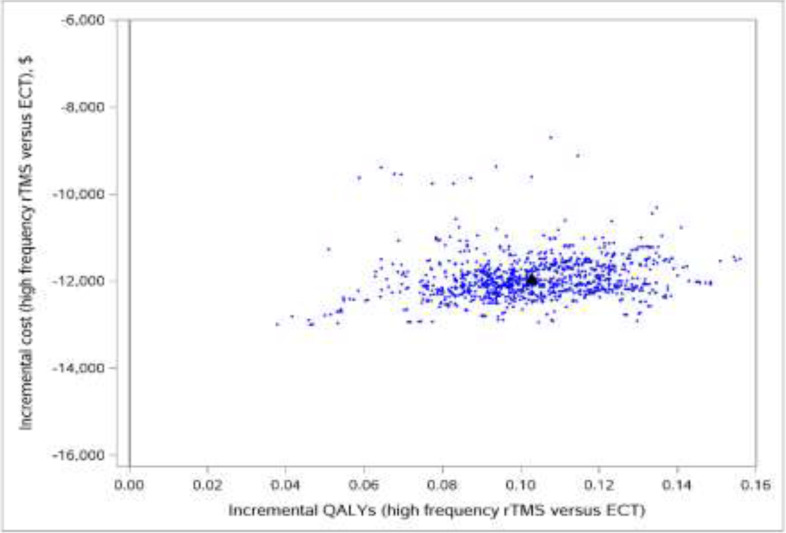
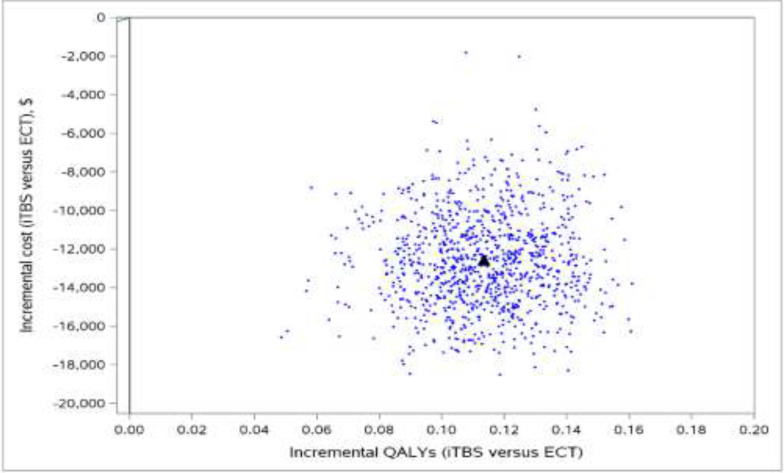
We included 58 primary studies, 9 systematic reviews, and 1 network meta-analysis in the clinical evidence review. Most rTMS modalities were more effective than sham treatment for all outcomes (GRADE: Moderate to High). All rTMS modalities were similar to one another in response and remission rates (GRADE: not reported) and were similar to electroconvulsive therapy (ECT) in response and remission rates (GRADE: Moderate). Moreover, in both the reference case and scenario analyses, two rTMS modalities (rTMS or iTBS), followed by ECT when patients did not respond to initial treatment, were less expensive and more effective than ECT alone. They were cost-effective compared with pharmacotherapy alone at a willingness-to-pay amount of $50,000 per quality-adjusted life-year (QALY). The annual budget impact of publicly funding rTMS would range from $9.3 million in year 1 to $15.76 million in year 5, for a total of $63.2 million over the next 5 years. People with TRD we spoke with reported that their experiences were generally favourable, and their attitudes toward rTMS were positive. Similarly, psychiatrists had positive attitudes toward and acceptance of rTMS. Our quantitative literature review on preferences revealed some gaps in psychiatrists’ knowledge of rTMS, which could have been influenced by their level of training on rTMS.
Conclusions
Most rTMS modalities are likely more effective than sham rTMS on all outcomes. All rTMS modalities are similar to ECT and to one another in response and remission rates. Compared with ECT alone, two rTMS modalities (high-frequency rTMS and iTBS), followed by ECT when necessary in a stepped care pathway, were less costly and more effective for managing adults with TRD. These types of rTMS (high-frequency rTMS and iTBS) were cost-effective compared with pharmacotherapy alone at a willingness-to-pay amount of $50,000 per QALY. Publicly funding rTMS (high-frequency rTMS and iTBS) for the treatment of adults with TRD in Ontario over the next 5 years would add $63.2 million in total costs. People with TRD had positive experiences and attitudes toward rTMS.
Objective
This health technology assessment evaluates the effectiveness, safety, and cost-effectiveness of repetitive transcranial magnetic stimulation (rTMS) for people with treatment-resistant depression (TRD). It also evaluates the budget impact of publicly funding rTMS and the experiences, preferences, and values of people with TRD.
Background
Health Condition
Major and Bipolar Depression
Major depression (also known as clinical depression, major depressive disorder, or unipolar depression) is a serious public health issue resulting in personal, societal, and economic burdens.1–3 Symptoms of depression are highly individual, but people often have persistent feelings of sadness, feelings of irritability, feelings of hopelessness, and difficulty feeling pleasure in most activities.1,4,5 They sometimes also have changes in energy, appetite, or sleeping patterns and are less able to concentrate. Some people with major depression have recurrent thoughts about self-harm or death.1 According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5),6 major depression is diagnosed if at least five specified symptoms are present during the same 2-week period and if the symptoms cause significant distress or impair occupational, social, or other important areas of function.6
Major depression develops from a complex interaction between various biological, psychological, and social factors. These can include a family or genetic history of depression, chronic health conditions, psychological or emotional vulnerability to depression, and life events or environmental stressors.4
Bipolar disorder (formerly known as manic-depressive disorder) is categorized as a mood disorder in the DSM-5.6 Bipolar disorder (types I and II) comprises both depression and mania. Both major and bipolar depression are included in this review.
Treatment-Resistant Depression
Pharmacotherapy is the common first-line treatment for depression. If available, psychotherapy can be used in conjunction with pharmacotherapy. Treatment-resistant depression is used to define a form of depression that does not improve despite the use of multiple and adequately dosed antidepressant medications. While many definitions are used to characterize treatment resistance, it is most often defined as an inadequate response to at least two appropriate courses of antidepressant medications.7,8 The definition of treatment failure can also vary, ranging from failure to achieve a response to failure to achieve full symptom remission.
Clinical Need and Target Population
Major depression is one of the most diagnosed mental illnesses in Canada. Each year, about 7% of people meet the diagnostic criteria for major depression, and approximately 13% to 15% of those will experience major depression for the rest of their lives.5,9,10 Bipolar disorder is less prevalent in Canada.
In 2012, bipolar disorder I and II lifetime prevalence was 0.87% (95% CI 0.67% to 1.07%) and 0.57% (95% CI 0.44% to 0.71%), respectively.11
The prevalence of TRD is difficult to ascertain, given the varied definitions of treatment failure. One small Ontario case series defined TRD as failure to respond to at least two antidepressants from different classes and estimated its prevalence among primary care patients at 24%.12 A randomized trial defined TRD as failing to achieve response after two courses of adequate treatment and estimated its prevalence at 35%.8 Using this figure, a previous Health Quality Ontario (now a part of Ontario Health) economic analysis estimated that 160,800 people 15 years of age or older in Ontario have major depression that is resistant to two courses of antidepressant treatment.13
Current Treatment Options
Pharmacotherapy and (if available) psychotherapy are common first-line treatments for depression. First-line pharmacotherapy treatment for major depression is antidepressants, whereas for bipolar depression it is mood stabilizers, antipsychotic drugs, or a combination of both. Antidepressant pharmacotherapy can include selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), atypical antidepressants, monoamine oxidase inhibitors (MAOIs), and tricyclic antidepressants.14 For some patients, it takes time to find the correct medication and dosage. Several strategies for pharmacological treatment exist: optimization of the antidepressant dose, “switching” within and between classes of compounds, or “combining” antidepressant therapies with each other or with, for example, antipsychotic therapies.15 Pharmacotherapy can take about 4 to 8 weeks to show full effect.
Various types of psychotherapy can allow patients to move toward a healthier emotional state and overcome negative emotions, such as sadness and anger. Types of psychotherapy include cognitive behavioural therapy, psychoanalysis, and psychodynamic therapies. Psychotherapy can be delivered face to face, in a group, or via the Internet (iCBT).16
If symptoms of depression continue despite antidepressant trials and psychotherapy or other treatments, it is critical that physicians first ensure that patients have had an adequate dose of medication for long enough to take effect and then re-evaluate the diagnosis before labelling the condition as TRD.
People diagnosed with TRD might continue antidepressants and psychotherapy in addition to neurostimulation treatments. Electroconvulsive therapy (ECT) is considered the most effective neurostimulation treatment for severe major depression that has not responded to any other treatment.17 The technique uses a machine to send brief electrical stimuli to the brain to induce a seizure. Anesthesia and muscle relaxants are needed to prevent muscle spasm, pain, or injury during ECT. In recent years, the technique has greatly improved and can more safely provide relief for patients with severe major depression.18
Health Technology Under Review
Repetitive transcranial magnetic stimulation is a noninvasive neurostimulation procedure that uses a series of focused magnetic field pulses to modulate the activity of nerve cells in the regions of the brain associated with mood regulation and depression. The magnetic field is created by a hand-held or helmet-like stimulating coil that is placed on the scalp over the target area. Various types of stimulating coils have been designed, each of which produces different magnetic field patterns. A circular coil (round coil) was the original design, but it could not deliver stimulation deep into the brain. Other types of coils have been designed to generate more focal and deeper stimulation. A double-cone coil conforms to the shape of the head to deliver deeper stimulation, and a figure 8 design (butterfly coil) produces a more focal pattern of activation. An H-coil has been designed for deep transcranial magnetic stimulation (TMS) to allow the depth of stimulation to target the subgenual anterior cingulate cortex.18
By repetitively stimulating neurons, the magnetic pulses change the function of the brain circuits involved, producing increases or decreases in brain activity. The level of neuronal activity depends on both the frequency and intensity of stimulation applied. Parameters for rTMS include stimulation intensity, frequency, pattern, and site. For major depression, rTMS has been applied using various modalities (protocols), including low-frequency (1 Hz) or high-frequency stimulation (10–20 Hz), unilateral or bilateral stimulation, intermittent theta burst stimulation (iTBS), continuous theta burst stimulation, and deep TMS.
Repetitive transcranial magnetic stimulation is thought to normalize hyperactive or hypoactive activity in the target brain regions that are associated with major depression and bipolar depression.19 The antidepressant effects of rTMS could be associated with many neurobiological changes in brain regions that are linked with the stimulated area. Brain imaging studies of the anatomic and functional brain activity show the left dorsolateral prefrontal cortex (DLPFC) receives input from specific sensory cortices and has dense interconnections with the subcortical areas involved in emotional modulation, such as the limbic area and the striatum.20,21 Among people with TRD, rTMS has the potential to improve symptoms when used alone or in addition to antidepressant medication.
According to clinical experts (A. Burhan, R. Milev, J. Downar, P. Giacobbe, J.G. Gagnon, teleconferences, June and July 2019), if the depression is categorized as treatment-resistant, both major depression and bipolar depression are treated with rTMS in Ontario. There is a small risk (about 3.1%) that those with bipolar depression will develop hypomania (a mood state characterized by persistent disinhibition and mood elevation) during rTMS treatment. This risk is similar to that of medications.22
Repetitive transcranial magnetic stimulation can be done in both an inpatient and outpatient setting, while patients are seated and awake without sedation. The initial treatment course comprises at least 20 to 30 sessions delivered once daily, five times weekly, in sessions ranging between 3 and 45 minutes (depending on the rTMS protocol used). The initial course of treatment can be for 4 to 6 weeks. People with TRD could need maintenance treatment at the discretion of the clinician. This might consist of one to two treatment sessions a week. No activities are restricted after the procedure.
Safety Guidelines and Contraindications
Clinical guidelines with respect to the margin of safety with rTMS were originally based on the evidence provided by Wassermann23 that was subsequently updated by Rossi et al.24 The US Food and Drug Administration cited the work by Wassermann23 and Rossi et al24 as a clinical guide to avoid stimulation parameters that fall outside safety recommendations and that can cause adverse events such as seizure or syncope.
According to the safety guideline by Rossi et al,24 the only absolute contraindication to rTMS is the presence of metallic hardware in close contact with the discharging rTMS coil. In such instances, there is a risk that these implanted devices will malfunction. Relative contraindications include the presence of a cardiac pacemaker or implantable defibrillator, a history of epilepsy, or a brain lesion (vascular, traumatic, neoplastic, infectious, or metabolic).
Regulatory Information
Health Canada approved the clinical use of rTMS in 2002, and four companies have multiple systems licensed for use (Table 1). Only one device was directly indicated for treatment of patients with major depression who are treatment-resistant. However, experts indicate that the others have been used for this treatment as well.18
Table 1:
rTMS Devices Licensed by Health Canada and Their Intended Use
| Manufacturer | Device Name | License Number | Intended Use |
|---|---|---|---|
| Brainsway Limited | Deep TMS System | 90504 | Indicated for treatment of depressive episodes in patients with major depression who have failed to benefit from or are intolerant to antidepressant drugs |
| Tonica Elektronik A/S | Magpro Compact Magnetic Stimulator | 12164 | For magnetic stimulation of the central nervous system |
| Magpro X100 Magnetic Stimulator System | 60608 | For noninvasive stimulation of nerves in the central and peripheral nervous systems. Used short-term to examine the physiology of motor pathways, ascertain the function of motor nerve stimulation, examine human cortical physiology, change muscle function in a therapeutic manner, and change brain activity in a therapeutic manner | |
| Magpro R30 Magnetic Stimulator | 68484 | Electrophysiologic aid for assessment, diagnosis, and prognosis and for monitoring diseases of the nervous system | |
| Magstim Company Limited | Magstim model 2002 | 70387 | Nerve stimulator that induces electrical current through electromagnetic pulses. Capable of stimulating neural tissue |
| Magstim Horizon TMS Therapy System | 102253 | ||
| Nexstim | Nexstim NBS System 5 | 102644 | Indicated for treatment of major depression |
| Nexstim NBT System 2 | 103949 |
Abbreviations: NBS, navigated brain stimulation; NBT, navigated brain therapy; rTMS, repetitive transcranial magnetic stimulation.
Ontario and Canadian Context
Repetitive transcranial magnetic stimulation is not a publicly funded service in Ontario. In 2016, a health technology assessment was done, and the Ontario Health Technology Advisory Committee (OHTAC) recommended that rTMS be publicly funded for patients with non-psychotic TRD only when ECT is not an option.18 This recommendation remains under review by the Ministry of Health. This recommendation is also supported by a Health Quality Ontario quality standard on major depression, which states that rTMS may be considered as an alternative treatment when ECT is contraindicated or not chosen by the patient.5 The 2016 guidelines from the Canadian Network for Mood and Anxiety Treatments state, “rTMS and ECT differ in mechanism, tolerability, and acceptability by patients and may be best understood as complementary rather than competing techniques. Likewise, rTMS response rates are poor in patients where ECT has failed. These findings indicate that rTMS should be considered prior to pursuing ECT.”17
Two rTMS modalities are often used in Ontario: high-frequency rTMS and iTBS. These two modalities are used most often because a recent noninferiority trial completed in Canada found that high-frequency rTMS and iTBS were equivalent.25 Based on this trial and the use of these modalities in the Ontario context, the economic analysis models an rTMS clinical pathway using these modalities.
Several clinics in Ontario offer rTMS. Some are private clinics where patients can pay out of pocket for access to the procedure, or through independent insurance coverage. Other clinics in Ontario offer rTMS through their hospital budget, or hospital donation funds and research grants.
In Canada, rTMS is publicly funded in Quebec, Saskatchewan, and Alberta.
Expert Consultation
We engaged with experts in the specialty of mental health to help inform our understanding of aspects of the health technology and our methodologies and to contextualize the evidence.
PROSPERO Registration
This health technology assessment has been registered in PROSPERO, the international prospective register of systematic reviews (CRD42020151553), available at https://www.crd.york.ac.uk/PROSPERO.
Clinical Evidence
Research Questions
Question 1: What are the effectiveness and safety of repetitive transcranial magnetic stimulation (rTMS) compared with sham rTMS for the treatment of adults with treatment-resistant depression (TRD)?
Question 2: What are the effectiveness and safety of rTMS compared with electroconvulsive therapy (ECT) for adults with TRD?
Question 3: What is the comparative effectiveness of various modalities of rTMS for adults with TRD?
Methods
Clinical Literature Search
We performed a clinical literature search on August 27, 2019, to retrieve studies published from January 2014 until the search date. We chose the year 2014 based on when the literature search was run for the previous rTMS health technology assessment conducted by Health Quality Ontario. We used the Ovid interface in the following databases: MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, the Health Technology Assessment database, the National Health Service Economic Evaluation Database (NHS EED), and PsycINFO. We used the EBSCOhost interface to search the Cumulative Index to Nursing & Allied Health Literature (CINAHL).
A medical librarian developed the search strategies using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. Methodological filters were used to limit retrieval to systematic reviews and meta-analyses. The final search strategy was peer-reviewed using the PRESS Checklist.26
We created database auto-alerts in MEDLINE, Embase, PsycINFO, and CINAHL and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites as well as clinical trial and systematic review registries. The grey literature search was updated January 2, 2020. See Appendix 1 for our literature search strategies, including all search terms.
To address research question 1, we identified primary studies through systematic reviews. For questions 2 and 3, we used only systematic reviews to address the research questions. Below are the inclusion and exclusion criteria for this clinical review. If the criteria pertain to only particular research questions, they are specified below.
Eligibility Criteria
STUDIES
Inclusion Criteria
English-language full-text publications
Studies published after January 2014
-
Question 1
– Randomized controlled trials (RCTs)
-
Questions 2 and 3
– Systematic reviews, meta-analyses, and network meta-analyses of comparative studies (i.e., RCTs)
Exclusion Criteria
Animal and in vitro studies
Abstracts, editorials, letters, case reports, and commentaries
-
Question 1
– Non-comparative studies, cross-over trials, observational studies
-
Questions 2 and 3
– Nonsystematic reviews, narrative reviews, RCTs, observational studies, noncomparative studies
– Studies for which the literature search was conducted before 2014
PARTICIPANTS
Inclusion Criteria
Adults (18 years of age and older) with TRD (major depression or bipolar depression)
Exclusion Criteria
Other conditions for which rTMS is used (e.g., obsessive–compulsive disorder, post-traumatic stress disorder)
Secondary major depression (e.g., post-stroke depression)
Adolescents (< 18 years of age)
INTERVENTIONS
Inclusion Criteria
-
Repetitive transcranial magnetic stimulation (with or without concomitant antidepressants)
– Any modality of rTMS (i.e., deep transcranial magnetic stimulation [TMS], intermittent theta burst stimulation [iTBS], continuous theta burst stimulation [cTBS], high or low frequency rTMS, unilateral or bilateral rTMS)
Exclusion Criteria
-
Question 1
– Studies that use treatment parameters that are not within safety guidelines
– Studies that administer fewer than 10 sessions for the initial course of treatment
-
Questions 2 and 3
– Systematic reviews that include studies that use treatment protocols that are not within safety guidelines for the intervention
– Systematic reviews that include studies that administer fewer than 10 sessions for the initial course of treatment
OUTCOME MEASURES
-
Changes from baseline depression scores (e.g., Hamilton Depression Rating Scale, Beck Depression Scale)
– Minimal clinically important difference is indicated to be between 2 and 3 on Hamilton Depression Rating Scale27
Remission rate (as defined by study)
Response rate (defined as ≥ 50% reduction in depression score)
Relapse rate (as defined by study)
Adverse events (as reported by study)
Acceptability (i.e., discontinuation of treatment)
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Covidence28 and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. The reviewer also examined the full-text articles and selected studies eligible for inclusion.
Data Extraction
We extracted relevant data at both the systematic review and primary study level on study characteristics and risk-of-bias items using a data form to collect information on the following:
Systematic review characteristics (study author, year, country, inclusion and exclusion characteristics for the population, intervention and comparators, databases searched, statistical analysis used, outcomes reported)
Clinical characteristics of population in primary studies (number of patients in each arm, mean age, definition of medication resistance, use of medication during trial)
Technical parameters of rTMS (frequency and intensity of stimulation, number of trains, train duration, duration of inter-train interval, number of sessions, number of pulses per session, number of total pulses)
Methods (e.g., study design, study duration, participant allocation, allocation sequence concealment, blinding, reporting of missing data, reporting of outcomes)
Outcomes (e.g., outcomes measured, definition of response and remission, number of participants for each outcome, number of participants missing for each outcome, outcome definition and source of information, unit of measurement, time points at which the outcomes were assessed)
We contacted study authors to provide clarification as needed.
Statistical Analysis
We assessed the overlap of studies in the included systematic reviews by using a study matrix and calculating the corrected covered area (CCA), a numerical measure by Pieper et al.29 The CCA is interpreted as slight (CCA 0–5), moderate (CCA 6–10), high (CCA 10–15), or very high (CCA > 15).
For question 1, we undertook meta-analyses for reported outcomes to determine the pooled estimate of effect of rTMS (any modality) compared with sham treatment, using Review Manager.30,31 For continuous scores such as “change in depression score,” we calculated the weighted mean difference; for binary data such as remission and response rates, we used risk ratios and risk differences as the pooled summary estimates because they accurately represented the data from the individual studies.
For the continuous outcome of “change in depression score,” both change scores and final scores are included in the analyses. On the basis of guidance from the Cochrane handbook,32 mixing outcomes is not a problem when it comes to a meta-analysis of mean differences because, in a randomized trial, mean differences based on changes from baseline can usually be assumed to be addressing exactly the same underlying intervention effects as analyses based on final measurements. Cochrane also advises to separate change scores and final scores into subgroups to avoid confusion, but the results of subgroups can legitimately be pooled.
We assessed the degree of statistical heterogeneity among studies using the I2 statistic for each outcome. An I2 > 50% was considered to be substantial heterogeneity. We used random− or fixed-effects models for meta-analysis following the guidance of the Cochrane handbook.32 Mental health research can involve heterogeneity, and focusing solely on statistical heterogeneity may oversimplify when it comes to complex, real-world data.33 Because previous systematic reviews have combined data and pooled estimates with substantial heterogeneity,18,34,35 we decided to combine estimates as well, but took the substantial heterogeneity into account in our critical appraisal of the evidence.
We completed two sensitivity analyses to establish trends in prespecified, clinically meaningful patient populations for the outcomes of “change in depression score” and “response rate“:
Type of depression (major depression vs. mixed population [major depression and bipolar depression])
Antidepressant status
Statistical analyses will be reported as originally presented in the systematic reviews for questions 2 and 3.
Critical Appraisal of Evidence
We assessed risk of bias of the systematic reviews using the Risk of Bias in Systematic Reviews (ROBIS) tool.36 We report the critical appraisal as reported by the systematic review authors. For the primary studies identified in the Canadian Agency for Drugs and Technologies in Health (CADTH) Rapid Response, we assessed the risk of bias using the Cochrane risk-of-bias tool for randomized studies.37
For the quality of the body of evidence, we prioritized our reporting using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.38 The GRADE system uses the body of evidence based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The overall rating reflects our certainty in the evidence. Where a comprehensive assessment of the body of evidence was not completed, we reported only risk of bias as reported by the systematic review authors.
If a primary study was captured in multiple systematic reviews, we used the most comprehensive, recent, and highest-quality systematic review according to the ROBIS assessment to report risk-of-bias assessment (Appendix 2). If the primary study was captured in only one systematic review, we used that review's quality assessment, regardless of ROBIS assessment.
Results
Clinical Literature Search
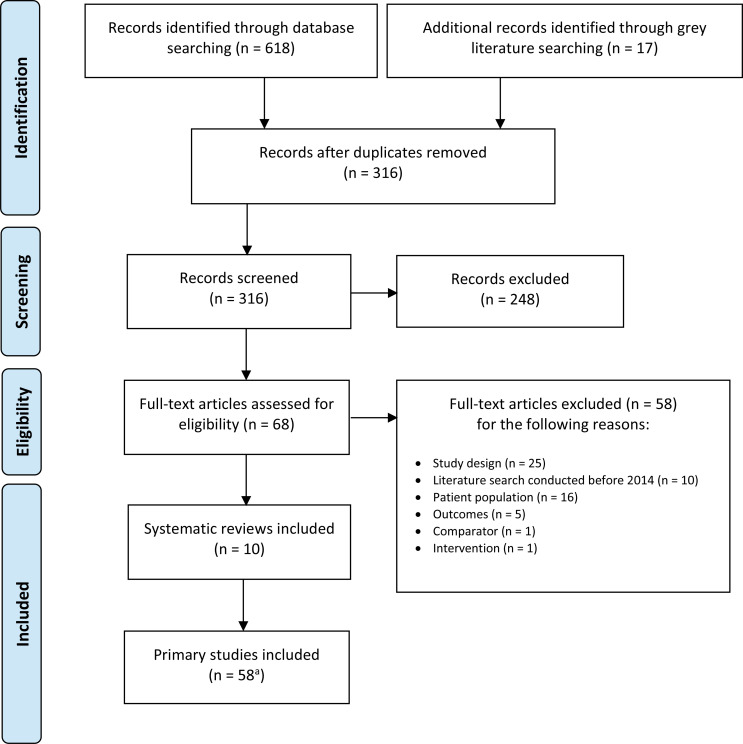
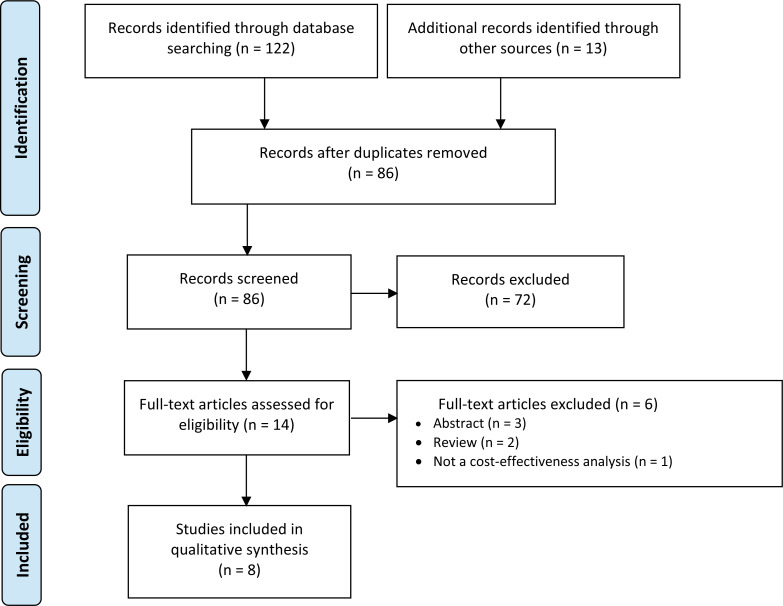
The database search of the clinical literature yielded 316 citations published between January 2014 and August 27, 2019, after duplicate records were removed. We identified 17 additional studies through grey literature. We identified 10 studies (9 systematic reviews and 1 network meta-analysis) that met our inclusion criteria. See Appendix 6 for a list of selected studies excluded after full-text review. Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)39 flow diagram for the clinical literature search.
Figure 1: PRISMA Flow Diagram—Clinical Search Strategy.
Source: Adapted from Moher et al.39
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
aPrimary studies identified through the 10 included systematic reviews.
Characteristics of Included Systematic Reviews
Nine systematic reviews and one network meta-analysis met the inclusion criteria for this clinical review. The included reviews were published between 2015 and 2019 and varied in their focus. Some included specific rTMS modalities, while others included any rTMS modality. Comparators for some reviews were just sham treatment, while others included other rTMS modalities and ECT. Some focused on major depression, while others included a mixed population of both major depression and bipolar depression (Table 2).
Table 2:
Characteristics of Included Systematic Reviews
| Author, Year Country | Literature Search Study Selection | No. of Studies | Population | Intervention | Comparators | Conclusion | ROBIS |
|---|---|---|---|---|---|---|---|
| Lepping et al, 201442 United Kingdom | Up to January 15, 2014 RCTs or non-RCTs (such as open-label or naturalistic trials) | 21a |
Inclusion: humans with a formal diagnosis of depression, irrespective of depression subtype or diagnostic criteria used Exclusion: studies where depression was not the primary diagnosis; adolescents or children |
Inclusion: rTMS as monotherapy or add-on therapy Exclusion: nonstandard rTMS (e.g., deep TMS or stimulation outside the DLPFC) |
Sham rTMS Another rTMS modality ECT | rTMS is superior to sham rTMS in treatment of TRD | High ROB |
| Zhang et al, 201543 China | Up to January 2014 RCTs | 10 |
Inclusion: adults diagnosed with major depression according to DSM or ICD, patients who met TRD criteria (defined by failure to respond to at least one course of adequate treatment for major depression) Exclusion: TRD patients with comorbid neurological disorders and psychotic disorders or specific types of depression (e.g., child and adolescent depression or postpartum depression) |
Inclusion: bilateral or unilateral rTMS | Sham rTMS Another rTMS modality | Clinical efficacy of bilateral rTMS was not significantly greater than of unilateral rTMS but is superior to sham TMS in people with TRD | Low ROB |
| Leggett et al, 201540 Canada |
Up to January 10, 2014 RCTs | 46a |
Inclusion: adults (≥ 18 years of ageb) who have had TRD (≥ 2 previous treatments) or bipolar or unipolar depression Exclusion: not TRD or do not report whether patients have TRD |
Inclusion: any form of rTMS | Sham rTMS Pharmacological therapyc Cognitive therapyc ECT Another modality of rTMS | rTMS is approximately twice as effective as sham TMS; however optimal rTMS modality remains unclear rTMS most likely as effective as ECT | Low ROB |
| Health Quality Ontario, 201618 Canada |
Up to March 1, 2015 RCTs | 30a |
Inclusion: studies with adults ≥ 18 years of age; at least 80% of patients were resistant to treatment (TRD population), studies that included unipolar patients only or that reported the proportion of bipolar patients as ≤ 20% Exclusion: Studies of depression due to specific conditions (i.e., post-stroke depression, postpartum depression) |
Inclusion: studies that applied HF rTMS (≥ 5 Hz) to left DLPFC (unilateral) and complied with safety guidelines; studies in which patients received at least 10 sessions of rTMS treatment Exclusion: studies with stimulation other than left DLPFC, used frequencies of rTMS outside range of this review, bilateral rTMS or bilateral vs. unilateral rTMS, sequential combined LF and HF rTMS, newer techniques (synchronized rTMS, pulsed rTMS, deep TMS, rTMS with priming stimulation) |
Sham rTMS ECT | rTMS has small short-term effect compared with sham TMS on improving depression scores Significantly more improvement in depression scores with ECT than with rTMS |
Low ROB |
| Nordenskjold et al, 201644 Sweden | Up to Nov 2014 Controlled studies with or without randomization | 1 | Inclusion: people with major depression or bipolar depression according to DSM or ICD criteria | Inclusion: H-coil deep TMS | Another treatmentc Sham deep TMS Different dose of deep TMSc | Evidence for deep TMS is considered insufficient for TRD | Low ROB |
| Berlim et al, 201745 Canada and United Kingdom | Jan 1, 2001, to Sept 6, 2016 RCTs, parallel or crossover trials | 5 | Inclusion: adults aged 18-75 years with a diagnosis of primary major depression (unipolar or bipolar) according to DSM or ICD criteria | Inclusion: Unilateral iTBS to the left DLPFC, unilateral cTBS to the right DLPFC, or consecutive iTBS/cTBS to the DLPFC given for ≥ 5 sessions either as monotherapy or as augmentation strategy for major depression | Sham TBS Pre-post active TBS | TBS (particularly cTBS and bilateral iTBS) is associated with substantial antidepressant effects, but researchers cannot draw definitive conclusions | Low ROB |
| Brunoni et al, 201746 Brazil and Canada |
Up to Oct 1, 2016 RCTs | 59 |
Inclusion: people with a primary diagnosis of an acute unipolar or bipolar depressive episode, including those who did not preclude comorbidities, such as anxiety or personality disorders Exclusion: studies with secondary mood disorders (e.g., post-stroke depression) |
Inclusion: LF rTMS over the right DLPFC, HF rTMS over the left DLPFC, bilateral rTMS (LF over the right and HF over the left DLPFC), TBS (including iTBS over the left DLPFC, cTBS over the right DLPFC, or bilateral TBS), pTMS over the right DLPFC, aTMS over the left DLPFC, sTMS, deep TMS over the left DLPFC, and sham. Also, 1 Hz or less and 5 Hz or more defined LF and HF, respectively Exclusion: studies performing more than 10 rTMS sessions, using frequencies of 2–4 Hz |
Sham rTMS Another rTMS modality | Few differences were found in clinical efficacy and acceptability between various rTMS modalities, favouring to some extent bilateral rTMS and priming LF rTMS, respectively | High ROB |
| University of Calgary, 201734 Canada |
Up to Jan 10, 2014 RCTs | 61a |
Inclusion: adults (18 years or older) diagnosed with unipolar or bipolar depression with TRD (had ≥ 2 treatments) Exclusion: not TRD or do not report whether patients have TRD, not unipolar or bipolar depression |
Inclusion: any form of rTMS Exclusion: not rTMS |
Sham rTMS ECT Cognitive therapyc Pharmaceuticalsc Another rTMS modality | rTMS is effective when compared with sham rTMS. Optimal frequency, location, and intensity of rTMS are unclear Effectiveness of rTMS compared with ECT is unclear | Low ROB |
| Mutz et al, 201835 United Kingdom | Up to May 1, 2018 RCTs, parallel or crossover trials | 33 |
Inclusion: adults aged 18–70 years, DSM or ICD diagnosis of major depression or bipolar disorder currently in a major episode Exclusion: primary diagnosis other than major depression or bipolar depression, studies limited to a specific subtype of depression |
Inclusion: Any form of rTMS | Sham rTMS | HF left DLPFC rTMS was associated with improved rates of response compared with sham in people with TRD | Low ROB |
| Sehatzadeh et al, 201941 Canada |
Up to Apr 3, 2017 RCTs | 23 |
Inclusion: people who did not respond to treatment with antidepressant medications (TRD) diagnosed with unipolar depression, study populations that had less than 20% bipolar patients Exclusion: people with depression due to specific conditions (i.e., post-stroke depression, postpartum depression) |
Inclusion: unilateral rTMS that applied HF rTMS to the left DLPFC, sequential bilateral rTMS that applied LF rTMS to the right DLPFC, and HF rTMS to the left DLPFC, had one treatment session daily and had at least 10 sessions Exclusion: novel rTMS interventions, studies that exceeded maximum allowed stimulation parameters set by safety guidelines |
Sham rTMS | rTMS has moderate antidepressant effects for people with unipolar TRD | Low ROB |
Abbreviations: aTMS, accelerated transcranial magnetic stimulation; cTBS, continuous theta burst stimulation; DLPFC, dorsolateral prefrontal cortex; DSM, Diagnostic and Statistical Manual of Mental Disorders; ECT, electroconvulsive therapy; HF, high frequency; ICD, International Classification of Diseases; iTBS, intermittent theta burst stimulation; LF, low frequency; pTMS, priming transcranial magnetic stimulation; RCT, randomized controlled trial; ROB, risk of bias; ROBIS, Risk of Bias in Systematic Reviews; rTMS, repetitive transcranial magnetic stimulation; sTMS, synchronized transcranial magnetic stimulation; TBS, theta burst stimulation; TMS, transcranial magnetic stimulation; TRD, treatment-resistant depression.
Includes rTMS vs. sham or another rTMS modality and rTMS vs. ECT studies if the review included that comparator.
Study also included youth but reported data separately.
Did not find any studies using this treatment as a comparator.
Two systematic reviews18,34 also had separate publications40,41 included in this overview of reviews. Both sets of reviews were included because there were discrepancies between the primary studies included across the reviews. We have highlighted where the initial review and the subsequent publication have the same results.
The 10 included reviews (9 systematic reviews and 1 network meta-analysis) are summarized in Table 2.
OVERLAP BETWEEN SYSTEMATIC REVIEWS
For question 1, rTMS versus sham, the reviews included 1 to 51 studies each, depending on their focus (number of studies generally reflected differences in inclusion and exclusion criteria). Approximately 73 unique studies were included in 9 systematic reviews and 1 network meta-analysis. To determine the amount of overlap of the primary studies included across the systematic reviews, we calculated the CCA for rTMS versus sham treatment. We found that coverage was very high—approximately 24.5%—meaning that the systematic reviews included many of the same primary studies.
For question 2, four systematic reviews used ECT as a comparator. The reviews included 5 to 7 studies each, totalling 10 unique studies that focused on rTMS versus ECT. We calculated the CCA for rTMS versus ECT and found that coverage was approximately 47%, which is considered very high.
Last, for question 3, three systematic reviews and one network meta-analysis compared rTMS with another rTMS modality. The reviews included 5 to 14 studies each, depending on their focus (number of studies generally reflected differences in inclusion and exclusion criteria). Approximately 22 unique studies were included across systematic reviews. We calculated the CCA and found that coverage was approximately 31.8%, which is considered very high.
Risk of Bias in the Included Studies
We used the ROBIS tool to assess risk of bias in the included systematic reviews. Eight of the 10 systematic reviews had low risk of bias. The risk of bias ratings of the included systematic reviews are presented in Appendix 2 (Table A1).
Seven of the 10 reviews assessed risk of bias using some variation of the Cochrane risk-of-bias tool for randomized studies. Most primary studies had low or unclear bias. Risk-of-bias ratings of the primary studies are presented in Appendix 2 (Table A2).
The GRADE levels were assessed in only two systematic reviews.18,41 We present only the assessment from the Health Quality Ontario 2016 systematic review,18 because this was the initial review. The assessment of the overall body of evidence is presented in Appendix 2 (Tables A3 and A4).
Question 1: What Are the Effectiveness and Safety of rTMS Compared With Sham rTMS for Treatment of Adults With TRD?
For this question, we will re-analyze the unique primary studies identified by the systematic reviews and the four additional RCTs47–50 comparing rTMS versus sham rTMS identified through a 2019 Rapid Response from CADTH that captured RCTs published after the search dates of the systematic reviews.51 Results of the systematic reviews can be found in Appendix 4 (Tables A6–A9).
Using systematic reviews and the 2019 CADTH51 report as a source for primary studies, we identified 73 unique studies of any rTMS modality compared with sham across the systematic reviews and 4 additional studies that were not captured in the systematic reviews.
Within the 77 primary studies, 19 studies were excluded. Three studies were crossover trials.52–54 One study was a conference abstract.55 Four studies were not the appropriate population (two studies were in a post-stroke population and the other two were not exclusively a treatment-resistant population).56–59 Six studies administered only five rTMS sessions,60–65 and one study66 administered both high-frequency and low-frequency rTMS to the same group. Two studies did not have outcomes of interest67,68 and two studies presented outcomes in ways that could not be analyzed (for example, median values69 or stratified by age instead of group assignment70). Last, two studies used the same data,71,72 so the most recent publication was used in the analyses.71 Therefore, 58 primary studies were included. Baseline characteristics of the included primary studies can be found in Appendix 5 (Table A10).
Results are stratified by type of rTMS modality (high-frequency left DLPFC, low-frequency right DLPFC, deep TMS, etc.) compared with sham treatment for each outcome.
CHANGE IN DEPRESSION SCORE
High-Frequency Left DLPFC rTMS Versus Sham rTMS
Four RCTs studied more than two groups. In the analysis we used the higher intensity (110%) from Bakim et al,73 the higher frequency (20 Hz) from Su et al,74 the once-daily rTMS session in both active and sham group,75 and the rTMS group that received treatment on their left DLPFC (as opposed to right DLPFC in both active and sham groups).76 We excluded one study from this review because the rTMS used was outside safety standards and exceeded the limit set by these guidelines for maximum duration of trains and number of pulses.77 We excluded another study where rTMS was given to United States veterans, because the sample had a high prevalence of post-traumatic stress disorder.50
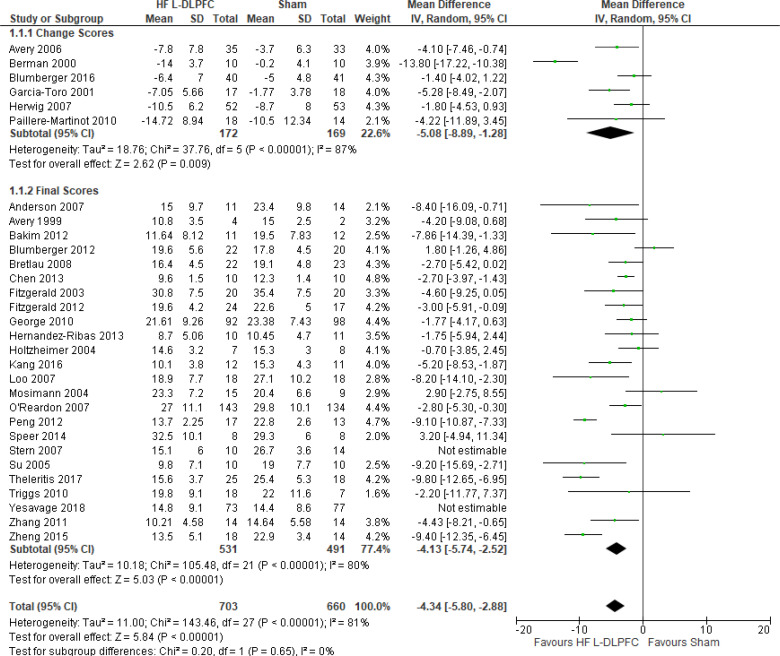
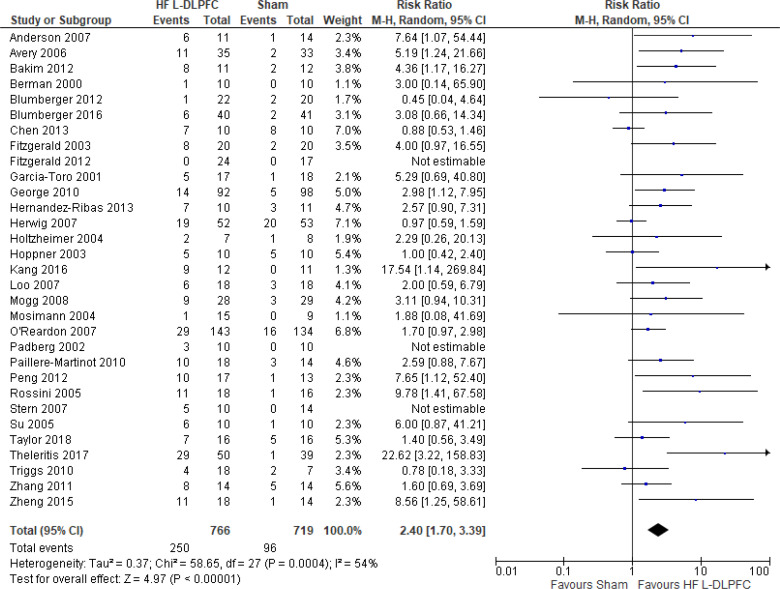
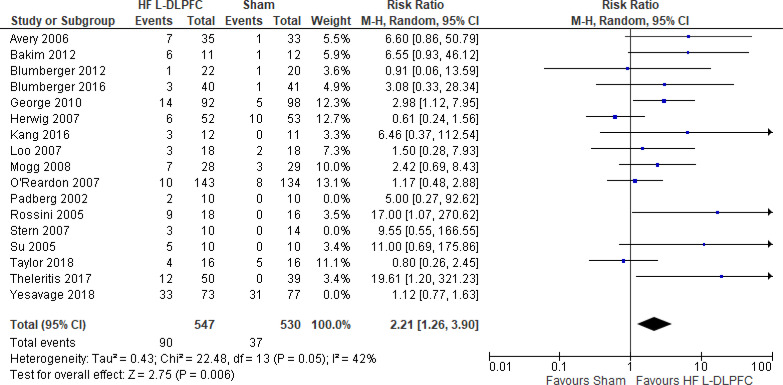
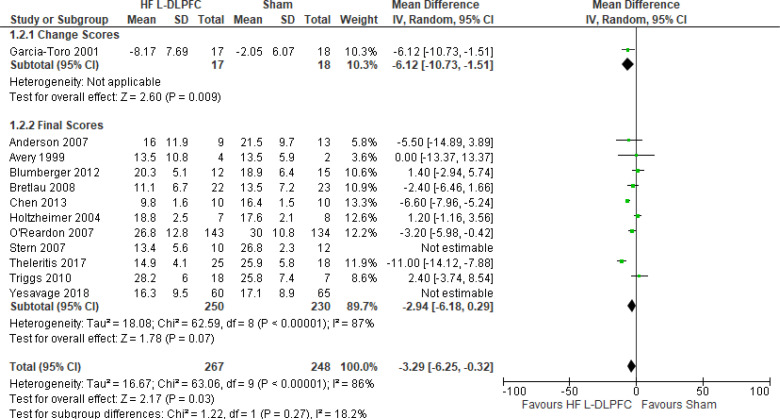
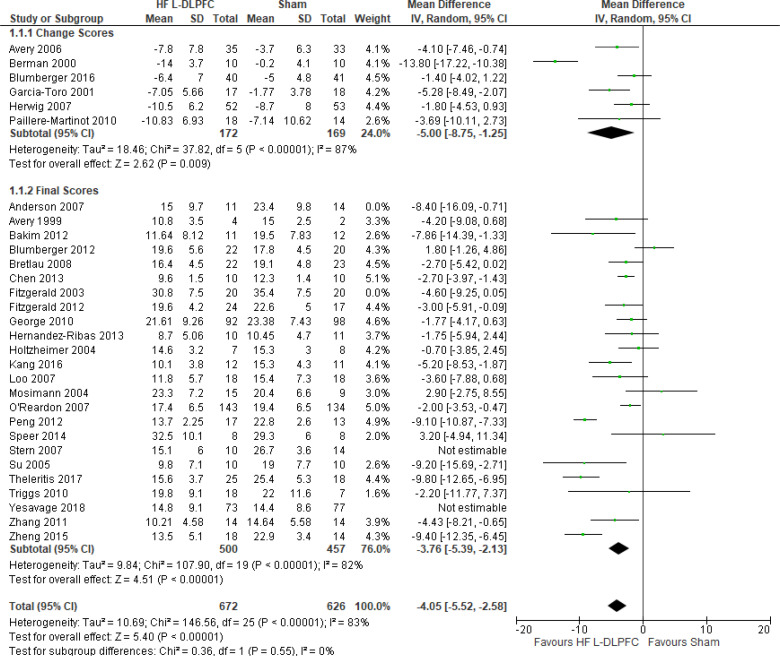
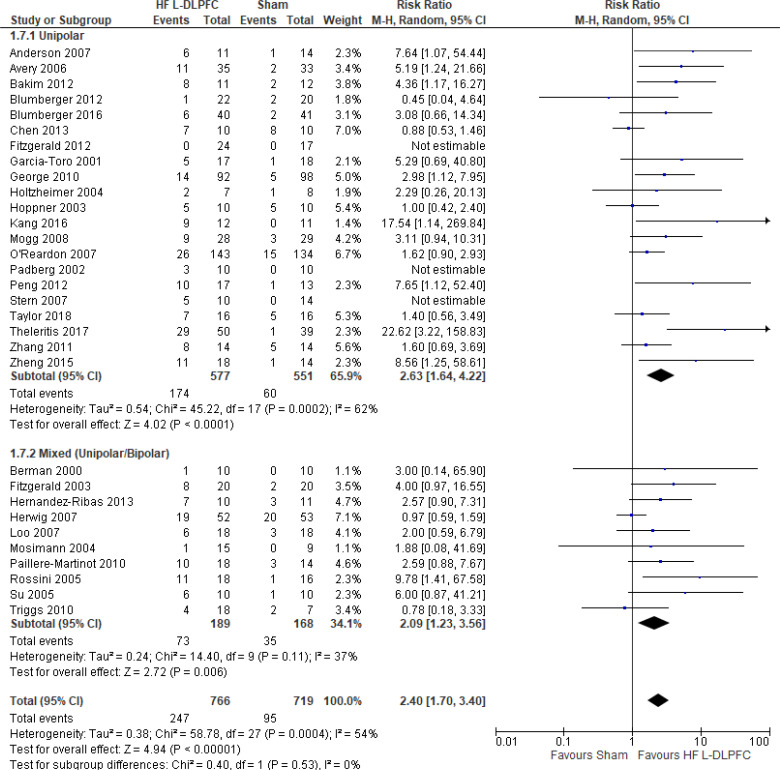
The effect of high-frequency rTMS applied to the left DLPFC on depression scores at end of the treatment phase (2–6 weeks) was examined by pooling data from 28 studies with 1,363 participants using a random-effects model (Figure 2). There were lower depression scores for those who received high-frequency left DLPFC rTMS compared with sham treatment (P < .00001).
Figure 2: High-Frequency Left DLPFC rTMS Versus Sham rTMS—Change in Depression Score at End of Treatment.
Sources: Anderson et al,78 Avery et al,79,80 Bakim et al,73 Berman et al,81 Blumberger et al,82,83 Bretlau et al,84 Chen et al,85 Fitzgerald et al,86,87 Garcia-Toro et al,88 George et al,89 Hernandez-Ribas et al,90 Herwig et al,91 Holtzheimer et al, 92 Kang et al,47 Loo et al,93 Mosimann et al,94 O'Reardon et al,95 Paillere Martinot et al,96 Peng et al,97 Speer et al,66 Stern et al,77 Su et al,74 Theleritis et al,75 Triggs et al,76 Yesavage et al,50 Zhang et al,98 Zheng et al.71
Abbreviations: CI, confidence interval; df, degrees of freedom; DLPFC, dorsolateral prefrontal cortex; HF, high frequency; IV, inverse variance; L, left; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation.
We conducted the same analyses for studies that included a longer follow-up (ranging from 3 weeks to 3 months). Ten studies included a longer follow-up; however, it should be noted that no rTMS maintenance treatments were given between the end of the acute treatment phase and the longer follow-up point. In this meta-analysis, there were still lower depression scores in the high-frequency left DLPFC rTMS group than in the sham group (P < .00001). Results can be found in Appendix 5, Figure A1.
We planned two subgroup analyses based on antidepressant status and type of depression. In both analyses, regardless of antidepressant status or type of depression, rTMS had lower depression scores than sham rTMS (P < .00001; Appendix 5, Figures A2 and A3).
We conducted one sensitivity analysis where we included only studies that used some version of the Hamilton Depression Rating Scale (HDRS). Three studies93,95,96 reported both Montgomery-Åsberg Depression Rating Scale (MADRS) and HDRS scores. For this analysis we used the scores from the HDRS. Regardless of the removal of two studies,78,86 people treated with high-frequency left DLPFC rTMS had lower depression scores than those treated with sham rTMS (P < .00001; Appendix 5, Figure A4).
The quality of the evidence for change in depression score using high-frequency left DLPFC rTMS was moderate (see Appendix 2, Table A3); it was downgraded for inconsistency because of high statistical heterogeneity (I2 = 81%).
Low-Frequency Right DLPFC rTMS Versus Sham rTMS
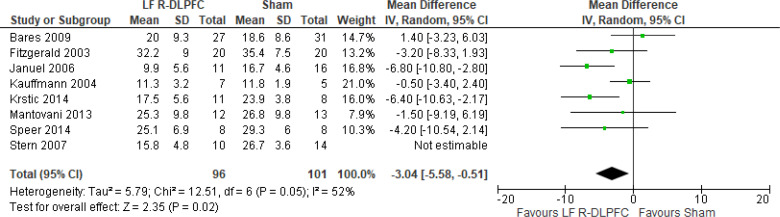
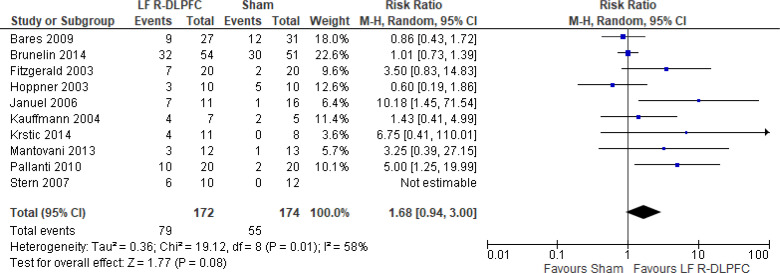
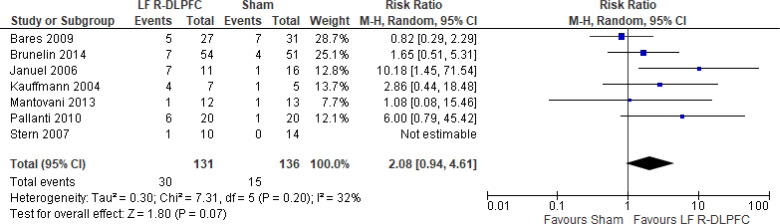
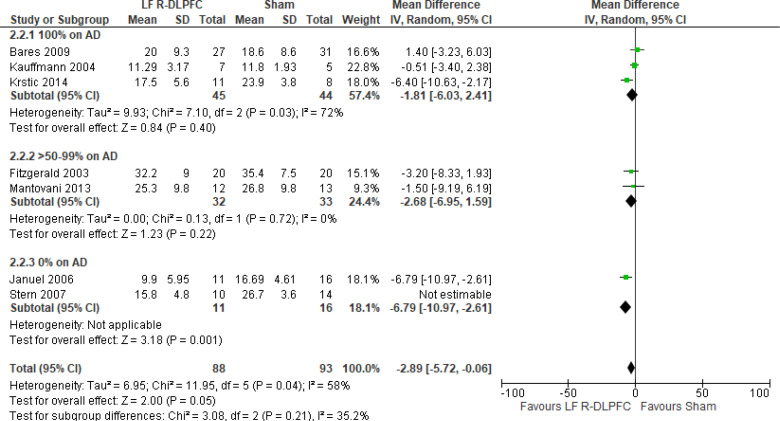
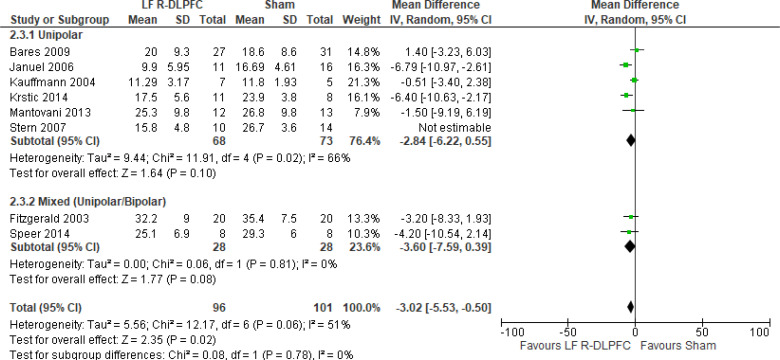
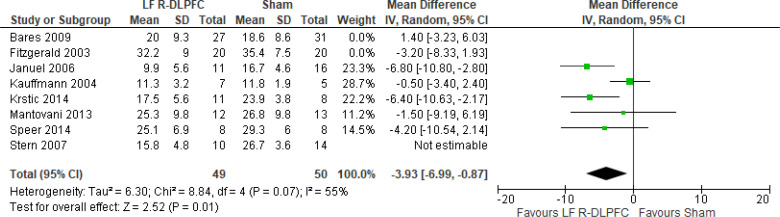
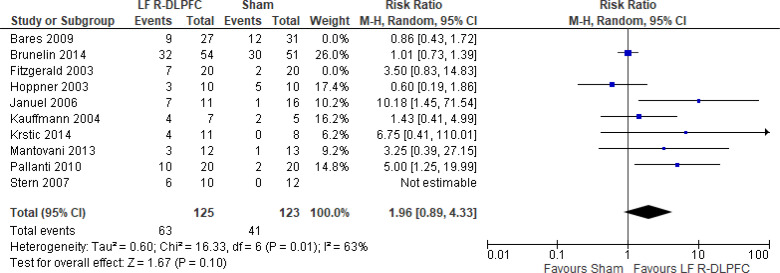
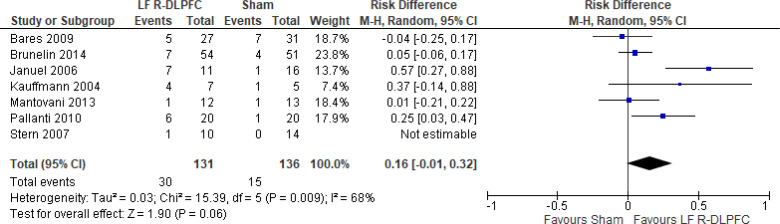
Of the studies we included in this review, we excluded one study because rTMS did not meet safety standards and exceeded the limit set by these guidelines for maximum duration of trains and number of pulses.77 The effect of low-frequency rTMS applied to the right DLPFC on change in depression scores at end of treatment phase (2–4 weeks) was examined by pooling data from seven studies with 197 participants using a random-effects model (Figure 3). Depression scores were lower for those who received low-frequency right DLPFC rTMS than for those who received sham treatment (P = .02).
Figure 3: Low-Frequency Right DLPFC rTMS Versus Sham rTMS—Change in Depression Score at End of Treatment.
Sources: Bares et al,99 Fitzgerald et al,86,100 Januel et al,101 Kauffmann et al,102 Krstic et al,103 Mantovani et al,104 Speer et al,105 Stern et al.77
Abbreviations: CI, confidence interval; df, degrees of freedom; DLPFC, dorsolateral prefrontal cortex; IV, inverse variance; LF, low frequency; R, right; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation.
Only one study103 had a longer-term follow-up (3 weeks: follow-up begins 1 week after the initial 2-week course of treatment ended). This study reported that the mean depression score in the rTMS group was 16.7 (standard deviation [SD] 5.7), compared with 25.2 (SD 4.5) in the sham group (P < .05).
We did two preplanned subgroup analyses based on antidepressant status and type of depression. Examining change in depression score by antidepressant status, we still found lower depression scores for patients who had low-frequency right DLPFC rTMS than for people who were not receiving antidepressants, but that effect disappeared in samples where more than half of subjects were receiving antidepressants. However, the overall effect estimate still favoured low-frequency right DLPFC rTMS regardless of antidepressant status (P = .05; Appendix 5, Figure A5). When we examined change in depression score by type of depression, the effect disappeared in the unipolar and mixed sample. However, the overall effect estimate still favoured low-frequency right DLPFC rTMS regardless of type of depression (P = .02; Appendix 5, Figure A6).
In one sensitivity analysis we included only studies that used some version of the Hamilton Depression Rating Scale. Regardless of our removal of two studies,86,99 people treated with low-frequency right DLPFC rTMS had lower depression scores than those treated with sham rTMS (P = .01; Appendix 5, Figure A7).
The quality of the evidence for change in depression score using low-frequency right DLPFC rTMS was moderate (see Appendix 2, Table A3); it was downgraded for inconsistency because of high statistical heterogeneity (I2 = 52%).
Bilateral rTMS Versus Sham rTMS
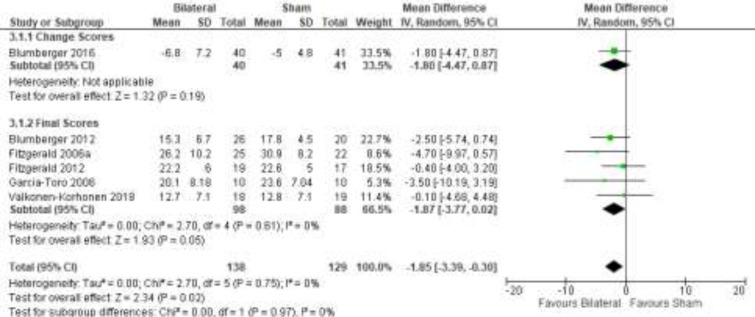
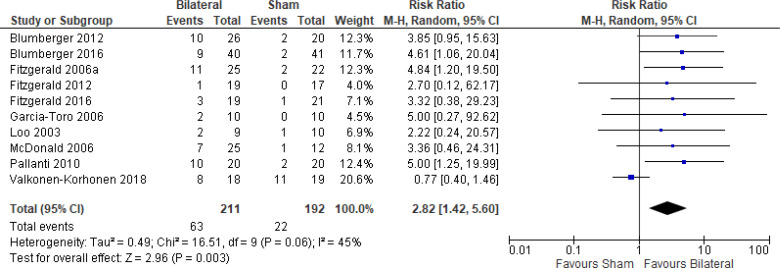
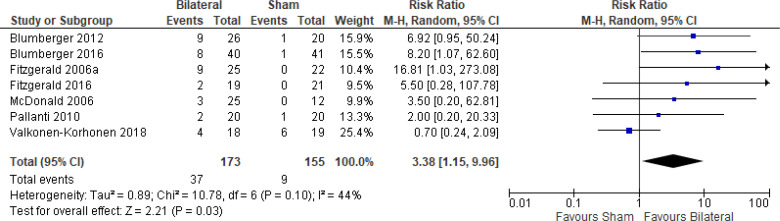
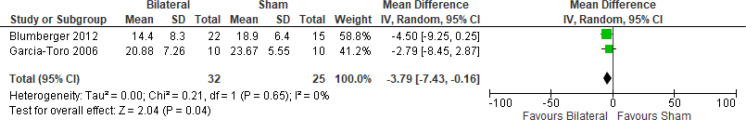
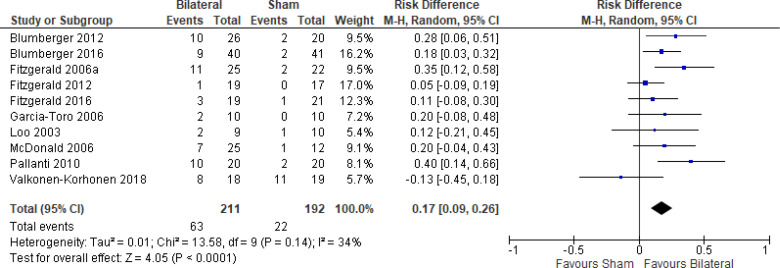
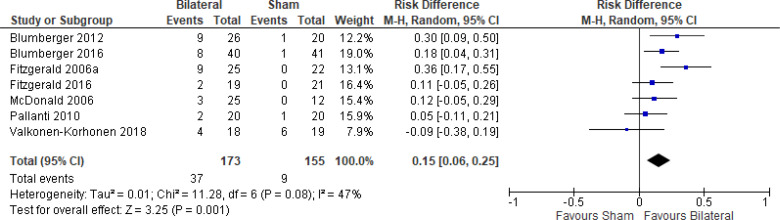
The effect of bilateral rTMS (combination of high and low frequency to the left and right DLPFC) on change in depression scores at end of treatment phase (2–6 weeks) was examined by pooling data from six studies with 267 participants using a random-effects model (Figure 4). Those who received bilateral rTMS had lower depression scores than those who received sham treatment (P = .02).
Figure 4: Bilateral rTMS Versus Sham rTMS—Change in Depression Score at End of Treatment.
Sources: Blumberger et al,82,83 Fitzgerald et al,87,106,107 Garcia-Toro et al,108 Valkonen-Korhonen et al.49
Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation.
We conducted the same analyses for studies that also included a longer follow-up (4 and 6 weeks). Two studies included a longer follow-up; however, it should be noted that no rTMS maintenance treatments were given between the end of the acute treatment phase and the longer follow-up point. In this meta-analysis, the bilateral rTMS group still had lower depression scores than the sham rTMS group (P = .04; Appendix 5, Figure A8).
We did not conduct the subgroup analyses for this outcome, because all samples from the included studies examining bilateral rTMS were receiving antidepressants. We also did not conduct a subgroup analysis for type of depression, because only one study106 included people with bipolar depression.
The quality of the evidence for change in depression score using bilateral rTMS was high (see Appendix 2, Table A3).
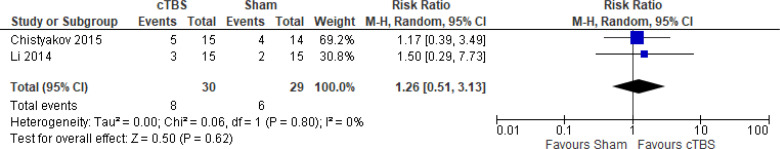
cTBS Versus Sham TBS
Only one study109 reported on change in depression scores as a mean percentage. Researchers found that cTBS had a mean reduction of 22.5% (range 13.3% to −70%) compared with sham TBS, which had a mean reduction of 17.4% ranging from 30% to −84.6% (P-value not reported).
The quality of the evidence for change in depression score using cTBS was moderate (see Appendix 2, Table A3); it was downgraded for imprecision because scores ranged from a beneficial to a non-beneficial effect.
iTBS Versus Sham TBS
Researchers in the one study109 that reported on change in depression scores as a mean percentage found that iTBS had a mean reduction of 42.3% (range 4.3% to −88.9%) compared with sham TBS, which had a mean reduction of 17.4% ranging from 30% to −84.6% (P = .002).
The quality of the evidence for change in depression score using iTBS was moderate (see Appendix 2, Table A3); it was downgraded for imprecision because scores ranged from a beneficial to a non-beneficial effect.
Bilateral TBS Versus Sham TBS
Researchers in the one study109 that reported on change in depression scores as a mean percentage found that bilateral TBS had a mean reduction of 52.5% (range −15% to −92.3%) compared with sham TBS, which had a mean reduction of 17.4% (range 30% to −84.6%; P = .002).
The quality of the evidence for change in depression score using bilateral TBS was moderate (see Appendix 2, Table A3); it was downgraded for imprecision because the scores ranged from a beneficial to a non-beneficial effect.
Deep TMS Versus Sham TMS
Only one study110 reported on change in depression scores at 4 and 8 weeks. Using the intention-to-treat analysis (ITT), mean depression score at 4 weeks for deep TMS was 14.08 (SD 8.99) compared with sham TMS (18.96, SD 9.83), with a difference of −4.88 (P = .03). At 8 weeks, the difference between deep TMS and sham TMS was −2.76 (P = .22).
The quality of the evidence for change in depression score using deep TMS was high (see Appendix 2, Table A3).
RESPONSE RATE
High-Frequency Left DLPFC rTMS Versus Sham rTMS
Four studies had more than two groups included in their RCT. In the analysis we used the higher intensity (110%) from Bakim et al,73 the higher frequency (20 Hz) from Su et al,74 and the rTMS group that received treatment on their left as opposed to right DLPFC in both active and sham groups.76 One study combined the response rate in their active groups (high-frequency left DLPFC once and twice daily) and sham groups (sham once and twice daily).75 We excluded two studies from this review because they provided rTMS outside of safety standards and exceeded the limit set by these guidelines for maximum duration of trains and number of pulses.77,111 In every study, response rate was defined as a 50% reduction in depression scores.
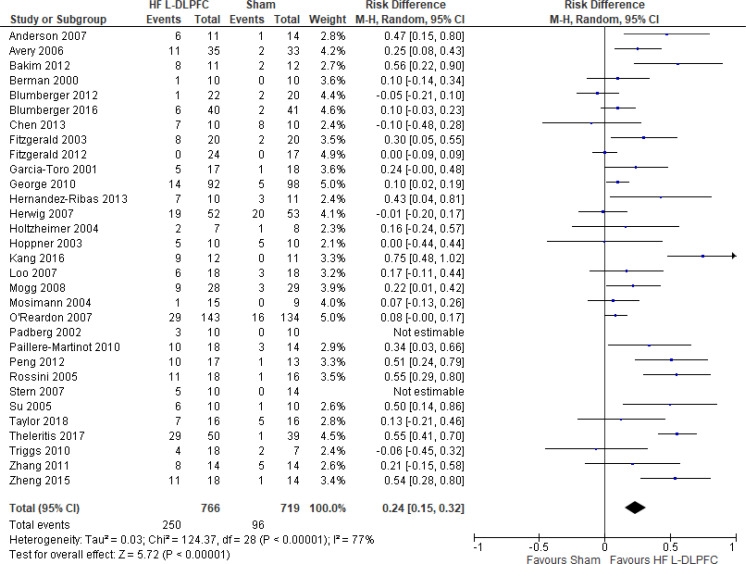
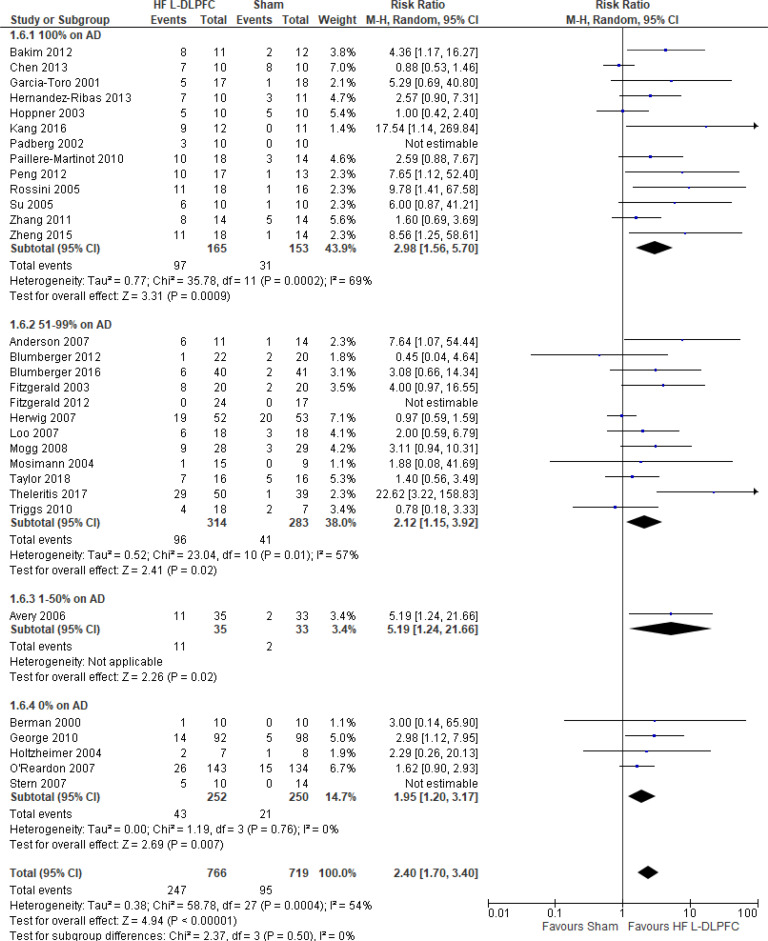
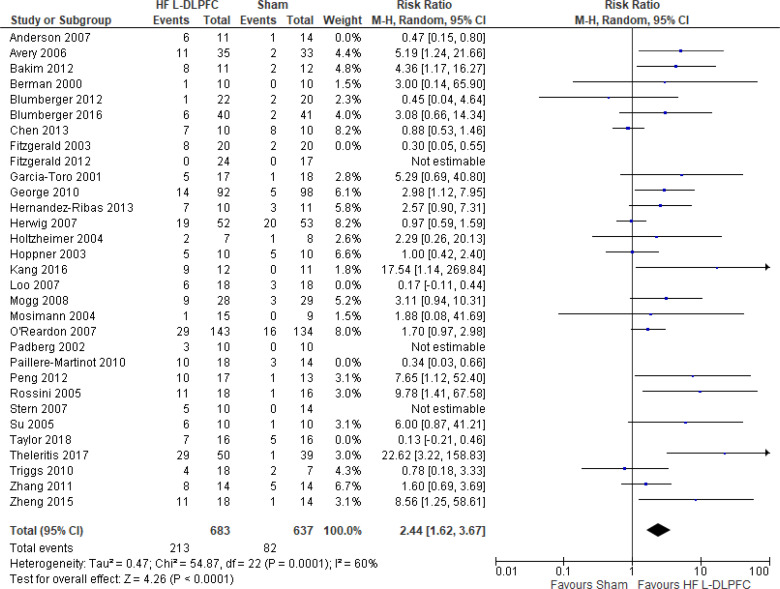
The effect of high-frequency rTMS applied to the left DLPFC on response rate at end of treatment phase (2–6 weeks) was examined by pooling data from 29 studies with 1,529 participants using a random-effects model (Figure 5). The difference in response rate favoured those who received high-frequency left DLPFC rTMS compared with those who received sham treatment (P < .00001). We also conducted a meta-analysis to show the risk difference between the two groups (Appendix 5, Figure A9). The meta-analysis showed that the absolute risk reduction was approximately 23% (95% confidence interval [CI] 15%–32%); therefore, the number needed to treat was 4.
Figure 5: High-Frequency Left DLPFC rTMS Versus Sham rTMS—Response Rate at End of Treatment.
Sources: Anderson et al,78 Avery et al,80 Bakim et al,73 Berman et al,81 Blumberger et al,82,83 Chen et al,85 Fitzgerald et al,86,87 Garcia-Toro et al,88 George et al,89 Hernandez-Ribas et al,90 Herwig et al,91 Holtzheimer et al,92 Hoppner et al,112 Kang et al47, Loo et al,93 Mogg et al,113 Mosimann et al,94 O'Reardon et al,95 Padberg et al,111 Paillere Martinot et al,96 Peng et al,97 Rossini et al,114 Stern et al,77 Su et al,74 Taylor et al,115 Theleritis et al,75 Triggs et al,76 Zhang et al,98 Zheng et al.71
Abbreviations: CI, confidence interval; df, degrees of freedom; DLPFC, dorsolateral prefrontal cortex; HF, high frequency; L, left; M-H, Mantel–Hansel test; rTMS, repetitive transcranial magnetic stimulation.
We conducted the same analyses for studies that included a longer follow-up (ranging from 3 weeks to 3 months). Seven studies included a longer follow-up; however, it should be noted that no rTMS maintenance treatments were given between the end of the acute treatment phase and the longer follow-up point. In this meta-analysis, there was still a higher response rate in the high-frequency left DLPFC rTMS group than in the sham group (P = .0004). Results can be found in Appendix 5, Figure A10.
We planned two subgroup analyses based on antidepressant status and type of depression. In both analyses, regardless of antidepressant status or type of depression, active rTMS had a better response rate than sham rTMS (P < .00001; Appendix 5, Figures A11 and A12).
We conducted one sensitivity analysis. Our analyses included only studies that used some version of the Hamilton Depression Rating Scale. O'Reardon95 reported response rate on both MADRS and HDRS. Despite our removal of five studies,78,86,93,96,115 people treated with high-frequency left DLPFC rTMS had better response rates than those treated with sham rTMS (P < .0001; Appendix 5, Figure A13).
The quality of the evidence for response rate using high-frequency left DLPFC was moderate (see Appendix 2, Table A3); it was downgraded for inconsistency because of high statistical heterogeneity (I2 = 54%).
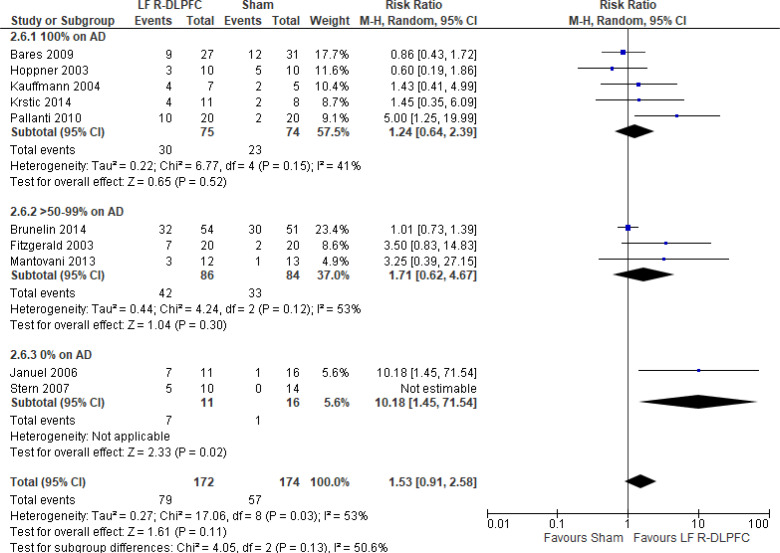
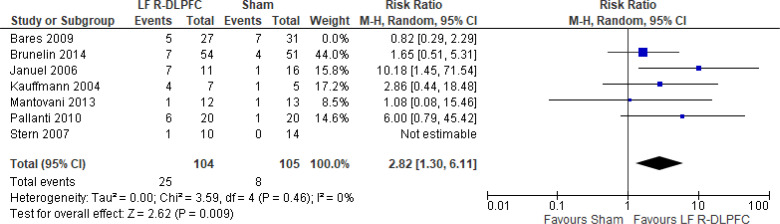
Low-Frequency Right DLPFC rTMS Versus Sham rTMS
One study116 included multiple groups; we report on the group that compared low-frequency right DLPFC rTMS without venlafaxine with sham rTMS with venlafaxine. We excluded one of the studies in this review because rTMS settings were outside safety standards and exceeded the limit set by these guidelines for maximum duration of trains and number of pulses.77
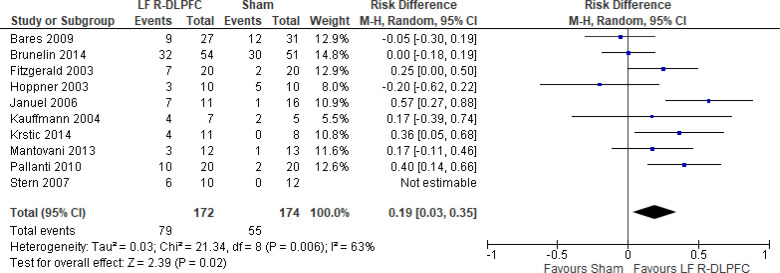
The effect of low-frequency rTMS applied to the right DLPFC on response rate at end of the treatment phase (2–4 weeks) was examined by pooling data from nine studies with 368 participants using a random-effects model (Figure 6). While there was a trend of better response rates for those who received low-frequency right DLPFC rTMS, there was no difference in response rates between those who received low-frequency right DLPFC rTMS and those who received sham treatment (P = .08). We also conducted a meta-analysis to show the risk difference between the two groups (Appendix 5, Figure A14). The meta-analysis showed that the absolute risk reduction was approximately 19% (95% CI 3%–35%); therefore, the number needed to treat was 5.
Figure 6: Low-Frequency Right DLPFC rTMS Versus Sham rTMS—Response Rate at End of Treatment.
Sources: Bares et al,99 Brunelin et al,116 Fitzgerald et al,86,100 Hoppner et al,112 Januel et al,101 Kauffmann et al,102 Krstic et al,103 Mantovani et al,104 Pallanti et al,117 Stern et al.77
Abbreviations: CI, confidence interval; df, degrees of freedom; DLPFC, dorsolateral prefrontal cortex; LF, low frequency; M-H, Mantel–Haenszel test; R, right; rTMS, repetitive transcranial magnetic stimulation.
No studies comparing low-frequency right DLPFC with sham reported longer follow-up.
We planned one subgroup analysis based on antidepressant status. Studies where people were not receiving antidepressants had better response rates; however, that effect disappeared in people receiving antidepressants (Appendix 5, Figure A15). We did not analyze subgroups for type of depression because only one study86 included people with bipolar depression.
We conducted one sensitivity analysis that included only studies that used some version of the HDRS. Despite our removal of two studies,86,99 we found people treated with low-frequency right DLPFC rTMS had response rates similar to those of sham treatment (P = .10; Appendix 5, Figure A16).
The quality of the evidence for response rate using low-frequency right DLPFC was moderate (see Appendix 2, Table A3); it was downgraded for inconsistency because of high statistical heterogeneity (I2 = 58%).
Bilateral rTMS Versus Sham rTMS
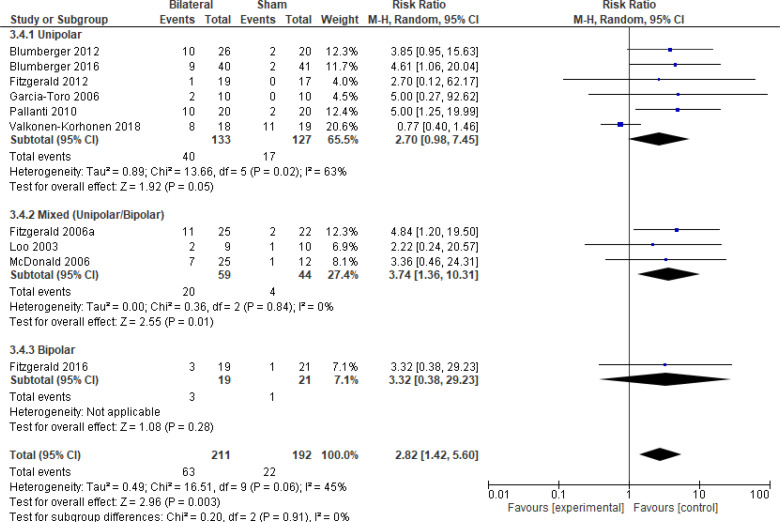
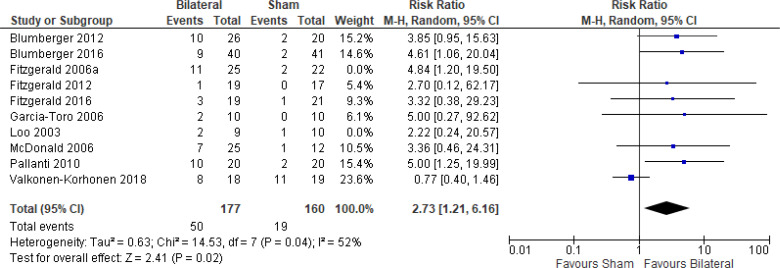
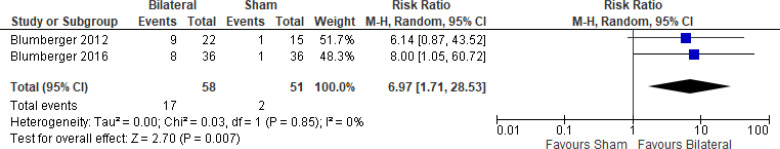
The effect of bilateral rTMS on response rate at end of treatment phase (2–6 weeks) was examined by pooling data from 10 studies with 403 participants using a random-effects model (Figure 7). The response rate favoured those who received bilateral rTMS compared with those who received sham rTMS (P = .003). We also conducted a meta-analysis to show the risk difference between the two groups (Appendix 5, Figure A17). The meta-analysis showed that the absolute risk reduction was approximately 17% (95% CI 9%–26%); therefore, the number needed to treat was 6.
Figure 7: Bilateral rTMS Versus Sham rTMS—Response Rate at End of Treatment.
Sources: Blumberger et al,82,83 Fitzgerald et al,87,106,107,118,119 Garcia-Toro et al,108 Loo et al,120 McDonald et al,121 Pallanti et al,117 Valkonen-Korhonen et al.49
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
We conducted the same analyses for studies that also had a longer follow-up (6 weeks). Two studies included a longer follow-up; however, it should be noted that no rTMS maintenance treatments were given between the end of the acute treatment phase and the longer follow-up. In this meta-analysis, there were still better response rates with bilateral rTMS than with sham rTMS (P = .008). Results can be found in Appendix 5, Figure A18.
We planned one subgroup analysis based on type of depression. This meta-analysis showed better response rates in studies that included only a population of people with major depression or a mixed population (major depression/bipolar depression), but that effect disappeared in the one study that included only people with bipolar depression. However, the overall effect estimate still favoured those who received bilateral rTMS compared with sham rTMS (P = .003; Appendix 5, Figure A19). We did not undertake a subgroup analysis on antidepressant status because only one study121 included people not receiving antidepressants.
We conducted one sensitivity analysis. We conducted a meta-analysis including only studies that used some version of the HDRS. Regardless of the removal of two studies,106,120 people treated with bilateral rTMS had better response rates than those treated with sham rTMS (P = .02; Appendix 5, Figure A20). No interventions in the included studies operated outside safety guidelines.
The quality of the evidence for response rate using bilateral rTMS was high (see Appendix 2, Table A3).
cTBS Versus Sham TBS
The effect of cTBS on response rate at end of treatment phase (2 weeks) was examined by pooling data from two studies with 59 participants using a random-effects model (Figure 8). There was no difference in response rate favouring those who received cTBS compared with those who received sham TBS (P = .62).
Figure 8: cTBS Versus Sham TBS—Response Rate at End of Treatment (2 Weeks).
Abbreviations: CI, confidence interval; cTBS, continuous theta burst stimulation; df, degrees of freedom; M-H, Mantel–Haenszel test; TBS, theta burst stimulation.
Li et al109 reported the response rate at 14-week follow-up. Two thirds (66.7%) of the cTBS group and half (50%) of the sham group remained responsive at 14 weeks.
There were not enough data to do any subgroup or sensitivity analyses for this rTMS modality.
The quality of the evidence for response rate using cTBS was moderate (see Appendix 2, Table A3); it was downgraded for imprecision because the CIs overlapped both the beneficial and non-beneficial effect.
iTBS Versus Sham TBS
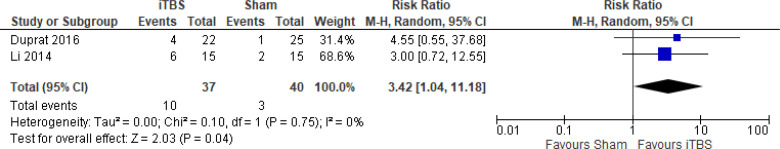
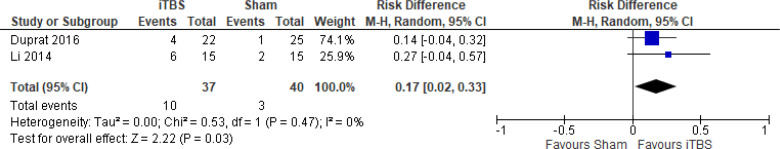
The effect of iTBS on response rate at end of treatment phase (1 and 2 weeks) was examined by pooling data from two studies with 77 participants using a random-effects model (Figure 9). It should be noted that Duprat et al124 administered 20 iTBS sessions in 1 week (accelerated). A difference in response rate favoured those who received iTBS compared with those who received sham treatment (P = .04). We also conducted a meta-analysis to show the risk difference between the two groups (Appendix 5, Figure A21). The meta-analysis showed that the absolute risk reduction was approximately 17% (95% CI 2%–33%); therefore, the number needed to treat was 6.
Figure 9: iTBS Versus Sham TBS—Response Rate at End of Treatment.
Abbreviations: CI, confidence interval; df, degrees of freedom; iTBS, intermittent theta burst stimulation; M-H, Mantel–Haenszel test; TBS, theta burst stimulation.
Li et al109 reported the response rate at 14-week follow-up. They found that 83.3% of the iTBS group and 50% of the sham group remained responsive at 14 weeks.
There were not enough data to do any subgroup or sensitivity analyses for this rTMS modality.
The quality of the evidence for response rate using iTBS was moderate (see Appendix 2, Table A3); it was downgraded for imprecision because the wide CIs overlapped both beneficial and non-beneficial effects.
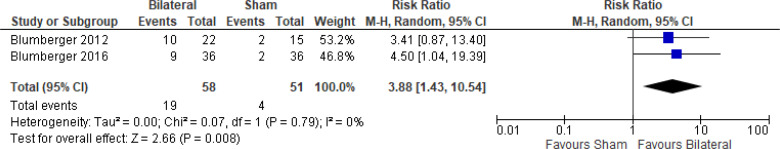
Bilateral iTBS Versus Sham iTBS
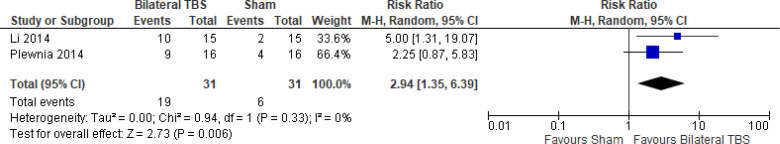
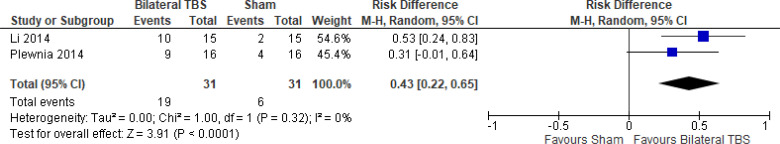
The effect of bilateral iTBS on response rate at the end of treatment phase (2 and 6 weeks) was examined by pooling data from two studies with 62 participants using a random-effects model (Figure 10). A difference in response rate favoured those who received bilateral TBS compared with those who received sham (P = .006). We also conducted a meta-analysis to show the risk difference between the two groups (Appendix 5, Figure A22). The meta-analysis showed that the absolute risk reduction was approximately 43% (95% CI 22%–65%); therefore, the number needed to treat was 2.
Figure 10: Bilateral TBS Versus Sham TBS—Response Rate at End of Treatment.
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel test; TBS, theta burst stimulation.
Li et al109 reported the response rate at 14-week follow-up. They found that two fifths (40%) of people in the bilateral TBS group and half (50%) of people in the sham group remained responsive at 14 weeks.
There were not enough data to do any subgroup or sensitivity analyses for this rTMS modality.
The quality of the evidence for response rate using bilateral TBS was high (see Appendix 2, Table A3).
Deep TMS Versus Sham TMS
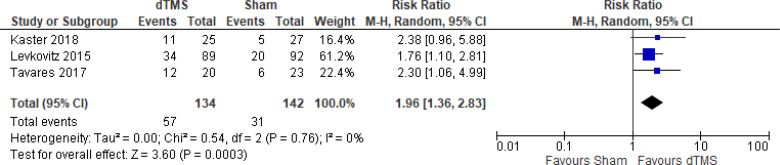
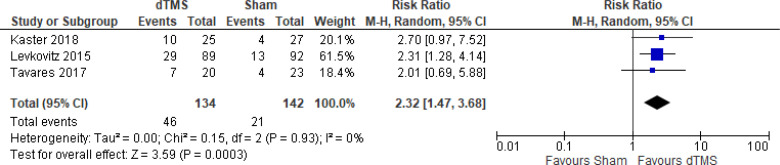
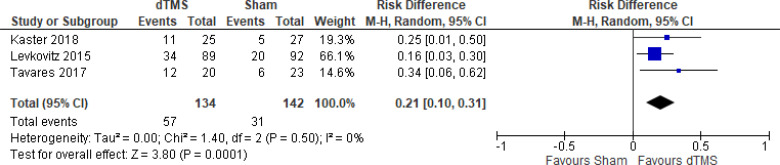
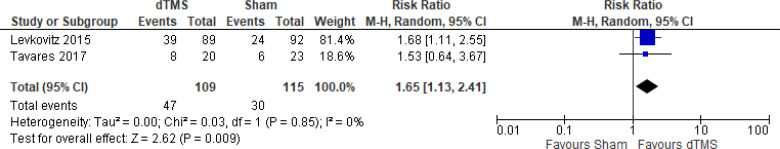
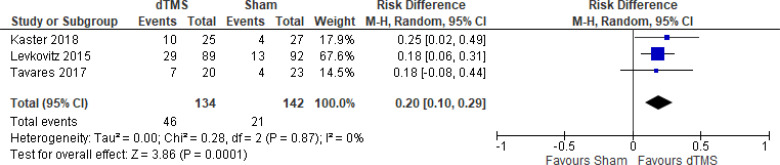
The effect of deep TMS on response rate at end of treatment (4–5 weeks) was examined by pooling data from three studies with 276 participants using a random-effects model (Figure 11). A difference in response rate favoured those who received deep TMS compared with those who received sham (P = .0003). We also conducted a meta-analysis to show the risk difference between the two groups (Appendix 5, Figure A23). The meta-analysis showed that the absolute risk reduction was approximately 21% (95% CI 10%–31%); therefore, the number needed to treat was 4.
Figure 11: Deep TMS Versus Sham TMS—Response Rate at End of Treatment.
Sources: Kaster et al,48 Levkovitz et al,126 Tavares et al.110
Abbreviations: CI, confidence interval; df, degrees of freedom; dTMS, deep transcranial magnetic stimulation; M-H, Mantel–Haenszel test; TMS, transcranial magnetic stimulation.
We conducted the same analyses for studies that also included a longer follow-up (8 and 16 weeks). Two studies included a longer follow-up; however, it should be noted that one study126 did administer maintenance treatment, which was deep TMS twice a week. In this meta-analysis, there were still better response rates in the deep TMS group than in the sham group (P = .009). Results can be found in Appendix 5, Figure A24.
There were not enough data to do any subgroup or sensitivity analyses for this rTMS modality.
The quality of the evidence for response rate using deep TMS was high (see Appendix 2, Table A3).
REMISSION RATE
High-Frequency Left DLPFC rTMS Versus Sham rTMS
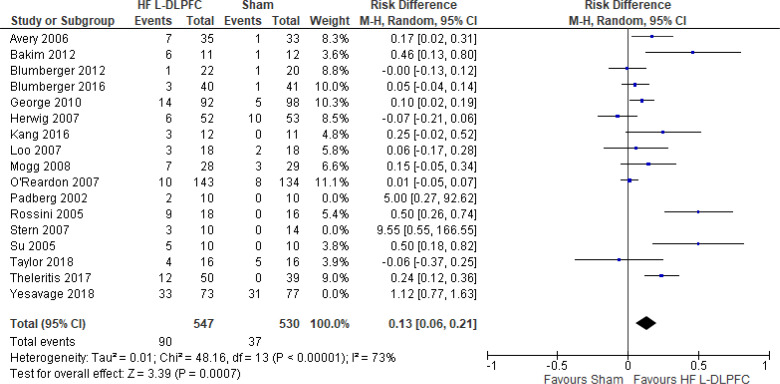
Four studies included more than two groups in their RCT. In the analysis we used the higher intensity (110%) from Bakim et al,73 the higher frequency (20 Hz) from Su et al,74 and the rTMS group that received treatment on their left DLPFC (as opposed to right DLPFC) in both active and sham groups.76 One study combined the response rate in their active groups (high-frequency left DLPFC once and twice daily) and sham groups (sham treatment once and twice a day).75 We excluded two of the studies in this review because one provided rTMS outside safety standards and exceeded the limit set by these guidelines for maximum duration of trains and number of pulses.77,111 In the other study rTMS was given to United States veterans where the sample had a high prevalence of post-traumatic stress disorder.50 The effect of high-frequency rTMS applied to the left DLPFC on remission rate at end of the treatment phase (2–6 weeks) was examined by pooling data from 14 studies with 1,077 participants using a random-effects model (Figure 12). A difference in remission rate favoured those who received high-frequency left DLPFC rTMS compared with those who received sham treatment (P = .006). We also conducted a meta-analysis to show the risk difference between the two groups (Appendix 5, Figure A25). The meta-analysis showed that the absolute risk reduction was approximately 13% (95% CI 6%–21%); therefore, the number needed to treat was 8.
Figure 12: High-Frequency Left DLPFC rTMS Versus Sham rTMS—Remission Rate at End of Treatment.
Sources: Avery et al,80 Bakim et al,73 Blumberger et al,82,83 George et al,89 Herwig et al,91 Kang et al,47 Loo et al,93 Mogg et al,113 O'Reardon et al,95 Padberg et al,111 Rossini et al,114 Stern et al,77 Su et al,74 Taylor et al,115 Theleritis et al,75 Yesavage et al.50
Abbreviations: CI, confidence interval; df, degrees of freedom; DLPFC, dorsolateral prefrontal cortex; HF, high frequency; L, left; M-H, Mantel– Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
We conducted the same analyses for studies that also included a longer follow-up (6 weeks). Three studies included a longer follow-up; however, no rTMS maintenance treatments were given between the end of the acute treatment phase and the longer follow-up point. In this meta-analysis, the high-frequency left DLPFC rTMS group still had better remission rates than the sham group (P = .01). Results can be found in Appendix 5, Figure A26.
We conducted one sensitivity analysis that included only studies that used some version of the HDRS. One study95 reported remission rates on both the MADRS and HDRS. Despite our removal of two studies,93,115 people treated with high-frequency left DLPFC rTMS had better remission rates than those treated with sham rTMS (P = .003; Appendix 5, Figure A27).
No subgroup analyses were planned for this outcome.
The quality of the evidence for remission rate using high-frequency left DLPFC rTMS was moderate (see Appendix 2, Table A3); it was downgraded for indirectness because various cut-points were used to define remission.
Low-Frequency Right DLPFC rTMS Versus Sham rTMS
One study116 included multiple groups; we assessed the group that was given low-frequency right DLPFC without venlafaxine versus sham treatment with venlafaxine. We excluded one of the studies in this review because rTMS did not meet safety standards and exceeded the limit set by these guidelines for maximum duration of trains and number of pulses.77 The effect of low-frequency rTMS applied to the right DLPFC on remission rate at end of treatment (2–6 weeks) was examined by pooling data from six studies with 291 participants using a random-effects model (Figure 13). There was no difference in remission rate when low-frequency right DLPFC rTMS was compared with sham treatment (P = .07). We also conducted a meta-analysis to show the risk difference between the two groups (Appendix 5, Figure A28). While there was still no difference between groups, the meta-analysis showed that the absolute risk reduction was approximately 16% (95% CI −0.01% to 32%); therefore, the number needed to treat was 6.
Figure 13: Low-Frequency Right DLPFC rTMS Versus rTMS—Remission Rate at End of Treatment.
Sources: Bares et al,99 Brunelin et al,116 Januel et al,101 Kauffmann et al,102 Mantovani et al,104 Pallanti et al,117 Stern et al.77
Abbreviations: CI, confidence interval; df, degrees of freedom; DLPFC, dorsolateral prefrontal cortex; LF, low frequency; M-H, Mantel–Haenszel test; R, right; rTMS, repetitive transcranial magnetic stimulation.
Only one study116 reported remission rates at a longer follow-up (unspecified). However, no rTMS maintenance treatments were given between the end of the acute treatment phase and the longer follow-up point. The remission rate was 22/54 in the rTMS group versus 22/51 in the sham group.
We conducted one sensitivity analysis, which included only studies that used some version of the HDRS. With our removal of one study,99 people treated with low-frequency right DLPFC rTMS had better remission rates than those treated with sham rTMS (P = .009; Appendix 5, Figure A29).
The quality of the evidence for remission rate using low-frequency right DLPFC rTMS was moderate (see Appendix 2, Table A3); it was downgraded for indirectness because various cut-points were used to define remission.
Bilateral rTMS Versus Sham rTMS
The effect of bilateral rTMS on remission rate at end of treatment (2–6 weeks) was examined by pooling data from seven studies with 328 participants using a random-effects model (Figure 14). The difference in remission rate favoured those who received bilateral rTMS compared with those who received sham treatment (P = .03). We also conducted a meta-analysis to show the risk difference between the two groups (Appendix 5, Figure A30). The meta-analysis showed that the absolute risk reduction was approximately 15% (95% CI 6%–25%); therefore, the number needed to treat was 7.
Figure 14: Bilateral rTMS Versus Sham rTMS—Remission Rate at End of Treatment.
Sources: Blumberger et al,82,83 Fitzgerald et al,106,107,118,119 McDonald et al,121 Pallanti et al,117 Valkonen-Korhonen et al.49
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
We conducted the same analyses for studies that also included a longer follow-up (6 weeks). Two studies included a longer follow-up; however, no rTMS maintenance treatments were given between the end of the acute treatment phase and the longer follow-up point. In this meta-analysis, the difference in remission rates still favoured the bilateral rTMS group versus the sham group (P = .007). Results can be found in Appendix 5, Figure A31.
There were not enough data to do any sensitivity analyses for this rTMS modality.
The quality of the evidence for remission rate using bilateral rTMS was moderate (see Appendix 2, Table A3); it was downgraded for indirectness because various cut-points were used to define remission. Continuous TBS Versus Sham TBS
No studies examined the effects of cTBS on remission rate.
iTBS Versus Sham TBS
One study124 reported on remission rates (defined as a score of ≤ 7 on the HDRS-17). ITBS was administered in this study at an accelerated rate: the intervention group received 20 sessions of iTBS in 1 week (5 days). The authors reported that 2 of 22 (9%) in the iTBS group and none of 25 (0%) in the sham group experienced remission.
The quality of the evidence for remission rate using iTBS was moderate (see Appendix 2, Table A3); it was downgraded for indirectness because the intervention was given unconventionally (generally iTBS is delivered 5 days per week and not at an accelerated pace).
Bilateral TBS Versus Sham TBS
One study125 reported on remission rates (defined as a score of ≤ 7 on the MADRS). At 6 weeks, 7/16 (44%) in the bilateral TBS group and 3/16 (19%) in the sham group experienced remission (P = .07).
The quality of the evidence for remission rate using bilateral TBS was high (see Appendix 2, Table A3).
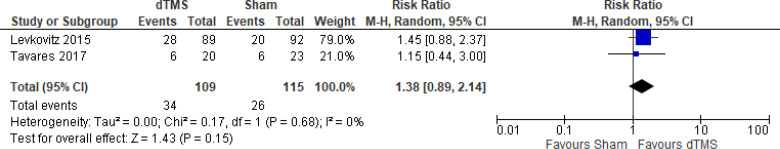
Deep TMS Versus Sham TMS
The effect of deep TMS on remission rate at end of the treatment phase (4–5 weeks) was examined by pooling data from three studies with 276 participants using a random-effects model (Figure 15). The difference in remission rate favoured those who received deep TMS compared with those who received sham treatment (P = .0003). We also conducted a meta-analysis to show the risk difference between the two groups (Appendix 5, Figure A32). The meta-analysis showed that the absolute risk reduction was approximately 20% (95% CI 10%–29%); therefore, the number needed to treat was 5.
Figure 15: Deep TMS Versus Sham TMS—Remission Rate at End of Treatment.
Sources: Kaster et al,48 Levkovitz et al,126 Tavares et al.110
Abbreviations: CI, confidence interval; df, degrees of freedom; dTMS, deep transcranial magnetic stimulation; M-H, Mantel–Haenszel test; TMS, transcranial magnetic stimulation.
We conducted the same analyses for studies that also included a longer follow-up (8 and 16 weeks). Two studies included a longer follow-up; however, one study126 did administer maintenance treatment, which was deep TMS twice weekly. In this meta-analysis, remission rates showed no difference between active and sham deep TMS (P = .15). Results can be found in Appendix 5, Figure A33.
The quality of the evidence for remission rate using deep TMS was high (see Appendix 2, Table A3).
RELAPSE RATE
One study80 reported on relapse rate at 6 months. Of respondents receiving high-frequency left DLPFC rTMS, 5 of 11 (45%) relapsed; in the sham group, half (50%) relapsed.
The quality of the evidence for remission rate using high-frequency left DLPFC was moderate (see Appendix 2, Table A3).
ACCEPTABILITY (DISCONTINUATION OF TREATMENT)
No studies comparing any modality of rTMS with sham reported on discontinuation of treatment.
ADVERSE EVENTS
Thirteen studies49,72,85,90,97,98,101,103,105,108,117,118,121 did not report on adverse events and side effects of treatment. Ten studies47,71,87,92,96,102,112,115,122,127 reported that there were no adverse events or side effects from active rTMS or sham rTMS. Two studies84,113 reported side effects on validated scales (e.g., Udvalg for Kliniske Unders⊘gelser [UKU] side effect scale, CSSES-Subjective Side Effects Schedule). Bretlau et al84 found that at 3 weeks, there was a significant difference in reduced length of sleep in the rTMS group compared with the sham group. At 12 weeks, the sham group had greater difficulty concentrating. Mogg et al113 found no significant difference in side effects between rTMS and sham groups.
Thirty-one studies reported rates of adverse events. Adverse events were similar between rTMS and sham groups. Most were minor events, where the most common events reported were headache and scalp discomfort (Appendix 5, Tables A11 and A12). The quality of the evidence for adverse events among all modalities of rTMS was moderate (see Appendix 2, Table A3); it was downgraded for indirectness because studies used various scales or counts to measure adverse events and some studies did not report adverse events separately for differing rTMS groups within one study.
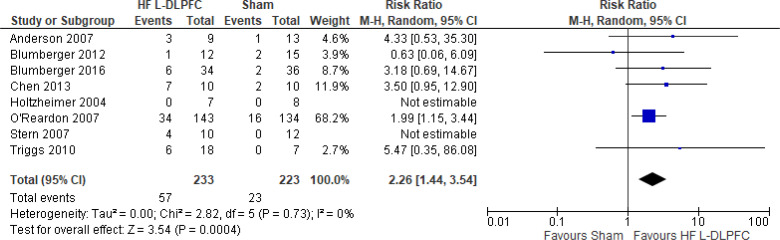
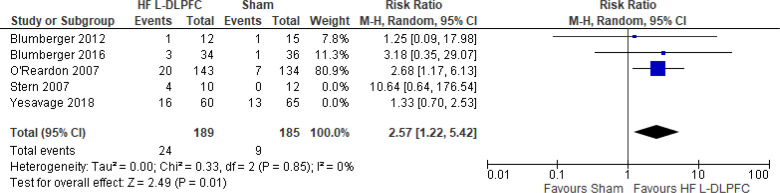
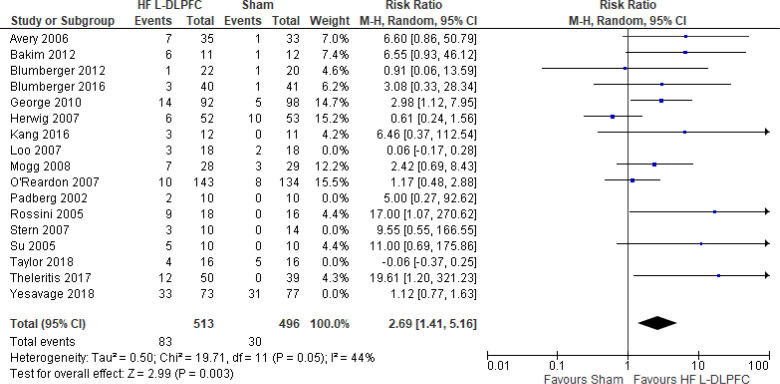
Question 2: What Are the Effectiveness and Safety of rTMS Compared With ECT for Treatment of Adults With TRD?
CHANGE IN DEPRESSION SCORE
Two systematic reviews18,42 evaluated the change in depression score when rTMS was compared with ECT. Both reviews compared high-frequency rTMS over the left dorsolateral prefrontal cortex (DLPFC) with ECT. Below are the results from those systematic reviews (Table 3). Both systematic reviews reported that ECT was more effective than rTMS at improving depression scores.
Table 3:
Change in Depression Scores for rTMS Versus ECT
| Author, Year | No. of Studies (Sample Size) | Results | Quality of Primary Studies | Conclusions |
|---|---|---|---|---|
| Lepping et al, 201442 | 5 (212) | “In those rTMS studies that used ECT as a comparator, ECT was more effective than rTMS which only reached a HDRS percentage reduction of 33.7%” | NR | No specific conclusions on this comparator |
| Health Quality Ontario, 201618 | 4 (185) | WMD −5.97 (95% CI −11.0 to −0.94, P = .020) I2 = 72.2% Favouring ECT SMD −0.67 (95% CI −1.23 to −0.10, P = .021) I2 = 70.6% Favouring ECT |
Moderate to high ROBa | “Trials of high-frequency rTMS of the DLPFC vs. ECT showed significantly more improvement in depression scores with ECT treatment than with rTMS treatment” |
Abbreviations: CI, confidence interval; DLPFC, dorsolateral prefrontal cortex; ECT, electroconvulsive therapy; HDRS, Hamilton Depression Rating Scale; NR, not reported; ROB, risk of bias; rTMS, repetitive transcranial magnetic stimulation; SMD, standardized mean difference; WMD, weighted mean difference.
The quality of the evidence for change in depression score comparing ECT with rTMS was moderate (see Appendix 2, Table A4); it was downgraded for risk of bias on the basis of the 2016 review.18
RESPONSE RATE
Three systematic reviews18,34,40 assessed response rate of high-frequency left DLPFC rTMS versus ECT using ≥ 50% reduction as the definition of response. Two of the systematic reviews34,40 reported the same results, so the most recent systematic review34 is presented below (Table 4). Both systematic reviews included one similar study,130 while the other two included studies differed; Grunhaus et al129 and Eranti et al128 in Health Quality Ontario's systematic review18 and Janicak et al133 and Rosa et al134 in the systematic reviews by Leggett and the University of Calgary. The systematic reviews found no difference in response rate between rTMS and ECT.
Table 4:
Response Rates for rTMS Versus ECT
| Author, Year | No. of Studies (Sample Size) | Results | Definition of Response Rate |
|---|---|---|---|
| University of Calgary, 201734 | 3 (104) | RR 1.09 (95% CI 0.79–1.48, P = .41) No difference | ≥ 50% reduction |
| Health Quality Ontario, 201618 | 3 (126) | RR 1.72 (95% CI 0.95–3.11, P = .72) I2 = 60.6% No difference | ≥ 50% reduction |
Abbreviations: CI, confidence interval; ECT, electroconvulsive therapy; RR, risk ratio; rTMS, repetitive transcranial magnetic stimulation.
The quality of the evidence for response rate comparing ECT with rTMS was moderate (see Appendix 2, Table A4); it was downgraded for risk of bias on the basis of the systematic review in 2016.18
REMISSION RATE
Three systematic reviews18,34,40 assessed remission rate of high-frequency left DLPFC rTMS versus ECT using various definitions depending on the depression scale used. Two of the systematic reviews34,40 reported the same results, so the most recent systematic review34 is presented in Table 5. The systematic reviews included two of the same studies130,131 in the analysis and one study that differed: Rosa et al134 in the systematic reviews by Leggett and the University of Calgary and Eranti et al128 in the systematic review by Health Quality Ontario. All studies found no difference in remission rates between rTMS and ECT.
Table 5:
Remission Rates for rTMS Versus ECT
| Author, Year | No. of Studies (Sample Size) | Results | Definition of Remission Rate |
|---|---|---|---|
| University of Calgary, 201734 | 3 (114) | RR 0.97 (95% CI 0.65–1.45, P = .87) No difference |
7–8 |
| Health Quality Ontario, 201618 | 3 (118) | RR 1.44 (95% CI 0.64–3.23, P = .375) I2 = 69.1% No difference |
NR |
Abbreviations: CI, confidence interval; ECT, electroconvulsive therapy; NR, not reported; RR, risk ratio; rTMS, repetitive transcranial magnetic stimulation.
The quality of the evidence for remission rate when ECT was compared with rTMS was moderate (see Appendix 2, Table A4); it was downgraded for risk of bias on the basis of the systematic review in 2016.18
RELAPSE RATE
One systematic review18 reported on relapse rate. Two primary studies128,135 included in the systematic review captured relapse rate at 6-month follow-up. The study by Eranti et al128 reported that 50% of patients relapsed during the first 6 months (rTMS group: 2/4 [50%] of remitters; ECT group: 6/12 [50%] of remitters; P = not significant). Dannon et al135 reported relapse was lower in the ECT group than in the rTMS group at 6 months (rTMS group: 4/9 [44.4%] of responders; ECT group: 4/16 [25%] of responders; P = not available).
The quality of evidence using GRADE was not assessed for relapse rate.
ADVERSE EVENTS
Two systematic reviews examined adverse events associated with rTMS and ECT. Results for adverse events were captured in different ways across primary studies. Results from the systematic reviews are captured in Table 6.
Table 6:
Adverse Events for rTMS Versus ECT in 2016 Systematic Review
| Author, Year | Time of Follow-Up | Side Effect Scores | Self-Rating of Cognitive Complaints | Cognition Scoresa |
|---|---|---|---|---|
| Eranti et al, 2007128 | Baseline | rTMS 13.2 (5.8) ECT 14.2 (4.7) | rTMS 2.1 (1.3) ECT 2.4 (1.2) | rTMS 85.3 (11.3) ECT 83.2 (11.1) |
| End of treatment | rTMS 9.7 (4.6) ECT 6.7 (6.4) | rTMS 1.5 (1.2) ECT 1.5 (1.4) | rTMS 84.7 (17.4) ECT 87.0 (14.8) | |
| 6 mo | rTMS 8.9 (4.7) ECT 7.1 (4.7) P = .02 Favouring ECT |
rTMS 2.1 (1.5) ECT 1.2 (1.4) P = .1 |
rTMS 84.8 (14.5) ECT 86.1 (17.3) P = .7 |
|
| Pridmore, 2000131 | Baseline | rTMS 8.1 (3.2) ECT 6.1 (3.6) P = .1 |
NA | NA |
| End of treatment | rTMS 3.9 (2.9) ECT 5.3 (4.3) P = .3 |
NA | NA | |
| Rate of Adverse Events | ||||
| Grunhaus et al, 2003130 | rTMS: 3 (15%) patients had headaches and 2 (10%) had sleep disturbance ECT: no adverse events occurred |
|||
| Grunhaus et al, 2000129 | rTMS: 5 (25%) had headaches ECT: no adverse events occurred |
|||
Abbreviations: ECT, electroconvulsive therapy; NA, not applicable; NR, not reported; rTMS, repetitive transcranial magnetic stimulation.
Total score is a maximum of 107.
Source: Health Quality Ontario.18
The University of Calgary's 2017 systematic review also reported on adverse events.34 The only adverse effects reported in the six included studies assessing rTMS versus ECT were pain or discomfort and headache. Three studies130,132,133 reported some of their patients had headaches; all reported that the headaches subsided quickly. Only one study130 reported rates of patient pain or discomfort. In this study, six participants in the rTMS arm reported pain or discomfort, and no patients in the ECT group reported pain or discomfort. None of the included studies reported serious adverse events such as cognitive impairment or seizure.
The quality of evidence using GRADE was not assessed for adverse events.
ACCEPTABILITY (DISCONTINUATION OF TREATMENT)
No studies comparing rTMS with ECT reported on discontinuation of treatment.
Question 3: What Is the Comparative Effectiveness of Various Modalities of rTMS for Adults With TRD?
CHANGE IN DEPRESSION SCORE
No systematic reviews evaluated change in depression scores when one rTMS modality was compared with another rTMS modality.
RESPONSE RATE
Three systematic reviews34,40,43 compared response rate for one rTMS modality with response rate for another rTMS modality. Two of the reviews34,40 used the same studies in their analyses, so the most recent systematic review34 is presented in Table 7. All systematic reviews comparing various modalities against each other found no difference in response rate.
Table 7:
Response Rates of Various rTMS Modalities
| Author, Year | No. of Studies (Sample Size) | Results | Definition of Response Rate |
|---|---|---|---|
| HF vs. LF rTMS | |||
| University of Calgary, 201734 | 11 (456) | RR 1.19 (95% CI 0.97-1.46, P = .86) I2 = NR No difference |
≥ 50% reduction |
| Unilateral vs. Bilateral rTMS | |||
| Zhang et al, 201543 | 6 (410) | RR 1.01 (95% CI 0.81-1.26, P = .93) I2 = 40% No difference |
≥ 50% reduction |
| University of Calgary, 201734 | 5 (455) | RR 1.15 (95% CI 0.85-1.56, P = .11) I2 = NR No difference |
≥ 50% reduction |
| High Intensity vs. Low Intensity rTMS | |||
| University of Calgary, 201734 | 3 (79) | RR 1.15 (95% CI 0.54-2.41, P = .09) I2 = NR No difference |
≥ 50% reduction |
Abbreviations: CI, confidence interval; HF, high frequency; LF, low frequency; NR, not reported; RR, risk ratio; rTMS, repetitive transcranial magnetic stimulation.
One network meta-analysis46 conducted a sensitivity analysis on treatment-resistant patients comparing rTMS modalities against other rTMS modalities and sham on the outcomes of response rate. Response rates did not differ between rTMS modalities. According to the surface area under the cumulative ranking curve, priming rTMS (84.5%) and bilateral rTMS (82.0%) were ranked in the two first positions for response rates.
The quality of evidence using GRADE was not assessed for response rate.
REMISSION RATE
Three systematic reviews34,40,43 examined remission rate of one rTMS modality compared with another rTMS modality. Two of the reviews34,40 used the same analysis, so the most recent systematic review34 is presented in Table 8. These two systematic reviews found no difference in remission rate for any combination.
Table 8:
Remission Rates of Various rTMS Modalities
| Author, Year | No. of Studies (Sample Size) | Results | Definition of Remission Rate |
|---|---|---|---|
| HF vs. LF rTMS | |||
| University of Calgary, 201734 | 6 (241) | RR 1.29 (95% CI 0.75-2.22, P = .36) I2 = NR No difference |
Range < 7 to ≤ 12 |
| Unilateral vs. Bilateral rTMS | |||
| Zhang et al, 201543 | 6 (428) | RR 0.77 (95% CI 0.52-1.16, P = .22) I2 = 9% No difference |
NR |
| University of Calgary, 201734 | 3 (369) | RR 1.18 (95% CI 0.71-1.96, P = .11) I2 = NR No difference |
Range ≤ 7 to ≤ 10 |
| High-Intensity vs. Low-Intensity rTMS | |||
| University of Calgary, 201734 | 3 (79) | RR 1.72 (95% CI 0.89-3.33, P = .50) I2 = NR No difference |
Range < 7 to ≤ 10 |
Abbreviations: CI, confidence interval; HF, high frequency; LF, low frequency; NR, not reported; RR, risk ratio; rTMS, repetitive transcranial magnetic stimulation.
The quality of evidence using GRADE was not assessed for remission rate.
RELAPSE RATE
No systematic reviews evaluated relapse rate by comparing one rTMS modality with another rTMS modality.
ADVERSE EVENTS
Only one systematic review34 compared adverse events for one rTMS modality with adverse events for another rTMS modality. No major adverse events were reported (Table 9).
Table 9:
Adverse Events for Compared rTMS Modalities
| Author, Year | Results |
|---|---|
| HF vs. LF rTMS | |
| Fitzgerald et al, 2009136 | HF rTMS: one participant experienced a headache LF rTMS: no reported adverse events |
| Su et al, 200574 | HF rTMS: one participant experienced a headache LF rTMS: one participant experienced a headache |
| Padberg et al, 2002111 | HF rTMS: three participants experienced pain; one participant experienced a headache LF rTMS: two participants experienced pain; one participant experienced a headache |
| Unilateral vs. Bilateral rTMS | |
| Blumberger et al, 201682 | Unilateral rTMS: one participant experienced headache and one experienced scalp pain Bilateral rTMS: No reported adverse events |
| Pallanti et al, 2010117 | Unilateral rTMS: one participant experienced a headache, two participants reported cognitive complaints Bilateral rTMS: one participant had a headache, one experienced scalp pain, three participants reported cognitive complaints |
| Fitzgerald et al, 201287 | Unilateral rTMS: one participant experienced headache, one had increased agitation Bilateral rTMS: one participant reported discomfort, one participant reported worsened pre-existing migraine condition |
| HI vs. LI rTMS | |
| Padberg et al, 2002111 | HI: two participants experienced an aversive tactile artifact,a two experienced discomfort LI: three participants experienced an aversive tactile artifact,a three experienced discomfort |
| Bakim et al, 201273 | HI: two participants experienced headaches LI: two participants experienced headaches |
| Rossini et al, 2010137 | HI: two participants experienced headaches LI: two participants experienced headaches |
Abbreviations: HF, high frequency; HI, high intensity; LF, low frequency; LI, low intensity; rTMS, repetitive transcranial magnetic stimulation.
Aversive tactile artifact: the defensiveness to the intervention.
The quality of evidence using GRADE was not assessed for adverse events.
ACCEPTABILITY (DISCONTINUATION OF TREATMENT)
One network meta-analysis46 conducted a sensitivity analysis on treatment-resistant patients comparing rTMS versus another rTMS modality and sham TMS on the outcome of acceptability. In the comparison of rTMS modalities, priming rTMS seemed to be slightly more acceptable than high-frequency rTMS (odds ratio [OR] 0.29, 95% CI 0.10–0.87), low-frequency rTMS (OR 0.27, 95% CI 0.09–0.80), synchronized rTMS (OR 0.23, 95% CI 0.07–0.77), and sham TMS (OR 0.27, 95% CI 0.09–0.80). No direct evidence compared priming rTMS to synchronized rTMS and sham treatment. According to the surface under the cumulative ranking curve, priming rTMS and bilateral rTMS were ranked in the two first positions for acceptability as well. The quality of evidence using GRADE was not assessed for discontinuation of treatment.
Ongoing Studies
A few ongoing studies compare rTMS with sham rTMS or other relevant comparators in people with TRD:
A randomized double-blind study from Canada is comparing various types of rTMS in people with TRD (NCT02778035). This study was projected to be completed in September 2019 (searched and did not find a completed RCT)
A randomized cross-over trial from France is comparing individualized rTMS with “conventional” rTMS and transcranial direct-current stimulation in people with TRD (NCT02863380). This study is projected to be completed in August 2021
A multicentre randomized open trial from the United States is comparing antidepressants with rTMS for people with TRD (NCT02977299). This study is projected to be completed in January 2022
We are aware of one ongoing systematic review comparing rTMS with sham rTMS or other relevant comparators in people with TRD:
A meta-analysis of rTMS in treating bipolar disorder: bilateral versus unilateral. PROSPERO 2018. CRD42018082165 is available from https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018082165 (searched and did not find completed review)
Discussion
In this review, we found that most rTMS modalities reduced depression scores and had higher response and remission rates than sham rTMS in people with TRD. Adverse events were similar between groups and were minor; headaches and scalp discomfort were the two most common adverse events. The most studied type of rTMS was high-frequency rTMS over the left DLPFC. We examined the outcomes separately by type of rTMS, and most (except cTBS) had a larger reduction in depression score and had higher response (except for low-frequency and cTBS) and remission rates (except for low-frequency rTMS, iTBS, and bilateral iTBS) compared with sham rTMS.
Low-frequency rTMS had slightly better reduction in depression scores but was not different from sham rTMS when examining response and remission rates. The results also varied when doing subgroup and sensitivity analyses. This variability could arise from the smaller sample sizes and quality of these studies.
We found high statistical heterogeneity in some analyses comparing high-frequency to low-frequency rTMS with sham rTMS. Higher statistical heterogeneity was also found across other systematic reviews (Appendix 4). When we tried to explore subgroup analyses, the statistical heterogeneity remained. This heterogeneity could be due to variation in the natural progression of major depression and the length of time people have had treatment-resistant depression across the studies, because in mental health research, the group of patients is often not homogenous. The statistical heterogeneity could also be attributed to differences in rTMS parameters used (e.g., differences in intensity and number of pulses within the category of high-frequency rTMS). We decided to pool estimates with substantial heterogeneity and considered this statistical heterogeneity in our critical appraisal of the evidence.
The studies included in this review had a brief follow-up (2–6 weeks), which makes it difficult to understand if rTMS is associated with a persistent response and remission rates, which is important for people with mental health issues. The THREE-D study shows that there are four trajectories of rTMS responders.138 These four categories include nonresponse (n = 43), rapid response (n = 73), lower baseline symptoms (n = 154), and higher baseline symptoms (n = 118)—which is considered “intermediate” because the groups show steady gains and no plateau by the end of treatment at 6 weeks. Authors found the following characteristics associated with response groups: clinician-rated depression severity on HRDS at baseline, self-rated depression severity on QIDS-SR (Quick Inventory of Depressive Symptomatology) score at baseline, age, and benzodiazepine use (present or absent). An inclusion criterion for studies in this review was a higher depression score cut-point at baseline (to reflect more severe depression), and studies did not always report anxiety medications participants were taking.
The lack of maintenance treatment (i.e., ongoing treatment beyond the initial course) makes it hard to understand the persistent effect of rTMS treatment. Only one study126 included a maintenance phase after an initial course of deep TMS treatment. Other studies included longer follow-up (at most 14 weeks) without including maintenance treatment. The guidelines on maintenance therapy are not well defined, but continual treatment could be important for people with TRD. Given the quantity of research on the short-term effects of rTMS, it could be important to examine the long-term effects of rTMS as well.
In our overview of reviews, we found no difference in outcomes when comparing various rTMS modalities. We also examined modalities of rTMS separately and found mostly favourable results for the types of rTMS examined in this review. These consistent results could mean that rTMS can be individualized to patients on the basis of their acceptance and tolerance of treatment.
Despite similar baseline scores between ECT and rTMS groups, ECT still had a larger reduction in depression scores than rTMS (−5.97),18 but there was no difference in response or remission rates.18,34 Baseline depression scores were numerically similar in studies comparing ECT with rTMS to scores in studies comparing rTMS with sham rTMS (approximately 20 points on the HDRS). Using the minimal clinically important difference on the HDRS (2–3),27 the most studied form of rTMS (high frequency) had approximately a 4-point difference compared with sham rTMS. As indicated in the 2016 Canadian Network for Mood and Anxiety Treatments guidelines and reflected in this review, given the different mechanism of action of rTMS, as well as its tolerance and acceptance by patients it may be reasonable to consider rTMS before pursuing ECT.1
Strengths and Limitations
Ours is the first overview of reviews to examine the effect of rTMS compared with sham rTMS, ECT, and other rTMS modalities. We did not conduct a search for primary studies to answer the clinical research question 1, but instead used the published systematic reviews and the CADTH 2019 rapid summary51 to identify primary studies. Given the CCA score examining the overlap of primary studies in systematic reviews, we believe that we identified most of the existing primary studies examining the effectiveness of rTMS modalities.
We did not investigate primary studies to address clinical research questions 2 (rTMS vs. ECT) and 3 (rTMS vs. other rTMS modalities). Because of this, we did not capture evidence that may have been published after 2018. We are unaware of recent primary studies comparing rTMS and ECT. With respect to the comparison of rTMS versus other rTMS modalities, we are aware of two studies25,139: one25 compared iTBS and high-frequency left DLPFC rTMS in a noninferiority trial, and the other139 compared accelerated rTMS with high-frequency left DLPFC rTMS. Similar to our review, neither of these studies found any differences in response and remission rates between the rTMS modalities.
Finally, the network meta-analysis included in our review of the evidence for question 3 (rTMS vs. other rTMS modalities) had a number of limitations and should be interpreted with caution. First, three of the eight network meta-analysis nodes (i.e., rTMS modalities) that formed the basis for its conclusions involved just a single study; the meta-analysis grouped several interventions into a single node (i.e., various types of TBS); it grouped several sham conditions into a single node (various sham techniques and treatment with and without concomitant antidepressants); and the authors emphasized ranking instead of treatment effects and uncertainty (with ranking, studies that have direct evidence with an active comparator but no sham comparator [e.g., priming rTMS] are likely to show greater effectiveness).140
Conclusions
At the end of short-term follow-up (generally 2–6 weeks), most rTMS modalities we examined (excluding cTBS) likely resulted in lower depression scores than sham rTMS (GRADE: Moderate to High). Most rTMS modalities (except for low-frequency and cTBS) likely resulted in higher response rates compared with sham rTMS (GRADE: Moderate to High). Three rTMS modalities examined (high frequency rTMS, bilateral rTMS, and deep TMS) likely resulted in higher remission rates than sham TMS (GRADE: Moderate to High). Adverse events were minor and similar in the rTMS and sham rTMS groups (GRADE: Moderate). Overall, none of the rTMS modalities was worse than sham rTMS.
Electroconvulsive therapy likely reduces depression scores (GRADE: Moderate), but probably results in no difference in response and remission rates compared with rTMS (GRADE: Moderate). Adverse events were no different for ECT or rTMS (GRADE: not reported).
When we compared rTMS modalities with one another, we found no difference in response or remission rates (GRADE: not reported). Adverse events were no different among rTMS modalities (GRADE: not reported).
Economic Evidence
Research Question
What is the cost-effectiveness of repetitive transcranial magnetic stimulation (rTMS) compared with pharmacotherapy and with electroconvulsive therapy (ECT) in adults with treatment-resistant depression (TRD)?
Methods
Economic Literature Search
We performed an economic literature search on August 27, 2019, to retrieve studies published from January 1, 2015, until the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic and costing filter applied.
We created database auto-alerts in MEDLINE, Embase, PsycINFO, and CINAHL, and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites, clinical trial and systematic review registries, and the Tufts Cost-Effectiveness Analysis Registry. See the Clinical Literature Search, above, for further details on methods used. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
STUDIES
Inclusion Criteria
English-language full-text publications
Studies published between January 1, 2015, and August 27, 2019
Cost–benefit analyses, cost-effectiveness analyses, cost-minimization analyses, or cost– utility analyses
Exclusion Criterion
Reviews, editorials, case reports, commentaries, and abstracts
POPULATION
INTERVENTIONS
Repetitive transcranial magnetic stimulation (37.5-minute, 10-Hz rTMS protocol [hereafter referred to as high-frequency rTMS]) and intermittent theta burst stimulation (iTBS)
OUTCOME MEASURES
Costs
Health outcomes (e.g., quality-adjusted life-years [QALYs])
Incremental costs
Incremental effectiveness
Incremental cost-effectiveness ratios (ICERs)
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Covidence54 and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. This reviewer then examined the full-text articles and selected studies eligible for inclusion.
Data Extraction
We extracted relevant data on study characteristics and outcomes to collect information about the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, analytic technique, perspective, time horizon, population, intervention[s], comparator[s])
Outcomes (e.g., health outcomes, costs, ICERs) We contacted study authors to provide clarification as needed.
Study Applicability and Limitations
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform the development of NICE's clinical guidelines.142 We modified the wording of the questions to remove references to guidelines and to make it specific to Ontario. Next, we separated the checklist into two sections. In the first section, we assessed applicability of each study to the research question (directly, partially, or inapplicable). In the second section, we assessed the limitations (minor, potentially serious, or very serious) of the studies we found to be directly applicable.
Results
Economic Literature Search
The economic literature search yielded 86 citations published between January 1, 2015, and August 27, 2019, after removing duplicates. We identified eight studies that met our inclusion criteria. Figure 16 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search.
Figure 16: PRISMA Flow Diagram—Economic Search Strategy.
Source: Adapted from Moher et al.39
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Overview of Included Economic Studies
All included studies examined the cost-effectiveness of high-frequency rTMS versus ECT or pharmacotherapy. No studies examined iTBS.
The most recently conducted cost-effectiveness study was published in 2019 by Fitzgibbon et al.143 In this study, the authors constructed a Markov microsimulation model to compare the costs and effects of high-frequency rTMS followed by ECT versus ECT alone over a person's lifetime. In the reference case analysis, the authors explored the effect of stepped care treatment, in which people with TRD would receive high-frequency rTMS as first-line treatment. Those who did not respond to rTMS could switch to ECT. In the reference case, the authors assumed that 35% of these people would switch to ECT. This analysis was conducted from a societal perspective (Ontario), in which indirect costs related to labour unproductivity and caregiver costs were included. The authors concluded that high-frequency rTMS (as part of the stepped care) dominated ECT alone (i.e., was less costly [savings of $46,094 in 2018 CAD] and more effective [gain of 0.96 QALYs]). The probabilistic sensitivity analysis (PSA) confirmed that there was a 100% chance that high-frequency rTMS would be more cost-effective than ECT.
Two cost-effectiveness studies were published in 2017 by Zhao et al144 and Voigt et al.145 Zhao et al compared high-frequency rTMS with ECT for people with TRD from a societal perspective (in Singapore).144 The authors constructed a Markov cohort model to simulate the costs and health outcomes of high-frequency rTMS and ECT over 1 year. At 1 year, high-frequency rTMS was associated with lower costs (in 2018 Singapore dollars [SGD]: $23,072 vs. $34,922) and lower QALYs (0.6862 vs. 0.7243). At Singapore's willingness-to-pay (WTP) of $70,000 SGD per QALY ($65,828/QALY [2017 CAD]),146 high-frequency rTMS was considered to be highly cost-effective compared with ECT. A subgroup analysis showed that high-frequency rTMS dominated ECT for treatment of nonpsychotic depressive patients in inpatient settings. Thus, compared with ECT, high-frequency rTMS was less costly ($21,835 SGD vs. $34,859 SGD) and more effective (total QALYs: 0.7361 vs. 0.7243 and remission rates: 70.3% vs. 67.9%). In contrast, when ECT was provided to outpatients, the ICER was much smaller (outpatient: $78,819 SGD per QALY vs. inpatient: $311,024 SGD per QALY). Similarly, the ICER per remission achieved was between one third and one quarter of the ICER calculated in the reference case analysis ($36,444 SGD per QALY vs. $143,811 SGD per QALY). These results were confirmed by sensitivity analyses: the relative risk for remission between high-frequency rTMS and ECT and hospitalization cost of ECT were the most influential drivers of cost-effectiveness. The PSA indicated that, at a WTP of $70,000 SGD/QALY ($65,828 CAD/QALY),146 rTMS had a 95% chance to be more cost-effective than ECT. However, when the WTP increased, the chance that high-frequency rTMS would be more cost-effective than ECT decreased.
Voigt et al145 developed a Markov cohort model to compare high-frequency rTMS with pharmacotherapy in people whose newly diagnosed major depression had failed one pharmacotherapy trial. The model followed patients over a lifetime and was conducted from the perspective of the US health care sector. High-frequency rTMS dominated pharmacotherapy in all age groups. However, the effectiveness of high-frequency rTMS was extrapolated over a lifetime without adjusting for possible decrements over time in health-related quality of life (i.e., utilities) or for the effectiveness of high-frequency rTMS.
One study from Iran was published in 2016.147 In this study, the authors’ economic evaluation compared high-frequency rTMS with ECT for people with TRD. The authors developed a decision tree model to simulate the costs and health outcomes of high-frequency rTMS and ECT over 7 months from the perspective of Iran's health care sector. The authors concluded that ECT was more cost-effective than high-frequency rTMS, given the high prevalence of TRD in Iran. Although the authors stated they conducted a cost-effectiveness analysis and calculated the ICER between high-frequency rTMS and ECT, we were unable to confirm results from the publication. In addition, no PSA was conducted to test the robustness of the findings on cost-effectiveness.
Two studies were published in 2015.148,149 Nguyen et al148 developed a Markov microsimulation model using a 2-month cycle and a time horizon of 3 years. They compared high-frequency rTMS with pharmacotherapy for people with TRD. The analysis was done from the perspective of Australia's health care sector. The analysis showed that high-frequency rTMS was more effective than antidepressants (1.25 vs. 1.18 QALYs) and slightly cheaper (in 2014 Australian dollars [AUD], $31,003 AUD vs. $31,190 AUD). Therefore, high-frequency rTMS dominated antidepressant medications. The PSA showed that, at a WTP of $50,000 AUD per QALY ($48,020/QALY [2015 CAD]),146 there was a 73% chance that rTMS would be more cost-effective than antidepressant medications.
The other 2015 study by Vallejo-Torres et al149 used a Markov cohort model to examine the cost-effectiveness of (1) ECT alone compared with high-frequency rTMS alone; and (2) ECT alone compared with high-frequency rTMS (followed by ECT alone) when rTMS failed in people with TRD. The study was conducted from the perspective of the Spanish National Health Service. The model simulated costs and health outcomes of TRD over 1 year. The authors used two utility measures in the analyses: McSad and EQ-5D (European Quality of Life questionnaire in five dimensions). When comparing ECT and high-frequency rTMS, ECT appeared to be less costly (in 2014 Euros: €16,690 vs. €16,858) and more effective (0.2137 vs. 0.1783 QALYs by McSad, or 0.4253 vs. 0.3988 QALYs by EQ-5D). Therefore, regardless of the utility weights that were applied, ECT dominated high-frequency rTMS. When comparing high-frequency rTMS followed by ECT with ECT alone (after depression had failed to improve with rTMS), the incremental cost between the two strategies was €3,589, while the incremental QALYs were 0.035 and 0.049, depending on the utility measure used. As a result, the ICER was €72,668 per QALY when using the McSad, or €103,953 per QALY when using the EQ-5D. At a WTP of €30,000 per QALY ($42,546/QALY 2015 CAD]),146 a commonly accepted WTP in Spain, high-frequency rTMS followed by ECT was not deemed cost-effective. The authors indicated that a longer time horizon was not considered because we lack evidence of the long-term effectiveness of rTMS.
HEALTH TECHNOLOGY ASSESSMENTS RELATED TO rTMS FROM TWO CANADIAN AGENCIES
We identified two Canadian health technology assessments, both published in 2016.13,150 The health technology assessment from Health Quality Ontario13 examined the short-term (6-month) cost-effectiveness of high-frequency rTMS for people with TRD using two decision tree models from the perspective of Ontario's Ministry of Health. One model compared high-frequency rTMS with ECT in people who were willing to be treated with either high-frequency rTMS or ECT. Another model compared high-frequency rTMS with pharmacotherapy alone in people who were ineligible for or refused ECT. In addition to the cost of the interventions, these analyses accounted for the cost of antidepressant medications, physician visits, and hospitalizations for people with TRD.
Results of Health Quality Ontario's cost-effectiveness analysis showed that high-frequency rTMS was more cost-effective than pharmacotherapy alone only if the WTP was higher than $98,242 per QALY. Further, high-frequency rTMS had lower costs ($5,272 vs. $5,960 [2014 CAD]) and smaller effects (0.31 vs. 0.32 QALYs) than ECT, and was cost-effective if the WTP was lower than $37,641 per QALY (Table 10). The PSA showed large uncertainty over the cost-effectiveness of high-frequency rTMS. The key drivers of cost-effectiveness were the effectiveness of high-frequency rTMS on response and remission outcomes. A lifetime horizon was not considered, owing to lack of evidence of the long-term effect of high-frequency rTMS.
Table 10:
Results of Economic Literature Review—Summary
| Author, Year, Country | Analytic Technique, Study Design, Perspective, Time Horizon | Population | Intervention(s) and Comparator(s) | Results | |||
|---|---|---|---|---|---|---|---|
| Health Outcomes | Costs | Cost-Effectiveness | Uncertainty | ||||
| Fitzgibbon et al, 2019 143 Canada |
Cost–utility analysis Microsimulation model Societal perspective Lifetime | Adults with TRD Age (mean) 42 y | rTMS followed by ECT vs. ECT alone |
Currency: 2018 CAD Incremental cost (rTMS vs. ECT): −$46,094 Discount rate: 1.5% |
rTMS vs. ECT: Dominant (lower cost and higher effectiveness) | PSA showed 100% certainty that rTMS dominated ECT and that model was robust Scenario analysis showed that more people with TRD switching to ECT if they did not initially respond to rTMS could increase cost savings |
|
| Zhao et al, 2018144 Singapore | Cost–utility analysis Markov decision analytic model Societal perspective 1 y | Adults with TRD Age (mean): 43 y |
rTMS vs. ECT | rTMS: 0.686 QALYs ECT: 0.724 QALYs Incremental QALYS (rTMS vs. ECT): −0.038 Discount rate: NA |
Currency: 2018 SGD (in 2019, $1 SGD = $0.97 CAD) rTMS: $23,072 SGD ECT: $34,922 SGD Incremental costs (rTMS vs. ECT): $11,850 SGD Discount rate: NA |
rTMS vs. ECT: $311,024 SGD /QALY ($302,241 CAD/QALY)b rTMS vs. ECT: cost-effective (lower costs but lower effects) |
Subgroup analysis, adults with nonpsychotic symptoms: cost-effective (lower costs and more effective) PSA confirmed robustness of model; rTMS had 95% chance of being more cost effective than ECT at WTP of $70,000 SGD/QALY |
| Voigt et al, 2017145 USA | Cost-utility analysis Markov model Health care sector perspective Lifetime | Adults newly diagnosed with major depression who failed to benefit from single-medication trial | rTMS vs. medication | Mid-20s rTMS: 15.22 Medication: 14.79 QALYs Incremental QALYs: 0.43 Mid-30s rTMS: 14.06 Medication: 13.62 Incremental QALYs: 0.44 Mid-40s rTMS: 12.26 QALYs Medication: 11.83 QALYs Incremental QALYs: 0.43 Mid-50s rTMS: 8.77 Medication: 8.45 QALYs Incremental QALYs: 0.32 Discount rate: 3% | Currency: 2018 USD Mid-20s rTMS: $278,103 Medication: $289,243 Incremental costs: −$11,140 Mid-30s rTMS: $257,686 Medication: $266,665 Incremental costs: −$8,979 Mid-40s rTMS: $226,126 Medication: $232,518 Incremental costs: −$6,392 Mid-50s rTMS: $164,769 Medication: $167,721 Incremental costs: −$2,952 Discount rate: 3% | rTMS vs. medication: dominant (lower cost and higher effectiveness) | rTMS vs. medication: cost-effective for all age groups PSA showed 36%–40% chance that rTMS would be more cost-effective than antidepressants |
| Ghiasvand et al, 2016147 Iran | Cost–utility analysis Decision tree model Health care sector perspective 7 mo | Adults with major depression | rTMS vs. ECT | rTMS: 1,184,001 improved patients ECT: 5,462,036 improved patients Incremental improved patients: −4,278,035 Discount rate: NA | Currency: 2016 Rials rTMS: 11,015,000 Rials ($376 USD) ECT: 11,742,700 ($397.70 USD) Incremental costs: −727,700 Rials Discount rate: NA | rTMS vs. ECT: 1,194,419 Rials ($40.00 USD) | Uncertainty was not addressed in report |
| Nguyen et al, 2015148 Australia | Cost–utility analysis Markov microsimulation decision analytic model 3 y Health care sector perspective | Adults with TRD | rTMS vs. medication | rTMS: 1.25 QALYs Medication: 1.18 QALYs Incremental QALYs: 0.07 Discount rate: 5% |
Currency: 2015 AUD rTMS: $31,003 Medication: $31,190 Incremental costs: −$87 Discount rate: 5% |
rTMS vs. medication: dominant | rTMS vs. medication: cost-effective, dominant (lower costs, more effective) PSA showed that, at WTP of $50,000 AUD/QALY, there was a 73% chance that rTMS would be more cost-effective than ECT |
| Vallejo-Torres et al, 2015149 Spain | Markov decision analytic model 1 y Health care sector perspective | Adults with TRD | ECT alone vs. rTMS alone vs. rTMS followed by ECT alone | ECT: 0.4253 QALYs rTMS: 0.3988 QALYs rTMS followed by ECT: 0.4598 QALYs Incremental QALYs (ECT vs. rTMS): 0.0265 Incremental QALYs (rTMS vs. rTMS followed by ECT): −0.061 Discount rate: NA |
Currency: 2015 Euros ECT: €16,690 rTMS: €16,858 rTMS followed by ECT: €20,279 Incremental costs (ECT vs. rTMS): −€168 Incremental costs (rTMS vs. rTMS followed by ECT): €3,589 | ECT vs. rTMS: dominant rTMS vs. rTMS followed by ECT: €103,953/QALY | PSA showed that, at WTP of €30 000/QALY, ECT alone had a 70% chance of being cost-effective |
| Health Quality Ontario, 201613 Canada |
Decision tree models 6 mo Health care sector perspective (Ministry of Health and Long-Term Care) | Adults with TRD | rTMS vs. sham (medications) ECT vs. rTMS | rTMS: 0.30 QALYs Medication: 0.28 QALYs Incremental QALYs: 0.02 ECT vs. rTMS ECT: 0.32 QALYs rTMS: 0.31 QALYs Incremental QALYs: 0.01 Discount rate: NA | Currency: 2014 CAD rTMS: $5,132 Medication: $2,978 Incremental costs: $2,154 ECT vs. rTMS ECT: $5,960 rTMS: $5,272 Incremental costs: $688 Discount rate: none | rTMS vs. medication: $98,242/QALY ECT vs. rTMS: $37,641/QALY | PSA showed that, at WTP of $50,000/QALY, there is a 45% chance that rTMS would be more cost-effective than ECT PSA showed that, at WTP of $50,000/QALY, there is a 2% chance that rTMS would be more cost-effective than pharmacotherapy alone |
| University of Calgary, 201634 Canada (Alberta) | Decision tree models 3–6-wk Health care sector perspective (Alberta) | Adults with TRD | rTMS vs. standard medication therapy (pharmacotherapy alone) ECT vs. rTMS | rTMS vs. medication Remission rTMS: 0.38 QALYs Medication: 0.34 QALYs Incremental QALYs: 0.04 Response rTMS: 0.42 QALYs Medication: 0.35 QALYs Incremental QALYs: 0.07 ECT vs. rTMS Remission ECT: 0.54 QALYs rTMS: 0.53 QALYs Incremental QALYs: 0.01 Response ECT: 0.57 QALYs rTMS: 0.59 QALYs Incremental QALYs: −0.02 Discount rate: NA | Currency: 2014 CAD rTMS vs. medication rTMS: $952 Medication: $45 Incremental costs: $907 ECT vs. rTMS ECT: $3,324 rTMS: $952 Incremental costs: $2,372 Discount rate: NA | rTMS vs. medication Remission: $20,203/QALY Response: $13,084/QALY ECT vs. rTMS Remission: $328,325/QALY Response: dominated | PSA for response outcome showed a 98.2% chance that ECT would be more costly and less effective than rTMS PSA for remission outcome showed an 84.5% chance that rTMS would be most cost-effective |
Abbreviations: AUD, Australian dollar; ECT, electroconvulsive therapy; ICER, incremental cost-effectiveness ratio; NA, not applicable; NR, not recorded; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life-year; rTMS, high-frequency repetitive transcranial magnetic stimulation; SGD, Singaporean dollar; TRD, treatment-resistant depression; WTP, willingness to pay.
The second health technology assessment was conducted by the Health Technology Assessment Unit at the University of Alberta.150 The authors investigated the cost-effectiveness of (1) high-frequency rTMS compared with ECT, and (2) high-frequency rTMS compared with pharmacotherapy, in people with TRD, from the perspective of Alberta's Ministry of Health. A decision tree model was constructed to follow adults with TRD over 3 to 6 months. This was the longest duration of follow-up reported in randomized controlled trials assessing the clinical effectiveness of rTMS. Remission and relapse were the two main clinical outcomes captured in the model. For the response outcome, high-frequency rTMS generated more QALYs (0.59 vs. 0.57 QALYs) and was less expensive ($952 vs. $3,324 [2014 CAD]) than ECT; therefore, high-frequency rTMS dominated ECT. For the remission outcome, high-frequency rTMS generated QALYs (0.53 vs. 0.54 QALYs) almost equal to those of ECT at a much lower cost ($952 vs. $3,324). It was, therefore, found to be cost-effective compared with ECT. Last, compared with standard care (pharmacotherapy), high-frequency rTMS was cost-effective.
Applicability and Limitations of the Included Studies
Appendix 7 provides results of the quality appraisal checklist for economic evaluations applied to the included studies. Three Canadian studies13,143,150 were deemed partially applicable to the research question; two studies from Spain and Australia148,149 were also considered partially applicable (Appendix 7, Table A13). Two other studies were not applicable.145,147 It was unclear whether one study144 from Singapore was applicable (see Appendix 7, Table A13).
All eight studies were conducted in adults with TRD except for one study145 that included people with newly diagnosed major depression. Further, the studies included relevant comparators and effectiveness data from recently published randomized controlled trials. The three directly applicable studies143,150,151 were conducted using the perspective of Ontario's Ministry of Health and Alberta's Ministry of Health. They also used PSAs. One study applied a 1.5% discount rate as recommended by CADTH152 and two applied no discount rate, as the time horizon was less than a year. Two other studies144,149 had a 1-year time horizon and no discount rate was applied. One study148 applied a 3-year time horizon and used a discount rate of 5%, and one study145 applied a lifetime horizon and used a discount rate of 3%.
An assessment of the limitations of the Ontario− and Alberta-based studies is presented in Appendix 7 (Table A14). All studies had only minor limitations. No study examined the cost-effectiveness of iTBS over high-frequency rTMS or control treatments. Ontario-based studies included relevant costs and used Ontario sources (i.e., Ontario Health Insurance Plan billing codes, Canadian Institute for Health Information Patient Cost Estimator) to estimate the costs and resource use associated with rTMS, ECT, and pharmacotherapy. The Alberta-based study used Alberta sources (i.e., the Alberta Interactive Drug Benefit List, The Alberta Health Services job board) to estimate the costs and resource use associated with rTMS, ECT, and pharmacotherapy. The Ontario-based cost–utility by Fitzgibbon et al143 used a lifetime horizon and was conducted from the societal perspective. The two health technology assessments used a 6-month time horizon13,150 and were conducted from the perspective of Ontario's Ministry of Health and Long-Term Care (now Ministry of Health) and Alberta's Ministry of Health.
Discussion
Our economic evidence review identified eight studies that assessed the cost-effectiveness of high-frequency rTMS compared with pharmacotherapy and ECT in treatment of adults with TRD. The studies have conflicting conclusions on the potential economic value of high-frequency rTMS. These differences could be attributed to a variety of factors, such as different comparators, different health outcomes, variability in settings, analytic perspective, or time horizon.
The results comparing high-frequency rTMS with ECT also varied across studies. The most recent published study by Fitzgibbon et al143 concluded that high-frequency rTMS followed by ECT in a stepped care pathway for people with TRD, who initially did not respond to rTMS, would generate cost savings compared with ECT alone. This Canadian study was conducted from a societal perspective and applied a lifetime horizon. In contrast, the study by Vallejo-Torres et al149 found that rTMS followed by ECT was not more cost-effective than ECT alone. The opposite results might be explained by the differences in perspectives and time horizon and the proportion of TRD patients progressing to ECT. The latter was conducted by the Spanish Health Service over 1 year.149 Another study by Zhao et al144 concluded that rTMS was highly cost-effective compared with ECT at Singapore's WTP of $70,000 SGD per QALY ($69,181/QALY [2017 CAD]). The study was conducted from the societal perspective. In an Iranian study by Ghiasvand et al,147 rTMS was less cost-effective than ECT. In the Ontario study, rTMS was cost-effective compared with ECT if the WTP is less than $37,641 per QALY. In contrast, in the Alberta study, rTMS was cost-effective compared with pharmacotherapy when considering both remission and response (ICER ≤ $50,000/QALY)150; rTMS was more cost-effective than ECT when considering remission, and dominated ECT when considering response.
The study by Fitzgibbon et al143 and the Ontario and Alberta health technology assessments150,151 were partially applicable to our context and our research questions. The two health technology assessments did not explore differences between frequencies of rTMS (i.e., high-frequency rTMS vs. iTBS) and used short-term horizons. The study by Fitzgibbon et al143 also did not explore the differences between frequencies of rTMS (i.e., high-frequency rTMS vs. iTBS), applied a lifetime horizon, and was conducted from a societal perspective. Therefore, the health technology assessments conducted in Ontario and Alberta might not have adequately captured the full costs and consequences associated with long-term use of rTMS and relapse.13,150 On the contrary, by applying a lifetime horizon, the study by Fitzgibbon et al143 might overestimate the long-term effects of rTMS, given the lack of clinical data.
Conclusions
Our review of the literature identified eight published cost-effectiveness studies that compared high-frequency rTMS with pharmacotherapy or with ECT in treatment of adults with TRD. Two Canadian studies were conducted from the perspective of the provincial ministries of health13,150 and one Canadian study was conducted from the societal perspective.143 The two health technology assessments showed that rTMS was cost-effective compared with pharmacotherapy and might be cost-effective compared with ECT.13,150 These studies also applied a short-term horizon; thus, the long-term effects of rTMS were not captured.13,150 The study by Fitzgibbon et al143 used a lifetime time horizon and compared high-frequency rTMS followed by ECT with ECT alone for people with TRD who did not initially respond to rTMS. The results showed that high-frequency rTMS followed by ECT was associated with cost savings compared with ECT alone. This study also did not consider pharmacotherapy alone as a comparator. Notably, none of the studies explored the economic impact of various frequencies of rTMS, such as high-frequency rTMS (37.5-minute, 10-Hz rTMS protocol) versus iTBS, which has been commonly used in Ontario; a recent noninferiority trial completed in Canada found that high-frequency rTMS and iTBS were equivalent.25 Considering all these factors, it was deemed important to conduct a primary economic evaluation in the context of Ontario that follows the TRD clinical pathway over a longer time.
Primary Economic Evaluation
We identified three published Canadian economic evaluations that partially addressed our research questions.13,143,150 However, each of these studies had limitations. For example, two health technology assessments constructed a simple decision tree analysis over 6 months, which did not capture long-term effects of repetitive transcranial magnetic stimulation (rTMS).13,150 In contrast, the economic evaluation by Fitzgibbon et al143 constructed a Markov microsimulation model over a lifetime horizon but from a societal perspective. No studies compared the effectiveness of intermittent theta burst stimulation (iTBS; followed by electroconvulsive therapy [ECT] in a stepped care pathway) with that of ECT alone or with that of pharmacotherapy alone. As mentioned in the clinical evidence section, high-frequency rTMS and iTBS are used most in Ontario because a recent noninferiority trial completed in Canada found that high-frequency rTMS and iTBS were equivalent.25 Based on the findings of the noninferiority trial and the use of these modalities in the Ontario context, we conducted a full primary economic evaluation in which we adapted the model by Fitzgibbon et al143 and modeled an rTMS clinical pathway using these modalities.
Research Question
From the perspective of the Ontario Ministry of Health, what is the cost-effectiveness of the following treatments for adults with TRD:
High-frequency rTMS (followed by ECT if initial treatment with rTMS fails) compared with pharmacotherapy alone or with ECT alone
ITBS (followed by ECT if initial treatment with iTBS fails) compared with pharmacotherapy alone or with ECT alone
Methods
The information presented in this report follows the reporting standards set out by the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement.153
Analysis
We conducted a cost–utility analysis to measure the costs and quality-adjusted life-years (QALYs) of high-frequency rTMS or iTBS within a stepped care pathway compared with pharmacotherapy alone and ECT alone.
We conducted the reference case and scenario analyses. Our reference case analysis adhered to the Canadian Agency for Drugs and Technologies in Health (CADTH) guidelines152 when appropriate and represents the most likely set of input parameters and model assumptions. Our scenario analyses explored how results are affected by varying input parameters and model assumptions.
Target Population
Our target population was adults 18 years of age and older with TRD (unipolar). People who failed to respond to two appropriately dosed antidepressants were considered to have TRD.154
Perspective
We conducted this analysis from the perspective of the Ontario Ministry of Health.
Interventions and Comparators
We compared four strategies in total: two interventions and two comparators.
Two intervention strategies were considered within a stepped care pathway:
High-frequency rTMS: In this pathway those who failed to respond initially to rTMS could switch to ECT, representing a stepwise clinical trajectory from less to more invasive treatments (hereafter shortened as high-frequency rTMS followed by ECT)
ITBS: In this pathway, those who failed to respond initially to iTBS could switch to ECT, representing a stepwise clinical trajectory from less to more invasive treatments (hereafter shortened as iTBS followed by ECT)
The two comparators were ECT alone and pharmacotherapy alone.
We chose high-frequency rTMS (followed by ECT for those who initially fail to benefit from rTMS) because this treatment pathway is recommended by the 2016 CANMAT (Canadian Network for Mood and Anxiety Treatments) guidelines.17 We chose iTBS (followed by ECT for those who initially fail to benefit from iTBS) because this novel mode of rTMS treatment has similar clinical effectiveness to high-frequency rTMS (see Clinical Evidence section) and a shorter treatment duration (3 minutes).
We used ECT and pharmacotherapy alone as the two comparators because these two strategies represent usual care in the treatment of adults with TRD. Table 11 summarizes the interventions, comparators, and outcomes evaluated in our economic model.
Table 11:
Interventions, Comparators, and Outcomes Evaluated in the Primary Economic Model
| Intervention | Comparator | Population | Outcome |
|---|---|---|---|
| High-frequency rTMS (within stepped care)a | Pharmacotherapy alone; ECT alone | Adults with TRD | Cost, QALYs, ICER |
| iTBS (within stepped care)b | Pharmacotherapy alone; ECT alone | Adults with TRD | Cost, QALYs, ICER |
Abbreviations: ECT, electroconvulsive therapy; ICER, incremental cost-effectiveness ratio; iTBS, intermittent theta burst stimulation; QALYs, quality-adjusted life-year; rTMS, repetitive transcranial magnetic stimulation; TRD, treatment-resistant depression.
In this pathway, those who failed to respond initially to high-frequency rTMS could switch to ECT, representing a stepwise clinical trajectory from less to more invasive treatment (shortened as high-frequency rTMS followed by ECT).
In this pathway, those who failed to respond initially to iTBS could switch to ECT, representing a stepwise clinical trajectory from less to more invasive treatment (shortened as iTBS followed by ECT).
Discounting and Time Horizon
For our reference case, we used a 3-year time horizon to capture the effectiveness of various treatments and the chronic, relapsing nature of TRD. This assumption is in line with available clinical evidence and short-term effectiveness of rTMS. In our scenario analyses, we applied a 1-year and lifetime time horizon. In accordance with CADTH guidelines,152 we applied an annual discount rate of 1.5% to both costs and QALYs, incurred after the first year.
Model Structure
We adapted the model by Fitzgibbon et al143 by modifying its interventions and comparators. A schematic of the model is presented in Figure 17.
Figure 17: Model Structure.
Source: Adapted from model by Fitzgibbon et al.143
Abbreviations: ECT, electroconvulsive therapy; iTBS: Intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation. ∗Treatment options include high-frequency rTMS, ECT, iTBS, and pharmacotherapy alone.
The simulated cohort included 10,000 persons, whose age when entering the model was distributed according to the 2017 adult age distribution of the Ontario population.155 A person with TRD would receive high-frequency rTMS, ECT, iTBS, or pharmacotherapy alone and would transition through the following model health states: remission, acute treatment (a temporary health state that could be repeated), maintenance treatment, severe depression, and death (death due to severe depression or other causes). The model was run in 6-month cycles (intervals) for a maximum of 3 years (6 cycles).
We assumed that people with TRD who received high-frequency rTMS or iTBS for management of TRD would follow a stepped care pathway. Each course of rTMS or iTBS included 30 sessions in acute treatment and 12 sessions in maintenance treatment, as indicated by Fitzgibbon et al.143
When adults with TRD initially received either high-frequency rTMS or iTBS as first-line treatment, they either responded or failed to respond to these therapies. In accordance with standard clinical practice, we assumed the following:
Those who failed to respond to high-frequency rTMS or iTBS could switch to ECT. In the reference case, only 35% of people with TRD would switch to ECT143
-
Those who responded to high-frequency rTMS or iTBS could either achieve remission or respond without remission:
– Those who achieved remission could either remain in remission or could relapse. If they relapsed, they would enter the acute treatment health state and would receive treatment until the maximum limit was reached (i.e., three courses of acute treatment for high-frequency rTMS and iTBS over the 3-year time horizon)
– Those who responded without remission would receive maintenance treatment. Those who relapsed from maintenance treatment would receive another round of acute treatment, again, until the maximum limit was reached (i.e., three courses of high-frequency rTMS treatment)
Adults who received ECT alone for treatment of TRD could respond or fail to respond to this therapy. Each course of acute ECT treatment included 15 sessions, as indicated by Fitzgibbon et al.143 In accordance with standard clinical practice, we assumed the following:
Those who failed to respond initially to ECT would transition to severe depression (i.e., pharmacologic treatment solely; no rTMS) for the remainder of follow-up
-
Those who responded to ECT could either achieve remission or respond without remission:
– Those who achieved remission could either remain in remission or could relapse. If they relapsed, they would enter the acute ECT health state and would receive one more course of acute ECT treatment (i.e., the maximum limit)
-
– Those who responded without remission would receive maintenance treatment. Those who relapsed from maintenance would receive one more course of acute ECT treatment over the 3-year time horizon
Those who had no response to the second ECT course would transition to the severe depression health state and would remain in this health state for the rest of follow-up unless they transitioned to the death state
For pharmacotherapy alone, we modelled the following health states: remission, severe depression, and death. Adults with TRD who received pharmacotherapy alone could respond or fail to respond to the treatment:
Those who failed to benefit from pharmacotherapy would transition to the severe depression health state for the remainder of the simulation
-
Those who responded to pharmacotherapy would achieve remission (simplifying assumption) and would either remain in this health state or relapse
– If patients relapsed, they would transition to the severe depression health state for the rest of follow-up unless they transitioned to the death state
COMPARISON OF ORIGINAL MODEL AND OUR MODIFIED MODEL
Similarities
The overall clinical pathway for high-frequency rTMS and ECT treatment in the modified model followed the pathway in the model used by Fitzgibbon et al.143 The clinical pathway for iTBS was assumed to be similar to the pathway described for high-frequency rTMS (email and oral communication with D. Blumberger, MD, on October 30, 2019; see Figure 17).
The number of sessions in acute and maintenance treatment states for high-frequency rTMS, ECT, and iTBS in the modified model remained the same as those used in the Ontario model (see Figure 17).143 For high-frequency rTMS and iTBS, one course of acute treatment consisted of 42 sessions and a course of maintenance treatment consisted of 12 sessions. For ECT,143 a course of acute treatment included 15 sessions, and a course of maintenance treatment consisted of 6 sessions (email communication with D. Blumberger, MD, on October 22, 2019)
Differences
For interventions, we classified rTMS as either high-frequency rTMS or iTBS (both modelled as stepped care pathways). The Ontario model143 included only high-frequency rTMS
-
For comparators, in addition to ECT alone (which was the only comparative strategy in the Ontario model143), we included pharmacotherapy alone
– Our microsimulation imposed a maximum limit of three courses of acute treatment for high-frequency rTMS or iTBS and one course of acute treatment for ECT over the 3-year time horizon (email communication with D. Blumberger, MD, on December 10, 2019). In the Ontario model,143 the maximum limit was 12 courses of acute treatment for high-frequency rTMS and four courses of acute treatment for ECT over a person's lifetime
Main Assumptions
Because our model was an adaptation of the model by Fitzgibbon et al,143 all assumptions related to modeling high-frequency rTMS and ECT were consistent and could be found in their research.143 Given that our model included pharmacotherapy alone and iTBS as two additional treatment strategies, and given that our time horizon was 3 years, we made the following assumptions:
-
The maximum limit of acute treatment for rTMS and iTBS was three courses (42 sessions per course). The maximum limit of acute treatment courses for ECT was one course, which included 15 sessions:
– Those who failed to respond initially to iTBS could switch to ECT; this pathway represented a stepwise clinical trajectory from less to more invasive treatment
– All patients who responded to treatment without attaining remission would receive maintenance therapy
35% of people with TRD would switch to ECT given no initial response to high-frequency rTMS or iTBS
For pharmacotherapy alone, those who relapsed from remission would transition to the severe depression health state
Clinical Outcomes and Utility Parameters
We populated our cost-effectiveness model with the clinical and utility parameters described below. These parameters are associated with the natural and clinical course of severe depression and with the treatment effects of high-frequency rTMS, ECT, iTBS, and pharmacotherapy alone.
The main clinical outcomes used in the model are remission, response without remission, relapse, and death. Input parameters related to these outcomes are presented in Table 12.
Table 12:
Model Input Parameters Used in Reference Case Analysis
| Model Parameters | Mean (95% CI) | Distributiona | Reference |
|---|---|---|---|
| rTMS | |||
| Probability of response | 0.418 (0.296–0.552) | Beta | HQO13 |
| Probability of remission given response | 0.481 (0.395–0.586) | Beta | HQO13 |
| Sustained response | |||
| At 6 mo | 0.529 (0.403–0.650) | Beta | Jelovac et al156 and |
| At 12 mo | 0.463 (0.326–0.607) | Beta | Fitzgibbon et al143 |
| Percentage of TRD patients who would switch to ECT after no initial response to rTMS | 0.35 | Not variedc | Fitzgibbon et al143 |
| ECT | |||
| Probability of response | 0.666 (0.633–0.698) | Beta | Ross et al157 |
| Probability of remission given response | 0.509 (0.474–0.544) | Beta | Ross et al157 |
| Probability of remission without response | 0.183 (0.111–0.280) | Beta | Ross et al157 |
| Relapse | |||
| Without maintenance therapy | 0.377 (0.307–0.452) | Beta | Jelovac et al156 |
| With maintenance therapy | 0.308 (0.194–0.433) | Beta | Ross et al157 |
| Pharmacotherapy Alone | |||
| Risk ratio of probability of response (rTMS vs. pharmacotherapy) | 2.4 (1.70–3.40) | Lognormal | Clinical review |
| Probability of response | 0.174 (0.162–0.174) | Calculation | |
| Risk ratio of probability of remission (rTMS vs. pharmacotherapy) | 2.21 (1.26–3.90) | Lognormal | Clinical review |
| Probability of remission given response | 0.218 (0.150–0.313) | Calculation | |
| iTBS | |||
| Estimated adjusted difference in response (rTMS vs. iTBS) | 0.0183 (−0.066 to 0.102) | Blumberger et al25 | |
| Probability of response | 0.436 (0.231–0.654) | Beta | Calculation |
| Estimated adjusted difference in remission (rTMS vs. iTBS) | 0.052 (−0.024 to 0.128) | Blumberger et al25 | |
| Probability of remission Sustained responseb | 0.533 (0.371–0.714) | Beta | Calculation |
| At 6 mo | 0.529 (0.403–0.650) | Beta | Jelovac et al156 and |
| At 12 mo | 0.463 (0.326–0.607) | Beta | Fitzgibbon et al143 |
| All Treatment Strategies | |||
| Increase in mortality due to severe depression | 29% | Not variedd | Fitzgibbon et al,143 Chang et al,158 Chesney et al,159 Cuijpers et al160 |
Abbreviations: ECT, electroconvulsive therapy; HQO, Health Quality Ontario; iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation; TRD, treatment-resistant depression.
Distribution was assigned and calculated by Briggs et al.161
Under the assumption that values for iTBS are the same as for rTMS.
This assumption was not varied in the reference case analysis based on Fitzgibbon et al.143 However, this value was varied in the scenario analysis from 0 to 100%.
This assumption was not varied in the reference case analysis based on Fitzgibbon et al.143 However, this value was varied in the scenario analysis by ±25%
HEALTH STATE UTILITIES
We quantified health outcomes as QALYs. Because we adapted the model by Fitzgibbon et al,143 we applied the same health state utility values (Table 13).
Table 13:
Utilities Used in the Economic Model
| Utility Measures | Mean (SE) | Distribution | Reference |
|---|---|---|---|
| Health State | |||
| Remission | 0.80 (0.03) | Beta | HQO,13 Sapin et al,162 Zhao et al,144 Fitzgibbon et al143 |
| Responder without remission/maintenance | 0.72 (0.04) | Beta | HQO,13 Sapin et al,162 Zhao et al,144 Fitzgibbon et al143 |
| Severe depression | 0.30 (0.03) | Beta | HQO,13 Sapin et al,162 Zhao et al,144 Fitzgibbon et al143 |
| Acute Treatment | |||
| ECT | 0.55 (0.05) | HQO,13 Sapin et al,162 Zhao et al,144 Fitzgibbon et al143 | |
| rTMS | 0.63 (0.05) | Beta | HQO,13 Sapin et al,162 Zhao et al,144 Fitzgibbon et al143 |
| iTBS | 0.63 (0.05) | Beta | HQO,13 Sapin et al,162 Zhao et al,144 Fitzgibbon et al143 |
Abbreviations: ECT, electroconvulsive therapy; HQO, Health Quality Ontario; iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation; SE, standard error.
Cost Parameters
Costs related to various treatments and relevant calculations are presented in Tables 14 to 16.
Table 14:
Material Cost Components Included in Estimation of Per-Session Cost of iTBSa
| Resource Itemsb | USD ($) | CAD ($) | Reference/Remarks |
|---|---|---|---|
| Core equipment (A) | $73,000 | $97,228 | Manufacturer |
| Yearly maintenance (B) | $2,500 | $3,330 | Expert opinion |
| Coil I | $19,000 | $25,306 | Manufacturer |
| Material cost per yearc E= (A + C)/D + B | $20,900 | $27,836 | Calculated |
| Numbers of sessions per year (F) | $5,280 | $5,280 | Expert opinion |
| Cost of machine per sessiond (G = E/F) | $3.96 | $5.27 | Calculated |
Abbreviations; ECT, electroconvulsive therapy; iTBS, intermittent theta burst stimulation; OHIP, Ontario Health Insurance Plan; OSB, Ontario Schedule of Benefits; rTMS, repetitive transcranial magnetic stimulation.
- Cost of core equipment and coil was converted from US dollars into Canadian dollars using the exchange rate of $1 USD = $1.33 CAD (Bank of Canada rate on October 1, 2019). To get the yearly amortization, the cost of acquiring core equipment and coil was divided by number of years of amortization for equipment and coil.
Amortization over years (D), according to expert opinion.
Yearly cost incurred for iTBS was calculated as the sum of yearly amortization of core equipment, coil, and yearly maintenance.
Material cost per session for iTBS was calculated as the sum of yearly amortization of core equipment, coil, and yearly maintenance divided by total sessions of iTBS yearly.
Source: Mendlowitz et al.165
Table 16:
Overall Costs Used in the Economic Model
| Parameters | Mean (SE), $ | Distributiona | Reference |
|---|---|---|---|
| rTMS | |||
| Acute phase | 4,600 (1,150) | Gamma | HQO13 |
| Maintenance phase | 1,314 (328.5) | Gamma | HQO13 |
| ECT | |||
| Acute phase | 13,618 (3,404) | Gamma | Fitzgibbon et al143 |
| Maintenance phase | 2,838 (710) | Gamma | Fitzgibbon et al143 |
| iTBS | |||
| Acute phase | 4,268 (1,067) | Gamma | Mendlowitz et al165 and calculation |
| Maintenance phase | 1,219 (305) | Gamma | Mendlowitz et al165 and calculation |
| All Treatments | |||
| Severe depressionb | 890 (227) | Gamma | ODF,167 HQO13 |
| Remission | 226 (56.5) | Gamma | ODF167 |
| Antidepressant drugs | 226 (56.5) | Gamma | Fitzgibbon et al143 |
Abbreviations: ECT, electroconvulsive therapy; HQO, Health Quality Ontario; iTBS, intermittent theta burst stimulation; ODF, Ontario Drug Formulary; rTMS, repetitive transcranial magnetic stimulation; SE, standard error.
Severe depression costs include cost of physician visits and costs of antidepressant drugs.
Distribution was assigned and calculated by Briggs et al.161
Costs of ECT were taken from Fitzgibbon et al.143 The authors derived these costs from various sources, such as the Canadian Institute for Health Information (CIHI)163 and the Ontario Case Costing Initiative (OCCI)164 (see Table 16).
Costs for rTMS were obtained from Health Quality Ontario's 2016 report.13 The per-session rTMS cost was estimated at $109.52; this cost included the cost of nursing time, equipment, and psychiatric expertise (for detailed calculations, see the 2016 report). We calculated acute and maintenance treatment costs by multiplying the per-session rTMS cost with the number of sessions classified by the type of treatment (i.e., 30 sessions for acute treatment and 12 sessions for maintenance treatment; see Table 16).
Costs for acute and maintenance treatment with iTBS were calculated as the cost per iTBS session multiplied by the number of sessions (i.e., 30 sessions for acute treatment and 12 sessions for maintenance treatment). The per-session cost of iTBS included the following components:
Material cost, which includes the core equipment, coil, and maintenance service (see Table 14)
Professional cost (i.e., nursing time and psychiatric expertise; see Table 15)
Table 15:
Professional Cost Incurred for Each iTBS Session
| Resource Items | Costs, $ | Data Sources and Comments |
|---|---|---|
| Psychiatric expert (A) | 85.92 | Based on weighted average of OHIP fee codes (54% outpatient/46% inpatient from IntelliHealth data): OSB G479 $92.60 for outpatient, OSB G478 $80.30 for inpatient. We assumed the OHIP fee for iTBS would be equivalent to that of ECT |
| Nurse/technician time (B) | 10.43 | Based on hourly rate of $41.70 for nurse in Ontario: 15 minutes technical time estimated in expert consultation |
| Cost of machine per session (C) | 5.27 | Calculated in Table 14 |
| Per-session cost (A + B + C) | 101.62 | Calculation |
Abbreviations; ECT, electroconvulsive therapy; iTBS, intermittent theta burst stimulation; OHIP, Ontario Health Insurance Plan; OSB, Ontario Schedule of Benefits.
The material cost was taken from Mendlowitz et al.165 The authors conducted a study using patient-level data from a large randomized controlled non-inferiority trial (THREE-D),25 using a US health care payer's perspective. The cost of core equipment, coil, and maintenance service was subsequently converted from US dollars into Canadian dollars using the exchange rate of $1 USD = $1.3318 CAD.166
To calculate the professional cost per session of iTBS, we used the same approach described in the 2016 report by Health Quality Ontario.13 We assumed that utilization of resources for iTBS (i.e., psychiatrist's time, nurses’ time) would be the same as that assumed for rTMS (email communication with D. Blumberger, MD, on October 22, 2019; see Table 15).
The cost of treatment for severe depression was taken from various sources.13,143 For example, the cost of physician visits was taken from the 2016 health technology assessment.13 The cost of antidepressants was taken from Fitzgibbon et al.143 The costs of antidepressant medication were estimated as the average of all antidepressants listed in the Ontario Drug Formulary167 and were factored into the health states assuming that all patients with TRD, regardless of their health state, would continue with lifelong pharmacotherapy.
Table 16 presents overall costs itemized by category and by treatment: high-frequency rTMS, ECT, iTBS, and pharmacotherapy alone.
Internal Validation
Formal internal validation was conducted by the secondary health economist. This included testing the mathematical logic of the model and checking for errors and accuracy of parameter inputs and equations.
Analysis
We conducted the reference case PSA by running 1 million simulations (1,000 × 1,000 persons), which simultaneously captured the uncertainty in model parameters and sampling error due to variability between patients. We calculated mean costs and mean QALYs for each intervention and then the mean incremental costs, mean incremental QALYs, and incremental cost-effectiveness ratios (ICERs) as cost per QALY gained for:
High-frequency rTMS (followed by ECT in a stepped care pathway) versus ECT alone and pharmacotherapy alone
ITBS (followed by ECT in a stepped care pathway) versus ECT alone and pharmacotherapy alone
The results of the PSA were presented on a cost-effectiveness acceptability curve. We presented uncertainty quantitatively as the probability that an intervention is cost-effective at specific willingness-to-pay (WTP) values. We also present uncertainty qualitatively against WTP of $50,000 CAD per QALY, in one of five categories defined by the Ontario Decision Framework168: highly likely to be cost-effective (80%–100% probability of being cost-effective), moderately likely to be cost-effective (60%–79% probability), uncertain if cost-effective (40%–59% probability), moderately likely not to be cost-effective (20%–39% probability), or highly likely not to be cost-effective (0–19% probability).
SCENARIO ANALYSES
We also conducted scenario analyses to assess model structure assumptions. The scenario analyses are presented in Table 17. We explored the following:
Table 17:
Variables in Scenario Analyses
| Scenario | Reference Case | Scenario Analyses |
|---|---|---|
| Stepped care pathway is not an option | 35% of people with TRD would switch to ECT if they did not initially respond to rTMS or iTBS | People with TRD would not receive ECT as second treatment if they did not initially respond to rTMS or iTBS and would transition to severe depression health state with sham therapy |
| Switching to ECT (stepped care pathway is an option) | 35% of people with TRD would switch to ECT if depression did not initially respond to rTMS or iTBS | All people with TRD would receive ECT as second treatment if they did not initially respond to rTMS or iTBS |
| Time horizon | 3 y | 1 y or lifetime |
| Discount rate | 1.5% | 3% |
| Increase in mortality due to severe depression | 29% | ±25% |
Abbreviations: ECT, electroconvulsive therapy; iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation; TRD, treatment-resistant depression.
A stepped care pathway is not an option
All people with TRD who did not initially respond to rTMS would switch to ECT
Duration of the model time horizon
Discount rate
Change in the rate of mortality due to severe depression
Results
Our economic evaluation estimated the cost–utility of high-frequency rTMS and iTBS, compared with ECT alone and pharmacotherapy alone in treatment of adults with TRD.
Reference Case Analysis
In the reference case analysis, in which 35% of people with TRD would follow a stepped care pathway and switch to ECT if they did not initially respond to high-frequency rTMS, we found that high-frequency rTMS would be dominant (i.e., less costly and more effective) over ECT alone (Table 18).
Table 18:
Cost–Utility Analyses Comparing High-Frequency rTMS with ECT and Pharmacotherapy Alone in Adults With Treatment-Resistant Depression—Reference Case Analysis
| Strategy | Average Total Cost ($), Mean (95% CI) | Incremental Cost ($),a Mean (95% CI) | Average Total Effect in QALYs, Mean (95% CI) | Incremental Effect in QALYs,b Mean (95% CI) | ICER, $/QALY |
|---|---|---|---|---|---|
| High-Frequency rTMS vs. ECT | |||||
| High-frequency rTMS (stepped care pathway) | 13,858 (12,372–14,548) | − 11,938 (−12,765 to −10,990)c | 1.7337 (1.5002–1.7977) | 0.1028 (0.0643–0.1369) | Dominantd |
| ECT alone | 25,796 (23,508–26,666) | — | 1.6309 (1.4078–1.6883) | — | — |
| High-Frequency rTMS vs. Pharmacotherapy Alone | |||||
| High-frequency rTMS (stepped care pathway) | 13,858 (12,372–14,548) | 12,343 (11,097–13,003) | 1.7337 (1.5002–1.7977) | 0.5397 (0.4685–0.5848) | 22,868 |
| Pharmacotherapy alone | 1,515 (1,272–1,570) | — | 1.1939 (1.0267–1.2403) | — | — |
Abbreviations: CI, confidence interval; ECT, electroconvulsive therapy; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; rTMS, repetitive transcranial magnetic stimulation; TRD, treatment-resistant depression.
Incremental cost = Average cost (strategy B) − Average cost (strategy A).
Incremental effect = Average effect (strategy B) − Average effect (strategy A).
Negative costs indicate cost savings.
Dominant is less costly and more effective.
When high-frequency rTMS was compared with pharmacotherapy alone, an additional $12,343 would be spent to gain 0.5397 QALYs. This translates to an ICER of $22,868 per QALY gained. At a WTP of $50,000 per QALY, high-frequency rTMS (followed by ECT in a stepped care pathway) would be cost-effective compared with pharmacotherapy alone (see Table 18).
In the reference case analysis, in which 35% people with TRD would follow a stepped care pathway and switch to ECT if they did not respond to iTBS, we found that iTBS would be dominant over ECT alone (i.e. less costly and more effective than ECT alone; Table 19).
Table 19:
Cost–Utility Analyses Comparing iTBS With ECT and Pharmacotherapy Alone in Adults With TRD—Reference Case Analysis
| Strategy | Average Total Cost ($), Mean (95% CI) | Incremental Cost ($),a Mean (95% CI) | Average Total Effect in QALYs, Mean (95% CI) | Incremental Effect in QALYs,b Mean (95% CI) | ICER, $/QALY |
|---|---|---|---|---|---|
| iTBS vs. ECT | |||||
| iTBS (stepped care pathway) | 13,217 (8,975–18,408) | −12,579 (−16,875 to −7,504)c | 1.7444 (1.5094–1.8112) | 0.1135 (0.0745–0.1468) | Dominantd |
| ECT alone | 25,796 (23,508–26,666) | — | 1.6309 (1.4078–1.6883) | — | — |
| iTBS vs. Pharmacotherapy Alone | |||||
| iTBS (stepped care pathway) | 13,217 (8,975–18,408) | 11,703 (7,519–16,878) | 1.7444 (1.5094–1.8112) | 0.5505 (0.4792–0.5987) | 21,259 |
| Pharmacotherapy alone | 1,515 (1,272–1,570) | — | 1.1939 (1.0267–1.2403) | — | — |
Abbreviations: CI, confidence interval; ECT, electroconvulsive therapy; ICER, incremental cost-effectiveness ratio; iTBS, intermittent theta burst stimulation; QALY, quality-adjusted life-year; TRD, treatment-resistant depression.
Incremental cost = Average cost (strategy B) − Average cost (strategy A).
Incremental effect = Average effect (strategy B) − Average effect (strategy A).
Negative costs indicate cost savings.
Dominant is less costly and more effective.
When iTBS is compared with pharmacotherapy alone, an additional $11,703 would be spent to gain 0.5505 QALYs. This translates to an ICER of $21,259 per QALY gained. At a WTP of $50,000 per QALY, iTBS (followed by ECT in a stepped care pathway) would be cost-effective compared with pharmacotherapy alone (see Table 19).
Cost-Effectiveness Acceptability Curve
Figures 18 and 19 represent the uncertainty around the estimated ICER generated in the PSAs for high-frequency rTMS or iTBS (followed by ECT alone in a stepped care pathway) versus pharmacotherapy alone. In both analyses, rTMS and iTBS were highly likely to be cost-effective compared with pharmacotherapy alone at a WTP of $50,000 per QALY gained and above.
Figure 18: Cost-Effectiveness Acceptability Curve as a Function of Willingness to Pay Comparing High-Frequency rTMS With Pharmacotherapy Alone.
Abbreviations: QALY, quality-adjusted life-year; rTMS, repetitive transcranial magnetic stimulation.
Figure 19: Cost-Effectiveness Acceptability Curve as a Function of Willingness to Pay Comparing iTBS With Pharmacotherapy Alone.
Abbreviations: iTBS, intermittent theta burst stimulation; QALY, quality-adjusted life-year.
The scatter plots represent the uncertainty around the estimated ICER generated in the PSAs, when comparing (1) high-frequency rTMS and iTBS (followed by ECT alone in a stepped care approach) versus ECT alone, are presented in Appendix 8, Figures A34 and A35, and (2) high-frequency rTMS and iTBS (followed by ECT alone in a stepped care approach) versus pharmacotherapy alone are presented in Appendix 8, Figures A36 and A37.
Scenario Analyses
Table 20 presents results of various scenario analyses comparing (1) high-frequency rTMS (stepped care pathway) and (2) iTBS (stepped care pathway) with ECT alone or pharmacotherapy alone.
Table 20:
Scenario Analysis Results
| Scenario | High-Frequency rTMS vs. ECT | High-Frequency rTMS vs. Pharmacotherapy Alone | iTBS vs. ECT | iTBS vs. Pharmacotherapy Alone |
|---|---|---|---|---|
| Reference case (stepped care, 35% switching to ECT) | ICER ($/QALY): dominanta | ICER ($/QALY): 22,868 | ICER: dominanta | ICER ($/QALY): 21,259 |
| Δ C = −$11,938 | Δ C = $12,343 | Δ C = −$12,579 | Δ C = $11,703 | |
| Δ E (mean) = 0.1028 QALYs | Δ E (mean) = 0.5397 QALYs | Δ E (mean) = 0.1135 QALYs | Δ E (mean) = 0.5505 QALYs | |
| No switching to ECT (no stepped care) | ICER ($/QALY): dominanta | ICER ($/QALY): 23,157 | ICER ($/QALY): dominanta | ICER ($/QALY): 21,181 |
| Δ C = −$14,134 | Δ C = −$10,147 | Δ C = −$14,736 | Δ C = $9,545 | |
| Δ E (mean) = 0.0012 QALYs | Δ E (mean) = 0.4382 QALYs | Δ E (mean) = 0.0137 QALYs | Δ E (mean) = 0.4507 QALYs | |
| 100% switching to ECT (stepped care) | ICER ($/QALY): dominanta | ICER ($/QALY): 22,517 | ICER($/QALY): dominanta | ICER ($/QALY): 21,322 |
| Δ C = −$7,876 | Δ C = $16,405 | Δ C = −$8,587 | Δ C = $15,694 | |
| Δ E (mean) = 0.2991 QALYs | Δ E (mean) = 0.7285 QALYs | Δ E (mean) = 0.2991 QALYs | Δ E (mean) = 0.7360 QALYs | |
| 1-y time horizon | ICER ($/QALY): dominanta | ICER($/QALY): 37,630 | ICER($/QALY): dominanta | ICER($/QALY): 34,853 |
| Δ C = −$11,422 | Δ C = $7,669 | Δ C = −$11,857 | Δ C = $7,235 | |
| Δ E (mean) = 0.0068 QALYs | Δ E (mean) = 0.2038 QALYs | Δ E (mean) = 0.0106 QALYs | Δ E (mean) = 0.2076 QALYs | |
| Lifetime time horizon | ICER($/QALY): dominanta | ICER($/QALY): 15,880 | ICER($/QALY): dominanta | ICER($/QALY): 14,833 |
| Δ C = −$13,109 | Δ C = $32,155 | Δ C = −$14,711 | Δ C = $30,552 | |
| Δ E (mean) = 0.6101 QALYs | Δ E (mean) = 2.0249 QALYs | Δ E (mean) = 0.6449 QALYs | Δ E (mean) = 2.0597 QALYs | |
| Discount rate: 3% | ICER($/QALY): dominanta | ICER($/QALY): 23,045 | ICER($/QALY): dominanta | ICER($/QALY): 21,422 |
| Δ C = −$11,914 | Δ C = $12,191 | Δ C = −$12,548 | Δ C = $11,557 | |
| Δ E (mean) = 0.0997 QALYs | Δ E (mean) = 0.5290 QALYs | Δ E (mean) = 0.1102 QALYs | Δ E (mean) = 0.5395 QALYs | |
| Mortality rate due to severe depression decreased by 25% | ICER ($QALY): dominanta | ICER ($/QALY): 23,123 | ICER ($/QALY): dominanta | ICER ($/QALY): 21,490 |
| Δ C = −$11,938 | Δ C = $12,334 | Δ C = −$12,579 | Δ C = $11,693 | |
| Δ E (mean) = 0.1028 QALYs | Δ E (mean) = 0.5334 QALYs | Δ E (mean) = 0.01135 QALYs | Δ E (mean) = 0.5441 QALYs | |
| Mortality rate due to severe depression increased by 25% | ICER ($QALY): dominanta | ICER ($/QALY): 22,630 | ICER ($/QALY): dominanta | ICER ($/QALY): 21,043 |
| Δ C = −$11,938 | Δ C = $12,352 | Δ C = −$12,579 | Δ C = $11,711 | |
| Δ E (mean) = 0.1028 QALYs | Δ E (mean) = 0.5458 QALYs | Δ E (mean) = 0.01135 QALYs | Δ E (mean) = 0.5565 QALYs |
Abbreviations: ECT, electroconvulsive therapy; ICER, incremental cost-effectiveness ratio; iTBS, intermittent theta burst stimulation; QALY, quality-adjusted life-year; rTMS, repetitive transcranial magnetic stimulation; TRD, treatment-resistant depression.
Dominant is less costly and more effective.
High-frequency rTMS and iTBS were dominant over ECT in all scenarios. High-frequency rTMS and iTBS were cost effective compared with pharmacotherapy alone in all scenarios.
Discussion
Our analysis investigated the cost-effectiveness of: (1) high-frequency rTMS (in a stepped care pathway with ECT) compared with ECT alone and with pharmacotherapy alone; and (2) iTBS (in a stepped care pathway with ECT) compared with ECT alone and with pharmacotherapy alone, from the perspective of Ontario's Ministry of Health. The resulting information helped us better understand the cost-effectiveness of two rTMS modalities and their budget impact for Ontario.
Our reference-case model consisted of a stepped-care approach when only 35% patients with TRD could switch to ECT if they did not initially respond to either high-frequency rTMS or iTBS as first-line treatments. Results showed that both high-frequency rTMS and iTBS would be dominant (less costly and more effective) over ECT alone. In other words, high-frequency rTMS and iTBS would potentially save more than ECT. These cost savings might come from avoiding inpatient charges, because most patients receiving high-frequency rTMS or iTBS are treated at rTMS outpatient clinics. It was important that analyses were done from the perspective of the Ontario Ministry of Health, in which only direct medical costs were considered. Depression is the leading cause of disability. Had the analyses been done from a societal perspective, high-frequency rTMS or iTBS would have been even less costly than ECT, because rTMS does not have cognitive adverse effects and does not require anesthesia, as ECT does.169
When we compared high-frequency rTMS or iTBS with pharmacotherapy alone, reference case results showed that both high-frequency rTMS and iTBS would be cost-effective at a WTP of $50,000 per QALY. In all scenario analyses, high-frequency rTMS or iTBS dominated (were less costly and more effective than) ECT alone in treatment of people with TRD.
In all analyses (reference case and scenarios), results demonstrated that high-frequency rTMS or iTBS would be more cost-effective than pharmacotherapy alone at a WTP of $50,000 per QALY. The best outcomes were produced when all TRD patients would start with high-frequency rTMS or iTBS as the first-line treatment and then would transition to ECT if there was no response. This suggests that a stepped-care approach is preferred for treatment of TRD. The important role of stepped care in managing TRD suggests that, regardless of expansion of rTMS clinics in the future, ECT centres should still exist. The most cost-effective treatment seems to be rTMS used as the first step followed by ECT alone as the second step. Moreover, rTMS may not always be effective, and ECT remains another option.
Our model also had some limitations, which included the assumptions that patients with TRD who responded initially to rTMS treatment could not switch to ECT. Also, our model had a 3-year follow-up period owing to a lack of clinical data, even though TRD is a chronic illness requiring a longer time horizon to capture the clinical effects of rTMS. However, we conducted a cost-effective analysis over a lifetime horizon and results remained robust. As well, disutility associated with either treatment was not included because data were unavailable. Had disutility been considered in the model, high-frequency rTMS and iTBS would have been even more cost-effective than ECT, because rTMS therapies could have fewer adverse events than ECT.
Nevertheless, our analysis had several strengths. Our model was constructed to include iTBS as a treatment for people with TRD in Ontario. To our knowledge, no cost-effectiveness study has investigated the cost-effectiveness of iTBS in comparison with high-frequency rTMS, ECT, or pharmacotherapy.
Conclusions
Our reference case results showed that high-frequency rTMS and iTBS in a stepped care approach involving ECT when necessary were cost-saving compared with ECT alone and were cost-effective compared with pharmacotherapy alone at a WTP of $50,000 per QALY. Among four treatments (i.e., high-frequency rTMS, iTBS, ECT, and pharmacotherapy alone), iTBS appeared to be most cost-effective. Indeed, cost-effectiveness results showed that the stepped-care approach (high-frequency rTMS or iTBS followed by ECT if necessary) was highly likely to be cost-effective for the treatment of people with TRD.
Budget Impact Analysis
Research Question
What is the potential 5-year budget impact for Ontario's Ministry of Health of publicly funding repetitive transcranial magnetic stimulation (rTMS; high-frequency rTMS, and intermittent theta burst stimulation [iTBS]) in adults with treatment-resistant depression (TRD)?
Methods
Analytic Framework
We estimated the 5-year budget impact of publicly funding rTMS (high-frequency rTMS and iTBS) in adults with TRD who would not receive electroconvulsive therapy (ECT) using the cost difference between two scenarios: (1) current clinical practice without public funding for rTMS (the current scenario, where clinics in Ontario offer rTMS treatment through their hospital budget, hospital donation funds, or research grants) and (2) anticipated clinical practice with public funding for rTMS (the new scenario, where clinics have more funding to expand their treatment capacity (high-frequency rTMS and iTBS). Figure 20 presents the schematic for the budget impact model.
Figure 20: Schematic Model of Budget Impact.
Abbreviations: iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation; TRD, treatment-resistant depression.
aWe estimated the number of people with TRD living in Ontario who could be treated by the current capacity of rTMS clinics in Ontario. We did not estimate the overall volume of people with TRD in Ontario because the potential demand for rTMS could exceed what can be realistically provided through the existing rTMS infrastructure.
bIn the current scenario (since rTMS was not publicly funded and patients with TRD did not choose electroconvulsive therapy as treatment), we assumed that the cost in the current scenario was zero.
cIn the new scenario, we estimated the cost incurred by operating rTMS machines at current levels of capacity and future uptake in the next 5 years.
dBudget impact would be incremental cost incurred to operate rTMS machines at current levels of capacity and future uptake in the next 5 years, assuming that rTMS (both high-frequency rTMS and iTBS) is to be publicly funded.
It is important to note that current publicly funded rTMS clinics are operated under the informal public funding structure (e.g., through hospital global budget, hospital donation funds, and research grants). In this analysis, we are aiming to calculate the budget required to formally institute public funding for rTMS (funding does not come from informal channels).
We conducted a reference case analysis and scenario analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. Our scenario analyses explored how results are affected by varying input parameters and model assumptions.
Key Assumptions
There is currently no public funding for rTMS machines in Ontario
A treatment is classified either as a long treatment (i.e., high-frequency rTMS) or a brief treatment (i.e., iTBS). According to expert opinion (email communication with D. Blumberger, MD, on October 20, 2019), a brief treatment is 3 minutes and a long treatment is 37.5 minutes
All future rTMS clinics (or facilities looking to start an rTMS program) would procure machines that would have the capacity to run both long treatments (high-frequency rTMS) and brief treatments (iTBS). Necessary training of physicians and technicians would be provided
All new machines to be purchased by existing rTMS clinics in the next 5 years can deliver both long treatments (i.e., high-frequency rTMS) and brief treatments (i.e., iTBS)
If a machine can provide both long treatments and brief treatments, for simplicity, we assumed that the machine would be used only for brief treatments. Long treatments would be provided only as needed
-
All rTMS machines in all existing and future (looking-to-start) clinics were assumed to run at the following capacity:
– For long treatments (i.e., high-frequency rTMS), a machine would deliver 8 sessions daily (email communication with Dr. Blumberger on October 20, 2019)
– For brief treatments (i.e., iTBS), a machine would deliver 22 sessions daily (email communication with Dr. Blumberger, on October 20, 2019)
-
In the reference case analysis, we included only the existing rTMS clinics in Ontario. Therefore, we estimated the budget impact of operating existing rTMS clinics at current levels of capacity and future uptake in the next 5 years:
– In the current scenario, rTMS was not publicly funded and patients with TRD did not choose ECT as a treatment; thus, we assumed that cost in the current scenario was zero
– In the new scenario, we estimated the cost incurred by operating rTMS machines at the current levels of capacity and future uptake in the next 5 years
In the scenario analysis, we included both the existing and future (looking-to-start) rTMS clinics in Ontario. Therefore, we estimated the budget impact of operating both existing and future (looking-to-start) rTMS clinics in the next 5 years
We assumed that all adults with TRD who did not receive ECT would be eligible for and willing to undergo rTMS as an alternative treatment for less severe forms of TRD or as a treatment for people who refused ECT
The numbers of ECT suites remain the same, regardless of possible funding and expansion of rTMS clinics
A clinic that had only one rTMS machine would provide brief treatments solely (i.e., iTBS only; this assumption is based on email communication with D. Blumberger, MD, on October 30, 2019)
Target Population
The target population was adults with TRD who are eligible for rTMS. The potential demand for rTMS could exceed what can be realistically provided through the existing rTMS infrastructure. Therefore, for our budget impact analysis, we incorporated this constraint and considered only the capacity of rTMS clinics to provide this treatment. Consequently, we did not estimate the budget based on the overall number of people with TRD living in Ontario.
Current Intervention Mix
As mentioned above (see Key Assumptions), we assumed no use of rTMS in the current scenario.
Uptake of the New Intervention and New Intervention Mix
CURRENT rTMS CLINICS AND THEIR EXPECTED CAPACITY EXPANSION
Currently, eight public clinics, which are all part of acute care or mental health hospitals in Ontario, are providing rTMS treatment to adults with TRD (Table A15). Through expert consultation, we obtained the following:
Numbers of rTMS machines, categorized by long and brief treatments, at existing rTMS clinics in Ontario in the current year
Expected numbers of rTMS machines, categorized by long and brief treatments, at existing rTMS clinics in the future
These details are presented in Table 21. Additional details of estimated numbers of rTMS machines in each rTMS clinic in Ontario in the next 5 years are provided in Appendix 8, Table A15.
Table 21:
Estimated Numbers of rTMS Machines Over 5 Years, by Type of Treatment in Current rTMS Clinics in Ontario—Reference Case Analysis
| Treatment in Existing rTMS Clinics | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Long treatments (high-frequency rTMS)a | 3 | 3 | 3 | 3 | 3 |
| Brief treatments (iTBS)b | 16 | 19 | 24 | 24 | 28 |
Abbreviation: iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation.
Long treatment: defined as 37.5-minute session; an rTMS machine could provide 8 long-treatment sessions daily (communication with D. Blumberger, MD, on October 30, 2019).
Brief treatment: defined as 3-minute session; an rTMS machine could provide 22 brief treatment sessions daily (communication with D. Blumberger, MD, on October 30, 2019).
ESTIMATING rTMS TREATMENT CAPACITY
The capacity of an rTMS machine is defined by the number of sessions it can complete or the number of adults with TRD a machine can treat daily:
If long treatments (i.e., high-frequency rTMS) were being offered, we assumed that a machine could run up to 8 sessions daily (email communication with D. Blumberger, MD, on October 30, 2019)
If brief treatments (i.e., iTBS) were being offered, a conservative estimate would be that a machine would run 22 sessions daily (email communication with D. Blumberger, MD, on October 20, 2019)
We assumed that each machine would run for 5 days weekly over 48 weeks each year. Our calculations for the total number of sessions a machine could operate each year are outlined below:
-
Number of sessions yearly, per rTMS machine = number of sessions daily × numbers of working days/week × numbers of working weeks/year
– Long treatment (i.e., high-frequency rTMS): Number of sessions yearly, per rTMS machine = 8 sessions daily × 5 days weekly × 48 weeks yearly = 1,920 sessions yearly
– Brief treatment (i.e., iTBS): Number of sessions yearly, per iTBS machine = 22 sessions daily × 5 days weekly × 48 weeks yearly = 5,280 sessions yearly
Appendix 10, Tables A15 and A16, estimate numbers of sessions classified by long treatments (i.e., high-frequency rTMS) and brief treatments (i.e., iTBS) operated by existing and future (planning to start) rTMS clinics in Ontario within the next 5 years.
Resources and Costs
We obtained the cost per session of high-frequency rTMS from the 2016 health technology assessment.13 The cost per session of iTBS was calculated in the primary economic section. Per-session costs for high-frequency rTMS and iTBS are summarized in Table 22.
Table 22:
Summary of Per-Session Costs by High-Frequency rTMS and iTBS
| Treatment Duration | Per-Session Cost ($) |
|---|---|
| Long treatment (high-frequency rTMS) | 109.52 |
| Brief treatment (iTBS) | 101.62 |
Abbreviations: iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation.
In addition to the cost of running treatment sessions, there is a yearly cost for training physicians and technicians, who need to be trained by the Centre for Addiction and Mental Health to operate machines in rTMS clinics in Ontario.
COST OF TRAINING PHYSICIANS
There is no need to account for costs of training physicians because existing physician training courses count toward their continuing medical education requirements that physicians must meet and the cost is not attributable to the Ministry of Health.
COST OF TRAINING TECHNICIANS
According to expert opinion (email communication with D. Blumberger, MD, on November 5, 2019), technicians’ training is based on demand, which means when technicians must be trained to run iTBS machines, a training course would be organized.
We assumed that one course to train 10 technicians would be delivered yearly. The cost of this training is estimated at about $5,000 per technician. We also accounted for the salary paid to technicians while attending the course. The average hourly wage of a technician was assumed to be $28, with additional costs associated with benefits (28% of the hourly wage; email communication with D. Blumberger, MD, on December 11, 2019). Thus, the overall salary equals $35.84 hourly. Assuming a normal working day consists of 7.5 hours, the total wage paid to a technician daily would equal $268.80. Consequently, the salary paid for a 15-day training course (didactic and hands-on for 5 days, supervised delivery for 10 days) to train 10 technicians would be about $40,320 (Table 23). Overall, the total yearly training course to train technicians would cost about $90,320. This amount was added to the annual budget of running rTMS machines in Ontario.
Table 23:
Costs for Training: Technicians
| Resources | Unit | Data Sources and Comments |
|---|---|---|
| Hourly wage of techniciana | $35.84 | Based on hourly rate of $28 plus 28% benefits for technician in Ontariob |
| Working hours daily | 7.5 h | Estimated |
| Days per training course | 15 d | Didactic and hands-on training for 5 days, supervised delivery for 10 daysb |
| Technicians trained per course | 10 persons | Based on demand and assuming 10 technicians per course |
| Training fee per technician | $5,000 | Expert opinionb |
| Total cost per training coursec | $90,320 | Calculated |
Hourly wage = $28 × (1 + 28%) = $35.84.
Information supplied in email communication with D. Blumberger, MD, on December 11, 2019.
Total cost per training course = hourly wage of $35.84 × 7.5 hours daily × 15 days × 10 persons + $50,000 for training course = $90,320.
COST OF TREATING ADULTS WITH TRD
On average, an adult with TRD would receive 42 treatment sessions (acute and maintenance phases) of either high-frequency rTMS or iTBS (email communication with D. Blumberger, MD, on March 10, 2020). The average cost incurred by a person who receives either high-frequency rTMS or iTBS was calculated as follows:
Average cost per adult with TRD treated by rTMS (acute and maintenance phases) = Total treatment sessions (acute and maintenance phases; see Table 23) per adult with TRD × Cost per treatment session
Therefore, it costs about $4,600 per adult with TRD to be treated by high-frequency rTMS and about $4,268 per adult with TRD to be treated by iTBS.
Internal Validation
The secondary health economist conducted formal internal validation. This process included checking for errors and ensuring accuracy of parameter inputs and equations in the budget impact analysis.
Analysis
REFERENCE CASE ANALYSIS
In the reference case analysis, we calculated the required budget to publicly fund rTMS in the existing rTMS clinics for adults with TRD in Ontario. The budget impact would be the incremental cost incurred to operate rTMS machines at current levels of capacity and the future uptake in the next 5 years assuming that rTMS (both high-frequency rTMS and iTBS) is to be publicly funded.
Given these assumptions, we calculated the total budget required to fund rTMS in Ontario by estimating the total cost incurred by all rTMS machines in the province operating during a year:
Total cost of rTMS sessions yearly = Number of rTMS machines in all sites × Number of rTMS sessions/year per rTMS machine∗ × Cost per rTMS session
∗For brief treatment, number of sessions per rTMS machine yearly = 5,280 sessions. For long treatment, number of sessions per rTMS machine yearly = 1,920 sessions
We also calculated the number of adults with TRD to be treated in the next 5 years by rTMS (high-frequency rTMS and iTBS), based on the total budgets and per-person cost of treatment estimated for high-frequency rTMS ($4,600) and iTBS ($4,268):
Number of adults with TRD treated by rTMS (high-frequency rTMS and iTBS) during acute and maintenance phases = Total budget required to fund rTMS (high-frequency rTMS and iTBS) ÷ Per-person cost of treatment (acute and maintenance phases)
SCENARIO ANALYSES
We examined the possibility of increasing access to rTMS treatment over time. Thus, we explored two scenarios.
Scenario 1: Existing and Future (Looking-to-Start) rTMS Clinics
Another five rTMS clinics are estimated to be set up in the next 5 years in Ontario (see Table 21). For this calculation, we assumed that in future (looking-to-start) rTMS clinics, all machines would provide only brief treatments (i.e., iTBS). Long treatments (i.e., high-frequency rTMS) would be provided only if needed. Through expert consultation, we obtained information on the number of rTMS machines planned for long and brief treatments in future rTMS clinics in Ontario (Table 24). Detailed estimates of numbers of rTMS machines in each rTMS clinic in Ontario anticipated to open in the next 5 years are provided in Appendix 8, Table A15.
Table 24:
Estimated Number of rTMS Machines Over 5 Years in Existing and Future rTMS Clinics in Ontario—Scenario Analysis by Type of Treatment
| Type of Treatmenta | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Long (high-frequency rTMS) | 3 | 3 | 3 | 3 | 3 |
| Brief (iTBS) | 16 | 24 | 29 | 30 | 34 |
Abbreviations: iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation.
Long treatment is defined as 37.5-minute sessions and brief treatment is defined as 3-minute sessions. Each rTMS machine could deliver 8 long sessions or 22 brief sessions daily.
Source: personal communication with D. Blumberger, MD, on October 30, 2019.
Scenario 2: Exclusion of Capital Cost (i.e., Purchase and Maintenance Cost of High-Frequency rTMS or iTBS Machines)
We acknowledge that the capital cost (i.e., purchase and maintenance cost of high-frequency rTMS or iTBS) might be a big component of the budget impact. Therefore, to explore the impact of this factor, in Scenario 2, we excluded the capital cost (i.e., the cost of high-frequency rTMS or iTBS) from our calculation of the required budget to operate machines in (1) existing rTMS clinics, and (2) existing and future (looking-to-start) rTMS clinics in Ontario. Detailed estimates of cost per session by high-frequency rTMS or iTBS without the cost of the machine are provided in Appendix 11, Table A18, and the total budgets required to operate existing and future (looking-to-start) rTMS clinics in Ontario are presented in Appendix 11, Table A19.
Scenario 3: Extreme Diffusion of rTMS
In Scenario 3, extreme rTMS diffusion (suggested through expert consultation), rTMS was assumed to be available at all centres that have ECT facilities, with three treatment suites per site so as to provide the highest possible access given our existing infrastructure. Thus, we estimated 50 ECT suites and 150 rTMS suites for this analysis.
Results
Reference Case
Table 25 presents the costs incurred to operate rTMS machines at current levels of capacity and the future uptake in the next 5 years, assuming that rTMS (both high-frequency rTMS and iTBS) is to be publicly funded (new scenario). This cost included the costs to operate rTMS machines in all existing rTMS clinics in Ontario and the cost of training technicians to operate high-frequency rTMS and iTBS machines.
Table 25:
Budget Impact Analysis of Adopting rTMS in Current and Future rTMS Clinics in Ontario
| Scenarios | Year 1 ($) | Year 2 ($) | Year 3 ($) | Year 4 ($) | Year 5 ($) | Total ($) |
|---|---|---|---|---|---|---|
| Current scenario | 0 | 0 | 0 | 0 | 0 | 0 |
| Costs specific to new scenarios | ||||||
| Long treatments (high-frequency rTMS)a | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 | 3.15 |
| Brief treatments (iTBS) in reference caseb | 8.58 | 10.19 | 12.88 | 12.88 | 15.02 | 59.56 |
| Brief treatments (iTBS) in scenario analysis | 8.58 | 12.88 | 15.56 | 16.10 | 18.24 | 71.36 |
| Training costs | 0.09 | 0.09 | 0.10 | 0.10 | 0.11 | 0.49 |
| Total costs (new scenario) in reference case analysisc | 9.30 | 10.92 | 13.60 | 13.61 | 15.76 | 63.20 |
| Total costs (new scenario) in scenario analysisc | 9.30 | 13.60 | 16.29 | 16.83 | 18.98 | 75.01 |
| Budget impact in reference cased | 9.30 | 10.92 | 13.60 | 13.61 | 15.76 | 63.20 |
| Budget impact in scenario analysisd | 9.30 | 13.60 | 16.29 | 16.83 | 18.98 | 75.01 |
Abbreviations: iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation.
Long treatment is defined as a 37.5-minute session. An rTMS machine could run 8 sessions per day (communication with D. Blumberger, MD, on October 30, 2019). All costs expressed in 2019 Canadian dollars.
Brief treatment is defined as a 3-minute session. An rTMS machine could run 22 sessions per day (communication with D. Blumberger, MD, on October 30, 2019). All costs are expressed in 2019 Canadian dollars.
Total costs (new scenario) = Long treatments (high-frequency rTMS) + Brief treatments (iTBS) + Training costs.
Budget impact = New scenario − Current scenario.
The budget impact of adopting rTMS would range from $9.3 million to $15.76 million yearly over the next 5 years, yielding a cumulative 5-year budget of $63.2 million (see Table 25).
We estimate a total of 14,640 adults with TRD would be treated by rTMS (high-frequency and iTBS) over the next 5 years at Ontario's current rTMS clinics (Table 26).
Table 26:
Adults With TRD Expected To Be Treated by High-Frequency rTMS and iTBS in Next 5 Years in Ontario
| Treatment | Adults With TRD Treated by rTMS | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| High-frequency rTMS | 137 | 137 | 137 | 137 | 137 | 686 |
| Reference Case | ||||||
| iTBS | 2,011 | 2,389 | 3,017 | 3,017 | 3,520 | 13,954 |
| Reference case totals treated with rTMS | 2,149 | 2,526 | 3,154 | 3,154 | 3,657 | 14,640 |
| Scenario Analysis | ||||||
| iTBS | 2,011 | 3,017 | 3,646 | 3,771 | 4,274 | 16,720 |
| Scenario analysis totals treated with rTMS | 2,149 | 3,154 | 3,783 | 3,908 | 4,411 | 17,405 |
Abbreviations: iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation; TRD, treatment-resistant depression.
Scenario Analysis
SCENARIO 1: INCLUDING CURRENT AND FUTURE (LOOKING-TO-START) rTMS CLINICS
Table 25 presents the costs incurred to operate rTMS machines of both current and future rTMS clinics in Ontario in the next 5 years assuming that rTMS (both high-frequency rTMS and iTBS) is to be publicly funded (new scenario).
The budget required to run all current and future rTMS clinics in Ontario in the next 5 years would range from $9.3 million to $18.98 million in Ontario in the next 5 years, yielding a 5-year total budget of $75 million (see Table 25).
There would be about 2,149 adults with TRD to be treated by rTMS (high-frequency and iTBS) in year 1 and about 4,411 adults with TRD to be treated in year 5, with a total of 17,405 adults to be treated by rTMS in the next 5 years (Table 26).
SCENARIO 2: EXCLUSION OF THE CAPITAL COST (I.E., PURCHASE AND MAINTENANCE COST OF rTMS MACHINES)
When the purchase and maintenance costs of rTMS machines are excluded, the budget required to operate machines in existing rTMS clinics in Ontario would range from $8.84 million in year 1 to $14.96 million in year 5, yielding a 5-year total budget of $60 million. Similarly, the budget required to operate machines in both existing and future (looking-to-start) rTMS clinics in Ontario in the next 5 years would range from $8.84 million to $18.02 million in Ontario, yielding a 5-year total budget of $71.20 million (see Appendix 11 Table A19).
SCENARIO 3: EXTREME DIFFUSION OF rTMS
In the case of extreme diffusion of rTMS, the number of rTMS machines would be 150 (see Table 26). Assuming these machines could run both long (high-frequency rTMS) treatments and brief (iTBS) treatments, the budget required to operate 150 rTMS machines would range from $31.5 million if all machines were to run long treatments to $80.4 million if all machines were to run brief treatments. The costs were higher for iTBS because the machines used for iTBS could run more sessions.
Discussion
In the reference-case analysis, our estimated budget impact was based on funding only existing rTMS clinics in Ontario in the next 5 years. This was a conservative approach, because we do not know how many clinics would be set up in the next 5 years. In the budget impact analysis, we also included both capital costs (i.e., purchase and maintenance costs of rTMS machines) and variable costs (e.g., training, labour). In addition, expanding rTMS clinics requires the development of infrastructure and the training of human resources (e.g., technicians); thus, estimating the budget to fund only existing rTMS clinics and their expansion capacity in the next 5 years seemed reasonable.
Our reference case analyses showed that when considering both capital costs (i.e., purchase and maintenance cost of rTMS machines) and variable costs (e.g., training, labour), funding rTMS (high-frequency and iTBS) would require $9.3 million to $15.76 million each year over the next 5 years. This estimate was based on a realistic capacity of current and future (looking-to-start) rTMS clinics. We also explored the budget impact when excluding capital costs and including only variable costs. In this scenario, the budget required to fund rTMS machines in Ontario's existing rTMS clinics would range from $8.84 million in year 1 to $14.96 million in year 5, for a total of $60 million over 5 years. Similarly, funding rTMS machines in both existing and future (looking-to-start) rTMS clinics in Ontario, the required budget (including only variable costs) would range from $8.84 million in year 1 to $18.02 million in year 5, or a total of $71.02 million over 5 years.
In a more ambitious approach, our scenario analyses explored the possibility of setting up new rTMS clinics in the province. In an extreme scenario, we estimated the budget required to set up rTMS clinics in all 50 existing ECT centres in Ontario at a ratio of 3:1.
In the extreme diffusion of rTMS, additional costs would range from $31.5 million to $80.4 million. In this case, three rTMS clinics would be available in each ECT centre in Ontario (totalling 150 rTMS clinics). In the case of extreme diffusion, it is reasonable to assume that rTMS centres would have an adequate pool of TRD patients to treat, given the relatively high prevalence of major depression and TRD in Ontario.
Considering the cost of operating sessions in treatment of TRD, iTBS appeared to be the best choice, as the machine would provide briefer sessions and treat more patients with TRD, while maintaining effects equal to those of high-frequency rTMS machines. It is important to note that the introduction of iTBS in treatment of adults with TRD does not offset expenses incurred by use of ECT. Electroconvulsive therapy continues to be an effective treatment option. Further, results from the primary economic evaluation have shown that treatment of TRD using high-frequency rTMS or iTBS would be most cost-effective when used as first-line treatment and in a stepped care approach with ECT. If patients fail to respond to rTMS, they could continue with ECT as the second-line treatment.
A potential limitation of our analysis is that the budget impact captured only the total costs of high-frequency rTMS and iTBS while ignoring potential cost savings from reducing the treatment cost of ECT. As mentioned above, our budget impact calculation did not imply the replacement of ECT treatment for several reasons: (1) we modelled the stepped care pathway in which rTMS is used as a first step followed by ECT (a stepwise clinical trajectory from less to more invasive treatments; in the reference case, we assumed that only 35% of TRD patients would switch to ECT if they did not respond to high-frequency rTMS or iTBS initially) that will ensure better disease management; (2) the experts we consulted emphasized that the use of rTMS in treatment-resistant depression would not phase out the use of ECT.
Our budget impact analysis suggests that publicly funding rTMS should happen in phases, as it requires the infrastructure to be in place and the training of human resources.
Conclusions
To publicly fund rTMS in the next 5 years in Ontario, annual costs would increase by $9.3 million in year 1 to $15.76 million in year 5, yielding a total 5-year budget of about $63.2 million.
Preferences and Values Evidence
Objective
The objective of this analysis was to explore the underlying values, needs, and priorities of adults who have lived experience with repetitive transcranial magnetic stimulation (rTMS) as a treatment for treatment-resistant depression (TRD), as well as how they decided to seek this treatment.
Background
Exploring patient preferences and values provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat that health condition. It includes the effect of the condition and its treatment on the person with the health condition, their family and other caregivers, and the person's personal environment. Engagement also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., outcomes important to those with lived experience that are not reflected in the literature).170–172 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, preferences, priorities, and values of those with lived experience in Ontario are often inadequately explored in the published literature, we may speak directly with people who live with a given health condition, including those with experience of the technology or intervention we are exploring.
For this analysis, we examined in two ways the perspectives and values of those with lived experience with TRD and who might have experience with rTMS and their providers:
A review by Ontario Health of the quantitative evidence on patient and provider preferences and values
Direct engagement by Ontario Health with people who have these conditions through interviews
Quantitative Evidence
Research Questions
What are patients’ and providers’ relative preference for rTMS compared with other treatments i.e., electroconvulsive therapy (ECT), antidepressants, or psychotherapy)
What is the relative importance of key attributes of rTMS?
What trade-offs between attributes of rTMS are people willing to make?
Methods
LITERATURE SEARCH
We performed a literature search for quantitative evidence of preferences on September 6, 2019, to retrieve studies published from database inception until the search date. We used the Ovid interface to search MEDLINE and the EBSCOhost interface to search the Cumulative Index to Nursing & Allied Health Literature (CINAHL).
The search was based on the population and intervention of the clinical search strategy, with a methodological search filter applied to limit retrieval to quantitative evidence of preferences and values (modified from Selva et al173). We further modified the search filter to include additional key terms relevant to psychological and emotional outcomes and patient satisfaction. The final search strategy was peer reviewed using the PRESS Checklist.26
We created database auto-alerts in MEDLINE and CINAHL and monitored them for the duration of the assessment period. See Appendix 1 for our literature search strategies, including all search terms.
ELIGIBILITY CRITERIA
Studies
Inclusion Criteria
English-language full-text publications
Studies published until September 6, 2019
Randomized controlled trials (RCTs), observational studies that use utility measures (e.g., standard gamble, time trade-off, health utility index), and non-utility measures (e.g., decision aids, surveys)
Exclusion Criteria
Studies where results for outcomes of interest cannot be extracted
Qualitative studies, editorials, commentaries, case reports, conferences abstracts, letters
Animal and in vitro studies
Participants
Inclusion Criteria
Adults (18 years of age and older) with TRD (unipolar and bipolar)
Providers who treat people with TRD (unipolar and bipolar)
Exclusion Criteria
Other conditions for which rTMS is used (e.g., obsessive–compulsive disorder, post-traumatic stress disorder)
Intervention
Any modality of rTMS
Comparators
Electroconvulsive therapy
Antidepressants
Psychotherapy
No comparator
Outcome Measures
Key attributes of treatment
Barriers to treatment
Trade-offs
LITERATURE SCREENING
A single reviewer conducted an initial screening of titles and abstracts using Covidence and then obtained the full text of studies that appeared eligible for the review according to the inclusion criteria. This reviewer then examined the full-text articles and selected studies eligible for inclusion.
DATA EXTRACTION
We extracted relevant data on study characteristics using a data form to collect information about the following:
Source (e.g., citation information, contact details, study type)
Methods (e.g., study design, study duration, participant recruitment)
Outcomes (e.g., outcomes measured, outcome definition and source of information, unit of measurement, upper and lower limits [for scales], time points at which the outcomes were assessed)
STATISTICAL ANALYSIS
After determining that a meta-analysis to provide an overall statistical summary of the effect estimate was inappropriate for a broad summary of the evidence on quantitative preferences, we chose a descriptive approach using text or tables.
CRITICAL APPRAISAL OF EVIDENCE
We assessed the quality of evidence using the Purpose, Respondents, Explanation, Findings, Significance (PREFS) checklist.174
Results
LITERATURE SEARCH
The literature search of the quantitative evidence of preferences and values yielded 183 citations published from inception until September 6, 2019. We identified one additional study from auto-alerts. In total, we included three relevant studies in our review. Figure 21 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the literature search for quantitative evidence of preferences and values.
Figure 21: PRISMA Flow Diagram—Quantitative Evidence of Preferences and Values Search Strategy.
Source: Adapted from Moher et al.39
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
CHARACTERISTICS OF INCLUDED STUDIES
We did not find any studies that quantified patient and provider preferences and values of rTMS. However, we found three studies examining provider and patient experience, knowledge, attitudes, and acceptability of rTMS. One study175 surveyed patients who received rTMS while the other two studies176,177 surveyed psychiatrists. Table 27 shows the characteristics of the included studies. Tables 28 and 29 show the characteristics of the patient and psychiatrist populations.
Table 27:
Characteristics of Included Quantitative Studies on Patients’ and Providers’ Preferences for rTMS
| Author, Year Country | Study Design | Study Methods | Participants |
|---|---|---|---|
| Walter et al, 2001175 Australia (Tasmania) | Questionnaire | 60-item survey constructed by authors. Fifty-five items offered a set choice of responses and 5 items were open-ended. Several items were adapted from an instrument that Walter et al developed for ECT studies. The survey covered demographic features (e.g., age, sex, relationship status, occupation), experience with rTMS (e.g., fears, provision of information, perceived effectiveness, side effects, comparisons with other treatments), knowledge (e.g., about the nature and technical aspects of rTMS) and attitudes (e.g., whether rTMS is humane or cruel and whether recipients would recommend it to others) | All participants received rTMS. Patients all had a DSM-IV major depressive episode that had failed to respond to adequate trials of medication. All patients had received antidepressants; some had also been treated with antipsychotics, anxiolytics, and mood stabilizers |
| AlHadi et al, 2017176 Saudi Arabia | Questionnaire | Developed a new questionnaire based on other studies that have measured knowledge of and attitudes toward ECT. The questionnaire's 3 sections cover demographic information, knowledge, and attitudes. The knowledge section had 21 items that evaluated aspects of rTMS knowledge. These items had 3 response options: “yes,” “no,” and “I don't know.” The attitude section had 13 items, including both positive and negative attitude statements. These items used a 5-point Likert scale with options of “strongly agree,” “agree,” “neutral,” “disagree,” and “strongly disagree” | Questionnaire was e-mailed to approximately 300 psychiatrists, and those who responded were included in the study (convenience sampling), resulting in a response rate of 33%. The e-mail list was obtained from several sources, primarily the Saudi Psychiatric Association, the Saudi Commission for Health Specialties, and personal communication. Junior residents are first− and second-year residents in the psychiatry training program; senior residents are third− and fourth-year residents in the program |
| Bourla et al, 2020177 France | Questionnaire | All questions were designed during 3 focus groups that included psychiatrists and sociologists and were cross-validated by a sample of psychiatrists working at a hospital in Paris. Questions were inspired by previous studies. Questionnaire had 3 sections: epidemiological data, acceptability of rTMS and influencing factors (25 items), and a blank field for qualitative data. For each variable, participants responded to the questions on a Likert-type scale ranging from 1 to 6 (or ranging from “absolutely not” to “I agree totally”). For some questions (items 2–5) a more specific response was allowed (“yes,” “no,” “I don't know,” or “more sustainable,” “less,” “same,” etc.). For item 1, we asked practitioners to consider the most useful treatment option (ECT, rTMS, tDCS, antidepressants, or psychotherapy) in various situations (mild, moderate, severe, etc.). An “overall acceptability” score was implemented using four specific variables highly representative of each domain (items 1, 8, 9, 11) allowing assignment of respondents to three groups: low, moderate, or high acceptability | Study focused on a population of psychiatrists working in France. They ranged from residents to senior psychiatrists who worked in psychiatric facilities, general or university hospitals, or private practice. 475 psychiatrists participated in the study |
Abbreviations: ECT, electroconvulsive therapy; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct-current stimulation; TMS, transcranial magnetic stimulation.
Table 28:
Patients and Their Treatment in Included Studies
| Author, Year Participants, N | Age, Years | AD Status n (%) | Type of rTMS | No. of rTMS Courses, n (%) | Last rTMS Session, n (%) | Had ECT, n (%) | Hospital Admission n (%) |
|---|---|---|---|---|---|---|---|
| Walter et al, 2001175 48 patients | 49 (range 23–79) | Using medication 44 (92) | HF left DLPFC 100% MT, 10 Hz in 5-s trains and 20 Hz in 2-s trains, 20–30 trains were given per session, 5 d/wk. One course was 10–15 sessions | 1: 31 (65) 2: 8 (16) 3: 3 (3) > 3: 6 (16) | 1 year ago: 18 (38) 1–2 years: 15 (31) > 2 years: 15 (31) | 31 (65) | Inpatient: 27 (56) Outpatient: 21 (44) |
Abbreviations: AD, antidepressant; DLPFC, dorsolateral prefrontal cortex; ECT, electroconvulsive therapy; HF, high frequency; MT, motor threshold; rTMS, repetitive transcranial magnetic stimulation.
Table 29:
Characteristics of Psychiatrists in Included Studies
| Author, Year | N | Age, Years | Education, n (%) | Subspecialty, n (%) | Place of Work, n (%) | rTMS in Place of Work, n (%) | Experience With rTMS, (%) |
|---|---|---|---|---|---|---|---|
| AlHadi et al, 2017176 | 96 psychiatrists 78 men (81%) | 36.99 (SD 7.84) | Junior resident 14 (15) Senior resident 11 (11) Specialist (registrar) 23 (24) Consultant 48 (50) | General 62 (64.6) Mood 1 (1) Anxiety 2 (2) Child or adolescent 8 (8) Psychosomatic 7 (7) Psychotherapy 2 (2) Geriatrics 3 (3) Addiction 7 (7) Other 4 (4) | General hospital 37 (39) Teaching hospital 20 (21) Psychiatric 39 (41) | Yes 8 (8) No 88 (92) | Yes 8 (8) No 88 (92) |
| Bourla et al, 2020177 | 475 psychiatrists | 205 (43.1%) | Resident 176 (37) Hospital practitioner 142 (29.8) Private practitioner 72 (15.1) Post-resident (assistant) 66 (19.9) Professor 17 (3.6) NA 3 (0.6) | Adult psychiatry 371 (77.9) Child psychiatry 52 (10.9) Addiction medicine 24 (5) Geriatric psychiatry 21 (4.4) Forensic psychiatry 5 (1.1) Other 3 (0.6) | (Except residents) University hospital 99 (33) Public psychiatry (sectored) 98 (32.7) Private practice 46 (15.3) Clinic 27 (9) General hospital 25 (8.3) NA 5 (1.6) | NR | NR |
Abbreviations: NA, not applicable; NR, not recorded; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation.
All studies used a questionnaire to assess patient and provider experience, knowledge, and attitudes with respect to rTMS, as well as the acceptability of rTMS. The number of participants ranged from 48 to 475 and represented people from Australia and psychiatrists from France and Saudi Arabia.
Patients had TRD and experience with rTMS. Psychiatrists were not required to have practical experience with rTMS to participate in the studies.
We assessed the quality of the evidence using the PREFS checklist.174 All studies had similar quality. No studies directly studied “preferences,” none compared respondents to nonrespondents, and some significance tests were used, but most outcomes were given as proportions and counts.
EXPERIENCE WITH rTMS
Walter et al175 examined patients’ experiences with rTMS and its side effects. More than half of participants in the study believed rTMS was helpful for their TRD (30/48, 63%; Table 30).
Table 30:
Results for Patient Experience With rTMS
| Author, Year | N | Measurement Method | Results |
|---|---|---|---|
| Walter et al, 2001175 | 48 | Questionnaire | Experience of rTMS
Experience of side effects for rTMS, ECT,a and medications, respectively
|
Abbreviations: ECT, electroconvulsive therapy; NS, not significant; rTMS, repetitive transcranial magnetic stimulation.
Captures only patients who had experience with ECT (n = 31).
All side effects (e.g., muscle aches, nausea, confusion, memory impairment) except headaches were less common with rTMS than with ECT.
KNOWLEDGE OF rTMS
All three studies assessed knowledge of rTMS. Walter et al175 asked broader questions of patients about when rTMS was first used in practice, the mechanism, and indications for rTMS. AlHadi et al176 proposed various statements and participants had to correctly identify which were true and which false. Bourla et al177 asked seven questions about contraindications, side effects, and where rTMS fits on the clinical pathway (Table 31).
Table 31:
Patients’ and Psychiatrists’ Knowledge of rTMS
| Author, Year | N | Measurement Method | Results |
|---|---|---|---|
| Walter et al, 2001175 | 48 patients | Questionnaire |
|
| AlHadi et al, 2017176 | 96 psychiatrists | Questionnairea |
|
| Bourla et al, 2020177 | 475 psychiatrists | Questionnaire |
|
Abbreviations: AD, antidepressant; ECT, electroconvulsive therapy; F, false; FDA, Food and Drug Administration; rTMS, repetitive transcranial magnetic stimulation; T, true.
All knowledge statements were either true or false; percentage of people who answered correctly is shown.
ATTITUDES TOWARD rTMS
Two studies measured attitudes toward treatment. Walter et al175 used statements to allow patients to compare rTMS with ECT and medications. AlHadi et al176 asked psychiatrist's questions on a 5-point Likert scale ranging from “strongly disagree” to “strongly agree” around rTMS status in clinical care for patients with depressive conditions and whether psychiatrists would use rTMS. Table 32 summarizes attitudes toward rTMS.
Table 32:
Patients’ and Psychiatrists’ Attitudes Toward rTMS
| Author, Year | N | Measurement Method | Results |
|---|---|---|---|
| Walter et al, 2001175 | 48 patients | Questionnaire | Patient attitudes towards rTMS, ECT, and medications,a respectively
Would agree to rTMS if recommended in the future 44 (92%)
Would recommend rTMS to friends and family if it had been advised by a doctor, either immediately or if other treatments did not work 42 (87%) Patients who believed rTMS helped “a lot” (23/48)
|
| AlHadi et al, 2017176 | 96 psychiatrists | Questionnaireb | Overall findings
Results of questionnaire
|
Abbreviations: A, agree; D, disagree; ECT, electroconvulsive therapy; N, neutral; rTMS, repetitive transcranial magnetic stimulation; SA, strongly agree; SD, strongly disagree.
P values are differences between ECT and rTMS (no differences in attitude comparing rTMS with medication).
AlHadi et al176 calculated scores by assigning scores of 0 to 4 based on whether attitude statement was positive or negative.
ACCEPTABILITY OF rTMS
One study177 examined psychiatrists’ acceptability of rTMS evaluating the usefulness, usability, easiness and a benefit-risk equation. This allowed authors to calculate an overall acceptability score. An overall acceptability score was implemented using four specific variables highly representative of each domain (items 1, 8, 9, 11), allowing authors to classify respondents in three groups: low, moderate, or high acceptability. Table 33 summarizes results on acceptability of rTMS.
Table 33:
Results for Acceptability of rTMS
| Domain | Notes | Results |
|---|---|---|
| Usefulness | — | What treatment would you use for the following?
Do you think rTMS has a faster effect than AD, psychotherapy, or ECT?
Do you think rTMS has a more lasting effect than AD, psychotherapy, or ECT?
86.4% believed rTMS could be helpful for a patient reluctant to use medication |
| Usability | — | 86.5% of the sample considered themselves ready to integrate rTMS into their usual therapeutic approach Indications for which psychiatrists would prescribe rTMSa
Would you use rTMS for patients who had a severe depressive episode? 60% |
| Easiness | Measured on a Likert-scale (1–6) to estimate ease of using rTMS | 48.07% of participants believed rTMS was simple to use (> 3) |
| Benefit-risk equation | — | 81.27% thought benefit of rTMS outweighs riska For treatment of a depressive episode
Do you think rTMS is riskier than no treatment? 13% agreed or strongly agreed |
| Overall acceptability score | Composed of items 1, 8, 9, and 11 1: What therapeutic option is the most useful treatment (question captured in usefulness domain) 8: If you were to suffer from a severe depressive episode, would you use this technique for yourself? 9: Do you think that this technique is easy to use? 11: Do you think the benefit outweighs the risk? |
47.2% have high acceptance 40.6% have moderate acceptance 12.1% have low acceptance |
Abbreviations: AD, antidepressants; ECT, electroconvulsive therapy; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; TMS, transcranial magnetic stimulation.
Percentages are those who responded “strongly agree” or “agree”
Discussion
Outcomes included in this quantitative evidence review were attitudes toward, experience with, knowledge of, and acceptance of rTMS. The included studies did not report on patient preferences assessing the key attributes, trade-offs, and barriers to rTMS.
Only one study by Walter et al175 surveyed patients who had previously received rTMS. Most patients reported that rTMS was helpful (63%) for their TRD, and side effects experienced by patients aligned with clinical literature (e.g., headaches). Patients also expressed positive attitudes toward the treatment. Most of the sample (92%) reported that they would agree to rTMS if recommended in the future and would recommend it to friends and family (87%).
Two studies176,177 surveyed psychiatrists’ knowledge, attitudes, and acceptance of rTMS treatment. Many psychiatrists did not have practical experience with rTMS (8%–28%) but did have some theoretical training (21%–54%). The amount of practical and theoretical training could have influenced the knowledge gaps that psychiatrists reported. Only 58%176 stated rTMS had shown significant results in drug-resistant depression, and 53% incorrectly stated that rTMS is more effective than ECT in treating depression. Many psychiatrists thought seizures were a common side effect of rTMS (32.8%). However, most generally had positive attitudes toward and acceptance of rTMS. In general, we found that patients and psychiatrists have positive attitudes toward and high acceptance of rTMS for TRD.
Limitations
Patients were within an undefined population with TRD (did not specify treatment-resistant definition used to classify patients). AlHadi et al176 did not have a representative population. More than 80% of the sample of psychiatrists were male. Bourla et al177 had a larger, more representative sample.
Studies used different questions to measure similar outcomes. All questionnaires were adapted from previous questionnaires that measured the same outcomes for various treatments (e.g., ECT) and were not validated. The use of different questions for measuring outcomes makes comparisons between studies difficult.
Conclusions
The evidence on patients’ and psychiatrists’ experience, knowledge, attitudes, and acceptance of rTMS was examined from 3 published studies. Patients generally had favourable experiences and positive attitudes toward rTMS. Similarly, psychiatrists had positive attitudes toward and acceptance of rTMS. Studies reported gaps in psychiatrists’ knowledge of rTMS (see Table 31), which could be influenced by the level of training on rTMS.
Direct Patient Engagement
Methods
PARTNERSHIP PLAN
The partnership plan for this health technology assessment focused on consultation to examine the experiences of those with lived experience with rTMS as a treatment for TRD and those of their families and other caregivers. We engaged people via phone interviews and through written responses.
We used a qualitative interview, as this method of engagement allowed us to explore the meaning of central themes in the experiences of people with TRD, as well as those of their families and caregivers.178 The sensitive nature of exploring people's experiences of a health condition and their quality of life are other factors that support our choice of an interview method.
PARTICIPANT OUTREACH
We used an approach called purposive sampling,179–182 which involves actively reaching out to people with direct experience of the health condition and health technology or intervention being reviewed. We approached a variety of partner organizations, including the Centre for Addiction and Mental Health and the Ontario Brain Institute, to spread the word about this engagement activity and to contact people with TRD and their family members or caregivers, including those with experience of rTMS.
Inclusion Criteria
We sought to speak with adults with lived experience of rTMS as therapy for TRD. Participants did not need to have direct experience with rTMS to participate.
Exclusion Criteria
We did not set exclusion criteria.
Participants
For this project, we spoke with 26 participants who had TRD living in Ontario and 9 family members. Of the 26 participants with TRD, 19 had used rTMS previously while the other 7 were seeking rTMS or currently waiting for treatment.
APPROACH
At the beginning of the interview, we explained the role of our organization, the purpose of this health technology assessment, the risks of participation, and how participants’ personal health information would be protected. We gave this information to participants both verbally and in a letter of information (Appendix 11) if requested. We then obtained participants’ verbal consent before starting the interview. With participants’ consent, we audio-recorded and then transcribed the interviews.
Interviews lasted approximately 20 to 60 minutes. The interview was loosely structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.183 Questions focused on the impact of depression and the quality of life of people with depression, their experiences with treatments to manage or treat their condition, their decision-making about rTMS, their perceptions of the benefits or limitations of rTMS, and the potential effect of having this technology more widely available on Ontario. For family members and caregivers, questions focused on their perceptions of the effect of depression and treatment on quality of life of the person with depression, as well as the effect of the person's health condition and treatment on family members and caregivers themselves. See Appendix 12 for our interview guide.
DATA EXTRACTION AND ANALYSIS
We used a modified version of a grounded-theory method to analyze interview transcripts. The grounded-theory approach allowed us to organize and compare information on experiences across participants. This method consists of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.184,185 We used the qualitative data analysis software program NVivo56 to identify and interpret patterns in the data. The patterns we identified allowed us to highlight the effect of TRD, decision-making around rTMS, and how treatment affected the people with depression, family members, and caregivers we interviewed.
Results
IMPACT OF DEPRESSION
Interview participants emphasized the difficulties they and their loved ones faced dealing with the mental health challenges of depression. Many had suffered from depression for many years, though the cause could be uncertain and varied across interviews. Some participants believed their depression had a familial origin or grew from a specific incident, but many were unsure of the cause of their depression or when exactly it began. Some participants indicated that their depression began as a young adult or even as a child, while others experienced depression for the first time as adults:
I have been living with mental illness since my adolescence and am 3 years into this current episode of depression.
I went to my doctor's office for a different appointment, and I saw a pamphlet that said, “If you have 5 of these 20 symptoms you might have depression.” I had 19 of them, and it clicked that this is what it could be, that there's actually nothing wrong with me, that something is actually going wrong. Because I obviously was trying to find reasons because I felt sad all the time, and I had no reason to feel sad because my life was so good.
Participants’ journeys with depression reflected the unique and personal nature of mental health and its challenges. Features of their depression, such as symptoms, severity, or cycles, varied for each person. However, while the lived experience of each person was unique and personal, there were commonalities when describing how depression affected their lives or the lives of family members. Participants consistently reported on serious problems in their lives due to TRD. These problems not only affected them, but affected family members and caregivers as well, changing relationships and family dynamics:
Mental illness has had a major impact on most areas of my life. My marriage fell apart for a while (my husband moved out), and my daughters were under a lot of stress and experienced anxiety as a result of my illness.
And I went downhill from that point, and [a family member] began, basically, caring for me because I had become effectively bedridden and, at times, even suicidal. Suicidal ideation, not suicidal effectuation, but suicidal ideation. And I remained in that state for almost 4 years.
In describing their history of depression, many interview participants specifically reported on activities and events that were negatively affected by their depression, reducing their quality of life. Some activities or interactions that previously had brought pleasure were avoided owing to the depression. Examples included attendance at school or work, performance of common and simple tasks, or interactions with family members and friends:
I had to drop out of my PhD program—withdraw, sorry, is the correct word—and then was on disability because I was … you know, this was very, very severe depression and a very treatment-resistant depression.
I live in a constant state of overwhelm. Tasks that for most people are easy, almost automatic, such as getting out of bed, showering, leaving the house, taking care of basic household chores, and making simple decisions are difficult and cause much stress, procrastination, and worry. I have no motivation and can't enjoy things I used to, mostly because I overthink and can't make decisions, so don't end up doing anything. There is a horrible, uncomfortable, and distressing feeling that is very hard to put into words. It's like being both paralyzed and panicked at the same time.
[Depression] impacts my life a lot. I'm not going out of my house. I try to hide it when people are around me, but I don't seek people out. … It gets to be a very lonely life.
Several participants described their situation as demoralizing, feeling very disheartened at their near-constant state of depression and its effects in many areas of their lives:
I've had 13 years of gaps in my career (due to depression). To not be working—it's demeaning, it's demoralizing.
In addition, it is costly because I was unable to work and relying on work benefits, which was extremely stressful. … There is also still a lot of stigma that I felt when I told my employer why I was ill and needed to go off leave. I had lost hope and [made] a serious suicide attempt.
THERAPIES FOR DEPRESSION
People seeking ways to manage depression most frequently report treatment with pharmacotherapy. Trying a variety of medications at different doses over several years was not uncommon. These attempts were necessary to search for an effective and long-lasting therapy for their depression. While some medications were effective for a short time, many participants were ultimately forced to find new medications or dosages when the first became ineffective or they developed unwanted side effects:
[A]fter all the alternative treatments, we went back on medication and there was new medication that had come out. [W]e tried that, and it seemed to give me a little bit of a lift, but again it was still problematic.
Medications, we tried many, many, many medication trials for many months at a time, and nothing ever seemed to work. I seem to be really sensitive to the side effects of them, to the point where I ended up having a bunch of gastrointestinal issues.
I was like 94 pounds, and they thought I was really sick; no, just depression. So, they kind of went back and said, “Okay, well, you've been on different medications before; let's try you on this.” That wasn't working. “Let's try you on something else.” Wasn't working. “Let's try …”—I [used] 5 different medications and 12 different dosages between August 2018 and May 2019.
This seemingly continuous cycle of searching and trying new pharmacotherapies had emotional consequences for patients and their families. Many commented on the frustration they felt at continually searching for effective treatments, a feeling of dependence on medications, and the desire for a more permanent or reliable treatment. A few participants also spoke about how cost could affect the types of medications they could try or the ability to afford consistent treatment:
So yes, it has been frustrating because I've never had something that has worked long term very, very well for me.
Yeah, it's kind of like trial and error. Sometimes it works and sometimes it doesn't. I've been on multiple medications so [I'm] interested in looking at other things.
The medications that I'm on currently, that I found seem to be … the most helpful, are quite expensive, and I don't have coverage right now. Even with … basic coverage, they're still really expensive because they're some of the newer types of medication that I'm on and I've reacted the best to.
The personal and unique nature of depression led patients to attempt multiple different therapies to manage and treat their symptoms. Participants reported that their search for effective and tolerable treatment could take years. Many occasionally visited various health care practitioners to address their own needs. Some of these therapies were successful for a short time, providing relief and improvement.
However, participant recruitment for this report targeted those who were seeking (or had used) rTMS treatment. This targeted approach means that the participants we interviewed would be unlikely to have found other effective therapies for managing their depression, a bias in our sample. During interviews, both patients and family members spoke of these other therapies and their impact, providing context for the ultimate decision to try rTMS or to consider it for future treatment. Examples of these alternate treatments for depression include meditation, yoga, and other relaxation techniques, talk and group therapy, as well as more intensive psychotherapy:
I went through [cognitive behavioural therapy], didn't really notice any significant improvement. I did the training, … but I didn't find myself very motivated to engage with the actual at-home therapies. Afterward, I was directed towards a … sort of combined art therapy and group therapy program.
I was referred to a … stress reduction program through meditation. … I've actually very recently done another program like that, a wellness program with a strong focus on meditation, both of which I found very useful. And I'm trying to think what else I've done; … I have tried yoga, tried tai chi. And those provide a sense of relief while I'm doing them, but it doesn't last.
Participants noted that receiving these alternative treatments could sometimes be a challenge; cost and access were two barriers that were most often mentioned. These barriers could reduce the potential effectiveness of these treatments by delaying treatment, limiting the number of sessions or the overall time that could be spent treating their depression:
Right now I'm at the stage I can't afford a therapist, so I've been seeing a social worker. So, she's the one who … brought up rTMS to me. We're going to be talking about that with my psychiatrist, who again I can't be seeing regularly; he said he can help me now, but once I'm kind of under control that he won't be able to follow me regularly.
She put us on an urgent list for SickKids, for their Anxiety Clinic, which was great except that that was October. [T]hey called and their earliest appointment was March. So I was like, “Well, my son may not be here by the time March comes because he's threatening to hurt himself.” So, again, [treatment] comes down to like, money and coverage, which I don't have.
Despite these challenges, participants expressed their strong interest in trying alternatives and the value they placed on the availability of multiple options for managing their depression. Participants often reported that a combination of medication and other therapies could be most effective, and their decision-making weighed values such as convenience and cost with effectiveness to find something that worked:
I find that, with depression, or at least with me, it can't just be one thing. You have to try multiple things at the same time.
But I think [for] many of the people that would be seeking a treatment, … it's lower on their priority list to be convenient than it is to have access to something that would actually bring relief.
I've been on medication for years for depression and anxiety and in therapy. So I've just kind of been interested in looking at other things.
rTMS TREATMENT
Participants’ familiarity with rTMS before they sought the technology or accessed the treatment itself varied. Some participants reported knowing very little about rTMS before their first session and having trouble finding accurate information, while others were very familiar with the technology before using it. And information about rTMS could come from multiple sources: their trusted physician, friends or colleagues, or their own research online:
It was like the heavens had opened and I said, “Oh my God, hi [doctor]. You can read my story here.” I had no idea. Nobody's talked to me about this. I Googled this myself based on a friend's recommendation.
I guess I felt like I was fairly proactive with it. So I saw that there was a research project going on, and I asked for a referral to it. That's how I became involved; it was just sort of hearing [about] it on CBC, and then that prompted a little bit later the Internet search.
I couldn't even tell you when I first heard about it. It's been on my radar for a long time, because again, you know, my psychiatrist and I were always, always looking for anything else out there that might be a possibility.
It was sort of the first I've heard of it and at first, it sounds kind of scary; you're in the waiting room and you can hear the machine going, which I didn't know what it was.
So, it sounds scary at first, but then once I learnt a little bit more about it, it kind of reminds me [of] when you go to the physiotherapist or chiropractor and they do acupuncture and they put those electrodes on you.
Some participants acknowledged that they were hesitant owing to the unusual nature of the device and a lack of general awareness of the procedure:
[Speaking to the doctor] totally not only worked on my son in terms of explaining what was happening, but also my husband because he was very nervous about all this, and he's like, “you found this off the Internet; what if this is some crazy goofball thing? Why isn't it [covered by the Ontario Health Insurance Plan]? This is going to fry our kid's brain.” My husband was very nervous.
It seems a little wonky. … I didn't have a lot of faith [in] it when I first walked in.
However, while there was some hesitation, participants were generally open to trying rTMS treatment, relying on the advice of physicians and friends for reassurance about starting treatment. Additionally, participants expressed appreciation that this was a new method to try that purported to have minimal side effects:
If it works for the patient, then [my psychiatrist] supports it. And so I completely trust her. So when she suggested [rTMS], I said, “OK yes, where do I go? What do I do?” And I didn't have any type of hesitation.
So my doctor at that point at [hospital] suggested rTMS and said that it doesn't carry the same intense side effects as [electroconvulsive therapy, or ECT]—obviously because there's no anesthetic, because you're not having a full seizure, etc.—so that was encouraging.
And when [my doctor] indicated that this was a protocol that had been successfully tested in the [United States] and been accepted as a therapeutic modality, I decided that I would allow myself to see if this would do something for me.
Expectations of rTMS
As a treatment for depression, participants said their expectations for success from rTMS were modest. Given that most participants had struggled with managing and treating their depression for many years through many methods, expectations for rTMS were tempered and moderate. Participants generally appreciated the opportunity to access a new method that could perhaps help them with their depression:
Well, I can't think about [rTMS] as something that I need, because the chances of … I can't put such high hopes on it working. I mean, it may work, which would be lovely. But it may not. And, if you pin your hopes on … each thing working, that's not going to work out so well.
Quite a few respondents mentioned that they understood the success rates of rTMS, acknowledging that it is not guaranteed to work for everyone. This served to temper their expectations before receiving treatment:
[S]ome of the doctors … say that rTMS works for some patients, but it doesn't seem to work for other patients, and they don't know why.
He said that he's had about 10 patients [receive rTMS] and one had fantastic results and half of the others had good results. The other half had no results. No effect. So it's actually a pretty good [ratio].
I knew that there was an 80% chance that I would have some relief and a 20% chance that I would do fairly well through the program. So I felt like those were decent odds for the investment of my time into it. So I was very aware of … I don't know if those statistics have changed since then.
Expectations of rTMS could be more complicated for those with familiarity or experience with ECT as a treatment for depression. Many participants were familiar with the general nature of ECT, but there was a great deal of fear and stigma attached to this type of treatment. Participants mentioned that the pain and side effects associated with ECT influenced their expectations of rTMS, noting the differences between the two therapies. For those who had experienced ECT in the past, this contrast could be even more stark and was strongly emphasized during interviews:
I wanted to try rTMS because other treatments were not fully effective and rTMS does not have negative side effects once you stop treatment. I was determined to try and get my mental health to a place where I did not feel defeat, shame, and suicidal thoughts on a regular basis, so it was worth it—and again, it is a much, much gentler and [more] tolerable treatment than ECT.
rTMS was presented to me sort of as the modern alternative to ECT. I think I function well enough that electroconvulsive whatever isn't [needed]. I get the impression from what I've been reading that rTMS is the humane modern version [of ECT].
I'm not sure which direction they're going to recommend that I try, but ECT definitely sounds a lot more invasive and it's definitely more [intimidating] seeing it in the media, … whereas rTMS, you can just go in and come out and it's not a big deal. Whereas to go under full anesthetic, that's pretty intense.
Procedure and Access to rTMS
Participants reported a variety of protocols associated with their initial rTMS treatment. Some participants had multiple sessions daily, while others received the therapy only once each day. Generally participants would have sessions every day for several weeks, but scheduling variability was common depending on patients, their condition, the location of the rTMS clinic, and the physician's protocols:
I went daily for 1 week and then did the treatments for the next—it turned out to be 7 weeks for me just because there were holidays in there. So, I had to go to the hospital for six treatments a day for 5 days, so I was at the hospital all day for the first week.
I started with treatments 5 days a week for 5 weeks, and then the treatments were reduced to finally being at the maintenance level.
Every weekday for 6 weeks, he is to go in and have rTMS, and he needs some place to stay and he stays with my wife and [me]. This is his third time at [the hospital], and it's likely that there will be a fourth.
Despite differing protocols, participants consistently reported that some disruption and accommodation was necessary to commit to this therapy. The relatively limited availability of rTMS across Ontario meant that some participants reported having to travel more than 2 hours each way for each session, adding stress and disruption to their lives and those of family members. With multiple sessions weekly, this could be a fairly large inconvenience and a challenge for people to accommodate in their busy lives. Other participants were more fortunate to live or work closer to a rTMS facility, easing the burden of access:
If I do end up receiving the treatment, it will take me at least 2 hours to get to [the hospital] (train/subway/bus) for the 15-minute treatment and of course another 2 hours to get home. I will need 20–30 treatments. This travel will cause me much added stress, as I find it incredibly difficult to leave my house. Such a shame it is not offered at the hospital 10 minutes up the road where I live.
I think it was almost three or four times a week at the beginning. And of course my son can't drive, so my husband and I are taking time off work to go pick up my son and bring him down, bring him back.
And then, because I [rely on Ontario Disability Support Program benefits], I had to figure out how am I going to do this? How do I drive every day? Do I find a place to stay down there? How is this going to work, that kind of thing.
Beyond travel, access to rTMS could also be limited to the high demand and low capacity at a particular clinic. Some participants reported that they could not receive the number of sessions they desired or had to wait before being able to access services at a particular rTMS clinic:
I went through the second protocol again; I think there was a down-time. At that period, I think [the clinic director] only had three chairs or two chairs that he had in his clinic and he had hundreds of people and so he had a limited amount of chair time that was available, so I had to wait an extended period of time before I could restart on that new protocol.
I was on a wait list for over a year to access a psychiatrist and referred to Toronto Western Hospital for rTMS treatment. Unfortunately, I do not fit into any of their ongoing studies and learned this treatment is not yet covered by OHIP but have been put on a compassionate wait list to get the treatment in 3 to 4 months. I am absolutely desperate for care.
That was their expectation: that I would be able to get in within a couple of days because I was returning [for another round of treatment]. So when I contacted them, I was hoping to get back in in a couple of days, but the popularity of rTMS has grown so much that I was on the waiting list for 2 to 2$f12 months in the end, waiting to get back in.
Depending on the rTMS protocol for each participant, reported side effects varied. While some participants did not mention feeling any sensation during treatment, others commented on sensations of pain, tightness, or vibration throughout the head or into the jaws and teeth. A few participants also expressed concern about the long-term side effects of rTMS treatment, being unsure of any consequences they could face in future years. Typically, these side effects were not enough to dissuade participants from completing their rTMS sessions, but they were mentioned as downsides to the treatment:
I didn't even have the facial pain or the headaches. … I didn't have any significant side effects.
It was like a sharp … it reminded me of (because I used to knit), knitting needles when you first put them together, that kind of sound. But if you put that very, very quickly on your skin, a whole bunch of them, that's what it felt like to me. Like thick knitting needles, a whole bunch of them, very quickly going up and down on my head.
Now, of course, like with many therapies, I don't know what the 10-year or the 20-year outcome will be, whether or not there are some long-term negative outcomes that could be introduced or collateral outcomes that we're not seeing as yet.
I was surprised that it was painful, to be honest. It got less painful as time went on, but I was really surprised that it was painful.
Effects of rTMS
As mentioned previously, expectations for rTMS were relatively modest. Many participants knew that there was a good chance they would see only mild improvement to their depression, if any. Among patients and family members we interviewed, many experienced greater benefit from rTMS than they had expected. However, we also heard from participants who did not experience any benefit from rTMS treatment:
And so there was a sense of, “Well, what's wrong with me?” kind of thing. What did I do? What's going on with me? So I initially felt a lot of self-blame and realized that this is new, and this technique and this technology obviously doesn't work for everybody.
And I went through, I think it was an 8-week course at that time. It was four times a week that I was going, and at the end of the first protocol, I really did not notice any significant improvement.
Participants who reported improvement in their mental health after rTMS treatment commented that benefits did not necessarily appear right away. Often it would be several sessions—and occasionally multiple protocol attempts—before any improvement in their depression was noted:
And then … probably 2 or 3 weeks into that [new] protocol, a couple of things began to become apparent. One, I was experiencing a much different wake-up in the morning. I'd get up in the morning and I'd feel like, “Oh, let's get something done today.”
By the second or third week [of treatment], he started to say, “I think I need to take a shower.” And we're like, “Oh my God!” He's like, “I need to brush my teeth.” We're like, “Oh my God!” Then he's actually said, “Could I have a friend over?” We almost fell over [in surprise]. He said, “I can feel it in my brain; … I can do more.”
When it came to the nature of the improvement, participants often described the lifting of a weight off their shoulders and the disappearance of negative thoughts. Often activities of daily living were easier to do and could be done with greater energy. Some participants mentioned a change in sleep pattern or greater appetite. Participants reported that these differences were not only noticeable by participants, but by friends, family members, and health care providers as well and could have huge impact on their emotions and their day-to-day life:
It's almost like a switch goes off and I wake up one morning and realize that something isn't there, that despair isn't … as heavy as it was. It's a realization that there's a little bit more space in your mind for maybe a touch of joy. There's just less heaviness. I describe it as a switch to most people because I'm not sure how else to describe it, but it's not that something more is there, it's something less is there. And less despair.
You stop noticing that the skies are blue, that the trees are green. But all of a sudden you start noticing that again, which is pretty remarkable.
rTMS has been the game changer for my son, but so has medication, so has the fact that he's now 16, so has whatever. But without rTMS, I actually don't know if my son would be here.
And it's not even that I slept last night, it's that I had more energy. And then by the [last day of treatment], I was actually talking to somebody and I said, “I didn't go home from treatment and have to go crawl straight into bed. I actually stayed up. I had more energy.”
For some participants, this success had followed years of treatment with many unsuccessful therapies. Many participants who observed a benefit through management of their depression expressed gratitude for the availability of this treatment, while acknowledging that it wouldn't necessarily be as successful for everyone and that it could be hard for some in the province to access it:
It [worked]. It did. I mean, thank God it did. And it won't for everybody but there are a lot of kids who it will [help]. But if people never even heard about it, then it can't. And then even if they hear about it, if they can't get to it, then … it just seems silly that we have this treatment that will work for quite a good number of people and we're not offering it.
I consider myself fortunate over the last few years to have had this option.
For some participants, the specific benefit to receiving rTMS was its effectiveness and lack of side effects when compared directly with ECT. We spoke to several participants who had used both treatments, and the ability to access rTMS on their own, its lack of requirement for anesthesia, and the association of ECT with memory loss were all factors that weighed in favour of rTMS:
It definitely does the same thing as ECT, not as strong as ECT, but it doesn't wreak havoc with the side effects … like ECT did.
[For rTMS] that's actually a huge benefit; that's a huge thing, the fact that you can drive yourself home, that independence. It's a huge plus. [If you would] have to get somebody to drive you home every day for a month, that would be a big challenge. But the fact that you can do this on your own and that you're independent is huge. That's a big plus.
Ongoing Treatment With rTMS
While many participants reported on the improvement in their depression they observed after treatment with rTMS, they did not expect rTMS to be curative. Participants expected that regular rTMS therapy would be necessary to maintain their mental health and prevent recurrent depression. Participants hoped to be placed on a regular “maintenance” regimen of rTMS treatment. Protocols for this maintenance regimen were unique to each patient and depended on their circumstances and how they responded to treatment:
And so, I think after that juncture, because I had had such a strong positive outcome, I went onto a maintenance program. I was showing up once every 2 weeks.
I now stay on maintenance. … I actually drive there 2 days a week, and that seems to be the magic number.
Depending on the timing of this treatment and the logistics necessary to access it, regular maintenance could be challenging. Several participants expressed their concern and worry that they would be unable to continue to get access to rTMS for their ongoing treatment. They acknowledged the high demand for the treatment and the number of people who might be struggling with more severe cases of depression. However, knowing the effectiveness and the difference that rTMS had made in their own cases, many participants described their worry about losing treatment and their desire to maintain treatment as long as necessary into the future:
I certainly felt very different from the beginning to the end, and it's lasted a long time for me, too … about 18 months or so before I needed it again. I was told at the time that because I had had such a strong positive reaction previously, [we could hope] that would happen again.
It feels like at some point [the doctor] might say, “I've got other people I've got to help.” And it's like, I get it, but don't sacrifice my son for that. Now he didn't say that, but I've got to tell you we live in fear.
Now that I have completed rTMS, I am concerned about how difficult it is to … get this treatment if my severe symptoms return and I need it again. Quite simply, rTMS saved my life.
Discussion
Through interviews and written submissions from people with lived-experience with TRD, we were able to explore the preferences, values, and decision-making around different treatments for depression, including rTMS. We spoke with adult patients who had used rTMS, patients who were seeking rTMS, and family members of patients who have used this treatment. This robust engagement allowed us to include and consider multiple perspectives on this technology.
All participants clearly expressed the toll that mental health challenges such as depression can have on their lives and those of family members. This background and the lived experience with types of treatments over several years provided clear context regarding the decision to seek rTMS treatment and the expectations and accommodations necessary to access this treatment.
The modest availability of rTMS throughout southern Ontario allowed us to engage with many people who had direct access and experience with the devices and their results. However, the limitation of availability of rTMS to urban centres did allow for information to be collected around the context of accessing this treatment for those who live more rurally and must travel to receive treatment.
Given the clinical outcomes expected from rTMS used for depression, we can infer that the sample population we interviewed was biased toward people who achieved a significant decrease in depression-related symptoms and therefore perceived rTMS as effective treatment for their depression. Many participants reported that rTMS improved their mental health and their quality of life, finding it greatly reduced their depression for an extended period.
Conclusions
Depression can have a large negative effect on patients and families. Patients often try many treatments to manage their unique condition to find a reliable and effective therapy. While relatively unknown initially, rTMS was seen as an effective treatment by most participants, who found it reduced depression symptoms with only occasional mild side effects. Ongoing access to rTMS and its therapeutic benefit could be a challenge owing to its limited availability and high demand in Ontario.
Preferences and Values Evidence Conclusions
The quantitative evidence of preferences and values showed that patients generally had favourable experiences and positive attitudes toward rTMS. Similarly, psychiatrists had positive attitudes toward and acceptance of rTMS. The published quantitative literature reported some gaps in psychiatrists’ knowledge of rTMS, such as identification of populations that would benefit from rTMS and potential contraindications for rTMS; these gaps in knowledge might be explained by the level of training on rTMS.
Both direct patient engagement and quantitative evidence of preferences and values indicated that patients had favourable attitudes toward rTMS and saw it as an effective treatment. While the direct patient engagement did not interview psychiatrists, patients stated that psychiatrists gave them measured expectations of rTMS.
Conclusions of the Health Technology Assessment
Major depression can usually be treated successfully with antidepressants, psychotherapy, or both. However, in some cases, these treatments are ineffective. Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive neurostimulation treatment that delivers magnetic pulses to stimulate the area of the brain associated with mood regulation and can be another treatment option.
Multiple ways to deliver rTMS (i.e., rTMS modalities) were considered in this health technology assessment. Most rTMS modalities examined (excluding cTBS) likely resulted in lower depression scores than sham rTMS (GRADE: Moderate to High). Most rTMS modalities (except for low-frequency and cTBS) likely resulted in higher response rates compared with sham TMS (GRADE: Moderate to High). Of the seven rTMS modalities examined, three (high-frequency rTMS, bilateral rTMS, and deep TMS) likely resulted in higher remission rates than sham TMS (GRADE: Moderate to High). Adverse events were minor and similar in the rTMS and sham rTMS groups (GRADE: Moderate). Overall, none of the rTMS modalities was worse than sham rTMS.
Electroconvulsive therapy likely reduces depression scores (GRADE: Moderate), but probably results in no difference in response and remission rates compared with rTMS (GRADE: Moderate). Adverse events were no different for ECT or rTMS (GRADE: not reported).
When we compared rTMS modalities with one another, we found no difference in response or remission rates (GRADE: not reported). Adverse events were no different among rTMS modalities (GRADE: not reported).
Our review of the literature identified eight published cost-effectiveness studies that compared high-frequency rTMS with pharmacotherapy or with ECT in treatment of adults with TRD. High-frequency rTMS followed by ECT when necessary was associated with cost savings compared with ECT alone.
Our reference case results showed that high-frequency rTMS and iTBS in a stepped care approach were cost-effective compared with pharmacotherapy alone at a willingness to pay of $50,000 per quality-adjusted life-year (QALY) and were cost-saving compared with ECT alone. Among four treatments (i.e., high-frequency rTMS, iTBS, ECT, and pharmacotherapy alone), iTBS appeared to be most cost-effective. The clinical evidence has shown that iTBS was as effective as high-frequency rTMS in treatment of people with TRD. Compared with high-frequency rTMS, operating sessions of iTBS are associated with lower costs and similar effects. Also, an iTBS session is briefer than a high-frequency rTMS session; therefore, it could be possible to treat more people with TRD each day if iTBS were used instead of high-frequency rTMS. Electroconvulsive therapy could be an option for people with TRD who do not respond to either high-frequency rTMS or iTBS. A stepped-care approach (high-frequency rTMS or iTBS followed by ECT if needed) is a cost-effective treatment for people with TRD.
To publicly fund rTMS in the next 5 years in Ontario, annual costs would start at $9.3 million and increase to $15.75 million, yielding a total 5-year budget of about $63.2 million.
Patients generally had favourable experiences and positive attitudes toward rTMS. Similarly, psychiatrists had positive attitudes toward and acceptance of rTMS. We found some gaps in psychiatrists’ knowledge of rTMS, such as identification of populations that would benefit from rTMS and potential contraindications for rTMS; these gaps might be explained by the level of training on rTMS.
Depression can have a large, negative impact on patients and families. Patients with depression often attempt many treatments to find a reliable and effective therapy. While relatively unknown initially, rTMS was seen as an effective treatment by most participants, who found it reduced depression symptoms with only occasional mild side effects. Ongoing access to rTMS and its therapeutic benefit could be a challenge due to its limited availability and high demand in Ontario.
Acknowledgments
This report was developed by a multidisciplinary team from Ontario Health. The clinical epidemiologist was Anna Lambrinos, the medical librarian was Caroline Higgins, the primary health economist was Hong Anh Tu, the secondary health economist was Olga Gajic, and the patient and public partnership analyst was David Wells.
The medical editor was Elizabeth Jean Betsch. Others involved in the development and production of this report were Jamie Turnbull, Claude Soulodre, Susan Harrison, Saleemeh Abdolzahraei, Elisabeth Smitko, Sarah McDowell, Vivian Ng, Andrée Mitchell, Charles de Mestral, and Nancy Sikich.
We thank the following people for lending their expertise to the development of this report:
Daniel Blumberger, Temetry Centre for Therapeutic Brain Intervention
Amer Burhan, St. Joseph's Health Care London
Jeff Daskalakis, Temetry Centre for Therapeutic Brain Intervention
Jonathan Downar, Toronto Western Hospital
Jean-Guy Gagnon, HeadWay Clinic
Peter Giacobbe, Sunnybrook Hospital
Roumen Milev, Providence Care
William Wai Lun Wong, University of Waterloo
We also thank our lived experience participants who generously gave their time to share their stories with us for this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those we consulted.
Abbreviations
- CADTH
Canadian Agency for Drugs and Technologies in Health
- CCA
Corrected covered area
- CI
Confidence interval
- CINAHL
Cumulative Index to Nursing & Allied Health Literature
- cTBS
Continuous theta burst stimulation
- DLPFC
Dorsolateral prefrontal cortex
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- ECT
Electroconvulsive therapy
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- HDRS
Hamilton Depression Rating Scale
- ICER
Incremental cost-effectiveness ratio
- iTBS
Intermittent theta burst stimulation
- MADRS
Montgomery-Åsberg Depression Rating Scale
- NICE
National Institute for Health and Care Excellence
- OR
Odds ratio
- PREFS
Purpose, Respondents, Explanation, Findings, and Significance
- PRESS
Peer Review of Electronic Search Strategies
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- PSA
Probabilistic sensitivity analysis
- QALY
Quality-adjusted life-year
- ROBIS
Risk of Bias in Systematic Reviews
- rTMS
Repetitive transcranial magnetic stimulation
- SD
Standard deviation
- SGD
Singaporean dollar
- TBS
Theta burst stimulation
- TMS
Transcranial magnetic stimulation
- TRD
Treatment-resistant depression
- WTP
Willingness to pay
Glossary
- Adverse event
An adverse event is an unexpected medical problem that happens during treatment for a health condition. Adverse events may be caused by something other than the treatment.
- Budget impact analysis
A budget impact analysis estimates the financial impact of adopting a new health care intervention on the current budget (i.e., the affordability of the new intervention). It is based on predictions of how changes in the intervention mix will impact the level of health care spending for a specific population. Budget impact analyses are typically conducted for a short-term period (e.g., 5 years). The budget impact, sometimes referred to as the net budget impact, is the estimated cost difference between the current scenario (i.e., the anticipated amount of spending for a specific population without using the new intervention) and the new scenario (i.e., the anticipated amount of spending for a specific population following the introduction of the new intervention).
- Cohort model
In economic evaluations, a cohort model is used to simulate what happens to a homogeneous cohort (group) of patients after receiving a specific health care intervention. The proportion of the cohort who experiences certain health outcomes or events is estimated, along with the relevant costs and benefits. In contrast, a microsimulation model follows the course of individual patients.
- Corrected covered area
The corrected covered area measures the degree of overlap in primary studies across systematic reviews.
- Cost-effective
A health care intervention is considered cost-effective when it provides additional benefits, compared with relevant alternatives, at an additional cost that is acceptable to a decision-maker based on the maximum willingness-to-pay value.
- Cost-effectiveness acceptability curve
In economic evaluations, a cost-effectiveness acceptability curve is a graphical representation of the results of a probabilistic sensitivity analysis. It illustrates the probability of health care interventions being cost-effective over a range of willingness-to-pay values. Willingness-to-pay values are plotted on the horizontal axis of the graph, and the probability of the intervention of interest and its comparator(s) being cost-effective at corresponding willingness-to-pay values is plotted on the vertical axis.
- Cost-effectiveness analysis
Used broadly, “cost-effectiveness analysis” may refer to an economic evaluation used to compare the benefits of two or more health care interventions with their costs. It may encompass several types of analysis (e.g., cost-effectiveness analysis, cost–utility analysis). Used more specifically, “cost-effectiveness analysis” may refer to a type of economic evaluation in which the main outcome measure is the incremental cost per natural unit of health (e.g., life-year, symptom-free day) gained.
- Cost-effectiveness plane
In economic evaluations, a cost-effectiveness plane is a graph used to show the differences in cost and effectiveness between a health care intervention and its comparator(s). Differences in effects are plotted on the horizontal axis, and differences in costs are plotted on the vertical axis.
- Cost-minimization analysis
In economic evaluations, a cost-minimization analysis compares the costs of two or more health care interventions. It is used when the intervention of interest and its relevant alternative(s) are determined to be equally effective.
- Cost–utility analysis
A cost–utility analysis is a type of economic evaluation used to compare the benefits of two or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years, which capture both the quality and quantity of life. In a cost–utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Decision tree
A decision tree is a type of economic model used to assess the costs and benefits of two or more alternative health care interventions. Each intervention may be associated with different outcomes, which are represented by distinct branches in the tree. Each outcome may have a different probability of occurring and may lead to different costs and benefits.
- Discounting
Discounting is a method used in economic evaluations to adjust for the differential timing of the costs incurred and the benefits generated by a health care intervention over time. Discounting reflects the concept of positive time preference, whereby future costs and benefits are reduced to reflect their present value. The health technology assessments conducted by Health Quality Ontario (now a part of Ontario Health) use an annual discount rate of 1.5% for both future costs and future benefits.
- Disutility
A disutility is a decrease in utility (i.e., a decrease in preference for a particular health outcome) typically resulting from a particular health condition (e.g., experiencing a symptom or complication).
- Dominant
A health care intervention is considered dominant when it is more effective and less costly than its comparator(s).
- Health-related quality of life
Health-related quality of life is a measure of the impact of a health care intervention on a person's health. It includes the dimensions of physiology, function, social life, cognition, emotions, sleep and rest, energy and vitality, health perception, and general life satisfaction.
- Health state
A health state is a particular status of health (e.g., sick, well, dead). A health state is associated with some amount of benefit and may be associated with specific costs. Benefit is captured through individual or societal preferences for the time spent in each health state and is expressed in quality-adjusted weights called utility values. In a Markov model, a finite number of mutually exclusive health states are used to represent discrete states of health.
- Incremental cost
The incremental cost is the additional cost, typically per person, of a health care intervention versus a comparator.
- Incremental cost-effectiveness ratio (ICER)
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a health care consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental cost-effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- Markov model
A Markov model is a type of decision-analytic model used in economic evaluations to estimate the costs and health outcomes (e.g., quality-adjusted life-years gained) associated with using a particular health care intervention. Markov models are useful for clinical problems that involve events of interest that may recur over time (e.g., stroke). A Markov model consists of mutually exclusive, exhaustive health states. Patients remain in a given health state for a certain period of time before moving to another health state based on transition probabilities. The health states and events modelled may be associated with specific costs and health outcomes.
- Microsimulation model
In economic evaluations, a microsimulation model (e.g., an individual-level or patient-level model) is used to simulate the health outcomes for a heterogeneous group of patients (e.g., patients of different ages or with different sets of risk factors) after receiving a particular health care intervention. The health outcomes and health events of each patient are modelled, and the outcomes of several patients are combined to estimate the average costs and benefits accrued by a group of patients. In contrast, a cohort model follows a homogeneous cohort of patients (e.g., patients of the same age or with the same set of risk factors) through the model and estimates the proportion of the cohort who will experience specific health events.
- Ministry of Health perspective
The perspective adopted in economic evaluations determines the types of costs and health benefits to include. Ontario Health develops health technology assessment reports from the perspective of Ontario's Ministry of Health. This perspective includes all costs and health benefits attributable to the Ministry of Health, such as treatment costs (e.g., drugs, administration, monitoring, hospital stays) and costs associated with managing adverse events caused by treatments. This perspective does not include out-of-pocket costs incurred by patients related to obtaining care (e.g., transportation) or loss of productivity (e.g., absenteeism).
- Monte Carlo simulation
Monte Carlo simulation is an economic modelling method that derives parameter values from distributions rather than fixed values. The model is run several times, and in each iteration, parameter values are drawn from specified distributions. This method is used in microsimulation models and probabilistic sensitivity analysis.
- Natural history
The natural history of a disease is the progression of a disease over time in the absence of any health care intervention.
- Probabilistic sensitivity analysis (PSA)
A probabilistic sensitivity analysis (PSA) is used in economic models to explore uncertainty in several parameters simultaneously and is done using Monte Carlo simulation. Model inputs are defined as a distribution of possible values. In each iteration, model inputs are obtained by randomly sampling from each distribution, and a single estimate of cost and effectiveness is generated. This process is repeated many times (e.g., 10,000 times) to estimate the number of times (i.e., the probability) that the health care intervention of interest is cost-effective.
- Quality-adjusted life-year (QALY)
The quality-adjusted life-year (QALY) is a generic health outcome measure commonly used in cost–utility analyses to reflect the quantity and quality of life-years lived. The life-years lived are adjusted for quality of life using individual or societal preferences (i.e., utility values) for being in a particular health state. One year of perfect health is represented by one quality-adjusted life-year.
- Reference case
The reference case is a preferred set of methods and principles that provide the guidelines for economic evaluations. Its purpose is to standardize the approach of conducting and reporting economic evaluations, so that results can be compared across studies.
- Risk difference
Risk difference is the difference in the risk of an outcome occurring between one health care intervention and an alternative intervention.
- Scenario analysis
A scenario analysis is used to explore uncertainty in the results of an economic evaluation. It is done by observing the potential impact of different scenarios on the cost-effectiveness of a health care intervention. Scenario analyses include varying structural assumptions from the reference case.
- Sensitivity analysis
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Societal perspective
The perspective adopted in an economic evaluation determines the types of costs and health benefits to include. The societal perspective reflects the broader economy and is the aggregation of all perspectives (e.g., health care payer and patient perspectives). It considers the full effect of a health condition on society, including all costs (regardless of who pays) and all benefits (regardless of who benefits).
- Standard gamble
In economic evaluations, standard gamble is a direct method of measuring people's preferences for various health states. In a standard gamble, respondents are asked about their preference for either (a) remaining in a certain health state for the rest of their life, or (b) a gamble scenario in which there is a chance of having optimal health for the rest of one's life but also a chance of dying immediately. Respondents are surveyed repeatedly, with the risk of immediate death varying each time (e.g., 75% chance of optimal health, 25% chance of immediate death) until they are indifferent about their choice. The standard gamble is considered the gold standard for eliciting preferences as it incorporates individual risk attitudes, unlike other methods of eliciting preferences.
- Time horizon
In economic evaluations, the time horizon is the time frame over which costs and benefits are examined and calculated. The relevant time horizon is chosen based on the nature of the disease and health care intervention being assessed, as well as the purpose of the analysis. For instance, a lifetime horizon would be chosen to capture the long-term health and cost consequences over a patient's lifetime.
- Time trade-off
In economic evaluations, time trade-off is a direct method of measuring people's preferences for various health states. In a time-trade off, respondents are asked about their preference for either (a) living with a chronic health condition for a certain amount of time, followed by death, or (b) living in optimal health but for less time than in scenario (a). That is, respondents decide how much time in good health they would be willing to “trade off” for more time spent in poorer health. Respondents are surveyed repeatedly, with the amount of time spent in optimal health varying each time until they are indifferent about their choice.
- Utility
A utility is a value that represents a person's preference for various health states. Typically, utility values are anchored at 0 (death) and 1 (perfect health). In some scoring systems, a negative utility value indicates a state of health valued as being worse than death. Utility values can be aggregated over time to derive quality-adjusted life-years, a common outcome measure in economic evaluations.
- Willingness-to-pay (WTP) value
A willingness-to-pay (WTP) value is the monetary value a health care consumer is willing to pay for added health benefits. When conducting a cost–utility analysis, the willingness-to-pay value represents the cost a consumer is willing to pay for an additional quality-adjusted life-year. If the incremental cost-effectiveness ratio is less than the willingness-to-pay value, the health care intervention of interest is considered cost-effective. If the incremental cost-effectiveness ratio is more than the willingness-to-pay value, the intervention is considered not to be cost-effective.
Appendices
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Search date: August 27, 2019
Databases searched: Medline, Embase, Cochrane Database of Systematic Reviews, CRD Health Technology Assessment, NHS Economic Evaluation, PsycINFO, CINAHL
Database: EBM Reviews – Cochrane Database of Systematic Reviews < 2005 to August 21, 2019>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2019 Week 34>, Ovid MEDLINE(R) ALL <1946 to August 26, 2019>, PsycINFO <1967 to August Week 3 2019>
Search strategy:
-
1
Depression/ (473609)
-
2
exp Depressive Disorder/ (542337)
-
3
(depressi∗ or TRD or MDD or dysthymi∗ or melancholia∗ or involutional psychos∗ or unipolar∗).ti,ab,kf. (1131316)
-
4
Bipolar Disorder/ (114714)
-
5
(bipolar∗ or (manic∗ adj2 (depress∗ or disorder∗ or psychos#s or state∗))).ti,ab,kf. (194628)
-
6
or/1-5 (1439037)
-
7
Transcranial Magnetic Stimulation/ (39531)
-
8
(((transcranial∗ or trans-cranial∗) adj2 magnetic∗) or TMS or rTMS or aTMS or pTMS or sTMS or dTMS).ti,ab,kf. (62805)
-
9
((theta∗ adj3 stimul∗) or TBS or cTBS or iTBS or blTBS).ti,ab,kf. (11765)
-
10
(coil∗ adj2 (double cone∗ or figure or butterfly or H)).ti,ab,kf. (923)
-
11
(Deep TMS∗ or Magpro∗ or Magstim∗).ti,ab,kf. (626)
-
12
or/7-11 (79770)
-
13
6 and 12 (10156)
-
14
(Systematic Reviews or Meta Analysis).pt. (104128)
-
15
Systematic Review/ or Systematic Reviews as Topic/ or Meta-Analysis/ or exp Meta-Analysis as Topic/ or exp Technology Assessment, Biomedical/ (553900)
-
16
((systematic∗ or methodologic∗) adj3 (review∗ or overview∗)).ti,ab,kf. (408297)
-
17
(meta analy∗ or metaanaly∗ or met analy∗ or metanaly∗ or meta review∗ or metareview∗ or health technolog∗ assess∗ or HTA or HTAs or (technolog∗ adj (assessment∗ or overview∗ or appraisal∗))).ti,ab,kf. (416788)
-
18
(evidence adj (review∗ or overview∗ or synthes#s)).ti,ab,kf. (15516)
-
19
(review of reviews or overview of reviews).ti,ab,kf. (1529)
-
20
umbrella review∗.ti,ab,kf. (638)
-
21
GRADE Approach/ (203)
-
22
((pool∗ adj3 analy∗) or published studies or published literature or hand search∗ or handsearch∗ or manual search∗ or ((database∗ or systematic∗) adj2 search∗) or reference list∗ or bibliograph∗ or relevant journals or data synthes∗ or data extraction∗ or data abstraction∗).ti,ab,kf. (441027)
-
23
(medline or pubmed or medlars or embase or cinahl or web of science or ovid or ebsco∗ or scopus).ab. (447532)
-
24
cochrane.ti,ab,kf. (185593)
-
25
(meta regress∗ or metaregress∗).ti,ab,kf. (18998)
-
26
(((integrative or collaborative or quantitative) adj3 (review∗ or overview∗ or synthes∗)) or (research adj3 overview∗)).ti,ab,kf. (31636)
-
27
(cochrane or (health adj2 technology assessment) or evidence report or systematic review∗).jw. (61535)
-
28
((comparative adj3 (efficacy or effectiveness)) or relative effectiveness or ((indirect or indirect treatment or mixed-treatment) adj comparison∗)).ti,ab,kf. (45350)
-
29
or/14-28 (1223785)
-
30
13 and 29 (1087)
-
31
exp Animals/ not Humans/ (17764786)
-
32
30 not 31 (698)
-
33
Case Reports/ or Comment.pt. or Editorial.pt. or (Letter not (Letter and Randomized Controlled Trial)).pt. or Congress.pt. (5315345)
-
34
32 not 33 (682)
-
35
34 use medall,cleed (277)
-
36
13 use coch,clhta (23)
-
37
35 or 36 (300)
-
38
limit 37 to english language [Limit not valid in CDSR; records were retained] (280)
-
39
limit 38 to yr=“2014 −Current” (183)
-
40
depression/ (473609)
-
41
treatment resistant depression/ or major depression/ or chronic depression/ or depressive psychosis/ or dysthymia/ or melancholia/ or involutional depression/ (288127)
-
42
(depressi∗ or TRD or MDD or dysthymi∗ or melancholia∗ or involutional psychos∗ or unipolar∗).tw,kw. (1151057)
-
43
exp bipolar disorder/ (127394)
-
44
(bipolar∗ or (manic∗ adj2 (depress∗ or disorder∗ or psychos#s or state∗))).tw,kw. (197924)
-
45
or/40-44 (1452676)
-
46
exp transcranial magnetic stimulation/ (41227)
-
47
(((transcranial∗ or trans-cranial∗) adj2 magnetic∗) or TMS or rTMS or aTMS or pTMS or sTMS or dTMS).tw,kw,dv. (64144)
-
48
((theta∗ adj3 stimul∗) or TBS or cTBS or iTBS or blTBS).tw,kw,dv. (11837)
-
49
(coil∗ adj2 (double cone∗ or figure or butterfly or H)).tw,kw,dv. (942)
-
50
(Deep TMS∗ or Magpro∗ or Magstim∗).tw,kw,dv. (1489)
-
51
or/46-50 (80726)
-
52
45 and 51 (10419)
-
53
Systematic review/ or “systematic review (topic)”/ or exp Meta Analysis/ or “Meta Analysis (Topic)”/ or Biomedical Technology Assessment/ (547380)
Annotation: Added Systematic review/ or “systematic review (topic)”/ for thoroughness, but these may add many results. Will monitor
-
54
(meta analy∗ or metaanaly∗ or health technolog∗ assess∗ or systematic review∗).hw. (542060)
-
55
((systematic∗ or methodologic∗) adj3 (review∗ or overview∗)).tw,kw. (420337)
-
56
(meta analy∗ or metaanaly∗ or met analy∗ or metanaly∗ or meta review∗ or metareview∗ or health technolog∗ assess∗ or HTA or HTAs or (technolog∗ adj (assessment∗ or overview∗ or appraisal∗))).tw,kw. (444705)
-
57
(evidence adj (review∗ or overview∗ or synthes#s)).tw,kw. (15949)
-
58
(review of reviews or overview of reviews).tw,kw. (1727)
-
59
umbrella review∗.tw,kw. (682)
-
60
((pool∗ adj3 analy∗) or published studies or published literature or hand search∗ or handsearch∗ or manual search∗ or ((database∗ or systematic∗) adj2 search∗) or reference list∗ or bibliograph∗ or relevant journals or data synthes∗ or data extraction∗ or data abstraction∗).tw,kw. (472993)
-
61
(medline or pubmed or medlars or embase or cinahl or web of science or ovid or ebsco∗ or scopus).ab. (447532)
-
62
cochrane.tw,kw. (189216)
-
63
(meta regress∗ or metaregress∗).tw,kw. (19929)
-
64
(((integrative or collaborative or quantitative) adj3 (review∗ or overview∗ or synthes∗)) or (research adj3 overview∗)).tw,kw. (32894)
-
65
(cochrane or (health adj2 technology assessment) or evidence report or systematic review∗).jw. (61535)
-
66
((comparative adj3 (efficacy or effectiveness)) or relative effectiveness or ((indirect or indirect treatment or mixed-treatment) adj comparison∗)).tw,kw. (47095)
-
67
or/53-66 (1258784)
-
68
52 and 67 (1201)
-
69
(exp animal/ or nonhuman/) not exp human/ (10406402)
-
70
68 not 69 (1200)
-
71
Case Report/ or Comment/ or Editorial/ or (letter.pt. not (letter.pt. and randomized controlled trial/)) or conference abstract.pt. (10708295)
-
72
70 not 71 (1074)
-
73
limit 72 to english language [Limit not valid in CDSR; records were retained] (987)
-
74
limit 73 to yr=“2014 −Current” (591)
-
75
74 use emez (263)
-
76
major depression/ (176381)
-
77
treatment resistant depression/ or dysthymic disorder/ (14469)
-
78
(depressi∗ or TRD or MDD or dysthymi∗ or melancholia∗ or involutional psychos∗ or unipolar∗).ti,ab,id. (1132101)
-
79
exp bipolar disorder/ (127394)
-
80
(bipolar∗ or (manic∗ adj2 (depress∗ or disorder∗ or psychos#s or state∗))).ti,ab,id. (194343)
-
81
or/76-80 (1293678)
-
82
transcranial magnetic stimulation/ (39531)
-
83
(((transcranial∗ or trans-cranial∗) adj2 magnetic∗) or TMS or rTMS or aTMS or pTMS or sTMS or dTMS).ti,ab,id. (62571)
-
84
((theta∗ adj3 stimul∗) or TBS or cTBS or iTBS or blTBS).ti,ab,id. (11623)
-
85
(coil∗ adj2 (double cone∗ or figure or butterfly or H)).ti,ab,id. (920)
-
86
(Deep TMS∗ or Magpro∗ or Magstim∗).ti,ab,id. (626)
-
87
or/82-86 (79328)
-
88
81 and 87 (9514)
-
89
(Systematic Review or Meta Analysis).md. (39451)
-
90
meta analysis/ (278192)
-
91
((systematic∗ or methodologic∗) adj3 (review∗ or overview∗)).ti,ab,id. (407496)
-
92
(meta analy∗ or metaanaly∗ or met analy∗ or metanaly∗ or meta review∗ or metareview∗ or health technolog∗ assess∗ or HTA or HTAs or (technolog∗ adj (assessment∗ or overview∗ or appraisal∗))).ti,ab,id. (415719)
-
93
(evidence adj (review∗ or overview∗ or synthes#s)).ti,ab,id. (15391)
-
94
(review of reviews or overview of reviews).ti,ab,id. (1512)
-
95
umbrella review∗.ti,ab,id. (626)
-
96
((pool∗ adj3 analy∗) or published studies or published literature or hand search∗ or handsearch∗ or manual search∗ or ((database∗ or systematic∗) adj2 search∗) or reference list∗ or bibliograph∗ or relevant journals or data synthes∗ or data extraction∗ or data abstraction∗).ti,ab. (439968)
-
97
(medline or pubmed or medlars or embase or cinahl or web of science or ovid or ebsco∗ or scopus).ab. (447532)
-
98
cochrane.ti,ab. (185561)
-
99
(meta regress∗ or metaregress∗).ti,ab. (18943)
-
100
(((integrative or collaborative or quantitative) adj3 (review∗ or overview∗ or synthes∗)) or (research adj3 overview∗)).ti,ab. (31591)
-
101
((comparative adj3 (efficacy or effectiveness)) or relative effectiveness or ((indirect or indirect treatment or mixed-treatment) adj comparison∗)).ti,ab. (44815)
-
102
or/89-101 (1142513)
-
103
88 and 102 (967)
-
104
case report/ or editorial.dt. or comment reply.dt. or letter.dt. (4542735)
-
105
103 not 104 (942)
-
106
limit 105 to english language [Limit not valid in CDSR; records were retained] (862)
-
107
limit 106 to yr=“2014 −Current” (531)
-
108
107 use psyb (111)
-
109
39 or 75 or 108 (557)
-
110
109 use medall (164)
-
111
109 use emez (263)
-
112
109 use psyb (111)
-
113
109 use coch (2)
-
114
109 use clhta (17)
-
115
109 use cleed (0)
-
116
remove duplicates from 109 (321)
Database: CINAHL
| S1 (MH “Depression”) | 94,826 |
| S2 (MH “Dysthymic Disorder”) | 408 |
| S3 (depressi∗ or TRD or MDD or dysthymi∗ or melancholia∗ or involutional psychos∗ or unipolar∗) | 154,701 |
| S4 (MH “Bipolar Disorder”) | 10,524 |
| S5 (bipolar∗ or (manic∗ N2 (depress∗ or disorder∗ or psychosis or psychoses or state∗))) | 16,547 |
| S6 S1 OR S2 OR S3 OR S4 OR S5 | 165,219 |
| S7 (MH “Transcranial Magnetic Stimulation”) | 926 |
| S8 (((transcranial∗ or trans-cranial∗) N2 magnetic∗) or TMS or rTMS or aTMS or pTMS or sTMS or dTMS) | 4,575 |
| S9 ((theta∗ N3 stimul∗) or TBS or cTBS or iTBS or blTBS) | 832 |
| S10 (coil∗ N2 (double cone∗ or figure or butterfly or H)) | 46 |
| S11 (Deep TMS∗ or Magpro∗ or Magstim∗) | 25 |
| S12 S7 OR S8 OR S9 OR S10 OR S11 | 5,319 |
| S13 S6 AND S12 | 624 |
| S14 (PT “Meta Analysis”) or (PT “Systematic Review”) | 86,930 |
| S15 (MH “Systematic Review”) OR (MH “Meta Analysis”) | 92,943 |
| S16 ((systematic∗ or methodologic∗) N3 (review∗ or overview∗)) | 117,327 |
| S17 (meta analy∗ or metaanaly∗ or met analy∗ or metanaly∗ or meta review∗ or metareview∗ or health technolog∗ assess∗ or HTA or HTAs or (technolog∗ N1 (assessment∗ or overview∗ or appraisal∗))) | 73,884 |
| S18 (evidence N2 (review∗ or overview∗ or synthes#s))) | 18,637 |
| S19 ((review or overview) N2 reviews) | 6,140 |
| S20 umbrella review∗ | 165 |
| S21 ((pool∗ N3 analy∗) or published studies or published literature or hand search∗ or handsearch∗ or manual search∗ or ((database∗ or systematic∗) N2 search∗) or reference list∗ or bibliograph∗ or relevant journals or data synthes∗ or data extraction∗ or data abstraction∗) | 77,356 |
| S22 AB(medline or pubmed or medlars or embase or cinahl or web of science or ovid or ebsco∗ or scopus) | 70,331 |
| S23 cochrane | 42,678 |
| S24 (meta regress∗ or metaregress∗) | 2,727 |
| S25 (((integrative or collaborative or quantitative) N3 (review∗ or overview∗ or synthes∗)) or (research N3 overview∗)) | 7,811 |
| S26 SO(cochrane or (health N2 technology assessment) or evidence report or systematic review∗) | 10,635 |
| S27 ((comparative N3 (efficacy or effectiveness)) or relative effectiveness or ((indirect or indirect treatment or mixed-treatment) N1 comparison∗)) | 6,770 |
| S28 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 | 224,192 |
| S29 S13 AND S28 | 97 |
| S30 PT(Case Study or Commentary or Editorial or Letter or Proceedings) | 974,123 |
| S31 S29 NOT S30 | 90 |
| S32 Narrow by Language: – english | 89 |
| S33 Limiters – Published Date: 20140101-20191231 | 61 |
Economic Evidence Search
Search date: August 27, 2019
Databases searched: Medline, Embase, Cochrane Database of Systematic Reviews, CRD Health Technology Assessment, NHS Economic Evaluation, PsycINFO, CINAHL
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <July 2019>, EBM Reviews −Cochrane Database of Systematic Reviews <2005 to August 21, 2019>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2019 Week 34>, Ovid MEDLINE(R) ALL <1946 to August 26, 2019>, PsycINFO <1967 to August Week 3 2019>
Search strategy:
-
1
Depression/ (483870)
-
2
exp Depressive Disorder/ (553103)
-
3
(depressi∗ or TRD or MDD or dysthymi∗ or melancholia∗ or involutional psychos∗ or unipolar∗).ti,ab,kf. (1197380)
-
4
Bipolar Disorder/ (117095)
-
5
(bipolar∗ or (manic∗ adj2 (depress∗ or disorder∗ or psychos#s or state∗))).ti,ab,kf. (201948)
-
6
or/1-5 (1511599)
-
7
Transcranial Magnetic Stimulation/ (40803)
-
8
(((transcranial∗ or trans-cranial∗) adj2 magnetic∗) or TMS or rTMS or aTMS or pTMS or sTMS or dTMS).ti,ab,kf. (67716)
-
9
((theta∗ adj3 stimul∗) or TBS or cTBS or iTBS or blTBS).ti,ab,kf. (12503)
-
10
(coil∗ adj2 (double cone∗ or figure or butterfly or H)).ti,ab,kf. (1100)
-
11
(Deep TMS∗ or Magpro∗ or Magstim∗).ti,ab,kf. (764)
-
12
or/7-11 (85238)
-
13
6 and 12 (11449)
-
14
economics/ (274137)
-
15
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (834389)
-
16
economics.fs. (422791)
-
17
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).ti,ab,kf. (1044753)
-
18
exp “costs and cost analysis”/ (621567)
-
19
(cost or costs or costing or costly).ti. (278724)
-
20
cost effective∗.ti,ab,kf. (341933)
-
21
(cost∗ adj2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kf. (230766)
-
22
models, economic/ (12786)
-
23
markov chains/ or monte carlo method/ (82688)
-
24
(decision adj1 (tree∗ or analy∗ or model∗)).ti,ab,kf. (46598)
-
25
(markov or markow or monte carlo).ti,ab,kf. (137002)
-
26
quality-adjusted life years/ (40147)
-
27
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (79625)
-
28
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).ti,ab,kf. (127445)
-
29
or/14-28 (2773989)
-
30
13 and 29 (355)
-
31
30 use medall,cctr,coch,clhta (99)
-
32
13 use cleed (5)
-
33
31 or 32 (104)
-
34
exp Animals/ not Humans/ (17764795)
-
35
33 not 34 (104)
-
36
Case Reports/ or Comment.pt. or Editorial.pt. or (Letter not (Letter and Randomized Controlled Trial)).pt. or Congress.pt. (5319195)
-
37
35 not 36 (102)
-
38
limit 37 to english language [Limit not valid in CDSR; records were retained] (86)
-
39
limit 38 to yr=“2014 −Current” (45)
-
40
depression/ (483870)
-
41
treatment resistant depression/ or major depression/ or chronic depression/ or depressive psychosis/ or dysthymia/ or melancholia/ or involutional depression/ (298340)
-
42
(depressi∗ or TRD or MDD or dysthymi∗ or melancholia∗ or involutional psychos∗ or unipolar∗).tw,kw. (1220668)
-
43
exp bipolar disorder/ (129784)
-
44
(bipolar∗ or (manic∗ adj2 (depress∗ or disorder∗ or psychos#s or state∗))).tw,kw. (205526)
-
45
or/40-44 (1528701)
-
46
exp transcranial magnetic stimulation/ (42499)
-
47
(((transcranial∗ or trans-cranial∗) adj2 magnetic∗) or TMS or rTMS or aTMS or pTMS or sTMS or dTMS).tw,kw,dv. (69112)
-
48
((theta∗ adj3 stimul∗) or TBS or cTBS or iTBS or blTBS).tw,kw,dv. (12582)
-
49
(coil∗ adj2 (double cone∗ or figure or butterfly or H)).tw,kw,dv. (1119)
-
50
(Deep TMS∗ or Magpro∗ or Magstim∗).tw,kw,dv. (1630)
-
51
or/46-50 (86242)
-
52
45 and 51 (11751)
-
53
Economics/ (274137)
-
54
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (130267)
-
55
Economic Aspect/ or exp Economic Evaluation/ (456004)
-
56
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).tw,kw. (1075543)
-
57
exp “Cost”/ (581562)
-
58
(cost or costs or costing or costly).ti. (278724)
-
59
cost effective∗.tw,kw. (354755)
-
60
(cost∗ adj2 (util∗ or efficac∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kw. (241929)
-
61
Monte Carlo Method/ (64654)
-
62
(decision adj1 (tree∗ or analy∗ or model∗)).tw,kw. (50755)
-
63
(markov or markow or monte carlo).tw,kw. (142440)
-
64
Quality-Adjusted Life Years/ (40147)
-
65
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (83540)
-
66
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).tw,kw. (148320)
-
67
or/53-66 (2396633)
-
68
52 and 67 (387)
-
69
68 use emez (202)
-
70
(exp animal/ or nonhuman/) not exp human/ (10406417)
-
71
69 not 70 (201)
-
72
Case Report/ or Comment/ or Editorial/ or (letter.pt. not (letter.pt. and randomized controlled trial/)) or conference abstract.pt. (10731956)
-
73
71 not 72 (142)
-
74
limit 73 to english language [Limit not valid in CDSR; records were retained] (135)
-
75
limit 74 to yr=“2014 −Current” (50)
-
76
major depression/ (176385)
-
77
treatment resistant depression/ or dysthymic disorder/ (14967)
-
78
(depressi∗ or TRD or MDD or dysthymi∗ or melancholia∗ or involutional psychos∗ or unipolar∗).ti,ab,id. (1198165)
-
79
exp bipolar disorder/ (129784)
-
80
(bipolar∗ or (manic∗ adj2 (depress∗ or disorder∗ or psychos#s or state∗))).ti,ab,id. (201663)
-
81
or/76-80 (1364171)
-
82
transcranial magnetic stimulation/ (40803)
-
83
(((transcranial∗ or trans-cranial∗) adj2 magnetic∗) or TMS or rTMS or aTMS or pTMS or sTMS or dTMS).ti,ab,id. (67482)
-
84
((theta∗ adj3 stimul∗) or TBS or cTBS or iTBS or blTBS).ti,ab,id. (12361)
-
85
(coil∗ adj2 (double cone∗ or figure or butterfly or H)).ti,ab,id. (1097)
-
86
(Deep TMS∗ or Magpro∗ or Magstim∗).ti,ab,id. (764)
-
87
or/82-86 (84796)
-
88
81 and 87 (10796)
-
89
economics/ or economy/ (371796)
-
90
pharmacoeconomics/ or health care economics/ (191088)
-
91
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).tw. (1048656)
-
92
exp “costs and cost analysis”/ (621567)
-
93
cost∗.ti. (300004)
-
94
cost effective∗.tw. (349246)
-
95
(cost∗ adj2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab. (228597)
-
96
markov chains/ (21448)
-
97
(decision adj1 (tree∗ or analy∗ or model∗)).tw. (49602)
-
98
(markov or markow or monte carlo).tw. (139545)
-
99
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (82774)
-
100
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).tw. (145619)
-
101
or/89-100 (2317560)
-
102
88 and 101 (286)
-
103
102 use psyb (34)
-
104
case report/ or editorial.dt. or comment reply.dt. or letter.dt. (4542739)
-
105
103 not 104 (30)
-
106
limit 105 to english language [Limit not valid in CDSR; records were retained] (26)
-
107
limit 106 to yr=“2014 −Current” (16)
-
108
39 or 75 or 107 (111)
-
109
108 use medall (31)
-
110
108 use emez (50)
-
111
108 use cctr (10)
-
112
108 use coch (0)
-
113
108 use clhta (3)
-
114
108 use cleed (1)
-
115
108 use psyb (16)
-
116
remove duplicates from 108 (70)
Database: CINAHL
| S1 (MH “Depression”) | 94,826 |
| S2 (MH “Dysthymic Disorder”) | 408 |
| S3 (depressi∗ or TRD or MDD or dysthymi∗ or melancholia∗ or involutional psychos∗ or unipolar∗) | 154,701 |
| S4 (MH “Bipolar Disorder”) | 10,524 |
| S5 (bipolar∗ or (manic∗ N2 (depress∗ or disorder∗ or psychosis or psychoses or state∗))) | 16,547 |
| S6 S1 OR S2 OR S3 OR S4 OR S5 | 165,219 |
| S7 (MH “Transcranial Magnetic Stimulation”) | 926 |
| S8 (((transcranial∗ or trans-cranial∗) N2 magnetic∗) or TMS or rTMS or aTMS or pTMS or sTMS or dTMS) | 4,575 |
| S9 ((theta∗ N3 stimul∗) or TBS or cTBS or iTBS or blTBS) | 832 |
| S10 (coil∗ N2 (double cone∗ or figure or butterfly or H)) | 46 |
| S11 (Deep TMS∗ or Magpro∗ or Magstim∗) | 25 |
| S12 S7 OR S8 OR S9 OR S10 OR S11 | 5,319 |
| S13 S6 AND S12 | 624 |
| S14 (MH “Economics”) | 12,749 |
| S15 (MH “Economic Aspects of Illness”) | 8,582 |
| S16 (MH “Economic Value of Life”) | 592 |
| S17 MH “Economics, Dental” | 121 |
| S18 MH “Economics, Pharmaceutical” | 2,046 |
| S19 MW “ec” | 165,569 |
| S20 (econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗) | 264,105 |
| S21 (MH “Costs and Cost Analysis+”) | 105,152 |
| S22 TI cost∗ | 48,650 |
| S23 (cost effective∗) | 36,692 |
| S24 AB (cost∗ N2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)) | 28,154 |
| S25 (decision N1 (tree∗ or analy∗ or model∗)) | 7,320 |
| S26 (markov or markow or monte carlo) | 5,147 |
| S27 (MH “Quality-Adjusted Life Years”) | 4,047 |
| S28 (QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs) | 9,959 |
| S29 ((adjusted N1 (quality or life)) or (willing∗ N2 pay) or sensitivity analys?s) | 15,967 |
| S30 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 | 357,446 |
| S31 S13 AND S30 | 28 |
| S32 PT(Case Study or Commentary or Editorial or Letter or Proceedings) | 974,123 |
| S33 S31 not S32 | 28 |
| S34 Narrow by Language: – english | 28 |
| S35 Limiters – Published Date: 20140101-20191231 | 10 |
Quantitative Evidence of Preferences and Values Search
Search date: September 06, 2019
Databases searched: MEDLINE, CINAHL
Database: Ovid MEDLINE(R) ALL <1946 to September 05, 2019>
Search strategy:
-
1
Depression/ (111339)
-
2
exp Depressive Disorder/ (104998)
-
3
(depressi∗ or TRD or MDD or dysthymi∗ or melancholia∗ or involutional psychos∗ or unipolar∗).ti,ab,kf. (374907)
-
4
Bipolar Disorder/ (39073)
-
5
(bipolar∗ or (manic∗ adj2 (depress∗ or disorder∗ or psychos#s or state∗))).ti,ab,kf. (65407)
-
6
or/1-5 (468297)
-
7
Transcranial Magnetic Stimulation/ (10627)
-
8
(((transcranial∗ or trans-cranial∗) adj2 magnetic∗) or TMS or rTMS or aTMS or pTMS or sTMS or dTMS).ti,ab,kf. (22745)
-
9
((theta∗ adj3 stimul∗) or TBS or cTBS or iTBS or blTBS).ti,ab,kf. (4104)
-
10
(coil∗ adj2 (double cone∗ or figure or butterfly or H)).ti,ab,kf. (284)
-
11
(Deep TMS∗ or Magpro∗ or Magstim∗).ti,ab,kf. (165)
-
12
or/7-11 (27555)
-
13
6 and 12 (2741)
-
14
Attitude to Health/ (81990)
-
15
Health Knowledge, Attitudes, Practice/ (104872)
-
16
Patient Participation/ (24331)
-
17
Patient Preference/ (7449)
-
18
Attitude of Health Personnel/ (116878)
-
19
∗Professional-Patient Relations/ (11250)
-
20
∗Physician-Patient Relations/ (34464)
-
21
Choice Behavior/ (31237)
-
22
(choice or choices or value∗ or valuation∗ or knowledg∗).ti. (250823)
-
23
(preference∗ or expectation∗ or attitude∗ or acceptab∗ or point of view).ti,ab. (548761)
-
24
((patient∗1 or user∗1 or men or women or personal or provider∗ or practitioner∗ or professional∗1 or (health∗ adj2 worker∗) or clinician∗ or physician∗ or doctor∗ or psychiatrist∗ or nurs∗) adj2 (participation or perspective∗ or perception∗ or misperception∗ or perceiv∗ or view∗ or understand∗ or misunderstand∗ or value∗1 or knowledg∗)).ti,ab. (149822)
-
25
health perception∗.ti,ab. (2568)
-
26
∗Decision Making/ (39882)
-
27
(patient∗1 or user∗1 or men or women or personal or provider∗ or practitioner∗ or professional∗1 or (health∗ adj2 worker∗) or clinician∗ or physician∗ or doctor∗ or psychiatrist∗ or nurs∗).ti. (2547771)
-
28
26 and 27 (8361)
-
29
(decision∗ and mak∗).ti. (27093)
-
30
(decision mak∗ or decisions mak∗).ti,ab. (130127)
-
31
29 or 30 (131604)
-
32
(patient∗1 or user∗1 or men or women or personal or provider∗ or practitioner∗ or professional∗1 or (health∗ adj2 worker∗) or clinician∗ or physician∗ or doctor∗ or psychiatrist∗ or nurs∗).ti,ab. (7939057)
-
33
31 and 32 (83873)
-
34
(discrete choice∗ or decision board∗ or decision analy∗ or decision-support or decision tool∗ or decision aid∗ or latent class∗ or decision∗ conflict∗ or decision∗ regret∗).ti,ab. (31406)
-
35
Decision Support Techniques/ (19188)
-
36
(health and utilit∗).ti. (1379)
-
37
(gamble∗ or prospect theory or health utilit∗ or utility value∗ or utility score∗ or utility estimate∗ or health state or feeling thermometer∗ or best-worst scaling or time trade-off or TTO or probability trade-off).ti,ab. (12361)
-
38
(preference based or preference score∗ or preference elicitation or multiattribute or multi attribute).ti,ab. (2594)
-
39
or/14-25,28,33-38 (1225312)
-
40
13 and 39 (116)
-
41
limit 40 to english language (108)
Database: CINAHL
| # Query | Results |
| S1 (MH “Depression”) | 94,959 |
| S2 (MH “Dysthymic Disorder”) | 408 |
| S3 (depressi∗ or TRD or MDD or dysthymi∗ or melancholia∗ or involutional psychos∗ or unipolar∗) | 154,933 |
| S4 (MH “Bipolar Disorder”) | 10,535 |
| S5 (bipolar∗ or (manic∗ N2 (depress∗ or disorder∗ or psychosis or psychoses or state∗))) | 16,569 |
| S6 S1 OR S2 OR S3 OR S4 OR S5 | 165,466 |
| S7 (MH “Transcranial Magnetic Stimulation”) | 929 |
| S8 (((transcranial∗ or trans-cranial∗) N2 magnetic∗) or TMS or rTMS or aTMS or pTMS or sTMS or dTMS) | 4,588 |
| S9 ((theta∗ N3 stimul∗) or TBS or cTBS or iTBS or blTBS) | 835 |
| S10 (coil∗ N2 (double cone∗ or figure or butterfly or H)) | 46 |
| S11 (Deep TMS∗ or Magpro∗ or Magstim∗) | 25 |
| S12 S7 OR S8 OR S9 OR S10 OR S11 | 5,335 |
| S13 S6 AND S12 | 624 |
| S14 (MH “Attitude to Health”) | 39,968 |
| S15 (MH “Health Knowledge”) | 25,846 |
| S16 (MH “Consumer Participation”) | 17,668 |
| S17 (MH “Patient Preference”) | 364 |
| S18 (MH “Attitude of Health Personnel”) | 38,879 |
| S19 (MM “Professional-Patient Relations”) | 11,823 |
| S20 (MM “Physician-Patient Relations”) | 14,461 |
| S21 (MM “Nurse-Patient Relations”) | 13,271 |
| S22 TI (choice or choices or value∗ or valuation∗ or knowledg∗) | 84,554 |
| S23 (preference∗ or expectation∗ or attitude∗ or acceptab∗ or point of view) | 380,449 |
| S24 ((patient or patients or user or users men or women or personal or provider∗ or practitioner∗ or professional or professionals or (health∗ N2 worker∗) or clinician∗ or physician∗ or doctor∗ or psychiatrist∗ or nurs∗) N2 (participation or perspective∗ or perception∗ or misperception∗ or perceiv∗ or view∗ or understand∗ or misunderstand∗ or value or values or knowledg∗)) | 700,010 |
| S25 health perception∗ | 3,745 |
| S26 (MH “Decision Making, Shared”) | 1,019 |
| S27 (MH “Decision Making, Patient”) | 13,531 |
| S28 (MH “Decision Making, Family”) | 3,637 |
| S29 (MM “Decision Making”) | 20,154 |
| S30 TI (patient or patients or user or users men or women or personal or provider∗ or practitioner∗ or professional or professionals or (health∗ N2 worker∗) or clinician∗ or physician∗ or doctor∗ or psychiatrist∗ or nurs∗) | 1,104,810 |
| S31 S29 AND S30 | 3,818 |
| S32 TI (decision∗ and mak∗) | 15,678 |
| S33 (decision mak∗ or decisions mak∗) | 131,692 |
| S34 S32 OR S33 | 131,893 |
| S35 (patient or patients or user or users or men or women or personal or provider∗ or practitioner∗ or professional or professionals or (health∗ N2 worker∗) or clinician∗ or physician∗ or doctor∗ or psychiatrist∗ or nurs∗) | 3,039,213 |
| S36 S34 AND S35 | 91,893 |
| S37 (discrete choice∗ or decision board∗ or decision analy∗ or decision support or decision tool∗ or decision aid∗ or latent class∗ or decision∗ conflict∗ or decision∗ regret∗) | 23,830 |
| S38 (MH “Decision Support Techniques”) | 5,827 |
| S39 TI (health and utilit∗) | 818 |
| S40 (gamble∗ or prospect theory or health utilit∗ or utility value∗ or utility score∗ or utility estimate∗ or health state or feeling thermometer∗ or best worst scaling or time trade off or TTO or probability trade off) | 14,265 |
| S41 (preference based or preference score∗ or preference elicitation or multiattribute or multi attribute) | 1,296 |
| S42 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S31 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 | 1,026,020 |
| S43 S13 AND S42 | 114 |
| S44 Narrow by Language: – english | 112 |
Grey Literature Search
Search Date: August 30, 2019; updated January 2, 2020
Websites searched:
HTA Database Canadian Repository, Alberta Health Evidence Reviews, BC Health Technology Assessments, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, Centre Hospitalier de l'Universite de Quebec-Universite Laval, Health Technology Assessment Database, Epistemonikos, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Council of Australian Governments Health Technologies, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, Health Technology Wales, Oregon Health Authority Health Evidence Review Commission, Veterans Affairs Health Services Research and Development, Italian National Agency for Regional Health Services (AGENAS), Australian Safety and Efficacy Register of New Interventional Procedures −Surgical (ASERNIP-S), Belgian Health Care Knowledge Centre, Ludwig Boltzmann Institute for Health Technology Assessment, Ministry of Health Malaysia Health Technology Assessment Section, Swedish Agency for Health Technology Assessment and Assessment of Social Services, PROSPERO, EUnetHTA, Tuft's Cost-Effectiveness Analysis Registry
Keywords used: transcranial AND magnetic, rTMS, TMS, aTMS, pTMS, sTMS, dTMS, theta burst, theta bursts, TBS, cTBS, iTBS, blTBS, (resistant AND depression), TRD, (major AND depression), coil, bipolar
Results from clinical search: (included in PRISMA): 17
Results from economic search: (included in PRISMA): 13
Ongoing systematic reviews (PROSPERO/EUnetHTA): 7
Results from search update: 0
Appendix 2: Critical Appraisal of Clinical Evidence
Table A1:
Risk of Biasa Among Systematic Reviews (ROBIS Tool)
| Author, Year | Phase 2 | Phase 3 | |||
|---|---|---|---|---|---|
| Study Eligibility Criteria | Identification and Selection of Studies | Data Collection and Study Appraisal | Synthesis and Findings | Risk of Bias in Review | |
| Lepping et al, 201442 | Low | High | High | High | High |
| Leggett et al, 201540 | Low | Low | Low | Low | Low |
| Zhang et al, 201543 | Low | Low | Low | Low | Low |
| Health Quality Ontario, 201618 | Low | Low | Low | Low | Low |
| Nordenskjold et al, 201644 | Low | Low | Unclear | Low | Low |
| Berlim et al, 201745 | Low | Low | High | Low | Low |
| Brunoni et al, 201746 | High | Low | Low | High | High |
| University of Calgary, 201734 | Low | Low | Low | Low | Low |
| Mutz et al, 201835 | Low | High | Low | Low | Low |
| Sehatzadeh et al, 201941 | Low | Low | Low | Low | Low |
Abbreviation: ROBIS, Risk of Bias in Systematic Reviews.
Possible risk of bias levels: low, high, unclear.
Table A2:
Risk of Biasa Among Randomized Controlled Trials (Cochrane Risk-of-Bias Tool)
| Author, Year | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias | SR Source |
|---|---|---|---|---|---|---|---|---|
| rTMS vs. Another rTMS Modality or Sham Treatment | ||||||||
| Aguirre et al, 201170 | Unclear | Unclear | Unclear | Low | Low | Low | NR | Brunoni et al, 201746 |
| Anderson et al, 200778 | Unclear | Low | Unclear | Low | Low | Low | NR | Brunoni et al, 201746 |
| Avery et al, 199979 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | Mutz et al, 201835 |
| Avery et al, 200680 | Low | Unclear | Low | Unclear | Low | Low | Unclear | Mutz et al, 201835 |
| Baeken et al, 201352 | Low | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Bakim et al, 201273 | Low | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Bares et al, 200999 | Low | Low | Low | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Berman et al, 200081 | Unclear | Unclear | Low | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Blumberger et al, 201283 | Low | Unclear | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Blumberger et al, 201682 | Low | Unclear | Low | Low | Low | Low | NR | Brunoni et al, 201746 |
| Bortolomasi et al, 2007127 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Boutros et al, 2002186 | Low | Unclear | High | Low | Low | Low | High | Mutz et al, 201835 |
| Bretlau et al, 200884 | Unclear | Unclear | Low | Unclear | Low | Low | Unclear | University of Calgary, 201734 |
| Brunelin et al, 2014116 | Low | Low | Unclear | Low | Low | Low | NR | Brunoni et al, 201746 |
| Chen et al, 201385 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | Mutz et al, 201835 |
| Chistyakov et al, 2015122 | Unclear | Unclear | Low | Low | Low | Low | Unclear | NA |
| Conca et al, 2002187 | Low | High | Low | Low | Unclear | Unclear | NR | Zhang et al, 201543b |
| Concerto et al, 201569 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | Mutz et al, 201835 |
| Dell'Osso et al, 2015188 | Unclear | Unclear | High | Unclear | High | Low | NR | Brunoni et al, 201746 |
| Duprat et al, 2016124 | Low | Unclear | Unclear | Unclear | Low | Low | Unclear | Mutz et al, 201835 |
| Eche et al, 2012189 | Unclear | Unclear | High | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Eschweiler et al, 200053 | Unclear | Unclear | Low | Low | Low | Low | NR | Brunoni et al, 201746 |
| Fitzgerald et al, 200386 | Unclear | Low | Low | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Fitzgerald et al, 2006a107 | Low | Low | Low | Low | Low | Low | Low | Mutz et al, 201835 |
| Fitzgerald et al, 2006b106 | Low | Unclear | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Fitzgerald et al, 2007190 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Fitzgerald et al, 2008191 | Low | Unclear | Unclear | Low | Low | Low | NR | Brunoni et al, 201746 |
| Fitzgerald et al, 2009136 | Low | Low | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Fitzgerald et al, 2011192 | Low | Unclear | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Fitzgerald et al, 201287 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Fitzgerald et al, 2013193 | Low | Unclear | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Fitzgerald et al, 2016119 | Low | Unclear | Low | Low | Low | Low | NR | Brunoni et al, 201746 |
| Garcia-Toro et al, 200188 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Garcia-Toro et al, 2006108 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | University of Calgary, 201734 |
| George et al, 201089 | Low | Unclear | Low | Low | Low | Low | Low | Mutz et al, 201835 |
| Hernandez-Ribas et al, 201390 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Herwig et al, 200791 | Low | Unclear | Unclear | Low | Low | Low | NR | Brunoni et al, 201746 |
| Holtzheimer et al, 200492 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Hoppner et al, 2003112 | Low | High | Unclear | Low | Low | Low | NR | Health Quality Ontario, 201618 |
| Isenberg et al, 2005194 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Januel et al, 2006101 | Low | Unclear | Unclear | Low | High | Low | NR | Brunoni et al, 201746 |
| Jorge et al, 200457 | Unclear | Unclear | Low | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Jorge et al, 200856 | Unclear | Unclear | Low | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Karamustafalioglu et al, 201055 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | NR | Brunoni et al, 201746 |
| Kauffmann et al, 2004102 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Kimbrell et al, 199954 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Klein et al, 199958 | Low | Unclear | Unclear | Low | Low | Low | NR | Brunoni et al, 201746 |
| Krstic et al, 2014103 | Unclear | Unclear | Unclear | Low | Low | Low | NR | Brunoni et al, 201746 |
| Levkovitz et al, 2015126 | Low | Low | Low | Low | Low | Low | Low | Mutz et al, 201835 |
| Li et al, 2014109 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Lisanby et al, 200967 | Unclear | Unclear | Low | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Loo et al, 199968 | Unclear | Unclear | Low | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Loo et al, 2003120 | Unclear | Unclear | Low | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Loo et al, 200793 | Unclear | Unclear | Low | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Manes et al, 200160 | Unclear | Unclear | Low | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Mantovani et al, 2013104 | Unclear | Unclear | Low | Low | Low | Low | Unclear | University of Calgary, 201734 |
| McDonald et al, 2006121 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Miniussi et al, 200561 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Mogg et al, 2008113 | Low | Low | Low | Low | Low | Low | Low | Health Quality Ontario, 201618 |
| Mosimann et al, 200494 | Unclear | Unclear | Unclear | Low | Low | Low | NR | Brunoni et al, 201746 |
| Moller et al, 200662 | Low | Low | Low | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Moser et al, 200263 | Unclear | Unclear | Low | Low | Low | Low | Unclear | University of Calgary, 201734 |
| O'Reardon et al, 200795 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Pallanti et al, 2010117 | Low | Low | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Paillere Martinot et al, 201096 | Low | Low | Low | Low | Low | Low | Low | Mutz et al, 201835 |
| Peng et al, 201297 | Unclear | Unclear | Low | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Plewnia et al, 2014125 | Low | Unclear | Low | Unclear | Low | Low | Low | NA |
| Prasser et al, 201559 | Unclear | Unclear | Unclear | Low | Low | Low | NR | Brunoni et al, 201746 |
| Padberg et al, 199964 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Padberg et al, 2002111 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Pascual-Leone et al, 199665 | Unclear | Unclear | Unclear | Low | Low | High | Unclear | University of Calgary, 201734 |
| Richieri et al, 2012195 | High | Unclear | Unclear | High | Low | Low | Unclear | University of Calgary, 201734 |
| Rossini et al, 2005114 | Low | Unclear | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Rossini et al, 2010137 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Rybak et al, 2005196 | Unclear | High | Low | Low | Unclear | Unclear | NR | Zhang et al, 201543b |
| Speer et al, 200966 | Unclear | Unclear | Unclear | Low | Low | High | Unclear | University of Calgary, 201734 |
| Speer et al, 2014105 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Stern et al, 200777 | Unclear | Unclear | Low | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Su et al, 200574 | Unclear | Unclear | Unclear | Low | Low | Low | Unclear | Mutz et al, 201835 |
| Tavares et al, 2017110 | Low | Low | Low | Low | Low | Low | Low | Mutz et al, 201835 |
| Taylor et al, 2018115 | Low | Low | High | Low | High | Low | High | Mutz et al, 201835 |
| Theleritis et al, 201775 | Low | Low | Low | Low | Low | Low | Low | Mutz et al, 201835 |
| Triggs et al, 201076 | Low | Unclear | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Zhang et al, 201198 | Low | Unclear | Unclear | Low | Low | Low | NR | Brunoni et al, 201746 |
| Zheng et al, 201072 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | Mutz et al, 201835 |
| Zheng et al, 201571 | Unclear | Unclear | Unclear | Low | Low | Low | NR | Brunoni et al, 201746 |
| rTMS vs. ECT | ||||||||
| Dannon et al, 2002135 | Unclear | Unclear | High | Low | Low | Low | Unclear | Health Quality Ontario, 201618 |
| Eranti et al, 2007128 | Low | Low | High | Low | Low | Low | Unclear | Health Quality Ontario, 201618 |
| Grunhaus et al, 2000129 | Unclear | Low | High | Low | Low | Low | Unclear | Health Quality Ontario, 201618 |
| Grunhaus et al, 2003130 | Low | Unclear | High | Low | Unclear | Low | Unclear | University of Calgary, 201734 |
| Janicak et al, 2002133 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | University of Calgary, 201734 |
| Keshtkar et al, 2011132 | Low | Unclear | High | High | Low | Low | Unclear | University of Calgary, 201734 |
| Pridmore, 2000a197 | Unclear | Unclear | Low | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Pridmore et al, 2000b131 | High | High | High | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Rosa et al, 2006134 | Low | Unclear | Unclear | Low | Low | Low | Unclear | University of Calgary, 201734 |
| Wang et al, 2004198 | Low | Unclear | Unclear | Unclear | Low | Low | Unclear | NA |
| RCTs From CADTH 2019 (rTMS Vs. Sham) | ||||||||
| Kang et al, 201647 | Unclear | Low | Low | Low | Low | Low | Unclear | NA |
| Kaster et al, 201848 | Low | Low | Low | Low | Low | Low | Unclear | NA |
| Valkonen-Korhonen et al, 201849 | Low | Low | Low | Unclear | Low | Low | Unclear | NA |
| Yesavage et al, 201850 | Low | Low | Low | Low | Low | Low | Unclear | NA |
Abbreviations: CADTH, Canadian Agency for Drugs and Technologies in Health; ECT, electroconvulsive therapy; NA, not applicable; NR, not reported; RCT, randomized controlled trial; rTMS, repetitive transcranial magnetic stimulation; SR, systematic review.
Possible risk of bias levels: low, high, and unclear.
Used another tool to assess risk of bias. Translated results for Cochrane Risk of Bias tool.
Table A3:
GRADE Evidence Profile for the Comparison of rTMS and Sham Treatment
| No. of Studies (Design) | Risk of Biasa | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| HF Left DLPFC | |||||||
| Change in Depression Score | |||||||
| 30 (RCTs) | No serious limitations | Serious limitations (−1)b | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Response Rate | |||||||
| 31 (RCTs) | No serious limitations | Serious limitations (−1)b | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Remission Rate | |||||||
| 17 (RCTs) | No serious limitations | No serious limitations | Serious limitations (−1)c | No serious limitations | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| LF Right DLPFC | |||||||
| Change in Depression Score | |||||||
| 8 (RCTs) | No serious limitations | Serious limitations (−1)b | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Response Rate | |||||||
| 10 (RCTs) | No serious limitations | Serious limitations (−1)b | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Remission Rate | |||||||
| 7 (RCTs) | No serious limitations | No serious limitations | Serious limitations (−1)c | No serious limitations | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Bilateral | |||||||
| Change in Depression Score | |||||||
| 6 (RCTs) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕⊕ High |
| Response Rate | |||||||
| 10 (RCTs) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕⊕ High |
| Remission Rate | |||||||
| 7 (RCTs) | No serious limitations | No serious limitations | Serious limitations (−1)c | No serious limitations | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| cTBS | |||||||
| Change in Depression Score | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)d | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Response Rate | |||||||
| 2 (RCTs) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)d | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| iTBS | |||||||
| Change in Depression Score | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)d | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Response Rate | |||||||
| 2 (RCTs) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)e | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Remission Rate | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | Serious limitations (−1)f | No serious limitations | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Bilateral TBS | |||||||
| Change in Depression Score | |||||||
| 1 (RCTs) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)d | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Response Rate | |||||||
| 2 (RCTs) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕ High |
| Remission Rate | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕⊕ High |
| Deep TMS | |||||||
| Change in Depression Score | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕⊕ High |
| Response Rate | |||||||
| 3 (RCTs) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕⊕ High |
| Remission Rate | |||||||
| 3 (RCTs) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕⊕ High |
| Overall rTMS Relapse Rate | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Overall rTMS Adverse Events | |||||||
| 31 (RCTs) | No serious limitations | No serious limitations | Serious limitations (−1)g | No serious limitations | Undetected | No other considerations | ⊕⊕⊕ Moderate |
Abbreviations: DLPFC, dorsolateral prefrontal cortex; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HF, high frequency; LF, low frequency; RCT, randomized controlled trial; rTMS, repetitive transcranial magnetic stimulation; TBS, theta burst stimulation; TMS, transcranial magnetic stimulation.
Many RCTs had low or unclear risk of bias; however, we thought that it did not warrant downgrading.
Substantial statistical heterogeneity (I2 > 50%).
Different cut-points used to define remission.
Wide range of scores or confidence intervals overlap both beneficial and non-beneficial treatment effects.
Wide confidence intervals.
ITBS was given at accelerated pace, which is unlike current clinical practice.
Adverse events were always secondary outcomes; not many events occurred; and adverse events were measured using scales or counts.
Table A4:
GRADE Evidence Profile for the Comparison of rTMS and ECT
| No. of Studies (Design) | Risk of Bias | Inconsistencya | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Change in Depression Scores | |||||||
| 4 (RCTs) | Serious limitations | No serious limitations | No serious limitations | No serious limitations | No serious limitations | NA | ⊕⊕⊕ Moderate |
| Remission Rate | |||||||
| 3 (RCTs) | Serious limitations | No serious limitations | No serious limitations | No serious limitations | No serious limitations | NA | ⊕⊕⊕ Moderate |
| Response Rate | |||||||
| 3 (RCTs) | Serious limitations | No serious limitations | No serious limitations | No serious limitations | No serious limitations | NA | ⊕⊕⊕ Moderate |
Abbreviations: ECT, electroconvulsive therapy; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; NA, not applicable; RCT, randomized controlled trial; rTMS, repetitive transcranial magnetic stimulation.
Heterogeneity in results was mostly due to different applications of ECT among studies.
No GRADE for comparison of one rTMS modality and another modality.
Appendix 3: Critical Appraisal of Quantitative Evidence of Preferences and Values
Table A5:
Critical Appraisal of Quantitative Evidence of Preferences and Values
| Author, Year | Purpose of Study Is the purpose of the study in relation to preferences clearly stated? | Respondents Are respondents similar to nonrespondents? | Explanation Are methods of assessing preferences clearly explained? | Findings Were all respondents included in reported findings and analysis of preference results? | Significance Were significance tests used to assess preference results? |
|---|---|---|---|---|---|
| Walter et al, 2001175 | No: to examine the experience, knowledge, and attitudes of recipients regarding rTMS treatment | Unclear: No assessment of differences between respondents and nonrespondents | Yes: questions and answers are listed in text, and mode of questions and scales used for answers are explained | Yes: all respondents who completed multiple preference questions were included in analysis | Yes/No: some questions were analyzed with P values and some were given as just proportions and counts |
| AlHadi et al, 2017176 | No: to assess psychiatrists’ knowledge of and attitudes toward rTMS and determine contributing factors | Unclear: No assessment of difference between respondents and nonrespondents | Yes: questions and answers are listed in tables, and mode of questions and scales used for answers are explained | Yes: all respondents who completed multiple preference questions were included in analysis | Yes/No: some questions were analyzed with P values and some were given as just proportions and counts |
| Bourla et al, 2020177 | No: to assess psychiatrists’ acceptance of rTMS by using four domains (usefulness, usability, ease, and risk) | Unclear: No assessment of differences between respondents and nonrespondents | Yes: questions and answers are listed in tables and text, and mode of questions and scales used for answers are explained | Yes: all respondents who completed multiple preference questions were included in analysis | Yes/No: some questions were analyzed with P values and some were given as just proportions and counts |
Abbreviation: rTMS, repetitive transcranial magnetic stimulation.
Appendix 4: Results from Systematic Reviews for Question 1
Change in Depression Score
Five systematic reviews reported on this outcome. All five systematic reviews used the Hamilton Depression Rating Scale at the end of treatment (variation in follow-up time) to determine change in depression score. Below are the results by repetitive transcranial magnetic stimulation (rTMS) modality.
Table A6:
Change in Depression Scores for rTMS Modalities Versus Sham Treatment
| Author, Year | No. of Studies (Sample Size) | Results | Conclusions |
|---|---|---|---|
| HF Left DLPFC | |||
| Lepping et al, 201442 | 10 (120) | MD 6.18 (SD 4.48, P = NR) I2 = NR Favouring rTMS |
“Our results confirm a statistical superiority of rTMS over sham rTMS in the treatment of TRD” |
| Health Quality Ontario, 201618 | 15 (NR) | WMD 2.31 (95% CI 1.19–3.42, P < .001) I2 = 19.8% Favouring rTMS SMD 0.33 (95% CI 0.17–0.5, P < .001) I2 = 14.7% |
“On average, rTMS reduced depression scores by about 2 points more than sham rTMS” |
| Sehatzadeh et al, 201941 | 18 (NR) | WMD 3.36 (95% CI 1.85–4.88, P = .00) I2 = 62.4% Favouring rTMS |
“Our study suggests that rTMS has moderate antidepressant effects and appears to be promising in the short-term treatment of patients with unipolar TRD” |
| Bilateral rTMS | |||
| Sehatzadeh et al, 201941 | 4 (NR) | WMD 2.67 (95% CI 0.83–4.51, P = .5) I2 = 0% No difference |
— |
| All TBS modalitiesa | |||
| Berlim et al, 201745 | 6 (118 + 103) | Hedge's g = 1.0 (95% CI 0.3–1.7, P = .003) I2 = 82% Favouring TBS Removal of two trials that contributed tolarge heterogeneity: Hedge's g = 0.5 (95% CI 0.1–0.8, P = .004) I2 = 0% Favouring TBS |
“Significant and large-sized different in outcome favouring the active procedure” |
| Deep TMS | |||
| Nordenskjold et al, 201644 | 1 (89 + 92) | Deep TMS −6.17 (−7.78 to −4.55) Sham deep TMS −3.94 (−5.58 to −2.29) P = .05 Favouring deep TMS |
“Scientific support for deep TMS is considered insufficient for TRD” |
Abbreviations: CI, confidence interval; cTBS, continuous theta burst stimulation; DLPFC, dorsolateral prefrontal cortex; HF, high frequency; iTBS, intermittent theta burst stimulation; MD, mean difference; NR, not reported; TMS, repetitive transcranial magnetic stimulation; SD, standard deviation; SMD, standardized mean difference; TBS, theta burst stimulation; TMS, transcranial magnetic stimulation; TRD, treatment-resistant depression; WMD, weighted mean difference.
Includes two unilateral cTBS, two unilateral iTBS, three bilateral TBS.
SUBGROUP ANALYSES
Health Quality Ontario in 201618 and Sehatzadeh et al in 201941 did many subgroup analyses on this outcome. The rTMS modality examined was high-frequency rTMS on the left dorsolateral prefrontal cortex. Researchers explored the difference of various technical parameters and the use of concomitant antidepressants. A frequency of 20 Hz was the only technical parameter that was significant; rTMS with concomitant antidepressants was also significant. Table A7 shows results for each subgroup analysis.
Table A7:
Subgroup Analyses for Change in Depression Score
| Author, Year | Subgroup | Results | |
|---|---|---|---|
| Sehatzadeh et al, 201941 | Antidepressants |
With medication (n = 14 studies) WMD 3.64 (95% CI 1.52–5.76, P = .00) I2 = 69.6% Favouring rTMS |
Without medication (n = 5 studies) WMD 2.47 (95% CI 0.90–4.05, P = .3) I2 = 15.3% No difference |
| Sehatzadeh et al, 201941 | Frequency |
20 Hz (n = 8 studies) WMD 6.05 (95% CI 2.46–9.64, P = .00) I2 = 77.5% Favouring rTMS |
< 20 Hz (n = 10 studies) WMD 2.11 (95% CI 1.10–3.12, P = .69) I2 = 0% Favouring rTMS |
| Health Quality Ontario, 201618 | Frequency |
20 Hz (n = 6 studies) WMD 4.96 (95% CI 1.15–8.76, P = .01) I2 = 58% |
10 Hz (n = 7 studies) WMD 1.93 (95% CI 0.74–3.12, P = .00) I2 = 0% < 10 Hz (n = 2 studies) WMD 2.03 (95% CI −2.07 to 6.12, P = .332) I2 = 31.8% |
| Sehatzadeh et al, 201941 | Intensity | > 100% MT (n = 7 studies) WMD 2.39 (95% CI 1.28–3.50, P = NR) I2 = 0% |
< 100% MT (n = 11 studies) WMD: 4.08 (95% CI 1.30–6.87, P = NR) I2 = 0% |
| Health Quality Ontario, 201618 | No. of sessions |
30 sessions (n = 2 studies) WMD 3.17 (95% CI −0.28 to 6.62, P = .072) I2 = 36.3% |
15–20 sessions (n = 5 studies) WMD 2.17 (95% CI 0.69–3.64, P = .004) I2 = 1.5% 10 sessions (n = 8 studies) WMD 2.81 (95% CI 0.07–5.56, P = .04) I2 = 39.5% |
| Sehatzadeh et al, 201941 | No. of sessions |
15–30 sessions (n = 9 studies) WMD: 3.51 (95% CI 1.43–5.59, P = NR) I2 = 75.2% |
< 15 sessions (n = 9 studies) WMD 3.09 (95% CI 0.74–5.44, P = NR) I2 = 36.5% |
| Health Quality Ontario, 201618 | No. of pulses | > 16,000 (n = 8 studies) WMD 2.22 (95% CI 1.11–3.33, P = .000) I2 = 0% |
10,000-16,000 (n = 4 studies) WMD 3.41 (95% CI −1.13 to 7.96, P = .141) I2 = 45.7% ≤ 10,000 (n = 3 studies) WMD 4.49 (95% CI −1.85 to 10.83, P = .165) I2 = 54.3% |
Abbreviations: CI, confidence interval; MT, motor threshold; NR, not reported; rTMS, repetitive transcranial magnetic stimulation; WMD, weighted mean difference.
Response Rate
Eight systematic reviews examined the response rate of any rTMS modality versus sham TMS. Response rates were always defined as ≥ 50% reduction in score on the depression score used. Below are the results by rTMS modality.
Table A8:
Response Rates for rTMS Modalities Versus Sham Treatment
| Author, Year | No. of Studies (Active + Sham TMS) | Results | Definition of Response Rate |
|---|---|---|---|
| All rTMS Modalitiesa | |||
| University of Calgary, 201734b | 31 (NR) | RR 2.35 (95% CI 1.70-3.25, P = .025) I2 = 36.1% Favouring rTMS |
≥ 50% reduction |
| HF Left DLPFC | |||
| Health Quality Ontario, 201618 | 18 (NR) | RR 1.72 (95% CI 1.1-2.62, P = .011) I2 = 46.4% Favouring rTMS |
≥ 50% reduction |
| Mutz et al, 201835 | 25 (566) | OR 4.31 (95% CI 2.66-6.99, P = NR) I2 = NR Favouring rTMS |
≥ 50% reduction |
| Sehatzadeh et al, 201941 | 17 (NR) | RR 2.00 (95% CI 1.26-3.19, P = .01) I2 = 50.4% Favouring rTMS NNT = 8 |
≥ 50% reduction |
| Bilateral rTMS | |||
| Zhang et al, 201543 | 7 (NR) | RR 3.29 (95% CI 1.69-6.38, P = .004) I2 = 0% Favouring rTMS |
≥ 50% reduction |
| Sehatzadeh et al, 201941 | 7 (NR) | RR 3.55 (95% CI 1.87-6.76, P = .86) I2 = 0% Favouring rTMS NNT = 6 |
≥ 50% reduction |
| All TBS modalitiesc | |||
| Berlim et al, 201745 | 6 (118 + 103) | OR 2.7 (95% CI 1.4-5.3, P = .005) I2 = 0% Favouring TBS |
≥ 50% reduction |
| iTBS | |||
| Mutz et al, 201835 | 2 (NR) | OR 4.70 (95% CI 1.14-19.38, P = NR) I2 = 0% Favouring iTBS |
≥ 50% reduction |
| cTBS | |||
| Mutz et al, 201835 | 1 (NR) | OR 1.63 (95% CI 0.23-11.46, P = NR) I2 = NR No difference |
≥ 50% reduction |
| Deep TMS | |||
| Nordenskjold et al, 201644 | 1 (89 + 92) | Deep TMS 37.0% Sham deep TMS 27.8% P = .03 Favouring deep TMS |
≥ 50% reduction |
| Mutz et al, 201835 | 2 (NR) | OR 1.69 (95% CI 1.00-2.85, P = NR) I2 = 0% Favouring deep TMS |
≥ 50% reduction |
Abbreviations: CI, confidence interval; cTBS, continuous theta burst stimulation; DLPFC, dorsolateral prefrontal cortex; HF, high frequency; iTBS, intermittent theta burst stimulation; NNT, number needed to treat; NR, not reported; OR, odds ratio; RR, risk ratio; rTMS, repetitive transcranial magnetic stimulation; TBS, theta burst stimulation; TMS, transcranial magnetic stimulation.
Includes high-frequency, low-frequency, and bilateral rTMS.
Same results as results of Leggett et al.40
Includes two unilateral cTBS, two unilateral iTBS, and three bilateral TBS.
One network meta-analysis46 conducted a sensitivity analysis on treatment-resistant patients comparing rTMS versus another rTMS modality and sham TMS on the outcome of response rate. The following strategies were more effective than sham TMS: priming rTMS (OR 5.70, 95% CI 2.86-11.35), bilateral rTMS (OR 5.21, 95% CI 3.27-8.30), high-frequency rTMS (OR 4.16, 95% CI 3.27-6.92), TBS (OR 3.12, 95% CI 1.14-8.55), accelerated rTMS (OR 2.25, 95% CI 0.17-29.77), and low-frequency rTMS (OR 3.91, 95% CI 2.49-6.14). It is worth noting that priming rTMS had no direct evidence compared with sham. According to the surface under the cumulative ranking curve, priming rTMS (84.5%) and bilateral rTMS (82.0%) were ranked in the two first positions for response rates.
Remission Rate
Eight systematic reviews examined the remission rate of any rTMS modality versus sham TMS. The definition of remission rates varied across included primary studies dependent on the measure used. Below are remission rates by rTMS modality.
Table A9:
Remission Rates for rTMS Modalities Versus Sham Treatment
| Author, Year | No. of Studies (Active + Sham TMS) | Results | Definition of Remission Rate |
|---|---|---|---|
| All rTMS Modalitiesa | |||
| University of Calgary, 201734b | 18 (NR) | RR 2.24 (95% CI 1.53-3.27, P = .441) I2 = 1.1% No difference |
Range ≤ 7 to ≤ 10 |
| HF Left DLPFC | |||
| Health Quality Ontario, 201618 | 11 (NR) | RR 2.20 (95% CI 1.44-3.38, P < .001) I2 = 0% Favouring rTMS |
Range ≤ 7 to ≤ 10 |
| Mutz et al, 201835 | 25 (566) | OR 3.04 (95% CI 1.72-5.37, P = NR) I2 = NR Favouring rTMS |
NR |
| Sehatzadeh et al, 201941 | 13 (NR) | RR 2.33 (95% CI 1.52-3.58, P = .86) I2 = 0% NNT = 11 |
Range ≤ 7 to ≤ 10 |
| Bilateral rTMS | |||
| Zhang et al, 201543 | 5 (NR) | RR 0.50 (95% CI 0.19-1.31, P = .16) I2 = 0% No difference |
NR |
| Sehatzadeh et al, 201941 | 6 (NR) | RR 5.54 (95% CI 1.96-15.61, P = .63) I2 = 0% Favouring rTMS NNT = 8 |
Range ≤ 7 to ≤ 10 |
| All TBS Modalitiesc | |||
| Berlim et al, 201745 | 6 (118 + 103) | OR 1.9 (95% CI 0.9-4.5, P = .11) I2 = 0% No difference |
≤ 7 |
| iTBS | |||
| Mutz et al, 201835 | 1 (NR) | “No evidence for antidepressant efficacy compared to sham” | NR |
| Deep TMS | |||
| Nordenskjold et al, 201644 | 1 (89 + 92) | Deep TMS 30.4% Sham deep TMS 15.8% P = .02 Favouring deep TMS |
NR |
| Mutz et al, 201835 | 2 (NR) | OR 2.24 (95% CI 1.24-4.06, P = NR) I2 = 0% Favouring deep TMS |
NR |
Abbreviations: CI, confidence interval; cTBS, continuous theta burst stimulation; DLPFC, dorsolateral prefrontal cortex; HF, high frequency; iTBS, intermittent theta burst stimulation; MD, mean difference; NNT, number needed to treat; NR, not reported; OR, odds ratio; RR, risk ratio; rTMS, repetitive transcranial magnetic stimulation; SMD, standardized mean difference; TBS, theta burst stimulation; TMS, transcranial magnetic stimulation; TRD, treatment-resistant depression; WMD, weighted mean difference.
Includes high-frequency, low-frequency, and bilateral rTMS.
Same results as results of Leggett et al.40
Includes two unilateral cTBS, two unilateral iTBS, and three bilateral TBS.
Relapse Rate
One systematic review18 reported on relapse rate. However, only one primary study80 within the review reported relapse rate. The systematic review reported that about half of patients (rTMS group: 5/11 responders [45.5%]; sham group: 1/2 responders [50%]) had relapsed at 6-month follow-up.
Adverse Events
Three systematic reviews reported on adverse events.18,34,41 In the Health Quality Ontario18 review in 2016, studies that reported on adverse events found headache and scalp discomfort are most frequently reported and that rates are higher in patients treated with rTMS than in patients treated with sham TMS. Thirteen studies reported the rate of headache ranges from 0 to 60% in the rTMS group and 0 to 50% in the sham group. Nine studies reported the rate of scalp pain or discomfort ranges from 4.5% to 78.9% in the rTMS group and 0 to 21% in the sham group. Six studies reported the rate of gastrointestinal problems ranges from 5% to 22% in the rTMS group and 0 to 22% in the sham group. Four studies reported the rate of eye problems (eye pain, conjunctivitis, or tearfulness) ranges from 5.6% to 21% in the rTMS group and 0 to 1.9% in the sham group. Four studies reported the rate of muscle twitching ranges from 5.5% to 20.6% in the rTMS group and 0 to 3.2% in the sham group. Other reported adverse events were vertigo or dizziness, insomnia, muscle pain, fatigue, difficulty concentrating, anxiety or panic episode, hypomania, tinnitus, skin pain, facial pain, depersonalization, paranoid thoughts, crying, getting worse, suicidal thoughts, and syncope (fainting).
Sehatzadeh et al41 reported no seizures. A variety of minor adverse events occurred: the most frequent adverse events are headache (rTMS 0–60%, sham 0–50%), scalp pain or discomfort (rTMS 4.5%–79%, sham 0–21%), gastrointestinal problems (rTMS 5%–22%, sham 0–22%), eye problems (rTMS 5.6%–21%, sham 0–1.9%), muscle twitching (rTMS 0–20.6%, sham 0–3.2%), vertigo or dizziness (rTMS 0–16.7%, sham 2%–14%), insomnia (rTMS 4.5%–7.6%, sham 0–10%), and tinnitus (rTMS 0–11%, sham 0–3%).
The University of Calgary34 also reported a variety of adverse events. As in the other two systematic reviews, the most frequent adverse event was pain or discomfort and headache. Ten studies reported that some patients had headaches; all reported that the headaches subsided quickly. Although headaches were more common in the rTMS groups (in one study, 60% of the rTMS group reported having headaches), they also occurred in the sham groups (up to 50% of the control group reported headaches). Nine studies reported rates of patient discomfort or pain. In six of these studies, discomfort and pain were reported in both the rTMS and sham groups; the remaining three studies reported pain and discomfort only in the active group. None of the included studies assessed serious adverse events such as cognitive impairment, seizures, or thoughts of suicide.
Acceptability (Discontinuation of Treatment)
Two systematic reviews captured acceptability.35,45 Berlim et al45 found no difference (OR 0.70, 95% CI 0.3–1.9, P = .50) in dropout rates at the end of the study between TBS and sham TBS. Mutz et al35 found “no significant differences in drop-out rates for any treatment modalities.”
Appendix 5: Results and Additional Analyses from Question 1
Table A10:
Baseline Characteristics of Primary Studies Included for Question 1
| Study, Year N | Follow-Up | Mean Age (SD) | TRD Definition | AD Status | Type of Depression | Depression Scale | Baseline Depression Score | % MT | Frequency (Hz) | Sessions (N) | Total Pulses (N) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HF Left DLPFC | |||||||||||
| Anderson et al, 200778 rTMS 11 Sham 14 | 4 wk (EOT) 12 wk | rTMS 48 (8) Sham 46 (12) | NR | Add-on 89% | Unipolar | MADRS | rTMS 26.7 (3.6) Sham 27.7 (7.1) | 110 | 10 | 12 | 12,000 |
| Avery et al, 199979 rTMS 4 Sham 2 | 2 wk (EOT) 4 wk | rTMS 44.25 (NR) Sham 45 (NR) | ≤ 2 | Add-on rTMS 50% Sham 50% | Unipolar/bipolar | HDRS | rTMS 21.3 (6.7) Sham 19.5 (8.1) | 80 | 10 | 10 | NR |
| Avery et al, 200680 rTMS 35 Sham 33 | 4 wk (EOT) 6 wk | rTMS 44.3 (10.3) Sham 44.2 (9.7) | ≤ 2 | Add-on rTMS 31% Sham 27% | Unipolar | HDRS-17 | rTMS 23.5 (3.9) Sham 23.5 (2.9) | 110 | 10 | 15 | 24,000 |
| Bakim et al, 201273 80% MT 12 110% MT 11 Sham 12 |
6 wk (EOT) | 80% MT 38.75 (9.96) 110% MT 43.09 (8.18) Sham 44.41 (10.22) |
≤ 2 | Add-on 100% | Unipolar | HDRS-17 | 80% MT 23.08 (3.63) 110% MT 24.09 (2.77) Sham 25.58 (3.82) |
80 110 |
20 | 30 | 24,000 |
| Berman et al, 200081 rTMS 10 Sham 10 | 2 wk (EOT) | rTMS 45.2 (NR) Sham 39.4 (NR) | ≤ 1 | No AD 100% | Unipolar/bipolar | HDRS-25 | rTMS 37.1 (NR) Sham 37.3 (NR) | 80 | 20 | 10 | NR |
| Blumberger et al, 201283 rTMS 22 Sham 20 | 3 wk (EOT) 6 wk | rTMS 48.9 (13.4) Sham 45.8 (13.4) |
≤ 2 | Add-on rTMS 77.2% Sham 50% | Unipolar | HDRS-17 | rTMS 26.0 (3.3) Sham 25.2 (3.6) | 100 or 120a | 10 | 15 | 21,750 |
| Blumberger et al, 201682 rTMS 40 Sham 41 | 3 wk (EOT) 6 wk | rTMS 46.5 (14.1) Sham 48.1 (12.0) | ≤ 2 | Add-on rTMS 90% Sham 95.1% | Unipolar | HDRS-17 | rTMS 26.0 (3.4) Sham 25.5 (3.6) | 120 | 10 | 15 | 31,500 |
| Bortolomasi et al, 2007127b rTMS 12 Sham 7 | 4 wk (EOT) 12 wk | rTMS 45–56 Sham 44–53 | NR | Add-on rTMS 99% Sham 100% | Unipolar/bipolar | HDRS-24 | rTMS 25.17 (NR) Sham NR | 90 | 20 | 20 | 16,000 |
| Boutros et al, 2002186b rTMS 12 Sham 9 | 2 wk (EOT) | rTMS 49.5 (8) Sham 52 (7) | ≤ 2 | No AD 100% | Unipolar/bipolar | HDRS-25 | rTMS 34.4 (10.1) Sham 31.7 (4.9) | 80 | 20 | 10 | 8,000 |
| Bretlau et al, 200884 rTMS 22 Sham 23 | 3 wk (EOT) 12 wk | rTMS 53.1 (10.1) Sham 57.8 (10.0) | ≤ 1 | Add-on 100%c | Unipolar | HDRS-17 | rTMS 25.3 (3.0) Sham 24.7 (3.2) | 90 | 8 | 15 | 19,200 |
| Chen et al, 201385 rTMS 10 Sham 10 | 2 wk (EOT) 4 wk | rTMS 44.1 (4.4) Sham 47.3 (3.5) | ≤ 2 | Add-on 100% | Unipolar | HDRS-17 | rTMS 23.5 (1.9) Sham 24.9 (1.9) | 90 | 20 | 10 | NR |
| Fitzgerald et al, 200386 rTMS 20 Sham 20 | 2 wk (EOT) 4 wk | rTMS 42.4 (9.8) Sham 49.15 (14.24) | ≤ 2 | Add-on 76.7% | Unipolar/bipolar | MADRS | rTMS 36.05 (7.55) Sham 35.75 (8.14) | 100 | 10 | 10 | 10,000 |
| Fitzgerald et al, 201287 rTMS 24 Sham 17 | 3 wk (EOT) 6 wk | rTMS 43.4 (12.7) Sham 44.9 (15.7) | ≤ 2 | Add-on 60% | Unipolar | HDRS-17 | rTMS 23.7 (3.8) Sham 22.9 (2.1) | 120 | 10 | NR | NR |
| Garcia-Toro et al, 200188 rTMS 17 Sham 18 | 2 wk (EOT) 4 wk | rTMS 51.5 (15.9) Sham 50 (11) | ≤ 2 | Add-on 100% | Unipolar | HDRS-21 | rTMS 27.11 (6.65) Sham 25.6 (4.92) | 90 | 20 | 10 | NR |
| George et al, 201089 rTMS 92 Sham 98 | 3 wk (EOT) | rTMS 47.7 (10.6) Sham 46.5 (12.3) | 1–4 | No AD 100% | Unipolar | HDRS-24 | rTMS 26.3 (5.0) Sham 26.5 (4.8) | 120 | 20 | NR | NR |
| Hernandez-Ribas et al, 201390 rTMS 10 Sham 11 | 3 wk (EOT) | rTMS 42.6 (5.56) Sham 50.1 (8.11) | ≤ 1 | Add-on 100% | Unipolar/bipolar | HDRS-21 | rTMS 19.7 (3.8) Sham 16.55 (2.4) | 100 | 15 | 15 | 22,500 |
| Herwig et al, 200791 rTMS 52 Sham 53 | 3 wk (EOT) | rTMS 50 (15) Sham 49 (13) | ≤ 2 | Add-on rTMS 92.3% Sham 88.7% | Unipolar/bipolar | HDRS-21 | rTMS 24.7 (5.4) Sham 22.8 (4.8) | 110 | 10 | 15 | 30,000 |
| Holtzheimer et al, 200492 rTMS 7 Sham 8 | 2 wk (EOT) 3 wk |
rTMS 40.4 (8.5) Sham 45.4 (4.9) | ≤ 2 | No AD 100% | Unipolar | HDRS-17 | rTMS 22.7 (5.3) Sham 20.8 (6.3) | 110 | 10 | 10 | 16,000 |
| Hoppner et al, 2003112 rTMS 10 Sham 10 | 2 wk (EOT) | rTMS 60.36 (NR) Sham 56.44 (NR) | ≤ 1 | Add-on 100% | Unipolar | HDRS-21 | NR | 90 | 20 | 10 | NR |
| Kang et al, 201647 rTMS 13 Sham 11 | 2 wk (EOT) | rTMS 32.8 (19.1) Sham 52.2 (20.1) | ≤ 1 | Add-on 100% | Unipolar | HDRS-17 | rTMS 24.1 (6.4) Sham 20.0 (4.6) | 110 | 10 | 10 | 10,000 |
| Loo et al, 200793 rTMS 18 Sham 18 | 2 wk (EOT) | rTMS 49.8 (2.5) Sham 45.7 (15.0) | ≤ 2 | Add-on 58.3% | Unipolar/bipolar | MADRS HDRS-17 | MADRS rTMS 29.5 (3.9) Sham 32.6 (4.3) HDRS-17 rTMS 19.2 (3.7) Sham 20.9 (4.2) | 110 | 10 | 20d | 30,000 |
| Mogg et al, 2008113 rTMS 28 Sham 29 | 2 wk (EOT) | rTMS 55 (18) Sham 52 (15.5) | ≤ 2 | Add-on rTMS 93.1% Sham 86.7% | Unipolar | HDRS-17 | rTMS 20.5 (4.4) Sham 21.6 (4.7) | 110 | 10 | 10 | 10,000 |
| Moismann et al, 200494 rTMS 15 Sham 9 | 2 wk (EOT) | rTMS 60 (13.4) Sham 64.4 (13.0) | ≤ 2 | Add-on 95.8% | Unipolar/bipolar | HDRS-21 | rTMS 28.5 (4.6) Sham 24.5 (7.3) | 100 | 20 | 10 | NR |
| O'Reardon et al, 200795 rTMS 155 Sham 146 | 4 wk (EOT) 6 wk | rTMS 47.9 (11.0) Sham 48.7 (10.6) | ≤ 1 | No AD 100% | Unipolar | MADRS HDRS-17 HDRS-24 | MADRS rTMS 32.8 (6.0) Sham 33.9 (5.7) HDRS-17 rTMS 22.6 (3.3) Sham 22.9 (3.5) HDRS-24 rTMS 30.1 (5.0) Sham 30.5 (4.9) | 120 | 10 | 30 | 90,000 |
| Padberg et al, 2002111 100% MT 10 90% MT 10 Sham 10 | 2 wk (EOT) | 100% MT 62.1 (4.6) 90% MT 60.3 (4.1) Sham 52.7 (5.7) | ≤ 2 | Add-on 100% | Unipolar | HDRS-21 MADRS | HRSD-21 100% MT 23.6 (1.9) 90% MT 21.9 (1.8) Sham 24.4 (2.1) MADRS 100% MT 28.7 (2.0) 90% MT 28.2 (2.5) Sham 30.4 (2.0) | 100 90 |
10 | 10 | 15,000 |
| Paillere Martinot et al, 201096 rTMS 18 Sham 14 | 2 wk (EOT) | rTMS 48.19 (7.77) Sham 46.57 (10.27) | ≤ 2 | Add-on 100% | Unipolar/bipolar | MADRS HDRS-21 | MADRS rTMS 32 (7.78) Sham 34.57 (6.07) HDRS-21 rTMS 26 (6.4) Sham 25.93 (6.65) | 90 | 10 | 10 | 16,000 |
| Peng et al, 201297 rTMS 17 Sham 13 | 4 wk (EOT) | rTMS 27.41 (6.14) Sham 26.38 (3.45) | ≤ 2 | Add-on 100%c | Unipolar | HDRS-17 | rTMS 24.7 (3.0) Sham 24.5 (3.3) | 110 | 20 | 15 | 60,000 |
| Rossini et al, 2005114 100% MT 18 80% MT 19 Sham 17 | 2 wk (EOT) | 100% MT 57.4 (8.7) 80% MT 54.0 (11.2) | |||||||||
| Sham 56.3 (12.6) | ≤ 2 | Add-on 100% | Unipolar/bipolar | HDRS-21 | 100% MT 28.8 (3.1) 80% MT 28.6 (2.7) Sham 28.7 (2.1) | 100 80 |
15 | 10 | NR | ||
| Speer et al, 2014105 rTMS 8 Sham 8 | 3 wk (EOT) | rTMS 41.3 (14.5) Sham 44.9 (9.1) | ≤ 2 | NR | Unipolar/bipolar | HDRS-28 | rTMS 35.8 (10.6) Sham 24.0 (4.6) | 110 | 20 | 15 | 24,000 |
| Stern et al, 200777 rTMS 10 Sham 15 | 2 wk (EOT) 4 wk | rTMS 53.2 (12) Sham 53.3 (9) | NR | No AD 100% | Unipolar | HDRS-21 | rTMS 27.8 (3.2) Sham 27.4 (2.9) | 110 | 10 | 10 | NR |
| Su et al, 200574 HF 20 Hz 10 HF 5 Hz 10 Sham 10 | 2 wk (EOT) | HF 20 Hz 43.6 (12.0) HF 5 Hz 43.2 (10.6) Sham 42.6 (11.0) | ≤ 2 | Add-on 100% | Unipolar/bipolar | HDRS-21 | HF 20-Hz 23.2 (7.5) HF 5-Hz 26.5 (5.2) Sham 22.7 (4.7) | 100 | 20 5 |
10 | 16,000 |
| Taylor et al, 2018115 rTMS 16 Sham 16 | 4 wk (EOT) | rTMS 46.9 (10.7) Sham 44.13 (11.1) | ≤ 1 | Add-on 93.8% | Unipolar | MADRS HDRS-17 |
MADRS rTMS 25.4 (5.7) Sham 21.9 (3.1) HRSD-17 rTMS: 16.0 (3.9) Sham 13.1 (2.3) |
120 | 10 | 20 | 60,000 |
| Theleritis et al, 201775 rTMS 25 Sham 18 | 3 wk (EOT) 4 wk | rTMS 39.1 (10.1) Sham 38 (9.9) | ≤ 2 | Add-on rTMS 56% Sham 50% | Unipolar | HDRS-17 | rTMS 30.6 (3.2) Sham 29.4 (3.2) | 100 | 20 | 15 | 24,000 |
| Triggs et al, 201076 rTMS 18 Sham 7 | 2 wk (EOT) 12 wk | rTMS 41.9 (14.1) Sham 41.9 (14.1) | ≤ 2 | Add-on 97.9% | Unipolar/bipolar | HDRS-24 | rTMS 28.2 (6) Sham 27.7 (3.5) | 100 | 5 | 10 | 20,000 |
| Yesavage et al, 201850e rTMS 81 Sham 83 | 6 wk (EOT) 24 wk | rTMS 55.6 (12.2) Sham 54.8 (12.6) | ≤ 2 | Add-on 100% | Unipolar | HDRS-24 | rTMS 26.2 (4.9) Sham 27.5 (5.1) | 120 | 10 | 30 | 120,000 |
| Zhang et al, 201198 rTMS 14 Sham 14 | 4 wk (EOT) | rTMS 50.8 (13.3) Sham 43.8 (13.9) | ≤ 2 | Add-on 100% | Unipolar | HDRS-17 | rTMS 20.07 (2.92) Sham 20.21 (4.21) | 110 | 10 | 20 | 30,000 |
| Zheng et al, 201571 rTMS 18 Sham 14 | 4 wk (EOT) | rTMS 26.9 (6.4) Sham 26.9 (4.3) | ≤ 2 | Add-on 100% | Unipolar | HDRS-17 | rTMS 23.1 (3.6) Sham 23.6 (3.6) | 100 110 |
15 | 20 | 60,000 |
| LF Right DLPFC | |||||||||||
| Bares et al, 200999 rTMS 27 Sham 31 | 4 wk (EOT) | rTMS 45.4 (11.7) Sham 44.2 (11.6) |
≤ 1 | Add-on 100%f | Unipolar | MADRS | rTMS 27.5 (4.1) Sham 26.7 (4.0) | 100 | 1 | 20 | 12,000 |
| Brunelin et al, 2014116g rTMS w/V50 rTMS w/o V54 Sham w/V 51 | 2–6 wk (EOT) Endpoint (unspecified) | rTMS w/V 54.2 (11.9) rTMS w/o V 53.3 (11.3) Sham w/V 56.2 (9.9) | ≤ 1 | Add-on | Unipolar | HDRS-17 | rTMS w/V 26.1 (3.9) rTMS w/o V 25.8 (3.6) Sham w/V 25.8 (3.4) | 120 | 1 | 10–30 | 3,600–10,800 |
| Fitzgerald et al, 200386 rTMS 20 Sham 20 | 2 wk (EOT) 4 wk |
rTMS 45.55 (11.49) Sham 49.15 (14.24) | ≥ 2 | Add-on 76.7% | Unipolar/bipolar | MADRS | rTMS 37.70 (8.36) Sham 35.75 (8.14) | 100 | 1 | 10 | 3,000 |
| Hoppner et al, 2003112 rTMS 10 Sham 10 | 2 wk (EOT) | rTMS 52 (NR) Sham 56.44 (NR) | ≥ 1 | Add-on 100% | Unipolar | HDRS-21 | NR | 110 | 1 | 10 | NR |
| Januel et al, 2006101 rTMS 11 Sham 16 | 4 wk (EOT) | rTMS 38.64 (11.16) Sham 37.19 (11.67) | ≥ 2 | No AD 100% | Unipolar | HDRS-17 | rTMS 21.73 (3.52) Sham 22.5 (2.73) | 90 | 1 | 16 | NR |
| Kauffmann et al, 2004102 rTMS 7 Sham 5 | 2 wk (EOT) | NR | ≥ 2 | Add-on 100% | Unipolar | HDRS-21 | rTMS 21.86 (2.31) Sham 18.2 (2.2) | NR | 1 | 10 | NR |
| Krstic et al, 2014103 rTMS 11 Sham 8 | 2 wk (EOT) 3 wk | rTMS 50.7 (7.3) Sham 46.1 (8.5) |
≥ 2 | Add-on 100% | Unipolar | HDRS | rTMS 30.1 (3.53) Sham 28 (2.74) | 110 | 1 | 10 | 3,000 |
| Mantovani et al, 2013104 rTMS 12 Sham 13 | 4 wk (EOT) | rTMS 40.2 (10) Sham 39.8 (13.3) |
NR | Add-on rTMS 83.3% Sham 76.9% | Unipolarh | HDRS-24 | rTMS 31.9 (6.5) Sham 31.1 (8.3) | 110 | 1 | 20 | 36,000 |
| Pallanti et al, 2010117 rTMS 20 Sham 20 | 3 wk (EOT) | rTMS 51.2 (12.53) Sham 47.85 (9.12) |
≥ 2 | Add-on 100% | Unipolar | HDRS | rTMS 27.95 (5.89) Sham 29.05 (3.54) | 110 | 1 | 15 | 6,300 |
| Speer et al, 2014105 rTMS 8 Sham 8 | 3 wk (EOT) | rTMS 39.6 (9) Sham 44.9 (9.1) | ≥ 2 | NR | Unipolar/bipolar | HDRS-28 | rTMS 28.6 (7.6) Sham 24 (4.6) | 110 | 1 | 15 | 24,000 |
| Stern et al, 200777 rTMS 10 Sham 15 | 2 wk (EOT) 4 wk | rTMS 52.8 (9.5) Sham 53.3 (9) | NR | No AD 100% | Unipolar | HDRS-21 | rTMS 27.9 (3.8) Sham 27.4 (2.9) | 110 | 1 | 10 | NR |
| Bilateral rTMS | |||||||||||
| Blumberger et al, 201283 rTMS 26 Sham 20 | 3 wk (EOT) 6 wk | rTMS 58.0 (12.5) Sham 45.8 (13.4) | ≤ 2 | Add-on rTMS 69.2% Sham 50% | Unipolar | HDRS-17 | rTMS 25.1 (3.8) Sham 25.2 (3.6) | 100 or 120a | 10/1 | 15 | 18,225 |
| Blumberger et al, 201682 rTMS 40 Sham 41 | 3 wk (EOT) 6 wk | rTMS 46.4 (12.5) Sham 48.1 (12.0) | ≤ 2 | Add-on rTMS 100% Sham 95.1% | Unipolar | HDRS-17 | rTMS 24.1 (3.2) Sham 25.5 (3.6) | 120 | 10/1 | 15 | 31,500 |
| Fitzgerald et al, 2006106 rTMS 25 Sham 25 | 2 wk (EOT) | rTMS 46.8 (10.7) Sham 43.7 (10.2) |
≤ 2 | Add-on rTMS 92% Sham 84% | Unipolar/bipolar | MADRS | rTMS 34.0 (5.9) Sham 34.1 (5.2) | 110 100 |
10/1 | 10 | NR |
| Fitzgerald et al, 201287 rTMS 19 Sham 17 | 3 wk (EOT) 6 wk | rTMS 40.5 (15.5) Sham 44.9 (15.7) | ≤ 2 | Add-on 60% | Unipolar | HDRS-17 | rTMS 24.3 (3.6) Sham 22.9 (2.1) | 120 | 10/1 | NR | NR |
| Fitzgerald et al, 2016119 rTMS 23 Sham 23 | 4 wk (EOT) | rTMS 46.3 (12.6) Sham 49.7 (11.0) | ≤ 2 | Add-on 100% | Bipolar | HDRS-17 | rTMS 23.2 (4.0) Sham 23.0 (5.1) | 110 | 10/1 | 20 | 40,000 |
| Garcia-Toro et al, 2006108 rTMS 10 Sham 10 | 2 wk (EOT) 4 wk | rTMS 48.5 (13.3) Sham 47.2 (11.8) | ≤ 2 | Add-on 100% | Unipolar | HDRS | rTMS 27.3 (4.9) Sham 25.1 (7.3) | 110 | 20/1 | 10 | NR |
| Loo et al, 2003120 rTMS 9 Sham 10 | 3 wk (EOT) | rTMS 54.9 (18.0) Sham 48.4 (10.9) | ≤ 1 | Add-on rTMS 77.7% Sham 70% | Unipolar/bipolar | MADRS | rTMS 38.4 (6.3) Sham 33.1 (5.2) | 90 | 15/15 | 15 | NR |
| McDonald et al, 2006121 rTMS 25 Sham 12 | 2 wk (EOT) | rTMS 49 (41–55) Sham 54 (47–64)i |
> 3 | No AD 100% | Unipolar/bipolar | HDRS-17 | NR | 110 | 10/1 | 10 | 16,000 |
| Pallanti et al, 2010117 rTMS 20 Sham 20 |
3 wk (EOT) | rTMS 47.6 (12.33) Sham 47.85 (9.12) | ≥ 2 | Add-on 100% | Unipolar | HDRS | rTMS 28.75 (6.01) Sham 29.05 (3.54) | 100/110 | 10/1 | 15 | 21,300 |
| Valkonen-Korhonen et al, 201849 rTMS 18 Sham 19 | 6 wk (EOT) | rTMS 37.1 (11.1) Sham 36.4 (15.3) | ≥ 2 | Add-on 100% | Unipolar | HDRS | rTMS 27.7 (6.9) Sham 25.9 (5.4) | 110 | 10/1 | 30 | 5,700 |
| cTBS | |||||||||||
| Chistyakov et al, 2015122 rTMS 15 Sham 14 | 2 wk (EOT) | rTMS 39.8 (12.4) Sham 33.9 (13.7) | NR | Add-on 88.4%j | Unipolar | HDRS-21 | rTMS 26.7 (3.9) Sham 24.8 (3.2) | 100 | 3-pulse 50-Hz bursts every 200 ms (5 Hz) in uninterrupted bursts | 10 | 36,000 |
| Li et al, 2014109 rTMS 15 Sham 15 | 2 wk (EOT) 14 wk | rTMS 49.2 (NR) Sham 46.9 (NR) | ≥ 2 |
Add-on rTMS 66.6% Sham 86.6% |
Unipolar | HDRS-17 | rTMS 24.3 (5.5) Sham 23.8 (3.2) | 80 | 3-pulse 50-Hz bursts every 200 ms (5 Hz) in uninterrupted bursts | 10 | 18,000 |
| iTBS | |||||||||||
| Duprat et al, 2016124 rTMS 22 Sham 25 | 1 wk (EOT)k | NR | ≤ 1 | No AD 100% | Unipolar | HDRS-17 | NR | 110 | 3-pulse burst (Hz NR) | 20 | 32,400 |
| Li et al, 2014109 rTMS 15 Sham 15 | 2 wk (EOT) 14 wk | rTMS 42.4 (NR) Sham 46.9 (NR) | ≥ 2 | Add-on rTMS 73.3% Sham 86.6% | Unipolar | HDRS-17 | rTMS 23.1 (3.9) Sham 23.8 (3.2) | 80 | 3-pulse 50-Hz bursts every 200 ms (5 Hz) in a 2-s train every 10 s | 10 | 18,000 |
| Bilateral TBS (combination of cTBS and iTBS) | |||||||||||
| Plewnia et al, 2014125 rTMS 16 Sham 16 | 6 wk (EOT) | rTMS 46.9 (13.2) Sham 49.0 (13.6) | ≥ 2 | Add-on 100% | Unipolar | MADRS HDRS | MADRS rTMS 26.8 (7.1) Sham 26.6 (7.1) HDRS rTMS 23.6 (5.3) Sham 22.2 (5.9) | NR | cTBS: 3-pulse 50-Hz bursts every 200 ms (5 Hz) uninterrupted iTBS: 3-pulse 50-Hz bursts every 200 ms (5 Hz) in a 2-s train every 10 s | 30 | NR |
| Li et al, 2014109 rTMS 15 Sham | 2 wk (EOT) 14 wk | rTMS 42.5 (NR) Sham 46.9 (NR) | ≥ 2 | Add-on rTMS 73.3% Sham 86.6% | Unipolar | HDRS-17 | rTMS 23.1 (3.9) Sham 23.8 (3.2) | 80 | cTBS: 3-pulse 50-Hz bursts every 200 ms (5 Hz) in uninterrupted bursts iTBS: 3-pulse 50-Hz bursts every 200 ms (5 Hz) in a 2-s train every 10 s |
10 | 18,000 |
| Deep TMS | |||||||||||
| Kaster et al, 201848 rTMS 25 Sham 27 | 4 wk (EOT) | rTMS 65 (5.5) Sham 65.4 (5.5) | ≥ 1 adequate or ≥ 2 inadequate doses | Add-on rTMS 72% Sham 63% | Unipolar | HDRS-24 | rTMS 25.8 (4) Sham 27.6 (4.1) | 120 | 18 | 20 | 120,240 |
| Levkovitz et al, 2015126 rTMS 101 Sham 111 | 4 wk (EOT) 12 wk | rTMS 45.1 (11.7) Sham 47.6 (11.6) | 1-4 | No AD 100% | Unipolar | HDRS-21 | rTMS 23.5 (4.3) Sham 23.4 (3.7) | 120 | 18 | 20 (acute phase) 22 (maintenance phase) | 39,600 |
| Tavares et al, 2017110 rTMS 25 Sham 25 | 4 wk (EOT) 8 wk | rTMS 43.5 (12) Sham 41.2 (8.9) | ≥ 2 | Add-on rTMS 88% Sham 87.5% | Bipolar | HDRS-17 | rTMS 25.8 (5.25) Sham 25.3 (3.76) | 120 | 18 | 20 | 39,600 |
Abbreviations: AD, antidepressant; cTBS, continuous theta burst stimulation; DLPFC, dorsolateral prefrontal cortex; EOT, end of trial; HDRS, Hamilton Depression Rating Scale; HF, high frequency; iTBS, intermittent theta burst stimulation; LF, low frequency; MADRS, Montgomery-Åsberg Depression Rating Scale; MT, motor threshold; NR, not recorded; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation; TBS theta burst stimulation; TRD, treatment-resistant depression; V, venlafaxine.
Dependent on age: 100 MT given to patients who were ≤ 60 years of age and 120 MT given to patients who were > 60 years of age.
Not included in analyses because authors did not provide standard deviations of final mean depression scores, and response and remission rates were not reported.
All patients took escitalopram.
dAdministered rTMS twice daily.
Many patients in the sample had post-traumatic stress disorder, which could be a moderator variable.
Sham group received venlafaxine.
One rTMS group and the sham group received venlafaxine.
Patients also had comorbid anxiety.
Age is given as median (range).
73% of rTMS group and 50% of sham group changed their medication within week before intervention.
Gave rTMS sessions four times daily.
High-Frequency Left DLPFC rTMS Versus Sham rTMS
Figure A1: Change in Depression Score for High-Frequency Left DLPFC rTMS Versus Sham rTMS at Follow-Up (3 Weeks to 3 Months).
Sources: Anderson et al,78Avery et al,79Blumberger et al,83Bretlau et al,84Chen et al,85Garcia-Toro et al,88Holtzheimer et al,92O'Reardon et al,95Stern et al,77Theleritis et al,75Triggs et al,76Yesavage et al.50
Abbreviations: CI, confidence interval; df, degrees of freedom; HF, high frequency; IV, inverse variance; L-DLPFC, left dorsolateral prefrontal cortex; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation.
Figure A2: Change in Depression Score for High-Frequency Left DLPFC rTMS Versus Sham rTMS at End of Treatment by Antidepressant Status.
Sources: Anderson et al,78Avery et al,79,80Bakim et al,73 Berman et al,81Blumberger et al,82,83Bretlau et al,84Chen et al,85Fitzgerald et al,86,87Garcia-Toro et al,88George et al,89Hernandez-Ribas et al,90Herwig et al,91Holtzheimer et al,92Kang et al,47Loo et al,93Mosimann et al,94O'Reardon et al,95Paillere Martinot et al,96Peng et al,97Stern et al,77Su et al,74Theleritis et al,75Triggs et al,76Yesavage et al,50Zhang et al,98Zheng et al.71
Abbreviations: AD, antidepressant; CI, confidence interval; df, degrees of freedom; HF, high frequency; IV, inverse variance; L-DLPFC, left dorsolateral prefrontal cortex; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation.
Figure A3: Change in Depression Score for High-Frequency Left DLPFC rTMS Versus Sham rTMS at End of Treatment by Type of Depression.
Sources: Anderson et al,78Avery et al,79,80Bakim et al,73 Berman et al,81Blumberger et al,82,83Bretlau et al,84Chen et al,85Fitzgerald et al,86,87Garcia-Toro et al,88George et al,89Hernandez-Ribas et al,90Herwig et al,91Holtzheimer et al,92Kang et al,47Loo et al,93Mosimann et al,94O'Reardon et al,95Paillere Martinot et al,96Peng et al,97Speer et al,105Stern et al,77Su et al,74Theleritis et al,75Triggs et al,76Yesavage et al,50Zhang et al,98Zheng et al.71
Abbreviations: CI, confidence interval; df, degrees of freedom; HF, high frequency; IV, inverse variance; L-DLPFC, left dorsolateral prefrontal cortex; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation.
Figure A4: Change in Depression Score for High-Frequency Left DLPFC rTMS Versus Sham rTMS at End of Treatment With Studies That Used Hamilton Depression Rating Scale.
Sources: Anderson et al,78Avery et al,79,80Bakim et al,73 Berman et al,81Blumberger et al,82,83Bretlau et al,84Chen et al,85Fitzgerald et al,86,87Garcia-Toro et al,88George et al,89Hernandez-Ribas et al,90Herwig et al,91Holtzheimer et al,92Kang et al,47Loo et al,93Mosimann et al,94 O'Reardon et al,95 Paillere Martinot et al,96 Peng et al,97 Speer et al,105Stern et al,77Su et al,75Theleritis et al,74Triggs et al,76Yesavage et al,50Zhang et al,98Zheng et al.71
Abbreviations: CI, confidence interval; df, degrees of freedom; HF, high frequency; IV, inverse variance; L-DLPFC, left dorsolateral prefrontal cortex; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation.
Figure A5: Change in Depression Score for Low-Frequency Right DLPFC rTMS Versus Sham rTMS at End of Treatment by Antidepressant Status.
Sources: Bares et al,99Fitzgerald et al,100Januel et al,101Kauffmann et al,102Krstic et al,103Mantovani et al,104Stern et al.77
Abbreviations: AD, antidepressant; CI, confidence interval; df, degrees of freedom; IV, inverse variance; LF, low frequency; R-DLPFC, right dorsolateral prefrontal cortex; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation.
Figure A6: Change in Depression Score for Low-Frequency Right DLPFC rTMS Versus Sham rTMS at End of Treatment by Type of Depression.
Sources: Bares et al,99Fitzgerald et al,100Januel et al,101Kauffmann et al,102Krstic et al,103Mantovani et al,104Speer et al,105Stern et al.77
Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; LF, low frequency; R-DLPFC, right dorsolateral prefrontal cortex; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation.
Figure A7: Change in Depression Score for Low-Frequency Right DLPFC rTMS Versus Sham rTMS at End of Treatment With Studies That Used Hamilton Depression Rating Scale.
Sources: Bares et al,99Fitzgerald et al,86,100Januel et al,101Kauffmann et al,102Krstic et al,103Mantovani et al,104Speer et al,105Stern et al.77
Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; LF, low frequency; R-DLPFC, right dorsolateral prefrontal cortex; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation.
Figure A8: Change in Depression Score for Bilateral rTMS Versus Sham rTMS at Follow-Up (4 and 6 Weeks).
Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation.
High-Frequency Left DLPFC rTMS Versus Sham rTMS
Figure A9: Risk Difference for Response Rate of High-Frequency Left DLPFC rTMS Versus Sham rTMS at End of Treatment.
Sources: Anderson et al,78Avery et al,80Bakim et al,73 Berman et al,81Blumberger et al,82,83Chen et al,85Fitzgerald et al,86,87,100Garcia-Toro et al,88George et al,89Hernandez-Ribas et al,90Herwig et al,91Holtzheimer et al,92Hoppner et al,112Kang et al,47Loo et al,93Mogg et al,113Mosimann et al,94O'Reardon et al,95Padberg et al,111Paillere Martinot et al,96Peng et al,97Rossini et al,114Stern et al,75Su et al,74Taylor et al,115Theleritis et al,75Triggs et al,76Zhang et al,98Zheng et al.71
Abbreviations: CI, confidence interval; df, degrees of freedom; HF, high frequency; L-DLPFC, left dorsolateral prefrontal cortex; M-H, Mantel– Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
Figure A10: Response Rate for High-Frequency Left DLPFC rTMS Versus Sham rTMS at Follow-Up (3 Weeks to 3 Months).
Sources: Anderson et al,78Blumberger et al,82,83Chen et al,85Holtzheimer et al,92O'Reardon et al,95Stern et al,77Triggs et al.76
Abbreviations: CI, confidence interval; df, degrees of freedom; HF, high frequency; L-DLPFC, left dorsolateral prefrontal cortex; M-H, Mantel–Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
Figure A11: Response Rate for High-Frequency Left DLPFC rTMS Versus Sham rTMS at End of Treatment by Antidepressant Status.
Sources: Anderson et al,78Avery et al,80Bakim et al,73 Berman et al,81Blumberger et al,82,83Chen et al,85Fitzgerald et al,86,87,100Garcia-Toro et al,88George et al,89Hernandez-Ribas et al,90Herwig et al,91Holtzheimer et al,92Hoppner et al,112Kang et al,47Loo et al,93Mogg et al,113Mosimann et al,94O'Reardon et al,95Padberg et al,111Paillere Martinot et al,94Peng et al,95Rossini et al,114 Stern et al,75Su et al,74Taylor et al,115Theleritis et al,75Triggs et al,76Zhang et al,98Zheng et al.71
Abbreviations: AD, antidepressant; CI, confidence interval; df, degrees of freedom; HF, high frequency; L-DLPFC, left dorsolateral prefrontal cortex; M-H, Mantel–Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
Figure A12: Response Rate for High-Frequency Left DLPFC rTMS Versus Sham rTMS at End of Treatment by Type of Depression.
Sources: Anderson et al,78Avery et al,80Bakim et al,73 Berman et al,81Blumberger et al,82,83Chen et al,85Fitzgerald et al,86,87,100Garcia-Toro et al,88George et al,89Hernandez-Ribas et al,90Herwig et al,91Holtzheimer et al,92Hoppner et al,112Kang et al,47Loo et al,93Mogg et al,113Mosimann et al,94O'Reardon et al,95Padberg et al,111Paillere Martinot et al,94Peng et al,95Rossini et al,114 Stern et al,75Su et al,74Taylor et al,115Theleritis et al,75Triggs et al,76Zhang et al,98Zheng et al.71
Abbreviations: CI, confidence interval; df, degrees of freedom; HF, high frequency; L-DLPFC, left dorsolateral prefrontal cortex; M-H, Mantel– Haenszel test’ rTMS, repetitive transcranial magnetic stimulation.
Figure A13: Response Rate for High-Frequency Left DLPFC rTMS Versus Sham rTMS With Studies That Used Hamilton Depression Rating Scale.
Sources: Anderson et al,78Avery et al,80Bakim et al,73 Berman et al,81Blumberger et al,82,83Chen et al,85Fitzgerald et al,86,87,100Garcia-Toro et al,88George et al,89Hernandez-Ribas et al,90Herwig et al,91Holtzheimer et al,92Hoppner et al,112Kang et al,47Loo et al,93Mogg et al,113Mosimann et al,94O'Reardon et al,95Padberg et al,111Paillere Martinot et al,96Peng et al,97Rossini et al,114Stern et al,75Su et al,74Taylor et al,115Theleritis et al,75Triggs et al,76Zhang et al,98Zheng et al.71
Abbreviations: CI, confidence interval; df, degrees of freedom; HF, high frequency; L-DLPFC, left dorsolateral prefrontal cortex; M-H, Mantel– Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
Low-Frequency Right DLPFC rTMS Versus Sham rTMS
Figure A14: Risk Difference for Low-Frequency Right DLPFC rTMS Versus Sham rTMS on Response Rate at End of Treatment.
Sources: Bares et al,99Brunelin et al,116Fitzgerald et al,86Hoppner et al,112Januel et al,101Kauffmann et al,102Krstic et al,103Mantovani et al,104Pallanti et al,117Stern et al.77
Abbreviations: CI, confidence interval; df, degrees of freedom; LF, low frequency; M-H, Mantel–Haenszel test; R-DLPFC, right dorsolateral prefrontal cortex; rTMS, repetitive transcranial magnetic stimulation.
Figure A15: Response Rate for Low-Frequency Right DLPFC rTMS Versus Sham rTMS at End of Treatment by Antidepressant Status.
Sources: Bares et al,99Brunelin et al,116Fitzgerald et al,86Hoppner et al,112Januel et al,101Kauffmann et al,102Krstic et al,103Mantovani et al,104Pallanti et al,117Stern et al.77
Abbreviations: AD, antidepressant therapy; CI, confidence interval; df, degrees of freedom; LF, low frequency; M-H, Mantel–Haenszel test; R-DLPFC, right dorsolateral prefrontal cortex; rTMS, repetitive transcranial magnetic stimulation.
Figure A16: Response Rate for Low-Frequency Right DLPFC rTMS Versus Sham rTMS at End of Treatment With Studies That Used Hamilton Depression Rating Scale.
Sources: Bares et al,99Brunelin et al,116Fitzgerald et al,86Hoppner et al,112Januel et al,101Kauffmann et al,102Krstic et al,103Mantovani et al,104Pallanti et al,117Stern et al.77
Abbreviations: CI, confidence interval; df, degrees of freedom; LF, low frequency; M-H, Mantel–Haenszel test; R-DLPFC, right dorsolateral prefrontal cortex; rTMS, repetitive transcranial magnetic stimulation.
Bilateral rTMS Versus Sham rTMS
Figure A17: Risk Difference for Bilateral rTMS Versus Sham rTMS on Response Rate at End of Treatment.
Sources: Blumberger et al,82,83Fitzgerald et al,87,106,119Garcia-Toro et al,108Loo et al,120McDonald et al,121Pallanti et al,117Valkonen-Korhonen et al.49
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
Figure A18: Response Rate for Bilateral rTMS Versus Sham rTMS at 6-Week Follow-Up.
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
Figure A19: Response Rate for Bilateral rTMS Versus Sham rTMS at End of Treatment by Type of Depression.
Sources: Blumberger et al,82,83Fitzgerald et al,87,106,107Garcia-Toro et al,108Loo et al,120McDonald et al,121Pallanti et al,117Valkonen-Korhonen et al.49
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
Figure A20: Response Rate for Bilateral rTMS Versus Sham rTMS at End of Treatment With Studies Using Hamilton Depression Rating Scale.
Sources: Blumberger et al,82,83Fitzgerald et al,87,106,107,118,119Garcia-Toro et al,108Loo et al,120McDonald et al,121Pallanti et al,117Valkonen-Korhonen et al.49
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
iTBS Versus Sham TBS
Figure A21: Risk Difference for iTBS Versus Sham TBS on Response Rate at End of Treatment.
Abbreviations: CI, confidence interval; df, degrees of freedom; iTBS, intermittent theta burst stimulation; M-H, Mantel–Haenszel test; TBS, theta burst stimulation.
Bilateral TBS Versus Sham TBS
Figure A22: Risk Difference for Bilateral TBS Versus Sham TBS on Response Rate at End of Treatment.
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel test; TBS, theta burst stimulation.
Deep TMS Versus Sham TMS
Figure A23: Risk Difference for Deep TMS Versus Sham TMS on Response Rate at End of Treatment.
Sources: Kaster et al,48Levkovitz et al,126Tavares et al.110
Abbreviations: CI, confidence interval; df, degrees of freedom; dTMS, deep transcranial magnetic stimulation; M-H, Mantel–Haenszel test.
Figure A24: Response Rate for Deep TMS Versus Sham TMS at Follow-Up (8 and 16 Weeks).
Abbreviations: CI, confidence interval; df, degrees of freedom; dTMS, deep transcranial magnetic stimulation; M-H, Mantel–Haenszel test.
High-Frequency Left DLPFC rTMS Versus Sham rTMS
Figure A25: Risk Difference for High-Frequency Left DLPFC rTMS Versus Sham rTMS on Remission Rate at End of Treatment.
Sources: Avery et al,80Bakim et al,73 Blumberger et al,82,83George et al,89Herwig et al,91Kang et al,47Loo et al,93Mogg et al,113O'Reardon et al,95Padberg et al,111Rossini et al,114Stern et al,77Su et al,74Taylor et al,115Theleritis et al,75Yesavage et al.50
Abbreviations: CI, confidence interval; df, degrees of freedom; HF, high frequency; L-DLPFC, left dorsolateral prefrontal cortex; M-H, Mantel– Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
Figure A26: Remission Rate for High-Frequency Left DLPFC rTMS Versus Sham rTMS at 6-Week Follow-Up.
Sources: Blumberger et al,82,83O'Reardon et al,95Stern et al,77Yesavage et al.50
Abbreviations: CI, confidence interval; df, degrees of freedom; HF, high frequency; L-DLPFC, left dorsolateral prefrontal cortex; M-H, Mantel–Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
Figure A27: Remission Rate for High-Frequency Left DLPFC rTMS Versus Sham rTMS at End of Treatment for Studies Using Hamilton Depression Rating Scale.
Sources: Avery et al,80Bakim et al,73 Blumberger et al,82,83George et al,89Herwig et al,91Kang et al,47Loo et al,93Mogg et al,113O'Reardon et al,95Padberg et al,111Rossini et al,114Stern et al,77Su et al,74Taylor et al,115Theleritis et al,75Yesavage et al.50
Abbreviations: CI, confidence interval; df, degrees of freedom; HF, high frequency; L-DLPFC, left dorsolateral prefrontal cortex. M-H, Mantel– Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
Low-Frequency Right DLPFC rTMS Versus Sham rTMS
Figure A28: Risk Difference for Low-Frequency Right DLPFC rTMS Versus Sham rTMS on Remission Rate at End of Treatment.
Sources: Bares et al,99Brunelin et al,116Januel et al,101Kauffmann et al,102Mantovani et al,104Pallanti et al,117Stern et al.77
Abbreviations: CI, confidence interval; df, degrees of freedom; LF, low frequency; M-H, Mantel–Haenszel test; R-DLPFC, right dorsolateral prefrontal cortex; rTMS, repetitive transcranial magnetic stimulation.
Figure A29: Remission Rate for Low-Frequency Right DLPFC rTMS Versus Sham rTMS at End of Treatment With Studies Using the Hamilton Depression Rating Scale.
Sources: Bares et al,99Brunelin et al,116Januel et al,101Kauffmann et al,102Mantovani et al,104Pallanti et al,117Stern et al.77
Abbreviations: CI, confidence interval; df, degrees of freedom; LF, low frequency; M-H, Mantel–Haenszel test; R-DLPFC, right dorsolateral prefrontal cortex; rTMS, repetitive transcranial magnetic stimulation.
Bilateral rTMS Versus Sham rTMS
Figure A30: Risk Difference for Bilateral rTMS Versus Sham rTMS on Remission Rate at End of Treatment.
Sources: Blumberger et al,82,83Fitzgerald et al,106,107,118,119McDonald et al,121Pallanti et al,117Valkonen-Korhonen et al.49
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
Figure A31: Remission Rate for Bilateral rTMS Versus Sham rTMS at 6-Week Follow-Up.
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel–Haenszel test; rTMS, repetitive transcranial magnetic stimulation.
Deep TMS Versus Sham rTMS
Figure A32: Risk Difference for Deep TMS Versus Sham TMS on Remission Rate at End of Treatment.
Sources: Kaster et al48, Levkovitz et al,126Tavares et al.110
Abbreviations: CI, confidence interval; df, degrees of freedom; dTMS, deep transcranial magnetic stimulation; M-H, Mantel–Haenszel test; TMS, transcranial magnetic stimulation.
Figure A33: Remission Rate for Deep TMS Versus Sham TMS at Follow-Up (8 and 16 Weeks).
Abbreviations: CI, confidence interval; df, degrees of freedom; dTMS, deep transcranial magnetic stimulation; M-H, Mantel–Haenszel test; TMS, transcranial magnetic stimulation.
Table A11:
Adverse Events Reported by Included Primary Studies (Part 1)
| Author, Year | Headache | Scalp Discomfort | Paina | Fatigue | Dizzinessb | Insomniac | Eye Problemsd | Nasal Problemse | Ear Problemsf | Mouth Problemsg | GI Issuesh |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anderson et al, 200778 | rTMS 2/11 Sham 0/14 | ||||||||||
| Avery et al, 199979 | rTMS 4/4 Sham 2/2 | ||||||||||
| Avery et al, 200680 | rTMS 11/33 Sham 1/30 | ||||||||||
| Bakim et al, 201273 | rTMS 4/23 Sham 1/12 | rTMS 2/23 Sham 0/12 | |||||||||
| Berman et al, 200081 | rTMS 6/10 Sham 5/10 | ||||||||||
| Blumberger et al, 201283 | rTMSi 1/22 Sham 0/15 | rTMSi 1/22 Sham 0/15 | |||||||||
| Blumberger et al, 201682j | Bi 7/40 Uni 7/40 Sham 7/41 | Bi 7/40 Uni 10/40 Sham 3/41 | Bi 2/40 Uni 2/40 Sham 1/41 | Bi 1/40 Uni 0/40 Sham 0/41 | Bi 3/40 Uni 2/40 Sham 1/41 | Bi 1/40 Uni 0/40 Sham 0/41 | Bi 1/40 Uni 0/40 Sham 0/41 | Bi 1/40 Uni 2/40 Sham 1/41 | |||
| Boutros et al, 2002186 | rTMS 8/12 Sham 5/9 | rTMS 3/12 Sham 1/9 | rTMS 1/12 Sham 0/9 | ||||||||
| Fitzgerald et al, 200386j | rTMS 6/40 Sham 0/40 | rTMS 2/40 Sham 1/40 | |||||||||
| Fitzgerald et al, 2006a106 | rTMS 5/25 Sham 2/22 | rTMS 3/25 Sham 0/22 | |||||||||
| Garcia-Toro et al, 200188 | rTMS 6/17 Sham 0/18 | ||||||||||
| George et al, 201089k | rTMS 29/92 Sham 23/98 | rTMS 17/92 Sham 10/98 | rTMS 0/92 Sham 1/98 | rTMS 5/92 Sham 4/98 | rTMS 2/92 Sham 0/98 | rTMS 7/92 Sham 10/98 | rTMS 6/92 Sham 3/98 | ||||
| Herwig et al, 200791 | rTMS 3/52 Sham 1/53 | rTMS 1/52 Sham 2/53 | rTMS 0/52 Sham 1/53 | rTMS 1/52 Sham 0/53 | |||||||
| Kaster et al, 201848 | rTMS 14/25 Sham 10/27 | rTMS 4/25 Sham 0/27 | rTMS 1/25 Sham 0/27 | rTMS 1/25 Sham 0/27 | rTMS 2/25 Sham 0/27 | rTMS 1/25 Sham 1/27 | rTMS 1/25 Sham 1/27 | ||||
| Levkovitz et al, 2015126 | rTMS 27/89 Sham 21/92 | rTMS 8/89 Sham 2/92 | rTMS 0/89 Sham 3/92 | rTMS 2/89 Sham 4/92 | |||||||
| Li et al, 2014109 | cTBS 1/15 iTBS 3/15 Bi TBS 1/15 Sham 2/15 | cTBS 1/15 iTBS 2/15 Bi TBS 5/15 Sham 1/15 | cTBS 0/15 iTBS 2/15 Bi TBS 1/15 Sham 0/15 | ||||||||
| Loo et al, 2003120 | rTMS 3/9 Sham 0/10 | rTMS 5/9 Sham 0/10 | rTMS 1/9 Sham 0/10 | ||||||||
| Loo et al, 200793 | rTMS 8/18 Sham 0/18 | rTMS 15/18 Sham 0/18 | rTMS 1/18 Sham 0/18 | rTMS 4/18 Sham 0/18 | rTMS 0/18 Sham 1/18 | ||||||
| Mantovani et al, 2013104 | rTMS 2/12 Sham 3/13 | rTMS 1/12 Sham 2/13 | rTMS 2/12 Sham 2/13 | ||||||||
| Mogg et al, 2008113 | rTMS 0/28 Sham 1/29 | rTMS 0/28 Sham1/29 | |||||||||
| Mosimann et al, 200494 | rTMS 0/15 Sham 2/9 | rTMS 0/15 Sham 1/9 | rTMS 3/15 Sham 0/9 | rTMS 2/15 Sham 0/9 | rTMS 1/15 Sham 2/9 | ||||||
| O'Reardon et al, 200795 | rTMS 18/165 Sham 2/158 | rTMS 84/165 Sham 12/158 | rTMS 10/165 Sham 3/158 | rTMS 12/165 Sham 1/158 | |||||||
| Plewnia et al, 2014125 | rTMS 2/16 Sham 3/16 | rTMS 1/16 Sham 0/16 | rTMS 1/16 Sham 0/16 | ||||||||
| Rossini et al, 2005114l | 100% 2/18 80% 2/18 Sham 0/16 | 100% 3/18 80% 0/18 Sham 0/16 | |||||||||
| Su et al, 200574 | rTMS 2/10 Sham 1/10 | ||||||||||
| Tavares et al, 2017110 | rTMS 9/20 Sham 10/23 | rTMS 5/20 Sham 0/23 | rTMS 11/20 Sham 10/23 | rTMS 5/20 Sham 2/23 | |||||||
| Theleritis et al, 201775m | rTMS 3/25 Sham 1/18 | rTMS 7/25 Sham 1/18 | |||||||||
| Triggs et al, 201076 | rTMS 7/18 Sham 3/7 | rTMS 6/18 Sham 1/7 | rTMS 1/18 Sham 0/7 | rTMS 5/18 Sham 2/7 | rTMS 3/18 Sham 1/7 | rTMS 1/18 Sham 1/7 | rTMS 1/18 Sham 0/7 | rTMS 4/18 Sham 0/7 | |||
| Yesavage et al, 201850 | rTMS 15/73 Sham 16/77 | rTMS 8/73 Sham 8/77 | |||||||||
| Total | rTMS 183/784 Sham 116/616 | rTMS 93/620 Sham 21/573 | rTMS 137/563 Sham 34/517 | rTMS 14/190 Sham 7/146 | rTMS 18/404 Sham 6/326 | rTMS 15/279 Sham 16/238 | rTMS 20/250 Sham 3/229 | rTMS 10/98 Sham 8/104 | rTMS 7/140 Sham 3/102 | rTMS 16/285 Sham 2/235 | rTMS 22/370 Sham 8/290 |
Abbreviations: Bi, bilateral; cTBS, continuous theta burst stimulation; GI, gastrointestinal; iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation; TBS, theta burst stimulation; Uni, unilateral.
Includes skin pain, back pain, facial pain, sore hip, muscle aches, neck pain and stiffness, scraping feeling, dermatitis, burning sensation.
Includes light-headedness, feeling “high,” vertigo.
Includes difficulty sleeping, nightmares.
Includes eye pain, tearfulness, conjunctivitis, corneal abrasion, crying.
Includes sinusitis, nasopharyngitis.
Includes tinnitus, difficulty hearing.
Includes toothache, metallic taste, aphthous ulcer.
Includes nausea, flu, vomiting.
Adverse events in unilateral high-frequency left dorsolateral prefrontal cortex group.
Adverse events reported separately for each group (bilateral rTMS, unilateral high-frequency rTMS, sham).
Combined adverse events in both active rTMS groups (high frequency and low frequency).
Also reported “other” adverse events (rTMS 18/92, sham 15/98).
Adverse events reported by group (100% motor threshold, 80% motor threshold, sham).
Adverse events reported in active rTMS and sham TMS groups that received 1 treatment session daily.
Table A12:
Adverse Events Reported by Included Primary Studies (Part 2)
| Adverse Events | Facial Muscle Twitching | Agitationa | Worsening Mood, Anxiety, Depressionb | Difficulty Concentratingc | Impaired Cognition | Impaired Memory | Falls | Lactation | Tremor | Aversive Tactile Artifact | Unpleasant Feelings | Suicidal Thoughts |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blumberger et al, 201682 | Bi 0/40 Uni 1/40 Sham 0/41 | Bi 3/40 Uni 3/40 Sham 1/41 | Bi 1/40 Uni 0/40 Sham 0/41 | Bi 0/40 Uni 0/40 Sham 1/41 | Bi 0/40 Uni 0/40 Sham 1/41 | |||||||
| Boutros et al, 2002186 | rTMS 5/12 Sham 0/9 | |||||||||||
| Brunelin et al, 2014116d | rTMS 5/104 Sham 7/51 | |||||||||||
| George et al, 201089 | rTMS 0/92 Sham 1/98 | rTMS 6/92 Sham 8/98 | ||||||||||
| Kaster et al, 201848 | rTMS 0/25 Sham 1/27 | |||||||||||
| Levkovitz et al, 2015126 | rTMS 0/89 Sham 2/92 | |||||||||||
| Loo et al, 2003120 | rTMS 3/9 Sham 0/10 | |||||||||||
| Loo et al, 200793 | rTMS 3/18 Sham 0/18 | rTMS 1/18 Sham 0/18 | rTMS 1/18 Sham 0/18 | |||||||||
| Mantovani et al, 2013104 | rTMS 1/12 Sham 2/13 | rTMS 1/12 Sham 1/13 | rTMS 1/12 Sham 1/13 | |||||||||
| Mosimann et al, 200494 | rTMS 1/15 Sham 0/9 | |||||||||||
| O'Reardon et al, 200795 | rTMS 34/165 Sham 5/158 | |||||||||||
| Padberg et al, 2002111e | 100% 2/10 90% 3/10 Sham 0/10 | 100% 2/10 90% 3/10 Sham 0/10 | ||||||||||
| Plewnia et al, 2014125 | rTMS 1/16 Sham 0/16 | |||||||||||
| Tavares et al, 2017110 | rTMS 6/20 Sham 5/23 | |||||||||||
| Triggs et al, 201076 | rTMS 1/18 Sham 0/7 | |||||||||||
| Yesavage et al, 201850 | rTMS 8/73 Sham 3/77 | rTMS 3/73 Sham 7/77 | rTMS 3/73 Sham 4/77 | |||||||||
| Total | rTMS 38/293 Sham 6/281 | rTMS 2/98 Sham 0/59 | rTMS 29/490 Sham 22/414 | rTMS 12/44 Sham 7/45 | rTMS 1/12 Sham 1/13 | rTMS 1/12 Sham 1/13 | rTMS 3/73 Sham 7/77 | rTMS 1/80 Sham 0/41 | rTMS 1/96 Sham 1/57 | rTMS 5/20 Sham 0/10 | rTMS 3/20 Sham 0/10 | rTMS 4/168 Sham 5/127 |
Abbreviations: Bi, bilateral; rTMS, repetitive transcranial magnetic stimulation; Uni, unilateral.
Includes anger.
Includes racing thoughts.
Includes confusion.
Combined rTMS with and without venlafaxine.
Adverse events reported by group (100% motor threshold, 90% motor threshold, sham).
Appendix 6: Selected Excluded Studies—Clinical Evidence
For transparency, we provide a list of studies that readers might have expected to see but that did not meet the inclusion criteria, along with the primary reason for exclusion.
| Citation | Primary Reason for Exclusion |
|---|---|
| Bauer M, Severus E, Kohler S, Whybrow PC, Angst J, Moller HJ. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. Part 2: maintenance treatment of major depressive disorder—update 2015. World J Biol Psychiatry. 2015;16(2):76-95 | Study design (guideline) |
| Berlim M, van den Eynde F, Tovar-Perdormo S, Daskalakis ZJ. Response, remission and drop-out rated following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized double-blind and sham controlled trials. Psychol Med. 2014;44(2):225-39 | Literature search conducted in 2012 |
| Beuzon G, Timour Q, Saoud M. Predictors of response to repetitive transcranial magnetic stimulation (rTMS) in the treatment of major depressive disorder. Encephale. 2017;43(1):3-9 | Study design (not a systematic review) |
| Blue Cross Blue Shield Association; Kaiser Foundation Health Plan; Southern California Permanente Medical Group. Transcranial magnetic stimulation for depression. Technol Eval Cent Assess Program Exec Summ. 2014 Jan;28(9):1-4 | Study design (not a systematic review) |
| Blue Cross Blue Shield Association. Transcranial magnetic stimulation for depression (structured abstract). Chicago (IL): The Association. TEC Assessment. 2014:28(9). Available from: https://www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=32014000914&UserID=0 | Study design (structured abstract) |
| Brunoni AR, Sampaio-Junior B, Moffa AH, Aparicio LV, Gordon P, Klein I, et al. Noninvasive brain stimulation in psychiatric disorders: a primer. Braz J Psychiatry. 2019;41(1):70-81 | Study design (overview) |
| Brunoni AR, Chaimani A, Moffa AH, Razza LB, Gattaz WF, Wagner F, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis: correction. JAMA Psychiatry. 2017;74(4):424 | Study design (correction to original article) |
| Canadian Agency for Drugs and Technologies in Health. Transcranial magnetic stimulation for the treatment of adults with PTSD, GAD, or depression: a review of clinical effectiveness and guidelines (structured abstract). 2016;(4) | Study design (structured abstract) |
| Cao X, Deng C, Su X, Guo Y. Response and remission rates following high-frequency vs. low-frequency repetitive transcranial magnetic stimulation (rTMS) over right DLPFC for treating major depressive disorder (MDD): a meta-analysis of randomized, double-blind trials. Front Psychiatry. 2018;9:413 | Patient population |
| Chen JJ, Liu Z, Zhu D, Li Q, Zhang H, Huang H, et al. Bilateral vs. unilateral repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomized controlled trials. Psychiatry Res. 2014;219(1):51-7 | Literature search conducted in 2013 |
| Chen JJ, Zhao LB, Liu YY, Fan S, Xie P. Comparative efficacy and acceptability of electroconvulsive therapy versus repetitive transcranial magnetic stimulation for major depression: a systematic review and multiple-treatments meta-analysis. Behav Brain Res. 2017;320:30-6 | Patient population |
| Chen L, Chung SW, Hoy KE, Fitzgerald PB. Is theta burst stimulation ready as a clinical treatment for depression? Exp Rev Neurotherapeut. 2019;19(11):1-14 | Study design (not a systematic review) |
| Dobek CE, Blumberger DM, Downar J, Daskalakis ZJ, Vila-Rodriguez F. Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion. Neuropsychiatric Dis Treat. 2015;11:2975-87 | Patient population |
| Feifel D, Roth Y, Pell GS, Zangen A, Brunoni AR, Gattaz W, et al. Network meta-analysis in mental health research. JAMA Psychiatry. 2017;74(8):850-2 | Study design (comment) |
| Fitzgerald PB, Hoy KE, Anderson RJ, Daskalakis ZJ. A study of the pattern of response to rTMS treatment in depression. Depress Anxiety. 2016;33(8):746-53 | Outcomes measured |
| Flynn K. Transcranial magnetic stimulation (TMS) for depression (structured abstract). 2016;(4). Available from: https://www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=32010001496&UserID=0 | Study design (structured abstract) |
| Galletly CA, Loo CK, Malhi G, Mitchell PB, Fitzgerald P. Why repetitive transcranial magnetic stimulation should be available for treatment resistant depression. Aust N Z J Psychiatry. 2015;49(2):182-3 | Study design (comment) |
| Gaynes BN, Lloyd SW, Lux L, Gartlehner G, Hansen RA, Brode S, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J Clin Psychiatry. 2014; 75(5):477-89 | Literature search conducted in 2013 |
| Gaynes BN, Lux L, Lloyd S, Hansen RA, Gartlehner G, Thieda P, et al. Nonpharmacologic interventions for treatment-resistant depression in adults (structured abstract). 2016;(4). Available from: https://www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=32011001375&UserID=0 | Study design (structured abstract) |
| Gellersen HM, Kedzior KK. Antidepressant outcomes of high-frequency repetitive transcranial magnetic stimulation (rTMS) with F8-coil and deep transcranial magnetic stimulation (DTMS) with H1-coil in major depression: a systematic review and meta-analysis. BMC Psychiatry. 2019;19(1):139 | Study design (not all studies had a control group) |
| Gellersen HM, Kedzior KK. An update of a meta-analysis on the clinical outcomes of deep transcranial magnetic stimulation (DTMS) in major depressive disorder (MDD). Zeitschrift für Psychologie 2018;226(1):30-44 | Study design (not all studies had a control group) |
| Hauer L, Sellner J, Brigo F, Trinka E, Sebastianelli L, Saltuari, L, et al. Effects of repetitive transcranial magnetic stimulation over prefrontal cortex on attention in psychiatric disorders: a systematic review. J Clin Med. 2019;8(4):27 | Outcomes measured |
| HAYES, Inc. Transcranial magnetic stimulation (TMS) to enhance pharmacotherapy for depression (structured abstract). 2016(4). Available from: https://www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=32014000477&UserID=0 | Study design (structured abstract) |
| HAYES, Inc. Transcranial magnetic stimulation for treatment-resistant depression (structured abstract). 2016(4). Available from https://www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=32014000476&UserID=0 | Study design (structured abstract) |
| HAYES, Inc. Transcranial magnetic stimulation for major depression (structured abstract). 2016(4). Available from: https://www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=32010001390&UserID=0 | Study design (structured abstract) |
| Iimori T, Nakajima S, Miyazaki T, Tarumi R, Ogyu K, Wada M. Effectiveness of the prefrontal repetitive transcranial magnetic stimulation on cognitive profiles in depression, schizophrenia, and Alzheimer's disease: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:31-40 | Outcomes measured |
| Kedzior KK, Azorina V, Reitz SK. More female patients and fewer stimuli per session are associated with the short-term antidepressant properties of repetitive transcranial magnetic stimulation (rTMS): a meta-analysis of 54 sham-controlled studies published between 1997-2013. Neuropsychiatric Dis Treatment. 2014;10:727-56 | Literature search conducted in 2013 |
| Kedzior KK, Gellersen HM, Brachetti AK, Berlim MT. Deep transcranial magnetic stimulation (DTMS) in the treatment of major depression: an exploratory systematic review and meta-analysis. J Affect Dis. 2015;187:73-83 | Study design (not all studies had a control group) |
| Kedzior KK, Gierke L, Gellersen HM, Berlim MT. Cognitive functioning and deep transcranial magnetic stimulation (DTMS) in major psychiatric disorders: a systematic review. J Psychiatric Res. 2016;75:107-15 | Outcomes measured |
| Kedzior KK, Reitz SK. Short-term efficacy of repetitive transcranial magnetic stimulation (rTMS) in depression—reanalysis of data from meta-analyses up to 2010. BMC Psychology. 2014;2(1):39 | Literature search conducted in 2008 |
| Kedzior KK, Reitz SK, Azorina V, Loo CK. Durability of the antidepressant effect of the high-frequency repetitive transcranial magnetic stimulation (rTMS) in the absence of maintenance treatment in major depression: a systematic review and meta-analysis of 16 double-blind, randomized, sham-controlled trials. Depress Anxiety. 2015;32(3):193-203 | Literature search conducted in 2013 |
| Kedzior KK, Schuchinsky M, Gerkensmeier I, Loo CK. Challenges in comparing the acute cognitive outcomes of high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) vs. electroconvulsive therapy (ECT) in major depression: a systematic review. J Psychiatric Res. 2017;91:14-17 | Outcomes measured |
| Kiebs M, Hurlemann R, Mutz J. Repetitive transcranial magnetic stimulation in non-treatment-resistant depression. Br J Psychiatry. 2019;215(2):445-6 | Patient population |
| Kisely S, Li A, Warren N, Siskind D. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468-80 | Intervention used |
| Lepping P, Schonfeldt-Lecuona C, Sambhi R, Lanka S, Lane S, Whittington R, et al. “A systematic review of the clinical relevance of repetitive transcranial magnetic stimulation”: Corrigendum. Acta Psychiatr Scand. 2014;130(5):341 | Study design (correction to original article) |
| Lewis G. Transcranial magnetic stimulation for depression. Lancet. 2018;391(10131):1639-40 | Study design (comment) |
| Liu B, Zhang Y, Zhang L, Li L. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342 | Literature search conducted in 2013 |
| Marques RC, Vieira L, Marques D, Cantilino A. Transcranial magnetic stimulation of the medial prefrontal cortex for psychiatric disorders: a systematic review. Braz J Psychiatry 2019;30:30 | Patient population |
| McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1):16cs10905 | Study design (consensus recommendations) |
| McGirr A, Berlim MT. Clinical usefulness of therapeutic neuromodulation for major depression: a systematic meta-review of recent meta-analyses. Psychiatric Clin North Am. 2018;41(3):485-503 | Patient population |
| Medical Services Advisory Committee. Repetitive transcranial magnetic stimulation (rTMS) for the treatment of depression (structured abstract). 2016(4). Available from: https://www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=32015000580&UserID=0 | Literature search conducted in 2013 |
| Micallef-Trigona B. Comparing the effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in the treatment of depression: a systematic review and meta-analysis. Depress Res Treat. 2014;1-8. | Literature search conducted in 2013 |
| Moyer ML, Cristancho MA, O'Reardon JP. Clinical efficacy of transcranial magnetic stimulation in depression. Title: A Clinical Guide to Transcranial Magnetic Stimulation. Holtzheimer PE, McDonald W, editors. Oxford, UK: Oxford University Press, 2014:17-31 | Study design (book chapter) |
| Mutz J, Vipulananthan V, Carter B, Hurlemann R, Fu CH, Young AH. Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis. BMJ. 2019;364:1079 | Patient population |
| Papadimitropoulou K, Vossen C, Karabis A, Donatti C, Kubitz N. Comparative efficacy and tolerability of pharmacological and somatic interventions in adult patients with treatment-resistant depression: a systematic review and network meta-analysis. Current Med Res Opin. 2017;33(4):701-11 | Comparator used |
| Perera T, George MS, Grammar G, Janicak PG, Pascual-Leone A, Wirecki TS. The clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-46 | Study design (systematic review used to inform guidelines was included) |
| Pohar R, Farrah K. Repetitive transcranial magnetic stimulation for patients with depression: a review of clinical effectiveness, cost- effectiveness and guidelines—an update. Ottawa (ON): CADTH. 2019:6:28. | Study design (not a systematic review) |
| Qin B, Chen H, Gao W, Zhao LB, Zhao MJ, Qin H, et al. Effectiveness of high-frequency repetitive transcranial magnetic stimulation in patients with depression and Parkinson's disease: a meta-analysis of randomized, controlled clinical trials. Neuropsychiatric Dis Treat. 2018;14:273-84 | Patient population |
| Ren J, Li H, Palaniyappan L, Wang J, Li C, Rossini PM. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Progr Neuropsychopharmacol Biol Psychiatry. 2014;51:181-9 | Literature search conducted in 2013 |
| Rodriguez M, Luis J, Barbanoj M, Schlaepfer J, Te C, et al. Transcranial magnetic stimulation for treating depression (structured abstract) 2018(11). Available from: https://www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=10000003493&UserID=0 | Patient population |
| Roth Y, Pell GS, Zangen A. Network meta-analysis in mental health research. JAMA Psychiatry. 2017;74(8):851 | Study design (comment) |
| Sarkar S, Grover S. A systematic review and meta-analysis of trials of treatment of depression from India. Ind J Psychiatry. 2014;56(1):29-38 | Patient population |
| Senova S, Cotovio G, Pascual-Leone A, Oliveira-Maia, AJ. Durability of antidepressant response to repetitive transcranial magnetic stimulation: systematic review and meta-analysis. Brain Stimul. 2019;12(1):119-28 | Patient population |
| Sonmez AI, Camsari DD, Nandakumar AL, Voort JL, Kung S, Lewis C, et al. Accelerated TMS for depression: a systematic review and meta-analysis. Psychiatry Res. 2019;273:770-81 | Patient population |
| Teng S, Guo Z, Peng H, Xing G, Chen H, He B, et al. High-frequency repetitive transcranial magnetic stimulation over the left DLPFC for major depression: session-dependent efficacy: a meta-analysis. Eur Psychiatry. 2017;41:75-84 | Patient population |
| Voigt J, Carpenter L, Leuchter A. A systematic literature review of the clinical efficacy of repetitive transcranial magnetic stimulation (rTMS) in non-treatment resistant patients with major depressive disorder. BMC Psychiatry. 2019;19(1):13 | Patient population |
| Wei Y, Zhu J, Pan S, Su H, Li H, Wang J. Meta-analysis of the efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. Shanghai Arch Psychiatry. 2017;29(6):328-42 | Patient population |
| Zis P, Shafique F, Hadjivassiliou M, Blackburn D, Venneri A, Iliodromiti S, et al. Safety, tolerability, and nocebo phenomena during transcranial magnetic stimulation: a systematic review and meta-analysis of placebo-controlled clinical trials. Neuromodulation. 2020;23(3):291-300 | Patient population |
Appendix 7: Results of Applicability and Limitation Checklists for Studies Included in the Economic Literature Review
Table A13:
Assessment of the Applicability of Studies Evaluating the Cost-Effectiveness of rTMS
| Author, Year, Country | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system studied sufficiently similar to Ontario? | Were the perspectives clearly stated? If yes, what were they? | Are all direct effects included? Are all other effects included where they are material? | Are all future costs and outcomes discounted? If yes, at what rate? | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall Judgmenta |
|---|---|---|---|---|---|---|---|---|---|
| Fitzgibbon et al, 2019143 (Ontario, Canada) | Yes | Yes (Partially) rTMS followed by ECT versus ECT alone | Yes | Yes (perspective of Ontario Ministry of Health) | Yes | Yes (discount rate of 1.5%) | Yes | Yes | Partially applicable |
| HQO, 2014 (published 2016),13 Canadad | Yes | Yes (Partially) rTMS vs. ECT rTMS vs. sham | Yes | Yes (perspective of Ontario's Ministry of Health and Long-Term Care) | Yes | No (time horizon was 6 mo) | Yes | No | Partially applicable |
| University of Calgary, 2014,34 Canada | Yes | Yes rTMS vs. ECT rTMS vs. sham | Yes | Yes (perspective of Alberta's Ministry of Health) | Yes | No (time horizon was 3–6 mo) | Yes | No | Partially applicable |
| Zhao et al, 2017, Singapore144 | Yes | Yes rTMS vs. ECT | Partially | Yes (Societal perspective) | Yes | No (1-y time horizon) | Yes | Yes | Unclear |
| Voigt et al, 2017, USA145 | Partially (adults newly diagnosed with major depression, who failed to benefit from single-medication trial) | Yes (Partially) rTMS vs. medication | Yes | Yes (perspective of health care sector) | Yes | Yes (lifetime horizon) | Yes | Yes | Not applicable |
| Nguyen et al, 2015, Australia148 | Yes | Yes (partially) rTMS vs. medication | Yes | Yes (perspective of health care sector) | Yes | Yes (3-y time horizon) | Yes | No | Partially applicable |
| Vallejo-Torres et al, 2015, Spain149 | Yes | Yes (partially) ECT alone vs. rTMS alone vs. rTMS followed by ECT alone | Yes | Yes (perspective of health care sector) | Yes | No (time horizon was 1 y) | Yes | No | Partially applicable |
| Ghiasvand et al, 2016, Iran147 | Yes (partially, adults with major depression) | Yes (partially) rTMS vs. ECT | No | Yes (perspective of health care sector) | Yes | No (time horizon was 7 mo) | No | No | Not applicable |
Abbreviations: ECT, electroconvulsive therapy; HQO, Health Quality Ontario; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; rTMS, repetitive transcranial magnetic stimulation; TRD, treatment-resistant depression.
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Overall judgment could be “directly applicable,” “partially applicable,” or “not applicable.”
Table A14:
Assessment of the Limitations of Canada-Based Studies Evaluating the Cost-Effectiveness of rTMS
| Author, Year, Country | Does the model structure adequately reflect the nature of the health condition under evaluation? | Is the time horizon sufficiently long to reflect all important differences in costs and outcomes? | Are all important and relevant health outcomes included? | Are the clinical inputsa obtained from the best available sources? | Do the clinical inputsa match the estimates contained in the clinical sources? | Are all important and relevant (direct) costs included in the analysis? | Are the estimates of resource use obtained from the best available sources? | Are the unit costs of resources obtained from the best available sources? | Is an appropriate incremental analysis presented, or can it be calculated from the reported data? | Are all important and uncertain parameters subjected to appropriate sensitivity analysis? | Is there a potential conflict of interest? | Overall Judgmentb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fitzgibbon et al, 2019,143 Canada (Ontario) | Yes (6-mo cycle for total of 164 cycles of a lifetime model. Main clinical states (acute treatment, remission, relapse, maintenance, and death were included | Yes. Lifetime horizon was applied | Yes | Yes (didn't use relative effects, but all efficacy inputs were obtained from meta-analysis of RCTs) | Yes | Yes | Yes (used Ontario sources) | Yes | Yes | Yes | No | Minor limitations |
| Health Quality Ontario, 2014 (published 2016),13 Canadad | Yes (12 monthly cycles due to unavailability of valid long-term clinical data, main clinical states (e.g., acute treatment with adverse events, remission, relapse, and death) | No. Time horizon is 1 y due to unavailability of long-term clinical data. No long-term costs and outcomes were captured | Yes | Yes (didn't use relative effects, but all efficacy inputs were obtained from meta-analysis of RCTs) | Yes | Yes | Yes (used Ontario sources) | Yes | Yes | Yes | No | Minor limitations |
| University of Calgary, 201434 (Alberta), Canada | Yes (3- to 6- week time horizon and, given unavailability of valid long-term data on effectiveness, only response and remission data were included) | No. Time horizon is 3–6 weeks (longest duration of follow-up reported in RCTs assessing clinical effectiveness). No long-term costs and outcomes were captured | Yes | Yes (relative effects of rTMS were obtained from meta-analysis of RCTs) | Yes | Yes | Yes (used Alberta sources) | Yes | Yes | Yes | No | Minor limitations |
Abbreviations: RCT, randomized controlled trial; rTMS, repetitive transcranial magnetic stimulation.
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Clinical inputs include relative treatment effects, natural history, and utilities.
Overall judgment could be “minor limitations,” “potentially serious limitations,” or “very serious limitations.”
Appendix 8: Scatter Plots
Figures A34 and A35 represent the uncertainty around the estimated incremental cost-effectiveness ratios (ICERs) generated in the probabilistic sensitivity analysis (PSA) when comparing high-frequency repetitive transcranial magnetic stimulation (rTMS; in a stepped care approach) versus electroconvulsive therapy (ECT) and intermittent theta burst stimulation (iTBS; in a stepped care approach) versus ECT.
Figure A34 shows a spread of the simulated ICERs across the cost-effectiveness plane and uncertainty around the ICER estimate (high-frequency rTMS versus ECT).
Figure A34: Scatter Plot of Simulated Pairs of Incremental Costs and Effects in the Cost-Effectiveness Plane: High-Frequency rTMS Versus ECT.
Abbreviations: ECT, electroconvulsive therapy; QALY, quality-adjusted life-year; rTMS, repetitive transcranial magnetic stimulation.
Figure A35 shows spread of the simulated ICERs across the cost-effectiveness plane and uncertainty around the ICER estimate (iTBS vs. ECT).
Figure A35: Scatter Plot of Simulated Pairs of Incremental Costs and Effects in the Cost-Effectiveness Plane: High-Frequency rTMS Versus ECT.
Abbreviations: ECT, electroconvulsive therapy; iTBS, intermittent theta burst stimulation; QALY, quality-adjusted life-years.
Figures A36 and A37 represent uncertainty around the estimated ICERs generated in the PSA.
Figure A36 shows spread of the simulated ICERs across the cost-effectiveness plane and uncertainty around the ICER estimate (high-frequency rTMS vs. pharmacotherapy alone).
Figure A36: Scatter Plot of Simulated Pairs of Incremental Costs and Effects in the Cost-Effectiveness Plane: High-Frequency rTMS Versus Pharmacotherapy Alone.
Abbreviations: QALY, quality-adjusted life-year; rTMS, repetitive transcranial magnetic stimulation.
Figure A37 shows a spread of the simulated ICERs across the cost-effectiveness plane and uncertainty around the ICER estimate (iTBS versus pharmacotherapy alone).
Figure A37: Scatter Plot of Simulated Pairs of Incremental Costs and Effects in the Cost-Effectiveness Plane: iTBS Versus Pharmacotherapy Alone.
Abbreviations: iTBS, intermittent theta burst stimulation; QALY, quality-adjusted life-year.
Appendix 9: Estimated Number of rTMS Machines Over the Next 5 Years
Table A15:
Estimated Number of rTMS Machines in Current and Future rTMS Clinics Over 5 Years in Ontario
| rTMS Clinics | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HF rTMS | iTBS | HF rTMS | iTBS | HF rTMS | iTBS | HF rTMS | iTBS | HF rTMS | iTBS | |
| Public (Clinical and Research) | ||||||||||
| London (Parkwood Institute)a | 1 | 1 | 1 | 2 | 1 | 3 | 1 | 3 | 1 | 5 |
| Toronto (Sunnybrook)b | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 2 |
| Toronto (Toronto Western Hospital, University Health Network)c | 4 | 1 | 4 | 1 | 4 | 2 | 4 | 2 | 4 | 2 |
| Toronto Centre for Addiction and Mental Healthd | — | 4 | — | 4 | — | 5 | — | 5 | — | 5 |
| Hamilton (St Joseph's Hospitale | 2 | 0 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 |
| Kingston (Providence Care)f | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 3 |
| Ottawa (Ottawa Hospital)g | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 |
| Markham (Markham-Stouffville Hospital)h | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Looking to Start an rTMS Program | ||||||||||
| Toronto (Humber River) | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Peterborough (Peterborough Regional Health Centre) | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Whitby (Ontario Shores) | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 2 |
| Thunder Bay (Thunder Bay Regional Health Sciences Centre) | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Sudbury (Health Sciences North) | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
Abbreviations: HF, high-frequency; iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation.
Two machines are used for brief treatments (email communication with A. Burhan, MD, on December 9, 2019, and December 27, 2019).
Deep rTMS machine provides long treatment (email communication with P. Giacobbe, MD, on December 9, 2019, and January 7, 2020).
All rTMS machines are used for brief treatments (email communication with J. Downar, MD, on December 23, 2019).
All rTMS machines are used for brief treatments (email communication with D. Blumberger, MD, on December 27, 2019). Some rTMS machines can deliver both long and brief treatment and can be placed in either column.
Two rTMS machines provide long treatments (communication with Dr. McCabe, MD, on December 12 and 13, 2019, and January 9, 2020, and with G. Hasey, MD, on January 15, 2020).
All rTMS machines are used for brief treatments (email communication with R. Milev, MD, on December 9 and 27, 2019).
The only rTMS machine can provide both long and brief treatments. For our calculations, we assumed brief treatments would be provided (email communication with D. Trembley, MD, on January 8, 2020).
Email communication with D. Blumberger, MD, on December 10, 2019.
Appendix 10: Estimated Numbers of rTMS Sessions Over Next 5 Years
Table A16:
Estimated Number of rTMS Sessions Over 5 Years, Classified by Type of Treatment in Current rTMS Clinics in Ontario: Reference Case Analysis
| Current rTMS Clinics (Reference Case) | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Long treatments (high-frequency rTMS)a | 5,760 | 5,760 | 5,760 | 5,760 | 5,760 |
| Brief treatments (iTBS)b | 84,480 | 100,320 | 126,720 | 126,720 | 147,840 |
Abbreviations: iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation.
Long treatment is defined as a 37.5-minute session. Long session per rTMS machine was defined as 8 sessions that a machine can operate daily (communication with D. Blumberger, MD, on October 30, 2019).
Brief treatment is defined as a 3-minute session. Brief sessions per rTMS machine was defined as 22 sessions that a machine can operate daily (communication with D. Blumberger, MD, on October 30, 2019).
Table A17:
Estimated Number of rTMS Sessions Over 5 Years, Classified by Types of Treatment in Current and Future rTMS Clinics in Ontario: Scenario Analysis
| Current and Future (Looking-to-Start) rTMS Clinics (Scenario Analysis) | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Long treatments (high-frequency rTMS)a | 5,760 | 5,760 | 5,760 | 5,760 | 5,760 |
| Brief treatments (iTBS)b | 84,480 | 126,720 | 153,120 | 158,400 | 179,520 |
Abbreviations: iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation.
Long treatment is defined as a 37.5-minute session. Each rTMS machine was considered capable of delivering 8 sessions daily (communication with D. Blumberger, MD, on October 30, 2019).
Brief treatment was defined as a 3-minute session. Each rTMS machine was considered capable of delivering 22 sessions daily (communication with D. Blumberger, MD, on October 30, 2019).
Appendix 11: Budget Impact Analysis of Adopting rTMS in Ontario When Capital Cost is Excluded
Table A18:
Summary of Per-Session Costs by High-Frequency rTMS and iTBS When Capital Cost is Excluded
| Treatment Duration | Per-Session Cost ($) |
|---|---|
| Long treatment (high-frequency rTMS) | 106.77 |
| Brief treatment (iTBS) | 96.35 |
Abbreviations: iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation.
Table A19:
Budget Impact Analysis of Adopting rTMS in Existing and Future (Looking-to-Start) rTMS Clinics in Ontario When Capital Cost is Excluded
| Scenarios | Year 1 ($) | Year 2 ($) | Year 3 ($) | Year 4 ($) | Year 5 ($) | Total ($) |
|---|---|---|---|---|---|---|
| Current scenario | 0 | 0 | 0 | 0 | 0 | 0 |
| Costs specific to new scenarios | ||||||
| Long treatments (high-frequency rTMS)a | 0.61 | 0.61 | 0.61 | 0.61 | 0.61 | 3.05 |
| Brief treatments (iTBS) in existing rTMS clinics onlyb | 8.14 | 9.66 | 12.21 | 12.21 | 14.24 | 56.46 |
| Brief treatments (iTBS) in both existing and future (looking-to-start) rTMS clinics | 8.14 | 12.21 | 14.75 | 15.26 | 17.30 | 67.66 |
| Training costs | 0.09 | 0.09 | 0.1 | 0.1 | 0.11 | 0.49 |
| Total costs (new scenario) incurred from existing rTMS clinics onlyc | 8.84 | 10.36 | 12.92 | 12.92 | 14.96 | 60.00 |
| Total costs (new scenario) incurred from both existing and future (looking-to-start) rTMS clinicsc | 8.84 | 12.91 | 15.46 | 15.97 | 18.02 | 71.20 |
| Budget impact to fund existing rTMS clinics onlyd | 8.84 | 10.36 | 12.92 | 12.92 | 14.96 | 60.00 |
| Budget impact to fund both existing and future (looking-to-start) rTMS clinicsd | 8.84 | 12.91 | 15.46 | 15.97 | 18.02 | 71.20 |
Abbreviations: iTBS, intermittent theta burst stimulation; rTMS, repetitive transcranial magnetic stimulation.
Long treatment is defined as a 37.5-minute session. An rTMS machine could run 8 sessions per day (communication with D. Blumberger, MD, on October 30, 2019). All costs expressed in 2019 Canadian dollars.
Brief treatment is defined as a 3-minute session. An rTMS machine could run 22 sessions per day (communication with D. Blumberger, MD, on October 30, 2019). All costs are expressed in 2019 Canadian dollars.
Total costs = Long treatments (high-frequency rTMS) + Brief treatments (iTBS) + Training costs.
Budget impact = New scenario – Current scenario.
Appendix 12: Letter of Information∗
Health Quality Ontario is now a part of Ontario Health
Appendix 13: Interview Guide†
Health Quality Ontario is now a part of Ontario Health.
Author contributions
This report was developed by a multidisciplinary team from Ontario Health. The clinical epidemiologist was Anna Lambrinos, the medical librarian was Caroline Higgins, the primary health economist was Hong Anh Tu, the secondary health economist was Olga Gajic, and the patient and public partnership analyst was David Wells.
Key Messages
What Is This Health Technology Assessment About?
Major depression is one of the most often diagnosed mental illnesses in Canada. It is a serious public health issue and can reduce social, emotional, physical, and mental function. Effects can vary, but people often have feelings of sadness, feelings of irritability, feelings of hopelessness, and difficulty taking pleasure in most activities.
Generally, people can be successfully treated with antidepressants, psychotherapy, or both. However, some people with major depression do not respond to these treatments. If people are diagnosed with treatment-resistant depression (TRD), other treatments can be tried. Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive treatment that delivers magnetic pulses to stimulate the area of the brain associated with mood regulation.
This health technology assessment looked at how safe, effective, and cost-effective rTMS is for people with TRD. It also looked at the budget impact of publicly funding rTMS and at the experiences, preferences, and values of people with TRD.
What Did This Health Technology Assessment Find?
Compared with sham treatment, most rTMS modalities led to lower depression scores and better response and remission rates. There was no difference in response or remission rates when comparing rTMS modalities with each other. Electroconvulsive therapy led to a greater reduction in depression scores than rTMS, but there was no difference in response or remission rates between the two treatments. Adverse events were minor and did not differ between rTMS and comparators. Publicly funding rTMS (high-frequency rTMS and intermittent theta burst stimulation) for the treatment of adults with TRD in Ontario over the next 5 years would result in additional costs ranging from $9.3 million in year 1 to $15.76 million in year 5, for a total of $63.2 million over the next 5 years. People with TRD had positive experiences and attitudes toward rTMS.
Contributor Information
Ontario Health (Quality):
Anna Lambrinos, Caroline Higgins, Hong Anh Tu, Olga Gajic, and David Wells
About Us
Ontario Health is an agency of the Government of Ontario. Our mandate is to connect and coordinate our province's health care system in ways that have not been done before to help ensure that Ontarians receive the best possible care. We work to support better health outcomes, patient experiences, provider experiences and value for money spent.
For more information about Ontario Health, visit ontariohealth.ca
References
- (1).Lam RW, McIntosh D, Wang J, Enns MW, Kolivakis T, Michalak EE, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 1. Disease burden and principles of care. Can J Psychiatry. 2016;61(9):510–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Ratnasingham SC, Rehm J, Manson H, Kurdyak PA. Opening eyes, opening minds: the Ontario burden of mental illness and addictions report. An ICES/PHO Report [Internet]. Toronto (ON): Institute for Clinical Evaluative Sciences and Public Health Ontario; 2012. Available from: https://www.publichealthontario.ca/-/media/documents/opening-eyes.pdf?la=en [Google Scholar]
- (3).Steensma C, Loukine L, Orpana H, McRae L, Vachon J, Mo F, et al. Describing the population health burden of depression: health-adjusted life expectancy by depression status in Canada. Health Promot Chronic Dis Prev Can. 2016;36(10):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).The Centre for Addiction and Mental Health. Depression [Internet]. Ottawa (ON): The Centre; 2019. [cited 2020 February 10]. Available from: https://www.camh.ca/en/health-info/mental-illness-and-addiction-index/depression [Google Scholar]
- (5).Health Quality Ontario. Major depression. Care for adults and adolescents [Internet]. Toronto (ON): Queen's Printer for Ontario; 2016. Available from: https://www.hqontario.ca/portals/0/documents/evidence/quality-standards/qs-depression-clinical-guide-1609-en.pdf [Google Scholar]
- (6).American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington (DC): The Association; 2013. [Google Scholar]
- (7).Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649–59. [DOI] [PubMed] [Google Scholar]
- (8).Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry. 2007;68 (Suppl 8):17–25. [PubMed] [Google Scholar]
- (9).Kennedy SH, Lam RW, Parikh SV, Patten SB, Ravindran AV. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. J Affect Disord. 2009;117 (Suppl 1):S1–S64. [DOI] [PubMed] [Google Scholar]
- (10).Public Health Agency of Canada. What is depression? [Internet]. Ottawa (ON): The Agency; 2016. [updated 2014 Dec 22; cited 2016 Jan]. Available from: http://www.phac-aspc.gc.ca/cdmc/mi-mm/depression-eng.php [Google Scholar]
- (11).McDonald KC, Bulloch AGM, Duffy A, Bresee L, Williams JVA, Lavorato DH, et al. Prevalence of bipolar I and II disorder in Canada. Can J Psychiatry. 2015;60(3):151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Rizvi SJ, Grima E, Tan M, Rotzinger S, Lin P, McIntyre RS, et al. Treatment-resistant depression in primary care across Canada. Can J Psychiatry. 2014;59(7):349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Health Quality Ontario. Repetitive transcranial magnetic stimulation for treatment-resistant depression: an economic analysis. Ont Health Technol Assess Ser. 2016;16(6):1–51. [PMC free article] [PubMed] [Google Scholar]
- (14).Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61(9):540–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Papadimitropoulou K, Vossen C, Karabis A, Donatti C, Kubitz N. Comparative efficacy and tolerability of pharmacological and somatic interventions in adult patients with treatment-resistant depression: a systematic review and network meta-analysis. Curr Med Res Opin. 2017;33(4):701–11. [DOI] [PubMed] [Google Scholar]
- (16).Health Quality Ontario. Internet-delivered cognitive behavioural therapy for major depression and anxiety disorders: a health technology assessment. Ont Health Technol Assess Ser. 2019;19(6):1–199. [PMC free article] [PubMed] [Google Scholar]
- (17).Milev RV, Giacobbe P, Kennedy SH, Blumberger DM, Daskalakis ZJ, Downar J, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61(9):561–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Health Quality Ontario. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis of randomized controlled trials. Ont Health Technol Assess Ser. 2016;16(5):1–66. [PMC free article] [PubMed] [Google Scholar]
- (19).Downar J, Blumberger D, Daskalakis Z. Repetitive transcranial magnetic stimulation: an emerging treatment for medication-resistant depression. CMAJ. 2016;188(16):1175–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ferry AT, Ongur D, An X, Price JL. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425(3):447–70. [DOI] [PubMed] [Google Scholar]
- (21).Yeterian EH, Pandya DN. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J Comp Neurol. 1991;312(1):43–67. [DOI] [PubMed] [Google Scholar]
- (22).Xia G, Gajwani P, Muzina DJ, Kemp DE, Gao K, Ganocy SJ, et al. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119–30. [DOI] [PubMed] [Google Scholar]
- (23).Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16. [DOI] [PubMed] [Google Scholar]
- (24).Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of T. M. S. Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683–92. [DOI] [PubMed] [Google Scholar]
- (26).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (27).Canadian Agency for Drugs and Technologies in Health. Validity and minimal clinically important difference of outcome measures. 2016 Nov. In: Aripiprazole (Abilify): depression, major depressive disorder (MDD) [Internet]. Ottawa (ON): The Agency. Available from: https://www.ncbi.nlm.nih.gov/books/NBK409740/table/T25/ [PubMed] [Google Scholar]
- (28).Covidence systematic review software [Internet]. Melbourne (Australia): Veritas Health Innovation; 2019. [cited 2020 February 10]. Available from: https://www.covidence.org/home [Google Scholar]
- (29).Pieper D, Antoine SL, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67(4):368–75. [DOI] [PubMed] [Google Scholar]
- (30).Cochrane Collaboration. Review Manager meta-analysis software [Internet]. Copenhagen: The Collaboration; 2020. [cited 2020 June 1]. Available from: https://community.cochrane.org/editorial-and-publishing-policy-resource/information-technology/review-manager-revman. [Google Scholar]
- (31).The Nordic Cochrane Centre. Review Manager meta-analysis software. Copenhagen: The Cochrane Collaboration; 2011. [Google Scholar]
- (32).Higgins JPT, Green S. (editors). Cochrane Handbook for systematic reviews of interventions [Internet]: The Cochrane Collaboration; 2011. [cited 2020 June 1]. Available from: www.handbook.cochrane.org [Google Scholar]
- (33).Nunes A, Trappenberg T, Alda M. We need an operational framework for heterogeneity in psychiatric research. J Psychiatry Neurosci. 2020;45(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Health Technology Assessment Unit. Repetitive transcranial magnetic stimulation for treatment resistant depression: a health technology assessment [Internet]. Calgary (AB): University of Calgary; 2017. [Google Scholar]
- (35).Mutz J, Edgcumbe DR, Brunoni AR, Fu CHY. Efficacy and acceptability of non-invasive brain stimulation for the treatment of adult unipolar and bipolar depression: a systematic review and meta-analysis of randomised sham-controlled trials. Neurosci Biobehav Rev. 2018;92:291–303. [DOI] [PubMed] [Google Scholar]
- (36).Whiting P, Savovic J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Higgins JP, Altman DG, G⊘tzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Br Med J. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. [DOI] [PubMed] [Google Scholar]
- (39).Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Leggett LE, Soril LJ, Coward S, Lorenzetti DL, MacKean G, Clement FM. Repetitive transcranial magnetic stimulation for treatment-resistant depression in adult and youth populations: a systematic literature review and meta-analysis. Prim Care Companion CNS Disord. 2015;17(6):10.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Sehatzadeh S, Daskalakis ZJ, Yap B, Tu HA, Palimaka S, Bowen JM, et al. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression: a meta-analysis of randomized controlled trials over 2 decades. J Psychiatry Neurosci. 2019;44(3):151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Lepping P, Schonfeldt-Lecuona C, Sambhi RS, Lanka SV, Lane S, Whittington R, et al. A systematic review of the clinical relevance of repetitive transcranial magnetic stimulation. Acta Psychiatr Scand. 2014;130(5):326–41. [DOI] [PubMed] [Google Scholar]
- (43).Zhang YQ, Zhu D, Zhou XY, Liu YY, Qin B, Ren GP, et al. Bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis of randomized controlled trials. Braz J Med Biol Res. 2015;48(3):198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Nordenskjold A, Martensson B, Pettersson A, Heintz E, Landen M. Effects of Hesel-coil deep transcranial magnetic stimulation for depression–a systematic review. Nord J Psychiatry. 2016;70(7):492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Berlim MT, McGirr A, Rodrigues Dos Santos N, Tremblay S, Martins R. Efficacy of theta burst stimulation (TBS) for major depression: an exploratory meta-analysis of randomized and sham-controlled trials. J Psychiatr Res. 2017;90:102–9. [DOI] [PubMed] [Google Scholar]
- (46).Brunoni AR, Chaimani A, Moffa AH, Razza LB, Gattaz WF, Daskalakis ZJ, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry. 2017;74(2):143–52. [DOI] [PubMed] [Google Scholar]
- (47).Kang JI, Lee H, Jhung K, Kim KR, An SK, Yoon KJ, et al. Frontostriatal connectivity changes in major depressive disorder after repetitive transcranial magnetic stimulation: a randomized sham-controlled study. J Clin Psychiatry. 2016;77(9):e1137–e43. [DOI] [PubMed] [Google Scholar]
- (48).Kaster TS, Daskalakis ZJ, Noda Y, Knyahnytska Y, Downar J, Rajji TK, et al. Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: a prospective randomized controlled trial. Neuropsychopharmacology. 2018;43(11):2231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Valkonen-Korhonen M, Leinola H, Kononen M, Niskanen E, Purhonen M, Pakarinen M, et al. Bifrontal active and sham rTMS in treatment-resistant unipolar major depression. Nord J Psychiatry. 2018;72(8):586–92. [DOI] [PubMed] [Google Scholar]
- (50).Yesavage JA, Fairchild JK, Mi Z, Biswas K, Davis-Karim A, Phibbs CS, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Pohar R, Farrah K. Repetitive transcranial magnetic stimulation for patients withdDepression: a review of clinical effectiveness, cost-effectiveness and guidelines–an update. CADTH Rapid Response Reports. 2019;6:28. [PubMed] [Google Scholar]
- (52).Baeken C, Vanderhasselt MA, Remue J, Herremans S, Vanderbruggen N, Zeeuws D, et al. Intensive HF-rTMS treatment in refractory medication-resistant unipolar depressed patients. J Affect Disord. 2013;151(2):625–31. [DOI] [PubMed] [Google Scholar]
- (53).Eschweiler GW, Wegerer C, Schlotter W, Spandl C, Stevens A, Bartels M, et al. Left prefrontal activation predicts therapeutic effects of repetitive transcranial magnetic stimulation (rTMS) in major depression. Psychiatry Res. 2000;99(3):161–72. [DOI] [PubMed] [Google Scholar]
- (54).Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wassermann EM, et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry. 1999;46(12):1603–13. [DOI] [PubMed] [Google Scholar]
- (55).Karamustafalioglu O, Ozcelik B, Uzun U, Tankaya O, Alpak G, Cengiz Y. Augmentative repetitive transcranial magnetic stimulation treatment in medication resistant major depression. Int J Neuropsychopharmacol. 2010;13:152. [Google Scholar]
- (56).Jorge RE, Moser DJ, Acion L, Robinson RG. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268–76. [DOI] [PubMed] [Google Scholar]
- (57).Jorge RE, Robinson RG, Tateno A, Narushima K, Acion L, Moser D, et al. Repetitive transcranial magnetic stimulation as treatment of poststroke depression: a preliminary study. Biol Psychiatry. 2004;55(4):398–405. [DOI] [PubMed] [Google Scholar]
- (58).Klein E, Kreinin I, Chistyakov A, Koren D, Mecz L, Marmur S, et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry. 1999;56(4):315–20. [DOI] [PubMed] [Google Scholar]
- (59).Prasser J, Schecklmann M, Poeppl TB, Frank E, Kreuzer PM, Hajak G, et al. Bilateral prefrontal rTMS and theta burst TMS as an add-on treatment for depression: a randomized placebo controlled trial. World J Biol Psychiatry. 2015;16(1):57–65. [DOI] [PubMed] [Google Scholar]
- (60).Manes F, Jorge R, Morcuende M, Yamada T, Paradiso S, Robinson RG. A controlled study of repetitive transcranial magnetic stimulation as a treatment of depression in the elderly. Int Psychogeriatr. 2001;13(2):225–31. [DOI] [PubMed] [Google Scholar]
- (61).Miniussi C, Bonato C, Bignotti S, Gazzoli A, Gennarelli M, Pasqualetti P, et al. Repetitive transcranial magnetic stimulation (rTMS) at high and low frequency: an efficacious therapy for major drug-resistant depression? Clin Neurophysiol. 2005;116(5):1062–71. [DOI] [PubMed] [Google Scholar]
- (62).Moller AL, Hjaltason O, Ivarsson O, Stefansson SB. The effects of repetitive transcranial magnetic stimulation on depressive symptoms and the P(300) event-related potential. Nord J Psychiatry. 2006;60(4):282–5. [DOI] [PubMed] [Google Scholar]
- (63).Moser DJ, Jorge RE, Manes F, Paradiso S, Benjamin ML, Robinson RG. Improved executive functioning following repetitive transcranial magnetic stimulation. Neurology. 2002;58(8):1288–90. [DOI] [PubMed] [Google Scholar]
- (64).Padberg F, Zwanzger P, Thoma H, Kathmann N, Haag C, Greenberg BD, et al. Repetitive transcranial magnetic stimulation (rTMS) in pharmacotherapy-refractory major depression: comparative study of fast, slow and sham rTMS. Psychiatry Res. 1999;88(3):163–71. [DOI] [PubMed] [Google Scholar]
- (65).Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348(9022):233–7. [DOI] [PubMed] [Google Scholar]
- (66).Speer AM, Benson BE, Kimbrell TK, Wassermann EM, Willis MW, Herscovitch P, et al. Opposite effects of high and low frequency rTMS on mood in depressed patients: relationship to baseline cerebral activity on PET. J Affect Disord. 2009;115(3):386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Lisanby SH, Husain MM, Rosenquist PB, Maixner D, Gutierrez R, Krystal A, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522–34. [DOI] [PubMed] [Google Scholar]
- (68).Loo C, Mitchell P, Sachdev P, McDarmont B, Parker G, Gandevia S. Double-blind controlled investigation of transcranial magnetic stimulation for the treatment of resistant major depression. Am J Psychiatry. 1999;156(6):946–8. [DOI] [PubMed] [Google Scholar]
- (69).Concerto C, Lanza G, Cantone M, Ferri R, Pennisi G, Bella R, et al. Repetitive transcranial magnetic stimulation in patients with drug-resistant major depression: a six-month clinical follow-up study. Int J Psychiatry Clin Pract. 2015;19(4):252–8. [DOI] [PubMed] [Google Scholar]
- (70).Aguirre I, Carretero B, Ibarra O, Kuhalainen J, Martinez J, Ferrer A, et al. Age predicts low-frequency transcranial magnetic stimulation efficacy in major depression. J Affect Disord. 2011;130(3):466–9. [DOI] [PubMed] [Google Scholar]
- (71).Zheng H, Jia F, Guo G, Quan D, Li G, Wu H, et al. Abnormal anterior cingulate N-acetylaspartate and executive functioning in treatment-resistant depression after rTMS therapy. Int J Neuropsychopharmacol. 2015;18(11):pyv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Zheng H, Zhang L, Li L, Liu P, Gao J, Liu X, et al. High-frequency rTMS treatment increases left prefrontal myo-inositol in young patients with treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1189–95. [DOI] [PubMed] [Google Scholar]
- (73).Bakim B, Uzun UE, Karamustafalioglu O, Ozcelik B, Alpak G, Tankaya O, et al. The combination of antidepressant drug therapy and high-frequency repetitive transcranial magnetic stimulation in medication-resistant depression. Bull Clin Psychopharmacol. 2012;22(3):244–63. [Google Scholar]
- (74).Su TP, Huang CC, Wei IH. Add-on rTMS for medication-resistant depression: a randomized, double-blind, sham-controlled trial in Chinese patients. J Clin Psychiatry. 2005;66(7):930–7. [DOI] [PubMed] [Google Scholar]
- (75).Theleritis C, Sakkas P, Paparrigopoulos T, Vitoratou S, Tzavara C, Bonaccorso S, et al. Two versus one high-frequency repetitive transcranial magnetic stimulation session per day for treatment-resistant depression: a randomized sham-controlled trial. J ECT. 2017;33(3):190–7. [DOI] [PubMed] [Google Scholar]
- (76).Triggs WJ, Ricciuti N, Ward HE, Cheng J, Bowers D, Goodman WK, et al. Right and left dorsolateral pre-frontal rTMS treatment of refractory depression: a randomized, sham-controlled trial. Psychiatry Res. 2010;178(3):467–74. [DOI] [PubMed] [Google Scholar]
- (77).Stern WM, Tormos JM, Press DZ, Pearlman C, Pascual-Leone A. Antidepressant effects of high and low frequency repetitive transcranial magnetic stimulation to the dorsolateral prefrontal cortex: a double-blind, randomized, placebo-controlled trial. J Neuropsychiatry Clin Neurosci. 2007;19(2):179–86. [DOI] [PubMed] [Google Scholar]
- (78).Anderson IM, Delvai NA, Ashim B, Ashim S, Lewin C, Singh V, et al. Adjunctive fast repetitive transcranial magnetic stimulation in depression. Br J Psychiatry. 2007;190:533–4. [DOI] [PubMed] [Google Scholar]
- (79).Avery DH, Claypoole K, Robinson L, Neumaier JF, Dunner DL, Scheele L, et al. Repetitive transcranial magnetic stimulation in the treatment of medication-resistant depression: preliminary data. J Nerv Ment Dis. 1999;187(2):114–7. [DOI] [PubMed] [Google Scholar]
- (80).Avery DH, Holtzheimer PE, III, Fawaz W, Russo J, Neumaier J, Dunner DL, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2006;59(2):187–94. [DOI] [PubMed] [Google Scholar]
- (81).Berman RM, Narasimhan M, Sanacora G, Miano AP, Hoffman RE, Hu XS, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry. 2000;47(4):332–7. [DOI] [PubMed] [Google Scholar]
- (82).Blumberger DM, Maller JJ, Thomson L, Mulsant BH, Rajji TK, Maher M, et al. Unilateral and bilateral MRI-targeted repetitive transcranial magnetic stimulation for treatment-resistant depression: a randomized controlled study. J Psychiatry Neurosci. 2016;41(4):E58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Blumberger DM, Mulsant BH, Fitzgerald PB, Rajji TK, Ravindran AV, Young LT, et al. A randomized double-blind sham-controlled comparison of unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant major depression. World J Biol Psychiatry. 2012;13(6):423–35. [DOI] [PubMed] [Google Scholar]
- (84).Bretlau LG, Lunde M, Lindberg L, Unden M, Dissing S, Bech P. Repetitive transcranial magnetic stimulation (rTMS) in combination with escitalopram in patients with treatment-resistant major depression: a double-blind, randomised, sham-controlled trial. Pharmacopsychiatry. 2008;41(2):41–7. [DOI] [PubMed] [Google Scholar]
- (85).Chen SJ, Chang CH, Tsai HC, Chen ST, Lin C. Superior antidepressant effect occurring 1 month after rTMS: add-on rTMS for subjects with medication-resistant depression. Neuropsychiatr Dis Treat. 2013;9:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Fitzgerald PB, Brown TL, Marston NA, Daskalakis ZJ, De Castella A, Kulkarni J. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003;60(10):1002–8. [DOI] [PubMed] [Google Scholar]
- (87).Fitzgerald PB, Hoy KE, Herring SE, McQueen S, Peachey AV, Segrave RA, et al. A double blind randomized trial of unilateral left and bilateral prefrontal cortex transcranial magnetic stimulation in treatment resistant major depression. J Affect Disord. 2012;139(2):193–8. [DOI] [PubMed] [Google Scholar]
- (88).Garcia-Toro M, Mayol A, Arnillas H, Capllonch I, Ibarra O, Crespi M, et al. Modest adjunctive benefit with transcranial magnetic stimulation in medication-resistant depression. J Affect Disord. 2001;64(2-3):271–5. [DOI] [PubMed] [Google Scholar]
- (89).George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507–16. [DOI] [PubMed] [Google Scholar]
- (90).Hernandez-Ribas R, Deus J, Pujol J, Segalas C, Vallejo J, Menchon JM, et al. Identifying brain imaging correlates of clinical response to repetitive transcranial magnetic stimulation (rTMS) in major depression. Brain Stimul. 2013;6(1):54–61. [DOI] [PubMed] [Google Scholar]
- (91).Herwig U, Fallgatter AJ, Hoppner J, Eschweiler GW, Kron M, Hajak G, et al. Antidepressant effects of augmentative transcranial magnetic stimulation: randomised multicentre trial. Br J Psychiatry. 2007;191:441–8. [DOI] [PubMed] [Google Scholar]
- (92).Holtzheimer PE, III, Russo J, Claypoole KH, Roy-Byrne P, Avery DH. Shorter duration of depressive episode may predict response to repetitive transcranial magnetic stimulation. Depress Anxiety. 2004;19(1):24–30. [DOI] [PubMed] [Google Scholar]
- (93).Loo CK, Mitchell PB, McFarquhar TF, Malhi GS, Sachdev PS. A sham-controlled trial of the efficacy and safety of twice-daily rTMS in major depression. Psychol Med. 2007;37(3):341–9. [DOI] [PubMed] [Google Scholar]
- (94).Mosimann UP, Schmitt W, Greenberg BD, Kosel M, Muri RM, Berkhoff M, et al. Repetitive transcranial magnetic stimulation: a putative add-on treatment for major depression in elderly patients. Psychiatry Res. 2004;126(2):123–33. [DOI] [PubMed] [Google Scholar]
- (95).O'Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–16. [DOI] [PubMed] [Google Scholar]
- (96).Paillere Martinot ML, Galinowski A, Ringuenet D, Gallarda T, Lefaucheur JP, Bellivier F, et al. Influence of prefrontal target region on the efficacy of repetitive transcranial magnetic stimulation in patients with medication-resistant depression: a [(18)F]-fluorodeoxyglucose PET and MRI study. Int J Neuropsychopharmacol. 2010;13(1):45–59. [DOI] [PubMed] [Google Scholar]
- (97).Peng H, Zheng H, Li L, Liu J, Zhang Y, Shan B, et al. High-frequency rTMS treatment increases white matter FA in the left middle frontal gyrus in young patients with treatment-resistant depression. J Affect Disord. 2012;136(3):249–57. [DOI] [PubMed] [Google Scholar]
- (98).Zhang XH, Wang LW, Wang JJ, Liu Q, Fan Y. Adjunctive treatment with transcranial magnetic stimulation in treatment resistant depression: a randomized, double-blind, sham-controlled study. Shanghai Arch Psychiatry. 2011;23(1):17–24. [Google Scholar]
- (99).Bares M, Kopecek M, Novak T, Stopkova P, Sos P, Kozeny J, et al. Low frequency (1-Hz), right prefrontal repetitive transcranial magnetic stimulation (rTMS) compared with venlafaxine ER in the treatment of resistant depression: a double-blind, single-centre, randomized study. J Affect Disord. 2009;118(1-3):94–100. [DOI] [PubMed] [Google Scholar]
- (100).Fitzgerald P. Is it time to introduce repetitive transcranial magnetic stimulation into standard clinical practice for the treatment of depressive disorders? Aust N Z J Psychiatry. 2003;37(1):5–11; discussion 2-4. [DOI] [PubMed] [Google Scholar]
- (101).Januel D, Dumortier G, Verdon CM, Stamatiadis L, Saba G, Cabaret W, et al. A double-blind sham controlled study of right prefrontal repetitive transcranial magnetic stimulation (rTMS): therapeutic and cognitive effect in medication free unipolar depression during 4 weeks. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(1):126–30. [DOI] [PubMed] [Google Scholar]
- (102).Kauffmann CD, Cheema MA, Miller BE. Slow right prefrontal transcranial magnetic stimulation as a treatment for medication-resistant depression: a double-blind, placebo-controlled study. Depress Anxiety. 2004;19(1):59–62. [DOI] [PubMed] [Google Scholar]
- (103).Krstic J, Buzadzic I, Milanovic SD, Ilic NV, Pajic S, Ilic TV. Low-frequency repetitive transcranial magnetic stimulation in the right prefrontal cortex combined with partial sleep deprivation in treatment-resistant depression: a randomized sham-controlled trial. J ECT. 2014;30(4):325–31. [DOI] [PubMed] [Google Scholar]
- (104).Mantovani A, Aly M, Dagan Y, Allart A, Lisanby SH. Randomized sham controlled trial of repetitive transcranial magnetic stimulation to the dorsolateral prefrontal cortex for the treatment of panic disorder with comorbid major depression. J Affect Disord. 2013;144(1-2):153–9. [DOI] [PubMed] [Google Scholar]
- (105).Speer AM, Wassermann EM, Benson BE, Herscovitch P, Post RM. Antidepressant efficacy of high and low frequency rTMS at 110% of motor threshold versus sham stimulation over left prefrontal cortex. Brain Stimul. 2014;7(1):36–41. [DOI] [PubMed] [Google Scholar]
- (106).Fitzgerald PB, Benitez J, de Castella A, Daskalakis ZJ, Brown TL, Kulkarni J. A randomized, controlled trial of sequential bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression. Am J Psychiatry. 2006;163(1):88–94. [DOI] [PubMed] [Google Scholar]
- (107).Fitzgerald PB, Huntsman S, Gunewardene R, Kulkarni J, Daskalakis ZJ. A randomized trial of low-frequency right-prefrontal-cortex transcranial magnetic stimulation as augmentation in treatment-resistant major depression. Int J Neuropsychopharmacol. 2006;9(6):655–66. [DOI] [PubMed] [Google Scholar]
- (108).Garcia-Toro M, Salva J, Daumal J, Andres J, Romera M, Lafau O, et al. High (20-Hz) and low (1-Hz) frequency transcranial magnetic stimulation as adjuvant treatment in medication-resistant depression. Psychiatry Res. 2006;146(1):53–7. [DOI] [PubMed] [Google Scholar]
- (109).Li CT, Chen MH, Juan CH, Huang HH, Chen LF, Hsieh JC, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137(Pt 7):2088–98. [DOI] [PubMed] [Google Scholar]
- (110).Tavares DF, Myczkowski ML, Alberto RL, Valiengo L, Rios RM, Gordon P, et al. Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology. 2017;42(13):2593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Padberg F, Zwanzger P, Keck ME, Kathmann N, Mikhaiel P, Ella R, et al. Repetitive transcranial magnetic stimulation (rTMS) in major depression: relation between efficacy and stimulation intensity. Neuropsychopharmacology. 2002;27(4):638–45. [DOI] [PubMed] [Google Scholar]
- (112).Hoppner J, Schulz M, Irmisch G, Mau R, Schlafke D, Richter J. Antidepressant efficacy of two different rTMS procedures. High frequency over left versus low frequency over right prefrontal cortex compared with sham stimulation. Eur Arch Psychiatry Clin Neurosci. 2003;253(2):103–9. [DOI] [PubMed] [Google Scholar]
- (113).Mogg A, Pluck G, Eranti SV, Landau S, Purvis R, Brown RG, et al. A randomized controlled trial with 4-month follow-up of adjunctive repetitive transcranial magnetic stimulation of the left prefrontal cortex for depression. Psychol Med. 2008;38(3):323–33. [DOI] [PubMed] [Google Scholar]
- (114).Rossini D, Lucca A, Zanardi R, Magri L, Smeraldi E. Transcranial magnetic stimulation in treatment-resistant depressed patients: a double-blind, placebo-controlled trial. Psychiatry Res. 2005;137(1-2):1–10. [DOI] [PubMed] [Google Scholar]
- (115).Taylor SF, Ho SS, Abagis T, Angstadt M, Maixner DF, Welsh RC, et al. Changes in brain connectivity during a sham-controlled, transcranial magnetic stimulation trial for depression. J Affect Disord. 2018;232:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Brunelin J, Jalenques I, Trojak B, Attal J, Szekely D, Gay A, et al. The efficacy and safety of low frequency repetitive transcranial magnetic stimulation for treatment-resistant depression: the results from a large multicenter French RCT. Brain Stimul. 2014;7(6):855–63. [DOI] [PubMed] [Google Scholar]
- (117).Pallanti S, Bernardi S, Di Rollo A, Antonini S, Quercioli L. Unilateral low frequency versus sequential bilateral repetitive transcranial magnetic stimulation: is simpler better for treatment of resistant depression? Neuroscience. 2010;167(2):323–8. [DOI] [PubMed] [Google Scholar]
- (118).Fitzgerald PB, Hoy KE, Anderson RJ, Daskalakis ZJ. A study of the pattern of response to rTMS treatment in depression. Depress Anxiety. 2016;33(8):746–53. [DOI] [PubMed] [Google Scholar]
- (119).Fitzgerald PB, Hoy KE, Elliot D, McQueen S, Wambeek LE, Daskalakis ZJ. A negative double-blind controlled trial of sequential bilateral rTMS in the treatment of bipolar depression. J Affect Disord. 2016;198:158–62. [DOI] [PubMed] [Google Scholar]
- (120).Loo CK, Mitchell PB, Croker VM, Malhi GS, Wen W, Gandevia SC, et al. Double-blind controlled investigation of bilateral prefrontal transcranial magnetic stimulation for the treatment of resistant major depression. Psychol Med. 2003;33(1):33–40. [DOI] [PubMed] [Google Scholar]
- (121).McDonald WM, Easley K, Byrd EH, Holtzheimer P, Tuohy S, Woodard JL, et al. Combination rapid transcranial magnetic stimulation in treatment refractory depression. Neuropsychiatr Dis Treat. 2006;2(1):85–94. [PMC free article] [PubMed] [Google Scholar]
- (122).Chistyakov AV, Kreinin B, Marmor S, Kaplan B, Khatib A, Darawsheh N, et al. Preliminary assessment of the therapeutic efficacy of continuous theta-burst magnetic stimulation (cTBS) in major depression: a double-blind sham-controlled study. J Affect Disord. 2015;170:225–9. [DOI] [PubMed] [Google Scholar]
- (123).Li H, Wang J, Li C, Xiao Z. Repetitive transcranial magnetic stimulation (rTMS) for panic disorder in adults. Cochrane Database Syst Rev. 2014(9):CD009083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Duprat R, Desmyter S, Rudi de R, van Heeringen K, Van den Abbeele D, Tandt H, et al. Accelerated intermittent theta burst stimulation treatment in medication-resistant major depression: a fast road to remission? J Affect Disord. 2016;200:6–14. [DOI] [PubMed] [Google Scholar]
- (125).Plewnia C, Pasqualetti P, Grosse S, Schlipf S, Wasserka B, Zwissler B, et al. Treatment of major depression with bilateral theta burst stimulation: a randomized controlled pilot trial. J Affect Disord. 2014;156:219–23. [DOI] [PubMed] [Google Scholar]
- (126).Levkovitz Y, Isserles M, Padberg F, Lisanby SH, Bystritsky A, Xia G, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. 2015;14(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Bortolomasi M, Minelli A, Fuggetta G, Perini M, Comencini S, Fiaschi A, et al. Long-lasting effects of high frequency repetitive transcranial magnetic stimulation in major depressed patients. Psychiatry Res. 2007;150(2):181–6. [DOI] [PubMed] [Google Scholar]
- (128).Eranti S, Mogg A, Pluck G, Landau S, Purvis R, Brown RG, et al. A randomized, controlled trial with 6-month follow-up of repetitive transcranial magnetic stimulation and electroconvulsive therapy for severe depression. Am J Psychiatry. 2007;164(1):73–81. [DOI] [PubMed] [Google Scholar]
- (129).Grunhaus L, Dannon PN, Schreiber S, Dolberg OH, Amiaz R, Ziv R, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry. 2000;47(4):314–24. [DOI] [PubMed] [Google Scholar]
- (130).Grunhaus L, Schreiber S, Dolberg OT, Polak D, Dannon PN. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry. 2003;53(4):324–31. [DOI] [PubMed] [Google Scholar]
- (131).Pridmore S, Bruno R, Turnier-Shea Y, Reid P, Rybak M. Comparison of unlimited numbers of rapid transcranial magnetic stimulation (rTMS) and ECT treatment sessions in major depressive episode. Int J Neuropsychopharmacol. 2000;3(2):129–34. [DOI] [PubMed] [Google Scholar]
- (132).Keshtkar M, Ghanizadeh A, Firoozabadi A. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for the treatment of major depressive disorder; a randomized controlled clinical trial. J ECT. 2011;27(4):310–4. [DOI] [PubMed] [Google Scholar]
- (133).Janicak PG, Dowd SM, Martis B, Alam D, Beedle D, Krasuski J, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659–67. [DOI] [PubMed] [Google Scholar]
- (134).Rosa MA, Gattaz WF, Pascual-Leone A, Fregni F, Rosa MO, Rumi DO, et al. Comparison of repetitive transcranial magnetic stimulation and electroconvulsive therapy in unipolar nonpsychotic refractory depression: a randomized, single-blind study. Int J Neuropsychopharmacol. 2006;9(6):667–76. [DOI] [PubMed] [Google Scholar]
- (135).Dannon PN, Dolberg OT, Schreiber S, Grunhaus L. Three and six-month outcome following courses of either ECT or rTMS in a population of severely depressed individuals–preliminary report. Biol Psychiatry. 2002;51(8):687–90. [DOI] [PubMed] [Google Scholar]
- (136).Fitzgerald PB, Hoy K, Daskalakis ZJ, Kulkarni J. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26(3):229–34. [DOI] [PubMed] [Google Scholar]
- (137).Rossini D, Lucca A, Magri L, Malaguti A, Smeraldi E, Colombo C, et al. A symptom-specific analysis of the effect of high-frequency left or low-frequency right transcranial magnetic stimulation over the dorsolateral prefrontal cortex in major depression. Neuropsychobiology. 2010;62(2):91–7. [DOI] [PubMed] [Google Scholar]
- (138).Kaster TS, Downar J, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, et al. Trajectories of response to dorsolateral prefrontal rTMS in major depression: a THREE-D study. Am J Psychiatry. 2019;176(5):367–75. [DOI] [PubMed] [Google Scholar]
- (139).Fitzgerald PB, Hoy KE, Elliot D, Susan McQueen RN, Wambeek LE, Daskalakis ZJ. Accelerated repetitive transcranial magnetic stimulation in the treatment of depression. Neuropsychopharmacology. 2018;43(7):1565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Brunoni AR, Gattaz W, Chaimani A. Network meta-analysis in mental health treatment research—reply. JAMA Psychiatry. 2017;74(8):851–2. [DOI] [PubMed] [Google Scholar]
- (141).Pearson C, Janz T, Ali J. Mental and substance use disorders in Canada. Health at a glance. Ottawa (ON): Statistics Canada; 2013. September. [Google Scholar]
- (142).National Institute for Health and Care Excellence. Process and methods guides. Appendix I: Quality appraisal checklist–economic evaluations [Internet]. London: The Institute; 2012. [cited 2016 Jan]. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations [Google Scholar]
- (143).Fitzgibbon KP, Plett D, Chan BCF, Hancock-Howard R, Coyte PC, Blumberger DM. Cost-utility analysis of electroconvulsive therapy and repetitive transcranial magnetic stimulation for treatment-resistant depression in Ontario. Can J Psychiatry. 2019:706743719890167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Zhao YJ, Tor PC, Khoo AL, Teng M, Lim BP, Mok YM. Cost-effectiveness modeling of repetitive transcranial magnetic stimulation compared to electroconvulsive therapy for treatment-resistant depression in Singapore. Neuromodulation. 2018;21(4):376–82. [DOI] [PubMed] [Google Scholar]
- (145).Voigt J, Carpenter L, Leuchter A. Cost effectiveness analysis comparing repetitive transcranial magnetic stimulation to antidepressant medications after a first treatment failure for major depressive disorder in newly diagnosed patients—a lifetime analysis. PLoS One. 2017;12(10):e0186950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Bank of Canada. Daily exchange rates [Internet]. Ottawa (ON): Bank of Canada; 2020. [cited 2020 Mar 23]. Available from: https://www.bankofcanada.ca/rates/exchange/daily-exchange-rates/ [Google Scholar]
- (147).Ghiasvand H, Moradi-Joo M, Abolhassani N, Ravaghi H, Raygani SM, Mohabbat-Bahar S. Economic evaluation of resistant major depressive disorder treatment in Iranian population: a comparison between repetitive Transcranial Magnetic Stimulation with electroconvulsive. Med J Islam Repub Iran. 2016;30:330. [PMC free article] [PubMed] [Google Scholar]
- (148).Nguyen KH, Gordon LG. Cost-effectiveness of repetitive transcranial magnetic stimulation versus antidepressant therapy for treatment-resistant depression. Value Health. 2015;18(5):597–604. [DOI] [PubMed] [Google Scholar]
- (149).Vallejo-Torres L, Castilla I, Gonzalez N, Hunter R, Serrano-Perez P, Perestelo-Perez L. Cost-effectiveness of electroconvulsive therapy compared to repetitive transcranial magnetic stimulation for treatment-resistant severe depression: a decision model. Psychol Med. 2015;45(7):1459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Health Technology Assessment Unit, University of Calgary. Repetitive transcranial magnetic stimulation for treatment resistant depression. Health Technol Assess Database. 2016(4). [PMC free article] [PubMed] [Google Scholar]
- (151).Health Quality Ontario. Repetitive transcranial magnetic stimulation for treatment-resistant depression: an economic analysis [structured abstract]. Health Technol Assess Database. 2016(4). [PMC free article] [PubMed] [Google Scholar]
- (152).Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada. 4th ed [Internet]. Ottawa (ON): The Agency; 2017. [cited 2018 Jan]. Available from: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf [Google Scholar]
- (153).Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–50. [DOI] [PubMed] [Google Scholar]
- (154).Conway CR, George MS, Sackeim HA. Toward an evidence-based, operational definition of treatment-resistant depression: when enough is enough. JAMA Psychiatry. 2017;74(1):9–10. [DOI] [PubMed] [Google Scholar]
- (155).Statistics Canada. Life Tables, Canada, Provinces and Territories 1980/1982 to 2014/2016. [Internet]. Ottawa (ON): Statistics Canada; 2018. Available from: https://www150.statcan.gc.ca/n1/pub/84-537-x/84-537-x2018002-eng.htm [Google Scholar]
- (156).Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Ross EL, Zivin K, Maixner DF. Cost-effectiveness of electroconvulsive therapy vs pharmacotherapy/psychotherapy for treatment-resistant depression in the United States. JAMA Psychiatry. 2018;75(7):713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Chang CK, Hayes RD, Broadbent M, Fernandes AC, Lee W, Hotopf M, et al. All-cause mortality among people with serious mental illness (SMI), substance use disorders, and depressive disorders in southeast London: a cohort study. BMC Psychiatry. 2010;10:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry. 2014;13(2):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. 2014;171(4):453–62. [DOI] [PubMed] [Google Scholar]
- (161).Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- (162).Sapin C, Fantino B, Nowicki ML, Kind P. Usefulness of EQ-5D in assessing health status in primary care patients with major depressive disorder. Health Qual Life Outcomes. 2004;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Canadian Institute for Health Information. Patient cost estimator [Internet]. Toronto (ON): The Institute; 2019. [cited 2019 Jan 18]. Available from: https://www.cihi.ca/en/patient-cost-estimator [Google Scholar]
- (164).Ontario Ministry of Health and Long-Term Care. Ontario Case Costing Initiative (OCCI) [Internet]. 2017. [cited 2018 Dec 4]. Available from: https://www.ontario.ca/data/ontario-case458
- (165).Mendlowitz AB, Shanbour A, Downar J, Vila-Rodriguez F, Daskalakis ZJ, Isaranuwatchai W, et al. Implementation of intermittent theta burst stimulation compared to conventional repetitive transcranial magnetic stimulation in patients with treatment resistant depression: a cost analysis. PLoS One. 2019;14(9):e0222546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Bank of Canada. Annual exchange rates [Internet]. Ottawa (ON): Bank of Canada; 2019. [cited 2019 Dec 1]. Available from: https://www.bankofcanada.ca/rates/exchange/ [Google Scholar]
- (167).Ontario Drug Formulary. 28:16:04 Psychotherapeutic agents and antidepressants [Internet]. 2018. [cited 2018 Dec 1]. Available from: https://www.formulary.health.gov.on.ca/formulary/results.xhtml?class$f14281604000&fbclid$f14IwAR2qtljHalBMxN6_QuGRkHSEp1ZKQuR8bkeXEjYn_V_gFZtDy_7q93Z3CYE.
- (168).Krahn M, Miller F, Bayoumi A, Brooker AS, Wagner F, Winsor S, et al. Development of the Ontario decision framework: a values based framework for health technology assessment. Int J Technol Assess Health Care. 2018;34(3):290–9. [DOI] [PubMed] [Google Scholar]
- (169).Simpson KN, Welch MJ, Kozel FA, Demitrack MA, Nahas Z. Cost-effectiveness of transcranial magnetic stimulation in the treatment of major depression: a health economics analysis. Adv Ther. 2009;26(3):346–68. [DOI] [PubMed] [Google Scholar]
- (170).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (171).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (172).Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. Apr [cited 2018 Apr 30]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (173).Selva A, Sola I, Zhang Y, Pardo-Hernandez H, Haynes RB, Martinez Garcia L, et al. Development and use of a content search strategy for retrieving studies on patients’ views and preferences. Health Qual Life Outcomes. 2017;15(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Joy SM, Little E, Maruthur NM, Purnell TS, Bridges JF. Patient preferences for the treatment of type 2 diabetes: a scoping review. Pharmacoeconomics. 2013;31(10):877–92. [DOI] [PubMed] [Google Scholar]
- (175).Walter G, Martin J, Kirkby K, Pridmore S. Transcranial magnetic stimulation: experience, knowledge and attitudes of recipients. Aust N Z J Psychiatry. 2001;35(1):58–61. [DOI] [PubMed] [Google Scholar]
- (176).AlHadi AN, AlShiban AM, Alomar MA, Aljadoa OF, AlSayegh AM, Jameel MA. Knowledge of and attitude toward repetitive transcranial magnetic stimulation among psychiatrists in Saudi Arabia. J ECT. 2017;33(1):30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Bourla A, Chaneac E, Poulet E, Haffen E, Ogorzelec L, Guinchard C, et al. Acceptability, attitudes and knowledge towards Transcranial Magnetic Stimulation (TMS) among psychiatrists in France. Encephale. 2020;46(2):88–95. [DOI] [PubMed] [Google Scholar]
- (178).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage; 1996. [Google Scholar]
- (179).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage; 1999. p. 33–45. [Google Scholar]
- (180).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods. Thousand Oaks (CA): Sage; 1994. p. 23–41. [Google Scholar]
- (181).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage; 2002. [Google Scholar]
- (182).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage; 1998. [Google Scholar]
- (183).Health Technology Assessment International. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2018 Apr 30]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (184).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (185).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage; 1994. p. 273–85. [Google Scholar]
- (186).Boutros NN, Gueorguieva R, Hoffman RE, Oren DA, Feingold A, Berman RM. Lack of a therapeutic effect of a 2-week sub-threshold transcranial magnetic stimulation course for treatment-resistant depression. Psychiatry Res. 2002;113(3):245–54. [DOI] [PubMed] [Google Scholar]
- (187).Conca A, Di Pauli J, Beraus W, Hausmann A, Peschina W, Schneider H, et al. Combining high and low frequencies in rTMS antidepressive treatment: preliminary results. Hum Psychopharmacol. 2002;17(7):353–6. [DOI] [PubMed] [Google Scholar]
- (188).Dell'Osso B, Oldani L, Camuri G, Dobrea C, Cremaschi L, Benatti B, et al. Augmentative repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of poor responder depressed patients: a comparison study between high and low frequency stimulation. Eur Psychiatry. 2015;30(2):271–6. [DOI] [PubMed] [Google Scholar]
- (189).Eche J, Mondino M, Haesebaert F, Saoud M, Poulet E, Brunelin J. Low- vs high-frequency repetitive transcranial magnetic stimulation as an add-on treatment for refractory depression. Front Psychiatry. 2012;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Fitzgerald PB, Sritharan A, Daskalakis ZJ, de Castella AR, Kulkarni J, Egan G. A functional magnetic resonance imaging study of the effects of low frequency right prefrontal transcranial magnetic stimulation in depression. J Clin Psychopharmacol. 2007;27(5):488–92. [DOI] [PubMed] [Google Scholar]
- (191).Fitzgerald PB, Hoy K, McQueen S, Herring S, Segrave R, Been G, et al. Priming stimulation enhances the effectiveness of low-frequency right prefrontal cortex transcranial magnetic stimulation in major depression. J Clin Psychopharmacol. 2008;28(1):52–8. [DOI] [PubMed] [Google Scholar]
- (192).Fitzgerald PB, Hoy K, Gunewardene R, Slack C, Ibrahim S, Bailey M, et al. A randomized trial of unilateral and bilateral prefrontal cortex transcranial magnetic stimulation in treatment-resistant major depression. Psychol Med. 2011;41(6):1187–96. [DOI] [PubMed] [Google Scholar]
- (193).Fitzgerald PB, Hoy KE, Singh A, Gunewardene R, Slack C, Ibrahim S, et al. Equivalent beneficial effects of unilateral and bilateral prefrontal cortex transcranial magnetic stimulation in a large randomized trial in treatment-resistant major depression. Int J Neuropsychopharmacol. 2013;16(9):1975–84. [DOI] [PubMed] [Google Scholar]
- (194).Isenberg K, Downs D, Pierce K, Svarakic D, Garcia K, Jarvis M, et al. Low frequency rTMS stimulation of the right frontal cortex is as effective as high frequency rTMS stimulation of the left frontal cortex for antidepressant-free, treatment-resistant depressed patients. Ann Clin Psychiatry. 2005;17(3):153–9. [DOI] [PubMed] [Google Scholar]
- (195).Richieri R, Boyer L, Padovani R, Adida M, Colavolpe C, Mundler O, et al. Equivalent brain SPECT perfusion changes underlying therapeutic efficiency in pharmacoresistant depression using either high-frequency left or low-frequency right prefrontal rTMS. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):364–70. [DOI] [PubMed] [Google Scholar]
- (196).Rybak M, Bruno R, Turnier-Shea Y, Pridmore S. An attempt to increase the rate and magnitude of the antidepressant effect of transcranial magnetic stimulation (TMS): a pilot study. German J Psychiatry. 2005;8(4):60–5. [Google Scholar]
- (197).Pridmore S. Substitution of rapid transcranial magnetic stimulation treatments for electroconvulsive therapy treatments in a course of electroconvulsive therapy. Depress Anxiety. 2000;12(3):118–23. [DOI] [PubMed] [Google Scholar]
- (198).Wang XM, Yang DB, Yu YF, Huang H, Zhao XQ. A controlled study of the treatment of repetitive transcranial magnetic stimulation in patients with major depression. Chin J Clin Rehab. 2004;8(9):1770–1. [Google Scholar]