Abstract
Microplastics (MPs) with sizes < 5 mm are found in various compositions, shapes, morphologies, and textures that are the major sources of environmental pollution. The fraction of MPs in total weight of plastic accumulation around the world is predicted to be 13.2% by 2060. These micron-sized MPs are hazardous to marine species, birds, animals, soil creatures and humans due to their occurrence in air, water, soil, indoor dust and food items. The present review covers discussions on the damaging effects of MPs on the environment and their removal techniques including biodegradation, adsorption, catalytic, photocatalytic degradation, coagulation, filtration and electro-coagulation. The main techniques used to analyze the structural and surface changes such as cracks, holes and erosion post the degradation processes are FTIR and SEM analysis. In addition, reduction in plastic molecular weight by the microbes implies disintegration of MPs. Adsorptive removal by the magnetic adsorbent promises complete elimination while the biodegradable catalysts could remove 70–100% of MPs. Catalytic degradation via advanced oxidation assisted by or radicals generated by peroxymonosulfate or sodium sulfate are also adequately covered in addition to photocatalysis. The chemical methods such as sol–gel, agglomeration, and coagulation in conjunction with other physical methods are discussed concerning the drinking water/wastewater/sludge treatments. The efficacy, merits and demerits of the currently used removal approaches are reviewed that will be helpful in developing more sophisticated technologies for the complete mitigation of MPs from the environment.
Keywords: Microplastics, Biodegradation, Adsorption, Photocatalytic degradation, Physical, Electrocoagulation
1. Introduction
Plastics are synthetic materials prepared from organic polymers such as polyethylene, polyvinylchloride (PVC), polyethylene terephthalate (PET), and nylon that are generally treated with chemical additives to transform them into useful plastics [1]. The occurrence of plastics is ubiquitous in our routine life, such as combs, water-bottles, shopping bags, even complex items like airplanes and spaceships. Plastics comprise synthetic or semi-synthetic organics that molded into objects of various shapes and sizes. Resistance towards moisture, light, and temperature, in addition to cost-effectiveness, durability, and ease of manufacture, are the significant factors in the primary usage of plastics for various purposes [2].
Modern society is witnessing a steep escalation in global population along with rapid urbanization [3–6], leading to several societal and environmental challenges such as increasing energy demand, environmental pollution, and global warming [7–16]. The annual global production of plastics is around 150 million tons [17], and by around 2060, the plastic build-up in the environment is predicted to reach 155 to 265 million tons [18]. The prevalent use of plastics leads to huge volumes of debris, and around 12 billion metric tons of plastic wastes may possibly be generated by 2050 if this growth rate is continued [19], leading to substantial environmental pollution issues. Plastics are recyclable materials but only < 5% are recovered [20]. The plastic debris gets accumulated in rivers, oceans, and landfills, leading to massive damage to water, soil, and air and causing a threat to living beings [21,22].
MPs are the particles of synthetic organic polymers having a size of < 5 mm have emerged as dangerous pollutants [23,24] and this topic has caught the attention of researchers in the eighties and nineties [25,26]. Lately, much attention has been paid to MPs and their related health impacts [27–36]. MPs of diverse compositions include conventional polymers such as polyethylene, acrylics, polyamides, nylon, polyesters, polypropylene, polystyrene, and polyesters [37] along with some specialized industrial polymers [38,39], but the fraction of polyethylene is much higher [40]. MPs have varying shapes and morphologies such as fibers, foils, films, foams, sheets, fragments, pellets, and spheres [37,41,42], all of which are found in the environment reaching from land to air to aquatic systems.
MPs can be classified according to their sources. The primary MPs are deliberately manufactured with the sizes in micron-scale range, which are used in the preproduction pellets, air blasting, granulates, and microbeads for cosmetic and personal care products such as scrub and toothpaste. The secondary MPs arise from the fragmentation (weathering, photolysis, abrasion or microbial disintegration) of macro-plastics in the land and aquatic surroundings such as discarded plastics, fishing nets, urban discharge and wastewater/drinking water treatment plants effluents as shown in Fig. 1 [43–45].
Fig. 1.
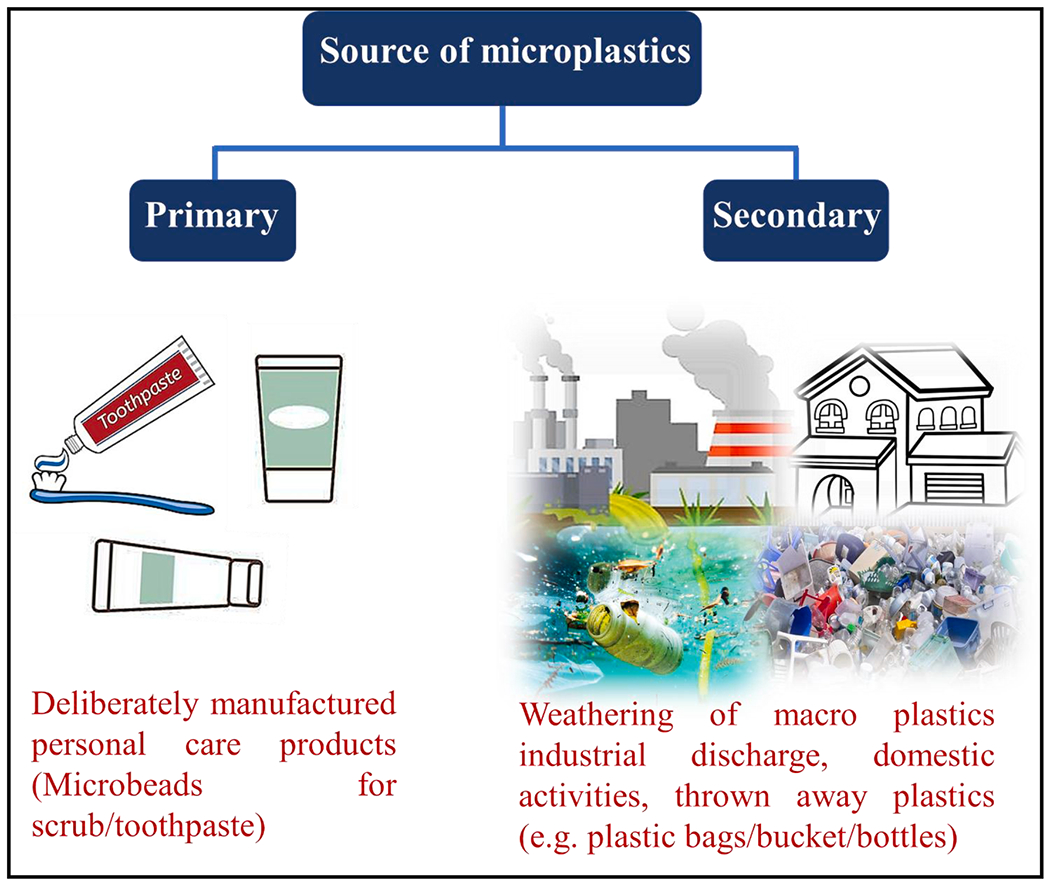
Different sources of pollution due to MPs in the environment.
MPs are prevalent in lakes [46–49], rivers [50,51], estuaries [52,53], oceans [54,55], lagoons [56], and beaches [57,58] in addition to indoor and outdoor air [59–62]. Around 80% of plastic litter can be found in marine surroundings originated from the terrestrial environment [63]. The coastal line debris largely consists of domestic plastics, plastic water/soda bottles, bags, fishing gear, and polybags [47] as these can be produced by daily routine activities such as opening of plastics bottles by scissoring, tearing by hands, and cutting through knife or even manual twisting producing about 0.46–250 MPs/cm [18]. MPs easily get transported into aquatic and terrestrial domains through the atmosphere [64–66]. Recently, COVID-19 disposable face masks (produced from polymers) have also added to the environmental pollution as these are the likely sources of MPs [67].
Wastewater and drinking water treatment plants contribute significantly to MPs [37,45,68–70]. Fig. 2(A–B) shows the presence of microbeads obtained from personal care products, while Fig. 2(C–D) displays the fragments collected after the break-down of larger plastics from a wastewater treatment plant [70]. In Europe and the US, the amount of MPs transported from wastewater treatment plants to biosolids is higher than the quantity existing in seawater [71]. Fig. 3(a–b) demonstrates different types and colors of MPs extracted from liquid fraction of a secondary wastewater treatment plant situated on Clyde River in Glasgow, which consisted of flakes and fibers with a smaller proportion of films, beads, and foam in different colors [45].
Fig. 2.

(A-B): Microbeads obtained from personal care products and (C-D) fragments from the break-down of larger plastics and synthetic textile fibers acquired from a wastewater treatment plant [70] (reproduced with permission from Elsevier).
Fig. 3.
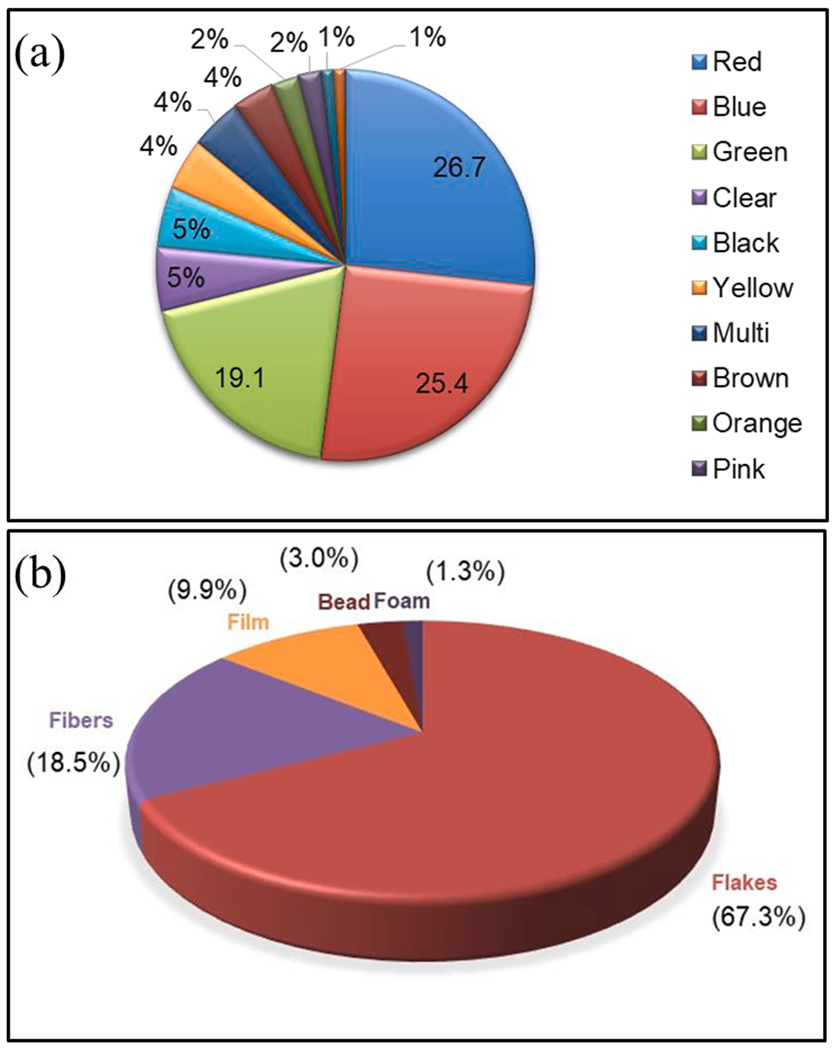
Different (a) types and (b) colors of MPs extracted from liquid fraction from a secondary wastewater treatment plant near the River Clyde, Glasgow [32].
MPs in the sediments are generally in the range (0–3146 particles/kg) of dry weight sediment [72] with their concentrations in sewage sludge ranging from 1.0 to 56.4 MPs/g of dry sludge. Apart from aquatic and air media, soil biotas have also been identified as the prime reservoir of MPs, predominantly in the agio domain [73] to the extent of 700 to 4000 plastic particles/kg of the soil [74]. In Europe, the annual load of MPs in agro-systems range from 63,000 to 430,000 tons [75] and in soil media they can erode and leach out to groundwater, which may be ingested by the soil earthworms. Digging mammals may also be responsible for the translocation of MPs and their abrasion to nanoplastics [76]. This review attempts to address the environmental issues as well as the methods used to mitigate MPs from the wastewater sources. Their health damaging effects as well as the techniques used to mitigate these MPs will be discussed.
2. Environmental effects of MPs
MPs are detrimental due to their high durability, stability, small size, and lightweight; their micron size would allow them to be swallowed by aquatic organisms, animals, and birds, finally ending up in human food items causing harmful effects [77–80]. The hydrophobic nature, relatively large surface area/volume ratio, and extraordinary vector-capacity of MPs cause adsorption of numerous toxic contaminants viz., polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAH), polybrominated diphenyl ethers (PBDEs), dichlorodiphenyltri-chloroethane (DDT), endocrine disrupting compounds (EDCs), various pharmaceuticals [81–87] and heavy metals [88–93]. For instance, acetyl tri-n-butyl citrate is the predominant additive leaching from polyethylene MPs [94] and retention of polyethylene MPs in the waste activated sludge potentially affects the performance of anaerobic digestion systems [71].
Plastics additives such as plasticizer, flame retardant, antioxidant, lubricants, anti-inflammatory agents, and UV stabilizers are responsible for causing toxic effects on the environment [95–99]. Bisphenol-A (BPA) used even in low concentrations in plastics is considered as an EDC, exhibiting adverse effects even at low concentrations in both humans and animals [100–102]. Besides, phthalates, which are used as plasticizers for improving the flexibility of plastics, are quite dangerous since they are categorized as EDCs, leading to significant reproductive disorders [103,104]. The MPs have been identified in about 220 types of aquatic creatures such as mussels, crabs, seabirds, and oysters [105–109]. The dangers of the ingestion of MPs include growth impediment, physical impairment, and histological variations in the intestines as well as changes in the lipid metabolism along with the behavioral fluctuations [110]. Furthermore, endocrine disruption and neurotransmission dysfunction of marine species due to MPs has been reported due to their genotoxicity [111,112]. MPs have been found in the digestive tracts of both vertebrates and invertebrates [23,113] since significant levels of bisphenols have been found in the liver and muscle of fish [100].
The pollutants associated with MPs have a substantial effect on the immunity system of marine mussels [114], but their toxicity effects on humans have not yet been fully understood [115]. MPs can be present in a wide range of food items and drinks such as milk [116], honey, sugar [117], sea salt [118] and a significant amount (118 ± 88 MPs/L > 5 μm and approximately 6292 ± 10,521 MPs/L > 1 μm [37]) of MPs has been detected in bottled waters [119,120]. Even in drinking water supply at the metro stations in Mexico City, MPs (5 ± 2 to 91 ± 14 MPs/L) were present comprising of polyesters and epoxy resin [121]. MPs were also detected in beverages such as cold tea (11 ± 5.26 MPs/L; only fibers; size < 1 mm), soft drinks (40 ± 24.53 MPs/L; only fibers; size ~ 0.1–3 mm), energy drinks (14 ± 5.79 MPs/L; mostly fibers; 70% had size < 1 mm), and beer (152 ± 50.97 MPs/L) [122].
Zhang et al. [123] collected dust samples from twelve countries, including India (during 2010–2014) to test the presence of MPs using HPLC-MS/MS to find that PET-based MPs were present (29 to 110,000 μg/g in all the 286 samples). Free terephthalic acid monomer was observed in most of the samples with median concentrations varying from 2.0 to 34 μg/g, while moderately high concentrations of polycarbonate-based MPs were detected in the Indian dust samples. Free BPA was detected in all the samples at concentrations < 0.05 to 36 μg/g. The daily intake of MPs can be assessed by eq. (1).
| (1) |
where CD, M and BW are the concentrations of MPs in the dust (μg g−1), dust-ingestion rate (g day−1) and body weight (kg), respectively depending on the age-group and geographical locations. The median ‘estimated daily intake’ of PET-based MPs evaluated for the infants for all the samples (age < 1 year) was quite substantial (4000 to 150,000 ng/kg-bw/day), which was about 10,000 ng/kg-bw/day for the Indian indoor dust in case of infants [123]. Table 1 summarizes the sources, composition, shapes, and characteristics of various types of MPs identified in the literature.
Table 1.
Different sources, compositions, shapes, and characteristics of various types of MPs.
| Types and characteristics of MPs | Refs. | |
|---|---|---|
| Primary MPs | Source: (manufactured deliberately) preproduction pellets, air blasting, plastic granulates, microbeads for cosmetic and personal care products (scrub, toothpaste) | [44,124–126] |
| Secondary MPs | Source: (degradation of macro-plastics) Fragmentation, weathering, photolysis, microbial disintegration of fishing nets, textile, urban discharge, and wastewater treatment plant effluent | [44,124,127] |
| Composition | Polyethylene, polystyrene, acrylic, polyamide, nylon, polyester, polypropylene, polyester, acrylic | [37,127] |
| Shape and morphology | fibers, foils, films, sheet, foams, fragments, pellets, microbeads, spheres, primarily irregular shape | [37,127,128] |
| Source of contact with living beings | Marine, lakes, terrestrial, atmospheric, agricultural land, indoor dust, seafood, drinking water, wastewater treatment plant discharge | [52,59,64,70,74,105,119,120,129,130] |
Studies have established close relationship with the size, shape, and morphology of the MPs to their toxicity and adsorption capabilities [128,131–133]. MPs with the diameters < 130 μm were known to be translocated into human tissues [19], but those found in aquatic environments have irregular shapes owing to weathering and fragmentation. Choi et al. [128] compared the effect of spherical and irregular shapes of MPs on sheep’s head minnow (Cyprinodon variegatus) to find that irregular shapes have reduced their swimming behavior compared to spherical shapes. Mazurais et al. [134] suggested that MPs identified in the digestive tracts of marine fish are incredibly variable concerning their shape and roughness, while a 7-day exposure study of zebra fishes to polystyrene MPs showed varying effects on the size of the MPs [135]. The MPs of ~ 5 μm size were found in the liver, gill, and gut, whereas those having ~ 20 μm were found only in the fish gill as well as the gut. The polypropylene MP fibers were reported to be more noxious compared to spherical particles towards Hyalella azteca (freshwater amphipod) due to longer residence time of the fibers in the gut [136]. Fig. 4 displays the extent of pollution due to MPs.
Fig. 4.
Effect of pollution due to MPs on the environment and living beings.
These studies suggest the magnitude of contamination of MPs in the environment and their associated risks, and hence, it is necessary to develop the cost-effective methodologies for the mitigation of MPs. However, their complete removal is a challenging task due to their small size and continuous breakdown; hence, methods used alleviate MPs are still inadequate.
3. Techniques used for the eradication of MPs
Several analytical techniques have been employed for the mitigation of MPs (Fig. 5), which will be briefly discussed here.
Fig. 5.
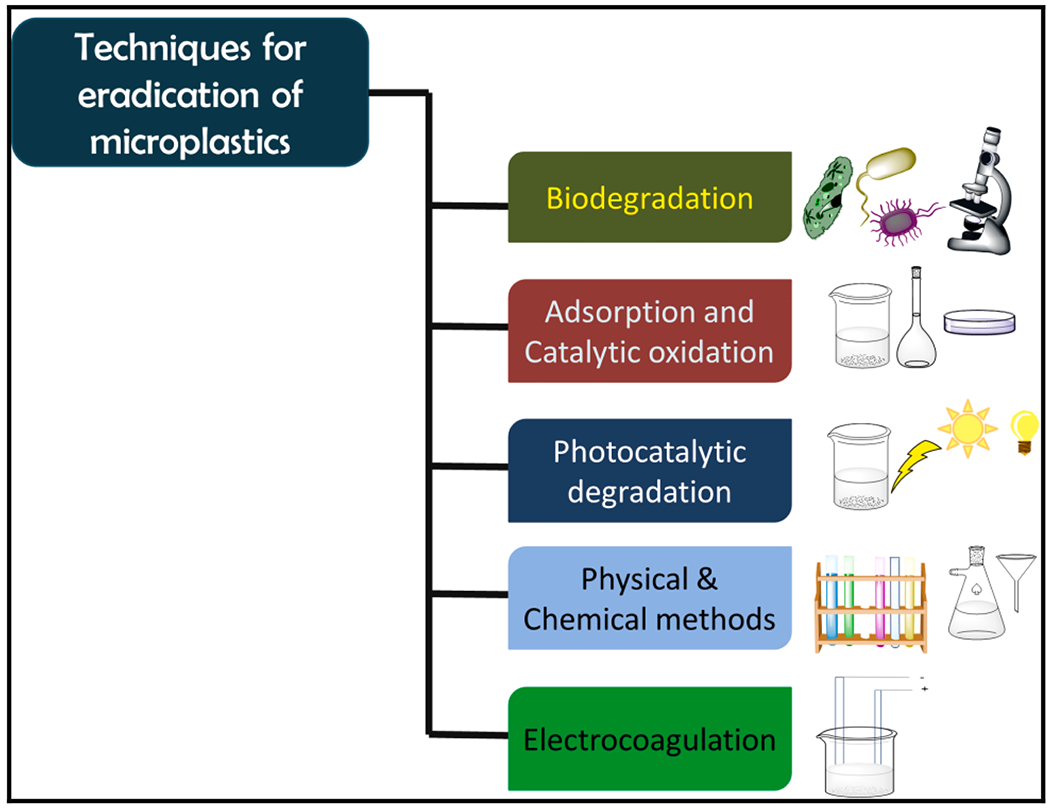
Various techniques used for the mitigation of MPs.
3.1. Biodegradation
Microorganisms are responsible for causing the disintegration of organic polymers into simple CO2, CH4, water, and inorganic substances. This approach is greatly affected by the environmental conditions, including temperature, sunlight, and humidity since microorganisms are adaptable to diverse environmental conditions and are responsible for the transformation of soil buried MPs [137,138]. Microorganisms such as Bacillus sp. [138,139], Rhodococcus sp. [124], Pseudomonas aeruginosa [140], Aspergillus clavatus [141] and Fusarium, Penicillium, Phanerochaete, Acremonium [17] have been examined for the biodegradation of MPs, where these microbes utilize the MPs as the exclusive source of nutrients from the soils, landfills, sediments, and compost [137].
The microorganisms adhere to the plastics’ surface to initiate the biodegradation by creating biofilms [142,143]. Even though hydrophobic nature of MPs becomes a hindrance interfering with the colonization of microbes and biofilm formation, microbial enzymes can encourage adhesion of microorganisms to the plastic surface by enhancing its hydrophilicity. Later, the bacteria act on the plastics by the excretion of extracellular enzymes, which would eventually stimulate oxidation or hydrolysis processes [17,53,124,144]. The extracellular enzymes can work only on the surface of the plastics as they are too bulky for penetration into the plastics since biodegradation is typically a surface-erosion process [141] commencing from a few days to weeks [124]. The microbes consequently contribute to a decrease in polymer molecular weight, while at the same time, inducing physicochemical tanges in the polymer structure [145]. Enzymes such as laccases, langanese peroxides or lignin peroxidases are responsible for hydrolyzing high-density polyethylene (HDPE) MPs [17], whereas hydrolases such as esterases, lipases, cutinases manifest the activity to induce the egradation of PET [146]. Nonetheless, utilizing enzymes for the catalysis purpose during biodegradation is quite an arduous and expensive process as the cell of microbes contain a wide-ranging engines, which could interfere with the desired reaction [141].
The breaking of -C–C- bonds in hyper-thermal settings, apparently increase the biodegradation of plastics [147]. The pretreatment of MPs by UV light could help in reducing the hydrophobic characteristics due to the introduction of −OH and C = O groups, and subsequently, the compatibility of MPs with the microorganisms is enhanced. Thermal and chemical oxidation also would help to augment biodegradation as well as the presence of metal ions in MPs, which generate the free radicals on the polymer surface, thereby reacting with O2 producing carbonyl groups, and eventually decreasing the hydrophobicity of MPs [124]. FTIR can be used to study the changes in the surface of the MPs by detecting functional groups after the degradation, while scanning electron microscopy (SEM) can be used to analyze the surface changes such as cracks, holes, and erosions.
Literature reports on the biodegradation of MPs by the microorganisms are summarized in Table 2. Auta et al. [138] isolated the bacterial strains of Bacillus cereus, Bacillus gottheilii, Bacillus cibi, Acinetobacter schindleri, Bacillus pseudomycoides, Bacillus stratosphericus, Bacillus aquimaris, and Stenotrophomonas maltophilia from the mangrove sediments, out of which only the first two grew on synthetic media comprising of diverse MPs. The growth curves of Bacillus cereus and Bacillus gottheilii during the biodegradation are displayed in Fig. 6 (a–b). The weight loss for polyethylene, polyethylene terephthalate, and polystyrene by Bacillus cereus were found to be 1.6%, 6.6%, and 7.4%, respectively, while by Bacillus gottheilii, the results were 6.2%, 3.0%, and 5.8%, respectively, and 3.6% for polypropylene. In a study [124], bacteria Bacillus sp. strain 27 and Rhodococcus sp. strain 36 were isolated from the mangrove sediments utilizing polypropylene MPs. As a consequence of incubation for 40 days, the plastic mass was reduced by 4.0% due to Bacillus sp. strain 27 and 6.4% due to Rhodococcus sp. strain 36, thus validating the biodegradation of MPs. Gong et al. [148] employed a combinatorial technique based on whole-cell biocatalysts to study the degradation of polyethylene terephthalate MPs via Comamonas testosterone F6 where the size of the plastics was reduced remarkably in alkaline conditions compared to neutral pH.
Table 2.
Biodegradation of various MPs using different microorganisms.
| Microplastic | Microorganism | Polymer molecular weight reduction | Time | Reference |
|---|---|---|---|---|
| Polyethylene | Zalerion maritimum | 56.7% ± 2.9% | 14 days | [137] |
| Polyethylene | Bacillus cereus | 1.6% | 40 days | [138] |
| Polyethylene terephthalate | Bacillus cereus | 6.6% | 40 days | [138] |
| Polystyrene | Bacillus cereus | 7.4% | 40 days | [138] |
| Polyethylene | Bacillus gottheilii | 6.2% | 40 days | [138] |
| Polyethylene terephthalate | Bacillus gottheilii | 3.0% | 40 days | [138] |
| Polystyrene | Bacillus gottheilii | 5.8% | 40 days | [138] |
| Polypropylene | Bacillus gottheilii | 3.6% | 40 days | [138] |
| Polypropylene | Bacillus sp. strain 27 | 4.0% | 40 days | [124] |
| Polypropylene | Rhodococcus sp. strain 36 | 6.4% | 40 days | [124] |
| Polyethylene terephthalate | Comamonas testosterone F6 | - | 48 h | [148] |
| Polyethylene | Bacillus sp. | 14.7% | 60 days | [145] |
| Polyethylene | Paenibacillus sp. | 22.8% | 60 days | [145] |
| HDPE | Aspergillus flavus strain PEDX3 | 3.9025 ± 1.18% | 28 days | [17] |
| Polystyrene | hyperthermophilic bacteria | 43.7% | 45 days | [147] |
Fig. 6.
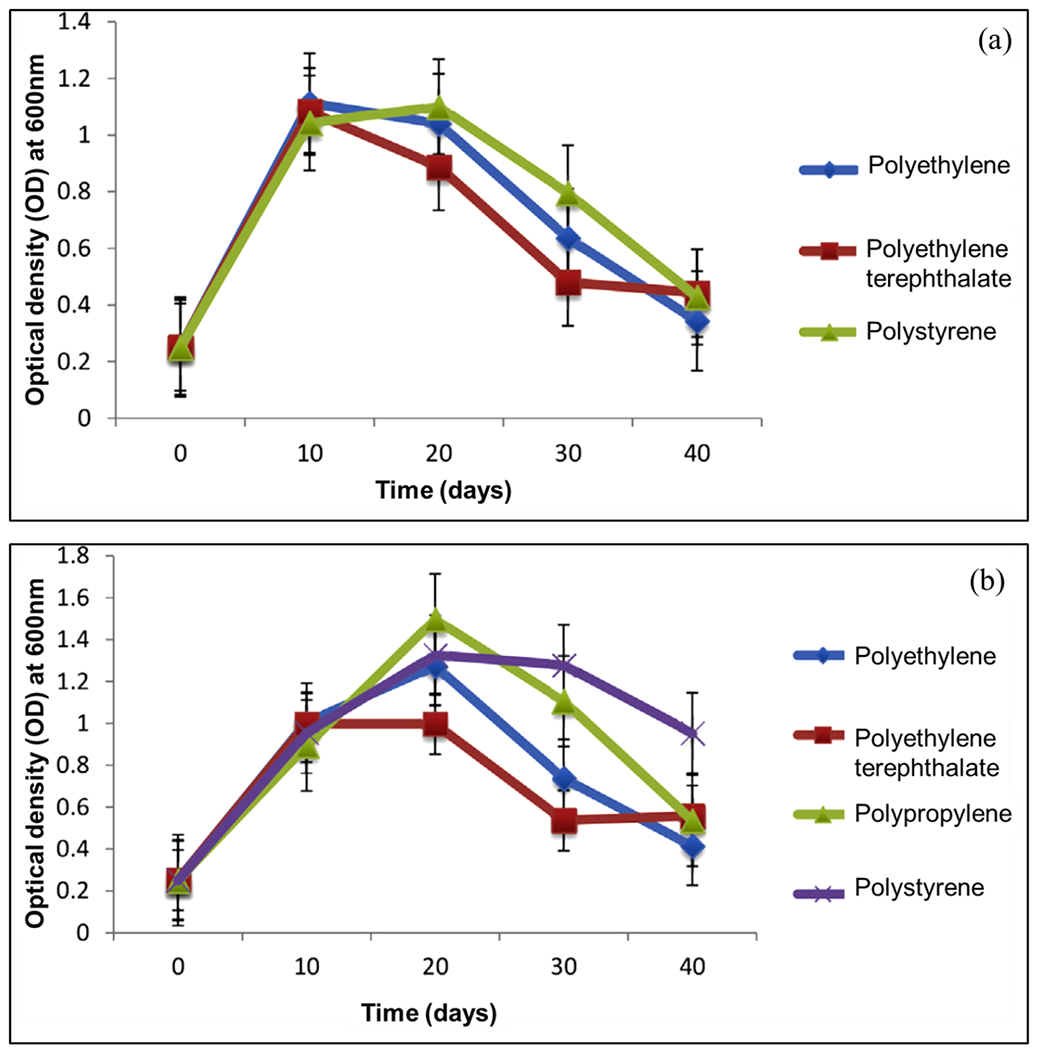
Growth curves of (a) Bacillus cereus and (b) Bacillus gottheilii during biodegradation studies [138] (reproduced with permission from Elsevier).
Paço et al. [137] developed marine fungus Zalerion maritimum in a minimum growth for the degradation of polyethylene pellets. The total period of incubation was 28 days and biodegradation was confirmed by a decrease in size and mass of the MPs pellet. The maximum of fungi biomass was grown in the initial 7 days because the growth media contained nutrients, and the deterioration of MPs was almost negligible at this time. The highest degradation rate of MPs was between 7 and 14 days, during this time polymer mass was reduced by ~ 56.7% after 14 days. FTIR-ATR and NMR confirmed the changes in molecular structures. Zhang et al. [17] isolated the fungus Aspergillus flavus strain PEDX3 from the guts of wax moth Galleria mellonella to study the biodegradation of HDPE-based MPs. After an incubation of 28 days, approximately 3.9% weight loss of the polymer was observed. In another study, laccase-like multicopper oxidases genes (AFLA_006190 and AFLA_053930) were identified to be the potential degradation enzymes. Park et al. [145] employed mesophilic mixed bacterial culture to investigate the degradation of polyethylene MPs, where it was observed that Bacillus sp. and Paenibacillus sp. decreased the polymer molecular weight by 14.7% after 60 days of incubation in addition to a reduction in mean particle sizes by 23%.
Chen et al. [147] proposed a hyper-thermophilic composting technique for in situ degradation of MPs based on the sludge by achieving 43.7% degradation after 45 days of the treatment and 7.3% degradation of lab-scale polystyrene MPs at 70 °C in 56 days. Here, high temperature introduced functional groups such as C = O or C – O, which could decrease the hydrophobicity of polystyrene-based MPs. Li et al. [149] employed fungicide prothioconazole to study the degradation of polyethylene and biodegradable poly(butylene adipate-co-terephthalate) MPs. Prothioconazole stimulated the degradation process as well as repressed the adsorption of metals such as Cr, As, Pb, and Ba by the MPs, but only the adsorption of Cu was observed. In another study [150], periphytic biofilm was employed to investigate the biodegradation of three structurally dissimilar MPs (polypropylene, polyethylene, and PET) using different carbon sources such as glucose, peptone, and glucose + peptone. Glucose was found to escalate the biodegradation of all the MPs in comparison to natural biofilm after 60 days. On the other hand, peptone and glucose + peptone have shown the inhibiting effects.
Cunha et al. [151] utilized the fact that microalgae excretes exopolymer substances (EP) with a potential of forming hetero-aggregates with MPs and compared marine (Tetraselmis sp. and Gloeocapsa sp.) and freshwater (Microcystis panniformis and Scenedesmus sp.) microalgae by exposing them to diverse types of MPs to reveal that aggregates constituted of microalgae-EP (homo-aggregates) or microalgae-EP-MPs (hetero-aggregates). The hetero-aggregation was dependent on the size and yield for the production of EP, which was species-specific. However, freshwater microalgae manifested lesser ability towards the MPs aggregation. Tetraselmis sp. exhibited higher capacity towards aggregation of low as well as high-density MPs, somewhat restricted by the size of MPs. Gloeocapsa sp. on the other hand, showed an exceptional EP production and supreme aggregation proficiencies. Microalgae have been recognized to possess high biocompatibility with a potential to treat MPs. Arossa et al. [152] claimed that the surface of aquatic biota could help to eradicate MPs from the water column and giant clams (Tridacna maxima) was investigated as sinks for the MPs since they ingest it. The contribution of clams’ shells to eliminate MPs was ~ 66%, but ingestion is not a convincing approach to treat MPs. These above findings suggest that flexibility, ease, and safety of utilizing biodegradation in large-scale may be an advantage besides low operating costs, but the method may not be reproducible, since controlling the environmental conditions is difficult.
3.2. Adsorption and catalytic oxidation
Adsorption process has been investigated for wastewater treatment [82], but adsorbents oriented towards MPs removal specifically are limited. Sun et al. [153] developed a robust compressive sponge using chitin and graphene oxide for the removal of MPs, which effectively adsorbed the diverse types of MPs at pH 6–8. High adsorption capacities were observed even after three sorption cycles (polystyrene ~ 89.8%; carboxylate-modified polystyrene ~ 72.4%; amine-modified polystyrene ~ 88.9%). At pH 10, a poor efficiency (43.7%) was observed because OH− could compete with the negatively charged polystyrene amid the exchange sites. Electrostatic interactions are responsible among the amino groups of chitin and carboxyl-groups in carboxylate-modified polystyrene along in addition to the interactions between the carboxyl group of graphene oxide and amine-modified polystyrene. H-bonding also would occur among the oxygen-containing functional groups onto the adsorbent and amino/carboxyl groups of polymers in addition to π – π interactions between graphene oxide and aromatic rings of the polymers in the MPs. Chitin, being a polysaccharide consisting of a glycosidic bond, is fully biodegradable in the soil via lysozyme and chitinase [154] and hence, sponge could also undergo biodegradation by the microorganisms present in the soil. Graphene oxide is an excellent adsorbent because of the large number of oxygen atoms on the surface (epoxy and hydroxyl) in addition to carboxyl groups [155].
Pollutant adsorption via biochar as an adsorbent, derived from various sources has been widely explored [156,157]. Adsorption of MPs by the biochar and activated biochar was investigated [158], wherein Pinus sylvestrus and Picea spp. barks were used to obtain biochar by pyrolysis at 475 °C for 3 h. The steam activation of biochar was done at diverse conditions using low and high (1.1 and 5 L min−1) N2 gas flows along with varying water flow rates. Polyethylene MPs of different sizes (10 μm, 2–3 mm), shapes, and fleece fibers were tested for adsorption using spherical polyethylene microbeads (10 μm), cylindrical, smooth PE pieces (2–3 mm) as well as fleece fibers. MPs were immobilized or retained between the biochar particles depending on the particle size, and activated biochar showed the massive potential for the recovery of MPs. The larger particles retained completely (complete retention was reported for polyethylene MPs, whereas almost 100% retention of fleece fibers), while MPs in μm size (10-μm spherical microbead) did not show efficient adsorption as larger meso-and macropore contents were helpful for the exclusion of the tiniest MPs.
Misra et al. [159] synthesized a magnetic nanoparticle composite based on the adsorption of polyoxometalate ionic liquid (PIL) onto core–shell particles of Fe2O3/SiO2 that are superparamagnetic and microporous. The viscous coating of PIL on the superparamagnetic nanoparticles facilitated the binding of MPs along with the contaminants (other microbial, organic or inorganic) from wastewater. Complete removal of polystyrene beads (size = 1 μm; concentration = 1 g/L) was achieved using an adsorbent concentration of 10 g/L and these MPs were recovered using a permanent magnet. The use of biodegradable and magnetic adsorbents was thus quite convincing, nevertheless, more research is needed to understand the intricacies of the adsorption route for the removal of MPs.
Advanced oxidation of contaminants with the aid of an efficient catalyst functionalized by radical generating species has drawn much attention in degrading the organic materials [160,161]. Kang et al. [162] fabricated magnetic nitrogen-doped carbon nano-springs for the advanced sulfate oxidation of cosmetic MPs under hydrothermal conditions. Firstly, one-pot pyrolysis was used to encapsulate manganese carbide nanoparticles in the helically N-doped carbon nanotubes, which was then functionalized with peroxymonosulfate (POMS) having an asymmetric structure. About 54% weight loss of MPs was reported after 8 h of pyrolysis and at hydrothermal temperatures of 800 and 160 °C, respectively. The hydrothermal condition simultaneously offered high pressure as well as physical ripping of the polymer commencing its degradation. FTIR and SEM validated the results as ketonic and hydroxy functional groups as well as a considerable number of cavities were observed in the treated MPs. The N-doping into a honeycomb of sp2 carbons created more active sites and facilitated POMS activation [163], since POMS () was proficient to generate reactive oxygen species such as and having E0 = 3.1 and 2.7 V vs normal hydrogen electrode for advanced oxidation to proceed. Moreover, manganese and other transition metals were recognized as POMS activators to generate [164] and this system has efficiently mineralized MPs without causing any toxic impact on the aquatic microbes [162].
MPs are progressively covered by a layer of organic and inorganic matter upon exposure to aquatic environment, providing an accessible surface for the occupation of microbes forming a biofilm [17,165]. The sequential development of a conditioning layer consisting of bacteria can adsorb metals. In a stimulating study, Ye et al. [166] proposed a strategy for removing the adsorbed metals from the MPs via decomposition of an organic layer by advanced oxidation initiated by radicals. The authors synthesized porous, ferromagnetic biochar with upgraded graphitization by treatment of straw with potassium ferrate and subsequent annealing at 900 °C for 2 h under N2 flow. The biochar-based catalyst with enhanced graphitization was capable of mineralizing organic pollutants into CO2 and H2O with the help of active sites of defects apart from the useful functional groups [167]. Biochar demonstrated a significant adsorption capacity for metal ions and hence, acted as a powerful tool to decrease the transportable form of metal by reimmobilizing metals that are disconnected from the surface of the MPs [166]. The biochar and sodium persulfate system manifested nearly 60% of lead removal after the treatment of Pb-adsorbed MPs (adsorption capacity ~ 31.29 mg/g), which is much higher when no sodium persulfate was used (adsorption capacity ~ 7.07 mg/g), but the reaction was inhibited under saline conditions due to the presence of radical scavenger Cl− in the system [166]. MPs were also agglomerated in a highly saline system as assessed by the lesser electrostatic repulsion as well as a steric effect with the double layer compression hampering the contact between the surface of MPs and the active radicals [168].
Overall, the reusability and cost-effectiveness of adsorbent/catalyst are the major factors in determining its feasibility and application at large scales. More straightforward separation techniques and synthesis routes with minimal cost ought to be designed to accomplish a versatile catalyst. Therefore, an exhaustive probe is needed for this methodology to eliminate the MPs.
3.3. Photocatalytic degradation
Photocatalytic degradation is a redox process in which semiconductor photocatalyst absorbs photons of suitable wavelength (visible/UV) and electrons (e−) in the valence band get excited to the conduction band leaving the positive holes (h+) behind. The e− and h+ react with the adsorbed water and oxygen, giving rise to free radicals such as superoxide radicals () and hydroxyl radicals (•OH) [169,170]. These active species further react with organic polymers to disintegrate them, leading to the breakage of polymeric chains and even complete mineralization. Semiconductors such as TiO2, ZnO, ZnS, WO3, ZrO2, and g-C3N4 have been employed for the photocatalytic wastewater treatment [169–174]. Of all these, TiO2 has been widely explored owing to its easy-availability, non-toxicity, and economical nature [175]. The general mechanism of photocatalytic degradation for the removal of organic pollutants is illustrated in Fig. 7.
Fig. 7.
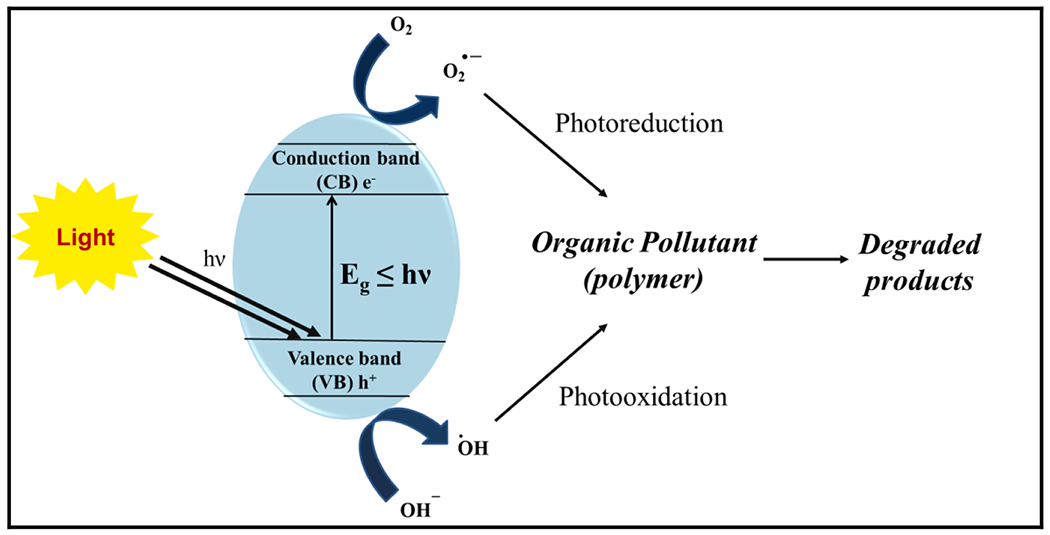
General mechanism of photocatalytic degradation of organic pollutants using photocatalysts under the suitable light source.
Photocatalytic treatment of polyethylene, polystyrene, and propylene plastics under various light sources has been done using TiO2-embedment technique. Here, composite films were formed by mixing the polymer with TiO2 using an organic solvent such as cyclohexane and tetrahydrofuran [176–179]. Photocatalytic degradation of polyethylene employing polypyrrole/ TiO2 composite as the photocatalyst was also investigated [180], but not many reports are available specifically on the photocatalytic degradation of MPs. Tofa et al. [181,182] employed ZnO nanorods and Pt nanoparticles–deposited ZnO nanorods for the photocatalytic degradation of low-density polyethylene (LDPE) MPs in water under the visible light irradiation. Functional groups such as carbonyl (1700–1760 cm−1), hydroperoxide (3600–3610 cm−1), peroxides (1100–1300 cm−1), and unsaturated groups (880–920 cm−1) appeared during the photo-degradation as confirmed by FTIR. The SEM images revealed that the surface of LDPE without illumination was smooth, excluding a few manufacturing defects and spots. After the photo-degradation, increased brittleness in addition to wrinkles, cracks and cavities onto polymer surface was observed owing to the formation of oxygenated groups and volatile organic compounds, thus validating chemical transformation of the polymer. The ZnO-Pt catalysts showed 13% higher probability for the oxidation of LDPE than the ZnO nano-rods, suggesting the breaking of LDPE MPs under artificial sunlight.
Sekino et al. [183] investigated the structure of MPs by the photocatalysis utilizing TiO2-based micro and nanodevices. Wang et al. [184] investigated Au@Ni@TiO2 based micromotors to eradicate the polystyrene MPs suspended in wastewater under UV irradiation provided by a micromotor propulsion by the photochemical reactions in water and H2O2 instigated by e−-h+ generation. The non-applicability of this system apart from a lack of selectivity has been the shortcoming of this approach. The requirement of low H2O2 concentrations for encouraging phoretic interactions enabled the movement of the micromotor.
Ariza-Tarazona et al. [185] investigated the degradation of HDPE MPs extracted from a commercial facial scrub using two different semiconductor photocatalysts based on N-TiO2. The first catalyst was fabricated via a green synthesis route using an extra pallial fluid of fresh blue mussels (Mytilus edulis). The second catalyst was developed via a conventional sol–gel process using urea and tri-block copolymer (Pluronic). The photocatalytic reaction was carried out for 20 h under the visible light and protein-derived catalyst exhibited a high capability to facilitate photo-degradation in both solid as well as aqueous media. At the same time, sol–gel derived displayed good proficiency for promoting the mass loss of MPs in aqueous media. The results revealed that in the solid phase, protein-derived catalyst degraded HDPE with a rate constant of 12.2 × 10−4/h with a mass loss of 1.1%, while in an aqueous phase, the mass loss and rate constant values were 6.4% and 38.2 × 10−4/h, respectively. It was suggested that surface area of the catalyst in addition to the interactions between the catalyst and MPs, played a crucial role as validated by SEM and FTIR during the structural changes.
In another study [186], the effects of pH and temperature on the photocatalytic degradation of HDPE MPs were investigated. The C, N-TiO2 photocatalyst was fabricated via green bioinspired synthesis by utilizing extra-pallial fluid of Mytilus edulis. The protein-derived photocatalyst was obtained by doping extra-pallial fluid onto titanium (IV) butoxide. The photocatalytic experiment was carried out by adding 200 mg each of MPs and C, N-TiO2 catalyst into a 50 mL buffer solution to obtain an average mass loss of ~ 71.77% at pH 3 and at ~ 0 °C after the continuous stirring for 50 h under the visible light illumination. The photocatalysis at low temperature enhanced the surface area of MPs via fragmentation, but low pH facilitated the generation of hydroperoxide radicals.
The mechanism is explained by Eqs. 2–7; first, OH• radicals generated from the photocatalyst (C, N-TiO2) commenced the polymer degradation giving rise to polyethylene alkyl radicals (Eq. (2)). After reacting with O2, an alkyl radical generated peroxy radicals (Eq. (3)), which then extracted H-atom from the polymer generating hydroperoxide (Eq. (4)). The hydroperoxide then generated much more active oxy and OH• radicals after the cleavage of weak O–O bond (Eq. (5)), which extracted hydrogen from the polymer chains (Eq. (6)), suggesting the role of hydroperoxide. The process can speed up depending on the ease of H-atom removal (from other polymer chains) by the radicals (Eq. (5)) and speed of termination of free radicals by the recombination in addition to disproportionation. Then increase in the concentration of hydroperoxide radicals accelerated the reaction; the ratio of peroxy radicals and hydroperoxide was then determined by the equilibrium (Eq. (7)). As per Le Chatelier’s Principle, increase in the concentration of H+ ions would encourage backward reaction by accelerating the generation of hydroperoxide. Therefore, pH 3 favors MPs degradation as the system is rich in H+ ions, and also colloidal nanoparticles will then interact better with the MPs. Thus, a combined effect of pH and temperature was responsible for achieving better photo-degradation efficiency.
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
The above study suggests the benefits of photocatalytic degradation with no requirement of external chemicals except for a solid efficient photocatalyst. Moreover, natural and abundant solar light can be utilized for the mineralization process, but lack of selectivity, difficulty in regenerating catalysts and inefficiency of degradation of MPs might limit the application of photo-degradation for scale-up applications.
3.4. Chemical and physical methods
Chemical methods include sol–gel process, agglomeration, and ozonation for the elimination of MPs, while the physical techniques include sedimentation and filtration. Usually, chemical and physical techniques are used jointly for wastewater treatment.
3.4.1. Sol-gel process
The sol–gel method is an appealing chemical technique for the treatment of MPs. Herbort and Schuhen [187] utilized the idea of host–guest relationship for the treatment of MPs and suggested to use inventive inorganic–organic composite silica gels. The removal steps comprised the fabrication of an inclusion unit, a bioinspired element of the whole molecule, and subsequently, the fabrication of a capture unit, which is capable of bonding with the substance to be included through the functional groups. These units are ultimately combined to give rise to inclusion compound. The presence of alkoxysilyl helped in obtaining the desired 3-D network and through the organized network structure, structured composite silica gels were obtained via the sol–gel process. Later, interaction of the capture unit and inclusion compound bioinspiration took place to facilitate the inclusion of inert compounds, while the hydrophobic stressors captured were easily isolated by a separation technique such as a sand trap.
The silica gel was found to be better than the activated carbon for the separation of MPs due to the increase in compared to granulated activated carbon. The bioinspired functionalized hybrids can encapsulate MPs from wastewater in a sustainable manner as the formation of silane occurs via hydrolysis as well as condensation to form macromolecular network. Notably, high or low pH values would facilitate faster gelation due to higher rate of hydrolysis and condensation. Alkoxysilanes such as tetraethoxysilane react slowly in neutral pH [188], but n-alkyl-substituted chlorosilanes give rise to silanols to release hydrochloric acid as they are highly active with water [189]. Monochlorosilanes are thus likely to dimerize, whereas dichlorosilanes produce long-chain oligomers [190], but in case of n-alkyltrichlorosilanes, three reactive groups can help to establish 3-D networks [191], which are appropriate for treating industrial wastewater as the sol–gel process manifests pH-induced reaction [192].
Herbort et al. [193] synthesized the functionalized molecular precursors in an inert nitrogen atmosphere that were utilized to form bioinspired alkoxy-silyl hybrids through which hybrid functionalized molecules act as linkage reagents between the MPs. Therefore, agglomeration of MPs via the sol–gel method includes the formation of 3-D agglomerates, which can be eradicated by using a filtration technique. In another study [192], pH-induced agglomeration followed by the elimination of polyethylene and polypropylene MPs (250– 350 μM) from the water was investigated in a two-step process based on a physicochemical technique, wherein particle size could grow independent of the pH due to the aqueous environment by the addition of trichlorosilane-substituted Si derivatives. Thus formed Si-based MPs aggregates (size ~ 2–3 cm) can be removed by filtration (sand traps). In the agglomeration process, diverse alkyl groups have a strong influence on the aptness of alkyltrichlorosilanes since they can affect the hydrolysis and condensation kinetics in water, ultimately the affinity to MPs. The intermediate chain length between C-3 and C-5 was found to be suitable for this approach [194].
3.4.2. Coagulation and filtration
The formation of enlarged particles through coagulation makes the separation process more accessible and these techniques would allow the coagulants to bind minuscule particles through the complexation by the ligand exchange process [195]. Skaf et al. [196] studied the degradation of microspheres and microfibers by coagulation method using alum (concentration ~ 5–10 mg/L Al), and found the microspheres solution with an initial turbidity of 16 NTU (Nephelometric Turbidity Units), which was reduced to < 1.0 NTU post treatment. During coagulation, the addition of alum gives rise to H2SO4 and cationic Al-containing species affecting the pH and zeta potential. Sweep flocculation was suggested as the governing mechanism for removing the microspheres, but occurrence of 20 mg/L of surfactants in the solution did not affect coagulation performance of the microspheres at a relatively lower dose of alum, but a damaging influence would have been prevalent at high particle loadings and alum-dose. On the other hand, the stability of polyethylene microfibers was profoundly affected by the surfactants, but the fibers were efficiently removed using the coagulation.
An efficient separation by the classical filtration is arduous and inconvenient because of the enormous water volumes with little solid-concentrations in addition to extremely minute particle sizes [159]. Ultrafiltration (UF), which consumes less energy, has a high separation efficiency and most importantly, the process requires a relatively compact size of the plant [197,198]. Coagulation and UF membranes have been a brilliant choice because of the excellent effluent quality of the discharge produced after the treatment. Enfrin et al. [198] studied the degree of fouling of a commercial UF poly(sulfone) membrane caused by the MPs in addition to nanoplastics having the sizes in the range of 13–690 nm. After 48 h, >25% of the MPs were adsorbed onto the surface of the membrane and hydrophobic interactions besides the surface repulsive forces might have decreased the rate of adsorption. Ultrafiltration, combined with coagulation, is one of the primary wastewater treatment techniques employed currently in wastewater treatment plants [64,199].
Coagulation of MPs by Fe, Al, and the alum-based coagulants has been widely explored [40,195,196,200] in which polyethylene MPs having various sizes were removed by employing aluminum chloride and ferric chloride. Ma et al. [40,195] used Al- and Fe-based salts and observed that coagulation could eliminate MPs that are expected to float. The Al-based salts also showed a better performance compared to Fe-based salts. Smaller the PE particle size, the higher would be the removal efficiency and hence, MPs of < 0.5 mm exhibited a maximum removal efficiency (~40%) at a high dose of AlCl3 (~ 270 to 405 mg/L). Additionally, pH 6 was also reported to show maximum removal efficiency, but low removal efficiency was observed even with a high Al-based salt dosage of 15mM (below 40%). Further, ionic strength and turbidity level showed little effect on the removal efficiency. The addition of anionic polyacrylamide played a key role owing to the generation of positive Fe- and Al-based floes in neutral conditions. Also, for all the particle sizes, removal efficiencies utilizing FeCl3 were quite low (<15%). In conclusion, the merits of coagulation and agglomeration lie in the fact that small MPs can be easily omitted. In addition, there are simpler mechanical devices and manageable operational conditions however, the drawback of coagulation is that it requires extra chemicals as well as excessive use of Al-based coagulation might increase Al-salt residue in drinking water, which might be risky for living beings.
3.4.3. Dynamic membrane processes
Conventionally separation of floes created after the coagulation is done by sedimentation though the process requires high maintenance costs and equipment budget [201 ]. Dynamic membrane techniques have been widely used for wastewater treatment for over many years [202,203]; the supporting membrane, a mesh with a large pore-sizes can be inexpensive. The already existing impurities in wastewater can be utilized for the formation of a filtration layer without the need for any extra chemicals. Its appealing attributes include economic nature, low-energy consumption, small filtration resistance, low trans-membrane pressure (TMP), besides easy cleaning and the entire filtration process can be performed without using the pumps [204]. Moreover, the membrane can be easily cleaned through surface brushing [205], air scouring [206], and water backwashing [207].
Li et al. [204] developed a dynamic membrane onto a 90 μm supporting mesh by filtering synthetic wastewater, where turbidity of the effluent was decreased to < 1 NTU during 20 min of filtration. TMP and total filtration resistance displayed a linear increase with a high correlation coefficient as the filtration time increased. The escalation in influent flux besides the influent particle concentration correlated with rising TMP and filtration resistance in addition to quick decrease in turbidity of the effluent due to the instantaneous formation of dynamic membrane over the supporting mesh.
3.4.4. Membrane bioreactor
Lares et al. [208] investigated the efficacy of a municipal wastewater treatment plant (located in Mikkeli, Finland) to remove MPs from the wastewater by collecting sludge samples once a biweekly for a period of three-month sampling campaigns; the plant operated based on the conventional activated sludge technique and a pilot-scale membrane bioreactor (MBR). The MBR had a superior efficacy of exclusion of MPs of ~ 99.4% in comparison to conventional activated sludge process (98.3%). In another investigation [209], it was examined that the exclusion performance of MBR was instantaneously reduced by the addition of PVC MPs into the system, and the process was improved after operating for a couple of days. The contamination due to MPs could lead to a greater or even irreversible membrane fouling.
3.4.5. Agitation method
Mekaru [210] compared the agitation methods namely, rotation mixing, shaking, and flowing to no agitation (for 7 days at 23 °C) to comprehend the degradation of polystyrene MPs to nanoplastics. The extent of deterioration as well as aggregation was assessed via nanoparticle tracking analysis, while the length of physical abrasion was gauged by FESEM analysis (Fig. 8). These experiments suggested that aggregation repression needs ample volume of the sample. Also, lesser the rotation speed, the more was the suppression of particle-aggregation. Shaking was reckoned as the most apposite method for the agitation of particles when the biochemical degradability of particles in a solvent was sought. Additionally, agitating particles via rotation mixing is the most balanced approach for the validation of biochemical as well as physical degradability at the same time. It is thus indispensable to choose proper conditions in addition to suitable agitation methodology based on the natural environmental degradation of polystyrene MPs to nanoplastics, which was replicated. The study advocated the importance of discussion of plastic debris contamination in aquatic media from the degradation of micro to nano sizes as well as from recrystallization following the degradation.
Fig. 8.
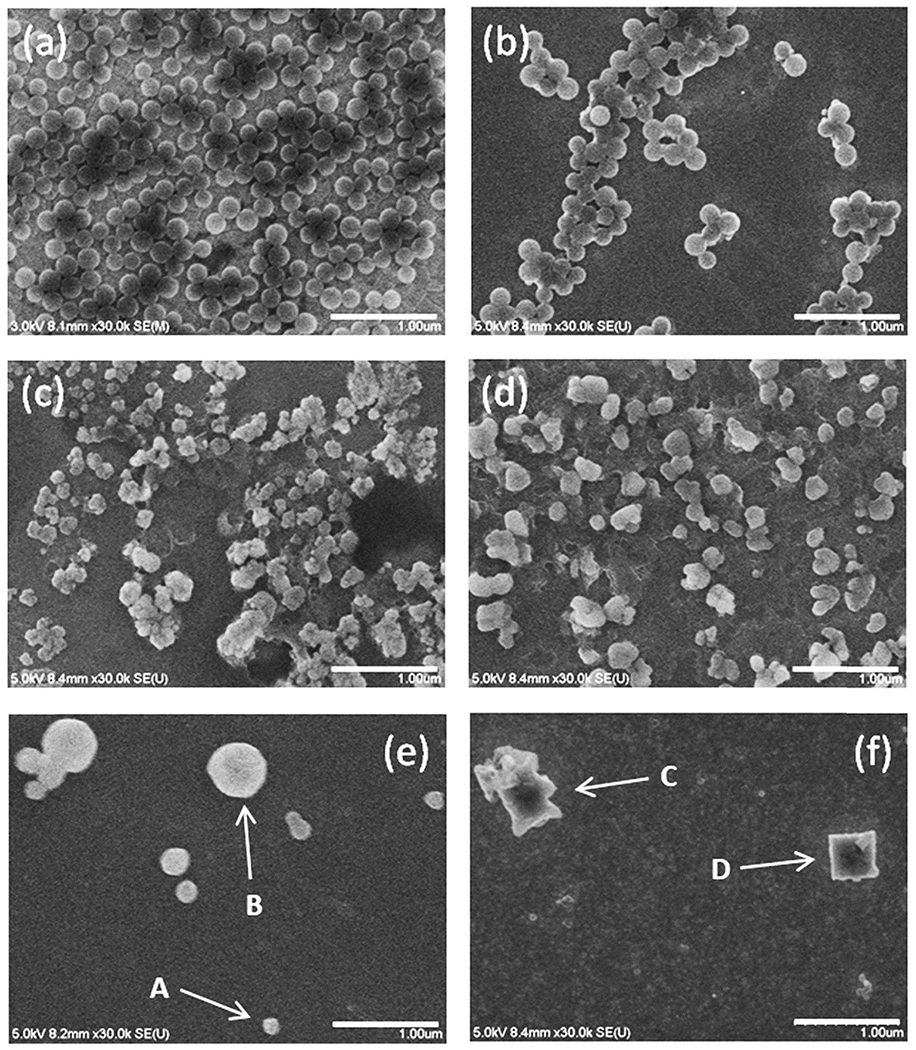
FESEM images of polystyrene particles (a) before and after incubation by (b) standing with no agitation, (c) rotating, (d) shaking, and flowing using (e) tubing and (f) intelligent pumps for 7 days [210] (reproduced with permission from ACS).
Overall, the physical and chemical methods showed promising ability for the treatment of MPs in wastewater using the combined physical techniques along with biological processes. However, more focus on the proper design of the methods and infrastructure is a pre-requisite for large-scale use.
3.5. Electrocoagulation
Electrocoagulation has been an appealing technique in the domain of wastewater treatment as it has been used widely for the remediation of wastewater containing dyes [211], heavy metals [212], dairy industry effluents [213], phosphate [214], Cr (VI) [215], and herbicides [216]. The assets of coagulation, floatation and electrochemical methods have been incorporated in the electro-coagulation [212,213]. The electrocoagulation consists of three significant events viz., electrolytic reactions on the electrode surface, coagulant formation in the medium, and adsorption of colloidal/soluble contaminants through a coagulant and finally, separation by sedimentation because of the formation of hydrogen bubbles by the cathode that assists the separation of particles [213]. This process employed metal cations as sacrificial anodes, such as Fe or Al electrodes [212]. Upon applying current to the electrodes, the oxidation of metal anode and release of metal cations in the solution took place following the formation of metal hydroxide coagulants, leading to destabilizing surface charges of the pollutants disintegrating the colloid [217]. This makes the pollutant and coagulant to be in close proximity by approaching towards each other, therefore allowing the van der Waals forces to play a role. In the meantime, coagulant creates a sludge layer that captures suspended particles/pollutants. The simultaneous oxidation of water into oxygen and the generation of electrolytic gases such as H2 occurs in the system [212,217,218]. The electrolytic reactions mechanisms are explained by Eq. 8–12 [217].
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
Despite being used for wastewater treatment for a long time, not much has been investigated regarding the electrocoagulation of MPs. In one of the first reports, Perren et al. [217] explored the effectiveness of electrocoagulation for the removal of microbeads by carrying out the investigations in a 1-liter stirred-tank batch reactor for 60 min for synthetic wastewater constituting polyethylene microbeads of varying concentrations. The effects of initial pH (pH 3, 5, 7.5, and 10), conductivity and current density were explored. Fig. 9 displays schematics of the reactor setup used by the authors to obtain a removal efficacy of > 89% at pH values ranging from 3 to 10; nevertheless, efficacy at pH 3 and 10 was much smaller compared to pH 5 as well as pH 7.5. A maximum of 99% removal was observed at pH 7.5, justifying that in neutral pH, there is a favorable formation of the coagulant. The influence of conductivity was examined by the variation of NaCl concentration (2–8 g/L that corresponds to 7.44–13.75 mS/cm) in the sample to report that by increasing the concentration of Cl− ions efficacy was minimum while removing MPs, which is contrary to the reports claiming the reliance on dye-removal efficacy of Cl− ions [219]. The formation of HOC1 helped in dye-degradation, but in with microbeads, HOCl occurred in about 60 min to cause significant degradation, but current density did not influence the removal efficacy of electrocoagulation cell. However, the operation with a current density of 11 A/m2 improved the energy-efficiency of the cell. For the working of the reactor, lesser energy demand was required due to greater water conductivity [217] and this procedure was simple as the metal electrodes were employed to produce the coagulant [220].
Fig. 9.
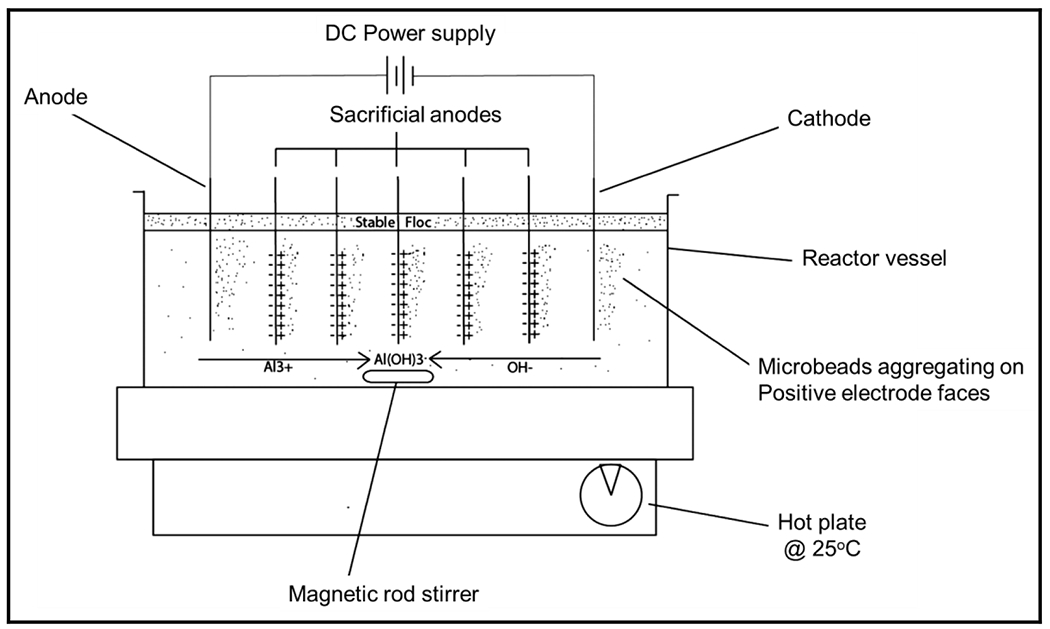
Schematic diagram of the reactor setup used for electrocoagulation of microbeads [217] (reproduced with permission from ACS).
Electrochemical techniques are relatively economical for the tertiary treatment since these are not dependent on chemicals or microorganisms and moreover, they are energy-efficient and flexible with the least chance of secondary pollution. However, the recurrent requirement of replacing sacrificial anode and electricity-requirement are the limitations of electrocoagulation. There is thus a considerable scope for this technique and more investigations are needed for the treatment of MPs and nanoplastics using electrocoagulation. Table 3 summarizes the reports on the removal of MPs by various techniques.
Table 3.
Various techniques used for the removal of MPs.
| Microplastic | Technique | Adsorbent/Catalyst/Membrane/Coagulant | Experimental conditions | Outcome | Ref. |
|---|---|---|---|---|---|
| Polystyrene | Adsorption | Chitin and graphene oxide based sponge | Time = 24 h | Maximum removal = 92.2% | [153] |
| Carboxylate-modified polystyrene | Adsorption | Chitin and graphene oxide based sponge | Time = 24 h | Maximum removal ~ 74.9% | [153] |
| Amine-modified polystyrene | Adsorption | Chitin and graphene oxide based sponge | Time = 24 h | Maximum removal ~ 90.2% | [153] |
| Polystyrene beads | Adsorption | Polyoxometalate ionic liquid adsorbed on the core–shell particles of Fe2O3/SiO2 | Microbeads concentration = 1 g/L | Maximum removal = 100% | [159] |
| Polyethylene | Advanced sulfate oxidation | Functionalized carbon nanosprings | Time = 8 h; pyrolysis temperature = 800 °C; hydrothermal temperature = 160 °C | Weight loss ~ 54% | [162] |
| HDPE | Photocatalytic degradation | Protein derived N-TiO2 | Time = 20 min; visible light; aqueous phase; | Weight loss ~ 6.4% | [185] |
| HDPE | Photocatalytic degradation | C, N-TiO2 | Time = 50 h; visible light; pH 3; Temperature ~ 0 °C | Weight loss ~ 71.77% | [186] |
| Microspheres (Rayon, Polyethylene, Polyester) | Coagulation | Alum | Microsphere concentration = 5 mg/L; Alum dose = 4.8 mg/L Al | Reduction in NTU = 97% | [196] |
| Polyethylene | Coagulation + Ultrafiltration | Coagulant: AlCl3.6H2O (15 mM) | MPs particle size < 0.5 mm | Removal = 36.89% | [40] |
| Polyethylene | Ultrafiltration | Poly(sulfone) membrane | Time = 48 h | Removal ~ 25% | [198] |
| Polyethylene microbeads | Electrocoagulation | Parallel, bipolar electrode setup | pH 7.5 | Maximum removal = 99.24% | [217] |
3.6. Removal of MPs in treatment plants
Various research groups have investigated the removal techniques used in wastewater and drinking water treatment plants. The combination of several methods was used in the plants to achieve excellent results and these data on the treatment of MPs in real wastewater as well as drinking water treatment plants of different regions are summarized in Table 4.
Table 4.
Treatment of MPs in various wastewater/drinking water treatment plants with diverse treatment techniques in different regions.
| Location of treatment plant | Treatment techniques | Influent (MPs/L) | Effluent (MPs/L) | Removal efficiency | Ref. |
|---|---|---|---|---|---|
| MBR (primary effluent) | 6.9 (±1.0) | 0.005 (±0.004) | 99.9% | ||
| Discfilter (pore size = 10 μm) (secondary effluent) | 0.5 (±0.2) | 0.3 (±0.1) | 40.0% | [70] | |
| Finland | Discfilter (pore size = 20 μm) (secondary effluent) | 2.0 (±1.3) | 0.03 (±0.01) | 98.5% | |
| Rapid sand filters (secondary effluent) | 0.7 (±0.1) | 0.02 (±0.007) | 97.1% | ||
| Dissolved air flotation (secondary effluent) | 2.0 (±0.07) | 0.1 (±0.04) | 95% | ||
| Finland | Primary, conventional activated sludge | 57.6 | 1 | 98.3% | [208] |
| Finland | Primary, MBR | 57.6 | 0.4 | 99.3% | |
| Czech Republic | Coagulation, sand filtration | 1473 ± 34 | 443 ± 10 | 70% | [37] |
| Czech Republic | Coagulation, sedimentation, GAC filtration | 1812 ± 35 | 338 ± 76 | 81% | [37] |
| Czech Republic | Coagulation, flotation, sand filtration, GAC filtration. | 3605 ± 497 | 628 ± 28 | 82% | [37] |
| China | aerated grit treatment, primary, secondary, A2O, denitrification, ultra-filtration, ozonation and UV | 12.03 ± 1.29 | 0.59 ± 0.22 | > 95% | [222] |
| South Korea (Plant A) | Primary, Secondary, coagulation, ozone | 4200 | 33 | 99.2% | [127] |
| South Korea(Plant B) | Primary, Secondary, coagulation, membrane disc-filter | 31,400 | 297 | 99.1% | [127] |
| South Korea(Plant C) | Primary, Secondary, coagulation, rapid sand filtration | 5840 | 66 | 98.9% | [127] |
| China | Coagulation/flocculation, sedimentation, sand filtration and advanced treatment units, ozonation combined with the GAC filtration | 6614 ± 1132 | 930 ± 72 | 82.1 – 88.6% | [69] |
Hidayaturrahman and Lee [127] examined the removal of MPs (microbeads and fragments) from various stages in three wastewater treatment plants. They investigated the performance of tertiary treatment carried out via coagulation and diverse techniques such as ozone (A), membrane disc-filter (B) in addition to rapid sand filtration (C). The primary as well as secondary treatment processes were reported to efficiently eliminate MPs to the extents of ~ 75% and 91.9%, respectively. The removal efficacy escalated to > 98% post the tertiary treatment. On the other hand, ozonation reduced to 89.9% of the MPs in the plant A along with the generation of least number of MPs in the final discharge. Similarly, membrane disc-filter and rapid sand filtration eliminated 79.4% and 73.8% of MPs, respectively. It was suggested that Al-based coagulant performed excellent in the tertiary treatment of MPs and higher the number of MPs indicated a greater removal efficiency, while excess doses of coagulant lead to reduced productivity.
Wang et al. [69] used a combination of coagulation with sedimentation and granular activated carbon (GAC) filtration, which exhibited an efficacy of ~ 40.5–54.5% predominantly for the fibers in a drinking water treatment plant. On the other hand, coagulation with GAC displayed 56.8–60.9% efficacy, mostly for small-sized MPs. On the other hand, ozonation had a negative impact on the removal efficiency at increasing percentage of MPs.
Talvite et al. [70] investigated the exclusion of MPs from the discharge of four diverse wastewater treatment plants using MBR for the treatment of primary effluent, while several tertiary treatment techniques (disc filter, rapid sand filtration, and dissolved air flotation) were employed to treat the secondary effluent. MBR succeeded in removing 99.9% of MPs at the decreasing concentrations of MPs from 6.9 to 0.005 MPs/L. The tertiary techniques (rapid sand filter, dissolved air flotation, and disc filter) exhibited the removal efficiency (reduction in MPs concentration) of 97% (from 0.7 to 0.02 MPs/L), 95% (from 2.0 to 0.1 MPs/L), and 40–98.5% (from 0.5 to 2.0 to 0.03–0.3 MPs/L), respectively.
Talvitie et al. [221] examined the contributing methods for the elimination of micro litter removal in the course of several wastewater treatment steps viz., mechanical, chemical, and biological in addition to a biologically active filter (BAF) to suggest that majority of micro litter got eliminated by a pre-treatment. The biological treatment alleviated the concentration of micro litter, while the gross retention capacity of the plant was > 99% post the secondary treatment.
Yang et al. [222] examined the removal of MPs from a discharge in China’s Gaobeidian sewage treatment plant using aerated grit chamber followed by a primary sedimentation tank and secondary sedimentation tank. Subsequently, A2O (anaerobic, anoxic, and aerobic treatments) and eventually advanced treatment processes (denitrification, ultrafiltration, ozonation, and UV) were employed. The sampling sequence of the plant is displayed in Fig. 10 and the overall hydraulic retention time was 4–12 h, while sludge retention was up to 5–15 days. The concentration of MPs found in the influent was ~ 12.03 ± 1.29 items/L and among the identified MPs, 18 kinds of different plastics of ten colors were detected, of which PET (42.26%) and polyester (19.1%) were the most abundant. The microfibers had an average size of ~ 1110.72 ± 862.95 μm, whereas only 14.08% of microparticles were present with an average size of 681.46 ± 528.73 μm. The primary treatment stage of aerated grit eliminated almost 58.84% of MPs, while the removal efficacy was increased to 71.67% after the advanced treatment techniques by mitigating the concentrations of MPs up to > 95%; 0.59 ± 0.22 items/L of MPs were identified in the regained waters.
Fig. 10.
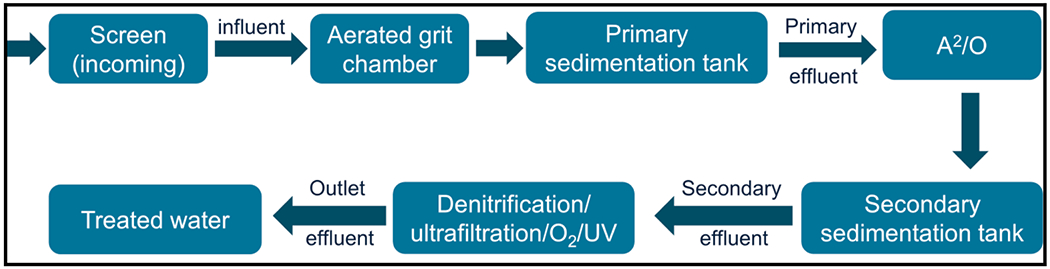
Sampling sequence for MPs from Gaobeidian sewage treatment plant in China [222].
The reports mentioned above suggest that the use of combined physical, chemical techniques with biological processes can manifest an exceptional efficiency for treating MPs in wastewater and drinking water treatment plants.
4. Discussion
The occurrence of MPs (size < 5 mm) is ubiquitous in the environment as these have been identified in significant amounts in oceans, beaches, rivers, agricultural lands, soil ecosystems, and even in remote lakes, indoor dust as well as drinking water sources. MPs are generally classified as primary and secondary based on their origin; primary MPs are deliberately manufactured such as microbeads for personal care products, while secondary MPs are the result of fragmentation of macro-plastics in terrestrial and aquatic media. Wastewater and drinking water treatment plants are also a substantial source of MPs as these pollute a broad range of foodstuffs and drinks, including bottled waters, milk, honey, sugar, beer, and sea salt. Moreover, micron range size of MPs allow them to be inhaled or swallowed by aquatic organisms, soil creatures, animals, birds, and even human beings. The hydrophobic nature, large surface area/volume ratio, and astonishing vector-capacity of MPs would result in adsorption of several harmful pollutants such as heavy metals, EDCs, PCBs, PAH, PBDEs, and DDT. The ingested MPs can translocate from phytoplankton as well as zooplankton to the food chain ending up in human food, leading to detriment at a more substantial level. The effect of the presence of MPs in the environment may affect the biodiversity and the ecosystem. Once the MPs enter into the human system through ingestion/inhalation, they possibly may lead to a localized immune response, suggesting the advanced level research to precisely understand the damaging effects of prolonged exposure of MPs on the humans
Various techniques have been explored for the removal of MPs. After the application of a suitable method, MPs can be characterized by FTIR and SEM to elucidate the functional groups attached structural changes, and surface changes such as cracks, holes, and erosions. In biodegradation methods, microorganisms such as bacteria and fungi (Bacillus sp., Rhodococcus sp., Pseudomonas aeruginosa, Aspergillus clavatus, Fusarium, Penicillium) have been investigated. The microbes utilize the plastics/polymer materials as the sole source of nutrients, thereby showing a reduction in polymer molecular weight, indicating the chain disintegration. Microalgae have been recognized to have high biocompatibility and potential for treating the MPs and the use of bio-nanocomposites as well as magnetic adsorbents promises excellent MPs-removal efficacy. Catalytic oxidation of MPs via advanced oxidation facilitated by or are also widely introduced in the presence of radical-generating species such as peroxymonosulfate or sodium sulfate. In photocatalytic degradation, semiconductor catalyst of optimum bandgap such as TiO2 and ZnO in the presence of a light source of suitable energy generates the active species like and that can react with the organic polymers to disintegrate them.
Chemical and physical methods are often used to achieve the best results. The sol–gel method by the utilization of bio-inspired alkoxy-silyl hybrid through which functionalized molecules work as linkages between the MPs chains are used. The 3-D agglomerates of MPs thus obtained can be eliminated using the cost-effective filtration methods. Most wastewater and drinking water treatment plants employ the coagulation/flocculation combined with other effective membrane-based techniques such as ultrafiltration, dynamic membrane, membrane bioreactor, and advanced treatment processes such as denitrification and ozonation for the mitigation of MPs. The electrocoagulation technique is relatively economical and better suited for the tertiary treatment, which does not depend on the chemicals or microorganisms, but utilizes floatation, coagulation as well as electrochemical methods. MPs are effectually eradicated in wastewater and drinking water treatment plants where the combination of all such techniques can be used for accomplishing the best results. Nevertheless, complete removal of MPs via utilization of current technologies from the environment is quite a challenging task from the perspective of large-scale applications.
5. Conclusions and future outlook
This study presents an overview of the dangers associated with the MPs and the currently used techniques for their mitigation. Literature reports validate the grievousness of plastic-pollution that has penetrated the safe four walls of our houses. MPs are all around us ranging from air we breathe to the water we drink. The effects of inhalation/ingestion of MPs on living beings could be deleterious and a complete elimination of MPs is quite a difficult task owing to their minute sizes. Moreover, most of the existing studies have investigated MPs with size > 20 μm, but smaller MPs are also abundant in nature.
Future explorations for the mitigation of MPs should emphasize on better analyses techniques and regulating the source of MPs. Better infrastructure, product design, arrangement and sequence of methods for the precise identification of MPs. Continuous and persistent research is need of the day on plastic regulation and approaches for their complete removal. Awareness regarding the waste-management and health-risks related to plastics at large-scale units as well as the individual level is imperative to wipe out the problems from the core to create a benign environment.
Acknowledgments
Ms. Surbhi Sharma is highly grateful to the UGC, New Delhi, India, for a research fellowship. The U.S. Environmental Protection Agency funded and collaborated in the work described here. It has been subjected to the Agency’s review and has approved for publication. Note that approval does not signify that the contents necessarily reflect the views of the Agency. Mention of trade names, products, or services does not convey official EPA approval, endorsement, or recommendation.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Wagner M, Lambert S, Freshwater Microplastics, Springer International Publishing, Cham, 2018, 10.1007/978-3-319-61615-5. [Google Scholar]
- [2].Da Costa JP, Nunes AR, Santos PSM, Girão AV, Duarte AC, Rocha-Santos T, Degradation of polyethylene microplastics in seawater: Insights into the environmental degradation of polymers, Journal of Environmental Science and Health, Part A 53 (9) (2018) 866–875, 10.1080/10934529.2018.1455381. [DOI] [PubMed] [Google Scholar]
- [3].Srivastava RK, Shetti NP, Reddy KR, Aminabhavi TM, Sustainable energy from waste organic matters via efficient microbial processes, Sci. Total Environ. 722 (2020) 137927, 10.1016/j.scitotenv.2020.137927. [DOI] [PubMed] [Google Scholar]
- [4].Mehta A, Mishra A, Basu S, Shetti NP, Reddy KR, Saleh TA, Aminabhavi TM, Band gap tuning and surface modification of carbon dots for sustainable environmental remediation and photocatalytic hydrogen production – A review, J. Environ. Manage. 250 (2019) 109486, 10.1016/j.jenvman.2019.109486. [DOI] [PubMed] [Google Scholar]
- [5].Sharma S, Basu S, Shetti NP, Aminabhavi TM, Waste-to-energy nexus for circular economy and environmental protection: Recent trends in hydrogen energy, Sci. Total Environ. 713 (2020) 136633, 10.1016/j.scitotenv.2020.136633. [DOI] [PubMed] [Google Scholar]
- [6].Sharma S, Kundu A, Basu S, Shetti NP, Aminabhavi TM, Sustainable environmental management and related biofuel technologies, J. Environ. Manage. 273 (2020) 111096, 10.1016/j.jenvman.2020.111096. [DOI] [PubMed] [Google Scholar]
- [7].Mishra A, Basu S, Shetti NP, Reddy KR, Aminabhavi TM, Photocatalysis of Graphene and Carbon Nitride-Based Functional Carbon Quantum Dots, in, Nanoscale Mater. Water Purif, Elsevier; (2019) 759–781, 10.1016/B978-0-12-813926-4.00035-5. [DOI] [Google Scholar]
- [8].Reddy NL, Rao VN, Vijayakumar M, Santhosh R, Anandan S, Karthik M, Shankar MV, Reddy KR, Shetti NP, Nadagouda MN, Aminabhavi TM, A review on frontiers in plasmonic nano-photocatalysts for hydrogen production, Int. J. Hydrogen Energy 44 (21) (2019) 10453–10472, 10.1016/j.ijhydene.2019.02.120. [DOI] [Google Scholar]
- [9].Reddy NR, Bharagav U, Kumari MM, Cheralathan KK, Shankar MV, Reddy KR, Saleh TA, Aminabhavi TM, Highly efficient solar light-driven photocatalytic hydrogen production over Cu/FCNTs-titania quantum dots-based heterostructures, J. Environ. Manage. 254 (2020) 109747, 10.1016/j.jenvman.2019.109747. [DOI] [PubMed] [Google Scholar]
- [10].Mishra A, Shetti NP, Basu S, Reddy KR, Aminabhavi TM, Recent developments in ionic liquid-based electrolytes for energy storage supercapacitors and rechargeable batteries, Green Sustain. Process Chem. Environ. Eng. Sci. (2020) 199–221, 10.1016/B978-0-12-817386-2.00007-X. [DOI] [Google Scholar]
- [11].Mishra A, Shetti NP, Basu S, Raghava Reddy K, Aminabhavi TM, Carbon Cloth-based Hybrid Materials as Flexible Electrochemical Supercapacitors, Chem Electro Chem 6 (23) (2019) 5771–5786, 10.1002/celc.201901122. [DOI] [Google Scholar]
- [12].Mishra A, Mehta A, Basu S, Maiode SJ, Shetti NP, Shukia SS, Nadagouda MN, Aminabhavi TM, Electrode materials for lithium-ion batteries, Materials Science for Energy Technologies 1 (2) (2018) 182–187, 10.1016/j.mset.2018.08.001. [DOI] [Google Scholar]
- [13].Srivastava RK, Shetti NP, Reddy KR, Aminabhavi TM, Biofuels, biodiesel and biohydrogen production using bioprocesses. A review, Environ Chem Lett 18 (4) (2020) 1049–1072, 10.1007/s10311-020-00999-7. [DOI] [Google Scholar]
- [14].Navakoteswara Rao V, Lakshmana Reddy N, Mamatha Kumari M, Ravi P, Sathish M, Kuruvilla KM, Preethi V, Reddy KR, Shetti NP, Aminabhavi TM, Shankar MV, Photocatalytic recovery of H2 from H2S containing wastewater: Surface and interface control of photo-excitons in Cu2S@TiO2 core-shell nanostructures, Appl. Catal. B 254 (2019) 174–185, 10.1016/j.apcatb.2019.04.090. [DOI] [Google Scholar]
- [15].Mishra A, Basu S, Shetti NP, Reddy KR, Metal oxide nanohybrids-based low-temperature sensors for NO2 detection: a short review, J Mater Sci: Mater Electron 30 (9) (2019) 8160–8170, 10.1007/s10854-019-01232-0 [DOI] [Google Scholar]
- [16].Reddy KR, Reddy CHV, Nadagouda MN, Shetti NP, Jaesool S, Aminabhavi TM, Polymeric graphitic carbon nitride (g-C3N4)-based semiconducting nanostructured materials: Synthesis methods, properties and photocatalytic applications, J. Environ. Manage. 238 (2019) 25–40, 10.1016/j.jenvman.2019.02.075. [DOI] [PubMed] [Google Scholar]
- [17].Zhang J, Gao D, Li Q, Zhao Y, Li L.i., Lin H, Bi Q, Zhao Y, Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella, Sci. Total Environ. 704 (2020) 135931, 10.1016/j.scitotenv.2019.135931. [DOI] [PubMed] [Google Scholar]
- [18].Sobhani Z, Lei Y, Tang Y, Wu L, Zhang X, Naidu R, Megharaj M, Fang C, Microplastics generated when opening plastic packaging, Sci Rep 10 (1) (2020), 10.1038/s41598-020-61146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE, Human Consumption of Microplastics, Environ. Sci. Technol. 53 (12) (2019) 7068–7074, 10.1021/acs.est.9b01517.s001. [DOI] [PubMed] [Google Scholar]
- [20].Guo J-J, Huang X-P, Xiang L, Wang Y-Z, Li Y-W, Li H, Cai Q-Y, Mo C-H, Wong M-H, Source, migration and toxicology of microplastics in soil, Environ. Int. 137 (2020) 105263, 10.1016/j.envint.2019.105263. [DOI] [PubMed] [Google Scholar]
- [21].Rochman CM, Browne MA, Halpern BS, Hentschel BT, Hoh E, Karapanagioti HK, Rios-Mendoza LM, Takada H, Teh S, Thompson RC, Classify plastic waste as hazardous, Nature 494 (7436) (2013) 169–171, 10.1038/494169a. [DOI] [PubMed] [Google Scholar]
- [22].Mrosovsky N, Ryan GD, James MC, Leatherback turtles: The menace of plastic, Mar. Pollut. Bull. 58 (2) (2009) 287–289, https://doi.0rg/lO.lOl6/j.marpolbul.2008.10.018. [DOI] [PubMed] [Google Scholar]
- [23].Bayo J, Olmos S, López-Castellanos J, Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors, Chemosphere 238 (2020) 124593, 10.1016/j.chemosphere.2019.124593. [DOI] [PubMed] [Google Scholar]
- [24].Carpenter EJ, Smith KL, Plastics on the Sargasso Sea Surface, Science 175 (4027) (1972) 1240–1241, 10.1126/science:175.4027.1240. [DOI] [PubMed] [Google Scholar]
- [25].Aminabhavi TM, Balundgi RH, Cassidy PE, A Review on Biodegradable Plastics, Polymer-Plastics Technology and Engineering 29 (3) (1990) 235–262, 10.1080/03602559008049843. [DOI] [Google Scholar]
- [26].Cassidy PE, Aminabhavi TM, Enhanced Environmental Degradation of Plastics, Journal of Macromolecular Science, Part C 21 (1) (1981) 89–133, 10.1080/00222358108080926. [DOI] [Google Scholar]
- [27].Pirsaheb M, Hossini H, Makhdoumi P, Review of microplastic occurrence and toxicological effects in marine environment: Experimental evidence of inflammation, Process Saf. Environ. Prot. 142 (2020) 1–14, 10.1016/j.psep.2020.05.050. [DOI] [Google Scholar]
- [28].Dioses-Salinas DC, Pizarro-Ortega CI, De-la-Torre GE, A methodological approach of the current literature on microplastic contamination in terrestrial environments: Current knowledge and baseline considerations, Sci. Total Environ. 730 (2020) 139164, 10.1016/j.scitotenv.2020.139164. [DOI] [PubMed] [Google Scholar]
- [29].Hüffer T, Praetorius A, Wagner S, von der Kammer F, Hofmann T, Microplastic Exposure Assessment in Aquatic Environments: Learning from Similarities and Differences to Engineered Nanoparticles, Environ. Sci. Technol. 51 (5) (2017) 2499–2507, 10.1021/acs.est.6b04054. [DOI] [PubMed] [Google Scholar]
- [30].Waldschläger K, Lechthaler S, Stauch G, Schüttrumpf H, The way of microplastic through the environment – Application of the source-pathway-receptor model (review), Sci. Total Environ. 713 (2020) 136584, 10.1016/j.scitotenv.2020.136584. [DOI] [PubMed] [Google Scholar]
- [31].Bellasi A, Binda G, Pozzi A, Galafassi S, Volta P, Bettinetti R, Microplastic Contamination in Freshwater Environments: A Review, Focusing on Interactions with Sediments and Benthic Organisms, Environments 7 (2020) 30, 10.3390/environments7040030. [DOI] [Google Scholar]
- [32].Laskar N, Kumar U, Plastics and microplastics: A threat to environment, Environ. Technol. Innovation 14 (2019) 100352, 10.1016/j.eti.2019.100352. [DOI] [Google Scholar]
- [33].Burns EE, Boxall ABA, Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps: Microplastics in the environment, Environ Toxicol Chem 37 (11) (2018) 2776–2796, 10.1002/etc.4268. [DOI] [PubMed] [Google Scholar]
- [34].Reimonn G, Lu T, Gandhi N, Chen W-T, Review of Microplastic Pollution in the Environment and Emerging Recycling Solutions, J. Renew. Mater. 7 (2019) 1251–1268. 10.32604/jrm.2019.08055. [DOI] [Google Scholar]
- [35].González-Pleiter M, Tamayo-Belda M, Pulido-Reyes G, Amariei G, Leganés F, Rosal R, Fernández-Piñas F, Secondary nanoplastics released from a biodegradable microplastic severely impact freshwater environments, Environ. Sci.: Nano 6 (5) (2019) 1382–1392, 10.1039/C8EN01427B. [DOI] [Google Scholar]
- [36].Yu Y, Zhou D, Li Z, Zhu C, Advancement and Challenges of Microplastic Pollution in the Aquatic Environment: a Review, Water Air Soil Pollut 229 (5) (2018), 10.1007/s11270-018-3788-z. [DOI] [Google Scholar]
- [37].Pivokonsky M, Cermakova L, Novotna K, Peer P, Cajthaml T, Janda V, Occurrence of microplastics in raw and treated drinking water, Sci. Total Environ. 643 (2018) 1644–1651, 10.1016/j.scitotenv.2018.08.102. [DOI] [PubMed] [Google Scholar]
- [38].Cassidy PE, Aminabhavi TM, Reddy VS, Heat-Resistant Polymers, in: Kirk-Othmer Encycl. Chem. Technol, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2000. 10.1002/0471238961.0805012003011919.a01. [DOI] [Google Scholar]
- [39].Cassidy PE, Aminabhavi TM, Reddy VS, Fitch III JW, Polymers derived from 2-phenyl-1,1,1,3,3,3-hexafluoropropan-2-ol and its derivatives, Eur. Polym. J. 31 (4) (1995) 353–361, 10.1016/0014-3057(94)00159-6. [DOI] [Google Scholar]
- [40].Ma B, Xue W, Hu C, Liu H, Qu J, Li L, Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment, Chem. Eng. J. 359 (2019) 159–167, 10.1016/j.cej.2018.ll.155. [DOI] [Google Scholar]
- [41].Chubarenko I, Bagaev A, Zobkov M, Esiukova E, On some physical and dynamical properties of microplastic particles in marine environment, Mar. Pollut. Bull. 108 (1–2) (2016) 105–112, 10.1016/j.marpolbul.2016.04.048. [DOI] [PubMed] [Google Scholar]
- [42].Conkle JL, Báez Del Valle CD, Turner JW, Are We Underestimating Microplastic Contamination in Aquatic Environments? Environ. Manage. 61 (1) (2018) 1–8, 10.1007/s00267-017-0947-8. [DOI] [PubMed] [Google Scholar]
- [43].Ziajahromi S, Neale PA, Rintoul L, Leusch FDL, Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based micro plastics, Water Res. 112 (2017) 93–99, 10.1016/j.watres.2017.01.042. [DOI] [PubMed] [Google Scholar]
- [44].Anderson AG, Grose J, Pahl S, Thompson RC, Wyles KJ, Microplastics in personal care products: Exploring perceptions of environmentalists, beauticians and students, Mar. Pollut. Bull. 113 (1–2) (2016) 454–460, 10.1016/j.marpolbul.2016.10.048. [DOI] [PubMed] [Google Scholar]
- [45].Murphy F, Ewins C, Carbonnier F, Quinn B, Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment, Environ. Sci. Technol. 50 (11) (2016) 5800–5808, 10.1021/acs.est.5b05416.s001. [DOI] [PubMed] [Google Scholar]
- [46].Su L, Xue Y, Li L, Yang D, Kolandhasamy P, Li D, Shi H, Microplastics in Taihu Lake, China, Environ. Pollut. 216 (2016) 711–719, 10.1016/j.envpol.2016.06.036. [DOI] [PubMed] [Google Scholar]
- [47].Free CM, Jensen OP, Mason SA, Eriksen M, Williamson NJ, Boldgiv B, High-levels of microplastic pollution in a large, remote, mountain lake, Mar. Pollut. Bull. 85 (1) (2014) 156–163, 10.1016/j.marpolbul.2014.06.001. [DOI] [PubMed] [Google Scholar]
- [48].Anderson PJ, Warrack S, Langen V, Challis JK, Hanson ML, Rennie MD, Microplastic contamination in Lake Winnipeg, Canada, Environ. Pollut. 225 (2017) 223–231, 10.1016/j.envpol.2017.02.072. [DOI] [PubMed] [Google Scholar]
- [49].Sruthy S, Ramasamy EV, Microplastic pollution in Vembanad Lake, Kerala, India: The first report of microplastics in lake and estuarine sediments in India, Environ. Pollut. 222 (2017) 315–322, 10.1016/j.envpol.2016.12.038. [DOI] [PubMed] [Google Scholar]
- [50].Mani T, Hauk A, Walter U, Burkhardt-Holm P, Microplastics profile along the Rhine River, Sci Rep 5 (1) (2016), 10.1038/srep17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tibbetts J, Krause S, Lynch I, Sambrook Smith G, Abundance, Distribution, and Drivers of Microplastic Contamination in Urban River Environments, Water. 10 (2018) 1597, 10.3390/w10111597. [DOI] [Google Scholar]
- [52].Cohen JH, Internicola AM, Mason RA, Kukulka T, Observations and Simulations of Microplastic Debris in a Tide, Wind, and Freshwater-Driven Estuarine Environment: the Delaware Bay, Environ. Sci. Technol. 53 (24) (2019) 14204–14211, 10.1021/acs.est.9b04814.s002. [DOI] [PubMed] [Google Scholar]
- [53].Wu N, Zhang Y, Zhao Z, He J, Li W, Li J, Xu W, Ma Y, Niu Z, Colonization characteristics of bacterial communities on microplastics compared with ambient environments (water and sediment) in Haihe Estuary, Sci. Total Environ. 708 (2020) 134876, 10.1016/j.scitotenv.2019.134876. [DOI] [PubMed] [Google Scholar]
- [54].Wright SL, Thompson RC, Galloway TS, The physical impacts of microplastics on marine organisms: A review, Environ. Pollut. 178 (2013) 483–492, 10.1016/j.envpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- [55].Cutroneo L, Reboa A, Besio G, Borgogno F, Canesi L, Canuto S, Dara M, Enrile F, Forioso I, Greco G, Lenoble Veronique, Malatesta A, Mounier S, Petrillo M, Rovetta R, Stocchino A, Tesan J, Vagge G, Capello M, Microplastics in seawater: sampling strategies, laboratory methodologies, and identification techniques applied to port environment, Environ Sci Pollut Res 27 (9) (2020) 8938–8952, 10.1007/s1l356-020-07783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Abidli S, Toumi H, Lahbib Y, Trigui El Menif N, The First Evaluation of Micro plastics in Sediments from the Complex Lagoon-Channel of Bizerte (Northern Tunisia), Water Air Soil Pollut 228 (7) (2017), 10.1007/s11270-017-3439-9. [DOI] [Google Scholar]
- [57].Stolte A, Forster S, Gerdts G, Schubert H, Microplastic concentrations in beach sediments along the German Baltic coast, Mar. Pollut. Bull. 99 (1-2) (2015) 216–229, 10.1016/j.marpolbul.2015.07.022. [DOI] [PubMed] [Google Scholar]
- [58].Veerasingam S, Saha M, Suneel V, Vethamony P, Rodrigues AC, Bhattacharyya S, Naik BG, Characteristics, seasonal distribution and surface degradation features of microplastic pellets along the Goa coast, India, Chemosphere 159 (2016) 496–505, 10.1016/j.chemosphere.2016.06.056. [DOI] [PubMed] [Google Scholar]
- [59].Prata JC, Castro JL, da Costa João.P., Duarte AC, Cerqueira Mário, Rocha-Santos T, An easy method for processing and identification of natural and synthetic microfibers and microplastics in indoor and outdoor air, MethodsX 7 (2020) 100762, 10.1016/j.mex.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wright SL, Ulke J, Font A, Chan KLA, Kelly FJ, Atmospheric microplastic deposition in an urban environment and an evaluation of transport, Environ. Int. 136 (2020) 105411, 10.1016/j.envint.2019.105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dris R, Gasperi J, Mirande Cécile, Mandin C, Guerrouache M, Langlois V, Tassin B, A first overview of textile fibers, including micro plastics, in indoor and outdoor environments, Environ. Pollut. 221 (2017) 453–458, 10.1016/j.envpol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- [62].Zhang Q, Zhao Y, Du F, Cai H, Wang G, Shi H, Microplastic Fallout in Different Indoor Environments, Environ. Sci. Technol. 54 (11) (2020) 6530–6539, 10.1021/acs.est.0c00087.s001. [DOI] [PubMed] [Google Scholar]
- [63].Dris R, Gasperi J, Rocher V, Saad M, Renault N, Tassin B, Microplastic contamination in an urban area: a case study in Greater Paris, Environ. Chem. 12 (5) (2015) 592, 10.1071/EN14167. [DOI] [Google Scholar]
- [64].Liu K, Wu T, Wang X, Song Z, Zong C, Wei N, Li D, Consistent Transport of Terrestrial Microplastics to the Ocean through Atmosphere, Environ. Sci. Technol. 53 (18) (2019) 10612–10619, 10.1021/acs.est.9b03427.s002. [DOI] [PubMed] [Google Scholar]
- [65].Siegfried M, Koelmans AA, Besseling E, Kroeze C, Export of microplastics from land to sea. A modelling approach, Water Res. 127 (2017) 249–257, 10.1016/j.watres.2017.10.011. [DOI] [PubMed] [Google Scholar]
- [66].Syafei AD, Nurasrin NR, Assomadi AF, Boedisantoso R, Microplastic Pollution in the Ambient Air of Surabaya, Indonesia, Curr. World Environ 14 (2) (2019) 290–298, 10.12944/CWE.14.2.13. [DOI] [Google Scholar]
- [67].Fadare OO, Okoffo ED, Covid-19 face masks: A potential source of microplastic fibers in the environment, Sci. Total Environ. 737 (2020) 140279, 10.1016/j.scitotenv.2020.140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mintenig SM, Int-Veen I, Löder MGJ, Primpke S, Gerdts G, Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging, Water Res. 108 (2017) 365–372, 10.1016/j.watres.2016.11.015. [DOI] [PubMed] [Google Scholar]
- [69].Wang Z, Lin T, Chen W, Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP), Sci. Total Environ. 700 (2020) 134520, 10.1016/j.scitotenv.2019.134520. [DOI] [PubMed] [Google Scholar]
- [70].Talvitie J, Mikola A, Koistinen A, Setälä O, Solutions to microplastic pollution – Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies, Water Res. 123 (2017) 401–407, 10.1016/j.watres.2017.07.005. [DOI] [PubMed] [Google Scholar]
- [71].Wei W, Huang Q-S, Sun J, Dai X, Ni B-J, Revealing the Mechanisms of Polyethylene Microplastics Affecting Anaerobic Digestion of Waste Activated Sludge, Environ. Sci. Technol. 53 (16) (2019) 9604–9613, 10.1021/acs.est.9b02971.s001. [DOI] [PubMed] [Google Scholar]
- [72].Maes T, Van der Meulen MD, Devriese LI, Leslie HA, Huvet A, Frère L, Robbens J, Vethaak AD, Microplastics Baseline Surveys at the Water Surface and in Sediments of the North-East Atlantic, Front. Mar. Sci. 4 (2017), 10.3389/fmars.2017.00135. [DOI] [Google Scholar]
- [73].Piehl S, Leibner A, Löder MGJ, Dris R, Bogner C, Laforsch C, Identification and quantification of macro- and microplastics on an agricultural farmland, Sci Rep 8 (1) (2018), 10.1038/s41598-018-36172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Boots B, Russell CW, Green DS, Effects of Microplastics in Soil Ecosystems: Above and Below Ground, Environ. Sci. Technol. 53 (19) (2019) 11496–11506, 10.1021/acs.est.9b03304. [DOI] [PubMed] [Google Scholar]
- [75].Nizzetto L, Futter M, Langaas S, Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 50 (20) (2016) 10777–10779, 10.1021/acs.est.6b04140. [DOI] [PubMed] [Google Scholar]
- [76].Rillig MC, Ziersch L, Hempel S, Microplastic transport in soil by earthworms, Sci Rep 7 (1) (2017), 10.1038/s41598-017-01594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zhu D, Bi Q-F, Xiang Q, Chen Q-L, Christie P, Ke X, Wu L-H, Zhu Y-G, Trophic predator-prey relationships promote transport of microplastics compared with the single Hypoaspis aculeifer and Folsomia Candida, Environ. Pollut. 235 (2018) 150–154, 10.1016/j.envpol.2017.12.058. [DOI] [PubMed] [Google Scholar]
- [78].Dawson A, Huston W, Kawaguchi S, King C, Cropp R, Wild S, Eisenmann P, Townsend K, Bengtson Nash S, Uptake and Depuration Kinetics Influence Microplastic Bioaccumulation and Toxicity in Antarctic Krill (Euphausia superba), Environ. Sci. Technol. 52 (5) (2018) 3195–3201, 10.1021/acs.est.7b05759.s001. [DOI] [PubMed] [Google Scholar]
- [79].Karami A, Romano N, Galloway T, Hamzah H, Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus), Environ. Res. 151 (2016) 58–70, 10.1016/j.envres.2016.07.024. [DOI] [PubMed] [Google Scholar]
- [80].Zeng EY, Microplastic contamination in aquatic environments: An emerging matter of environmental urgency, Elsevier; (2018), 10.1016/C2016-0-04784-8. [DOI] [Google Scholar]
- [81].Reddy AVB, Moniruzzaman M, Aminabhavi TM, Polychlorinated biphenyls (PCBs) in the environment: Recent updates on sampling, pretreatment, cleanup technologies and their analysis, Chem. Eng. J. 358 (2019) 1186–1207, 10.1016/j.cej.2018.09.205. [DOI] [Google Scholar]
- [82].Rathi A, Basu S, Barman S, Adsorptive removal of fipronil from its aqueous solution by modified zeolite HZSM-5: Equilibrium, kinetic and thermodynamic study, J. Mol. Liq. 283 (2019) 867–878, 10.1016/j.molliq.2019.02.140. [DOI] [Google Scholar]
- [83].Imran M, Das KR, Naik MM, Co-selection of multi-antibiotic resistance in bacterial pathogens in metal and microplastic contaminated environments: An emerging health threat, Chemosphere 215 (2019) 846–857, 10.1016/j.chemosphere.2018.10.114. [DOI] [PubMed] [Google Scholar]
- [84].Koelmans AA, Bakir A, Burton GA, Janssen CR, Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies, Environ. Sci. Technol. 50 (7) (2016) 3315–3326, 10.1021/acs.est.5b06069.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Velzeboer I, Kwadijk CJAF, Koelmans AA, Strong Sorption of PCBs to Nanoplastics, Microplastics, Carbon Nanotubes, and Fullerenes, Environ. Sci. Technol. 48 (9) (2014) 4869–4876, 10.1021/es405721v. [DOI] [PubMed] [Google Scholar]
- [86].Carr SA, Liu J, Tesoro AG, Transport and fate of microplastic particles in wastewater treatment plants, Water Res. 91 (2016) 174–182, 10.1016/j.watres.2016.01.002. [DOI] [PubMed] [Google Scholar]
- [87].Razanajatovo RM, Ding J, Zhang S, Jiang H, Zou H, Sorption and desorption of selected pharmaceuticals by polyethylene microplastics, Mar. Pollut. Bull. 136 (2018) 516–523, 10.1016/j.marpolbul.2018.09.048. [DOI] [PubMed] [Google Scholar]
- [88].Zou J, Liu X, Zhang D, Yuan X, Adsorption of three bivalent metals by four chemical distinct microplastics, Chemosphere 248 (2020) 126064, 10.1016/j.chemosphere.2020.126064. [DOI] [PubMed] [Google Scholar]
- [89].Turner A, Holmes L, Thompson RC, Fisher AS, Metals and marine microplastics: Adsorption from the environment versus addition during manufacture, exemplified with lead, Water Res. 173 (2020) 115577, 10.1016/j.watres.2020.115577. [DOI] [PubMed] [Google Scholar]
- [90].Lang M, Yu X, Liu J, Xia T, Wang T, Jia H, Guo X, Fenton aging significantly affects the heavy metal adsorption capacity of polystyrene microplastics, Sci. Total Environ. 722 (2020) 137762, [DOI] [PubMed] [Google Scholar]
- [91].Hodson ME, Duffus-Hodson CA, Clark A, Prendergast-Miller MT, Thorpe KL, Plastic Bag Derived-Microplastics as a Vector for Metal Exposure in Terrestrial Invertebrates, Environ. Sci. Technol. 51 (8) (2017) 4714–4721, 10.1021/acs.est.7b00635.s001. [DOI] [PubMed] [Google Scholar]
- [92].Holmes LA, Turner A, Thompson RC, Adsorption of trace metals to plastic resin pellets in the marine environment, Environ. Pollut. 160 (2012) 42–48, 10.1016/j.envpol.2011.08.052. [DOI] [PubMed] [Google Scholar]
- [93].Gao F, Li J, Sun C, Zhang L, Jiang F, Cao W, Zheng L, Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment, Mar. Pollut. Bull. 144 (2019) 61–67, 10.1016/j.marpolbul.2019.04.039. [DOI] [PubMed] [Google Scholar]
- [94].Suhrhoff TJ, Scholz-Böttcher BM, Qualitative impact of salinity, UV radiation and turbulence on leaching of organic plastic additives from four common plastics — A lab experiment, Mar. Pollut. Bull. 102 (1) (2016) 84–94, 10.1016/j.marpolbul.2015.11.054. [DOI] [PubMed] [Google Scholar]
- [95].Beltifa A, Feriani A, Machreki M, Ghorbel A, Ghazouani L, Di Bella G, Van Loco J, Reyns T, Mansour HB, Plasticizers and bisphenol A, in packaged foods sold in the Tunisian markets: study of their acute in vivo toxicity and their environmental fate, Environ Sci Pollut Res 24 (28) (2017) 22382–22392, 10.1007/s11356-017-9861-0. [DOI] [PubMed] [Google Scholar]
- [96].Wade MG, Kawata A, Rigden M, Caldwell D, Holloway AC, Toxicity of Flame Retardant Isopropylated Triphenyl Phosphate: Liver, Adrenal, and Metabolic Effects, Int J Toxicol 38 (4) (2019) 279–290, 10.1177/1091581819851502. [DOI] [PubMed] [Google Scholar]
- [97].Nakata H, Shinohara R-I, Nakazawa Y, Isobe T, Sudaryanto A, Subramanian A, Tanabe S, Zakaria MP, Zheng GJ, Lam PKS, Kim EY, Min B-Y, We S-U, Viet PH, Tana TS, Prudente M, Frank D, Lauenstein G, Kannan K, Asia–Pacific mussel watch for emerging pollutants: Distribution of synthetic musks and benzotriazole UV stabilizers in Asian and US coastal waters, Mar. Pollut. Bull. 64 (10) (2012) 2211–2218, 10.1016/j.marpolbul.2012.07.049. [DOI] [PubMed] [Google Scholar]
- [98].Jang M, Shim WJ, Han GM, Rani M, Song YK, Hong SH, Styrofoam Debris as a Source of Hazardous Additives for Marine Organisms, Environ. Sci. Technol. 50 (10) (2016) 4951–4960, 10.1021/acs.est.5b05485.s001. [DOI] [PubMed] [Google Scholar]
- [99].Rani M, Shim WJ, Han GM, Jang M, Song YK, Hong SH, Benzotriazole-type ultraviolet stabilizers and antioxidants in plastic marine debris and their new products, Sci. Total Environ. 579 (2017) 745–754, [DOI] [PubMed] [Google Scholar]
- [100].Barboza Luís.G.A., Cunha SC, Monteiro C, Fernandes José.O., Guilhermino Lúcia, Bisphenol A and its analogs in muscle and liver of fish from the North East Atlantic Ocean in relation to microplastic contamination. Exposure and risk to human consumers, J. Hazard. Mater. 393 (2020) 122419, 10.1016/j.jhazmat.2020.122419. [DOI] [PubMed] [Google Scholar]
- [101].Carchia E, Porreca I, Almeida PJ, D’Angelo F, Cuomo D, Ceccarelli M, De Felice M, Mallardo M, Ambrosino C, Evaluation of low doses BPA-induced perturbation of glycemia by toxicogenomics points to a primary role of pancreatic islets and to the mechanism of toxicity, Cell Death Dis 6 (10) (2015) el959, 10.1038/cddis.2015.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Molkenthin M, Olmez-Hanci T, Jekel MR, Arslan-Alaton I, Photo-Fenton-like treatment of BPA: Effect of UV light source and water matrix on toxicity and transformation products, Water Res. 47 (14) (2013) 5052–5064, 10.1016/j.watres.2013.05.051. [DOI] [PubMed] [Google Scholar]
- [103].Xu Z, Xiong X, Zhao Y, Xiang W, Wu C, Pollutants delivered every day: Phthalates in plastic express packaging bags and their leaching potential, J. Hazard. Mater. 384 (2020) 121282, 10.1016/j.jhazmat.2019.121282. [DOI] [PubMed] [Google Scholar]
- [104].Posnack NG, The Adverse Cardiac Effects of Di(2-ethylhexyl)phthalate and Bisphenol A, Cardiovasc Toxicol 14 (4) (2014) 339–357, 10.1007/s12012-014-9258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Jinhui S, Sudong X, Yan N, Xia P, Jiahao Q, Yongjian X, Effects of microplastics and attached heavy metals on growth, immunity, and heavy metal accumulation in the yellow seahorse, Hippocampus kuda Bleeker, Mar. Pollut. Bull. 149 (2019) 110510, 10.1016/j.marpolbul.2019.110510. [DOI] [PubMed] [Google Scholar]
- [106].Van Cauwenberghe L, Claessens M, Vandegehuchte MB, Janssen CR, Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats, Environ. Pollut. 199 (2015) 10–17, 10.1016/j.envpol.2015.01.008. [DOI] [PubMed] [Google Scholar]
- [107].Jahan S, Strezov V, Weldekidan H, Kumar R, Kan T, Sarkodie SA, He J, Dastjerdi B, Wilson SP, Interrelationship of microplastic pollution in sediments and oysters in a seaport environment of the eastern coast of Australia, Sci. Total Environ. 695 (2019) 133924, 10.1016/j.scitotenv.2019.133924. [DOI] [PubMed] [Google Scholar]
- [108].Álvarez G, Barros Álvaro, Velando A, The use of European shag pellets as indicators of microplastic fibers in the marine environment, Mar. Pollut. Bull. 137 (2018) 444–448, 10.1016/j.marpolbul.2018.10.050. [DOI] [PubMed] [Google Scholar]
- [109].Welden NAC, Cowie PR, Environment and gut morphology influence microplastic retention in langoustine, Nephrops norvegicus, Environ. Pollut. 214 (2016) 859–865, 10.1016/j.envpol.2016.03.067. [DOI] [PubMed] [Google Scholar]
- [110].Jovanović B, Ingestion of microplastics by fish and its potential consequences from a physical perspective: Potential Consequences of Fish Ingestion of Microplastic, Integr Environ Assess Manag 13 (3) (2017) 510–515, 10.1002/ieam.l913. [DOI] [PubMed] [Google Scholar]
- [111].Avio CG, Gorbi S, Regoli F, Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea, Marine Environmental Research 111 (2015) 18–26, 10.1016/j.marenvres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- [112].Rochman CM, Kurobe T, Flores I, Teh SJ, Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment, Sci. Total Environ. 493 (2014) 656–661, 10.1016/j.scitotenv.2014.06.051. [DOI] [PubMed] [Google Scholar]
- [113].Foley CJ, Feiner ZS, Malinich TD, Höök TO, A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates, Sci. Total Environ. 631-632 (2018) 550–559, 10.1016/j.scitotenv.2018.03.046. [DOI] [PubMed] [Google Scholar]
- [114].Pittura L, Avio CG, Giuliani ME, D’Errico G, Keiter SH, Cormier B, Gorbi S, Regoli F, Microplastics as Vehicles of Environmental PAHs to Marine Organisms: Combined Chemical and Physical Hazards to the Mediterranean Mussels, Mytilus galloprovincialis, Front. Mar. Sci. 5 (2018), 10.3389/fmars.2018.00103. [DOI] [Google Scholar]
- [115].Schwabl P, Köppel S, Königshofer P, Bucsics T, Trauner M, Reiberger T, Liebmann B, Detection of Various Microplastics in Human Stool: A Prospective Case Series, Ann Intern Med 171 (7) (2019) 453, 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- [116].Kutralam-Muniasamy G, Pérez-Guevara F, Elizalde-Martínez I, Shruti VC, Branded milks – Are they immune from microplastics contamination? Sci. Total Environ. 714 (2020) 136823, 10.1016/j.scitotenv.2020.136823. [DOI] [PubMed] [Google Scholar]
- [117].Liebezeit G, Liebezeit E, Non-pollen particulates in honey and sugar, Food Additives & Contaminants: Part A 30 (12) (2013) 2136–2140, 10.1080/19440049.2013.843025. [DOI] [PubMed] [Google Scholar]
- [118].Kim J-S, Lee H-J, Kim S-K, Kim H-J, Global Pattern of Microplastics (MPs) in Commercial Food-Grade Salts: Sea Salt as an Indicator of Seawater MP Pollution, Environ. Sci. Technol. 52 (21) (2018) 12819–12828, 10.1021/acs.est.8b04180.s001. [DOI] [PubMed] [Google Scholar]
- [119].Schymanski D, Goldbeck C, Humpf H-U, Fürst P, Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water, Water Res. 129 (2018) 154–162, 10.1016/j.watres.2017.11.011. [DOI] [PubMed] [Google Scholar]
- [120].Zuccarello P, Ferrante M, Cristaldi A, Copat C, Grasso A, Sangregorio D, Fiore M, Oliveri Conti G, Exposure to microplastics (< 10 μm) associated to plastic bottles mineral water consumption: The first quantitative study, Water Res. 157 (2019) 365–371, 10.1016/j.watres.2019.03.091. [DOI] [PubMed] [Google Scholar]
- [121].Shruti VC, Pérez-Guevara F, Kutralam-Muniasamy G, Metro station free drinking water fountain- A potential “microplastics hotspot” for human consumption, Environ. Pollut. 261 (2020) 114227, 10.1016/j.envpol.2020.114227. [DOI] [PubMed] [Google Scholar]
- [122].Shruti VC, Pérez-Guevara F, Elizalde-Martínez I, Kutralam-Muniasamy G, First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks - Future research and environmental considerations, Sci. Total Environ. 726 (2020) 138580, 10.1016/j.scitotenv.2020.138580. [DOI] [PubMed] [Google Scholar]
- [123].Zhang J, Wang L, Kannan K, Microplastics in house dust from 12 countries and associated human exposure, Environ. Int. 134 (2020) 105314, 10.1016/j.envint.2019.105314. [DOI] [PubMed] [Google Scholar]
- [124].Auta HS, Emenike CU, Jayanthi B, Fauziah SH, Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment, Mar. Pollut. Bull. 127 (2018) 15–21, 10.1016/j.marpolbul.2017.11.036. [DOI] [PubMed] [Google Scholar]
- [125].Coppock RL, Cole M, Lindeque PK, Queirós AM, Galloway TS, A small-scale, portable method for extracting microplastics from marine sediments, Environ. Pollut. 230 (2017) 829–837, 10.1016/j.envpol.2017.07.017. [DOI] [PubMed] [Google Scholar]
- [126].Napper IE, Bakir A, Rowland SJ, Thompson RC, Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics, Mar. Pollut. Bull. 99 (1-2) (2015) 178–185, 10.1016/j.marpolbul.2015.07.029. [DOI] [PubMed] [Google Scholar]
- [127].Hidayaturrahman H, Lee T-G, A study on characteristics of microplastic in wastewater of South Korea: Identification, quantification, and fate of microplastics during treatment process, Mar. Pollut. Bull. 146 (2019) 696–702, 10.1016/j.marpolbul.2019.06.071. [DOI] [PubMed] [Google Scholar]
- [128].Choi JS, Jung Y-J, Hong N-H, Hong SH, Park J-W, Toxicological effects of irregularly shaped and spherical microplastics in a marine teleost, the sheepshead minnow (Cyprinodon variegatus), Mar. Pollut. Bull. 129 (1) (2018) 231–240, 10.1016/j.marpolbul.2018.02.039. [DOI] [PubMed] [Google Scholar]
- [129].Liu C, Li J, Zhang Y, Wang L, Deng J, Gao Y, Yu L, Zhang J, Sun H, Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure, Environ. Int. 128 (2019) 116–124, 10.1016/j.envint.2019.04.024. [DOI] [PubMed] [Google Scholar]
- [130].Dehghani S, Moore F, Akhbarizadeh R, Microplastic pollution in deposited urban dust, Tehran metropolis, Iran,, Environ Sci Pollut Res 24 (25) (2017) 20360–20371, 10.1007/s11356-017-9674-1. [DOI] [PubMed] [Google Scholar]
- [131].Gray AD, Weinstein JE, Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio) : Uptake and retention of microplastics in grass shrimp, Environ Toxicol Chem 36 (11) (2017) 3074–3080, 10.1002/etc.3881. [DOI] [PubMed] [Google Scholar]
- [132].Lee K-W, Shim WJ, Kwon OY, Kang J-H, Size-Dependent Effects of Micro Polystyrene Particles in the Marine Copepod Tigriopus japonicus, Environ. Sci. Technol. 47 (19) (2013) 11278–11283, 10.1021/es401932b. [DOI] [PubMed] [Google Scholar]
- [133].Qiao R, Deng Y, Zhang S, Wolosker MB, Zhu Q, Ren H, Zhang Y, Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish, Chemosphere 236 (2019) 124334, 10.1016/j.chemosphere.2019.07.065. [DOI] [PubMed] [Google Scholar]
- [134].Mazurais D, Ernande B, Quazuguel P, Severe A, Huelvan C, Madec L, Mouchel O, Soudant P, Robbens J, Huvet A, Zambonino-Infante J, Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae, Marine Environmental Research 112 (2015) 78–85, 10.1016/j.marenvres.2015.09.009. [DOI] [PubMed] [Google Scholar]
- [135].Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, Ding L, Ren H, Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver, Environ. Sci. Technol. 50 (7) (2016) 4054–4060, 10.1021/acs.est.6b00183.s001. [DOI] [PubMed] [Google Scholar]
- [136].Au SY, Bruce TF, Bridges WC, Klaine SJ, Responses of Hyalella azteca to acute and chronic microplastic exposures : Effects of Microplastic Exposure on Hyalella azteca, Environ Toxicol Chem 34 (11) (2015) 2564–2572, 10.1002/etc.3093. [DOI] [PubMed] [Google Scholar]
- [137].Paço A, Duarte Kátia, da Costa João.P., Santos Patrícia.S.M., Pereira R, Pereira ME, Freitas AC, Duarte AC, Rocha-Santos TAP, Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum, Sci. Total Environ. 586 (2017) 10–15, 10.1016/j.scitotenv.2017.02.017. [DOI] [PubMed] [Google Scholar]
- [138].Auta HS, Emenike CU, Fauziah SH, Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation, Environ. Pollut. 231 (2017) 1552–1559, 10.1016/j.envpol.2017.09.043. [DOI] [PubMed] [Google Scholar]
- [139].Harshvardhan K, Jha B, Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India, Mar. Pollut. Bull. 77 (1-2) (2013) 100–106, 10.1016/j.marpolbul.2013.10.025. [DOI] [PubMed] [Google Scholar]
- [140].Jeon HJ, Kim MN, Functional analysis of alkane hydroxylase system derived from Pseudomonas aeruginosa E7 for low molecular weight polyethylene biodegradation, Int. Biodeterior. Biodegrad. 103 (2015) 141–146, 10.1016/j.ibiod.2015.04.024. [DOI] [Google Scholar]
- [141].Gajendiran A, Krishnamoorthy S, Abraham J, Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil, 3, Biotech. 6 (2016) 1–6, 10.1007/s13205-016-0394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Rummel CD, Jahnke A, Gorokhova E, Kühnel D, Schmitt-Jansen M, Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment, Environ. Sci. Technol. Lett. 4 (7) (2017) 258–267, 10.1021/acs.estlett.7b00164.s001. [DOI] [Google Scholar]
- [143].Harrison JP, Hoellein TJ, Sapp M, Tagg AS, Ju-Nam Y, Ojeda JJ, Microplastic-Associated Biofilms: A Comparison of Freshwater and Marine Environments, in, Microplastic Contam. Aquat. Environ. An Emerg. Matter Environ. Urgency (2018) 181–201, 10.1007/978-3-319-61615-5_9. [DOI] [Google Scholar]
- [144].Gilan I, Hadar Y, Sivan A, Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber, Appl. Microbiol. Biotechnol. 65 (2004) 97–104, 10.1007/s00253-004-1584-8. [DOI] [PubMed] [Google Scholar]
- [145].Park SY, Kim CG, Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site, Chemosphere 222 (2019) 527–533, 10.1016/j.chemosphere.2019.01.159. [DOI] [PubMed] [Google Scholar]
- [146].Han X, Liu W, Huang J-W, Ma J, Zheng Y, Ko T-P, Xu L, Cheng Y-S, Chen C-C, Guo R-T, Structural insight into catalytic mechanism of PET hydrolase, Nat Commun 8 (1) (2017), 10.1038/s41467-017-02255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Chen Z, Zhao W, Xing R, Xie S, Yang X, Cui P, Lü J, Liao H, Yu Z, Wang S, Zhou S, Enhanced in situ biodegradation of microplastics in sewage sludge using hyperthermophilic composting technology, J. Hazard. Mater. 384 (2020) 121271, 10.1016/j.jhazmat.2019.121271. [DOI] [PubMed] [Google Scholar]
- [148].Gong J, Kong T, Li Y, Li Q, Li Z, Zhang J, Biodegradation of Microplastic Derived from Poly(ethylene terephthalate) with Bacterial Whole-Cell Biocatalysts, Polymers (Basel). 10 (2018) 1326, 10.3390/polym10121326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Li R, Liu Y, Sheng Y, Xiang Q, Zhou Y, Cizdziel JV, Effect of prothioconazole on the degradation of microplastics derived from mulching plastic film: Apparent change and interaction with heavy metals in soil, Environ. Pollut. 260 (2020) 113988, 10.1016/j.envpol.2020.113988. [DOI] [PubMed] [Google Scholar]
- [150].Shabbir S, Faheem M, Ali N, Kerr PG, Wang L-F, Kuppusamy S, Li Y, Periphytic biofilm: An innovative approach for biodegradation of microplastics, Sci. Total Environ. 717 (2020) 137064, 10.1016/j.scitotenv.2020.137064. [DOI] [PubMed] [Google Scholar]
- [151].Cunha César, Faria M, Nogueira N, Ferreira A, Cordeiro N, Marine vs freshwater microalgae exopolymers as biosolutions to microplastics pollution, Environ. Pollut. 249 (2019) 372–380, 10.1016/j.envpol.2019.03.046. [DOI] [PubMed] [Google Scholar]
- [152].Arossa S, Martin C, Rossbach S, Duarte CM, Microplastic removal by Red Sea giant clam (Tridacna maxima), Environ. Pollut. 252 (2019) 1257–1266, 10.1016/j.envpol.2019.05.149. [DOI] [PubMed] [Google Scholar]
- [153].Sun C, Wang Z, Chen L, Li F, Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups, Chem. Eng. J. 393 (2020) 124796, 10.1016/j.cej.2020.124796. [DOI] [Google Scholar]
- [154].Duan B, Chang C, Ding B, Cai J, Xu M, Feng S, Ren J, Shi X, Du Y, Zhang L, High strength films with gas-barrier fabricated from chitin solution dissolved at low temperature, J. Mater. Chem. A 1 (5) (2013) 1867–1874, 10.1039/C2TA00068G. [DOI] [Google Scholar]
- [155].Sheng G, Huang C, Chen G, Sheng J, Ren X, Hu B, Ma J, Wang X, Huang Y, Alsaedi A, Hayat T, Adsorption and co-adsorption of graphene oxide and Ni(II) on iron oxides: A spectroscopic and microscopic investigation, Environ. Pollut. 233 (2018) 125–131, 10.1016/j.envpol.2017.10.047. [DOI] [PubMed] [Google Scholar]
- [156].Choudhary M, Kumar R, Neogi S, Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+2 and Ni+2 from water, J. Hazard. Mater. 392 (2020) 122441, 10.1016/j.jhazmat.2020.122441. [DOI] [PubMed] [Google Scholar]
- [157].Regkouzas P, Diamadopoulos E, Adsorption of selected organic micro-pollutants on sewage sludge biochar, Chemosphere 224 (2019) 840–851, 10.1016/j.chemosphere.2019.02.165. [DOI] [PubMed] [Google Scholar]
- [158].Siipola V, Pflugmacher S, Romar H, Wendling L, Koukkari P, Low-Cost Biochar Adsorbents for Water Purification Including Microplastics Removal, Appl. Sci. 10 (2020) 788, 10.3390/app10030788. [DOI] [Google Scholar]
- [159].Misra A, Zambrzycki C, Kloker G, Kotyrba A, Anjass MH, Franco Castillo I, Mitchell SG, Giütel R, Streb C, Water Purification and Microplastics Removal Using Magnetic Polyoxometalate-Supported Ionic Liquid Phases (magPOM-SILPs), Angew. Chem. Int. Ed. 59 (4) (2020) 1601–1605, 10.1002/anie.201912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Tang L, Liu Y, Wang J, Zeng G, Deng Y, Dong H, Feng H, Wang J, Peng B, Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism, Appl. Catal. B 231 (2018) 1–10, 10.1016/j.apcatb.2018.02.059. [DOI] [Google Scholar]
- [161].Khaghani R, Kakavandi B, Ghadirinejad K, Dehghani Fard E, Asadi A, Preparation, characterization and catalytic potential of γ-Fe2O3@AC mesoporous heterojunction for activation of peroxymonosulfate into degradation of cyfluthrin insecticide, Microporous Mesoporous Mater. 284 (2019) 111–121, 10.1016/j.micromeso.2019.04.013. [DOI] [Google Scholar]
- [162].Kang J, Zhou L, Duan X, Sun H, Ao Z, Wang S, Degradation of Cosmetic Microplastics via Functionalized Carbon Nanosprings, Matter 1 (3) (2019) 745–758, 10.1016/j.matt.2019.06.004. [DOI] [Google Scholar]
- [163].Duan X, Sun H, Wang Y, Kang J, Wang S, N-Doping-Induced Nonradical Reaction on Single-Walled Carbon Nanotubes for Catalytic Phenol Oxidation, ACS Catal. 5 (2) (2015) 553–559, 10.1021/cs5017613. [DOI] [Google Scholar]
- [164].Zhou D, Chen L, Li J, Wu F, Transition metal catalyzed sulfite auto-oxidation systems for oxidative decontamination in waters: A state-of-the-art minireview, Chem. Eng. J 346 (2018) 726–738, 10.1016/j.cej.2018.04.016. [DOI] [Google Scholar]
- [165].Zhang L, Zhang J, Zeng G, Dong H, Chen Y, Huang C, Zhu Y, Xu R, Cheng Y, Hou K, Cao W, Fang W, Multivariate relationships between microbial communities and environmental variables during co-composting of sewage sludge and agricultural waste in the presence of PVP-AgNPs, Bioresour. Technol. 261 (2018) 10–18, 10.1016/j.biortech.2018.03.089. [DOI] [PubMed] [Google Scholar]
- [166].Ye S, Cheng M, Zeng G, Tan X, Wu H, Liang J, Shen M, Song B, Liu J, Yang H, Zhang Y, Insights into catalytic removal and separation of attached metals from natural-aged microplastics by magnetic biochar activating oxidation process, Water Res. 179 (2020) 115876, 10.1016/j.watres.2020.115876. [DOI] [PubMed] [Google Scholar]
- [167].Zhang P, Tan X, Liu S, Liu Y, Zeng G, Ye S, Yin Z, Hu X, Liu N, Catalytic degradation of estrogen by persulfate activated with iron-doped graphitic biochar: Process variables effects and matrix effects, Chem. Eng. J. 378 (2019) 122141, 10.1016/j.cej.2019.122141. [DOI] [Google Scholar]
- [168].Li S, Liu H, Gao R, Abdurahman A, Dai J, Zeng F, Aggregation kinetics of microplastics in aquatic environment: Complex roles of electrolytes, pH, and natural organic matter, Environ. Pollut. 237 (2018) 126–132, 10.1016/j.envpol.2018.02.042. [DOI] [PubMed] [Google Scholar]
- [169].Sharma S, Basu S, Highly reusable visible light active hierarchical porous WO3/SiO2 monolith in centimeter length scale for enhanced photocatalytic degradation of toxic pollutants, Sep. Purif. Technol. 231 (2020) 115916, 10.1016/j.seppur.2019.115916. [DOI] [Google Scholar]
- [170].Aanchal, Barman S, Basu S, Complete removal of endocrine disrupting compound and toxic dye by visible light active porous g-C3N4/H-ZSM-5 nanocomposite, Chemosphere 241 (2020) 124981, 10.1016/j.chemosphere.2019.124981. [DOI] [PubMed] [Google Scholar]
- [171].Monga D, Basu S, Enhanced photocatalytic degradation of industrial dye by g-C3N4/TiO2 nanocomposite: Role of shape of TiO2, Adv. Powder Technol. 30 (5) (2019) 1089–1098, 10.1016/j.apt.2019.03.004. [DOI] [Google Scholar]
- [172].Reddy CV, Reddy IN, Ravindranadh K, Reddy KR, Shetti NP, Kim D, Shim J, Aminabhavi TM, Copper-doped ZrO2 nanoparticles as high-performance catalysts for efficient removal of toxic organic pollutants and stable solar water oxidation, J. Environ. Manage. 260 (2020) 110088, 10.1016/j.jenvman.2020.110088. [DOI] [PubMed] [Google Scholar]
- [173].Venkata Reddy C, Reddy KR, Shetti NP, Mishra A, Basu S, Recent Progress in TiO2- and ZnO-Based Nanostructured Hybrid Photocatalysts for Water Purification and Hydrogen Generation, Nanoscale Mater. Water Purif. (2019) 815–843, 10.1016/b978-0-12-813926-4.00039-2. [DOI] [Google Scholar]
- [174].Reddy CV, Reddy IN, Harish VVN, Reddy KR, Shetti NP, Shim J, Aminabhavi TM, Efficient removal of toxic organic dyes and photoelectrochemical properties of iron-doped zirconia nanoparticles, Chemosphere 239 (2020) 124766, 10.1016/j.chemosphere.2019.124766. [DOI] [PubMed] [Google Scholar]
- [175].Dong S, Feng J, Fan M, Pi Y, Hu L, Han X, Liu M, Sun J, Sun J, Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: a review, RSC Adv. 5 (19) (2015) 14610–14630, 10.1039/C4RA13734E. [DOI] [Google Scholar]
- [176].Shang J, Chai M, Zhu Y, Solid-phase photocatalytic degradation of polystyrene plastic with TiO2 as photocatalyst, J. Solid State Chem. 174 (1) (2003) 104–110, https://doi.org/10.l016/S0022-4596(03)00183-X. [Google Scholar]
- [177].Thomas RT, Nair V, Sandhyarani N, TiO2 nanoparticle assisted solid phase photocatalytic degradation of polythene film: A mechanistic investigation, Colloids Surf., A 422 (2013) 1–9, 10.1016/j.colsurfa.2013.01.017. [DOI] [Google Scholar]
- [178].Thomas RT, Sandhyarani N, Enhancement in the photocatalytic degradation of low density polyethylene-TiO2 nanocomposite films under solar irradiation, RSC Adv. 3 (33) (2013) 14080, 10.1039/c3ra42226g. [DOI] [Google Scholar]
- [179].Verma R, Singh S, Dalai MK, Saravanan M, Agrawal VV, Srivastava AK, Photocatalytic degradation of polypropylene film using TiO2-based nanomaterials under solar irradiation, Mater. Des. 133 (2017) 10–18, 10.1016/j.matdes.2017.07.042. [DOI] [Google Scholar]
- [180].Li S, Xu S, He L, Xu F, Wang Y, Zhang L, Photocatalytic Degradation of Polyethylene Plastic with Polypyrrole/TiO 2 Nanocomposite as Photocatalyst, Polymer-Plastics Technology and Engineering 49 (4) (2010) 400–406, 10.1080/03602550903532166. [DOI] [Google Scholar]
- [181].Tofa TS, Kunjali KL, Paul S, Dutta J, Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods, Environ Chem Lett 17 (3) (2019) 1341–1346, 10.1007/s10311-019-00859-z. [DOI] [Google Scholar]
- [182].Tofa TS, Ye F, Kunjali KL, Dutta J, Enhanced Visible Light Photodegradation of Microplastic Fragments with Plasmonic Platinum/Zinc Oxide Nanorod Photocatalysts, Catalysts. 9 (2019) 819, 10.3390/catal9100819. [DOI] [Google Scholar]
- [183].Sekino T, Takahashi S, Takamasu K, Fundamental Study on Nanoremoval Processing Method for Microplastic Structures Using Photocatalyzed Oxidation, Key Eng. Mater. 523–524 (2012) 610–614, 10.4028/www.scientific.net/KEM.523-524.610. [DOI] [Google Scholar]
- [184].Wang L, Kaeppler A, Fischer D, Simmchen J, Photocatalytic TiO 2 Micromotors for Removal of Microplastics and Suspended Matter, ACS Appl. Mater. Interfaces 11 (36) (2019) 32937–32944, 10.1021/acsami.9b06128.s010. [DOI] [PubMed] [Google Scholar]
- [185].Ariza-Tarazona MC, Villarreal-Chiu JF, Barbieri V, Siligardi C, Cedillo-González EI, New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor, Ceram. Int. 45 (7) (2019) 9618–9624, 10.1016/j.ceramint.2018.10.208. [DOI] [Google Scholar]
- [186].Ariza-Tarazona MC, Villarreal-Chiu JF, Hernández-López JM, De la Rosa J. Rivera, Barbieri V, Siligardi C, Cedillo-González EI, Microplastic pollution reduction by a carbon and nitrogen-doped TiO2: Effect of pH and temperature in the photocatalytic degradation process, J. Hazard. Mater. 395 (2020) 122632, 10.1016/j.jhazmat.2020.122632. [DOI] [PubMed] [Google Scholar]
- [187].Herbort AF, Schuhen K, A concept for the removal of microplastics from the marine environment with innovative host-guest relationships, Environ Sci Pollut Res 24 (12) (2017) 11061–11065, 10.1007/s11356-016-7216-x. [DOI] [PubMed] [Google Scholar]
- [188].Ratola N, Ramos S, Homem V, Silva JA, Jiménez-Guerrero P, Amigo JM, Santos L, Alves A, Using air, soil and vegetation to assess the environmental behaviour of siloxanes, Environ Sci Pollut Res 23 (4) (2016) 3273–3284, 10.1007/s11356-015-5574-4. [DOI] [PubMed] [Google Scholar]
- [189].Hurkes N, Ehmann HMA, List M, Spirk S, Bussiek M, Belaj F, Pietschnig R, Silanol-Based Surfactants: Synthetic Access and Properties of an Innovative Class of Environmentally Benign Detergents, Chem. Eur. J. 20 (30) (2014) 9330–9335, 10.1002/chem.201402857. [DOI] [PubMed] [Google Scholar]
- [190].Neumeyer F, Auner N, One-Step Synthesis of Siloxanes from the Direct Process Disilane Residue, Chem. Eur. J. 22 (48) (2016) 17165–17168, 10.1002/chem.201603842. [DOI] [PubMed] [Google Scholar]
- [191].Al-Oweini R, El-Rassy H, Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R″Si(OR′)3 precursors, J. Mol. Struct. 919 (1-3) (2009) 140–145, 10.1016/j.molstruc.2008.08.025. [DOI] [Google Scholar]
- [192].Herbort AF, Sturm MT, Schuhen K, A new approach for the agglomeration and subsequent removal of polyethylene, polypropylene, and mixtures of both from freshwater systems – a case study, Environ Sci Pollut Res 25 (15) (2018) 15226–15234, 10.1007/s11356-018-1981-7. [DOI] [PubMed] [Google Scholar]
- [193].Herbort AF, Sturm MT, Fiedler S, Abkai G, Schuhen K, Alkoxy-silyl Induced Agglomeration: A New Approach for the Sustainable Removal of Microplastic from Aquatic Systems, J Polym Environ 26 (11) (2018) 4258–4270, 10.1007/s10924-018-1287-3. [DOI] [Google Scholar]
- [194].Sturm MT, Herbort AF, Horn H, Schuhen K, Comparative study of the influence of linear and branched alkyltrichlorosilanes on the removal efficiency of polyethylene and polypropylene-based microplastic particles from water, Environ Sci Pollut Res 27 (10) (2020) 10888–10898, 10.1007/s11356-020-07712-9. [DOI] [PubMed] [Google Scholar]
- [195].Ma B, Xue W, Ding Y, Hu C, Liu H, Qu J, Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment, J. Environ. Sci. 78 (2019) 267–275, 10.1016/j.jes.2018.10.006. [DOI] [PubMed] [Google Scholar]
- [196].Skaf DW, Punzi VL, Rolle JT, Kleinberg KA, Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation, Chem. Eng. J. 386 (2020) 123807, 10.1016/j.cej.2019.123807. [DOI] [Google Scholar]
- [197].Mintenig SM, Bäuerlein PS, Koelmans AA, Dekker SC, van Wezel AP, Closing the gap between small and smaller: towards a framework to analyse nano-and microplastics in aqueous environmental samples, Environ. Sci.: Nano 5 (7) (2018) 1640–1649, 10.1039/C8EN00186C. [DOI] [Google Scholar]
- [198].Enfrin M, Lee J, Le-Clech P, Dumée LF, Kinetic and mechanistic aspects of ultrafiltration membrane fouling by nano- and microplastics, J. Membr. Sci. 601 (2020) 117890, 10.1016/j.memsci.2020.117890. [DOI] [Google Scholar]
- [199].Zhou Z, Yang Y, Li X, Gao W, Liang H, Li G, Coagulation efficiency and flocs characteristics of recycling sludge during treatment of low temperature and micro-polluted water, J. Environ. Sci. 24 (6) (2012) 1014–1020, 10.1016/S1001-0742(11)60866-8. [DOI] [PubMed] [Google Scholar]
- [200].Zhang Y, Diehl A, Lewandowski A, Gopalakrishnan K, Baker T, Removal efficiency of micro- and nanoplastics (180 nm–125 μm) during drinking water treatment, Sci. Total Environ. 720 (2020), 137383, 10.1016/j.scitotenv.2020.137383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Xu C, Gao B, Cao B, Cheng D, Yue Q, Evaluation of dynamic membrane formation and filtration models at constant pressure in a combined coagulation/dynamic membrane process in treating polluted river water, Water Sci. Technol. Water Supply. (2013), 10.2166/ws.2013.599. [DOI] [Google Scholar]
- [202].Huang B-C, Guan Y-F, Chen W, Yu H-Q, Membrane fouling characteristics and mitigation in a coagulation-assisted microfiltration process for municipal wastewater pretreatment, Water Res. 123 (2017) 216–223, 10.1016/j.watres.2017.06.080. [DOI] [PubMed] [Google Scholar]
- [203].Hu Y, Yang Y, Wang XC, Hao Ngo H, Sun Q, Li S, Tang J, Yu Z, Effects of powdered activated carbon addition on filtration performance and dynamic membrane layer properties in a hybrid DMBR process, Chem. Eng. J. 327 (2017) 39–50, 10.1016/j.cej.2017.06.072. [DOI] [Google Scholar]
- [204].Li L, Xu G, Yu H, Xing J, Dynamic membrane for micro-particle removal in wastewater treatment: Performance and influencing factors, Sci. Total Environ. 627 (2018) 332–340, 10.1016/j.scitotenv.2018.01.239. [DOI] [PubMed] [Google Scholar]
- [205].Yi Z, Shibin X, Feng H, Dong X, Lingwei K, Zhenbin W, Phosphate removal of acid wastewater from high-phosphate hematite pickling process by in-situ self-formed dynamic membrane technology, Desalin. Water Treat. 37 (1-3) (2012) 77–83, 10.1080/19443994.2012.661257. [DOI] [Google Scholar]
- [206].Salerno C, Vergine P, Berardi G, Pollice A, Influence of air scouring on the performance of a Self Forming Dynamic Membrane BioReactor (SFD MBR) for municipal wastewater treatment, Bioresour. Technol. 223 (2017) 301–306, 10.1016/j.biortech.2016.10.054. [DOI] [PubMed] [Google Scholar]
- [207].Xue N, Xia J, Huang X, Fouling control of a pilot scale self-forming dynamic membrane bioreactor for municipal wastewater treatment, Desalin. Water Treat. 18 (1-3) (2010) 302–308, 10.5004/dwt.2010.1812. [DOI] [Google Scholar]
- [208].Lares M, Ncibi MC, Sillanpää M, Sillanpää M, Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology, Water Res. 133 (2018) 236–246, 10.1016/j.watres.2018.01.049. [DOI] [PubMed] [Google Scholar]
- [209].Li L, Liu D, Song K, Zhou Y, Performance evaluation of MBR in treating microplastics polyvinylchloride contaminated polluted surface water, Mar. Pollut. Bull. 150 (2020) 110724, 10.1016/j.marpolbul.2019.110724. [DOI] [PubMed] [Google Scholar]
- [210].Mekaru H, Effect of Agitation Method on the Nanosized Degradation of Polystyrene Microplastics Dispersed in Water, ACS Omega 5 (7) (2020) 3218–3227, 10.1021/acsomega.9b03278.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Nandi BK, Patel S, Effects of operational parameters on the removal of brilliant green dye from aqueous solutions by electrocoagulation, Arabian J. Chem. 10 (2017) S2961–S2968, 10.1016/j.arabjc.2013.11.032. [DOI] [Google Scholar]
- [212].Kim T, Kim T-K, Zoh K-D, Removal mechanism of heavy metal (Cu, Ni, Zn, and Cr) in the presence of cyanide during electrocoagulation using Fe and Al electrodes, J. Water Process Eng. 33 (2020) 101109, 10.1016/j.jwpe.2019.101109. [DOI] [Google Scholar]
- [213].Akansha J, Nidheesh PV, Gopinath A, Anupama KV, Suresh Kumar M, Treatment of dairy industry wastewater by combined aerated electrocoagulation and phytoremediation process, Chemosphere 253 (2020) 126652, 10.1016/j.chemosphere.2020.126652. [DOI] [PubMed] [Google Scholar]
- [214].Tian Y, He W, Zhu X, Yang W, Ren N, Logan BE, Improved Electrocoagulation Reactor for Rapid Removal of Phosphate from Wastewater, ACS Sustainable Chem. Eng. 5 (1) (2017) 67–71, 10.1021/acssuschemeng.6b01613. [DOI] [Google Scholar]
- [215].Pan C, Troyer LD, Catalano JG, Giammar DE, Dynamics of Chromium(VI) Removal from Drinking Water by Iron Electrocoagulation, Environ. Sci. Technol. 50 (24) (2016) 13502–13510, 10.1021/acs.est.6b03637.s001. [DOI] [PubMed] [Google Scholar]
- [216].Raschitor A, Llanos J, Cañizares P, Rodrigo MA, Improved electrolysis of colloid-polluted wastes using ultrasounds and electrocoagulation, Sep. Purif. Technol. 231 (2020) 115926, 10.1016/j.seppur.2019.115926. [DOI] [Google Scholar]
- [217].Perren W, Wojtasik A, Cai Q, Removal of Microbeads from Wastewater Using Electrocoagulation, ACS Omega 3 (3) (2018) 3357–3364, 10.1021/acsomega.7b02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Azerrad SP, Isaacs M, Dosoretz CG, Integrated treatment of reverse osmosis brines coupling electrocoagulation with advanced oxidation processes, Chem. Eng. J. 356 (2019) 771–780, 10.1016/j.cej.2018.09.068. [DOI] [Google Scholar]
- [219].Pirkarami A, Olya ME, Removal of dye from industrial wastewater with an emphasis on improving economic efficiency and degradation mechanism, Journal of Saudi Chemical Society 21 (2017) S179–S186, 10.1016/j.jscs.2013.12.008. [DOI] [Google Scholar]
- [220].Zeboudji B, Drouiche N, Lounici H, Mameri N, Ghaffour N, The Influence of Parameters Affecting Boron Removal by Electrocoagulation Process, Sep. Sci. Technol. 48 (8) (2013) 1280–1288, 10.1080/01496395.2012.731125. [DOI] [Google Scholar]
- [221].Talvitie J, Mikola A, Setälä O, Heinonen M, Koistinen A, How well is microlitter purified from wastewater? – A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant, Water Res. 109 (2017) 164–172, 10.1016/j.watres.2016.11.046. [DOI] [PubMed] [Google Scholar]
- [222].Yang L, Li K, Cui S, Kang Y, An L, Lei K, Removal of microplastics in municipal sewage from China’s largest water reclamation plant, Water Res. 155 (2019) 175–181, 10.1016/j.watres.2019.02.046. [DOI] [PubMed] [Google Scholar]


