Abstract
Introduction
To our knowledge, the diagnostic value of the sP-Selectin level in the diagnosis of COVID-19 disease has not yet been investigated. In this study, we aimed to assess this by evaluating the relationship between sP-Selectin level and the clinical severity of COVID-19 infections.
Methods
A total of 80 patients (50 with mild to moderate and 30 with severe COVID-19 pneumonia), and 60 non-symptomatic healthy volunteers participated in the study. Following serum isolation, sP-Selectin levels were assessed by Enzyme-Linked Immunosorbent Assay (ELISA) method.
Results
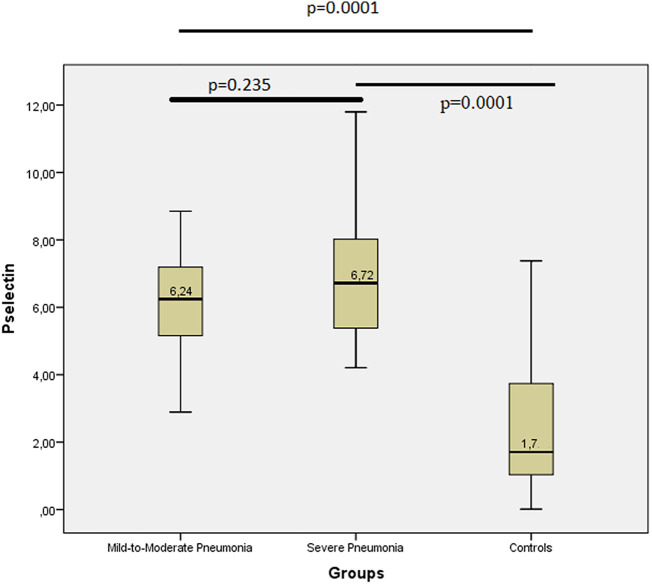
The serum sP-Selectin level was 1.7 ng/ml in the control group (1–3.78); 6.24 ng/ml (5.14–7.23) in mild-to-moderate pneumonia group; and 6.72 ng/ml (5.36–8.03) in the severe pneumonia group. Serum sP-Selectin levels in both mild-to-moderate pneumonia and severe pneumonia groups were found to be higher than the control group, with statistical significance (p = 0.0001 and p = 0.0001, respectively).
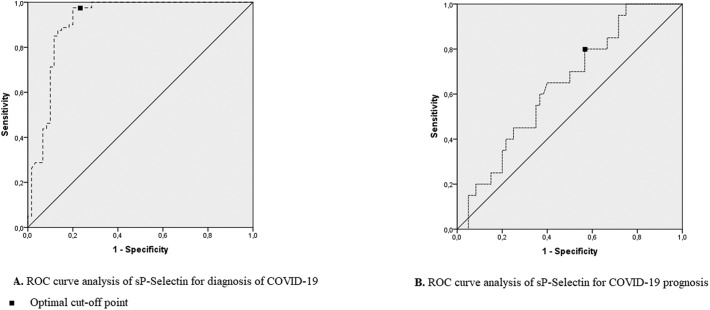
Receiver operating characteristic analysis (ROC) showed greater area under the curve (AUC) for the serum sP-Selectin levels of the COVID-19 patients (AUC = 0.913, 95% CI = 0.857–0.969; p = 0.0001). The serum sP-Selectin level was found to be 97.5% sensitive and 80% specific at 4.125 ng/ml level for diagnosis (p = 0.0001). The serum sP-Selectin level was found to be 76.9% sensitive and 51.9% specific at the level of 6.12 ng/ml (p = 0.005) to predict the need for intensive care treatment.
Conclusion
This study showed that sP-Selectin can be used as a valuable biomarker in both diagnosing and predicting the need for intensive care treatment of COVID-19 infection.
Keywords: Serum soluble P-selectin levels, COVID-19 infection, Pneumonia
1. Introduction
COVID-19 is a life-threatening disease with high risk of transmission. It started in Wuhan, China, in 2019 and affected almost 115 million people worldwide by March 4th, 2021. It was declared a pandemic by the World Health Organization (WHO), with almost 400-600 k new cases daily, and it is responsible for more than 2.5 million deaths [1]. COVID-19 disease causes clinical manifestations, ranging from asymptomatic cases to severe pneumonia, and even to sepsis, multiorgan failure, and acute respiratory distress syndrome (ARDS) [2].
Many diagnostic and prognostic biomarkers associated with COVID-19 disease have been revealed and investigated in the literature. These include D-dimer, C-Reactive protein (CRP), neutrophil count, leukocyte count, lymphocyte ratio, neutrophil-to-lymphocyte ratio, ferritin, procalcitonin and others not routinely tested in the diagnosis and clinical prognosis of COVID-19 [[2], [3], [4], [5], [6], [7]].
Soluble P-selectin (sP-Selectin) is a 140 kD molecule that mediates the interaction of stimulated endothelial cells or platelets with the white blood cells on the vascular surface and is also called as antigen CD62, platelet activation-dependent granule-outer membrane protein (PADGEM) or granule membrane protein 140 [8]. It has been found that, when combined with its ligand PSGL-1, it triggers platelet release and aggregation and mediates the adhesion of platelets to vascular endothelial cells [9].
Angiotensin converting enzyme 2 (ACE2), with which the SARS-CoV-2 virus interacts to facilitate cell use, is significantly expressed in endothelial cells, making endothelial cells an important target of the SARS-CoV-2 virus. Previous studies have found widespread thrombosis and microangiopathy in the pulmonary vascular bed in COVID-19 patients [10]. Damage caused by viral injury and hyper-inflammatory processes in the COVID-19 vascular bed leads to dysfunction in endothelial cells [11]. Endothelial dysfunction might result in the expression of P-selectin and tissue factor (TF), thus promoting platelet recruitment and aggregation. Subsequent accumulation of mononuclear cells provides a platform for the initiation of plasma coagulation by triggering prothrombin's cleavage to thrombin and fibrin formation. The molecular interaction between P-selectin-PSGL-1 complex and endothelial cells rapidly triggers TF exposure on monocytes, which may represent a mechanism by which platelets and mononuclear cells contribute to disproportionate intravascular micro-thrombosis in SARS-CoV-2 [[12], [13], [14]].
It has been reported that P-Selectin inhibition may be important in reducing acute lung injury in mice [15]. It has also been suggested that P-selectin blockage may be effective in reducing the severity of COVID-19 disease related ARDS [16]. The relationship between serum sP-Selectin level and COVID-19 infection has been examined in a few studies. Venter et al. reported high serum sP-Selectin levels in COVID-19 patients in their study, which included only 30 patients and 10 controls [17]. In a study conducted by Goshua et al., the levels of markers that play a role in endothelial cell and platelet activation in COVID-19 infection were examined, and the sP-Selectin level was detected to be higher in patients treated in the intensive care unit compared to patients who did not require intensive care [18]. More recently, other studies have examined the level of sP-Selectin in severe and non-severe COVID-19 patient groups as well as the sP-Selectin levels and clinical diagnostic values in critically ill patients in survivor and non-survivor groups [[19], [20], [21]].
.Previous studies have examined sP-Selectin level as a biomarker in the diagnosis and prognosis of COVID-19 in a wider population. In line with these studies, this study aimed to investigate the value of sP-Selectin level in the diagnosis of patients in the emergency department and predicting the need for intensive care.
2. Methods
2.1. Study type
This is a prospective case-control study, and its protocol was approved by the Pamukkale University Ethics Committee (Numbered:E-60116787-020-9701). All procedures carried out on patients were in compliance with the Helsinki Declaration.
2.2. Study p03opulation
A total of 80 patients (50 with mild to moderate and 30 with severe COVID-19 pneumonia), and 60 non-symptomatic healthy volunteers were enrolled in the study. The patients' SARS-CoV-2 infection was confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) assay from nasopharyngeal swab specimen.
The healthy group included volunteers who had similar age and gender characteristics as the patient population, who did not have a history of known disease or drug use, who did not have any recent or current infectious symptoms, and who had accompanied patients to the hospital (e.g., they were relatives of patients who were admitted for non-infectious reasons).
2.3. Inclusion criteria
2.3.1. Patient groups
Patients who were diagnosed with COVID-19 infection according to WHO guidelines after clinical evaluation in the emergency department (ED), whose diagnosis was confirmed by RT-PCR assay, and who provided a written informed consent form were included in the study [22].
2.3.2. Control group
This group included participants who did not have a known disease, infectious and thrombotic symptoms, did not use drugs, and consented to participate in the study.
2.4. Exclusion criteria
The exclusion criteria for the patient groups included a history of acute pulmonary embolism or deep venous thrombosis; kidney, liver or heart failure; a history of chronic inflammatory disease; pregnancy; and antiplatelet, anti-aggregant or anti-coagulant drug use.
Two patients were excluded from the study because of a history of pulmonary embolism; 25 patients because of a diagnosis of heart failure; 50 patients because of the use of antiplatelet, anti-aggregate or anticoagulant drugs; and 3 patients because of multiple exclusion criteria.
2.5. Clinical evaluation
The patients were assessed clinically as mild-to-moderate pneumonia or severe pneumonia in accordance with the COVID-19 diagnosis and treatment guidelines of the Turkish Ministry of Health [23].
2.5.1. Severe pneumonia
All enrolled patients had severe pneumonia (respiratory rate >30, SpO2 < 90% on room air, PaO2/FiO2 < 300, or lung infiltrates >50%) [23].
2.5.2. Mild-to-moderate pneumonia
Patients who did not meet the criteria for severe pneumonia but had pneumonic infiltrations in thorax CT scans were included in this group.
In some studies in the literature on COVID-19 pneumonia conducted to evaluate the need for ICU treatment, the mortality of patients with a CURB-65 score of 3 was found to increase and based on other studies in which the CUB-65 cut off value was found to be ≥2.5 in terms of mortality and invasive mechanical ventilation requirement, patients with a CURB65 score of 3 were evaluated as patients requiring ICU treatment [[24], [25], [26], [27]]. Relying on these findings, patients in this study were divided into two groups as ICU need and non-ICU need.
2.6. Data collection
Medical history, socio-demographic data, vital findings (fever, blood pressure, sPO2, respiratory rate), complete blood count (CBC), blood urea nitrogen (BUN), creatinine, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), C-reactive protein (CRP), D-dimer, CK-MB, hsTnT and blood gas analysis, pneumonia grade on computed tomography (CT) scan, and comorbid diseases were recorded in the data set.
2.7. Blood collection and sP-Selectin level measurement
Serum sP-Selectin levels were assessed with 3 cc of blood samples taken into a dry tube from the patients and healthy volunteers when they were admitted to ED, and the blood samples were centrifuged at 5000 rpm for 15 min. Following the serum isolation, sP-Selectin levels were assessed by Enzyme-Linked Immunosorbent Assay (ELISA) method, using a commercial kit (P-Selectin ELISA Kit, Elabscience, E-EL-H0917, USA), according to instructions of the manufacturer.
2.8. Data analysis
An initial power analysis revealed that at least 66 individuals (min. 22 for each cohort) were needed to achieve 95% power at a 95% confidence interval, with an assumption that the estimated effect size between the control, mild-to-moderate, and severe pneumonia groups in terms of sP-Selectin levels would be medium-high (f = 0.5). SPSS package program was used to analyze the dataset.
The continuous variables were expressed as median (IQR) and mean ± standard deviation. The sP-Selectin levels were given as median (IQR) and 95% CI. Categorical variables were provided as N (%). Kolmogorov-Smirnov tests were used to test the normality of distribution of the parameters.
Kruskal-Wallis analyses or Mann-Whitney U tests were used for independent nonparametric group comparisons. Spearman correlation analysis was used for testing the correlations between the parameters. Categorical variables were compared using Fisher's Exact-test and Chi-square tests. Discriminant performance sP-Selectin levels were assessed using Receiver Operating Characteristic (ROC) curve analysis. The intersection level of the highest sensitivity and the highest specificity points were assessed as cut-off point on the ROC analysis. p < 0.05 was defined as the significance level for all analyses.
3. Results
Individuals in both the study and control groups were sex and age matched (p = 0.392 and p = 0.158 respectively).
The mean duration of symptoms was 4.95 ± 1.08 days and 6.48 ± 1.82 days in mild-to-moderate pneumonia and severe pneumonia groups, respectively. The symptom duration was statistically significantly higher in the severe pneumonia group than in the mild-to-moderate group (p = 0.03).
In the severe pneumonia group, the CURB-65 score, heart rate, and respiratory rate were found to be higher than the mild-to-moderate pneumonia group (p = 0.0001 for all comparisons). While the sPO2 levels in the severe pneumonia group were found to be significantly higher than the mild-to-moderate pneumonia group (p = 0.0001). There was no statistically significant difference between the groups in terms other vital parameters (p > 0.05 for all comparisons). The distribution of comorbid diseases was similar in the patient groups (p > 0.05 for all comparisons; Table 1 ).
Table 1.
Clinical datas and comorbidity datas of the patient groups.
| Mild-to-moderate pneumonia (N = 50) |
Severe pneumonia (N = 30) |
p-Value | |||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) |
Mean ± SD | Median (IQR) |
||
| Symptom Duration (Day) | 4.95 ± 1.08 | 2 (2–5.5) |
6.48 ± 1.82 | 4 (2.25–6.75) |
p=0.032 |
| CURB-65 Score | 1.68 ± 0.79 | 2 (1–2) |
2.81 ± 1.14 | 3 (2–4) |
p=0.00011 |
| Fever (°C) | 36.93 ± 0.77 | 36.85 (36.5–37.1) |
36.83 ± 1.43 | 36.8 (36.05–37.55) |
p=0.5951 |
| Heart Rate (beat/min) | 88.66 ± 14.55 | 90 (82.25–96) |
110.1 ± 20.16 | 110 (95.5–122:5) |
p=0.00012 |
| Respiratory Rate | 24.42 ± 5.4 | 24 (20.75–27.25) |
33.2 ± 7.48 | 33 (27–39.5) |
p=0.00012 |
| sPO2 | 92.96 ± 3.99 | 93.5 (90–96) |
75.62 ± 13.73 | 80 (68.5–85) |
p=0.00011 |
| SBP (mm/Hg) | 138.3 ± 23.7 | 139.5 (118.25–156.7) |
136.89 ± 26.7 | 136 (114.5–153) |
p=0.812 |
| DBP (mm/Hg) | 75.3 ± 13.61 | 74 (66–86.5) |
73.44 ± 15.18 | 76 (63–82) |
p=0.5782 |
| Comorbidities N (%) | |||||
| Hypertension | 22 (44%) | 14 (46.6%) | p=0.8163 | ||
| Hyperlipidemia | 3 (6%) | 1 (3.3%) | p=14 | ||
| CAD | 7 (14%) | 6 (20%) | p=0.5393 | ||
| Diabetes Mellitus | 21(42%) | 15 (50%) | p=0.6433 | ||
SBP, systolic blood pressure; DBP, diastolic blood pressure.
p-Values are derived from Mann Whitney U test.
p-Values are derived from Student's t-test.
p-Values are derived from chi square test.
p-Values are derived from Fisher's exact-test.
The serum sP-Selectin level was 1.7 ng/ml in the control group [IQR (1–3.78) and 95% CI (1.94–3.09)]; 6.24 ng/ml [IQR (5.14–7.23) and 95% CI (5.92–6.8)] in the mild-to-moderate pneumonia group; and 6.72 ng/ml [IQR (5.36–8.03) and 95% CI (6.27–7.79)] in the severe pneumonia group. The serum sP-Selectin levels in both the mild-to-moderate pneumonia and severe pneumonia groups were found to be higher than the control group, with statistical significance (p = 0.0001 for both comparisons) (Fig. 1 ).
Fig. 1.
sP-Selectin levels of the groups.
Median values of the serum sP-Selectin levels were given in the middle of the boxes.
With the help of the power analysis, the effect size of the sP-Selectin concentrations for the differences between the patient and control groups was high (f = 2.5), while the observed power level found for this effect size was 95%, and the reliability was 100%.
The complete blood count, biochemistry parameters, and blood gas analysis parameters for the patient groups are given in Table 2 .
Table 2.
Laboratory parameters of the patient groups.
| Mild-to moderate pneumonia (N = 50) |
Severe pneumonia (N = 30) |
p Value | |||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) |
Mean ± SD | Median (IQR) |
||
| WBC (K/μl) | 6.26 ± 2.71 | 5.62 (4.85–6.85) |
11.42 ± 6.25 | 9.19 (7.17–15.1) |
0.0001 |
| Hemoglobin (g/dl) | 13.22 ± 1.39 | 13.4 (12.4–14.12) |
13.5 ± 1.48 | 13.15 (12.08–14.67) |
p=0.3951 |
| Platelete Count (K/μl) | 204.6 ± 59.4 | 192.5 (169–245.25) |
256.5 ± 94.6 | 233 (188.2–327.75) |
p=0.0031 |
| NLR | 3.49 ± 1.95 | 3.22 (1.88–4.38) |
9.52 ± 7.57 | 7.95 (4.03–9.82) |
0.0001 |
| ESR | 39.16 ± 26.1 | 36 (20.25–56.25) |
43.37 ± 21.25 | 45 (23.5–55) |
0.23 |
| CRP (mg/l) | 68.12 ± 107.5 | 40.7 (14–91.7) |
147.35 ± 79.7 | 138.6 (77.7–196.6) |
0.0001 |
| BUN | 39.7 ± 20.25 | 34 (27.75–48.5) |
56.23 ± 29.02 | 49.5 (36.25–70.25) |
0.004 |
| Creatinine | 1.31 ± 2 | 0.88 (0.8–1.13) |
1.32 ± 0.87 | 1.11 (0.89–1.42) |
0.014 |
| AST | 39.91 ± 15.47 | 27 (22–35) |
62.22 ± 56.5 | 44 (30–79) |
0.0001 |
| ALT | 20.77 ± 10.92 | 18 (12–26.5) |
46.18 ± 54.31 | 26 (22–49) |
0.001 |
| T. Bilurubin | 0.45 ± 0.21 | 0.42 (0.28–0.56) |
1.05 ± 2.02 | 0.64 (0.52–0.88) |
0.0001 |
| D-Dimer (ng/mL) | 972.9 ± 1348.4 | 580 (297.5–1097.5) |
3759.3 ± 4569 | 2000 (895–4435) |
0.0001 |
| hsTnT (μg/l) | 51.93 ± 254.7 | 12.31 (6.93–20.42) |
50.86 ± 65.85 | 25.9 (18.67–58.5) |
0.0001 |
| CKMB | 2.56 ± 4.1 | 1.4 (0.91–2.81) |
6.55 ± 9.65 | 2.89 (1.84–7.02) |
0.0001 |
| pH | 7.4 ± 0.04 | 7.41 (7.37–7.45) |
7.34 ± 0.2 | 7.41 (7.36–7.44) |
0.792 |
| pCO2 | 38.83 ± 8.59 | 39.5 (35.12–44.3) |
37.7 ± 8.52 | 38.6 (33.15–41.15) |
0.434 |
| Lactate | 1.75 ± 0.8 | 1.75 (1.22–2.1) |
4.12 ± 4.01 | 2.8 (1.8–4.6) |
0.0001 |
| HCO3 | 23.29 ± 3.51 | 24 (22.45–24.5) |
22.13 ± 4.33 | 22.6 (20.55–24.5) |
0.169 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; ESR, Erythrocyte Sedimentation Rate; CRP, C-Reactive Protein; BUN, blood urea nitrogen; AST, aspartate transaminase; ALT, alanine transaminase; hsTnT, high sensitive troponin T; CK-MB, Creatinine kinase MB.
p-Values are derived from Kruskal-Wallis test.
p-Values are derived from Student's t Test.
The area under the curve (AUC) for the serum sP-Selectin levels of the COVID-19 patients was found to be greater than the AUC for the control group (AUC = 0.913, 95% CI = 0.857–0.969; p = 0.0001) according to ROC analysis. Furthermore, the sP-Selectin level was found to be 97.5% sensitive and 80% specific at a level of 4.125 ng/ml for diagnosis (p = 0.0001; Fig. 2A).
Fig. 2.
A. ROC curve analysis of sP-Selectin for diagnosis of COVID-19 Optional cut-off point.
B. ROC curve analysis of sP-Selectin for COVID-19 prognosis.
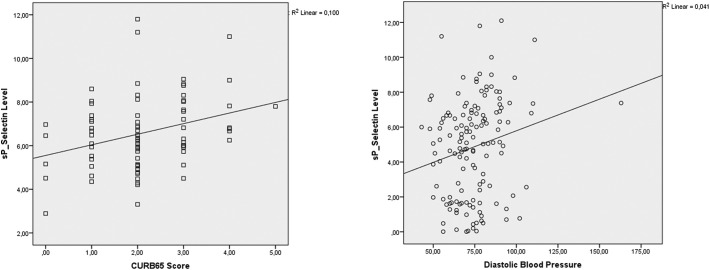
The serum sP-Selectin levels and CURB-65 scores of the patients were mild and positively correlated (rho = 0.292; R2 = 0.1 and p = 0.01), and the sP-Selectin levels and diastolic blood pressure of the patients were also mild and positively correlated (rho = 0.244; R2 = 0.04 and p = 0.03) (Fig. 3 ).
Fig. 3.
Scatter plot graphics of the sP-selectin and CURB-65 score and diastolic blood pressure.
The patient group was divided into those with a CURB65 score of 3 and above and those with a CURB65 score below 3, and the serum sP-Selectin levels were evaluated in terms of the need for intensive care. ROC analysis showed greater AUC for the serum sP-Selectin levels of the COVID-19 patients who need ICU treatment compared to non-ICU treatment patients (AUC = 0.696, 95% CI = 0.577–0.816; p = 0.005). Furthermore, the sP-Selectin level was found to be 76.9% sensitive and 51.9% specific at a level of 6.12 ng/ml (p = 0.005) to predict the need for intensive care treatment (Fig. 2B). Correlations between sP-Selectin levels and clinical and laboratory parameters were given in Table 3 .
Table 3.
Correlations between sP-Selectin levels and laboratory and clinical parameters.
| sP-Selectin | ||
|---|---|---|
| CURB65 Score | rho | 0.292 |
| p Value | 0.01 | |
| Fever | rho | −0.013 |
| p Value | 0.911 | |
| Heart Rate | rho | 0.29 |
| p Value | 0.009 | |
| Breathe Rate | rho | 0.164 |
| p Value | 0.148 | |
| sPO2 | rho | −0.168 |
| p Value | 0.139 | |
| SBP | rho | 0.165 |
| p Value | 0.147 | |
| DBP | rho | 0.244 |
| p Value | 0.03 | |
| WBC Count | rho | 0.1 |
| p Value | 0.377 | |
| Hemoglobin | rho | 0.078 |
| p Value | 0.494 | |
| Platelete Count | rho | 0.062 |
| p Value | 0.587 | |
| NLR | rho | 0.104 |
| p Value | 0.359 | |
| ESR | rho | −0.007 |
| p Value | 0.958 | |
| CRP | rho | 0.038 |
| p Value | 0.744 | |
| BUN | rho | 0.191 |
| p Value | 0.089 | |
| Creatinine | rho | 0.129 |
| p Value | 0.253 | |
| AST | rho | 0.086 |
| p Value | 0.471 | |
| ALT | rho | 0.093 |
| p Value | 0.413 | |
| T.Bilurubin | rho | 0.047 |
| p Value | 0.679 | |
| D-Dimer | rho | 0.026 |
| p Value | 0.818 | |
| hsTnT | rho | 0.111 |
| p Value | 0.334 | |
| CK-MB | rho | 0.052 |
| p Value | 0.648 | |
| pH | rho | −0.175 |
| p Value | 0.142 | |
| pCO2 | rho | 0.057 |
| p Value | 0.637 | |
| Lactate | rho | 0.225 |
| p Value | 0.064 | |
| HCO3 | rho | −0.106 |
| p Value | 0.375 | |
p and rho values are derived from Spearman Correlation test. SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; ESR, Erythrocyte Sedimentation Rate; CRP, C-Reactive Protein; BUN, blood urea nitrogen; AST, aspartate transaminase; ALT, alanine transaminase; hsTnT, high sensitive troponin T; CK-MB, creatinine kinase MB.
4. Discussion
This study examined the diagnostic and predictive values of serum soluble sP-Selectin levels in COVID-19 disease and found higher serum sP-Selectin levels in mild-to-moderate and severe pneumonia groups compared to the control group.
Although the definitive diagnosis of COVID-19 disease is the RT-PCR test, many routine and non-routine biological and biochemical parameters have been examined for their diagnostic values and clinical outcomes [[2], [3], [4], [5], [6], [7]]. In the Cochrane database, 21 studies that include the laboratory data of 56,585 non-COVID-19 patients and 14,126 COVID-19 patients were reviewed. It was reported that the median sensitivities of CRP was 66%, IL-6 was 73%, and LDH was 77%, while the median specificity of creatinine kinase was 94% [28]. In a study conducted by Peng et al., the monocyte/lymphocyte ratio showed an acceptable efficiency in discriminating patients from healthy individuals but not in discriminating between influenza pneumonia and COVID-19 pneumonia [29].
In our study, the serum sP-Selectin levels were found to be 97.5% sensitive and 80% specific in the diagnosis of COVID-19 at the cut-off level of 4.125 ng/ml, which may suggest a significant contribution to the clinical diagnosis of patients.
Compared to the average sensitivity of markers indicated in the Cochrane database, sP-Selectin appears to be a more powerful marker for diagnosis, although its specificity was 80%, which was relatively lower than the other markers.
sP-Selectin plays an important role in platelet aggregation. Previous studies have examined its role in thrombotic events by activating the procoagulant status in the release of tissue factor as well as many cytokines by binding with sP-Selectin glycoprotein ligand [[30], [31], [32]]. P-selectin (CD62P) is stored in the Weibel-Palade bodies of endothelial cells and can be taken to the cell surface within minutes following stimulation by inflammatory mediators such as histamine, thrombin or leukotrienes. Its expression on platelets may increase following the secretion of α-granules in response to activation [33].
P-selectin plays a role in the inflammatory response and has been shown to be essential in the host defense in Klebsiella pneumonia in mice. It has also been demonstrated that P-Selectin inhibition reduces severe acute lung injury in compromised mice [15,34]. Some studies in COVID-19 patients have assessed the role of P-selectin in lung infections. Venter et al. reported that serum sP-Selectin level was lower in COVID-19 patients compared to a control group [17]. In a study conducted by Goshua et al., the level of P-selectin was detected to be higher especially in ICU patients compared to a control group, and it was stated that sP-Selectin could be a valuable biomarker in predicting the severity of COVID-19 infection [18]. In addition, Goshua et al. revealed the occurrence of endotheliopathy in COVID-19 disease [18]. More recently, Comer et al. found higher levels of sP-Selectin in patients with COVID-19 infection. Moreover, a higher sP-Selectin level was found in severe COVID-19 patients compared to the non-severe COVID group [35]. In another study, although Agrati et al. found serum sP-Selectin level to be high in COVID-19 patients, there was no difference in sP-Selectin level in ICU and Non-ICU groups [19].
In their study, Spadaro et al. found no statistically significant differences between survivor and non-survivor patient groups in terms of sP-Selectin level in 31 patients followed up with mechanical ventilation [20]. Unlike this study, Vasiliou et al. found the sP-Selectin level to be higher in non-survivor critically ill patients than in the survivor patient population [21].
The large study universe of our study seems to provide important outcomes for COVID-19 disease. Higher sP-Selectin levels were identified in patients compared to the control group, which supports the view that regardless of the radiological severity (mild-moderate or severe pneumonia), endotheliopathy and thrombosis tendency were increased in patients with inflammatory response. In addition, despite the fact that the cut-off values used to predict the need for ICU treatment vary between commercial kits, the sP-Selectin level used in our study demonstrated 76.9% of sensitivity and 51.9% of specificity.
Limitations.
This study has some limitations. We did not measure continuously, and we have no data about potential continuous change in sP-Selectin levels. We did not follow up with the patients regarding mortality.
5. Conclusion
Many markers are being investigated in the diagnosis of COVID-19 and in predicting the prognosis of the infection. This study showed that sP-Selectin level can be used as a valuable biomarker in both diagnosing and predicting the need for intensive care treatment.
In addition, this study found an increase in sP-Selectin level, which is one of the indicators of endotheliopathy and thrombosis tendency, in COVID-19 pneumonia. Therefore, it should be noted that patients with COVID-19 disease have a tendency to thrombosis.
Declaration of competing interest
The authors declare that they have no conflicts of interests.
Acknowledgment
There is no funding statement for this study.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ Available at:
- 2.Yilmaz A., Sabirli R., Seyit M., et al. Association between laboratory parameters and CT severity in patients infected with COVID-19: a retrospective, observational study. Am. J. Emerg. Med. 2021;42:110–114. doi: 10.1016/j.ajem.2021.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aceti A., Margarucci L.M., Scaramucci E., et al. Serum S100B protein as a marker of severity in COVID-19 patients. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-75618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozen M., Yilmaz A., Cakmak V., et al. D-dimer as a potential biomarker for disease severity in COVID-19. Am. J. Emerg. Med. 2021;40:55–59. doi: 10.1016/j.ajem.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seyit M., Avci E., Nar R., et al. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. Am. J. Emerg. Med. 2020;40:110–114. doi: 10.1016/j.ajem.2020.11.058. (S0735-6757(20)31188-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soraya G.V., Ulhaq Z.S. Crucial laboratory parameters in COVID-19 diagnosis and prognosis: an updated meta-analysis. Med Clin (Engl Ed) 2020;155:143–151. doi: 10.1016/j.medcle.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Tardón N., Abbes A.P., Gerrits A., Slingerland R.J., den Besten G. Laboratory parameters as predictors of mortality in COVID-19 patients on hospital admission. J Lab Med. 2020;44:357–359. [Google Scholar]
- 8.Pasquali A., Trabetti E., Romanelli M.G., et al. Detection of a large deletion in the P-selectin (SELP) gene. Mol. Cell. Probes. 2010;24:161–165. doi: 10.1016/j.mcp.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Mayadas T.N., Johnson R.C., Rayburn H., Hynes R.O., Wagner D.D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 10.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;5395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson S.P., Darbousset R., Schoenwaelder S.M. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906–918. doi: 10.1182/blood-2018-11-882993. [DOI] [PubMed] [Google Scholar]
- 14.Ivanov I.I., Apta B.H.R. BonnaAM, Harper MT. platelet P-selectin triggers rapid surface exposure of tissue factor in monocytes. Sci. Rep. 2019;9:13397. doi: 10.1038/s41598-019-49635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Xiang D., Gao F., Yao H., Ye Q., Wang Y. The inhibition of P-selectin reduced severe acute lung injury in immunocompromised mice. Oxidative Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/8430465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neri T., Nieri D., Celi A. P-selectin blockade in COVID-19-related ARDS. Am J Physiol Lung Cell Mol Physiol. 2020;318:L1237–L1238. doi: 10.1152/ajplung.00202.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venter C., Bezuidenhout J.A., Laubscher G.J., et al. Erythrocyte, platelet, serum ferritin, and P-selectin pathophysiology implicated in severe hypercoagulation and vascular complications in COVID-19. Int. J. Mol. Sci. 2020;21:8234. doi: 10.3390/ijms21218234. Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-Centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrati C., Bordoni V., Sacchi A., et al. Elevated P-selectin in severe Covid-19: considerations for therapeutic options. Mediterr J Hematol Infect Dis. 2021;13 doi: 10.4084/MJHID.2021.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spadaro S., Fogagnolo A., Campo G., et al. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID-19 ICU patients. Crit. Care. 2021;25 doi: 10.1186/s13054-021-03499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassiliou A.G., Keskinidou C., Jahaj E., et al. ICU admission levels of endothelial biomarkers as predictors of mortality in critically ill COVID-19 patients. Cells. 2021;10:186. doi: 10.3390/cells10010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization . World Health Organization; 2020. Clinical Management of COVID-19: Interim Guidance, 27 May 2020 (No. WHO/2019-nCoV/clinical/2020.5) Accessed October 22, 2020. [Google Scholar]
- 23.Turkish Ministery of Health COVID-19 Diasnosis and Treatment Guideline. https://covid19.saglik.gov.tr/Eklenti/39061/0/COVID19rehberieriskinhastatedavisipdf.pdf Accessed at:
- 24.Nguyen Y., Corre F., Honsel V., et al. Applicability of the CURB-65 pneumonia severity score for outpatient treatment of COVID-19. J. Inf. Secur. 2020;81:e96–e98. doi: 10.1016/j.jinf.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Artero A., Madrazo M., Fernández-Garcés M., et al. Severity scores in COVID-19 pneumonia: a multicenter, retrospective, cohort study. J. Gen. Intern. Med. 2021;11:1–8. doi: 10.1007/s11606-021-06626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng P., Wu H., Yang J., et al. Pneumonia scoring systems for severe COVID-19: which one is better. Virol. J. 2021;18 doi: 10.1186/s12985-021-01502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley P., Frost F., Tharmaratnam K., et al. Utility of established prognostic scores in COVID-19 hospital admissions: multicentre prospective evaluation of CURB-65, NEWS2 and qSOFA. BMJ Open Respir. Res. 2020;7 doi: 10.1136/bmjresp-2020-000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stegeman I., Ochodo E.A., Gulei F., et al. Routine laboratory testing to determine if a patient has COVID-19. Cochrane Database Syst. Rev. 2020;(11) doi: 10.1002/14651858.CD013787. Accessed 26 March 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng J., Qi D., Yuan G., Deng X., Mei Y., Feng L., Wang D. Diagnostic value of peripheral hematologic markers for coronavirus disease 2019 (COVID-19): a multicenter, cross-sectional study. J. Clin. Lab. Anal. 2020;34:e23475. doi: 10.1002/jcla.23475. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merten M., Thiagarajan P. P-selectin in arterial thrombosis. Z. Kardiol. 2004;93:855–863. doi: 10.1007/s00392-004-0146-5. [DOI] [PubMed] [Google Scholar]
- 31.Antonopoulos C.N., Sfyroeras G.S., Kakisis J.D., Moulakakis K.G., Liapis C.D. The role of soluble P selectin in the diagnosis of venous thromboembolism. Thromb. Res. 2014;133:17–24. doi: 10.1016/j.thromres.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Borgel D., Bianchini E., Lasne D., Pascreau T., Saller F. Inflammation in deep vein thrombosis: a therapeutic target? Hematology. 2019;24:742–750. doi: 10.1080/16078454.2019.1687144. [DOI] [PubMed] [Google Scholar]
- 33.Kelly M., Hwang J.M., Kubes P. Modulating leukocyte recruitment in inflammation. J. Allergy Clin. Immunol. 2007;120:3–10. doi: 10.1016/j.jaci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 34.de Stoppelaar S.F., Van’t Veer C., Roelofs J.J., et al. Platelet and endothelial cell P-selectin are required for host defense against Klebsiella pneumoniae-induced pneumosepsis. J. Thromb. Haemost. 2015;13:1128–1138. doi: 10.1111/jth.12893. [DOI] [PubMed] [Google Scholar]
- 35.Comer S.P., Cullivan S., Szklanna P.B., et al. COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001109. [DOI] [PMC free article] [PubMed] [Google Scholar]