Abstract
The reactivation of human JC polyoma virus (JCPyV) results in lytic infection of oligodendrocytes and neuronal cells. The corresponding clinical picture is called progressive multifocal leukoencephalopathy (PML) and results mostly from a disease-related or drug-induced immunosuppression. The opportunistic brain infection leads to a progressive demyelination of multiple areas of the central nervous system. Patients can present with various neurological deficits ranging from slight motoric symptoms to marked aphasia or reduced vigilance. Currently, there is no effective causal therapy for PML. Survival depends on the ability to achieve timely immune reconstitution. If the immune system cannot be restored, PML progresses rapidly and often ends fatally within months. Recently, some evidence for positive response has been reported in patients treated with immune checkpoint inhibitor therapy. Here, we provide a case series of three PML patients with underlying hematological malignancies who were treated with anti-PD-1-antibody pembrolizumab at Hannover Medical School. All patients received an extensive diagnostic follow-up including cerebrospinal fluid analysis, brain imaging, and lymphocyte-phenotyping via flow cytometry. Our patients had very different outcomes, with the only patient showing a specific anti-JCPyV immune response in the sense of an increased JCPyV antibody index clearly benefiting most from the treatment. Our results partly support the hypothesis that anti-PD-1 therapy may represent a promising treatment option for patients with PML. However, there is a current lack of pre-therapeutic stratification regarding the therapeutic response rates. Before larger studies can be initiated to further evaluate the efficacy of anti-PD-1 antibodies in PML, it is imperative to develop a reliable strategy for selecting suitable patients.
Keywords: flow cytometry, immune checkpoint inhibitor, Progressive multifocal leukencephalopathy, PD-1
Introduction
Progressive multifocal leukoencephalopathy (PML) is an opportunistic infection of the brain caused by reactivation of human JC polyoma virus (JCPyV) leading to a lytic infection of oligodendrocytes and neuronal cells.1 Typically, PML presents with multifocal areas of demyelination particularly in the deep and juxtacortical white matter.2 It is assumed that the virus establishes low-level persistent or latent infections in the kidney and in bone marrow-derived cells largely due to inefficient viral replication in these cell types.3 The major tropism of JCPyV for human glial cells is not fully understood, but host cell- and species-specific transcription and replication factors seem to be responsible for contributing to robust viral replication in this cell type.4 PML and other JCPyV related diseases such as granule cell neuronopathy and JCPyV encephalopathy almost exclusively occur in immunocompromised patients. The spectrum of immunosuppressive conditions related to PML is broad and heterogenous. In general, PML is a relatively rare disease, but it represents the most frequent opportunistic infection of the brain in therapy-related immunosuppression.5 The clinical outcome in terms of mortality and long-term neurologic morbidity depends on the underlying immunosuppressive condition and the possibility to reverse this, that is, whether immunosuppressive treatments can be stopped or the immune deficiency be counterbalanced. In general, post-transplantation PML seems to have a higher case fatality rate compared with PML in HIV patients on highly-active antiretroviral therapy or in multiple sclerosis patients treated with natalizumab.6 The in vivo diagnosis of PML is based on the clinical presentation, imaging findings, and the detection of JCPyV DNA in the cerebrospinal fluid (CSF).7,8 There is no efficient therapy available. Several approaches such as treatment with mirtazapine or mefloquine have been examined with poor results.9 Survival depends on the ability to achieve timely immune reconstitution. Thus, withdrawal of immunosuppressive drugs or, rather, provision of antiretroviral therapy are the only methods to show a survival benefit so far.7 More recently, some evidence for positive response has been reported in patients treated with immune checkpoint inhibitor therapy.10–15 The blockade of checkpoint molecules such as PD-1, PD-L1, and CTLA-4, with monoclonal antibodies, has enabled the development of breakthrough therapies in oncology. Several observations suggest that the PD-1 pathway may be of relevance in PML as the percentage of PD-1 positive T-cells is elevated in blood and CSF of PML patients compared with healthy individuals.10 Based on this observation, anti-PD-1 therapy was assumed to have the potential to stimulate T-cells and consecutively clear JCPyV from the central nervous system (CNS) of PML patients. However, besides the positive reports, there are also PML patients in whom the anti-PD-1 therapy did not show any effect.16 It is generally recognized that rapid reconstitution of T-cell-mediated immunity in immunocompromised patients is the most promising strategy to treat reactivation of latent viral diseases. Loss of T-cell effector function and consecutive immune exhaustion appears to be the underlying mechanism for the onset of PML in immunocompromised patients. Here, we present three cases of patients who suffered from hematological malignancies and were treated with chemotherapy and/or rituximab. Subsequently diagnosed with PML, all patients received anti-PD-1 therapy at the Department of Neurology at Hannover Medical School. We demonstrate that a distinct immune reaction following pembrolizumab treatment for PML can result in a favorable clinical outcome.
Methods
Patients and research ethics
After application for an off-label use of medicine patients were treated as part of an individual therapeutic attempt due to their progressive disease. Therefore, the study did not require an ethical board approval. All patients or a legally authorized representative gave written informed consent for off-label use and separately for publication of patient information and images.
Lymphocyte-phenotyping
Phenotypic analyses from patients’ blood were performed by multicolor flow cytometry of Ficoll-Hypaque-separated cell samples utilizing directly labeled mAb as described previously.17 Briefly, 5 × 105 to 1 × 106 cells were incubated with murine mAb against the appropriate antigens at an optimal dilution for 20 min at 4°C. The following antibodies were used for this study: CD3 PE-Cy7, CD4 PerCP, CD8 PB, CD20 PE (BD Biosciences, Heidelberg, Germany), CD56 APC, CD279 (PD-1) AF488. If not otherwise stated antibodies were purchased from BioLegend (London, UK). Corresponding isotype-matched antibodies were used as controls. Non-specific binding was eliminated by mixing the samples with a 1:5 solution of a commercial human IgG (Octagam; Octapharma, Langenfeld, Germany). After 20 min incubation samples were washed three times in PBS/BSA, and at least 5 × 104 cells per appropriate gate were analyzed using a FACSCanto II flow cytometer with Diva software (Becton Dickinson, Heidelberg, Germany) (gating strategy: see Supplemental material Figure 1). Offline data analysis was performed by using FCS Express software V6 (Denovo Software, Glendale, CA, USA).
MRI
Regarding the internally performed imaging all patients were examined by brain magnetic resonance imaging (MRI) using a whole-body MR system operating at 1.5 or 3 Tesla. The protocol included 3D fluid attenuated inversion recovery, axial T2-weighted, diffusion-weighted imaging, susceptibility weighted imaging, and T1-weighted sequences before and after single dose intravenous gadolinium administration.
Anti-JCPyV antibody detection, calculation of CSF JCPyV antibody index, and JCPyV DNA detection
JC polyoma virus (JCV) DNA by quantitative polymerase chain reaction (qPCR) and the JCPyV antibody index (AIJCV) in CSF were determined at the Institute of Virology, University of Düsseldorf, Germany as recently described.18–20 qPCR for the detection of JCV DNA was performed amplifying a 78 bp product in the large T antigen region. The AIJCV describes the ratio of the pathogen-specific antibody concentration in CSF to that in serum divided by the respective immunoglobulin concentration (IgG) in CSF to the corresponding immunoglobulin concentration in serum. For the AIJCV, serum and CSF were tested in an enzyme-linked immunosorbent assay using a JCV-VP1 protein fused to glutathione S-transferase as antigen, and the AIJCV was calculated. The calculation of the AIJCV required the additional determination of albumin and total IgG from serum and CSF.
Case 1
A 78-year-old male suffering from concomitant diseases such as arterial hypertension and extrinsic allergic alveolitis was diagnosed with mantle cell lymphoma stage IV. Pulmonary involvement, splenomegaly, and lymphadenopathy were observed and the patient was treated with rituximab and bendamustine for 5 months. Six months after treatment initiation, radiological staging revealed complete remission. In January 2020, 2 months after rituximab/bendamustine was completed, the patient complained about subacute, subtle dysarthria and presented with left facial nerve palsy [modified Rankin Scale (mRS): 1]. Brain MRI showed a T2-hyperintense lesion in the left frontotemporal juxtacortical and deep white matter. Initial CSF analysis remained inconspicuous regarding JCPyV detection and standard CSF parameters (Table 1). The diagnosis of PML was ultimately made by brain biopsy 2 weeks later. As symptoms were progressive, the patient presented to our clinic approximately 2 weeks after PML diagnosis in order to evaluate potential treatment options. In the meantime, he developed dysphagia and psychomotor slowing (mRS: 3). Brain MRI exhibited progressive T2 hyperintense lesions (Table 2) without contrast enhancement in T1-postcontrast weighted sequence. A further lumbar puncture with CSF analysis showed a cell count of 5/µl while protein and lactate concentrations were inconspicuous (411 mg/l and 1.8 mmol/l, respectively) and no oligoclonal bands (OCBs) were detectable. Using PCR, a weak positive detection of JCPyV DNA was obtained (<500 c/ml). AIJCV18 remained negative (Table 1). One month after PML diagnosis, he received the first cycle of pembrolizumab therapy (2 mg/kg bodyweight). After the first infusion, his symptoms slightly worsened: dysarthria improved, however, increasing left hemiparesis was observed. Correspondingly, brain MRI showed an increase of PML lesion load without contrast-enhancement (Table 2). Two months after initiation of pembrolizumab treatment there was an increase in the CSF-JCPyV viral load (780 c/ml), until 4 weeks later a reduction in virus copies (200 c/ml) was observed (Table 1). AIJCV remained negative throughout the whole treatment period (Table 1). In total, the patient received three infusions of pembrolizumab at intervals of 4 weeks each and a fourth infusion 6 weeks thereafter. Despite reduction of the viral load, there was a steady deterioration of the patient’s clinical condition, resulting in complete paralysis of both legs and the left hand, dysphagia, dysarthria, and reduced vigilance (mRS: 5). Brain MRI showed a marked increase in the lesion load (Table 2) and minimal indications of an immune reconstitution inflammatory syndrome in the sense of discreet contrast enhancement at the margins of the T2-lesions. Due to the significant deterioration under pembrolizumab therapy, the decision was finally made to evaluate further treatment options (e.g. JCPyV specific T-cells).
Table 1.
CSF results of all three patients treated with pembrolizumab.
| Number of LPs: | Patient 1 |
Patient 2 |
Patient 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (external) | 2 | 3 | 4 | 1 (external) | 2 | 3 | 1 (external) | 2 | 3 | |
| Time since first symptoms (weeks) | 2 | 6 | 13 | 17 | 1 | 4 | 16 | 8 | 12 | 17 |
| CSF cells/µl | 3 | 5 | 4 | 4 | 5 | 8 | 36 | 1 | 3 | 12 |
| CSF protein (mg/l) | 370 | 410 | 480 | 500 | 820 | 1390 | 860 | 300 | 330 | 420 |
| Qalbumin | 5.1 | 5.8 | 6.4 | 8.6 | N/A | 21.6 | 15.6 | 4.3 | 4.2 | 4.9 |
| CSF lactate (mmol/l) | 1.6 | 1.8 | 1.6 | 1.6 | 1.2 | 1.7 | 1.7 | 1.6 | n.d. | 2.0 |
| OCBs | Negative | Negative | Negative | Negative | N/A | Positive (type 3) | Positive (type 3) | Negative | Positive (type 2a) | Negative |
| JCPyV PCR (c/ml) | Negative | <500 | 780 | 220 | Negative | Negative | Negative | 500 | 90.000 | 190.000 |
| AIJCV | n.d. | Negative | Negative | Negative | n.d. | 5.2 | 6.3 | n.d. | Negative | Negative |
AIJCV, JCPyV antibody index; CSF, cerebrospinal fluid; JCPyV, JC polyoma virus; LP, lumbar puncture; N/A, not applicable; n.d., not done; OCB, oligoclonal band; PCR, polymerase chain reaction.
Table 2.
Clinical characteristics, brain MRI, and clinical course of patient 1 during pembrolizumab treatment.
| Patient 1 | ||||
|---|---|---|---|---|
| Baseline clinical characteristics | Brain MRI and clinical course | |||
| Age at PML diagnosis (years) | 78 |
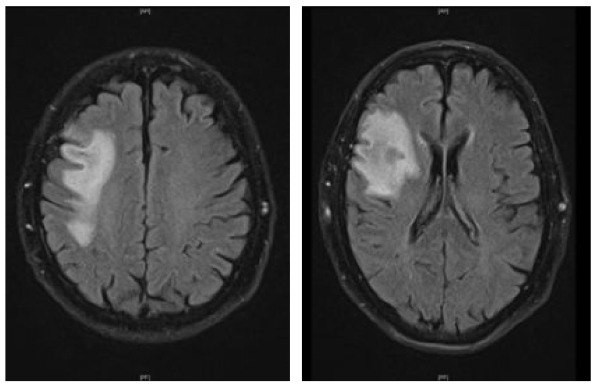
|
|
|
| Sex | Male | |||
| Underlying diagnosis | Non-Hodgkin lymphoma in remission | |||
| Latency initial hematologic malignancy and PML | 9 months | |||
| Immunosuppressive treatment | Rituximab, bendamustine | |||
| Neurological deficits | Left-sided facial nerve palsy, psychomotor slowing, dysarthria | Left-sided facial nerve palsy, psychomotor slowing, dysarthria | Left-sided facial nerve palsy, psychomotor slowing, paresis of left hand, dysarthria, dysphagia | Left-sided facial nerve palsy, psychomotor slowing, left hemiparesis, dysarthria, dysphagia |
| Modified Rankin Scale | 3 | 3 | 4 | 4 |
| Total no. of pembrolizumab infusions | 4 | |||
MRI, magnetic resonance imaging; PML, progressive multifocal leukoencephalopathy.
Case 2
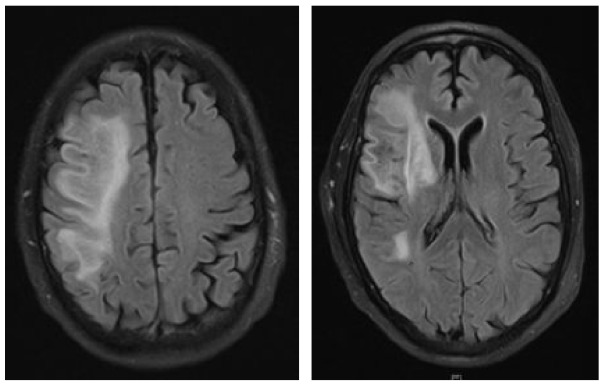
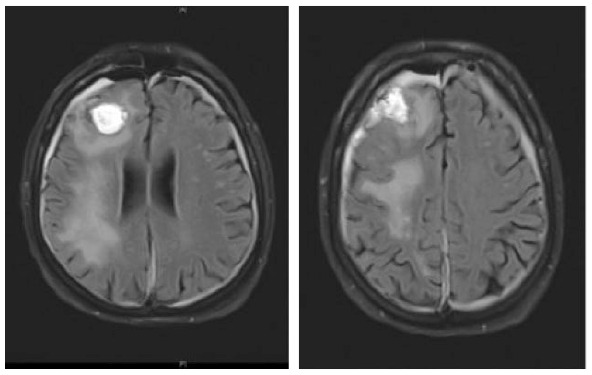
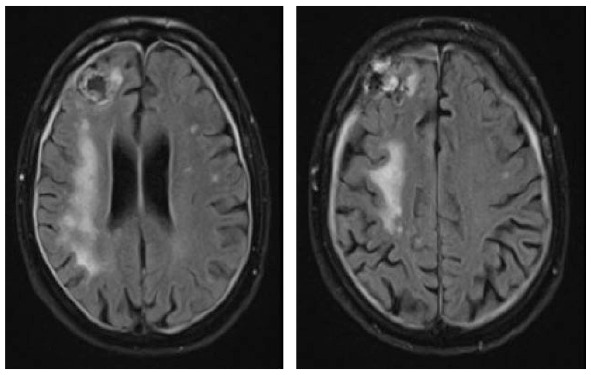
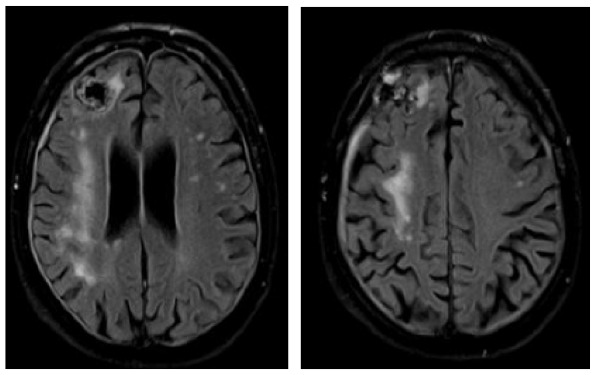
A 73-year-old male was diagnosed with immunocytoma 22 years ago and was treated with chemotherapy including mitoxantrone, melphalan, and prednisone, which led to a complete remission of his hematological disease. Due to hypogammaglobulinemia additional therapy with intravenous immunoglobulins was initiated 19 years after the initial diagnosis, the last infusion being administered 1 month before the first neurological symptoms occurred. A paresis and disturbance of fine motor skills of his left hand were the reasons why the patient presented at an external hospital. Initial brain MRI exhibited large T2-hyperintense lesions in the deep and juxtacortical white matter of the right frontal, temporal and parietal lobe (Table 3). Retrospectively, half a year before a right temporal pachymeningeal thickening had been present on MRI. As CSF analysis remained benign (5 cells/µl, CSF protein 820 mg/l, lactate 1.2 mmol/l, JCPyV PCR negative) (Table 1), a brain biopsy from the right frontal lobe was performed. Histological examination of the tissue revealed lymphoid infiltrates, demyelinating changes, and polymorphic glial cells, which nuclei showed positive staining for JC virus. PML was diagnosed, treatment with mefloquine and mirtazapine was initiated, and the patient was transferred to our clinic for further treatment. At that time, he presented with a central facial palsy and with a moderate hemiparesis on the left side (mRS: 2). Follow-up brain MRI scans revealed size-progressive lesions supporting the histopathology based diagnosis of PML. Another brain MRI 1 month later showed a constant expansion of white matter lesions, and contrast enhancement was slightly increased (Table 3). The first CSF analysis at our department revealed mild pleocytosis (8 cells/µl), a disturbed blood–CSF barrier (CSF protein 1386 mg/l, Qalbumin: 21.6), and positive OCBs. No JCPyV DNA was detectable, but AIJCV was elevated (5.2; normal value ⩽1.5) (Table 1). A hematological co-evaluation revealed indications for a recurrence of non-Hodgkin’s lymphoma (NHL) (clonal B-cell population with kappa light chain restriction within the bone marrow). However, no lymphoma cells as a sign of CNS involvement were found in the CSF. Due to persistent immunoglobulin deficiency, a total of 60 g of intravenous immunoglobulins were substituted. Therapy with pembrolizumab (2 mg/kg bodyweight) was initiated 1 month after PML diagnosis and about 6 weeks after the onset of the first neurological symptoms. In total, the patient received three infusions of pembrolizumab at intervals of 4 weeks each. Already after the first infusion, the symptoms improved significantly and the patient was almost free of complaints. Accordingly, brain MRI images showed mild reduction in lesion size (Table 3) and decrease of contrast enhancement. When the patient presented for the third infusion he was completely free of symptoms. JCPyV DNA was still not detectable (Table 1), but AIJCV was persistently elevated to 6.3. Brain MRI showed a decrease in PML-associated lesions (Table 3).
Table 3.
Clinical characteristics, brain MRI, and clinical course of patient 2 during pembrolizumab treatment. The right frontal signal changes are due to brain biopsy.
| Patient 2 | ||||
|---|---|---|---|---|
| Baseline clinical characteristics | Brain MRI and clinical course | |||
| Age at PML diagnosis (years) | 73 |
|
|
|
| Sex | Male | |||
| Underlying diagnosis | Non-Hodgkin lymphoma, relapsing | |||
| Latency initial hematologic malignancy and PML | 22 years | |||
| Immunosuppressive treatment | Mitoxantrone, melphalan, prednisone | |||
| Neurological deficits | Left-sided facial nerve palsy, left-sided hand extensor paresis | Left-sided facial nerve palsy, moderate hemiparesis on the left side | Minimal left-sided hand extensor paresis | No neurological deficits |
| Modified Rankin Scale | 2 | 2 | 1 | 0 |
| Total no. of pembrolizumab infusions | 3 | |||
MRI, magnetic resonance imaging; PML, progressive multifocal leukoencephalopathy.
Case 3
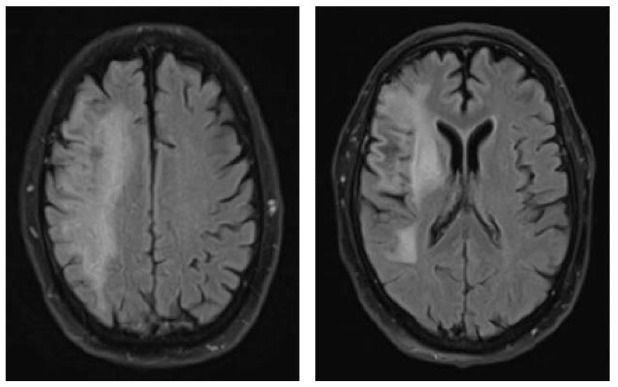
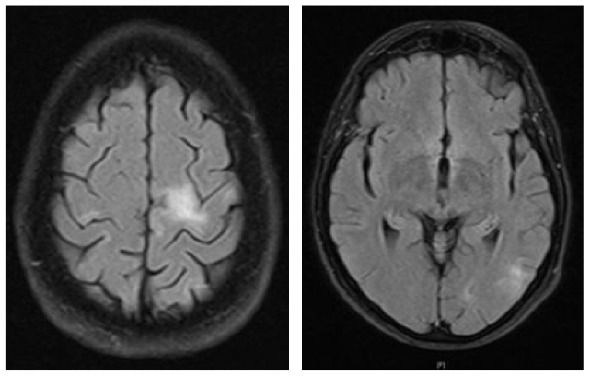
A 70-year-old female was diagnosed with B-cell NHL (follicular lymphoma grade 2). She was treated with rituximab and bendamustine combination therapy (six cycles) for 5 months. Rituximab maintenance therapy was continued for another 9 months. This regimen led to a complete remission of the hematological disease. The patient’s comorbidities included Arnold–Chiari-malformation with previous decompression of the foramen magnum without residual neurological deficits and arterial hypertension. Nine months after completing rituximab/bendamustine combination therapy while rituximab maintenance therapy was still ongoing, the patient reported the first subtle neurological symptoms such as fine motor skill disturbance of the right hand (mRS: 1). Brain MRI revealed multiple cortical grey matter lesions and white matter lesions predominantly of the left hemisphere but also of the right hemisphere (Table 4). These lesions were initially misinterpreted at an external institution as vascular lesions (subacute strokes). Therefore, further cardiovascular diagnostic work-up was performed and the patient was transferred to a rehabilitation clinic. Within the following weeks, the patient presented with a rapid worsening of neurological symptoms including a severe hemiparesis on the right side, motor aphasia, and need for wheelchair use (mRS: 4). A follow-up brain MRI 6 weeks after the initial scan showed a significant increase in lesion volumes (Table 4). Based on the lesion morphology and evolution, PML was suspected and diagnosis was confirmed by the detection of JCPyV DNA in the CSF (500 c/ml) (Table 1). During treatment with mirtazapine, neurological symptoms further progressed and the patient was admitted to our hospital about 10 weeks after initial symptoms occurred. At this time, she was nearly bedridden and required constant help (mRS: 5). Another follow-up brain MRI showed further worsening of PML lesions and CSF JCPyV PCR revealed a viral load of 90.000 c/ml (Table 1). Therapy with pembrolizumab (2 mg/kg bodyweight) was initiated 5 weeks after PML diagnosis and three months after the first neurological symptoms. No improvement of the clinical condition was observed. When the patient was admitted for the second treatment cycle, she was no longer able to express herself independently. The third CSF analysis showed lymphocytic pleocytosis (12 cells/µl) with activated lymphocytes. MRI showed a clear progression in the size of demyelinating lesions with isolated discreet contrast enhancement as a possible sign of a response to therapy in “immune reconstitution inflammatory syndrome (IRIS)”. In addition, viral load further increased to 190.000 c/ml (Table 1), indicating disease progression. However, IRIS could not be excluded as an additional cause of deterioration, since an increase in viral load can also be observed in IRIS.19 A second pembrolizumab cycle was administered, but the patient deteriorated and died within 3 weeks.
Table 4.
Clinical characteristics, brain MRI, and clinical course of patient 3 during pembrolizumab treatment.
| Patient 3 | ||||
|---|---|---|---|---|
| Baseline clinical characteristics | Brain MRI and clinical course | |||
| Age at PML diagnosis (years) | 70 |
|
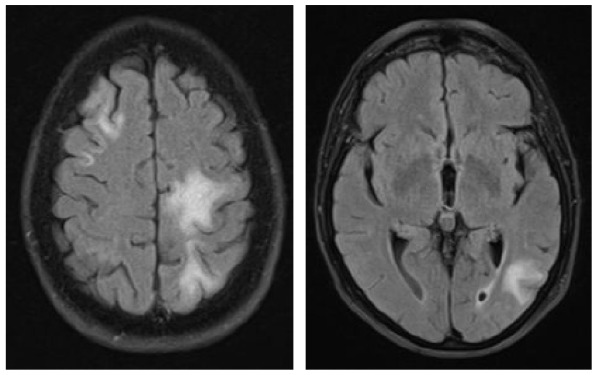
|
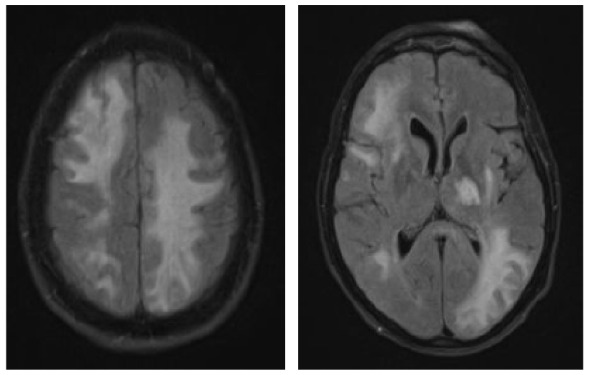
|
| Sex | Female | |||
| Underlying diagnosis | Non-Hodgkin lymphoma in remission | |||
| Latency initial hematologic malignancy and PML | 2.5 years | |||
| Immunosuppressive treatment | Rituximab, bendamustine | |||
| Neurological deficits | Right-sided hemiparesis, aphasia, right-sided facial nerve palsy | Fine motor skill disturbance of the right hand | Right-sided hemiparesis, aphasia, right-sided facial nerve palsy | Right-sided hemiparesis, aphasia, right-sided facial nerve palsy, dysphagia, bedridden patient |
| Modified Rankin Scale | 1 | 1 | 4 | 5 |
| Total no. of pembrolizumab infusions | 2 | |||
MRI, magnetic resonance imaging; PML, progressive multifocal leukoencephalopathy.
Laboratory results
Results of CSF analysis in PML patients
Table 1 shows the results of follow-up CSF analyses in all three patients. Three lumbar punctures were performed in patients 2 and 3, whereas patient 1 received four punctures during the observation period. AIJCV remained negative in patients 1 and 3 during the entire observation period. For patient 2 an increase of the AIJCV was observed in the second and third lumbar puncture. Additionally, OCBs (type 3) were detected. The increase of AIJCV as a specific immune response to JCPyV infection corresponded with an improvement of his clinical symptoms. Regarding standard CSF parameters, no abnormalities appeared in patient 1 except for a slightly disturbed blood–CSF-barrier (Qalbumin: 8.6) in the last lumbar puncture. Patient 2 presented with an increasing pleocytosis as a possible indication of immune activation. Patient 3 as well showed a slight pleocytosis at last lumbar puncture corresponding with slight contrast enhancement in brain MRI.
Analysis of lymphocyte subsets at different time points
Table 5 illustrates the course of the lymphocyte count and the respective lymphocyte subsets over time. Patient 1 presented with lymphocytopenia from the beginning, while lymphocyte cell counts of patient 2 and patient 3 were within normal range. CD20+ cells were not detectable at any time in patients 1 and 3. Both patient 1 and patient 3 showed a relative reduction of CD4+ T helper cells, while in patient 2 the proportion of these cells increased considerably during therapy. Patient 3 also experienced a significant decrease in the relative proportion of CD8+ cytotoxic T cells. No other pronounced changes in the lymphocyte subsets could be detected.
Table 5.
Whole lymphocyte counts and percentage of CD4+, CD8+, CD56+, and CD20+ lymphocytes of all three patients at different time points.
| Patient | Date | Whole lymphocytes (per µl) | CD3+CD4+
(% WLs) |
CD3+CD8+
(% WLs) |
CD20+
(% WLs) |
CD56+CD3−
(% WLs) |
|---|---|---|---|---|---|---|
| 1 | 1 week prior to first infusion | 490 | 26.3 | 17 | 0 | 51.1 |
| 1 day prior to second infusion | 380 | 21.5 | 11.6 | 0 | 60.3 | |
| 2 weeks after second infusion | 700 | 12.2 | 8.1 | 0 | 71.2 | |
| 2 | 1 week prior to first infusion | 2421 | 17.3 | 12.8 | 56.5 | 3.8 |
| 1 day prior to second infusion | 2530 | 26.9 | 19.8 | 39.9 | 9.6 | |
| 2 weeks after second infusion | 1640 | 41.2 | 14.6 | 28.4 | 10.9 | |
| 3 | 1 day prior to first infusion | 1200 | 26.1 | 32.2 | 0 | 9.6 |
| 2 weeks after first infusion | 2120 | 17 | 16.1 | 0.2 | 6.9 |
WL, whole lymphocyte.
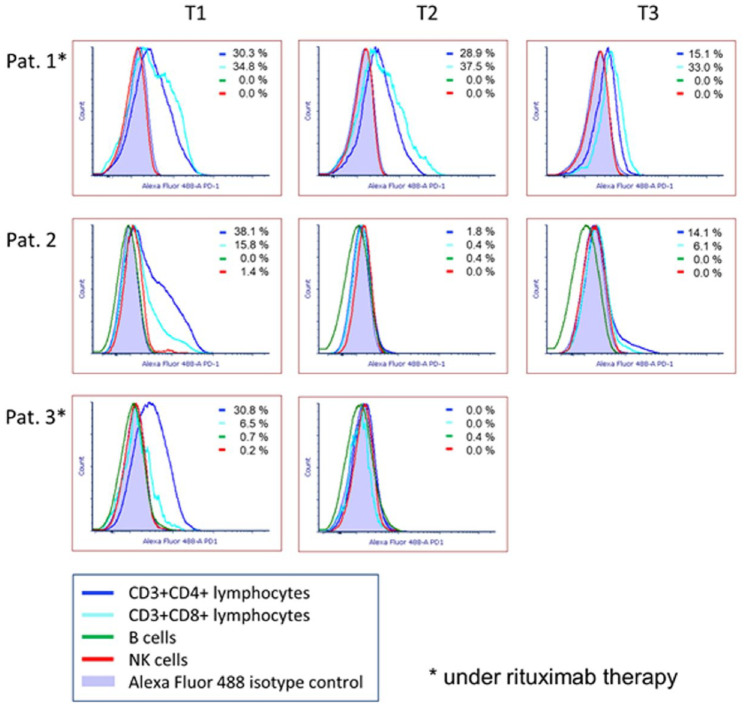
Analysis of PD-1 expression via flow cytometry
Figure 1 displays the proportion of PD-1 positive cells in CD4+ T-cells, CD8+ T-cells, B-cells, and NK cells, isolated from patients’ blood. In the case of patients 1 and 2, PD-1-expression was analyzed prior to the first pembrolizumab-infusion (T1) as well as one day before the second (T2) and the third (T3) infusion, respectively. Measurements in patient 3 were conducted before and shortly after the first infusion.
Figure 1.
Flow cytometry analysis of PD-1 expression in T cells, B cells, and NK cells isolated from patients’ blood.
Timing of measurements: T1, prior to the first pembrolizumab-infusion; T2, 1 day before the second infusion; T3, 1 day before third infusion.
Pat., patient.
We see that all patients exhibited a relatively high proportion of PD-1 positive CD4+ T-cells prior to the first pembrolizumab treatment. In patient 1, the proportion of PD-1 positive cells decreased steadily, but remained at a rather high level. In patients 2 and 3, PD-1 positive CD4+ T-cells were almost completely absent after the first infusion, but in the case of patient 2 they repopulated after the second treatment cycle. CD8+ T-cells of patient 1 showed a constantly high proportion of PD-1 positive cells (33.0–37.5%) during the entire analysis period. In patients 2 and 3, no PD-1 positive CD8+ T-cells were detectable after the first pembrolizumab infusion. One day prior to the third infusion a repopulation of PD-1 positive CD8+ T-cells was observed in patient 2. None of the patients exhibited a relevant proportion of PD-1 positive B-cells or NK cells, neither before nor during therapy. It has to be added that patient 1 had lymphopenia (380–700/µl) throughout the entire examination period. Due to rituximab therapy, no B-cells were detectable in this patient (data not shown).
Discussion
We describe three patients who were treated with pembrolizumab due to PML. During therapy the patients differed significantly regarding their clinical disease course. Patient 1 exhibited gradual deterioration of neurological symptoms and increase of lesion load over time. However, his JCPyV viral load within the CSF was slightly decreased. While patient 2 showed a gradual improvement of symptoms and could be discharged without complaints after the third infusion, patient 3 experienced rapid clinical and imaging deterioration up to death despite two pembrolizumab infusions. In contrast to patient 2, who exhibited a specific immune response to JCPyV infection, no AIJCV was detectable in patients 1 and 3. Patient 3, who had the worst outcome compared with the other patients, presented with the highest viral load. This observation is in line with finding that a high viral load at diagnosis negatively correlates with PML outcome.21 The fact that patient 1 exhibited a markedly reduced lymphocyte count as a result of rituximab therapy may be the reason for the limited therapeutic effect in his case. Former studies in patients with head and neck squamous cell carcinomas and advanced non-small cell lung cancer have demonstrated that patients with lower lymphocyte count appeared to have significantly less clinical benefit from anti-PD-1 therapy.22,23 The same might be true for the effect of pembrolizumab in clearing JCPyV from the CNS of PML patients. Long-term rituximab therapy and consecutive depletion of CD20+ cells as seen in patient 1 and patient 3 may be a reason for negative AIJCV.
Mechanisms of T-cell activation via PD-1 inhibition have not been elucidated in full detail yet. It is known that PD-1 is predominantly expressed on T-cells and its interaction with PD-L1 and PD-L2 expressed on antigen-presenting cells sends a negative signal to T-cells, which can lead to T-cell exhaustion.24 T-cell exhaustion or dysfunction is seen as a key mechanism contributing to impaired T-cell responses against tumors and also against viral pathogens.25 Consequently, restoring T-cell activity via anti-PD-1 treatment is not only efficient in cancer therapy but may also provide a therapeutic option for chronic viral infections such as PML.10
Interestingly, fluorescence activated cell sorting (FACS) analysis of lymphocytes in patient 2, the only patient with a pronounced positive effect of pembrolizumab, initially showed a significant reduction of PD-1 positive cells. This effect was consistent with the results of Cortese and colleagues, who observed that pembrolizumab treatment was associated with a decrease in detection of PD-1 on lymphocytes in peripheral blood and CSF.10 Following the second infusion, a repopulation of those cells was observed. The initial decrease of PD-1 on lymphocytes may be explained by the fact that antibody binding to cell surface receptors, such as PD-1, leads to cross-linking and internalization of the receptors with consecutive cell activation and proliferation.26 Activated T-cells with internalized PD-1 receptor are no longer detectable by flow cytometry analysis. As a restriction in case 2 contrast enhancement was slightly increased on MRI prior to the initiation of therapy as a possible indication of beginning immune reconstitution.
Our observations suggest that pembrolizumab may have the potential to effectively treat PML in individual cases. However, we could not reproduce the positive results of the first published case series by Cortese and colleagues.10 As with Pawlitzki and colleagues16 two of our three patients must be considered as therapy failures. In addition, it must be noted that in the case of patient 2, who showed positive outcome, the assessment of treatment response might have been influenced by the use of immunoglobulins. The biggest problem at present is the correct stratification of patients regarding the possible success of anti-PD-1 therapy. In general, the balance between disease burden and the strength of T-cell reinvigoration appears to define the likelihood of success of anti-PD-1 therapy in PML.27 Having a sufficient number of functional lymphocytes prior to the start of anti-PD-1 therapy seems to be an important condition for the treatment’s success. Analysis of PD-1 expression via flow cytometry hereby is helpful to estimate the impact of PD-1 expression on lymphocytes. Previous studies have demonstrated that depletion of B cells can have a negative effect on the function of CD8+ cytotoxic T cells.28–30 This is a possible explanation for the fact that rituximab-treated patients did not benefit from pembrolizumab therapy. At the same time, an increase in AIJCV as a sign of a specific B-cell response could indicate a positive course of the disease. Therefore, persisting or increasing AIJCV may not only serve as an indicator for immune activation, but might also be a parameter for predicting treatment success. However, the role of the CD8+ cytotoxic T cells themselves is more crucial in this infection. CD4+ T helper cells also seem to play an important role, as their proportion in patient 2 increased significantly during therapy, especially compared with the other patients. Additionally, the increase of AIJCV in patient 2 has to be interpreted with caution as he had received intravenous immunoglobulins before. Prior studies on JC-specific immune response have underlined the important role of cellular immunity,31,32 but it has also been shown that higher JCV-specific IgG activity at diagnosis and a rise in JCV IgG levels was associated with better survival.33 In the future, further biomarkers will be needed to facilitate the individual prognosis of the therapeutic response for each patient. One conceivable option would be to measure T-cell activation via flow cytometry, for example through analysis of CD69 expression on T-cells34 or measuring the spatiotemporal calcium mobilization,35 in order to estimate the chance of therapeutic efficacy before initiating anti-PD-1 therapy in PML.
In summary, anti-PD-1 therapy represents a potential innovative treatment option for patients with PML. Currently, there is a lack of pre-therapeutic stratification regarding the response to therapy. Should it be possible to select patients more specifically before treatment initiation, larger, multi-center studies will be necessary to determine the success of anti-PD-1 therapy more precisely.
Supplemental Material
Supplemental material, sj-tif-1-tan-10.1177_1756286421993684 for PD-1-inhibitor pembrolizumab for treatment of progressive multifocal leukoencephalopathy by Nora Möhn, Mike P. Wattjes, Ortwin Adams, Sandra Nay, Daria Tkachenko, Friederike Salge, Johanne Heine, Kaweh Pars, Günter Höglinger, Gesine Respondek, Martin Stangel, Thomas Skripuletz, Roland Jacobs and Kurt-Wolfram Sühs in Therapeutic Advances in Neurological Disorders
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Sandra Nay  https://orcid.org/0000-0002-4986-1181
https://orcid.org/0000-0002-4986-1181
Martin Stangel  https://orcid.org/0000-0003-2504-5398
https://orcid.org/0000-0003-2504-5398
Kurt-Wolfram Sühs  https://orcid.org/0000-0002-8077-481X
https://orcid.org/0000-0002-8077-481X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Nora Möhn, Department of Neurology, Hannover Medical School, Hannover, Germany.
Mike P. Wattjes, Department of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany
Ortwin Adams, Department of Virology, University Hospital Düsseldorf, Düsseldorf, Germany.
Sandra Nay, Department of Neurology, Hannover Medical School, Hannover, Germany.
Daria Tkachenko, Department of Neurology, Hannover Medical School, Hannover, Germany.
Friederike Salge, Department of Neurology, Hannover Medical School, Hannover, Germany.
Johanne Heine, Department of Neurology, Hannover Medical School, Hannover, Germany.
Kaweh Pars, Department of Neurology, European Medical School, University Oldenburg, Oldenburg, Germany.
Günter Höglinger, Department of Neurology, Hannover Medical School, Hannover, Germany.
Gesine Respondek, Department of Neurology, Hannover Medical School, Hannover, Germany.
Martin Stangel, Department of Neurology, Hannover Medical School, Hannover, Germany.
Thomas Skripuletz, Department of Neurology, Hannover Medical School, Hannover, Germany.
Roland Jacobs, Department of Rheumatology & Clinical Immunology, Hannover Medical School, Hannover, Germany.
Kurt-Wolfram Sühs, Department of Neurology, Hannover Medical School, Carl-Neuberg-Str. 1, Hannover, 30625, Germany.
References
- 1. Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol 2010; 9: 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gheuens S, Wuthrich C, Koralnik IJ. Progressive multifocal leukoencephalopathy: why gray and white matter. Annu Rev Pathol 2013; 8: 189–215. [DOI] [PubMed] [Google Scholar]
- 3. Major EO, Amemiya K, Elder G, et al. Glial cells of the human developing brain and B cells of the immune system share a common DNA binding factor for recognition of the regulatory sequences of the human polyomavirus, JCV. J Neurosci Res 1990; 27: 461–471. [DOI] [PubMed] [Google Scholar]
- 4. Ferenczy MW, Marshall LJ, Nelson CD, et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev 2012; 25: 471–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maas RP, Muller-Hansma AH, Esselink RA, et al. Drug-associated progressive multifocal leukoencephalopathy: a clinical, radiological, and cerebrospinal fluid analysis of 326 cases. J Neurol 2016; 263: 2004–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mateen FJ, Muralidharan R, Carone M, et al. Progressive multifocal leukoencephalopathy in transplant recipients. Ann Neurol 2011; 70: 305–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN neuroinfectious disease section. Neurology 2013; 80: 1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kartau M, Sipila JO, Auvinen E, et al. Progressive multifocal leukoencephalopathy: current insights. Degener Neurol Neuromuscul Dis 2019; 9: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pavlovic D, Patera AC, Nyberg F, et al. Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord 2015; 8: 255–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cortese I, Muranski P, Enose-Akahata Y, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med 2019; 380: 1597–1605. [DOI] [PubMed] [Google Scholar]
- 11. Beck ES, Cortese I. Checkpoint inhibitors for the treatment of JC virus-related progressive multifocal leukoencephalopathy. Curr Opin Virol 2020; 40: 19–27. [DOI] [PubMed] [Google Scholar]
- 12. Walter O, Treiner E, Bonneville F, et al. Treatment of progressive multifocal leukoencephalopathy with nivolumab. N Engl J Med 2019; 380: 1674–1676. [DOI] [PubMed] [Google Scholar]
- 13. Hoang E, Bartlett NL, Goyal MS, et al. Progressive multifocal leukoencephalopathy treated with nivolumab. J Neurovirol 2019; 25: 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rauer S, Marks R, Urbach H, et al. Treatment of progressive multifocal leukoencephalopathy with pembrolizumab. N Engl J Med 2019; 380: 1676–1677. [DOI] [PubMed] [Google Scholar]
- 15. Goereci Y, Schweitzer F, Wellstein A, et al. Clearance of JC polyomavirus from cerebrospinal fluid following treatment with interleukin-2 and pembrolizumab in an individual with progressive multifocal leukoencephalopathy and no underlying immune deficiency syndrome. Eur J Neurol 2020; 27: 2375–2377. [DOI] [PubMed] [Google Scholar]
- 16. Pawlitzki M, Schneider-Hohendorf T, Rolfes L, et al. Ineffective treatment of PML with pembrolizumab: exhausted memory T-cell subsets as a clue? Neurol Neuroimmunol Neuroinflamm 2019; 6: e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tufa DM, Chatterjee D, Low HZ, et al. TNFR2 and IL-12 coactivation enables slanDCs to support NK-cell function via membrane-bound TNF-α. Eur J Immunol 2014; 44: 3717–3728. [DOI] [PubMed] [Google Scholar]
- 18. Warnke C, von Geldern G, Markwerth P, et al. Cerebrospinal fluid JC virus antibody index for diagnosis of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2014; 76: 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan IL, McArthur JC, Clifford DB, et al. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology 2011; 77: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ryschkewitsch CM, Jensen P, Hou J, et al. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. Journal of virological methods 2004; 121: 217–221. [DOI] [PubMed] [Google Scholar]
- 21. Blankenbach K, Schwab N, Hofner B, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in Germany. Neurology 2019; 92: e2232–e2239. [DOI] [PubMed] [Google Scholar]
- 22. Ho WJ, Yarchoan M, Hopkins A, et al. Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J Immunother Cancer 2018; 6: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cho Y, Park S, Byun HK, et al. Impact of treatment-related lymphopenia on immunotherapy for advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2019; 105: 1065–1073. [DOI] [PubMed] [Google Scholar]
- 24. Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious diseases. European journal of immunology. 2017; 47: 765–79. [DOI] [PubMed] [Google Scholar]
- 25. McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol 2019; 37: 457–495. [DOI] [PubMed] [Google Scholar]
- 26. Ikushima H, Munakata Y, Ishii T, et al. Internalization of CD26 by mannose 6-phosphate/insulin-like growth factor II receptor contributes to T cell activation. Proc Natl Acad Sci U S A 2000; 97: 8439–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017; 545: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo L, Kapur R, Aslam R, et al. CD20+ B-cell depletion therapy suppresses murine CD8+ T-cell-mediated immune thrombocytopenia. Blood 2016; 127: 735–738. [DOI] [PubMed] [Google Scholar]
- 29. Fiocca Vernengo F, Beccaria CG, Araujo Furlan CL, et al. CD8+ T cell immunity is compromised by anti-CD20 treatment and rescued by interleukin-17A. mBio 2020; 11: e00447-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen H, Whitmire JK, Fan X, et al. A specific role for B cells in the generation of CD8 T cell memory by recombinant Listeria monocytogenes. J Immunol 2003; 170: 1443–1451. [DOI] [PubMed] [Google Scholar]
- 31. Du Pasquier RA, Clark KW, Smith PS, et al. JCV-specific cellular immune response correlates with a favorable clinical outcome in HIV-infected individuals with progressive multifocal leukoencephalopathy. J Neurovirol 2001; 7: 318–322. [DOI] [PubMed] [Google Scholar]
- 32. Du Pasquier RA, Kuroda MJ, Zheng Y, et al. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain 2004; 127: 1970–1978. [DOI] [PubMed] [Google Scholar]
- 33. Khanna N, Wolbers M, Mueller NJ, et al. JC virus-specific immune responses in human immunodeficiency virus type 1 patients with progressive multifocal leukoencephalopathy. J Virol 2009; 83: 4404–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cibrian D, Sanchez-Madrid F. CD69: from activation marker to metabolic gatekeeper. Eur J Immunol 2017; 47: 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cerveira J, Begum J, Di Marco Barros R, et al. An imaging flow cytometry-based approach to measuring the spatiotemporal calcium mobilisation in activated T cells. J Immunol Methods 2015; 423: 120–130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-tan-10.1177_1756286421993684 for PD-1-inhibitor pembrolizumab for treatment of progressive multifocal leukoencephalopathy by Nora Möhn, Mike P. Wattjes, Ortwin Adams, Sandra Nay, Daria Tkachenko, Friederike Salge, Johanne Heine, Kaweh Pars, Günter Höglinger, Gesine Respondek, Martin Stangel, Thomas Skripuletz, Roland Jacobs and Kurt-Wolfram Sühs in Therapeutic Advances in Neurological Disorders