Abstract
The clinical performance of saliva compared with nasopharyngeal swabs (NPSs) has shown conflicting results in healthcare and community settings. In the present study, a total of 429 matched NPS and saliva sample pairs, collected in either healthcare or community setting, were evaluated. Phase-1 (protocol U) tested 240 matched NPS and saliva sample pairs; phase 2 (SalivaAll protocol) tested 189 matched NPS and saliva sample pairs, with an additional sample homogenization step before RNA extraction. A total of 85 saliva samples were evaluated with both protocols. In phase-1, 28.3% (68/240) samples tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from saliva, NPS, or both. The detection rate from saliva was lower compared with that from NPS samples (50.0% versus 89.7%). In phase-2, 50.2% (95/189) samples tested positive for SARS-CoV-2 from saliva, NPS, or both. The detection rate from saliva was higher compared with that from NPS samples (97.8% versus 78.9%). Of the 85 saliva samples evaluated with both protocols, the detection rate was 100% for samples tested with SalivaAll, and 36.7% with protocol U. The limit of detection with SalivaAll protocol was 20 to 60 copies/mL. The pooled testing approach demonstrated a 95% positive and 100% negative percentage agreement. This protocol for saliva samples results in higher sensitivity compared with NPS samples and breaks the barrier to using pooled saliva for SARS-CoV-2 testing.
The outbreak of coronavirus disease 2019 [COVID-19; caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] is an ongoing pandemic that has caused substantial social, economic, and public health strain. Since its identification in the region of Wuhan, China, 105,962,538 confirmed cases with >2,313,136 COVID-19–related deaths have been reported globally (https://coronavirus.jhu.edu/map.html, last accessed February 7, 2021). Testing, coupled with measures such as social distancing, masking, maintaining personal hygiene, contact tracing, quarantine, travel restrictions, and lockdowns, have hitherto been the most widely adopted strategies to contain this pandemic.1 Regional policies have been primarily dictated by the positivity rate in the respective region(s). Thus, rapid and accurate detection of SARS-CoV-2 is the foremost and likely most essential component in controlling this outbreak and has immediate clinical, epidemiologic, and policy implications. Various clinical specimens such as bronchoalveolar lavage, sputum, saliva, nasopharyngeal swabs (NPSs), oropharyngeal swabs (OPSs), feces, and blood have been evaluated for detection of SARS-CoV-2 virus.2 NPS and OPS samples are the current standard upper respiratory tract specimens recommended for COVID-19 diagnostic testing. However, the collection of NPS samples poses challenges such as exposure risk to healthcare workers, supply chain constraints pertaining to swabs and personal protective equipment, and difficulties with self-collection. Inappropriate sampling may lead to false-negative results.3, 4, 5 Amidst the several other sample types under investigation for COVID-19 testing, saliva samples are of significant interest owing to their ease of collection, and ability to alleviate some of the challenges associated with NPS sampling. True saliva is defined as the naturally collecting clear liquid that accumulates in the mouth. However, saliva from patients can be confounded with the presence of mucus or blood, thereby rendering it difficult to process in the laboratory. Several reports evaluating the clinical performance of saliva compared with NPS/OPS samples have demonstrated conflicting results. In a healthcare setting, studies have demonstrated comparable6, 7, 8, 9 and even higher sensitivity of saliva10/early morning saliva collection11 compared with NPS samples, as well as reported higher viral titer values in saliva.9 Conversely, deep-throat saliva12 and typical saliva samples have also been demonstrated to be less sensitive compared with NPS samples in both healthcare13 and community settings.14 Furthermore, saliva samples are difficult to pipet by the testing personnel, which leads to increased processing time.15
Although different collection devices, media, sample handling, extraction procedure, and real time PCR methods may have accounted for these discrepancies, a critical facet of the saliva samples was investigated that, if accounted for, renders the saliva samples more sensitive than NPS samples. In this analysis, the saliva samples initially demonstrated a lower sensitivity compared with NPS samples. When a simple processing step using the bead mill homogenizer was introduced, the saliva samples showed higher sensitivity compared with NPS samples. Not only did the saliva processing become significantly easier after using the homogenizer, but the saliva samples could also be validated with a five-sample pooling strategy. The SalivaAll protocol is sensitive and detects up to 20 copies/mL, facilitating utility in saliva sample pooled testing, which is critical in SARS-CoV-2 mass surveillance. This single-site study identified both increased and decreased sensitivity of saliva as a diagnostic sample type, consistent with the discrepancies reported in the literature, that were largely believed to be the result of processing challenges. We contend that with appropriate management of these processing challenges, saliva samples are more sensitive compared with the NPS samples.
Materials and Methods
Study Site and Ethics
This single-center diagnostic study was conducted at the Augusta University (Augusta, GA). This site is a Clinical Laboratory Improvement Amendments–accredited laboratory for high-complexity testing and is one of the main SARS-CoV-2 testing centers in the state of Georgia.
Patient Specimens and Setting
The study evaluated 429 matched NPS and saliva samples (sample pairs) collected from 344 individuals in either a healthcare or a community setting. Of the 344 individuals, 95 matched clinical specimen pairs were collected in healthcare setting from individuals at either a medical nursing home or Augusta University Medical Center, both in Georgia. In the community setting, 249 matched clinical specimen pairs were collected from drive-through collection centers in different regions of Georgia that include Augusta, Albany, and Atlanta. As a standard protocol in both settings, NPSs from individuals were collected by a healthcare worker using a sterile flocked swab placed in a sterile tube containing 3 mL of viral transport medium (Specimen Collection and Transport System BD Sterile, catalog number 220531; Becton Dickinson, Franklin Lakes, NJ). Before collecting the NPS samples, the individuals were instructed to provide saliva samples by spitting into a sterile container (DNA/RNA Shield Saliva Sputum Collection Kit – DX, catalog number R1210-E; Zymo Research, Irvine, CA) over which the healthcare worker added 2 mL of viral transport medium. All samples were stored at 4°C temperature and transported to the SARS-CoV-2 testing facility at Augusta University within 24 hours of sample collection for further processing.
Assay for the Detection of SARS-CoV-2
The assay is based on nucleic acid extraction followed by TaqMan-based real time PCR assay to conduct in vitro transcription of SARS-CoV-2 RNA, DNA amplification, and fluorescence detection [Food and Drug Administration (FDA) Emergency Use Authorization assay by PerkinElmer Inc., Waltham, MA]. The assay targets specific genomic regions of SARS-CoV-2: nucleocapsid (N) gene and ORF1ab. The TaqMan probes for the two amplicons are labeled with FAM and ROX fluorescent dyes, respectively, to generate target-specific signals. The assay includes an RNA internal control (IC; bacteriophage MS2) to monitor the processes from nucleic acid extraction to fluorescence detection. The IC probe is labeled with VIC fluorescent dye to differentiate its fluorescent signal from SARS-CoV-2 targets. The samples were resulted as positive or negative based on the cycle threshold (Ct) values specified by the manufacturer (Supplemental Table S1).
Phase 1 Study and Sample Processing (Protocol U)
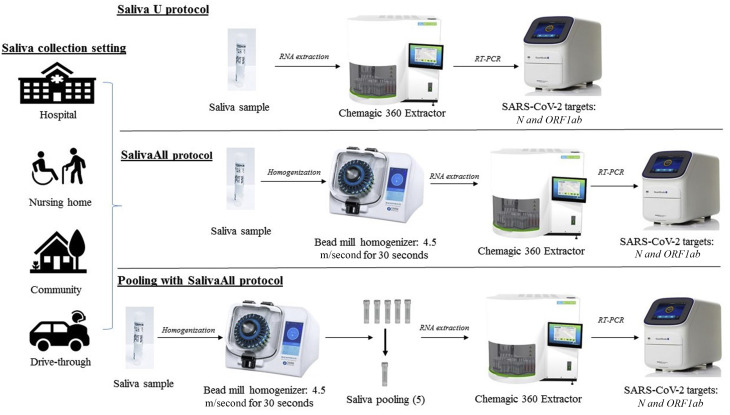
In phase 1 of this study, 240 matched NPS and saliva sample pairs were tested prospectively for SARS-CoV-2 RNA by real time PCR. Of the 240 samples, 95 were collected in a healthcare setting and 145 were collected in a community setting. In brief, all samples were vortexed, and an aliquot of 300 μL from each sample (NPS or saliva), positive and negative controls, was added to respective wells in a 96-well plate. To each well, 5 μL IC, 4 μL poly(A) RNA, 10 μL proteinase K, and 300 μL lysis buffer 1 were added. The plate was placed on a semi-automated instrument (Chemagic 360 Instrument; PerkinElmer Inc.) following the manufacturer's protocol. The nucleic acid was extracted in a 96-well plate, with an elution volume of 60 μL. From the extraction plate, 10 μL of extracted nucleic acid and 5 μL of PCR master mix were added to the respective wells in a 96-well PCR plate. The PCR method was set up as per the manufacturer's protocol on Quantstudio 3 or 5 (Thermo Fisher Scientific, Waltham, MA). The samples were classified as positive or negative, depending on the Ct values specified by the manufacturer (Figure 1 ).
Figure 1.
Schematic overview of sample processing and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) assay workflow, depicting main steps. Matched nasopharyngeal swab (NPS) and saliva sample pairs collected in healthcare and community setting were tested and validated as follows. Top row: NPS or saliva samples were processed with protocol U for nucleic acid extraction using a semi-automated instrument, followed by real time PCR for N and ORF1ab gene targets and internal control (IC) used as extraction and real time PCR IC. Middle row: Saliva samples were processed with SalivaAll protocol that included a saliva homogenization step using a bead mill homogenizer before RNA extraction and downstream processing. Bottom row: Saliva samples were homogenized using a bead mill homogenizer (SalivaAll protocol) before pooling samples with a five-sample pooling strategy for SARS-CoV-2 testing.
Phase 2 Study and Sample Processing (SalivaAll)
In phase 2 of this study, 189 matched NPS and saliva sample pairs were tested for SARS-CoV-2. Of the 189 samples, 40 were collected in healthcare and 149 were collected in a community setting. More importantly, 85 samples previously evaluated with protocol U were re-evaluated with SalivaAll protocol to determine the effect of bead homogenization. In SalivaAll protocol, an additional processing step was added for saliva samples that included aliquoting saliva samples from the collection tubes into Omni tubes (2 mL reinforced tubes, SKU 19-628D; Omni International, Kennesaw, GA), which were then placed in Omni bead mill homogenizer (Bead Ruptor Elite, SKU: 19-040E; Omni International). The samples were homogenized at 4.5 m/second for 30 seconds. From the Omni tubes, an aliquot of 300 μL homogenized saliva samples was added to 96-well plate and downstream processing was performed as described in protocol U. The NPS sample processing remained the same as in protocol U (Figure 1). However, an additional study was done with 189 NPS samples with both protocol U and SalivaAll protocol to determine if bead homogenization would affect the clinical sensitivity in NPS samples.
Studies
The limit of detection (LoD) studies were conducted as per the FDA guidelines (https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas, last accessed September 9, 2020) using two different reference materials [FDA Emergency Use Authorization assay by PerkinElmer Inc. and AccuPlex SARS-CoV-2 Molecular Controls Kit - Full Genome, Material number 0505-0159 (SeraCare, Milford, MA)]. Briefly, SARS-CoV-2 reference control materials were spiked into the negative saliva samples to serve as positive samples at 180, 60, and 20 copies/mL concentrations, and were processed with SalivaAll protocol. The lowest concentration detected in all three triplicates was determined as the preliminary LoD. To confirm the LoD, 20 replicates of preliminary LoD were analyzed and deemed as confirmed if at least 19 of 20 replicates were detected. The PerkinElmer Inc. and SeraCare reference control materials consisted of encapsulated synthetic RNA with concentration of 1000 and 5000 copies/mL, respectively.
Pooling Saliva Samples for Mass Population Screening
A five-sample pooling strategy was evaluated as per FDA guidelines. Briefly, 20 previously confirmed positive saliva samples were identified to generate 20 positive pools, each comprising of one positive and four negative samples. The Ct values of positive samples ranged from N of 9.8 and ORF1ab of 17.3 to N of 38.4 and ORF1ab of undetermined, with 25% of positive samples having high Ct values (32.0 to 38.4). Similarly, 20 negative sample pools were generated, composed of five negative samples. All saliva samples were first processed with Omni bead mill homogenizer, as described in SalivaAll protocol, before pooling for SARS-CoV-2 real time PCR testing (Figure 1). Of note, each pool was generated by aliquoting 60 μL from each of the five homogenized samples to make 300 μL for extraction.
Data Analysis
Data were analyzed for descriptive statistics and presented as number (percentage) for categorical variables and means ± SD for continuous variables. Ct values were compared using paired t-test. Regression analysis with slope and intercept along with a 95% CI was determined in the pooling sample study.
Results
Comparison of SARS-CoV-2 Detection In Saliva and NPS [Phase 1 Study: Protocol U(unprocessed)]
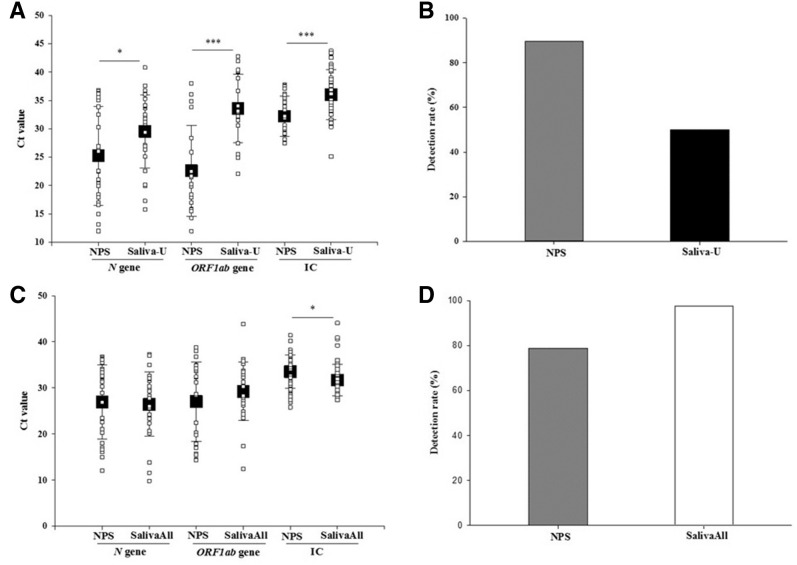
Of the 240 matched sample pairs, 28.3% (68/240) tested positive for SARS-CoV-2 from saliva, NPS, or both. The detection rate for SARS-CoV-2 was significantly higher in NPS compared with saliva testing [89.7% (61/68) versus 50.0% (34/68); P < 0.001]. The concordance for positive results between the two tests was only 39.7% (virus detected in both saliva and NPS in 27/68). Of the 68 positive samples, 50% (34/68) were positive in NPS but not in saliva, whereas only 10.2% (7/68) were positive in saliva but not in NPS. The Ct values for N (25.2 ± 8.7 versus 29.5 ± 6.4; P < 0.05), ORF1ab (22.6 ± 7.9 versus 33.5 ± 6.0; P < 0.001), and IC (32.2 ± 3.5 versus 36.0 ± 4.4; P < 0.001) were significantly lower in NPS compared with saliva samples, respectively (Figure 2, A and B). Overall, IC (extraction and real time PCR control) was detected in 95% (228/240) NPS samples, and 80.4% (193/240) saliva samples.
Figure 2.
A: Boxplots of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Ct value of both N and ORF1ab genes of all the positive specimens in phase 1 study. The Ct value of both genes was lower in the nasopharyngeal swab (NPS) than saliva samples. Also included Ct values of internal control (IC) of all the samples in both specimens. B: Bar graph depicting detection rate with NPS and saliva samples in phase 1 study. C: Boxplots of SARS-CoV-2 Ct value of both N and ORF1ab genes of all the positive specimens in phase 2 study. The Ct value of both genes was lower in the saliva than NPS samples. Also included Ct values of IC of all the samples in both specimens. D: Bar graph depicting detection rate with NPS and saliva samples in phase 2 study. Data are given as means ± SD (A and C). ∗P < 0.05, ∗∗∗P < 0.001. Saliva-U, unprocessed saliva samples.
Comparison of SARS-CoV-2 Detection between Saliva and NPS (Phase 2 Study: SalivaAll)
Of the 189 matched sample pairs tested in phase 2, 50.2% (95/189) tested positive for SARS-CoV-2 from saliva, NPS, or both. The detection rate for SARS-CoV-2 was significantly higher in saliva compared with that in NPS [97.8% (93/95) versus 78.9% (75/95); P < 0.001]. The concordance for positive results between the two tests was 76.8% (virus detected in both saliva and NPS in 73/95). Of the 95 positive samples, 21.0% (20/95) were positive in saliva but not in NPS, whereas only 2.1% (2/95) were positive in NPS but not in saliva. The Ct values for N (26.9 ± 8.0 versus 26.4 ± 6.9) and ORF1ab (27.0 ± 8.6 versus 29.2 ± 6.3) were comparable, whereas the IC values (33.4 ± 3.6 versus 31.6 ± 3.4; P < 0.05) were significantly higher in NPS compared with saliva samples, respectively (Figure 2, C and D). Overall, IC (extraction and real time PCR control) was detected in all NPS and saliva samples, except in one saliva sample. The results were comparable and no difference was observed in clinical sensitivity in the additional study that processed 189 NPS samples via protocol U and the bead mill homogenization (SalivaAll protocol).
Comparison of SARS-CoV-2 Detection in Saliva Samples (Protocol U versus SalivaAll)
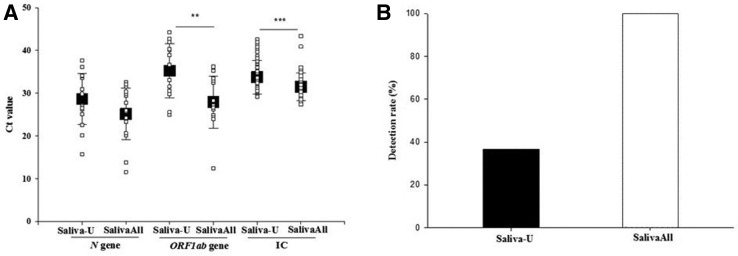
Eighty-five saliva samples, of which 47% (40/85) were positive, were tested with both protocols. Of these, 57.6% (49/85) tested positive for SARS-CoV-2 with protocol U, SalivaAll, or both. At 100% (49/49), the detection rate was significantly higher in samples tested with SalivaAll compared with 36.7% (18/49) tested with protocol U (P < 0.001). The concordance for positive results between the two protocols was 36.7% (18/49). The Ct values for N (25.1 ± 6.0 versus 28.6 ± 5.9) were lower, whereas at (27.9 ± 6.0 versus 35.2 ± 6.3; P < 0.01) and IC (31.2 ± 2.7 versus 33.7 ± 3.9; P < 0.001), ORF1ab values were significantly lower with SalivaAll compared with protocol U, respectively (Figure 3 ).
Figure 3.
A: Boxplots of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Ct value of both N and ORF1ab genes of all the positive saliva specimens detected with protocol U and SalivaAll. Ct value of both genes was lower with SalivaAll compared with protocol U. Also included Ct values of internal control (IC) of all the samples with both protocols. B: Bar graph depicting the detection rate in the saliva sample with protocol U and SalivaAll. Data are given as means ± SD (A). ∗∗P < 0.01, ∗∗∗P < 0.001. Saliva-U, unprocessed saliva samples.
Limit of Detection
In the preliminary LoD study, all replicates were detected at the three tested concentrations with the PerkinElmer Inc. reference material, and two of three replicated were detected at 20 copies/mL with SeraCare material. The LoD was determined to be 20 and 60 copies/mL with PerkinElmer Inc. and SeraCare material, respectively, and all 20 replicates were detected (Table 1 ).
Table 1.
SARS-CoV-2 Limit of Detection Using SalivaAll Protocol with PerkinElmer Inc. Material
| SARS-CoV-2 (triplicates), copies/mL | N gene, mean Ct ± SD | ORF1ab gene, mean Ct ± SD |
|---|---|---|
| 20 | 36.7 ± 0.6 | 35.1 ± 1.3 |
| 60 | 33.6 ± 0.15 | 33.7 ± 0.4 |
| 180 | 32.7 ± 0.19 | 32.6 ± 0.2 |
Limit of detection confirmation (20 copies/mL): 20/20 replicates detected.
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Pooling Saliva Samples for Mass Population Screening
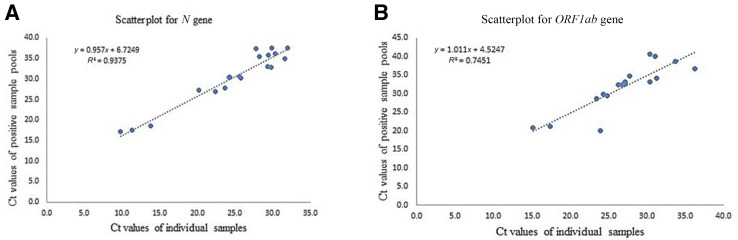
The five-sample pooling strategy was evaluated by comparing the results of the 20 positive and negative pools with individual sample testing results. The pooled testing results demonstrated a 95% positive and a 100% negative agreement. The N and ORF1ab gene Ct values were compared between pooled and individual testing. The shift in Ct value was found to be significant, with pooled testing toward higher Ct values; nonetheless, the pools containing positive samples with viral loads close to the assay's LoD (ie, weak positives) were accurately detected (Table 2 and Figure 4 ).
Table 2.
Performance of Saliva Pooling with SalivaAll Protocol
| Study design: SalivaAll | Composition of saliva pools | Concordance |
|---|---|---|
| Positive pools (20) | 1 Positive + 4 negative (individually tested samples) | 19/20 |
| Negative pools (20) | 5 Negative (individually tested samples) | 20/20 |
Positive percentage agreement = 95%. Negative percentage agreement = 100%.
Figure 4.
A: The Ct value comparison of N gene with individual testing versus pool testing. B: The Ct value comparison of ORF1ab gene with individual testing versus pool testing. The equation represents the trendline regression equation and the R2 value.
Discussion
An optimal sample type and easier collection method for the detection of SARS-CoV-2 are primary requirements in the global effort to control this pandemic. NPS and OPS samples are the currently recommended sample types for COVID-19 diagnostic testing. Testing for SARS-CoV-2 has relied primarily on these samples, but the associated challenges with NPS/OPS sampling have engendered a need to evaluate several other sample types, of which saliva has remained an attractive alternate. However, there are conflicting reports in the literature on the clinical performance of saliva compared with NPS/OPS samples. In a healthcare setting, initial reports from To et al6 , 7 demonstrated comparable sensitivity of saliva samples (86.9% and 91.7%) compared with NPS samples. Similarly, independent investigations from Azzi et al8 and Yoon et al9 demonstrated a 100% concordance of saliva with NPS samples, with Yoon et al9 reporting high viral titer values in saliva. In addition, Wyllie et al10 and Rao et al11 demonstrated higher sensitivity of saliva and particularly early morning saliva compared with NPS samples, respectively. Conversely, Lai et al12 and Jamal et al13 reported lower sensitivity of deep-throat saliva (68.7% versus 80.9%) and saliva samples (72% versus 89%) compared with NPS samples, respectively. Furthermore, Becker et al14 reported a 30% to 50% lower sensitivity of saliva compared with NPS samples in the community setting.
The phase 1 study, comprising 240 matched NPS and saliva sample pairs (protocol U), demonstrated lower sensitivity of saliva samples compared with NPS samples. The detection rate in saliva samples was significantly lower compared with that in NPS samples. In addition, the Ct values for N, ORF1ab, and IC (extraction and PCR control) were significantly higher in saliva compared with that in the NPS samples. Furthermore, a high percentage of saliva samples yielded invalid results. A significant challenge in the wet laboratory revolved around accurate pipetting of saliva samples because most of them were viscous, even after intensive vortexing. The viscous gel-like consistency not only caused issues with pipetting but also led to a high percentage of invalid results. This was demonstrated as a significantly high Ct value drift in the IC, suggesting that the lower sensitivity of saliva samples is most likely the result of processing and sometimes pre-analytical issue(s). Several factors that might have led to the lower sensitivity of saliva samples were contemplated, including inaccurate pipetting of sample volume into the extraction plate for nucleic acid extraction, the viscous gel-like texture of saliva samples preventing appropriate mixing with viral transport media, and leading to the pipetting of primarily saliva or media into extraction plate for nucleic acid extraction, and the likelihood that the higher viscosity of saliva might also impair sample lysis for nucleic acid extraction.
The underlying issue associated with these challenges emerged to be the gel-like consistency of saliva samples. This significant pre-analytic concern was addressed by adding a simple step before processing samples for nucleic acid extraction. The saliva samples were homogenized using a bead mill homogenizer at a speed of 4.5 m/second for 30 seconds (SalivaAll). The homogenization rendered the saliva samples uniform in viscosity and consistency, making it easier to pipet for the downstream assay. Thus, in phase 2 of the study, with 189 matched NPS and saliva sample pairs, adding this single preprocessing step rendered saliva samples more sensitive compared with NPS samples. The detection rate was higher in saliva samples, with significantly lower Ct value for IC, compared with NPS samples. Of the 85 saliva samples processed both in phase 1 and phase 2 studies, the detection rate was significantly higher in samples processed with the homogenization step (SalivaAll) compared with the samples processed without it (protocol U). The Ct values in the same saliva samples were lower for the N gene, and significantly lower for ORF1ab and IC in samples processed with SalivaAll compared with protocol U. These results demonstrate that the sensitivity of the saliva samples observed in the phase 1 study was lower because of inadequate sample processing. The saliva samples were found to be more sensitive compared with NPS samples, with the addition of this simple processing step. Furthermore, the LoD of 20 copies/mL with the PerkinElmer Inc. material and 60 copies/mL with the SeraCare material confirmed the sensitivity of saliva samples as the LoD was determined to be similar to that for the FDA Emergency Use Authorization comparator test for NPS samples. The LoD for the assay in NPS samples was determined to be 20 copies/mL with PerkinElmer Inc. positive control and SeraCare reference material. Viscosity measurement studies were performed to demonstrate the effect of homogenization on saliva samples. Uunprocessed saliva samples (Saliva-U) had a viscosity ranging from 176 centipoise (cP) to 677 cP (between the viscosity of olive oil and honey), compared to that of homogenized samples (SalivaAll), which had a viscocity of 2.1 cP to 3.1 cP, a viscosity close to that of water (1 cP). These findings highlight and explain the difficulty the saliva samples posed during pipetting and in the extraction procedure, which made uniform mixing of reagents challenging. The homogenization of saliva samples led to a uniform and less viscous sample and allowed adequate extraction of the nucleic acids in the extraction procedure, leading to higher sensitivity of the assay.16
Furthermore, saliva processing became significantly easier, leading to successful validation of saliva samples with a five-sample pooling strategy. The pooled testing results demonstrated a positive percentage agreement of 95% (19/20 pools showing positive results), with one pool that contained the sample with high Ct (N: 38.4; ORF1ab: undetermined) being undetectable. The negative percentage agreement was found to be 100%. The feasibility and accuracy of a sample pooling approach with NPS samples for wide-scale population screening for COVID-19 has been demonstrated.17 Herein, the utility and potential benefits of the sample pooling approach were extended for population screening with saliva samples. Considering the evolving epidemiology of COVID-19 and the reopening of educational and professional institutions, travel, tourism, and social activities, monitoring SARS-CoV-2 is expected to remain a critical public health need. Therefore, the use of a noninvasive collection method and easily accessible samples such as saliva, will enhance screening and surveillance activities.
In conclusion, this study has demonstrated that the saliva samples are more sensitive than NPS samples if collected and processed appropriately before extraction and PCR. The study evaluated matched NPS and saliva sample pairs collected in both healthcare and community settings. In the community setting, no control was exercised regarding food or drink restriction and time of sample collection as most of the community-collected samples came from drive-through facilities that operate from morning until evening. The spectrum of disease also varied from asymptomatic to severely ill patients, and the study was not biased for a particular group. However, it must be highlighted that homogenization is an additional step in the workflow and will increase the processing time compared with NPS samples. In addition, similar approaches to homogenize the saliva samples should be evaluated by laboratories intending to implement saliva testing in different regions of the world, where this particular homogenizer might not be available. Furthermore, the study has two limitations: the Ct value in this study reflects viral load but not the viral copies per mL, and the sample collection was not controlled for a specific day of illness. Nonetheless, despite these limitations, this study presents a significant and clinically validated approach to the utilization of saliva samples for COVID-19 testing, individually or as pooled samples.
Acknowledgment
We thank Dr. Brooks Keel, President, Augusta University and Augusta University Health Sciences, for strategic, science-based, data-driven leadership in all matters COVID-19, and constant support and encouragement at every step of this work.
Footnotes
Disclosures: R.K. has received honoraria, travel funding, and research support from Illumina, Asuragen, Qiagen, and BMS. M.H. holds stock options at PerkinElmer Inc. This project has been funded by the National Institute of Allergy and Infectious Diseases, a component of the National Institutes of Health, Department of Health and Human Services (75N93019C00052).
Supplemental material for this article can be found at http://doi.org/10.1016/j.jmoldx.2021.04.005.
Supplemental Data
References
- 1.Bedford J., Enria D., Giesecke J., Heymann D.L., Ihekweazu C., Kobinger G., Lane H.C., Memish Z., Oh M.D., Schuchat A., Ungchusak K. COVID-19: towards controlling of a pandemic. Lancet. 2020;395:1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piras A., Rizzo D., Uzzau S., De Riu G., Rubino S., Bussu F. Inappropriate nasopharyngeal sampling for SARS-CoV-2 detection is a relevant cause of false-negative reports. Otolaryngol Head Neck Surg. 2020;163:459–461. doi: 10.1177/0194599820931793. [DOI] [PubMed] [Google Scholar]
- 4.Ali F., Sweeney D.A. No one likes a stick up their nose: making the case for saliva-based testing for COVID-19. Clin Infect Dis. 2020;72:e357–e358. doi: 10.1093/cid/ciaa1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanson K.E., Barker A.P., Hillyard D.R., Gilmore N., Barrett J.W., Orlandi R.R., Shakir S.M. Self-collected anterior nasal and saliva specimens versus health care worker-collected nasopharyngeal swabs for the molecular detection of SARS-CoV-2. J Clin Microbiol. 2020;58:e01824-20. doi: 10.1128/JCM.01824-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., Yip C.C., Cai J.P., Chan J.M., Chik T.S., Lau D.P. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.To K.K., Tsang O.T., Yip C.C., Chan K.H., Wu T.C., Chan J.M., Leung W.S., Chik T.S., Choi C.Y., Kandamby D.H., Lung D.C. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71:841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azzi L., Carcano G., Gianfagna F., Grossi P., Dalla Gasperina D., Genoni A., Fasano M., Sessa F., Tettamanti L., Carinci F., Maurino V. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020;81:e45–e50. doi: 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon J.G., Yoon J., Song J.Y., Yoon S.Y., Lim C.S., Seong H., Noh J.Y., Cheong H.J., Kim W.J. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J Korean Med Sci. 2020;35:e195. doi: 10.3346/jkms.2020.35.e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyllie A.L., Fournier J., Casanovas-Massana A., Campbell M., Tokuyama M., Vijayakumar P., Geng B., Muenker M.C., Moore A.J., Vogels C.B., Petrone M.E. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020;383:1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao M., Rashid F.A., Sabri F.S., Jamil N.N., Zain R., Hashim R., Amran F., Kok H.T., Samad M.A., Ahmad N. Comparing nasopharyngeal swab and early morning saliva for the identification of SARS-CoV-2. Clin Infect Dis. 2020;72:e352–e356. doi: 10.1093/cid/ciaa1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai C.K., Chen Z., Lui G., Ling L., Li T., Wong M., Ng R.W., Tso E.Y., Ho T., Fung K.S., Ng S.T. Prospective study comparing deep-throat saliva with other respiratory tract specimens in the diagnosis of novel coronavirus disease (COVID-19) J Infect Dis. 2020;222:1612–1619. doi: 10.1093/infdis/jiaa487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamal A.J., Mohammad M., Coomes E., Powis J., Li A., Paterson A., Anceva-Sami S., Barati S., Crowl G., Faheem A., Farooqi L. Sensitivity of nasopharyngeal swabs and saliva for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;72:1064–1066. doi: 10.1093/cid/ciaa848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker D., Sandoval E., Amin A., De Hoff P., Leonetti N., Lim Y.W., Elliott C., Laurent L., Grzymski J., Lu J. Saliva is less sensitive than nasopharyngeal swabs for COVID-19 detection in the community setting. medRxiv. 2020 doi: 10.1101/2020.05.11.20092338. [DOI] [Google Scholar]
- 15.Landry M.L., Criscuolo J., Peaper D.R. Challenges in use of saliva for detection of SARS-CoV-2 RNA in symptomatic outpatients. J Clin Virol. 2020;130:104567. doi: 10.1016/j.jcv.2020.104567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahajpal N.S., Mondal A.K., Ananth S., Njau A., Ahluwalia P., Newnam G., Lozoya-Colinas A., Hud N.V., Kota V., Ross T.M., Reid M.D., Fulzele S., Chaubey A., Hegde M., Rojiani A.M., Kolhe R. SalivaSTAT: direct-PCR and pooling of saliva samples collected in healthcare and community setting for SARS-CoV-2 mass surveillance. Diagnostics. 2021;11(5) doi: 10.3390/diagnostics11050904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahajpal N.S., Mondal A.K., Njau A., Ananth S., Jones K., Ahluwalia P.K., Ahluwalia M., Jilani Y., Chaubey A., Hegde M., Kota V. Proposal of reverse transcription-PCR–based mass population screening for SARS-CoV-2 (COVID-19) J Mol Diagn. 2020;22:1294–1299. doi: 10.1016/j.jmoldx.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.