Key Points
Question
What are the long-term safety and efficacy of onasemnogene abeparvovec in infants with spinal muscular atrophy type 1?
Findings
In this ongoing, long-term follow-up safety study of 13 infants with symptomatic spinal muscular atrophy type 1 treated with a single low or therapeutic dose of onasemnogene abeparvovec in the START phase 1 clinical trial, a favorable safety profile was observed for up to 6.2 years after dosing. For patients who received the therapeutic dose (which subsequently became the approved dose), onasemnogene abeparvovec provided sustained, durable efficacy, with all patients alive and without the need for permanent ventilation.
Meaning
These results support the favorable long-term safety profile of onasemnogene abeparvovec and provide evidence of sustained clinical durability of the therapeutic dose.
This ongoing follow-up study of the START phase 1 clinical trial assesses the long-term safety and efficacy of onasemnogene abeparvovec in infants with spinal muscular atrophy type 1.
Abstract
Importance
This ongoing study assesses long-term safety and durability of response in infants with spinal muscular atrophy (SMA) type 1 after dosing with onasemnogene abeparvovec gene replacement therapy.
Objective
The primary objective of this ongoing study is to assess safety. The secondary objective is to determine whether developmental milestones achieved in the START phase 1 clinical trial were maintained and new milestones gained.
Design, Setting, and Participants
This study is an ongoing, observational, follow-up study for continuous safety monitoring for 15 years in patients from the START phase I study (conducted May 5, 2014, through December 15, 2017) at Nationwide Children’s Hospital in Columbus, Ohio. Participants were symptomatic infants with SMA type 1 and 2 copies of SMN2 previously treated with an intravenous dose of onasemnogene abeparvovec (low dose, 6.7 × 1013 vg/kg; or therapeutic dose, 1.1 × 1014 vg/kg) in START. Thirteen of 15 original START patients are included in this analysis; 2 patients’ families declined follow-up participation. Data were analyzed from September 21, 2017, to June 11, 2020.
Exposures
Median time since dosing of 5.2 (range, 4.6-6.2) years; 5.9 (range, 5.8-6.2) years in the low-dose cohort and 4.8 (range, 4.6-5.6) years in the therapeutic-dose cohort.
Main Outcomes and Measures
The primary outcome measure was the incidence of serious adverse events (SAEs).
Results
At data cutoff on June 11, 2020, 13 patients treated in START were enrolled in this study (median age, 38.9 [range, 25.4-48.0] months; 7 females; low-dose cohort, n = 3; and therapeutic-dose cohort, n = 10). Serious adverse events occurred in 8 patients (62%), none of which resulted in study discontinuation or death. The most frequently reported SAEs were acute respiratory failure (n = 4 [31%]), pneumonia (n = 4 [31%]), dehydration (n = 3 [23%]), respiratory distress (n = 2 [15%]), and bronchiolitis (n = 2 [15%]). All 10 patients in the therapeutic-dose cohort remained alive and without the need for permanent ventilation. Prior to baseline, 4 patients (40%) in the therapeutic-dose cohort required noninvasive ventilatory support, and 6 patients (60%) did not require regular ventilatory support, which did not change in long-term follow-up. All 10 patients treated with the therapeutic dose maintained previously acquired motor milestones. Two patients attained the new milestone of “standing with assistance” without the use of nusinersen.
Conclusions and Relevance
The findings of this ongoing clinical follow-up of patients with SMA type 1 treated with onasemnogene abeparvovec supports the long-term favorable safety profile up to 6 years of age and provides evidence for sustained clinical durability of the therapeutic dose.
Trial Registration
ClinicalTrials.gov Identifier: NCT03421977.
Introduction
Spinal muscular atrophy (SMA) is an autosomal recessive, neuromuscular disorder characterized by the dysfunction and loss of alpha motor neurons in the spinal cord, resulting in progressive muscle atrophy, weakness, and paralysis. Spinal muscular atrophy has a global disease incidence of approximately 1 in 12 000 and an overall carrier frequency of 1 in 54, which ranges from approximately 1 in 50 persons for White and Asian populations, to 1 in 100 persons in populations of African or Afro-American ancestry.1,2 Approximately 95% of all individuals with SMA have a homozygous deletion of survival motor neuron (SMN) 1.3 Spinal muscular atrophy is classified into 4 clinical groups (types 1 to 4) based on age at onset and maximum motor milestones achieved.3,4 Spinal muscular atrophy type 1 is the most severe and common form of the disease, accounting for 60% of cases. Individuals with SMA type 1 have onset of weakness in the first 6 months of infancy and never achieve the ability to sit independently. Prior to the availability of current therapies, SMA type 1 was associated with death or need for permanent ventilation (≥16 hours per day of noninvasive ventilation support for ≥14 days in the absence of an acute reversible illness or perioperatively) by 2 years of age.5 Median survival is 6 to 8 months in most survival studies of treatment-naive patients with SMA type 1.6,7
Onasemnogene abeparvovec is a single intravenous infusion of a recombinant adeno-associated virus serotype 9 vector-based gene therapy8 designed to deliver a full-length functional copy of the human SMN gene via a self-complementary adeno-associated virus serotype 9 vector8 that crosses the blood-brain barrier.9 The efficacy and safety of onasemnogene abeparvovec in patients with SMA type 1 was assessed in a 2-year, open-label, phase 1 study (START) conducted from May 5, 2014, through December 15, 2017.10,11 Data demonstrated that a 1-time infusion of onasemnogene abeparvovec low dose (6.7 × 1013 vg/kg of body weight) in 3 patients or therapeutic dose (1.1 × 1014 vg/kg) in 12 patients resulted in longer survival than observed in historical controls. Two of 3 patients in the low-dose cohort and all patients in the therapeutic-dose cohort were alive without the need for permanent ventilation at 24 months of age.8,10 Patients in the therapeutic-dose cohort also had significantly improved motor function and motor milestone achievements compared with historical cohorts. Eleven of 12 patients sat unassisted for ≥5 seconds, and 2 children pulled to a stand, stood, and walked independently.
Onasemnogene abeparvovec was generally well tolerated in these patients. The START long-term follow-up study (START LTFU hereafter) was designed to evaluate long-term serious adverse events (SAEs) and adverse events of special interest (AESIs) (ie, gene therapy–related adverse events, liver function enzyme elevations, new incidences of a malignancy or hematologic disorders, and new incidences or exacerbations of existing neurologic or autoimmune disorders). Of the known, identified potential risks related to onasemnogene abeparvovec, including hepatotoxicity, thrombocytopenia, cardiac adverse events, and events suggestive of neuronopathy, elevations in aminotransferase concentrations occurred in the time period proximate to administration.12 The question to be answered in the long-term follow-up of START is whether these elevations result in long-term sequelae. More recently, thrombotic microangiopathy has been identified as a new safety signal, with an apparent temporal relationship to time of administration.13 Thrombotic microangiopathy events have been observed 2 to 3 weeks after initiation of therapy. Given this new safety signal, ongoing monitoring is needed from all sources of safety data, including this and other long-term follow-up studies.
These long-term study data will help in the assessment of any potential long-term consequences (with reference to hematologic or autoimmune events). In addition, any new incidences of malignancy and new incidences or exacerbations of existing neurologic or autoimmune disorders are monitored. Although the aforementioned potential safety risks occur within a narrow window after administration, the long-term risks associated with onasemnogene abeparvovec are unknown to date.
To continue monitoring the safety and efficacy of onasemnogene abeparvovec in these patients, we undertook a long-term follow-up study (START LTFU) involving patients who had completed the phase 1 START study. Enrollment criteria were described in the original clinical trial.10 Here, we report interim results of this follow-up extension study (data cutoff on June 11, 2020).
Methods
Study Design and Participants
The START LTFU is an ongoing, observational, long-term follow-up study of patients with a genetically confirmed diagnosis of SMA type 1, homozygous SMN1 exon 7 deletions, and 2 copies of SMN2 who received a low dose (6.7 × 1013 vg/kg) or a therapeutic dose (2.0 × 1014 vg/kg, subsequently determined to be 1.1 × 1014 vg/kg by direct measurement using the Digital Droplet polymerase chain reaction method). This dose was used in subsequent studies. Patients who received onasemnogene abeparvovec in the START study10 were eligible to enter the START LTFU. The primary objective of this ongoing analysis is to evaluate the long-term safety of onasemnogene abeparvovec, and the secondary objective is to determine whether the efficacy milestones achieved in START are maintained and whether new milestones are gained. The study consists of an initial 5-year phase, in which patients are assessed in the clinic annually, followed by annual phone assessments for an additional 10 years.
The study protocol (Supplement 1) was approved by the institutional review board at Nationwide Children’s Hospital in Columbus, Ohio. Study procedures were performed in accordance with principles of the Declaration of Helsinki14 and were consistent with the Good Clinical Practice Guidelines of the International Conference on Harmonization, applicable regulatory requirements, and Novartis Gene Therapies’ policy on bioethics. Written informed consent was obtained from the parents or legal guardians of the children at the last visit of the START study.
Assessments
The primary outcome measures were the determination of safety on the basis of SAEs or AESIs. These AESIs included gene therapy–related delayed adverse events; liver function enzyme elevations; new malignancies; new incidence or exacerbation of a pre-existing neurologic disorder, or prior rheumatologic or other autoimmune disorder; or new incidence of hematologic disorder. Efficacy outcomes included motor-milestone achievement and assessment of ventilation. Milestone achievement was classified according to the World Health Organization Multicenter Growth Study definitions or Bayley Scales of Infant and Toddler Development, 3rd ed. New developmental milestones (ie, those that the patient had not previously achieved in the START study) were documented by video evidence captured either at the site or by a parent or legal guardian.
At each study visit in the initial 5-year phase of the study, we collected safety data through physical examinations, clinical laboratory evaluations, medical histories, and adverse event reporting. In addition, efficacy evaluations focused on pulmonary assessment (ie, ventilation) and a review of developmental milestones. As applicable, patients’ records from their local physicians were reviewed by the investigators. In the event of serious situations, such as the COVID-19 pandemic, that may have limited or prevented on-site study visits, alternative methods of conducting study assessments (eg, phone calls, virtual contacts or visits by site staff or home nursing service to the participant’s home) could be implemented. During the 10-year observational phase, caregivers and patients will be contacted at least once a year, and site staff will review a yearly questionnaire designed to elicit information regarding medical histories, SAEs, and clinical outcomes.
Detailed patient records include reports of SAEs related or unrelated to the study, AESIs, and concomitant medications, including other SMA treatments. Severity grades were defined per protocol: grade 3 (severe or medically significant but not immediately life-threatening, hospitalization or prolongation of hospitalization indicated, disabling, or limiting self-care activities of daily living) and grade 4 (life-threatening consequences or urgent intervention indicated). Upper limits of normal for laboratory values included platelets (<75.0 × 109/L), neutrophils (<1.5 × 109/L), alanine aminotransferase (>3.0 × upper limit of normal), and aspartate aminotransferase (>3.0 × upper limit of normal).
Statistical Analysis
No formal statistical testing was planned. Only descriptive statistics were performed. The statistical analysis plan is included in Supplement 2. Analyses for the present study were conducted from September 21, 2017, to June 11, 2020.
Results
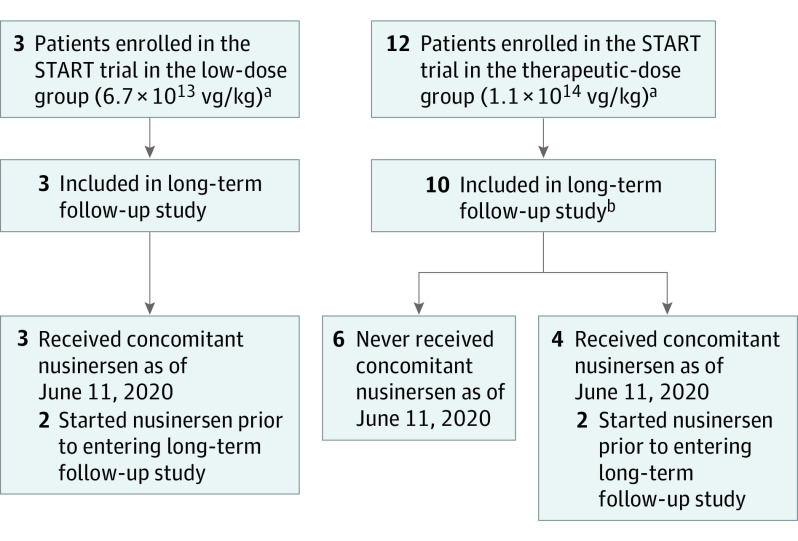
The first patient enrolled in the START LTFU on September 21, 2017. At the time of data cutoff (June 11, 2020), 13 of the original 15 patients had been enrolled in the long-term follow-up study, including 3 patients from the low-dose cohort and 10 from the therapeutic-dose cohort of the START study (Figure 1). Two patients’ families declined to continue participation into the START LTFU. These patients continued to be followed at the Nationwide Children’s Hospital Spinal Muscular Atrophy Clinic in Columbus, Ohio. Key demographic details and baseline clinical characteristics are summarized in Table 1. Seven patients (54%) were female, 12 (92%) were White, and 1 (8%) was Hispanic/Latino. At the baseline visit in the START LTFU, patient age ranged from 25.4 to 48 months (median, 38.9 months), and body weight ranged from 10.0 to 14.7 kg (median, 12.0 kg).
Figure 1. Disposition of Patients in the START LTFU Study.
aNo patient was treated with concomitant nusinersen during the START phase 1 trial.
bTwo patients who did not enroll in the START long-term follow-up study are being followed up at the Nationwide Children’s Hospital Spinal Muscular Atrophy Clinic in Columbus, Ohio. Note that no visits were conducted in the START LTFU clinical trial from December 31, 2019, through June 11, 2020.
Table 1. Demographic Details and Baseline Clinical Characteristics.
| Parameter | No. (%) | ||
|---|---|---|---|
| Low-dose cohort (n = 3) | Therapeutic-dose cohort (n = 10) | All (N = 13) | |
| Age at start of long-term study, mo | |||
| Mean (SD) | 45.5 (2.4) | 33.7 (7.7) | 36.4 (8.5) |
| Median (range) | 45.3 (43.2-48.0) | 31.9 (25.4-46.3) | 38.9 (25.4-48.0) |
| Sex | |||
| Male | 1 (33) | 5 (50) | 6 (46) |
| Female | 2 (67) | 5 (50) | 7 (54) |
| Race | |||
| White | 3 (100) | 9 (90) | 12 (92) |
| Multiple | 0 | 1 (10) | 1 (8) |
| Ethnicity | |||
| Hispanic or Latino | 0 | 2 (20) | 2 (15) |
| Not Hispanic or Latino | 3 (100) | 8 (80) | 11 (85) |
| Weight at baseline, kg | |||
| Mean (SD) | 13.3 (1.4) | 11.9 (1.3) | 12.2 (1.4) |
| Median (range) | 13.0 (12.0-14.7) | 12.0 (10.0-14.6) | 12.0 (10.0-14.7) |
Safety
As of this data cutoff, the maximum follow-up was 6.2 years after dosing. Serious adverse events were reported for 8 patients (62%): 1 patient in the low-dose cohort and 7 patients in the therapeutic-dose cohort (Table 2). The most frequently reported SAEs were related to the underlying SMA disease process: acute respiratory failure (n = 4; terminology required by the US Food and Drug Administration to indicate that a child was hospitalized with a respiratory illness requiring invasive ventilation, but does connote the severest degree of respiratory decompensation or distress), pneumonia (n = 4), dehydration (n = 3), respiratory distress (n = 2), and bronchiolitis (n = 2). No SAE led to study discontinuation, and all were considered by the investigators to be unrelated to onasemnogene abeparvovec therapy. All SAEs were considered severe—all were grade 3, except for three grade 4 events in 1 patient in the low-dose cohort precipitated by an episode of hypoxemic respiratory failure due to a mucous plug that led to cardiac arrest, requiring resuscitation and intubation. The patient was subsequently extubated, and returned to baseline status.
Table 2. Serious Adverse Events by Patient in the START Long-term Follow-up Studya,b.
| System organ class or preferred term | No. (%) | ||
|---|---|---|---|
| Low-dose cohort (n = 3) | Therapeutic-dose cohort (n = 10) | All (N = 13) | |
| Patients with ≥1 SAE | 1 (33) | 7 (70) | 8 (62) |
| Infections and infestations | 1 (33) | 6 (60) | 7 (54) |
| Pneumonia | 1 (33) | 3 (30) | 4 (31) |
| Bronchiolitis | 0 | 2 (20) | 2 (15) |
| Bronchitis | 0 | 1 (10) | 1 (8) |
| Gastroenteritis | 0 | 1 (10) | 1 (8) |
| Gastroenteritis viral | 0 | 1 (10) | 1 (8) |
| Osteomyelitis | 0 | 1 (10) | 1 (8) |
| Pneumonia bacterial | 0 | 1 (10) | 1 (8) |
| Pneumonia viral | 0 | 1 (10) | 1 (8) |
| Urinary tract infections | 0 | 1 (10) | 1 (8) |
| Metabolism and nutrition disorders | 1 (33) | 4 (40) | 5 (38) |
| Dehydration | 0 | 3 (30) | 3 (23) |
| Hypernatremia | 1 (33) | 0 | 1 (8) |
| Hypoglycemia | 0 | 1 (10) | 1 (8) |
| Ketoacidosis | 0 | 1 (10) | 1 (8) |
| Respiratory, thoracic, and mediastinal disorders | 1 (33) | 3 (30) | 4 (31) |
| Acute respiratory failure | 1 (33) | 3 (30) | 4 (31) |
| Respiratory distress | 1 (33) | 1 (10) | 2 (15) |
| Dyspnea | 0 | 1 (10) | 1 (8) |
| Respiratory failure | 1 (33) | 0 | 1 (8) |
| Gastrointestinal disorders | 1 (33) | 1 (10) | 2 (15) |
| Gastrointestinal hemorrhage | 1 (33) | 0 | 1 (8) |
| Pneumatosis intestinalis | 0 | 1 (10) | 1 (8) |
| Vomiting | 0 | 1 (10) | 1 (8) |
| Cardiac disorders | 1 (33) | 0 | 1 (8) |
| Cardiac arrest | 1 (33) | 0 | 1 (8) |
| Injury, poisoning, and procedural complications | 0 | 1 (10) | 1 (8) |
| Wound | 0 | 1 (10) | 1 (8) |
| Musculoskeletal and connective tissue disorder | 0 | 1 (10) | 1 (8) |
| Scoliosis | 0 | 1 (10) | 1 (8) |
| Skin and subcutaneous tissue disorders | 0 | 1 (10) | 1 (8) |
| Decubitus ulcer | 0 | 1 (10) | 1 (8) |
Abbreviation: SAE, serious adverse event.
All adverse events were coded in accordance with the Medical Dictionary of Regulatory Activities (MedDRA®) coding dictionary (version 20.1).
Patients and event categories may not be mutually exclusive.
No AESIs have been reported in the study to date. No AESIs were associated with liver enzyme elevations, hematology values, new malignancies, or autoimmune disorders. Upon review of laboratory data, we observed no trends in mean change from baseline for platelet or neutrophil counts. In addition, no patients shifted to a low value from baseline to the minimum platelet (75.0 × 109/L) or neutrophil count. No patient shifted from a low or normal baseline alanine aminotransferase concentration to a high maximum value during the study. One patient shifted from a low or normal baseline aspartate aminotransferase concentration to a high maximum value. This value remained high at the final value. No patients in the START LTFU met any of the criteria for potential hepatotoxicity or thrombotic microangiopathy at any time point.
Efficacy
As of June 11, 2020, the median time since dosing was 5.2 (range, 4.6-6.2) years in the overall population, 5.9 (range, 5.8-6.2) years in the low-dose cohort, and 5.0 (range, 4.6-5.6) years in the therapeutic-dose cohort (Table 3). All 10 patients in the therapeutic-dose cohort (eFigure in Supplement 3) were alive and did not require permanent ventilation; all 3 of the patients in the low-dose cohort remain alive, and 2 of these 3 remain free of permanent ventilation. At baseline, 6 of the 10 patients in the therapeutic-dose cohort did not require regular ventilatory support. No patient in this cohort has initiated new mechanical respiratory support to date during the follow-up study. The remaining 4 patients in this cohort had required noninvasive ventilatory support in the START study and maintained this requirement in the START LTFU, with no decline in their respiratory status or increased need for baseline respiratory support.
Table 3. Follow-up Times Since Dosing, Ages at Dosing, and Current Patient Ages.
| Variable | Low-dose cohort (n = 3) | Therapeutic-dose cohort (n = 10) | All (N = 13) |
|---|---|---|---|
| Age at dosing, y | |||
| Mean (SD) | 0.5 (0.1) | 0.3 (0.1) | 0.3 (0.2) |
| Median (range) | 0.5 (0.5-0.6) | 0.2 (0.1-0.5) | 0.3 (0.1-0.6) |
| Age on June 11, 2020, y | |||
| Mean (SD) | 6.5 (0.2) | 5.2 (0.5) | 5.5 (0.7) |
| Median (range) | 6.4 (6.4-6.7) | 5.0 (4.7-6.1) | 5.5 (4.7-6.7) |
| Time since dosing as of June 11, 2020, y | |||
| Mean (SD) | 6.0 (0.2) | 5.0 (0.4) | 5.2 (0.6) |
| Median (range) | 5.9 (5.8-6.2) | 4.8 (4.6-5.6) | 5.2 (4.6-6.2) |
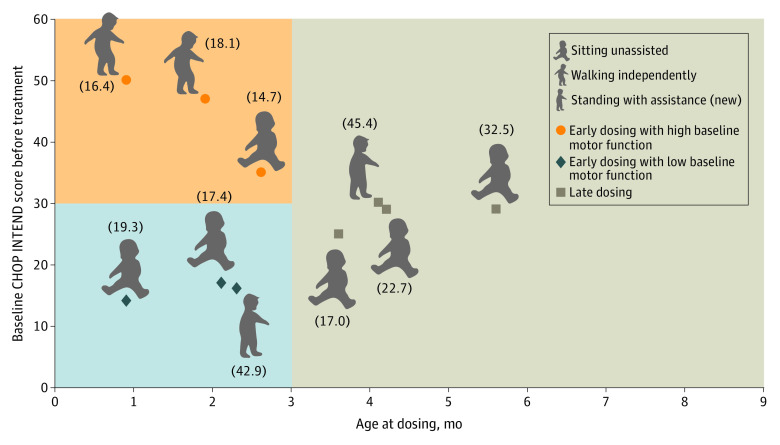
Two of the 10 patients in the therapeutic-dose cohort attained the new video-confirmed motor milestone of standing with assistance since completing the START study (Figure 2),15 both of which were confirmed by a central reviewer. In the remaining 8 patients of the cohort, all motor milestones attained in the START study were maintained, with no regression or loss of function. The ages at which these milestones were achieved are also provided in Figure 2.
Figure 2. Greatest Development Milestones Achieved During the START Long-term Follow-up Study.
Milestones are shown for the 10 patients in the therapeutic-dose cohort who received dosing early and had low baseline motor function (blue quadrant), those who received dosing early and had high motor function (orange quadrant), and those who received dosing late (gray quadrant). Values in parentheses are the age at which the milestone was achieved (in months). Patients were grouped according to baseline motor function (Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders scores <20 points [low] or ≥20 points [high]) and age at dosing (<3 months [early] or ≥3 months [late]).5 This figure was adapted with author permission from Lowes LP et al.15
As of June 11, 2020, 7 of the 13 patients were receiving concomitant nusinersen (all 3 patients in the low-dose cohort and 4 of the 10 patients in the therapeutic-dose cohort) in an attempt to maximize benefit and not because of a loss in motor function or perceived regression. Six patients in the therapeutic-dose cohort were recorded as receiving no further treatment for SMA apart from onasemnogene abeparvovec more than 4 years after onasemnogene abeparvovec dosing. The 2 patients in the therapeutic-dose cohort, who achieved the new milestones in the START LTFU, did not receive nusinersen at any point.
Discussion
In the phase 1 START study, a single intravenous dose of onasemnogene abeparvovec gene replacement therapy extended survival, as evidenced by a median age at the last pulmonary assessment of 30.8 months for the low-dose cohort and 25.7 months for the therapeutic-dose cohort, and improved motor function in patients with SMA type 1.10 The current study provides long-term follow-up data in patients from the START study. To our knowledge, and based on a search of the literature, we believe this to be longest follow-up of gene therapy published to date.
In this first analysis of data from this ongoing START LTFU study, onasemnogene abeparvovec had a favorable safety profile for up to 6.2 years after dosing, with no new safety concerns or treatment-related SAEs reported during long-term follow-up. The SAEs reported during long-term follow-up were consistent with those previously observed with onasemnogene abeparvovec. No AESIs have been reported in the study, including liver function enzyme elevations, new incidences of malignancy or hematologic disorders, and new incidences or exacerbations of existing neurologic or autoimmune disorders.
Onasemnogene abeparvovec, at the therapeutic dose, maintained a durable response in patients up to 5.6 years after dosing, as of the June 11, 2020, data cutoff, which is unmatched in the natural history of patients with SMA. All 10 patients in the therapeutic-dose cohort and 2 of the 3 patients in the low-dose cohort were alive and did not require permanent ventilation at the time of this analysis. To date, no patient has initiated new mechanical respiratory support or required increased baseline respiratory support during long-term follow-up. In addition, motor milestones achieved in the START study have been maintained during long-term follow-up, with 2 patients in the therapeutic-dose cohort attaining the new milestone of standing with assistance, which can only be attributed to the long-term clinical durability of onasemnogene abeparvovec, as neither patient had received additional SMN protein-producing therapies.
The timing of drug intervention is critical in treating SMA, regardless of disease severity.3 In patients with more severe forms of SMA, irreversible loss of motor neurons starts in the prenatal period and progresses rapidly thereafter.16 In addition, preclinical studies have suggested that drug intervention in the presymptomatic or early symptomatic period is likely to produce the best response to therapy.3,17,18,19 In the original START study, 2 children who received early dosing (ie, within 3 months of birth) with onasemnogene abeparvovec achieved the motor milestone of sitting unassisted within a timeline consistent with that in healthy children (4-9 months).15 In the current long-term follow-up study, all patients in the early and late dosing groups maintained the motor milestones gained in the original START study, and 1 patient in each group has acquired the new milestone of standing with support. These data highlight the long-lasting durability of SMA treatment, the importance of identifying affected infants in the early period, and the need for reliable and validated newborn screening assays.3
In addition to this long-term follow-up study, the efficacy and safety of onasemnogene abeparvovec is being evaluated in several ongoing or recently completed phase 3 studies, including the STR1VE-US,20 STR1VE-EU,21 STR1VE-AP,22 and SPR1NT23 studies. Also underway is the phase 4, observational, long-term follow-up LT-002 study,24 which is enrolling patients with SMA types 1, 2, or 3 who have participated in previous onasemnogene abeparvovec intravenous studies other than START. Results of these studies are pending.
Limitations
This follow-up study is limited by the small sample size of the patient population and confounded by treatment with nusinersen for several patients. However, given that the 2 patients who acquired the new motor milestone of standing with assistance did not receive nusinersen at any time, this benefit can be attributed solely to onasemnogene abeparvovec. In addition, rather than all adverse events, only SAEs and AESIs were collected. The study could also be limited by the ongoing COVID-19 pandemic, which has caused disruptions to patients, researchers, and institutions and could affect future follow-up and data collection.25 Of note, no visits were conducted in START LTFU, from December 31, 2019, through June 11, 2020.
Conclusions
In this analysis of data from the ongoing, long-term follow-up of the START clinical trial, a single intravenous dose of onasemnogene abeparvovec in patients with SMA type 1 continued to have a monitorable and manageable safety profile for up to 6.2 years after dosing. To date, no treatment-related SAEs or AESIs have been reported during long-term follow-up. Onasemnogene abeparvovec provides sustained and durable efficacy in patients up to 6.2 years after dosing. Anticipated results from completed and ongoing phase 3 and 4 studies will further confirm the efficacy and safety of onasemnogene abeparvovec. Current evidence demonstrates that onasemnogene abeparvovec continues to have a favorable benefit-risk profile for the treatment of pediatric patients with SMA.
Trial Protocol
Statistical Analysis Plan
eFigure 1. Probability of permanent ventilation-free survival
eReferences
References
- 1.Sugarman EA, Nagan N, Zhu H, et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur J Hum Genet. 2012;20(1):27-32. doi: 10.1038/ejhg.2011.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verhaart IEC, Robertson A, Wilson IJ, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy—a literature review. Orphanet J Rare Dis. 2017;12(1):124. doi: 10.1186/s13023-017-0671-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glascock J, Sampson J, Haidet-Phillips A, et al. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. J Neuromuscul Dis. 2018;5(2):145-158. doi: 10.3233/JND-180304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercuri E, Finkel RS, Muntoni F, et al. ; SMA Care Group . Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103-115. doi: 10.1016/j.nmd.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 5.Finkel RS, McDermott MP, Kaufmann P, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810-817. doi: 10.1212/WNL.0000000000000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolb SJ, Coffey CS, Yankey JW, et al. ; NeuroNEXT Clinical Trial Network on behalf of the NN101 SMA Biomarker Investigators . Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82(6):883-891. doi: 10.1002/ana.25101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijngaarde CA, Stam M, Otto LAM, et al. Population-based analysis of survival in spinal muscular atrophy. Neurology. 2020;94(15):e1634-e1644. doi: 10.1212/WNL.0000000000009248 [DOI] [PubMed] [Google Scholar]
- 8.AveXis, Inc . ZOLGENSMA® (onasemnogene abeparvovec-xioi). 2021. Accessed April 13, 2021. https://www.fda.gov/media/126109/download
- 9.Foust KD, Wang X, McGovern VL, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28(3):271-274. doi: 10.1038/nbt.1610 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713-1722. doi: 10.1056/NEJMoa1706198 [DOI] [PubMed] [Google Scholar]
- 11.Gene transfer clinical trial for spinal muscular atrophy type 1. ClinicalTrials.gov identifer: NCT02122952. Updated May 10, 2019. Accessed April 8, 2021. https://clinicaltrials.gov/ct2/show/NCT02122952
- 12.Chand D, Mohr F, McMillan H, et al. Hepatotoxicity following administration of onasemnogene abeparvovec (AVXS-101) for the treatment of spinal muscular atrophy. J Hepatol. 2021;74(3):560-566. doi: 10.1016/j.jhep.2020.11.001 [DOI] [PubMed] [Google Scholar]
- 13.Chand DH, Zaidman C, Arya K, et al. Thrombotic microangiopathy following onasemnogene abeparvovec for spinal muscular atrophy: a case series. J Pediatr. 2021;231;265-268. doi: 10.1016/j.jpeds.2020.11.054 [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 15.Lowes LP, Alfano LN, Arnold WD, et al. Impact of age and motor function in a phase 1/2A study of infants with SMA type 1 receiving single-dose gene replacement therapy. Pediatr Neurol. 2019;98:39-45. doi: 10.1016/j.pediatrneurol.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 16.Swoboda KJ, Prior TW, Scott CB, et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57(5):704-712. doi: 10.1002/ana.20473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrar MA, Park SB, Vucic S, et al. Emerging therapies and challenges in spinal muscular atrophy. Ann Neurol. 2017;81(3):355-368. doi: 10.1002/ana.24864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Vivo DC, Bertini E, Swoboda KJ, et al. ; NURTURE Study Group . Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29(11):842-856. doi: 10.1016/j.nmd.2019.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul GR, Gushue C, Kotha K, Shell R. The respiratory impact of novel therapies for spinal muscular atrophy. Pediatr Pulmonol. 2021;56(4):721-728. doi: 10.1002/ppul.25135 [DOI] [PubMed] [Google Scholar]
- 20.Day JW, Finkel RS, Chiriboga CA, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(4):284-289. doi: 10.1016/S1474-4422(21)00001-6 [DOI] [PubMed] [Google Scholar]
- 21.Single-dose gene replacement therapy clinical trial for participants with spinal muscular atrophy type 1 (STRIVE-EU). ClinicalTrials.gov identifier: NCT03461289. Updated April 2, 2021. Accessed April 15, 2021. https://clinicaltrials.gov/ct2/show/NCT03461289
- 22.Single-dose gene replacement therapy using for patients with spinal muscular atrophy type 1 with one or two SMN2 copies. ClinicalTrials.gov identifier: NCT03837184. Updated March 30, 2021. Accessed April 8, 2021. https://clinicaltrials.gov/ct2/show/NCT03837184
- 23.Pre-symptomatic study of intravenous onasemnogene abeparvovec-xioi in spinal muscular atrophy (SMA) for patients with multiple copies of SMN2 (SPR1NT). ClinicalTrials.gov identifier: NCT03505099. Updated March 30, 2021. Accessed April 8, 2021. https://clinicaltrials.gov/ct2/show/NCT03505099
- 24.Long-term follow-up study of patients receiving onasemnogene abeparvovec-xioi. ClinicalTrials.gov identifier: NCT04042025. Updated March 30, 2021. Accessed April 8, 2021. https://clinicaltrials.gov/ct2/show/NCT04042025
- 25.van Dorn A. COVID-19 and readjusting clinical trials. Lancet. 2020;396(10250):523-524. doi: 10.1016/S0140-6736(20)31787-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eFigure 1. Probability of permanent ventilation-free survival
eReferences