Abstract
The defining feature of the eukaryotic cell, the nucleus, is bounded by a double envelope. This envelope and the nuclear pores within it play a critical role in separating the genome from the cytoplasm. It also presents cells with a challenge. How are cells to remodel the nuclear compartment boundary during mitosis without compromising nuclear function? In the two billion years since the emergence of the first cells with a nucleus, eukaryotes have evolved a range of strategies to do this. At one extreme, the nucleus is disassembled upon entry into mitosis and then reassembled anew in the two daughter cells. At the other, cells maintain an intact nuclear compartment boundary throughout the division process. In this review, we discuss common features of the division process that underpin remodelling mechanisms, the topological challenges involved and speculate on the selective pressures that may drive the evolution of distinct modes of division.
Keywords: Mitosis, Nuclear envelope, Nuclear division, Nuclear pore complex, Lamina, Eukaryogenesis
All eukaryotes organise their genome within a nucleus, a secure data storage centre and transcription hub, that is separated from the cytoplasm by a nuclear envelope [1]. The outer membrane of this nuclear envelope (NE) is continuous with the endoplasmic reticulum (ER) and is connected to the inner membrane of the NE by a series of pores formed at sites of high membrane curvature. These nuclear pores are scaffolded by nuclear pore complexes (NPCs), massive structures (in humans, around 110 MDa comprising over a thousand subunits) that regulate the shuttling of soluble material between nuclear and cytoplasmic compartments and membrane traffic between the inner and outer membranes [2]. As a result, although physically continuous with the outer NE and the ER, the inner NE forms a distinct, metabolically active [3] compartment with a unique lipidome [4] and proteome [5,6], whose identity is determined at least in part by its close physical proximity to the DNA [7].
Competing theories have been proposed to explain how the NE evolved during eukaryogenesis from the symbiosis of an archaeal and a bacterial cell, both of which were devoid of internal membranes. Such topological models aim to explain the unique organisation and asymmetry of the NE [8]. Collectively, they can be classified into ‘outside-in’ models, which envision the nucleus arising as a specialised extension of internal ER membranes that were brought together at pores to generate a continuous NE, and ‘inside-out’ models, which view the NE as a remnant of the original folded archaeal plasma membrane [9]. Whatever the path of eukaryogenesis, 2 billion years later, modern eukaryotic organisms cannot survive without a nucleus [10].
Nevertheless, the presence of a nucleus presents cells with a challenge. Each time a cell divides, it must duplicate and split its nuclear compartment into two. Although the mechanism by which this is done remains one of the least well-understood aspects of division, decades of experiments in animal and fungal models [11] have suggested that there are two, fundamentally different, ways to remodel the NE during mitosis:
-
1.
Dismantle the nucleus and then reconstruct new compartments around the DNA when cells leave mitosis
-
2.
Divide the entire intact nuclear compartment into two.
These strategies have long been termed ‘open’ and ‘closed’ mitosis, respectively. In recent years, however, the value of this binary classification has been brought into question as it has become increasingly clear that the majority of eukaryotes engage in a process that lies somewhere between these two theoretical extremes [12, 13, 14]. In fact, truly ‘open’ and ‘closed’ topologies may not even exist (Figure 1). Thus, remnants of the NE appear to interact with the mitotic spindle in the most open mitoses [15], while canonical examples of closed mitosis appear to exhibit transient, local, ‘opening’ during the final act of nuclear division [16,17] (Figure 1). In addition, closely related species [18,19] and even different cell types within the same organism [20] can exhibit remarkable differences in the degree of nuclear ‘opening’ during mitosis, and perturbing single genes can have a major impact on mitotic strategy [19,21] (Figure 2). Taken together, these various data highlight the plasticity of NE remodelling, mirrored by the plasticity of the NE itself [22]. This makes it possible to leave behind the old questions of classification and focus instead on the fundamental structural challenges that cells must overcome to successfully duplicate their nuclear compartment during mitosis. In doing so, one would aim to identify universal features of the process that are conserved across eukaryotes and unique adaptations restricted to certain lineages.
Figure 1.
A schematic illustrating the full range of possible mitotic nuclear remodelling strategies, starting from completely open at the top to completely closed at the bottom. The compartment barrier is highlighted by labelling the nucleoplasm in blue and the cytoplasm in white; chromosomes are in dark grey and spindle microtubules in brown; other key cellular structures are labelled on the diagram. The two theoretical extremes have not been experimentally observed in any eukaryote to date. The blue bars indicate either local or global fenestration or ‘opening’ of the nuclear envelope, leading to a disruption of the barrier between the nucleoplasm and cytoplasm. In closed or semiclosed mitosis, the first local opening event allows the microtubule-nucleating centres to access the nucleoplasm; the second local opening drives nuclear division.
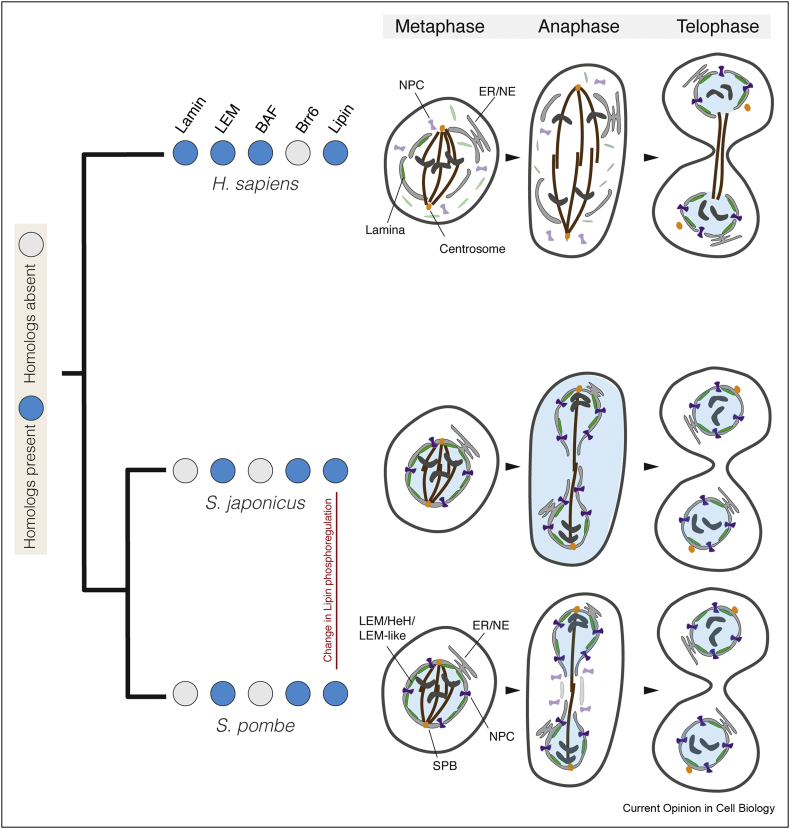
Figure 2.
Plasticity in nuclear remodelling across well-studied model systems. The compartment barrier is highlighted by labelling the nucleoplasm in blue and the cytoplasm in white; chromosomes are in dark grey and spindle microtubules in brown; other key cellular structures are labelled on the diagram. The presence (blue) or absence (grey) of homologues of an illustrative subset of the key protein or domain families involved in controlling nuclear remodelling is shown as a phylogenetic profile (filled circles) for each species. Differences in mitotic mode (closed or open) can be observed at large evolutionary distances, for example, between humans and fission yeast (illustrated by distance on the tree and low phylogenetic profile similarity), but also between closely related species, for example, S. pombe and S. japonicus (illustrated by proximity on the tree and high phylogenetic profile similarity). A divergence in lipin phosphorylation during mitosis [19] is thought to be responsible for the tearing of the nuclear envelope and loss of integrity in S. japonicus mitosis that is not observed during local NE breakdown [16,17] in its sister species S. pombe.
Prophase: compartment mixing
Eukaryotes generally rely on a microtubule-based spindle to segregate their chromosomes and cytoplasmic organelles [23] and, in many cases, to position the cleavage furrow [24]. Because microtubule-nucleating centres and the pool of tubulin itself play important roles in organising the interphase cytoplasm, the relevant proteins are excluded from the nucleus for most of the cell cycle [25]. This changes upon entry into mitosis, when tubulin and the microtubule-nucleating machinery need to gain access to the nucleus to construct a spindle. In all eukaryotes, this requires a loosening of the compartment barrier to enable a degree of mixing between the cytoplasm and nucleoplasm. Even in an open mitosis, this appears to be a tightly regulated process. Thus, tubulin is imported into the nucleus at the onset of NE disassembly [26,27], a process that is triggered by changes in NPC composition, structure and transport properties at the onset of prophase, downstream of the mitotic kinases CDK1 and PLK1 [28]. Importantly, this is a common feature of both open and closed mitoses [29].
If the microtubule-nucleating centre is not already embedded in the NE, as it is in budding yeast [30], the poles of the spindle or spindle microtubules must penetrate the NE through local or global envelope remodelling for them to gain access to the kinetochores of the condensed mitotic chromosomes. In an animal cell open mitosis, this is made possible through the phosphorylation and stepwise disassembly of NPCs and the NE [31] (Figure 2). In budding and fission yeasts, although this happens at different points in the cell cycle, the spindle pole body is imported through a transient pore in the NE in a poorly understood process [30] regulated by nucleoporins, SUN/KASH proteins [32] and the protein Brr6 [33] and likely requires subsequent envelope sealing (Figure 2).
Metaphase: decoupling the DNA and spindle from the membrane
In metaphase, the DNA must become completely uncoupled from the overlying NE to freely segregate and partition into the two daughter nuclei at the end of mitosis [34]. This is the case in all eukaryotes. If this does not happen, such as when the function of NE-localised REEP proteins is compromised [35], serious chromosome segregation, cell division and nuclear morphology defects ensue [34]. The failure of DNA to disengage from nuclear pore complexes can also lead to its mis-segregation [36].
In a closed or partially closed mitosis, the architecture of the NE must somehow persist in the absence of attachments to DNA. Conversely, during an open or semiopen mitosis, the loss of contacts between the DNA and the overlying inner NE, together with loss of laminar integrity [37], likely contributes to the breakdown in coherence of the double membrane compartment barrier. It is important that the ensuing compartment mixing does not permit cytoplasmic structures, including membranes and organelles, to get in the way of spindle assembly or chromosome separation. Membranes derived from the NE and ER [31], as well as a poorly understood ‘proteinaceous matrix’ [38], function to ensure that cells retain a privileged nuclear space despite having lost their continuous NE. This has been observed in dividing HeLa cells (Figure 2) — a classic model of open mitosis — and in a variety of other systems without a fully intact nuclear compartment [39].
Anaphase: partitioning
In both open and closed mitosis, once the chromosomes are properly attached to spindle microtubules and the spindle assembly checkpoint is satisfied, the clipping of the cohesive bridges that connect sister chromatids initiates anaphase [40]. Sister chromatids are then free to move to opposite poles of the spindle through the action of microtubule disassembly and the movement of kinetochore-anchored dynein motors towards the spindle poles, as spindle poles separate [23].
Importantly, although this process separates the DNA, cells must also find a way of coupling chromosome segregation to duplication of the nuclear compartment. Thus, as cells exit mitosis, the double membrane of the NE must be split into two without compromising the integrity of the ER lumen, which is topologically equivalent to the cell exterior. This requires NE expansion driven by ER lipid synthesis [41] — without which the NE can rupture — as seen in the fission yeast S. japonicus, where it leads to the transient loss of the compartment barrier at the site of division [18,19] (Figure 2). In most cells, however, division of the nuclear compartment is triggered by the disassembly of nuclear pores — sites at which the inner and outer NE wrap around to meet each other. This leads to loss of the nuclear compartment boundary and to fenestration of the envelope. In animal cells, NPC disassembly is initiated at the onset of mitosis [42,43], as it is in the unusual partially open mitosis of Ustilago maydis [44,45]. In the closed mitosis of the fission yeast Schizosaccharomyces pombe, where spindle elongation drives the formation of intact daughter nuclei connected only by a narrow bridge, nuclear division requires remodelling of the NE at a single site — the bridge midzone [16]. At the centre of the bridge, a subset of NPCs disassemble in a process analogous to that occurring at prophase in animal cells, fenestrating the membrane and exposing the spindle to the cytoplasm [16,17] as part of a local NE breakdown (Figure 2). As this makes clear, it is the timing and localisation of this NPC disassembly that determine whether a mitosis is more open or closed. However, it is not yet known how these differences are regulated.
Telophase and cytokinesis: compartment demixing
Once the continuous NE has been compromised, the ER and/or NE must be segregated between daughter cells at cytokinesis [46,47] — a process that requires the ER to be reshaped [48]. The compartment barrier must then be re-established. In an open mitosis, where there is extensive mixing of the nucleoplasm and cytoplasm, this requires the active exclusion of the cytoplasm — achieved in part by the surfactant-like Ki-67 at the surface of chromosomes [49]. As nuclei reform, traffic across nuclear pores, driven by Ran-GTP, must then do the work of demixing the nucleoplasm and cytoplasm [50]. In S. pombe closed mitosis, the compartment barrier is maintained during local NE breakdown at least partially by the inner nuclear component Les1 [16], limiting the portion of NE that needs to be sealed to the tips of the stalk membranes and sites of spindle pole body insertions.
Across all systems studied thus far, this process of membrane sealing is a result of the action of the ESCRTIII system acting in coordination with the inner NE protein Lem2/LEM2 [∗51, ∗52, 53, 54, 55, ∗56]. In mammalian cells, ESCRTIII polymers and LEM2 act together with the microtubule disassembly factor Spastin to remodel and seal the envelope as spindle microtubules are disassembled [54,55]. In Drosophila cells, this entire process has been reported to proceed from the nuclear poles towards the midcell [57]. At the same time, as the nuclear compartment is re-established, functional NPCs must be assembled and inserted into the double envelope [58]. In many systems, this process is coordinated with the reassembly of the nuclear lamina [59,60], the disassembly of the spindle and the initiation of cytokinesis — a careful choreography that can be thrown into disarray if chromosomes have not segregated properly [61,62].
Outlook
The discovery of common themes and molecular mechanisms (ESCRTIII, NPC disassembly) uniting open and closed nuclear remodelling strategies makes it easier to define the conserved aspects of the process of nuclear division. It also raises its own questions. If the regulatory differences between open and closed mitosis are relatively superficial, why do cells choose one remodelling strategy over the other? One major difficulty in addressing this question lies in interpreting the likely selective pressures arising from the conflicting roles of the nucleus during interphase and mitosis.
The interphase NE is essential for eukaryotic cell survival. It is the barrier that separates the cytoplasm and nucleoplasm and that enables RNA processing before translation. Thus, even a transient loss of the nuclear compartment boundary in interphase can have disastrous consequences for a eukaryotic cell, for example, by exposing the DNA to cytoplasmic nucleases [63].
At the same time, the nuclear compartment must be duplicated at division — a process that, as detailed earlier, relies on breaking the compartment barrier, at least transiently. Because of this, cells with an open mitosis must ensure that the DNA is adequately protected from cytoplasmic nucleases when the compartment boundary dissolves and must quickly re-establish the compartment boundary upon mitotic exit. Furthermore, while chromosome segregation and cell division may be relatively easy to coordinate in an open mitosis, as cells exit mitosis, the NE and NPCs must now be reassembled around the segregated DNA without losing stray chromosomes [59,64]. This would seem to be even more challenging in syncytia, where the ER must be partitioned between a large number of nuclei, something that may require additional mechanisms to limit contact between spindles [65].
Conversely, although freed from the constraints imposed by compartment mixing, cells undergoing a closed mitosis must ensure that the polarity and structure of the NE is maintained even in the absence of contacts between the chromatin and the NE and NPCs. Furthermore, the NE in cells undergoing a closed mitosis has to survive contortions in shape, area and shear as nuclei are remodelled to give rise to two daughter nuclei [11]. This in turn may constrain the size of the spindle. If so, one would expect an increase in genome size to be associated with a move to a more open mitosis. In addition, cells undergoing a closed mitosis have to coordinate nuclear division with organelle segregation and cytokinesis, a feat that might involve complex cross-compartment choreography — as is the case in budding yeast cells that require additional machinery to ensure that the anaphase spindle bridges the bud neck beforecell division [66]. Finally, even in cases of closed mitosis, the nucleus must be transiently and locally permeabilised either once or twice — first to insert the microtubule organising centre into the envelope, if it is not already embedded in the NE, and then again to actually split the nucleus — all the while keeping the nuclear compartment intact and insulated from the local permeabilisation process. Thus, the benefits of a closed mitosis may not be as great as were once imagined.
Speculatively, then, a need for greater nuclear autonomy and protection from cytoplasmic threats could shift the balance in favour of a closed mitosis. In contrast, an open mitosis would offer an easier way to coordinate chromosome segregation with the segregation of cytoplasmic organelles and cytokinesis, while also providing additional degrees of freedom with respect to spindle geometry and genome size.
Understanding how such selection pressures have acted to shape the mitotic strategies of living species will depend on identifying organisms in which the modes of nuclear division are flexible and depend on cell state (e.g., developmental stage or environmental conditions) and/or on reconstructing the evolutionary trajectories that led up to specific modes of nuclear division. In the latter case, this will require a more comprehensive description of nuclear remodelling mechanisms across poorly studied eukaryotes. Based upon the current data, although a change in the mode of nuclear division appears straightforward to trigger or evolve [12], in all known examples, this appears to occur in one direction (closed to open). In these cases, it will be interested to determine how cells then compensate for the additional compartment mixing — something that can be directly tested. While this might suggest an early origin for closed mitosis, the case is far from clear [9].
While such questions can be hard to answer, gaining a clearer picture of the likely evolutionary history of the nuclear compartment and nuclear division should be seen as much more than an exercise in the study of the history of life on earth. A better understanding of the evolution of nuclear division would be expected to have far-reaching implications for biology today: for example, by shedding light on the prevalence of syncytial divisions, by identifying ways of selectively killing parasites such as Plasmodium and Giardia which rely on closed nuclear division [67,68] and understanding the sensitivity of cells to transient nuclear rupture or delayed nuclear reformation at division. Fortunately, given recent advances in microscopy (including both live cell imaging and electron tomography) and in sampling the diversity of eukaryotic life, we might not be that far from a deeper understanding of the origins and core functional roles of the one organelle that all eukaryotes share.
Author contributions
Both authors contributed equally to the conceptualisation, content and drafting of this review.
Conflict of interest statement
Nothing declared.
Acknowledgements
The authors would like to thank Martin Raff, Ishier Raote, Eelco Tromer, Pedro Pereira, Felix Mikus, Hiral Shah, Agathe Chaigne and Siân Culley for helpful feedback on this manuscript. GD and BB would like to acknowledge University College London, the Medical Research Council and the European Molecular Biology Laboratory for core support, as well as the Wellcome Trust (203276/Z/16/Z).
This review comes from a themed issue on Cell Nucleus
Edited by Jane Skok and Daniel Gerlich
Contributor Information
Gautam Dey, Email: gautam.dey@embl.de.
Buzz Baum, Email: bbaum@mrc-lmb.cam.ac.uk.
References
- De Magistris P., Antonin W. The dynamic nature of the nuclear envelope. Curr Biol. 2018;28:R487–R497. doi: 10.1016/j.cub.2018.01.073. [DOI] [PubMed] [Google Scholar]; Excellent recent review of nuclear envelope dynamics, focussing on how cells solve the various topological challenges of mitosis as well as NPC insertion and assembly by remodelling the nuclear envelope.
- 2.Hampoelz B., Andres-Pons A., Kastritis P., Beck M. Structure and assembly of the nuclear pore complex. Annu Rev Biophys. 2019;48:515–536. doi: 10.1146/annurev-biophys-052118-115308. [DOI] [PubMed] [Google Scholar]
- Romanauska A., Köhler A. The inner nuclear membrane is a metabolically active territory that generates nuclear lipid droplets. Cell. 2018;174:700–715. doi: 10.1016/j.cell.2018.05.047. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]; First demonstration of a unique metabolic activity traced to the inner nuclear envelope: enzymes directed to the inner nuclear envelope drive the synthesis of lipid droplets through lipid transfer regulated by Seipins.
- Bahmanyar S., Schlieker C. Lipid and protein dynamics that shape nuclear envelope identity. Mol Biol Cell. 2020;31:1315–1323. doi: 10.1091/mbc.E18-10-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]; Timely review of recent advances in understanding lipid and protein dynamics that distinguish the nuclear envelope from the rest of the endoplasmic reticulum.
- Smoyer C.J., Jaspersen S.L. Patrolling the nucleus: inner nuclear membrane-associated degradation. Curr Genet. 2019 doi: 10.1007/s00294-019-00971-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Specific proteasomal activity clears misfolded and mis-localised proteins from the inner nuclear envelope and maintains the integrity of the nuclear proteome. A recent review focussed mostly on findings in yeast.
- 6.Smoyer C.J., Katta S.S., Gardner J.M., Stoltz L., McCroskey S., Bradford W.D., McClain M., Smith S.E., Slaughter B.D., Unruh J.R. Analysis of membrane proteins localizing to the inner nuclear envelope in living cells. JCB (J Cell Biol) 2016;215:575–590. doi: 10.1083/jcb.201607043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerreiro I., Kind J. Spatial chromatin organization and gene regulation at the nuclear lamina. Curr Opin Genet Dev. 2019;55:19–25. doi: 10.1016/j.gde.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baum D.A. A comparison of autogenous theories for the origin of eukaryotic cells. Am J Bot. 2015;102:1954–1965. doi: 10.3732/ajb.1500196. [DOI] [PubMed] [Google Scholar]
- 9.Baum D.A., Baum B. An inside-out origin for the eukaryotic cell. BMC Biol. 2014;12:76. doi: 10.1186/s12915-014-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lusk C.P., King M.C. The nucleus: keeping it together by keeping it apart. Curr Opin Cell Biol. 2017;44:44–50. doi: 10.1016/j.ceb.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ungricht R., Kutay U. Mechanisms and functions of nuclear envelope remodelling. Nat Rev Mol Cell Biol. 2017;18:229–245. doi: 10.1038/nrm.2016.153. [DOI] [PubMed] [Google Scholar]
- 12.Makarova M., Oliferenko S. Mixing and matching nuclear envelope remodeling and spindle assembly strategies in the evolution of mitosis. Curr Opin Cell Biol. 2016;41:43–50. doi: 10.1016/j.ceb.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Souza C.P.C., Osmani S.A. Mitosis, not just open or closed. Eukaryot Cell. 2007;6:1521–1527. doi: 10.1128/EC.00178-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sazer S., Lynch M., Needleman D. Deciphering the evolutionary history of open and closed mitosis. Curr Biol: CB. 2014;24:R1099–R1103. doi: 10.1016/j.cub.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson V.J., Jafferali M.H., Vijayaraghavan B., Figueroa R.A., Hallberg E. Mitotic spindle assembly and γ-tubulin localisation depend on the integral nuclear membrane protein Samp1. J Cell Sci. 2018:131. doi: 10.1242/jcs.211664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey G., Culley S., Curran S., Schmidt U., Henriques R., Kukulski W., Baum B. Closed mitosis requires local disassembly of the nuclear envelope. Nature. 2020;585:119–123. doi: 10.1038/s41586-020-2648-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; First demonstration of local nuclear envelope breakdown to divide nuclei at the end of fission yeast mitosis, and the identification of a protein that maintains the integrity of daughter nuclei during the process of local NEB. See also (17).
- 17.Expósito-Serrano M., Sánchez-Molina A., Gallardo P., Salas-Pino S., Daga R.R. Selective nuclear pore complex removal drives nuclear envelope division in fission yeast. Curr Biol. 2020;30:3212–3222. doi: 10.1016/j.cub.2020.05.066. e2. [DOI] [PubMed] [Google Scholar]
- 18.Gu Y., Yam C., Oliferenko S. Divergence of mitotic strategies in fission yeasts. Nucleus-Austin. 2012;3:220–225. doi: 10.4161/nucl.19514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makarova M., Gu Y., Chen J.-S., Beckley J.R., Gould K.L., Oliferenko S. Temporal regulation of Lipin activity diverged to account for differences in mitotic programs. Curr Biol. 2016;26:237–243. doi: 10.1016/j.cub.2015.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsani K.R., Karess R.E., Dostatni N., Doye V. In vivo dynamics of Drosophila nuclear envelope components. MBoC. 2008;19:3652–3666. doi: 10.1091/mbc.E07-11-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemudupati M., Johns M., Osmani S.A. The mode of mitosis is dramatically modified by deletion of a single nuclear pore complex gene in Aspergillus nidulans. Fungal Genet Biol. 2019;130:72–81. doi: 10.1016/j.fgb.2019.04.010. [DOI] [PubMed] [Google Scholar]; Disrupting the Nup84-120 complex in Aspergillus has a dramatic effect on the morphology of the nuclear envelope during mitosis and the site of nuclear division.
- 22.Koreny L., Field M.C. Ancient eukaryotic origin and evolutionary plasticity of nuclear lamina. Genome Biology and Evolution. 2016;8:2663–2671. doi: 10.1093/gbe/evw087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vukušić K., Buđa R., Tolić I.M. Force-generating mechanisms of anaphase in human cells. J Cell Sci. 2019;132 doi: 10.1242/jcs.231985. [DOI] [PubMed] [Google Scholar]
- 24.Kotak S. Mechanisms of spindle positioning: lessons from worms and mammalian cells. Biomolecules. 2019;9:80. doi: 10.3390/biom9020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzerová K., Bellinvia E., Martinek J., Sikorová L., Dostál V., Libusová L., Bokvaj P., Fischer L., Schmit A.C., Nick P. Tubulin is actively exported from the nucleus through the Exportin1/CRM1 pathway. Sci Rep. 2019;9:5725. doi: 10.1038/s41598-019-42056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Residual tubulin in the nucleus, trapped by a reforming nuclear envelope at mitotic exit, must be exported into the cytoplasm – here shown in a recent study in tobacco plants.
- 26.Okada N., Sato M. Spatiotemporal regulation of nuclear transport machinery and microtubule organization. Cells. 2015;4:406–426. doi: 10.3390/cells4030406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi H., Kimura K., Kimura A. Localized accumulation of tubulin during semi-open mitosis in the Caenorhabditis elegans embryo. Mol Biol Cell. 2012;23:1688–1699. doi: 10.1091/mbc.E11-09-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linder M.I., Köhler M., Boersema P., Weberruss M., Wandke C., Marino J., Ashiono C., Picotti P., Antonin W., Kutay U. Mitotic disassembly of nuclear pore complexes involves CDK1- and PLK1-mediated phosphorylation of key interconnecting nucleoporins. Dev Cell. 2017;43:141–156. doi: 10.1016/j.devcel.2017.08.020. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Souza C.P.C., Osmani A.H., Hashmi S.B., Osmani S.A. Partial nuclear pore complex disassembly during closed mitosis in Aspergillus nidulans. Curr Biol. 2004;14:1973–1984. doi: 10.1016/j.cub.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 30.Jaspersen S.L., Ghosh S. Nuclear envelope insertion of spindle pole bodies and nuclear pore complexes. Nucleus. 2012;3:226–236. doi: 10.4161/nucl.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellenberg J., Siggia E.D., Moreira J.E., Smith C.L., Presley J.F., Worman H.J., Lippincott-Schwartz J. Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J Cell Biol. 1997;138:1193–1206. doi: 10.1083/jcb.138.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Gardner J.M., Yu Z., Smith S.E., McKinney S., Slaughter B.D., Unruh J.R., Jaspersen S.L. Yeast centrosome components form a noncanonical LINC complex at the nuclear envelope insertion site. J Cell Biol. 2019;218:1478–1490. doi: 10.1083/jcb.201809045. [DOI] [PMC free article] [PubMed] [Google Scholar]; Molecular analysis of the toroidal structure that enables the insertion of the budding yeast spindle pole body into the locally fenestrated nuclear envelope.
- 33.Tamm T., Grallert A., Grossman E.P.S., Alvarez-Tabares I., Stevens F.E., Hagan I.M. Brr6 drives the Schizosaccharomyces pombe spindle pole body nuclear envelope insertion/extrusion cycle. J Cell Biol. 2011;195:467–484. doi: 10.1083/jcb.201106076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion L., Pawar S., Luithle N., Ungricht R., Kutay U. Dissociation of membrane–chromatin contacts is required for proper chromosome segregation in mitosis. Mol Biol Cell. 2019;30:427–440. doi: 10.1091/mbc.E18-10-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]; Elegant study in human cells that uses a synthetic membrane-chromatin tether to show that a failure to dissociate the chromatin from the NE leads to impaired chromosome segregation, cytokinesis and nuclear morphology defects.
- 35.Schlaitz A.-L., Thompson J., Wong C.C.L., Yates J.R., Heald R. REEP3/4 ensure endoplasmic reticulum clearance from metaphase chromatin and proper nuclear envelope architecture. Dev Cell. 2013;26:315–323. doi: 10.1016/j.devcel.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denoth-Lippuner A., Krzyzanowski M.K., Stober C., Barral Y. Role of SAGA in the asymmetric segregation of DNA circles during yeast ageing. eLife. 2014;3 doi: 10.7554/eLife.03790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wesolowska N., Avilov I., Machado P., Geiss C., Kondo H., Mori M., Lénárt P. Actin assembly ruptures the nuclear envelope by prying the lamina away from nuclear pores and nuclear membranes in starfish oocytes. eLife. 2020;9 doi: 10.7554/eLife.49774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao C., Rath U., Maiato H., Sharp D., Girton J., Johansen K.M., Johansen J. A nuclear-derived proteinaceous matrix embeds the microtubule spindle apparatus during mitosis. Mol Biol Cell. 2012;23:3532–3541. doi: 10.1091/mbc.E12-06-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schweizer N., Pawar N., Weiss M., Maiato H. An organelle-exclusion envelope assists mitosis and underlies distinct molecular crowding in the spindle region. J Cell Biol. 2015;210:695–704. doi: 10.1083/jcb.201506107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhlmann F., Lottspeich F., Nasmyth K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature. 1999;400:37–42. doi: 10.1038/21831. [DOI] [PubMed] [Google Scholar]
- 41.Takemoto A., Kawashima S.A., Li J.-J., Jeffery L., Yamatsugu K., Elemento O., Nurse P. Nuclear envelope expansion is crucial for proper chromosomal segregation during a closed mitosis. J Cell Sci. 2016;129:1250–1259. doi: 10.1242/jcs.181560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dultz E., Zanin E., Wurzenberger C., Braun M., Rabut G., Sironi L., Ellenberg J. Systematic kinetic analysis of mitotic dis- and reassembly of the nuclear pore in living cells. J Cell Biol. 2008;180:857–865. doi: 10.1083/jcb.200707026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lénárt P., Rabut G., Daigle N., Hand A.R., Terasaki M., Ellenberg J. Nuclear envelope breakdown in starfish oocytes proceeds by partial NPC disassembly followed by a rapidly spreading fenestration of nuclear membranes. J Cell Biol. 2003;160:1055–1068. doi: 10.1083/jcb.200211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Straube A., Weber I., Steinberg G. A novel mechanism of nuclear envelope break-down in a fungus: nuclear migration strips off the envelope. EMBO J. 2005;24:1674–1685. doi: 10.1038/sj.emboj.7600644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theisen U., Straube A., Steinberg G. Dynamic rearrangement of nucleoporins during fungal “open” mitosis. MBoC. 2008;19:1230–1240. doi: 10.1091/mbc.E07-02-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poteryaev D., Squirrell J.M., Campbell J.M., White J.G., Spang A. Involvement of the actin cytoskeleton and homotypic membrane fusion in ER dynamics in Caenorhabditis elegans. MBoC. 2005;16:2139–2153. doi: 10.1091/mbc.E04-08-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang D., Vjestica A., Oliferenko S. The cortical ER network limits the permissive zone for actomyosin ring assembly. Curr Biol. 2010;20:1029–1034. doi: 10.1016/j.cub.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 48.Kumar D., Golchoubian B., Belevich I., Jokitalo E., Schlaitz A.-L. REEP3 and REEP4 determine the tubular morphology of the endoplasmic reticulum during mitosis. MBoC. 2019;30:1377–1389. doi: 10.1091/mbc.E18-11-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuylen-Haering S., Petrovic M., Hernandez-Armendariz A., Schneider M.W.G., Samwer M., Blaukopf C., Holt L.J., Gerlich D.W. Chromosome clustering by Ki-67 excludes cytoplasm during nuclear assembly. Nature. 2020;587:285–290. doi: 10.1038/s41586-020-2672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent paper that fills a key gap in our understanding of how cells carrying out an open mitosis re-establish the nucleocytoplasmic compartment barrier. The surfactant-like protein Ki-67 coats and clusters chromosomes as cells exit mitosis, driving cytoplasmic components out of the reforming nucleus.
- 50.Wente S.R., Rout M.P. The nuclear pore complex and nuclear transport. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper G.H., Sprenger S., Teis D., Oliferenko S. ESCRT-III/Vps4 controls heterochromatin-nuclear envelope attachments. Dev Cell. 2020;53:27–41. doi: 10.1016/j.devcel.2020.01.028. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Along with (52–56), this paper demonstrates a role for the ESCRTIII complex and the inner nuclear protein Lem2/LEM2 in sealing holes in the nuclear envelope at the end of mitosis. This work was carried out in fission yeast; others have demonstrated similar phenotypes in mammalian cells.
- von Appen A., LaJoie D., Johnson I.E., Trnka M.J., Pick S.M., Burlingame A.L., Ullman K.S., Frost A. LEM2 phase separation promotes ESCRT-mediated nuclear envelope reformation. Nature. 2020;582:115–118. doi: 10.1038/s41586-020-2232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; The ability of the LEM2 protein to phase separate in contact with microtubules plays a key role in ESCRTIII nuclear envelope resealing.
- 53.Gu M., LaJoie D., Chen O.S., von Appen A., Ladinsky M.S., Redd M.J., Nikolova L., Bjorkman P.J., Sundquist W.I., Ullman K.S. LEM2 recruits CHMP7 for ESCRT-mediated nuclear envelope closure in fission yeast and human cells. Proc Natl Acad Sci Unit States Am. 2017;114:E2166–E2175. doi: 10.1073/pnas.1613916114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olmos Y., Perdrix-Rosell A., Carlton J.G. Membrane binding by CHMP7 coordinates ESCRT-III-dependent nuclear envelope reformation. Curr Biol. 2016;26:2635–2641. doi: 10.1016/j.cub.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vietri M., Schink K.O., Campsteijn C., Wegner C.S., Schultz S.W., Christ L., Thoresen S.B., Brech A., Raiborg C., Stenmark H. Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature. 2015;522:231–235. doi: 10.1038/nature14408. [DOI] [PubMed] [Google Scholar]
- Penfield L., Shankar R., Szentgyörgyi E., Laffitte A., Mauro M.S., Audhya A., Müller-Reichert T., Bahmanyar S. Regulated lipid synthesis and LEM2/CHMP7 jointly control nuclear envelope closure. J Cell Biol. 2020:219. doi: 10.1083/jcb.201908179. [DOI] [PMC free article] [PubMed] [Google Scholar]; The ability of ESCRTIII proteins to seal holes in the nuclear envelope depends also on coupled lipid synthesis, shown here in C. elegans oocytes.
- 57.Roubinet C., White I.J., Baum B. Asymmetric nuclear division of neural stem cells contributes to the formation of sibling nuclei with different identities. bioRxiv. 2020 doi: 10.1101/2020.08.29.272724. [DOI] [Google Scholar]
- 58.Otsuka S., Steyer A.M., Schorb M., Hériché J.-K., Hossain M.J., Sethi S., Kueblbeck M., Schwab Y., Beck M., Ellenberg J. Postmitotic nuclear pore assembly proceeds by radial dilation of small membrane openings. Nat Struct Mol Biol. 2018;25:21–28. doi: 10.1038/s41594-017-0001-9. [DOI] [PubMed] [Google Scholar]
- 59.Liu S., Kwon M., Mannino M., Yang N., Renda F., Khodjakov A., Pellman D. Nuclear envelope assembly defects link mitotic errors to chromothripsis. Nature. 2018;561:551–555. doi: 10.1038/s41586-018-0534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Pellman D. The coordination of nuclear envelope assembly and chromosome segregation in metazoans. Nucleus. 2020;11:35–52. doi: 10.1080/19491034.2020.1742064. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lagging chromosomes disrupt the coordination between nuclear and cell division; animal cells assemble defective nuclear envelopes into micronuclei that house mis-segregating chromosomes, driving catastrophic DNA damage; see also (59).
- 61.Norden C., Mendoza M., Dobbelaere J., Kotwaliwale C.V., Biggins S., Barral Y. The NoCut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell. 2006;125:85–98. doi: 10.1016/j.cell.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 62.Mendoza M., Norden C., Durrer K., Rauter H., Uhlmann F., Barral Y. A mechanism for chromosome segregation sensing by the NoCut checkpoint. Nat Cell Biol. 2009;11:477–483. doi: 10.1038/ncb1855. [DOI] [PubMed] [Google Scholar]
- 63.Raab M., Gentili M., Belly H de, Thiam H.-R., Vargas P., Jimenez A.J., Lautenschlaeger F., Voituriez R., Lennon-Duménil A.-M., Manel N. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science. 2016;352:359–362. doi: 10.1126/science.aad7611. [DOI] [PubMed] [Google Scholar]
- 64.Samwer M., Schneider M.W.G., Hoefler R., Schmalhorst P.S., Jude J.G., Zuber J., Gerlich D.W. DNA cross-bridging shapes a single nucleus from a set of mitotic chromosomes. Cell. 2017;170:956–972. doi: 10.1016/j.cell.2017.07.038. e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bobinnec Y., Marcaillou C., Morin X., Debec A. Dynamics of the endoplasmic reticulum during early development of Drosophila melanogaster. Cell Motil. 2003;54:217–225. doi: 10.1002/cm.10094. [DOI] [PubMed] [Google Scholar]
- 66.Varshney N., Sanyal K. Nuclear migration in budding yeasts: position before division. Curr Genet. 2019;65:1341–1346. doi: 10.1007/s00294-019-01000-x. [DOI] [PubMed] [Google Scholar]
- 67.White M.W., Suvorova E.S. Apicomplexa cell cycles: something old, borrowed, lost, and new. Trends Parasitol. 2018;34:759–771. doi: 10.1016/j.pt.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harding C.R., Frischknecht F. The riveting cellular structures of apicomplexan parasites. Trends Parasitol. 2020 doi: 10.1016/j.pt.2020.09.001. [DOI] [PubMed] [Google Scholar]