Abstract
Establishing bioequivalence (BE) for dermatological drug products by conducting comparative clinical end point studies can be costly and the studies may not be sufficiently sensitive to detect certain formulation differences. Quantitative methods and modeling, such as physiologically‐based pharmacokinetic (PBPK) modeling, can support alternative BE approaches with reduced or no human testing. To enable PBPK modeling for regulatory decision making, models should be sufficiently verified and validated (V&V) for the intended purpose. This report illustrates the US Food and Drug Administration (FDA) approval of a generic diclofenac sodium topical gel that was based on a totality of evidence, including qualitative and quantitative sameness and physical and structural similarity to the reference product, an in vivo BE study with PK end points, and, more importantly, for the purposes of this report, a virtual BE assessment leveraging dermal PBPK modeling and simulation instead of a comparative clinical end point study in patients. The modeling approach characterized the relationship between systemic (plasma) and local (skin and synovial fluid) diclofenac exposure and demonstrated BE between the generic and reference products at the presumed site of action. Based on the fit‐for‐purpose modeling principle, the V&V process involved assessing observed data of diclofenac concentrations in skin tissues and plasma, and the overall performance of the modeling platform for relevant products. Using this case as an example, this report provides current scientific considerations on good practices for model V&V and the establishment of BE for dermatological drug products when leveraging PBPK modeling and simulation for regulatory decision making.
INTRODUCTION
Locally acting drug products deliver the active ingredient active ingredient at or near the site of action. These products include, for example, orally inhaled, nasal, ophthalmic, and dermatological drug products and oral products that act locally in the gastrointestinal tract. 1 To demonstrate bioequivalence (BE) between a brand name product (reference) and its generic (test), there must be an “absence of significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study.” 2 However, “for a drug that is not intended to be absorbed into the bloodstream,” the Agency may consider establishing “alternative, scientifically valid methods to show bioequivalence if the alternative methods are expected to detect a significant difference between the drug and the listed drug in safety and therapeutic effect.” 3
The Agency makes recommendations for BE studies based on the current scientific understanding of a particular drug product captured in product‐specific guidances (PSGs) that are publicly available and updated at regular intervals. 4 For locally acting drug products applied on the skin as semisolid dosage forms, BE approaches include drug product characterization studies on the product microstructure, vasoconstrictor studies, BE studies with pharmacokinetic (PK) end points, and comparative clinical end point BE studies. 1 , 5 , 6 Comparative clinical end point BE studies specifically can be costly, time‐consuming, and less sensitive at detecting formulation differences between a test and a reference product due to the absence of established dose‐response relationship and modest efficacy for the active pharmaceutical ingredient (API) that exerts its pharmacological activity following skin application. 1 , 6 , 7
Physiologically‐based pharmacokinetic (PBPK) modeling and simulation is a methodology that describes the PKs of the active ingredient in the body in a quantitative and mechanistic manner. It has been widely used to inform the development of and regulatory decision making for new drugs, including dose selection (first‐in‐human) and optimization, interspecies extrapolations, dose adjustments in specific populations (pregnancy, pediatrics, and organ impairment), and assessment of drug‐drug interactions. 8 , 9 , 10 , 11 For generic drug products, mechanistic PBPK models can guide the establishment of clinically relevant product quality attributes, support the development of PSGs, and provide a risk assessment following formulation changes, etc. 12 , 13 , 14 , 15 PBPK models with enhanced population simulation capabilities can be used to support virtual BE (VBE) assessments. 16 , 17 , 18 , 19 , 20 Previously, mechanistic dermal PBPK models have been used to describe the permeation through the skin for environmental and other chemicals and perform risk‐based assessments. 21 , 22 For locally acting drug products, including those applied on the skin, although administered at or close to the site of action, quantification of active ingredient amounts at the site of action for BE assessments is often not feasible. Therefore, for these products, PBPK models can be leveraged to predict local drug exposure by integrating knowledge on the characterization of a drug product, the physiology or pathophysiology at or near the site of action, and the population information of healthy volunteers or patients receiving the drug product and to establish a link with systemic exposure if measurable. 20 , 21 , 23 , 24
This report summarizes the approval of an Abbreviated New Drug Application (ANDA) for a generic diclofenac sodium topical gel, 1% (referencing Voltaren topical gel, 1%), where for the first time a VBE assessment leveraging dermal PBPK modeling and simulation supported by a totality of evidence approach resulted in approval of the ANDA and discusses the lessons learned from this submission.
DERMAL PBPK MODELING AND SIMULATION TO SUPPORT BE ASSESSMENT AND APPROVAL OF ABBREVIATED NEW DRUG APPLICATION
Regulatory background
Voltaren (diclofenac sodium) topical gel, 1% (New Drug Application [NDA] 022122, reference product) was approved by the US Food and Drug Administration (FDA) for the relief of the pain of osteoarthritis of joints amenable to topical treatment, such as the knees and hands. 25 , 26 Since then, the Agency has approved 6 generic products that are considered therapeutically equivalent to Voltaren topical gel, 1%. 27 The current PSG for diclofenac sodium topical gel, 1%, recommends 2 studies for establishing BE between the test and the reference products (i.e., an in vivo BE study with PK end points in healthy volunteers and an in vivo comparative clinical end point BE study in men and women with osteoarthritis of the knee). 28 These recommendations reflect the Agency’s current thinking under the consideration that diclofenac may reach the site of action following topical application directly as well as by redistribution from the systemic circulation. 29
The FDA approved a generic diclofenac sodium topical gel, 1%, under ANDA 211253 on May 16, 2019, during its first review cycle. This was the first ANDA approval for which a PBPK model supported the BE assessment. 16 The applicant utilized the pre‐ANDA program to obtain the Agency’s feedback on a proposed alternative to the BE approach recommended in the PSG, which they incorporated in its final submission. 30 The Agency provided additional feedback on the applicant’s modeling approach through a discipline review letter and during the mid‐review cycle meeting. 30
Compared with the reference product, the applicant’s product contained no difference in inactive ingredients or other aspects of the formulation that may have the potential to significantly alter the local or systemic availability of the active ingredient, was physically and structurally similar, and had an equivalent rate of diclofenac release compared with the reference product evaluated using a validated in vitro release test (IVRT)—elements not part of the current PSG. The applicant conducted the PSG‐recommended in vivo BE study with PK end points but did not perform the PSG‐recommended comparative clinical end point study. Instead, the applicant developed a dermal PBPK model for a VBE assessment based on drug exposure at the presumed site of action between the reference and the test products.
Methodology for dermal PBPK model development for diclofenac sodium topical gel, 1%
The multi‐phase multi‐layer (MPML) MechDermA model implemented within the Simcyp Simulator (version 17; Certara, Princeton, NJ, USA) was utilized for the quantitative description of diclofenac absorption through the skin. Briefly, the structure of the MPML MechDermA model is modified from the physiologically relevant compartmental model introduced by Shatkin and Brown. 31 , 32 In addition to the stratum corneum (SC) and viable epidermis compartments described in the original publication, the modified model also includes dermis, subcutis, and deeper tissue (muscle) compartments to describe API absorption. The SC is the outer layer of the skin that is composed of multiple layers of corneocytes surrounded by a lipid layer. It serves as the primary barrier between the body and the environment. The heterogeneity of the SC has been captured in the platform. Longitudinal solute diffusion within and between the different skin layers is modeled after Fick’s Law based on the physicochemical properties of the API. Quantitative Structure‐Activity Relationship (QSAR) models are used to inform model parameters based on API properties, such as lipophilicity, ionization state, solubility, and molecular weight, among others. Other physiological processes and anatomic structures, such as protein binding, SC hydration state, absorption via skin appendages (i.e., hair follicles), and impact of skin surface pH on absorption, are accounted for in the MPML MechDermA model. Formulation attributes accounted for in the model include formulation pH, API solubility, viscosity, vehicle drying rate (evaporation), particle and droplet size (distribution) in the case of multiphase formulations, and drug release rate for transdermal delivery systems (TDS). Finally, intersubject variability is modeled by introducing variability on physiological parameters, such as skin layer thickness across genders, races, and age groups, and intrasubject variability is considered for model simulations by predicting the percutaneous kinetics of an active ingredient at different application areas (arms, legs, head, and back).
The applicant measured certain formulation parameters of the reference product formulation (Voltaren topical gel, 1%) and the test formulation, such as formulation pH, viscosity, and droplet size. Sensitivity analysis conducted by the applicant on all formulation attributes identified the formulation pH, viscosity, and droplet size as influential; these attributes were experimentally determined by the applicant. When additional formulation attributes were required for model building but were not available in the literature or determined experimentally by the applicant, the values for these model parameters were either calculated or the default values were used with no further justification.
Following application of the reference product, quantifiable amounts of diclofenac are present in the systemic circulation. 33 To describe the systemic disposition of diclofenac following the dermal application of the diclofenac sodium topical gel, 1%, the dermal PBPK model developed for the diclofenac sodium topical gel, 1%, was coupled with a minimal PBPK model. Publicly available information on the physicochemical properties and the PK characteristics of diclofenac were considered for the model development. 34 The performance of the systemic disposition model was evaluated using clinical PK data (systemic exposure) of diclofenac following intravenous and oral administration prior to being coupled with the MPML MechDermA model.
In addition to the model structure described above, considering that synovial fluid is the presumed site of action of the product, 29 the thickness of the muscle compartment was modified by the applicant to result in an approximate synovial fluid (tissue) volume as reported in the literature.
Table 1 provides a general list of key information and data sources considered for the development and validation of the PBPK model for diclofenac sodium topical gel, 1%, developed in the MPML MechDermA model in the Simcyp Simulator, version 17. Information required for the development and validation of the model components describing the systemic disposition (systemic disposition model) and the permeation through the skin (skin permeation) of the active ingredient is indicated.
TABLE 1.
Data sources and key information considered for the development and validation of the PBPK model for diclofenac sodium topical gel, 1%, developed in MPML MechDermA within the Simcyp Simulator, version 17
| Data source | Model development | Model validation | ||
|---|---|---|---|---|
| Systemic disposition | Skin permeation | Systemic disposition | Skin permeation | |
| Drug substance | ||||
| Physicochemical properties (MW, lipophilicity, ionization status, etc.) | X | X | ||
| ADME properties (protein binding, blood to plasma ratio, tissue distribution, and elimination) | X | |||
| Skin ADME properties (protein binding, tissue distribution and sequestration, metabolism, and handling by transporter proteins) | X | |||
| Clinical PK (plasma/blood) profiles following intravenous administration a | X | X | ||
| Drug product | ||||
| In vitro physicochemical characterization of the drug product | X | |||
| Formulation pH, API solubility (aqueous or oil phase), droplet size, rheological properties (viscosity) | X | |||
| Formulation composition | X | |||
| Evaporation (drying rate or vehicle volume loss profile) b | X | |||
| In vivo percutaneous PK studies (dMD) | X c | X | ||
| Synovial fluid sampling | X c | X | ||
| Clinical (plasma/blood) PK profiles following skin application | X | |||
Abbreviations: ADME, absorption, distribution, metabolism, elimination; API, active pharmaceutical ingredient; dMD, dermal microdialysis; MPML, multi‐phase multi‐layer; MW, molecular weight; PBPK, physiologically‐based pharmacokinetic; PK, pharmacokinetic.
If not available, clinical PK profiles following oral administration may be considered.
Not included in the current model.
Model refinement by the Agency.
Methodology on verification and validation of dermal PBPK model for diclofenac sodium topical gel, 1%
The applicant implemented an innovative two‐level approach toward verifying and validating the fit‐for‐purpose dermal PBPK model developed for diclofenac sodium topical gel, 1%. Briefly, the first level validation focused on assessing the performance of the developed models for topical products with diclofenac sodium as the API. The dermal PBPK model for diclofenac sodium topical gel, 1%, incorporated drug‐product specific formulation attributes generated by the applicant as part of its product characterization program and was validated leveraging clinical PK data supporting the ANDA submission. The second level V&V aimed at assessing the performance of the platform that was used (i.e., the MPML MechDermA model within the Simcyp Simulator, version 17). The performance of the platform was evaluated by validating a significant number of PBPK models for dermatological products using literature data sources. The dermal PBPK models supporting the second level validation included dermatological products with APIs other than diclofenac sodium, as these were covered under the first level validation. The overview of the model validation process is presented in Figure 1. The performance acceptance criteria utilized by the Agency are detailed below in Considerations on the performance acceptance criteria for dermal PBPK models.
FIGURE 1.
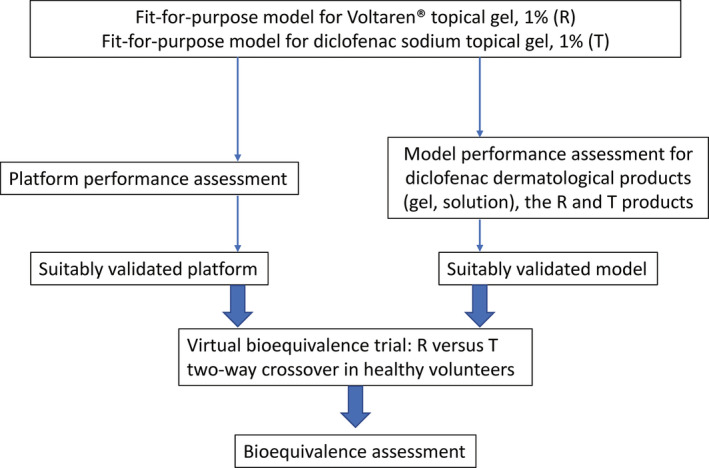
Overview of the validation methodology proposed by the applicant in support of their fit‐for‐purpose dermal physiologically‐based pharmacokinetic models for Voltaren topical gel, 1% (reference, R) and for the generic diclofenac sodium topical gel, 1% (test, T)
Performance assessment of dermal PBPK models developed for diclofenac sodium topical drug products in the MPML MechDermA, Simcyp Simulator, version 17
Various literature sources 29 , 35 , 36 , 37 , 38 were leveraged by the applicant and the Agency toward validating the dermal PBPK models developed for diclofenac sodium topical drug products, including solution and gel (emulsion) formulations. Simulation scenarios captured model performance following a single or multiple product application. Model predictions were generated for a range of applied doses at multiple application sites that included the backs and knees among others and were compared with corresponding observed data. The applicant validated its product‐specific models developed for the reference and the test products by utilizing an independent dataset from the in vivo BE study with PK end points supporting its submission (Figure 2). The “bottom‐up” approach described in this paper, resulted in overprediction of the distribution and elimination phase of diclofenac following the application of the diclofenac sodium topical gel, 1%, as shown on Figure 2, although observed exposure PK parameters such as the maximum concentration (Cmax) and area under the concentration versus time curve (AUC) were predicted reasonably well (Table 2). The noted discrepancy between predicted and observed PK profiles is consistent with dermal absorption being the rate limiting step for diclofenac disposition. Sensitivity analysis indicated the diclofenac partition coefficient from the vehicle (diclofenac sodium topical gel, 1%) to the SC (KpSClip:vehicle) and the diclofenac partition coefficient from the SC and to the viable epidermis (KpSC:VE) as impactful model parameters. In the absence of experimental data, these parameters were informed by the relevant QSAR models embedded into the MPML MechDermA platform in the applicant’s proposed model. Optimization of these parameters by the Agency resulted in improved model predictions, as shown in Figure S1. The Agency concluded on the acceptability of the fit‐for‐purpose model for diclofenac sodium topical gel that the applicant proposed for the following reasons: (i) BE between the reference and test products is established based on systemic PK profiles. Further modifying skin permeation model parameters, such as KpSClip:vehicle and KpSC:VE, would impact the PK profiles for both reference and test products to the same extent, but would not impact the outcome of the VBE assessment, (ii) there was no observed data available to inform KpSClip:vehicle or KpSC:VE, therefore QSAR‐generated predictions may be sufficient, (iii) model flexibility, which considers study‐to‐study variation, is desirable; the Agency extended the model validation process, leveraging publicly available data on the reference product. 26
FIGURE 2.
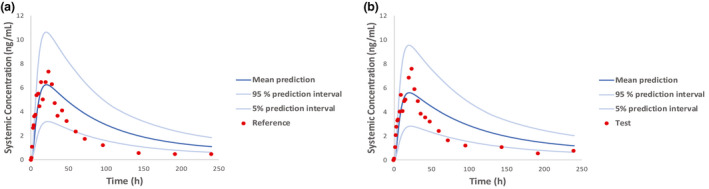
Observed mean plasma pharmacokinetic profiles (red circles) versus population predictions (mean and 5/95% prediction intervals) following application of the reference (a) and the test (b) drug product. Predictions were generated leveraging dermal physiologically‐based pharmacokinetic models developed for Voltaren topical gel, 1% (reference) and its genetic (test)
TABLE 2.
Observed and predicted plasma diclofenac Cmax and AUC following the application of the application of the reference and the test drug product
| Parameter | R | T | ||
|---|---|---|---|---|
| Cmax, ng/ml | AUC, ng·hr/ml | Cmax, ng/ml | AUC, ng·hr/ml | |
| Predicted/observed ratio | 0.82 | 1.77 | 0.70 | 1.74 |
Predictions were generated leveraging dermal physiologically‐based pharmacokinetic models developed for Voltaren topical gel, 1% (reference) and its genetic (test).
Abbreviations: AUC, area under the concentration/amount curve from time zero to the last measurable concentration; Cmax, maximum concentration; R, reference drug product; T, test drug product.
Model predictions on local exposure were validated, leveraging literature sources in which diclofenac amounts were quantified using dermal microdialysis (dMD) and skin biopsy among others. 29 , 35 , 36 High variability was observed with measurements of skin diclofenac amounts that typically involved single time sampling of the subcutis (n = 2 studies), muscle (n = 1 study), or synovial fluid (n = 1 study), as documented in the literature 29 , 35 , 36 , 39 and shown in Figure 3. Due to the lack of agreement between model predictions and quantified drug amounts in the skin (Figure 3), the performance of the dermal PBPK model for diclofenac sodium was not considered satisfactory. Therefore, the applicant’s dermal PBPK model for diclofenac sodium in dermatological drug products was considered to be partially validated. To gain confidence in using the model for the intended purpose of predicting local tissue diclofenac concentrations, the Agency refined the model using publicly available information, as described in detail below in Refinement of the dermal PBPK model.
FIGURE 3.
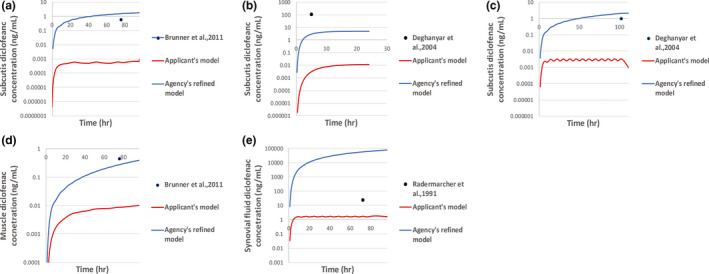
Observed diclofenac amounts quantified in subcutis (a, b, c), the muscle (d) and the synovial fluid (e) (black circles) following application of diclofenac sodium dermatological products versus model‐generated predictions generated by simulating the study design conditions provided in the respective literature sources
Performance assessment of the modeling platform, MPML MechDermA in the Simcyp Simulator, version 17
Evaluating the performance of the MPML MechDermA modeling platform was critical for the overall credibility of the quantitative tool. As shown in Table 3, dermal PBPK models for more than 10 active ingredients were developed utilizing the MPML MechDermA model for a variety of dermatological products including TDS and topical drug products (e.g., topical solutions, creams, ointments, and gels). The active ingredients of the selected dermatological products covered a wide range of values in terms of their physicochemical properties (lipophilicity and ionization potential) and PK characteristics (protein binding, extent of distribution in the human body, route of elimination, and blood‐to‐plasma partitioning among others). Active ingredients that were similar to diclofenac in physicochemical properties, pharmacological activity, and site of action were included. The diverse selection of active ingredients was useful toward validating the performance of the platform intended to model the local and systemic diclofenac bioavailability.
TABLE 3.
Overview of the platform performance assessment conducted by the applicant in support of the dermal PBPK model for diclofenac sodium topical gel, 1%, developed in MPML MechDermA within the Simcyp Simulator, version 17
| Active ingredient a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Dosage form/products b | TDS | TDS | TDS | Solution | TDS | Cream | Gel ointment cream | TDS cream |
Gel Solution Nanoparticles TDS |
Gel | Solution |
| Verification matrix | Plasma | Plasma | Plasma | Plasma | Plasma | IVPT | Plasma | Plasma |
Plasma Synovial fluid Subcutis Muscle Dermis Stratum corneum |
Plasma Synovial fluid |
Skin biopsy (stratum corneum, viable epidermis, dermis) |
| Number of literature sources for validation of the systemic disposition PBPK model | 4 | 1 | 1 | c | 1 | c | 3 | c | 4 | 2 | c |
| Number of literature sources for validation of the dermal PBPK model | 8 | 2 | 1 | 1 | 1 | 1 | 1/1/1 | 1/1 | 6/1/1/1 | 2 | 1 |
Abbreviations: IVPT, in vitro permeation testing; MPML, multi‐phase multi‐layer; PBPK, physiologically‐based pharmacokinetic; TDS, transdermal delivery system.
The selected active ingredients differed in terms of their physicochemical properties (lipophilicity and ionization potential) and pharmacokinetic characteristics (protein binding, extent of distribution in the human body, route of elimination, and blood‐to‐plasma partitioning among others). More specifically, the molecular weight, logP (lipophilicity), blood to plasma ratio, fraction unbound in plasma, volume of distribution at steady‐state and total systemic clearance of the selected active pharmaceutical ingredients ranged from 162 to 468 g/mol, from −1.6 to 6.4, from 0.55 to 1.107, from 0.003 to 0.95, from 0.123 L/Kg to 48.8 L/Kg and from 1.6 L/h to 71.5 L/h, respectively. The selected active ingredients were acids, bases, and ampholytes.
Product‐specific dermal PBPK models were developed for each of these dosage forms.
Not provided in the submission or refers to a drug substance that is not given by other than the topical route.
Topical drug products, including single phase systems (solutions, gels) and complex dosage forms, such as gels (emulsions), creams, and ointments, were selected for the performance assessment. The complex pharmaceutical formulations of the modeled drug products presented the platform with the necessary challenge for assessing its predictive power under relevant scenarios. However, information on formulation attributes was not always readily available for certain products in literature sources or for those approved by regulatory agencies outside the United States. This lack of information necessitated assumptions in the model development process and was acknowledged as a limitation by the applicant and the Agency.
Three active ingredients were studied in multiple dosage forms for which individual dermal PBPK models were developed. For two active ingredients, simulated amounts were compared with the observed amounts in multiple skin layers, including SC, dermis, subcutis, muscle, and synovial fluid. Data obtained with validated in vitro permeation testing (IVPT) were also leveraged toward platform performance assessment.
Model validation for each of the developed dermal PBPK models involved the comparison between the predicted local (SC, dermis, subcutis, and muscle) and/or systemic PK profiles with the corresponding observed PK profiles retrieved from the literature. A considerable number of independent literature sources was used for model validation (Table 3). For most of the developed models, model predictions were in good agreement with observed data. The performance of the MPML MechDermA platform was deemed overall satisfactory, and the platform was considered appropriate for the development of fit‐for‐purpose dermal PBPK model for diclofenac sodium topical gel, 1%.
Considerations on the performance acceptance criteria for dermal PBPK models
Currently, there are no established or formally recognized acceptance criteria for adequate model performance by the Agency. The “2‐fold” criterion on mCmax and AUC was referenced in this case for performance assessment of the dermal PBPK models developed for diclofenac sodium topical drug products and of dermal PBPK models developed for drug products supporting platform performance assessment. 24 , 40 , 41 , 42 , 43 Overall, a good agreement between the predicted and observed PK profiles was observed (i.e., the overall shape of the PK profile, model‐predicted absorption phase, and prediction of the time at Cmax [Tmax] value were deemed satisfactory). Additional considerations on the acceptance criteria included the quality of the data used for building and assessing the performance of the model, the credibility of the model assumptions, the regulatory impact of the decision associated with the model, and the level of confidence on the overall modeling approach.
Refinement of the dermal PBPK model for diclofenac sodium, topical gel 1% performed by the Agency
The proposed model was expected to establish a reliable link between systemic (plasma) and local (skin tissue and synovial fluid) diclofenac concentrations/amounts. However, model predictions locally were not satisfactory per the Agency’s review, as noted previously. Therefore, the Agency conducted sensitivity analyses and identified model parameters with the potential to impact local, but not systemic, diclofenac exposure. These parameters were modified (manual parameter optimization) by leveraging observed data and implementing a “middle‐out” approach. 29 , 35 , 36
More specifically, when protein binding in the subcutis and muscle tissue was assumed to be equal to plasma protein binding (protein fraction unbound was modified from the default value of 1 and set equal to 0.003), model output improved compared with the applicant’s original model output. Partitioning coefficients to the subcutis (partition coefficient from the dermis to the subcutis was modified from the default value of 10−5 to 100) and muscle/synovial fluid (partition coefficient from the subcutis to the muscle modified from the default value of 10−5 to 100 and partition coefficient from the subcutis to the blood modified from the default value of 10−5 to 10) were described in an empirical manner by refining the relevant model parameters leveraging experimental data from literature sources (Figure 3). The impact of these optimized parameters on the systemic diclofenac concentration was found to be minimal. Model refinement was challenging considering high variability associated with the observed data and considerations on model structural identifiability. Following refinement, the model was able to both reliably predict systemic diclofenac concentrations and reasonably predict the local diclofenac amounts.
The applicant modified the muscle compartment to model diclofenac partitioning into the synovial fluid, the presumed pharmacological site of action. Although the repurposing of a tissue compartment is feasible within the MPML MechDermA model and attractive suggesting model/platform flexibility, it is important to be cautious of the underlying assumptions and limitations of this approach. The lack of agreement between observed data and model predictions on diclofenac amounts in the presumed site of action (Figure 3e), the synovial fluid, even after model refinement can be attributed to the differences in physiology between the muscle and the synovial fluid; the muscle compartment was modified to mimic the synovial fluid only in terms of its volume without changes in the physiology. For instance, protein expression, diclofenac partitioning and diffusion, and extent of vascularization may be some of the differences between the muscle and the synovial fluid physiology and its interplay with diclofenac that were not taken into account. 44 , 45 Finally, a suboptimal model performance may be attributed to not accounting for the disease (osteoarthritis) pathophysiology—namely, the increase in the synovial fluid volume and local blood flow commonly reported in patients with knee osteoarthritis due to inflammation; model predictions generated in virtual healthy volunteers were compared with observed data collected in patients. 29 , 46 , 47 Although developing and validating a disease model for knee osteoarthritis was outside the scope of dermal PBPK model for diclofenac sodium topical gel supporting the ANDA submission and its assessment by the Agency, sensitivity analysis performed by the Agency showed that both synovial fluid volume and local blood flow have the potential to impact diclofenac amounts in the synovial fluid.
During the review process, the Agency assessed the capability of the model/MPML MechDermA platform to predict the skin diclofenac amounts that may result from the redistribution of diclofenac from the systemic circulation in the skin as these may interfere with the local BE assessment. The Agency leveraged literature sources on drug products that provided measurements on drug amounts in the skin (skin biopsy or dMD) resulting from redistribution from the blood following oral or i.v. API administration. 29 , 39 , 48 , 49 The platform was able to predict local amounts resulting from redistribution of various APIs, including diclofenac reasonably well.
Agency‐conducted sensitivity analysis found that drug product changes following skin application, such as vehicle evaporation, may impact skin permeation. Although the applicant provided data showing comparable drying profiles between its test and the reference product, the applicant did not account for drug product evaporation post‐application in its dermal PBPK model. As the formulation drying rate may impact local and systemic bioavailability, incorporating this type of experimentally generated data into the proposed dermal PBPK model is expected to increase its predictive power. To that end, the Agency leveraged the proposed model and conducted a risk‐based assessment under the consideration that drying profiles were very similar between the reference and the test products.
Application of the dermal PBPK model developed for diclofenac sodium topical gel, 1%, reference and test drug products toward a bioequivalence assessment
One of the unique capabilities of PBPK modeling and simulation tools is accounting for variability on anatomy, physiology, demographics, and drug product parameters rendering them useful tools toward generating predictions for a virtual population of healthy volunteers or patients. 17 , 18 , 19 , 20
The fit‐for‐purpose refined dermal PBPK model for diclofenac sodium topical gel, 1%, was leveraged to predict systemic and local (skin) exposure of diclofenac following the administration of the reference and the test drug products in virtual healthy volunteers (Figure 1). As shown in Figure 4, the population predictions for the reference and test drug products were informed by the differing formulation attributes (drug product microstructure) and the variability assigned to model parameters (physiology, PK, and drug product parameters) within the Simcyp Simulator. Assuming a crossover study design similar to the in vivo BE study with PK end points that supported the submission, systemic and skin PK parameters were estimated from the population predictions for the two products. BE was formally assessed in the plasma and several skin layers including the dermis and the synovial fluid with Phoenix (version 8; Certara). The two products were found to be bioequivalent. A summary of the results of the BE statistical analysis is provided in Table 4.
FIGURE 4.
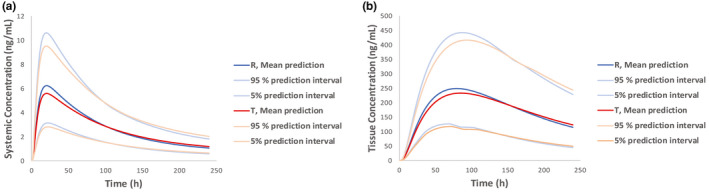
Population predictions (mean and 5/95% prediction intervals) of systemic (a) and local (b) exposure following application of the reference and the test drug product generated leveraging dermal physiologically‐based pharmacokinetic models for Voltaren topical gel, 1%, and its genetic (test)
TABLE 4.
Summary of bioequivalence assessment results performed leveraging the simulated plasma and synovial fluid PK profiles generated with the dermal PBPK models developed for the R and T drug products
| Parameter | R | T | N | Ratio | Lower 90% PI | Upper 90% PI |
|---|---|---|---|---|---|---|
| Plasma | ||||||
| Cmax, ng/ml | 5.87 | 5.56 | 78 | 0.90 | 81.29 | 98.77 |
| AUC, ng·hr/ml | 638.40 | 630.56 | 78 | 0.98 | 88.63 | 107.39 |
| Synovial fluid | ||||||
| Amax, μg | 230.13 | 222.47 | 78 | 0.93 | 85.06 | 102.68 |
| AUC, μg·hr/ml | 37,888.28 | 37,362.20 | 78 | 0.97 | 87.7 | 107.83 |
Predictions were generated leveraging dermal PBPK models developed for Voltaren topical gel, 1% (reference) and its genetic (test).
Abbreviations: Amax, maximum amount in the tissue; AUC, area under the concentration/amount curve from time zero to the last measurable concentration; Cmax, maximum concentration; PBPK, physiologically‐based pharmacokinetic; PK, pharmacokinetic; PI, prediction interval; N, sample size; R, reference drug product; T, test drug product.
DISCUSSION
The regulatory recommendations for demonstrating BE for generic diclofenac sodium topical gel, 1%, to the reference product involve a comparative clinical end point BE study coupled with an in vivo BE study with PK end points. 28 The recommendation is based on the potential for redistribution of the diclofenac plasma concentrations obtained following skin application from the blood into the skin contributing to the observed pharmacological effect. Considering comparative clinical end point BE studies tend to be the least sensitive approach to detect formulation differences between a reference and test product, the Agency encourages generic drug applicants to use alternative BE approaches, such as modeling and simulation.
The overall BE assessment for the generic diclofenac sodium topical gel, 1%, discussed in this report included: (i) evaluation of formulation sameness according to which no difference in inactive ingredients or other aspects of the formulation relative to the reference product with the potential to significantly alter the local or systemic availability of the active ingredient was noted between the reference and the test products; (ii) the two products (reference and test) were found to be physically and structurally similar and to have equivalent rates of diclofenac release evaluated using a suitably validated IVRT; (iii) the test product was BE to the reference product in terms of systemic exposure assessed in an acceptable in vivo study with PK end points; and (iv) the developed dermal PBPK model was suitably validated to demonstrate that the reference and test products are bioequivalent at or close to the presumed site of action (skin and synovial fluid) through a local VBE assessment. The latter mitigated the risk of not conducting the PSG‐recommended in vivo BE study with comparative clinical end points.
The proposed dermal PBPK model for diclofenac sodium topical gel, 1%, incorporated information on formulation attributes obtained following the in vitro characterization of the reference and the test product. Relevant model parameters were informed, and model assumptions were supported by experimental data retrieved from the published literature, and proprietary data were generated by the applicant. The capability of the model to generate local and systemic diclofenac exposure predictions following the application of dermatological products that were sensitive to formulation differences was adequately validated. The first stage of the V&V process was aimed at demonstrating the predictive power of the model developed for diclofenac sodium topical gel, 1%, in describing observed clinical data of local and systemic diclofenac exposure. In this step, the developed models reasonably described literature data and data generated by the applicant on diclofenac skin permeation and plasma exposure following application of various diclofenac dermatological products in addition to the test and the reference products. For locally acting drug products, the overall performance of the modeling platform used for model development constitutes an integral part of the V&V process. To demonstrate satisfactory performance for the modeling platform utilized in this case, a significant number of PBPK models for dermatological products were developed by the applicant and validated using literature data sources. The developed models captured reasonably well local and systemic bioavailability for active ingredients of varying physicochemical properties and PK characteristics. The predictive power of the MechDermA model was validated by assessing model predictions for simple or more complex semisolid topical products and TDSs for which the impact of dispensing and application methodologies and product metamorphosis on skin permeation varies. This novel, two‐level V&V process is relevant for locally acting drug products for which validation of model predictions on local bioavailability may be challenging due to feasibility or data availability issues. Notably, the overall V&V process proposed by the applicant was resource‐intensive as it involved the development and validation of a high number of dermal PBPK models, but it was crucial in establishing confidence in the predictive power of the platform and in the developed models for the reference and test products. Collectively, acceptable performance across all developed dermal PBPK models increased confidence in the platform used for model development, the assumptions, and the model structure itself. Importantly, the collection of dermatological drug products under the two stages of the V&V process was useful in assessing the impact of formulation attributes as these were captured in the platform on local and systemic bioavailability; the latter is desirable for a fit‐for‐purpose dermal PBPK model for diclofenac sodium topical gel, 1%, developed to support a BE approach. The Agency extended the model and platform V&V process on assessing the capability of the MPML MechDermA platform to predict the API concentration in the skin resulting from redistribution from the systemic circulation as this may interfere with a VBE assessment.
The Agency refined the submitted model to improve local exposure predictions at or near to the presumed site of action (synovial fluid) 29 with considerations of high variability associated with observed data and model structure identifiability issues. Optimization of diclofenac protein binding and distribution in the deeper tissues, such as the subcutis and the simulated synovial fluid/muscle parameters, improved model outcomes. Satisfactory model performance locally was deemed necessary for the proposed fit‐for‐purpose model to establish a link between systemic and local exposure considering the PSG‐recommended comparative clinical end point BE study was not performed. The fully validated refined model was found adequate for a VBE assessment.
Further improvement in the model‐generated predictions of local and systemic diclofenac bioavailability may be achieved by accounting for additional formulation components. Incorporating the rheological properties of the drug product in the proposed dermal PBPK model, and accounting for the effects of inactive ingredients on the API permeation through the skin and the changes in the thermodynamic activity of the drug product post‐application may lead to more realistic modeling outputs. Additionally, a more detailed model structure that captures how diclofenac skin disposition may be impacted by protein binding, blood flow, and lymph flow, especially in the dermis, may improve model predictions in the skin and the systemic circulation. To that end, the FDA has initiated external research projects under the Generic Drug User Fee Amendments I and II regulatory science program. 16 , 40
CONCLUSION
Approval of the generic diclofenac sodium topical gel, 1%, discussed here provides a successful example of the utilization of novel quantitative tools and modeling in support of an alternative BE approach for dermatological generic drug products. PBPK modeling supported an alternative BE approach that did not include a comparative clinical end point BE study for a generic diclofenac sodium topical gel, 1%. To demonstrate the credibility of its modeling approach, the applicant applied a novel V&V process in its regulatory submission. The benefit of applying PBPK models to support regulatory decision making, especially for locally acting products, warrants further investment, collaboration, and a joint effort from the Agency, industry, and academia.
FUNDING INFORMATION
No funding was received for this work.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTION
All authors contributed to the organization and writing of the paper. E.T. contributed to the modeling analysis.
FDA DISCLAIMER
The opinions expressed in this paper are those of the authors and should not be interpreted as the position of the US Food and Drug Administration.
Supporting information
Figure S1
Acknowledgments
The authors would like to thank scientists from the Office of Research and Standards and the Office of Bioequivalence at the Office of Generic Drugs, Center of Drug Evaluation and Research, the US Food and Drug Administration for their support and helpful discussions in relevant scientific areas. The authors also thank the scientists involved in the development of the dermal PBPK model that supported the ANDA approval discussed here. The authors gratefully acknowledge Dr. Robert Lionberger for insightful comments and discussion. Finally, the authors would like to acknowledge Dr. Mingjiang Xu for proofing and editing the manuscript.
References
- 1. Lionberger RA. FDA critical path initiatives: opportunities for generic drug development. AAPS J. 2008;10:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. CFR. ‐ Code of Federal Regulations Title 21, Volume 5, Revised as of April 1, 2019. Available from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=320.23 [Accessed 02/23/2021]
- 3. United States Food and Drug Administration. 21CFR320.23. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=320.23 [Accessed 02/23/2020]
- 4. FDA . Product‐Specific Guidances for Generic Drug Development. Available from: https://www.fda.gov/drugs/guidances‐drugs/product‐specific‐guidances‐generic‐drug‐development [Accessed 02/23/2021]
- 5. Raney SG, Luke MC. A new paradigm for topical generic drug products: Impact on therapeutic access. J Am Acad Dermatol. 2020;82:1570–1571. [DOI] [PubMed] [Google Scholar]
- 6. Lionberger RA. Innovation for generic drugs: science and research under the generic drug user fee amendments of 2012. Clin Pharmacol Ther. 2019;105:878–885. [DOI] [PubMed] [Google Scholar]
- 7. Raney SG, Franz TJ, Lehman PA, Lionberger R, Chen ML. Pharmacokinetics‐based approaches for bioequivalence evaluation of topical dermatological drug products. Clin Pharmacokinet. 2015;54:1095–1106. [DOI] [PubMed] [Google Scholar]
- 8. Zhao P, Zhang L, Grillo JA, et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther. 2011;89:259–267. [DOI] [PubMed] [Google Scholar]
- 9. Grimstein M, Yang Y, Zhang X, et al. Physiologically based pharmacokinetic modeling in regulatory science: an update from the U.S. food and drug administration's office of clinical pharmacology. J Pharm Sci. 2019;108:21–25. [DOI] [PubMed] [Google Scholar]
- 10. Huang SM, Abernethy DR, Wang Y, Zhao P, Zineh I. The utility of modeling and simulation in drug development and regulatory review. J Pharm Sci. 2013;102:2912–2923. [DOI] [PubMed] [Google Scholar]
- 11. Sager JE, Yu J, Ragueneau‐Majlessi I, Isoherranen N. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos. 2015;43:1823–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang X, Duan J, Kesisoglou F, et al. Mechanistic oral absorption modeling and simulation for formulation development and bioequivalence evaluation: report of an FDA public workshop. CPT Pharmacometrics Syst Pharmacol. 2017;6:492–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McAllister M, Flanagan T, Boon K, et al. Developing clinically relevant dissolution specifications for oral drug products‐industrial and regulatory perspectives. Pharmaceutics. 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heimbach T, Suarez‐Sharp S, Kakhi M, et al. Dissolution and translational modeling strategies toward establishing an in vitro‐in vivo link‐a workshop summary report. AAPS J. 2019;21:29. [DOI] [PubMed] [Google Scholar]
- 15. Zhao L, Kim MJ, Zhang L, Lionberger R. Generating model integrated evidence for generic drug development and assessment. Clin Pharmacol Ther. 2019;105:338–349. [DOI] [PubMed] [Google Scholar]
- 16. FY2019 GDUFA Research Report: Locally acting physiologically‐based pharmacokinetic modeling: summary of FY2019 activities. Available from: https://www.fda.gov/media/135187/download#page=35 [Accessed 02/23/2021].
- 17. Mitra A, Petek B, Bajc A, Velagapudi R, Legen I. Physiologically based absorption modeling to predict bioequivalence of controlled release and immediate release oral products. Eur J Pharm Biopharm. 2019;134:117–125. [DOI] [PubMed] [Google Scholar]
- 18. Loisios‐Konstantinidis I, Cristofoletti R, Fotaki N, Turner DB, Dressman J. Establishing virtual bioequivalence and clinically relevant specifications using in vitro biorelevant dissolution testing and physiologically‐based population pharmacokinetic modeling. case example: Naproxen. Eur J Pharm Sci. 2020;143:105170. [DOI] [PubMed] [Google Scholar]
- 19. Fan J, Zhang X, Zhao L. Utility of physiologically based pharmacokinetic absorption modeling to predict the impact of salt‐to‐base conversion on prasugrel HCl product bioequivalence in the presence of proton pump inhibitors. AAPS J. 2017;19:1479–1486. [DOI] [PubMed] [Google Scholar]
- 20. Doki K, Darwich AS, Patel N, Rostami‐Hodjegan A. Virtual bioequivalence for achlorhydric subjects: The use of PBPK modelling to assess the formulation‐dependent effect of achlorhydria. Eur J Pharm Sci. 2017;109:111–120. [DOI] [PubMed] [Google Scholar]
- 21. Karrer C, Roiss T, von Goetz N, Gramec Skledar D, Peterlin Masic L, Hungerbuhler K. Physiologically based pharmacokinetic (PBPK) modeling of the bisphenols BPA, BPS, BPF, and BPAF with new experimental metabolic parameters: comparing the pharmacokinetic behavior of BPA with its substitutes. Environ Health Perspect. 2018;126:77002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dancik Y, Troutman JA, Jaworska J. A framework incorporating the impact of exposure scenarios and application conditions on risk assessment of chemicals applied to skin. Silico Pharmacol. 2013;1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le Merdy M, Fan J, Bolger MB, et al. Application of mechanistic ocular absorption modeling and simulation to understand the impact of formulation properties on ophthalmic bioavailability in rabbits: a case study using dexamethasone suspension. AAPS J. 2019;21:65. [DOI] [PubMed] [Google Scholar]
- 24. Puttrevu SK, Arora S, Polak S, Patel NK. Physiologically based pharmacokinetic modeling of transdermal selegiline and its metabolites for the evaluation of disposition differences between healthy and special populations. Pharmaceutics. 2020;12:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Voltaren gel (diclofenac sodium topical gel), 1%. US FDA drug product label. Revised Feb 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022122s011lbl.pdf [Accessed 02/23/2021]
- 26. Voltaren gel (diclofenac sodium topical gel), 1%. NDA 022122. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=022122 [Accessed 02/23/2021]
- 27. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Available from: https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm?resetfields=1 [Accessed 03/04/2020]
- 28. Product‐specific guidance on Diclofenac Sodium, Topical Gel, 1%. Recommended Feb 2011; Revised Jul 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Diclofenac%20Sodium_draft_Topical%20gel_RLD%2022122_RC07‐18.pdf [Accessed 02/23/2021]
- 29. Radermacher J, Jentsch D, Scholl MA, Lustinetz T, Frolich JC. Diclofenac concentrations in synovial fluid and plasma after cutaneous application in inflammatory and degenerative joint disease. Br J Clin Pharmacol. 1991;31:537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. GDUFA II Commitment Letter: GDUFA reauthorization performance goals and program enhancements Fiscal years 2018‐2022. Available from: https://www.fda.gov/media/101052/download [Accessed 03/04/2020].
- 31. Shatkin JA, Brown HS. Pharmacokinetics of the dermal route of exposure to volatile organic chemicals in water: a computer simulation model. Environ Res. 1991;56:90–108. [DOI] [PubMed] [Google Scholar]
- 32. Polak S, Ghobadi C, Mishra H, et al. Prediction of concentration‐time profile and its inter‐individual variability following the dermal drug absorption. J Pharm Sci. 2012;101:2584–2595. [DOI] [PubMed] [Google Scholar]
- 33. Voltaren (diclofenac sodium) topical gel, 1%. NDA022122, Clinical Pharmacology and Biopharmaceutics Review. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022122_ClinPharmR.pdf [Accessed 02/23/2021]
- 34. PubChem Diclofenac Sodium . Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Diclofenac‐sodium#section=Canonical‐SMILES [Accessed 02/23/2021]
- 35. Brunner M, Davies D, Martin W, Leuratti C, Lackner E, Muller M. A new topical formulation enhances relative diclofenac bioavailability in healthy male subjects. Br J Clin Pharmacol. 2011;71:852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dehghanyar P, Mayer BX, Namiranian K, Mascher H, Muller M, Brunner M. Topical skin penetration of diclofenac after single‐ and multiple‐dose application. Int J Clin Pharmacol Ther. 2004;42:353–359. [DOI] [PubMed] [Google Scholar]
- 37. Sioufi A, Pommier F, Boschet F, Godbillon J, Lavoignat D, Salliere D. Percutaneous absorption of diclofenac in healthy volunteers after single and repeated topical application of diclofenac Emulgel. Biopharm Drug Dispos. 1994;15:441–449. [DOI] [PubMed] [Google Scholar]
- 38. Seth BL. Comparative pharmacokinetics and bioavailability study of percutaneous absorption of diclofenac from two topical formulations containing drug as a solution gel or as an emulsion gel. Arzneimittelforschung. 1992;42:120–122. [PubMed] [Google Scholar]
- 39. Brunner M, Dehghanyar P, Seigfried B, Martin W, Menke G, Muller M. Favourable dermal penetration of diclofenac after administration to the skin using a novel spray gel formulation. Br J Clin Pharmacol. 2005;60:573–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao L, Seo P, Lionberger R. Current scientific considerations to verify physiologically‐based pharmacokinetic models and their implications for locally acting products. CPT Pharmacometrics Syst Pharmacol. 2019;8:347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peters SA, Dolgos H. Requirements to establishing confidence in physiologically based pharmacokinetic (PBPK) models and overcoming some of the challenges to meeting them. Clin Pharmacokinet. 2019;58:1355–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shebley M, Sandhu P, Emami Riedmaier A, et al. Physiologically based pharmacokinetic model qualification and reporting procedures for regulatory submissions: a consortium perspective. Clin Pharmacol Ther. 2018;104:88–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kuemmel C, Yang Y, Zhang X, et al. Consideration of a credibility assessment framework in model‐informed drug development: potential application to physiologically‐based pharmacokinetic modeling and simulation. CPT Pharmacometrics Syst Pharmacol. 2020;9:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kraus VB, Stabler TV, Kong SY, Varju G, McDaniel G. Measurement of synovial fluid volume using urea. Osteoarthritis Cartilage. 2007;15:1217–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tamer TM. Hyaluronan and synovial joint: function, distribution and healing. Interdiscip Toxicol. 2013;6:111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O'Neill TW, Parkes MJ, Maricar N, et al. Synovial tissue volume: a treatment target in knee osteoarthritis (OA). Ann Rheum Dis. 2016;75:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bielecka‐Grzela S, Klimowicz A. Application of cutaneous microdialysis to evaluate metronidazole and its main metabolite concentrations in the skin after a single oral dose. J Clin Pharm Ther. 2003;28:465–469. [DOI] [PubMed] [Google Scholar]
- 49. Erdogan F, Ergun H, Gokay NS, Gulmez SE, Bolay B, Tulunay FC. The diffusion of nimesulide gel into synovial fluid: a comparison between administration routes. Int J Clin Pharmacol Ther. 2006;44:270–275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


