Abstract
Background
Prior reports on a possible female survival advantage in both surgical and nonsurgical cohorts of patients with lung cancer are conflicting. Previously reported differences in survival after lung cancer surgery could be the result of insufficient control for disparities in risk factor profiles in men and women.
Research Question
Do women who undergo pulmonary resections for lung cancer have a better prognosis than men when taking a wide range of prognostic factors into account?
Study Design and Methods
We performed a nationwide population-based observational cohort study analyzing sex-specific survival after pulmonary resections for lung cancer. We identified 6356 patients from the Swedish National Quality Register for General Thoracic Surgery and performed individual-level record linkage to other national health-data registers to acquire detailed information regarding comorbidity, socioeconomic status, and vital status. Inverse probability of treatment weighting was used to account for differences in baseline characteristics. The association between female sex and all-cause mortality was assessed with Cox regression models, and flexible parametric survival models were used to estimate the absolute survival differences with 95% CIs. We also estimated the difference in restricted mean survival time.
Results
We observed a lower risk of death in women compared with men (hazard ratio, 0.73; 95% CI, 0.67-0.79). The absolute survival difference at 1, 5, and 10 years was 3.0% (95% CI, 2.2%-3.8%), 10% (95% CI, 7.0%-12%), and 12% (95% CI, 8.5%-15%), respectively. The restricted mean survival time difference at 10 years was 0.84 year (95% CI, 0.61-1.07 years). The findings were consistent across several subgroups.
Interpretation
Women who underwent pulmonary resections for lung cancer had a significantly better prognosis than men. The survival advantage was evident regardless of age, common comorbidities, socioeconomic status, lifestyle factors, physical performance, type and extent of surgery, tumor characteristics, and stage of disease.
Trial Registry
ClinicalTrials.gov; No.: NCT03567538; URL: www.clinicaltrials.gov
Key Words: epidemiology (pulmonary), lung cancer, sex, thoracic surgery
Abbreviations: ThoR, Swedish National Quality Register for General Thoracic Surgery; VATS, video-assisted thoracic surgery
FOR EDITORIAL COMMENT, SEE PAGE 1719
Differences in cancer incidence and survival between women and men are known, and the reasons for this are not understood fully.1, 2, 3, 4 In Sweden, women have been found to have a lower incidence and less excess mortality than men for most cancers that affect both sexes.3 Female sex has been suggested as a risk factor for lung cancer.5, 6, 7 By contrast, several reports exist of a female survival advantage in both surgical and nonsurgical cohorts,8, 9, 10, 11, 12, 13, 14 but the results are conflicting.15,16 In a recent Swedish nationwide cohort, women with non-small cell lung cancer consistently were found to have a better prognosis than men.11 Diagnostic and treatment intensity was analyzed, and no evidence of differences in clinical management was found. However, the female survival advantage was most pronounced in early-stage lung cancer, that is, patients who were more likely to have undergone surgical treatment. To investigate the association between female sex and better prognosis further, we performed a nationwide population-based study analyzing sex-specific survival after pulmonary resection for lung cancer. We identified the study population from the Swedish National Quality Register for General Thoracic Surgery (ThoR), which contains detailed information on patient characteristics and surgical procedures.
Methods
In this nationwide population-based observational cohort study, the reporting followed the Strengthening the Reporting of Observational Studies in Epidemiology and the Reporting of Studies Conducted Using Observational Routinely Collected Health Data guidelines for observational studies using routinely collected data.17,18 The study was approved by the Swedish Ethical Review Authority and the need for informed consent was waived (Identifier: 2017/1435-31).
Study Population
The ThoR register was used to identify the study population.19 The ThoR register was started in 2008 and contains detailed information on patient characteristics and surgical procedures for patients who have undergone general thoracic surgery in Sweden. From 2009 through 2011, approximately 50% of all patients who underwent thoracic surgery in Sweden were included. During 2011 and 2012, seven of eight hospitals reported to the register, and complete coverage of all eight thoracic surgery departments in Sweden was achieved in 2013.
Data Collection
The unique personal identity number that is assigned to all Swedish residents20 was used to link information from the ThoR register to other nationwide health-care registers. The record linkage was performed by the Swedish National Board of Health, and the study database subsequently was anonymized. Relevant information on previous medical history was retrieved from the National Patient Register.21 Information on educational level, household composition, and household disposable income was obtained from the Longitudinal Integration Database for Insurance and Labor Market Studies.22
Outcomes
The outcome measure was time to death from any cause. The Swedish Population Register was used to ascertain vital status and date of death.23
Definitions
Smoking was divided into four categories: never (never actively smoked), former (smoking cessation more than 1 month before surgery), current (active smoker or smoking cessation within 1 month of surgery), and unknown. Performance status was defined according to the Eastern Cooperative Oncology Group/World Health Organization.24 Information on previous or concurrent medical conditions was obtained from the ThoR register or the National Patient Register using International Classification of Diseases codes.21 The extent of surgery was classified into two categories: sublobar resection vs lobectomy, bilobectomy, or pneumectomy.
Statistical Analysis
Baseline characteristics were described with frequencies and percentages for categorical variables and means and SDs for continuous variables. Time to event was calculated as the time in days from the date of surgery to the date of death from any cause or end of follow-up (January 12, 2019). All variables reported in Table 1 were used in the estimation of propensity scores using generalized boosted regression modeling.25,26 We used the scores to develop weights for inverse probability of treatment weighting. We examined the distribution of weights and found no patients with extreme weights, and therefore, we decided that trimming was not necessary. Balance between the groups was assessed by standardized mean differences. An absolute standardized difference of ≤ 0.1 was considered an ideal balance.27 All subsequent analyses were conducted in the weighted sample. Cox proportional hazards regression was used to estimate hazard ratios and 95% CIs for the association between female sex and all-cause mortality, using male sex as the reference category. The Cox models was stratified by hospital and year of surgery. We constructed survival curves using the Kaplan-Meier method. We used flexible parametric survival models to obtain survival proportions at specified time points during follow-up together with absolute survival differences with 95% CI.28 We estimated the difference in restricted mean survival time in men and women. The restricted mean survival time is a robust measure that represents the mean event-free survival time in a prespecified period.29,30 The statistical analyses were performed with Stata version 16.1 software (StataCorp LP) and included the use of the stpm228 program and R version 3.6.3 software (R Foundation for Statistical Computing) and the twang26 package.
Table 1.
Baseline Characteristics of Patients Who Underwent Pulmonary Resections for Lung Cancer in Sweden From 2008 Through 2017 Before and After IPTW
| Variable | Unweighted |
IPTW |
||||
|---|---|---|---|---|---|---|
| Men | Women | SMD | Mena | Womena | SMD | |
| No. of patients | 2,865 | 3,671 | … | 5,991.69 | 6,164.69 | … |
| Age, y | 67.6 ± 9.2 | 66.8 ± 9.1 | 0.090 | 67.1 ± 9.2 | 67.1 ± 9.1 | 0.008 |
| BMI, kg/m2 | 26.2 ± 4.3 | 25.9 ± 5.3 | 0.070 | 26.0 ± 4.6 | 26.0 ± 4.9 | 0.003 |
| Household composition | … | … | 0.174 | … | … | 0.029 |
| 2 adults, no children | 1,350 (47.1) | 1,482 (40.4) | … | 2,629.5 (43.9) | 2,673.9 (43.4) | … |
| 1 adult, no children | 1,019 (35.6) | 1,615 (44.0) | … | 2,359.0 (39.4) | 2,505.4 (40.6) | … |
| 1-2 adults and ≥1 child(ren) | 496 (17.3) | 574 (15.6) | … | 1,003.2 (16.7) | 985.3 (16.0) | … |
| Education, y | … | … | 0.135 | … | … | 0.021 |
| < 10 | 1,089 (38.0) | 1,162 (31.7) | … | 2,109.8 (35.2) | 2,110.3 (34.2) | … |
| 10-12 | 1,222 (42.7) | 1,700 (46.3) | … | 2,644.1 (44.1) | 2,773.7 (45.0) | … |
| > 12 | 554 (19.3) | 809 (22.0) | … | 1,237.8 (20.7) | 1,280.7 (20.8) | … |
| Household disposable income, kSEK | 343 ± 347 | 327 ± 405 | 0.040 | 333 ± 296 | 331 ± 386 | 0.006 |
| Smoking status | … | … | 0.214 | … | … | 0.042 |
| Never smoker | 409 (14.3) | 814 (22.2) | … | 1,079.4 (18.0) | 1,198.6 (19.4) | … |
| Former smoker | 1,586 (55.4) | 1,774 (48.3) | … | 3,084.0 (51.5) | 3,134.4 (50.8) | … |
| Current smoker | 818 (28.6) | 1,001 (27.3) | … | 1,717.9 (28.7) | 1,705.8 (27.7) | … |
| Unknown | 52 (1.8) | 82 (2.2) | … | 110.4 (1.8) | 125.8 (2.0) | … |
| Alcohol dependency | 228 (8.0) | 132 (3.6) | 0.188 | 348.4 (5.8) | 298.5 (4.8) | 0.043 |
| Preoperative predicted FEV1 < 80% | 1,040 (41.4) | 1,195 (36.5) | 0.101 | 2,052.0 (38.8) | 2,088.1 (38.1) | 0.013 |
| Performance status | … | … | 0.151 | … | … | 0.027 |
| 0 | 1,643 (57.5) | 2,357 (64.3) | … | 3,627.0 (60.5) | 3,803.7 (61.7) | … |
| 1 | 1,131 (39.6) | 1,247 (34.0) | … | 2,215.6 (37.0) | 2,223.0 (36.1) | … |
| 2+ | 83 (2.9) | 61 (1.7) | … | 149.0 (2.5) | 137.9 (2.2) | … |
| Hypertension | 1,116 (39.0) | 1,225 (33.4) | 0.116 | 2,148.0 (35.9) | 2,230.1 (36.2) | 0.007 |
| Ischemic heart disease | 675 (23.6) | 408 (11.1) | 0.333 | 1,032.2 (17.2) | 944.4 (15.3) | 0.052 |
| Atrial fibrillation | 329 (11.5) | 210 (5.7) | 0.207 | 531.1 (8.9) | 476.6 (7.7) | 0.041 |
| Hyperlipidemia | 430 (15.0) | 372 (10.1) | 0.147 | 746.4 (12.5) | 725.6 (11.8) | 0.021 |
| Heart failure | 210 (7.3) | 128 (3.5) | 0.171 | 324.3 (5.4) | 293.9 (4.8) | 0.029 |
| COPD | 458 (16.0) | 648 (17.7) | 0.045 | 964.6 (16.1) | 1,046.6 (17.0) | 0.024 |
| Diabetes mellitus | 507 (17.7) | 374 (10.2) | 0.218 | 852.8 (14.2) | 770.6 (12.5) | 0.051 |
| Prior stroke/TIA | 305 (10.6) | 252 (6.9) | 0.134 | 535.6 (8.9) | 492.4 (8.0) | 0.034 |
| Peripheral vascular disease | 315 (11.0) | 169 (4.6) | 0.240 | 477.9 (8.0) | 408.5 (6.6) | 0.052 |
| Chronic kidney disease | 70 (2.4) | 59 (1.6) | 0.059 | 116.0 (1.9) | 133.9 (2.2) | 0.017 |
| Preoperative radiotherapy | 95 (3.4) | 90 (2.5) | 0.053 | 170.6 (2.9) | 153.2 (2.6) | 0.023 |
| Preoperative chemotherapy | 133 (4.8) | 140 (3.9) | 0.043 | 263.0 (4.5) | 259.1 (4.3) | 0.009 |
| Preoperative PET scanning | 2,353 (88.9) | 3,007 (88.1) | 0.024 | 4,895.4 (88.4) | 5,055.9 (88.5) | 0.004 |
| Lobectomy/pneumonectomy | 2,268 (79.2) | 2,803 (76.4) | 0.068 | 4,712.3 (78.6) | 4,777.4 (77.5) | 0.028 |
| VATS | 510 (17.8) | 907 (24.7) | 0.169 | 1,219.3 (20.3) | 1,360.3 (22.1) | 0.042 |
| Extended resectionb | 135 (4.7) | 126 (3.4) | 0.065 | 264.5 (4.4) | 241.3 (3.9) | 0.025 |
| Lymph node sampling | 2,329 (84.2) | 2,926 (82.2) | 0.052 | 4,828.7 (83.1) | 4,950.2 (82.9) | 0.006 |
| Microscopic residual disease | 173 (6.0) | 169 (4.6) | 0.064 | 322.3 (5.4) | 302.8 (4.9) | 0.021 |
| Postoperative histopathologic findings | … | … | 0.316 | … | … | 0.062 |
| Squamous cell carcinoma | 638 (22.3) | 457 (12.4) | … | 1053.5 (17.6) | 976.6 (15.8) | … |
| Adenocarcinoma | 1,441 (50.3) | 2,087 (56.9) | … | 3,185.5 (53.2) | 3,364.7 (54.6) | … |
| Carcinoid | 154 (5.4) | 383 (10.4) | … | 455.5 (7.6) | 533.0 (8.6) | … |
| Other | 335 (11.7) | 390 (10.6) | … | 695.4 (11.6) | 679.9 (11.0) | … |
| Unknown | 297 (10.4) | 354 (9.6) | … | 601.8 (10.0) | 610.5 (9.9) | … |
| Stage of diseasec | … | … | 0.206 | … | … | 0.042 |
| IA | 966 (33.7) | 1595 (43.4) | … | 2,271.0 (37.9) | 2,437.8 (39.5) | … |
| IB | 655 (22.9) | 705 (19.2) | … | 1,291.2 (21.5) | 1,281.7 (20.8) | … |
| IIA | 366 (12.8) | 396 (10.8) | … | 702.2 (11.7) | 722.8 (11.7) | … |
| IIB | 319 (11.1) | 315 (8.6) | … | 611.0 (10.2) | 575.5 (9.3) | … |
| IIIA | 377 (13.2) | 440 (12.0) | … | 755.3 (12.6) | 780.2 (12.7) | … |
| IIIB+ | 182 (6.4) | 220 (6.0) | … | 361.1 (6.0) | 366.5 (5.9) | … |
Data are presented as No. (%) or mean ± SD, unless otherwise indicated. IPTW = inverse probability of treatment weighting; kSEK = 1,000 Swedish Krona; SMD = standardized mean difference; TIA = transient ischemic attack; VATS = video-assisted thoracic surgery.
The overall numbers of patients in each group are not necessarily integers owing to inverse probability of treatment weighting.
If any structure other than the lung or lymph nodes was included in the resection (eg, pericardium, diaphragm, thoracic wall).
Pathologic stage.
Missing Data
Data were complete for most variables, including exposure and outcome, but the following variables had missing data: preoperative predicted FEV1 (11.4%), preoperative PET scanning (7.3%), BMI (6.2%), lymph node sampling (3.2%), preoperative radiotherapy (2.7%), and preoperative chemotherapy (2.7%). For variables with missing data, the weights were constructed also to balance rates of missingness in both groups.25,26
Results
The study population consisted of 6,536 patients (56% women, 44% men) who underwent pulmonary resection for lung cancer in Sweden from 2008 through 2017. The mean age was 67 years for women and 68 years for men. Women were more likely never to have smoked and less likely to have comorbidities (including alcohol dependency), with the exception of COPD. At the time of surgery, women were found to have a better functional status and greater pulmonary function than men, and women underwent minimally invasive and sublobar resections more often than men. Adenocarcinoma was more common in women and squamous cell carcinoma was found more often in men. Compared with men, women had a higher educational level, a lower income, and more often lived alone. Baseline characteristics according to sex before and after inverse probability of treatment weighting are presented in Table 1. The distribution of baseline characteristics was well balanced after inverse probability of treatment weighting, and the standardized mean difference was < 0.1 in all variables (Table 1, e-Fig 1).
Survival
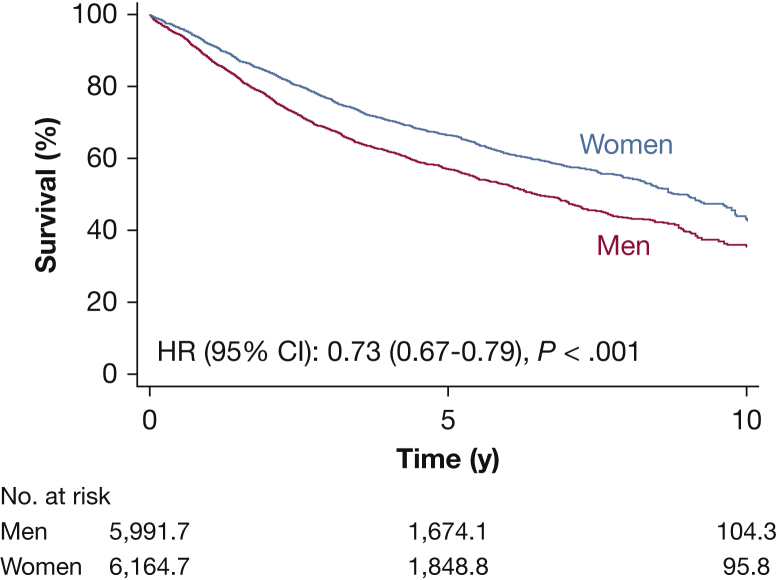
Women consistently were found to achieve better survival than men, and the survival gap increased over the years (Fig 1, Table 2). We observed a lower risk of death in women compared with men (hazard ratio, 0.73; 95% CI, 0.67-0.79). The absolute survival difference at 1, 5, and 10 years was 3.0% (95% CI, 2.2%-3.8%), 10% (95% CI, 7.0%-12%), and 12% (95% CI, 8.5%-15%), respectively. The restricted mean survival time at 10 years was 6.8 years (95% CI, 6.7-7.0 years) and 6.0 years (95% CI, 5.8-6.2 years) for women and men, respectively. The restricted mean survival time difference at 10 years was 0.84 year (95% CI, 0.61-1.07 years) (e-Fig 2). The overall survival was fairly stable over the study period, and the sex-specific 3-year survival according to year of surgery showed a consistent pattern of better survival for women compared with men (e-Fig 3). Early mortality, defined as death within 30 days of surgery, was 1.4% in men vs 0.7% in women (P = .010).
Figure 1.
Kaplan-Meier estimated survival curve plotted against time from surgery and stratified according to sex. Male patients are the reference group. The numbers of patients at risk are not necessarily integers owing to inverse probability of treatment weighting. HR = hazard ratio.
Table 2.
Survival According to Sex in Patients Who Underwent Pulmonary Resection for Lung Cancer in Sweden From 2008 Through 2017a
| Time From Surgery, y | Survival |
Survival Difference | |
|---|---|---|---|
| Men | Women | ||
| 1 | 89 (88-90) | 92 (91-92) | 3.0 (2.2-3.8) |
| 5 | 56 (54-58) | 66 (64-68) | 10 (7.0-12) |
| 10 | 36 (33-39) | 48 (45-50) | 12 (8.5-15) |
Data are presented as percentage (95% CI).
After inverse probability of treatment weighting.
Age, Histopathologic Findings, and Stage
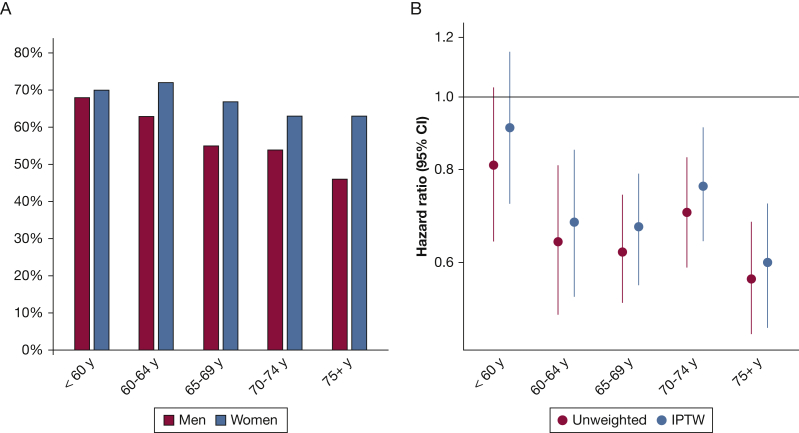
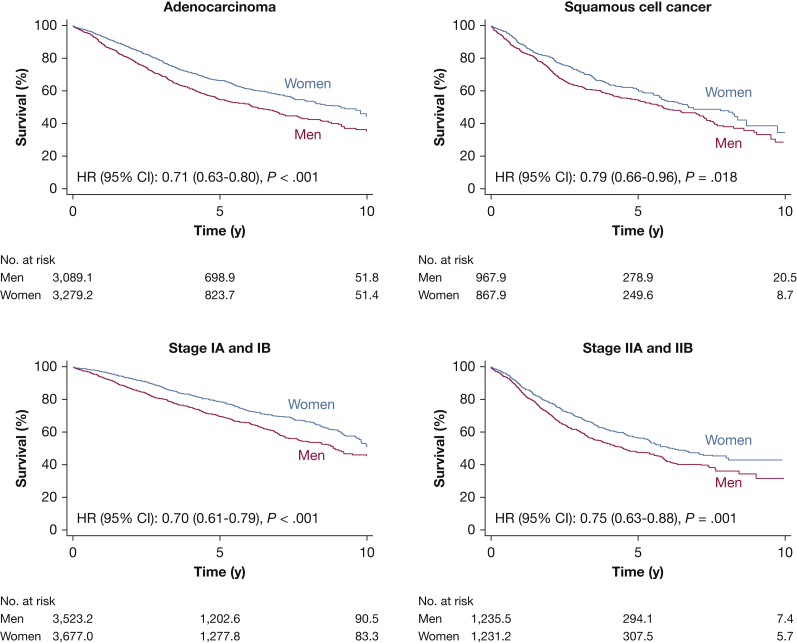
Separate analyses in subsets of patients according to age categories, histopathologic findings, and pathologic stage revealed the same pattern, with few exceptions. Women showed a better 5-year survival compared with men in all age categories, except in patients younger than 60 years (Fig 2, Table 3). Figure 3 shows that the female survival advantage was seen in patients with adenocarcinoma as well as in patients with squamous cell carcinoma, although the survival difference was less pronounced in patients with squamous cell carcinoma. Women with stage I-II disease showed better survival than men with an approximately 9% absolute survival difference at 5 years in both stages (Fig 3, Table 3).
Figure 2.
A, Bar graph showing survival at 5 years in age categories according to sex. B, Hazard ratios and 95% CIs for the association between female sex and all-cause mortality in the unweighted (red) and weighted (blue) population in different age categories. Male patients are the reference group. IPTW = inverse probability of treatment weighting.
Table 3.
Survival According to Sex in Different Subsets of Patients Who Underwent Pulmonary Resection for Lung Cancer in Sweden From 2008 Through 2017a
| Variable | 5-y Survival |
Survival Difference | |
|---|---|---|---|
| Men | Women | ||
| Age categories, y | |||
| <60 | 68 (64-73) | 70 (66-74) | 1.7 (−4.4 to 7.8) |
| 60-64 | 63 (58-68) | 72 (68-76) | 9.4 (3.2-16) |
| 65-69 | 55 (51-59) | 67 (64-71) | 12 (7.2-17) |
| 70-74 | 54 (50-58) | 63 (59-67) | 8.9 (3.3-14) |
| 75+ | 46 (42-52) | 63 (59-67) | 16 (10-23) |
| Histopathologic findings | |||
| Squamous cell carcinoma | 52 (48-56) | 59 (55-64) | 7.3 (1.1-13) |
| Adenocarcinoma | 55 (52-58) | 66 (64-69) | 11 (7.3-15) |
| Stage of disease | |||
| IA and IB | 70 (67-72) | 78 (76-80) | 8.6 (5.6-12) |
| IIA and IIB | 47 (43-51) | 56 (52-60) | 9.1 (3.5-15) |
Data are presented as percentage (95% CI).
After inverse probability of treatment weighting.
Figure 3.
Kaplan-Meier estimated survival curves plotted against time from surgery and stratified according to sex in different subsets of patients. A, Adenocarcinoma. B, Squamous cell carcinoma. C, Stage IA and IB disease. D, Stage IIA and IIB disease. The numbers of patients at risk are not necessarily integers owing to inverse probability of treatment weighting. HR = hazard ratio.
Discussion
In this nationwide population-based study of patients undergoing pulmonary resection for lung cancer, women consistently were found to have a better prognosis than men. The female survival advantage was independent of differences in baseline characteristics such as comorbidities, physical frailty, socioeconomic status, and tumor characteristics.
The female survival advantage was evident regardless of age, except in patients who were 60 years or younger, which has been reported previously.31 It was suggested that the lack of an evident survival difference in the younger age category may be explained by differences in life expectancy between women and men. By contrast, a Norwegian study, analyzing sex-specific long-term survival after lung cancer surgery and taking median expected lifetime into account, found that female sex was associated independently with better outcome.8 The reasons for the lack of a female survival advantage in the younger age category in our cohort remain uncertain. Factors such as more aggressive treatment regimens in younger patients and lifestyle choices, before and after an early diagnosis of lung cancer, may influence survival.32,33
Life expectancy is affected by smoking patterns in the general population, and tobacco smoking is one of the most important risk factors of disease burden and mortality.34, 35, 36 In the present cohort, women more often were never smokers, whereas men were more likely to be former smokers, which is in accord with previous reports.11,15,37 An evaluation of lung cancer risk in young Swedish women found first-hand and second-hand exposure to tobacco smoke to be the greatest risk factor also for nonsmokers when taking other risk factors such as lifestyle, environmental exposures, and personal and family medical history into account.38 A shorter lung cancer latency in younger women, compared with older women, also was reported. Since the 1980s, overall daily tobacco smoking incidence has decreased in Sweden, and in 2018, 7% of the Swedish population reported daily tobacco smoking, with no evident difference between the sexes.34 However, smoking rates have been higher in women than in men from the early 1990s. The gradual decline in smoking prevalence has been steeper in men than in women, especially among younger individuals. In a prospective evaluation of lung cancer risk and survival, women were found to have a prevalence OR of 1.9 (95% CI, 1.5-2.5) and a hazard ratio of fatal outcome of 0.48 (95% CI, 0.25-0.89) compared with men of equal age and exposure to tobacco smoke.7 The authors concluded that women might have an increased susceptibility to tobacco carcinogens, a notion supported by some39 and contradicted by others.40 Thus, differences in smoking prevalence, disease latency, and susceptibility to the harmful effects of tobacco smoke may be contributing explanatory factors for the lack of a female survival advantage in the younger patients.
A comparison of the contribution of smoking-related deaths to differences in life expectancy among the Nordic countries, using lung cancer mortality as proxy, has been conducted.41 The largest differences in life expectancy were seen between Denmark and Sweden and the smallest were between Norway and Sweden. Men had a 0.39-year shorter life expectancy (0.25 year [64%] attributable to smoking) and women had a 0.06-year shorter life expectancy (0.07 year [116%] attributable to smoking) in Norway compared with Sweden.
The average length of life in Sweden has increased more in men than in women, and from 1980 to 2019, the survival gap diminished from 6.1 to 3.4 years.42 Using lung cancer mortality as a proxy, the contribution of smoking-related deaths to the narrowing survival gap was estimated to be 0.6 year (38%) from 1997 through 2016.43 Thus, the survival gap diminished by 2.7 years in four decades,42 which is roughly a decrease of 0.68 year per decade. The contribution of smoking-related deaths to the narrowing survival gap could be estimated to be approximately 0.3 year per decade. Together with the finding that the female survival advantage is independent of life expectancy in Norway,8 and considering the small differences in life expectancy between Sweden and Norway,41 our finding of a restricted mean survival time difference at 10 years of 0.84 year (95% CI, 0.61-1.07 years) in favor of women may suggest a female survival advantage in patients undergoing surgery for lung cancer in Sweden that cannot be explained fully by differences in life expectancy and smoking patterns in the general population.
Socioeconomic disparities have been shown to influence lung cancer survival.44, 45, 46 Women in this cohort achieved a higher educational level than men, which has been linked to a better prognosis.46 However, women had lower incomes and more often lived alone, factors that have been associated with poorer survival.44,45 Socioeconomic status also has been linked to lifestyle behaviors such as alcohol consumption.47,48 In Sweden, excessive alcohol consumption has been found to be higher among those with an intermediate level of education as compared with those with the highest or the lowest educational levels.47 From 2006 through 2018, the prevalence of excessive alcohol consumption was higher in men than in women (20% vs 13%) when taking age, educational level, region, and country of birth into account. In the current cohort, men showed a higher prevalence of alcohol dependency than women. Moderate alcohol consumption has been associated with a modest decrease in lung cancer risk, whereas high alcohol consumption has been associated with an increased risk of lung cancer and lung adenocarcinoma.49 By contrast, squamous cell carcinoma was associated inversely with any level of alcohol drinking. Alcohol dependency also has been associated with an increased risk of postoperative complications in patients undergoing lung cancer surgery50,51; however, the results are conflicting regarding the effect on mortality.50, 51, 52, 53 Taken together, efforts aiming to reduce excessive alcohol consumption and to prevent dependency may have the potential to decrease morbidity and mortality in patients with lung cancer, particularly among men.
Cardiovascular disease has been associated with increased lung cancer risk and mortality.54, 55, 56, 57 By contrast, dyslipidemia has been associated with a lower risk of lung cancer,58 and the use of statins has been associated with reduced lung cancer risk and mortality.59,60 In the present cohort, both manifest cardiovascular disease and risk factors thereof were seen more often in men than in women. Thus, with the exception of a higher prevalence of hyperlipidemia and a slightly lower prevalence of COPD, men in the present cohort showed to a higher extent than women several factors that may affect survival in patients with lung cancer negatively.
TNM stage has been suggested to be the most important prognostic factor in non-small cell lung cancer.61,62 It also has been proposed that TNM staging should be sex specific because men seem to have a poorer prognosis than women within each stage.14 Similar to Radkiewicz et al,11 we found an absolute survival difference favoring women of 11%, 7.3%, 8.6%, and 9.1% for patients with adenocarcinoma, squamous cell carcinoma, stage I disease, and stage II disease, respectively.
It has been suggested that the female survival advantage may be attributed to factors other than a high prevalence of adenocarcinoma and early-stage disease among women.63 Watanabe et al63 analyzed postoperative recurrence patterns of non-small cell lung cancer and found that women show delayed times of peak recurrence compared with men. This was evident for stages IB through IIIB and was more pronounced in patients with squamous cell carcinoma. A sex-specific analysis of the risk of stroke after lung cancer diagnosis showed an increased risk of stroke within 1 year after diagnosis for men as compared with 2 years for women.64 Delayed postdiagnosis complications and postoperative tumor recurrence in women may have an influence on survival differences in patients with lung cancer.
It has been speculated that survival differences in lung cancer likely are explained by biological differences between women and men.11,63 Radkiewicz et al11 found no evidence of unequal treatment between women and men in patients with early-stage lung cancer in Sweden. However, the crude analysis of baseline characteristics in the present cohort support the notion that differences in clinical management exist31 and may contribute to sex-specific differences in survival in patients undergoing lung cancer surgery. Preoperative PET scanning, intraoperative lymph node sampling, and lobectomy were more common in men, whereas video-assisted thoracic surgery (VATS) was more common in women.
VATS for early-stage lung cancer has been associated with reduced postoperative pain and complication rates and improved recovery, quality of life, and long-term survival compared with open surgery.65, 66, 67 The adoption of VATS anatomic resections for lung cancer initially was gradual. In recent years, the technique has gained widespread acceptance within the thoracic community.68 The adoption of new surgical techniques entails a learning curve,69 and therefore, it is plausible that this period also includes patient selection. Hence, it is possible that patients with fewer comorbidities, better functional status, greater pulmonary function, and small tumors (ie, often women) to some extent were more likely to have been selected for VATS resection. Regarding extent of surgery and survival, lobectomy is still considered to be the standard surgical treatment for lung cancer. Anatomic segmentectomy is deemed superior to wedge resections and is considered acceptable for early types of adenocarcinoma.70 Women in the present cohort underwent segmentectomies slightly more often than men (4.6% vs 3.2%); however, segmentectomies constituted only 4% of the total number of operations. Thus, women undergoing VATS and segmentectomies to a greater extent than men theoretically could be contributory factors to better prognosis. The slightly less frequent use of preoperative PET scanning and intraoperative lymph node sampling in women could be interpreted as indicative of a greater rate of inadequate staging in women, possibly negatively influencing survival.70,71
Taken together, differences in clinical management of patients with lung cancer may exist, some potentially favoring survival in women and others favoring survival in men. Health-care decisions inadvertently may be influenced by sociocultural conceptions and norms, which might lead to unequal clinical management, as reviewed by Hay et al.72 Traditional norms within society influence priorities within health services, and inequalities in health care affect both sexes. For example, perceiving men as strong and in less need of care can lead to a lesser focus on men’s health, despite generally a higher health risk and shorter life expectancy. Norms related to masculinity have been associated with behavioral risks such as substance use and delayed health seeking. Valuing women based on their reproductive capacity in conjunction with the sometimes unrecognized higher risk of health burdens resulting from ageing can lead to worse care of women compared with men. Futures studies prospectively exploring possible sex-specific differences in lifestyle factors, sociocultural conditions, and clinical management of patients with lung cancer are needed. Educational efforts to increase awareness among health-care personnel of inequalities in health care may help to mitigate disparities in clinical management.
Strengths of this study include the nationwide population-based design and the use of national high-quality registers with high coverage and validity, minimizing the risk of selection and misclassification bias. The Swedish National Registers offer detailed prognostic information and complete and accurate follow-up. In addition, the ThoR register contains detailed individual-level information on baseline characteristics, including perioperative parameters. The lack of information on potential confounding factors such as smoking intensity, diet, physical activity, social support, as well as other unknown prognostic factors was an important limitation of our study. However, under the assumption that factors indicating a healthy lifestyle, as well as access to social support, may be more prevalent among women and may affect survival positively, it can be speculated that these limitations would not change the basic conclusions of the present study. Moreover, we did not have information on adjuvant treatment, cause of death, and when and to what extent implementation of VATS and enhanced recovery protocols took place during the study period. These are all factors that might have affected survival; however, because of the high concordance between our results and previous reports on lung cancer-specific survival in the Swedish population, in conjunction with the nationwide population-based study design, we believe our results to be robust.
Interpretation
Women who underwent pulmonary resection for lung cancer had significantly better prognosis than men. The survival advantage was evident regardless of age, common comorbidities, socioeconomic status, lifestyle factors, physical performance, type and extent of surgery, tumor characteristics, and stage of disease.
Acknowledgments
Author contributions: U. S. and V. J. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. E. S., U. S., and V. J. contributed substantially to the study design, data analysis and interpretation, and writing of the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the ThoR steering committee for providing data for this study.
Additional information: The e-Figures can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Sartipy and Jackson contributed equally to this manuscript.
FUNDING/SUPPORT: This work was supported by the Swedish Heart-Lung Foundation [Grants 20180400 and 20190533 to U. S.], the Åke Wiberg Foundation [Grant M18-0016 to U. S.], the Karolinska Institutet Foundations and Funds [Grant 2018-01530 (V. J.)], and Region Stockholm (ALF project) [Grant 20180114 to U. S.].
Supplementary Data
References
- 1.Afshar N., English D.R., Thursfield V. Differences in cancer survival by sex: a population-based study using cancer registry data. Cancer Causes Control. 2018 doi: 10.1007/s10552-018-1079-z. ;29(11):1059-1069. [DOI] [PubMed] [Google Scholar]
- 2.Micheli A., Ciampichini R., Oberaigner W. The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer. 2009;45(6):1017–1027. doi: 10.1016/j.ejca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Radkiewicz C., Johansson A.L.V., Dickman P.W., Lambe M., Edgren G. Sex differences in cancer risk and survival: a Swedish cohort study. Eur J Cancer. 2017;84:130–140. doi: 10.1016/j.ejca.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Edgren G., Liang L., Adami H.O., Chang E.T. Enigmatic sex disparities in cancer incidence. Eur J Epidemiol. 2012;27(3):187–196. doi: 10.1007/s10654-011-9647-5. [DOI] [PubMed] [Google Scholar]
- 5.Wakelee H.A., Wang W., Schiller J.H. Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J Thorac Oncol. 2006;1(5):441–446. [PubMed] [Google Scholar]
- 6.Zang E.A., Wynder E.L. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst. 1996;88(3-4):183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 7.International Early Lung Cancer Action Program. Henschke C.I., Yip R., Miettinen O.S. Women’s susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA. 2006;296(2):180–184. doi: 10.1001/jama.296.2.180. [DOI] [PubMed] [Google Scholar]
- 8.Batevik R., Grong K., Segadal L., Stangeland L. The female gender has a positive effect on survival independent of background life expectancy following surgical resection of primary non-small cell lung cancer: a study of absolute and relative survival over 15 years. Lung Cancer. 2005;47(2):173–181. doi: 10.1016/j.lungcan.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita F.L., Ito Y., Morishima T., Miyashiro I., Nakayama T. Sex differences in lung cancer survival: long-term trends using population-based cancer registry data in Osaka, Japan. Jpn J Clin Oncol. 2017;47(9):863–869. doi: 10.1093/jjco/hyx094. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura H., Ando K., Shinmyo T. Female gender is an independent prognostic factor in non-small-cell lung cancer: a meta-analysis. Ann Thorac Cardiovasc Surg. 2011;17(5):469–480. doi: 10.5761/atcs.oa.10.01637. [DOI] [PubMed] [Google Scholar]
- 11.Radkiewicz C., Dickman P.W., Johansson A.L.V., Wagenius G., Edgren G., Lambe M. Sex and survival in non-small cell lung cancer: a nationwide cohort study. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0219206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagerup C.M., Smastuen M., Johannesen T.B., Helland A., Brustugun O.T. Sex-specific trends in lung cancer incidence and survival: a population study of 40,118 cases. Thorax. 2011;66(4):301–307. doi: 10.1136/thx.2010.151621. [DOI] [PubMed] [Google Scholar]
- 13.Visbal A.L., Williams B.A., Nichols F.C., III Gender differences in non-small-cell lung cancer survival: an analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg. 2004;78(1):209–215. doi: 10.1016/j.athoracsur.2003.11.021. discussion 215. [DOI] [PubMed] [Google Scholar]
- 14.Wainer Z., Wright G.M., Gough K. Sex-dependent staging in non-small-cell lung cancer; analysis of the effect of sex differences in the eighth edition of the tumor, node, metastases staging system. Clin Lung Cancer. 2018;19(6):e933–e944. doi: 10.1016/j.cllc.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Bugge A., Kongerud J., Brunborg C., Solberg S., Lund M.B. Gender-specific survival after surgical resection for early stage non-small cell lung cancer. Acta Oncol. 2017;56(3):448–454. doi: 10.1080/0284186X.2016.1253862. [DOI] [PubMed] [Google Scholar]
- 16.Jubelirer S.J., Varela N.L., Welch C.A., Emmett M.K. Does sex make a difference in survival of patients undergoing resection for early stage non-small cell lung cancer (NSCLC)? W V Med J. 2009;105(4):18–22. [PubMed] [Google Scholar]
- 17.Benchimol E.I., Smeeth L., Guttmann A. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10) doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E., Altman D.G., Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 19.ThoR The Swedish National Quality Register for General Thoracic Surgery. Uppsala Clinical Research Center website. https://www.ucr.uu.se/thor/
- 20.Ludvigsson J.F., Otterblad-Olausson P., Pettersson B.U., Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludvigsson J.F., Andersson E., Ekbom A. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludvigsson J.F., Svedberg P., Olen O., Bruze G., Neovius M. The Longitudinal Integrated Database for Health Insurance and Labour Market Studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34(4):423–437. doi: 10.1007/s10654-019-00511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludvigsson J.F., Almqvist C., Bonamy A.K. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–136. doi: 10.1007/s10654-016-0117-y. [DOI] [PubMed] [Google Scholar]
- 24.Oken M.M., Creech R.H., Tormey D.C. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 25.Griffin B.A., Ridgeway G., Morral A. Toolkit for Weighting and Analysis of Nonequivalent Groups (TWANG). Rand Corporation website. 2014. http://www.rand.org/statistics/twang Accessed June 27, 2020.
- 26.Ridgeway G., McCaffrey D., Morral A., Griffin B.A., Burgette L. twang: Toolkit for Weighting and Analysis of Nonequivalent Groups. 2017. The Comprehensive R Archive Network website. https://CRAN.R-project.org/package=twang Accessed June 27, 2020.
- 27.Austin P.C., Stuart E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert P.C., Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9(2):265–290. [Google Scholar]
- 29.Kim D.H., Uno H., Wei L.J. Restricted mean survival time as a measure to interpret clinical trial results. JAMA Cardiol. 2017;2(11):1179–1180. doi: 10.1001/jamacardio.2017.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Royston P., Parmar M.K. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152. doi: 10.1186/1471-2288-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minami H., Yoshimura M., Miyamoto Y., Matsuoka H., Tsubota N. Lung cancer in women: sex-associated differences in survival of patients undergoing resection for lung cancer. Chest. 2000;118(6):1603–1609. doi: 10.1378/chest.118.6.1603. [DOI] [PubMed] [Google Scholar]
- 32.Arnold B.N., Thomas D.C., Rosen J.E. Lung cancer in the very young: treatment and survival in the National Cancer Data Base. J Thorac Oncol. 2016;11(7):1121–1131. doi: 10.1016/j.jtho.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y.B., Pan X.F., Chen J. Combined lifestyle factors, incident cancer, and cancer mortality: a systematic review and meta-analysis of prospective cohort studies. Br J Cancer. 2020;122(7):1085–1093. doi: 10.1038/s41416-020-0741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Public Health Agency of Sweden Public health development. Public Health Agency of Sweden website. https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/tolkad-rapportering/folkhalsans-utveckling/levnadsvanor/tobaksrokning-daglig/
- 35.The Institute for Health Metrics and Evaluation (IHME) Country profiles—Sweden 2017. Institute for Health Metrics and Evaluation website. www.healthdata.org/sweden Accessed June 27, 2020.
- 36.Global Burden of Disease Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida Y., Murayama T., Sato Y., Suzuki Y., Saito H., Nomura Y. Gender differences in long-term survival after surgery for non-small cell lung cancer. Thorac Cardiovasc Surg. 2016;64(6):507–514. doi: 10.1055/s-0035-1558995. [DOI] [PubMed] [Google Scholar]
- 38.Fritz I., Olsson H. Lung cancer in young women in southern Sweden: a descriptive study. Clin Respir J. 2018;12(4):1565–1571. doi: 10.1111/crj.12712. [DOI] [PubMed] [Google Scholar]
- 39.Powell H.A., Iyen-Omofoman B., Hubbard R.B., Baldwin D.R., Tata L.J. The association between smoking quantity and lung cancer in men and women. Chest. 2013;143(1):123–129. doi: 10.1378/chest.12-1068. [DOI] [PubMed] [Google Scholar]
- 40.Yu Y., Liu H., Zheng S. Gender susceptibility for cigarette smoking-attributable lung cancer: a systematic review and meta-analysis. Lung Cancer. 2014;85(3):351–360. doi: 10.1016/j.lungcan.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Ostergren O., Martikainen P., Tarkiainen L., Elstad J.I., Bronnum-Hansen H. Contribution of smoking and alcohol consumption to income differences in life expectancy: evidence using Danish, Finnish, Norwegian and Swedish register data. J Epidemiol Community Health. 2019;73(4):334–339. doi: 10.1136/jech-2018-211640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Statistics Sweden Average length of life. Statistics Sweden website. https://www.scb.se/hitta-statistik/sverige-i-siffror/manniskorna-i-sverige/medellivslangd-i-sverige/
- 43.Ostergren O., Martikainen P. The contribution of smoking-related deaths to the gender gap in life expectancy in Sweden between 1997 and 2016. Scand J Public Health. 2020;48(3):346–349. doi: 10.1177/1403494819848278. [DOI] [PubMed] [Google Scholar]
- 44.Dalton S.O., Steding-Jessen M., Jakobsen E. Socioeconomic position and survival after lung cancer: influence of stage, treatment and comorbidity among Danish patients with lung cancer diagnosed in 2004-2010. Acta Oncol. 2015;54(5):797–804. doi: 10.3109/0284186X.2014.1001037. [DOI] [PubMed] [Google Scholar]
- 45.Sachs E., Jackson V., Sartipy U. Household disposable income and long-term survival after pulmonary resections for lung cancer. Thorax. 2020 doi: 10.1136/thoraxjnl-2019-214321. ;75(9):764-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willen L., Berglund A., Bergstrom S. Educational level and management and outcomes in non-small cell lung cancer. A nationwide population-based study. Lung Cancer. 2019;131:40–46. doi: 10.1016/j.lungcan.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Public Health Agency of Sweden Public health development. Public Health Agency of Sweden website. https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/tolkad-rapportering/folkhalsans-utveckling/resultat/levnadsvanor/alkohol-riskkonsumtion/ Accessed June 27, 2020.
- 48.Beard E., Brown J., West R., Kaner E., Meier P., Michie S. Associations between socio-economic factors and alcohol consumption: a population survey of adults in England. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0209442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Troche J.R., Mayne S.T., Freedman N.D., Shebl F.M., Abnet C.C. The association between alcohol consumption and lung carcinoma by histological subtype. Am J Epidemiol. 2016;183(2):110–121. doi: 10.1093/aje/kwv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paull D.E., Updyke G.M., Davis C.A., Adebonojo S.A. Complications and long-term survival for alcoholic patients with resectable lung cancer. Am J Surg. 2004;188(5):553–559. doi: 10.1016/j.amjsurg.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 51.Graf S.A., Zeliadt S.B., Rise P.J., Backhus L.M., Zhou X.H., Williams E.C. Unhealthy alcohol use is associated with postoperative complications in veterans undergoing lung resection. J Thorac Dis. 2018;10(3):1648–1656. doi: 10.21037/jtd.2018.02.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neuenschwander A.U., Pedersen J.H., Krasnik M., Tonnesen H. Impaired postoperative outcome in chronic alcohol abusers after curative resection for lung cancer. Eur J Cardiothorac Surg. 2002;22(2):287–291. doi: 10.1016/s1010-7940(02)00263-4. [DOI] [PubMed] [Google Scholar]
- 53.Green A., Hauge J., Iachina M., Jakobsen E. The mortality after surgery in primary lung cancer: results from the Danish Lung Cancer Registry. Eur J Cardiothorac Surg. 2016;49(2):589–594. doi: 10.1093/ejcts/ezv107. [DOI] [PubMed] [Google Scholar]
- 54.Armenian S.H., Xu L., Ky B. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol. 2016;34(10):1122–1130. doi: 10.1200/JCO.2015.64.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X., Hemminki K., Forsti A., Sundquist K., Sundquist J., Ji J. Cancer risk in patients with type 2 diabetes mellitus and their relatives. Int J Cancer. 2015;137(4):903–910. doi: 10.1002/ijc.29440. [DOI] [PubMed] [Google Scholar]
- 56.Strongman H., Gadd S., Matthews A. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394(10203):1041–1054. doi: 10.1016/S0140-6736(19)31674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zoller B., Ji J., Sundquist J., Sundquist K. Risk of coronary heart disease in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. 2012;48(1):121–128. doi: 10.1016/j.ejca.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 58.Tseng C.H. Diabetes but not insulin increases the risk of lung cancer: a Taiwanese population-based study. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang W.Y., Li C.H., Lin C.L., Liang J.A. Long-term statin use in patients with lung cancer and dyslipidemia reduces the risk of death. Oncotarget. 2016;7(27):42208–42215. doi: 10.18632/oncotarget.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon Y.J., You N.Y., Lee J.W., Kim J., Kang H.T. High receipt of statins reduces the risk of lung cancer in current smokers with hypercholesterolemia: the National Health Insurance Service-Health Screening Cohort. Clin Lung Cancer. 2019;20(2):e177–e185. doi: 10.1016/j.cllc.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 61.Woodard G.A., Jones K.D., Jablons D.M. Lung cancer staging and prognosis. Cancer Treat Res. 2016;170:47–75. doi: 10.1007/978-3-319-40389-2_3. [DOI] [PubMed] [Google Scholar]
- 62.Chansky K., Sculier J.P., Crowley J.J. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol. 2009;4(7):792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe K., Sakamaki K., Nishii T. Gender differences in the recurrence timing of patients undergoing resection for non-small cell lung cancer. Asian Pac J Cancer Prev. 2018;19(3):719–724. doi: 10.22034/APJCP.2018.19.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen P.C., Muo C.H., Lee Y.T., Yu Y.H., Sung F.C. Lung cancer and incidence of stroke: a population-based cohort study. Stroke. 2011;42(11):3034–3039. doi: 10.1161/STROKEAHA.111.615534. [DOI] [PubMed] [Google Scholar]
- 65.Al-Ameri M., Bergman P., Franco-Cereceda A., Sartipy U. Video-assisted thoracoscopic versus open thoracotomy lobectomy: a Swedish nationwide cohort study. J Thorac Dis. 2018;10(6):3499–3506. doi: 10.21037/jtd.2018.05.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bendixen M., Jorgensen O.D., Kronborg C., Andersen C., Licht P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17(6):836–844. doi: 10.1016/S1470-2045(16)00173-X. [DOI] [PubMed] [Google Scholar]
- 67.Dziedzic R., Marjanski T., Binczyk F., Polanska J., Sawicka W., Rzyman W. Favourable outcomes in patients with early-stage non-small-cell lung cancer operated on by video-assisted thoracoscopic surgery: a propensity score-matched analysis. Eur J Cardiothorac Surg. 2018;54(3):547–553. doi: 10.1093/ejcts/ezy101. [DOI] [PubMed] [Google Scholar]
- 68.Cao C., Frick A.E., Ilonen I. European questionnaire on the clinical use of video-assisted thoracoscopic surgery. Interact Cardiovasc Thorac Surg. 2018;27(3):379–383. doi: 10.1093/icvts/ivy062. [DOI] [PubMed] [Google Scholar]
- 69.Li X., Wang J., Ferguson M.K. Competence versus mastery: the time course for developing proficiency in video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg. 2014;147(4):1150–1154. doi: 10.1016/j.jtcvs.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 70.Postmus P.E., Kerr K.M., Oudkerk M. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 71.Pawelczyk K., Blasiak P., Szromek M., Nowinska K., Marciniak M. Assessment of adequacy of intraoperative nodal staging and factors influencing the lack of its compliance with recommendations in the surgical treatment of non-small cell lung cancer (NSCLC) J Thorac Dis. 2018;10(8):4902–4911. doi: 10.21037/jtd.2018.07.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hay K., McDougal L., Percival V. Disrupting gender norms in health systems: making the case for change. Lancet. 2019;393(10190):2535–2549. doi: 10.1016/S0140-6736(19)30648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.