Abstract
The relationship between serum uric acid and risk of stroke is still controversial. Therefore, we conducted a meta-analysis based on the cohort study to explore the relationship between serum uric acid and risk of stroke, and further illuminate whether there is a linear or non-linear relationship between them. We manually searched the database including Cochrane, PubMed, Embase, Web of Science, and selected cohort studies focusing on the relationship between serum uric acid and stroke risk. Random effect model was used for statistical analysis. Twenty-one cohort studies involving 818,098 participants were included. The pooled relative risk for the high-vs-low categories was 1.22 (95% CI: 1.15–1.30). In addition, there was a non-linear dose-response relationship between uric acid and stroke risk. Serum uric acid was in the range of 3–5 mg/dl, with the lowest risk of stroke. In conclusion, high serum uric acid level increases the risk of stroke, with a non-linear dose-response relationship.
Keywords: stroke, uric acid, meta-analysis, cohort study
Introduction
Stroke is the fifth leading cause of death in the United States and the second leading cause of death in the world.(1) Although there are many advanced methods to treat stroke at present, the complications and sequelae are rarely improved. Therefore, we should focus on secondary prevention to reduce the common risk factors of stroke, such as hypertension, hyperlipidemia, and diabetes.(2) In addition, some studies have focused on other biomarkers, such as serum uric acid. Uric acid is a product of purine metabolism and is considered to be the main cause of gout. In addition, the increase of uric acid is also related to the occurrence of stroke, which is confirmed by the meta-analysis published in 2018, but it only proves that stroke is positively related to uric acid.(3) It does not conduct dose-response analysis to discuss whether there is a linear or non-linear relationship between uric acid and stroke risk, and it only discusses the relationship between hemorrhagic stroke and uric acid, without assessing the relationship between the risk of ischemic stroke and uric acid.
Therefore, in order to further explore the relationship between uric acid levels and stroke risk, we conducted a random effect dose-response meta-analysis of cohort studies with a view to potentially finding ways to reduce stroke risk.(4–24)
Materials and Methods
Search strategy
Our meta-analysis was conducted and reported in line with the standards specified by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) under the registration with the International Prospective Register of Systematic Reviews (CRD42020172870).(25)
There are four databases searched for this analysis, including Web of Science, Embase, Cochrane, and PubMed. The terms used for PubMed search are as follows: (stroke [Mesh] OR cerebrovascular accident OR Cerebrovascular Apoplexy OR Acute Stroke OR Brain Vascular Accident OR Cerebral Stroke OR Acute Cerebrovascular Accident) AND (cohort OR prospective OR follow-up OR cohort studies [Mesh]) AND (uric acid [Mesh] OR 2,6,8-Trihydroxypurine OR Potassium Urate OR Urate OR Ammonium Acid Urate OR Sodium Urate Monohydrate OR Monosodium Urate Monohydrate OR Monosodium Urate OR Sodium Acid Urate). Apart from that, the references made in the literature were also used for manual retrieval. When the data obtained from the included articles was insufficient for our meta-analysis, contact was made with the author of the articles for more information.
Study selection
The inclusion of all studies was performed independently by two reviewers in line with the predefined inclusion and exclusion criteria. Any uncertainty was resolved through discussion with a third reviewer. The study was deemed qualified for inclusion if the following criteria can be met: (a) the outcome of the original study was stroke; (b) the exposure of interest was uric acid; (c) Hazard ratio (HR), relative risk (RR) or odds ratio (OR) and 95% confidence interval (95% CI) were provided; (d) cohort studies; (e) human studies. The exclusion criteria are as follows: (a) letter, review, supplementary article; (b) case study, cross-sectional study or randomized controlled trial.
Data extraction
The data was extracted by two reviewers. Any disagreement was addressed through discussion with a third reviewer. The data extracted from the included studies contains the name of the first author, location, follow-up years, the baseline level of uric acid, the number of participants, age, the number of cases, adjusted confounding factors, and effect size (RR and 95% CIs). The quality of studies was assessed by two investigators using the Newcastle-Ottawa scale (NOS).(26) The studies rated more than 7 stars were treated as being of high quality.
Statistical analysis
In order to determine the correlation between plasma uric acid levels and stroke incidence, the reported RRs and 95% CI were taken as effect size, with ORs and HRs considered as the equivalent of RRs. In the study of reported uric acid concentration, the conversion from mmol/L to mg/dl was obtained after dividing the value by 16.81 (1 mg/dl = 59.48 mmol/L). If the average uric acid value for each category was not obtained, the midpoint of each uric acid level was extracted. If the highest or lowest category was not definitive, the width of the interval was assumed as identical to that in the closest category.(27) The studies providing less than three categories of uric acid levels or neither cases nor participants were excluded from the dose-response meta-analysis. The random-effects model was applied to estimate the correlation between uric acid and stroke incidence. The Cochran Q test was conducted to detect heterogeneity, in which p<0.1 was treated as significant, with I2 taken to indicate the size of heterogeneity.(28,29) Sensitivity analysis was carried out by removing one study at a time, before the rest of the studies was evaluated. Funnel plots and Egger’s tests were performed to assess publication bias.(30) Additionally, the restricted cubic spline models with three knots (10, 50, and 90%) were applied to estimate the potential non-linear trend between blood uric acid and stroke risk. Finally, a subgroup analysis was conducted of stroke risk. The analysis was subject to adjustment depending on the age, sex, female proportion, number of participants, the number of cases, location, follow-up time, and the quality of research, as well as adjusted and stroke subtype.
In our meta-analysis, stata14.0 software was applied to carry out statistical analysis. The p value less than 0.05 was treated as statistically significant.
Results
Study characteristics
Our search identified 960 studies, 939 were excluded, and the rest 21 studies were included in our meta-analysis. The detailed flow chart is shown in Fig. 1. There are 21(4–24) cohort studies with 818,098 participants. One study included only women,(12) four studies included only men.(5,8,11,22) Seven studies focused on fatal stroke.(8,11,12,13,15,20,22) All but three studies were considered of high quality (Table 1 for quality assessment results). Nine studies were included in the dose-response,(5,9,8,11,12,13,15,16,25) and the rest eleven were excluded for providing less than three categories of uric acid level, or lack of a specific number of stroke patients.
Fig. 1.
Flow chart of study selection.
Table 1.
Quality assessment
| Study | Selection |
Comparability* |
Outcome/exposure |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 1 | 2** | 3 | |||
| Sakata, 2001 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | |||
| Jee, 2004 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ||||
| Chien, 2005 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | |||
| Gerber, 2005 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | |||
| Bos, 2006 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | |||
| Hozawa, 2006 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | |||
| Baba, 2007 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ||||
| Strasak, 2008 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |||
| Strasak, 2008 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | |||
| Holme, 2009 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ||||
| Storhaug, 2013 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | ||
| Kamei, 2016 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ||||
| Zhang, 2016 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | ||
| Norvik, 2017 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ||||
| Lazzeroni, 2017 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ||||
| Tscharre, 2018 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | |||
| Grossman, 2019 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |||
| Tu, 2019 | ☆ | ☆ | ☆ | ☆☆ | ☆ | |||||
| Lim, 2020 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ||
| Ninad, 2020 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | |||
| Li, 2020 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | |||
Uric acid level and risk of stroke
Twenty-one cohort studies with a total of 818,098 participants (Table 2) were included in the meta-analysis on the relationship between the highest and lowest uric acid levels and the incidence risk of stroke. The pooled RR was 1.22 (95% CI: 1.15–1.30) (Fig. 2), with significant heterogeneity (I2 = 60.2%, p<0.001). Through a careful review of included 21 studies, we found that the outcome of seven studies only included fatal stroke. After the removal of these seven articles, there was no significant change in the pooled results (RR = 1.23, 95% CI: 1.15–1.32), but the heterogeneity no longer existed (I2 = 19.1%, p = 0.230) (Fig. 3).
Table 2.
Characteristics of studies included
| Study | Location | No. of participants | Event | No. of cases | Age (mean years unless otherwise indicated) | Percentage of women (%) | Follow-up years | Covariates | RR (95% CI) high vs low |
|---|---|---|---|---|---|---|---|---|---|
| Sakata, 2001 | Japan | 8,172 | fatal stroke | 174 | 30 | 56 | 14 | age, body mass index, systolic blood pressure, use of antihypertensive agents, serum total cholesterol level, serum creatinine level, serum glucose level, smoking status, alcohol intake, and left ventricular hypertrophy. | Male: 1.71 (0.92–3.17), Female: 1.12 (0.46–2.74) |
| Jee, 2004 | Korean | 22,698 | stroke | 192 | 53.5 | 0 | 6.5 | age, diabetes, hypertension, hypercholesterolaemia and smoking status. | 1.10 (0.71–1.72) |
| Chien, 2005 | China | 3,602 | stroke | 155 | 35 | 52.7 | 11 | age, systolic blood pressure, body mass index, diabetes, low density cholesterol, high density cholesterol. | Male: 1.13 (0.88–1.46), Female: 1.32 (1.00–1.73) |
| Gerber, 2005 | Israel | 9,125 | fatal stroke | 292 | 40 | 0 | 23 | age, body mass index, systolic blood pressure, diabetes, serum cholesterol, smoking and left ventricular function. | 1.2 (0.81–1.78) |
| Bos, 2006 | The Netherlands | 4,385 | ischemic stroke and hemorrhagic stroke | 381 | 69 | 64.6 | 8.4 | age and sex, systolic blood pressure, total cholesterol, high-density lipoprotein, ever smoking, diuretic use, and waist/hip ratio diabetes mellitus. | 1.50 (1.05–2.14) |
| Hozawa, 2006 | US | 13,413 | ischemic stroke | 267 | 53.9 | 57.3 | 12.6 | age, sex, race, education, systolic blood pressure, diabetes mellitus, anti-hypertensive medication, cigarette smoking status, ethanol intake, serum albumin, von Willebrand factor, body mass index, and low cholesterol. | 1.49 (1.00–2.23) |
| Baba, 2007 | Japan | 2,024 | stroke | 84 | 63 | 61.7 | 8 | — | 1.07 (0.53–2.16) |
| Strasak, 2008 | Austrian | 83,683 | fatal stroke | 645 | 41.6 | 0 | 13.6 | age, body mass index, systolic and diastolic blood pressure, total cholesterol, triglycerides, glucose, smoking status. | 1.59 (1.23–2.04) |
| Strasak, 2008 | Austrian | 28,613 | fatal stroke | 776 | 62.3 | 100 | 15.2 | age, mass index, systolic and diastolic blood pressure, total cholesterol, triglycerides, gamma-glutamyltransferase glucose. | 1.37 (1.09–1.74) |
| Holme, 2009 | Sweden | 417,734 | fatal stroke | 162,76 | 57.5 | 47 | 11.8 | age, total cholesterol, triglyceride, hypertension. | Male: 1.26 (1.19–1.34), Female: 1.41 (1.31–1.53) |
| Storhaug, 2013 | Norway | 5,700 | ischemic stroke | 1,514 | 65 | 52.7 | 12 | age, body mass index (BMI), systolic blood pressure (SBP) and diastolic blood pressure (DBP). HDL-cholesterol and total cholesterol. use of diuretics and other antihypertensive medication. current smoking and physical activity renal factors added. | 1.24 (1.11–1.38) |
| Kamei, 2016 | Japan | 155,322 | stroke | 2,081 | 56.5 | 61 | 2 | age, obesity, hypertension, diabetes, dyslipidemia, smoking, alcohol consumption, glomerular filtration rate, and proteinuria. | Male: 1.26 (1.04–1.54), Female: 1.21 (1.00–1.48) |
| Zhang, 2016 | Japan | 36,313 | fatal stroke | 594 | 62 | 57 | 10 | body mass index, smoking status, ethanol intake, systolic blood pressure and total cholesterol. | Male: 1.19 (0.84–1.68), Female: 1.46 (0.98–2.19) |
| Norvik, 2017 | Norway | 2,940 | stroke | 271 | 59.7 | 49.6 | 18 | sex, age, body mass index, mean systolic blood pressure, mean diastolic blood pressure, total cholesterol, triglycerides, estimated glomerular filtration rate, haemoglobin A1c, current smoking, physical activity, and use of antihypertensive medication. | 1.13 (1.02–1.25) |
| Lazzeroni, 2017 | Italy | 1,440 | stroke | 32 | 67 | 29 | 2.9 | — | 1.1 (0.30–2.10) |
| Tscharre, 2018 | Austria | 1,215 | ischemic stroke | 39 | 62.9 | 33.6 | 5.5 | age, gender, Cardiogenic shock Glomerular filtration rate, C-reactive protein, per 1 mg/L increase Arterial hypertension hyperlipidaemia, peripheral artery disease prior stroke or tia, prior revascularisation heart failure beta blocker angiotensin converting enzyme inhibitor diuretics, high-intensity statin, number of affected coronary vessels, use of drug eluting stent | 1.10 (0.49–2.06) |
| Grossman, 2019 | Israel | 8,822 | fatal stroke | 292 | 40 | 0 | 18 | age, baseline uric acid, blood pressure, diabetes mellitus, ischemic heart disease. | 0.97 (0.71–1.34) |
| Tu, 2019 | China | 3,243 | stroke | 122 | 70.8 | 45 | 3 | age, gender, smoking and drinking habits, along with baseline body mass index, systolic and diastolic blood pressure and baseline Glomerular filtration rate, triglycerides, total cholesterol. | 2.32 (1.56–3.45) |
| Lim, 2020 | China | 3,202 | ischemic stroke | 64 | 68.8 | 16.9 | 5.4 | age, sex, hypertension, diabetes, body mass index, renal function, statin and diuretics use. | 1.61 (0.84–3.09) |
| Ninad, 2020 | US | 1,854 | stroke | 903 | 45 | 49.4 | 4 | age, gender, hypertension, diabetes mellitus, smoking, atrial fibrillation, left ventricular hypertrophy, coronary artery disease, aspirin, lipid-lowering medications, warfarin, estimated glomerular filtration rate, and high-density lipoprotein level. | 1.06 (0.78–1.45) |
| Li, 2020 | Japan | 13,420 | stroke | 1,018 | 59.5 | 61 | 23.1 | age, community, body mass index, cigarette smoking status, alcohol intake status, systolic blood pressure, atrial fibrillation, serum total cholesterol, serum triglycerides, estimated glomerular filtration rate, diabetes mellitus, antihypertensive medication use, and in women, menopausal status total cholesterol, serum triglycerides, estimated glomerular filtration rate, diabetes mellitus, antihypertensive medication use, and in women, menopausal status. | 1.45 (1.07–1.96) |
Fig. 2.
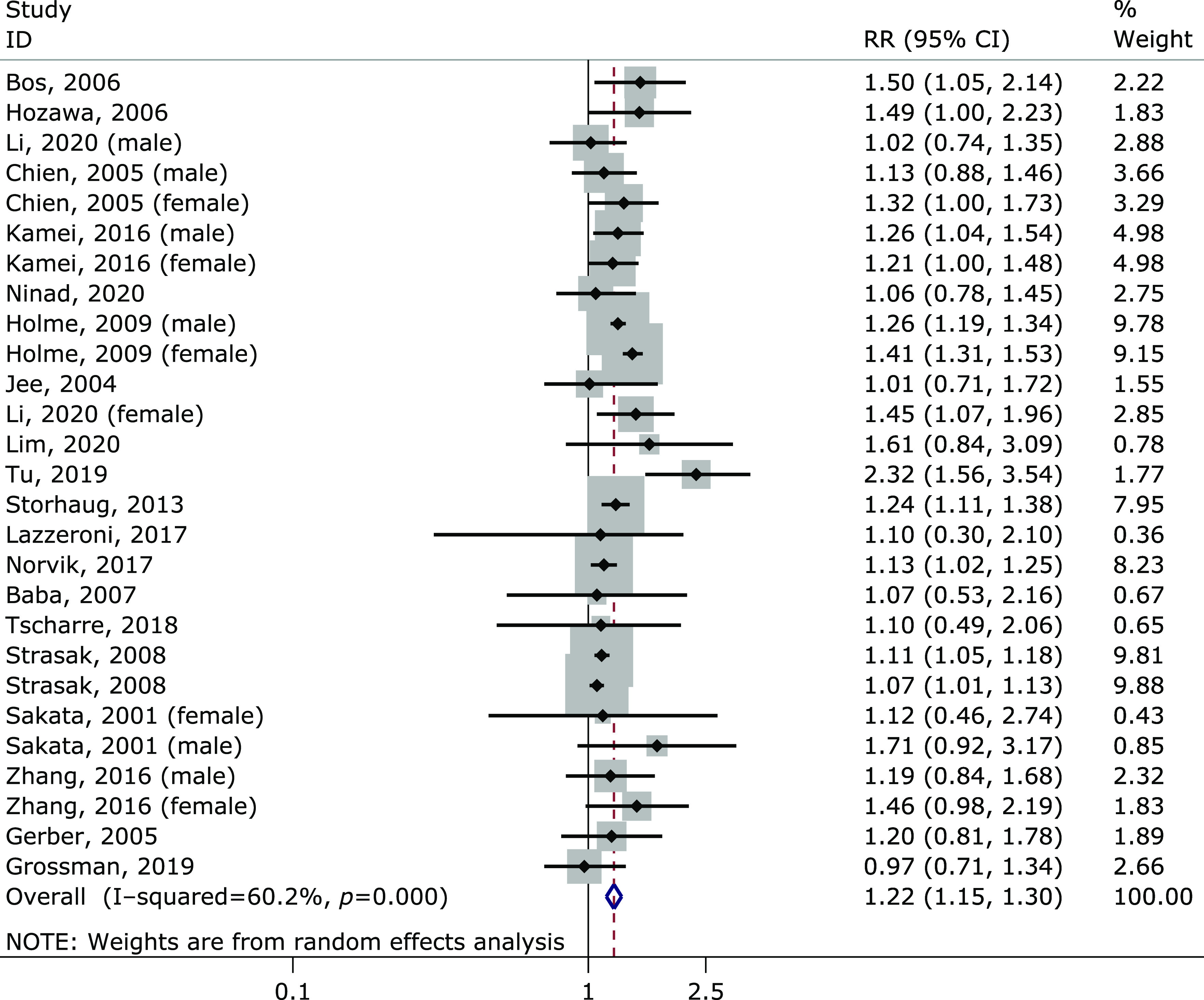
Forest plot of the meta-analysis of highest vs lowest uric acid level and stroke risk.
Fig. 3.
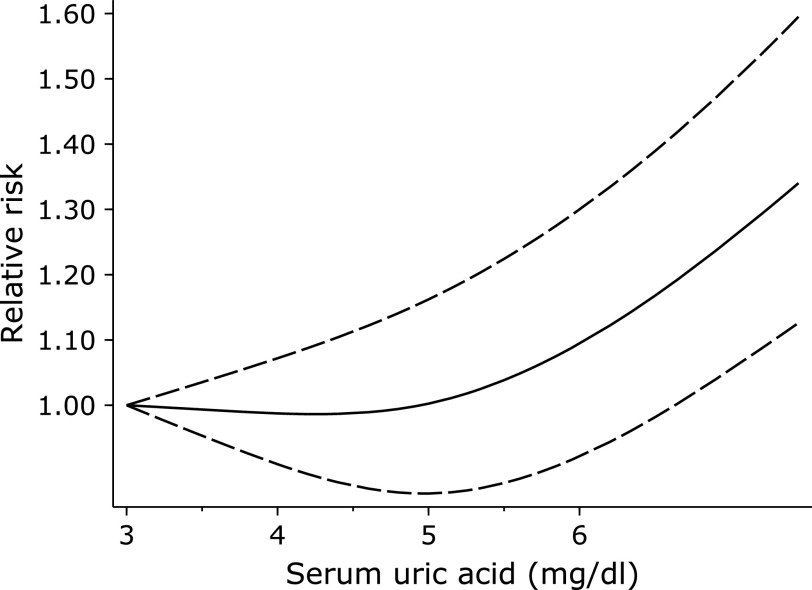
The non-linear trend showed that when serum uric acid was in the range of 3–5 mg/dl, the incidence rate of stroke is lowest.
Sensitivity analysis showed the pooled RRs (range 1.19–1.24) were influenced by no single study. In Egger test, no evidence showed that publication bias existed in this study (p = 0.137).
We excluded thirteen of the twenty-one studies in the dose-response meta-analysis due to providing less than three categories of uric acid level, or an insufficient reporting of either cases or participants. There was a non-linear relationship between plasma uric acid and stroke risk (p = 0.002). The non-linear trend showed that when serum uric acid was in the range of 3–5 mg/dl, the incidence rate of stroke reduced to the lowest (Fig. 3).
Subgroup analysis
We conducted a subgroup analysis of stroke risk based on age, female proportion, number of participants, number of cases, location, follow-up time, study quality, adjustment, stroke subtype, and sex. The results of subgroup analysis showed that, except for the United States studies and non adjustment group studies, other parts showed that they had an impact on stroke risk (Table 3).
Table 3.
Subgroup analysis of the risk of stroke
| No. of studies | RR (95% CI) | Heterogeneity p | p | ||
|---|---|---|---|---|---|
| Serum UA level | |||||
| 1 | Age (mean/median) | ||||
| >50 | 19 | 1.26 (1.17–1.35) | 0.001 | <0.001 | |
| ≤50 | 8 | 1.12 (1.06–1.18) | 0.7 | <0.001 | |
| 2 | Percentage of women | ||||
| >45 | 16 | 1.24 (1.14–1.34) | 0.001 | <0.001 | |
| ≤45 | 11 | 1.20 (1.08–1.34) | 0.006 | 0.001 | |
| 3 | No. of participants | ||||
| >15,000 | 9 | 1.21 (1.11–1.32) | 0.001 | <0.001 | |
| ≤15,000 | 18 | 1.23 (1.14–1.34) | 0.2 | <0.001 | |
| 4 | No. of cases | ||||
| >500 | 8 | 1.21 (1.11–1.33) | 0.001 | <0.001 | |
| ≤500 | 19 | 1.22 (1.13–1.32) | 0.3 | <0.001 | |
| 5 | Location | ||||
| United States | 2 | 1.23 (0.88–1.71) | 0.2 | 0.226 | |
| Europe | 12 | 1.20 (1.11–1.29) | 0.001 | <0.001 | |
| Aisa | 13 | 1.28 (1.16–1.41) | 0.4 | <0.001 | |
| 6 | Follow-up time | ||||
| >10 | 15 | 1.20 (1.12–1.29) | 0.001 | <0.001 | |
| ≤10 | 12 | 1.29 (1.14–1.44) | 0.3 | <0.001 | |
| 7 | Quality | ||||
| >7 | 20 | 1.22 (1.14–1.30) | 0.001 | <0.001 | |
| ≤7 | 7 | 1.26 (1.08–1.47) | 0.1 | 0.003 | |
| 8 | Adjusted | ||||
| Yes | 25 | 1.22 (1.15–1.30) | 0.001 | <0.001 | |
| No | 2 | 1.08 (0.61–1.91) | 0.9 | 0.791 | |
| 9 | Subtype of stroke | ||||
| Ischemic stroke | 16 | 1.34 (1.24–1.45) | 0.2 | <0.001 | |
| Hemorrhagic stroke | 9 | 1.41 (1.12–1.77) | 0.01 | 0.004 | |
| 10 | Sex | ||||
| Male | 13 | 1.20 (1.12–1.29) | 0.001 | <0.001 | |
| Female | 11 | 1.27 (1.30–1.47) | 0.001 | <0.001 | |
Discussion
A meta-analysis of 21 cohort studies involving 818,098 participants confirmed a dose-response relationship between plasma uric acid and stroke. The results showed obvious heterogeneity. Through a careful review of 21 studies included, we found that the results of 7 studies included only fatal strokes. After removing these seven substances, the combined results did not change significantly (RR = 1.23, 95% CI: 1.15–1.32), but the heterogeneity was no longer present (I2 = 19.1%, p = 0.230) (Fig. 4). We hypothesized that these seven studies, which included only fatal stroke as a result, mainly contributed to heterogeneity.
Fig. 4.
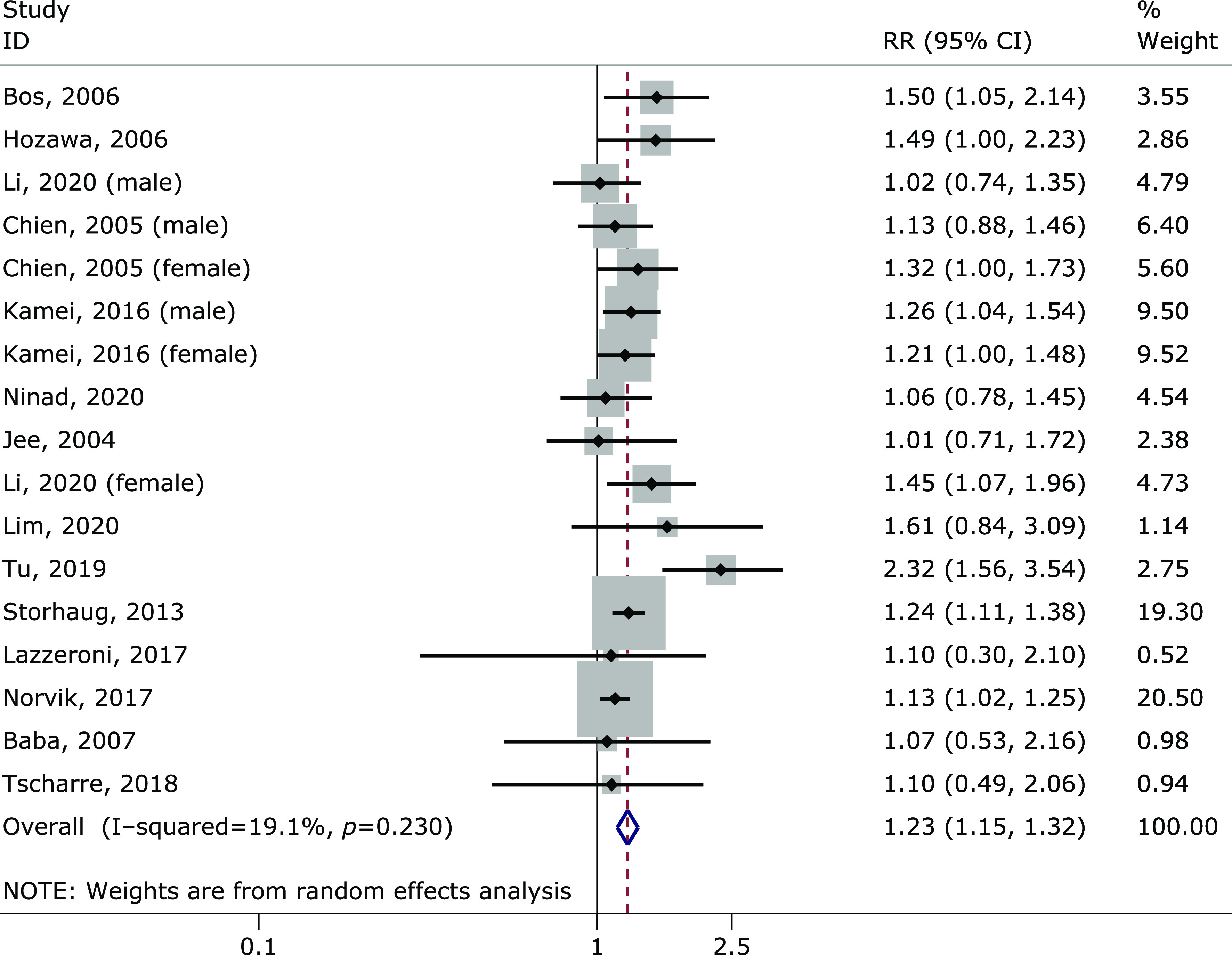
Meta-analysis forest plot after removal of only fatal stroke as outcome.
Stroke is considered to be a multifactor disease. Some studies have shown that uric acid can lead to endothelial dysfunction,(31) which leads to atherosclerotic endothelial damage and atherosclerotic plaque formation.(32) Lifestyle interventions and many widely used drugs, such as allopurinol, can be used to effectively treat hyperuricemia.(33) However, there are no published randomized controlled trials to determine whether lowering uric acid reduces the risk of stroke.
Compared with previous meta-analysis, this meta-analysis has several outstanding advantages. Firstly, new research is added to the current meta analysis. Second, we only included cohort studies, and most of the studies were of high quality. In this way, selection bias and recall bias will be greatly reduced. Of course, our meta-analysis has some limitations. First, we found significant heterogeneity in the studies included in the analysis. After excluding studies that had only fatal stroke outcomes, the results did not change significantly and heterogeneity no longer existed. Secondly, the confounding variables of each study are different, and there may be risk factors not considered. Therefore, in this analysis, the most adjusted RR results were included. Finally, the possible limitation is due to language bias. We tried to minimize this bias by searching four electronic databases without language constraints. However, some articles published in non-English languages may not appear in the International Journal Database.
In conclusion, there was a positive correlation between plasma uric acid and the risk of stroke, as well as a non-linear dose-response relationship. Maintaining low plasma uric acid levels may reduce the risk of stroke.
Funding
This work was supported by a China Medical University Major Clinical Medicine Construction Project in 2018 (111-3110118036).
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Dong Y, Qi X, Huang C, Hou L. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta-analysis. Stroke 2013; 44: 1833–1839. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Z, Liang Y, Lin J, et al. Serum uric acid concentrations and risk of intracerebral hemorrhage: a systematic review and meta-analysis. Atherosclerosis 2018; 275: 352–358. [DOI] [PubMed] [Google Scholar]
- 4.Sakata K, Hashimoto T, Ueshima H, Okayama A; NIPPON DATA 80 Research Group. Absence of an association between serum uric acid and mortality from cardiovascular disease: NIPPON DATA 80, 1980–1994. National Integrated Projects for Prospective Observation of Non-communicable Diseases and its Trend in the Aged. Eur J Epidemiol 2001; 17: 461–468. [DOI] [PubMed] [Google Scholar]
- 5.Jee SH, Lee SY, Kim MI. Serum uric acid and risk of death from cancer, cardiovascular disease or all causes in men. Eur J Cardiovasc Prev Rehabil 2004; 11: 185–191. [DOI] [PubMed] [Google Scholar]
- 6.Chien KL, Hsu HC, Sung FC, Su TC, Chen MF, Lee YT. Hyperuricemia as a risk factor on cardiovascular events in Taiwan: The Chin-Shan Community Cardiovascular Cohort Study. Atherosclerosis 2005; 183: 147–155. [DOI] [PubMed] [Google Scholar]
- 7.Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke 2006; 37: 1503–1507. [DOI] [PubMed] [Google Scholar]
- 8.Gerber Y, Tanne D, Medalie JH, Goldbourt U. Serum uric acid and long-term mortality from stroke, coronary heart disease and all causes. Eur J Cardiovasc Prev Rehabil 2006; 13: 193–198. [DOI] [PubMed] [Google Scholar]
- 9.Hozawa A, Folsom AR, Ibrahim H, Nieto FJ, Rosamond WD, Shahar E. Serum uric acid and risk of ischemic stroke: the ARIC study. Atherosclerosis 2006; 187: 401–407. [DOI] [PubMed] [Google Scholar]
- 10.Baba T, Amasaki Y, Soda M, et al. Fatty liver and uric acid levels predict incident coronary heart disease but not stroke among atomic bomb survivors in Nagasaki. Hypertens Res 2007; 30: 823–829. [DOI] [PubMed] [Google Scholar]
- 11.Strasak A, Ruttmann E, Brant L, et al. Serum uric acid and risk of cardiovascular mortality: a prospective long-term study of 83 683 Austrian men. Clinical Chemistry 2008; 54: 273–284. [DOI] [PubMed] [Google Scholar]
- 12.Strasak AM, Kelleher CC, Brant LJ, et al. Serum uric acid is an independent predictor for all major forms of cardiovascular death in 28,613 elderly women: a prospective 21-year follow-up study. Int J Cardiol 2008; 125: 232–239. [DOI] [PubMed] [Google Scholar]
- 13.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417,734 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). J Intern Med 2009; 266: 558–570. [DOI] [PubMed] [Google Scholar]
- 14.Storhaug HM, Norvik JV, Toft I, et al. Uric acid is a risk factor for ischemic stroke and all-cause mortality in the general population: a gender specific analysis from The Tromso Study. BMC Cardiovasc Disord 2013; 13: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Iso H, Murakami Y, et al. Serum uric acid and mortality form cardiovascular disease: EPOCH-JAPAN study. J Atheroscler Thromb 2016; 23: 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamei K, Konta T, Hirayama A, et al. Associations between serum uric acid levels and the incidence of nonfatal stroke: a nationwide community-based cohort study. Clin Exp Nephrol 2017; 21: 497–503. [DOI] [PubMed] [Google Scholar]
- 17.Norvik JV, Schirmer H, Ytrehus K, et al. Uric acid predicts mortality and ischaemic stroke in subjects with diastolic dysfunction: the Tromso Study 1994–2013. ESC Heart Fail 2017; 4: 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazzeroni D, Bini M, Camaiora U, et al. Serum uric acid level predicts adverse outcomes after myocardial revascularization or cardiac valve surgery. Eur J Prev Cardiol 2018; 25: 119–126. [DOI] [PubMed] [Google Scholar]
- 19.Tscharre M, Herman R, Rohla M, et al. Uric acid is associated with long-term adverse cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Atherosclerosis 2018; 270: 173–179. [DOI] [PubMed] [Google Scholar]
- 20.Grossman C, Grossman E, Goldbourt U. Uric acid variability at midlife as an independent predictor of coronary heart disease and all-cause mortality. PLoS ONE 2019; 14: e0220532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tu W, Wu J, Jian G, et al. Asymptomatic hyperuricemia and incident stroke in elderly Chinese patients without comorbidities. Eur J Clin Nutr 2019; 73: 1392–1402. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhary NS, Bridges SL Jr, Saag KG, et al. Severity of hypertension mediates the association of hyperuricemia with stroke in the REGARDS case cohort study. Hypertension 2020; 75: 246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Muraki I, Imano H, et al. Serum uric acid and risk of stroke and its types: the Circulatory Risk in Communities Study (CIRCS). Hypertens Res 2020; 43: 354–356. [DOI] [PubMed] [Google Scholar]
- 24.Lim SS, Yang YL, Chen SC, et al. Association of variability in uric acid and future clinical outcomes of patient with coronary artery disease undergoing percutaneous coronary intervention. Atherosclerosis 2020; 297: 40–46. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 26.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 27.Zhong C, Zhong X, Xu T, Xu T, Zhang Y. Sex-specific relationship between serum uric acid and risk of stroke: a dose-response meta-analysis of prospective studies. J Am Heart Assoc 2017; 6: e005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods 2017; 8: 5–18. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meschia JF, Worrall BB, Rich SS. Genetic susceptibility to ischemic stroke. Nat Rev Neurol 2011; 7: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humphries SE, Morgan L. Genetic risk factors for stroke and carotid atherosclerosis: insights into pathophysiology from candidate gene approaches. Lancet Neurol 2004; 3: 227–235. [DOI] [PubMed] [Google Scholar]
- 33.Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 2016; 118: 620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]