Abstract
Withdrawal from opioid painkillers can produce short‐lived physical symptoms and protracted psychological symptoms including anxiety and depressive‐like states that often lead to opioid misuse and opioid use disorder (OUD). Studies testing the hypothesis that opioid withdrawal potentiates the reinforcing effects of opioid self‐administration (SA) are largely inconclusive and have focused on males. Although some clinical evidence indicates that women are more likely than men to misuse opioids to self‐medicate, preclinical studies in both sexes are lacking. Based on clinical reports, we hypothesized that withdrawal from escalating‐dose morphine injections that approximates a prescription painkiller regimen would lead to increased oxycodone SA to a greater extent in female compared to male rats. After escalating‐dose morphine (5–30 mg/kg or vehicle, twice/day for 12 days), rats underwent a 2‐week abstinence period during which withdrawal signs were measured. The impact of this treatment was assessed on oxycodone SA acquisition, maintenance, dose response, and progressive ratio responding, with additional analyses to compare sexes. We found that both sexes expressed somatic withdrawal, whereas only males demonstrated hyperalgesia in the warm water tail flick assay. During SA acquisition, males with prior morphine exposure took significantly more oxycodone than females. Finally, females with prior morphine exposure demonstrated the lowest motivation to SA oxycodone in the progressive ratio test. Contrary to our initial hypothesis, our findings suggest that prior opioid exposure increases vulnerability to initiate misuse more in males and decreases the reinforcing efficacy of oxycodone in females.
Keywords: hyperalgesia, intravenous self‐administration, opioid use disorder, progressive ratio, sex differences, somatic withdrawal
Escalating‐dose, noncontingent morphine treatment produces dependence in male and female rats. Withdrawal experience potentiates acquisition of oxycodone self‐administration in males and diminishes maintenance of oxycodone self‐administration in females. These findings demonstrate an impact of biological sex throughout opioid dependence and voluntary intake that will help guide mechanistic understanding of sex differences in opioid use disorder.

1. INTRODUCTION
The 2018 National Survey on Drug Use and Health reported that 9.9 million people aged 12 and older misused prescription opioids in the past year, suggesting that prescription opioid misuse contributes to the current opioid epidemic. 1 Although the majority of people who misuse prescription opioids or subsequently develop opioid use disorder (OUD) do not have a legitimate prescription, the evolution of misuse often develops after a prescribed treatment regimen of opioid painkillers. 2 In adults, data suggest that the most typical motive for initiating opioid misuse is pain relief, but this often shifts to a motivation to manage withdrawal and negative affect, 3 experience euphoria, or to sleep. 2
Chronic opioid treatment leads to dependence, defined by an abstinence‐induced withdrawal syndrome that includes both an acute physical syndrome (24–48 h postwithdrawal) and a negative affective syndrome of dysphoria, anxiety, irritability, and preoccupation with cravings to use opioids. 4 Negative affect is observed with acute withdrawal but typically has a protracted and debilitating course in humans 5 and animal models. 6 Although the opioid withdrawal syndrome is considered to be one of the primary causes of continued opioid misuse, precipitators of relapse, and barriers to positive treatment outcomes, there is conflicting data in support of the idea that negative reinforcement motivates opioid self‐administration (SA) and relapse. 7 On the one hand, the opponent process theory 8 suggests that the motivation to take opioids transitions from positive to negative reinforcement as tolerance to the rewarding effects of the drug along with the emergence of withdrawal states grows. 9 On the other hand, there is evidence that induction of opioid withdrawal is associated with reduced drug‐taking and drug seeking, 10 and that, in humans, exposure to drug‐related cues can produce withdrawal signs but relatively weak craving for drug. 11 A seminal study showed that spontaneous withdrawal from repeated morphine injections increased SA of the short‐acting opioid remifentanil, whereas continued morphine treatment reduced SA compared to control rats that did not receive any noncontingent morphine. 12 However, these studies have been conducted exclusively in males.
Gender plays an important role in prescription opioid misuse. Prevalence rates for prescription opioid misuse between men and women are often found to be similar—a finding that contrasts with the consistently higher prevalence of abuse in men compared to women observed across most other substances of abuse, including heroin. 13 In general, women who are drug‐dependent express greater negative emotional states such as stress and depression, 14 , 15 and these are more likely to trigger craving and relapse in women than men. 16 , 17 There is evidence that women are more likely to initiate opioid misuse and develop OUD if they are first prescribed opioid painkillers. 15 Furthermore, women report misusing opioids to self‐medicate negative affect more than men. 15 Together, these studies suggest that opioid withdrawal plays a greater role in triggering misuse and OUD in women, but there is no conclusive biological evidence supporting this clinical narrative.
Preclinical data on how previous exposure to opioid dependence contributes to drug‐taking behavior are limited. Furthermore, there is little data on how biological sex contributes to risk for opioid misuse after a painkiller prescription. The overall goal of the current study was to test the hypothesis that exposure to, and withdrawal from, chronic, noncontingent morphine injections—meant to generically approximate a clinical regimen of prescription opioids—would increase opioid SA behavior to a greater extent in female compared to male rats. To test this hypothesis, adult male and female Sprague‐Dawley rats were treated for 12 days with an escalating‐dose regimen of morphine followed by 14 days of abstinence. Morphine was chosen as a representative opioid because it shares key pharmacological and physiological actions with other prescription opioids, and there is a rich literature on effects of chronic morphine in rodent models. 18 Withdrawal‐associated parameters were measured throughout this period, and rats were then implanted with jugular vein catheters and allowed to SA oxycodone, one of the most commonly abused prescription opioids, 19 under a range of conditions aimed to assess acquisition, total intake, drug sensitivity, and motivation to work for oxycodone. These studies are important because they address factors (prior opioid exposure and sex) that may contribute to risk for prescription opioid misuse and development of OUD.
2. METHODS AND MATERIALS
2.1. Animals
Adult male (275–300 g; N = 54) and female (220–225 g; N = 66) Sprague‐Dawley rats (Charles River Laboratory, Wilmington, MA) were used, comprising five cohorts. Each cohort represented each of four treatment groups (male, vehicle; male, morphine; female, vehicle; female, morphine). Upon arrival, rats were group‐housed (three rats/cage) and were acclimated for 1 week in a 12‐h light‐dark cycle (lights on 07:00) with food and water ad libitum. All rats remained singly housed following surgeries and during SA studies. All guidelines recommended by the Animal Care and Use Committee of McLean Hospital and by the National Institutes of Health guide for the care and use of Laboratory animals were followed.
2.2. Chronic morphine regimen
To approximate the noncontingent nature of an opioid painkiller prescription, male and female rats were injected with escalating‐dose morphine (or saline) twice a day for 12 days (10:00 am and 4:00 pm). Morphine (morphine sulphate; NIDA Drug Supply Program) was dissolved in saline (0.09%) and administered intraperitoneally (i.p.) as day (d) am, pm: d1: 5, 5; d2: 10, 10; d3: 10, 10; d4: 10, 10; d5: 10, 10; d6: 10, 20; d7: 20, 20; d8: 20, 20; d9: 20, 30; d10: 30, 30; d11: 30, 30; d12: 30, 30 mg/kg (see Figure 1A,B). Body weights were measured before each injection. This dosing was based on modified versions of published regimens in rats using escalating doses up to 140 mg/kg 20 , 21 and chronic injections of a constant dose of 10 mg/kg. 22 In a pilot study, we found that 10%–20% of male rats died in response to morphine doses higher than 30 mg/kg.
FIGURE 1.
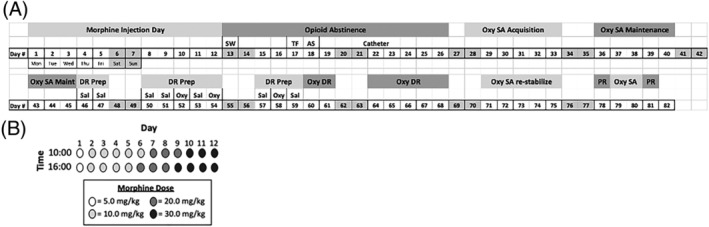
Experimental design of the study. A, Timeline of behaviors and jugular catheter surgeries (“catheter”, d22). Days are numbered sequentially starting with the first day of morphine injections and ending on the last day of PR. B, Escalating dose morphine regimen: 2 injections per day (10:00 and 16:00) for 12 days (12d), with doses increasing from 5 to 30 mg/kg/injection, IP. AS, acoustic startle; DR, dose response; inj, injection; Morph, morphine; Oxy, oxycodone; Prep, Preparation; Prog Ratio, progressive ratio; SA, self‐administration; SW, somatic withdrawal; TF, tail flick; Veh, vehicle
2.3. Somatic withdrawal
Somatic withdrawal signs were scored on withdrawal d1, 16 h following the last morphine injection. After 15‐min habituation to the behavior room, each rat was placed in a Plexiglas cylindrical tank with bedding. Rats' behavior was digitally recorded for 15 min, and videos were later scored by an experimenter blind to treatment condition. The number of instances of the following behaviors was recorded once every 15 s throughout the 15‐min session, as described in Chartoff et al 23 : grooming, ptosis, and wet dog shakes. Body weights were also recorded for a subset of the total rats from withdrawal days 3–7 and 14. See Supporting Information for more detail.
2.4. Tail flick and acoustic startle assays
To determine if quick, noninvasive behavioral assays could be used to detect protracted abstinence signs, rats underwent the warm water tail flick (TF) assay on withdrawal day 5 and the acoustic startle (AS) test on withdrawal day 7. Latency for a rat to flick its tail out of the warm water is a measure of thermal nociception, and opioid withdrawal can be associated with hyperalgesia, reflected as a decrease in TF latency after chronic morphine treatment. 24 AS amplitude has been positively correlated with anxiogenesis, raising the possibility that this test could detect opioid withdrawal‐induced anxiety‐like states. 25
The TF assay was conducted as in Page et al. 26 Rats were habituated to experimental handling for 10 min the day prior to testing and habituated to the testing room for 1 h on test day. Rats were held with a towel, and the bottom 1 inch of the rat's tail was dipped in a water bath (52°C; Fisher Scientific). The latency for the rat to flick its tail out of the water was recorded, with a cut‐off time of 15 s to prevent tissue damage. After testing, rats were returned to their home cages. On each test day, TF latency was measured twice, with only the second measurement used for data analysis. See Supporting Information for more detail.
The AS test was conducted as in Meloni et al. 27 Briefly, rats were placed in startle cages (Med Associates) and given a 5‐min acclimation period followed by the presentation of two habituating startle stimuli (100 dB, 30‐s interstimulus interval [ISI]). Rats were then presented with 100 startle stimuli at each of three different intensities (95, 100, and 105 dB) in a semirandom order with a 30‐s ISI.
2.5. Oxycodone self‐administration studies
2.5.1. Intravenous catheter implantation
On day 10 of morphine withdrawal, rats were anesthetized with 80 mg/kg ketamine and 8 mg/kg xylazine and implanted with chronic indwelling silastic intravenous jugular catheters as described in Mavrikaki et al. 28 After surgery, rats were injected once with ketoprofen (5 mg/kg, SC) to reduce pain. Catheter patency was tested at least once per week by i.v. infusion of 150‐ to 200‐μl methohexital sodium (2 mg in 200 μl for the males and 1.5 mg in 150 μl for the females, i.v.) and assessing loss of righting reflex. If the latency to loss of righting was greater than 10 s, the catheter was not considered patent, and the rat was not tested. Attrition due to faulty catheters is indicated in Section 4 by the number of rats that completed each phase of the SA studies.
2.5.2. Oxycodone SA
Five days after catheter surgery, rats began oxycodone SA training. SA sessions were conducted 5 days/week in operant conditioning chambers (Med Associates, St. Albans, VT) as described in Mavrikaki et al 28 and in the Supporting Information. Each session began with the onset of the house light, and levers were extended to signal drug availability. Each infusion (4 s) was signaled by the onset of a cue light over the active lever and offset of the house light. A 6‐s timeout period was initiated at the start of each infusion, and during that period, any additional presses on the active lever had no consequences. Once the timeout period was over, the cue light turned off and the house light turned on to signal drug availability. All rats were fed ad libitum when in their home cages.
SA testing began with a 5‐day acquisition phase of 1‐h SA sessions under a fixed ratio 1 (FR1) schedule of reinforcement. The 1‐h sessions were followed by 8 days of 2‐h maintenance sessions at FR1. Under these FR1 schedules, an active lever press resulted in an infusion of 0.06 mg/kg oxycodone. Inactive lever presses were counted but had no outcome. After 8 days of 2 h/day SA maintenance sessions, those rats with patent, catheters that displayed consistent SA behavior were moved to the dose response phase. To initiate dose‐response testing, oxycodone was replaced with saline for four consecutive sessions to ensure that rats could discriminate oxycodone from saline and to minimize nonspecific responses to saline. In the dose‐response test, rats self‐administered oxycodone (0.0003, 0.003, 0.01, 0.03, 0.1, and 0.3 mg/kg/infusion), presented in random order, in daily 2‐h trials.
After the dose response, rats continued to SA oxycodone in daily (M‐F) 2‐h sessions for an additional 5 days to restabilize before being tested under progressive ratio (PR) conditions. Rats were tested twice (separated by 2 days of 2‐h, FR1 training SA) in 4‐h PR oxycodone (0.06 mg/kg/infusion) sessions following this schedule of ratios: 1, 1, 2, 2, 2, 3, 3, 4, 4, 5, 5, 6, 6, 7, 7, 8, 9, 9, 10, 11, 11, 12, 13, 14, 14, and so forth, as established for oxycodone. 29 The final ratio completed for a drug infusion during the PR session is defined as the break point, which reflects the motivation of the animal to self‐administer a drug. 30 The data shown in Figure 6 represent the average infusions, active lever presses, and break points for the two PR sessions.
FIGURE 6.
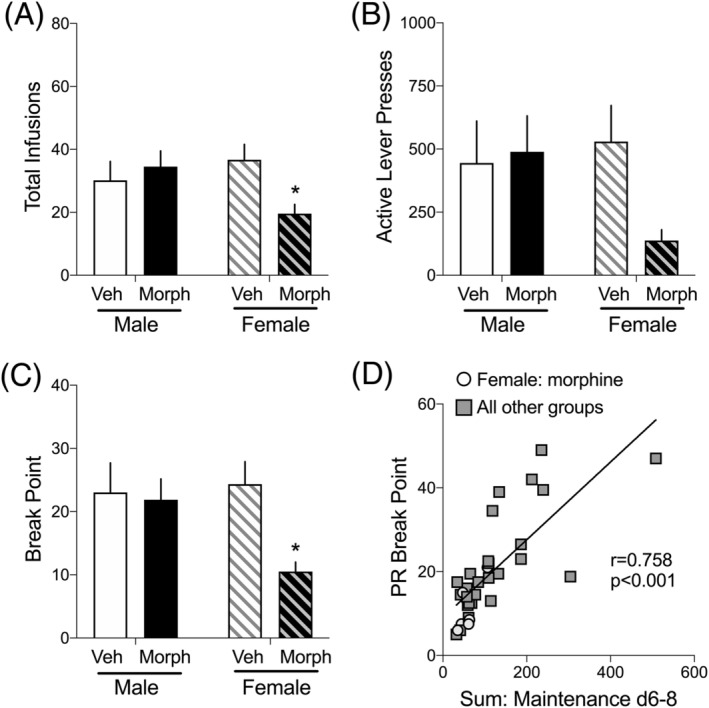
Effects of previous morphine experience and withdrawal on motivation to SA oxycodone in the progressive ratio (PR) test. Data are the average (+SEM) of 2d of 2 h/days PR testing. A, Total number of infusions, B, active lever presses, and C, break point, which is the last ratio completed to earn a reinforcer. N = 7–12 rats/group. *p < 0.05 compared to Veh of the same sex. D, Pearson r correlation was conducted on the sum of infusions on maintenance days 8–10 compared to the PR break point. A significant positive correlation was found (r = 0.758) such that the more oxycodone self‐administered at the end of the maintenance phase of SA, the higher the PR break point. Data from females previously exposed to morphine and withdrawal are represented with grey circles, whereas all other groups are represented with dark squares. Linear regression analysis was also conducted: R 2 = 0.574, p < 0.001
3. STATISTICAL ANALYSIS
Statistics were performed using GraphPad Prism 8. Time course analyses (Figures 2, 4, and 5) were analyzed with 3‐way mixed effects analysis (sex × treatment × time) with repeated measures on time. Grouped analyses (Figures 3, 4, and 6) were analyzed with 2‐way ANOVA (sex × treatment). Correlation analysis (Figure 6D) was analyzed with Pearson r correlation. In the event of significant 2‐ or 3‐factor/variable interactions in the 3‐way mixed effects analyses, subsequent combined 2‐way ANOVAs or post hoc Bonferroni tests, respectively, were done. In the event of significant interactions in the 2‐way ANOVAs, post hoc Bonferroni tests were done.
FIGURE 2.
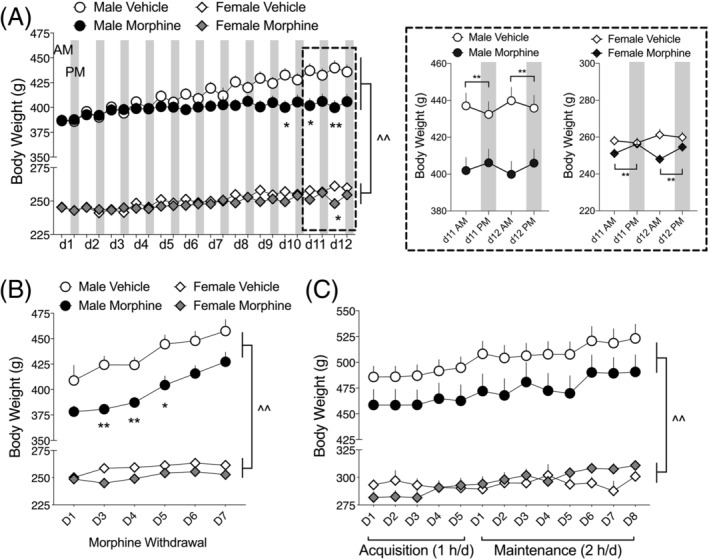
Body weight in male and female rats over the course of morphine injections, withdrawal, and oxycodone SA. A, Vehicle‐treated, but not morphine‐treated, male rats gain weight during the 12d morphine/vehicle injection regimen. Female rats, regardless of treatment, gain a nominal amount of weight. Inset (dashed box): A daily pattern of weight changes emerges at the end of the 12d morphine injection regimen. Morphine‐treated male and female rats gain a small amount of weight during the day (from AM to PM measurements), whereas vehicle‐treated rats tend to lose a small amount of weight during the day. N = 25–33 per group. Each day shows body weights prior to the two injections: White background is AM injection, grey background is PM injection. B, Body weights remain significantly decreased in morphine‐treated male rats on withdrawal days 3, 4, and 5, although weights tend to be lower in morphine‐withdrawn males throughout the withdrawal period. No change in body weights is observed in female rats during withdrawal. N = 25–33 per group. C, Body weights of male rats previously exposed to morphine remain qualitatively lower throughout the oxycodone SA tests, but oxycodone SA does not in itself alter body weights in either males or females. N = 15–18 per group. *p < 0.05, **p < 0.01 comparing within‐sex body weights on specific days or between two timepoints indicated by brackets; ^^ p < 0.01 main effect of sex
FIGURE 4.
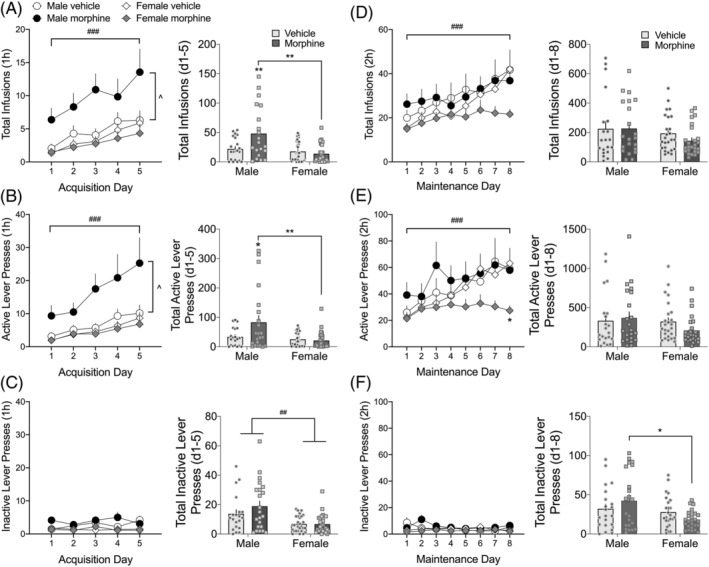
Effects of previous morphine experience and withdrawal on oxycodone self‐administration (SA; 0.06 mg/kg/infusion, FR1 schedule of reinforcement). (A–C) Prior morphine experience and withdrawal potentiate acquisition of oxycodone SA in male, but not female, rats. Total number of oxycodone infusions (A), active lever presses (B), and inactive lever presses (C) in 1‐h daily sessions. (D–F) Prior morphine experience and withdrawal reduce the amount of oxycodone SA in female, but not male, rats during the last 4 days of the 2‐h/days maintenance phase. Total number of oxycodone infusions (D), active lever presses (E), and inactive lever presses (F) in 2‐h daily sessions. *p < 0.05 comparing female morphine to female vehicle; ### p < 0.001 main effect of time; ^ p < 0.05 main effect of treatment. Insets: For each graph (A–F), data are also expressed as the total of infusions or lever presses over either the acquisition or maintenance phases of SA. *p < 0.05. **p < 0.01 comparing groups indicated with brackets; ## p < 0.01 main effect of sex. Data are expressed as mean ± SEM; N = 20–26/group
FIGURE 5.
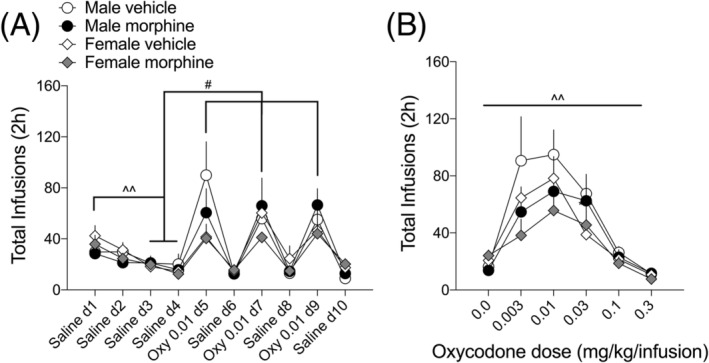
Effects of previous morphine experience and withdrawal on oxycodone dose–response in male and female rats. A, In preparation for the dose response test, rats went through a small extinction procedure in which saline was substituted for oxycodone for 4 days until the number of infusions (saline d3, 4) was significantly less than those on saline d1 (^^ p < 0.01). Subsequently, rats self‐administered either oxycodone (0.01 mg/kg/infusion) or saline on alternate days for a total of 6 days to demonstrate that rats could behaviorally differentiate between saline and oxycodone (# p < 0.05 comparing infusions on oxycodone days to saline d3, 4). B, In the dose response test, rats self‐administered one dose/day of oxycodone (0.003–0.3 mg/kg/infusions; one dose/trial/day), presented in random order. Data are presented as the total number of oxycodone infusions during each days' 2‐h dose–response test. ^^ p < 0.01 main effect of dose. N = 7–14 rats/group
FIGURE 3.
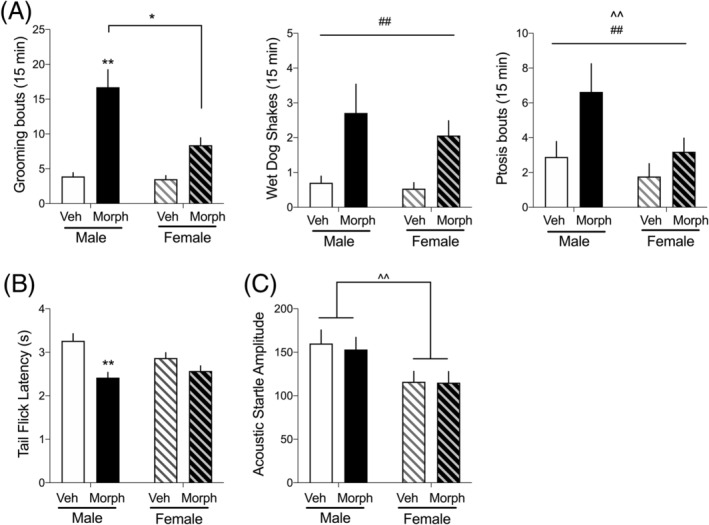
Spontaneous morphine withdrawal and protracted withdrawal effects on tail flick (TF) latency and acoustic startle (AS) amplitude. A, from left to right, panels represent the observed number of grooming, wet dog shakes, and ptosis bouts observed in the 15‐min recording sessions, with morphine‐withdrawn rats showing more of each withdrawal sign. N = 25–32 per group. B, Latency to withdraw tail from warm water bath (TF assay) measured on withdrawal day 5. Male, but not female, rats previously exposed to chronic morphine show a decrease in TF latency N = 19–21 per group. C, Amplitude of acoustic startle response measured on withdrawal day 7. Regardless of treatment, female rats showed lower startle amplitudes because of their lower body weights. N = 25–30 rats per group. *p < 0.05, **p < 0.01 compared to vehicle treated of the same sex or groups indicated with brackets; ^ p < 0.05, ^^ p < 0.01 main effect of sex; ## p < 0.01 main effect of treatment
4. RESULTS
4.1. Escalating‐dose, noncontingent morphine produces acute and protracted withdrawal signs in males and females
During the 12 days of escalating‐dose morphine (or vehicle) injections, body weights were measured twice each day: just prior to the am and pm injections. The number of rats in each group were Male, Vehicle: 25; Male, Morphine: 29; Female, Vehicle: 33; Female, Morphine: 33. A three‐way mixed effects analysis on body weights (sex × treatment × time) revealed a significant sex × treatment × time interaction (F 23,2,654 = 14.18, p < 0.0001) (Figure 2A), with male rats having significantly greater body weights than female rats, regardless of treatment. Independent two‐way ANOVAs for males and females over time showed significant treatment × time interactions for males (F 23,1,472 = 9.06, p < 0.0001) and females (F 23,1,182 = 28.65, p < 0.0001), which can be observed in Figure 2A as morphine‐treated rats—particularly males—gaining significantly less weight over the 12‐day morphine regimen. Further examining the daily changes in body weights over the last 2 days of the morphine treatment regimen, there were significant treatment × time interactions for both males (F 3,192 = 29.86, p < 0.0001) and females (F 3,150 = 13.79, p < 0.0001). Bonferroni post hoc tests showed that vehicle‐treated male and female rats showed dips in body weights in the PM measurements, whereas morphine‐treated rats showed an opposite trend: increased body weights in the PM measurements.
Body weights were also tracked during morphine withdrawal and throughout the acquisition and maintenance phases of oxycodone intravenous SA. A three‐way mixed effects analysis on body weights (sex × treatment × withdrawal day) revealed a significant sex × treatment × withdrawal day interaction (F 5,359 = 3.84, p < 0.01) (Figure 2B), with male rats having significantly greater body weights than female rats, regardless of treatment. Independent two‐way mixed effects analyses for males and females over time showed significant treatment × withdrawal day interactions for males (F 5,161 = 5.27, p < 0.001) and females (F 5,188 = 4.85, p < 0.001). Bonferroni post hoc tests show that morphine‐withdrawn male, but not female, rats weighed significantly less than control rats during the first 5 days of withdrawal. In males, body weights of morphine‐withdrawn rats remained lower than controls for the remainder of the experiment (Figure 2B,C), but the differences were not statistically significant on a day‐by‐day basis. Oxycodone SA began after the 14‐day morphine withdrawal period. Weights are reported throughout the acquisition (SA: 1 h/day) and training (SA: 2 h/day) periods. There was not a significant sex × treatment × SA day interaction (F 12,590 = 0.905, ns) (Figure 2C), but there was a significant sex × SA day interaction (F 12,590 = 8.45, p < 0.001). These data indicate that body weights continued to increase over time, but there were no differences between morphine‐treated and controls for each sex during the SA period.
The day after the last morphine injection, spontaneous somatic withdrawal signs were scored. The number of rats in each group wer Male, Vehicle: 25; Male, Morphine: 26; Female, Vehicle: 30; Female, Morphine: 32. For each of the somatic withdrawal behaviors reported, the incidence was significantly higher in morphine‐treated compared to vehicle‐treated rats (Figure 3A). In addition, males generally showed higher levels of somatic withdrawal signs than females. Withdrawal‐induced grooming depended on a sex × treatment interaction (F 1,109 = 9.45, p < 0.001), wet dog shakes and ptosis depended on main effects of treatment (wet dog shakes: F 1,109 = 15.52, p < 0.0001; Ptosis: F 1,109 = 6.53, p < 0.05), and ptosis also depended on a main effect of sex (F 1,109 = 5.13, p < 0.05).
During the 14‐day withdrawal period, tail flick (TF) latency was recorded on withdrawal day 5, and acoustic startle (AS) amplitude was recorded on withdrawal day 7. These noninvasive and rapid behavioral tests were used to assess the presence of protracted withdrawal signs. For TF latencies, there was a sex × treatment interaction (F 1,77 = 4.26, p < 0.05); Figure 3B), with males, but not females, previously treated with morphine having a significantly shorter tail withdrawal latency than males previously treated with saline. This decrease in latency is consistent with opioid withdrawal‐induced hyperalgesia. 24 For AS amplitude, there was a main effect of sex (F 1,109 = 9.18, p < 0.01; Figure 3C), with males having a greater AS response than females, regardless of morphine treatment. Importantly, this is because males weigh more than females and thus displace the load cell on the startle platform more when they startle.
4.2. Withdrawal from escalating‐dose, noncontingent morphine is associated with greater initial oxycodone self‐administration in males
After 14 days of withdrawal from the escalating‐dose morphine injection regimen, rats were put in the SA operant chambers for the first time and allowed to SA oxycodone for 1 h/days for 5 days (acquisition). The number of rats in each group that completed the acquisition and maintenance SA phases was Male, Vehicle: 20; Male, Morphine: 21; Female, Vehicle: 26; Female, Morphine: 25. The number of oxycodone infusions during acquisition (Figure 4A) depended on a sex × treatment interaction (F 1,92 = 5.85, p < 0.05). Subsequent two‐way mixed effects analyses for both males and females revealed that morphine‐withdrawn males but not females, self‐administered significantly more oxycodone than vehicle‐treated males during acquisition (F 1,39 = 4.54, p < 0.05). Similarly, the number of active lever presses (Figure 4B) depended on a sex × treatment interaction (F 1,92 = 5.00, p < 0.05), with morphine‐withdrawn males, but not females, pressing the active lever significantly more than vehicle‐treated males (F 1,40 = 4.00, p < 0.05). Presses on the inactive lever averaged 5 or fewer per group per day, and there was a main effect of sex (F 1,92 = 15.97, p < 0.001; Figure 4C), with males pressing the inactive lever more than females—regardless of prior treatment. Taken together, these data show that, over time, all rats increased the amount of oxycodone self‐administered, and morphine‐withdrawn males took the most drug of any group. To summarize the findings over the 5‐day acquisition period, data are also represented as the sum of infusions, active lever presses, or inactive lever presses (see Figure 4A–C insets). Morphine‐withdrawn males, but not females, SA more oxycodone and press the active lever more than vehicle‐treated males and morphine‐withdrawn females (infusions: sex × treatment interaction, F 1,91 = 8.08, p < 0.01; active lever: sex × treatment interaction, F 1,92 = 5.34, p < 0.05).
After the 5‐day oxycodone SA acquisition period, rats went through an 8‐day, FR1, 2 h/days SA maintenance regimen. The number of oxycodone infusions and active lever presses during maintenance (Figure 4D,E) depended on treatment × time interactions (infusions: F 7,561 = 3.44, p < 0.01; active lever presses; F 7,561 = 3.54, p < 0.01). As with the acquisition phase, presses on the inactive lever (Figure 4F) were minimal, yet there was a main effect of sex (F 1,90 = 7.46, p < 0.01), with males pressing the inactive lever more than females. To summarize the findings over the 8‐day maintenance period, data are also represented as the sum of infusions, active lever presses, or inactive lever presses (see Figure 4D–F insets). Although the time course data clearly show that oxycodone infusions and active lever presses increased over time in all groups except morphine‐withdrawn females, the sum of infusions and active lever presses over the entire 8‐day period do not reveal any significant effects (insets).
4.3. Prior exposure to non‐contingent morphine does not significantly alter the dose‐effect function for oxycodone SA
After the 8 days of oxycodone SA maintenance, rats were put through 4 days of saline extinction, in which active lever presses led to a saline, rather than an oxycodone, infusion. This was followed by three rounds of alternating saline and oxycodone (0.01 mg/kg/infusion; the oxycodone dose that produces maximum operant responding under our conditions 28 SA in order to establish the ability of the rats to behaviorally distinguish between saline and oxycodone. This 10‐day procedure (Figure 5A) was important for the subsequent dose‐effect determinations because it demonstrated that rats would press the active lever very little for saline and press the lever significantly more for oxycodone. The effects of this saline extinction and saline versus oxycodone discrimination procedure depended on time (F 2.1,97.95 = 13.45, p < 0.0001). All treatment groups responded similarly such that the total infusions self‐administered on saline extinction days 3 and 4 were significantly less than those self‐administered on day 1 (p < 0.01), regardless of treatment. Likewise, the total infusions self‐administered on oxycodone (0.01 mg/kg/infusion) days 5, 7, and 9 were more than saline extinction days 3 and 4. Given the ability of all treatment groups to distinguish between saline and oxycodone, the rats were then allowed to SA increasing doses of oxycodone in 1‐h sessions per day to establish a dose‐effect function (Figure 5B). The number of oxycodone infusions depended on dose (F 2.2,86.51 = 21.08, p < 0.0001), regardless of treatment group. Although it is visually clear from Figure 5B that morphine‐withdrawn females had qualitatively blunted responses in the dose‐effect experiment, there were no significant interactions (sex × treatment [F 1,40 = 0.123, ns] or sex × treatment × dose [F 5,197 = 0.051, ns]) nor a main effect of treatment (F 1,40) = 1.62, ns). The number of rats in each group that completed the dose response experiment were Male, Vehicle: 14; Male, Morphine: 13; Female, Vehicle: 10; Female, Morphine: 7.
4.4. Prior exposure to escalating‐dose, noncontingent morphine is associated with reduced responding in the progressive ratio test in females
The final test of the impact of noncontingent morphine injections followed by 14 days of withdrawal on oxycodone SA behavior in male and female rats was the progressive ratio (PR) test, which is indicative of the motivation to SA a drug. 31 The number of rats in each group that completed the PR tests were Male, Vehicle: 8; Male, Morphine: 9; Female, Vehicle: 12; Female, Morphine: 7. Five days after the dose response test, rats were tested in two independent, 4‐h PR sessions (oxycodone: 0.06 mg/kg/infusion) separated by 2 days of 2‐h, FR1 SA. Total infusions, active lever presses, and break points were averaged for the two PR sessions. Break point is defined as the last FR completed in the 4‐h session. The number of oxycodone infusions and break points depended on sex × treatment interactions (Infusions: F 1,31 = 4.20, p < 0.05; Figure 6A, break points: F 1,31 = 4.31, p < 0.05; Figure 6C), with Bonferroni post hoc tests showing a significant decrease in infusions in morphine‐experienced compared to vehicle control females. In contrast, a two‐way ANOVA of active lever presses was not significant (F 1,31 = 2.29, ns; Figure 6B). The finding that females previously exposed to morphine have lower PR responses compared to other groups is consistent with this group's lower oxycodone intake in the last half of the 10‐day training phase (Figure 4D,E). To ensure that the subset of rats on which PR testing was done showed SA behaviors similar to those shown in Figure 4, we graphed oxycodone acquisition and maintenance SA data for just the rats tested in PR (Figure S1) and found the same pattern of results. To further investigate the relationship between oxycodone SA in the last few days of SA training and PR break point, a Pearson r correlation test was conducted on the sum of infusions on training days 8–10 compared to the PR break point (Figure 6D). There was a strong positive correlation (r = 0.758, p < 0.001), such that the more oxycodone self‐administered in the last 3 days of training correlated with higher break points in the PR test—regardless of treatment group. We distinguished morphine‐withdrawn female data points (open circles) as a visual aid to show that both the sum of infusions during training and PR break points were at the lowest end of the graph. Due to the length of time rats had indwelling jugular catheters, a total of 35 rats still had patent catheters for the PR tests. To ensure that the SA data collected from these 35 rats were representative of the data presented for oxycodone SA acquisition and maintenance, we graphed these data for only those 35 rats used in PR (see Figure S1).
5. DISCUSSION
This study demonstrates that prior exposure to chronic, escalating dose morphine treatment followed by withdrawal results in potentiated acquisition of oxycodone SA in males but decreased maintenance of, and motivation for, oxycodone SA in females. Evidence for this is that when first allowed to SA oxycodone, morphine‐withdrawn male rats pressed the active lever and took significantly more infusions than control males or females. In contrast, female rats that had prior morphine and withdrawal experience self‐administered qualitatively less oxycodone across the 8‐day maintenance period following acquisition, and significantly less on the eighth day of oxycodone maintenance relative to other treatment groups. In addition, female rats previously exposed to morphine showed the least motivation to work for a unit dose of oxycodone in the PR paradigm. Although not significant, morphine‐exposed females also tended to SA less oxycodone per unit dose in the dose response paradigm. Spontaneous somatic withdrawal signs were observed in both male and female rats the day after the last morphine injection, demonstrating morphine dependence in both sexes. However, only morphine‐treated males failed to gain weight during the escalating dose morphine regimen and subsequent withdrawal period. In addition, withdrawal‐induced hyperalgesia was observed in males but not females. Broadly, these findings are contrary to our hypothesis that morphine‐withdrawn female rats would be more vulnerable to the reinforcing efficacy of oxycodone as measured with intravenous SA. This hypothesis of gender/sex differences in addictive‐like behavior was based primarily on clinical literature 2 , 32 as well as some preclinical studies, 33 , 34 , 35 although see Caine et al. 36 Despite these findings, there have been relatively few studies that directly compare male and female addiction‐like behaviors and those that do have typically used psychostimulants under a variety of experimental conditions. As such, our current findings combined with our previously published work 28 showing that drug‐naïve males SA more oxycodone than females provide important additions to our knowledge on male and female vulnerability to the reinforcing effects of prescription opioids.
It is well established that opioid withdrawal reduces body weight in male rats, 37 and our data corroborate this. It is notable that neither daily morphine treatments nor withdrawal modulated overall body weights in females, which is consistent with prior studies. 38 Close inspection of daily body weight fluctuations at the end of the escalating dose morphine regimen showed that morphine treated male and female rats gained weight between the AM and PM injections (all morphine treatments were done during the day) that was then lost overnight. This contrasts with saline‐treated rats, which gained weight overnight. Although the impact of opioids on food intake and body weight was not the focus of this study, our findings are consistent with studies showing that acute opioid treatment stimulates feeding behavior 39 and appears independent of biological sex.
Both male and female rats showed significant spontaneous somatic withdrawal signs the day after the last morphine injection. These were less robust than those observed after naloxone‐precipitated withdrawal, 40 but specific withdrawal‐associated behaviors such as increased grooming, wet dog shakes, and ptosis were significantly increased in morphine rats. On balance, somatic withdrawal signs were greater in males, suggesting a contribution of biological sex to their magnitude. 38 Opioid withdrawal unmasks neuroadaptations in the brain, 18 and our studies suggest that sex modulates these neuroadaptations in a circuit‐ and cellular‐specific manner.
Repeated exposure to opioids can induce the paradoxical response of opioid‐induced hyperalgesia, a condition in people characterized by increased pain sensitivity. 41 We found that only morphine‐treated males exhibited hyperalgesia‐like behavior in the TF assay when measured on withdrawal day 5, which is broadly consistent with findings by Holtman and Wala 42 in which males, but not females, showed hyperalgesia 2 h after low‐dose oxycodone administration. Previous research has shown that the underlying mechanisms of opioid‐induced hyperalgesia can be sex‐specific, 43 although it is not known if hyperalgesia promotes of discourages opioid misuse. The broad conclusion that males show more robust morphine withdrawal signs compared to females is consistent with increasing findings that opioid painkillers are typically more potent in males. Numerous studies suggest that this is due to brain region‐specific sex differences in MOR density 44 rather than pharmacokinetics. As such, in our study, it would not be feasible to equalize morphine doses to generate identical behavioral outputs because each behavior likely results from different brain circuits and different combinations of MOR densities and functional activities.
Over the last several decades, numerous studies have investigated whether opioid dependence and withdrawal are required for subsequent opioid SA, because clinical evidence suggests chronic opioid administration is associated with increased risk for opioid misuse and OUD. 2 As such, numerous approaches have been taken to test the interaction between opioid dependence/withdrawal and SA. Consistent with the opponent process theory of addiction, 6 , 8 it has been shown that spontaneous withdrawal from noncontingent heroin does, whereas naloxone‐precipitated withdrawal does not, reinstate lever pressing in rats with heroin SA experience. 10 Likewise, morphine‐dependent rats show increased escalation of heroin SA compared to control rats when allowed 8‐h access to heroin SA. 9 On the other hand, there is evidence showing a lack of interaction between opioid dependence and SA behavior. 45 Indeed, there is a considerable body of evidence supporting a proponent process of addiction, 7 in which the essential motivator for drug SA is prior experience of drug reward or a drug‐associated cue. Early studies showed that opioid‐seeking behavior is primed by opioid administration and not by the opponent effects associated with opioid withdrawal. 46 Taken together, it is likely that both opponent and proponent processes contribute to maintenance of opioid misuse and relapse. In the current study, previous morphine exposure potentiated acquisition of oxycodone SA in males, but not females. During the 8 days of maintenance SA, morphine‐treated males showed a trend for increased, whereas morphine‐treated females did not increase, oxycodone SA. This latter finding in females could be due to a reduction in tolerance, a reduction in the reinforcing efficacy of oxycodone, an increase in sensitivity to oxycodone reward, or an increase in the sensitivity to the aversive effects of oxycodone. Our finding that females previously exposed to morphine tended to SA less oxycodone in the dose response and showed a decrease in PR break point suggests a reduced motivation to work for drug driven, perhaps, by increased sensitivity to the negative effects of drug. A caveat of our PR data is that the test was conducted over 1 month after jugular catheters were implanted. As such, the number of rats in each PR treatment group was lower than that for the initial acquisition and maintenance phases of SA (see Section 4 for breakdown of rat numbers). As noted in the Supporting Information, there were a number of reasons for attrition. However, to ensure the SA behavior of the rats used in the PR test was similar to that for the initial SA tests, we graphed and analyzed the acquisition and maintenance data for the rats used in the PR test (Figure S1). The same trends were observed in both the full cohort of rats (Figure 2) and the PR rats (Figure S1).
The results of this study are the first to demonstrate a number of sex differences in interactions between non‐contingent opioid exposure and SA behavior, which we predict will provide a platform for subsequent, mechanistic studies. Our study design was longitudinal, so it is not yet possible to directly attribute the individual effects of morphine treatments, morphine abstinence, or oxycodone self‐administration to our findings. Rather, the combination of these experiences contributed to a potentiation of initial oxycodone SA in males but not females. Importantly, we used a “generic” opioid (morphine) to induce dependence and a “specific” opioid (oxycodone) to test measures of addictive‐like behavior. Our intention was to focus attention on the specific problem of oxycodone abuse after noncontinent experience with a “generic” opioid. Morphine and oxycodone are both full agonists at MORs, activation of which produces the drugs' powerful analgesic and rewarding effects. 47 , 48 Oxycodone has a lower affinity for MORs and crosses the blood‐brain barrier more readily than morphine. 49 However, dependence to both drugs readily develops, resulting in similar withdrawal effects upon abstinence. There are numerous studies in the fields of pain and stress touching on the molecular and cellular mechanisms underlying sex differences in opioid responses. Applying some of this knowledge to preclinical models of OUD is essential. For example, mu opioid receptor (MOR) levels and function are decreased in pain‐ and stress‐related regions such as the periaqueductal grey and locus coeruleus of females compared to males, with evidence that estrogen plays a role in decreasing MOR levels. 44 In addition, chronic morphine has been associated with selective internalization of MOR in male, but not female, locus coeruleus 50 as well as an estrogen‐potentiated switch of MOR coupling from Gαi/o to Gβs proteins in females. This latter effect is thought to contribute to sex differences in opioid withdrawal signs and hyperalgesia. 51 Regardless, our findings suggest that clinical studies reporting that women more than men tend to misuse prescription opioids to self‐medicate aversive states do not necessarily reflect underlying biological processes. Further preclinical studies in rodent models of addictive‐like behavior are warranted to understand these biological processes.
AUTHOR CONTRIBUTIONS
MM, TL, NC, and SP performed the behavioral assays and reviewed/edited the manuscript. MM and EC analyzed the results and wrote the manuscript. All authors critically reviewed content and approved the final version for publication.
Supporting information
Figure S1. Effects of previous morphine experience and withdrawal on oxycodone self‐administration (SA; 0.06 mg/kg/infusion, FR1 schedule of reinforcement) in the subset of rats (N = 35) that underwent progressive ratio (PR). (A) Total number of oxycodone infusions during acquisition of oxycodone SA were greater in male, but not female, rats previously exposed to morphine. The number of infusions depended on a Sex X Treatment X Time interaction [F(4,123) = 2.94, p < 0.05], and when data were consolidated by Sex, there was a significant main effect of Sex [F (1,33)=8.41, p < 0.01]. No significant pairwise effects were observed using Bonferroni's post hoc test. (B) Total number of oxycodone infusions during maintenance of oxycodone SA were qualitatively decreased in female, but not male, rats previously exposed to morphine, as in Figure 4D. However, there was not a significant interaction or main effect of sex in this subset of rats. N = 7 male, vehicle; 9 male, morphine; 12 female, vehicle; 7 female, morphine.
ACKNOWLEDGEMENT
This study was supported by Department of Defense (DoD) funding W81XWH‐17‐1‐0004 and W81XWH‐13‐2‐0075 (EHC).
Mavrikaki M, Lintz T, Constantino N, Page S, Chartoff E. Chronic opioid exposure differentially modulates oxycodone self‐administration in male and female rats. Addiction Biology. 2021;26:e12973. 10.1111/adb.12973
REFERENCES
- 1. SAMHSA . Results from the 2018 National Survey on Drug Use and Health: summary of national findings. In: NSDUH NSoDUaH , ed. HHS Publication No. PEP19–5068, NSDUH Series H‐54. Substance Abuse and Mental Health Services Administration; 2019. [Google Scholar]
- 2. McHugh RK, Nielsen S, Weiss RD. Prescription drug abuse: from epidemiology to public policy. J Subst Abuse Treat. 2015;48(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeffery DD, Babeu LA, Nelson LE, Kloc M, Klette K. Prescription drug misuse among U.S. active duty military personnel: a secondary analysis of the 2008 DoD survey of health related behaviors. Mil Med. 2013;178(2):180‐195. [DOI] [PubMed] [Google Scholar]
- 4. Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51(1–2):23‐47. [DOI] [PubMed] [Google Scholar]
- 5. Dole VP, Nyswander ME, Kreek MJ. Narcotic blockade. Arch Intern Med. 1966;118(4):304‐309. [PubMed] [Google Scholar]
- 6. Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29‐53. [DOI] [PubMed] [Google Scholar]
- 7. Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39(2):254‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solomon RL, Corbit JD. An opponent‐process theory of motivation: I. Temporal dynamics of affect. Psychol Rev. 1974;81(2):119‐145. [DOI] [PubMed] [Google Scholar]
- 9. Walker JR, Chen SA, Moffitt H, Inturrisi CE, Koob GF. Chronic opioid exposure produces increased heroin self‐administration in rats. Pharmacol Biochem Behav. 2003;75(2):349‐354. [DOI] [PubMed] [Google Scholar]
- 10. Shaham Y, Rajabi H, Stewart J. Relapse to heroin‐seeking in rats under opioid maintenance: the effects of stress, heroin priming, and withdrawal. J Neurosci. 1996;16(5):1957‐1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Childress AR, McLellan AT, O'Brien CP. Role of conditioning factors in the development of drug dependence. Psychiatr Clin North am. 1986;9(3):413‐425. [PubMed] [Google Scholar]
- 12. Cooper ZD, Truong YN, Shi YG, Woods JH. Morphine deprivation increases self‐administration of the fast‐ and short‐acting mu‐opioid receptor agonist remifentanil in the rat. J Pharmacol Exp Ther. 2008;326(3):920‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Back SE, Payne RL, Simpson AN, Brady KT. Gender and prescription opioids: findings from the National Survey on Drug Use and Health. Addict Behav. 2010;35(11):1001‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3(1):1‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McHugh RK, Devito EE, Dodd D, et al. Gender differences in a clinical trial for prescription opioid dependence. J Subst Abuse Treat. 2013;45(1):38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fox HC, Hong KI, Siedlarz KM, et al. Sex‐specific dissociations in autonomic and HPA responses to stress and cues in alcohol‐dependent patients with cocaine abuse. Alcohol Alcohol. 2009;44(6):575‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McHugh RK, Sugarman DE, Meyer L, Fitzmaurice GM, Greenfield SF. The relationship between perceived stress and depression in substance use disorder treatment. Drug Alcohol Depend. 2020;207:1–4, 107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chartoff EH, Connery HS. It's MORe exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front Pharmacol. 2014;5:1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(Suppl 1):S4‐S7. [DOI] [PubMed] [Google Scholar]
- 20. Acquas E, Di Chiara G. Depression of mesolimbic dopamine transmission and sensitization to morphine during opiate abstinence. J Neurochem. 1992;58(5):1620‐1625. [DOI] [PubMed] [Google Scholar]
- 21. Diana M, Pistis M, Muntoni A, Gessa G. Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. J Pharm Exp Ther. 1995;272(2):781‐785. [PubMed] [Google Scholar]
- 22. Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK‐801. Science. 1991;251(4989):85‐87. [DOI] [PubMed] [Google Scholar]
- 23. Chartoff EH, Mague SD, Barhight MF, Smith AM, Carlezon WA Jr. Behavioral and molecular effects of dopamine D1 receptor stimulation during naloxone‐precipitated morphine withdrawal. J Neurosci. 2006;26(24):6450‐6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roeckel LA, Le Coz GM, Gaveriaux‐Ruff C, Simonin F. Opioid‐induced hyperalgesia: cellular and molecular mechanisms. Neuroscience. 2016;338:160‐182. [DOI] [PubMed] [Google Scholar]
- 25. Rothwell PE, Thomas MJ, Gewirtz JC. Protracted manifestations of acute dependence after a single morphine exposure. Psychopharmacology (Berl). 2012;219(4):991‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Page S, Mavrikaki MM, Lintz T, et al. Behavioral pharmacology of novel kappa opioid receptor antagonists in rats. Int J Neuropsychopharmacol. 2019;22(11):735‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA Jr. Behavioral and anatomical interactions between dopamine and corticotropin‐releasing factor in the rat. J Neurosci. 2006;26(14):3855‐3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E. Oxycodone self‐administration in male and female rats. Psychopharmacology (Berl). 2017;234(6):977‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS. Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharmacol. 2004;12(3):163‐172. [DOI] [PubMed] [Google Scholar]
- 30. Roberts DC, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(8):1614‐1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57(3):441‐447. [DOI] [PubMed] [Google Scholar]
- 32. Chartoff EH, McHugh RK. Translational studies of sex differences in sensitivity to opioid addiction. Neuropsychopharmacology. 2016;41(1):383‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lynch WJ. Sex differences in vulnerability to drug self‐administration. Exp Clin Psychopharmacol. 2006;14(1):34‐41. [DOI] [PubMed] [Google Scholar]
- 34. Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self‐administered cocaine and heroin in rats. Psychopharmacology (Berl). 1999;144(1):77‐82. [DOI] [PubMed] [Google Scholar]
- 35. Roth ME, Casimir AG, Carroll ME. Influence of estrogen in the acquisition of intravenously self‐administered heroin in female rats. Pharmacol Biochem Behav. 2002;72(1–2):313‐318. [DOI] [PubMed] [Google Scholar]
- 36. Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self‐administration in female and male rats. Neuropsychopharmacology. 2004;29(5):929‐942. [DOI] [PubMed] [Google Scholar]
- 37. Houshyar H, Gomez F, Manalo S, Bhargava A, Dallman MF. Intermittent morphine administration induces dependence and is a chronic stressor in rats. Neuropsychopharmacology. 2003;28(11):1960‐1972. [DOI] [PubMed] [Google Scholar]
- 38. Papaleo F, Contarino A. Gender‐ and morphine dose‐linked expression of spontaneous somatic opiate withdrawal in mice. Behavioural Brain Research. 2006;170 (1):110‐118. 10.1016/j.bbr.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 39. Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76(3):365‐377. [DOI] [PubMed] [Google Scholar]
- 40. Russell SE, Puttick DJ, Sawyer AM, et al. Nucleus accumbens AMPA receptors are necessary for morphine‐withdrawal‐induced negative‐affective states in rats. J Neurosci. 2016;36(21):5748‐5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as a reward: hedonic and neural responses to safety from pain. PLoS ONE. 2011;6(4):e17870. 10.1371/journal.pone.0017870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Holtman JR, Jr. , Wala EP. Characterization of the antinociceptive effect of oxycodone in male and female rats. Pharmacol Biochem Behav. 2006;83(1):100‐108. [DOI] [PubMed] [Google Scholar]
- 43. Juni A, Cai M, Stankova M, et al. Sex‐specific mediation of opioid‐induced hyperalgesia by the melanocortin‐1 receptor. Anesthesiology. 2010;112(1):181‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Loyd DR, Wang X, Murphy AZ. Sex differences in micro‐opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci. 2008;28(52):14007‐14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Vry J, Donselaar I, Van Ree JM. Intraventricular self‐administration of heroin in the rat: reward seems dissociated from analgesia and physical dependence. Eur J Pharmacol. 1989;161(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 46. Stewart J, Wise RA. Reinstatement of heroin self‐administration habits: morphine prompts and naltrexone discourages renewed responding after extinction. Psychopharmacologia. 1992;108(1–2):79‐84. [DOI] [PubMed] [Google Scholar]
- 47. Kalso E. Oxycodone. J Pain Symptom Manage. 2005;29(5 Suppl):S47‐S56. [DOI] [PubMed] [Google Scholar]
- 48. Matthes HW, Maldonado R, Simonin F, et al. Loss of morphine‐induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu‐opioid‐receptor gene. Nature. 1996;383(6603):819‐823. [DOI] [PubMed] [Google Scholar]
- 49. Lalovic B, Kharasch E, Hoffer C, Risler L, Liu‐Chen LY, Shen DD. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther. 2006;79(5):461‐479. [DOI] [PubMed] [Google Scholar]
- 50. Enman NM, Reyes BAS, Shi Y, Valentino RJ, Van Bockstaele EJ. Sex differences in morphine‐induced trafficking of mu‐opioid and corticotropin‐releasing factor receptors in locus coeruleus neurons. Brain Res. 2019;1706:75‐85. [DOI] [PubMed] [Google Scholar]
- 51. Crain SM, Shen KF. Opioids can evoke direct receptor‐mediated excitatory effects on sensory neurons. Trends Pharmacol Sci. 1990;11(2):77‐81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effects of previous morphine experience and withdrawal on oxycodone self‐administration (SA; 0.06 mg/kg/infusion, FR1 schedule of reinforcement) in the subset of rats (N = 35) that underwent progressive ratio (PR). (A) Total number of oxycodone infusions during acquisition of oxycodone SA were greater in male, but not female, rats previously exposed to morphine. The number of infusions depended on a Sex X Treatment X Time interaction [F(4,123) = 2.94, p < 0.05], and when data were consolidated by Sex, there was a significant main effect of Sex [F (1,33)=8.41, p < 0.01]. No significant pairwise effects were observed using Bonferroni's post hoc test. (B) Total number of oxycodone infusions during maintenance of oxycodone SA were qualitatively decreased in female, but not male, rats previously exposed to morphine, as in Figure 4D. However, there was not a significant interaction or main effect of sex in this subset of rats. N = 7 male, vehicle; 9 male, morphine; 12 female, vehicle; 7 female, morphine.


