SUMMARY
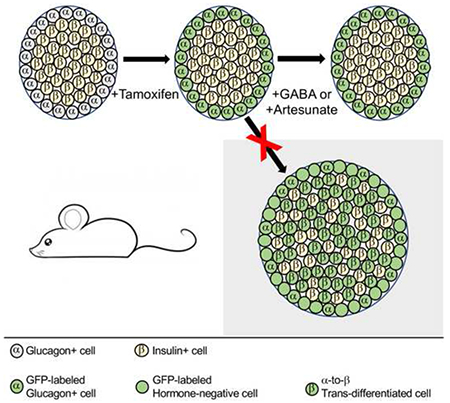
Recent reports identified activation of the GABA signaling pathway as a means to induce trans-differentiation of pancreatic α cells into β cells. These reports followed several previous studies that found that α cells were particularly well-suited to conversion into β cells in mice, but only after nearly complete β cell loss or forced overexpression of key transcriptional regulators. The possibility of increasing β cell number via reprograming of α cells with a small molecule is enticing, as this could be a potential new pharmacologic therapy for diabetes. Here, we employed rigorous genetic lineage tracing of α cells, using Glucagon-CreERT2;Rosa-LSL-eYFP mice, to evaluate if activation of GABA signaling caused α-to-β cell reprogramming. In contrast to previous reports, we found that even after long-term treatment of mice with artesunate or GABA, neither α-to-β cell trans-differentiation nor insulin secretion were stimulated, putting into question whether these agents represent a viable path to a novel diabetes therapy.
Graphical Abstract
INTRODUCTION
Diabetes mellitus results from an absolute (type 1) or relative (type 2) deficiency of insulin-secreting pancreatic β cells. Thus, there is much interest in identifying methods to enhance insulin secretion and increase β cell number, by stimulating β cell proliferation or differentiation from other cell types. Over the past decade, multiple studies have shown that pancreatic α cells can trans-differentiate into β cells or β-like cells after deletion of the α cell-specific transcription factor Arx (Chakravarthy et al., 2017; Courtney et al., 2013; Wilcox et al., 2013), over-expression of transcription factors necessary for β cell differentiation such as Pax4 (Collombat et al., 2009) or Pdx1 and Mafa (Matsuoka et al., 2017), or nearly absolute β cell ablation (Thorel et al., 2010). α and β cells arise from a common endocrine progenitor during development but have divergent differentiation pathways and ultimately produce hormones with opposing effects on plasma glucose regulation, with insulin lowering plasma glucose and glucagon increasing plasma glucose, as reviewed by (van der Meulen and Huising, 2015). Histone methylation (Bramswig et al., 2013) and chromatin accessibility (Ackermann et al., 2016) data reveal that α cells are uniquely “poised” to activate expression of β cell-specific transcripts. Translating these findings into potential therapeutic approaches to induce α-to-β cell trans-differentiation in patients with diabetes is thus an exciting emerging field.
Activation of the γ-aminobutyric acid (GABA) signaling pathway, both directly by GABA (Ben-Othman et al., 2017) and indirectly by the anti-malarial drug class of artemisinins (Li et al., 2017), was recently reported to induce α-to-β cell trans-differentiation in zebrafish, rodent, and human islets. Long-term GABA administration in rodents also was reported to stimulate β cell neogenesis by generation of endocrine progenitor cells and differentiation through a transient glucagon-expressing state (Ben-Othman et al., 2017). In addition, these small molecule compounds also enhanced islet glucose-stimulated insulin secretion, which was attributed to the presumed increase in β-cell number. However, the dramatic in vivo results were surprising given the relatively small changes in Insulin expression observed in vitro.
Some of these findings were recently challenged by a study of murine and human islets cultured in vitro with the artemisinin artemether, which failed to show evidence for α-to-β cell transdifferentiation, but rather revealed pan-endocrine cell de-differentiation and suppression of glucose-stimulated insulin secretion (van der Meulen et al., 2018). Here, we used in vivo genetic α cell lineage tracing with a tamoxifen-inducible system (Figure 1A) to specifically determine whether long-term systemic treatment of mice with GABA or with the artemisinin artesunate (Figure 1B) induces α-to-β cell trans-differentiation, as was suggested by (Ben-Othman et al., 2017) and (Li et al., 2017).
Figure 1. Experimental Design, Glucose Tolerance and Body Weight After Treatment with Artesunate or GABA.
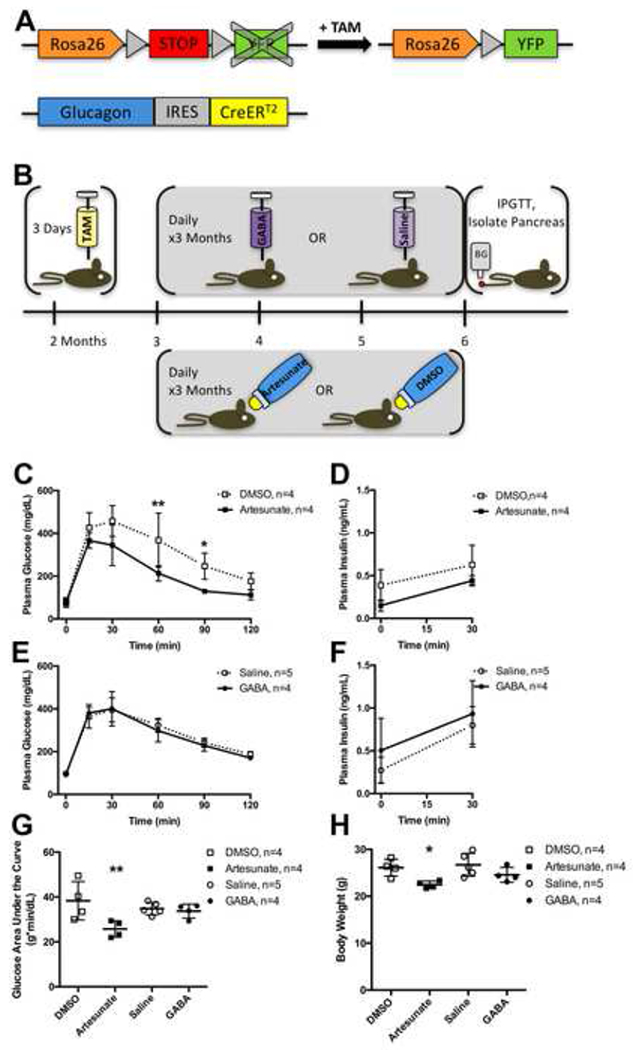
(A) Glucagon-CreERT2;Rosa-LSL-eYFP/+ mice were treated with tamoxifen (TAM) to induce Cre-mediated recombination of the floxed STOP cassette in the Rosa26 locus, resulting in YFP expression in α cells.
(B) 2 month old male mice were injected with TAM daily for 3 days, then 1 month later were treated with either daily injections of GABA or saline vehicle, or with artesunate or DMSO vehicle in the drinking water, for 3 months, then the mice underwent fasting intraperitoneal glucose tolerance test (IPGTT) and were killed for pancreas isolation.
(C) Plasma glucose concentrations during fasting intraperitoneal glucose tolerance test (IPGTT) in Glucagon-CreERT2;Rosa-LSL-eYFP/+ male mice performed at end of experimental timeline, 6 months of age, after treatment with artesunate (solid line with filled squares) or DMSO vehicle (dotted line with open squares). See also Figure S2.
(D) Plasma insulin concentrations during fasting IPGTT after treatment with artesunate or DMSO vehicle.
(E) Plasma glucose concentrations during fasting IPGTT after treatment with GABA (solid line with filled circles) or saline vehicle (dotted line with open circles).
(F) Plasma insulin concentrations during fasting IPGTT after treatment with GABA or saline vehicle.
(G) Plasma glucose concentration area under the curve during IPGTT.
(H) Body weight at time of IPGTT.
Data are presented as mean ± standard deviation. Data points from individual mice are presented in (G-H). * represents P < 0.05 and ** represents P < 0.01 by t-test with Holm-Sidak correction for multiple comparisons (C-F) or by one-way ANOVA with Sidak correction for multiple comparisons (G-H).
RESULTS
Glucose Tolerance and Body Weight After Treatment with Artesunate or GABA
Mice treated with artesunate for three months demonstrated faster glucose clearance than control mice treated with DMSO, with significantly lower plasma glucose levels at 60 and 90 minutes during intraperitoneal glucose tolerance tests (IPGTT; Figures 1C, S2). However, fasting and 2-hour ending plasma glucose levels were similar between the two groups. Furthermore, no significant differences in plasma insulin concentrations were observed between artesunate-treated versus DMSO control mice at fasting or 30 minutes during IPGTT (Figure 1D). No significant differences in plasma glucose (Figure 1E) or insulin concentrations (Figure 1F) were observed between GABA-treated versus saline control mice during fasting IPGTT. Mice treated with artesunate had overall reduced plasma glucose concentrations throughout IPGTT (Figure 1G) and reduced body weight (Figure 1H) at the time of the IPGTT at the end of the study (6 months of age), compared to all other study groups.
No Evidence of α-to-β Cell Trans-differentiation After Artesunate or GABA Treatment in vivo
Glucagon-CreERT2;Rosa-LSL-eYFP/+ mice were treated with tamoxifen to indelibly label α cells and any of their descendants with yellow fluorescent protein (YFP) expression at two months of age. This was followed by a one month chase period prior to artesunate or GABA treatment to permit clearance of tamoxifen (Reinert et al., 2012) and loss of nuclear-localized Cre protein in α cells (Figure S1). The Glucagon-CreERT2 specifically labels 95% of α cells in this protocol (Ackermann et al., 2017) and allows for robust α cell lineage tracing to accurately quantify trans-differentiation of mature α cells to β cells. Only a small number of Insulin and YFP co-expressing cells were present in all groups of mice, after treatment with DMSO (data not shown), saline (Figures 2A–2F), GABA (Figures 2G–2L), or artesunate (Figures 2M–2R) for 3 months. Next, we quantified the percentage of these Insulin+/YFP+ cells, which are the result of α-to-β cell reprogramming, among all treatment and control groups (Figure 2S). The fractions of these Insulin+/YFP+ cells were not different between treatment and control groups, indicating that neither GABA nor artesunate treatment stimulated the naturally occurring, slow trans-differentiation process in vivo.
Figure 2. No Lineage Tracing Evidence of α-to-β Cell Trans-differentiation After Artesunate or GABA Treatment.
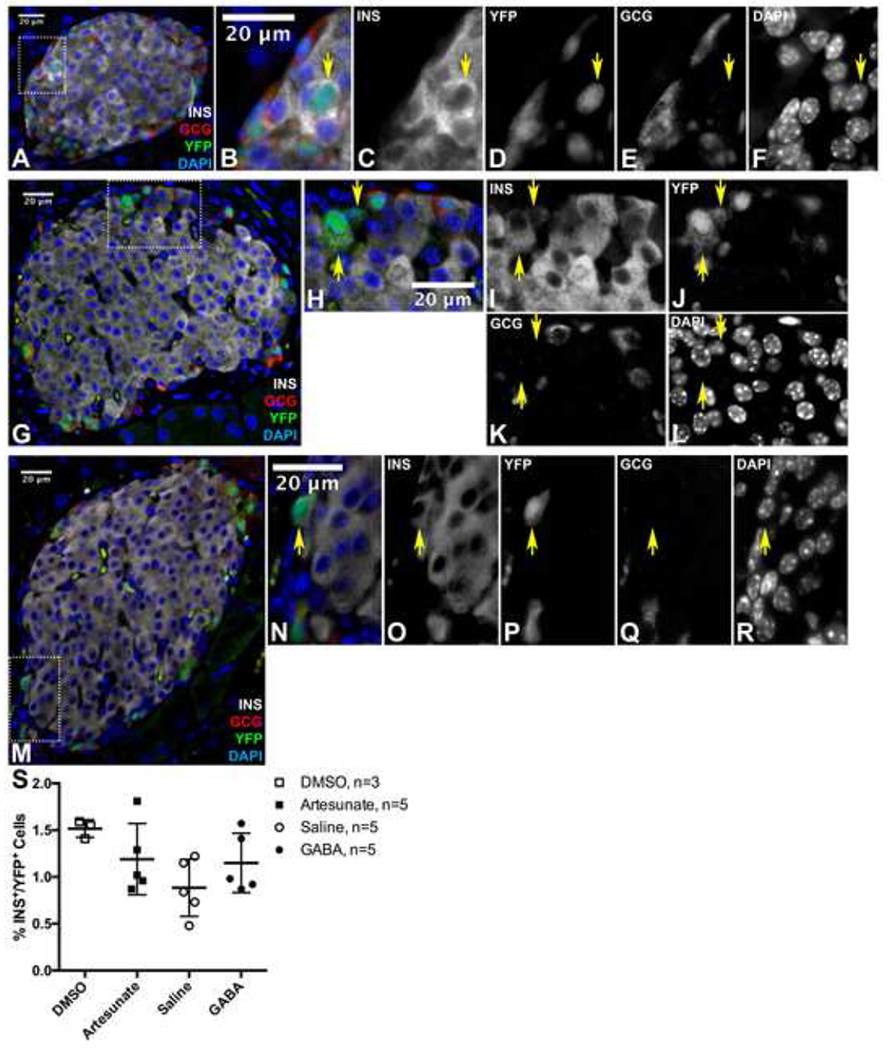
(A) Representative immunofluorescent image of islet from a saline-treated mouse.
(B-F) are magnified view of area outlined in (A).
(G) Representative immunofluorescent image of islet from a GABA-treated mouse.
(H-L) are magnified view of area outlined in (G).
(M) Representative immunofluorescent image of islet from an artesunate-treated mouse.
(N-R) are magnified view of area outlined in (M).
Yellow arrows indicate Insulin+/YFP+ cells. Scale bars represent 20 μm. INS = Insulin, GCG = Glucagon
(S) Quantification of % YFP+ cells that are also lnsulin+, as depicted in (A-M). Data points from individual mice are presented with mean ± standard deviation. No significant differences were detected by one-way ANOVA with Sidak correction for multiple comparisons.
No Evidence of Islet Neogenesis or Increased β Cell Number After Artesunate or GABA Treatment
Ben-Othman and colleagues had suggested islet neogenesis from duct-resident progenitor cells as the mechanism by which GABA treatment increases β-cell number via a short-lived α-cell intermediate (Ben-Othman et al., 2017). To address the issue of islet neogenesis, we quantified islet number, islet area, β cell area per islet, islet size distribution, and number of YFP+ cells per islet in all treatment groups (Figure 3). There were no significant differences in islet number (Figure 3A) or proportion of pancreatic area comprised of islets (Figure 3B), as determined by Chromogranin A immunolabeling. There were also no significant differences in the sizes of β cell clusters, as determined by Insulin immunolabeling (Figures 3C–3D), in mice treated with artesunate or GABA compared to control groups. Lastly, there were no significant differences in the number of YFP+ cells per islet among the treatment and control groups (Figure 3E).
Figure 3. No Evidence of Islet Neogenesis or Increased β Cell Number After Artesunate or GABA Treatment.
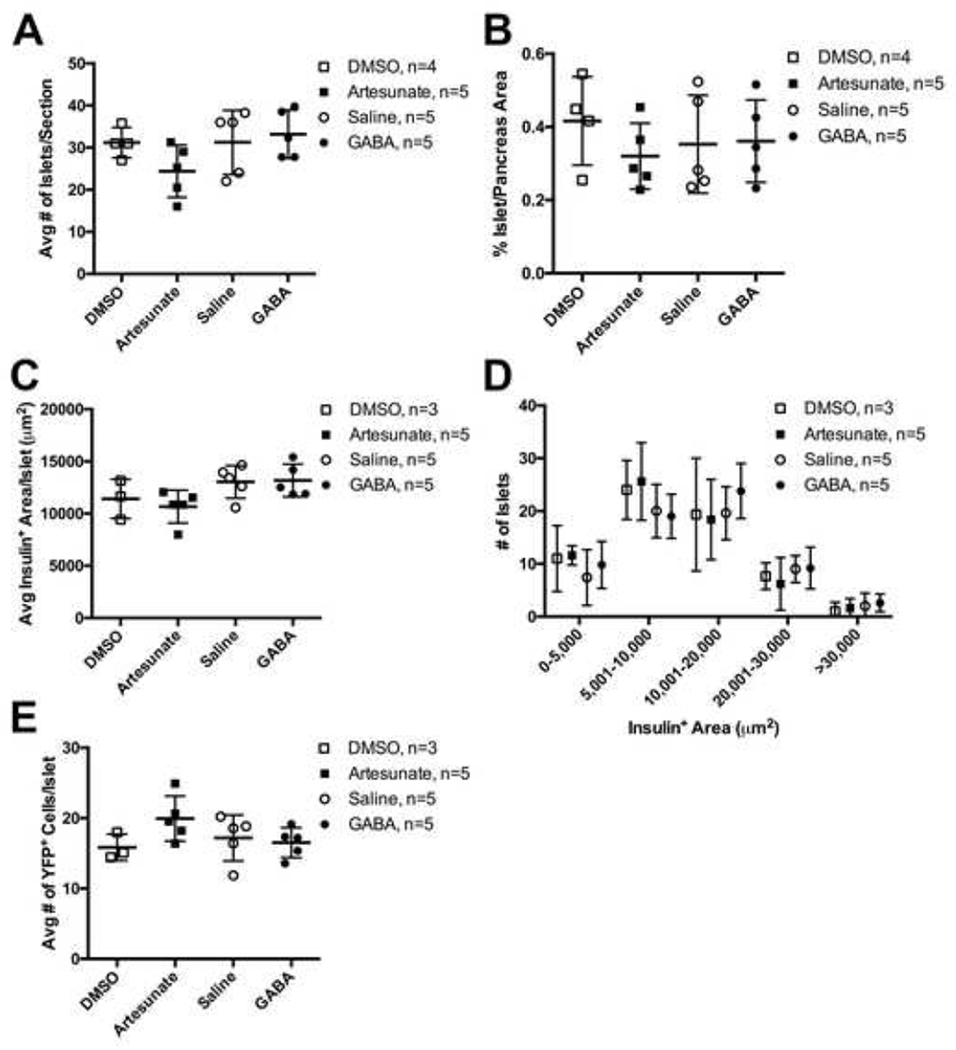
(A) Average number of Chromogranin A+ islets per section.
(B) Percent of Chromogranin A+ area per pancreatic section.
(C) Average lnsulin+ area per islet on all sections used for lineage tracing analysis.
(D) Distribution of Insulin+ area per islet on all sections used for lineage tracing analysis.
(E) Average number of YFP+ cells per islet on all sections used for lineage tracing analysis.
Data points from individual mice are presented with mean ± standard deviation. No significant differences were detected by one-way ANOVA with Sidak correction for multiple comparisons.
DISCUSSION
The main findings of this study are that (1) neither GABA nor artesunate induce α-to-β cell trans-differentiation in mice, (2) long-term GABA or artesunate treatment of mice does not alter insulin secretion, and (3) long-term GABA administration in mice does not alter glucose tolerance. These results contradict recent reports that the GABA signaling pathway, activated by GABA or artemisinins, represses α cell identity and promotes β cell differentiation (Ben-Othman et al., 2017; Li et al., 2017). Of note, a recent in vitro study by van der Meulen and colleagues also found no evidence for trans-differentiation of α-cells to β cells by the artemisinin artemether (van der Meulen et al., 2018).
The lineage tracing results reported here indicate that a small number of α cells trans-differentiate into β cells over the lifetime of a mouse at baseline. Our data on the frequency of these natural trans-differentiation events are congruent to those observed in Glucagon-CreERT2;Rosa-LSL-eYFP/+ mice 4 months after tamoxifen injection (van der Meulen et al., 2017), the same time point as evaluated in the current study. Our study shows no evidence that artesunate or GABA treatment stimulates α-to-β cell trans-differentiation. The Glucagon-CreERT2 mouse line and tamoxifen-inducible lineage tracing protocol used in this study specifically labels α cells in adult mice prior to GABA or artesunate administration, unlike the constitutive Glucagon-Cre mouse line that was used by (Ben-Othman et al., 2017). Therefore, we are able to detect direct mature α-to-β cell trans-differentiation, whereas the lineage tracing results reported by (Ben-Othman et al., 2017) may reflect β cell neogenesis through a transient glucagon-expressing immature cell state, which is supported by their other experiments contained in their study that utilized Ngn3-CreER mice and Ngn3 shRNA. Although we found no indirect evidence of islet or β cell neogenesis, based on the size and number of islets and β cell clusters, we did not aim to test this possibility directly. Because evidence for human β cell neogenesis in adulthood is limited, inducing α-to-β cell trans-differentiation is a more attractive therapeutic option for treating diabetes, but our results, along with those of van der Meulen et al. (2018), suggest that stimulating the GABA pathway is not sufficient to cause direct trans-differentiation.
It is possible that the discrepant results observed between this study and those previously-reported may be due to the fact that different mouse strains were used. Other potential differences in mouse diet or housing conditions could also contribute, although this is less likely. It is possible that genetic or environmental modifiers affected response to artesunate and GABA treatment. However, if this is the case, it puts into question the universal applicability of GABA pathway agonists in treating diverse human patients with diabetes.
The difference in glucose tolerance results observed between artesunate-treated and DMSO control mice in our study most likely reflects the fact that the artesunate-treated mice gained significantly less weight than the three other groups during the three-month treatment period. The underlying cause of reduced weight gain in the artesunate-treated mice is unclear and may be due to reduced food or water intake related to drug administration, or off-target effects of artesunate in other tissues, such as fat. We did not observe increased plasma insulin concentrations in these mice compared to the other groups; in fact, the plasma insulin concentrations in the artesunate-treated mice were slightly lower. No specific effect of artesunate on glucose tolerance in non-diabetic mice has previously been reported, although streptozotocin-induced diabetic rats treated with the artemisinin artemether were described to have improved fasting glucose and glucose tolerance compared to untreated diabetic rats (Li et al., 2017). Conversely, GABA was reported to improve glucose tolerance and enhance glucose-stimulated insulin secretion in mice treated with 3 months of daily GABA injections, the same protocol used in this study, compared to saline-treated control mice (Ben-Othman et al., 2017). Our study did not reproduce these findings following the same drug exposure protocols as reported by the prior study. This discrepancy could possibly be the result of differences in animal housing conditions or differential responses by specific inbred mouse strains used by Ben-Othman and colleagues. Nevertheless, our results put into question the robustness of the proposed GABA-induced trans-differentiation protocol.
By direct lineage tracing of mature α cells in mice, following the same protocols used by (Li et al., 2017) and (Ben-Othman et al., 2017), we were unable to reproduce several of their findings. In conclusion, the data presented here do not support a role for GABA signaling or artemisinins in stimulating α-to-β cell trans-differentiation, increasing β cell number, or improving β cell function.
Limitations of Study
By design, this study only evaluated direct trans-differentiation of mature α cells into β cells. The study was not designed to analyze β cell neogenesis specifically, although we used indirect measurements to assess β cell and islet neogenesis. The mice used in these experiments, which we recently generated as a new and robust model for α cell lineage tracing, were from a mixed strain background different than that used by Ben-Othman et al., 2017. Other potential differences in animal housing conditions, food intake, and water intake may have also contributed to some of the discrepancies observed between our results and those of prior studies.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Klaus H. Kaestner (kaestner@pennmedicine.upenn.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Eight week old Gcg-CreERT2;Rosa-LSL-eYFP/+ male mice were used for all experiments. These mice were generated from v6.5 embryonic stem cells (Ackermann et al., 2017), which are a C57BL/6 and 129S4/SvJae hybrid cell line, and they were back-crossed to C57BL/6 for two or three generations prior to inbreeding to generate the experimental mice. Littermates were randomly assigned to the treatment or control group for each experiment, and the groups were housed separately during the treatment period. Mice were fed standard chow diet (Lab Diet 5010) throughout the study. All mouse protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
METHOD DETAILS
Tamoxifen Treatment
At 8 weeks of age, mice were given daily intraperitoneal injections of 4-hydroxy-tamoxifen (TAM) 0.1 mg/g body weight for 3 days. Tamoxifen was made fresh each day, mixed with corn oil at 20 mg/mL in a light-safe tube and rotated for 2 hours at room temperature prior to injection. Four weeks after the third tamoxifen injection, mice were allocated to the treatment or control groups.
Artesunate Treatment
Mice were given artesunate 1 mg/mL in drinking water, made fresh daily, for 3 months. Artesunate 100 mg was first dissolved in 2 mL dimethyl sulfoxide (DMSO), then 100 mL tap water was added. 2% DMSO in tap water was used in the control group.
GABA Treatment
Mice were given intraperitoneal injections of GABA 250 μg/kg body weight daily for 3 months. GABA was diluted in 0.9% sodium chloride at 0.025 mg/mL, made fresh weekly, aliquoted into light-safe tubes, and stored at room temperature. Equivalent volume saline injections were used in the control group.
Intraperitoneal Glucose Tolerance Tests
Mice were fasted for 16 hours overnight, then subjected to intraperitoneal glucose tolerance test (IPGTT) with dextrose 2 mg/g body weight in normal saline. Tail blood was used to measure glucose concentration with glucose meter at 0, 15, 30, 60, 90, and 120 minutes. Tail blood was also collected at 0 and 30 minutes for plasma insulin measurement. The blood was collected in heparinized tubes, which were temporarily stored on ice before centrifugation at 2,000 xg for 5 minutes at 4°C, after which the plasma supernatant was transferred to new tubes and stored at −20°C. Mice were fed at the end of the IPGTT, then killed the following day for pancreas isolation.
Insulin Enzyme-Linked Immunosorbent Assay (ELISA)
Insulin concentration was measured from 5 μL of each plasma sample collected at 0 and 30 minutes during IPGTT, following the manufacturer’s protocol for the Crystal Chem Ultra Sensitive Mouse Insulin ELISA Kit Low Range Assay.
Histology
Isolated pancreata were weighed and fixed in 4% paraformaldehyde overnight at 4°C, then washed in 1X phosphate-buffered saline (PBS) and either dehydrated in escalating ethanol series to xylene prior to embedding in paraffin, or immersed in 30% sucrose overnight at 4°C and embedded in optimum cutting temperature (OCT) compound prior to freezing for cryosectioning. Sections 5 μm thick and 50 μm apart were adhered to glass slides. Paraffin sections underwent antigen retrieval with 1X R Buffer A diluted in milli-Q water in a pressure cooker for 1 hour. Immunolabeling with anti-Cre and goat anti-Glucagon antibodies required cryosections. Sections were permeabilized with 1X PBS + 0.1% Tween-20 for 5 minutes at room temperature, then blocked with CAS-Block for 10 minutes at room temperature prior to incubation with primary antibodies in CAS-Block overnight at 4°C (anti-GFP 1:200, rabbit anti-Glucagon 1:200, anti-insulin 1:500, anti-Cre 1:10,000, goat anti-Glucagon 1:100, rabbit anti-Chromogranin A 1:500). After washing with 1X PBS + 0.1% Tween-20, sections were incubated with secondary antibodies (all 1:250) in CAS-Block at room temperature for 3 hours. Sections were then washed and incubated with DAPI (4’,6-diamidino-2-phenylindole) for 1 minute at room temperature to counterstain DNA prior to mounting with coverslips. Images were obtained using a Nikon Eclipse 80i widefield microscope with 40X objective and iVision software. At least 2 sections per mouse (range 2-12 sections) were analyzed, allowing for identification of approximately 1,000 YFP+ cells per mouse (range 821-1,564 cells).
QUANTIFICATION AND STATISTICAL ANALYSIS
Cell Counts and Islet Measurements
Images were analyzed using FIJI software. Individual channels overlaid with the DAPI channel were used for manual counting of YFP+ cells, then YFP+/Insulin+ cells, then YFP+/Insulin+/Glucagon+ cells. The Insulin channel was used for manual outlining and measurement of Insulin+ islet areas. Chromogranin A labeling was used to quantify islet number and islet mass.
Statistical Analyses
Data are presented as mean ± standard deviation. P < 0.05 was considered significant. Data were analyzed using Prism software. Statistical details for each experiment can be found in the figure legends. All experimental and control groups were designed to have 5 mice. One mouse allocated to the DMSO treatment group died following tamoxifen treatment. IPGTT results from one artesunate-treated mouse and from one GABA-treated mouse were excluded from the reported dataset, based on a technical complication resulting in failure of dextrose injection to increase plasma glucose concentration; these two samples were identified as outliers by the ROUT method with Q = 1%.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-Glucagon | Santa Cruz Biotechnology | Cat# sc-13091, RRID: AB_641026 |
| Goat polyclonal anti-Glucagon | Santa Cruz Biotechnology | Cat# sc-7780, RRID: AB_641025 |
| Goat polyclonal anti-GFP | Abcam | Cat# ab6673, RRID: AB_305643 |
| Guinea pig polyclonal anti-Insulin | Dako | Cat# A0564, RRID: AB_10013624 |
| Guinea pig polyclonal anti-Insulin | Invitrogen | Cat# 180067 |
| Rabbit polyclonal anti-Cre | Millipore | Cat# 69050, RRID: AB_11212994 |
| Rabbit polyclonal anti-Chromogranin A | ImmunoStar | Cat# 20085, RRID: AB_572227 |
| Donkey anti-goat Cy2-conjugated IgG (H+L) | Jackson ImmunoResearch Laboratories | Cat# 705-225-147, RRID: AB_2307341 |
| Donkey anti-rabbit Cy3-conjugated IgG (H+L) | Jackson ImmunoResearch Laboratories | Cat# 711-165-152, RRID: AB_2307443 |
| Donkey anti-guinea pig Cy5-conjugated IgG (H+L) | Jackson ImmunoResearch Laboratories | Cat# 706-175-148, RRID: AB_2340462 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Tamoxifen | Sigma-Aldrich | Cat# T5648 |
| Corn oil | Sigma-Aldrich | Cat# C8267 |
| Gamma-aminobutyric acid | Sigma-Aldrich | Cat# A5835 |
| Artesunate | Tokyo Chemical Industry | Cat# A2191 |
| Dimethyl Sulfoxide | Fisher Scientific | Cat# D128 |
| 0.9% Sodium chloride | Fisher Scientific | Cat# BP358-10 |
| DAPI | Sigma-Aldrich | Cat# D9542 |
| Dextrose | Fisher Scientific | Cat# BP350-1 |
| 32% Paraformaldehyde | Electron Microscopy Sciences | Cat# 15714 |
| 10X R Buffer A | Electron Microscopy Sciences | Cat# 62706-10 |
| Tween-20 | Fisher Scientific | Cat# BP337 |
| CAS-Block | Thermo Fisher Scientific | Cat# 008120 |
| Critical Commercial Assays | ||
| Ultra Sensitive Mouse Insulin ELISA Kit | Crystal Chem | Cat# 90080 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Gcg-CreERT2: B6;129S4-Gcgem1(Cre/ERT2)Khk/Mmjax | Ackermann et al., 2017 | JAX-042277, RRID:MMRRC_042277-JAX |
| Mouse: Rosa-LSL-eYFP: B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J | Jackson Laboratory | JAX-006148, RRID: IMSR_JAX:006148 |
| Software and Algorithms | ||
| Prism 6.0h | GraphPad | https://www.graphpad.com |
| iVision | BioVision | N/A |
| FIJI | http://fiji.sc | N/A |
| Photoshop CC 2017.0.1 | Adobe | N/A |
| Other | ||
| Breeze 2 glucometer | Bayer | N/A |
| Pressure Cooker | Electron Microscopy Sciences | Cat# 62700-10 |
ACKNOWLEDGMENTS
We thank the University of Pennsylvania Molecular Pathology and Imaging Core (supported by P30 DK050306 and P01 DK049210). This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants UC4 DK104119 and R01 DK088383 to KHK. The authors have no relevant conflicts of interest.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests
REFERENCES
- Ackermann AM, Wang Z, Schug J, Naji A, and Kaestner KH (2016). Integration of ATAC-seq and RNA-seq identifies human alpha cell and beta cell signature genes. Mol Metab 5, 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann AM, Zhang J, Heller A, Briker A, and Kaestner KH (2017). High-fidelity Glucagon-CreER mouse line generated by CRISPR-Cas9 assisted gene targeting. Mol Metab 6, 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Othman N, Vieira A, Courtney M, Record F, Gjernes E, Avolio F, Hadzic B, Druelle N, Napolitano T, Navarro-Sanz S, et al. (2017). Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-like Cell Neogenesis. Cell 168, 73–85 e11. [DOI] [PubMed] [Google Scholar]
- Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, Streeter PR, Naji A, Grompe M, and Kaestner KH (2013). Epigenomic plasticity enables human pancreatic alpha to beta cell reprogramming. J Clin Invest 123, 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy H, Gu X, Enge M, Dai X, Wang Y, Damond N, Downie C, Liu K, Wang J, Xing Y, et al. (2017). Converting Adult Pancreatic Islet alpha Cells into beta Cells by Targeting Both Dnmt1 and Arx. Cell Metab 25, 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, and Mansouri A (2009). The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 138, 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M, Gjernes E, Druelle N, Ravaud C, Vieira A, Ben-Othman N, Pfeifer A, Avolio F, Leuckx G, Lacas-Gervais S, et al. (2013). The Inactivation of Arx in Pancreatic α-Cells Triggers Their Neogenesis and Conversion into Functional β-Like Cells. PLoS Genet 9, e1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Casteels T, Frogne T, Ingvorsen C, Honore C, Courtney M, Huber KV, Schmitner N, Kimmel RA, Romanov RA, et al. (2017). Artemisinins Target GABAA Receptor Signaling and Impair alpha Cell Identity. Cell 168, 86–100 e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka TA, Kawashima S, Miyatsuka T, Sasaki S, Shimo N, Katakami N, Kawamori D, Takebe S, Herrera PL, Kaneto H, et al. (2017). Mafa Enables Pdx1 to Effectively Convert Pancreatic Islet Progenitors and Committed Islet alpha-Cells Into beta-Cells In Vivo. Diabetes 66, 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert RB, Kantz J, Misfeldt AA, Poffenberger G, Gannon M, Brissova M, and Powers AC (2012). Tamoxifen-Induced Cre-loxP Recombination Is Prolonged in Pancreatic Islets of Adult Mice. PloS one 7, e33529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, and Herrera PL (2010). Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 464, 1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen T, and Huising MO (2015). Role of transcription factors in the transdifferentiation of pancreatic islet cells. J Mol Endocrinol 54, R103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen T, Lee S, Noordeloos E, Donaldson CJ, Adams MW, Noguchi GM, Mawla AM, and Huising MO (2018). Artemether Does Not Turn Alpha Cells into Beta Cells. Cell Metab 27, 218–225 e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen T, Mawla AM, DiGruccio MR, Adams MW, Nies V, Dólleman S, Liu S, Ackermann AM, Cáceres E, Hunter AE, et al. (2017). Virgin Beta Cells Persist throughout Life at a Neogenic Niche within Pancreatic Islets. Cell Metabolism 25, 911–926.e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CL, Terry NA, Walp ER, Lee RA, and May CL (2013). Pancreatic α-Cell Specific Deletion of Mouse Arx Leads to α-Cell Identity Loss. PloS one 8, e66214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


