Abstract
Convalescent plasma therapy (CPT) is one of the well-known therapeutic protocols for treating infectious diseases that do not have special treatment or vaccine. Several documents confirm the clinical efficacy of this therapy for treating bacterial and viral infections. A comprehensive systematic search was conducted by August 2020 using global databases including PubMed, Scopus, Embase, Cochrane library, Google scholar, medRxiv and bioRxiv. The Joanna Briggs Institute critical appraisal checklist was used to evaluate the included studies. Using the Comprehensive Meta-Analysis software version 2.2 (Biostat, Englewood, NJ, USA), the pooled data analysis process was performed. A total of 15 eligible articles were enrolled in the current quantitative synthesis. The statistical analysis showed that clinical improvement in the group of patients who had received convalescent plasma was significantly increased compared with the control group (OR: 2.23; 1.12-4.45 with 95% CIs; p value: 0.022; Q-value: 6.11; I2: 83.64; Eggers p value: 0.064; Beggs p value: 0.093). Furthermore, the rate of hospital discharge had increased in patients receiving CPT (OR: 2.92; 1.48-5.77 with 95% CIs; p value: 0.002; Q-Value: 4.32; I2: 53.80; Eggers p value: 0.32; Beggs p value: 0.50). Because there is currently no fully effective antiviral drug against the virus and it will take time to confirm the effectiveness of new drugs, CPT can be used as an alternative treatment strategy to improve the severe clinical manifestations of COVID-19.
Keywords: Convalescent plasma, MERS-CoV, meta-analysis, SARS-CoV-1, SARS-CoV-2
Background
The family Coronaviridae is known as one of the most important etiologic factors for severe acute respiratory diseases for 21st-century human beings. The members of this family are enveloped, single-stranded, positive-sense RNA viruses (26-32 kbp length), and also have many spikes proteins on their surfaces that mediate virus entry into the cells [1,2]. Coronaviruses have a broad spectrum of the host including avian species, humans and several mammals such as bats, camels, mice, cats, dogs and anteaters [3]. Among them, several coronaviruses (CoVs) such as 229E, OC43, NL63, HKU1, severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1), MERS-CoV and more recently SARS-CoV-2 can infect human [4]. Before 2002, CoVs were known only as of the causes of common cold; however, in the 21st century, the large pandemic of betacoronavirus revealed that these RNA viruses can cause the life-threatening severe respiratory diseases [5]. In 2002, the first pandemic, severe acute respiratory syndrome (SARS) was caused by SARS-CoV-1, so that had the mortality rate about 10% [6]. The second pandemic, Middle East respiratory syndrome (MERS), was occurred by MERS-CoV in 2012, with mortality rate about 35% [7]. In December 2019, a new member of betacoronavirus, 2019 novel coronavirus (2019-nCoV) emerged in Wuhan, China, causing a severe pneumonia, that was called coronavirus disease 2019 (COVID-19) [8]. The clinical manifestations of COVID-19 are fever, dyspnoea, myalgia and invasive multilobular lesions (in chest radiological findings), and these are much like SARS and MERS diseases [5,9]. The nucleotide sequence of the 2019-nCoV genome is very similar (88%) with both bat-SL-CoVZC45 and bat-SL-CoVZXC21 genomes. In addition, the phylogenetic analysis revealed that the similarity of its genome with both SARS-CoV-1 and MERS-CoV is 79% and 50%, respectively [10]. SARS-CoV-2 is a highly contagious virus and rapidly spread worldwide. In March 2020, the World Health Organization announced the COVID-19 pandemic, and nowadays more than 13 million cases are infected by this virus [11]. Despite the rapid spread of the virus, so far there is no vaccine or drug approved by the Food and Drug Administration (FDA) against COVID-19 [12]. The lack of a protective vaccine and yet the need to avoid of spread of virus has been led to use of alternative strategies such as convalescent plasma therapy (CPT) for the treatment of patients [13]. Historically, passive immunisation is as one the therapeutic protocols against infectious agents, and first was used in 1880s [14]. In passive immunisation, the individuals who recover from an infectious disease are investigated, and the convalescent plasma (CP) with high titres of neutralising antibodies is used for other similar patients, so that leads to reduce the clinical sings, treatment duration and mortality rate [15]. As per review of literature, CP has been used for treating diseases such as diphtheria, Spanish influenza, Ebola, West Nile fever, SARS and MERS, and has been satisfactory results in amelioration and reduction of mortality rate [[16], [17], [18], [19], [20]]. Owing to advantages such as clinical efficacy, viral therapy, reducing death and low side effects, CPT is considered as a suitable therapeutic option in complications such infectious diseases, immune deficiencies, allergies and autoimmune diseases [14,21,22]. Furthermore, based on studies, it is demonstrated that CP has the satisfying results in improving and increasing the survival of COVID-19 patients [23]. CPT is one of the most reliable therapeutic options during the outbreaks of infectious agents, in particular in the absence of the appropriate vaccine [13]. Recently, FDA has announced that CP can be used as a trustworthy way in cases of widespread outbreak of COVID-19 [24]. In the present meta-analysis, we fulfilled a comprehensive evaluation study about the effects of CP on clinical improvement, increase of discharged cases, as well as reducing mortality of infected patients by viruses SARS-CoV-1, MERS-CoV and SARS-CoV-2.
Methods
Search strategy
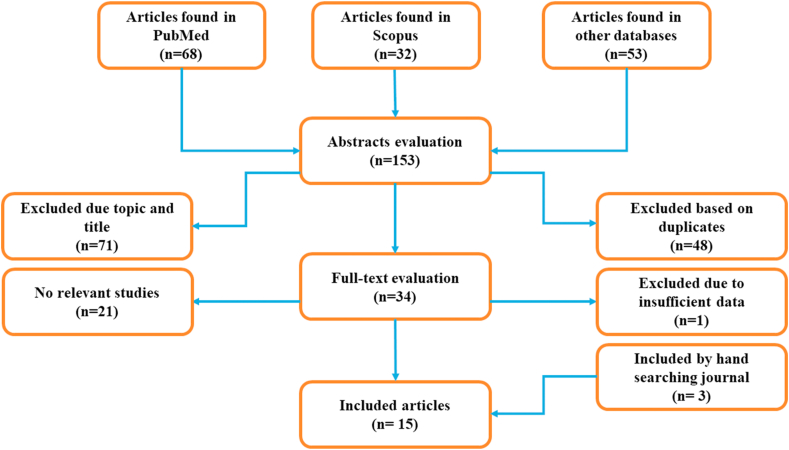
Comprehensive systematic search was conducted independently by two authors (MK1 and MK2) using several databases including PubMed, Scopus, Embase, Cochrane library, Google scholar, medRxiv and bioRxiv. Our search strategy was based on MeSH and using keywords such as “convalescent plasma”, “COVID-19”, “SARS-CoV-1”, “MERS-CoV”, “SARS-CoV-2” and “Coronavirus”. Next, we retrieved all relevant articles (up to August 2020) about the evaluation of CP effect on infected patients by SARS-CoV-1, MERS-CoV and SARS-CoV-2. The inclusion criteria were as follows: 1) articles containing the characteristics such as clinical improvement, viral therapy, mortality rate, the number of discharged cases and adverse event rate, in CP therapy patients; 3) patients infected by SARS-CoV-1, MERS-CoV and SARS-CoV-2; 3) articles containing the full text; 4) English articles. Also, duplicate studies were considered as exclusion criteria (Fig. 1). We reviewed all potentially relevant articles, and finally disagreements were resolved through discussion.
Fig. 1.
The flowchart of search strategy.
Quality assessment and data extraction
The Joanna Briggs Institute critical appraisal checklist was used for the evaluation of included studies. The main findings and characteristics of included studies include first author, country, viral aetiology, number of patients, CP dosages, outcome endpoint, number of improved patients, number of death, viral therapy rate, adverse event rate, number of cases weaned from the mechanical ventilation, number of discharged cases, therapeutic received drugs and reference number. The information is summarised in Table 1 [[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]]. The process of extracting the required data was also performed by both authors (MK1 and MK2).
Table 1.
Characteristics of included studies
| First author | Country | Viral Etiology | Patients | CP dose | Outcome endpoint | Clinical improvement | Death | Viral therapy | Adverse event | Weaned ventilation | Discharge | Drugs | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li | China | SARS-CoV-2 | 52 case 51 control |
S-RBD–IgG 1:640 200 ml |
28 days | 27/52 22/51 |
15.7% 24% |
87.2% 37.5% |
2/0 | NA | 51% 36% |
Antiviral, antibacterial, antifungal, interferon, steroids | [25] |
| Shen | China | SARS-CoV-2 | 5 | IgG (1:1000) 200-250 ml |
12 days | 3 | 0 | 5 | NA | 3 | 3 | Lopinavir/ritonavir, favipiravir, interferon alfa-1b, arbidol, darunavir | [26] |
| Joyner | USA | SARS-CoV-2 | 5000 | IgG 500 ml |
7 days | NA | 602 | NA | 36 | NA | NA | NA | [27] |
| Zeng | China | SARS-CoV-2 | 6/15 | IgG 300 ml |
22 days | 6 | 5/6 14/15 |
6/3 | 0 | NA | 1/1 | NA | [28] |
| Ahn | Korea | SARS-CoV-2 | 2 | IgG 500 ml |
26 days | 2 | 0 | 2 | 0 | 2 | 2 | Lopinavir/ritonavir, hydroxychloroquine, methylprednisolone, antibiotics | [29] |
| Ye | China | SARS-CoV-2 | 6 | IgG 400-600 ml |
33 days | 5 | 0 | 2 | 0 | NA | 3 | Arbidol, levofloxacin | [30] |
| Zhang | China | SARS-CoV-2 | 4 | IgG 200-400 ml |
NA | 4 | 0 | 4 | 0 | 2 | 4 | Arbidol, lopinavir-ritonavir, interferon alpha | [31] |
| Duan | China | SARS-CoV-2 | 10 | IgG 1:640 200 ml |
20 days | 10 | 0 | 7 | 0 | 3 | 10 | Arbidol, remdesivir, ribavirin, peramivir, antibacterial | [32] |
| Ko | Korea | MERS-CoV | 3 | IgG 1:80 |
3 days | 3 | 0 | 2 | NA | 3 | 3 | NA | [33] |
| Chun | Korea | MERS-CoV | 1 | IgG 500 ml |
NA | 1 | 0 | NA | 1 | NA | 1 | Ribavirin, lopinavir/ritonavir, interferon alpha | [34] |
| Wong | China | SARS-CoV-1 | 1 | IgG 200 ml |
NA | 1 | 0 | NA | 0 | NA | 0 | Cefotaxime, levofloxacin, oseltamivir, ribavirin | [35] |
| Yeh | Taiwan | SARS-CoV-1 | 3 | IgG 1:640 500 ml |
NA | 3 | 0 | 2 | NA | 1 | 3 | Lopinavir, ritonavir, methylprednisolone | [36] |
| Soo | China | SARS-CoV-1 | 19/21 | IgG 600-900 ml |
22 days | 14/4 | 0/5 | NA | 0 | NA | 74% 19% |
Ribavirin, methylprednisolone |
[37] |
| Kong | China | SARS-CoV-1 | 1 | IgG 500 ml |
7 days | 1 | 0 | NA | NA | 1 | 1 | Steroids, antiviral | [38] |
| Cheng | China | SARS-CoV-1 | 40 | IgG 600-900 ml |
22 days | 40 | 13 | NA | 0 | NA | 26 | Cefotaxime, levofloxacin, ribavirin, prednisolone, methylprednisolone | [39] |
Quantitative synthesis
Using the Comprehensive Meta-Analysis software version 2.2 (Biostat, Englewood, NJ, USA), the pooled data analysis was performed. In the present study, the cases who had received CP therapy were evaluated from aspects such as clinical improvement, discharged from hospital, weaned from mechanical ventilation, viral therapy, adverse events and mortality rate. For assessing the mentioned information, we used from event rate with 95% confidence intervals (CIs). Also, for evaluating, the clinical efficacy of CP therapy was used from the odds ratio (OR) with 95% CIs as well. Using the random-effects model, the pooled OR was estimated. It is noteworthy that, based on the Dersimonian and Laird method and random-effects model, the high heterogeneity cases included I2 index >25% and Cochrane Q test p value ≤ 0.05.
Results
In the present meta-analysis, from all fifteen studies (5240 participants), eight studies were on SARS-CoV, while the other two and five were about MERS-CoV and SARS-CoV-1, respectively. The studies had been conducted in four countries China, Korea, Taiwan and the United States (USA). From all of the infected patients, 5151 were infected by SARS-CoV-2, while four patients were infected by MERS-CoV, as well as eighty-five were infected by SARS-CoV-1. The patients had received 200-900 mL of CP in addition to medication with steroids, antibiotics, anti-fungi drugs and also anti-viral drugs containing lopinavir/ritonavir, favipiravir, IFN-alpha 1b, arbidol, darunavir, ribavirin, remdesivir and peramivir. However, the parameters such as IgG titre, therapeutic regimens, disease status and outcome endpoint varied in different patients.
Overall, the results indicate that the CPT has good clinical effects on the patients infected by CoVs (SARS-CoV-1, MERS-CoV and SARS-CoV-2). Except for antiviral effects, the recovery rate in patients that had received CP was satisfying; adverse events or even mortality rate was low as well. The statistical analysis showed that clinical improvement in the group of patients who received CP significantly increased compared with the control group (OR: 2.23; 1.12-4.45 with 95% CIs; p value: 0.022; Q-value: 6.11; I2: 83.64; Eggers p value: 0.064; Beggs p value: 0.093). Furthermore, discharge rate of patients from hospital in the group that were received CPT had increased (OR: 2.92; 1.48-5.77 with 95% CIs; p value: 0.002; Q-Value: 4.32; I2: 53.80; Eggers p value: 0.32; Beggs p value: 0.50).
In the subgrouping analysis, we determined the efficacy of CPT in each patient separately. The statistical analysis on the eighty-five patients infected by SARS-CoV-1 demonstrated that the parameters such as clinical improvement, weaning from mechanical ventilation and discharging from the hospital in patients under the CPT significantly had increased (Table 2). On the other hand, the rate of mortality had a significant decrease in patients who had received CP compared with the control group (OR: 0.077; 0.004-1.497 with 95% CIs; p value: 0.090; Q-value: 0.00; I2: 0.00; p value: 1.00).
Table 2.
Summarised events rate
| Viral etiology | Clinical improvement |
Death |
Virological cure |
Adverse event |
Weaned ventilation |
Discharge |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event rate (95% CIs) | Heterogeneity | Publication bias | Event rate (95% CIs) | Heterogeneity | Publication bias | Event rate (95% CIs) | Heterogeneity | Publication bias | Event rate (95% CIs) | Heterogeneity | Publication bias | Event rate (95% CIs) | Heterogeneity | Publication bias | Event rate (95% CIs) | Heterogeneity | Publication bias | |
| Total CoVs | 66.5% (56.7-75) p value: 0.001 |
Q: 18.28 I2: 39.85 p value: 0.075 |
Eggers p value: 0.04 Beegs p value: 0.50 |
12.2% (11.3-13.1) p value: 0.001 |
Q: 29.98 I2: 59.98 p value: 0.003 |
Eggers p value: 0.34 Beegs p value: 0.25 |
13.1% (12.2-14.1) p value: 0.001 |
Q: 158.96 I2: 93.70 p value: 0.001 |
Eggers p value: 0.001 Beegs p value: 0.46 |
1% (0.7-1.3) p value: 0.001 |
Q: 28.98 I2: 68.94 p value: 0.001 |
Eggers p value: 0.001 Beegs p value: 0.10 |
49.7% (38.6-0.8) p value: 0.95 |
Q: 4.75 I2: 0.00 p value: 0.57 |
Eggers p value: 0.24 Beegs p value: 0.18 |
52.3% (44.4-0.2) p value: 0.56 |
Q: 20.31 I2: 45.85 p value: 0.041 |
Eggers p value: 0.081 Beegs p value: 0.15 |
| SARS-CoV 1 | 81% (64.9-91.4) p value: 0.001 |
Q: 5.04 I2: 40.52 p value: 0.169 |
Eggers p value: 0.14 Beegs p value: 0.50 |
27.7% (17.1-41.5) p value: 0.002 |
Q: 4.62 I2: 35.15 p value: 0.020 |
Eggers p value: 0.76 Beegs p value: 0.50 |
66.7% (15.4-56.6) p value: 0.57 |
Q: 8.96 I2: 5.00 p value: 0.1 |
Eggers p value: NA Beegs p value: NA |
1.7% (0.2-1.13) p value: 0.001 |
Q: 0.14 I2: 0.00 p value: 0.70 |
Eggers p value: NA Beegs p value: NA |
40.0% (10-80) p value: 0.65 |
Q: 1.15 I2: 0.00 p value: 0.97 |
Eggers p value: NA Beegs p value: NA |
67.9% (55.5-8.2) p value: 0.006 |
Q: 1.35 I2: 0.00 p value: 0.71 |
Egger p value: 0.33 Beegs p value: 0.15 |
| MERS-CoV | 85.6% (41.6-98) p value: 0.10 |
Q: 0.02 I2: 0.00 p value: 0.87 |
Eggers p value: NA Beegs p value: NA |
14.4% (2-58.4) p value: 0.100 |
Q: 5.04 I2: 40.52 p value: 0.169 |
Eggers p value: NA Beegs p value: NA |
66.7% (15.4-95.7) p value: 0.57 |
Q: 0.00 I2: 0.00 p value: 1.0 |
Eggers p value: NA Beegs p value: NA |
26% (5-69) p value: 0.276 |
Q: 0.215 I2: 0.00 p value: 0.643 |
Eggers p value: NA Beegs p value: NA |
55.3% (12-91) p value: 0.845 |
Q: 2.698 I2: 62.931 p value: 0.100 |
Eggers p value: NA Beegs p value: NA |
85.6% (41-98) p value: 0.100 |
Q: 0.24 I2: 0.02 p value: 0.876 |
Eggers p value: NA Beegs p value: NA |
| SARS-CoV 2 | 60.4% (48-71) p value: 0.082 |
Q: 10.141 I2: 40.83 p value: 0.119 |
Eggers p value: 0.002 Beegs p value: 0.183 |
12.1% (11–13) p value: 0.001 |
Q: 12.147 I2: 42.371 p value: 0.096 |
Eggers p value: 0.24 Beegs p value: 0.19 |
80.6% (69-88) p value: 0.001 |
Q: 9.153 I2: 34.44 p value: 0.165 |
Eggers p value: 0.46 Beegs p value: 0.27 |
0.9% (0.7-1.2) p value: 0.001 |
Q: 18.391 I2: 67.37 p value: 0.05 |
Eggers p value: 0.001 Beegs p value: 0.08 |
46.7% (26-68) p value: 0.77 |
Q: 2.70 I2: 0.00 p value: 0.44 |
Eggers p value: 0.003 Beegs p value: 0.15 |
54.5% (43-65) p value: 0.44 |
Q: 9.514 I2: 36.93 P value: 0.147 |
Eggers p value: 0.154 Beegs p value: 0.11 |
In the group infected by MERS-CoV, we analysed the information of four patients. Although owing to the low sample size, we did not achieve significant results; however, using CPT had satisfying results in improving the clinical manifestations (Table 2). Interestingly, statistical analysis of information of 5151 COVID-19 patients confirmed the efficacy of CPT during the treatment. Improvement of clinical symptoms in patients who had received CP was more compared with the control group, although we observe the significant changes (OR: 1.66; 0.78-3.53 with 95% CIs; p value: 0.183; Q-value: 2.613; I2: 61.72; p value: 0.106). Negative virus test for this disease in recipients of CP had the meaningful change (OR: 2.59; 1.65-3.52 with 95% CIs; p value: 0.001; Q-value: 0.67; I2: 0.00; p value: 0.41). To discharge the patients in this group significantly increased as well (OR: 2.02; 0.94-4.35 with 95% CIs; p value: 0.07; Q-value: 0.049; I2: 0.00; p value: 0.824). As well as, based on statistical analysis results, CP could be significantly decreased the mortality rate in COVID-19 patients (OR: 0.311; 0.12-0.76 with 95% CIs; p-value: 0.011; Q-value: 0.009; I2: 0.00; p-value: 0.923). The mentioned data are listed in Table 2.
Discussion
So far, several genera of family Coronaviridae such as 229E, OC43, NL63, HKU1, SARS-CoV-1 and MERS-CoV have been known; however, in 2019, a new genus of this family, SARS-CoV-2, was considered as the etiologic agent of COVID-19 [40,41]. The first outbreak of COVID-19 occurred in more than 800 health care workers in Wuhan, China; however, the disease rapidly was spread in other countries, in particular Thailand, Japan, South Korea and the USA [5,42,43]. Today, various studies have shown that some underlying factors such as old age, pregnancy, cancer, diabetes, hypertension, as well as AIDS are specifically considered for the fatal outcomes and severity of this disease [44]. At the moment, several drugs such as teicoplanin, hydroxychloroquine, remdesivir, lopinavir, oseltamivir, ribavirin, favipiravir and tocilizumab are used for the treatment of COVID-19 [10,45]. Currently, regarding the importance of COVID-19 on one hand, and lack of the protective vaccine or effective drug, on the other hand, COVID-19 convalescent plasma (CCP) therapy can be considered as one of the fundamental ways for the treatment of this disease [46]. Studies show that the transfusion of plasma from patients who have recovered from COVID-19 infection to other SARS-CoV-2-infected patients can be led to their treatment without the occurrence of severe adverse events [47]. As per a study that Shen et al. conducted on five critically ill patients, following plasma transfusion, body temperature normalised during 3 days in four patients, the viral load became negative within two weeks after CCP therapy in all patients, and also, and 3 patients were weaned from mechanical ventilation [26]. Ye et al. conducted a study on six COVID-19 patients who had been admitted to Wuhan Huoshenshan Hospital from 11th February to 12th March 2020, and among them, there was a patient with Sjögren syndrome as well. They observed that the treatment of patients with CP had satisfying outcomes in all of them. Although the exact mechanism of action of CPT is not understood, they proposed that the antibodies IgM and IgG can directly neutralise the SARS-CoV-2, and probably, the anti-inflammatory contents of CP prevent cytokine storms [48]. In another study, Ahn et al. estimated the viral load of two cases that were affected by COVID-19 and acute respiratory distress syndrome by rRT-PCR technique, before and after CPT. In both cases, the value of the cycle threshold (Ct) had changed, which indicated a reduction in viral load after CP transfusion. In case 1 Ct changed from 24.98 on day 10 to 33.96 on day 20 after plasma transfusion, and in case 2 Ct changed from 20.51 on day 5 to 36.33 on day 9 after plasma transfusion [29]. Li et al. (2020) realised that CPT could be led to the negative conversion of SARS-CoV-2 in PCR (OR: 11.39; 3.91-33.18 with 95% CIs; p-value: 0.01) [49]. Based on studies, the exception with itching or skin rash and sometimes the little increase in body temperature, so far no serious adverse events have been reported with CPT [50]. Our present study had several limitations including 1) low population sample size, 2) evaluating limit published articles till August 2020, 3) significant heterogeneity in some cases, 4) presence of publication bias, 5) inaccessibility to raw data to evaluate several variables such as non-uniform details on interactions of medications, comorbidities, risk factors, morbidity, complications and randomised data. However, further investigations are necessary to examine the clinical benefit of CPT in CoVs with more sample size as well as randomised controlled studies to reduce certain sources of bias.
Conclusion
Based on different studies, it seems that in the absence of full effective antiviral drug or vaccine, CPT is an appropriate alternative for the treatment of patients infected by CoVs. The results of the present meta-analysis showed a reasonable conclusion about the clinical efficacy of CPT against COVID-19. In this study, we showed that CPT can be considered as a candidate for treating the patients who were infected by CoVs. This therapeutic protocol is an effective solution for characteristics such as clinical improvement, weaning from mechanical ventilation, hospital discharge, viral treatment and also prevention of mortality.
Ethics approval and consent to participate
Not applicable (this article was provided based on research in global databases).
Consent to publish
Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Transparency declaration
There is no conflict of interest among all authors.
Funding
We have not received any funding for this research.
Authors' contributions
-
1.
MK1 has contributed to the design of the work and analysis of data.
-
2.
MK2 has drafted the work and substantively revised it.
All authors read and approved the final manuscript.
Acknowledgements
The authors appreciate both Mashhad University of Medical Sciences and Jiroft University of Medical Sciences.
Editor: Michel Drancourt
Abbreviations
- CoVs
Coronaviruses
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- MERS
Middle East respiratory syndrome
- 2019-nCoV
2019 novel coronavirus
- COVID-19
Coronavirus disease 2019
- WHO
World Health Organization
- FDA
Food and Drug Administration
- CPT
Convalescent plasma therapy
- CP
Convalescent plasma
- JBI
Joanna Briggs Institute
- CMA
Comprehensive Meta-Analysis
- CIs
Confidence intervals
- OR
Odds Ratio
- USA
United States
- ARDS
Acute respiratory distress syndrome
References
- 1.Su S., Wong G., Shi W., Liu J., Lai A.C., Zhou J. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trend Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zare H., Aryan E., Meshkat Z., Gheybi F., Neshani A., Ghazvini K. Development of biosensors for the detection of COVID-19. Nanomed Res J. 2021;6(1):11–16. [Google Scholar]
- 3.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatrics. 2020:1–6. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehr A.R., Perlman S. Springer; 2015. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 7.de Groot R.J., Baker S.C., Baric R.S., Brown C.S., Drosten C., Enjuanes L. Commentary: Middle east respiratory syndrome coronavirus (mers-cov): announcement of the coronavirus study group. J Virol. 2013;87(14):7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yousefi B., Valizadeh S., Ghaffari H., Vahedi A., Karbalaei M., Eslami M. A global treatments for coronaviruses including COVID-19. J Cell Physiol. 2020 doi: 10.1002/jcp.29785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilder-Smith A., Chiew C.J., Lee V.J. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev. 2020:102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marano G., Vaglio S., Pupella S., Facco G., Catalano L., Liumbruno G.M. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14(2):152. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahani L., Singh S., Khardori N.M. Immunotherapy in clinical medicine: historical perspective and current status. Med Clin. 2012;96(3):421–431. doi: 10.1016/j.mcna.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Casadevall A., Pirofski L-a. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luke T.C., Hoffman S.L. Blood products for Spanish influenza: a future H5N1 treatment? Ann Inter Med. 2007;146(9):687. doi: 10.7326/0003-4819-146-9-200705010-00020. [DOI] [PubMed] [Google Scholar]
- 17.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.-M., Lim W.S. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojas M., Monsalve D.M., Pacheco Y., Acosta-Ampudia Y., Ramírez-Santana C., Ansari A.A. Ebola virus disease: an emerging and re-emerging viral threat. J Autoimmun. 2020;106:102375. doi: 10.1016/j.jaut.2019.102375. [DOI] [PubMed] [Google Scholar]
- 19.Planitzer C.B., Modrof J., Kreil T.R. West Nile virus neutralization by US plasma-derived immunoglobulin products. J Infect Dis. 2007;196(3):435–440. doi: 10.1086/519392. [DOI] [PubMed] [Google Scholar]
- 20.Jean S.-S., Lee P.-I., Hsueh P.-R. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherer Y., Levy Y., Shoenfeld Y. IVIG in autoimmunity and cancer–efficacy versus safety. Expet Opin Drug Saf. 2002;1(2):153–158. doi: 10.1517/14740338.1.2.153. [DOI] [PubMed] [Google Scholar]
- 22.Katz U., Achiron A., Sherer Y., Shoenfeld Y. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun Rev. 2007;6(4):257–259. doi: 10.1016/j.autrev.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Food U. 2020. Recommendations for investigational COVID-19 convalescent plasma. [Google Scholar]
- 25.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joyner M.J., Wright R.S., Fairweather D., Senefeld J.W., Bruno K.A., Klassen S.A. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9) doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng Q.-L., Yu Z.-J., Gou J.-J., Li G.-M., Ma S.-H., Zhang G.-F. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis. 2020;222(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Kor Med Sci. 2020;35(14) doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020;92(10):1890–1901. doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang B., Liu S., Tan T., Huang W., Dong Y., Chen L. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158(1):e9–e13. doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Nat Acad Sci. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko J.-H., Seok H., Cho S.Y., Ha Y.E., Baek J.Y., Kim S.H. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23(7):617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- 34.Chun S., Chung C.R., Ha Y.E., Han T.H., Ki C.-S., Kang E.-S. Possible transfusion-related acute lung injury following convalescent plasma transfusion in a patient with Middle East respiratory syndrome. Ann Lab Med. 2016;36(4):393. doi: 10.3343/alm.2016.36.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong V., Dai D., Wu A., Sung J. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. 2003;9(3):199–201. [PubMed] [Google Scholar]
- 36.Yeh K.-M., Chiueh T.-S., Siu L., Lin J.-C., Chan P.K., Peng M.-Y. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56(5):919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soo Y., Cheng Y., Wong R., Hui D., Lee C., Tsang K. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong L. Severe acute respiratory syndrome (SARS). Transfusion and apheresis science. Off J World Apher Assoc: Off J Eur Soc Haemapheresis. 2003;29(1):101. doi: 10.1016/S1473-0502(03)00109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng Y., Wong R., Soo Y., Wong W., Lee C., Ng M. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Decaro N., Lorusso A. Novel human coronavirus (SARS-CoV-2): a lesson from animal coronaviruses. Vet Microbiol. 2020:108693. doi: 10.1016/j.vetmic.2020.108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakhki R.K., Kakhki M.K., Neshani A. COVID-19 target: a specific target for novel coronavirus detection. Gene Rep. 2020;20:100740. doi: 10.1016/j.genrep.2020.100740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haynes B., Messonnier N.E., Cetron M.S. 2020. First travel-related case of 2019 novel coronavirus detected in United States. press release, Tuesday, January 21, 2020. [Google Scholar]
- 44.Sahu K.K., Mishra A.K., Raturi M., Lal A. Current Perspectives of convalescent plasma therapy in COVID-19. Acta Bio Medica: Atenei Parmensis. 2020;91(4) doi: 10.23750/abm.v91i4.10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghazvini K., Karbalaei M., Keikha M. What are the clinical benefits of tocilizumab for COVID-19 patients? Evidence from available case-control studies. Le Pharmacien Hospitalier & Clinicien. 2020 [Google Scholar]
- 46.Xia X., Li K., Wu L., Wang Z., Zhu M., Huang B. Improved clinical symptoms and mortality on severe/critical COVID-19 patients utilizing convalescent plasma transfusion. Blood. 2020 doi: 10.1182/blood.2020007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020 doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tiberghien P., de Lamballerie X., Morel P., Gallian P., Lacombe K., Yazdanpanah Y. 2020. Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how? Vox sanguinis. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.