Abstract
Previous studies have documented the utility of a transdiagnostic internalizing factor in predicting important future outcomes (e.g., subsequent mental disorder diagnoses). To date, however, no study has investigated whether an internalizing factor predicts mortality risk. Also, while previous studies of mortality risk have emphasized its associations with particular internalizing disorders, no study has assessed how the transdiagnostic internalizing factor vs. disorder‐specific variance differently predict that risk. The primary aims of this study were to explore: a) whether the internalizing factor predicts mortality risk, b) whether particular internalizing psychopathologies uniquely predict mortality risk over and beyond the transdiagnostic internalizing factor, and c) whether there is a significant interaction of internalizing with self‐reported health in the prediction of mortality risk. We utilized a large national sample of American adults from the Midlife in the United States (MIDUS), a longitudinal study that examined midlife development of individuals across multiple waves between 1995 and 2015. Data were analyzed for the 6,329 participants who completed the phone interview and self‐administered questionnaire in MIDUS 1 (1995‐1996) and were then followed up until October 31, 2015 or until death. To investigate the association between internalizing and mortality risk, we used the semi‐parametric proportional hazards Cox model, where survival time was regressed on a latent internalizing factor. Overall findings indicate that a transdiagnostic internalizing factor significantly predicts mortality risk over a 20‐year period (hazard ratio, HR=1.12, 95% CI: 1.05‐1.16, p<0.01) and that internalizing outperforms disorder‐specific variance (e.g., depression‐specific variance) in the prediction of that risk. Further, there was a significant interaction between transdiagnostic internalizing and self‐reported health, whereby internalizing psychopathology had a specific association with early death for individuals with excellent self‐reported health condition (HR=1.50, 95% CI: 1.17‐1.84, p<0.05). This highlights the clinical utility of using the transdiagnostic internalizing factor for prediction of an important future outcome, and supports the argument that internalizing psychopathology can be a meaningful liability to explore in public health practice.
Keywords: Internalizing factor, mortality, transdiagnostic prediction, diagnosis‐based prediction, major depressive disorder, generalized anxiety disorder, panic disorder, neuroticism
Numerous studies have reported that individuals with depressive or anxiety symptoms are at higher risk of experiencing various negative physical health conditions subsequently, compared with individuals without those symptoms. For example, depressive symptoms are associated with greater decline in physical performance in the later stages of life 1 , increased risk of developing various forms of cardiovascular disease2, 3, and excessive risk of developing some forms of cancer 4 . Furthermore, childhood separation anxiety symptoms predict poor physical health in later stages of development 5 ; generalized anxiety disorder (GAD) symptoms are associated with risk for coronary heart disease 6 ; and GAD and post‐traumatic stress disorder symptoms longitudinally predict shorter leukocyte telomere length (a biomarker for age‐related diseases) 7 .
Not surprisingly, a number of studies have reported the association of depression and/or anxiety with a higher risk of mortality8, 9, 10. For example, using survival analysis, a study investigated mortality rate in a large Danish population‐based cohort (N=5,103,699), reporting that individuals with unipolar depression had a higher risk of early death 11 . Several studies also found that individuals with anxiety symptoms were exposed to a higher risk of premature death12, 13. Additionally, some studies have indicated that individuals with a higher level of neuroticism, a personality trait with a close relation to mood and anxiety disorders 14 , also have a higher mortality risk15, 16.
Although informative, a major limitation of prior research is that it mainly focused on how particular categorical diagnostic constructs were associated with mortality risk, while there has been growing evidence supporting the value of a dimensional conceptualization of psychopathology17, 18, 19, 20, 21. According to this latter approach, each mental disorder can be conceptualized as a manifestation of relatively few underlying transdiagnostic dimensions, which account for the co‐occurrence among various disorders (i.e., comorbidity). For example, major depressive disorder (MDD) and GAD tend to co‐occur more frequently than it is expected by chance 22 . This may indicate that they are highly correlated through the transdiagnostic internalizing factor. Indeed, numerous studies have reported that the internalizing factor accounts for the commonalities among various mood and anxiety disorders22, 23, 24, 25.
This framework provides the opportunity to investigate how the transdiagnostic internalizing factor, compared to particular forms of internalizing pathology (e.g., diagnostic categories), is associated with mortality risk 26 . A few prior studies have suggested a possible association of the common variance among various internalizing disorders with that risk15, 27, 28. For example, Mirza et al27 reported that the relationship between anxiety symptoms and mortality risk was no longer significant after adjusting for comorbid depressive symptoms. This finding seems to suggest that it is the common variance that anxiety shares with depression which leads to higher mortality, and that the anxiety disorder‐specific variance may not predict mortality risk meaningfully once comorbid depression is controlled for.
There are several advantages of using the transdiagnostic internalizing factor as a predictor. Previous research has shown notable structural invariance of internalizing across different samples 29 , high long‐term stability of internalizing over time 30 , and notable predictive validity for important future outcomes (e.g., subsequent mental disorder diagnoses)30, 31, 32. Given these findings, transdiagnostic internalizing could be a reliable and strong predictor of mortality risk.
It is also probable that the anticipated relationship between transdiagnostic internalizing and mortality risk is moderated by other factors. A possible moderator is one's self‐reported health, given some prior studies suggesting that the association of depression and neuroticism with mortality risk varied depending on one's self‐reported health condition11, 15.
Taking all the research discussed above into consideration, major limitations of the prior literature are that: a) no study has investigated whether or not a transdiagnostic internalizing dimension meaningfully predicts mortality risk, and b) previous studies have focused on the associations of individual diagnostic constructs with that risk, leaving it unclear whether these constructs have a general or a specific and unique association with early mortality8, 9, 11, 28, 33. This underscores the necessity to compare the prediction of mortality risk from various internalizing disorders' shared variance (i.e., transdiagnostic internalizing) versus the specific (unique) variance of each disorder, to ascertain which is a more robust predictor.
The primary aims of the current study were: a) to investigate whether the transdiagnostic internalizing factor predicts mortality risk in a longitudinal probability sample of American adults, b) to compare the utility of the transdiagnostic internalizing factor versus disorder‐specific variance in the prediction of that risk, and c) to examine whether self‐rated physical health moderates the association between internalizing and early mortality.
METHODS
Participants
This study utilized a large national sample of American adults from the Midlife in the United States (MIDUS) 34 , which is a longitudinal study examining midlife development of individuals across multiple waves. Our study initially utilized information on the 7,108 participants who were recruited in the initial survey at MIDUS 1 (1995‐1996). In order to be included in the final sample, participants needed to complete the MIDUS 1 phone interview and self‐administered questionnaire, which yielded the final analytic sample of 6,329 individuals (mean age: 46.77±12.92 years; 52.64% females; 88.04% White, 4.90% African American).
These individuals were followed up until October 31, 2015 or until death. A total of 1,234 people were deceased during the study period (i.e., from 1995 to 2015). The mean survival time for all participants was 19.23±4.16 years. The mean survival time for decedents was 11.50±5.28 years.
Measures
To model a transdiagnostic internalizing factor, we included continuous symptom scores for MDD, GAD, panic disorder and neuroticism. Past 12‐month MDD, GAD and panic disorder symptoms were measured using the Composite International Diagnostic Interview ‐ Short Form (CIDI‐SF) version 10, whose good diagnostic reliability and validity have been reported by numerous studies35, 36, 37. Neuroticism was assessed using the relevant subscale of the Midlife Development Inventory Personality Scales, whose internal consistency has been found to be good (Cronbach's alpha = .74) 38 .
We chose six covariates based on the following criteria: a) whether a given covariate had been previously identified to likely influence mortality risk, and b) whether there was a large enough response rate for a given covariate (more than 6,000 responses). Based on these criteria, the six covariates chosen were: age (a standardized variable), age squared, sex (a binary variable), education level (ranged from 1 to 12, with larger numbers indicating higher educational levels), experienced severe health condition (a continuous variable ranged from 0 to 3, where higher scores indicate more severe physical health condition), and heart disease family risk (a binary variable).
Analyses
To investigate the association between the transdiagnostic internalizing factor and mortality risk, we used the semi‐parametric proportional hazards Cox model. This model makes fewer assumptions about the distribution of survival time than do parametric models (e.g., Weibull, exponential models), enabling one to estimate regression coefficients and hazard ratios (HRs) even though the baseline hazard is not specified. This advantage makes it a practical and reasonable choice.
To model the transdiagnostic internalizing factor, we used confirmatory factor analysis (CFA), including four indicators (MDD, GAD, panic disorder, and neuroticism) assessed at MIDUS 1. This internalizing model (estimated from these same data and indicator variables) was previously identified as invariant across the different age cohorts and stable over time 39 . After modeling internalizing, we saved the factor scores to include them in the main Cox regression model.
The factor score approach may raise an issue of factor indeterminacy. In order to mitigate this concern, we further checked the factor determinacy index, which was calculated by the correlation between the estimated and true factor scores (ranging from 0 to 1; the higher the better representation of the true factor scores).
We performed survival analyses using maximum likelihood estimation with robust standard errors (MLR), with the latent internalizing variable standardized to have a variance of 1 and a mean of 0. All analyses were performed in Mplus version 8.0.
In order to compare the predictive validity of internalizing versus disorder‐unique variance, we parameterized an explicit residual variance factor for each of the three internalizing disorders and neuroticism (i.e., the unique variance remaining in each indicator after the common variance is accounted for by the latent internalizing variable). We then saved the factor scores from transdiagnostic internalizing and the four construct residual factors, and regressed survival time on both internalizing and the residual factor scores simultaneously.
RESULTS
The key assumption that Cox regression poses is that each predictor's multiplicative effect on the hazards function remains constant over time (proportional hazards assumption)40, 41. We tested this assumption by assessing time‐by‐covariates interaction, which has been proven to be powerful for detecting non‐proportionality 42 . This method involved creating the interaction term of internalizing x survival function time, including it in a Cox model with internalizing, and testing the significance of the interaction term. The result showed that the interaction term was not significant (HR=1.01, 95% CI: 0.99‐1.01), indicating that the proportional hazards assumption was met.
We first examined how each of the four indicators assessed at MIDUS 1 (MDD, GAD, panic disorder, and neuroticism) was associated with mortality risk by use of hierarchical regression. A set of four two‐stage hierarchical regression models were conducted where all covariates were entered at stage 1 and each of the indicators was entered one at a time at stage 2. Results showed that MDD, GAD and neuroticism significantly predicted mortality risk in this framework, while panic disorder did not (see Table 1).
Table 1.
Hierarchical regression analysis of individual internalizing pathologies predicting mortality risk (regression coefficients with 95% CI)
| Stage 1 | Stage 2 | ||||
|---|---|---|---|---|---|
| MDD | GAD | PAN | NEURO | ||
| Age | 8.39 (8.19‐8.59) *** | 8.69 (8.49‐8.89) *** | 8.52 (8.32‐8.73) *** | 8.47 (8.27‐8.67) *** | 8.66 (8.45‐8.86) *** |
| Age squared | 0.94 (0.66‐1.21) | 0.94 (0.66‐1.21) | 0.94 (0.66‐1.21) | 0.94 (0.66‐1.21) | 0.94 (0.66‐1.20) |
| Sex | 0.75 (0.63‐0.88) *** | 0.74 (0.62‐0.86) *** | 0.75 (0.63‐0.87) *** | 0.75 (0.63‐0.87) *** | 0.75 (0.63‐0.87) *** |
| Education level | 0.82 (0.76‐0.88) *** | 0.82 (0.76‐0.88) *** | 0.82 (0.76‐0.88) *** | 0.82 (0.76‐0.88) *** | 0.82 (0.76‐0.88) *** |
| Experienced physical illness | 1.52 (1.45‐1.60) *** | 1.51 (1.43‐1.58) *** | 1.52 (1.44‐1.59) *** | 1.52 (1.44‐1.59) *** | 1.50 (1.43‐1.58) *** |
| Heart disease family risk | 1.23 (1.12‐1.35) ** | 1.23 (1.12‐1.35) ** | 1.23 (1.12‐1.35) ** | 1.23 (1.12‐1.35) ** | 1.23 (1.12‐1.35) ** |
| MDD | 1.06 (1.02‐1.09) ** | ||||
| GAD | 1.08 (1.02‐1.15) * | ||||
| PAN | 1.03 (0.96‐1.09) | ||||
| NEURO | 1.12 (1.02‐1.21) * | ||||
MDD – major depressive disorder, GAD – generalized anxiety disorder, PAN – panic disorder, NEURO – neuroticism
p<0.05,
p<0.01,
p<0.001
We then explored whether the transdiagnostic internalizing factor predicted mortality risk. Our CFA model of internalizing showed an excellent fit to the data: root mean square error of approximation (RMSEA) = .008; comparative fit index (CFI) = .999; Tucker‐Lewis index (TLI) = .998. The factor determinacy index for our CFA model was .78, which mirrored the recommended threshold of 0.80 to indicate that a model is “adequate for most scientific purposes” 43 . The Cox regression analysis showed that internalizing significantly and positively predicted mortality risk (HR=1.12, 95% CI: 1.05‐1.16, p<0.01), after adjusting for age, age squared, sex, education level, experienced severe health condition, and heart disease family risk (see Table 2).
Table 2.
Results for Cox regression models of the effect of change in internalizing on mortality risk
| Predictor | Hazard ratio (95% CI) |
|---|---|
| Age | 5.50 (5.29‐5.71)*** |
| Age squared | 0.95 (0.75‐1.16) |
| Sex | 1.28 (1.18‐1.37)*** |
| Education level | 0.86 (0.81‐0.91)*** |
| Experienced physical illness | 1.37 (1.31‐1.44)*** |
| Heart disease family risk | 1.18 (1.09‐1.28)*** |
| Internalizing | 1.12 (1.05‐1.16)** |
| AIC | 10588.07 |
| BIC | 10635.26 |
AIC – Akaike information criterion, BIC – Bayesian information criterion
p<0.01,
p<0.001
In order to compare the prediction of mortality risk from internalizing disorders' shared variance (i.e., transdiagnostic internalizing) and disorder‐specific variance, we regressed mortality on internalizing and the residual variance of MDD, GAD, panic disorder and neuroticism simultaneously in the Cox regression framework (see Figure 1). The results showed that internalizing significantly predicted mortality risk across all analyses (HR ranged from 1.11 to 1.14), while MDD (HR=1.02, 95% CI: 0.98‐1.06), GAD (HR=1.01, 95% CI: 0.94‐1.09), panic (HR=0.94, 95% CI: 0.88‐1.00), and neuroticism (HR=1.02, 95% CI: 0.92‐1.12) residuals did not (see Table 3).
Figure 1.
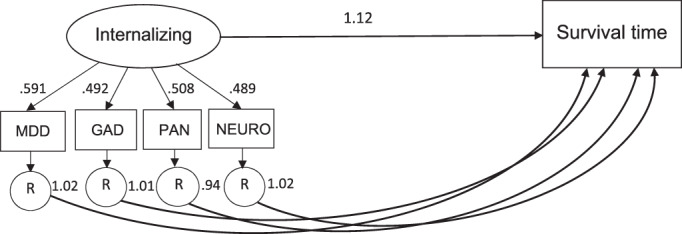
Comparative predictive validity analysis. Arrows flowing from the latent internalizing factor to its indicators represent factor loadings, which were all statistically significant at p<0.001. The arrow leading from internalizing to survival time represents the hazard ratio of the Cox regression model, which was significant at p<0.01. Arrows leading from each of the residual variance to survival time represent the hazard ratios of each Cox regression model, which were all non‐significant. MDD – major depressive disorder, GAD – generalized anxiety disorder, PAN – panic disorder, NEURO – neuroticism, R = residual variance.
Table 3.
Results of the comparative predictive validity analysis (hazard ratios with 95% CI)
| Models | ||||
|---|---|---|---|---|
| Internalizing vs. MDD | Internalizing vs. GAD | Internalizing vs. PAN | Internalizing vs. NEURO | |
| Age | 5.48 (5.28‐5.69)*** | 5.49 (5.28‐5.71)*** | 5.47 (5.26‐5.69)*** | 5.50 (5.29‐5.72)*** |
| Age squared | 0.95 (0.74‐1.17) | 0.95 (0.75‐1.17) | 0.95 (0.74‐1.17) | 0.95 (0.75‐1.17) |
| Sex | 1.27 (1.18‐1.37)*** | 1.27 (1.18‐1.37)*** | 1.27 (1.17‐1.37)*** | 1.28 (1.18‐1.38)*** |
| Education level | 0.86 (0.81‐0.92)*** | 0.86 (0.81‐0.92)*** | 0.86 (0.81‐0.92)*** | 0.86 (0.81‐0.92)*** |
| Experienced physical illness | 1.37 (1.31‐1.44)*** | 1.38 (1.31‐1.44)*** | 1.37 (1.31‐1.43)*** | 1.37 (1.31‐1.43)*** |
| Heart disease family risk | 1.18 (1.09‐1.28)*** | 1.18 (1.09‐1.28)*** | 1.18 (1.09‐1.28)*** | 1.18 (1.09‐1.28)*** |
| Internalizing | 1.11 (1.03‐1.18)** | 1.12 (1.04‐1.20)** | 1.14 (1.07‐1.22)*** | 1.12 (1.05‐1.20)** |
| MDD residual | 1.02 (0.98‐1.06) | |||
| GAD residual | 1.01 (0.94‐1.09) | |||
| PAN residual | 0.94 (0.88‐1.00) | |||
| NEURO residual | 1.02 (0.92‐1.12) | |||
MDD – major depressive disorder, GAD – generalized anxiety disorder, PAN – panic disorder, NEURO – neuroticism
p<0.01,
p<0.001
We then examined how the initial associations of MDD, GAD and neuroticism with mortality risk (panic disorder did not significantly predict that risk) were attenuated when adjusting for internalizing. Compared with the hierarchical regression analysis results, the comparative predictive validity analysis showed that the degrees to which MDD, GAD and neuroticism predicted mortality risk were attenuated, respectively, by 67.2%, 86.9% and 87.1%, when their shared variance captured in internalizing was accounted for.
To explore whether the association between internalizing and mortality risk was moderated by individuals' self‐reported health condition, we created an interaction term and included it in our Cox regression model following the method use by Gale et al15. We then compared the models with and without the interaction term. Results showed that the Bayesian information criterion (BIC) was lower for the model with the interaction term included (BIC=10622.6) than for the model without the interaction term (BIC=10635.3). BIC differences of 10 between two models indicate 150:1 posterior odds in favor of the model with superior (lower) BIC.
Given the statistical significance of the interaction term, we further analyzed the association of internalizing with mortality risk stratified by self‐rated physical health level (5‐point scale ranged from poor to excellent, with 5 being excellent). Results showed that internalizing significantly predicted mortality risk specifically among individuals whose self‐reported physical health was excellent (HR=1.50, 95% CI: 1.17‐1.84, p<0.05), but not in individuals with poorer self‐rated physical health (see Table 4).
Table 4.
Results for Cox regression models of the effect of change in internalizing on mortality risk stratified by self‐rated physical health (hazard ratios with 95% CI)
| Excellent health (N=1,217) | Very good health (N=2,506) | Good health (N=2,386) | Fair health (N=796) | Poor health (N=192) | |
|---|---|---|---|---|---|
| Age | 7.86 (7.21‐8.51)*** | 2.59 (2.36‐2.83)*** | 2.69 (2.50‐2.87)*** | 2.28 (2.04‐2.53)*** | 1.86 (1.57‐2.15)*** |
| Age squared | 1.16 (0.54‐1.78) | 0.98 (0.72‐1.23) | 1.09 (0.87‐1.32) | 0.91 (0.63‐1.20) | 0.77 (0.43‐1.12) |
| Sex | 1.11 (0.81‐1.4) | 1.14 (1.04‐1.24)** | 1.14 (1.05‐1.22)** | 1.22 (1.10‐1.34)** | 1.07 (0.88‐1.26) |
| Education level | 0.88 (0.75‐1.02) | 0.92 (0.82‐1.02) | 0.86 (0.77‐0.95)** | 0.97 (0.85‐1.09) | 1.02 (0.84‐1.19) |
| Experienced physical illness | 1.54 (1.33‐1.75)*** | 1.06 (1.00‐1.13)* | 1.19 (1.11‐1.26)*** | 1.19 (1.07‐1.31)** | 1.23 (1.03‐1.44)* |
|
Heart disease family risk |
1.54 (1.26‐1.82)** | 1.03 (0.93‐1.13) | 1.12 (1.03‐1.20)** | 1.05 (0.94‐1.16) | 1.01 (0.83‐1.18) |
| Internalizing | 1.50 (1.17‐1.84)* | 1.04 (0.93‐1.14) | 1.04 (0.93‐1.16) | 1.04 (0.89‐1.19) | 0.82 (0.59‐1.06) |
p<0.05,
p<0.01,
p<0.001
DISCUSSION
Internalizing and mortality risk
The primary aim of our study was to investigate the association between a transdiagnostic internalizing factor and mortality risk. Our findings show that higher levels of internalizing pathology are associated with a significantly increased mortality risk, even after adjusting for covariates known to affect that risk (e.g., age, sex, education level, heart disease family risk, experienced severe health condition). There was a 12.3% increase in mortality rate for every 1‐standard deviation unit increment in the internalizing factor level. These findings are consistent with the previously reported close link between individual internalizing disorders and high mortality rates8, 9, 10, 11, 28, 33, 44, but our study is the first to demonstrate that it is a transdiagnostic internalizing factor that predicts mortality risk, rather than the variance that is unique to MDD, GAD, panic, or neuroticism.
There are several possible explanations for why mortality rates are greater in individuals with higher levels of internalizing. One pathway is via maladaptive coping. Given that individuals with high internalizing experience frequent negative affect, they may attempt to manage their negative emotions via unhealthy coping, such as heavy drinking or drug abuse. Indeed, an internalizing pathway model has been proposed 45 , in which early and persistent internalizing symptoms lead individuals to use substances as a means of coping. Issues with substance and alcohol abuse tend to emerge after trauma exposure 46 , which also has additive negative effects on mental and physical health.
It is also possible that internalizing predicts mortality risk through physical inactivity. People with internalizing psychopathology tend to be physically inactive47, 48, which can lead to adverse physical health outcomes, eventually resulting in high mortality rates. Indeed, a number of prior studies indicated that physical inactivity is one of the main risk factors for cardiovascular disease49, 50, 51, and that engaging in regular activity can meaningfully reduce the risk of premature death52, 53.
In addition, people with high levels of internalizing are more likely to experience various adverse life outcomes, such as unemployment 54 , marital discord55, 56, poor social functioning57, 58, and poor quality of life57, 59, which may play a role as mediating factors in the relationship between internalizing and high mortality risk.
The superior predictive validity of the transdiagnostic internalizing factor
Contrary to the underlying assumption of traditional diagnostic systems that each mental disorder is a discrete entity, many internalizing disorders co‐occur more frequently than expected by chance. A significant relationship between a particular internalizing disorder and high mortality risk can be attributed to that disorder's unique variance or to the common variance that the disorder shares with other internalizing disorders (i.e., the transdiagnostic internalizing factor). To our knowledge, our study is the first to explore which of these two sources more significantly predicts mortality risk. Our findings show that, once the commonalities among the individual diagnostic constructs are accounted for by the internalizing factor, the unique variance that is specific to each construct no longer predicts mortality risk meaningfully. The significant associations between particular internalizing pathologies and mortality risk reported in prior studies may be therefore largely attributed to an underlying internalizing factor.
Of note, internalizing accounted for 34.93% of the variance in MDD (i.e., 65.07% of the variance was MDD‐specific), 24.21% in GAD (i.e., 75.79% of the variance was GAD‐specific), 25.81% in panic disorder (i.e., 74.19% of the variance was panic disorder‐specific), and 23.91% in neuroticism (i.e., 76.09% of the variance was neuroticism‐specific). Nevertheless, none of those disorder‐specific variances significantly predicted mortality risk.
It is worth further speculating about why mortality risk was mainly predicted by the commonalities among the internalizing pathologies rather than by the residual variance of each internalizing construct. Previous studies that looked at the stability of various diagnostic constructs reported a long‐term instability of mood and anxiety disorders60, 61, 62. Given the transitory nature of these pathologies, one's particular internalizing disorder symptoms may not be a strong predictor of long‐term outcomes. By contrast, a transdiagnostic internalizing dimension has high temporal stability and structural invariance over time29, 30, thus being a more reliable prospective predictor of long‐term outcomes such as death.
It was especially notable to find the insignificant association between neuroticism residual variance and mortality risk, after the common variance was saturated by the internalizing factor. While a number of prior studies have reported a significant connection between neuroticism and mortality risk15, 16, 28, our findings indicate that such significant effects may be largely accounted for by the common variance that neuroticism shares with other internalizing pathologies, and that neuroticism itself may not be a consistent predictor of mortality risk 63 .
The significant interaction between internalizing and self‐rated health condition
Of note, the meaningful association between internalizing and mortality risk was moderated by one's self‐reported health condition. That is, internalizing predicted mortality risk in individuals whose self‐reported physical health was excellent, but not in individuals with poorer self‐reported health. Individuals with excellent self‐rated physical health are exposed to a 50.2% increase in the risk of premature death for every 1‐standard deviation unit increment in the internalizing level, after adjusting for other covariates.
This significant interaction between internalizing and self‐reported health indicates that internalizing psychopathology may not confer additional risk of early death to those with poor physical health. However, if individuals are currently physically healthy, then internalizing psychopathology is more likely to have an effect on mortality risk. This is in line with prior findings that the association between individual internalizing pathologies and mortality risk was moderated by one's self‐reported health condition11, 15. It is likely that self‐reported health is a strong predictor of mortality, closely covarying with internalizing in our study.
Limitations
The current study was not without limitations. First, we included only four internalizing indicators (MDD, GAD, panic disorder, and neuroticism), since they were the only internalizing pathologies assessed in MIDUS 1. Future research needs to replicate our findings by including other internalizing disorder indicators. Second, the study was unable to test the association of a transdiagnostic externalizing factor with mortality risk, given that sufficient indicators were not available to model an externalizing factor in MIDUS. Third, although our final Cox regression model adjusted for many covariates, we were unable to control for some other covariates also known to influence mortality risk (e.g., body mass index, smoking status), due to large missing values for those variables. Fourth, given that the information regarding causes of death was not available in MIDUS, the current study was unable to further investigate the degree to which internalizing predicts mortality risk differently depending on the various causes of death. Last, our study included a binary sex variable as one of the covariates: future research could further explore how the association of internalizing with mortality risk differs in non‐binary individuals.
CONCLUSIONS
Our study is the first to identify the role of the transdiagnostic internalizing factor in predicting the risk of early death. Results show that one's level of internalizing meaningfully predicts mortality risk over a 20‐year period, and that internalizing outperforms disorder‐specific variance in the prediction of that risk. Moreover, the significant interaction between internalizing and physical health indicates that the former dimension is more likely to have an effect on early death for currently physically healthy individuals.
These findings highlight the clinical utility of using the transdiagnostic internalizing factor for prediction of an important future outcome, and support the argument that internalizing psychopathology can be a meaningful liability to incorporate into intervention and prevention research, and to explore in public health practice.
APPENDIX
The members of the Hierarchical Taxonomy of Psychopathology (HiTOP) Utility Workgroup include: Christopher C. Conway (Fordham University), Anna R. Docherty (University of Utah), Michael Dretsch (Walter Reed Army Institute of Research), Kelsie T. Forbush (University of Kansas), Vina M. Goghari (University of Toronto), Kristian E. Markon (University of Iowa), Stephanie N. Mullins‐Sweatt (Oklahoma State University), Brady Nelson (Stony Brook University), Thomas M. Olino (Temple University), and Tim Slade (University of Sydney).
REFERENCES
- 1. Penninx BW, Guralnik JM, Ferrucci L et al. Depressive symptoms and physical decline in community‐dwelling older persons. JAMA 1998;279:1720‐6. [DOI] [PubMed] [Google Scholar]
- 2. Frasure‐Smith N, Lespérance F, Talajic M. Depression and 18‐month prognosis after myocardial infarction. Circulation 1995;91:999‐1005. [DOI] [PubMed] [Google Scholar]
- 3. Ford DE, Mead LA, Chang PP et al. Depression is a risk factor for coronary artery disease in men: the precursors study. Arch Intern Med 1998;158:1422‐6. [DOI] [PubMed] [Google Scholar]
- 4. Gross AL, Gallo JJ, Eaton WW. Depression and cancer risk: 24 years of follow‐up of the Baltimore Epidemiologic Catchment Area sample. Cancer Causes Control 2010;21:191‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Battaglia M, Garon‐Carrier G, Côté SM et al. Early childhood trajectories of separation anxiety: bearing on mental health, academic achievement, and physical health from mid‐childhood to preadolescence. Depress Anxiety 2017;34:918‐27. [DOI] [PubMed] [Google Scholar]
- 6. Tully PJ, Cosh SM, Baune BT. A review of the affects of worry and generalized anxiety disorder upon cardiovascular health and coronary heart disease. Psychol Health Med 2013;18:627‐44. [DOI] [PubMed] [Google Scholar]
- 7. Shalev I, Moffitt TE, Braithwaite AW et al. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post‐traumatic stress disorder. Mol Psychiatry 2014;19:1163‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doyle F, Conroy R, McGee H. Differential predictive value of depressive versus anxiety symptoms in the prediction of 8‐year mortality after acute coronary syndrome. Psychosom Med 2012;74:711‐6. [DOI] [PubMed] [Google Scholar]
- 9. Miloyan B, Bulley A, Bandeen‐Roche K et al. Anxiety disorders and all‐cause mortality: systematic review and meta‐analysis. Soc Psychiatry Psychiatr Epidemiol 2016;51:1467‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Dijk MR, Utens EM, Dulfer K et al. Depression and anxiety symptoms as predictors of mortality in PCI patients at 10 years of follow‐up. Eur J Prev Cardiol 2016;23:552‐8. [DOI] [PubMed] [Google Scholar]
- 11. Laursen TM, Musliner KL, Benros ME et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord 2016;193:203‐7. [DOI] [PubMed] [Google Scholar]
- 12. Carriere I, Ryan J, Norton J et al. Anxiety and mortality risk in community‐dwelling elderly people. Br J Psychiatry 2013;203:303‐9. [DOI] [PubMed] [Google Scholar]
- 13. Denollet J, Maas K, Knottnerus A et al. Anxiety predicted premature all‐cause and cardiovascular death in a 10‐year follow‐up of middle‐aged women. J Clin Epidemiol 2009;62:452‐6. [DOI] [PubMed] [Google Scholar]
- 14. Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. J Abnorm Psychol 1994;103:103‐16. [PubMed] [Google Scholar]
- 15. Gale CR, Čukić I, Batty GD et al. When is higher neuroticism protective against death? Findings from UK Biobank. Psychol Sci 2017;28:1345‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mroczek DK, Spiro III A, Turiano NA. Do health behaviors explain the effect of neuroticism on mortality? Longitudinal findings from the VA Normative Aging Study. J Res Personal 2009;43:653‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Achenbach TM, Edelbrock CS. The classification of child psychopathology: a review and analysis of empirical efforts. Psychol Bull 1978;85:1275‐301. [PubMed] [Google Scholar]
- 18. Kotov R, Krueger RF, Watson D et al. The Hierarchical Taxonomy of Psychopathology (HiTOP): a dimensional alternative to traditional nosologies. J Abnorm Psychol 2017;126:454‐77. [DOI] [PubMed] [Google Scholar]
- 19. Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry 1999;56:921‐6. [DOI] [PubMed] [Google Scholar]
- 20. Krueger RF, Caspi A, Moffitt TE et al. The structure and stability of common mental disorders (DSM‐III‐R): a longitudinal‐epidemiological study. J Abnorm Psychol 1998;107:216‐27. [DOI] [PubMed] [Google Scholar]
- 21. Krueger RF, Kotov R, Watson D et al. Progress in achieving quantitative classification of psychopathology. World Psychiatry 2018;17:282‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krueger RF, Eaton NR. Structural validity and the classification of mental disorders. In: Kendler KS, Parnas J (eds). Philosophical issues in psychiatry II: Nosology. Oxford: Oxford University Press, 2012:199‐212. [Google Scholar]
- 23. Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annu Rev Psychol 1998;49:377‐412. [DOI] [PubMed] [Google Scholar]
- 24. Seeley JR, Kosty DB, Farmer RF et al. The modeling of internalizing disorders on the basis of patterns of lifetime comorbidity: associations with psychosocial functioning and psychiatric disorders among first‐degree relatives. J Abnorm Psychol 2011;120:308‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eaton NR, South SC, Krueger RF. The meaning of comorbidity among common mental disorders. In: Millon T, Krueger RF, Simonsen E (eds). Contemporary directions in psychopathology: scientific foundations of the DSM‐V and ICD‐11. New York: Guilford, 2010:223‐41. [Google Scholar]
- 26. Conway CC, Forbes MK, Forbush KT et al. A hierarchical taxonomy of psychopathology can transform mental health research. Perspect Psychol Sci 2019;14:419‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mirza S, Arfan Ikram M, Hofman A et al. Anxiety does not predict mortality. A population‐based study. World Psychiatry 2015;14:103‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mroczek DK, Spiro A. Personality change influences mortality in older men. Psychol Sci 2007;18:371‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eaton NR, Keyes KM, Krueger RF et al. An invariant dimensional liability model of gender differences in mental disorder prevalence: evidence from a national sample. J Abnorm Psychol 2012;121:282‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eaton NR, Krueger RF, Markon KE et al. The structure and predictive validity of the internalizing disorders. J Abnorm Psychol 2013;122:86‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim H, Eaton NR. The hierarchical structure of common mental disorders: connecting multiple levels of comorbidity, bifactor models, and predictive validity. J Abnorm Psychol 2015;124:1064‐78. [DOI] [PubMed] [Google Scholar]
- 32. Kessler RC, Ormel J, Petukhova M et al. Development of lifetime comorbidity in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry 2011;68:90‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boscarino JA. Posttraumatic stress disorder and mortality among US Army veterans 30 years after military service. Ann Epidemiol 2006;16:248‐56. [DOI] [PubMed] [Google Scholar]
- 34. Brim OG, Ryff CD, Kessler RC. How healthy are we?: A national study of well‐being at midlife. Chicago: University of Chicago Press, 2019. [Google Scholar]
- 35. Kessler RC, Abelson J, Demler O et al. Clinical calibration of DSM‐IV diagnoses in the World Mental Health (WMH) version of the World Health Organization (WHO) Composite International Diagnostic Interview (WMH‐CIDI). Int J Methods Psychiatr Res 2004;13:122‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kessler RC, Andrews G, Mroczek D et al. The World Health Organization Composite International Diagnostic Interview Short‐Form (CIDI‐SF). Int J Methods Psychiatr Res 1998;7:171‐85. [Google Scholar]
- 37. Kessler RC, Üstün TB. The World Mental Health (WMH) Survey Initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res 2004;13:93‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lachman ME, Weaver SL. The Midlife Development Inventory (MIDI) personality scales: scale construction and scoring. Waltham: Brandeis University, 1997. [Google Scholar]
- 39. Eaton NR, Krueger RF, Oltmanns TF. Aging and the structure and long‐term stability of the internalizing spectrum of personality and psychopathology. Psychol Aging 2011;26:987‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kleinbaum DG, Klein M. Survival analysis, 3rd ed. New York: Springer, 2010. [Google Scholar]
- 41. Hess KR. Assessing time‐by‐covariate interactions in proportional hazards regression models using cubic spline functions. Stat Med 1994;13:1045‐62. [DOI] [PubMed] [Google Scholar]
- 42. Ng'andu NH. An empirical comparison of statistical tests for assessing the proportional hazards assumption of Cox's model. Stat Med 1997;16:611‐26. [DOI] [PubMed] [Google Scholar]
- 43. Gorsuch RL. Factor analysis: classic edition. London: Taylor & Francis, 2014. [Google Scholar]
- 44. Kiecolt‐Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res 2002;53:873‐6. [DOI] [PubMed] [Google Scholar]
- 45. Hussong AM, Jones DJ, Stein GL et al. An internalizing pathway to alcohol use and disorder. Psychol Addict Behav 2011;25:390‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Forbes MK, Flanagan JC, Barrett EL et al. Smoking, posttraumatic stress disorder, and alcohol use disorders in a nationally representative sample of Australian men and women. Drug Alcohol Depend 2015;156:176‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. Int J Psychiatry Med 2011;41:15‐28. [DOI] [PubMed] [Google Scholar]
- 48. Strine TW, Mokdad AH, Dube SR et al. The association of depression and anxiety with obesity and unhealthy behaviors among community‐dwelling US adults. Gen Hosp Psychiatry 2008;30:127‐37. [DOI] [PubMed] [Google Scholar]
- 49. Carnethon MR. Physical activity and cardiovascular disease: how much is enough? Am J Lifestyle Med 2009;3(Suppl. 1):44S‐9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kohl HW. Physical activity and cardiovascular disease: evidence for a dose response. Med Sci Sports Exerc 2001;33:S472‐83. [DOI] [PubMed] [Google Scholar]
- 51. Ahmed HM, Blaha MJ, Nasir K et al. Effects of physical activity on cardiovascular disease. Am J Cardiol 2012;109:288‐95. [DOI] [PubMed] [Google Scholar]
- 52. Woodcock J, Franco OH, Orsini N et al. Non‐vigorous physical activity and all‐cause mortality: systematic review and meta‐analysis of cohort studies. Int J Epidemiol 2011;40:121‐38. [DOI] [PubMed] [Google Scholar]
- 53. Oguma Y, Shinoda‐Tagawa T. Physical activity decreases cardiovascular disease risk in women: review and meta‐analysis. Am J Prev Med 2004;26:407‐18. [DOI] [PubMed] [Google Scholar]
- 54. Borenstein M, Hedges L, Higgins J et al. Comprehensive Meta Analysis Version 2. Englewood: Biostat, 2005. [Google Scholar]
- 55. O'Leary KD, Christian JL, Mendell NR. A closer look at the link between marital discord and depressive symptomatology. J Soc Clin Psychol 1994;13:33‐41. [Google Scholar]
- 56. Trudel G, Goldfarb M. Marital and sexual functioning and dysfunctioning, depression and anxiety. Sexologies 2010;19:137‐42. [Google Scholar]
- 57. Comer JS, Blanco C, Hasin DS et al. Health‐related quality of life across the anxiety disorders. J Clin Psychiatry 2011;72:43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hecht H, von Zerssen D, Wittchen H‐U. Anxiety and depression in a community sample: the influence of comorbidity on social functioning. J Affect Disord 1990;18:137‐44. [DOI] [PubMed] [Google Scholar]
- 59. Sareen J, Jacobi F, Cox BJ et al. Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Arch Intern Med 2006;166:2109‐16. [DOI] [PubMed] [Google Scholar]
- 60. Andrews G. Should depression be managed as a chronic disease? BMJ 2001;322:419‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thompson KD, Leadbeater BJ, Ames ME. Reciprocal effects of internalizing and oppositional defiance symptoms on heavy drinking and alcohol‐related harms in young adulthood. Substance Abuse: Research and Treatment 2015;9(Suppl. 1):21‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wittchen H‐U. Natural course and spontaneous remissions of untreated anxiety disorders: results of the Munich Follow‐up Study (MFS). In: Hand I, Wittchen HU (eds). Panic and phobias 2. Berlin: Springer, 1988:3‐17. [Google Scholar]
- 63. Turiano NA, Graham EK, Weston S et al. Is healthy neuroticism associated with longevity? A coordinated integrative data analysis. Collabra: Psychology 2020;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]


