Abstract
The development and application of quantitative systems pharmacology models in neuroscience have been modest relative to other fields, such as oncology and immunology, which may reflect the complexity of the brain. Technological and methodological advancements have enhanced the quantitative understanding of brain physiology and pathophysiology and the effects of pharmacological interventions. To maximize the knowledge gained from these novel data types, pharmacometrics modelers may need to expand their toolbox to include additional mathematical and statistical frameworks. A session was held at the 10th annual American Conference on Pharmacometrics (ACoP10) to highlight several recent advancements in quantitative and systems neuroscience. In this mini‐review, we provide a brief overview of technological and methodological advancements in the neuroscience therapeutic area that were discussed during the session and how these can be leveraged with quantitative systems pharmacology modeling to enhance our understanding of neurological diseases. Microphysiological systems using human induced pluripotent stem cells (IPSCs), digital biomarkers, and large‐scale imaging offer more clinically relevant experimental datasets, enhanced granularity, and a plethora of data to potentially improve the preclinical‐to‐clinical translation of therapeutics. Network neuroscience methodologies combined with quantitative systems models of neurodegenerative disease could help bridge the gap between cellular and molecular alterations and clinical end points through the integration of information on neural connectomics. Additional topics, such as the neuroimmune system, microbiome, single‐cell transcriptomic technologies, and digital device biomarkers, are discussed in brief.
INTRODUCTION
After the 9th annual American Conference on Pharmacometrics in 2018, a Neuroscience Quantitative Systems Pharmacology (NeuroQSP) working group was formed in collaboration between the International Society of Pharmacometrics (ISoP) QSP Special Interest Group (QSP SIG) and C‐Path Neuro‐Pharmacometrics Community of Practice (NeuroCOP). The NeuroQSP working group organized a session, the subsequent year, at 10th annual American Conference on Pharmacometrics (ACoP10) titled, Quantitative Systems Pharmacology in Neuroscience: Novel Methodologies and Technologies. The overall goal of the session was to highlight some of the ongoing state‐of‐the‐art computational and experimental approaches in quantitative and systems neuroscience. The session offered a perspective on various techniques typically considered beyond the scope of traditional pharmacometrics approaches. Topics included an application of quantitative systems pharmacology (QSP) modeling, an experimental microphysiological model of the brain, and applications of network neuroscience and its combination with wearable readouts. In addition to the topics covered in the session, other advancements were briefly mentioned during the opening comments of the session relating to neuroimaging, digital device biomarkers, single‐cell transcriptomics, machine learning, microbiome, and neuroimmunology.
QSP approaches combine experimental and computational methods to understand the pharmacological effects of drugs on biological systems, which provides a framework for translational medicine. 1 QSP is a promising approach for drug discovery and development, particularly in the neuroscience therapeutic area due to the high drug attrition rates despite the wealth of information and significant advancements in basic research. 2 Experimental models in neuroscience have poor translatability due to many factors, including differences in brain physiology and disease etiology between animals and humans. 3 The lack of predictive biomarkers of disease state and nature of functional clinical end points create challenges. Clinical applications of magnetic resonance imaging (MRI) and positron emission tomography (PET) have enabled the ability to obtain data on brain networks and biodistribution of molecules in the brain allowing better understanding of drug exposure and target engagement in the central nervous system (CNS), which often remains unknown and assumed to be similar to exposures and engagement in surrogate compartments, such as blood or cerebral spinal fluid. Pharmaco‐electroencephalography (EEG) and pharmaco‐functional MRI methods are additional techniques to visualize pharmacodynamic (PD) effects of drugs on brain activity.
Novel in vitro preclinical models, such as microphysiological systems (MPS), better represent tissue physiology and may improve translation to humans. Novel biomedical devices are enabling the generation of digital biomarkers, which have great potential in the neuroscience space to supplement current metrics of sleep quality, cognition function, gait measurements, physical activity, vocal abnormalities, and behavioral changes. Figure 1 depicts select methodologies and technologies in the neuroscience domain that are further described in the paper.
FIGURE 1.
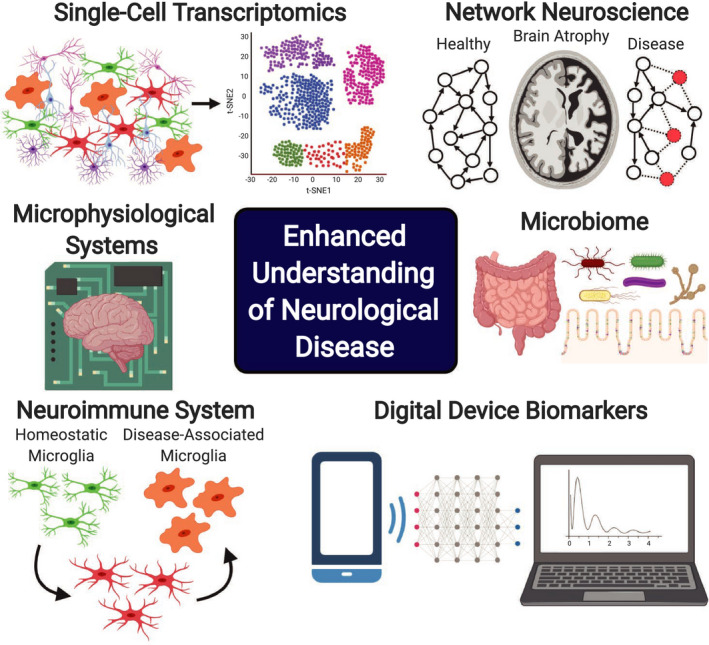
Select methodologies, technologies, and areas of research in neuroscience that can be leveraged and incorporated in Quantitative Systems Pharmacology modeling workflows to enhance the quantitative understanding of pathophysiological processes in neurological disease and therapeutic effects of pharmacological treatment strategies
As novel types of experimental data emerge, the pharmacometrics community may need to consider the development of new modeling workflows, such as network‐based, agent‐based, and machine learning methods that utilize less routine mathematical and/or statistical frameworks. Workflows and computational methods should be tailored to the specific data type and spatiotemporal resolution of the biological processes to be described. Integration of models representing different neurobiological scales from the cellular and molecular level to neural circuitry, brain, and ultimately functional and behavioral clinical end points remains a major challenge.
SESSION OVERVIEW
The first presentation, by Tatiana Karelina, introduced an Alzheimer’s disease (AD) QSP platform that had been developed and applied to study the mechanisms of disease etiology. Beta‐amyloid (Aβ) and tau are two key hallmarks and potential targets in AD. Previously developed models of Aβ/tau pathology were integrated and updated with intracellular processes implicated in AD to construct an AD QSP platform model. 4 Intracellular processes that contribute to Aβ and tau accumulation were included in the platform model as individual submodels. These submodels include the autophagy‐lysosomal system, proteasome, cholesterol and sphingolipid metabolism, and calpain biology. Submodels were individually developed, calibrated, and validated. A programming platform was developed for automatic integration of submodels into a single model framework.
The AD QSP model was used to describe the rate of disease progression and predict effects of therapeutic interventions. Specifically, the model captures the decreased activity of protein degradation processes and accumulation of pathological proteins. The model was validated using preclinical datasets of transgenic species with pharmacological activators of protein degradation, which are shown to halt the in vivo accumulation of Aβ and tau and described an accumulation of tau tangles in the limbic system prior to an extensive appearance of Aβ in the cerebral cortex in humans. Despite the potential interplay between Aβ and tau, model predictions support initial clinical observations that biomarkers of tau pathology are typically not sensitive to amyloid‐targeted therapy, which was observed clinically in patients with mild‐to‐moderate AD treated with a beta‐site cleavage enzyme (BACE) inhibitor. QSP model simulations in prodromal AD, predicted an 80% elimination of amyloid burden and approximately a 15–25% decrease in tau compared with placebo, over several years of treatment. Anti‐Aβ antibody therapies clinically investigated for AD exhibited decreased Aβ and tau burden, whereas BACE inhibitors only reduced Aβ. The QSP platform model reflects multiple biomarkers of neuronal functioning, but lacks any connection to clinical outcomes. The most imperative question is how to appropriately translate these predictions on the molecular level to capture neurophysiological end points.
The second presentation, by Murat Cirit, provided an assessment of drug‐induced toxicity in human embryonic stem cell‐derived brain MPS using targeted and untargeted molecular profiling. The workflow presented used a combined experimental brain MPS, QSP modeling, and machine learning algorithms to enable preclinical‐clinical translation. MPS can be utilized in a variety of preclinical applications, such as the assessment of pharmacokinetics (PKs), PDs, and toxicity studies. The brain MPS, described in 2015 by Schwartz et al., was applied to assess the toxicodynamic effects of a neurotoxic and non‐neurotoxic drug, bortezomib and tamoxifen, respectively. 5 , 6 Significant differences in metabolomic profiles between the two drugs were identified and mapped to metabolic pathways to identify mechanisms of toxicity. Bortezomib‐induced alterations to the cysteine pathway suggested a disruption of reduction‐oxidation balance leading to the accumulation of reactive oxygen species and subsequent oxidative stress. 6 These novel in vitro methods are expanding in complexity from a single organ of interest to studying multiple organs and the cross‐talk between different organs. For example, a multi‐MPS model, described as a “physiome‐on‐a‐chip,” was developed for up to 10 different organs and metrics of tissue function and integrity were quantified. 7 Considering that these human‐based MPS models provide high‐content organotypic data, MPS experiments can be used in QSP models to extrapolate in vitro findings to in vivo outcomes. MPS coupled with physiologically‐based PK (PBPK) and QSP models can be used to characterize drug metabolism, disposition to different organs, and evaluate drug‐drug interactions and effects on organs of interest.
The third presentation, by Sarah Muldoon, introduced the field of network neuroscience and the opportunity for developing personalized brain network models (BNMs) for improving our understanding of individual disease progression and response to therapeutic interventions. Network neuroscience models of the brain consist of a complex network, where nodes can represent neurons (at the microscale level) or brain regions (at the macroscopic level) and edges represent the structural or functional connectivity between nodes. 8 Personalized BNMs can be developed to study structural‐functional relationships in individual patient’s brains. 9 Underlying differences in the structural and functional connectivity of the brain has been shown to differentiate individual cognitive performance and classify healthy versus diseased individuals. 10 Examples for the application of personalized BNMs for understanding the pharmacological effects on altering disease progression are sparse. Stefanovski et al. (2019) developed personalized BNMs for patients with AD through a combination of PET data that measures amyloid burden and MRI to obtain information on structural connectivity. 11 The model reproduced known aberrant EEG alterations observed in patients with AD compared with healthy controls and predicted that intervention with an N‐methyl‐D‐aspartate receptor antagonist, such as memantine, could reverse EEG alterations. The spatial heterogeneity of Aβ burden through PET measurements was critical for model‐predicted EEG alterations in patients with AD, as a homogenously distributed mean value for Aβ burden did not produce simulations with EEG alterations.
Last, Justin Baker presented on computational phenotyping of psychiatric disorders using neuroimaging and digital devices. Intrinsic functional connectivity MRI is a powerful technology that has enabled the characterization of human brain organization. Baker et al. (2014) obtained functional connectivity profiles for 100 patients with psychotic illnesses and 100 appropriately matched healthy controls. 12 They found that individuals with a psychotic illness, relative to healthy controls, had significantly disrupted brain regions, especially the frontoparietal control network. In a subsequent study, a machine learning approach using MRI data of individual‐specific functional connectivity was able to predict positive and negative syndrome scale (PANSS) score of patients with schizophrenia, schizoaffective disorder, or bipolar disorder with psychosis. 13 Last, a perspective was provided on how the passive collection of data through smartphones and other digital devices could be used to identify significant differences in habitual behaviors to forecast episodes of relapse into a manic or depressed state.
HIGHLIGHT OF NOVEL METHODOLOGIES AND TECHNOLOGIES
Network neuroscience
Network neuroscience is a rapidly growing interdisciplinary field, which aims to leverage principles of graph theory to analyze complex data across all levels of neurological organization and temporal scales. 8 Network neuroscience seeks to bridge gaps between scales of biological organization and can include molecular networks, such as genes and biomolecules giving rise to particular networks of intracellular pathways in neurons and glia. Other implementations investigate how neuron‐level processes produce structural and functional circuits, and how they integrate in specific brain regions.
Microphysiological systems
MPSs have gained tremendous popularity as they account for complex cytoarchitecture of human physiology in vitro and, hence, provide more physiologically relevant experimental framework, which cannot be achieved with traditional in vitro methods. Neural circuits in a region of the brain develop in 3D space and interact through a complex network, which is a major limitation for the translatability between 2D in vitro experimental models and in vivo observations. Microphysiological models of neuronal networks have been developed to overcome some of these limitations. 14 A microphysiological model of the peripheral nerve has been used to evaluate neurotoxicity and chemotherapy‐induced peripheral neuropathy. In addition to nerve‐on‐a‐chip technology, mini‐brain is another microphysiological model that has been developed for neuroscience applications. 15 MPS of the brain, using patient‐derived neuron and glial cells from induced‐pluripotent stem cells, could potentially help to select better lead drug candidates and improve preclinical‐clinical translatability.
Multiorgan MPS mimic physiological interactions among organs and could represent a whole‐body response to pharmacological perturbations. Choice of a common media that is able to support all of the different organs is a challenge, as some cell types can be more sensitive than others and require specific nutrients and growth factors. Another challenge is the incorporation of a vascular network and endothelial layer to create a closed microvascular network separating blood and tissues. This would be particularly important for neuroscience therapeutics as the blood‐brain‐barrier highly regulates the transport of molecules into the CNS. Considering the design and predetermined parameters (flow rates and volumes) of the multi‐organ MPS, a PBPK modeling approach would be appropriate to describe PKs. As MPS become more high‐throughput and provide more complex biological readouts, a QSP modeling approach could be applied to describe PDs.
Single‐cell transcriptomics and neuroimmunology
Single‐cell transcriptomics has begun to revolutionize our understanding of the human brain. Recently, a single‐cell transcriptomics study identified 75 distinct cell types in the middle temporal gyrus of the human brain. 16 These cells were primarily inhibitory (45) and exhibitory (24) neurons, but also consisted of six non‐neuronal glial cells. Single‐cell transcriptomics experiments could help inform the development of QSP models by identifying unique cell populations, determining cell‐type abundance, and understanding differential gene expression among cell‐types. A foundational single‐cell transcriptomic study in neuroscience was by Keren‐Shaul et al. in 2017, where they identified a new type of microglia cell, termed disease‐associated microglia (DAM), which is associated with neurodegenerative diseases and exhibit a distinct phenotype. 17 DAMs are spatially located at sites of disease pathology and exhibit a protective phenotype, an upregulation of phagocytic‐related genes, regulation of the neuroimmune microenvironment, chemotaxis to sites of disease pathology, and barrier formation around pathological regions. Understanding the function of the neuroimmune system could lead to novel treatment strategies for neurodegenerative diseases.
Microbiome
Microorganisms that live in the human gut and brain have been gaining a lot of attention for the potential role they play in the development and progression of neurodegenerative diseases. Many analyses to date have primarily reported correlative findings, such as relationships between the abundance of specific bacteria taxa and AD biomarkers. 18 Recent preclinical experiments in mice showed the formation of pathological alpha‐synuclein in the gut and transneuronal spread to the brain via the gut‐brain‐axis, which subsequently led to the manifestation of a Parkinson’s disease phenotype, 19 supporting the hypothesis that Parkinson’s disease arises in the gastrointestinal tract. The role for the cross‐talk between the gut microbiome, immune system, and CNS on neurological function and disease has been extensively reviewed. 20
Digital device biomarkers
Technological advancements have enabled the ability to gather large amounts of data over an extended period of time. Data can be collected using various types of technology, such as wearables, invisibles, ingestibles, and smartphone applications. Wearables physically attach to the human body, such as a cell phone or smart watch. Invisibles are devices that remain in the vicinity, but are not physically attached to the body. The Emerald device, an invisible technology developed at the Massachusetts Institute of Technology, has been utilized in a pilot study to passively monitor the activity of patients with Parkinson’s disease. 21 Wearables and invisibles are able to track human activity, heart rate, breathing patterns, sleeping patterns, and obtain spatial information about physical location. These devices in particular have the opportunity to better understand day‐to‐day variability and trajectory of disease end points, allowing to better understand therapeutic drug effects in a more realistic clinical setting. Continuous datasets of certain metrics may enable a better understanding of signs leading to an event, such as a suicide attempt or a schizophrenic episode. Information obtained from digital devices could help to replace or supplement current clinical scores for neuropsychiatric and neurodegenerative diseases.
This leads to the relatively new concept of virtual decentralized patient‐centric clinical trials. Although continuous monitoring of day‐to‐day functional outcomes might reduce variability in clinical trials and decrease the burden on patients by skipping site visits, significant issues remain, such as regulatory acceptance and standardization, signal detection and data analysis, patient dropout rates, and privacy concerns. Metrics obtained from digital devices could help to replace or supplement current clinical scores for neuropsychiatric and neurodegenerative diseases where a number of observational studies are currently underway in Parkinson’s disease.
IMPLEMENTATION OF QSP MODELING IN DRUG DISCOVERY AND DEVELOPMENT
Omics studies, MPS experiments, neuroimaging, and digital devices can provide information on drug pharmacology and disease pathophysiology across multiple levels of biological organization and implemented throughout various stages of the drug discovery and development process (Figure 2). Appropriate computational and experimental methods will depend on the question of interest and scope of the project. For pipeline programs, individual computational models can be utilized to characterize a specific process or a multiscale model can be developed to characterize multiple processes that span a wide spatial scale. The technical details of integrating models that describe multiple levels of biological organization and different mathematical frameworks is outside the scope of this review. Differential equations, Boolean and logic‐based networks, agent‐based, network neuroscience modeling, and machine learning are some of the mathematical modeling frameworks that can be utilized to translate data obtained from the reviewed technologies into actionable knowledge.
FIGURE 2.
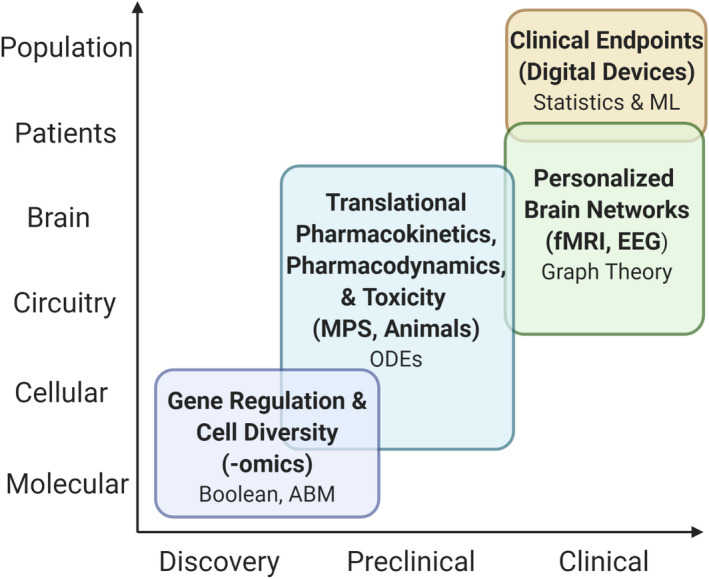
Select methodologies and technologies that provides pharmacological and pathophysiological information across various levels of neurobiological organization and can be implemented across several stages of the drug discovery and development process. Each level contains select modeling approaches that are appropriately suited to describe data obtained from the experimental methodologies listed in parentheses. ABM, agent‐based modeling; EEG, electroencephalography; fMRI, functional magnetic resonance imaging; ML, machine learning; MPS, microphysiological system; ODEs, ordinary differential equations
Graph theory and network neuroscience modeling
As described above, network neuroscience investigates how structural and functional connectomics links to patterns of behavior and functionally relevant scales. Structural information combined with functional imaging from MRI techniques can be combined with mathematical models of brain activity to develop personalized BNMs. 9 In other words, a network model can be developed for each individual patient, where nodes represent regions of the brain and edges are the structural connections between these regions. Mathematical equations are then used to simulate brain activity based on the observed connections in a given individual. The topological structure of this model has been used for investigating high‐level mechanisms related to amyloid and tau progression in AD and for differential diagnosis in dementia. 22 In the mouse brain, a network model, based on brain connectomics and endogenous levels of alpha‐synuclein, was developed to quantitatively understand the pathology spread of alpha‐synuclein. 23 Other example applications include understanding the rate of disease progression at the individual patient level, in epilepsy, 24 and its use as a PD biomarker of pharmacological interventions. 25
A primary goal of bringing principles of network neuroscience into the QSP modeling field is to leverage individual patient connectomics data to bridge the gap from cellular and molecular changes to clinical end points. As an example of QSP modeling with clinical readouts, a detailed biophysical model of a cortico‐striatal‐thalamo‐cortical network for motor symptoms in Parkinson’s disease was calibrated to the Unified Parkinson’s Disease Rating Scale (UPDRS) and generated a classifier for the prediction of motor side effects in clinical practice of schizophrenia patients on antipsychotic polypharmacy, which showed superior predictive value over traditional chlorpromazine equivalents. 26
Selecting and implementing appropriate mathematical and statistical frameworks to analyze data and describe the multiple levels of neurobiological complexity will be difficult. To our advantage, we can utilize resources from computational and systems neuroscience fields. For example, a tool for multiscale modeling of brain circuits, NetPyNE, and a repository for standardized models of neurons and circuits, Open Source Brain, were recently developed. 27 , 28
Ordinary differential equations
Differential equations are commonly used to develop models of various spatiotemporal scales to describe drug PDs and PDs. The PK/PD, PBPK, and QSP models are most commonly developed as a series of ordinary differential equations (ODEs). Stochastic and partial differential equations are less common. These models can be applied throughout several stages of drug discovery and development.
Boolean network modeling
Boolean and logic‐based network models have been used to describe gene regulation, cellular differentiation, and cell fate determination. 29 These models could be utilized to understand gene regulatory processes and the microenvironment that gives rise to cellular diversity, where different cell populations could be represented as different network attractors. Additionally, these models provide a qualitative understanding of the intracellular effects of pharmacological perturbations and genetic alterations. Single‐cell transcriptomic studies and biomolecular time courses would provide the necessary data to develop this type of model. However, these models are seldomly used in drug discovery.
Agent‐based modeling
Agent‐based models can be used to describe spatially resolved biological phenomena at the cellular and molecular level, such as chemotaxis, phagocytosis, and emergent behaviors. 30 Boolean network and agent‐based models could be integrated to describe multilevel organization. For example, a hybrid multiscale model that coupled an agent‐based model with logic‐based differential equations was developed to describe the role of fibroblast signaling and movement in cardiac fibrosis. 31 Scaling a hybrid multiscale model of this nature to the whole tissue level would be methodologically challenging and computationally expensive. This type of modeling framework could provide an alternative method, compared with ODEs, to describe pharmacological and pathophysiological systems. MPS and real‐time imaging experiments could offer rich data sets to inform models of this nature. These models could be applied to bridge drug discovery and preclinical development.
Statistics and machine learning
Statistical and machine learning methods are used to analyze large and complex data from digital devices, neuroradiological images, and omics studies. These methods could also be used to help guide the development of QSP models. For example, network inference methods using machine learning algorithms can identify important features and provide insights into complex topological structure, which would inform node selection and edge determination. 32 Connectomics data from MRI and PET imaging studies can inform the development of personalized BNMs and improve the spatial granularity. The Virtual Brain is a neuroinformatic modeling approach that utilizes individual patient brain connectivity data to build personalized BNMs in order to understand brain structure and function and its relation to disease state and response to therapeutic interventions. 24 Machine learning algorithms will play an instrumental role in digital biomarker data collection and analysis.
CONCLUSION
In this mini‐review, we have highlighted several novel methods and emerging technologies in the neuroscience domain that could be leveraged to supplement QSP approaches to enhance our understanding of neurological diseases and translation of new therapeutics. A primary objective of the conference session was to introduce the field of network neuroscience to a broader pharmacometrics community. Neuroscience QSP models often describe cellular/molecular processes and empirically link biomarker changes to clinical end points of interest, which neglects information of neuronal circuitry and leaves a large knowledge gap between cellular and patient levels of neurobiological organization. The integration of multiple spatial and temporal scales, bridging from intracellular signaling to neuronal network activity to individual patient outcomes, into a holistic mechanistic modeling framework could improve predictive power and facilitate CNS research and development. However, integrating these technologies with QSP modeling remains challenging and may require the utilization and careful selection of mathematical and statistical frameworks. We believe that the success rate for potential therapies under clinical investigation for the treatment of neurodegenerative diseases can be improved by integrating these new methods into current drug discovery and development paradigms.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
Funding information
No funding was received for this work.
REFERENCES
- 1. Sorger PK, Allerheiligen SRB, Abernethy DR, et al. Quantitative and systems pharmacology in the post‐genomic era: new approaches to discovering drugs and understanding therapeutic mechanisms. An NIH white paper by the QSP workshop group. Vol. 48. Bethesda, MD: NIH Bethesda, 2011.
- 2. Geerts H, Wikswo J, Graaf PH, et al. Quantitative systems pharmacology for neuroscience drug discovery and development: current status, opportunities, and challenges. CPT Pharmacometrics Syst Pharmacol. 2020;9:5‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geerts H. Of mice and men: bridging the translational disconnect in CNS drug discovery. CNS Drugs. 2009;23:915‐926. [DOI] [PubMed] [Google Scholar]
- 4. Karelina T, Demin O Jr, Demin O, Duvvuri S, Nicholas T. Studying the progression of amyloid pathology and its therapy using translational longitudinal model of accumulation and distribution of amyloid beta. CPT Pharmacometrics Syst Pharmacol. 2017;6(10):676‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwartz MP, Hou Z, Propson NE, et al. Human pluripotent stem cell‐derived neural constructs for predicting neural toxicity. Proc Natl Acad Sci. 2015;112:12516‐12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mina SG, Alaybeyoglu B, Murphy WL, et al. Assessment of drug‐induced toxicity biomarkers in the Brain Microphysiological System (MPS) using targeted and untargeted molecular profiling. Frontiers Big Data. 2019;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edington CD, Chen WLK, Geishecker E, et al. Interconnected microphysiological systems for quantitative biology and pharmacology studies. Sci Rep. 2018;8:4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bassett DS, Sporns O. Network neuroscience. Nat Neurosci. 2017;20:353‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bansal K, Nakuci J, Muldoon SF. Personalized brain network models for assessing structure‐function relationships. Curr Opin Neurobiol. 2018;52:42‐47. [DOI] [PubMed] [Google Scholar]
- 10. Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nat Rev Neurosci. 2015;16:159‐172. [DOI] [PubMed] [Google Scholar]
- 11. Stefanovski L, Triebkorn P, Spiegler A, et al. Linking molecular pathways and large‐scale computational modeling to assess candidate disease mechanisms and pharmacodynamics in Alzheimer’s disease. Front Computat Neurosci. 2019;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baker JT, Holmes AJ, Masters GA, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71:109‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang D, Li M, Wang M, et al. Individual‐specific functional connectivity markers track dimensional and categorical features of psychotic illness. Mol Psychiatry. 2018;25:2119‐2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frega M, Tedesco M, Massobrio P, Pesce M, Martinoia S. Network dynamics of 3D engineered neuronal cultures: a new experimental model for in‐vitro electrophysiology. Sci Rep. 2014;4:5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma AD, McCoy L, Jacobs E, et al. Engineering a 3D functional human peripheral nerve in vitro using the Nerve‐on‐a‐Chip platform. Sci Rep. 2019;9:8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hodge RD, Bakken TE, Miller JA, et al. Conserved cell types with divergent features in human versus mouse cortex. Nature. 2019;573:61‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keren‐Shaul H, Spinrad A, Weiner A, et al. A unique microglia type associated with restricting development of Alzheimer's disease. Cell. 2017;169:1276‐1290 e1217. [DOI] [PubMed] [Google Scholar]
- 18. Vogt NM, Kerby RL, Dill‐McFarland KA, et al. Gut microbiome alterations in Alzheimer's disease. Sci Rep. 2017;7:13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim S, Kwon SH, Kam TI, et al. Transneuronal propagation of pathologic alpha‐synuclein from the gut to the brain models Parkinson's disease. Neuron. 2019;103:627‐641.e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kabelac Z, Tarolli CG, Snyder C, et al. Passive monitoring at home: a pilot study in Parkinson disease. Digital Biomarkers. 2019;3:22‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franzmeier N, Neitzel J, Rubinski A, et al. Functional brain architecture is associated with the rate of tau accumulation in Alzheimer's disease. Nat Commun. 2020;11:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henderson MX, Cornblath EJ, Darwich A, et al. Spread of α‐synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nat Neurosci. 2019;22:1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Falcon MI, Jirsa V, Solodkin A. A new neuroinformatics approach to personalized medicine in neurology: The Virtual Brain. Curr Opin Neurol. 2016;29:429‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anticevic A, Gancsos M, Murray JD, et al. NMDA receptor function in large‐scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci USA. 2012;109:16720‐16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roberts P, Spiros A, Geerts H. A humanized clinically calibrated quantitative systems pharmacology model for hypokinetic motor symptoms in Parkinson's disease. Front Pharmacol. 2016;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gleeson P, Cantarelli M, Marin B, et al. Open source brain: a collaborative resource for visualizing, analyzing, simulating, and developing standardized models of neurons and circuits. Neuron. 2019;103:395‐411.e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dura‐Bernal S, Suter BA, Gleeson P, et al. NetPyNE, a tool for data‐driven multiscale modeling of brain circuits. Elife. 2019;8:e44494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bloomingdale P, Nguyen VA, Niu J, Mager DE. Boolean network modeling in systems pharmacology. J Pharmacokinet Pharmacodyn. 2018;45:159‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cosgrove J, Butler J, Alden K, et al. Agent‐based modeling in systems pharmacology. CPT Pharmacometrics Syst Pharmacol. 2015;4:615‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rikard SM, Athey TL, Nelson AR, et al. Multiscale coupling of an agent‐based model of tissue fibrosis and a logic‐based model of intracellular signaling. Front Physiol. 2019;10:1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Albert R. Network inference, analysis, and modeling in systems biology. Plant Cell. 2007;19:3327‐3338. [DOI] [PMC free article] [PubMed] [Google Scholar]


