Abstract
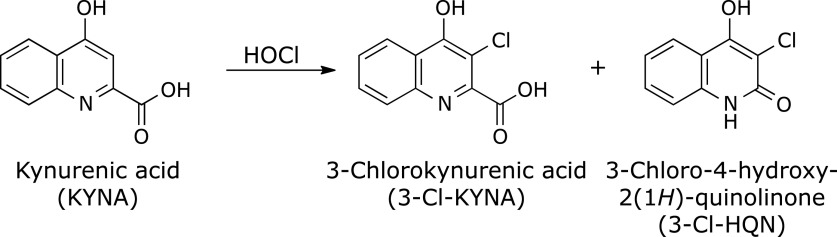
Kynurenic acid, a tryptophan metabolite, acts as antagonist or agonist of several receptors. Hypobromous acid (HOBr) and hypochlorous acid (HOCl) are generated by eosinophils and neutrophils. At inflammation sites, kynurenic acid may encounter HOBr and HOCl to generate products. When kynurenic acid was incubated with HOBr under neutral conditions, kynurenic acid generated a single product almost exclusively. This was identified as 3-bromokynurenic acid. Kynurenic acid reacted with HOCl, generating two products. The major product was identified as 3-chlorokynurenic acid with its oxidative decarboxylation product, 3-chloro-4-hydroxy-2(1H)-quinolinone as a by-product. Free amino acids suppressed the reactions of kynurenic acid with HOBr and HOCl. Taurine suppressed the HOCl reaction but not the HOBr reaction. An eosinophil peroxidase system containing H2O2, NaCl, and NaBr reacted with kynurenic acid, generating 3-bromokynurenic acid under mildly acidic conditions. Although a myeloperoxidase system containing H2O2 and NaCl reacted with kynurenic acid to generate 3-chlorokynurenic acid under mildly acidic conditions, the product was altered to 3-bromokynurenic acid by addition of NaBr to the system. These results suggest that 3-bromokynurenic acid and 3-chlorokynurenic acid may be generated from kynurenic acid at inflammation sites in humans, although their formation will be suppressed by coexistent amino acids.
Keywords: kynurenic acid, hypobromous acid, hypochlorous acid, myeloperoxidase, eosinophil peroxidase
Introduction
Kynurenic acid (KYNA) is a tryptophan metabolite generated by enzymes along the kynurenine pathway.(1) KYNA is an agonist of G-protein coupled GPR35 receptor, which presents predominantly on immune cells and in the gastrointestinal tract.(2) The serum level of KYNA was significantly elevated in patients with inflammatory bowel disease, compared to control subjects.(3) Several reports suggest that KYNA may have a positive influence on a number of pathologies of the gastrointestinal tract.(4) KYNA is also an antagonist of both the N-methyl-d-aspartate receptor and the α7 nicotinic acetylcholine receptor.(5,6) The brain level of KYNA was elevated in patients with schizophrenia and bipolar disorder.(7,8) KYNA possesses neuroprotective properties, since it blocks some of the neurotoxic effects of excitotoxins.(9) Recently, the relationship between inflammation and these disorders has received.(10,11) Eosinophils are a minor component of white blood cells, but are abundant in blood and tissues in various inflammatory disorders.(12) Eosinophils discharge eosinophil peroxidase (EPO), which catalyzes the oxidation of bromide ion (Br−) to hypobromous acid (HOBr) through hydrogen peroxide (H2O2) reduction.(13,14) Although the plasma concentrations are 39–84 µM for Br− and 100 mM for Cl−, EPO uses Br− selectively, resulting in HOBr.(15) The formed HOBr plays an important role in defense mechanisms against parasites. Meanwhile, neutrophils discharge myeloperoxidase (MPO), which catalyzes the oxidation of chloride ion (Cl−) to hypochlorous acid (HOCl) through hydrogen peroxide (H2O2) reduction.(16–18) Neutrophils are a major component of white blood cells. The HOCl formed by MPO plays a central role in host defense mechanisms against infection. Since HOCl can react with Br− to generate HOBr, a portion of the HOCl formed by the MPO system will react with Br− of the plasma concentration converting to HOBr.(19,20) Under inflammation conditions, KYNA may encounter HOBr and HOCl, and react with them. However, there is little information about the reactions of KYNA with HOBr and HOCl. In the present study, we examined the reactions of KYNA with reagents HOBr and HOCl, and with peroxidases EPO and MPO, using RP-HPLC, and found that both the reagents and the peroxidase systems readily react with KYNA to generate halogenated products.
Materials and Methods
Materials
KYNA was purchased from ChemCruz (Dallas, TX). NaBr (>99.99%), NaCl (>99.99%), KH2PO4, K2HPO4, MPO from human leukocytes, and EPO human recombinant protein were purchased from Sigma-Aldrich (St. Louis, MO). Other chemicals were obtained from Nacalai Tesque (Kyoto, Japan) or TCI (Tokyo). Bromide-free hypobromous acid (HOBr) was prepared by the addition of silver nitrate and subsequent distillation, as previously reported.(21) The concentration of HOBr was determined spectrophotometrically at 331 nm in 10 mM NaOH using a molar extinction coefficient of 315 M−1 cm−1.(22) Chloride-free sodium hypochlorite (NaOCl) was prepared by the method previously reported.(23) The concentration of NaOCl was determined spectrophotometrically at 290 nm using a molar extinction coefficient of 350 M−1 cm−1.(24)
HPLC and MS conditions
The HPLC system consisted of LC-10ADvp pumps and an SPD-M10Avp UV-vis photodiode-array detector (Shimadzu, Kyoto, Japan). For the RP-HPLC, an Inertsil ODS-3 octadecylsilane column of 4.6 × 250 mm and particle size 5 µm (GL Sciences, Tokyo, Japan) was used. The eluent was 20 mM ammonium acetate (pH 7.0) containing acetonitrile. The acetonitrile concentration was increased from 0 to 14% during 40 min in linear gradient mode. The column temperature was 40°C and the flow rate was 1 ml/min. The electrospray ionization time of flight mass spectrometry (ESI-TOF/MS) measurements were performed on a MicroTOF spectrometer (Bruker, Bremen, Germany) in negative mode.
Spectrometric data
Peak 1: 3-bromokynurenic acid (3-Br-KYNA). ESI-TOF/MS: m/z 222 and 224 (1:1), 266 and 268 (1:1). HR-ESI-TOF/MS: 265.946532 obsd (265.945829 calcd for C10H5BrNO3−). UV: λmax = 248, 320, 330 nm. 1H NMR (500 MHz, DMSO-d6): δ (ppm/TMS) 8.02 (d, J = 8.0 Hz, 1H), 7.61 (d, J = 8.6 Hz, 1H), 7.55 (dd, J = 7.7, 7.7 Hz, 1H), 7.24 (dd, J = 7.5, 7.5 Hz, 1H), 13C NMR (125 MHz, DMSO-d6): δ (ppm/TMS) 171.7, 161.0, 151.0, 138.2, 131.0, 124.9, 123.0, 122.8, 118.3, 99.3.
Peak 2: 3-chlorokynurenic acid (3-Cl-KYNA). ESI-TOF/MS: m/z 178 and 180 (3:1), 222 and 224 (3:1). HR-ESI-TOF/MS: 221.996068 obsd (221.996344 calcd for C10H5ClNO3−). UV: λmax = 246, 317, 331 nm. 1H NMR (500 MHz, DMSO-d6): δ (ppm/TMS) 8.06 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 8.5 Hz, 1H), 7.58 (dd, J = 7.5, 7.5 Hz, 1H), 7.27 (dd, J = 7.5, 7.6 Hz, 1H), 13C NMR (125 MHz, DMSO-d6): δ (ppm/TMS) 172.0, 162.0, 149.0, 138.2, 131.2, 124.8, 123.0, 122.8, 118.3, 109.0.
Peak 3: 3-chloro-4-hydroxy-2(1H)-quinolinone (3-Cl-HQN). ESI-TOF/MS: m/z 158, 194 and 196 (3:1). HR-ESI-TOF/MS: 193.9996 obsd (194.0007 calcd for C9H5ClNO2−). UV: λmax = 218, 300 nm. 1H NMR (500 MHz, DMSO-d6): δ (ppm/TMS) 7.81 (d, J = 8.0 Hz, 1H), 7.21 (dd, J = 7.5, 7.6 Hz, 1H), 7.03 (d, J = 8.5 Hz, 1H), 6.89 (dd, J = 7.5, 7.5 Hz, 1H), 13C NMR (125 MHz, DMSO-d6): δ (ppm/TMS) 167.3, 160.6, 137.5, 128.3, 124.3, 121.5, 118.9, 114.2, 99.6.
Quantitative procedures
The concentrations of the products were evaluated according to the integrated peak areas on RP-HPLC chromatograms detected at 240 nm and by the molecular extinction coefficients at 240 nm (ɛ240 nm). The ɛ240 nm value of 25,300 M−1 cm−1 was used for KYNA. The ɛ240 nm values of Peaks 1–3 were determined from the integration of proton signals of NMR and the HPLC peak area detected at 240 nm relative to that of KYNA in the mixed solution. The estimated ɛ240 nm values were 27,300 M−1 cm−1 for Peak 1, 28,600 M−1 cm−1 for Peak 2, and 22,700 M−1 cm−1 for Peak 3.
Results
Reaction with HOBr
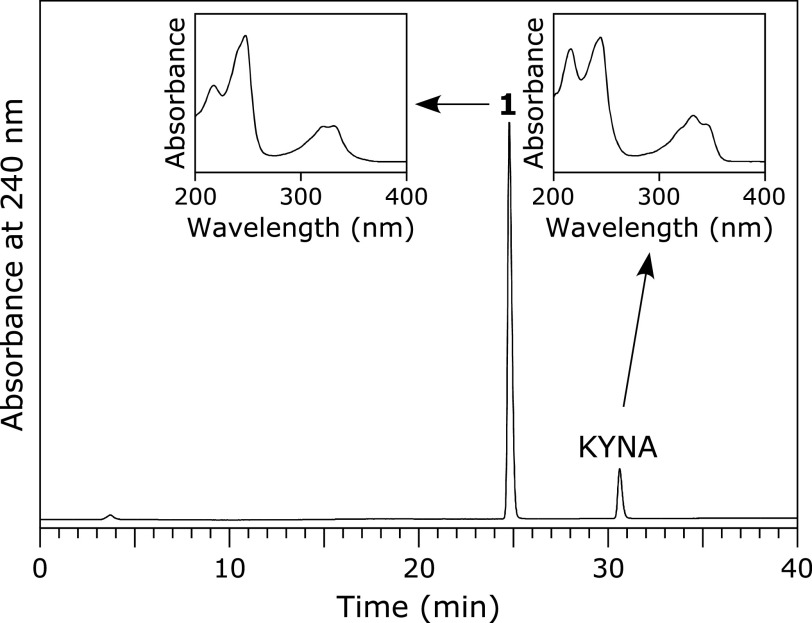
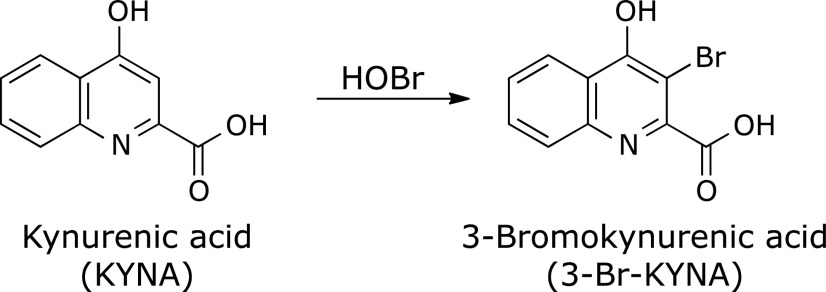
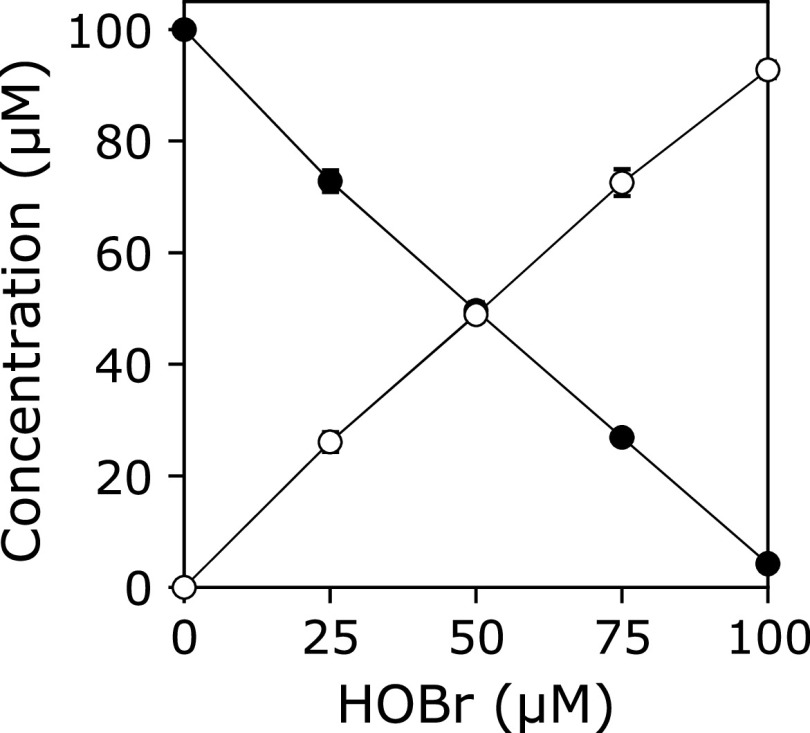
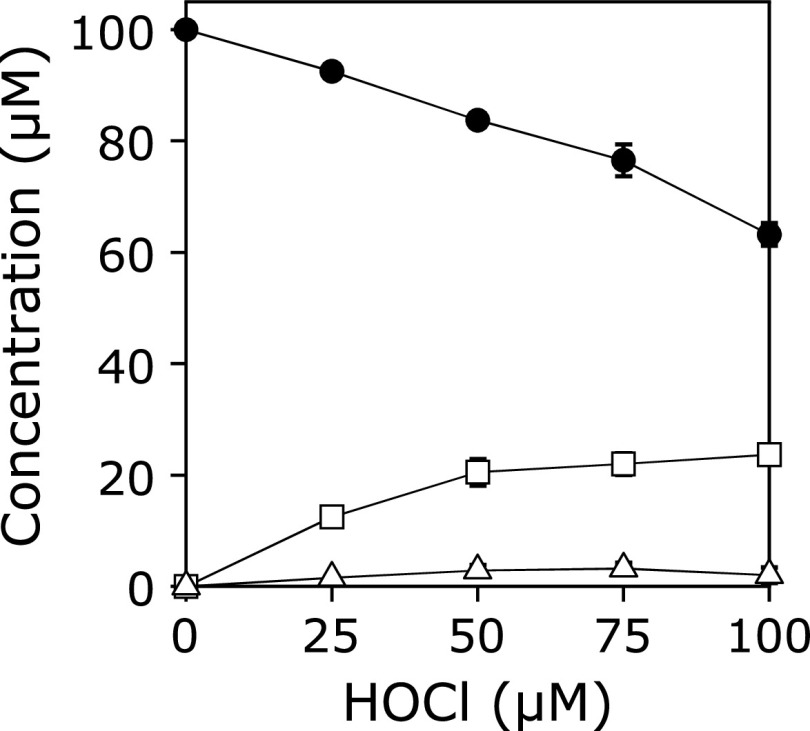
A solution of 100 µM KYNA was incubated with 100 µM HOBr in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. When the reaction mixture was analyzed by reversed-phase (RP) HPLC, a product peak, Peak 1, appeared in the chromatogram detected at 240 nm (Fig. 1). The product was isolated by RP-HPLC and identified using MS and NMR (1H-1d, 1H-1H COSY, 1H-13C HMQC, 1H-13C HMBC, 13C-1d). Peak 1 showed an ESI-TOF/MS spectrum with m/z = 222 and 224 (1:1) and 266 and 268 (1:1) in negative mode. High-resolution (HR) ESI-TOF/MS values of the molecular ion for Peak 1 agreed with the theoretical molecular mass for C10H5BrNO3− attributable to [KYNA + Br – 2H]− within 3 ppm. 1H NMR showed four aromatic protons coupled with each other on the 1H-1H COSY spectrum. 13C NMR showed nine aromatic carbons and a carbon attributable to a carboxy group. From these data, Peak 1 was identified as 3-bromokynurenic acid (3-Br-KYNA) (Scheme 1). Figure 2 shows the HOBr dose-dependence of the reaction of KYNA with HOBr when 100 µM KYNA and 0–100 µM HOBr were incubated in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. With increasing HOBr concentration, the consumptions of KYNA increased and the yield of 3-Br-KYNA increased. KYNA was converted to 3-Br-KYNA almost exclusively.
Fig. 1.
RP-HPLC chromatogram of a reaction mixture of KYNA with HOBr detected at 240 nm. A solution of 100 µM KYNA was incubated with 100 µM HOBr in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. The insets are the UV spectra of Peak 1 and KYNA.
Scheme 1.
Fig. 2.
HOBr dose-dependence of the concentration changes in KYNA and 3-Br-KYNA when a solution of 100 µM KYNA was incubated with 0–100 µM HOBr in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. KYNA (closed circle), 3-Br-KYNA (open circle). All the reaction mixtures were analyzed by RP-HPLC. Means ± SD (n = 3) are presented.
Reaction with HOCl
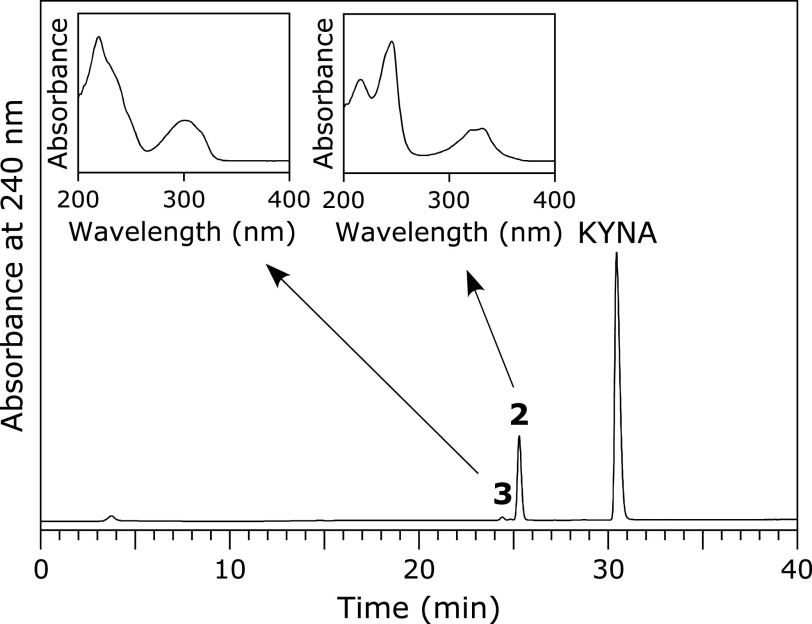
A solution of 100 µM KYNA was incubated with 100 µM HOCl in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. When the reaction mixture was analyzed by RP-HPLC, two product peaks, Peaks 2 and 3, appeared in the chromatogram detected at 240 nm (Fig. 3). The products were isolated by RP-HPLC and identified. Peak 2 showed an ESI-TOF/MS spectrum with m/z = 178 and 180 (3:1) and 222 and 224 (3:1) in negative mode. HR-ESI-TOF/MS values of the molecular ion for Peak 2 agreed with the theoretical molecular mass for C10H5ClNO3− attributable to [KYNA + Cl – 2H]− within 3 ppm. 1H NMR showed four aromatic protons correlated with each other on 1H-1H COSY. 13C NMR showed nine aromatic carbons and a carbon attributable to a carboxy group. Peak 2 was identified as 3-chlorokynurenic acid (3-Cl-KYNA) (Scheme 2). Peak 3 showed an ESI-TOF/MS spectrum with m/z = 194 and 196 (3:1) in negative mode. HR-ESI-TOF/MS values of the molecular ion for the Peak 3 agreed with the theoretical molecular mass for C9H5ClNO2− attributable to [KYNA + Cl – CO – 2H]− within 3 ppm. 1H NMR showed four aromatic protons correlated with each other on 1H-1H COSY. 13C NMR showed nine aromatic carbons but no carbon of a carboxy group. Peak 3 was identified as 3-chloro-4-hydroxy-2(1H)-quinolinone (3-Cl-HQN) (Scheme 2). Figure 4 shows the HOCl dose-dependence of the reaction of KYNA with HOCl. With increasing HOCl concentration, the consumptions of KYNA and both the yields of 3-Cl-KYNA and 3-Cl-HQN increased. The reactivity of HOCl to KYNA was lower than that of HOBr. The yield of 3-Cl-KYNA was higher than that of 3-Cl-HQN.
Fig. 3.
RP-HPLC chromatogram of a reaction mixture of KYNA with HOCl detected at 240 nm. A solution of 100 µM KYNA was incubated with 100 µM HOCl in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. The insets are the UV spectra of Peak 2 and Peak 3.
Scheme 2.
Fig. 4.
HOCl dose-dependence of the concentration changes in KYNA and products, 3-Cl-HQN and 3-Cl-KYNA, when a solution of 100 µM KYNA was incubated with 0–100 µM HOCl in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. KYNA (closed circle), 3-Cl-KYNA (open square), and 3-Cl-HQN (open triangle). All the reaction mixtures were analyzed by RP-HPLC. Means ± SD (n = 3) are presented.
Reaction with HOCl in the presence of NaBr
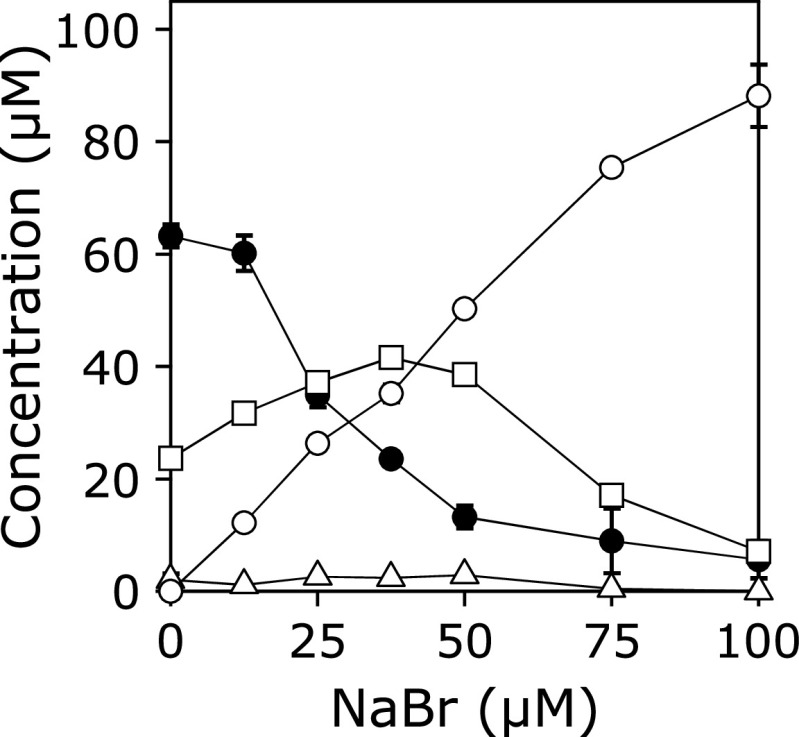
A solution of 100 µM KYNA was incubated with 100 µM HOCl in the presence of 0–100 µM NaBr in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min (Fig. 5). Consumption of KYNA and the yield of 3-Br-KYNA increased with increasing NaBr dose. At 100 µM NaBr, the major product was 3-Br-KYNA.
Fig. 5.
NaBr dose-dependence of the concentration changes in KYNA and products in the reaction with HOCl. A solution of 100 µM KYNA was incubated with 100 µM HOCl in the presence of 0–100 µM NaBr in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 30 min. KYNA (closed circle), 3-Br-KYNA (open circle), 3-Cl-KYNA (open square), and 3-Cl-HQN (open triangle). All the reaction mixtures were analyzed by RP-HPLC. Means ± SD (n = 3) are presented.
Effects of additives on the reaction with HOBr
To obtain information about the effects of coexistent compounds on the reactions of KYNA with HOBr, experiments were carried out in the presence of various additives. Table 1 shows the concentrations of KYNA and 3-Br-KYNA when a solution of 100 µM KYNA and 1 mM additives was incubated with 100 µM HOBr in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 24 h. Although ammonium chloride suppressed the reaction, methylamine, dimethylamine, trimethylamine, and tetramethylammonium chloride had no effect. Amino acids suppressed the reaction. Taurine had no effect. Ascorbic acid suppressed the reaction. Urea, glucose, and sodium acetate had no effect.
Table 1.
Effects of additives on the reactions of KYNA with HOBra)
| Additives | KYNA (µM) | 3-Br-KYNA (µM) |
|---|---|---|
| None | 4.0 ± 0.2 | 94.1 ± 1.2 |
| NH4Cl | 80.8 ± 1.6 | 22.4 ± 1.4 |
| CH3NH2/HCl | 1.2 ± 1.7 | 98.6 ± 0.8 |
| (CH3)2NH/HCl | 0.3 ± 0.4 | 97.9 ± 1.0 |
| (CH3)3N/HCl | 0.0 ± 0.0 | 99.7 ± 1.5 |
| (CH3)4NCl | 0.0 ± 0.0 | 97.8 ± 1.7 |
| Gly | 65.4 ± 1.3 | 32.1 ± 1.0 |
| Lys | 88.0 ± 0.2 | 9.2 ± 0.1 |
| Met | 93.4 ± 0.6 | 3.7 ± 0.2 |
| Cys | 93.1 ± 1.1 | 1.7 ± 0.1 |
| Taurine | 10.7 ± 1.6 | 86.8 ± 0.6 |
| Ascorbic acid | 72.7 ± 0.1 | 3.5 ± 0.2 |
| Urea | 9.6 ± 2.5 | 92.1 ± 2.6 |
| Glucose | 10.8 ± 0.4 | 90.2 ± 2.0 |
| CH3COONa | 1.9 ± 2.3 | 98.9 ± 2.0 |
a)Concentrations of KYNA and 3-Br-KYNA when a solution of 100 µM KYNA was incubated with 100 µM HOBr in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 24 h in the presence of the 1 mM additives. All the reaction mixtures were analyzed by RP-HPLC. Means ± SD (n = 3) are presented.
Effects of additives on the reaction with HOCl
To obtain information about the effects of coexistent compounds on the reactions of KYNA with HOCl, experiments were carried out in the presence of various additives (Table 2). Ammonium chloride, methylamine, and dimethylamine strongly suppressed the reaction, while trimethylamine and tetramethylammonium chloride showed little effect. In the case of tetramethylammonium chloride, the yield of 3-Cl-HQN was comparable to that of 3-Cl-KYNA, probably due to facilitation of the decarboxylation of 3-Cl-KYNA by tetramethylammonium ion, generating 3-Cl-HQN. Amino acids and taurine suppressed the reaction almost completely. Ascorbic acid suppressed the formations of 3-Cl-KYNA and 3-Cl-HQN almost completely, although a certain amount of KYNA was consumed. Urea, glucose, and sodium acetate showed little effect.
Table 2.
Effects of additives on the reactions of KYNA with HOCla)
| Additives | KYNA (µM) | 3-Cl-KYNA (µM) | 3-Cl-HQN (µM) |
|---|---|---|---|
| None | 66.3 ± 3.8 | 19.5 ± 0.5 | 3.1 ± 1.4 |
| NH4Cl | 94.4 ± 0.8 | 0.5 ± 0.1 | 0.0 ± 0.0 |
| CH3NH2/HCl | 97.0 ± 1.0 | 0.6 ± 0.2 | 0.0 ± 0.0 |
| (CH3)2NH/HCl | 92.4 ± 0.6 | 2.4 ± 0.2 | 0.0 ± 0.0 |
| (CH3)3N/HCl | 48.2 ± 1.5 | 21.7 ± 0.4 | 5.7 ± 0.1 |
| (CH3)4NCl | 59.5 ± 0.7 | 12.1 ± 0.1 | 10.9 ± 0.3 |
| Gly | 98.1 ± 0.4 | 0.2 ± 0.1 | 0.0 ± 0.0 |
| Lys | 97.7 ± 0.1 | 0.2 ± 0.2 | 0.0 ± 0.0 |
| Met | 97.3 ± 0.1 | 0.5 ± 0.1 | 0.0 ± 0.0 |
| Cys | 98.6 ± 1.2 | 0.2 ± 0.2 | 0.0 ± 0.0 |
| Taurine | 96.0 ± 3.0 | 0.7 ± 0.1 | 0.0 ± 0.0 |
| Ascorbic acid | 74.7 ± 0.1 | 0.3 ± 0.2 | 0.0 ± 0.0 |
| Urea | 58.6 ± 0.8 | 26.6 ± 0.7 | 2.6 ± 0.2 |
| Glucose | 59.5 ± 1.5 | 21.6 ± 0.4 | 3.3 ± 0.2 |
| CH3COONa | 59.0 ± 2.7 | 20.9 ± 0.6 | 3.8 ± 0.5 |
a)Concentrations of KYNA, 3Cl-KYNA, and 3-Cl-HQN when a solution of 100 µM KYNA was incubated with 100 µM HOCl in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 24 h in the presence of the 1 mM additives. All the reaction mixtures were analyzed by RP-HPLC. Means ± SD (n = 3) are presented.
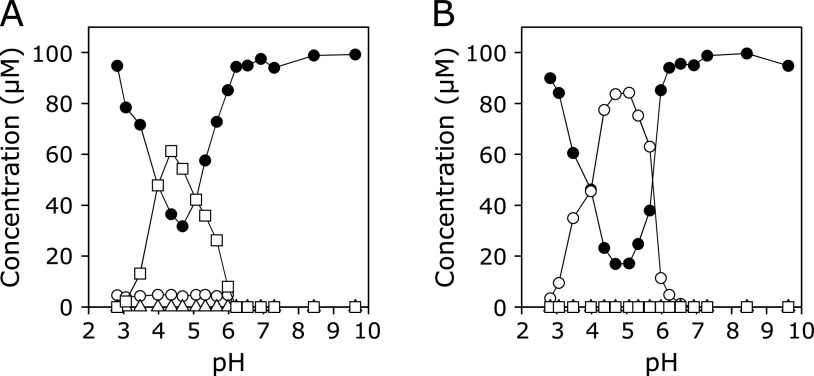
Reaction of KYNA with EPO systems
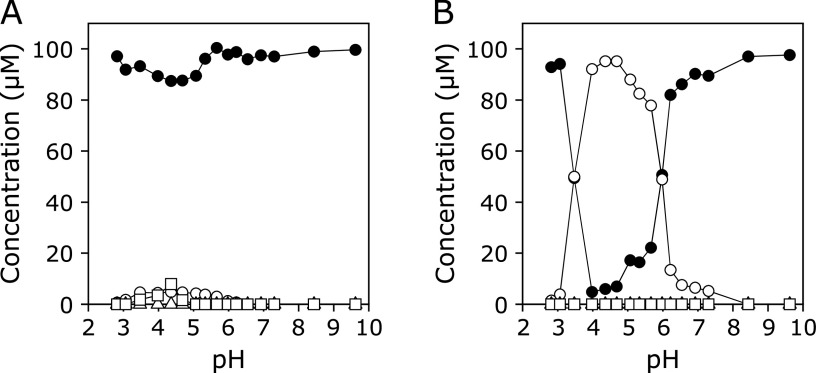
Figure 6A and B show the pH dependence of concentrations of KYNA and the products in the reaction of KYNA with an EPO/H2O2/Cl− system without or with Br−, respectively. A 100 µM KYNA solution with 100 µM H2O2, 100 mM NaCl, and 0.2 unit/ml EPO in 100 mM potassium phosphate buffer (pH 2.8–9.6) in the absence or presence of 100 µM NaBr was incubated at 37°C for 30 min, and the reaction was terminated by addition of 1 mM N-acetylcysteine (AcCys). In the absence of NaBr, the reactions were slow even under mildly acidic conditions. Small but comparable amounts of 3-Br-KYNA and 3-Cl-KYNA were generated. No reaction was observed above pH 7. In the presence of 100 µM NaBr, KYNA was consumed and 3-Br-KYNA formed under mildly acidic conditions. The maximum consumption of KYNA was observed at pH 4–5. At around pH 7, the reaction was still observed. 3-Cl-KYNA and 3-Cl-HQN were not generated over the pH range examined.
Fig. 6.
(A) pH dependence of concentrations of KYNA and products in the reaction of KYNA with an EPO/H2O2/Cl− system. A 100 µM KYNA solution with 100 µM H2O2 and 100 mM NaCl in the presence of 0.2 unit/ml EPO in 100 mM potassium phosphate buffer (pH 2.8–9.6) was incubated at 37°C for 30 min and the reaction was terminated by addition of 1 mM AcCys. (B) pH dependence of concentration of products in the reaction of KYNA with an EPO/H2O2/Cl− system. A 100 µM KYNA solution with 100 µM H2O2, 100 mM NaCl, and 100 µM NaBr in the presence of 0.2 unit/ml EPO in 100 mM potassium phosphate buffer (pH 2.8–9.6) was incubated at 37°C for 30 min and the reaction was terminated by addition of 1 mM AcCys. KYNA (closed circle), 3-Br-KYNA (open circle), 3-Cl-KYNA (open square), and 3-Cl-HQN (open triangle). All the reaction mixtures were analyzed by RP-HPLC.
Reaction of KYNA with MPO systems
Figure 7A and B show the pH dependence of concentrations of KYNA and the products in the reaction of KYNA with an MPO/H2O2/Cl− system with or without Br−, respectively. In the absence of NaBr, 3-Cl-KYNA was formed under mildly acidic conditions. Small amounts of 3-Br-KYNA were also generated, although 3-Cl-HQN was not detected. The maximum consumption of KYNA was observed at around pH 5. No reaction was observed above pH 7. In the presence of 100 µM NaBr, 3-Br-KYNA was formed under mildly acidic conditions. 3-Cl-KYNA and 3-Cl-HQN were not generated over the pH range examined. No reaction was observed above pH 7.
Fig. 7.
(A) pH dependence of concentrations of KYNA and products in the reaction of KYNA with an MPO/H2O2/Cl− system. A 100 µM KYNA solution with 100 µM H2O2 and 100 mM NaCl in the presence of 0.2 unit/ml MPO in 100 mM potassium phosphate buffer (pH 2.8–9.6) was incubated at 37°C for 30 min and the reaction was terminated by addition of 1 mM AcCys. (B) pH dependence of concentration of products in the reaction of KYNA with an MPO/H2O2/Cl−/Br− system. A 100 µM KYNA solution with 100 µM H2O2, 100 mM NaCl, and 100 µM NaBr in the presence of 0.2 unit/ml MPO in 100 mM potassium phosphate buffer (pH 2.8–9.6) was incubated at 37°C for 30 min and the reaction was terminated by addition of 1 mM AcCys. KYNA (closed circle), 3-Br-KYNA (open circle), 3-Cl-KYNA (open square), and 3-Cl-HQN (open triangle). All the reaction mixtures were analyzed by RP-HPLC.
Discussion
3-Br-KYNA and 3-Cl-KYNA were reportedly synthesized from an ethyl ester derivative of KYNA by treatment with Br2 and SO2Cl2, respectively, and subsequent hydrolysis.(25) The present study showed that 3-Br-KYNA and 3-Cl-KYNA were generated directly as the major products of the reaction of KYNA with HOBr and HOCl, respectively, under neutral conditions. We recently showed that rebamipide and methotrexate reacted with HOBr, resulting in corresponding brominated products as major products.(26,27) Methotrexate reacted with HOCl generating a corresponding chlorinated methotrexate, whereas rebamipide did not react with HOCl. Like methotrexate, KYNA reacted with both HOBr and HOCl. The reactivity of HOBr to KYNA was higher than that of HOCl (Fig. 2 and 4), probably due to the higher electrophilicity of HOBr compared to HOCl.(28) In the presence of NaBr, HOCl generated 3-Br-KYNA in addition to 3-Cl-KYNA, probably due to the formation of HOBr.(29) At serum concentration of Br− (about 60 µM), the yield of 3-Br-KYNA was higher than that of 3-Cl-KYNA (Fig. 5). Amines had enhanced on the reactions of KYNA with HOBr, whereas they suppressed the reactions with HOCl almost completely (Table 1 and 2). It has been reported that CH3NH2 and (CH3)2NH react with HOBr and HOCl to generated corresponding bromamines and chloramines, respectively, and that second-order rate constants for the reactions of phenol with bromamines of CH3NH2 and (CH3)2NH are several orders higher than those with chloramines.(30) Similarly, KYNA would react strongly with bromamines of them to generate 3-Br-KYNA, whereas KYNA would not react with the chloramines. Free amino acids of 1 mM suppressed the reactions of KYNA with both HOBr and HOCl. For HOCl, even Gly suppressed the reaction almost completely. Taurine suppressed the HOCl reaction but not the HOBr reaction. The reaction of KYNA in vivo with HOBr and especially HOCl should be suppressed by free amino acids and taurine, since the total concentration of free amino acids in human plasma is several mM and the concentration of taurine is high in neutrophils (19 mM) and eosinophils (15 mM).(31,32) An EPO/H2O2/Cl−/Br− system reacted with KYNA, generating 3-Br-KYNA exclusively under mildly acidic conditions (Fig. 6B). Although an MPO/H2O2/Cl− system reacted with KYNA to generate 3-Cl-KYNA under mildly acidic conditions with small amounts of 3-Br-KYNA (Fig. 7A), this was probably due to Br− as contaminant in NaCl or phosphate buffer. An MPO/H2O2/Cl−/Br− system of 100 µM NaBr generated 3-Br-KYNA almost exclusively (Fig. 7B).
Several studies have reported the concentrations of halogenated derivatives of tyrosine (Tyr), 3-bromotyrosine (3-Br-Tyr) and 3-chlorotyrosine (3-Cl-Tyr) in animals and humans, which were probably generated from Tyr by EPO and MPO. In wild-type mice, the concentration of free 3-Br-Tyr in the peritoneal fluid was comparable to that of free 3-Cl-Tyr. Treatment by cecal ligation and puncture greatly increased the concentrations of both 3-Br-Tyr and 3-Cl-Tyr, with the average concentration of 3-Cl-Tyr three times higher than that of 3-Br-Tyr.(33) In healthy volunteers, the concentration of 3-Cl-Tyr in proteins recovered from airways was five times higher than that of 3-Br-Tyr, while in severe asthmatics the concentrations of both 3-Br-Tyr and 3-Cl-Tyr were higher than in healthy volunteers; the average concentration of 3-Br-Tyr was seven times higher than that of 3-Cl-Tyr.(34) Another study showed that the concentration of free 3-Br-Tyr in human plasma of healthy male volunteers was three times higher than that of free 3-Cl-Tyr.(35) In contrast, only one study reported the concentrations of halogenated nucleosides in humans. The concentration of 8-bromo-2'-deoxyguanosine (8-Br-dGuo) in urine from healthy volunteers was similar to that of 8-chloro-2'-deoxyguanosine (8-Cl-dGuo), while urinary 8-Br-dGuo and 8-Cl-dGuo levels from diabetic patients were 8-fold the levels in healthy volunteers.(36) These reported results imply that both bromination and chlorination of living substances occur in healthy humans, and that inflammatory diseases greatly increase those levels. In humans, the concentration of KYNA is 0.003 µM in saliva, 0.004–0.060 µM in plasma, 0.14–1.58 µM in brain, and 4–40 µM in urine, whereas the concentration of Tyr ranges from 8 to 82 µM (median 30 µM) in plasma and 7 to 366 µM (median 64 µM) in urine.(4,37) The halogenated derivatives of Tyr are detected in human plasma.(35) The present study showed that in vitro EPO and MPO systems readily converted KYNA to its halogenated derivatives under mildly acidic conditions. There is a possibility that the halogenated derivatives of KYNA form in vivo, especially in urine, although the reaction rate constants of KYNA with HOBr and HOCl are not determined.
KYNA is an agonist of G-protein coupled GPR35 receptor, which presents predominantly on immune cells and in the gastrointestinal tract.(2) KYNA may have a positive influence on a number of pathologies of the gastrointestinal tract.(4) KYNA is also an antagonist of both the N-methyl-d-aspartate receptor and the α7 nicotinic acetylcholine receptor.(5,6) KYNA possesses neuroprotective properties, since it blocks some of the neurotoxic effects of excitotoxins.(9) It has been reported that halogenated analogues of KYNA, such as 7-chlorokynurenic acid, 5,7-dichlorokynurenic acid, and 5,7-dibromokynurenic acid, are selective antagonists at the glycine modulatory site of the N-methyl-d-aspartate receptor complex.(38,39) However, the biological activities of 3-Br-KYNA and 3-Cl-KYNA have not been clarified. And also, the formations of 3-Br-KYNA and 3-Cl-KYNA are unclear in humans. If 3-Br-KYNA and 3-Cl-KYNA formed in body fluids and show biological activities, they may affect the pathophysiologies of various diseases with inflammation.
The present study showed that KYNA readily reacts with reagents of HOBr and HOCl, and enzyme systems of EPO and MPO, resulting in halogenated products in vitro. These results suggest that 3-Br-KYNA and 3-Cl-KYNA may be generated from KYNA by EPO and MPO in inflammation sites in humans.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Badawy AA. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res 2017; 10: 1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, Simonavicius N, Wu X, et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem 2006; 281: 22021–22028. [DOI] [PubMed] [Google Scholar]
- 3.Forrest CM, Gould SR, Darlington LG, Stone TW. Levels of purine, kynurenine and lipid peroxidation products in patients with inflammatory bowel disease. Adv Exp Med Biol 2003; 527: 395–400. [DOI] [PubMed] [Google Scholar]
- 4.Turski MP, Turska M, Paluszkiewicz P, Parada-Turska J, Oxenkrug GF. Kynurenic acid in the digestive system-new facts, new challenges. Int J Tryptophan Res 2013; 6: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganong AH, Cotman CW. Kynurenic acid and quinolinic acid act at N-methyl-d-aspartate receptors in the rat hippocampus. J Pharmacol Exp Ther. 1986;236:293–299. [PubMed] [Google Scholar]
- 6.Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci 2001; 21: 7463–7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 2001; 313: 96–98. [DOI] [PubMed] [Google Scholar]
- 8.Olsson SK, Samuelsson M, Saetre P, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J Psychiatry Neurosci 2010; 35: 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zinger A, Barcia C, Herrero MT, Guillemin GJ. The involvement of neuroinflammation and kynurenine pathway in Parkinson’s disease. Parkinsons Dis 2011; 2011: 716859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci 2015; 9: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fries GR, Walss-Bass C, Bauer ME, Teixeira AL. Revisiting inflammation in bipolar disorder. Pharmacol Biochem Behav 2019; 177: 12–19. [DOI] [PubMed] [Google Scholar]
- 12.Rothenberg ME. Eosinophilia. N Engl J Med 1998; 338: 1592–1600. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Slungaard A. Role of eosinophil peroxidase in host defense and disease pathology. Arch Biochem Biophys 2006; 445: 256–260. [DOI] [PubMed] [Google Scholar]
- 14.Weiss SJ, Test ST, Eckmann CM, Roos D, Regiani S. Brominating oxidants generated by human eosinophils. Science 1986; 234: 200–203. [DOI] [PubMed] [Google Scholar]
- 15.Holzbecher J, Ryan DE. The rapid determination of total bromine and iodine in biological fluids by neutron activation. Clin Biochem 1980; 13: 277–278. [DOI] [PubMed] [Google Scholar]
- 16.Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukoc Biol 2013; 93: 185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz J, Kaminker K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys 1962; 96: 465–467. [DOI] [PubMed] [Google Scholar]
- 18.Kettle AJ, Winterbourn CC. Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep 1997; 3: 3–15. [DOI] [PubMed] [Google Scholar]
- 19.Thomas EL, Bozeman PM, Jefferson MM, King CC. Oxidation of bromide by the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase. Formation of bromamines. J Biol Chem 1995; 270: 2906–2913. [DOI] [PubMed] [Google Scholar]
- 20.Henderson JP, Byun J, Williams MV, Mueller DM, McCormick ML, Heinecke JW. Production of brominating intermediates by myeloperoxidase. A transhalogenation pathway for generating mutagenic nucleobases during inflammation. J Biol Chem 2001; 276: 7867–7875. [DOI] [PubMed] [Google Scholar]
- 21.Rosowsky A, Wright JE, Holden SA, Waxman DJ. Influence of lipophilicity and carboxyl group content on the rate of hydroxylation of methotrexate derivatives by aldehyde oxidase. Biochem Pharmacol 1990; 40: 851–857. [DOI] [PubMed] [Google Scholar]
- 22.Wajon JE, Morris JC. Rates of formation of N-bromo amines in aqueous solution. Inorg Chem 1982; 21: 4258–4263. [Google Scholar]
- 23.Hazen SL, Hsu FF, Gaut JP, Crowley JR, Heinecke JW. Modification of proteins and lipids by myeloperoxidase. Methods Enzymol 1999; 300: 88–105. [DOI] [PubMed] [Google Scholar]
- 24.Morris JC. The acid ionization constant of HOCl from 5 to 35°. J Phys Chem 1966; 70: 3798–3805. [Google Scholar]
- 25.Surrey AR, Cutler RA. The preparation of 3-halo-4-dialkylaminoalkylamino-quinoline derivatives. J Am Chem Soc 1946; 68: 2570–2574. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki T, Okuyama A. Reactions of rebamipide with hypobromous acid. Chem Pharm Bull 2019; 67: 1164–1167. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, Takeuchi R. Reactions of methotrexate with hypobromous acid and hypochlorous acid. Chem Pharm Bull 2019; 67: 1250–1254. [DOI] [PubMed] [Google Scholar]
- 28.Heeb MB, Criquet J, Zimmermann-Steffens SG, von Gunten U. Oxidative treatment of bromide-containing waters: formation of bromine and its reactions with inorganic and organic compounds—a critical review. Water Res 2014; 48: 15–42. [DOI] [PubMed] [Google Scholar]
- 29.Thomas EL, Bozeman PM, Jefferson MM, King CC. Oxidation of bromide by the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase. Formation of bromamines. J Biol Chem 1995; 270: 2906–2913. [DOI] [PubMed] [Google Scholar]
- 30.Heeb MB, Kristiana I, Trogolo D, Arey JS, von Gunten U. Formation and reactivity of inorganic and organic chloramines and bromamines during oxidative water treatment. Water Res 2017; 110: 91–101. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto H, Kondo K, Tanaka T, et al. Reference intervals for plasma-free amino acid in a Japanese population. Ann Clin Biochem 2016; 53 (Pt 3): 357–364. [DOI] [PubMed] [Google Scholar]
- 32.Learn DB, Fried VA, Thomas EL. Taurine and hypotaurine content of human leukocytes. J Leukoc Biol 1990; 48: 174–182. [PubMed] [Google Scholar]
- 33.Gaut JP, Yeh GC, Tran HD, et al. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Natl Acad Sci USA 2001; 89: 11961–11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacPherson JC, Comhair SA, Erzurum SC, et al. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol 2001; 166: 5763–5772. [DOI] [PubMed] [Google Scholar]
- 35.Gaut JP, Byun J, Tran HD, Heinecke JW. Artifact-free quantification of free 3-chlorotyrosine, 3-bromotyrosine, and 3-nitrotyrosine in human plasma by electron capture-negative chemical ionization gas chromatography mass spectrometry and liquid chromatography-electrospray ionization tandem mass spectrometry. Anal Biochem 2002; 300: 252–259. [DOI] [PubMed] [Google Scholar]
- 36.Asahi T, Kondo H, Masuda M, et al. Chemical and immunochemical detection of 8-halogenated deoxyguanosines at early stage inflammation. J Biol Chem 2010; 285: 9282–9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anesi A, Rubert J, Oluwagbemigun K, et al. Metabolic profiling of human plasma and urine, targeting tryptophan, tyrosine and branched chain amino acid pathways. Metabolites 2019; 9: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kemp JA, Foster AC, Leeson PD, et al. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-d-aspartate receptor complex. Proc Natl Acad Sci USA. 1988;85:6547–6550. doi: 10.1073/pnas.85.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster AC, Kemp JA, Leeson PD, et al. Kynurenic acid analogues with improved affinity and selectivity for the glycine site on the N-methyl-d-aspartate receptor from rat brain. Mol Pharmacol. 1992;41:914–922. [PubMed] [Google Scholar]