Abstract
Background
A Phase I, single-center investigation found that 8 weeks of antimycobacterial therapy improved sarcoidosis FVC. Safety and efficacy assessments have not been performed in a multicenter cohort.
Research Question
The objective of this study was to determine the safety and efficacy of antimycobacterial therapy on the physiological and immunologic end points of sarcoidosis.
Study Design and Methods
In a double-blind, placebo-controlled, multicenter investigation, patients with pulmonary sarcoidosis were randomly assigned to receive 16 weeks of concomitant levofloxacin, ethambutol, azithromycin, and rifabutin (CLEAR) or matching placebo to investigate the effect on FVC. The primary outcome was a comparison of change in percentage of predicted FVC among patients randomized to receive CLEAR or placebo in addition to their baseline immunosuppressive regimen. Secondary outcomes included 6-min walk distance (6MWD), St. George’s Respiratory Questionnaire (SGRQ) score, adverse events, and decrease in mycobacterial early secreted antigenic target of 6 kDa (ESAT-6) immune responses.
Results
The intention-to-treat analysis revealed no significant differences in change in FVC among the 49 patients randomized to receive CLEAR (1.1% decrease) compared with the 48 randomized to receive placebo (0.02% increase) (P = .64). Physiological parameters such as the change in 6MWD were likewise similar (P = .91); change in SGRQ favored placebo (–8.0 for placebo vs –1.5 for CLEAR; P = .028). The per-protocol analysis revealed no significant change in FVC at 16 weeks between CLEAR and placebo. There was no significant change in 6MWD (36.4 m vs 6.3 m; P = .24) or SGRQ (–2.3 vs –7.0; P = .14). A decline in ESAT-6 immune responses at 16 weeks was noted among CLEAR-treated patients (P = .0003) but not patients receiving placebo (P = .24).
Interpretation
Despite a significant decline in ESAT-6 immune responses, a 16-week CLEAR regimen provided no physiological benefit in FVC or 6MWD among patients with sarcoidosis.
Key Words: antimycobacterial therapy, ESAT-6, FVC, sarcoidosis
Abbreviations: 6MWD, 6-min walk distance; CLEAR, concomitant Levaquin, ethambutol, azithromycin, and rifamycin; ESAT-6, early secreted antigenic target of 6 kDa; SAE, severe adverse event; SGRQ, St. George’s Respiratory Questionnaire
Sarcoidosis is an idiopathic, granulomatous disease with limited therapeutic options.1,2 Current guidelines recommend various forms of immunosuppression as a mainstay of treatment, although these agents carry significant toxicities and have suboptimal efficacy. Corticosteroids have been proposed as the drug of choice for the treatment of pulmonary sarcoidosis, but toxicities are common. In addition, although antimalarial, cytotoxic, and biologic agents exhibit efficacy, relapse following tapering or discontinuation of these agents, as well as their side effect profiles, underscore the necessity for safer, more effective options.3,4
Although no definitive agent has been identified in sarcoidosis granulomas, independent laboratories have reported the presence of mycobacterial proteins and DNA in sarcoidosis lesions.5, 6, 7 In addition, several investigators have described immune responses against secreted mycobacterial virulence factors in patients with sarcoidosis.7, 8, 9 Immune responses against these mycobacterial antigens disappear with spontaneous clinical resolution of pulmonary sarcoidosis,8 as well as following administration of antimycobacterial therapy to patients with this disease.10 The clinical utility of antimycobacterial therapy was suggested by an 8-week, single-blind randomized trial of concomitant Levaquin, azithromycin, ethambutol, and rifabutin (CLEAR) in cutaneous sarcoidosis; an open-label trial similarly reported improved FVC, 6-min walk distance (6MWD), and early secreted antigenic target of 6 kDa (ESAT-6) responses in pulmonary sarcoidosis.10,11 Histologic evidence of granulomatous resolution following administration of the CLEAR regimen among patients with cutaneous sarcoidosis was also noted.11 A case report of resolution of ocular sarcoidosis with the same regimen has also been reported.12 We designed a Phase IIB study to further define the safety and efficacy of the CLEAR regimen in sarcoidosis patients with progressive pulmonary disease.
Patients and Methods
Trial Design and Objectives
This randomized, double-blind, placebo-controlled investigation compared a regimen of antimycobacterial therapy consisting of CLEAR vs a four-drug placebo regimen for 16 weeks. Each patient received 8 weeks of four drugs (induction phase), followed by 8 weeks of two drugs (consolidation phase). The primary end point was the absolute change in percentage of predicted FVC comparing baseline FVC vs FVC following completion of 16 weeks of therapy. The secondary end points included change in 6MWD, St. George’s Respiratory Questionnaire (SGRQ) score, adverse events of grades 1 to 5, and in ESAT-6-specific immune responses.
Protocol Development and Oversight
The study protocol was approved by the Vanderbilt University Medical Center Institutional Review Board by Health Sciences Committee 1 (#121532) and was registered at ClinicalTrials.gov.13 This study was conducted in accordance with the amended Declaration of Helsinki, and written informed consent was obtained from all patients. The Data and Safety Monitoring Board for this study reviewed data throughout the study and performed the single planned interim analysis for safety and efficacy after 50 randomized patients had completed their 16-week regimen.
Interventions
Patient were randomized to receive either an oral antibiotic regimen consisting of 8 weeks of daily levofloxacin 500 mg, ethambutol (1,200 mg for ≥ 50 kg; 800 mg for < 50 kg) once daily, azithromycin 250 mg, and rifabutin 300 mg vs a daily identical-appearing four-drug placebo regimen. For the last 8 weeks of the study, participants were given two of the four drugs based on their individual tolerance and toxicity during the first 8 weeks. The placebo regimen was administered in the same format. The levofloxacin, ethambutol, and azithromycin were paid for at full cost through the Vanderbilt Investigational Drug Pharmacy. Rifabutin was donated by the Pfizer Global Medical Grant Program (#53232269).
Population Eligibility and Randomization
Adults aged ≥ 18 years with the diagnosis of sarcoidosis as defined by the 1999 American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and Other Granulomatous Disorders statement on sarcoidosis were eligible for enrollment.1 Participants were also selected based on demonstration of pulmonary disease progression according to at least one of the following three criteria: (1) decline of absolute percentage of predicted FVC or diffusing capacity for carbon monoxide of at least 5% on serial measurements over 24 months; (2) radiographic progression in chest imaging on side-by-side comparison; or (3) decline in dyspnea score, as measured by using the Transition Dyspnea Index. Prior to randomization, participants were assessed for peripheral immune responses to ESAT-6 or evidence of peripheral anergy as defined by absence of responses to phytohemagglutinin. If either of those conditions were present, the subject was enrolled. Finally, participants were required to have evidence of parenchymal or nodal disease on chest radiograph. Site-specific pulmonologists who were unaffiliated with the CLEAR trial read all site-specific lung function tests.
Sample size was calculated for the primary end point: change from baseline of FVC percent predicted. Using data from participants with chronic pulmonary sarcoidosis treated with infliximab,14 we obtained the SD of 7.7 for the primary end point. A sample size of 51 completed participants per arm was needed to have 90% power to detect a 5% difference in change of FVC percent predicted from baseline.
The major exclusion criteria were as follows:
-
(1)
Inability to obtain consent.
-
(2)
Age < 18 years.
-
(3)
Female participants of childbearing potential not willing to use one of the following methods of birth control for the duration of the study and 90 days following study completion: condoms, sponge, foams, jellies, diaphragm, nonhormonal intrauterine device, a vasectomized sole partner, or abstinence. Note: Oral contraceptive pills are not effective birth control when taking rifamycin. A negative urine pregnancy test result at screening visit was required if the female subject was of childbearing potential.
-
(4)
FVC predicted value < 45%.
-
(5)
End-stage fibrotic pulmonary disease.
-
(6)
Significant underlying liver disease.
-
(7)
Allergy or intolerance to any of the antibiotics within the CLEAR regimen.
-
(8)
Allergy or intolerance to albuterol.
-
(9)
Poor venous access for obtaining blood samples.
-
(10)
History of active TB, close contact with a person with active TB within the 6 months prior to the screening visit, or a positive purified protein derivative skin test result.
-
(11)
Significant disorder, other than sarcoidosis, that would complicate the treatment evaluation (eg, respiratory, cardiac, neurologic, musculoskeletal, or seizure disorders).
-
(12)
Use of an investigational drug within 30 days prior to screening or within five half-lives of the agent, whichever is longer.
-
(13)
Currently receiving > 40 mg of prednisone.
-
(14)
Alanine aminotransferase or aspartate aminotransferase levels more than five times the upper limit of normal.
-
(15)
Leukopenia, as defined by a WBC count < 3.0 cells/mm3 or absolute neutrophil count < 1,000/μL.
-
(16)
Breastfeeding.
-
(17)
Color perception impairment as defined by the inability to differentiate colors per personal history or history of optic neuritis from any cause, including from sarcoidosis.
-
(18)
If the patient is on immunomodulators, they must be on a regimen for 3 months or longer and on a stable dose for > 4 weeks.
-
(19)
Family or personal history of long QT interval.
-
(20)
Most recent nuclear medicine scan or echocardiogram (if performed) showing cardiac ejection fraction < 35%.
-
(21)
Participant has persistent or active infection(s) requiring hospitalization or treatment with antibiotic, antiretroviral, or antifungal agents within 30 days prior to baseline. Minocycline and doxycycline are not considered antibiotics when used to treat sarcoidosis.
-
(22)
Any significant finding in the patient’s medical history or physical or psychiatric examination prior to or following randomization that, in the opinion of the investigator, would affect patient safety or compliance or ability to deliver the study drug according to protocol.
-
(23)
Taking medications that, in the opinion of the investigator, would affect patient safety when taken with the antibiotics of the CLEAR regimen.
-
(24)
History of or receiving treatment for pulmonary hypertension. Receiving biologic medication within the 6 months prior to screening visit.
Patients were assigned to receive the CLEAR or placebo regimen by using a block randomization stratified according to site and use of prednisone ≥ 10 mg or not. Randomization lists were generated and distributed to site pharmacies by a statistician at Vanderbilt University Medical Center not associated with the study.
Measurement of Treatment Completion
Patients were requested to bring all remaining doses of every trial drug to each visit for pill counts. Treatment completion for the per-protocol analysis was defined as administration of at least 90% of the doses within 16 weeks.
Measurement of Safety During Treatment
At each follow-up visit, participants were questioned and examined for adverse events. Suspected adverse events were investigated, managed, and reported according to a standardized protocol. Information about suspected adverse events was reviewed and graded by the site-specific principal investigator and clinical coordinator. The severity of adverse events was judged according to the Common Toxicity Criteria for Adverse Events version 4.0. The events were categorized as follows: an adverse event that was not related to a trial drug; an adverse event of grade 1 or 2 that was related to a trial drug (not serious); an adverse event of grade 3 or 4 that was related to a trial drug (generally considered to lead to trial drug discontinuation if related to a trial drug); or a grade 5 event (death) that was related to a trial drug. Each adverse event resulting in organ impairment, hospitalization, or death was defined as a serious adverse event (SAE). An SAE was reported and reviewed by the institutional review board, as well as the four-member Data Safety Monitoring Board. The board provided oversight regarding safety for continuation of the study.
Oversight
This trial was approved by the Vanderbilt University Human Research Protection Program and by the institutional review board at each participating site. All the authors vouch for the accuracy and completeness of the data and analyses presented and for the compliance of the trial to the protocol.
Statistical Analysis
This randomized Phase II clinical trial was conducted to determine if CLEAR elicits a statistically significant (two-sided value P < .05) improvement in respiratory performance, the 6MWD, SGRQ, and immune responses against ESAT-6. Ninety-seven patients were randomized to treatment from May 5, 2014, to December 13, 2018, of whom 49 patients were randomized to receive CLEAR and 48 to receive placebo. Data were cleaned and locked for final analysis on April 18, 2019. The primary end point was baseline to 16-week change in preinhaler FVC as percent predicted. We defined the 16-week postrandomization FVC percent predicted value measurement as the value closest to 16 weeks from randomization within a window of 12 to 20 weeks from randomization.
The primary comparison between the CLEAR and placebo arms was conducted on an intention-to-treat (as randomized) basis among patients with both baseline and 16-week outcomes (N = 97). Unless otherwise stated, mean, SE, and comparative P values were estimates with multiple imputation using predictive mean matching with aregImpute, as previously described.15,16 The multiple imputation data sets were summarized according to Rubin’s rules.17 Unless otherwise noted, figures represent the mean change score ± the SE computed by using Rubin’s rules for imputed data. Change scores for secondary end points were compared by using the linear model formulation of the two-sample test between groups over 100 imputed data sets. The paired Student t test for multiply imputed data was used to compare baseline and 16-week change scores within each treatment group at 8 and 16 weeks. All analyses were repeated for a per-protocol population of patients (n = 72). Analysis of ESAT-6 and adverse events was not based on imputed data. The ESAT-6 analysis was the only within-group comparison and was conducted by using a Student paired t test, comparing baseline with 16-week values within their randomization cohort. The number of subjects experiencing an SAE and separately an adverse event (adverse events not including SAEs) was compared between treatment arms by using the paired or unpaired Student t test.
Results
Trial Participants
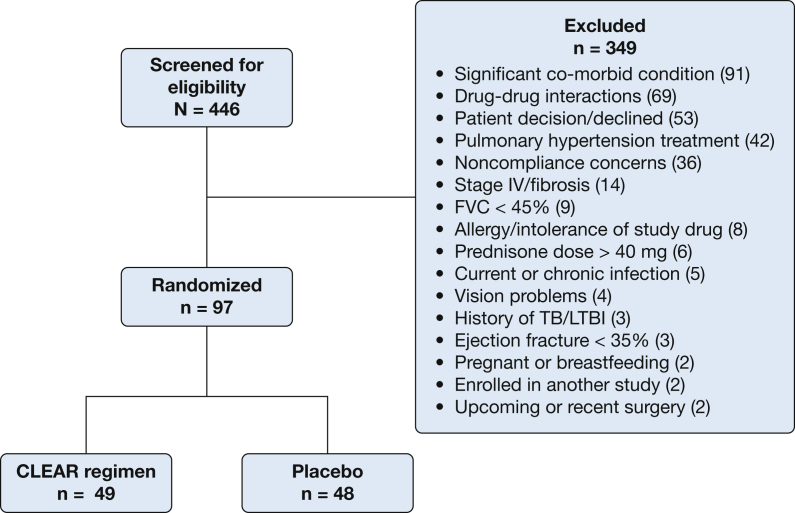
We screened 446 potential patients from May 2014 through December 2018, of whom 97 were enrolled and randomized to treatment (Fig 1). The most common reasons for exclusion from study participation were as follows: (1) significant comorbid conditions; (2) a history of a drug interaction between one of the CLEAR antibiotics with a medication that the patient was currently receiving; (3) patient declined to participate; (4) pulmonary hypertension treatment; (5) concerns for noncompliance with study visits; and (6) Scadding stage IV fibrosis detected on a chest radiograph.
Figure 1.
Consort Diagram of Phase IIB investigation of the efficacy of antimycobacterial therapy against progressive pulmonary sarcoidosis. CLEAR = concomitant Levaquin, ethambutol, azithromycin, and rifamycin; LTBI = latent TB infection.
The demographic and clinical characteristics of all study participants according to their randomization group are shown in Table 1. Of the 97 enrolled subjects, baseline characteristics were well balanced across treatment arms. Approximately one-half of the population (n = 50 [52%]) was female, and the majority were White (n = 68 [70%]), with approximately one-third African American (n = 28 [29%]). A possible balance exception was sex (59% women in the placebo group and 44% women in the CLEAR group). Although not a planned analysis, analysis of covariance of sex (P = .996) with treatment group (P = .903) on nonimputed data, as with their interaction (P = .678), suggests no confounding by sex.
Table 1.
Patient Demographic Characteristics According to Therapeutic Regimen
| Characteristic | CLEAR |
Placebo |
Combined |
|---|---|---|---|
| (n = 49) | (n = 48) | (N = 97) | |
| Age, mean ± SD, y | 54.5 ± 9.8 | 54.5 ± 9.8 | 54.5 ± 10 |
| Sex | |||
| Male | 20 (41%) | 27 (56%) | 47 (48%) |
| Female | 29 (59%) | 21 (44%) | 50 (52%) |
| Race | |||
| African American | 15 (31%) | 13 (27%) | 28 (29%) |
| White | 34 (69%) | 34 (71%) | 68 (70%) |
| Hawaiian or Pacific Islander | 0 (0) | 1 (2%) | 1 (1%) |
| Ethnicity | |||
| Non-Hispanic/nor Latino | 48 (98%) | 46 (96%) | 94 (97%) |
| Hispanic/Latino | 1 (2%) | 2 (4%) | 3 (3%) |
| Baseline end points | |||
| Preinhaler FVC % | 77.3 ± 13.7 | 75.5 ± 14.5 | 75.4 ± 14.1 |
| 6-min walk test, ma | 416.2 ± 140.7 | 416.4 ± 105.2 | 416.3 ± 123.5 |
| SGRQ activityb | 57.7 ± 23.2 | 55.5 ± 24.5 | 56.6 ± 23.8 |
| SGRQ impactb | 34.8 ± 22.2 | 32.9 ± 19.8 | 33.8 ± 20.9 |
| SGRQ symptomsb | 53.9 ± 23.8 | 50.5 ± 19.3 | 52.2 ± 21.7 |
| SGRQ totalb | 44.9 ± 21.1 | 42.7 ± 18.7 | 43.8 ± 19.9 |
| Immunosuppression | |||
| Patients on prednisone (mean dosage) | 23 (47%) (10 mg) | 19 (40%) (10 mg) | 42 (43%) (10 mg) |
| Patients on a DMA | 19 (39%) | 17 (35%) | 36 (37%) |
| Patients on a biologic agent | 1 (2%) | 1 (2%) | 2 (4%) |
| Patients on prednisone plus a DMA or biologic agent | 10 (20%) | 6 (13%) | 16 (16%) |
| Patients on any combination of prednisone, DMA, or biologic agent | 11 (22%) | 8 (17%) | 19 (20%) |
| ESAT-6 positivity | |||
| Yes | 39 (80%) | 36 (75%) | 75 (77%) |
| Equivocal | 10 (20%) | 12 (25%) | 25 (23%) |
CLEAR = concomitant Levaquin, ethambutol, azithromycin, and rifamycin; DMA = disease-modifying agent; ESAT-6 = early secreted antigenic target of 6 kDa.
N = 48 for CLEAR and placebo groups.
N = 48 and N = 47, for CLEAR and placebo groups, respectively.
The clinical data were analyzed via intention-to-treat and per protocol. No subjects were excluded from the intention-to-treat analysis. In the per-protocol analysis, 25 (12 in the active arm and 13 in the placebo arm) of the 97 patients were excluded because of failure to take > 4 weeks of the prescribed regimen (CLEAR, n = 8; placebo, n = 4), alteration in clinical immunosuppressive regimen while on study drugs (CLEAR, n = 1; placebo, n = 3), initiation of non-study antibiotics during study participation (CLEAR, n = 1; placebo, n = 3), and found to be receiving antibiotics with antimycobacterial therapy, such as trimethoprim-sulfamethoxazole, at the time of enrollment (CLEAR, n = 2; placebo, n = 3). The remaining 72 subjects were included in the per-protocol analysis.
Efficacy
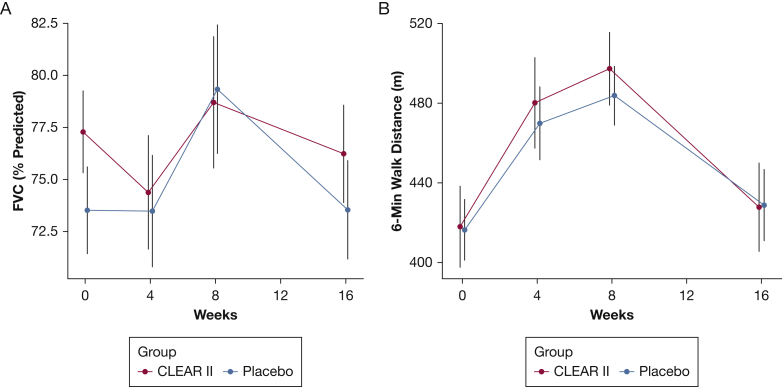
In the intention-to-treat analysis, there were no statistically significant differences in the primary end point (change from baseline to week 16 in pre-bronchodilator percent predicted FVC) between the CLEAR and placebo groups (CLEAR –1.06% vs placebo 0.02%; imputed two-sample Student t test, P = .64) (Fig 2A, Table 2). Eight-eight patients experienced follow-up during the 12- to 20-week postrandomization window. Median (interquartile range) and mean ± SD of time to the date of FVC percent measurement was 17 (16.1-18) weeks and 17.5 ± 1.9 weeks, respectively. Time to primary end point measurement was equivalent between treatment groups, with a median (interquartile range) of 17 (16.2-18) weeks for placebo and 17.2 (16.2-18.0) weeks for CLEAR II patients. Mean ± SD values were 17.5 ± 1.9 and 17.4 ± 1.8. Evaluation of other physiological parameters, such as 6MWD (placebo, 12.4 m; CLEAR, 9.8 m; imputed two-sample Student t test, P = .91) revealed no statistically significant differences (Fig 2B). A negative change in the SGRQ score reflects an improvement in quality of life. The SRGQ did reveal statistically and clinically significant differences in favor of the placebo group (placebo, –7.97; CLEAR II, –1.52; P = .028, minimal clinically important difference = 4.0).
Figure 2.
A-B, Graphic depiction of intention-to-treat assessment of physiological parameters such as FVC and 6-min walk distance in 97 subjects with sarcoidosis randomized to either the CLEAR regimen (active) or placebo. A, There were no statistically significant differences in the primary end point (change from baseline to week 16 in pre-bronchodilator percent predicted FVC) between 49 CLEAR and 48 placebo subjects (CLEAR –1.06% vs placebo 0.02%; imputed two-sample Student t test, P = .64). B, Evaluation of 6-min walk distance (placebo, 12.4 m; CLEAR, 9.8 m; imputed two-sample Student t test, P = 0.91) revealed no statistically significant difference. CLEAR = concomitant Levaquin, ethambutol, azithromycin, and rifamycin.
Table 2.
Physiological and Qualitative End Point Analyses
| Variable | Placebo | CLEAR | P Value |
|---|---|---|---|
| Intent-to-treat population | |||
| Preinhaler FVC % | 0.02 ± 1.47 | –1.06 ± 1.85 | .640 |
| 6-min walk distance | 12.40 ± 12.35 | 9.78 ± 19.31 | .908 |
| SGRQ activity | –7.15 ± 3.28 | –0.96 ± 2.87 | .162 |
| SGRQ impact | –7.45 ± 2.64 | –0.61 ± 2.78 | .057 |
| SGRQ symptoms | –10.15 ± 3.45 | –5.62 ± 3.22 | .331 |
| SGRQ total | –7.97 ± 2.01 | –1.52 ± 2.17 | .028 |
| Per-protocol group | |||
| Preinhaler FVC % | 0.42 ± 1.48 | –0.74 ± 1.75 | .616 |
| 6-min walk distance | 6.27 ± 11.99 | 36.35 ± 22.32 | .242 |
| SGRQ activity | –4.25 ± 2.39 | –1.45 ± 2.91 | .458 |
| SGRQ impact | –7.97 ± 2.51 | –1,10 ± 2.62 | .061 |
| SGRQ symptoms | –9.78 ± 3.60 | –8.07 ± 3.39 | .730 |
| SGRQ total | –6.97 ± 2.13 | –2.32 ± 2.32 | .141 |
Imputed mean ± SE of baseline to 16-week differences in measurements. P values are from imputation-based two-sample Student t test. CLEAR = concomitant Levaquin, ethambutol, azithromycin, and rifamycin; SGRQ = St. George’s Respiratory Questionnaire.
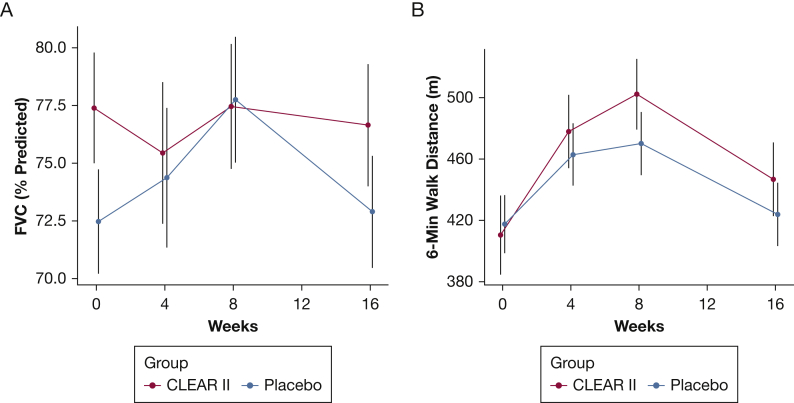
In the per-protocol analysis, 72 subjects were analyzed following removal of 25 patients prior to data analysis due to factors outlined in the protocol. Comparison of baseline vs week 16 end points of the remaining 72 patients revealed that there were no statistically differences in the primary end point (the change from baseline in percent predicted FVC) between 37 CLEAR-treated patients compared with 35 patients receiving placebo (CLEAR –0.74% vs placebo 0.42%; P = .62) (Fig 3A, Table 2). Evaluation of other physiological and qualitative end points revealed no significant differences; for example, there were no significant differences in the 6MWD of patients randomized to the CLEAR or placebo regimen (36.4 m vs 6.3 m; P = .242) (Fig 3B). The SGRQ also revealed no significant differences in the total score between the groups (placebo, –6.9; CLEAR, –2.3; imputed two-sample Student t test, P = .14). CLEAR-treated patients had less improvement in activity, impact, and symptoms compared with patients receiving placebo.
Figure 3.
A-B, Graphic depiction of per-protocol assessment of physiologic parameters, such as FVC and placebo in 97 patients with sarcoidosis randomized to either the CLEAR regimen (active) or placebo. A, Comparison of baseline vs week 16 end points of 72 subjects revealed that there were no statistically differences in the primary end point (change from baseline in percent predicted FVC) between 37 CLEAR subjects compared with 35 placebo subjects (CLEAR –0.74% vs placebo 0.42%; imputed two-sample Student t test, P = .62). B, Evaluation of 6-min walk distance revealed no significant difference among patients randomized to the CLEAR or the placebo regimen (36.4 m vs 6.3 m; imputed two-sample Student t test, P = .242). CLEAR = concomitant Levaquin, ethambutol, azithromycin, and rifamycin.
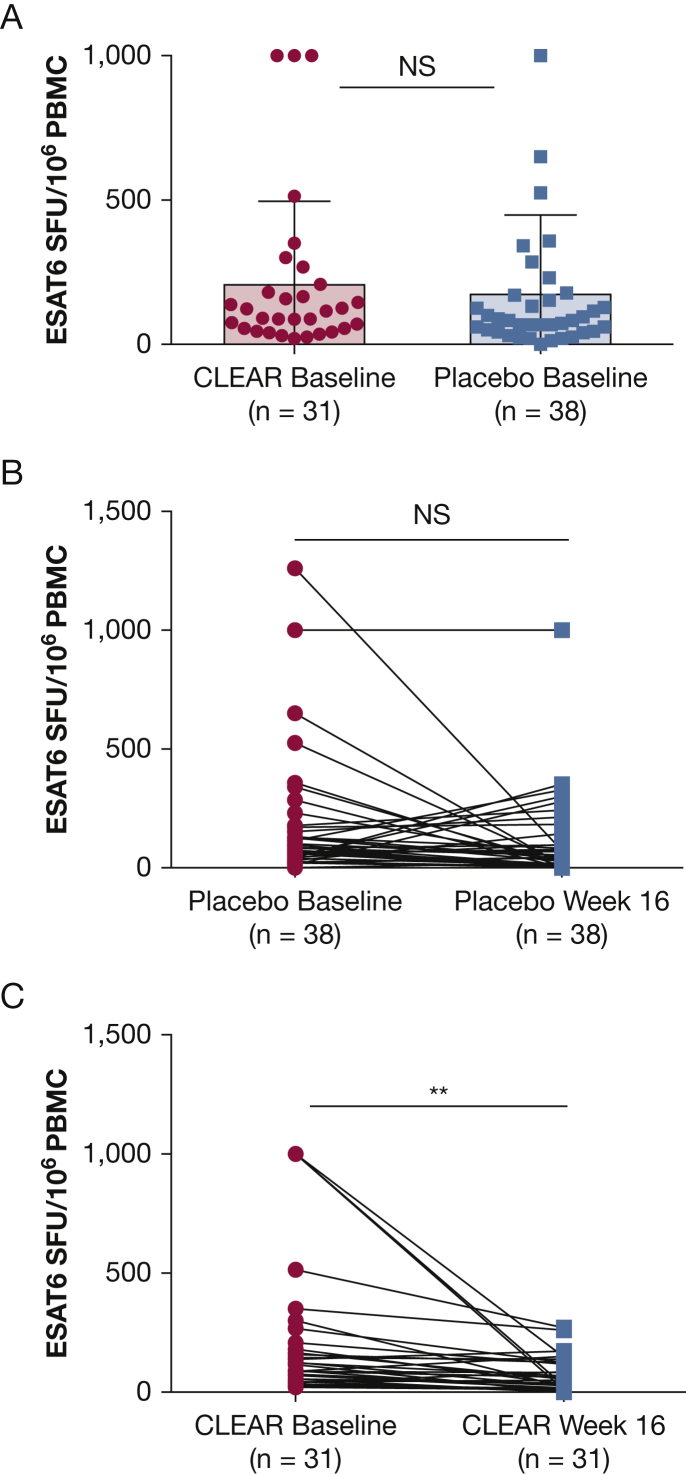
Although there was no significant baseline difference in ESAT-6-specific spot-forming units between the two groups (unpaired Student t test, P = .48) (Fig 4A), there was a significant difference in the spot-forming units following 16 weeks of therapy. There was no significant change in 38 subjects randomized to receive placebo (paired Student t test, P = .26) (Fig 4B); however, a significant decline in the ESAT-6 spot-forming units among the 31 patients randomized to the CLEAR regimen (paired Student t test, P = .0003) (Fig 4C). Only subjects for whom baseline and 16-week values were available were included in this analysis.
Figure 4.
Comparison of ESAT-6 immune responses at baseline (n = 69 subjects) (A), as well as baseline to 16 weeks among patients with sarcoidosis randomized to either the placebo (n = 38 subjects) (B) or CLEAR (n = 31 subjects) (C) regimen. ESAT-6 = early secreted antigenic target of 6 kDa; NS = not significant; PBMC = peripheral blood mononuclear cells; SFU = spot-forming units.
Safety
At each follow-up visit, participants were evaluated for adverse events. A total of 75 adverse events were noted in 39 subjects (e-Table 1). Any adverse event resulting in organ impairment, hospitalization, or death was defined as an SAE. The number of SAEs was similar for CLEAR (n = 4) and placebo (n = 3) (P = .72) (Table 3). Three of the four SAEs in the CLEAR cohort were believed to be related to study drugs; none was believed to be related in the placebo cohort. There were no deaths in this trial.
Table 3.
SAEs Throughout the Study
| SAE Number | Event | Group | Institution | Date | Anticipated | Caused by Therapy |
|---|---|---|---|---|---|---|
| 1 | Leukopenia | Active | Cincinnati | 08/14/2016 | Yes | Probable |
| 2 | Pneumonia | Active | Cincinnati | 11/06/2014 | Yes | Probable |
| 3 | Neurosarcoidosis | Active | Vanderbilt | 12/28/2016 | No | Not related |
| 4 | Hypotension | Active | AMC | 01/29/2018 | Yes | Probable |
| 5 | Sepsis from abscess | Placebo | Cleveland | 07/18/2017 | No | Not related |
| 6 | Postoperative infection | Placebo | Cleveland | 11/14/2014 | No | Unlikely |
| 7 | Pneumonia | Placebo | Cleveland | 06/04/2018 | No | Not related |
AMC = Albany Medical College; SAE = severe adverse event.
Discussion
In this randomized, double-blind, placebo-controlled trial of CLEAR therapy, we observed no benefit from the study intervention on pulmonary function, for both the intention-to-treat and per-protocol analyses. Most secondary end points, such as 6MWD and SGRQ, showed no significant improvement from CLEAR, and SGRQ was worse at the end of the CLEAR regimen. There was a significant decline in immune responses against ESAT-6 among the CLEAR-treated subjects, but no change was observed among those randomized to receive placebo (Figs 3, 4).
Although viable mycobacteria have been proposed to be causative agents for sarcoidosis, the current study provides no evidence to support that hypothesis. The current results are discordant from a randomized trial in which CLEAR therapy was beneficial for cutaneous sarcoidosis, and they also diverge from an uncontrolled report of CLEAR therapy.10,11 One explanation for the failure of CLEAR therapy is that the underlying hypothesis regarding the cause of sarcoidosis is incorrect. This explanation accords with the failure of prior studies to culture mycobacteria from sarcoidosis tissues.18,19
Other explanations for the failure of CLEAR therapy should also be considered. The inclusion criteria were designed to enroll a population with actively progressing sarcoidosis, but the precision of the longitudinal data supporting progression, and the long time window for demonstrating progression, are both problematic and may have biased the study population to include patients with relatively stable, adequately controlled disease.20 Progression in the placebo group on stable therapy would not be expected in a 16-week time frame. Active withdrawal of anti-sarcoidosis medications may be necessary to uncover relative treatment benefits if the effect size is modest, but this study required stable dosing throughout the treatment period. It is also possible that the treatment duration was too short to discern efficacy of therapy; medications such as methotrexate require up to 6 months to effect benefit in pulmonary sarcoidosis.21 Antibiotic therapy may not result in improved FVC; one study noted that these patients may continue to experience an FVC decline, but it occurs at a significantly lower rate than patients who were not successfully treated.22 Finally, FVC may be an insensitive marker of treatment effect; it has previously been shown to correlate poorly with symptoms and with chest imaging.23,24 However, the absence of any observable clinical benefits argues that the end point chosen here is likely not the cause of the negative result.
Immune responses against mycobacterial antigens, such as ESAT-6 and katG, are present in patients with active sarcoidosis disease.8,25,26 Independent investigators found that, using the ELISpot assay, antimycobacterial responses disappear with clinical resolution of sarcoidosis.8 Immune responses against ESAT-6 have also been detected with active or latent TB, as well as other nontuberculous mycobacteria infections27, 28, 29; these responses decline with effective antimycobacterial therapy.30, 31, 32 A significant decline in the ESAT-6 immune responses among subjects randomized to receive CLEAR (but not placebo) is provocative and of uncertain significance. It is unlikely that the decrease in ESAT-6 response in the CLEAR group is due to an immunosuppressant effect of one or more antibiotics in the treatment regimen because we previously reported improved immune function, including enhanced T-cell proliferative capacity and increased IL-2 and interferon-γ secretion, as well as augmented JAK-STAT signaling from sarcoidosis CD4+ T cells, following completion of the CLEAR regimen.11 The improvement in cellular immunity with CLEAR treatment of sarcoidosis could result in the augmented capacity to remove pathogenic microbial antigens such as ESAT-6. In addition, because the removal of microbial antigens such as ESAT-6 during treatment of mycobacterial infection reduces expression of profibrotic cytokines such as IL-17A, this mechanism may also mitigate the development of lung fibrosis during the treatment of sarcoidosis. The discrepancy between measurable declines in a virulence factor associated with active mycobacterial replication (ESAT-6) and the negative results of the current study remain unexplained.
It may be that mycobacterial antigens are important initial triggers of some cases of sarcoidosis, as suggested by persistence of mycobacterial antigens in sarcoidosis tissues,7 but viable infection is not integral to the perpetuation or progression of the disease. Also, because the study design did not obtain any samples to exclude the presence of infection in the subjects, it remains possible that the effects of CLEAR resulted in changes in the microbiome, which could then affect immune responses by changing the presence of microbial antigens. Future investigation regarding the impact of the CLEAR regimen on preventing further lung deterioration is warranted.
The current study did have some limitations. The number of subjects was relatively small. Twenty-five of the 97 patients were excluded from the per-protocol analysis, representing approximately 26% of the enrolled subjects. Twelve of those subjects (CLEAR, n = 8; placebo, n = 4) were excluded due to taking < 4 weeks of study medications, making it more difficult to assess the impact of 16 weeks of CLEAR therapy on the primary and secondary end points. Due to the pill burden and toxicities associated with a four-drug regimen, difficulty adhering to antimycobacterial therapy is well documented.33, 34, 35 Toxicities, such as myalgias and arthralgias from azithromycin and levofloxacin, as well as fatigue from rifabutin, may have affected the SGRQ score, masking our ability to clearly delineate an anti-sarcoidosis effect. Higher SGRQ scores were noted among CLEAR-treated patients experiencing these toxicities. Another limitation is the failure to include patients with disease for < 1 year. We did not include patients within 1 year of diagnosis because it was believed that differentiation of drug efficacy from spontaneous resolution would be too difficult. However, improvement in lung function among patients with TB is more likely if patients are < 40 years of age and do not have chronic sequelae.36 Future sarcoidosis clinical investigations should include patients within 1 year of their diagnosis, longer follow-up period assessment for potential steroid-sparing effect, and CT or PET scans. Several studies have shown that increased activity according to a PET scan is a useful predictor of treatment-responsive pulmonary sarcoidosis.37,38 PET scanning was not included in the current study because the information at time of study implementation was incomplete, and the cost of this as an exploratory analysis was prohibitive.
Conclusions
In a cohort of patients with progressive pulmonary sarcoidosis, the CLEAR therapy did not result in significant improvement in percent predicted FVC but, instead, was associated with significant declines in ESAT-6-specific immune responses.
Acknowledgments
Author contributions: W. P. D. had full access to all the data following study completion and assumes full responsibility for the integrity of the data and the accuracy of the data analysis. W. P. D., D. A. C., R. P. B., and G.R.B. were responsible for conception and design; K. A., D. A. C., R. P. B., M. A. J., E. D. C., E. J., and A. G. performed experiments; T. D., G. D. A., G. R. B., and W. P. D. contributed to analysis and interpretation; and W. P. D, D. A. C., R. P. B., M. A. J., E. D. C., E. J., T. D., K. A., A. G., A. K., A.S., and G. R. B. drafted the manuscript for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. P. B. received personal fees from Actelion and from Mallinckrodt during the conduct of the study. None declared (W. P. D., D. A. C., M. A. J., E. D. C., E. J., G. D. A., T. D., K. A., A. G., A. K., A. S., G. R. B.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors are grateful for the support of the patients with sarcoidosis and nursing personnel for this investigation.
Additional information: The e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
FUNDING/SUPPORT: The publication was supported in part by CTSA award UL1 TR002243 from the National Center for Advancing Translational Sciences (G. R. B.); R01 HL117074 (W. P. D.); Pfizer Global Medical Grant Program 53232269; and the Pierce Foundation.
Supplementary Data
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Grunewald J., Grutters J.C., Arkema E.V., Saketkoo L.A., Moller D.R., Muller-Quernheim J. Sarcoidosis. Nat Rev Dis Primers. 2019;5(1):45. doi: 10.1038/s41572-019-0096-x. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb J.E., Israel H.L., Steiner R.M., Triolo J., Patrick H. Outcome in sarcoidosis. The relationship of relapse to corticosteroid therapy. Chest. 1997;111(3):623–631. doi: 10.1378/chest.111.3.623. [DOI] [PubMed] [Google Scholar]
- 4.Vorselaars A.D., Verwoerd A., van Moorsel C.H., Keijsers R.G., Rijkers G.T., Grutters J.C. Prediction of relapse after discontinuation of infliximab therapy in severe sarcoidosis. Eur Respir J. 2014;43(2):602–609. doi: 10.1183/09031936.00055213. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell D.N. Mycobacteria and sarcoidosis. Lancet. 1996;348(9030):768–769. doi: 10.1016/S0140-6736(05)65205-1. [DOI] [PubMed] [Google Scholar]
- 6.Oswald-Richter K.A., Beachboard D.C., Seeley E.H. Dual analysis for mycobacteria and propionibacteria in sarcoidosis BAL. J Clin Immunol. 2012;32(5):1129–1140. doi: 10.1007/s10875-012-9700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Z., Marzilli L., Greenlee B.M. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med. 2005;201(5):755–767. doi: 10.1084/jem.20040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen E.S., Wahlstrom J., Song Z. T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol. 2008;181(12):8784–8796. doi: 10.4049/jimmunol.181.12.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmadzai H., Cameron B., Chui J.J., Lloyd A., Wakefield D., Thomas P.S. Peripheral blood responses to specific antigens and CD28 in sarcoidosis. Respir Med. 2012;106(5):701–709. doi: 10.1016/j.rmed.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Drake W.P., Richmond B.W., Oswald-Richter K. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30(3):201–211. [PMC free article] [PubMed] [Google Scholar]
- 11.Drake W.P., Oswald-Richter K., Richmond B.W. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. JAMA Dermatol. 2013;149(9):1040–1049. doi: 10.1001/jamadermatol.2013.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richmond B.W., Richter K., King L.E., Drake W.P. Resolution of chronic ocular sarcoidosis with antimycobacterial therapy. Case Rep Intern Med. 2014;1(2):5042. doi: 10.5430/crim.v1n2p216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institutes of Health Clinical Center. Phase II investigation of antimycobacterial therapy on progressive, pulmonary sarcoidosis. NCT02024555. ClinicalTrials.gov. National Institutes of Health; 2013. Updated July 9, 2020. https://clinicaltrials.gov/ct2/show/NCT02024555.
- 14.Baughman R.P., Drent M., Kavuru M. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 15.Jain N.B., Ayers G.D., Koudelkova H. Operative vs nonoperative treatment for atraumatic rotator cuff tears: a trial protocol for the arthroscopic rotator cuff pragmatic randomized clinical trial. JAMA Netw Open. 2019;2(8) doi: 10.1001/jamanetworkopen.2019.9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickinson R.N., Ayers G.D., Archer K.R. Physical therapy versus natural history in outcomes of rotator cuff tears: the Rotator Cuff Outcomes Workgroup (ROW) cohort study. J Shoulder Elbow Surg. 2019;28(5):833–838. doi: 10.1016/j.jse.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin D.B. Wiley-Interscience; Hoboken, NJ: 2004. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 18.Brown S.T., Brett I., Almenoff P.L. Recovery of cell wall-deficient organisms from blood does not distinguish between patients with sarcoidosis and control subjects. Chest. 2003;123(2):413–417. doi: 10.1378/chest.123.2.413. [DOI] [PubMed] [Google Scholar]
- 19.Milman N., Lisby G., Friis S., Kemp L. Prolonged culture for mycobacteria in mediastinal lymph nodes from patients with pulmonary sarcoidosis. A negative study. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21(1):25–28. [PubMed] [Google Scholar]
- 20.Moller D.R. Negative clinical trials in sarcoidosis: failed therapies or flawed study design? Eur Respir J. 2014;44(5):1123–1126. doi: 10.1183/09031936.00156314. [DOI] [PubMed] [Google Scholar]
- 21.Lower E.E., Baughman R.P. The use of low dose methotrexate in refractory sarcoidosis. Am J Med Sci. 1990;299(3):153–157. doi: 10.1097/00000441-199003000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Park H.Y., Jeong B.H., Chon H.R., Jeon K., Daley C.L., Koh W.J. Lung function decline according to clinical course in nontuberculous mycobacterial lung disease. Chest. 2016;150(6):1222–1232. doi: 10.1016/j.chest.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Baughman R.P., Drent M., Culver D.A. Endpoints for clinical trials of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29(2):90–98. [PubMed] [Google Scholar]
- 24.Baughman R.P., Nunes H., Sweiss N.J., Lower E.E. Established and experimental medical therapy of pulmonary sarcoidosis. Eur Respir J. 2013;41(6):1424–1438. doi: 10.1183/09031936.00060612. [DOI] [PubMed] [Google Scholar]
- 25.Oswald-Richter K., Sato H., Hajizadeh R. Mycobacterial ESAT-6 and katG are recognized by sarcoidosis CD4+ T cells when presented by the American sarcoidosis susceptibility allele, DRB1∗1101. J Clin Immunol. 2010;30(1):157–166. doi: 10.1007/s10875-009-9311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oswald-Richter K.A., Beachboard D.C., Zhan X. Multiple mycobacterial antigens are targets of the adaptive immune response in pulmonary sarcoidosis. Respir Res. 2010;11:161. doi: 10.1186/1465-9921-11-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyendak M.R., Park B., Null M.D. Mycobacterium tuberculosis specific CD8(+) T cells rapidly decline with antituberculosis treatment. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0081564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherrer S., Landolt P., Friedel U., Stephan R. Distribution and expression of ESAT-6 and CFP-10 in non-tuberculous mycobacteria isolated from lymph nodes of slaughtered cattle in Switzerland. J Vet Diagn Invest. 2019;31(2):217–221. doi: 10.1177/1040638718824074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston J.C., Chiang L., Elwood K. Mycobacterium kansasii. Microbiol Spectr. 2017;5(1) doi: 10.1128/microbiolspec.tnmi7-0011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C., Zhao Z., Fan J. Quantification of circulating Mycobacterium tuberculosis antigen peptides allows rapid diagnosis of active disease and treatment monitoring. Proc Natl Acad Sci U S A. 2017;114(15):3969–3974. doi: 10.1073/pnas.1621360114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattos A.M., de Almeida C.S., Franken K.L. Increased IgG1, IFN-gamma, TNF-alpha and IL-6 responses to Mycobacterium tuberculosis antigens in patients with tuberculosis are lower after chemotherapy. Int Immunol. 2010;22(9):775–782. doi: 10.1093/intimm/dxq429. [DOI] [PubMed] [Google Scholar]
- 32.Clifford V., Tebruegge M., Zufferey C. Mycobacteria-specific cytokine responses as correlates of treatment response in active and latent tuberculosis. J Infect. 2017;75(2):132–145. doi: 10.1016/j.jinf.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 33.De Schacht C., Mutaquiha C., Faria F. Barriers to access and adherence to tuberculosis services, as perceived by patients: a qualitative study in Mozambique. PLoS One. 2019;14(7) doi: 10.1371/journal.pone.0219470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Law S., Daftary A., O’Donnell M., Padayatchi N., Calzavara L., Menzies D. Interventions to improve retention-in-care and treatment adherence among patients with drug-resistant tuberculosis: a systematic review. Eur Respir J. 2019;53(1):1801030. doi: 10.1183/13993003.01030-2018. [DOI] [PubMed] [Google Scholar]
- 35.Wurie F.B., Cooper V., Horne R., Hayward A.C. Determinants of non-adherence to treatment for tuberculosis in high-income and middle-income settings: a systematic review protocol. BMJ Open. 2018;8(1) doi: 10.1136/bmjopen-2017-019287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apostu M., Mihaescu T. Respiratory functional changes in pulmonary tuberculosis [in Romanian] Pneumologia. 2013;62(3):148–157. [PubMed] [Google Scholar]
- 37.Vorselaars A.D., Crommelin H.A., Deneer V.H. Effectiveness of infliximab in refractory FDG PET-positive sarcoidosis. Eur Respir J. 2015;46(1):175–185. doi: 10.1183/09031936.00227014. [DOI] [PubMed] [Google Scholar]
- 38.Schimmelpennink M.C., Vorselaars A.D.M., van Beek F.T. Efficacy and safety of infliximab biosimilar Inflectra® in severe sarcoidosis. Respir Med. 2018;138S:S7–S13. doi: 10.1016/j.rmed.2018.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.