Abstract
Preventive approaches have latterly gained traction for improving mental health in young people. In this paper, we first appraise the conceptual foundations of preventive psychiatry, encompassing the public health, Gordon's, US Institute of Medicine, World Health Organization, and good mental health frameworks, and neurodevelopmentally‐sensitive clinical staging models. We then review the evidence supporting primary prevention of psychotic, bipolar and common mental disorders and promotion of good mental health as potential transformative strategies to reduce the incidence of these disorders in young people. Within indicated approaches, the clinical high‐risk for psychosis paradigm has received the most empirical validation, while clinical high‐risk states for bipolar and common mental disorders are increasingly becoming a focus of attention. Selective approaches have mostly targeted familial vulnerability and non‐genetic risk exposures. Selective screening and psychological/psychoeducational interventions in vulnerable subgroups may improve anxiety/depressive symptoms, but their efficacy in reducing the incidence of psychotic/bipolar/common mental disorders is unproven. Selective physical exercise may reduce the incidence of anxiety disorders. Universal psychological/psychoeducational interventions may improve anxiety symptoms but not prevent depressive/anxiety disorders, while universal physical exercise may reduce the incidence of anxiety disorders. Universal public health approaches targeting school climate or social determinants (demographic, economic, neighbourhood, environmental, social/cultural) of mental disorders hold the greatest potential for reducing the risk profile of the population as a whole. The approach to promotion of good mental health is currently fragmented. We leverage the knowledge gained from the review to develop a blueprint for future research and practice of preventive psychiatry in young people: integrating universal and targeted frameworks; advancing multivariable, transdiagnostic, multi‐endpoint epidemiological knowledge; synergically preventing common and infrequent mental disorders; preventing physical and mental health burden together; implementing stratified/personalized prognosis; establishing evidence‐based preventive interventions; developing an ethical framework, improving prevention through education/training; consolidating the cost‐effectiveness of preventive psychiatry; and decreasing inequalities. These goals can only be achieved through an urgent individual, societal, and global level response, which promotes a vigorous collaboration across scientific, health care, societal and governmental sectors for implementing preventive psychiatry, as much is at stake for young people with or at risk for emerging mental disorders.
Keywords: Young people, prevention, mental disorders, preventive psychiatry, psychosis, bipolar disorder, anxiety, depression, evidence‐based medicine, neurodevelopment, children, adolescents
According to the latest World Health Organization (WHO) Global Burden of Disease study, about one billion people of the total global population (7.5 billion) are affected by any mental disorder 1 , including psychotic, bipolar or common mental disorders such as depression and anxiety. Overall, about 50% of mental disorders start by the age of 14, and 75% start by the age of 242, 3. Young people account for 41% of the current global population (0‐14 years: 25.4% and 15‐24 years: 15.5% 4 ). Justifiably, mental disorders have been called “the chronic diseases of the young” 5 .
After their onset, mental disorders often persist, disrupting the capacity for young people to fulfil their potential6, 7, limiting access to mental 8 and physical9, 10, 11, 12 health care, and exposing them to poor education and reduced occupational opportunities 13 , stigma, social isolation, discrimination, and violation of human rights14, 15, 16. Young individuals suffering from mental disorders have higher morbidity and mortality risks for any reason (including suicide 17 ) than the general population, translating into a striking 10‐20 years reduction in life expectancy 18 .
The mental health of the younger generation, and indeed of our future, is already fragile and threatened by exceptional worldwide forces such as an ongoing pandemic, population migrations, economic uncertainties, the sustainability of ecosystems and climate changes 19 . An urgent individual, societal, and global level response is needed to reduce the incidence and burden of mental disorders in young people6, 20. Preventive approaches in psychiatry lagged behind somatic medicine 21 and emerged only a few decades ago, increasingly gaining traction. At the same time, future advancements require ongoing efforts to identify and overcome their limitations.
This paper addresses these issues, with a focus on reducing the incidence of psychotic, bipolar and common mental disorders. We first summarize the conceptual foundations of preventive psychiatry and then appraise the evidence supporting different preventive approaches in young people, as well as their current limitations. The knowledge reviewed is then used to develop a blueprint for future preventive research and practice to improve the mental health of young people.
DEFINING PREVENTIVE PSYCHIATRY
This section reviews core preventive psychiatry concepts and frameworks that hold relevance for assessing the evidence and limitations of prevention in young populations and informing future research.
Public health framework
“Possible measures of prevention” 22 for mental disorders have been advocated since the late 19th century. In the early 20th century, an individual with the lived experience of a mental disorder initiated the mental hygiene movement 23 , which generated new community practices for preventing mental disorders in young people 24 , establishing preliminary public health principles 25 of preventive psychiatry 26 . Therefore, historically, service users and the community have been key actors in the development of preventive psychiatry, a discipline which is closely intertwined with societal and cultural values.
Early work by Leavell and Clark (middle of 20th century) introduced a classification of prevention in medicine 27 , which was tailored on the pre‐pathogenesis (primary prevention: health promotion and specific protection) and pathogenesis (secondary and tertiary prevention) phases of syphilis 28 . Caplan, in 1964, classified prevention in mental health as follows: a) primary prevention, which “aims at reducing the incidence of new cases of mental disorder and disability in a population”; b) secondary prevention, which “aims at reducing the duration of cases (and therefore the prevalence) of mental disorders, which will inevitably occur in spite of the programs of primary prevention”; c) tertiary prevention, which “aims at reducing the community rate of residual defect, which is a sequel to acute mental illness” 29 .
In 1978, Strasser introduced a fourth level of “primordial prevention” to denote activities that prevented the penetration and appearance of risk factors (risk factors increase the likelihood of clinical events, while protective factors decrease this likelihood) into the population itself, as opposed to primary prevention which addresses risk factors to prevent diseases 30 . Finally, Bradford Hill defined nine criteria that may be considered in navigating the difficult question of causation versus plain association: strength of association, consistency across different situations, specificity and temporality between exposure and outcomes, biological gradient, biological plausibility, coherence with present knowledge, experiment (in laboratory and randomized trials), and analogy with similar classes of exposures and outcomes31, 32.
Gordon's framework
The original formulation of the public health framework was disease‐oriented, relying on mechanistic linearity of infectious diseases and identification of a clear‐cut biological onset. It also ignored epidemiological knowledge on statistical associations between risk/protective factors and clinical events, as well as multifactorial aetiopathologies with a long period of latency 33 . Furthermore, several disorders may be risk factors for other disorders, so all treatments could potentially be labelled as preventive interventions.
In 1983, Gordon 33 addressed these issues in the context of physical illnesses, reserving the term prevention for those individuals who were not “suffering from any discomfort or disability from the disease or disorder to be prevented”, thus excluding tertiary prevention as well as antecedents such as clinical high‐risk syndromes (see below). Furthermore, Gordon noted that the public health definitions of prevention had little correspondence to interventions offered, and proposed an alternative threefold classification based on the costs and benefits of delivering the intervention: a) universal prevention, “a measure that is desirable for everybody”, including actions for the general public which, in many cases, can be “applied without professional advice or assistance”; b) selective prevention, “a procedure [which] can be recommended only when the individual is a member of a subgroup of the population whose risk of becoming ill is above average”; c) indicated preventive measures, that “are advisable only for persons who, on examination, are found to manifest a risk factor, condition, or abnormality that identifies them, individually, as being at sufficiently high risk to require the preventive intervention” 33 .
As illustrated in Figure 1, while targeted approaches (i.e., selective and/or indicated) aim to reduce risk among those with the most to gain, and therefore reach a small proportion of the population, universal approaches aim to shift the risk profile of the whole population.
Figure 1.
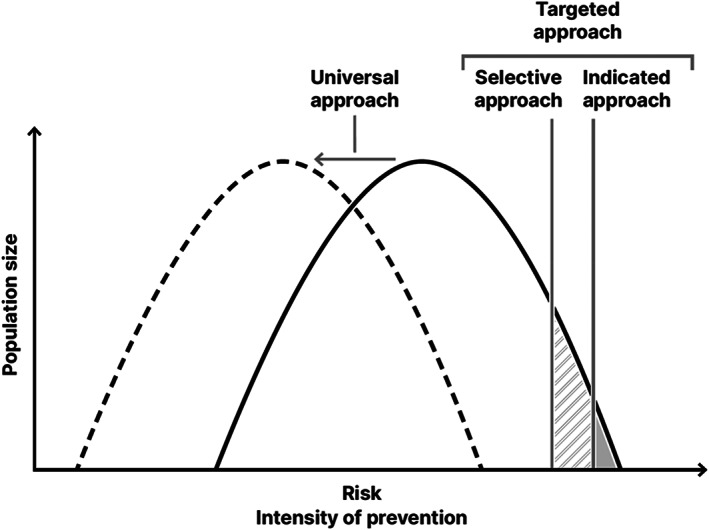
Universal, selective and indicated prevention. Selective and indicated approaches aim to reduce risk amongst those with the most to gain, and therefore reach a small proportion of the population. Universal approaches aim to shift the risk profile of the whole population.
US Institute of Medicine framework
Gordon's classification was not designed for use in mental disorders. In 1994, the US Institute of Medicine 34 noted that the definition of caseness is more difficult to establish in psychiatry than in somatic medicine, and that the presence of symptoms and dysfunctions is frequent even if diagnostic criteria (ICD/DSM) for mental disorder are not met. Prevention was thus refined as “reducing incidence, prevalence, recurrence of mental disorders, the time spent with symptoms, or the risk condition for a mental illness, preventing or delaying recurrences and also decreasing the impact of illness in the affected person, their families and the society” 34 . The Institute allowed indicated interventions to target antecedents of the disorder, such as clinical high‐risk syndromes 34 .
It was also acknowledged that, although some people receiving indicated preventive interventions may already have comorbid mental disorders, if they are selected into the intervention based on having early symptoms, then the intervention is still considered preventive 34 . Kessler and Price 35 later refined the concept as primary prevention of secondary psychiatric comorbidities.
The Institute also defined prevention screening to identify risk exposure at population level (for universal prevention efforts, e.g. poverty, violence, lack of health care) or at‐risk group/individual level (for selective prevention efforts, e.g. maternal depression or childhood abuse), or to identify core/distinctive characteristics in high‐risk individuals (for indicated prevention, e.g. attenuated symptoms, functional impairment or early phenotypic features). Core requisites of prevention screening are identifiable risk/protective factors linked to a disorder, availability of a validated screening tool, an effective intervention to address the identified factors and improve outcomes, solid guidelines on care pathways following screening, wide acceptability to the population, and dynamic implementation of screening procedures 34 .
WHO framework
In the current WHO framework (Table 1), universal, selective and indicated preventive interventions are all included within primary prevention 36 , and indicated approaches are allowed to target antecedents/clinical high‐risk syndromes (see below).
Table 1.
World Health Organization's classification of preventive approaches for mental disorders 36
| Public health classification of prevention | Gordon's classification of prevention 33 , modified by the US Institute of Medicine 34 |
|---|---|
| Primary prevention seeks to prevent the onset (incidence) of a disorder or illness. | |
| Universal prevention is defined as those interventions that are targeted at the general public or a whole population group that has not been identified on the basis of increased risk. | |
| Selective prevention targets individuals or subgroups of the population whose risk of developing a mental disorder is significantly higher than average, as evidenced by biological, psychological or social risk factors. | |
| Indicated prevention targets high‐risk people who are identified as having minimal but detectable signs or symptoms foreshadowing mental disorder, or biological markers indicating predisposition for mental disorders, but who do not meet diagnostic criteria for disorder at that time. | |
| Secondary prevention seeks to lower the rate of established cases of the disorder or illness in the population (prevalence) through early detection and treatment of diagnosable diseases. | |
| Tertiary prevention includes interventions that reduce disability, enhance rehabilitation and prevent relapses and recurrences of the illness. |
The WHO classifies the management of mental disorders as a continuum encompassing prevention (complementary universal, selective and indicated approaches), treatment (secondary prevention and early or standard treatment), and rehabilitation (tertiary prevention and long‐term care). The conceptual boundaries between preventive “interventions” (in “individuals”) and “treatments” (in “patients”), particularly in early management 37 , are porous at times and associated with several empirical, ethical and societal aspects.
Prevention of mental disorders vs. promotion of good mental health
The WHO broadly defines good mental health as “a state of well‐being in which the individual realizes his or her own abilities, can cope with the normal stresses of life, can work productively and fruitfully, and is able to make a contribution to his or her community” 36 . Therefore, mental health is much more than the absence of mental disorders.
Good mental health and mental disorder, although interrelated, are not on a one‐dimensional continuum. For example, empirical evidence has associated individual levels of creativity with psychotic or bipolar disorders38, 39, and this association has recently been confirmed at a genetic level 40 . Conversely, individuals without mental disorders do not necessarily have good mental health. Normally developing young people can display reactive mild anxiety or depression as physiological adaptive strategies aimed at harm avoidance and extinction of maladaptive behaviours 41 .
Therefore, mental health promotion can be implemented across all stages illustrated in Figure 2 (e.g., from healthy people to individuals affected with chronic mental disorders) 34 , and not only during the pre‐pathological phase (i.e., within primary preventive approaches, as suggested by Leavell and Clark 27 ). Promotion of good mental health could also be enhanced by improving physical health, given the close relatedness between these two domains 42 .
Figure 2.
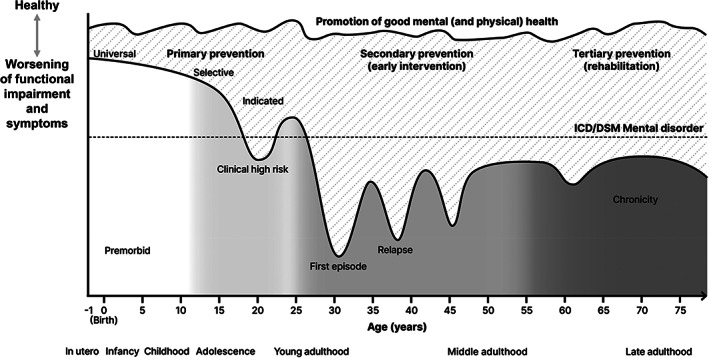
Neurodevelopmental continuum model for prevention of psychosis, bipolar disorder and common mental disorders, and promotion of good mental and physical health
Neurodevelopmental prevention of mental disorders in young people
As noted by Clark 28 , prevention “requires knowledge of the natural history” of a disease. Psychotic disorders are infrequent before the age of 14 43 ; their incidence peaks in the age group of 15‐35 and declines after the age of 35 44 . The average age of onset for bipolar disorder is 23 years, with a wide range (9 to 37) 45 . The median onset age is earlier for anxiety disorders (11 years of age) versus major depression (32 years) 2 . The range of the age of onset of depressive disorders is typically wider than for many other mental disorders 46 .
The pathophysiology of psychotic disorders is generally understood to originate from several genetic and non‐genetic risk/protective factors (and their interactions) that impact the neurodevelopment7, 47, 48. Early abnormalities of maturational changes appear from the ectodermal phase to the first year after birth (first‐wave hits) 49 . A further phase of significant neurobiological changes is from mid‐childhood through pubescence to mid‐20s (second‐wave hits) 47 , when the risk of disorder onset is the highest. Similar neurobiological models have been investigated for bipolar disorder50, 51 and depression 52 .
Clinical staging models 53 integrate these epidemiological and neurobiological findings (Figure 2) 47 . The clinical staging model for psychosis is the most established54, 55, but similar models have also emerged for bipolar56, 57, 58, 59, depressive60, 61 and anxiety62, 63, 64, 65 disorders. The premorbid stage starts during the perinatal period and is often asymptomatic and generally associated with preserved functioning (Figure 2). Accumulation of further risk factors from infancy to young adulthood could lead to the emergence of a clinical high‐risk stage (Figure 2), characterized by attenuated symptoms that do not meet the diagnostic threshold for mental disorders but are typically associated with some degree of functional impairment. These attenuated symptoms can then progress to a fully symptomatic mental disorder, and then persist into adulthood, especially if treated sub‐optimally, leading to a relapsing stage and eventually a chronic stage (Figure 2).
The period from the prenatal/perinatal phase to the onset of the first episode of the disorder may represent the most compelling window of preventive opportunity7, 55. By integrating the preventive framework within a neurodevelopmentally sensitive clinical staging model, primary prevention (universal, selective and indicated) and promotion of good mental (and physical) health 7 emerge as core strategies to target this critical window (Figure 2).
EVIDENCE SUPPORTING PRIMARY PREVENTION AND MENTAL HEALTH PROMOTION IN YOUNG PEOPLE
This section reviews the evidence supporting indicated, selective and universal preventive interventions and promotion of good mental health, reflecting the increasing width of these approaches from relatively small subgroups to the wider population (Figure 1).
Indicated preventive interventions
The available evidence supporting indicated preventive interventions for psychotic, bipolar and common mental disorders is summarized in Table 2.
Table 2.
Level of evidence for available indicated interventions to prevent (reduce the incidence) of psychotic, bipolar and common (depression/anxiety) mental disorders in young people
| Psychotic disorders | Bipolar disorder | Depression/Anxiety disorders | |
|---|---|---|---|
|
Target |
Clinical high risk for psychosis (CHR‐P) 72 **** |
Not available |
|
| Detection | |||
| Referral, risk enrichment | On suspicion of psychosis risk, 15% at 3 years 82 *** | On suspicion of bipolar risk | Screening in schools, universities or primary care114, 115*** |
| Screening instruments (sensitivity, specificity, positive predictive value, negative predictive value) | Several, but poor validation (67‐100%, 39‐100%, 24‐100%, 58‐100%) 83 ** |
BPSS‐AS‐P (data not available) 103 * |
Some, but none validated (data not available) 114 *** |
| Duration of attenuated symptoms | 709 days 72 **** | 107.9 months 98 *** | Not available |
| Mean age (SD) or range | 21 (3.2) years 72 **** | 16‐23 years103, 104, 105* | 18‐25 years 113 *** |
| Prognosis | |||
| Assessment instruments (accuracy) |
CAARMS 287 ***, SIPS288***, DSM‐5 APS 288 *** (0.90 pooled at 38 months) 74 *** Not recommended outside clinical samples 74 *** |
BPSS‐FP (data not available) 104 *, SIBARS (0.7 at 18 months) 105 * |
Not available |
| Transition risk | 17% at 1 year; 22% at 3 years (BLIPS>APS>GRD) 88 *** | 14% at 1 year 289 *; 23% at 2 years 105 * | Not available |
| Intervention | |||
| Type of intervention (efficacy) |
Needs‐based interventions, psychotherapy, pharmacotherapy, combinations (no evidence for superior efficacy in preventing psychosis or improving other outcomes)93, 94***,72,251**** |
Family‐focused therapy (reduced time to recovery, no effect on incidence of bipolar disorder) 106 * Individual psychotherapy (no efficacy on affective symptoms) 107 * |
Psychotherapy/psychoeducation (reduced severity of depressive/anxiety symptoms113, 115***, but not with digital psychoeducation 119 *** and not in humanitarian settings 120 ***; no evidence of effect on incidence of depressive/anxiety disorders113, 115***) |
* single study, ** systematic review, *** meta‐analysis, **** umbrella review. APS – attenuated psychotic symptoms, BLIPS – brief limited intermittent psychotic symptoms, BPSS‐AS‐P – Bipolar Prodrome Symptom Scale ‐ Abbreviated Screen for Patients, BPSS‐FP – Bipolar Prodrome Symptom Interview and Scale‐Full Prospective, CAARMS – Comprehensive Assessment of At Risk Mental States, GRD – genetic risk and deterioration syndrome, SIBARS – Semistructured Interview for Bipolar At Risk States, SIPS – Structured Interview for Psychosis‐Risk Syndromes.
Psychosis
Indicated prevention of psychosis originated in Australia about twenty‐five years ago 66 and subsequently gained traction globally, leading to the implementation of specialized services 67 taking care – according to a survey carried out in 2017‐2018 – of over than 22,000 young individuals across Western Europe (51.1%), North America (17.0%), East Asia (17.0%), Australia (6.4%), South America (6.4%) and Africa (2.1%)67. The consolidation of this paradigm in clinical practice has impacted national 68 and international 69 clinical guidelines and diagnostic manuals (e.g., DSM‐5 attenuated psychosis syndrome 70 ), although not everywhere 71 .
Young (typically 14‐35 years old, mean age 21 years 72 ) individuals at clinical high risk for psychosis (CHR‐P)73, 74 accumulate several risk factors for the disorder44, 75, 76, which can lead to functional impairments 77 and the emergence of attenuated psychotic symptoms 78 (which last on average 2 years 72 ). Because of these problems, these individuals often seek help 79 , including at specialized CHR‐P clinical services when available67, 80, 81.
Detection of CHR‐P individuals is unsystematic and mostly based on referrals made on suspicion of psychosis risk by several agencies and idiosyncratic sampling strategies. This recruitment phase nevertheless leads to substantial risk enrichment in help‐seeking samples 82 . Although several screening instruments for CHR‐P have been tested, their validation is currently limited 83 .
In CHR‐P clinics, help‐seeking individuals undergo a semi‐structured psychometric assessment with validated instruments, which deliver a group‐level estimate for predicting psychosis (i.e., at risk vs. not at risk) 74 . The CHR‐P criteria are robustly associated with psychosis onset (odds ratio, OR=9.32) 44 within high‐risk clinical samples (but not in the general population 84 ), while they cannot predict new cases of bipolar or common mental disorders85, 86.
In CHR‐P samples, most (~85%) individuals present with attenuated psychotic symptoms (APS), ~10% with short‐lived frank psychotic symptoms (brief and limited intermittent psychotic symptoms, BLIPS), and ~5% with schizotypal traits or a relative affected with psychosis coupled with functional decline (genetic risk and deterioration, GRD) 72 . Since most (68%) individuals with BLIPS also meet ICD‐10 criteria for an acute and transient psychotic disorder 87 , interventions in CHR‐P people extend beyond primary indicated prevention (for APS and GRD) into secondary prevention (for BLIPS). The overall risk of developing psychosis (22% at 3 years) differs across these three subgroups 88 .
Transition to psychosis is associated with clinically meaningful real‐world outcomes 89 and is modulated by baseline levels of attenuated positive psychotic (OR=2.56) and negative (OR=2.68) symptoms, while good functioning reduces the risk (OR=0.59) 44 .
Indicated prevention implemented in CHR‐P services (the NICE‐recommended intervention is cognitive behavioural therapy 68 ) has the potential to ameliorate presenting symptoms, delay or prevent the onset of psychosis, reduce health care access and duration of untreated psychosis (secondary prevention)55, 90. Furthermore, CHR‐P services routinely incorporate comprehensive needs‐based interventions focusing on psychosocial, vocational and familial requirements, as well as several public health initiatives such as outreach campaigns in collaboration with the local community (e.g., non‐governmental organizations, youth centres, schools, colleges, faith groups; low‐income, racial/ethnic, sexual and gender minorities) to foster mental health literacy (e.g., reducing illicit substances use, enhancing self‐coping strategies) and promote good mental (e.g., resilience, positive lifestyle behaviours) and physical health 72 .
Earlier meta‐analyses of randomized controlled trials suggested a significant preventive effect for psychological interventions91, 92. However, the most updated network meta‐analysis 93 found no robust evidence to favour any of these indicated interventions compared to each other or needs‐based interventions. A second independent pairwise meta‐analysis by the Cochrane group confirmed these findings, concluding that “there was no convincing unbiased, high‐quality evidence” to suggest that any type of intervention is more effective than others, including needs‐based interventions 94 (another meta‐analysis was recently published 95 , but used older data than the above‐mentioned ones93, 94).
Bipolar disorder
Indicated prevention in bipolar disorder was developed, following the CHR‐P template, only fifteen years ago96, 97, and is rapidly emerging98, 99, 100, 101. The supporting evidence lags behind that for CHR‐P99, 102.
Detection of a symptomatic clinical high risk for bipolar disorder is complicated by its inherent episodicity, long duration and the complex nature and definition of the disorder 98 . Individuals at clinical risk for bipolar disorder are represented by young help‐seeking clinical samples 97 (mean age 16‐23 years103, 104, 105), including a subset of CHR‐P individuals 105 , who present with attenuated bipolar‐risk features (which last on average 9 years 98 ). Self‐administered screening instruments have been developed, but require further validation 103 .
With respect to assessment, early manifestations – such as sleep disturbance, anxiety, irritability, cyclothymic features, manic or hypomanic symptoms, and depression – are non‐specific 100 . Emerging semi‐structured interviews can rate sub‐threshold manic, depressive and general symptoms 104 to define high‐risk subgroups in clinical samples: sub‐threshold mania, depression and cyclothymic features, genetic risk and depression, genetic risk and cyclothymic features, sub‐threshold mixed episode, mood swings 105 . The prospective validity of these instruments awaits validation, despite some promising pilot findings 105 .
Interventional research is in its infancy. Two randomized controlled trials conducted in young people presenting with genetic risk for (schizo)affective disorder and attenuated affective symptoms suggested a potential beneficial effect of family‐focused and cognitive behavioural therapy on time to recovery from attenuated symptoms 106 , but no efficacy in terms of reducing the severity of affective symptoms 107 or preventing the onset of bipolar disorder 106 .
Common mental disorders
Indicated prevention of depression and anxiety disorders in young people still represents a “blind spot in health care”108, 109, 110 and has been less investigated than selective/universal approaches 111 . There is also some degree of overlap with indicated prevention for bipolar disorder, because sub‐threshold/frank depressive episodes (especially the atypical phenotype) and cyclothymic features or genetic risk for depression coupled with bipolar‐like features are already subsumed in the clinical criteria for bipolar risk 112 .
Young people 113 at clinical high risk for depression/anxiety disorders have been detected through psychometric screening for sub‐threshold symptoms in schools, universities or primary care114, 115, typically following selective/universal screening 116 . However, results do not suggest that such screening is ready for wider use. Beyond these attempts, there are no established clinical high‐risk criteria to assess young people with an increased risk of depression (without bipolar risk features) or anxiety disorders and predict their outcomes.
Early meta‐analyses not focusing on young individuals showed that indicated psychological interventions, generally based on cognitive behavioural therapy, can reduce the incidence of depression114, 117, and that these interventions can be effectively delivered digitally in middle‐aged adults 118 . However, the most recent meta‐analysis focusing on young people with baseline sub‐threshold depression (along with selective/universal approaches) found that none of the included psychological intervention studies measured the incidence of emerging depression 113 . Another recent meta‐analysis confirmed that there is no evidence favouring digital psychoeducation over no intervention to improve depressive symptoms in young people 119 .
Meta‐regression analyses showed that psychological/psychoeducational interventions might be effective in reducing the severity of some anxiety symptoms in young people, but no conclusion could be drawn concerning prevention of the onset of anxiety disorders 115 . A meta‐analysis showed that indicated psychological/social interventions are not effective to prevent anxiety/depression in people living in low‐ and middle‐income countries affected by humanitarian crises 120 .
Selective preventive interventions
Selective preventive interventions in the premorbid stage of psychotic, bipolar and common mental disorders (summarized in Table 3) would require screening and reducing the exposures to identified detrimental factors in at‐risk groups before symptoms and help‐seeking behaviour manifest 76 .
Table 3.
Level of evidence for at‐risk group exposures and available selective interventions to prevent (reduce the incidence) of psychotic, bipolar and common (depression/anxiety) mental disorders in young people
| Psychotic disorders | Bipolar disorder | Depression/Anxiety disorders | |
|---|---|---|---|
|
At‐risk group exposures (association with the disorder) |
Genetic risk/protective factors: 22q11.2 deletion syndrome (prevalence 10‐41% 132 *, risk 37% at 32 months 135 *) Offspring (RR=7.54) 121 *** Twins (monozygotic concordance rate 40%) 123 * First‐degree relatives (one proband: OR=7.69; two probands: OR=11.11) 127 *** Non‐genetic risk/protective factors: Black‐Caribbean ethnicity in England (OR=4.87) 44 **** Ethnic minority in low ethnic density area (OR=3.71) 44 **** Second‐generation immigrants (OR=1.68) 44 **** Trait anhedonia (OR=4.41) 44 **** Minor physical anomalies (OR=5.30) 44 **** Premorbid IQ (OR=0.47) 44 **** Olfactory identification ability (OR=0.19) 44 **** Several prenatal/perinatal factors (OR=0.86 to 3.05) 150 *** Physical activity (OR=0.728) 235 **** Smoking (OR=1.99) 235 **** Peripheral biomarkers Decreased pyridoxal (vitamin B6) levels (data not available) 147 **** |
Genetic risk/protective factors: Offspring (RR=4.06) 121 *** Twins (monozygotic concordance rate 45%) 124 * First‐degree relatives (one proband: RR=6.10, two probands: RR=29.1) 128 * Non‐genetic risk/protective factors: Irritable bowel syndrome (OR=2.48) 144 **** Childhood adversity (OR=2.86) 144 **** Physical activity (OR=0.49) 235 **** Smoking (OR=1.46) 235 **** Poor sleep (OR=1.79) 235 **** Peripheral risk/protective biomarkers: Elevated awakening cortisol levels (g=0.25) 147 **** |
Genetic risk/protective factors: Offspring (depression: RR=2.38121***; anxiety: RR=1.76 122***) Twins (monozygotic concordance rate – depression: 46% 126 *; anxiety: 13‐73% 125 *) First‐degree relatives (anxiety: OR=4.1‐6.1129*; depression: one proband OR=2.14, two probands OR=3.23130***) Non‐genetic risk/protective factors: Sedentary behaviour (RR=1.25) 145 **** Sexual dysfunction (OR=2.71) 145 **** Four or five metabolic risk factors (OR=2.06) 145 **** Obesity (OR=1.35) 145 **** Job strain (OR=1.77) 145 **** Physical abuse in childhood (OR=1.98) 145 **** Early physical trauma (OR=2.59) 146 **** Physical activity (OR=0.837) 235 **** Smoking (OR=1.73) 235 **** Healthy diet (OR=0.77) 235 **** Poor sleep (OR=2.27) 235 **** |
| Type of intervention (efficacy) |
Screening for family history of psychotic disorder (data not available) 132 * Screening pregnant/postnatal women for emerging psychopathology (data not available) 148 *** |
Psychoeducation for young people at risk (improved affective symptoms but no evidence of effect on incidence of bipolar disorder) 155 *** |
Screening for family history of depression and psychoeducation (improved depressive symptoms and reduced incidence of depression in offspring) 136 *** Screening for post‐partum depression and psychoeducation/psychotherapy (inconclusive evidence) 152 *** Psychological interventions in women disclosing partner violence (improved anxiety but not depression) 153 *** Psychological/psychoeducation (improved anxiety symptoms 115 ***, but not as school‐based interventions 156 *** and not in humanitarian settings 120 ***; no evidence of effect in preventing depression/anxiety disorders 115 ***) Physical exercise in at‐risk youths (reduced severity of depression 157 *** and incidence of anxiety 174 ***) |
| Behavioural counselling to prevent illicit substance use in at‐risk adolescents and young adults (no evidence of efficacy) 154 *** | |||
* single study, ** systematic review, *** meta‐analysis, **** umbrella review. OR – odds ratio, RR – risk ratio
This approach would require robust aetiopathological knowledge of the association between specific genetic and non‐genetic factors and incidence of these disorders (and effective interventions). However, comprehensive explanatory pathophysiology is not established in psychiatry, and no singular putative causal factor fully meets Bradford Hill criteria, so that current diagnostic manuals (ICD‐11/DSM‐5) refer to mental syndromes (i.e., disorders) and not pathophysiological processes (i.e., diseases).
Genetic factors
Many genetic variants have been identified that modulate the risk for psychotic, bipolar or common mental disorders, but almost all of them have very small, and thus clinically unclear, effects for selective screening. Polygenic risk scores have been developed to overcome these limitations by analyzing genetic variants en masse 48, but the variance explained is still too small for implementation in selective prevention and does not provide singular neurobiological targets.
For example, offspring of patients affected with psychosis, bipolar disorder or depression have a greater risk of developing these disorders (32% by adulthood)121, 122. Monozygotic twins123, 124, 125, 126 and first‐degree relatives (depending on the number of probands)127, 128, 129, 130 also have an increased likelihood of developing these disorders. However, only 17.4% of the association between family history of psychosis and the disorder is mediated through a modelled polygenic risk score 131 . The only molecular risk factor for psychosis that may have a preventive relevance is the 22q11.2 deletion syndrome, which is characterized by high rates of schizophrenia (prevalence from 10% in adolescents to 41% in young adults) 132 .
Overall, familial vulnerability (along with 22q11.2 deletion syndrome) represents the most implementable target for selective screening intervention in health care 133 . It is more established for psychosis 134 , but it is emerging for bipolar disorder. One possible intervention could be monitoring and psychometric assessment for a CHR‐P/bipolar‐risk state when symptoms or functional disability develop 135 .
The associated preventive capacity is, however, limited: while a meta‐analysis found that selective psychoeducational interventions may have a small effect on reducing the severity and incidence of depression in the offspring of patients 136 , the preventive efficacy of other psychosocial interventions in young individuals with a familial vulnerability for psychotic 137 , bipolar 138 or anxiety disorders is currently unknown.
Non‐genetic factors
Similarly, non‐genetic factors have not yet entered selective screening139, 140. This situation is mostly due to the intrinsic complexity of the psyche itself 141 , and conflicting research findings that are characterized by several biases such as high heterogeneity, excess significance, selective reporting of statistically significant (i.e., “positive”) results and no adjustment for multiple confounders142, 143. Table 3 lists non‐genetic factors, along with their meta‐analytic strength of association (according to established criteria to classify the evidence) with psychotic 44 , bipolar 144 , depressive 145 and anxiety 146 disorders.
Among 733,316 measurements on 162 different peripheral biomarkers for psychosis, bipolar disorder and depression, only two were found to be reliably associated with these disorders 147 (see Table 3). Studies targeting inflammatory biomarkers using anti‐inflammatory therapies like aspirin 148 or targeting individual nutrients such as vitamin D 149 to prevent depression have not turned out to be effective approaches, at least in adults, dampening hopes in youth 133 .
Within risk/protective factors listed in Table 3 (their distinction from biomarkers may be challenging without clear pathophysiological knowledge), the majority exert their role before the age of 25 years, and some are potentially modifiable in vulnerable groups. For example, the evidence concerning several prenatal/perinatal risk factors laid the rationale for screening pregnant/postnatal women for emerging psychopathology in order to detect an incipient risk of psychosis or post‐partum depression150, 151. However, the risk may not be high enough to make such screening clinically useful. Furthermore, a meta‐analysis investigating psychological/ psychoeducational selective interventions (along with universal/indicated ones) to prevent post‐partum depression in pregnant/postnatal women 151 found considerable cost‐effectiveness uncertainty 152 .
Women disclosing current or recent intimate partner violence exposure represent another vulnerable group. A meta‐analysis found that selective psychological interventions can reduce their anxiety (but not depression) even in in low/middle‐income countries 153 .
Another potentially modifiable risk factor selectively targeted across psychotic, bipolar and common mental disorders has been the initiation of illicit and non‐medical drug use among adolescents and young adults. However, a recent meta‐analysis by the US Preventive Service Task Force found no evidence to favour selective (as well as population‐level/universal) behavioural counselling 154 .
Selective psychological/social interventions are not effective to prevent anxiety/depression in humanitarian settings 120 , and there is scarce preventive research in other vulnerable subgroups such as racial/ethnic, sexual and gender minorities.
Selective approaches have also been tested in various subgroups of at‐risk youths. A recent meta‐analysis reviewed the efficacy of selective (along with universal) interventions for young people (across different settings), finding that psychoeducation may be the most effective preventive intervention for improving affective symptoms (Hedges' g=0.6), but there was no efficacy on the incidence of mood disorders 155 . Another meta‐regression analysis showed that selective (as well as universal) psychological/psychoeducational interventions delivered across different settings (e.g., community schools and colleges, primary care clinics) might be effective in reducing some anxiety symptoms in young people, although findings were inconclusive regarding prevention of depression/anxiety disorders 115 . Recent sensitivity (network) meta‐analyses found little evidence that selective (and universal/indicated) school‐based educational interventions are effective for the prevention of common mental disorders in young people 156 .
Importantly, a recent umbrella review has documented that an exercise intervention may be effective in reducing depressive symptoms in at‐risk youths 157 . However, even the possible benefits at the level of symptoms may be due to selective reporting and other biases for what are largely subjective outcomes in unmasked trials.
Universal preventive interventions
As shown in Figure 1, universal preventive strategies (summarized in Table 4) would theoretically allow a population‐wide reduction in incidence/burden of psychosis, bipolar and common mental disorders in young people, producing wider societal‐level benefits compared to indicated/selective measures.
Table 4.
Level of evidence for population‐level exposures and available universal interventions to prevent (reduce the incidence) of psychotic, bipolar and common (depression/anxiety) mental disorders in young people
| Psychotic disorders | Bipolar disorder | Depression/Anxiety disorders | |
|---|---|---|---|
| Population‐level exposures (association with the disorder) |
Surrogate markers: psychotic experiences (risk of psychosis 0.5‐1 per year) 290 *** Neurodevelopmental biomarkers (data not available)164, 165, 167* |
Surrogate markers: K6/10 (data not available) 163 * | Surrogate markers: K6/10 (data not available) 163 * |
|
Social determinants of mental disorders (data not available) 173 **** Demographic (community diversity, population density, longevity, survival) Economic (economic recessions, economic inequalities, macroeconomic policy) Neighbourhood (infrastructure, neighbourhood deprivation, built environment settings) Environmental events (natural/industrial disasters, war or conflict, climate change, forced migration) Social/cultural (community social capital, social stability, culture) | |||
| Type of intervention (efficacy) |
Screening for psychotic experiences (data not available) 161 * Perinatal phosphatidylcholine (modulated biomarkers of neonatal brain development 164 *; fewer attention problems and less social withdrawal 165 *); perinatal folate acid (improved executive functioning) 167 *; vitamin D, polyunsaturated fatty acids (inconclusive evidence) 166 ** |
Screening for bipolar experiences (data not available) 163 * Psychoeducation for young people (improved affective symptoms but no evidence of effect on incidence of bipolar disorder) 155 *** |
Screening for depressive/anxiety experiences (data not available) 163 * Psychological/psychoeducation (improved anxiety symptoms 115 ***, but not as school‐based interventions 156 *** and not in humanitariansettings 120 ***; no evidence on preventing depression/anxiety disorders 115 ***) Public health strategies on school climate (improved depressive symptoms)171* Physical exercise (reduced incidence of anxiety disorders) 174 *** |
|
Reduction of gender‐based violence, child maltreatment, racial discrimination and xenophobia; basic income grants and improved employment; safe neighbourhoods; reductions in violence; early response to environmental events; action on protecting vulnerable ecosystems; improved education (data not available) 173 **** Behavioural counselling to prevent illicit substance use in adolescents and young adults (no evidence of efficacy) 154 *** | |||
* single study, ** systematic review, *** meta‐analysis, **** umbrella review. K6/10 – Kessler Distress Scale 6‐ or 10‐item
Universal strategies may take the form of a safe intervention that: a) decreases exposures to population‐level risk factors (most of the at‐risk group exposures listed in Table 3 could be, in principle, considered as well for population‐level universal approaches) and/or b) increases exposure to population‐level protective factors. However, pathophysiological knowledge is limited, and there is a lack of methods to readily assess the efficiency of such interventions.
In line with Gordon's observations, psychosis and bipolar disorder are characterized by a low incidence and long latency between exposures and the manifestation of the disorders (the latter point also applies to common mental disorders). Demonstrating an impact on the incidence of these disorders would be, if at all feasible, long and expensive 158 .
Decreasing exposures to population‐level risk factors
A possible avenue may be to use surrogate population‐level markers that may predict the effect of universal interventions on the incidence of disorders and that are convenient to measure. For example, “psychotic experiences” 159 are relatively frequent at the population level (prevalence about 8% in young adults aged 24 160 ) and can be measured through self‐administered questionnaires (e.g., Prodromal Questionnaire, PQ) 161 . These mostly transitory sub‐threshold manifestations are not to be conflated with clinical psychotic symptoms (see below) 162 , but could represent a potential surrogate marker of psychosis (risk of psychosis: 0.5‐1% per year 160 ). Other self‐administered instruments, such as the Kessler Psychological Distress Scale (6 or 10 items, K6/10) 163 , could theoretically be used as surrogate markers for bipolar, depressive and anxiety disorders. However, to date, there is no preventive capacity associated with these surrogate markers.
Similarly, neurodevelopmental surrogate biomarkers have been used to test dietary phosphatidylcholine supplementation in healthy pregnant women 164 . Phosphatidylcholine is an agonist at alpha‐7 nicotinic receptors, which are involved in the final maturation of GABA inhibitory synapses before birth, and have been implicated in schizophrenia 164 . A first randomized controlled trial confirmed the effect of perinatal phosphatidylcholine on an electrophysiological biomarker of foetal development 164 . A subsequent study demonstrated that, at 40 months, phosphatidylcholine impacted neurocognitive biomarkers, leading to fewer attention problems and less social withdrawal compared with the placebo group, thus potentially altering the risk of later development of psychosis 165 .
Another dietary intervention involved folic acid supplementation in pregnancy (folate is important in neurogenesis, cell growth and proliferation, and myelination), which has become one of the most important public health advances in medicine 166 . A randomized controlled trial demonstrated that folate supplementation could improve some neurocognitive biomarkers in children 8.5 years later 167 . Other compounds for use in pregnancy (vitamin D 168 , polyunsaturated fatty acids 166 ) have been suggested, but no randomized controlled trials have been conducted, and the overall evidence is inconclusive 166 .
Several other compounds have demonstrated hints of efficacy on experimental neurodevelopmental biomarkers (e.g., neonatal N‐acetylcysteine 62 , sulphoraphane 169 , modulation of microbiota 170 ) and are under investigation in humans (not listed in Table 3). However, these surrogate markers have not been well validated, and thus it is unknown whether they would indeed translate to preventive benefits. Furthermore, it is important to have fully pre‐registered protocols, including details on which biomarkers will be collected and how/when they will be analyzed. The large number of markers and analytical options allows for a situation where spurious “positive” results may emerge/be more likely published.
Beyond surrogate markers, universal psychoeducation and psychological interventions 115, 155, 156 have been frequently tested (blended with selective interventions, Table 3) for young people. Psychological interventions may improve affective symptoms 155 , while psychotherapy/psychoeducation may improve some anxiety symptoms 115 (but not as school‐based education intervention 156 ). Multi‐component public health and youth engagement strategies impacting the overall school climate (rather than individual behaviour change) may improve depressive symptoms (along with physical health outcomes)171, 172. However, there is no evidence that they can impact the incidence of depression/anxiety disorders 115 . As noted above, universal interventions were not effective to prevent illicit substance use in the general adolescent and young adult population 154 , or to prevent common mental disorders in humanitarian settings 120 .
To date, the most established population‐level exposures encompass social determinants of mental disorders, which have become the cornerstone of public health prevention. A large umbrella review has summarized about 300 (mostly observational) papers on social determinants of psychotic, bipolar and common mental disorders, and empirically linked them with the Sustainable Development Goals promoted by the United Nations Member States in 2015 (demographic, economic, neighbourhood, environmental events, social and cultural domains) 173 . For example, there is strong evidence that adverse social and economic circumstances – including poverty, income inequality, interpersonal and collective violence, and forced migration – are key risk determinants of psychotic disorders 173 .
The umbrella review identified several interventions that lie at the interface between universal, primordial and promotion approaches and could potentially lead to high benefit for young people: reduction of gender‐based violence, child maltreatment, racial discrimination and xenophobia, basic income grants and improved employment, safe neighbourhoods, reductions in violence, early response to environmental events, action on protecting vulnerable ecosystems, and improved education 173 . However, the review acknowledged that future trials should demonstrate the direct effect of these interventions on psychotic, bipolar or common mental disorders; furthermore, many implementation challenges remain unresolved 173 .
Increasing exposures to population‐level protective factors
Current evidence is mostly limited to the promotion of good mental health (reviewed below). Other approaches have focused on universal physical exercise interventions in young people, to foster resilience and additionally relieve the associated physical health burden. A recent umbrella review has demonstrated that an exercise intervention may be potentially effective in reducing the incidence of anxiety 174 in the general young population. Universal exercise interventions have also been suggested for psychotic 175 and bipolar 176 disorder. Interventions promoting positive lifestyle behaviours are under development (see below). However, there is not yet solid evidence demonstrating that these interventions can prevent psychotic, bipolar or common mental disorders (see below).
Promotion of good mental health
Promotion of good mental health (not summarized in Tables 2, 3, 4) has received less research attention than prevention of mental disorders, mostly because operationalization of outcomes have been fragmented 41 . Mental health promotion is also highly sensitive to different systems, cultures or clinical practices that differ in values. However, core domains of good mental health have been empirically proposed 177 , encompassing mental health literacy, attitude towards mental disorders, self‐perceptions and values, cognitive skills, academic/occupational performance, emotions, behaviours, self‐management strategies, social skills, family and significant relationships, physical health, sexual health, meaning of life, and quality of life 41 .
The consistency and magnitude of available interventions to promote good mental health in young people are similarly patchy and conflicting, comprising psychoeducation (including parent training)178, 179, psychotherapy180, 181, and less frequently physical therapy 182 , pet 183 or art 184 therapy.
A meta‐analysis appraised the efficacy of these interventions aimed to promote good mental health in asymptomatic young people 185 . Compared to controls, available interventions significantly improved mental health literacy (Hedges' g=0.685), emotions (g=0.541), self‐perceptions and values (g=0.490), quality of life (g=0.457), cognitive skills (g=0.428), social skills (g=0.371), physical health (g=0.285), sexual health (g=0.257), academic/occupational performance (g=0.211) and attitude towards mental disorders (g=0.177) 185 . Another recent umbrella review showed that positive psychology could increase subjective well‐being 186 . Although several interventions could be effective, evidence was of modest quality, and it is unknown whether these interventions can later impact the incidence of psychotic, bipolar or common mental disorders.
FUTURE DIRECTIONS OF RESEARCH AND PRACTICE
In this section, we integrate the conceptual frameworks with the evidence reviewed and suggest ten core ways toward advancing research and practice to prevent psychotic, bipolar and common mental disorders in young people.
Universal or targeted? Integrating preventive frameworks
An intense debate has lately centred on the antithesis between targeted and universal interventions for young people. Some authors 187 have split the field into proponents188, 189, 190, opponents191, 192, 193, and those with ambivalent attitudes 194 towards targeted interventions. A frequent criticism is that indicated prevention implemented in CHR‐P clinics should be replaced by universal/public health approaches, aimed for example to decrease cannabis use (an environmental risk factor for psychosis) in young people 195 . Similar criticisms are emerging for the indicated prevention of clinical high‐risk states for bipolar disorder196, 197. The overarching supporting argument is that targeted interventions represent a “prevention paradox” 187 , because they can only benefit a small minority of young people 198 .
It is true that CHR‐P clinics can currently detect only a minority of individuals who will later develop psychosis 199 (similarly, early intervention services can only detect about half of first episode cases 200 ), but research innovations to overcome this limitation are under development198, 201. Notably, this criticism overlooks the fundamental conceptual point illustrated in Figure 1: targeted approaches are expected a priori to target the tip of the iceberg of the population‐level risk, and are thus complementary and not antithetical to universal approaches. Furthermore, mainstreaming universal approaches to reduce cannabis abuse holds only theoretical foundation, because these approaches are not empirically effective in children, adolescents and young adults 154 .
Future research and clinical practice should better incorporate the continuum model for preventive psychiatry illustrated in Figure 2, which integrates universal, selective and indicated approaches to synergistically and complementarily maximize their efficiency in young people, and indeed across the age spectrum. For example, school‐based interventions to prevent anxiety and depression in children and young people are conceived as multilevel, systems‐based interventions 156 that encompass different modalities. Another example concerns the quest for effective suicide prevention initiatives in young people, where no single strategy clearly stands above the others, and combinations of individual‐ and population‐level strategies have been recommended 202 . A further example may be the implementation of a stepped or sequential assessment framework encompassing face‐to‐face CHR‐P or bipolar‐risk assessment (indicated prevention) following universal screening with self‐assessment instruments (e.g., PQ, K6/10) 203 , and the enhancement of public health approaches already partially implemented by CHR‐P services in the local community. Available meta‐analyses show that targeted and universal interventions can be blended together in young people to help preventing postnatal depression 152 or anxiety 115 .
In line with these arguments, the Lancet Commission on Global Mental Health called for a joint global initiative on preventive psychiatry integrating public health/universal and targeted approaches 173 . However, if single interventions are not effective, it is yet unclear how exactly their combination could be optimally effective.
Advancing multivariable, transdiagnostic, multi‐endpoint epidemiological knowledge
As noted by Leavell and Clark 27 , robust genetic and environmental epidemiological knowledge is required to inform evidence‐based preventive approaches. We have demonstrated above that this knowledge is currently limited, and several advancements are needed.
To date, non‐genetic factors have been mostly measured in univariate analyses that cannot control for their intercorrelation. Future epidemiological studies are required to augment polygenic risk prediction by collecting multiple non‐genetic exposures in the same individuals, using poly‐environmental risk scores (e.g., psychosis poly‐risk score, PPS) recently developed 204 , and exploring their interaction with lifestyle behaviours (see below).
Environmental exposures can be measured with digital health technologies (electronic medical records, mobile apps) 205 , but pose more challenges to measure massively: for example, measurement error, missing data and selection biases may be prominent, and operational definitions of environmental exposures may vary across and even within datasets. Collaborative harmonization efforts should mitigate these obstacles and integrate polygenetic and poly‐environmental information to better map the complex pathophysiology of psychotic, bipolar and common mental disorders.
Another area of future research is the identification of protective and resilience factors. To date, the disease‐centric model of research has inhibited the investigation of resilience factors that predict good outcomes (and that, therefore, cannot simply be defined as the inverse of risk factors). Shared definitions of good outcomes should also be developed, in particular with respect to promotion of good mental health, which is currently too fragmented. For example, in the CHR‐P field, there is a current refocus on good outcomes beyond psychosis onset (e.g., functional status, remission, quality of life 206 ). Importantly, these outcomes hold transdiagnostic potential to accommodate multi‐endpoint numerators across psychotic, bipolar and common mental disorders (as well as across physical health disorders) that are essential to justify the denominator of preventive (universal/selective/indicated) effort and cost. For example, social functioning is a shared domain across schizophrenia, depression and neurodegenerative disorders such as Alzheimer's disease 207 .
Transdiagnostic approaches have been suggested to complement current psychiatric nosography 208 , which is intrinsically limited, in particular in young people209, 210, by integrating clinical staging models and optimizing preventive efforts. However, to date, transdiagnostic approaches have been limited by several methodological caveats that should be addressed by future research.
First, there are frequently reporting inconsistencies (e.g., definition of the gold‐standard DSM/ICD diagnoses, outcome measures, and type of transdiagnostic approach) and low quality of studies, with few findings externally replicated 211 . Future studies could use the TRANSD recommendations, that may help improving the reporting of transdiagnostic research 212 . Second, while psychotic, bipolar and common mental disorders exhibit both multifinality (the same aetiological agents can result in different mental health disorders) and equifinality (multiple agents can lead to the same disorder), knowledge into shared risk/protective factors (Table 3) is still limited. The latter are mostly limited to social determinants of mental disorders, childhood adversity and familial vulnerability (and physical health/lifestyle behaviours discussed below). For example, risk of mood disorders is significantly increased among offspring of parents with schizophrenia (relative risk, RR=1.62), while the risk of schizophrenia is significantly increased in offspring of parents with bipolar disorder (RR=6.42) 121 . However, there is also evidence for diagnostic specificity: machine learning reclassification studies demonstrated a distinction between schizophrenia and mood disorders 213 ; treatment requirements and outcomes also differ 55 . Similarly, while early neurocognitive functioning has been suggested as a promising transdiagnostic biomarker 214 , some studies suggest that it is more specific to psychosis than to common mental disorders 215 .
No convincing evidence supports the existence of a truly transdiagnostic biomarker 147 . Evidence supporting a transdiagnostic clinical staging model that cuts across psychotic, bipolar and common mental disorders216, 217 is similarly limited to a few studies 218 , with scarce empirical validation 219 . There are also concerns that the natural course of bipolar 220 and depressive 221 disorders does not necessarily or consistently follow a clinical staging model. However, future research in this field is expected. For example, pervasively reduced neocortical thickness was recently found to be shared across psychotic and common mental disorders, representing a potentially transdiagnostic marker of general psychopathology (termed “p factor”) 222 . Thus, universal prevention of all these disorders may, in theory, overlap greatly.
Synergically preventing common and infrequent mental disorders
Refined transdiagnostic preventive approaches could facilitate targeting more prevalent common mental disorders to synergistically prevent the more infrequent psychotic and bipolar disorders, whose incidence may have been progressively declining 198 , although not everywhere 223 . Notably, the notion that psychotic symptoms are not infrequent but rather common among young individuals is caused by the trivialization of their contextual significance and operationalization, resulting in non‐specificity 224 . For example, surrogate markers, such as psychotic experiences, are frequently conflated with the APS of the CHR‐P state 225 (without explaining what makes a symptom truly “psychotic” 225 ). Unlike self‐assessed psychotic experiences, APS require detection by an experienced and trained clinician to distinguish pathological from non‐pathological phenomena 226 , and they are neither common features nor distributed continuously in the general population, accounting for only 0.3% of individuals 227 .
Overall, 66% of the incidence of clinical psychosis in the population is accounted for by preceding mood disorders 187 . This finding is not new: Conrad's phenomenological clinical‐stage model of psychosis onset 228 established early mood dysregulation as the underlying core feature. At the same time, a substantial proportion (37%) of the population‐level incidence of psychosis is explained by the CHR‐P stage, independently from mood disorders 187 . The majority of CHR‐P individuals have comorbid non‐psychotic mental disorders (which do not increase the risk for psychosis but tend to persist over time 229 ), mostly common mental disorders: 41% depressive disorders and 15% anxiety disorders72, 230. These findings demonstrate that the CHR‐P state is already partially transdiagnostic (some cases of psychosis may originate outside it 198 ), potentially capturing a psychosis dimension that emerges from anxiety or depressive disorders.
These considerations may inform the future configuration of preventive health care services. Conventional mental health services are not generally engineered to detect and prevent psychosis onset from anxiety or depressive disorders, as claimed by some authors195, 231. Young people at risk for psychosis or bipolar disorder typically present with blurred and unspecific symptoms that are too mild to fulfil the entry criteria of conventional mental health services. An alternative approach may be to enhance the transdiagnostic potential of current preventive (e.g., CHR‐P) services, implementing the detection of emerging bipolar and depressive (and anxiety) disorders and better integrating them with primary care to facilitate the prevention of physical health burden 3 . Such initiatives are emerging 232 .
Furthermore, the needs‐based support and the public health campaigns routinely offered by CHR‐P services could be expanded to better address the social determinants of psychotic and common mental disorders at the population level 233 . CHR‐P services also represent a successful global template for transitional mental health services and applied clinical research 232 that fully integrate between adolescence and young adulthood 67 . This overcomes the historical paediatric‐adult bifurcation, in which children and adolescent mental health services are usually cut at the age of 15 or 18 (the transitional period), when young people are most liable to mental disorders. This current two‐tier clinical research system is developmentally inappropriate (psychopathology and brain maturation see no abrupt transition among adolescence and early adulthood) to advance preventive psychiatry for young individuals, and leads many of them to fall through cracks.
To overcome these issues, broader youth‐friendly mental health services that ensure low‐threshold entry into pathways to care are currently advocated, but solid effectiveness evidence is still needed 3 , and caution is advised to not over‐pathologize the potentially non‐specific or transient occurrence of common mental health problems in young people 234 .
Preventing physical and mental health burden together
Despite the interconnectedness between mental and physical health problems (e.g., several shared risk factors) 42 , the severe physical health burden associated with emerging mental disorders in young people is not yet systematically incorporated in preventive approaches. A recent umbrella review of the top‐tier evidence has demonstrated that some lifestyle behaviours – such as low levels of physical activity, sleep disturbances, adverse dietary patterns, and tobacco smoking – are associated with an increased risk of psychotic, bipolar and depressive/anxiety disorders 235 . Future research could employ the TRANSD criteria to ascertain the transdiagnostic potential of lifestyle behaviours: for example, poor sleep is associated with bipolar disorder and depression/anxiety but not psychosis, while poor diet is associated with depressive disorders only 235 .
Prevention for these risk factors is currently driven by initiatives siloed in other non‐communicable disorders, such as cancer and obesity. However, these factors are also common across physical disorders: pursuing physical health and positive lifestyle behaviours is a tantalizing population‐level strategy for universal prevention, making sense for concurrently reducing the risk of many other physical diseases 42 . The numerator of cost and risk is thus offset by a denominator of multiple psychiatric and physical disease endpoints 236 .
Experience from smoking prevention suggests that similar public health population‐level interventions are far more effective than individual‐level approaches. However, current preventive capacity is limited 235 (e.g., selective/universal physical exercise may prevent common mental disorders157, 174, but these findings need to be consolidated), and future research should establish the most effective physical health/lifestyle interventions in young people.
Implementing stratified/personalized prognosis
Modern advancements in the field of individualized prediction modelling aim to consolidate stratified (tailored to subgroups) or precision (tailored to the individual subject) preventive psychiatry in young people 237 . Several individualized risk prediction models for forecasting the onset of psychosis, bipolar and depression/anxiety in young people 238 (see Table 5) have been externally validated in terms of prognostic accuracy, which is an essential step to address the extent to which predictions can be generalized to the data from plausibly related settings.
Table 5.
Externally validated, individualized prognostic models for forecasting the onset of psychotic, bipolar (BD), and major depressive (MD)/generalized anxiety (GAD) disorders in young people
| Outcome | Predictors | Development sample size (mean age, location); performance (measure) | External validation sample size (mean age, location); performance (measure) | |
|---|---|---|---|---|
|
Cannon et al291 |
Psychosis onset in CHR‐P | Age, family history, unusual thoughts and suspiciousness, lower verbal learning and memory performance, slower speed of processing, decline in social functioning |
596 (18.5, US); 0.71 (C‐index) 291 |
176 (16.6, US); 0.79 (AUC) 292 199 (19.1, China); 0.63 (AUC) 293 68 (18.59, US); 0.71 (AUC) 294 |
| Zhang et al295 | Psychosis onset in CHR‐P | Functional decline, positive symptoms, negative symptoms, general symptoms | 349 (20.4, China); 0.744 (AUC) 295 |
100 (age not available, China); 0.804 (AUC) 295 68 (18.59, US); 0.65 (AUC) 294 |
|
Fusar‐Poli et al199 |
Transdiagnostic psychosis onset in secondary mental health care patients |
Age, sex, ethnicity, age by gender, ICD‐10 index diagnosis |
33,820 (34.4, UK); 0.80 (C‐index) 199 |
54,716 (32.0, UK); 0.79 (C‐index) 199 13,702 (40.9, UK); 0.73 (C‐index) 296 33,710 (22.7, UK), 0.79 (C‐index) 297 2,430,333 (34.2, US); 0.68 (C‐index) 298 |
| Refined version including 14 symptoms extracted with natural language processing | 28,297 (34.8, UK); 0.86 (C‐index) 299 | 63,854 (33); 0.85 (C‐index) 299 | ||
| King et al300 |
MD onset in primary care |
Age, sex, country educational status, difficulties in work, history of depression in first‐degree relatives, experience of discrimination, lifetime major depression episode, mental quality of life, physical quality of life | 5,216 (48.9, UK, Spain, Slovenia, Portugal, The Netherlands); 0.79 (C‐index) 300 |
1,732 (47.0, Chile); 0.71(C‐index) 300 29,621 (43.8 US); 0.71(AUC) 301 |
| King et al302 |
GAD and MD onset in primary care |
Age, sex, country, difficulties in paid and unpaid work, history of depression in first‐degree relatives, follow‐up period, lifetime major depression episode, mental quality of life, physical quality of life | 4,905 (age not available, UK, Spain, Slovenia, Portugal); 0.75 (C‐index) 302 |
5,140 (age not available, Netherlands, Estonia, Chile); 0.71‐0.81 (C‐index) 302 24,626 (age not available, US); 0.62 (AUC) 303 |
| Birmhaer et al304 | Onset of BD‐I or BD‐II from sub‐threshold BD symptoms | Age, sex, mania, depression, anxiety, emotional lability, functioning, duration of BD, ethnicity, family history of BD | 140 (11.9, US); 0.71 (AUC) 304 | 58 (11.9, US); 0.75 (AUC) 304 |
| Raket et al201 | Onset of psychosis (schizophrenia) from primary and secondary care | Demographics and dynamic medical events (diagnoses, prescriptions, procedures, encounters and admissions, observations, and laboratory test results) |
102,030 (42, US); 0.856 (AUC) 201 |
4,770 (age not available, US); 0.799 (AUC) 201 |
CHR‐P – clinical high risk for psychosis, AUC – area under the curve
Despite these progresses, prognostic accuracy for most of these models is not sufficient to prove clinical utility and implementability across different scenarios 239 . In fact, a systematic review has found that only about 5% of the total pool of risk prediction models published in psychiatry is externally validated, and that only 0.2% are being considered for implementation (most models may not cross the implementation threshold, as they would not improve outcomes), highlighting a profound replication and translational gap 240 . For example, across all prognostic models reviewed in Table 5, only the transdiagnostic risk calculator has been piloted for real‐world implementation in clinical practice 241 .
To overcome these limitations, the next generation of research should prioritize further refinements and replications of existing algorithms. Given their complexity, the weighting of the predictors may vary considerably with context (e.g., adolescent vs. young adult, geographic contexts). For those models that may reach higher levels of proof for clinical utility, the implementation pathway is a perilous journey undermined by several obstacles, related to individuals involved (e.g., making their data available or accepting the outputs of the risk calculators), clinicians (e.g., adherence to the recommendations made by prediction models, communicating risks), providers (e.g., confidentiality of data, interpretability of outputs) and funders/organizations (implementing standard prediction procedures) 238 .
Implementation science itself is contested and complex, and there is no solid general implementation framework and practical guidance for preventive psychiatry. The next generation of research in this field should develop a coherent and pragmatic implementation framework and associated international infrastructures 177 .
The latter necessitate collaborative data sharing efforts and international, large‐scale, harmonized and multimodal (e.g., psychopathological, neurobiological, neurocognitive) clinical research databases, integrated with digital technologies (e.g., electronic medical records), as well as specific support from funders and stakeholders 237 . Harmonization is likely to be most successful for future datasets that are prospectively collected. However, efforts should also be made to standardize (to the extent possible) existing datasets that already include large amounts of data.
Establishing evidence‐based preventive interventions
Another area of future research is the development of evidence‐based preventive interventions to overcome the current divergence between “political” literature, which tends to deliver an overoptimistic message, and evidence‐based literature, which emphasizes methodological biases and the inconsistency of the available findings. For example, two independent meta‐analyses found no evidence (as opposed to evidence of absence) to favour specific interventions for preventing psychosis in CHR‐P individuals93, 94. Without providing any meta‐analytical counter‐evidence, some authors have complained that evidence needs to be contextualized, because the “potential for improvement is a key message for patients, families, and practitioners” 242 . The Cochrane authors replied that their meta‐analysis was not a criticism of the valuable preventive aims, but only scientific grading of the available evidence 243 .
Along these lines, Caplan first noted that, although there was little empirical evidence to support primary prevention and little knowledge of the aetiology of mental disorders, “there appears to be validity to the assumptions” 244 of primary prevention, which ought not to be suspended while awaiting the results of evidence‐based medicine. This tension extends beyond the CHR‐P paradigm: other evidence‐based syntheses have disconfirmed initial promising findings relating to the indicated/selective/universal prevention of anxiety and depression113, 115, 156 or reduction of substance abuse in young people 154 , and these debates are even more pronounced for public health approaches targeting social determinants of mental disorders. The goal to prevent psychotic, bipolar and common mental disease is noble, but this alone does not justify the use of interventions where there is no demonstrated effectiveness. Preventive breakthroughs that do not show cost‐effectiveness (see below) are also unlikely to be implemented in health care systems and in the general population, and this would be for good reasons. Future research is also needed to better customize the effectiveness of preventive interventions to several vulnerable groups such as refugees, prisoners, persons in humanitarian contexts; lesbian, gay, bisexual and transgender persons; persons who are being bullied or exposed to violence, and those who have recently been bereaved.
Future research should also explore methodological innovations. The lack of evidence to favour several preventive interventions113, 115, 154, 156 may indicate that a one‐size‐fits‐all approach is not effective and obfuscates the efficacy for specific subgroups of individuals. Future individual‐participant data level network meta‐analyses are under planning 245 and may help deconstructing the effect of different individual‐ or subgroup‐ level factors. As new interventions in this field are being tested at a rapid pace, living meta‐analyses may be particularly useful to update the emerging evidence. However, subgroup effect claims have a notoriously poor record of validation across medicine246, 247. Moreover, even if present, they would require very large sample sizes to be able to document and validate them in a rigorous fashion. Even large individual‐level meta‐analyses may not identify effect modification in most medical interventions 248 . Subgroup effects and intervention effect heterogeneity require rigorous documentation and validation before being adopted249, 250.
Another explanation for the lack of evidence may be that dilution of risk enrichment and infrequent events may have led to reduced statistical power to find a difference between a preventive intervention and a control group (e.g., more than 2,000 CHR‐P individuals are needed to detect a 50% reduction in risk to psychosis 251 ). Stratification algorithms to control for risk enrichment and inform trial recruitment are under development 252 , and harmonization of large‐scale datasets within international research consortia is expected to increase the statistical power.
Future meta‐analytical approaches could also exclude low‐quality studies instead of pooling all available data (which has frequently been advocated 242 ), most of which may be of insufficient quality253, 254, 255. Future interventional studies should investigate the efficacy of emerging preventive compounds (e.g., oxytocin, N‐acetylcysteine, cannabinoids), screening procedures (e.g., maternal screening, bipolar risk screening, screening in low/middle‐income countries) or refined psychoeducation interventions (e.g., for asymptomatic bipolar familial risk, reduction of alcohol and illicit substance abuse). Innovative adaptive trial designs 256 that integrate with stepped preventive care should also be considered.
Developing an ethical framework for preventive psychiatry
Preventive medicine in young people brings some ethical challenges. For example, the potential cost, inconvenience, social stigma and other harms of a false‐positive designation in young people may be high 257 . These concerns are corroborated by lack of valid biomarkers of risk (remarkably, there are no approved biomarkers in all of psychiatry) and adverse effects of antipsychotics258, 259 or other psychotropic agents. Antipsychotics are not recommended for preventing psychosis 68 , and these molecules are likely to be inappropriately prescribed to young people at risk outside preventive programmes 260 . Psychological/psychosocial interventions may also be associated with adverse effects. Population interventions to prevent substance abuse in children or common mental disorders in humanitarian settings have been shown to worsen outcomes 261 and to be not more acceptable than the waiting‐list condition 120 . Notably, similar ethical issues have been raised in preventive medicine: for example, handling pre‐diabetes (intermediate hyperglycaemia) has been challenged for the risk of false positives, as many people do not progress to diabetes 262 .
Another question is the extent to which sharing a risk designation with young people and their families may produce harmful stigma (non‐maleficence: first do no harm) or offer benefits (beneficence: helping the youth) and autonomy 263 . One perspective is that, in the absence of solid evidence for effectiveness and with potential for harm, preventive services may be seriously questioned. However, evidence shows that stigma is lower in service users than in their health care professionals 264 , and caused by the service user's experience of symptoms rather than induced by the clinician's designation 265 . Stigma seems also associated with at‐risk features even when no at‐risk label is attached 266 , and level of stigma in preventive services is comparable to that associated with depression 267 . Thus, sharing an at‐risk designation may not only be helpful (beneficence), but honour the ethical principle that young people have the right to receive information relevant to their health (autonomy) 268 , in particular given the very real morbidity (e.g., functional impairments of CHR‐P individuals 77 ), risks (e.g., up to 40% risk of developing persistent psychosis at 2 years for BLIPS 269 ), and their active help‐seeking behaviours.
A counter‐argument is that, if no effective preventive intervention can be provided, then knowing in advance may not be helpful outside clinical monitoring (which can reduce the duration of untreated disorder 90 ). However, young people accessing preventive (e.g., CHR‐P) services benefit from an integrated package of vocational, psychosocial and familial support interventions which would otherwise not be available to them. Prognostic communication in these services is nuanced, tailoring it to each individual, illustrating the varying outcomes that might be possible (remission/response, persistence, worsening), and that currently there is no certain way to distinguish those possibilities for any given individual 263 . Furthermore, service user groups are actively involved in designing dissemination materials and advising on service delivery 3 . The effectiveness of these approaches needs better study but, in principle, they may help young people endorse the need for several precision/preventive psychiatry concepts, including how testing may lead to tailored interventions 270 .
Future collaborative research should set up an ethical framework for implementing preventive psychiatry in young people, involving health care workers, policy makers, service users and their families, and putting emphasis on the subjective experience of the youth 271 . This call would realize the vision of predictive, preventive, personalized and participatory (“P4”) psychiatry and help ensure that future progresses occur in an ethically acceptable manner that optimizes benefits and minimizes harms for young people 268 .
Improving prevention through education and training
The recent systematic development of science‐based prognosis and prevention for young people should be reinforced by a comprehensive educational action targeting several stakeholders. Editors and reviewers of scientific journals should become aware that improving reproducibility standards is needed to maximize the efficiency and trustworthiness of preventive research for young people 237 . Power and other statistical issues need to be considered when interpreting the results of studies.
Another problem is that the current division of medical training leads to often contrasting approaches among adolescent versus adult health care workers, enhancing the cultural divide among the specialities. Innovative curricula could be developed to train new “transitional” health care workers, which could also incorporate core conceptual and methodological issues pertaining to the science of prognosis and preventive interventions (for example, universal/public health approaches may require economic or social understanding that extends beyond medical knowledge 33 ).
Future curricula should also rectify the ongoing erosion of psychiatric training on psychopathology and phenomenology, boosted by checklist and algorithmic approaches in the context of pressured health care, to avoid blurring the borders between pathology (e.g., psychotic symptoms) and variants of the normal (e.g., psychotic experiences) 272 in young people.
Furthermore, knowledge and resources in the prevention of mental disorders and mental health promotion in young people are unevenly distributed around the world, and global training initiatives are needed to support countries that are still lacking capacity and expertise. This goal could be achieved through international networks of collaborating research centres 273 . Finally, policy makers should be educated on the achievements and limitations surrounding the prevention of mental disorders in young people, and funders supported to design preventive calls.
Consolidating the cost‐effectiveness of preventive psychiatry
Due to high health care cost and impaired ability to work, psychotic, bipolar and common mental disorders in young people lead to a huge economic burden, estimated at US$ 16.3 trillion by 2030 (including neurological and substance use disorders), exceeding cardiovascular disease, chronic respiratory disease, cancer and diabetes, and accounting for more than half of the global economic burden attributable to non‐communicable diseases 274 . However, the current global median expenditure on mental health is low (only US$ 2.5 per person annually, accounting for less than 2% of government health expenditure globally 275 ), and this may be a major reason for the wide gap between young people's mental health needs and the provision of preventive interventions 275 .
Cost‐effectiveness of preventive interventions is essential to avoid adding pressure on already overstretched health care budgets. Available evidence indicates that cost‐effective preventive interventions may include perinatal screening‐plus‐intervention programmes 276 , stepped care for the prevention of anxiety (but not depression) 277 , and prevention of psychosis in CHR‐P individuals (savings of US$ 844 per prevented psychosis 278 ). However, economic evaluations of preventive approaches in young people remain relatively neglected 279 . Moreover, these cost‐effectiveness estimates may also be biased in favour of the tested interventions for various reasons (e.g., involvement of authors who are supportive of the interventions and/or practice them themselves, uncertain and inflated estimates of effectiveness, lack of reasonable estimates on most of the potential harms, difficulty to translate some harms, such as stigma, into quantitative parameters).
Future research should try to remedy some of these shortcomings and address economic evidence gaps in perinatal bipolar or anxiety disorders 275 , adolescent mental health 280 , interventions in low‐resource settings (cost‐effectiveness evidence may not be transferable across different countries) and youth mental health services 275 . Future economic preventive studies should also consider a long‐term time horizon, in the light of the potentially progressive nature of these disorders and the wider familial and societal impact outside health care. Finally, the interconnectedness of socio‐economic, religious, cultural, ethnic inequalities and cost‐effectiveness275 should be better addressed.
Decreasing inequalities to prevent mental disorders
Prevention of mental disorders in young people has not yet solidified as global research or programmatic focus 281 . We have demonstrated above that universal public health approaches targeting the social determinants of mental disorders hold the greatest potential for reducing the risk profile of the whole population. For example, the wide adoption of neo‐liberal economic policies and globalization has increased wealth inequality (e.g., in the US, the top 10% of the population averages nine times as much income as the bottom 90%), which is robustly associated with psychotic and depressive disorders 282 .
Effective actions may include reducing income inequality, such as progressive taxation policies and a basic universal income, in combination with promotion of good mental health and provision of packages of care with demonstrated effectiveness 283 . However, the effectiveness of poverty alleviation strategies is uncertain and requires further research; conversely, selective or indicated approaches in subgroups with mental health issues have the potential to improve economic outcomes 284 .
Future public health research will also require advancements in epidemiologic methods of causal inference, improvements in data quality and availability 233 , robust randomized controlled trials to demonstrate specific effectiveness on psychotic, bipolar and mental disorders, qualitative research to customize interventions around context/culture, and mixed‐method implementation science to assess the scaling up of interventions 173 .
Future public health approaches require committed and sustained efforts to address a range of other barriers, a strong health sector responsibility, and a vigorous leadership role in bringing society‐wide attention and cross‐government actions together. This point is particularly critical, given the experience of smoking prevention, whose success was predicated on successive hard‐fought public policy battles.
Governments should tackle unacceptable inequalities in young people's mental health 285 , and invest on improving the social determinants of their mental health: education, employment, social care, housing, criminal justice, poverty alleviation, social security/welfare benefits, community development, and immigration 275 . These inequalities are likely to increase with the current COVID‐19 pandemic, which will force to change mental health services, focusing even more on flexible systems that include prevention 286 .
Addressing these inequalities should be the shared responsibility of professionals across systems of care, representing the fundamental pillar of an individualized approach to youth mental health 233 . Such primordial‐like type of prevention argues for universal health care coverage and parity between physical and mental health 275 . It is hoped that more progress in this direction can be achieved in the decade to come, as much is at stake for young people at risk for and with emerging psychotic, bipolar and common mental disorders.
ACKNOWLEDGEMENTS
P. Fusar‐Poli is supported by the European Union Seventh Framework Program (PSYSCAN project), US National Institute of Mental Health (ProNET project) and Wellcome Trust (STEP project). M. Berk is supported by an Australian National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship (grant no. 1156072). C. Arango is funded by the Spanish Ministry of Science and Innovation (grant nos. SAM16PE07CP1, PI16/02012, PI19/024), co‐financed by European Regional Development Funds from the European Commission (grant no. B2017/BMD‐3740 AGES‐CM‐2), European Union Seventh Framework Program (EU‐GEI, PSYSCAN and METSY projects); and European Union H2020 Program (PRISM and AIMS‐2‐TRIALS projects).
FORUM – PREVENTION OF MENTAL DISORDERS IN YOUNG PEOPLE: RESEARCH EVIDENCE AND FUTURE DIRECTIONS
REFERENCES
- 1. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kessler RC, Berglund P, Demler O et al. Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:593‐602. [DOI] [PubMed] [Google Scholar]
- 3. Fusar‐Poli P. Integrated mental health services for the developmental period (0 to 25 years): a critical review of the evidence. Front Psychiatry 2019;10:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. United Nations . World population prospects 2019. https://population.un.org/wpp/DataQuery/. [Google Scholar]
- 5. Insel TR, Fenton WS. Psychiatric epidemiology: it's not just about counting anymore. Arch Gen Psychiatry 2005;62:590‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGorry P. Building the momentum and blueprint for reform in youth mental health. Lancet Psychiatry 2019;6:459‐61. [DOI] [PubMed] [Google Scholar]
- 7. Millan MJ, Andrieux A, Bartzokis G et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov 2016;15:485‐515. [DOI] [PubMed] [Google Scholar]
- 8. Wang PS, Angermeyer M, Borges G et al. Delay and failure in treatment seeking after first onset of mental disorders in the World Health Organization's World Mental Health Survey Initiative. World Psychiatry 2007;6:177‐85. [PMC free article] [PubMed] [Google Scholar]
- 9. Vancampfort D, Wampers M, Mitchell AJ et al. A meta‐analysis of cardio‐metabolic abnormalities in drug naive, first‐episode and multi‐episode patients with schizophrenia versus general population controls. World Psychiatry 2013;12:240‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu NH, Daumit GL, Dua T et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry 2017;16:30‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vancampfort D, Firth J, Schuch FB et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta‐analysis. World Psychiatry 2017;16:308‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Correll CU, Solmi M, Veronese N et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large‐scale meta‐analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017;16:163‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mojtabai R, Stuart EA, Hwang I et al. Long‐term effects of mental disorders on educational attainment in the National Comorbidity Survey ten‐year follow‐up. Soc Psychiatry Psychiatr Epidemiol 2015;50:1577‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stuart H. Fighting the stigma caused by mental disorders: past perspectives, present activities, and future directions. World Psychiatry 2008;7:185‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Puras D, Gooding P. Mental health and human rights in the 21st century. World Psychiatry 2019;18:42‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gerlinger G, Hauser M, De Hert M et al. Personal stigma in schizophrenia spectrum disorders: a systematic review of prevalence rates, correlates, impact and interventions. World Psychiatry 2013;12:155‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wasserman D, Cheng Q, Jiang GX. Global suicide rates among young people aged 15‐19. World Psychiatry 2005;4:114‐20. [PMC free article] [PubMed] [Google Scholar]
- 18. Chesney E, Goodwin GM, Fazel S. Risks of all‐cause and suicide mortality in mental disorders: a meta‐review. World Psychiatry 2014;13:153‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christensen H, Reynolds CFR, Cuijpers P. Protecting youth mental health, protecting our future. World Psychiatry 2017;16:327‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sommer IE, Arango C. Moving interventions from after to before diagnosis. World Psychiatry 2017;16:275‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arango C. Psychiatrists are experts when it comes to missing boats. Will prevention be the next one? Eur Arch Psychiatry Clin Neurosci 2019;269:869‐70. [DOI] [PubMed] [Google Scholar]
- 22. Robinson G. On the prevention and treatment of mental disorders. London: Longman, Brown, Green, Longmans & Roberts, 1859. [Google Scholar]
- 23. Parry M. From a patient's perspective: Clifford Whittingham Beers' work to reform mental health services. Am J Public Health 2010;100:2356‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anonymous. Mental hygiene in childhood. BMJ 1904;2:1414‐5. [PMC free article] [PubMed] [Google Scholar]
- 25. Emerson CP. Mental hygiene and its relation to public health. Am J Public Health 1925;15:1062‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. White WA. Preventive principles in the field of mental medicine. J Am Public Health Assoc 1911;1:82‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leavell H, Clark E. Preventive medicine for the doctor in his community: an epidemiologic approach, 1st ed. New York: McGraw‐Hill, 1958. [Google Scholar]
- 28. Clark EG. Natural history of syphilis and levels of prevention. Br J Vener Dis 1954;30:191‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caplan G, Grunebaum H. Perspectives on primary prevention. A review. Arch Gen Psychiatry 1967;17:331‐46. [DOI] [PubMed] [Google Scholar]
- 30. Strasser T. Reflections on cardiovascular diseases. Interdiscip Sci Rev 1978;3:225‐30. [Google Scholar]
- 31. Hofler M. The Bradford Hill considerations on causality: a counterfactual perspective. Emerg Themes Epidemiol 2005;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lucas RM, McMichael AJ. Association or causation: evaluating links between “environment and disease”. Bull World Health Organ 2005;83:792‐5. [PMC free article] [PubMed] [Google Scholar]
- 33. Gordon RS Jr. An operational classification of disease prevention. Public Health Rep 1983;98:107‐9. [PMC free article] [PubMed] [Google Scholar]
- 34. US Institute of Medicine . Reducing risks for mental disorders: frontiers for preventive intervention research. Washington: National Academies Press, 1994. [PubMed] [Google Scholar]
- 35. Kessler RC, Price RH. Primary prevention of secondary disorders: a proposal and agenda. Am J Community Psychol 1993;21:607‐33. [DOI] [PubMed] [Google Scholar]
- 36. World Health Organization . Prevention of mental disorders. Effective interventions and policy options. Geneva: Department of Mental Health and Substance Abuse, 2004. [Google Scholar]
- 37. Correll CU, Galling B, Pawar A et al. Comparison of early intervention services vs treatment as usual for early‐phase psychosis: a systematic review, meta‐analysis, and meta‐regression. JAMA Psychiatry 2018;75:555‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burkhardt E, Pfennig A, Breitling G et al. Creativity in persons at‐risk for bipolar disorder – A pilot study. Early Interv Psychiatry 2019;13:1165‐72. [DOI] [PubMed] [Google Scholar]
- 39. Parnas J, Sandsten KE, Vestergaard CH et al. Mental illness among relatives of successful academics: implications for psychopathology‐creativity research. World Psychiatry 2019;18:362‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keller MC, Visscher PM. Genetic variation links creativity to psychiatric disorders. Nat Neurosci 2015; 18:928‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fusar‐Poli P, Salazar de Pablo G, De Micheli A et al. What is good mental health? A scoping review. Eur Neuropsychopharmacol 2020;31:33‐46. [DOI] [PubMed] [Google Scholar]
- 42. Jacka FN, Mykletun A, Berk M. Moving towards a population health approach to the primary prevention of common mental disorders. BMC Med 2012;10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kessler RC, Amminger GP, Aguilar‐Gaxiola S et al. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry 2007;20:359‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Radua J, Ramella‐Cravaro V, Ioannidis JPA et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry 2018;17:49‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Joslyn C, Hawes DJ, Hunt C et al. Is age of onset associated with severity, prognosis, and clinical features in bipolar disorder? A meta‐analytic review. Bipolar Disord 2016;18:389‐403. [DOI] [PubMed] [Google Scholar]
- 46. Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health 2013;34:119‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jones PB. Adult mental health disorders and their age at onset. Br J Psychiatry 2013;202(Suppl. 54):s5‐10. [DOI] [PubMed] [Google Scholar]
- 48. Uher R, Zwicker A. Etiology in psychiatry: embracing the reality of poly‐gene‐environmental causation of mental illness. World Psychiatry 2017;16:121‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 2008;9:947‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kloiber S, Rosenblat JD, Husain MI et al. Neurodevelopmental pathways in bipolar disorder. Neurosci Biobehav Rev 2020;112:213‐26. [DOI] [PubMed] [Google Scholar]
- 51. Parellada M, Gomez‐Vallejo S, Burdeus M et al. Developmental differences between schizophrenia and bipolar disorder. Schizophr Bull 2017;43:1176‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hagan CC, Graham JM, Wilkinson PO et al. Neurodevelopment and ages of onset in depressive disorders. Lancet Psychiatry 2015;2:1112‐6. [DOI] [PubMed] [Google Scholar]
- 53. Fava GA, Kellner R. Staging: a neglected dimension in psychiatric classification. Acta Psychiatr Scand 1993;87:225‐30. [DOI] [PubMed] [Google Scholar]
- 54. McGorry PD, Hickie IB, Yung AR et al. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. Aust N Z J Psychiatry 2006;40:616‐22. [DOI] [PubMed] [Google Scholar]
- 55. Fusar‐Poli P, McGorry PD, Kane JM. Improving outcomes of first‐episode psychosis: an overview. World Psychiatry 2017;16:251‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Berk M, Post R, Ratheesh A et al. Staging in bipolar disorder: from theoretical framework to clinical utility. World Psychiatry 2017;16:236‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Salagre E, Dodd S, Aedo A et al. Toward precision psychiatry in bipolar disorder: staging 2.0. Front Psychiatry 2018;9:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kapczinski F, Magalhaes PV, Balanza‐Martinez V et al. Staging systems in bipolar disorder: an International Society for Bipolar Disorders task force report. Acta Psychiatr Scand 2014;130:354‐63. [DOI] [PubMed] [Google Scholar]
- 59. Berk M, Berk L, Dodd S et al. Stage managing bipolar disorder. Bipolar Disord 2014;16:471‐7. [DOI] [PubMed] [Google Scholar]
- 60. Verduijn J, Milaneschi Y, van Hemert AM et al. Clinical staging of major depressive disorder: an empirical exploration. J Clin Psychiatry 2015;76:1200‐8. [DOI] [PubMed] [Google Scholar]
- 61. Reneses B, Aguera‐Ortiz L, Sevilla‐Llewellyn‐Jones J et al. Staging of depressive disorders: relevance of resistance to treatment and residual symptoms. J Psychiatr Res 2020;129:234‐40. [DOI] [PubMed] [Google Scholar]
- 62. Bokma WA, Batelaan NM, Hoogendoorn AW et al. A clinical staging approach to improving diagnostics in anxiety disorders: is it the way to go? Aust N Z J Psychiatry 2020;54:173‐84. [DOI] [PubMed] [Google Scholar]
- 63. Clarke PJ, Hickie IB, Scott E et al. Clinical staging model applied to young people presenting with social anxiety. Early Interv Psychiatry 2012;6:256‐64. [DOI] [PubMed] [Google Scholar]
- 64. Fontenelle LF, Yucel M. A clinical staging model for obsessive‐compulsive disorder: is it ready for prime time? EClinicalMedicine 2019;7:65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Voshaar RC, Beekman AT, Pachana N. Clinical staging and profiling of late‐life anxiety disorders; the need for collaboration and a life‐span perspective. Int Psychogeriatr 2015;27:1057‐9. [DOI] [PubMed] [Google Scholar]
- 66. Yung AR, Yuen HP, McGorry PD et al. Mapping the onset of psychosis: the Comprehensive Assessment of At‐Risk Mental States. Aust N Z J Psychiatry 2005;39:964‐71. [DOI] [PubMed] [Google Scholar]
- 67. Kotlicka‐Antczak M, Podgorski M, Oliver D et al. Worldwide implementation of clinical services for the prevention of psychosis: the IEPA early intervention in mental health survey. Early Interv Psychiatry (in press). [DOI] [PubMed] [Google Scholar]
- 68. National Institute for Health and Care Excellence . Psychosis and schizophrenia in adults: prevention and management. London: National Institute for Health and Care Excellence, 2014. [PubMed] [Google Scholar]
- 69. Schmidt SJ, Schultze‐Lutter F, Schimmelmann BG et al. EPA guidance on the early intervention in clinical high risk states of psychoses. Eur Psychiatry 2015;30:388‐404. [DOI] [PubMed] [Google Scholar]
- 70. Fusar‐Poli P, Carpenter WT, Woods SW et al. Attenuated psychosis syndrome: ready for DSM‐5.1? Annu Rev Clin Psychol 2014;10:155‐92. [DOI] [PubMed] [Google Scholar]
- 71. Arango C, Bernardo M, Bonet P et al. When the healthcare does not follow the evidence: the case of the lack of early intervention programs for psychosis in Spain. Rev Psiquiatr Salud Ment 2017;10:78‐86. [DOI] [PubMed] [Google Scholar]
- 72. Fusar‐Poli P, Salazar de Pablo G, Correll C et al. Prevention of psychosis: advances in detection, prognosis and intervention. JAMA Psychiatry 2020;77:755‐65. [DOI] [PubMed] [Google Scholar]
- 73. Fusar‐Poli P. The Clinical High‐Risk State for Psychosis (CHR‐P), Version II. Schizophr Bull 2017;43:44‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fusar‐Poli P, Cappucciati M, Rutigliano G et al. At risk or not at risk? A meta‐analysis of the prognostic accuracy of psychometric interviews for psychosis prediction. World Psychiatry 2015;14:322‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oliver D, Reilly T, Baccaredda Boy O et al. What causes the onset of psychosis in individuals at clinical high risk? A meta‐analysis of risk and protective factors. Schizophr Bull (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fusar‐Poli P, Tantardini M, De Simone S et al. Deconstructing vulnerability for psychosis: meta‐analysis of environmental risk factors for psychosis in subjects at ultra high‐risk. Eur Psychiatry 2017;40:65‐75. [DOI] [PubMed] [Google Scholar]
- 77. Fusar‐Poli P, Rocchetti M, Sardella A et al. Disorder, not just state of risk: meta‐analysis of functioning and quality of life in people at high risk of psychosis. Br J Psychiatry 2015;207:198‐206. [DOI] [PubMed] [Google Scholar]
- 78. Fusar‐Poli P, Borgwardt S, Bechdolf A et al. The psychosis high‐risk state: a comprehensive state‐of‐the‐art review. JAMA Psychiatry 2013;70:107‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Falkenberg I, Valmaggia L, Byrnes M et al. Why are help‐seeking subjects at ultra‐high risk for psychosis help‐seeking? Psychiatry Res 2015;228:808‐15. [DOI] [PubMed] [Google Scholar]
- 80. Fusar‐Poli P, Estrade A, Spencer TJ et al. Pan‐London Network for Psychosis‐Prevention (PNP). Front Psychiatry 2019;10:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fusar‐Poli P, Byrne M, Badger S et al. Outreach and support in south London (OASIS), 2001‐2011: ten years of early diagnosis and treatment for young individuals at high clinical risk for psychosis. Eur Psychiatry 2013;28:315‐26. [DOI] [PubMed] [Google Scholar]
- 82. Fusar‐Poli P, Schultze‐Lutter F, Cappucciati M et al. The dark side of the moon: meta‐analytical impact of recruitment strategies on risk enrichment in the clinical high risk state for psychosis. Schizophr Bull 2016;42:732‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Addington J, Stowkowy J, Weiser M. Screening tools for clinical high risk for psychosis. Early Interv Psychiatry 2015;9:345‐56. [DOI] [PubMed] [Google Scholar]
- 84. Fusar‐Poli P. Why ultra high risk criteria for psychosis prediction do not work well outside clinical samples and what to do about it. World Psychiatry 2017;16:212‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Webb JR, Addington J, Perkins DO et al. Specificity of incident diagnostic outcomes in patients at clinical high risk for psychosis. Schizophr Bull 2015;41:1066‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fusar‐Poli P, Rutigliano G, Stahl D et al. Long‐term validity of the At Risk Mental State (ARMS) for predicting psychotic and non‐psychotic mental disorders. Eur Psychiatry 2017;42:49‐54. [DOI] [PubMed] [Google Scholar]
- 87. Fusar‐Poli P, Cappucciati M, De Micheli A et al. Diagnostic and prognostic significance of brief limited intermittent psychotic symptoms (BLIPS) in individuals at ultra high risk. Schizophr Bull 2017;43:48‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fusar‐Poli P, Cappucciati M, Borgwardt S et al. Heterogeneity of psychosis risk within individuals at clinical high risk: a meta‐analytical stratification. JAMA Psychiatry 2016;73:113‐20. [DOI] [PubMed] [Google Scholar]
- 89. Fusar‐Poli P, De Micheli A, Patel R et al. Real‐world clinical outcomes two years after transition to psychosis in individuals at clinical high risk: electronic health record cohort study. Schizophr Bull 2020;46:1114‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Oliver D, Davies C, Crossland G et al. Can we reduce the duration of untreated psychosis? A systematic review and meta‐analysis of controlled interventional studies. Schizophr Bull 2018;44:1362‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stafford MR, Jackson H, Mayo‐Wilson E et al. Early interventions to prevent psychosis: systematic review and meta‐analysis. BMJ 2013;346:f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stafford MR, Jackson H, Mayo‐Wilson E et al. Errata: Early interventions to prevent psychosis: systematic review and meta‐analysis. BMJ 2013;346:f762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Davies C, Cipriani A, Ioannidis JPA et al. Lack of evidence to favor specific preventive interventions in psychosis: a network meta‐analysis. World Psychiatry 2018;17:196‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bosnjak Kuharic D, Kekin I, Hew J et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev 2019;11:CD012236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Devoe DJ, Farris MS, Townes P et al. Interventions and transition in youth at risk of psychosis: a systematic review and meta‐analyses. J Clin Psychiatry 2020;81:17r12053. [DOI] [PubMed] [Google Scholar]
- 96. Bechdolf A, Ratheesh A, Wood SJ et al. Rationale and first results of developing at‐risk (prodromal) criteria for bipolar disorder. Curr Pharm Des 2012;18:358‐75. [DOI] [PubMed] [Google Scholar]
- 97. Bechdolf A, Nelson B, Cotton SM et al. A preliminary evaluation of the validity of at‐risk criteria for bipolar disorders in help‐seeking adolescents and young adults. J Affect Disord 2010;127:316‐20. [DOI] [PubMed] [Google Scholar]
- 98. Van Meter AR, Burke C, Youngstrom EA et al. The bipolar prodrome: meta‐analysis of symptom prevalence prior to initial or recurrent mood episodes. J Am Acad Child Adolesc Psychiatry 2016;55: 543‐55. [DOI] [PubMed] [Google Scholar]
- 99. Vieta E, Salagre E, Grande I et al. Early intervention in bipolar disorder. Am J Psychiatry 2018. 2018;175:411‐26. [DOI] [PubMed] [Google Scholar]
- 100. Faedda GL, Baldessarini RJ, Marangoni C et al. An International Society of Bipolar Disorders task force report: Precursors and prodromes of bipolar disorder. Bipolar Disord 2019;21:720‐40. [DOI] [PubMed] [Google Scholar]
- 101. Benarous X, Consoli A, Milhiet V et al. Early interventions for youths at high risk for bipolar disorder: a developmental approach. Eur Child Adolesc Psychiatry 2016;25:217‐33. [DOI] [PubMed] [Google Scholar]
- 102. Post RM, Goldstein BI, Birmaher B et al. Toward prevention of bipolar disorder in at‐risk children: potential strategies ahead of the data. J Affect Disord 2020;272:508‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Van Meter A, Guinart D, Bashir A et al. Bipolar Prodrome Symptom Scale ‐ abbreviated screen for patients: description and validation. J Affect Disord 2019;249:357‐65. [DOI] [PubMed] [Google Scholar]
- 104. Correll CU, Olvet DM, Auther AM et al. The Bipolar Prodrome Symptom Interview and Scale‐Prospective (BPSS‐P): description and validation in a psychiatric sample and healthy controls. Bipolar Disord 2014;16:505‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fusar‐Poli P, De Micheli A, Rocchetti M et al. Semistructured Interview for Bipolar At Risk States (SIBARS). Psychiatry Res 2018;264:302‐9. [DOI] [PubMed] [Google Scholar]
- 106. Miklowitz DJ, Schneck CD, Walshaw PD et al. Effects of family‐focused therapy vs enhanced usual care for symptomatic youths at high risk for bipolar disorder: a randomized clinical trial. JAMA Psychiatry 2020;77:455‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Leopold K, Bauer M, Bechdolf A et al. Efficacy of cognitive‐behavioral group therapy in patients at risk for serious mental illness presenting with subthreshold bipolar symptoms: results from a prespecified interim analysis of a multicenter, randomized, controlled study. Bipolar Disord 2020;22:517‐29. [DOI] [PubMed] [Google Scholar]
- 108. Davey CG, McGorry PD. Early intervention for depression in young people: a blind spot in mental health care. Lancet Psychiatry 2019;6:267‐72. [DOI] [PubMed] [Google Scholar]
- 109. Munoz RF, Beardslee WR, Leykin Y. Major depression can be prevented. Am Psychol 2012;67:285‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ebert DD, Cuijpers P. It is time to invest in the prevention of depression. JAMA Netw Open 2018;1:e180335. [DOI] [PubMed] [Google Scholar]
- 111. Hoare E, Callaly E, Berk M. Can depression be prevented? If so, how? JAMA Psychiatry (in press). [DOI] [PubMed] [Google Scholar]
- 112. Ratheesh A, Davey C, Hetrick S et al. A systematic review and meta‐analysis of prospective transition from major depression to bipolar disorder. Acta Psychiatr Scand 2017;135:273‐84. [DOI] [PubMed] [Google Scholar]
- 113. Breedvelt JJF, Kandola A, Kousoulis AA et al. What are the effects of preventative interventions on major depressive disorder (MDD) in young adults? A systematic review and meta‐analysis of randomized controlled trials. J Affect Disord 2018;239:18‐29. [DOI] [PubMed] [Google Scholar]
- 114. Cuijpers P, van Straten A, Smit F et al. Preventing the onset of depressive disorders: a meta‐analytic review of psychological interventions. Am J Psychiatry 2008;165:1272‐80. [DOI] [PubMed] [Google Scholar]
- 115. Moreno‐Peral P, Conejo‐Ceron S, Rubio‐Valera M et al. Effectiveness of psychological and/or educational interventions in the prevention of anxiety: a systematic review, meta‐analysis, and meta‐regression. JAMA Psychiatry 2017;74:1021‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hoare E, Thorp A, Bartholomeusz‐Raymond N et al. Opportunities in the Australian national education initiative for promoting mental health in schools. Lancet Child Adolesc Health 2020;4:11‐3. [DOI] [PubMed] [Google Scholar]
- 117. Cuijpers P, Koole SL, van Dijke A et al. Psychotherapy for subclinical depression: meta‐analysis. Br J Psychiatry 2014;205:268‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Buntrock C, Ebert DD, Lehr D et al. Effect of a web‐based guided self‐help intervention for prevention of major depression in adults with subthreshold depression: a randomized clinical trial. JAMA 2016;315:1854‐63. [DOI] [PubMed] [Google Scholar]
- 119. Garrido S, Millington C, Cheers D et al. What works and what doesn't work? A systematic review of digital mental health interventions for depression and anxiety in young people. Front Psychiatry 2019;10:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Papola D, Purgato M, Gastaldon C et al. Psychological and social interventions for the prevention of mental disorders in people living in low‐ and middle‐income countries affected by humanitarian crises. Cochrane Database Syst Rev 2020;9:CD012417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rasic D, Hajek T, Alda M et al. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta‐analysis of family high‐risk studies. Schizophr Bull 2014;40:28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lawrence PJ, Murayama K, Creswell C. Systematic review and meta‐analysis: anxiety and depressive disorders in offspring of parents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2019;58:46‐60. [DOI] [PubMed] [Google Scholar]
- 123. Pepper EJ, Pathmanathan S, McIlrae S et al. Associations between risk factors for schizophrenia and concordance in four monozygotic twin samples. Am J Med Genet B Neuropsychiatr Genet 2018;177:503‐10. [DOI] [PubMed] [Google Scholar]
- 124. Barnett JH, Smoller JW. The genetics of bipolar disorder. Neuroscience 2009;164:331‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hettema JM, Neale MC, Kendler KS. A review and meta‐analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry 2001;158:1568‐78. [DOI] [PubMed] [Google Scholar]
- 126. McGuffin P, Katz R, Watkins S et al. A hospital‐based twin register of the heritability of DSM‐IV unipolar depression. Arch Gen Psychiatry 1996;53:129‐36. [DOI] [PubMed] [Google Scholar]
- 127. Lo LE, Kaur R, Meiser B et al. Risk of schizophrenia in relatives of individuals affected by schizophrenia: a meta‐analysis. Psychiatry Res 2020;286:112852. [DOI] [PubMed] [Google Scholar]
- 128. Chen MH, Hsu JW, Huang KL et al. Risk and coaggregation of major psychiatric disorders among first‐degree relatives of patients with bipolar disorder: a nationwide population‐based study. Psychol Med 2019;49:2397‐404. [DOI] [PubMed] [Google Scholar]
- 129. Gottschalk MG, Domschke K. Genetics of generalized anxiety disorder and related traits. Dialogues Clin Neurosci 2017;19:159‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wilde A, Chan HN, Rahman B et al. A meta‐analysis of the risk of major affective disorder in relatives of individuals affected by major depressive disorder or bipolar disorder. J Affect Disord 2014;158:37‐47. [DOI] [PubMed] [Google Scholar]
- 131. Agerbo E, Sullivan PF, Vilhjalmsson BJ et al. Polygenic risk score, parental socioeconomic status, family history of psychiatric disorders, and the risk for schizophrenia: a Danish population‐based study and meta‐analysis. JAMA Psychiatry 2015;72:635‐41. [DOI] [PubMed] [Google Scholar]
- 132. Schneider M, Debbane M, Bassett AS et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry 2014;171:627‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Seidman LJ, Nordentoft M. New targets for prevention of schizophrenia: is it time for interventions in the premorbid phase? Schizophr Bull 2015;41:795‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Johnstone EC, Ebmeier KP, Miller P et al. Predicting schizophrenia: findings from the Edinburgh High‐Risk Study. Br J Psychiatry 2005;186:18‐25. [DOI] [PubMed] [Google Scholar]
- 135. Schneider M, Armando M, Pontillo M et al. Ultra high risk status and transition to psychosis in 22q11.2 deletion syndrome. World Psychiatry 2016;15:259‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Loechner J, Starman K, Galuschka K et al. Preventing depression in the offspring of parents with depression: a systematic review and meta‐analysis of randomized controlled trials. Clin Psychol Rev 2018;60:1‐14. [DOI] [PubMed] [Google Scholar]
- 137. Al‐Sawafi A, Lovell K, Renwick L et al. Psychosocial family interventions for relatives of people living with psychotic disorders in the Arab world: systematic review. BMC Psychiatry 2020;20:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Perich T, Mitchell PB. Psychological interventions for young people at risk for bipolar disorder: a systematic review. J Affect Disord 2019;252:84‐91. [DOI] [PubMed] [Google Scholar]
- 139. Fusar‐Poli P, Meyer‐Lindenberg A. Forty years of structural imaging in psychosis: promises and truth. Acta Psychiatr Scand 2016;134:207‐24. [DOI] [PubMed] [Google Scholar]
- 140. Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry 2012;17:1174‐9. [DOI] [PubMed] [Google Scholar]
- 141. Parnas J, Sass LA, Zahavi D. Rediscovering psychopathology: the epistemology and phenomenology of the psychiatric object. Schizophr Bull 2013;39:270‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ioannidis JP, Munafo MR, Fusar‐Poli P et al. Publication and other reporting biases in cognitive sciences: detection, prevalence, and prevention. Trends Cogn Sci 2014;18:235‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ioannidis JP. The mass production of redundant, misleading, and conflicted systematic reviews and meta‐analyses. Milbank Q 2016;94:485‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bortolato B, Kohler CA, Evangelou E et al. Systematic assessment of environmental risk factors for bipolar disorder: an umbrella review of systematic reviews and meta‐analyses. Bipolar Disord 2017;19:84‐96. [DOI] [PubMed] [Google Scholar]
- 145. Kohler CA, Evangelou E, Stubbs B et al. Mapping risk factors for depression across the lifespan: an umbrella review of evidence from meta‐analyses and Mendelian randomization studies. J Psychiatr Res 2018;103:189‐207. [DOI] [PubMed] [Google Scholar]
- 146. Fullana MA, Tortella‐Feliu M, Fernandez de la Cruz L et al. Risk and protective factors for anxiety and obsessive‐compulsive disorders: an umbrella review of systematic reviews and meta‐analyses. Psychol Med 2020;50:1300‐15. [DOI] [PubMed] [Google Scholar]
- 147. Carvalho AF, Solmi M, Sanches M et al. Evidence‐based umbrella review of 162 peripheral biomarkers for major mental disorders. Transl Psychiatry 2020;10:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Berk M, Woods RL, Nelson MR et al. Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. JAMA Psychiatry 2020;77:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sanders KM, Stuart AL, Williamson EJ et al. Annual high‐dose vitamin D3 and mental well‐being: randomised controlled trial. Br J Psychiatry 2011;198:357‐64. [DOI] [PubMed] [Google Scholar]
- 150. Davies C, Segre G, Estrade A et al. Prenatal and perinatal risk and protective factors for psychosis: a systematic review and meta‐analysis. Lancet Psychiatry 2020;7:399‐410. [DOI] [PubMed] [Google Scholar]
- 151. Jones I, Chandra PS, Dazzan P et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post‐partum period. Lancet 2014;384:1789‐99. [DOI] [PubMed] [Google Scholar]
- 152. Morrell CJ, Sutcliffe P, Booth A et al. A systematic review, evidence synthesis and meta‐analysis of quantitative and qualitative studies evaluating the clinical effectiveness, the cost‐effectiveness, safety and acceptability of interventions to prevent postnatal depression. Health Technol Assess 2016;20:1‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Keynejad RC, Hanlon C, Howard LM. Psychological interventions for common mental disorders in women experiencing intimate partner violence in low‐income and middle‐income countries: a systematic review and meta‐analysis. Lancet Psychiatry 2020;7:173‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. O'Connor E, Thomas R, Senger CA et al. Interventions to prevent illicit and nonmedical drug use in children, adolescents, and young adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2020;323:2067‐79. [DOI] [PubMed] [Google Scholar]
- 155. Salazar de Pablo G, De Micheli A, Solmi M et al. Universal and selective interventions to prevent poor mental health outcomes in young people: systematic review and meta‐analysis. Submitted for publication. [DOI] [PubMed]
- 156. Caldwell DM, Davies SR, Hetrick SE et al. School‐based interventions to prevent anxiety and depression in children and young people: a systematic review and network meta‐analysis. Lancet Psychiatry 2019;6:1011‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Hu MX, Turner D, Generaal E et al. Exercise interventions for the prevention of depression: a systematic review of meta‐analyses. BMC Public Health 2020;20:1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Szoke A, Kirkbride JB, Schurhoff F. Universal prevention of schizophrenia and surrogate endpoints at population level. Soc Psychiatry Psychiatr Epidemiol 2014;49:1347‐51. [DOI] [PubMed] [Google Scholar]
- 159. van Os J, Reininghaus U. Psychosis as a transdiagnostic and extended phenotype in the general population. World Psychiatry 2016;15:118‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Sullivan SA, Kounali D, Cannon M et al. A population‐based cohort study examining the incidence and impact of psychotic experiences from childhood to adulthood, and prediction of psychotic disorder. Am J Psychiatry 2020;177:308‐17. [DOI] [PubMed] [Google Scholar]
- 161. Karcher NR, Barch DM, Avenevoli S et al. Assessment of the Prodromal Questionnaire‐Brief Child Version for measurement of self‐reported psychoticlike experiences in childhood. JAMA Psychiatry 2018;75:853‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Yung AR, Lin A. Psychotic experiences and their significance. World Psychiatry 2016;15:130‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ferro MA. The psychometric properties of the Kessler Psychological Distress Scale (K6) in an epidemiological sample of Canadian youth. Can J Psychiatry 2019;64:647‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ross RG, Hunter SK, McCarthy L et al. Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am J Psychiatry 2013;170:290‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ross RG, Hunter SK, Hoffman MC et al. Perinatal phosphatidylcholine supplementation and early childhood behavior problems: evidence for CHRNA7 moderation. Am J Psychiatry 2016;173:509‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Li Y, Freedman R. Prospects for improving future mental health of children through prenatal maternal micronutrient supplementation in China. Pediatr Investig 2020;4:118‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Catena A, Munoz‐Machicao JA, Torres‐Espinola FJ et al. Folate and long‐chain polyunsaturated fatty acid supplementation during pregnancy has long‐term effects on the attention system of 8.5‐y‐old offspring: a randomized controlled trial. Am J Clin Nutr 2016;103:115‐27. [DOI] [PubMed] [Google Scholar]
- 168. McGrath J, Saari K, Hakko H et al. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res 2004;67:237‐45. [DOI] [PubMed] [Google Scholar]
- 169. Fujita Y, Fujita A, Ishima T et al. Dietary intake of glucoraphanin during pregnancy and lactation prevents the behavioral abnormalities in the offspring after maternal immune activation. Neuropsychopharmacol Rep 2020;40:268‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Codagnone MG, Spichak S, O'Mahony SM et al. Programming bugs: microbiota and the developmental origins of brain health and disease. Biol Psychiatry 2019;85:150‐63. [DOI] [PubMed] [Google Scholar]
- 171. Shinde S, Weiss HA, Varghese B et al. Promoting school climate and health outcomes with the SEHER multi‐component secondary school intervention in Bihar, India: a cluster‐randomised controlled trial. Lancet 2018;392:2465‐77. [DOI] [PubMed] [Google Scholar]
- 172. Shinde S, Weiss HA, Khandeparkar P et al. A multicomponent secondary school health promotion intervention and adolescent health: an extension of the SEHER cluster randomised controlled trial in Bihar, India. PLoS Med 2020;17:e1003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Lund C, Brooke‐Sumner C, Baingana F et al. Social determinants of mental disorders and the Sustainable Development Goals: a systematic review of reviews. Lancet Psychiatry 2018;5:357‐69. [DOI] [PubMed] [Google Scholar]
- 174. Schuch FB, Stubbs B, Meyer J et al. Physical activity protects from incident anxiety: a meta‐analysis of prospective cohort studies. Depress Anxiety 2019;36:846‐58. [DOI] [PubMed] [Google Scholar]
- 175. Bueno‐Antequera J, Munguia‐Izquierdo D. Exercise and schizophrenia. Adv Exp Med Biol 2020;1228:317‐32. [DOI] [PubMed] [Google Scholar]
- 176. Melo MC, Daher Ede F, Albuquerque SG et al. Exercise in bipolar patients: a systematic review. J Affect Disord 2016;198:32‐8. [DOI] [PubMed] [Google Scholar]
- 177. Fusar‐Poli P, Bauer M, Borgwardt S et al. European College of Neuropsychopharmacology Network on the Prevention of Mental Disorders and Mental Health Promotion (ECNP PMD‐MHP). Eur Neuropsychopharmacol 2019;29:1301‐11. [DOI] [PubMed] [Google Scholar]
- 178. Strunk CM, King KA, Vidourek RA et al. Effectiveness of the surviving the Teens® suicide prevention and depression awareness program: an impact evaluation utilizing a comparison group. Health Educ Behav 2014;41:605‐13. [DOI] [PubMed] [Google Scholar]
- 179. Witvliet M, van Lier PA, Cuijpers P et al. Testing links between childhood positive peer relations and externalizing outcomes through a randomized controlled intervention study. J Consult Clin Psychol 2009;77:905‐15. [DOI] [PubMed] [Google Scholar]
- 180. World Health Organization . Prevention and promotion in mental health. Geneva: World Health Organization, 2002. [Google Scholar]
- 181. Collins S, Woolfson L, Durkin K. Effects on coping skills and anxiety of a universal school‐based mental health intervention delivered in Scottish primary schools. Sch Psychol Int 2014;35:85‐100. [Google Scholar]
- 182. Sharp P, Caperchione C. The effects of a pedometer‐based intervention on first‐year university students: a randomized control trial. J Am Coll Health 2016;64:630‐8. [DOI] [PubMed] [Google Scholar]
- 183. Pendry P, Carr AM, Smith AN et al. Improving adolescent social competence and behavior: a randomized trial of an 11‐week equine facilitated learning prevention program. J Prim Prev 2014;35:281‐93. [DOI] [PubMed] [Google Scholar]
- 184. Rousseau C, Benoit M, Lacroix L et al. Evaluation of a sandplay program for preschoolers in a multiethnic neighborhood. J Child Psychol Psychiatry 2009;50:743‐50. [DOI] [PubMed] [Google Scholar]
- 185. Salazar de Pablo G, De Micheli A, Nieman D et al. Universal and selective interventions to promote good mental health in young people: systematic review and meta‐analysis. Eur Neuropsychopharmacol 2020;41:28‐39. [DOI] [PubMed] [Google Scholar]
- 186. Solanes A, Albajes‐Eizagirre A, Fullana M et al. Can we increase the subjective well‐being of the general population? An umbrella review of the evidence. Rev Psiquiatr Salud Ment 2021;14:50‐64. [DOI] [PubMed] [Google Scholar]
- 187. Guloksuz S, Pries LK, Ten Have M et al. Association of preceding psychosis risk states and non‐psychotic mental disorders with incidence of clinical psychosis in the general population: a prospective study in the NEMESIS‐2 cohort. World Psychiatry 2020;19:199‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Yung AR, Wood SJ, Malla A et al. The reality of at risk mental state services: a response to recent criticisms. Psychol Med 2021;51:212‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. McHugh MJ, McGorry PD, Yuen HP et al. The Ultra‐High‐Risk for psychosis groups: evidence to maintain the status quo. Schizophr Res 2018;195:543‐8. [DOI] [PubMed] [Google Scholar]
- 190. Schultze‐Lutter F, Klosterkotter J, Gaebel W et al. Psychosis‐risk criteria in the general population: frequent misinterpretations and current evidence. World Psychiatry 2018;17:107‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Perez J, Jones PB. Breaking the web: life beyond the at‐risk mental state for psychosis. Psychol Med (in press). [DOI] [PubMed] [Google Scholar]
- 192. Raballo A, Poletti M, Carpenter WT. Rethinking the psychosis threshold in clinical high risk. Schizophr Bull 2019;45:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Moritz S, Gaweda L, Heinz A et al. Four reasons why early detection centers for psychosis should be renamed and their treatment targets reconsidered: we should not catastrophize a future we can neither reliably predict nor change. Psychol Med 2019;49:2134‐40. [DOI] [PubMed] [Google Scholar]
- 194. McGorry PD, Hartmann JA, Spooner R et al. Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry. World Psychiatry 2018;17:133‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Ajnakina O, David AS, Murray RM. ‘At risk mental state’ clinics for psychosis – an idea whose time has come – and gone! Psychol Med 2019;49:529‐34. [DOI] [PubMed] [Google Scholar]
- 196. Malhi GS, Morris G, Hamilton A et al. Is “early intervention” in bipolar disorder what it claims to be? Bipolar Disord 2017;19:627‐36. [DOI] [PubMed] [Google Scholar]
- 197. Duffy A, Grof P. Commentary on McGorry et al.'s Debate on: “Is ‘early intervention’ in bipolar disorder what it claims to be?”. Bipolar Disord 2018;20:556‐7. [DOI] [PubMed] [Google Scholar]
- 198. Fusar‐Poli P, Sullivan SA, Shah JL et al. Improving the detection of individuals at clinical risk for psychosis in the community, primary and secondary care: an integrated evidence‐based approach. Front Psychiatry 2019;10:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Fusar‐Poli P, Rutigliano G, Stahl D et al. Development and validation of a clinically based risk calculator for the transdiagnostic prediction of psychosis. JAMA Psychiatry 2017;74:493‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Fusar‐Poli P, Oliver D, Spada G et al. The case for improved transdiagnostic detection of first‐episode psychosis: Electronic Health Record cohort study. Schizophr Res (in press). [DOI] [PubMed] [Google Scholar]
- 201. Raket L, Jaskolowski J, Kinon B et al. Dynamic ElecTronic hEalth reCord deTection (DETECT) of individuals at risk of a first episode of psychosis: a case‐control development and validation study. Lancet Digital Health 2020;2:E229‐39. [DOI] [PubMed] [Google Scholar]
- 202. Zalsman G, Hawton K, Wasserman D et al. Suicide prevention strategies revisited: 10‐year systematic review. Lancet Psychiatry 2016;3:646‐59. [DOI] [PubMed] [Google Scholar]
- 203. Schmidt A, Cappucciati M, Radua J et al. Improving prognostic accuracy in subjects at clinical high risk for psychosis: systematic review of predictive models and meta‐analytical sequential testing simulation. Schizophr Bull 2017;43:375‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Oliver D, Radua J, Reichenberg A et al. Psychosis Polyrisk Score (PPS) for the detection of individuals at‐risk and the prediction of their outcomes. Front Psychiatry 2019;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Oliver D, Spada G, Englund A et al. Real‐world digital implementation of the Psychosis Polyrisk Score (PPS): a pilot feasibility study. Schizophr Res 2020;226:176‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Petros N, Cullen AE, Fusar‐Poli P et al. Towards standardising the assessment of good outcome in people at clinical high risk for psychosis: a collaborative approach. Schizophr Res 2020;223:361‐2. [DOI] [PubMed] [Google Scholar]
- 207. Kas MJ, Penninx B, Sommer B et al. A quantitative approach to neuropsychiatry: the why and the how. Neurosci Biobehav Rev 2019;97:3‐9. [DOI] [PubMed] [Google Scholar]
- 208. Maj M. Why the clinical utility of diagnostic categories in psychiatry is intrinsically limited and how we can use new approaches to complement them. World Psychiatry 2018;17:121‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Reed GM, Sharan P, Rebello TJ et al. The ICD‐11 developmental field study of reliability of diagnoses of high‐burden mental disorders: results among adult patients in mental health settings of 13 countries. World Psychiatry 2018;17:174‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. First MB, Rebello TJ, Keeley JW et al. Do mental health professionals use diagnostic classifications the way we think they do? A global survey. World Psychiatry 2018;17:187‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Fusar‐Poli P, Solmi M, Brondino N, et al. Transdiagnostic psychiatry: a systematic review. World Psychiatry 2019;18:192‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Fusar‐Poli P. TRANSD recommendations: improving transdiagnostic research in psychiatry. World Psychiatry 2019;18:361‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Jauhar S, Krishnadas R, Nour MM et al. Is there a symptomatic distinction between the affective psychoses and schizophrenia? A machine learning approach. Schizophr Res 2018;202:241‐7. [DOI] [PubMed] [Google Scholar]
- 214. Caspi A, Houts RM, Ambler A et al. Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the Dunedin Birth Cohort Study. JAMA Netw Open 2020;3:e203221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Mollon J, David AS, Zammit S et al. Course of cognitive development from infancy to early adulthood in the psychosis spectrum. JAMA Psychiatry 2018;75:270‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Hickie IB, Hermens DF, Naismith SL et al. Evaluating differential developmental trajectories to adolescent‐onset mood and psychotic disorders. BMC Psychiatry 2013;13:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Hickie IB, Scott EM, Hermens DF et al. Applying clinical staging to young people who present for mental health care. Early Interv Psychiatry 2013;7:31‐43. [DOI] [PubMed] [Google Scholar]
- 218. Iorfino F, Scott EM, Carpenter JS et al. Clinical stage transitions in persons aged 12 to 25 years presenting to early intervention mental health services with anxiety, mood, and psychotic disorders. JAMA Psychiatry 2019;76:1167‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. de la Fuente‐Tomas L, Sanchez‐Autet M, Garcia‐Alvarez L et al. Clinical staging in severe mental disorders; bipolar disorder, depression and schizophrenia. Rev Psiquiatr Salud Ment 2019;12:106‐15. [DOI] [PubMed] [Google Scholar]
- 220. Duffy A, Malhi GS, Grof P. Do the trajectories of bipolar disorder and schizophrenia follow a universal staging model? Can J Psychiatry 2017;62:115‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Dodd S, Berk M, Kelin K et al. Treatment response for acute depression is not associated with number of previous episodes: lack of evidence for a clinical staging model for major depressive disorder. J Affect Disord 2013;150:344‐9. [DOI] [PubMed] [Google Scholar]
- 222. Romer AL, Elliott ML, Knodt AR et al. Pervasively thinner neocortex as a transdiagnostic feature of general psychopathology. Am J Psychiatry 2021;178:174‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Fusar‐Poli P, Palombini E, Davies C et al. Why transition risk to psychosis is not declining at the OASIS ultra high risk service: the hidden role of stable pretest risk enrichment. Schizophr Res 2018;192:385‐90. [DOI] [PubMed] [Google Scholar]
- 224. Parnas J, Henriksen MG. Epistemological error and the illusion of phenomenological continuity. World Psychiatry 2016;15:126‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Wigman JT, van Nierop M, Vollebergh WA et al. Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity – implications for diagnosis and ultra‐high risk research. Schizophr Bull 2012;38:247‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Fusar‐Poli P, Raballo A, Parnas J. What is an attenuated psychotic symptom? On the importance of the context. Schizophr Bull 2017;43:687‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Schultze‐Lutter F, Michel C, Ruhrmann S et al. Prevalence and clinical significance of DSM‐5‐attenuated psychosis syndrome in adolescents and young adults in the general population: the Bern Epidemiological At‐Risk (BEAR) study. Schizophr Bull 2014;40:1499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Mishara AL. Klaus Conrad (1905‐1961): delusional mood, psychosis, and beginning schizophrenia. Schizophr Bull 2010;36:9‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Rutigliano G, Valmaggia L, Landi P et al. Persistence or recurrence of non‐psychotic comorbid mental disorders associated with 6‐year poor functional outcomes in patients at ultra high risk for psychosis. J Affect Disord 2016;203:101‐10. [DOI] [PubMed] [Google Scholar]
- 230. Fusar‐Poli P, Nelson B, Valmaggia L et al. Comorbid depressive and anxiety disorders in 509 individuals with an at‐risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull 2014;40:120‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. van Os J, Guloksuz S. A critique of the “ultra‐high risk” and “transition” paradigm. World Psychiatry 2017;16:200‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Fusar‐Poli P, Spencer T, De Micheli A et al. Outreach and support in South‐London (OASIS) 2001‐2020: twenty years of early detection, prognosis and preventive care for young people at risk of psychosis. Eur Neuropsychopharmacol 2020;39:111‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Anglin DM, Galea S, Bachman P. Going upstream to advance psychosis prevention and improve public health. JAMA Psychiatry 2020;77:665‐6. [DOI] [PubMed] [Google Scholar]
- 234. Mennigen E, Bearden CE. Psychosis risk and development: what do we know from population‐based studies? Biol Psychiatry 2020;88:315‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Firth J, Solmi M, Wootton RE et al. A meta‐review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry 2020;19:360‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. O'Neil A, Jacka FN, Quirk SE et al. A shared framework for the common mental disorders and non‐communicable disease: key considerations for disease prevention and control. BMC Psychiatry 2015;15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Fusar‐Poli P, Hijazi Z, Stahl D et al. The science of prognosis in psychiatry: a review. JAMA Psychiatry 2018;75:1289‐97. [DOI] [PubMed] [Google Scholar]
- 238. Sanfelici R, Dwyer DB, Antonucci LA et al. Individualized diagnostic and prognostic models for patients with psychosis risk syndromes: a meta‐analytic view on the state of the art. Biol Psychiatry 2020;88:349‐60. [DOI] [PubMed] [Google Scholar]
- 239. Ioannidis JP, Tzoulaki I. What makes a good predictor?: the evidence applied to coronary artery calcium score. JAMA 2010;303:1646‐7. [DOI] [PubMed] [Google Scholar]
- 240. Salazar de Pablo G, Studerus E, Vaquerizo J et al. Implementing precision psychiatry: a systematic review of individualised prediction models for clinical practice. Schizophr Bull (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Oliver D, Spada G, Colling C et al. Real‐world implementation of precision psychiatry: transdiagnostic risk calculator for the automatic detection of individuals at‐risk of psychosis. Schizophr Res 2021;227:52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Nelson B, Amminger GP, Bechdolf A, et al. Evidence for preventive treatments in young patients at clinical high risk of psychosis: the need for context. Lancet Psychiatry 2020;7:378‐80. [DOI] [PubMed] [Google Scholar]
- 243. Kuharic DB, Kekin I, Hew J et al. Preventive treatments in patients at high risk of psychosis. Lancet Psychiatry 2020;7:384‐5. [DOI] [PubMed] [Google Scholar]
- 244. Wagenfeld MO. The primary prevention of mental illness: a sociological perspective. J Health Soc Behav 1972;13:195‐203. [PubMed] [Google Scholar]
- 245. Ebert DD, Buntrock C, Reins JA et al. Efficacy and moderators of psychological interventions in treating subclinical symptoms of depression and preventing major depressive disorder onsets: protocol for an individual patient data meta‐analysis of randomised controlled trials. BMJ Open 2018;8:e018582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Wallach JD, Sullivan PG, Trepanowski JF et al. Sex based subgroup differences in randomized controlled trials: empirical evidence from Cochrane meta‐analyses. BMJ 2016;355:i5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Wallach JD, Sullivan PG, Trepanowski JF et al. Evaluation of evidence of statistical support and corroboration of subgroup claims in randomized clinical trials. JAMA Intern Med 2017;177:554‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Schuit E, Li AH, Ioannidis JPA. How often can meta‐analyses of individual‐level data individualize treatment? A meta‐epidemiologic study. Int J Epidemiol 2019;48:596‐608. [DOI] [PubMed] [Google Scholar]
- 249. Schandelmaier S, Briel M, Varadhan R et al. Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta‐analyses. CMAJ 2020;192:E901‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Kent DM, Paulus JK, van Klaveren D et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement. Ann Intern Med 2020;172:35‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Fusar‐Poli P, Davies C, Solmi M et al. Preventive treatments for psychosis: umbrella review (just the evidence). Front Psychiatry 2019;11:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Fusar‐Poli P, Rutigliano G, Stahl D et al. Deconstructing pretest risk enrichment to optimize prediction of psychosis in individuals at clinical high risk. JAMA Psychiatry 2016;73:1260‐7. [DOI] [PubMed] [Google Scholar]
- 253. ESHRE Capri Workshop Group . Protect us from poor‐quality medical research. Hum Reprod 2018;33:770‐6. [DOI] [PubMed] [Google Scholar]
- 254. Berge E, Al‐Shahi Salman R, van der Worp HB et al. Increasing value and reducing waste in stroke research. Lancet Neurol 2017;16:399‐408. [DOI] [PubMed] [Google Scholar]
- 255. Correll CU, Rubio JM, Inczedy‐Farkas G et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta‐analytic evidence. JAMA Psychiatry 2017;74:675‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Pallmann P, Bedding AW, Choodari‐Oskooei B et al. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med 2018;16:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Malhi GS, Bell E, Hamilton A et al. Early intervention for risk syndromes: what are the real risks? Schizophr Res (in press). [DOI] [PubMed] [Google Scholar]
- 258. Solmi M, Fornaro M, Ostinelli EG et al. Safety of 80 antidepressants, antipsychotics, anti‐attention‐deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta‐review of 78 adverse effects. World Psychiatry 2020;19:214‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Correll CU, Rubio JM, Kane JM. What is the risk‐benefit ratio of long‐term antipsychotic treatment in people with schizophrenia? World Psychiatry 2018;17:149‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Catalan A, Salazar de Pablo G, Vaquerizo Serrano J et al. Annual research review: Prevention of psychosis in adolescents – systematic review and meta‐analysis of advances in detection, prognosis and intervention. J Child Psychol Psychiatry (in press). [DOI] [PubMed] [Google Scholar]
- 261. Berk M, Parker G. The elephant on the couch: side‐effects of psychotherapy. Aust N Z J Psychiatry 2009;43:787‐94. [DOI] [PubMed] [Google Scholar]
- 262. Tabak AG, Herder C, Rathmann W et al. Prediabetes: a high‐risk state for diabetes development. Lancet 2012;379:2279‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Beauchamp TL, Childress JF. Principles of biomedical ethics, 7th ed. Oxford: Oxford University Press, 2013. [Google Scholar]
- 264. Kim SW, Polari A, Melville F et al. Are current labeling terms suitable for people who are at risk of psychosis? Schizophr Res 2017;188:172‐7. [DOI] [PubMed] [Google Scholar]
- 265. Yang LH, Link BG, Ben‐David S et al. Stigma related to labels and symptoms in individuals at clinical high‐risk for psychosis. Schizophr Res 2015;168:9‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Anglin DM, Greenspoon MI, Lighty Q et al. Spontaneous labelling and stigma associated with clinical characteristics of peers ‘at‐risk’ for psychosis. Early Interv Psychiatry 2014;8:247‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Lee EH, Hui CL, Ching EY et al. Public stigma in China associated with schizophrenia, depression, attenuated psychosis syndrome, and psychosis‐like experiences. Psychiatr Serv 2016;67:766‐70. [DOI] [PubMed] [Google Scholar]
- 268. Lane NM, Hunter SA, Lawrie SM. The benefit of foresight? An ethical evaluation of predictive testing for psychosis in clinical practice. Neuroimage Clin 2020;26:102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Fusar‐Poli P, Cappucciati M, Bonoldi I et al. Prognosis of brief psychotic episodes: a meta‐analysis. JAMA Psychiatry 2016;73:211‐20. [DOI] [PubMed] [Google Scholar]
- 270. Blasco D, Stortz SW, Grivel MM et al. Naturalistic conceptions of genetic optimism and precision psychiatry among those at clinical high‐risk for psychosis. Early Interv Psychiatry (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Davison J, Scott J. Should we intervene at stage 0? A qualitative study of attitudes of asymptomatic youth at increased risk of developing bipolar disorders and parents with established disease. Early Interv Psychiatry 2018;12:1112‐9. [DOI] [PubMed] [Google Scholar]
- 272. Schultze‐Lutter F, Schmidt SJ, Theodoridou A. Psychopathology – a precision tool in need of re‐sharpening. Front Psychiatry 2018;9:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Saxena S, Jane‐Llopis E, Hosman C. Prevention of mental and behavioural disorders: implications for policy and practice. World Psychiatry 2006;5:5‐14. [PMC free article] [PubMed] [Google Scholar]
- 274. Bloom DE, Cafiero ET, Jané‐Llopis E et al. The global economic burden of noncommunicable diseases. Geneva: World Economic Forum, 2011. [Google Scholar]
- 275. Knapp M, Wong G. Economics and mental health: the current scenario. World Psychiatry 2020;19:3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Henderson C, Dixon S, Bauer A et al. Cost‐effectiveness of PoNDER health visitor training for mothers at lower risk of depression: findings on prevention of postnatal depression from a cluster‐randomised controlled trial. Psychol Med 2019;49:1324‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Ho FY, Yeung WF, Ng TH et al. The efficacy and cost‐effectiveness of stepped care prevention and treatment for depressive and/or anxiety disorders: a systematic review and meta‐analysis. Sci Rep 2016;6:29281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Aceituno D, Vera N, Prina AM et al. Cost‐effectiveness of early intervention in psychosis: systematic review. Br J Psychiatry 2019;215:388‐94. [DOI] [PubMed] [Google Scholar]
- 279. Arango C, Diaz‐Caneja CM, McGorry PD et al. Preventive strategies for mental health. Lancet Psychiatry 2018;5:591‐604. [DOI] [PubMed] [Google Scholar]
- 280. Patton GC, Sawyer SM, Santelli JS et al. Our future: a Lancet commission on adolescent health and wellbeing. Lancet 2016;387:2423‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Patel V, Chisholm D, Parikh R et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet 2016;387:1672‐85. [DOI] [PubMed] [Google Scholar]
- 282. Patel V, Burns JK, Dhingra M et al. Income inequality and depression: a systematic review and meta‐analysis of the association and a scoping review of mechanisms. World Psychiatry 2018;17:76‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Ridley M, Rao G, Schilbach F et al. Poverty, depression, and anxiety: causal evidence and mechanisms. Science (in press). [DOI] [PubMed] [Google Scholar]
- 284. Lund C, De Silva M, Plagerson S et al. Poverty and mental disorders: breaking the cycle in low‐income and middle‐income countries. Lancet 2011;378:1502‐14. [DOI] [PubMed] [Google Scholar]
- 285. Wahlbeck K. Public mental health: the time is ripe for translation of evidence into practice. World Psychiatry 2015;14:36‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Moreno C, Wykes T, Galderisi S et al. How mental health care should change as a consequence of the COVID‐19 pandemic. Lancet Psychiatry 2020;7:813‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Oliver D, Kotlicka‐Antczak M, Minichino A et al. Meta‐analytical prognostic accuracy of the Comprehensive Assessment of at Risk Mental States (CAARMS): the need for refined prediction. Eur Psychiatry 2018;49:62‐8. [DOI] [PubMed] [Google Scholar]
- 288. Salazar de Pablo G, Catalan A, Fusar‐Poli P. Clinical validity of DSM‐5 attenuated psychosis syndrome: advances in diagnosis, prognosis, and treatment. JAMA Psychiatry 2020;77:311‐20. [DOI] [PubMed] [Google Scholar]
- 289. Bechdolf A, Ratheesh A, Cotton SM et al. The predictive validity of bipolar at‐risk (prodromal) criteria in help‐seeking adolescents and young adults: a prospective study. Bipolar Disord 2014;16:493‐504. [DOI] [PubMed] [Google Scholar]
- 290. Linscott RJ, van Os J. An updated and conservative systematic review and meta‐analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med 2013;43:1133‐49. [DOI] [PubMed] [Google Scholar]
- 291. Cannon TD, Yu CH, Addington J et al. An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry 2016;173:980‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Carrion RE, Cornblatt BA, Burton CZ et al. Personalized prediction of psychosis: external validation of the NAPLS‐2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry 2016;173:989‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Zhang T, Li H, Tang Y et al. Validating the predictive accuracy of the NAPLS‐2 psychosis risk calculator in a clinical high‐risk sample from the SHARP (Shanghai At Risk for Psychosis) Program. Am J Psychiatry 2018;175:906‐8. [DOI] [PubMed] [Google Scholar]
- 294. Osborne KJ, Mittal VA. External validation and extension of the NAPLS‐2 and SIPS‐RC personalized risk calculators in an independent clinical high‐risk sample. Psychiatry Res 2019;279:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Zhang T, Xu L, Tang Y et al. Prediction of psychosis in prodrome: development and validation of a simple, personalized risk calculator. Psychol Med 2019;49:1990‐8. [DOI] [PubMed] [Google Scholar]
- 296. Fusar‐Poli P, Werbeloff N, Rutigliano G et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent national health service trust. Schizophr Bull 2019;45:562‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Puntis S, Oliver D, Fusar‐Poli P. Third external replication of an individualised transdiagnostic prediction model for the automatic detection of individuals at risk of psychosis using electronic health records. Schizophr Res 2021;228:403‐9. [DOI] [PubMed] [Google Scholar]
- 298. Oliver D, Wong CMJ, Bøg M et al. Transdiagnostic individualized clinically‐based risk calculator for the automatic detection of individuals at‐risk and the prediction of psychosis: external replication in 2,430,333 US patients. Transl Psychiatry 2020;10:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Irving J, Patel R, Oliver D et al. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr Bull (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. King M, Walker C, Levy G et al. Development and validation of an international risk prediction algorithm for episodes of major depression in general practice attendees: the PredictD study. Arch Gen Psychiatry 2008;65:1368‐76. [DOI] [PubMed] [Google Scholar]
- 301. Nigatu YT, Liu Y, Wang JL. External validation of the international risk prediction algorithm for major depressive episode in the US general population: the PredictD‐US study. BMC Psychiatry 2016;16:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. King M, Bottomley C, Bellon‐Saameno JA et al. An international risk prediction algorithm for the onset of generalized anxiety and panic syndromes in general practice attendees: predictA. Psychol Med 2011;41:1625‐39. [DOI] [PubMed] [Google Scholar]
- 303. Nigatu YT, Wang JL. External validation of the International Risk Prediction Algorithm for the onset of generalized anxiety and/or panic syndromes (The Predict A) in the US general population. J Anxiety Disord 2019;64:40‐4. [DOI] [PubMed] [Google Scholar]
- 304. Birmaher B, Merranko JA, Goldstein TR et al. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adolesc Psychiatry 2018;57:755‐63.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]


