Abstract
As the novel SARS-CoV-2 continues to infect numerous individuals worldwide, one of the leading approaches in dealing with the global health crisis is vaccination against the COVID-19. Due to recent reports, vaccination with ChAdOx1 nCov-19 (developed by Oxford and AstraZeneca) may result in a vaccine-induced catastrophic thrombotic thrombocytopenia disorder. Thus, as of March 16 of 2021, vaccination programs in 18 countries had been suspended until further examination, including Sweden, Germany and France. This disorder presents as extensive thrombosis in atypical sites, primarily in the cerebral venous, alongside thrombocytopenia and the production of autoantibody against platelet-factor 4 (PF4). PF4 autoantibody has the ability to binds the human FcRγIIA receptor of platelets and contribute to their aggregation. This rare adverse effect extremely resembles the clinical presentation of the classical immune-mediated HIT disorder, which occurs following exposure to heparin. Surprisingly, none of these patients had been pre-exposed to heparin before disease onset, leading to the hypothesis that a viral antigen from the vaccine had triggered the response. Importantly, COVID-19 had been associated with numerous autoimmune manifestations, including the production of pathogenic autoantibodies, new onset of autoimmune diseases and disorders. As the ChAdOx1 nCov-19 vaccination leads to the synthesis of specific SARS-CoV-2-proteins, they may trigger a production of PF4 autoantibody though molecular mimicry phenomena, while vaccination compounds lead to a rigorous bystander activation of immune cells. If existing, removing such homological sequences from the vaccine may eliminate this phenomenon. In contrast, it needs to be emphasized that the ChAdOx1 nCoV-19 vaccine was found to be safe and efficacious against symptomatic COVID-19 in randomized controlled trials, which included 23,848 participants from the UK, Brazil and South Africa.
Keywords: ChAdOx1 nCoV-19, Vaccination, Thrombosis, AstraZeneca, Thrombocytopenia, PF4, AstraZeneca vaccine, Hypercoagulation, Heparin-induced thrombocytopenia, HIT, SARS-CoV-2, COVID-19, Molecular mimicry, Hyperstimulation
Abbreviations: COVID-19, coronavirus disease 19; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HIT, Heparin-induced thrombocytopenia; Platelet factor 4, PF4
1. Introduction
As the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to infect numerous individuals worldwide, one of the leading approaches in dealing with the global health crisis is vaccination against the coronavirus disease 2019 (COVID-19). Simultaneously, many governments struggle with supplying sufficient quantities of vaccines for their population, others managed to achieve high levels of coverage. In Israel, so far, more than half out of the 9 million population had been vaccinated with the second Pfizer–BioNTech vaccine, leading to a rapid decrease in COVID-19 ill patients; in the United States, more than 202 million doses have been administered. With such extensive and swift vaccination processes, safety concerns must not be neglected.
So far, the FDA had approved three different vaccines: [1] BNT162b2 mRNA vaccine (Pfizer–BioNTech) [2] mRNA-1273 vaccine (Moderna) [3] Ad26.COV2.S adenovirus vaccine (Johnson & Johnson/Janssen) [1]. The European Union had also authorized the usage of these three vaccines, as well as the ChAdOx1 nCoV-19 vaccine (Oxford–AstraZeneca) [2]. Recent reports documented several patients from multiple medical centers that presented unusual thrombotic events, thrombocytopenia and autoimmune manifestations 1–3 weeks after receiving a single dose of the ChAdOx1 nCoV-19 vaccination, thus had safety concerns towards the vaccine. Many of those clinical presentations were fatal and resemble the rare heparin-induced thrombocytopenia (HIT) disorder, an autoimmune disorder that commonly occurs following exposure to heparin. The classic characteristics of HIT are the formation of thromboembolisms in atypical sites, thrombocytopenia and the production of platelet-activating autoantibodies directed against platelet factor 4 (PF4) [3,4]. Noteworthy, similar disorder was previously documented in COVID-19 patients, mostly in Severely ill patients with an absence of a pre-exposure to heparin [5,6].
The vaccination developed by Oxford and AstraZeneca, the ChAdOx1 nCoV-19, is composed of a recombinant chimpanzee adenoviral vector which is encoding the spike protein of SARS-CoV-2. Most ChAdOx1 nCoV-19 vaccination is taking place in European countries. Subsequent to the recent reports of the vaccine-induced catastrophic thrombotic thrombocytopenia, as of March 16 of 2021, 18 countries had suspended their use of the vaccine until further examination, including Sweden, Germany and France. The suspension of ChAdOx1 nCoV-19 vaccination had been done despite the guidance of the European Medicine Agency (EMA) and the World Health Organization (WHO), which confirmed that the association between ChAdOx1 nCoV-19 and HIT presentation is lower than the classical HIT disorder prevalence in the general population. Meanwhile, the United Kingdom carries out its vaccination program despite 19 confirmed fatal vaccine-induced catastrophic thrombotic thrombocytopenia cases of inoculated individuals, as too early April.
Importantly, As of 4 April 2021, 169 cases of cerebral venous sinus thrombosis and 53 cases of splanchnic vein thrombosis were reported to the EudraVigilance, the system that analyzes information on suspected adverse reactions to medicines that have been authorized in the European Economic Area (EEA). Nevertheless, numerous cases may have fallen below the radar. By this date, roughly 34 million people had been vaccinated in the EEA and UK. Cases of cerebral venous sinus thrombosis were found to be higher in ChAdOx1 nCoV-19 vaccinated individuals relative to other COVID-19 vaccines; for comparison: 35 possible cases of central nervous system thrombosis were identified among 54 million inoculated individuals by the Pfizer–BioNTech mRNA vaccine. Although this adverse reaction may be considered extremely rare, further examination could be necessary.
It needs to be emphasized that the ChAdOx1 nCoV-19 vaccine was found to be safe and efficacious against symptomatic COVID-19 in randomized controlled trials, which included 23,848 participants from the UK, Brazil and South Africa [7]. In this study, throughout 74,341 person-months of safety follow-up 175 severe adverse events occurred in 168 participants: 84 events in the ChAdOx1 nCoV-19 group and 91 in the control group [7]. Thus, advantages associated with ChAdOx1 nCoV-19 vaccination efficacious against symptomatic COVID-19 outbalance the risks involved, nevertheless, investigating specifically the vaccine-induced thrombotic thrombocytopenia syndrome is still essential in order to ensure the safety of inoculated individuals.
2. Methods
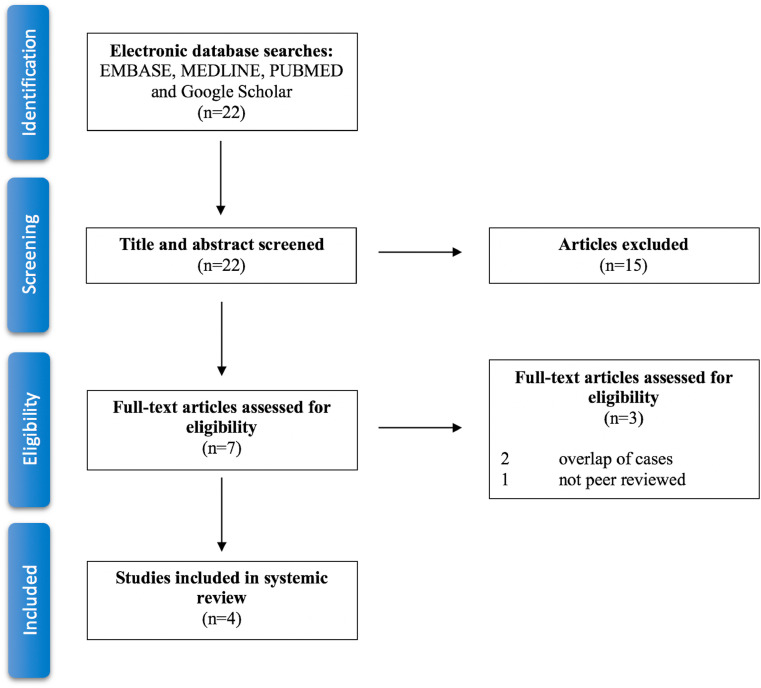
This systemic review was carried out using EMBASE, MEDLINE, PUBMED and Google Scholar databases in order to collect all the published articles related to the development of platelet factor 4 autoantibody and the presentation of heparin-induced thrombocytopenia in patients after ChAdOx1 nCoV-19 vaccination. This search was narrowed to original research articles, in order to avoid overlap of data. The search process occurred out on April 20, 2021 by the author, using the following terms in various combinations: “COVID-19”, “SARS-CoV-19”, “Heparin-induced thrombocytopenia”, “PF4”, “AstraZeneca vaccine” and “ChAdOx1 vaccine”. 22 published articles were identified. Out of those, 7 articles were relevant to this review. Two articles were removed due to overlap of cases with a different article, additionally to one that was not peer reviewed. Consequently, the search and sorting processes were finalized with 4 adequate articles, with the sum of 42 patients from the United Kingdom, Germany, Austria and Norway [[8], [9], [10], [11]] (Fig. 1).
Fig. 1.
Systematic review flow diagram. Flow diagram for the systematic review detailing the database searches, the number of abstracts screened and the full texts assessed.
3. Results
3.1. Patient characteristics
A sum of 41 ChAdOx1 nCov-19 induced catastrophic thrombotic thrombocytopenia cases are documented in peered reviewed journals, 39 of which in 3 articles published by ‘The New England Journal of Medicine’. All the examined individuals were in a medically stable condition at the time of vaccination and the vast majority had no preexisting prothrombotic condition in their medical history. Nonetheless, the initial rapid production of thrombosis accompanied by thrombocytopenia had developed 5–24 days after the first dose of vaccination with the ChAdOx1 nCov-19. 27 out of the 41 (65.8%) inoculated were females. The age range of the inspected patients was found to be relatively broad and unspecific (21–77 years old). By one of the examined articles, the mean age of 23 inoculated was 46 years of age [8].
3.2. Laboratory findings
Enzyme-linked immunosorbent assay (ELISA) for PF4 autoantibodies was positive in 36 of the 38 (94.7%) tested patients which were presented with vaccine-induced catastrophic thrombotic thrombocytopenia disorder. These autoantibodies are strongly associated with the classical HIT disorder and were found to activate platelet aggregation by binding to the FcRγIIA receptor of platelets [3,4]. Nonetheless, none of the patients had a pre-exposure to heparin before the onset of the illness, similar to the rare HIT-like disorder that severely-ill COVID-19 may develop [5,6]. All patients had a notable decline in platelet counts at diagnosis, with a proximate mean of 30,000–40,000 per cubic millimeter, with a range of approximately 10,000 to 110,000. Very high levels of d-dimers and low levels of fibrinogen had been documented. These laboratory findings corroborate the suggested systemic hypercoagulative state of the patients. Noteworthy that international normalized ratio (INR) and activated partial thromboplastin time (aPTT) was within the normal range in most patients. Likewise, in some patients, fibrinogen levels were found to be below the normal level, and yet normal in other patients.
3.3. Clinical findings
In time of admission, many of the symptoms reported by patients with vaccine-induced thrombotic thrombocytopenia included headaches, which began after the vaccination or a few days later, in some accompanied by fever. After laboratory and imaging tests were conducted, the patients were diagnosed with hypercoagulation that lead to thrombosis and thrombocytopenia. Numerous patients had more than a single site of thrombosis, most were found in atypical sites, primarily thrombosis that involved the cerebral venous. Additional common sites of thrombosis include the splanchnic, portal and hepatic veins, while some patients presented pulmonary emboli, arterial thrombosis and other sites of thromboembolism. 17 out of the 41 patients (41.4%) had died, mostly as a result of hemorrhagic or ischemic brain injury. Nevertheless, results of postmortem evaluation from one patient showed evidence of thrombosis in many small vessels, that were particularly found in the lungs, intestine and venous sinuses, accompanied by extensive intracerebral hemorrhage [8].
3.4. Treatment
Despite the fact that a relatively modest number of vaccine-induced catastrophic thrombotic thrombocytopenia patients and their treatment were documented, sufficient treatment appears to be similar to that which would be applied to a classical HIT disorder case.
Most importantly, despite thrombocytopenia, platelet transfusions would not be appropriate and should be avoided in order to abstain from additional immune-mediated platelet activation and hypercoagulation. As PF4 is an autoantibody responsible for platelet activation, Intravenous immunoglobulin (IVIg) had been shown to substantially improve patient coagulative stability and lead to a steady elevation in platelet count. Plasma exchange may be an effective treatment as well in removing PF4 autoantibodies if IVIg is unavailable. Nonetheless, long term implications of the PF4 autoantibodies remain unclear. Anticoagulation treatment might be harmful in some cases, due to the risk of hemorrhagic brain injury, particularly in patients with cerebral venous thrombosis. Treatment should be applied as soon as possible, due to high mortality rates, specifically in patients presenting with cerebral venous thrombosis. Therefore, immediate IVIg treatment should be considered in patients presenting with thrombosis, thrombocytopenia, very high d-dimer level after ChAdOx1 nCoV-19 vaccine. Treatment should not be delayed for examining the presence of PF4 autoantibodies through ELISA.
4. Discussion
COVID-19 is associated with numerous autoimmune manifestations, including the production of pathogenic autoantibodies, new onset of autoimmune diseases and additional immune-mediated disorders, such as immune-thyroid dysfunction, Guillain-Barré syndrome, Kawasaki disease and others [12]. The most common autoimmune manifestation in COVID-19 patient is the production of antiphospholipid antibodies (APLA), which includes development of the anticardiolipin (aCL), lupus anticoagulant (LAC) and beta2 glycoprotein I (β2GPI) antibodies [13]. APLA can bind to cell membrane and induce a hypercoagulative state, thereby contribute to thromboembolism formation. As COVID-19 patients commonly present with coagulation impairment, APLA seems to be a significant component. In a study conducted on 104 COVID-19 patients, 49/104 (47.1%) were found in positive for at least one of the APLA [14]. Yet, in contrast to LAC and β2GPI, thrombosis events in COVID-19 patients were significantly associated with aCL, which was found present in 35/104 (33.7%) of the patients [14].
Apart from the development of APLA, additional immune-mediated blood disorders were found to be associated with SARS-CoV-2 infection. Immune thrombocytopenia (ITP) secondary to SARS-CoV-2 infection had been documented in 45 cases [15]. Autoimmune hemolytic anemia (AIHA) and Evans syndrome (ES) were identified in COVID-19 patients, with the detection of both warm and cold IgG [[16], [17], [18]].
The immune-mediated blood disorders mentions are merely a modest fraction of the numerous autoimmune manifestations that were found to have an association with SARS-CoV-2 infection. These immune-mediated disorders may arise through a similar mechanism to that of the PF4 autoantibody production, described previously. In general, autoimmune manifestations secondary to SARS-CoV-2 infection are thought to arise in genetically predisposition individuals through two principal mechanisms: [1] molecular mimicry between human and components of SARS-CoV-2 leading to antigenic cross-reactivity phenomena and [2] the ability of SARS-CoV-2 to induce hyper-stimulation of the immune response.
Molecular mimicry is a well-established mechanism that could contribute to autoimmunity and is relevant to a wide variety of viruses [12,[19], [20], [21]]. The existence of molecular mimicry has high pathological potential, as an antibody‐mediated response against homological components of viruses may cross-react with self-antigens, possibly leading to the new-onset of autoimmune disease [12,[19], [20], [21]]. Current data describe many primary sequences as homological to human components, produced by a broad range of cells, thus establishing SARS-CoV-2 to be an additional virus that has molecular mimicry with humans [12,[21], [22], [23]]. Molecular mimicry may have a key role in the development of PF4 autoantibody, which binds to the human FcRγIIA receptor of platelets and contributes to their aggregation. Among these numerous homological sequences of SARS-CoV-2, there might be one that can trigger the production of PF4 autoantibody; This mechanism had been proposed to initiate the production of PF4 autoantibodies in some severely-ill COVID-19 patients, that were not pre-exposed to heparin [5,6]. In order to analyze the contribution of molecular mimicry as a mechanism contributing to the development of PF4 autoantibodies, we suggest analyzing primary sequences of the SARS-CoV-2 spike protein and PF4 for potential homology. Identify potential homology. Importantly, secondary and tetrameric folded forms of the proteins could originate additional structures that may shape homologous structures.
Mechanism of action of the ChAdOx1 nCov-19 vaccine includes a modified version of a chimpanzee adenovirus, known as ChAdOx1, that has the ability to insert artificial DNA into human cells. The objective is to generate synthesis of SARS-CoV-2 spike protein by the cells of the inoculated, which will lead to a rigorous bystander activation of immune cells. The existence of molecular mimicry between the vaccine-induced proteins of SARS-CoV-2 and human components might give rise to potential side effects by leading to the production of pathological autoantibodies, resulting in vaccine-induced autoimmunity [24]. In such a case, removing homologous sequences from the vaccination would prevent future production of PF4 antibodies by vaccinated individuals.
Additionally, as genetic disposition remains a leading factor of autoimmunity, researchers should also examine a potential association of non-classical MHC to the development of vaccine-induced catastrophic thrombotic thrombocytopenia disorder.
5. Conclusions
ChAdOx1 nCoV-19 vaccine (developed by Oxford and AstraZeneca) was found to be safe and efficacious against symptomatic COVID-19 in large randomized controlled trials, thus advantages associated with ChAdOx1 nCoV-19 vaccination efficacious against symptomatic COVID-19 outbalance the risks involved. Nevertheless, recent evidence suggests that vaccination with ChAdOx1 nCov-19 may result in a vaccine-induced catastrophic thrombotic thrombocytopenia disorder. This disorder presents as extensive thrombosis in atypical sites, thrombocytopenia and the production of autoantibody against platelet-factor 4. This rare adverse effect extremely resembles the clinical presentation of the immune-mediated heparin-induced thrombocytopenia disorder. Immediate treatment of IVIg should be considered in patients presenting with thrombosis, thrombocytopenia, very high d-dimer level after ChAdOx1 nCoV-19 vaccine. Implications of the autoantibody against platelet-factor 4, still remains unclear. Although seems to be rare, investigating the vaccine-induced thrombotic thrombocytopenia syndrome is essential in order to ensure the safety of inoculated individuals.
Funding
None.
References
- 1.Commissioner O of the. Learn more about COVID-19 vaccines from the FDA. 2021 Apr 20. https://www.fda.gov/consumers/consumer-updates/learn-more-about-covid-19-vaccines-fda FDA [Internet]-Available from:
- 2.COVID-19 vaccines European vaccination information portal. 2021 Apr 21. https://vaccination-info.eu/en/covid-19/covid-19-vaccines [Internet] Available from:
- 3.Arepally G.M., Ortel T.L. Heparin-induced thrombocytopenia. N. Engl. J. Med. 2006 Aug 24;355(8):809–817. doi: 10.1056/NEJMcp052967. [DOI] [PubMed] [Google Scholar]
- 4.Arepally G.M. Heparin-induced thrombocytopenia. Blood. The Journal of the American Society of Hematology. 2017 May 25;129(21):2864–2872. doi: 10.1182/blood-2016-11-709873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X., Zhang X., Xiao Y., Gao T., Wang G., Wang Z., Zhang Z., Hu Y., Dong Q., Zhao S., Yu L. Heparin-induced thrombocytopenia is associated with a high risk of mortality in critical COVID-19 patients receiving heparin-involved treatment. MedRxiv. 2020 Jan 1 [Google Scholar]
- 6.Riker R.R., May T.L., Fraser G.L., Gagnon D.J., Bandara M., Zemrak W.R., Seder D.B. Heparin‐induced thrombocytopenia with thrombosis in COVID‐19 adult respiratory distress syndrome. Research and practice in thrombosis and haemostasis. 2020 Jul;4(5):936–941. doi: 10.1002/rth2.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voysey M., Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021 Jan 9;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. 10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scully M., Singh D., Lown R., Poles A., Solomon T., Levi M., Goldblatt D., Kotoucek P., Thomas W., Lester W. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021 Apr 16 doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., Wiedmann M., Aamodt A.H., Skattør T.H., Tjønnfjord G.E., Holme P.A. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021 Apr 9 doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021 Apr 9 doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta P.R., Mangion S.A., Benger M., Stanton B.R., Czuprynska J., Arya R., Sztriha L.K. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination-a report of two UK cases. Brain Behav. Immun. 2021 Apr 12 doi: 10.1016/j.bbi.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dotan A., Muller S., Kanduc D., David P., Halpert G., Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021 Feb 19:102792. doi: 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cervera R. Antiphospholipid syndrome. Thromb. Res. 2017;151:S43–S47. doi: 10.1016/S0049-3848(17)30066-X. [DOI] [PubMed] [Google Scholar]
- 14.Le Joncour A., Frere C., Martin-Toutain I., Gougis P., Ghillani-Dalbin P., Maalouf G., Vieira M., Marcelin A.G., Salem J.E., Allenbach Y., Saadoun D. Antiphospholipid antibodies and thrombotic events in COVID-19 patients hospitalized in medicine ward. Autoimmun. Rev. 2021 Feb;20(2):102729. doi: 10.1016/j.autrev.2020.102729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharjee S., Banerjee M. Immune thrombocytopenia secondary to COVID-19: a systematic review. SN comprehensive clinical medicine. 2020 Sep 19 doi: 10.1007/s42399-020-00521-8. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazarian G., Quinquenel A., Bellal M., Siavellis J., Jacquy C., Re D., Merabet F., Mekinian A., Braun T., Damaj G., Delmer A. Autoimmune haemolytic anaemia associated with COVID‐19 infection. Br. J. Haematol. 2020 Jul;190(1):29–31. doi: 10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riker R.R., May T.L., Fraser G.L., Gagnon D.J., Bandara M., Zemrak W.R., Seder D.B. Heparin‐induced thrombocytopenia with thrombosis in COVID‐19 adult respiratory distress syndrome. Research and practice in thrombosis and haemostasis. 2020 Jul;4(5):936–941. doi: 10.1002/rth2.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M., Nguyen C.B., Yeung Z., Sanchez K., Rosen D., Bushan S. Evans syndrome in a patient with COVID‐19. Br. J. Haematol. 2020 Jul;190(2):e59–61. doi: 10.1111/bjh.16846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oldstone M.B. Molecular mimicry and immune-mediated diseases. FASEB J Off Publ Fed Am Soc Exp Biol. 1998 Oct;12(13):1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blank M., Barzilai O., Shoenfeld Y. Molecular mimicry and auto-immunity. Clin. Rev. Allergy Immunol. 2007 Feb 1;32(1):111–118. doi: 10.1007/BF02686087. [DOI] [PubMed] [Google Scholar]
- 21.Kanduc D., Shoenfeld Y. Molecular mimicry between sars-cov-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunological research. 2020 doi: 10.1007/s12026-020-09152-6. 4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angileri F., Légaré S., Marino Gammazza A., Conway de Macario E., Macario A.J., Cappello F. Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID‐19? Br. J. Haematol. 2020 Jul;190(2):e92–e93. doi: 10.1111/bjh.16883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucchese G., Flöel A. Molecular mimicry between SARS-CoV-2 and respiratory pacemaker neurons. Autoimmun. Rev. 2020 Jul 1 doi: 10.1016/j.autrev.2020.102556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segal Y., Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell. Mol. Immunol. 2018 Jun;15(6):586–594. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]