Key Points
Question
Do childhood asthma incidence rates across the US differ by demographic strata and parental history of asthma?
Findings
In this cohort study, including 11 404 children in the primary analysis, children with a family history of asthma had 2 to 3 times higher rates of asthma through age 4 years. The rates for boys declined with age, whereas rates among girls were steady across all ages; rates among non-Hispanic Black children were markedly higher than those of non-Hispanic White children during the preschool years and lower than White children after age 9 to 10 years.
Meaning
These findings suggest that asthma interventions developed to target early childhood may be important for the prevention of asthma among children with a family history of asthma and among Black children.
This cohort study uses data from the Environmental Influences on Child Health Outcomes consortium to assess whether US childhood asthma incidence rates differ based on parental asthma history and demographic strata.
Abstract
Importance
Asthma is the leading chronic illness in US children, but most descriptive epidemiological data are focused on prevalence.
Objective
To evaluate childhood asthma incidence rates across the nation by core demographic strata and parental history of asthma.
Design, Setting, and Participants
For this cohort study, a distributed meta-analysis was conducted within the Environmental Influences on Child Health Outcomes (ECHO) consortium for data collected from May 1, 1980, through March 31, 2018. Birth cohort data of children from 34 gestational weeks of age or older to 18 years of age from 31 cohorts in the ECHO consortium were included. Data were analyzed from June 14, 2018, to February 18, 2020.
Exposures
Caregiver report of physician-diagnosed asthma with age of diagnosis.
Main Outcome and Measures
Asthma incidence survival tables generated by each cohort were combined for each year of age using the Kaplan-Meier method. Age-specific incidence rates for each stratum and asthma incidence rate ratios by parental family history (FH), sex, and race/ethnicity were calculated.
Results
Of the 11 404 children (mean [SD] age, 10.0 [0.7] years; 5836 boys [51%]; 5909 White children [53%]) included in the primary analysis, 7326 children (64%) had no FH of asthma, 4078 (36%) had an FH of asthma, and 2494 (23%) were non-Hispanic Black children. Children with an FH had a nearly 2-fold higher incidence rate through the fourth year of life (incidence rate ratio [IRR], 1.94; 95% CI, 1.76-2.16) after which the rates converged with the non-FH group. Regardless of FH, asthma incidence rates among non-Hispanic Black children were markedly higher than those of non-Hispanic White children during the preschool years (IRR, 1.58; 95% CI, 1.31-1.86) with no FH at age 4 years and became lower than that of White children after age 9 to 10 years (IRR, 0.67; 95% CI, 0.50-0.89) with no FH. The rates for boys declined with age, whereas rates among girls were relatively steady across all ages, particularly among those without an FH of asthma.
Conclusions and Relevance
Analysis of these diverse birth cohorts suggests that asthma FH, as well as race/ethnicity and sex, were all associated with childhood asthma incidence rates. Black children had much higher incidences rates but only during the preschool years, irrespective of FH. To prevent asthma among children with an FH of asthma or among Black infants, results suggest that interventions should be developed to target early life.
Introduction
In 2018, the prevalence of asthma in US children younger than 18 years was 7.5%, with higher proportions in children aged 5 to 14 years (8.6%), boys (8.3%), non-Hispanic Black children (14.3%), Puerto Rican children (17.0%), children in the Northeast (8.9%), and in those with the lowest household incomes (10.2%).1 Treatments have been developed to enhance asthma control,2,3,4 but a cure remains elusive, making prevention imperative.5,6,7
The first step toward primary prevention in epidemiology is the evaluation of incidence rate data8; however, most descriptive data regarding childhood asthma are focused on prevalence or morbidity.9,10,11,12,13,14,15 An extensive search of the literature identified few childhood asthma studies that presented incidence rates; those that did were generally limited geographically and by sample size and calendar time, and they usually comprised populations that lacked diversity (eAppendix 1 and eTable 1 in Supplement 1). There have been numerous longitudinal US birth cohort studies of childhood asthma,16 but when incidence as a measure is presented, it is usually cumulative incidence (a risk estimate or proportion) rather than rates.17 The Environmental Influences on Child Health Outcomes (ECHO) program, a National Institutes of Health–funded consortium of child cohorts initiated in 2016, offers an opportunity to evaluate asthma incidence rates from multiple US prospective cohorts. Determining which subpopulations have the highest incidence rates has the promise of identifying high-priority subgroups of children for primary prevention of asthma.
Methods
Data Source
The ECHO cohorts were invited to participate in this distributed collective cohort analysis, which is a form of meta-analysis allowing for uniformity in variable definitions that were analyzed by each cohort and did not require pooling of actual data. All cohorts had institutional review board approval from their local institutions, and the work of the ECHO Data Analysis Center was approved through the Johns Hopkins Bloomberg School of Public Health Institutional Review Board. All participants gave written informed consent for participation in their specific cohorts. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Inclusion/Exclusion Criteria
The ECHO cohorts were invited to participate in this project if they had children in their cohort who were born at 34 weeks of gestational age or older with no evidence of chronic lung disease (eg, cystic fibrosis), had been followed until at least their fifth birthday, and had collected data on whether the children had been given a physician’s diagnosis of asthma. Data collected from May 1, 1980, through March 31, 2018, were included in this analysis.
A total of 32 of 81 invited eligible cohorts elected to participate and contributed data (eAppendix 2 in Supplement 1 contains the list of contributing cohorts). Thirteen cohorts enrolled participants in pregnancy or early infancy, with no other specific enrollment restriction. Five cohorts were restricted to children in which 1 or both biological parents reported a history of asthma or atopy. One cohort enrolled infants admitted to the hospital with physician-diagnosed bronchiolitis. The remainder had various enrollment restrictions not directly related to asthma, wheezing, or respiratory conditions.
Data Compilation
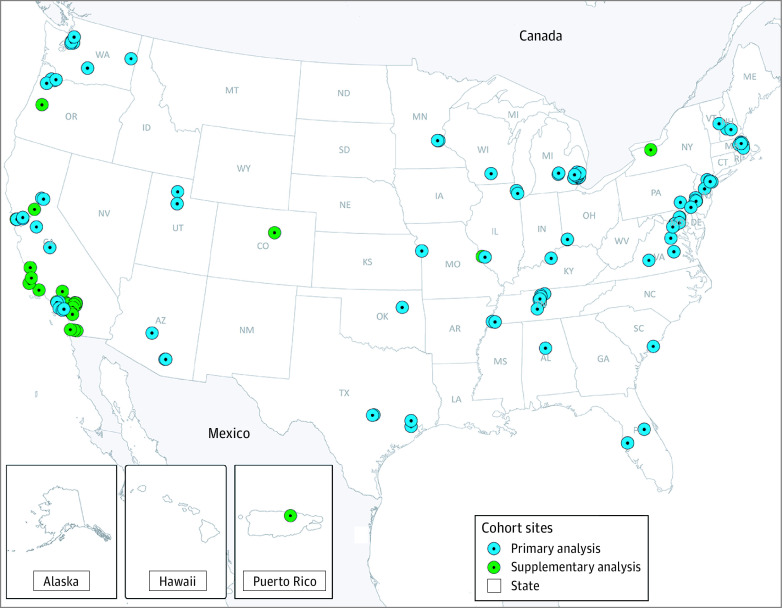
The Data Analysis Center provided cohorts with detailed instructions for data compilation and harmonization, a standardized data dictionary, programming code to derive the cohort-specific estimates, and code in R software, version 3.5 (R Foundation),18 all of which were used to perform on-site data checking and analyses. One cohort was excluded because only 8 children met eligibility criteria. For the primary analysis, 2 further exclusions were made: (1) data from 4 cohorts that did not enroll children until after age 1 year and (2) data from 5 cohorts that did not report cases of asthma through the first 5 years of life owing to data harmonization constraints. Therefore, 22 cohorts (11 404 children) contributed to the primary analysis, and all cohorts except the one with only 8 eligible children (ie, 31 cohorts) were included in the parallel supplementary analysis (24 635 children) (eAppendix 5 in Supplement 1). Figure 1 shows the location of all enrollment sites of the 31 cohorts contributing to the primary and supplementary analyses, which included children from 28 states and 30 states plus Puerto Rico, respectively.
Figure 1. Locations of Enrollment Sites for Children Included in the Primary and Supplementary Analyses.
Several Environmental Influences on Child Health Outcomes (ECHO) cohorts have multiple enrollment sites. Figure reprinted courtesy of ECHO, a program supported by the National Institutes of Health.
Outcome Definition
Parental report of a physician’s diagnosis of asthma and age at diagnosis were ascertained. If the exact age at diagnosis was unavailable, age was estimated using the midpoint of the age between the first indication of having an asthma diagnosis and the study visit before that first indication. For children diagnosed before 5 years of age, inclusion as a case required indication of ongoing asthma symptoms or medication use at age 5 years or later. Therefore, in this analysis, all participants with asthma diagnosed between ages 1 and 4 years represent children with ongoing asthma at age 5 years or older. This was done to avoid the inclusion of children who experienced early transient wheezing, often misdiagnosed as having asthma.19
Demographic Stratum Variables
Descriptive information was included in the analysis on the following child characteristics: family history (FH) of asthma, sex, race, ethnicity, year of birth, geographic region and urbanicity of enrollment site, and maternal education. Incidence rates were calculated by FH, sex, and race/ethnicity, as these variables have been important in prevalence studies. Family history was harmonized as reporting a history of asthma in either biological parent. Children were dichotomized as having any history, defined as a history in the mother, father, or both, vs no FH. Sex was reported as boy vs girl as recorded by the cohorts from caregiver reports. Child race and ethnicity were provided by the caregiver and harmonized as primary race/ethnicity being either non-Hispanic White (White), non-Hispanic Black (Black), Hispanic/Latinx, or non-Hispanic other race/ethnicity. However, asthma incidence rate comparisons were restricted to those who self-identified as non-Hispanic White or non-Hispanic Black owing to lack of further specificity of this information from some cohorts. Year of birth was classified in 5-year increments: 1980-1984, 1985-1989, 1990-1994, 1995-1999, 2000-2004, 2005-2009, and 2010-2014. Geographic region was identified as the location of the enrollment site, categorized by the regions defined by the US Census (eAppendix 3 in Supplement 1). Urbanicity of enrollment site was dichotomized as yes or no based on definitions from the US Census.20
Statistical Analysis
Output from each cohort included descriptive statistics for the study population as well as overall and stratum-specific survival tables. Each cohort provided cohort-level survival tables indicating asthma diagnoses at each age (with each stratum), thus including only person-years of observation for the time that the cohort had observed individuals. Survival tables were combined for each year of age, weighted by the inverse of the sample size for the cohort, and then survival probabilities were calculated using the frequently used Kaplan-Meier method.21
Age-specific incidence rates for each year of age within each stratum were calculated from the survival functions. Calculations were restricted to those cohorts that had at least 20 person-years at risk within each stratum. To smooth out variations in the incidence rates for each age, a cubic smoothing spline was fitted to the rates with the leave-one-out cross-validation method to find the best-fitted spline using the smooth.spline function in R.18,22 Asthma incidence rate ratios (IRRs) by age and by FH, sex, and race/ethnicity were calculated by comparing the corresponding predicted incidence rates from the smoothing splines. Confidence intervals were generated by first creating a pseudo-data set for each cohort given the number of children with the outcome and the total person-time observed within each age in each stratum. Bootstrapping 1000 times using resampling with replacement from each pseudo-data set was used to create a distribution of age-specific incidence estimates and IRRs, which were used to create the 95% CIs for each estimate.23
Several sensitivity analyses were done to confirm robustness of the findings. First, we conducted a series of leave-one-out analyses repeating the analysis excluding 1 cohort at a time to ensure that no single cohort significantly swayed the findings. Second, we analyzed the data restricting to those cohorts that provided data on children aged 10 years or older. Finally, we analyzed the data excluding the cohorts in which only children who were at high risk of asthma were enrolled. All analyses were done using R software, version 3.5 (R Foundation). Significance was set at P < .05, and all P values were 2-sided.
Results
Study Population
A total of 11 404 children (mean [SD] age, 10.0 [0.7] years; 5836 boys [51%]; 5909 White children [53%]) from just over 50 000 children in the ECHO program were included in the primary analysis (Table), whereas the secondary analysis included a broader population of 24 635 children (eTable 3 in Supplement 1). In the primary analysis, 7326 children (64%) had no FH of asthma, whereas 4078 children (36%) had a history of asthma in either 1 parent or both (any FH). This outcome was largely dependent on maternal history, because we had reliable reported asthma history for only 3324 of 11 404 fathers (29%). There was a fairly even split of boys (5836 of 11 404 [51%]) and girls (5568 of 11 404 [49%]), and 2494 children (23%) identified as non-Hispanic Black.
Table. Characteristics of Children Included in the Primary Analysis, Stratified by Family History of Asthma.
| Characteristic | No. (%) | ||
|---|---|---|---|
| All | No family history of asthma | Any family history of asthma | |
| Overall | 11 404 (100) | 7326 (100) | 4078 (100) |
| Family history of asthma | |||
| None | 7326 (64) | 7326 (100) | 0 |
| Both parents | 609 (5) | 0 | 609 (15) |
| Any (1 or both parents) | 4078 (36) | 0 | 4078 (100) |
| Sex | |||
| Boys | 5836 (51) | 3707 (51) | 2129 (52) |
| Girls | 5568 (49) | 3619 (49) | 1949 (48) |
| Race/ethnicity | |||
| Non-Hispanic White | 5909 (53) | 4103 (58) | 1806 (45) |
| Non-Hispanic Black | 2494 (23) | 1414 (20) | 1080 (27) |
| Othera | 896 (8) | 556 (8) | 340 (9) |
| Hispanic/Latino | 1797 (16) | 1025 (14) | 772 (19) |
| Birth year | |||
| 1980-1984 | 754 (7) | 576 (8) | 178 (4) |
| 1985-1989 | 700 (6) | 602 (8) | 98 (2) |
| 1990-1994 | 79 (1) | 42 (1) | 37 (1) |
| 1995-1999 | 891 (8) | 429 (6) | 462 (11) |
| 2000-2004 | 2360 (21) | 1514 (21) | 846 (21) |
| 2005-2009 | 1899 (16) | 876 (12) | 1023 (25) |
| 2010-2014 | 4721 (41) | 3287 (45) | 1434 (35) |
| Enrollment region | |||
| Northeast | 3483 (30) | 2120 (29) | 1363 (33) |
| Midwest | 2608 (23) | 1510 (21) | 1098 (27) |
| South | 3766 (33) | 2585 (35) | 1181 (29) |
| West | 1547 (14) | 1111 (15) | 436 (11) |
| Urbanb | 5412 (74) | 3225 (68) | 2187 (85) |
| Maternal education | |||
| ≥High school | 8340 (90) | 5468 (93) | 2872 (84) |
| <High school | 938 (10) | 411 (7) | 527 (16) |
Other includes non-Hispanic Native American, Pacific Islander, and Asian race/ethnicity, as well as individuals who indicated mixed or multiple races.
Urban areas were those areas classified as urbanized by the 2010 US Census, as well as counties/cities/standard metropolitan statistical areas with 2500 or more inhabitants according to the 2010 US Census criteria.
A higher proportion of children with any FH of asthma (compared with those with no FH) were Black (1080 of 4078 [27%] vs 1414 of 7326 [20%]), were born in the 2005 to 2009 time period (1023 of 4078 [25%] vs 876 of 7326 [12%]), and were enrolled at an urban study site (2187 of 4078 [85%] vs 3225 of 7326 [68%]). A lower proportion of children with any FH of asthma had a maternal education level of high school or above (2872 of 4078 [84%] vs 5468 of 7326 [93%]).
Asthma Incidence
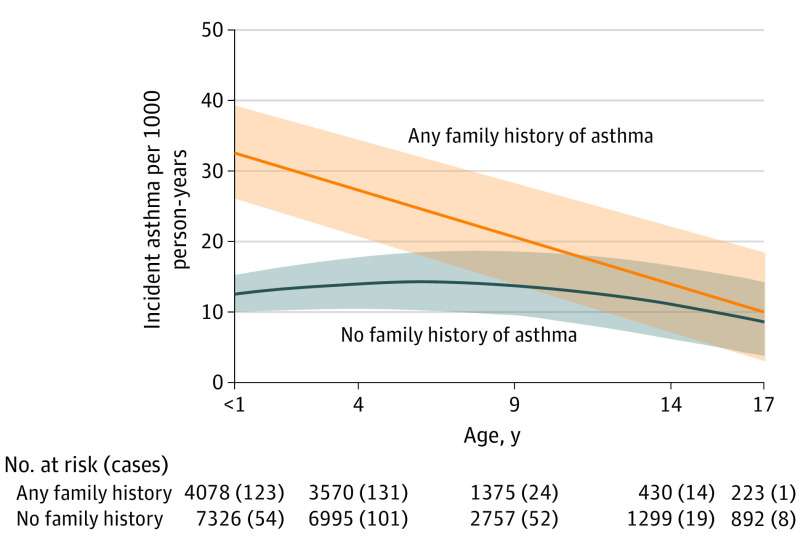
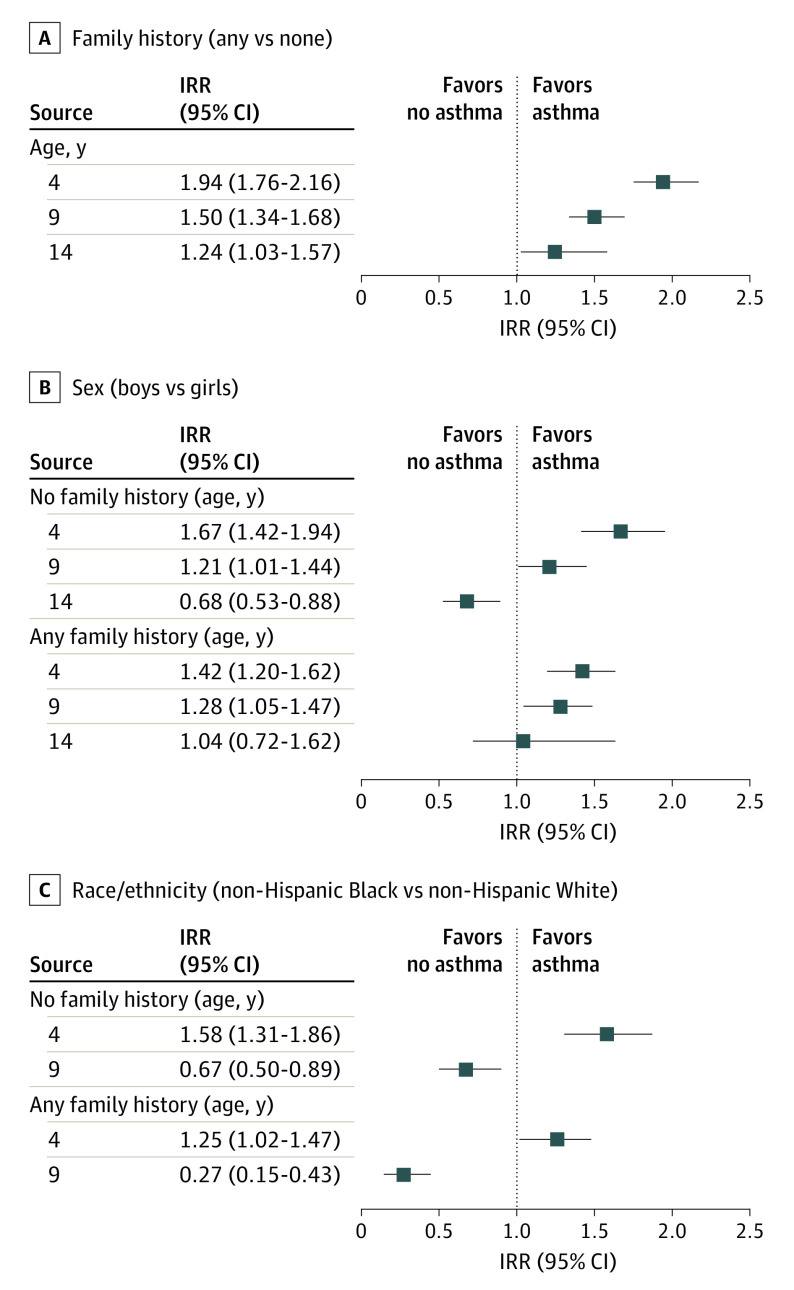
Among children with any FH of asthma, asthma incidence was highest in infancy, with an estimated 33 cases per 1000 person-years in children younger than 1 year of age, which steadily declined to 10 cases per 1000 person-years in children aged 17 years (Figure 2). In contrast, the incidence rate for children with no FH stayed relatively constant between birth and 17 years, at between approximately 9 and 14 cases per 1000 person-years, with no notable decline over time. Children with any FH of asthma had a nearly 2-fold increased risk of asthma at age 4 years compared with those with no FH (IRR, 1.94; 95% CI, 1.76-2.16) and continued to have a statistically significantly higher risk of asthma through age 14 years (IRR at age 14 years, 1.24; 95% CI, 1.03-1.57) (Figure 3). Therefore, all further analyses were stratified by FH of asthma.
Figure 2. Estimated Age-Specific Asthma Incidence Rates in Children Aged 1-17 Years Stratified by Family History of Asthma.
Numbers are shown for specific age groups only. Shaded area represents 95% CIs.
Figure 3. Incidence Rate Ratios (IRRs) of Asthma Diagnosis at Ages 4, 9, and 14 Years .
Panels compare family history (A), sex (B), and race/ethnicity (C) stratified by family history.
Sex was significantly associated with patterns of asthma incidence (Figure 4). For those with an FH of asthma, rates were higher for boys in the early years and declined with age in both boys and girls, but more so in boys, such that the rates were equal among boys and girls aged 14 to 17 years. For those without an FH of asthma, boys also had higher age-specific incidence rates at younger ages that declined at later ages, whereas girls had relatively steady rates, especially between 9 and 18 years of age. Confidence intervals overlapped for all these comparisons. Among the group with an FH of asthma, the IRR at age 4 years was higher among boys (1.42; 95% CI, 1.20-1.62), but the incidence rates among boys and girls were not significantly different at age 14 years (IRR, 1.04; 95% CI, 0.72-1.62) (Figure 3). Among the no FH group, the IRR in boys vs girls at age 4 years was 1.67 (95% CI, 1.42-1.94), whereas boys had a significantly lower incidence at age 14 years (IRR, 0.68; 95% CI, 0.53-0.88).
Figure 4. Estimated Age-Specific Asthma Incidence Rates in Children Aged 1-17 Years .
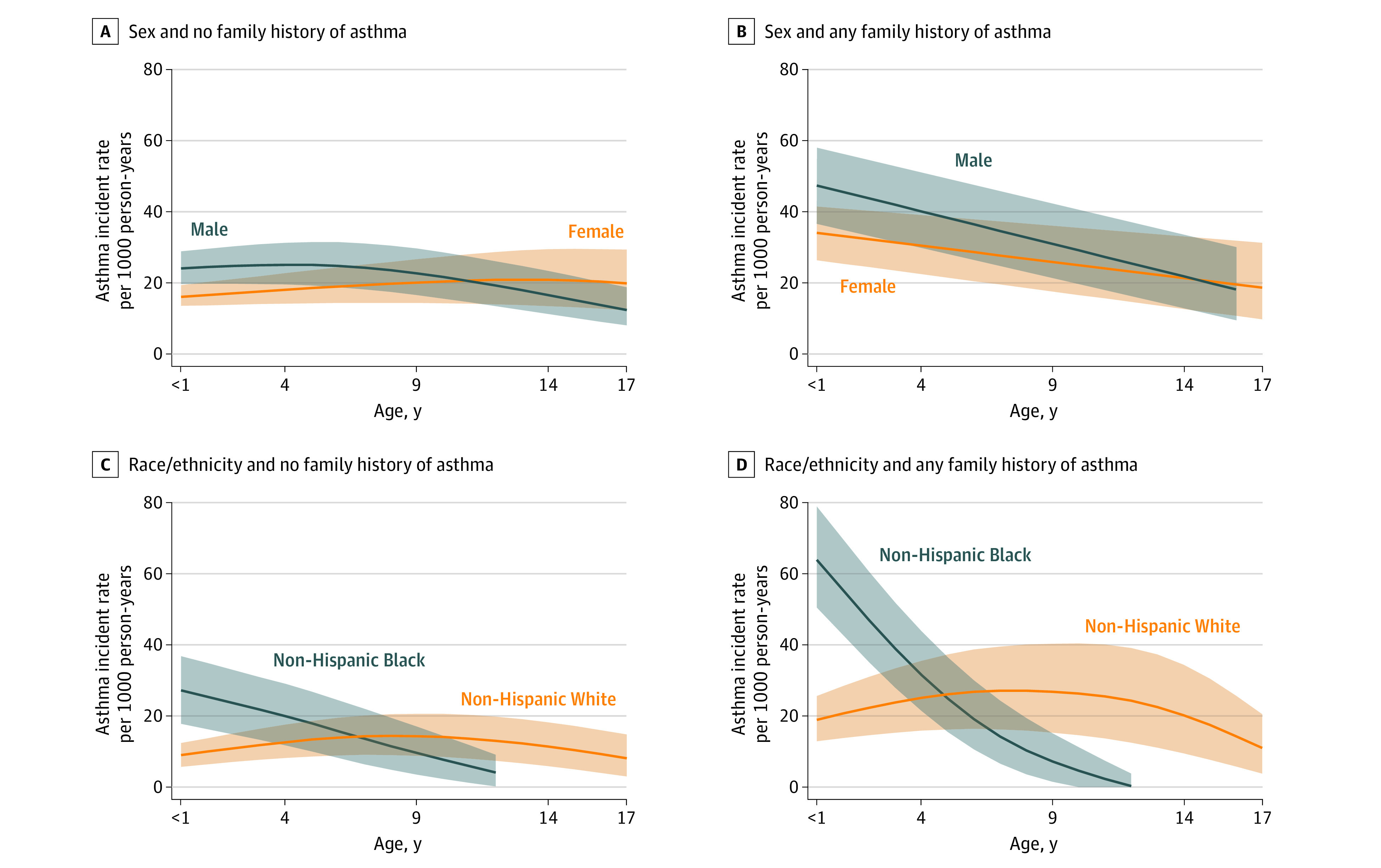
Panels compare child sex (A and B) or child race/ethnicity (C and D) stratified by family history. Shaded area represents 95% CIs.
Race/ethnicity were associated with asthma incidence rates in an age-dependent fashion (Figure 4C and D). Asthma incidence rates among Black children were higher up to age 4 years regardless of FH status, and the increased rate among younger Black children with any FH was the highest among any demographic (an estimated 64 cases per 1000 person-years at age younger than 1 year for those with any FH compared with 10 among White children with no FH).
At age 4 years, the IRR (Figure 3) for Black children vs White children was 1.25 (95% CI, 1.02-1.47) among those with any FH and 1.58 (95% CI, 1.31-1.86) among children with no FH. In contrast, Black children had significantly decreased incidence rates at age 9 years compared with White children in both the any FH and the no FH groups, with IRRs of 0.27 (95% CI, 0.15-0.43) and 0.67 (95% CI, 0.50-0.89), respectively.
Age-specific IRRs by geographic regions stratified by FH are provided in eTable 2 in Supplement 1. Compared with cohorts in the West, IRRs were significantly higher in the Midwest during the preschool years (at age 4, IRR, 1.34; 95% CI, 1.05-1.70 for children with no FH and IRR, 1.13; 95% CI, 0.90-1.43 for children with FH), and in the Northeast during midchildhood (at age 9 years, IRR, 1.40; 95% CI, 1.12-1.74 among children with no FH and IRR, 0.85; 95% CI, 0.67-1.10 among children with FH) but only among children without an FH.
The sensitivity analyses yielded results very similar to those in the primary analysis. Restricting to cohorts with follow-up through age 10 years and older, the IRRs were nearly identical to those reported in the primary analysis (eAppendix 4, eTable 3 in Supplement 1). The exceptions were that (1) the IRR for Black children vs White children aged 4 years with no FH was 1.26 (95% CI, 1.00-1.54), indicating that the direction of the effect was the same as in the primary analysis, but the result was not significant and (2) the IRR among those with any FH was 0.86 (95% CI, 0.54-1.13), indicating changed direction and decreased statistical precision such that the estimate was consistent with 1.0. These changes are likely due to the smaller sample size of Black children in the restricted cohort (fewer cohorts decades ago emphasized a diversity of study participants). Excluding the cohorts who enrolled based on the children being at high risk for asthma in another sensitivity analysis (eAppendix 4, eTable 4 in Supplement 1), the results did not materially change, with the exception of (1) a loss of magnitude in the boy-to-girl ratio in children aged 9 years with FH, probably largely owing to a smaller sample size, as well as (2) a loss of magnitude and precision in the ratio of Black children to White children in those aged 9 years without FH, also likely reflecting a loss of sample size. The results for the supplementary analyses, including all 31 cohorts (eAppendix 5 and eTable 5 in Supplement 1), were similar to the primary analysis (eAppendix 5 and eFigures 1-3 in Supplement 1).
Discussion
This cohort study involving multiple ECHO-funded cohorts provided a unique opportunity to investigate incident asthma in a demographically and geographically diverse population of thousands of children across the US. Our results suggest a strong association between parental history of asthma and childhood asthma incidence, as well as increased incidence rates in boys at younger ages. We reported 3 main findings: (1) parental history was associated with increased incidence rates primarily in the preschool years; (2) the sex differential crossover pattern commonly shown in prevalence data reflected a decreasing incidence rate trend in boys, with little change in girls’ incidence rates in the first 2 decades of life; and (3) Black children had higher associated incidence rates in the preschool years, particularly among children with a positive parental history of asthma, whereas White children had higher associated rates in later childhood.
A strong association between parental history of asthma and childhood asthma incidence rates was expected based on existing studies. This association was found even though this variable represented mainly maternal history, because paternal history was unfortunately unknown for the great majority of the children. The finding of higher rates of asthma at the youngest ages among those with a parental history suggests a possible genetic contribution.24 Among children with an FH, it is possible that early age diagnosis is sometimes prompted by clinician and parental heightened awareness. However, we required children with an early diagnosis to have evidence of ongoing asthma, which suggests that the observed association was not entirely explained by diagnosis bias.
Our findings were consistent with the hypothesis that asthma onset after age 10 years is more strongly influenced by environmental exposures. There is also the possibility that some environmental factors important for asthma development in a parent were present in the shared environment of the parent and child.
Previous studies have often included sex-specific rates, although usually not in a longitudinal fashion or simultaneously considering FH or race/ethnicity (eTable 1 in Supplement 1). During childhood, asthma prevalence25,26 or cumulative incidence27 and univariate incidence rates by sex28,29,30 have been observed to be higher in boys, with a reversal of this difference by adolescence or early adulthood.31 In the current analysis, this pattern was only modestly evident and then just among those without an FH, reflected mainly by a decline in boys but relatively steady incidence rates in girls from childhood through age 18 years. This finding suggests that a focus on puberty as a risk factor should be de-emphasized.27 This same pattern was suggested from sex-specific incidence rates reported in a Dutch study32 and in a recent MeDALL (Mechanisms of the Development of Allergy) meta-analysis of 5 European birth cohorts.33 Some study results have suggested that parental or personal atopy may have differential influences between boys and girls and child vs adolescent onset with respect to the development of wheeze.25,26,34
It is well documented that asthma prevalence in children younger than age 18 years is higher in Black children vs White children.35 An unexpected and more refined finding, enabled by the diversity of the cohorts, was the demonstration that Black children have significantly higher incidences rates than White children, but only in the preschool years, and those with an FH showed a dramatic decrease in incident asthma throughout the first decade of life. Potential explanations could include a stronger genetic association with asthma propensity in Black people36,37 or that young Black children are more genetically susceptible to or exposed to deleterious environmental factors, such as air pollution secondary to traffic or industry.38 Characteristics that have been shown to vary by race/ethnicity, such as low birth weight,17 increased exposure to selected indoor allergens39 and secondhand smoke,40 differences in the infant’s microbiome,41 diet,42 perinatal stress,43 or epigenetic associations due to environmental circumstances,44 probably contributed to disease inception among many other potential factors. It is also possible that parents of Black children may recognize asthma symptoms earlier and bring them to medical attention. Asthma that begins during infancy is more likely to be associated with low lung function and asthma exacerbations,45,46 which suggests that the early onset of disease in Black children may eventually be related to increased asthma morbidity and severity.
Limitations
As with all observational studies, there are limitations to be considered. Although these data represent children in more than 20 studies from over half the states in the US, this study was not derived from a national probability sample. Data were also not collected in a standardized fashion, which constrained our choices of analytic approaches. We relied on a parent or caregiver report of physician-diagnosed asthma as our defined outcome. Our age-specific incidence rates are modeled estimates, derived from meta-analyzed survival functions that were then smoothed. We were able to compute CIs; however, the derived CIs depend on data variance rather than directly on sample size. Therefore, the CI widths, although statistically appropriate, may appear counterintuitive to those relying on the usual number at risk as a rough guide for width magnitude. Finally, it should be considered that the incidence rate curves may be somewhat biased by race/ethnicity patterns related to health care,47 including less access to specialists and a possible propensity to diagnose asthma in Black children. It is also important to note that data were sparse or nonexistent for Black teenagers owing to less racial diversity in the earliest established birth cohorts that have been followed the longest, although the supplementary analyses that included a 29% increase in Black cohort members confirmed our results.
Conclusions
In summary, the results of this cohort study, which included cohorts from within the ECHO consortium, suggest that asthma FH, as well as race/ethnicity and sex, were all associated with childhood asthma incidence rates. Non-Hispanic Black children had significantly higher incidence rates in the preschool years than non-Hispanic White children, especially among children with a positive parental history. The implementation of solutions requires not only the development of effective interventions but also a better understanding of the populations who might benefit most from potential interventions, such as microbiome modification, closer medical surveillance, or eventual precision medicine approaches.
eAppendix 1. Published Studies on Incident Childhood Asthma
eTable 1. Key Characteristics of Published Studies With Information on Incident Asthma Rates in Children
eAppendix 2. Principal Investigators and Names of ECHO-Participating Cohorts Who Contributed to this Analysis
eAppendix 3. Asthma Incidence Comparisons Across Geographic Regions
eTable 2. Incidence Rate Ratios of Asthma Incidence by Age Across Geographic Regions, Compared to “West” as the Reference Region
eAppendix 4. Sensitivity Analyses
eTable 3. Comparison of Incidence Rate Ratios of Asthma Diagnosis at Ages 4, 9, and 14 Years by Family History, and Sex and Race Stratified by Family History Among the 22 Cohorts in the Primary Analysis vs the 12 Cohorts With Children Followed Through 10+ Years of Age
eTable 4. Comparison of Incidence Rate Ratios of Asthma Diagnosis at Ages 4, 9, and 14 Years by Family History, and Sex and Race Stratified by Family History Among the 22 Cohorts in the Primary Analysis vs Only the 18 Cohorts Where Eligibility Did Not Require Being at High Risk
eAppendix 5. Supplementary Analysis: 31 Contributing Cohorts
eTable 5. Baseline Characteristics of Children in the Supplementary Analyses
eFigure 1. Estimated Age-Specific Asthma Incidence Rates in Children <1 to 17 Years of Age Stratified by Family History
eFigure 2. Incidence Rate Ratios of Asthma Diagnoses at Ages <1, 4, 9, and 14 Years Comparing Family History, and Sex and Race/Ethnicity Stratified by Family History
eFigure 3. Estimated Age-Specific Asthma Incidence Rates in Children <1 to 17 Years of Age Stratified by Family History and Sex and Race/Ethnicity (n = 31 cohorts)
eReferences
Nonauthor Collaborators. Environmental Influences on Child Health Outcomes (ECHO) group members.
References
- 1.Centers for Disease Control and Prevention. 2018 National health interview survey (NHIS) data. Accessed February 10, 2020. https://www.cdc.gov/asthma/nhis/2018/table4-1.htm
- 2.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143-178. doi: 10.1183/09031936.00138707 [DOI] [PubMed]
- 3.Bousquet JJ, Schünemann HJ, Togias A, et al. ; ARIA Study Group; MASK Study Group . Next-generation ARIA care pathways for rhinitis and asthma: a model for multimorbid chronic diseases. Clin Transl Allergy. 2019;9:44. doi: 10.1186/s13601-019-0279-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poowuttikul P, Seth D. New concepts and technological resources in patient education and asthma self-management. Clin Rev Allergy Immunol. 2020;59(1):19-37. doi: 10.1007/s12016-020-08782-w [DOI] [PubMed] [Google Scholar]
- 5.Beasley R, Semprini A, Mitchell EA. Risk factors for asthma: is prevention possible? Lancet. 2015;386(9998):1075-1085. doi: 10.1016/S0140-6736(15)00156-7 [DOI] [PubMed] [Google Scholar]
- 6.Polk BI, Bacharier LB. Potential strategies and targets for the prevention of pediatric asthma. Immunol Allergy Clin North Am.2019;39(2):151-162. Medline:30954167 doi: 10.1016/j.iac.2018.12.010 [DOI] [PubMed]
- 7.Grant T, Brigham EP, McCormack MC. Childhood origins of adult lung disease as opportunities for prevention. J Allergy Clin Immunol Pract. 2020;8(3):849-858. doi: 10.1016/j.jaip.2020.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacMahon B, Pugh TF. Epidemiology: Principles and Methods. Little, Brown and Co; 1970. [Google Scholar]
- 9.Smith A, Serban N, Fitzpatrick A. Asthma prevalence among Medicaid-enrolled children. J Allergy Clin Immunol Pract. 2019;7(4):1207-1213.e4. doi: 10.1016/j.jaip.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes HK, Matsui EC, Tschudy MM, Pollack CE, Keet CA. Pediatric asthma health disparities: race, hardship, housing, and asthma in a national survey. Acad Pediatr. 2017;17(2):127-134. doi: 10.1016/j.acap.2016.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123(3):S131-S145. doi: 10.1542/peds.2008-2233C [DOI] [PubMed]
- 12.Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012;(35):1-58. [PubMed] [Google Scholar]
- 13.Rudd RA, Moorman JE. Asthma incidence: data from the national health interview survey, 1980-1996. J Asthma. 2007;44(1):65-70. doi: 10.1080/02770900601125896 [DOI] [PubMed] [Google Scholar]
- 14.Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital signs: asthma in children—United States, 2001-2016. MMWR Morb Mortal Wkly Rep. 2018;67(5):149-155. doi: 10.15585/mmwr.mm6705e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urquhart A, Clarke P. US racial/ethnic disparities in childhood asthma emergent health care use: national health interview survey, 2013-2015. J Asthma. 2020;57(5):510-520. doi: 10.1080/02770903.2019.1590588 [DOI] [PubMed] [Google Scholar]
- 16.Bousquet J, Gern JE, Martinez FD, et al. Birth cohorts in asthma and allergic diseases: report of a NIAID/NHLBI/MeDALL joint workshop. J Allergy Clin Immunol. 2014;133(6):1535-1546. doi: 10.1016/j.jaci.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson CC, Peterson EL, Joseph CL, Ownby DR, Breslau N. Birth weight and asthma incidence by asthma phenotype pattern in a racially diverse cohort followed through adolescence. J Asthma. 2015;52(10):1006-1012. doi: 10.3109/02770903.2015.1054405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team . The R project for statistical computing. Accessed December 31, 2019. https://www.R-project.org/
- 19.Guilbert TW, Mauger DT, Lemanske RF. Childhood asthma-predictive phenotype. J Allergy Clin Immunol Pract. 2014;2(6):664-670. doi: 10.1016/j.jaip.2014.09.010 [DOI] [PubMed]
- 20.National Archives. Qualifying urban areas for the 2010. census. Accessed December 31, 2019. https://www.govinfo.gov/content/pkg/FR-2012-03-27/pdf/2012-6903.pdf [Google Scholar]
- 21.Bland JM, Altman DG. Survival probabilities (the Kaplan-Meier method). BMJ. 1998;317(7172):1572. doi: 10.1136/bmj.317.7172.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arlot S, Celisse A. A survey of cross-validation procedures for model selection. Statist Surv. 2010;4:40-79. doi: 10.1214/09-SS054 [DOI] [Google Scholar]
- 23.Akritas M. Bootstrapping the Kaplan-Meier estimator. J Am Stat Assoc. 1986;81(396):1032-1038. doi: 10.1080/01621459.1986.10478369 [DOI] [Google Scholar]
- 24.Falconer DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet.1965;29(1):51-76. doi: 10.1111/j.1469-1809.1965.tb00500.x [DOI] [Google Scholar]
- 25.Almqvist C, Worm M, Leynaert B; working group of GA2LEN WP 2.5 Gender . Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63(1):47-57. doi: 10.1111/j.1398-9995.2007.01524.x [DOI] [PubMed] [Google Scholar]
- 26.Mandhane PJ, Greene JM, Cowan JO, Taylor DR, Sears MR. Sex differences in factors associated with childhood- and adolescent-onset wheeze. Am J Respir Crit Care Med. 2005;172(1):45-54. doi: 10.1164/rccm.200412-1738OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vink NM, Postma DS, Schouten JP, Rosmalen JG, Boezen HM. Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents' Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol.2010;126(3):498-504.e1-6. doi: 10.1016/j.jaci.2010.06.018 [DOI] [PubMed]
- 28.Anderson HR, Pottier AC, Strachan DP. Asthma from birth to age 23: incidence and relation to prior and concurrent atopic disease. Thorax. 1992;47(7):537-542. doi: 10.1136/thx.47.7.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson L. Incidence of asthma in Swedish teenagers: relation to sex and smoking habits. Thorax. 1995;50(3):260-264. doi: 10.1136/thx.50.3.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yunginger JW, Reed CE, O’Connell EJ, Melton LJ III, O’Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. incidence rates, 1964-1983. Am Rev Respir Dis. 1992;146(4):888-894. doi: 10.1164/ajrccm/146.4.888 [DOI] [PubMed] [Google Scholar]
- 31.Lawson JA, Janssen I, Bruner MW, Hossain A, Pickett W. Asthma incidence and risk factors in a national longitudinal sample of adolescent Canadians: a prospective cohort study. BMC Pulm Med. 2014;14:51. doi: 10.1186/1471-2466-14-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelkes M, Janssens HM, de Ridder MA, de Jongste JC, Sturkenboom MC, Verhamme KM. Time trends in the incidence, prevalence and age at diagnosis of asthma in children. Pediatr Allergy Immunol. 2015;26(4):367-374. doi: 10.1111/pai.12376 [DOI] [PubMed] [Google Scholar]
- 33.Hohmann C, Keller T, Gehring U, et al. Sex-specific incidence of asthma, rhinitis and respiratory multimorbidity before and after puberty onset: individual participant meta-analysis of five birth cohorts collaborating in MeDALL. BMJ Open Respir Res. 2019;6(1):e000460. doi: 10.1136/bmjresp-2019-000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melén E, Kere J, Pershagen G, Svartengren M, Wickman M. Influence of male sex and parental allergic disease on childhood wheezing: role of interactions. Clin Exp Allergy. 2004;34(6):839-844. doi: 10.1111/j.1365-2222.2004.01957.x [DOI] [PubMed] [Google Scholar]
- 35.Akinbami LJ, Moorman JE, Simon AE, Schoendorf KC. Trends in racial disparities for asthma outcomes among children 0 to 17 years, 2001-2010. J Allergy Clin Immunol. 2014;134(3):547-553.e5. doi: 10.1016/j.jaci.2014.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torgerson DG, Ampleford EJ, Chiu GY, et al. ; Mexico City Childhood Asthma Study (MCAAS); Children’s Health Study (CHS) and HARBORS study; Genetics of Asthma in Latino Americans (GALA) Study, Study of Genes-Environment and Admixture in Latino Americans (GALA2) and Study of African Americans, Asthma, Genes & Environments (SAGE) ; et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43(9):887-892. doi: 10.1038/ng.888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baye TM, Butsch Kovacic M, Biagini Myers JM, et al. Differences in candidate gene association between European ancestry and African American asthmatic children. PLoS One. 2011;6(2):e16522. doi: 10.1371/journal.pone.0016522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia E, Berhane KT, Islam T, et al. Association of changes in air quality with incident asthma in children in California, 1993-2014. JAMA.2019;321(19):1906-1915. doi: 10.1001/jama.2019.5357 [DOI] [PMC free article] [PubMed]
- 39.Celedón JC, Milton DK, Ramsey CD, et al. Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. J Allergy Clin Immunol. 2007;120(1):144-149. doi: 10.1016/j.jaci.2007.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics.2012;129(4):735-744. doi: 10.1542/peds.2011-2196 [DOI] [PubMed]
- 41.Levin AM, Sitarik AR, Havstad SL, et al. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci Rep. 2016;6:31775. doi: 10.1038/srep31775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lessard A, Turcotte H, Cormier Y, Boulet LP. Obesity and asthma: a specific phenotype? Chest. 2008;134(2):317-323. doi: 10.1378/chest.07-2959 [DOI] [PubMed] [Google Scholar]
- 43.Bose S, Chiu YM, Hsu HL, et al. Prenatal nitrate exposure and childhood asthma. influence of maternal prenatal stress and fetal sex. Am J Respir Crit Care Med.2017;196(11):1396-1403. doi: 10.1164/rccm.201702-0421OC [DOI] [PMC free article] [PubMed]
- 44.Chan MA, Ciaccio CE, Gigliotti NM, et al. DNA methylation levels associated with race and childhood asthma severity. J Asthma. 2017;54(8):825-832. doi: 10.1080/02770903.2016.1265126 [DOI] [PubMed] [Google Scholar]
- 45.Belgrave DC, Buchan I, Bishop C, Lowe L, Simpson A, Custovic A. Trajectories of lung function during childhood. Am J Respir Crit Care Med. 2014;189(9):1101-1109. doi: 10.1164/rccm.201309-1700OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oksel C, Granell R, Haider S, et al. Distinguishing wheezing phenotypes from infancy to adolescence. a pooled analysis of five birth cohorts. Ann Am Thorac Soc.2019;16(7):868-876. doi: 10.1513/AnnalsATS.201811-837OC [DOI] [PMC free article] [PubMed]
- 47.Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health. 2005;26(26):89-113. doi: 10.1146/annurev.publhealth.26.021304.144528 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Published Studies on Incident Childhood Asthma
eTable 1. Key Characteristics of Published Studies With Information on Incident Asthma Rates in Children
eAppendix 2. Principal Investigators and Names of ECHO-Participating Cohorts Who Contributed to this Analysis
eAppendix 3. Asthma Incidence Comparisons Across Geographic Regions
eTable 2. Incidence Rate Ratios of Asthma Incidence by Age Across Geographic Regions, Compared to “West” as the Reference Region
eAppendix 4. Sensitivity Analyses
eTable 3. Comparison of Incidence Rate Ratios of Asthma Diagnosis at Ages 4, 9, and 14 Years by Family History, and Sex and Race Stratified by Family History Among the 22 Cohorts in the Primary Analysis vs the 12 Cohorts With Children Followed Through 10+ Years of Age
eTable 4. Comparison of Incidence Rate Ratios of Asthma Diagnosis at Ages 4, 9, and 14 Years by Family History, and Sex and Race Stratified by Family History Among the 22 Cohorts in the Primary Analysis vs Only the 18 Cohorts Where Eligibility Did Not Require Being at High Risk
eAppendix 5. Supplementary Analysis: 31 Contributing Cohorts
eTable 5. Baseline Characteristics of Children in the Supplementary Analyses
eFigure 1. Estimated Age-Specific Asthma Incidence Rates in Children <1 to 17 Years of Age Stratified by Family History
eFigure 2. Incidence Rate Ratios of Asthma Diagnoses at Ages <1, 4, 9, and 14 Years Comparing Family History, and Sex and Race/Ethnicity Stratified by Family History
eFigure 3. Estimated Age-Specific Asthma Incidence Rates in Children <1 to 17 Years of Age Stratified by Family History and Sex and Race/Ethnicity (n = 31 cohorts)
eReferences
Nonauthor Collaborators. Environmental Influences on Child Health Outcomes (ECHO) group members.