Abstract
Many patients switch from efavirenz- to dolutegravir-based regimens. In a phase 1 dolutegravir-efavirenz interaction study, mean dolutegravir minimum concentration decreased by 60% and 85% among CYP2B6 normal and slow/intermediate metabolizers, respectively. Mean efavirenz half-life was 2.7 times greater in slow vs normal metabolizers. Slow metabolizers will experience more prolonged subtherapeutic dolutegravir concentrations.
Keywords: dolutegravir, efavirenz, pharmacogenetics
(See the Editorial Commentary by Morse on pages 1823–5.)
A once-daily tablet containing the nonnucleoside reverse transcriptase inhibitor (NNRTI) efavirenz plus 2 nucleoside reverse transcriptase inhibitors (NRTIs) has long been first-line therapy for adults living with human immunodeficiency virus type 1 (HIV-1) in low- and middle-income countries (LMICs). Second-line regimens following first-line treatment failures have typically involved a ritonavir-boosted HIV-1 protease inhibitor plus 2 NRTIs. With discounted pricing for LMICs, many countries are now transitioning to a once-daily tablet that contains dolutegravir plus 2 NRTIs not only as first-line therapy, but also as second-line therapy when prior regimens fail [1, 2]. Millions of HIV-positive individuals worldwide are now switching from efavirenz- to dolutegravir-based regimens.
In a clinical trial of virally suppressed patients who switched from non-dolutegravir regimens to dolutegravir plus 2 NRTIs, virologic suppression was well maintained [3]. That study included 172 individuals who switched from NNRTI-containing regimens. Since efavirenz has a low genetic barrier to resistance, HIV-1 in these individuals likely contained few if any NNRTI resistance mutations. Unfortunately, some patients who switch from efavirenz plus 2 NRTIs in those countries may be viremic with efavirenz resistance mutations (eg, K103N), especially given limited access to viral load and resistance testing in some countries.
Efavirenz decreases plasma dolutegravir exposure by inducing hepatic drug metabolizing enzymes. In study ING114005, in which 12 HIV-negative adults who received once-daily dolutegravir for 5 days, followed by efavirenz plus dolutegravir for 14 days, efavirenz reduced geometric mean plasma dolutegravir trough concentrations by 75% [4]. With efavirenz, the dolutegravir trough concentration was 0.22 μg/mL ± 0.17 microg/mL (geometric mean ± standard deviation), below the dolutegravir clinical target concentration of 0.3 μg/mL [5, 6]. Based on that study, 50 mg twice-daily dosing of dolutegravir is recommended if coadministered with efavirenz [7].
Efavirenz is metabolized primarily by cytochrome P450 2B6. Frequent CYP2B6 polymorphisms, especially CYP2B6 516G→T (rs3745274, *6 allele) and 983T→C (rs28399499, *9 allele), define slow metabolizer genotypes that predict increased plasma efavirenz exposure [8, 9]. Polymorphisms in CYP2B6 in combination explain approximately 35% of interindividual variability in plasma efavirenz exposure [8]. Slow metabolizer genotypes are present in approximately 30% of Asians, 25% of Africans, and 5% of Europeans.
For other medications similarly affected by efavirenz (eg, bedaquiline), reductions in plasma drug exposure are greatest among CYP2B6 slow metabolizers, presumably because higher efavirenz exposures lead to greater hepatic enzyme induction. A previous simulation modeling analysis by GlaxoSmithKline, which addressed concern that dolutegravir might require dose adjustment during the period following switch from efavirenz to dolutegravir (until CYP3A4 and UGT1A1 expression returned to baseline), concluded that no adjustment was necessary [5]. The authors concluded that at no time during the switch period would both dolutegravir and efavirenz concentrations simultaneously be below their respective clinical target concentration, regardless of CYP2B6 genotype.
Dolutegravir has a high genetic barrier to viral resistance. However, in studies of dolutegravir monotherapy in patients with prior excellent control of viremia, dolutegravir-resistant HIV-1 emerged in some individuals after 24 weeks [10, 11], demonstrating the importance of active concomitant antiretrovirals to protect against dolutegravir resistance. This suggests that, among viremic patients with efavirenz- and NRTI-resistant resistant HIV-1 who switch directly from efavirenz to dolutegravir plus 2 NRTIs, dolutegravir resistance may emerge in some individuals.
The present analyses used data from ING114005 to characterize the effect of CYP2B6 genotype on the pharmacokinetic (PK) interaction between efavirenz and dolutegravir. We consider implications for switching from efavirenz- to dolutegravir-containing regimens.
METHODS
Participants
Analyses were based on data from 12 HIV-negative adults who participated in ING114005. All received dolutegravir 50 mg every 24 hours for 5 days (period 1), followed by efavirenz 600 mg plus dolutegravir 50 mg every 24 hours for 14 days (period 2). De-identified data for PK parameters (maximum concentration [Cmax], minimum concentration [Cmin], clearance, area-under-the-curve to the end of the dosing interval [AUC0-tau], and concentration at the end of the dosing interval [Ctau], and half-life) for dolutegravir and efavirenz, race/ethnicity, and CYP2B6 genotype for rs3745274 (*1/*1, *1/*6 or *6/*6) were obtained through ClinicalStudyDataRequest.com. Analyses were approved by the Vanderbilt University Institutional Review Board.
Statistical Analyses
Associations were assessed by Wilcoxon rank-sum test, linear regression, or Spearman correlation as appropriate. Two-tailed P values < .05 were considered statistically significant.
RESULTS
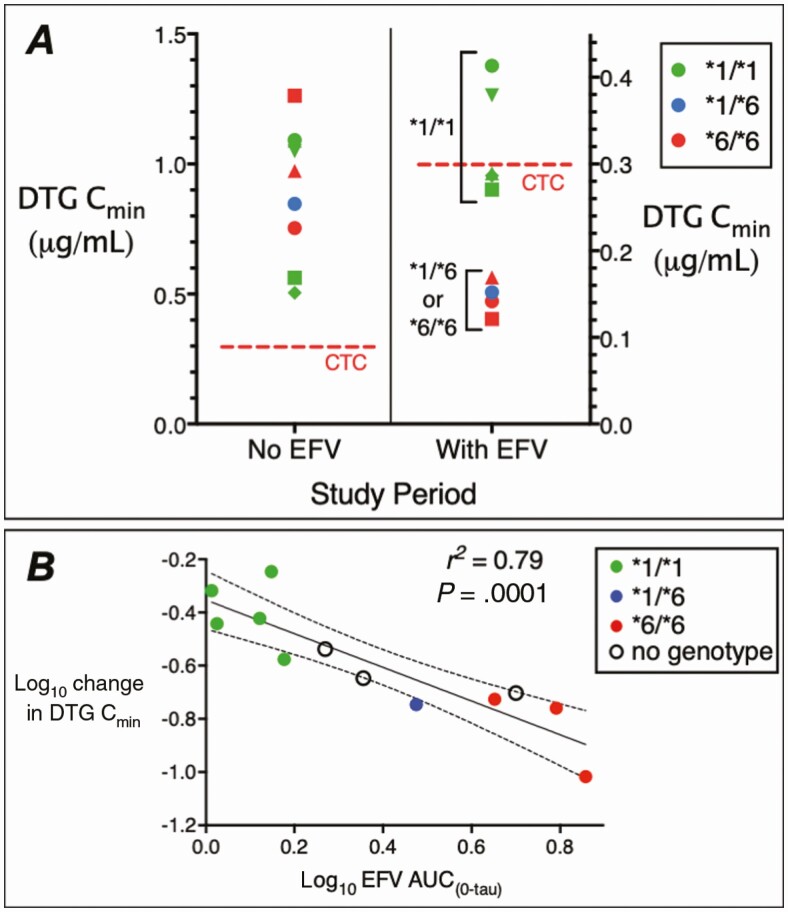
Of the 12 participants, 9 were evaluable for CYP2B6 genotype, including 8 self-identified as white, with 5 CYP2B6 normal (*1/*1), 1 intermediate (*1/*6), and 3 slow (*6/*6) metabolizers. During period 2, and comparing 4 slow/intermediate to 5 normal metabolizers, CYP2B6 genotype was significantly associated with every dolutegravir PK parameter except Cmax, both the absolute values (eg, period 2 CminP = .014) and change from period 1 to period 2 (eg, change in CminP = .014). Among CYP2B6 normal metabolizers, geometric mean dolutegravir Cmin decreased by 60%, from 812 ng/mL (95% confidence interval [CI], 502–1315 ng/mL) to 323 ng/mL (95% CI, 255–409 ng/mL). Among CYP2B6 slow and intermediate metabolizers, geometric mean dolutegravir Cmin decreased by 85%, from 941 ng/mL (95% CI, 661–1340 ng/mL) to 145 ng/mL (95% CI, 116–181 ng/mL) (Figure 1A). During period 1, CYP2B6 genotype was not associated with any dolutegravir PK parameter.
Figure 1.
Pharmacogenetics of the interaction of dolutegravir (DTG) with efavirenz (EFV). A, Plasma DTG minimum concentration (Cmin) values among 9 volunteers after 5 days of DTG 50 mg every 24 hours without EFV (left), and after 14 days of DTG 50 mg every 24 hours plus EFV 600 mg every 24 hours (right). The red dashed lines indicate the DTG clinical target concentration (CTC) of 0.3 μg/mL. Different marker shapes distinguish participants. Note the different y-axis scales. B, Relationship between log10 change in plasma DTG Cmin values and log10 plasma EFV area under the concentration-time curve (AUC0–tau) values. Each marker represents log10 change in plasma DTG Cmin values from period 1 to period 2 in each participant. The black line represents linear regression and the dashed lines represent 95% confidence intervals. Colored markers indicate CYP2B6 metabolizer genotypes as normal (*1/*1) in green, intermediate (*1/*6) in blue, slow (*6/*6) in red, and individuals who did not consent for genetic testing as open circles.
As expected, CYP2B6 genotype correlated with each efavirenz PK parameter (eg, CminP = .001, r2 = 0.89). Geometric mean efavirenz half-life was 2.7 times greater in CYP2B6 slow metabolizers (39.1 hours [95% CI, 17.1–89.5]) than in normal metabolizers (14.3 hours [95% CI, 12.6–16.1 hours]), and was 24.6 hours in the intermediate metabolizer. Efavirenz PK parameters correlated with period 2 dolutegravir PK parameters (eg, greater efavirenz AUC0-tau with greater period 1 to period 2 decrease in dolutegravir Cmin; P = .0008, r2 = 0.83) (Figure 1B). Among 3 participants without genetic data, dolutegravir Cmin from period 1 to period 2 decreased from 393 to 84 ng/mL, 426 to 89 ng/mL, and 2093 to 607 ng/mL. These individuals did not have particularly high efavirenz AUC0-tau values (Figure 1B).
Discussion
An important finding of these analyses is the strong association of the CYP2B6 slow metabolizer genotype (and resultant increased efavirenz exposure) with plasma dolutegravir concentrations that are below the dolutegravir clinical target concentration, when efavirenz and dolutegravir are coadministered. Dolutegravir has major advantages over efavirenz, including a higher genetic barrier to viral resistance, fewer drug-drug interactions, fewer central nervous system side effects, and lower manufacturing cost of generic production [2]. For individuals who switch directly from efavirenz- to dolutegravir-containing regimens when plasma viremia is well controlled, our findings are of little clinical concern, because at no time should both dolutegravir and efavirenz concentrations simultaneously fall below their respective clinical target concentrations [3, 5]. However, there may be cause for concern if direct switch occurs during uncontrolled viremia with efavirenz resistance, in which case efavirenz will not protect dolutegravir during its period of subtherapeutic exposure. This may be most relevant to black Africans and Southeast Asians, of whom approximately 1 in 4 are CYP2B6 slow metabolizers, as compared to approximately 1 in 20 white Europeans. Concomitant NRTIs will hopefully protect against dolutegravir resistance, assuming there is not also high-level NRTI resistance.
Our analysis had limitations. The sample size was small, with only 12 total participants and 9 evaluable for genetics. A larger sample size could better define how far efavirenz drives dolutegravir concentrations below its clinical target concentration, particularly among CYP2B6 slow metabolizers, and confirm this finding in other populations. Two participants lacking genotype data had the lowest dolutegravir Cmin values (84 and 89 ng/mL). A larger sample size could also better define the impact of efavirenz concentrations higher than those in this analysis, and with associated longer efavirenz half-lives (eg, among CYP2B6 slow metabolizers who carry 983T→C *9 alleles [8]). Pharmacokinetic data were not empirically collected after HIV-positive patients on chronic efavirenz switched to dolutegravir-containing regimens, who would ideally be studied longitudinally after switching.
Our findings reinforce that the safest strategy would be to switch directly from efavirenz- to dolutegravir-containing regimens when HIV-1 replication is well controlled [2]. If HIV-1 replication is not controlled and efavirenz-resistant HIV-1 is present, at least 2 options warrant consideration. One is to still switch directly to dolutegravir-containing regimens, consistent with current World Health Organization guidelines [1], realizing that this may select for dolutegravir-resistant virus but that risk may be low. Support for this approach comes from the Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING) study, in which patients failing efavirenz (or nevirapine) plus 2 NRTIs were randomized to switched to dolutegravir (or lopinavir/ritonavir) plus 2 NRTIs, and included research sites in Africa and Asia [12]. Among 312 participants randomized to dolutegravir, 78% switched from efavirenz. In a subgroup analysis, response rates were similar for participants receiving 2 fully active NRTIs as compared to dolutegravir with <2 fully active NRTIs. Among 11 participants who met virological withdrawal criteria, only 2 had treatment-emergent phenotypic resistance to dolutegravir.
An alternative approach would be to prescribe an extra 50-mg daily dose of dolutegravir for some time (perhaps 2 weeks) after the switch, taken approximately 12 hours after the dolutegravir fixed-dose combination tablet. However, this approach is likely impractical in most resource-limited settings. If CYP2B6 genotype data are available, one could target such interventions for slow metabolizers. Regardless, large surveillance datasets should monitor whether risk of dolutegravir-resistant HIV-1 is increased following switch from efavirenz- to dolutegravir-containing regimens among viremic individuals, especially among CYP2B6 slow metabolizers, and ideally informed by PK data.
Notes
Acknowledgments. The authors thank the 12 individuals who participated in ING114005; to ViiV for making study data available for analysis; and to colleagues at ViiV for their thoughtful suggestions on the manuscript.
Financial support. This work was supported by the National Institutes of Health (NIH) (grant numbers AI077505, TR000445, and AI069439 to D. W. H.) and by the Tennessee Center for AIDS Research (grant number P30 AI110527). E. P. A. received grant funding through the NIH International Maternal, Pediatric, Adolescent AIDS Clinical Trials (IMPAACT) network, which is supported in part by ViiV.
Potential conflicts of interest. The authors: No reported conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. Update of recommendations on first- and second-line antiretroviral regimens—policy brief. Available at: https://www.who.int/hiv/pub/arv/arv-update-2019-policy/en/. Accessed 16 March 2020.
- 2. Vitoria M, Hill A, Ford N, et al. . The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: what are the issues? AIDS 2018; 32:1551–61. [DOI] [PubMed] [Google Scholar]
- 3. Trottier B, Lake JE, Logue K, et al. . Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48-week, randomized, non-inferiority, open-label, phase IIIb study. Antivir Ther 2017; 22:295–305. [DOI] [PubMed] [Google Scholar]
- 4. Song I, Borland J, Chen S, et al. . Effects of enzyme inducers efavirenz and tipranavir/ritonavir on the pharmacokinetics of the HIV integrase inhibitor dolutegravir. Eur J Clin Pharmacol 2014; 70:1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Generaux G, Song I, Bowers B, Piscitelli S. A mechanistic SimCYP simulation evaluating dolutegravir and efavirenz pharmacokinetics following a switch from once-daily efavirenz to once-daily dolutegravir. In: 15th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy, Washington, DC, 19–21 May 2014. [Google Scholar]
- 6. van Lunzen J, Maggiolo F, Arribas JR, et al. . Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis 2012; 12:111–8. [DOI] [PubMed] [Google Scholar]
- 7. Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents.2018. Available at: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 20 June 2018.
- 8. Holzinger ER, Grady B, Ritchie MD, et al. . Genome-wide association study of plasma efavirenz pharmacokinetics in AIDS Clinical Trials Group protocols implicates several CYP2B6 variants. Pharmacogenet Genomics 2012; 22:858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desta Z, Gammal RS, Gong L, et al. . Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2B6 and efavirenz-containing antiretroviral therapy. Clin Pharmacol Ther 2019; 106:726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wijting I, Rokx C, Boucher C, et al. . Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non-inferiority trial. Lancet HIV 2017; 4:e547–54. [DOI] [PubMed] [Google Scholar]
- 11. Blanco JL, Rojas J, Paredes R, et al. . DOLAM Study Team . Dolutegravir-based maintenance monotherapy versus dual therapy with lamivudine: a planned 24 week analysis of the DOLAM randomized clinical trial. J Antimicrob Chemother 2018; 73:1965–71. [DOI] [PubMed] [Google Scholar]
- 12. Aboud M, Kaplan R, Lombaard J, et al. . Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis 2019; 19:253–64. [DOI] [PubMed] [Google Scholar]