Abstract
Background
Indicator condition (IC) guided testing for HIV is an effective way to identify undiagnosed people living with HIV, but studies suggest its implementation is lacking. This systematic review provides an overview of the adoption of IC-guided testing in Western countries.
Methods
Seven ICs were selected: tuberculosis (TB), malignant lymphoma, hepatitis B, hepatitis C, cervical/vulvar carcinoma/intraepithelial neoplasia grade 2+ (CC/CIN2+, VC/VIN2+), and peripheral neuropathy (PN). Embase and Ovid MEDLINE were searched up to November 20th, 2020. Publications of all types, using data from ≥2009, reporting on HIV test ratios in patients ≥18 years in all settings in Western countries were eligible. HIV test ratios and positivity were reported per IC. A random effects-model for proportions was used to calculate estimated proportions (ES) with 95% CIs. This study was registered at PROSPERO, registration number CRD42020160243.
Findings
Fifty-seven references, including 23 full-text articles and 34 other publications were included. Most (28/57) reported on HIV testing in TB. No reports on HIV testing in VC/VIN2+ or PN patients were eligible for inclusion. Large variation in HIV test ratios was observed between and within ICs, resulting from different testing approaches. Highest HIV test ratios (pooled ratio: 0·72, 95%CI 0·63–0·80) and positivity (0·05, 95% CI 0·03–0·06) were observed among TB patients, and lowest among CC/CIN2+ patients (pooled ES test ratio: 0·12, 95%CI 0·01–0·31, positivity: 0·00, 95%CI 0·00–0·00).
Interpretation
IC-guided HIV testing is insufficiently implemented in Western countries. The large variation in test ratios provides insight into priority areas for implementing routine IC-guided HIV testing in the future.
Funding
HIV Transmission Elimination in Amsterdam (H-TEAM) consortium and Aidsfonds (grant number P-42,702).
Research in context.
Evidence before this study
Identifying and treating people living with HIV is key to control the HIV epidemic. Opportunities to identify undiagnosed HIV through indicator condition guided testing are being missed in various healthcare settings. We have found no systematic review on the extent to which indicator condition guided testing has been adopted in Western countries
We searched Embase and Ovid MEDLINE for evidence published up to November 20th, 2020 for evidence on the extent to which indicator condition guided testing for HIV is implemented in all healthcare settings in the Western world. All publication types, including full-text peer reviewed articles, as well as abstracts, short communications, and correspondence reporting on HIV testing in a selection of seven indicator conditions in or after 2009 in all healthcare settings in Western Countries were eligible for inclusion. No language restrictions were applied. The search included terms for HIV testing, the selected indicator conditions, and the term ‘indicator condition’.
Added value of this study
This systematic review revealed that indicator condition guided testing for HIV is unevenly and insufficiently adopted across indicator conditions and healthcare settings in Western countries. We found that even in AIDS defining conditions, such as tuberculosis or cervical cancer, HIV testing strategies need to be further improved. Additionally, for some indicator conditions such as peripheral neuropathy, no evidence on HIV testing was identified, suggesting that in some conditions, this testing strategy might be lacking even more. Overall, this review revealed that adopting these strategies is an effective way to identify undiagnosed HIV, as in most conditions positivity percentages exceeded the established cost-effectivity threshold of 0·1%.
Implications of all the available evidence
Indicator condition guided testing for HIV remains insufficiently practiced, and results from settings where this strategy is best implemented reveal opportunities for improvement. Lessons on effective implementation can be learned from these settings, such as the high HIV test ratio observed when opt-out testing strategies are used. Adopting these strategies could lead to improved indicator condition guided HIV testing strategies across healthcare settings in Western countries.
Alt-text: Unlabelled box
1. Introduction
In our global efforts to complete the ‘last mile’ towards ending the HIV epidemic, timely diagnosis remains an important challenge. In the European Union/European Economic Area (EU/EEA), an estimated 14% of people living with HIV (PLHIV) is unaware of their diagnosis and late diagnosis (CD4 count <350 cells/mm3) is reported in almost half of all new cases. [1] These figures are of particular concern, as late presentation is associated with higher morbidity, mortality, and onward transmission of HIV. [2,3]
In the last decade, growing evidence on the potential role of indicator condition guided testing for HIV to improve timely testing has emerged. Indicator conditions (ICs) are defined as conditions that are either (1) AIDS-defining, (2) associated with an undiagnosed HIV prevalence of >0·1%, the cut-off for cost-effective screening for HIV, [4,5] or (3) conditions where failure to identify an HIV infection may have significant adverse implications for the patient. [6] In 2007 the World Health Organization recommended provider-initiated HIV testing in conditions that could indicate HIV infection, [7] and in 2014 the HIV in Europe-initiative published a guidance on IC-guided HIV testing in adults, [6] based on the HIV Indicator Diseases across Europe Study (HIDES) and subsequent HIDES II study, that were performed in Europe. [8,9]
In recent years numerous studies have shown that IC-guided HIV testing is an effective approach to identify undiagnosed PLHIV. [10], [11], [12], [13], [14], [15], [16], [17] Additionally, IC-guided testing has the advantage of bypassing barriers on both the patient and provider level, such as discussing sexual behavior and risk factors for HIV. [8] As a consequence, HIV guidelines recommend IC-guided testing as one of the strategies to reduce the proportion undiagnosed PLHIV. However, recent studies on implementation of IC-guided testing consistently show missed opportunities for earlier HIV diagnosis due to lack of adherence to- or absence of- local protocols on IC-guided testing, but no overview of the adoption of IC-guided testing has been reported. [18], [19], [20], [21], [22], [23], [24], [25]
The main objective of this systematic review was to assess the proportion of patients presenting with indicator conditions that are tested for HIV (i.e. the HIV test ratio). The secondary objective was to assess the outcomes of this testing strategy (i.e. the percentage positive).
2. Methods
2.1. Protocol and guidelines
The protocol for this review was published at PROSPERO (supplementary appendix 1), and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (supplementary appendix 2).
2.2. Review topics
Seven ICs from various medical specialties were selected for inclusion: tuberculosis (TB), cervical cancer (CC) or cervical intraepithelial neoplasia (CIN) grade 2+, vulvar cancer (VC) or vulvar intraepithelial neoplasia (VIN) grade 2+, malignant lymphoma, hepatitis B (HBV), hepatitis C (HCV), and peripheral neuropathy (PN). These ICs were selected as they are diagnosed and managed by various medical specialties (i.e. pulmonology, gynecology, hematology, gastroenterology/hepathology and neurology), ensuring a wide scope of the extent to which IC-guided testing is adopted, and they can all be objectively diagnosed using diagnostic tests.
2.3. Search strategy
With assistance of a clinical librarian, Ovid MEDLINE and Embase were searched for studies published up to November 20th, 2020. The search contained terms for HIV testing, the selected ICs, and the term ‘indicator condition’ (supplementary appendix 3). No language or date restrictions were applied. Additionally, all articles referring to the HIDES studies, [8,9] and abstracts identified in Embase were included for screening.
2.4. Selection criteria
Studies reporting HIV test ratios among patients ≥18 years (directly available or through calculation with presented data), all settings (e.g. primary care (PC), hospital care, registry surveillance), and all publication types (e.g. research article, abstract, correspondence) were eligible for inclusion. Only studies performed in Western countries (Western Europe, USA, Canada, Australia, New Zealand, and Japan) were included, as HIV epidemiology and the standard of healthcare are comparable in these countries. No language restrictions were applied. Studies among persons known HIV positive, with unconfirmed disease (e.g. suspected TB), or conditions not meeting the IC definition (e.g. latent TB infection), and studies with a sample size <10 per subgroup per IC were excluded. Studies with data on HIV testing before 2009 only were excluded, as IC-guided testing was globally implemented around 2009.
2.5. Selection process
Search results were exported through an EndNote database (version 19·1, Thomson Reuters, Philadelphia, USA) and duplicates were removed. All titles and abstracts were screened for inclusion by SJB, and 10% were independently screened by SHH. A maximum of 2·5% discrepancy was allowed for. Differences were resolved through discussion, and, if needed, SEG was consulted as a third reviewer to resolve differences of opinion. If after discussion the discrepancy remained >2·5%, all titles and abstracts would be screened by SHH. Subsequently, the full text of all selected references was assessed for eligibility by both reviewers. Differences were again resolved through discussion. For all eligible abstracts, subsequent full-text publications were searched for.
2.6. Data extraction
For data extraction, a form in Microsoft Excel (Version 2016, Microsoft Corporation, USA) was used. The form was piloted in the first 10% of eligible studies, and adjusted accordingly. Data extraction was independently performed by SJB and SHH and discrepancies were resolved through discussion, with consultation of SEG as a third reviewer, if needed. Type of publication (i.e. full-text peer-reviewed article or ‘other publication types’, including abstracts, short communications, and correspondence), first author, year, title, setting, aim, recruitment site, definition of the IC and of being HIV tested, inclusion and exclusion criteria, number of subjects, number tested for HIV, and data on the percentage positive were extracted, supplementary appendix 4. When HIV test ratios were presented separately by sex or time periods (e.g. before and after intervention), they were extracted separately. Missing data were requested from authors if needed.
2.7. Quality assessment
Risk of bias assessment per included full-text study was performed independently by SJB and SHH using an adaptation of the Joanna Briggs checklist for prevalence studies, with consultation of SEG as a third reviewer, if needed. [26] The item on statistical analysis was dropped as it was deemed not relevant, and an item on objective measurement of being HIV tested was added (supplementary appendix 5). Risk of bias was scored out of 10. Discrepancies were resolved by discussion. No risk of bias was assessed for the other publication types (including abstracts, short communications, and correspondence, as insufficient information was available in these publications.
2.8. Statistical analysis
HIV test ratios, percentage positive, and quality assessments per reference were reported by IC. Summary statistics across studies were reported as medians with interquartile ranges (IQR). Test ratios and positivity were pooled by IC, regardless of publication type and assessed risk of bias. A random effects-model for proportions by Nyaga et al. was used, [27] as considerable heterogeneity between studies was expected due to the broad inclusion criteria. No limit for heterogeneity as expressed by the I2 statistic was used. Results were reported as estimated proportions (ES) and 95% confidence intervals (CI) and displayed as forest plots. In sensitivity analyses, pooling of test ratio was performed using only low risk of bias full-text articles, and stratified by sex. A risk of bias score of ≥7/10 was chosen as cut-off for low risk by the researchers. Additionally, meta-regression analyses of the HIV test ratio per study by date of data collection (as a continuous variable) were performed to assess whether HIV test ratio varied by time, overall and by IC. For date of data collection, the midpoint of reported periods were taken. Permuted tests with an iteration of 1000 were used to confirm the findings. Analyses were performed using STATA 15 (StataCorp LLC, College Station, USA).
2.9. Role of funding sources
The funders of this study had no role in the study's design, conduct, analysis and interpretation of results, the writing of the report, or the decision to publish.
3. Results
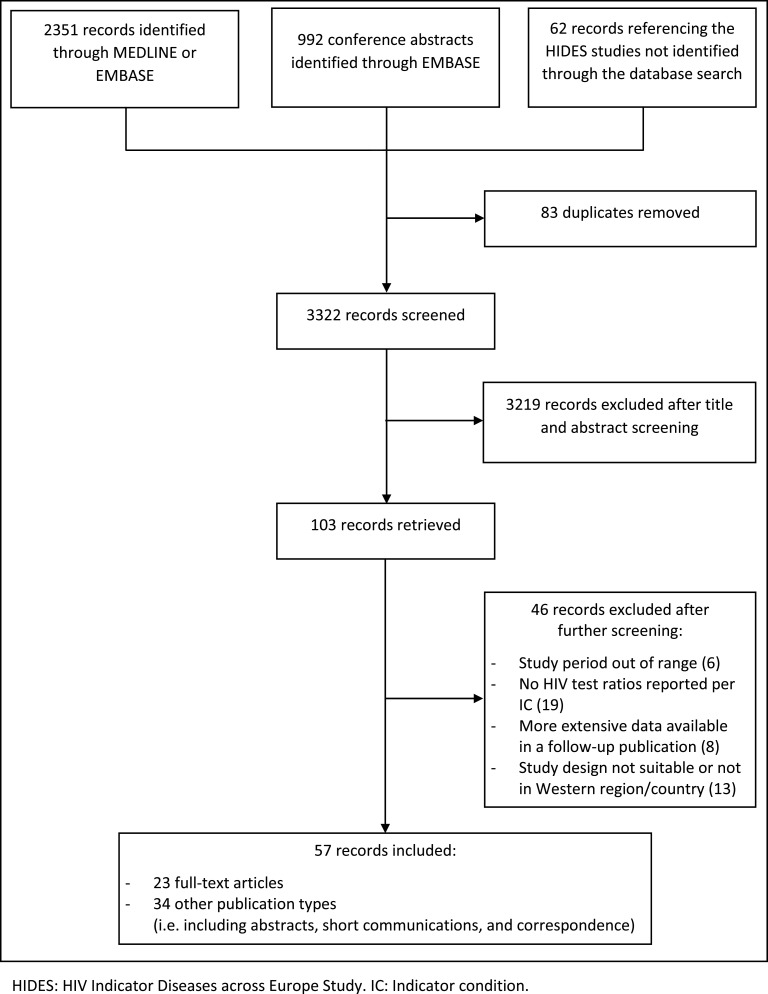
A total of 3405 records, including 992 abstracts and 62 records referencing the HIDES studies were identified through the search. Eighty-three were excluded because they were duplicates and 3219 based on title/abstract. Less than 2·5% discrepancy was found between the two screening authors during independent screening (5/341, 1·5%), which was resolved through discussion. Of the remaining 103 references, 46 were excluded based on full-text screening.
Of the 57 included references reporting on one or more IC, 23 were full-text articles and 34 were other publication types including abstracts, short communications, and correspondence (Fig. 1). Three of the 57 included citations reported on four or five of selected ICs, two reported on three ICs, ten reported on two ICs and 42 reported on one. Most included records (28/57) reported on HIV testing in TB patients (Table 1). No records on HIV testing in VC/VIN2+ patients or PN patients were eligible for inclusion. Most records were from the UK (24), followed by the USA (14) and Canada (5). Twenty-four records had included data from prior to 2009. There was considerable variation between records in how ‘tested for HIV’ was defined; 37% of studies (21) had defined a timeframe for being tested, using varying timeframes. Forty percent (23) described how HIV testing was defined, but did not define a timeframe, and 23% (13) described no definition of ‘HIV tested’, despite HIV test ratios being reported.
Fig. 1.
Search results and inclusions. HIDES: HIV Indicator Diseases across Europe Study. IC: Indicator condition.
Table 1.
Included records per indicator condition and publication type*.
| Number of included full-text publications | Number of included other publication types ** | Total included citations | |
|---|---|---|---|
| Tuberculosis | 16 | 12 | 28 |
| Hepatitis B | 3 | 9 | 12 |
| Hepatitis C | 5 | 13 | 18 |
| Hepatitis B or C | 2 | 1 | 3 |
| Cervical carcinoma or CIN2+ | 6 | 3 | 9 |
| Vulvar carcinoma or VIN2+ | 0 | 0 | 0 |
| Malignant lymphoma | 4 | 7 | 11 |
| Peripheral neuropathy | 0 | 0 | 0 |
| Total | 23 | 34 | 57 |
* Numbers add up more than the total number of included citations, as some reported on more than one indicator condition.
** (i.e. abstracts, short communications, and correspondence).
CIN: cervical intraepithelial neoplasia; VIN: vulvar intraepithelial neoplasia.
3.1. Tuberculosis
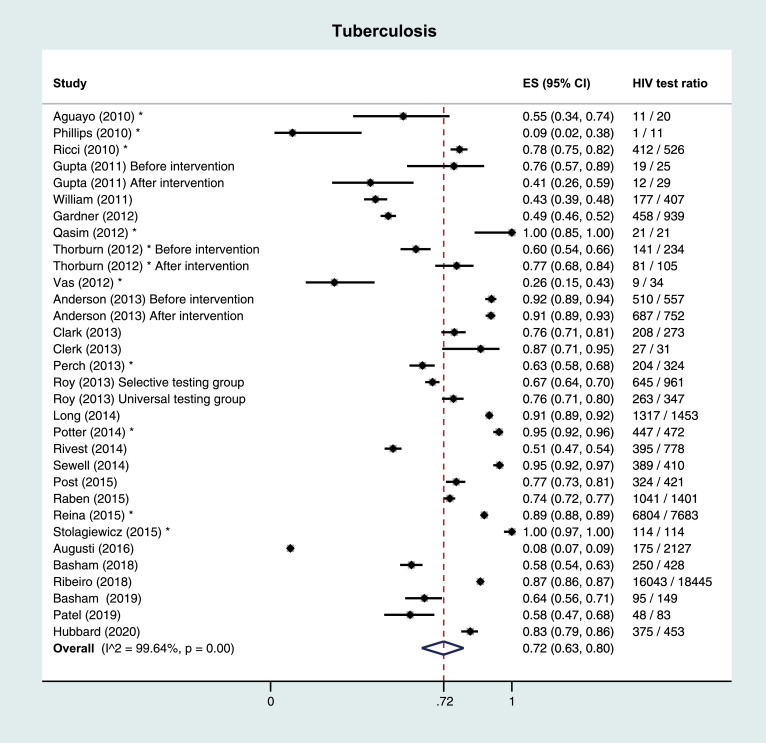
Of 16 included full-text articles on TB, eight were performed in a hospital/TB clinic setting, seven in the setting of a TB registry database, and one in the PC setting. Median number of study subjects was 603 (IQR 340–1355). HIV test ratios ranged from 44% to 95% in the hospital/registry setting, and was 8% in the PC setting. Median positivity percentage was 4·9% (IQR 4·4%−5·8%). Risk of bias was low; 77% of full-text references (13/16) had a low risk assessment (7/10 or higher). Across the 12 included other publication types, median number of subjects was 219 (IQR 28–463) and median HIV test ratio was 72% (IQR 56%−92%), Table 2.
Table 2.
Study characteristics, summary of findings and risk of bias by indicator condition.
| Tuberculosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Full-text articles | ||||||||
| Reference (year) | Design and setting | Included study period | Population and exclusion criteria | IC definition | HIV tested definition | HIV test ratio (%)** | Positivity ratio (%)** | Risk of bias score* |
| Anderson (2013) [33] | Retrospective cohort study in UK TB clinics – before cohort implementation | July 2009 - June 2010 | All TB cases of all ages from 5 London clinics were included | Patients notified as having TB disease | Uptake of HIV testing | Before: 510/557 (91·6%) | NA | 7/10 |
| Retrospective cohort study in UK TB clinics – after cohort implementation | July 2010 - December 2011 | After: 687/752 (91·4%) | ||||||
| Augusti (2016) [19] | Cross-sectional cohort in primary care, Spain | January 2010 - August 2012 | Patients aged 16–65 years were included; known HIV positive patients excluded | Using either their ICD-10 codes or a positive laboratory result | HIV test within 4 months of diagnosis date | Men: 112/1287 (8·7%) | Men: 0/112 (0%) | 9/10 |
| Women: 63/840 (7·5%) | Women: 1/63 (1·6%) | |||||||
| Basham (2018) [28] | Audit of a Canadian provincial tuberculosis program | 2008 - 2010 | All active TB cases of all ages in the TB registry | Active TB | HIV test recorded in TB registry database | 250/428 (58·4%) | 12/250 (4·8%) | 9/10 |
| Basham (2019) [39] | Audit of First Nations tuberculosis program in Canada | 2008 - 2012 | First Nations of all ages recorded in the TB registry | Recorded TB in registry | HIV test recorded in TB registry database | 95/149 (63·8%) | NA | 8/10 |
| Clark (2013) [40] | Retrospective cohort to assess HIV testing in TB surveillance database in US | 2008 - 2010 | Living patients with TB of all ages | Reported TB cases surviving with TB | Known (positive or negative) or unknown (refused testing/not offered testing) HIV status | 208/273 (76·2%) | 12/208 (5·8%) | 7/10 |
| Clerk (2013) [41] | Cross-sectional study on HIV testing in TB patients in the UK | NA | TB cases of all ages were included | Confirmed active TB cases | NA | 27/31 (87·1%) | NA | 6/10 |
| Gardner (2012) [42] | Retrospective cohort after implementation of opt-out HIV testing in US TB clinic | June 2010 - June 2011 | Excluded: Patients <14 years, known HIV positive, no chart available, diagnosed prior to study period | New TB cases presenting at the clinic | Tested for HIV in the clinic after presentation | 458/939 (48·8%) | 1/458 (0·2%) | 9/10 |
| Gupta (2011) [35] | First audit of IC-guided testing in UK general hospital | First audit: August 2008 - July 2009 | Patients of all ages testing positive for TB | Patients tested positive for tuberculosis | HIV testing was double checked using the electronic pathology records system and a separate database of HIV testing | First audit: 19/25 (76·0%) | NA | 7/10 |
| Re-audit of IC-guided testing in UK general hospital | Re-audit: August 2009 - June 2010 | Re-audit: 12/29 (41·4%) | ||||||
| Long (2014) [29] | Retrospective cohort of tuberculosis patients in Canada | 2003–2012 | Patients of all ages in the TB registry | Persons meeting the Canadian case definition for TB | Already known or newly diagnosed with HIV | 1317/1453 (90·6%) | 74/1317 (5·6%) | 8/10 |
| Post (2015) [43] | Retrospective cohort among patients with tuberculosis in Australia | 2009 | Patients of all ages with TB | Microbiologically confirmed TB and patients that were treated for TB without microbiological confirmation | HIV status was categorised as known or unknown (not tested or declined testing) | 2009: 56/80 (70·0%) | 2009: 3/56 (5·4%) | 6/10 |
| 2010 | 2010: 79/100 (79·0%) | 2010: 5/79 (6·3%) | ||||||
| 2011 | 2011: 72/98 (73·5%) | 2011: 4/72 (5·6%) | ||||||
| 2012 | 2012: 56/73 (76·7%) | 2012: 1/56 (1·8%) | ||||||
| 2013 | 2013: 61/70 (87·1%) | 2013: 3/61 (4·9%) | ||||||
| Raben (2015) [9] | Retrospective cohort on HIV testing in ICs in Europe | May 2013 | Patients in participating centres, >18 and <65 years of age, not known HIV positive, presenting within the previous year/last 100+ patients | Patients with tuberculosis | Participating centres reviewed retrospectively how many patients presenting with the IC were tested for HIV | 1041/1401 (74·3%) | 46/1041 (4·4%) | 2/10 |
| Ribeiro (2018) [44] | Retrospective cohort study on HIV screening of TB patients in Portugal | 2008 - 2014 | Notified TB cases of all ages in the Portuguese Tuberculosis Surveillance System | Notified TB | NA | Men: 10,629/12,115 (87·7%) | Men: NA | 7/10 |
| Women: 5414/6330 (85·5%) | Women: NA | |||||||
| Rivest (2014) [45] | Retrospective cohort on HIV-TB co-infection and predictors of HIV screening among incident TB cases in Canada | 2004 - 2009 | Incident TB cases of all ages reported to the TB reporting database | Cases confirmed by culture or diagnosed on the basis of clinical and radiological signs | HIV testing done from one month before to six months after date of TB diagnosis | Men: 226/422 (53·6%) | Men: 26/226 (11·5%) | 8/10 |
| Women: 169/356 (47·5%) | Women: 7/169 (4·1%) | |||||||
| Roy (2013) [36] | Cluster randomised controlled trial on the impact of implementing universal HIV testing in TB patients in the UK | September 2009 - March 2010 | Patients of all ages in centres using a selective HIV testing policy, not known HIV infected. | All patients seen and diagnosed with TB in participating centres | The date the HIV test was conducted was recorded and these patients were classified as having ‘‘accepted’’ the test | Women (selective testing): 269/417 (64·5%) | NA | 7/10 |
| Men (selective testing): 376/544 (69·1%) | ||||||||
| Patients of all ages in centres using a universal HIV testing policy, not known HIV infected. | Women (universal testing): 111/149 (74·5%%) | |||||||
| Men (universal testing): 152/198 (76·8%) | ||||||||
| Sewell (2014) [46] | Retrospective cohort in a UK TB clinic | January 2009 - July 2012 | TB patients of all ages at a TB medical outpatient service | Clinical or laboratory TB diagnosis | Tested < 3 months of attending the clinic or starting TB treatment | 389/410 (94·9%) | 27/389 (6·9%) | 9/10 |
| William (2011) [47] | Audit on HIV testing in TB patients after HIV testing guideline implementation in the UK | April 2008 - March 2009 | Patients <18 years, private patients, on chemoprophylaxis, non TB mycobacteria were excluded. | TB patients in the database | HIV testing in the six months prior to and following TB notification | Men: 101 / 214 (47·2%) | NA | 9/10 |
| Women: 76 / 193 (39·4%) | ||||||||
| Other publication types*** | ||||||||
| Reference (year) | Design and setting | Included study period | Population and exclusion criteria | IC definition | HIV tested definition | HIV test ratio (%) | Positivity ratio (%) | |
| Aguayo (2010) [48] | Retrospective study on Extrapulmonary TB in Spain | NA | NA | Extrapulmonary tuberculosis | NA | 11/20 (55·0%) | NA | |
| Hubbard (2020) [49] | Retrospective study on HBV and HCV prevalence in TB in a US hospital | September 2016 - May 2019 | Adult cases of active or latent TB | positive QuantiFERON-TB Gold In-Tube test | Tested for HIV | 375/453 (82·8%) | 22/375 (5·9%) | |
| Patel (2019) [50] | Retrospective study on mortality risk factors and delays in TB mortality cases in New Mexico, US | 2007 - 2017 | NA | TB mortality cases | Offered HIV testing | 48/83 (57·8%) | NA | |
| Perch (2013) [51] | Retrospective cohort on HIV testing in TB in Denmark | 2009 (Total study included 2007–2009) | Notified TB of all ages cases were included | All cases of notified tuberculosis in database | NA | 204/324 (63·0%) | 8/204 (3·9%) | |
| Phillips (2010) [52] | Audit of IC-guided testing in UK hospital | October 2008 - November 2009 | Patients of all ages with tuberculosis | Confirmed mycobacterium tuberculosis | HIV tested within the audit period | 1/11 (9·1%) | NA | |
| Potter (2014) [53] | Audit on HBV, HCV and HIV infection among new TB cases in UK | 2013 | Patients of all ages with active TB | Active tuberculosis | HIV screened | 447/472 (94·7%) | 15/447 (3·4%) | |
| Qasim (2012) [54] | Audit on diagnosis and management of TB patients in the UK | January 2009 - December 2010 | Patients with a positive Acid-Fast Bacillus test | A positive Acid-Fast Bacillus test | NA | 21/21 (100%) | NA | |
| Reina (2015) [55] | Cross sectional study on unknown HIV status in TB patients in Portugal | 2006 - 2012 | TB cases reported | Registered tuberculosis cases | Known HIV status | 6804/7683 (88·8%) | NA | |
| Ricci (2010) [56] | Audit on HIV testing and coinfection in TB patients in Italy | 2004 - 2009 | Patients with tuberculosis | Culture-confirmed cases of tuberculosis | Tested for HIV at any time | 412/526 (78·3%) | 67/412 (16·3%) | |
| Stolagiewicz (2015) [57] | Audit to quantify the local prevalence of HIV in patients with TB in the UK | 2014 | Patients diagnosed with or treated for TB | Diagnosed or treated for tuberculosis | Tested for HIV | 114/114 (100%) | 3/114 (2·6%) | |
| Thorburn (2012) [58] | Audit on HIV testing in TB patients in the UK | 2010 (before implementation multidisciplinary TB meeting) | Confirmed TB cases in 2010 | Confirmed TB cases | HIV tested in the year before or after TB diagnosis | 2010: 141/234 (60·3%) | 2010: 7/141 (5·0%) | |
| 2011 (after implementation multidisciplinary TB meeting) | Confirmed TB cases in 2011 | 2011: 81/105 (77·1%) | 2011: 2/81 (2·5%) | |||||
| Vas (2012) [59] | Audit on HIV testing in TB patients in a UK hospital | 2009 | Patients attending the chest clinic with TB | NA | Patients offered and accepted an HIV test | 9/34 (26·5%) | NA | |
| Hepatitis B | ||||||||
| Full-text articles | ||||||||
| Reference (year) | Design and setting | Included study period | Population and exclusion criteria | IC definition | HIV tested definition | HIV test ratio (%) | Positivity ratio (%) | Risk of bias score* |
| Augusti (2016) [19] | Cross-sectional cohort in primary care, Spain | January 2010 - August 2012 | Patients aged 16–65 years were included; known HIV positive patients excluded | Using either their ICD-10 codes or a positive laboratory result | HIV test within 4 months of diagnosis date | Men: 1792/6034 (29·7%) | Men: 27/1792 (1·5%) | 9/10 |
| Women: 1058/3712 (28·5%) | Women: 8/1058 (0·8%) | |||||||
| Gupta (2011) [35] | First audit of IC-guided testing in UK general hospital | First audit: August 2008 - July 2009 | Patients of all ages testing positive for HBV | Patients with a positive hepatitis B surface antigen test | HIV testing was double checked using the electronic pathology records system and a separate database of HIV testing | First audit: 6/27 (22·2%) | First audit: NA | 7/10 |
| Re-audit of IC-guided testing in UK general hospital | Re-audit: August 2009 - June 2010 | Re-audit: 10/44 (22·7%) | Re-audit: NA | |||||
| Hallager (2018) [31] | Retrospective cohort study on HIV coinfection among HBV and HCV patients in 18 hospitals in Denmark | January 2002 - July 2015 | Patients registered in the Danish hepatitis database of 16 years or older | Positive HBV surface antigen | HIV antibody/antigen tests performed before or within 6 months of database enrolment | 2287 / 3091 (74·0%) | 89/2287 (3·9%) | 9/10 |
| Other publication types*** | ||||||||
| Reference (year) | Design and setting | Included study period | Population and exclusion criteria | IC definition | HIV tested definition | HIV test ratio (%) | Positivity ratio (%) | |
| Deshpande (2015) [60] | Retrospective study on HIV testing in patients on Tenofovir monotherapy in Australia | January 2014 - June 2014 | Patients with HBV on Tenofovir | Medical record or pathology confirmed chronic HBV infection | HIV test recorded before start of Tenofovir monotherapy | 72/157 (45·9%) | NA | |
| Ireland (2018) [61] | Retrospective cross-sectional study on HIV testing in HBV patients in the UK | 2010 - 2014 | Patients of 15 years or over with HBV. Patients with known HIV and diagnosed with HBV at antenatal services were excluded | Hepatitis B virus (HBV) surface antigen positive | HIV tested on the same day or within 6 months following HBV diagnosis | 7315/16,086 (45·5%) | NA | |
| Lander (2014) [62] | Audit on HIV testing in HBV and HCV patients in a hepatitis clinic in the UK | September 2012 - August 2013 | HBV patients in the clinic | NA | Uptake of HIV testing in the clinic during the audit time period | 205/362 (56·6%) | NA | |
| Lynn (2014) [63] | Audit on HIV testing in HBV patients in the Rochester Epidemiology Project (REP) in the US | 1994 - 2010 | HBV patients in the REP cohort | NA | All HIV screening tests and their results | 273/607 (45·0%) | NA | |
| Pavlides (2011) [64] | Audit on HIV testing in HBV patients in the UK | October 2008 - September 2009 | HBV patients | HBV surface antigen positive | Whether these patients had an HIV test | 63/99 (63·6%) | 6/63 (9·5%) | |
| Perera (2011) [65] | Audit On HIV testing in HBV patients in the UK | NA | HBV patients in a teaching hospital | NA | HIV test performed | 53/88 (60·2%) | 4/53 (7·5%) | |
| Phillips (2010) [52] | Audit on HIV testing in indicator conditions in the UK | October 2008 - November 2009 | HBV patients at one hospital | Confirmed HBV infection | HIV tests taken within the same time period as inclusion | 2/32 (6·3%) | NA | |
| Su (2015) [66] | Audit on HBV treatment and care at an Asian health center in the US | 2012 | New patients presenting with chronic hepatitis B | NA | Offered HIV screening | 362/385 (94·0%) | NA | |
| Vas (2012) [59] | Audit on HIV testing in HBV patients in a hospital in the UK | 2009 | Patients attending the gastroenterology clinic with HBV | NA | Patients offered and accepted an HIV test | 2/25 (8·0%) | NA | |
| Hepatitis C | ||||||||
| Full-text articles | ||||||||
| Reference (year) | Design and setting | Included study period | Population and exclusion criteria | IC definition | HIV tested definition | HIV test ratio (%) | Positivity ratio (%) | Risk of bias score* |
| Augusti (2016) [19] | Cross-sectional cohort in primary care, Spain | January 2010 - August 2012 | Patients aged 16–65 years were included; known HIV positive patients excluded | Using either their ICD-10 codes or a positive laboratory result | HIV test within 4 months of diagnosis date | Men: 1995/6333 (31·5%) | Men: 67/1995 (1·1%) | 9/10 |
| Women: 828/3493 (23·7%) | Women: 18/828 (2·2%) | |||||||
| Bolther (2014) [30] | Cross-sectional cohort at a university hospital and outpatient clinics in Denmark | 1996 - 2011 | HCV patients of all ages; Patients no longer registered at the clinic were excluded | Chronic HCV patients with HCV RNA positive test outcome | HIV screening performance within 180 days of the HCV diagnosis | 360/624 (57·7%) | NA | 9/10 |
| Gupta (2011) [35] | First audit of IC-guided testing in UK general hospital | First audit: August 2008 - July 2009 | Patients of all ages testing positive for HCV | Patients with a positive hepatitis C antibody test | HIV testing was double checked using the electronic pathology records system and a separate database of HIV testing | First audit: 18/93 (19·4%) | First audit: NA | 7/10 |
| Re-audit of IC-guided testing in UK general hospital | Re-audit: August 2009 - June 2010 | Re-audit: 5/72 (6·9%) | Re-audit: NA | |||||
| Hallager (2018) [31] | Retrospective cohort study on HIV coinfection among HBV and HCV patients in 18 hospitals in Denmark | January 2002 - July 2015 | Patients registered in the Danish hepatitis database of 16 years or older | HCV-RNA before or within 6 months after enrolment in the database | HIV antibody/antigen tests performed before or within 6 months of enrolment in the database | 4400/5305 (82·9%) | 281/4400 (6·4%) | 9/10 |
| King (2019) [67] | Intervention study among patients with an IC admitted to an acute General Medicine Unit in Australia | July 2017 - October 2017 | Patients recently HIV tested, known HIV positive and with an alternative explanation for the IC were excluded | Hepatitis C antibody positive | Pathology lab data | 5 / 11 (45·5%) | NA | 4/10 |
| Other publication types*** | ||||||||
| Reference (year) | Design and setting | Included study period | Population and exclusion criteria | IC definition | HIV tested definition | HIV test ratio (%) | Positivity ratio (%) | |
| Cowan (2020) [68] | Retrospective review of testing and care of HCV mono- and HIV co-infected patients in a US emergency department | June 2018 - December 2019 | Patients aged 18 years or older with active HCV infection, triaged to the ED and able to provide consent for testing | HCV viral load positive | Known HIV status | 386/427 (90·4%) | 56/386 (14·5%) | |
| Fleischer (2018) [69] | Retrospective cohort on HIV testing in HCV patients in a US hospital | July 2015 - March 2017 | Patients with hepatitis C | HCV antibody positive | HIV antibody tested at any time | 252/445 (56·6%) | 6/252 (2·4%) | |
| Gilbert (2011) [70] | Audit to evaluate HIV testing in Canada | 2007 - 2009 | Patients diagnosed with HCV | NA | Tested for HIV within 3 months of diagnosis | 8183/15,981 (51·2%) | NA | |
| Ireland (2018) [61] | Retrospective cross-sectional study on HIV testing in HCV cases in the UK | 2010 - 2014 | Patients of 15 years or over with HCV. Patients with known HIV were excluded | HCV antibody positive | HIV tested on the same day or within 6 months following HCV diagnosis | 14,587/32,114 (45·4%) | NA | |
| Lander (2014) [62] | Audit on HIV testing in HBV and HCV patients in a hepatitis clinic in the UK | September 2012 - August 2013 | HCV patients in the clinic | NA | Uptake of HIV testing in the clinic during the audit time period | 40/72 (55·6%) | NA | |
| Lynn (2014) [63] | Audit on HIV testing in HCV patients in the Rochester Epidemiology Project in the US | 1994 - 2010 | HCV patients | NA | All HIV screening tests and their results | 553/965 (57·3%) | NA | |
| Oraka (2016) [71] | Retrospective cohort on prevalence of HIV testing among adults with HCV in the US | 1999 - 2014 | Patients with HCV aged 20–59 years | HCV RNA positive test result | Ever tested for HIV | 248/384 (64·6%) | NA | |
| Pavlides (2011) [64] | Audit on HIV testing in HCV patients in the UK | October 2008 - September 2009 | HCV patients | Positive hepatitis C antibody or PCR | Whether these patients had an HIV test | 51/102 (50·0%) | 6/51 (11·8%) | |
| Perera (2011) [65] | Audit on HIV testing in HCV patients in the UK | NA | HCV patients in a teaching hospital | NA | HIV test performed | 40/92 (43·5%) | 3/40 (7·5%) | |
| Phillips (2010) [52] | Audit on HIV testing in indicator conditions in the UK | October 2008 - November 2009 | HCV patients at one hospital | Confirmed HCV infection | HIV tests taken within the same time period as inclusion | 25/88 (28·4%) | NA | |
| Sterling (2017) [72] | Retrospective cohort on HIV testing in HCV patients in the PROP UP cohort in the US | NA | HCV patients on DAA therapy enrolled in the PROP UP study, not known HIV positive | NA | HIV tested at some point in their history or prior to initiating DAA therapy | 472/756 (62·4%) | NA | |
| Tunney (2018) [73] | Audit on management of HCV patients in the UK | March 2012 - March 2017 | HCV patients (acute, chronic or past resolved) at the clinic, not known HIV positive | NA | HIV status | 23/35 (65·7%) | NA | |
| Vas (2012) [59] | Audit on HIV testing in HCV patients in a hospital in the UK | 2009 | Patients attending the gastroenterology clinic with HCV | NA | Patients offered and accepted an HIV test | 1/29 (3·4%) | NA | |
| Hepatitis B or C | ||||||||
| Full-text articles | ||||||||
| Reference (year) | Design and setting | Included study period | Population and exclusion criteria | IC definition | HIV tested definition | HIV test ratio (%) | Positivity ratio (%) | Risk of bias score* |
| Cayuelas Redondo (2019) [34] | Prospective interventional study on IC-guided HIV testing with an electronic prompt in primary healthcare in Spain | 2013 (pre intervention) | Patients aged 18–65, with no known HIV infection, with acute or chronic hepatitis B or C | NA | HIV infection | Pre intervention: 2/26 (7·7%) | NA | 8/10 |
| July 2014 - May 2015 (intervention) | During intervention: 5/17 (29·4%) | |||||||
| June 2015 - May 2016 (post intervention) | Post intervention: 1/21 (4·8%) | |||||||
| Raben (2015) [9] | Retrospective cohort on HIV testing in ICs in Europe | May 2013 | Patients in participating centres, >18 and <65 years of age, not known HIV positive, presenting within the previous year/last 100+ patients | Patients with hepatitis B or C | Participating centres reviewed retrospectively how many patients presenting with the IC were tested for HIV | 2325/2681 (86·7%) | 23/2325 (1·0%) | 2/10 |
| Other publication types*** | ||||||||
| Reference (year) | Design and setting | Included study period | Population and exclusion criteria | IC definition | HIV tested definition | HIV test ratio (%) | Positivity ratio (%) | |
| Adlington (2014) [32] | An audit of HIV testing in acute medical patients with HIV clinical indicator conditions in the UK | January 2012 | Patients with hepatitis B or C | Hepatitis B or C, registered as ICD-10 code | Whether HIV test had been performed during admission | January 2012: 10/39 (25·6%) | NA | |
| January 2013 | January 2013: 7/41 (17·1%) | |||||||
| Cervical carcinoma or cervical intraepithelial neoplasia grade 2+ | ||||||||
| Full-text articles | ||||||||
| Reference (year) | Design and setting | Included study period | Population and exclusion criteria | IC definition | HIV tested definition | HIV test ratio (%) | Positivity ratio (%) | Risk of bias score* |
| Alldredge (2020) [74] | Retrospective cohort study on HIV screening in women with newly diagnosed invasive cervical cancer in a large comprehensive US gynecologic oncology practice | January 2007 - December 2017 | Women >18 years with invasive cervical cancer were included; cervical dysplasia, non-cervical or recurrent cancer and presenting at another specialty were excluded | International Classification of Diseases codes 180.9 and C53.9 for invasive cervical cancer | HIV-1/2 antibody or 4th generation p24 antigen test undertaken within 12 months before diagnosis, or within 30 days of the encounter. | 38 / 492 (7·7%) | 0/38 (0%) | 9/10 |
| Augusti (2016) [19] | Cross-sectional cohort in primary care, Spain | January 2010 - August 2012 | Patients aged 16–65 years were included; known HIV positive patients excluded | Using either their ICD-10 codes or a positive laboratory result | HIV test within 4 months of diagnosis date | 15/615 (2·4%) | 0/15 (0%) | 9/10 |
| Gupta (2011) [35] | First audit of IC-guided testing in UK general hospital | First audit: August 2008 - July 2009 | Patients of all age with cervical intraepithelial neoplasia | Patients of all ages with a positive pathology sample for CIN II or III | HIV testing was double checked using the electronic pathology records system and a separate database of HIV testing | First audit: 2/146 (1·4%) | NA | 7/10 |
| Re-audit of IC-guided testing in UK general hospital | Re-audit: August 2009 - June 2010 | Re-audit: 4/340 (1·2%) | ||||||
| Hwang (2015) [75] | Retrospective cohort on HIV testing in patients with cancer at the initiation of therapy at a large US comprehensive cancer center | January 2004 - April 2011 | Patients treated at a large comprehensive cancer center. Patients on oral chemotherapy and enrolled in clinical trials were excluded | Patients with cervical cancer who received systemic cancer therapy | HIV-1/2 antibody test and/or confirmatory Western blot testing after registration at the center. | 23 / 245 (9·4%) | 0/23 (0%) | 10/10 |
| McGee-Avila (2020) [76] | Retrospective study on patterns of HIV testing and determinants of non-receipt of HIV testing among women with cervical cancer in the New Jersey Medicaid program, US | January 2012 - December 2014 | Patients with cervical cancer aged 21–64 years. Cases identified postmortem, non-New Jersey residence at diagnosis and with previous primary cancer or known HIV positive were excluded. | Primary, histologically confirmed invasive cervical cancer | Tested at any point during the study period | 78/242 (32·2%) | NA | 10/10 |
| Tested 6 months before diagnosis to 6 months after diagnosis of cervical cancer | 33/242 (13·6%) | |||||||
| Raben (2015) [9] | Retrospective cohort on HIV testing in ICs in Europe | May 2013 | Patients in participating centres, >18 and <65 years of age, not known HIV positive, presenting within the previous year/last 100+ patients | Patients with cervical cancer | Participating centres reviewed retrospectively how many patients presenting with the IC were tested for HIV | 444/583 (76·2%) | 1/444 (0·2%) | 2/10 |
| Other publication types*** | ||||||||
| Reference (year) | Design and setting | Included study period | Population and exclusion criteria | IC definition | HIV tested definition | HIV test ratio (%) | Positivity ratio (%) | |
| Butler (2014) [77] | Retrospective cohort study on HIV testing in patients with CIN 2+ in the UK | July 2012 - June 2013 | Patients with CIN2+ at colposcopy, not known to be HIV positive | Cervical intraepithelial neoplasia grade 2 and above at colposcopy | The most recent HIV test at the service prior to their attendance for colposcopy (last 3 years) | 34/94 (36·2%) | NA | |
| Lebari (2012) [78] | Retrospective review of HIV testing in patients with AIDS defining malignancies in the UK | March 2007 - July 2011 | Patients referred or initially diagnosed with cervical cancer | NA | Tested for HIV | 1/64 (1·6%) | NA | |
| Mosimann (2014) [79] | Retrospective cohort study on HIV testing rates among patients treated for AIDS defining cancers and HL in Switzerland | January 2002 - July 2012 | Patients aged ≥ 18 years treated for invasive cervical cancer | Invasive cervical cancer | HIV tested within 90 days before and 90 days after the cancer diagnosis date | 6/57 (10·5%) | 0/6 (0%) | |
| Malignant lymphoma | ||||||||
| Full-text articles | ||||||||
| Reference (year) | Design and setting | Included study period | Population and exclusion criteria | IC definition | HIV tested definition | HIV test ratio (%) | Positivity ratio (%) | Risk of bias score* |
| Augusti (2016) [19] | Cross-sectional cohort in primary care, Spain | January 2010 - August 2012 | Patients aged 16–65 years were included; known HIV positive patients excluded | Using either their ICD-10 codes or a positive laboratory result | HIV test within 4 months of diagnosis date | Patients with HL 0/86 (0%) | Patients with HL NA | 9/10 |
| Men with NHL 6/250 (2·4%) | Men with NHL 1/6 (16·7%) | |||||||
| Women with NHL 6/214 (2·8%) | Women with NHL 0/6 (0%) | |||||||
| Gupta (2011) [35] | First audit of IC-guided testing in UK general hospital | First audit: August 2008 - July 2009 | Patients of all ages with lymphoma | Patients with a positive pathology sample for lymphoma | HIV testing was double checked using the electronic pathology records system and a separate database of HIV testing | First audit: 3/42 (7·1%) | NA | 7/10 |
| Re-audit of IC-guided testing in UK general hospital | Re-audit: August 2009 - June 2010 | Re-audit: 2/46 (4·3%) | ||||||
| Hwang (2015) [75] | Retrospective cohort on HIV testing in patients with cancer at the initiation of therapy at a large US comprehensive cancer center | January 2004 - April 2011 | Patients treated at a large comprehensive cancer center. Patients on oral chemotherapy and enrolled in clinical trials were excluded | Patients with NHL on systemic cancer therapy | HIV-1/2 antibody test and/or confirmatory Western blot testing after registration at the center. | NHL: 1439/1628 (88·4%) | NHL: 23/1439 (1·6%) | 10/10 |
| Patients with HL on systemic cancer therapy | HL: 322/356 (90·4%) | HL: 2/322 (0·6%) | ||||||
| Raben (2015) [9] | Retrospective cohort on HIV testing in ICs in Europe | May 2013 | Patients in participating centres, >18 and <65 years of age, not known HIV positive, presenting within the previous year/last 100+ patients | Patients with NHL | Participating centres reviewed retrospectively how many patients presenting with the IC were tested for HIV | 577/1274 (45·3%) | 21/577 (3·6%) | 2/10 |
| Other publication types*** | ||||||||
| Reference (year) | Design and setting | Included study period | Population and exclusion criteria | IC definition | HIV tested definition | HIV test ratio (%) | Positivity ratio (%) | |
| Bishin (2017) [80] | Longitudinal cohort study to assess treatment guidelines for diffuse large B-cell lymphoma in the US | 2005 - 2016 | All patients diagnosed and treated for diffuse large B-cell lymphoma | Diffuse large B-cell lymphoma | HIV serology testing | 165/179 (92·2%) | NA | |
| Bowman (2010) [81] | Cohort study on HIV testing in lymphoma patients in the UK | 6 month pilot period (date not reported) | All lymphoma patients seen in the 6 month pilot period at the study site | NA | NA | 27/214 (12·6%) | 0/27 (0%) | |
| Buxton (2011) [82] | Cross-sectional study to assess treatment in lymphoma patients in the UK | 2009 | All patients newly diagnosed with lymphoma | New lymphoma diagnosis | NA | 91/281 (32·4%) | 3/91 (3·3%) | |
| Datta (2015) [83] | Audit on treatment in Primary Central Nervous System lymphoma patients in the UK | 2008 - 2013 | All patients with Primary Central Nervous System lymphoma, excluding metastatic disease | Biopsy-proven Primary Central Nervous System lymphoma | HIV status | 1/20 (5%) | NA | |
| Davies (2018) [84] | Audit on HIV testing in lymphoma patients in the UK | 2016 - 2017 | All patients newly diagnosed with lymphoma | New lymphoma diagnosis | Tested for HIV at first clinic/specialist review | 101/135 (74·8%) | 0/101 (0%) | |
| Lebari (2012) [78] | Retrospective review of HIV testing in patients with AIDS defining malignancies in the UK | March 2007 - July 2011 | Patients referred or initially diagnosed with Non-Hodgkin's lymphoma | NA | Tested for HIV | 34/158 (21·5%) | NA | |
| Mosimann (2014) [79] | Retrospective cohort study on HIV testing rates among patients treated for AIDS defining cancers and HL in Switzerland | January 2002 - July 2012 | Patients aged ≥ 18 years treated for HL | Hodgkin's Lymphoma | HIV tested within 90 days before and 90 days after the cancer diagnosis date | HL: 79/133 (59·4%) | HL: 0/79 (0%) | |
| Patients aged ≥ 18 years treated for NHL | non-Hodgkin lymphoma | NHL: 392/653 (60·0%) | NHL: 4/392 (1·0%) | |||||
* Risk of bias was assessed for all included full-text references using an adapted version of the Joanna Briggs Institute checklist for prevalence studies, and scored out of 10. A risk of bias score of ≥7/10 was considered low risk by the researchers.
** If articles reported data on HIV test ratio and positivity ratio by subgroup (e.g. sex, before and after intervention), then the data of that article are provided by subgroup here.
*** Including abstracts, short communications, and correspondence.
CIN = cervical intraepithelial neoplasia; DAA = direct-acting antivirals; ED = emergency department; HBV = hepatitis B virus; HCV = hepatitis C virus; HL = Hodgkin's lymphoma; IC = indicator condition; ICD-10 = 10th revision of the International Classification of Diseases and Related Health Problems; NA = not reported/not applicable; NHL = Non-Hodgkin lymphoma; PCR = Polymerase chain reaction; REP = Rochester Epidemiology Project; RNA = ribonucleic acid; TB = tuberculosis.
3.2. Hepatitis B and C
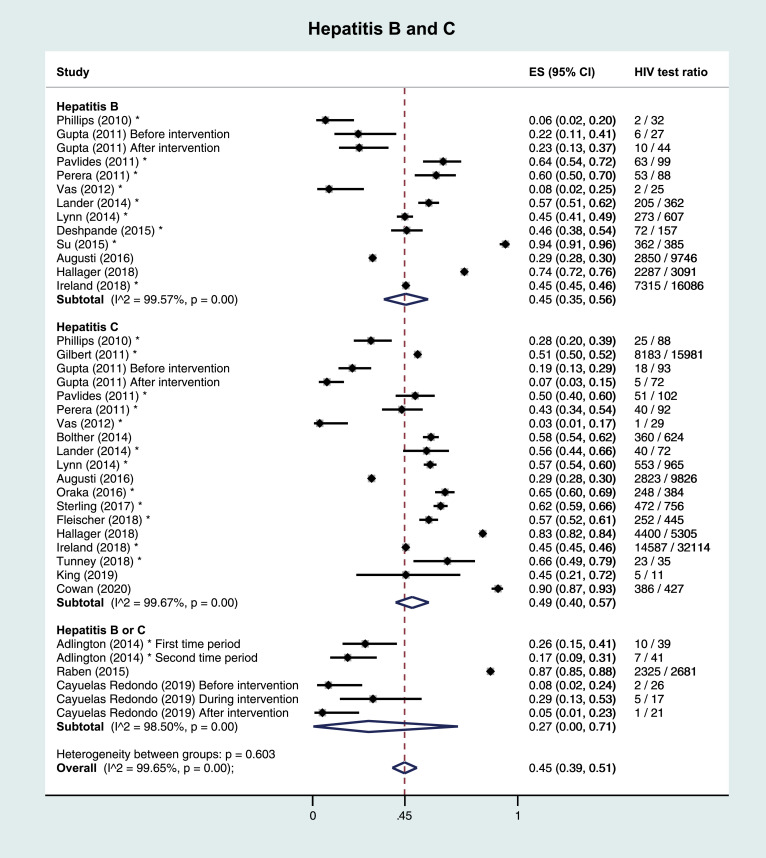
Of three full-text references on HBV, one was performed in the PC setting, and the other two in hospitals. Median number of subjects was 3091 (IQR 71–9746). HIV test ratios were 23% and 74% in the hospital setting, and 29% in the PC setting. Median positivity was 2·6% (IQR 1·2%−3·9%). No studies were scored high risk of bias. Across the nine included other publications, median number of subjects was 157 (IQR 88–385) and median HIV test ratio was 46% (IQR 45%−60%).
Of five full-text references on HCV, one was performed in the PC setting, and the others in hospitals. Median number of subjects was 624 (IQR 165–5305). HIV test ratios ranged from 14% to 83% in the hospital setting, and was 29% in the PC setting. Median positivity was 4·7% (IQR 3·0%−6·4%). One study was scored high risk of bias. Across the 13 included other publications, median number of subjects was 384 (IQR 88–756) and median HIV test ratio 56% (IQR 45%−62%).
Two full-text references and one abstract did not distinguish between HBV and HCV. The full-text studies reported HIV test ratios of 13% and 87%, the abstract reported 21%, Table 2.
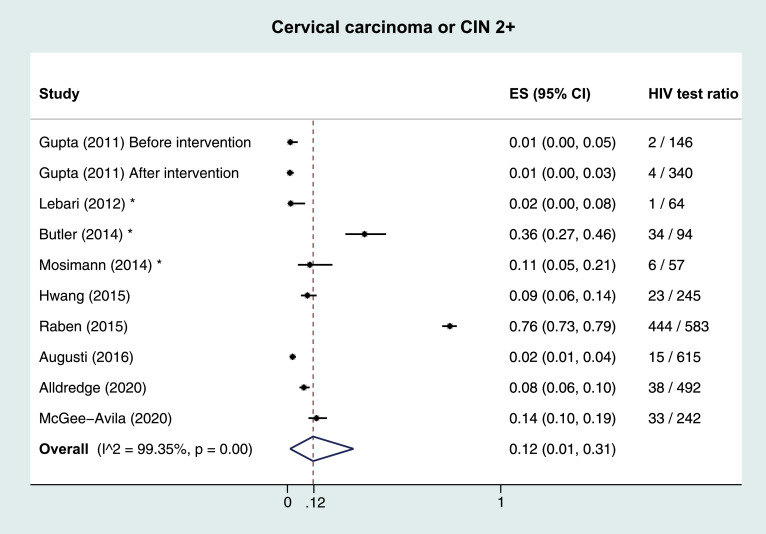
3.3. Cervical carcinoma or CIN2+
Of six full-text references on CC/CIN2+, one was performed in the PC setting, one in the context of a cancer surveillance program, and four in hospitals. Median number of subjects was 489 (IQR 245–583). The HIV test ratio was 2% in the PC setting. HIV test ratios ranged from 1% to 14% in four studies in the hospital/surveillance setting, and the fifth reported 76%, but risk of bias was deemed high. Of four studies reporting on positivity, three reported 0% and one 0·2% positivity. Three other publications were included, with a median number of 64 subjects (IQR 57–94) and median HIV test ratio of 11% (range 2%−36%), Table 2.
3.4. Malignant lymphoma
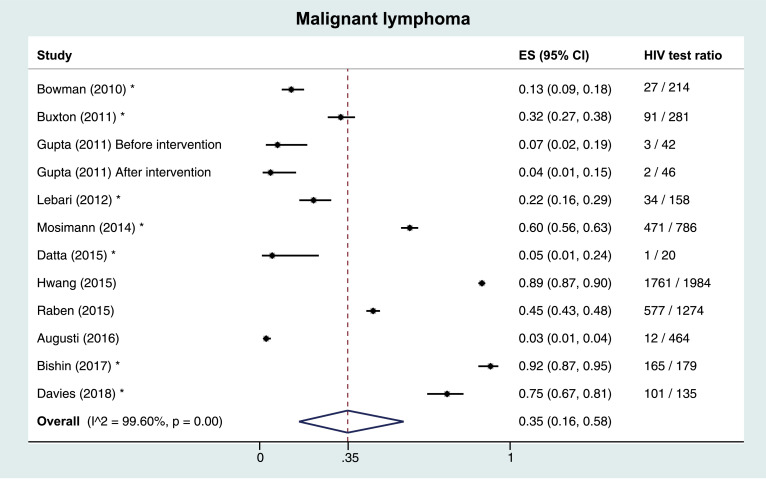
Of four full-text references on malignant lymphoma, one was performed in the PC setting, the others in hospitals. Median number of subjects was 869 (IQR 276–1629). HIV test ratios ranged from 6% to 89% in the hospital setting, and was 3% in the PC setting. Median positivity percentage was 3·6% (IQR 1·4%−8·3%). One study was high risk of bias. Across seven included other publications, median number of subjects was 179 (IQR 135–281) and median HIV test ratio was 32% (IQR 13%−75%), Table 2.
3.5. Pooled results
Meta-analyses of HIV test ratios by IC, including all publication types, regardless of risk of bias were performed. Heterogeneity between studies within ICs was very large, with the I2 test for heterogeneity exceeding 99% in all analyses. The overall estimated proportion (ES) tested for HIV was 0·49 (95% CI 0·43–0·54). By IC, this proportion was highest in TB; ES 0·72 (0·63–0·80), followed by HCV (ES 0·49, 0·40–0·57), HBV (ES 0·45, 0·35–0·56), malignant lymphoma (ES 0·35, 0·16–0·58) and studies reporting hepatitis B or C (ES 0·27, 0·0–0·71). Lowest ES were observed in CC/CIN2+ (ES 0·12, 0·01–0·31), Fig. 2. A sensitivity analysis including only low risk of bias full-text publications showed lower proportions, with an overall ES of 0·40 (0·29–0·52), and 0·68 (0·51–0·83), 0·38 (0·10–0·71), 0·37 (0·10–0·69), 0·21 (0·00–0·87), 0·12 (0·02–0·27), and 0·05 (0·02–0·09) for TB, HCV, HBV, malignant lymphoma, HBV/HCV, and CC/CIN2+, respectively. Five studies reported HIV test ratios stratified by sex; four on TB, and one on TB, HBV, HCV, and malignant lymphoma. When pooling studies among TB patients by sex, overall ES were similar in women (ES 0·49, 0·13–0·85) and men (ES 0·53, 0·15–0·89).
Fig. 2.
Pooled results and estimated proportion tested for HIV per indicator condition 2A: Tuberculosis 2B: Hepatitis B and C 2C: Malignant lymphoma 2D: Cervical carcinoma or cervical intraepithelial neoplasia grade 2+ * Other publication types than full-text articles (i.e. abstracts, short communications, and correspondence). CIN: cervical intraepithelial neoplasia. ES: estimated proportion.
Meta-analyses of HIV positivity by IC, including all publication types, regardless of risk of bias were performed. Heterogeneity between studies within ICs was large for most ICs (e.g. I2 test for heterogeneity 96% for HBV/HCV), but low for CC/CIN2+ (I2=0%). The overall estimated positivity ranged between 0% (CC/CIN2+) and 5% (TB) (supplementary appendix 6).
Meta-regression analyses showed no significant association between date of data collection and overall HIV test ratio (β=1·05%, 95%CI=−0·96%–3·06%, p = 0·30). When stratified by IC, a significant association was observed in studies on CC/CIN2+ only (β=6·14%, 95%CI=0·75%–11·53%, p = 0·03). However, this association was largely influenced by the most recent study, that reported the highest test ratio, but was also deemed high risk of bias. [9] In a sensitivity analysis excluding high risk of bias studies, this association was lost (β=0·47%, 95%CI=−2·86%–3·79%, p = 0·72).
4. Discussion
This systematic review provides an overview of the adoption of IC-guided testing in seven selected ICs in Western countries. Results show a large variation in HIV test ratios per IC, but overall HIV test ratios are low. The highest test ratios were observed in TB patients, followed by patients with HCV, HBV, and malignant lymphoma, respectively. Lowest test ratios were observed in patients with CC/CIN2+. No data on the extent of IC-guided testing in patients with VC/VIN2+ and PN was found.
Large differences in HIV test ratios between studies concerning the same IC were observed. Some outliers were studies with a high risk of bias, but among studies with low risk of bias, considerable variation was still observed. An explanation may be the difference in design of studies and how being tested for HIV was defined: some studies assessed evidence of any HIV testing, while others had a set timeframe around IC diagnosis to assess IC-guided testing. Another explanation could be the difference in setting between studies. For example, in malignant lymphoma, the lowest test ratio was observed in a study performed in the PC setting, while the highest ratio was observed in a study performed in a comprehensive cancer center. Variation was also observed within countries. Among TB patients in Canada, an audit performed in the province of Manitoba showed much lower HIV test ratios than one in Alberta (59% versus 91%, respectively [28,29]). This discrepancy is probably due to the ‘opt out’ HIV testing procedure for TB patients in Alberta, which was not used in Manitoba, suggesting its effectivity to optimize HIV testing. Two studies performed among HCV patients in Denmark also showed very different results, with an HIV test ratio of 58% in one university hospital, [30] compared to 83% in 18 Danish hospitals. [31] This discrepancy might be due to an increase in HIV test ratio over time, attributed to national efforts to increase HIV testing in risk groups, as the latter study concerned a later period (2002–2015) than the former (1996–2011). However, we found no association between data collection period and HIV test ratio in meta-regression analyses, suggesting that adherence to this testing strategy has not improved over time, and underlining the urgency of implementing strategies to improve IC-guided testing for HIV.
When comparing HIV test ratio before- and after interventions to increase HIV testing, some studies reported an improvement, [32,33] while others did not. [34,35] One UK study showed that HIV test ratios among patients with TB, HCV, cervical carcinoma and malignant lymphoma were lower in 2009–2010 than in 2008–2009 despite educational and promotional efforts by the researchers. [35] Studies showed that a universal HIV testing policy among TB patients yielded higher HIV test ratios than a selective testing policy based on risk-assessment, [36] a result in line with the high success rate of the ‘opt out’ testing procedure for TB patients in Alberta. [29]
HIV positivity was highest among TB patients, followed by HBV, HCV, and malignant lymphoma, respectively, but again large variation was observed. Among CC/CIN2+ patients, one study reported a positivity of 0·2%, [9] while positivity was 0% in the others. However, in view of the small number of studies and the low test ratios, this should not be interpreted as HIV screening not being cost-effective among CC/CIN2+ patients [4,5.]
This review confirms previous reporting on missed opportunities for earlier diagnosis through IC-guided testing. A barrier to optimal IC-guided testing might be the large number of ICs, the large variety in types of conditions and the many medical specialties involved. This variety requires tailored strategies to assure routine IC-guided testing is implemented across ICs. Moreover, an evaluation of IC-guided HIV testing recommendations in specialty guidelines in the UK and Europe revealed that the majority of IC guidelines do not recommend HIV testing, and physicians are not always aware of current HIV testing recommendations. [18,37] This is supported by the observation that the highest HIV test ratio were found in TB, HBV and HCV; HIV testing is recommended most prominently in the specialty guidelines for these conditions, and as pulmonologists and gastroenterologists commonly collaborate with infectious disease specialists, they may be more likely to focus on possible underlying HIV. Adoption of HIV testing in specialty guidelines and creating awareness of this strategy among involved specialties is an important first step to optimize testing. [38] As educational interventions to optimize testing showed varying results, additionally implementing previously proven successful strategies, such as opt-out testing or universal testing without detailed pre-test discussion, as described in the studies mentioned earlier, [29,36] is likely more effective than only educating involved medical professionals on IC-guided testing. In addition, sustained effect must be aimed for when designing interventions. For example, a digital case note prompt suggesting HIV testing when the patient has an IC diagnosis lead to a significant increase in HIV test ratios during the intervention period in two studies, but the effect was lost when the prompts were deactivated. [24,34] Thus, continuous implementation of a combination of the aforementioned strategies would likely be most effective.
A major strength of this review is the variety of settings and countries included. Second, by including not only published full-text articles, but also other publication types, we gained a more comprehensive picture of actual IC-guided HIV testing practices.
Although the retrospective design of included studies posed a potential risk of bias, most full-text studies were assessed as low risk of bias. We further addressed this possible limitation in a sensitivity analysis including only full-text articles with low risk of bias and found lower estimated proportions, suggesting that the IC-guided HIV test ratio outside study settings might be even lower. Very large heterogeneity was observed in the meta-analyses by IC, probably reflecting true heterogeneity across settings and Western countries. Thus, exact inferences on HIV test ratios by ICs could not be made, but conclusions can be drawn from the heterogeneity itself; testing practices are both inconsistently reported and inconsistently adopted. These findings should be considered when evaluating efforts to improve HIV testing strategies. Finally, a selection of only seven ICs was included in this review. Although not all ICs were included, it is unlikely that the HIV test ratios in other ICs will be much higher, as well-established and guideline-supported ICs such as TB and HCV were included in this study, and it is evident that even in those improvement is still needed.
This systematic review shows that a decade after its introduction, IC-guided testing for HIV is still insufficiently implemented in Western countries. Lessons on effective strategies from ICs with the highest test ratios, such as universal testing strategies, should be used to design effective implementation strategies for optimal IC-guided testing, to reduce underdiagnosis and late presentation of HIV.
Funding
The H-TEAM initiative, of which this review is a project, is being supported by Aidsfonds (Grant No. 2013169), Stichting Amsterdam Dinner Foundation, Bristol-Myers Squibb International Corp. (study number: AI424–541), Gilead Sciences Europe Ltd (Grant No. PA-HIV-PREP-16-0024), Gilead Sciences (protocol numbers: CO-NL-276-4222, CO-US-276-1712), Janssen Pharmaceutica (reference number: PHNL/JAN/0714/0005b/1912fde), M.A.C AIDS Fund, ViiV Healthcare (PO numbers: 3000268822, 3000747780) and ZonMw (Grant No. 522002003). This review is further funded by Aidsfonds (Grant No. P- 42702). The funders of the study had no role in the study design or execution.
Data sharing statement
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Contributors statement
Geerlings acquired financial support for the project leading to this publication. Geerlings, Schim van der Loeff, van Bergen and Bogers were involved in the conceptualisation and design of methodology of the study. Bogers performed the literature search. Bogers and Hulstein performed data curation including screening of search results, data extraction, and performing quality assessments. Bogers analysed the data and designed the figures. Schim van der Loeff performed quality controls and validation on all data analyses. De Bree and Reiss provided commentary and revisions on the original draft. All authors were involved in the interpretation of the data and the preparation of the final manuscript.
Declaration of Competing Interest
Dr. Bogers reports grants from Aidsfonds, grants from H-TEAM, during the conduct of the study; and this work is partially funded by H-TEAM, a consortium of all actors involved in hiv-care and prevention in Amsterdam, with the ultimate goal to pursue the end of new hiv-infections in Amsterdam. The H- team is sponsored by a mix of organisations, including the Municipality, AIDS-funds, and several farmaceutical companies (www.hteam.nl). There are no personal fees or payment involved. H-TEAM is not involved in the design or conduct of this work. S.H.H. Hulstein has nothing to disclose. Dr. Schim van der Loeff reports other from Merck, non-financial support from Stichting Pathologie Onderzoek en Ontwikkeling (SPOO), outside the submitted work. Dr. de Bree reports grants from AIDS Fonds, grants from Stichting AmsterdamDiner Foundation, grants from Gilead Sciences, grants from Janssen Pharmaceutica, grants from ViiV Healthcare, grants from ZonMW, grants from M.A.C AIDS Fund, during the conduct of the study; grants and other from Gilead Sciences, outside the submitted work. Dr. Reiss reports grants from AIDS Fonds, grants from Stichting AmsterdamDiner Foundation, grants from Gilead Sciences, grants from Janssen Pharmaceutica, grants from ViiV Healthcare, grants from ZonMW, grants from M.A.C AIDS Fund, during the conduct of the study; grants and other from Gilead Sciences, grants and other from ViiV Healthcare, grants and other from Merck, outside the submitted work. Dr. van Bergen reports grants from RIVM: national institute for public health and the environment, during the conduct of the study; and reports being a member of the board of the H-TEAM, a consortium of all actors involved in hiv-care and prevention in Amsterdam, with the ultimate goal to pursue the end of new hiv-infections in Amsterdam. The H- team is sponsored by a mix of organisations, including the Municipality, AIDS-funds, and several farmaceutical companies (www.hteam.nl). There are no personal fees or payment involved. Dr. Geerlings reports grants from Aidsfonds, during the conduct of the study.
Acknowledgements
We gratefully acknowledge René Spijker, MSc, for his collaboration and support in designing and conducting the search, and dr. Miranda Langendam for her collaboration and support. Finally, the authors thank the H-TEAM consortium (supplementary appendix 7).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100877.
Appendix. Supplementary materials
References
- 1.European Centre for Disease Prevention and Control . Copenhagen; Denmark: 2018. HIV/AIDS surveillance in Europe 2019 –2018 data.https://www.ecdc.europa.eu/sites/default/files/documents/hiv-surveillance-report-2019.pdf Accessed 17 November 2020. [Google Scholar]
- 2.Girardi E., Sabin C.A., Monforte A.D... Late diagnosis of HIV infection: epidemiological features, consequences and strategies to encourage earlier testing. J Acquir Immune Defic Syndr. 2007;46(Suppl 1):S3–S8. doi: 10.1097/01.qai.0000286597.57066.2b. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A., Lundgren J.D., Sabin M.L. Risk factors and outcomes for late presentation for HIV-positive persons in Europe: results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE) PLoS Med. 2013;10(9) doi: 10.1371/journal.pmed.1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders G.D., Bayoumi A.M., Sundaram V. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352(6):570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 5.Paltiel A.D., Weinstein M.C., Kimmel A.D. Expanded screening for HIV in the United States–an analysis of cost-effectiveness. N Engl J Med. 2005;352(6):586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 6.HIV in Europe . Copenhagen; Denmark: 2014. HIV indicator conditions: guidance for implementing hiv testing in adults in health care settings.http://www.hiveurope.eu/Portals/0/Documents/Guidance.pdf.pdf?ver=2014-01-29-113626-000 Accessed 13 October 2020. [Google Scholar]
- 7.World Health Organization . World Health Organization; Geneva, Switzerland: 2007. Guidance on provider-initiated HIV testing and counselling in health facilities. Report No.: ISBN 978 92 4 159556 8. [Google Scholar]
- 8.Sullivan A.K., Raben D., Reekie J. Feasibility and effectiveness of indicator condition-guided testing for HIV: results from HIDES I (HIV indicator diseases across Europe study) PLoS ONE. 2013;8(1):e52845. doi: 10.1371/journal.pone.0052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raben D., Mocroft A., Rayment M. Auditing HIV testing rates across Europe: results from the HIDES 2 Study. PLoS ONE. 2015;10(11) doi: 10.1371/journal.pone.0140845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menacho I., Sequeira E., Muns M. Comparison of two HIV testing strategies in primary care centres: indicator-condition-guided testing vs. testing of those with non-indicator conditions. HIV Med. 2013;14(Suppl 3):33–37. doi: 10.1111/hiv.12064. [DOI] [PubMed] [Google Scholar]
- 11.Read P., Armstrong-James D., Tong C.Y., Fox J... Missed opportunities for HIV testing–a costly oversight. QJM. 2011;104(5):421–424. doi: 10.1093/qjmed/hcq236. [DOI] [PubMed] [Google Scholar]
- 12.Scognamiglio P., Chiaradia G., De Carli G. The potential impact of routine testing of individuals with HIV indicator diseases in order to prevent late HIV diagnosis. BMC Infect Dis. 2013;13:473. doi: 10.1186/1471-2334-13-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wohlgemut J., Lawes T., Laing R.B... Trends in missed presentations and late HIV diagnosis in a UK teaching hospital: a retrospective comparative cohort study. BMC Infect Dis. 2012;12:72. doi: 10.1186/1471-2334-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Y.D., Garner S.E., Lau J.S.Y., Korman T.M., Woolley I.J. Prevalence of HIV indicator conditions in late presenting patients with HIV: a missed opportunity for diagnosis? QJM. 2019;112(1):17–21. doi: 10.1093/qjmed/hcy223. [DOI] [PubMed] [Google Scholar]
- 15.Levy I., Maor Y., Mahroum N. Missed opportunities for earlier diagnosis of HIV in patients who presented with advanced HIV disease: a retrospective cohort study. BMJ Open. 2016;6(11) doi: 10.1136/bmjopen-2016-012721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joore I.K., Arts D.L., Kruijer M.J. HIV indicator condition-guided testing to reduce the number of undiagnosed patients and prevent late presentation in a high-prevalence area: a case-control study in primary care. Sex Transm Infect. 2015;91(7):467–472. doi: 10.1136/sextrans-2015-052073. [DOI] [PubMed] [Google Scholar]
- 17.Ellis S., Curtis H., Ong E.L. British HIV Association. HIV diagnoses and missed opportunities. Results of the British HIV Association (BHIVA) National Audit. Clin Med (Lond) 2010;2012(5):430–434. doi: 10.7861/clinmedicine.12-5-430. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord E., Stockdale A.J., Malek R. Evaluation of HIV testing recommendations in specialty guidelines for the management of HIV indicator conditions. HIV Med. 2017;18(4):300–304. doi: 10.1111/hiv.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agusti C., Montoliu A., Mascort J. Missed opportunities for HIV testing of patients diagnosed with an indicator condition in primary care in Catalonia, Spain. Sex Transm Infect. 2016;92(5):387–392. doi: 10.1136/sextrans-2015-052328. [DOI] [PubMed] [Google Scholar]
- 20.Bull L., Rayment M... HIV-indicator-condition-driven HIV testing: clinically effective but still rarely implemented. Clin Med (Lond) 2016;16(2):175–179. doi: 10.7861/clinmedicine.16-2-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gullon A., Verdejo J., de Miguel R., Gomez A., Sanz J... Factors associated with late diagnosis of HIV infection and missed opportunities for earlier testing. AIDS Care. 2016;28(10):1296–1300. doi: 10.1080/09540121.2016.1178700. [DOI] [PubMed] [Google Scholar]
- 22.Page I., Phillips M., Flegg P., Palmer R... The impact of new national HIV testing guidelines at a district general hospital in an area of high HIV seroprevalence. J R Coll Phys Edinb. 2011;41(1):9–12. doi: 10.4997/JRCPE.2011.103. [DOI] [PubMed] [Google Scholar]
- 23.Ruutel K., Lemsalu L., Latt S... Monitoring HIV-indicator condition guided HIV testing in Estonia. HIV Med. 2018;19(Suppl 1):47–51. doi: 10.1111/hiv.12586. [DOI] [PubMed] [Google Scholar]
- 24.Youssef E., Sanghera T., Bexley A., Hayes M., Perry N., Dosekun O... HIV testing in patients presenting with indicator conditions in outpatient settings: offer and uptake rates, and educational and active interventions. Int J STD AIDS. 2018;29(13):1289–1294. doi: 10.1177/0956462418781681. [DOI] [PubMed] [Google Scholar]
- 25.Tominski D., Katchanov J., Driesch D. The late-presenting HIV-infected patient 30 years after the introduction of HIV testing: spectrum of opportunistic diseases and missed opportunities for early diagnosis. HIV Med. 2017;18(2):125–132. doi: 10.1111/hiv.12403. [DOI] [PubMed] [Google Scholar]
- 26.Munn Z., Moola S., Lisy K., Riitano D., Tufanaru C... Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 27.Nyaga V.N., Arbyn M., Aerts M... Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basham C.A., Elias B., Fanning A., Cook C., Orr P. Performance measurement of a Canadian provincial tuberculosis programme: manitoba, 2008-2012. Int J Tubercul Lung Dis. 2018;22(4):437–443. doi: 10.5588/ijtld.17.0508. [DOI] [PubMed] [Google Scholar]
- 29.Long R., Niruban S., Heffernan C. A 10-year population based study of 'opt-out' HIV testing of tuberculosis patients in Alberta, Canada: national implications. PLoS ONE. 2014;9(6):e98993. doi: 10.1371/journal.pone.0098993. [Electronic Resource] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolther M., Dalgaard L.S., Kristensen L.H., Tarp B.D., Jensen-Fangel S. Testing for hepatitis B virus and HIV in patients with chronic hepatitis C: screening performance and outcome. Scand J Infect Dis. 2014;46(10):686–692. doi: 10.3109/00365548.2014.929734. [DOI] [PubMed] [Google Scholar]
- 31.Hallager S., Lundh A., Ladelund S. The prevalence of human immunodeficiency virus coinfection among patients newly diagnosed with chronic hepatitis B or C in Denmark: a nationwide cohort study. Open Forum Infect Dis. 2018;5(12):ofy310. doi: 10.1093/ofid/ofy310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adlington K., Mackie N... An audit of HIV testing in acute medical patients with HIV clinical indicator conditions: are guidelines being ignored? HIV Med. 2014;15:102. (abstr) [Google Scholar]
- 33.Anderson C., White J., Abubakar I. Raising standards in UK TB control: introducing cohort review. Thorax. 2014;69(2):187–189. doi: 10.1136/thoraxjnl-2013-203751. [DOI] [PubMed] [Google Scholar]
- 34.Cayuelas Redondo L., Ruiz M., Kostov B. Indicator condition-guided HIV testing with an electronic prompt in primary healthcare: a before and after evaluation of an intervention. Sex Transm Infect. 2019;95(4):238–243. doi: 10.1136/sextrans-2018-053792. [DOI] [PubMed] [Google Scholar]
- 35.Gupta N.D., Lechelt M... Assessment of the implementation and knowledge of the UK national guidelines for HIV testing (2008) in key conditions at a UK district general hospital. Int J STD AIDS. 2011;22(2):102–104. doi: 10.1258/ijsa.2010.010218. [DOI] [PubMed] [Google Scholar]
- 36.Roy A., Anaraki S., Hardelid P. Universal HIV testing in London tuberculosis clinics: a cluster randomised controlled trial. Eur Respir J. 2013;41(3):627–634. doi: 10.1183/09031936.00034912. [DOI] [PubMed] [Google Scholar]
- 37.Martinez Sanz J., Perez Elias M.J., Muriel A. Outcome of an HIV education program for primary care providers: screening and late diagnosis rates. PLoS ONE. 2019;14(7) doi: 10.1371/journal.pone.0218380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabana M.D., Rand C.S., Powe N.R. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 39.Basham C.A., Elias B., Fanning A., Orr P... Tuberculosis among northern Manitoba First Nations, 2008-2012: program performance on- and off-reserve. Can J Public Health. 2019;8:08. doi: 10.17269/s41997-019-00231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark I.T., Lobato M.N., Gutierrez J., Sosa L.E... HIV status among patients with tuberculosis and HIV testing practices by Connecticut health care providers. J Int Assoc Provid AIDS Care. 2013;12(4):261–265. doi: 10.1177/2325957412473649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clerk N., Antunes G., Williams J., Dibble W. Improving the uptake of HIV testing in patients with tuberculosis. Br J Nurs. 2013;22(11):634–637. doi: 10.12968/bjon.2013.22.11.634. [DOI] [PubMed] [Google Scholar]
- 42.Gardner A., Naureckas C., Beckwith C., Losikoff P., Martin C., Carter E.J. Experiences in implementation of routine human immunodeficiency virus testing in a US tuberculosis clinic. Int J Tuberc Lung Dis. 2012;16(9):1241–1246. doi: 10.5588/ijtld.11.0628. [DOI] [PubMed] [Google Scholar]
- 43.Post J.J., Goldberg H., Kaufman G., Plit M., Ressler K.A., Ferson M.J... HIV testing rates and co-infection among patients with tuberculosis in south-eastern Sydney, 2008-2013. Med J Aust. 2015;202(5):255–257. doi: 10.5694/mja14.01490. [DOI] [PubMed] [Google Scholar]
- 44.Ribeiro L., Gomes M., Gaio R., Duarte R. HIV screening of tuberculosis patients in Portugal: what are we missing? Int J Tuberculos Lung Dis. 2018;22(10):1216–1219. doi: 10.5588/ijtld.17.0846. [DOI] [PubMed] [Google Scholar]
- 45.Rivest P., Sinyavskaya L., Brassard P. Burden of HIV and tuberculosis co-infection in Montreal, Quebec. Can J Public Health. 2014;105(4) doi: 10.17269/cjph.105.4269. e263-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sewell J., Capocci S., Johnson J. Expanded blood borne virus testing in a tuberculosis clinic. A cost and yield analysis. J Infect. 2015;70(4):317–323. doi: 10.1016/j.jinf.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 47.William S.T., Taylor R., Osman H... Changes in HIV testing rates among patients with tuberculosis in a large multiethnic city in the United Kingdom in 2008/09. HIV Med. 2010;1:57. [Google Scholar]
- 48.Aguayo C., Leon L., Sanchez A., Reguera A. Analysis of Extrapulmonary Tuberculosis (ETB) Registred in a Spanish hospital. Retrospective study 1999-2009. Intern Med J. 2010;40:66. (abstr) [Google Scholar]
- 49.Hubbard A., Garcia E., Chitnis A., Wong R. High prevalence of Hiv, Hbv, and Hcv Co-infection among adults with Tb infection: an urban safety-net hospital experience. Chest. 2020;158:A329. (abstr) [Google Scholar]
- 50.Patel R., Makaretz A., Fortune D. Factors associated with high mortality during TB treatment in New Mexico: 2007-2017. Am J Respir Crit Care Med Conf. 2019;199(9) (abstr) [Google Scholar]
- 51.Perch M., Andersen P., Kok-Jensen A... HIV testing of patients diagnosed with tuberculosis increased in Denmark during the period from 2007 to 2009. HIV Med. 2013;14:42–43. doi: 10.1111/hiv.12057. [DOI] [PubMed] [Google Scholar]
- 52.Phillips M., Page I., Sweeney J., Flegg P., Palmer R., Wasef W... A year on from UK national guidelines: an audit of HIV testing in patients diagnosed with a clinical indicator disease. HIV Med. 2010;1:111–112. (abstr) [Google Scholar]
- 53.Potter J.L., Hyams C., Shaukat M. Should screening for chronic viral hepatitis in patients with tuberculosis be introduced to nice guidelines? Thorax. 2014;69:A159. (abstr) [Google Scholar]
- 54.Qasim M., Nadama R., Adeloye O. American journal of respiratory and critical care medicine conference: American Thoracic Society International Conference, ATS. Vol. 185. 2012. Clinical study: diagnosis and management of tuberculosis patients as per national institute of clinical excellence (NICE) United Kingdom guidelines. (abstr) [Google Scholar]
- 55.Reina S., Silva C., Correia A.M., Duarte R... European respiratory journal conference: European respiratory society annual congress. Vol. 46. 2015. HIV screening in tuberculosis patients, in the northern region of Portugal. [DOI] [PubMed] [Google Scholar]
- 56.Ricci A., Buelli F., Pinsi G. Reported HIV-status in TB patients attended in a infectious diseases department in Brescia. Infection. 2010;38:68. (abstr) [Google Scholar]
- 57.Stolagiewicz N., Goodman A., Milburn H., Breen R... Are rates of HIV infection falling in patients with TB in inner London? HIV Med. 2015;16:42–43. (abstr) [Google Scholar]
- 58.Thorburn F... The impact of a multi-disciplinary meeting on the rates of HIV in testing in TB patients. HIV Med. 2012;13:64. (abstr) [Google Scholar]
- 59.Vas A., Morgan E., Padmakumar K., Bradley B., Williams M., Thornton-Chan J... HIV testing in TB and Hepatitis services in a district general hospital. HIV Med. 2012;13:65. (abstr) [Google Scholar]
- 60.Deshpande S., Ko T., Roberts E. Tenofovir therapy for hepatitis B may be commonly prescribed without HIV testing. J Acquir Immune Defic Syndr. 2016;71(2):e53–ee4. doi: 10.1097/QAI.0000000000000865. [DOI] [PubMed] [Google Scholar]
- 61.Ireland G., Ogaz D., Kirwan P. HIV testing in persons diagnosed with hepatitis B and C. HIV Med. 2018;19(3):e54–ee5. doi: 10.1111/hiv.12525. [DOI] [PubMed] [Google Scholar]
- 62.Lander M., Tohani A., Dias A., O'Connell R... Assessing HIV testing in hepatitis: an audit of HIV testing uptake in a specialist hepatology clinic in an area of high prevalence for hepatitis B and C. HIV Med. 2014;15:96–97. (abstr) [Google Scholar]
- 63.Lynn A.M., Larson J.J., Leise M.D... Population-based HIV screening rates in patients with viral hepatitis. Gastroenterology. 2014;146(5):S1003. (abstr) [Google Scholar]
- 64.Pavlides M., Madhotra R., Raza M.M... HIV testing in patients with hepatitis B and C infection in the United Kingdom. Clin Microbiol Infect. 2011;17:S802–S8S3. (abstr) [Google Scholar]
- 65.Perera S., Lallemant C., Gilleece Y., Verma S... HIV testing in patients with hepatitis B and/or C. HIV Med. 2011;12:41–42. (abstr) [Google Scholar]
- 66.Su C., Bannister N., Huang V... Evaluation of chronic hepatitis B treatment and assessment of chronic hepatitis B care at an Asian community health center in New York City, 2012. Hepatology. 2015;62 993A (abstr) [Google Scholar]
- 67.King K., Seah J., Cheng A., Whiting S., Hoy J... Missed Opportunities for HIV testing persist despite a single educational intervention: how can we close this evidence-practice gap? Intern Med J. 2019;5:05. doi: 10.1111/imj.14418. [DOI] [PubMed] [Google Scholar]
- 68.Cowan E., Hardardt J., Brandspiegel S., Calderon Y... Linkage outcomes for HIV/HCV Co-infected and HCV mono-infected patients participating in an emergency department screening program. Ann Emerg Med. 2020;76:S49. (abstr) [Google Scholar]
- 69.Fleisher I., Geboy A., Nichols W. HCV antibody positive patients in a large healthcare system: HIV testing rates and compliance with CDC guidelines. Gastroenterology. 2018;154:S1201. doi: 10.1371/journal.pone.0252412. (abstr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gilbert M., Barrios R., Wong E. Use of provincial laboratory and surveillance data to inform strategies for expanding provider-initiated HIV testing and to assess their impact. Can J Infect Dis Med Microbiol. 2011;22 86B (abstr) [Google Scholar]
- 71.Oraka E., Abara W., Pitasi M., Van Handel M., DiNenno E... Prevalence of HIV testing among adults with a hepatitis c diagnosis: findings from the national health and nutrition examination survey 1999-2014. Sex Transm Dis. 2016;43:S217. (abstr) [Google Scholar]
- 72.Sterling R.K., Amador J., Stewart P. HIV testing in patients starting HCV treatment and factors associated with adherence to AASLD-IDSA guidelines. Hepatology. 2017;66 531A-2A (abstr) [Google Scholar]
- 73.Tunney R., Saxon C. Characteristics and audit of the management of all new hepatitis C diagnoses made in a level 3 genitourinary medicine clinic over the last 5 years. HIV Med. 2018;19:S148. (abstr) [Google Scholar]
- 74.Alldredge J., Leaf M.C., Patel P. Prevalence and predictors of HIV screening in invasive cervical cancer: a 10 year cohort study. Int J Gynecol Cancer. 2020;30(6):772–776. doi: 10.1136/ijgc-2019-000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hwang J.P., Granwehr B.P., Torres H.A. HIV testing in patients with cancer at the initiation of therapy at a large US comprehensive cancer center. J Oncol Pract/Am Soc Clin Oncol. 2015;11(5):384–390. doi: 10.1200/JOP.2015.005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGee-Avila J.K., Doose M., Nova J., Kumar R., Stroup A.M., Tsui J... Patterns of HIV testing among women diagnosed with invasive cervical cancer in the New Jersey Medicaid Program. Cancer Causes Control. 2020;31(10):931–941. doi: 10.1007/s10552-020-01333-w. [DOI] [PubMed] [Google Scholar]
- 77.Butler M., Black C., Cumming J., Groom T., Fargie F... HIV testing in patients with cervical intraepithelial neoplasia grade 2 and above: a clinical indicator disease. HIV Med. 2014;15:105. (abstr) [Google Scholar]
- 78.Lebari D., Kaczmarski E... HIV testing in cancer: experience from a tertiary oncology hospital. HIV Med. 2012;13:34. (abstr) [Google Scholar]
- 79.Mosimann V., Cavassini M., Hugli O. Patients with AIDS-defining cancers are not universally screened for HIV: a 10-year retrospective analysis of HIV-testing practices in a Swiss university hospital. HIV Med. 2014;15(10):631–634. doi: 10.1111/hiv.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bishin A., Vishnu P., Mandelson M., Aboulafia D.M... Blood Conference: 59th Annual Meeting of the. Vol. 130. American Society of Hematology, ASH; 2017. Guidelines in clinical practice: appraisal of quality metrics in the management of newly diagnosed diffuse large b-cell lymphoma based on the American Society of hematology practice improvement module. (abstr) [Google Scholar]
- 81.Bowman C.A., Olarinde O., Wright J. Routine HIV testing in lymphoma patients - Overcoming the challenges. HIV Med. 2010;1:59–60. (abstr) [Google Scholar]
- 82.Buxton J.K., Davidson K.L., Davies J.M., Scott F.M., Tucker J. South East Scotland experience of HIV testing in patients newly diagnosed with lymphoma. J R College Phys Edinburgh. 2011;41(3):284. doi: 10.4997/JRCPE.2011.323. [DOI] [PubMed] [Google Scholar]
- 83.Datta S., Lee R., Webb T., Ismail A., Jung A... 5 years experience of primary central nervous system lymphoma (PCNSL): a retrospective pilot audit. Neurosurgery and psychiatry conference: association of British nSeurologists, ABNJ Neurol. 2015;86(11) (abstr) [Google Scholar]
- 84.Davies D., Ishimwe P., Roberts M. A single centre's experience of HIV testing in patients with lymphoma. HIV Med. 2018;19:S127. (abstr) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.